Research and Application of Green Technology Based on Microbially Induced Carbonate Precipitation (MICP) in Mining: A Review
Abstract
1. Introduction
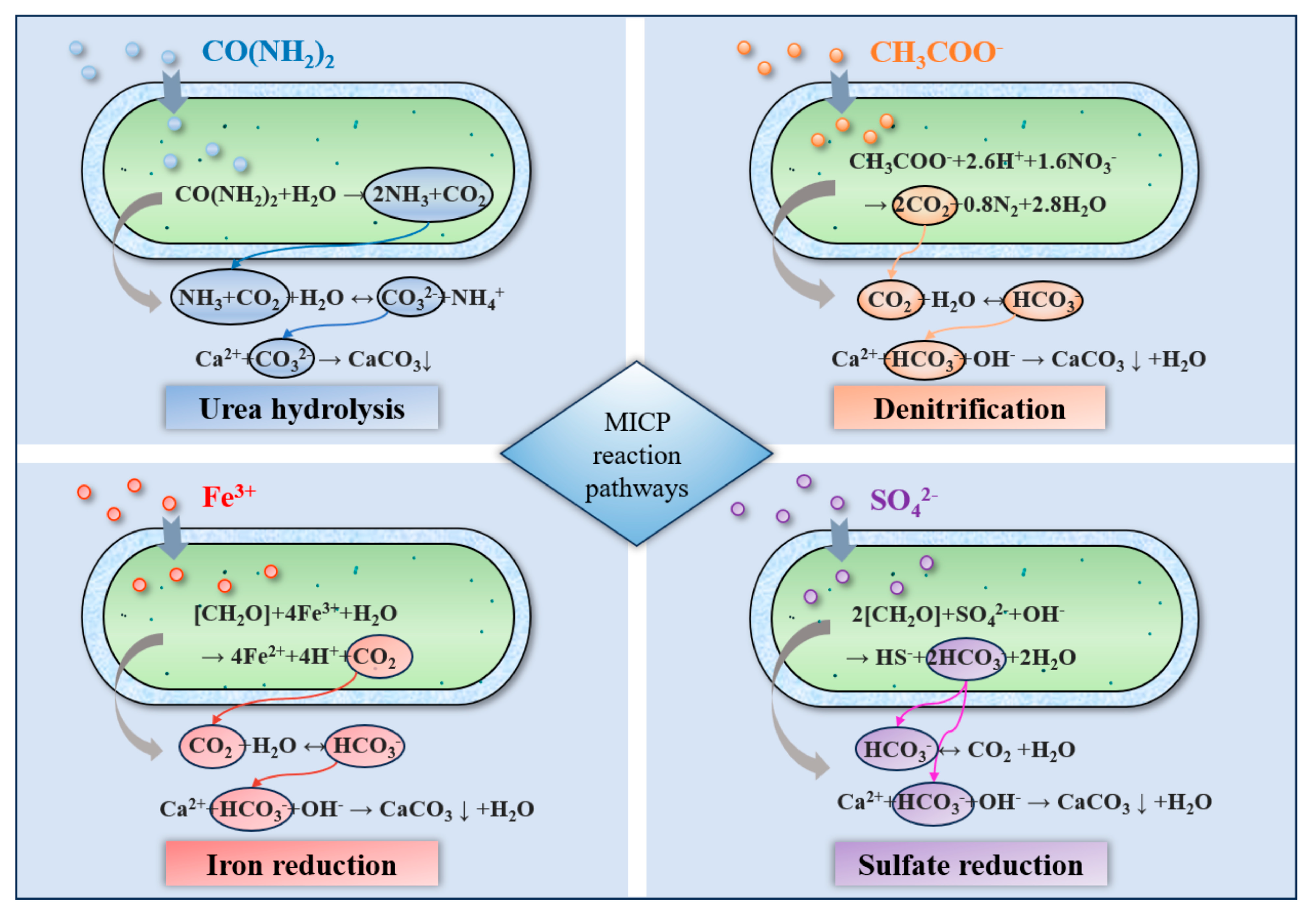
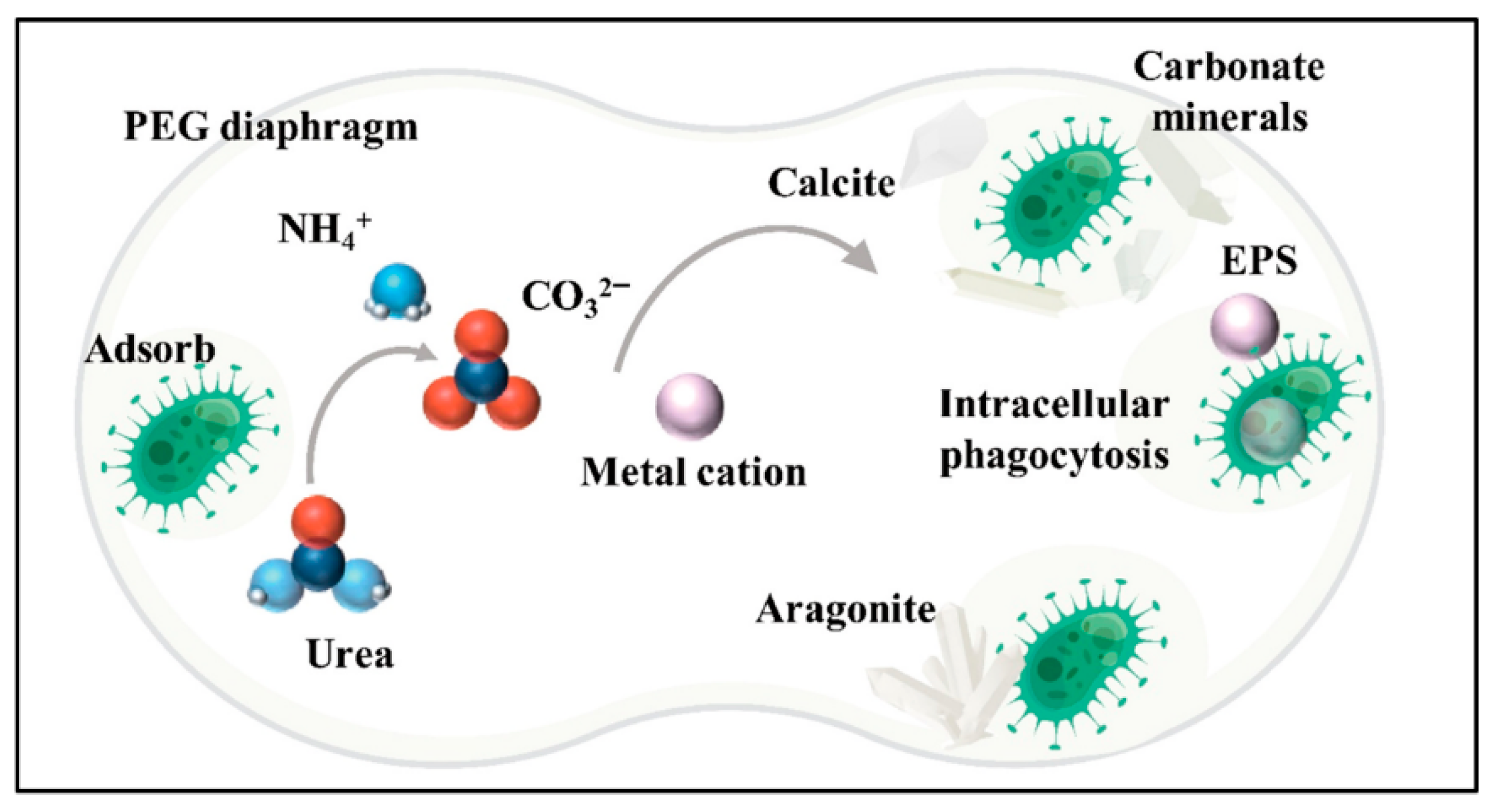
2. Solidification Mechanism
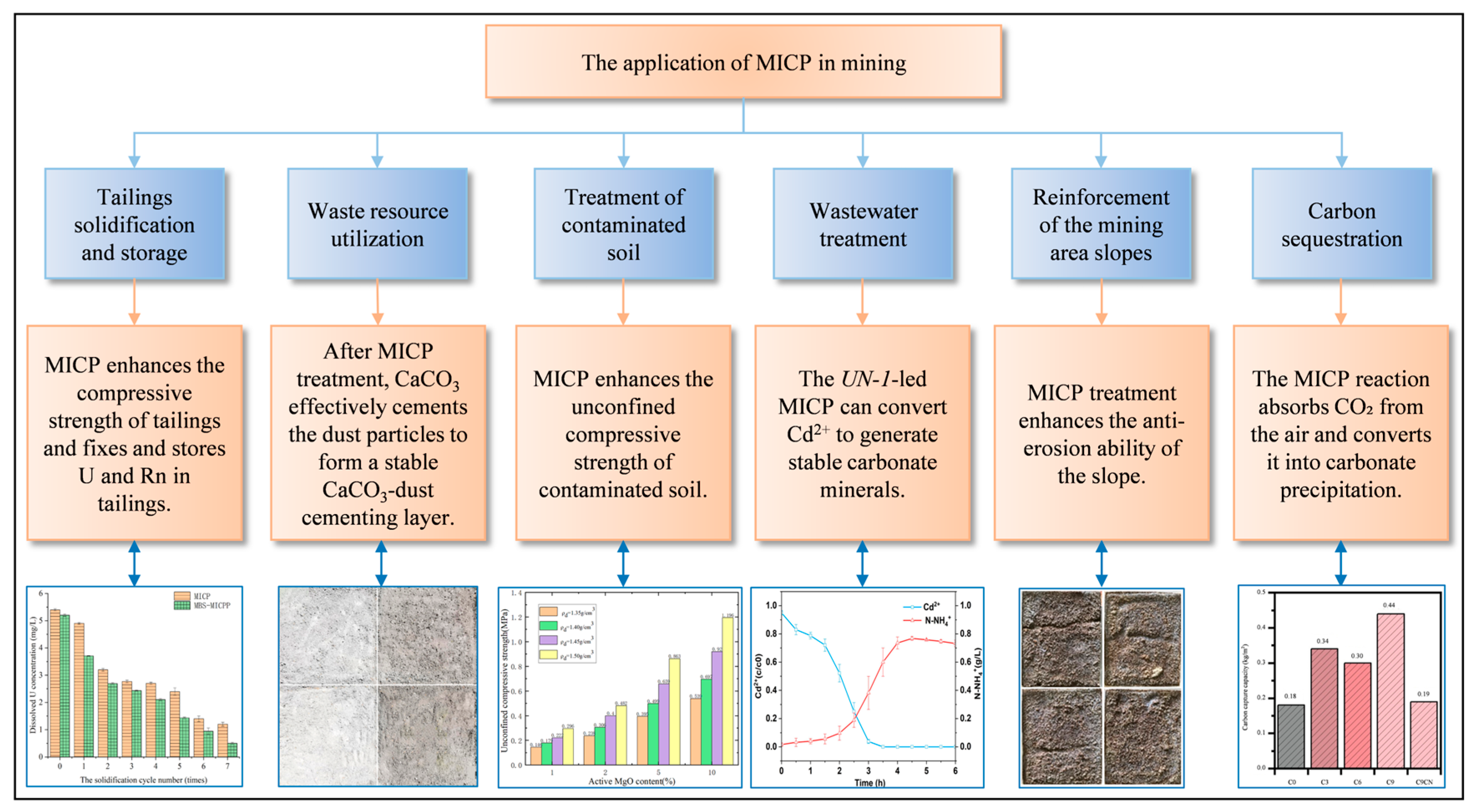
3. Laboratory Research
3.1. Tailings Solidification and Storage
3.2. Heavy Metal Immobilization

3.3. Waste Resource Utilization
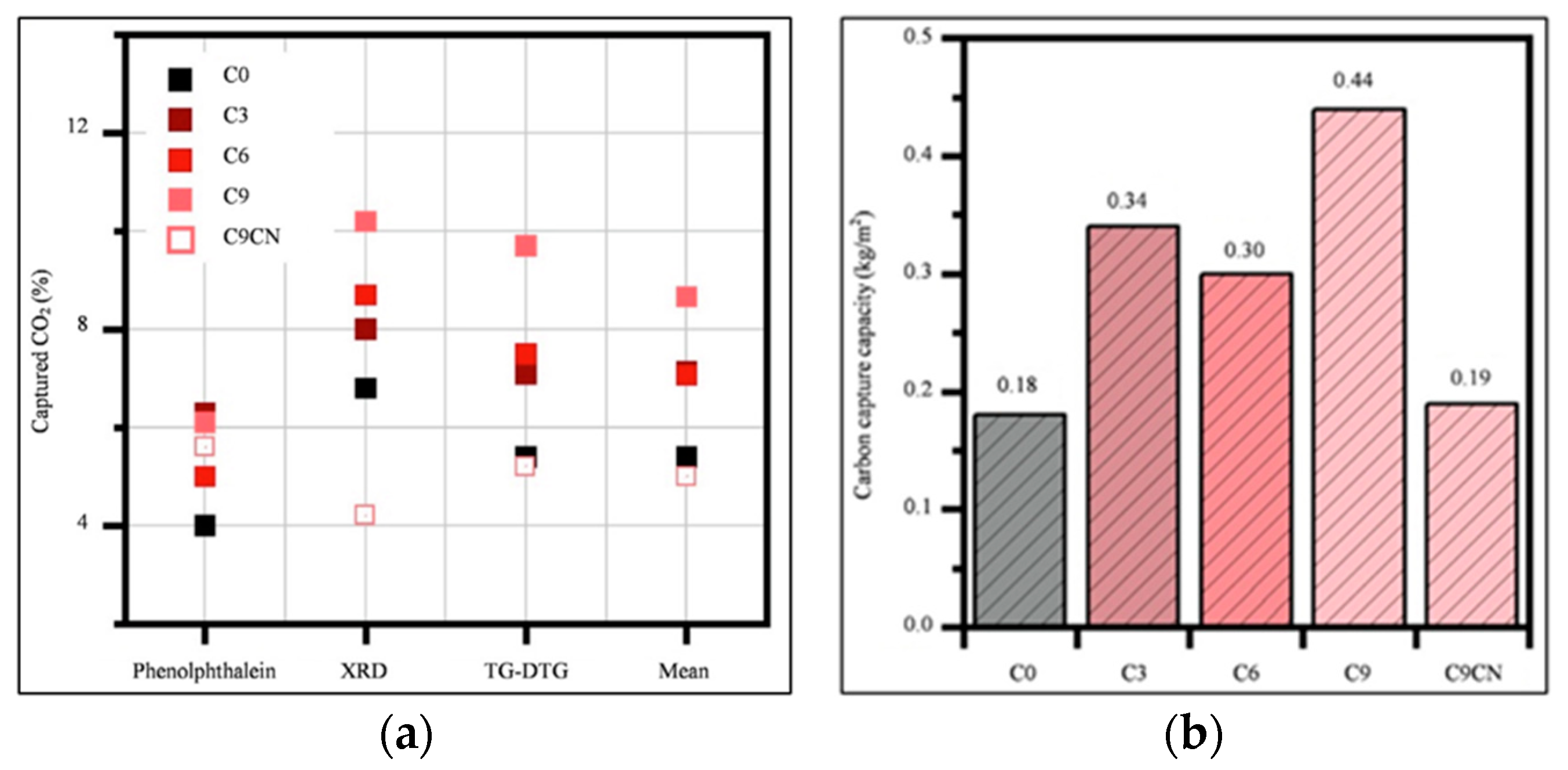
3.4. Carbon Sequestration
4. Field Applications Research
5. Influencing Factors
5.1. Solid Waste Characteristics
5.2. Bacterial Solution
5.3. Cementation Solution
| Bacteria | Cementation Solution | Test Sample | Solidification Effect |
|---|---|---|---|
| Sporosarcina pasteurrii [95] | Urea + CaCl2 | Calcareous sand | UCS peak strength 2.3 MPa |
| Urea + Soluble calcium | UCS peak strength 2.46 MPa | ||
| Sporosarcina pasteurii [100] | Urea + Ca(CH3COO)2 | Strongly weathered phyllite | UCS peak strength 6.78 MPa |
| Urea + Mg(CH3COO)2 | UCS peak strength 6.13 MPa | ||
| Urea + CaCl2 | UCS peak strength 7.45 MPa | ||
| Sporosarcina pasteurii [101] | Urea + CaCl2 | Industrial sand of 200–380 μm | UCS = 27.9 MPa |
| Urea + Ca(NO3)2 4(H2O) | UCS = 27.6 MPa | ||
| Urea + Ca(CH3COO)2 H2O | UCS = 26 MPa | ||
| Sporosarcina pas-teurii [91] | Urea + CaCl2 | Granite residual soil | When the phase pressure is 100 kPa, the shear strength is 80.32 kPa. |
| Urea + Ca(CH3COO)2 | When the phase pressure is 100 kPa, the shear strength is 102.71 kPa. | ||
| Deionized water | When the phase pressure is 100 kPa, the shear strength is 111.72 kPa. | ||
| Sporosarcina pas-teurii [92] | Urea + Raw clam shells | Sand column | The apparent porosity is 9.13%. |
| Urea + Ca(NO3)2 | The apparent porosity is 9.53%. | ||
| Urea + CaCl2 | The apparent porosity is 13.42%. |
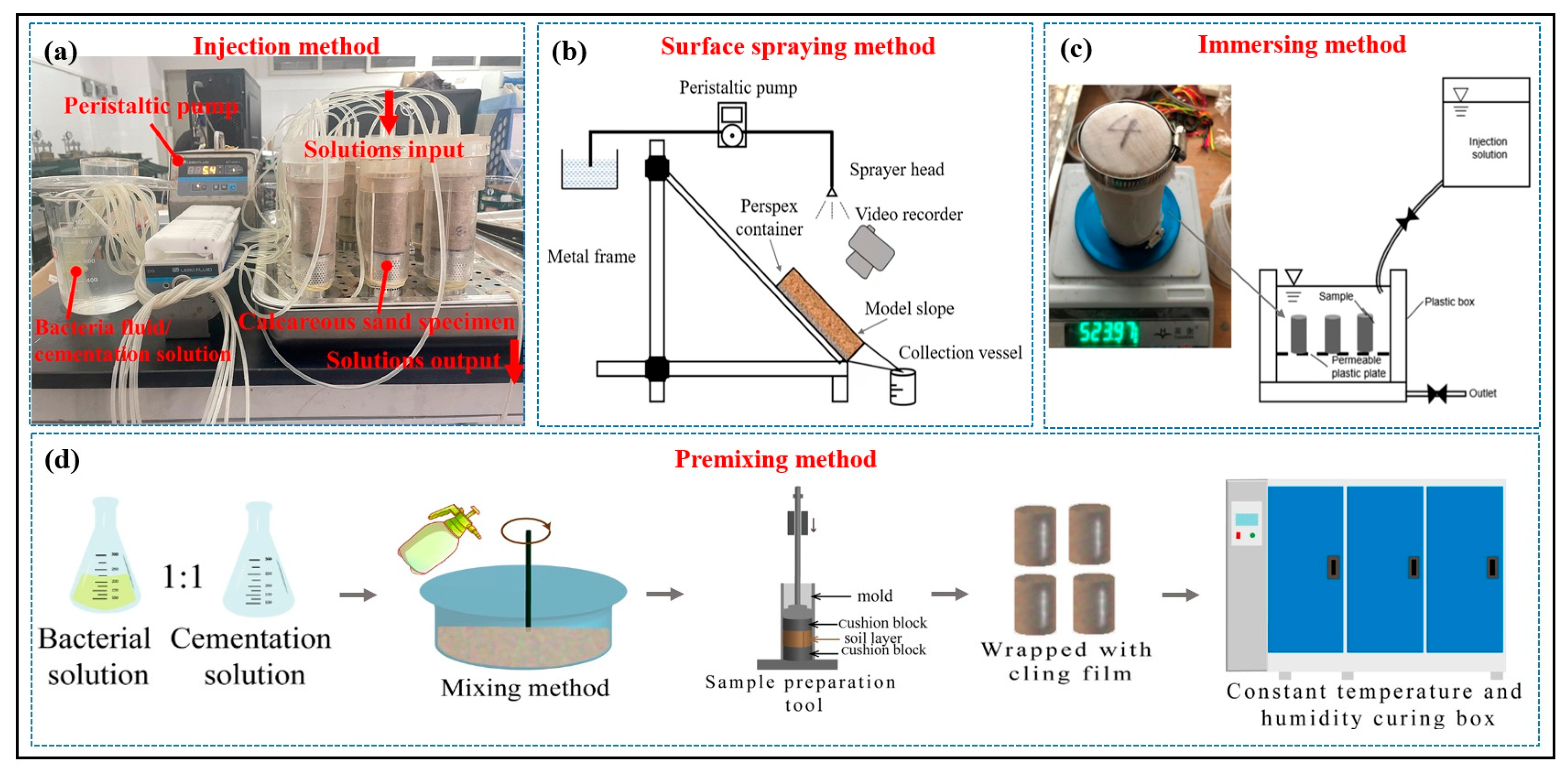
5.4. Grouting Method
5.4.1. Injection Method
5.4.2. Surface Spraying Method
5.4.3. Immersion Method
5.4.4. Premixing Method
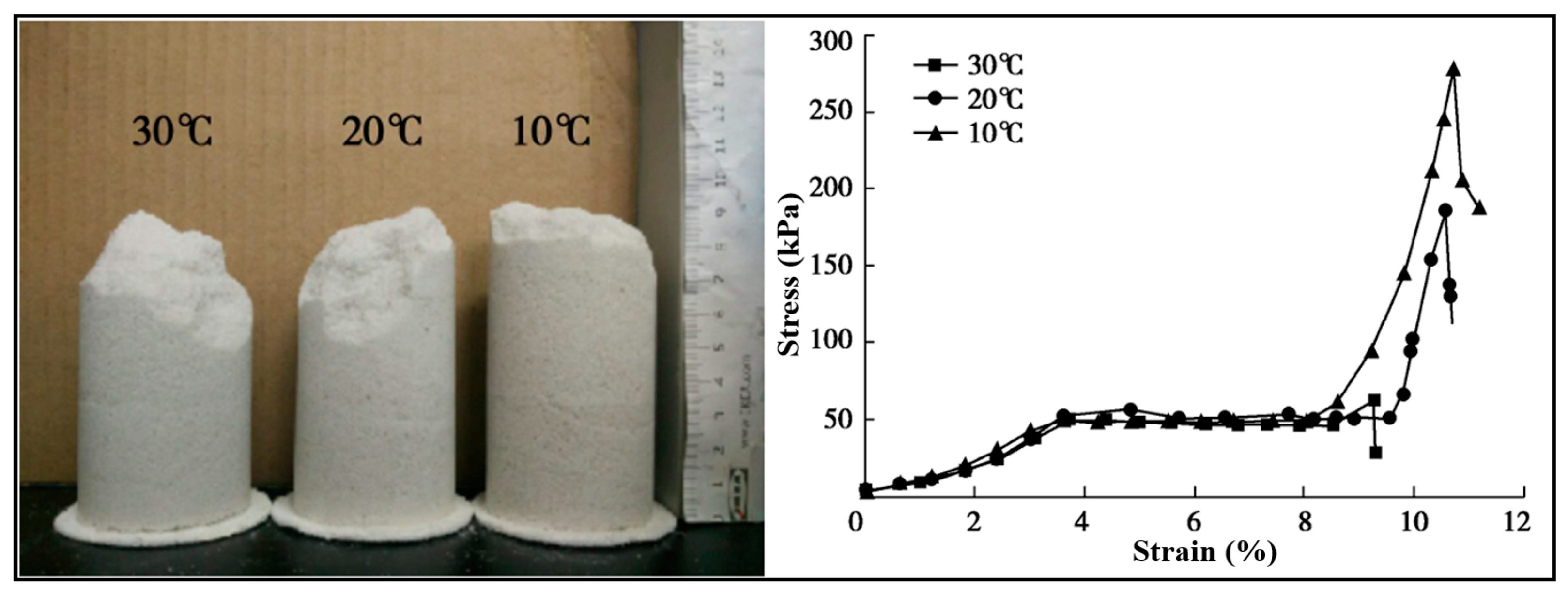
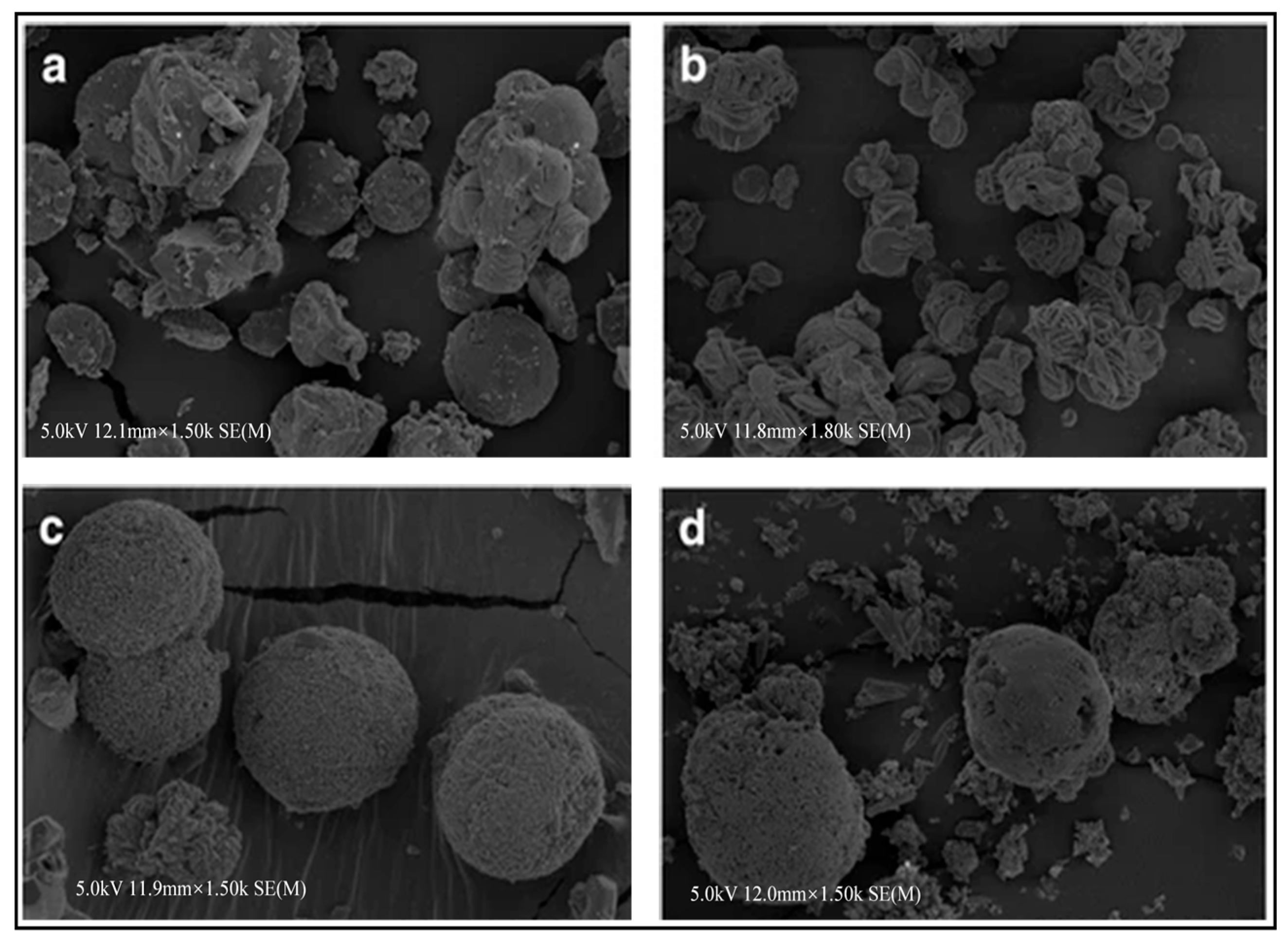
5.5. Temperature
5.6. pH
6. Improvement Methods
6.1. Self-Healing Technology
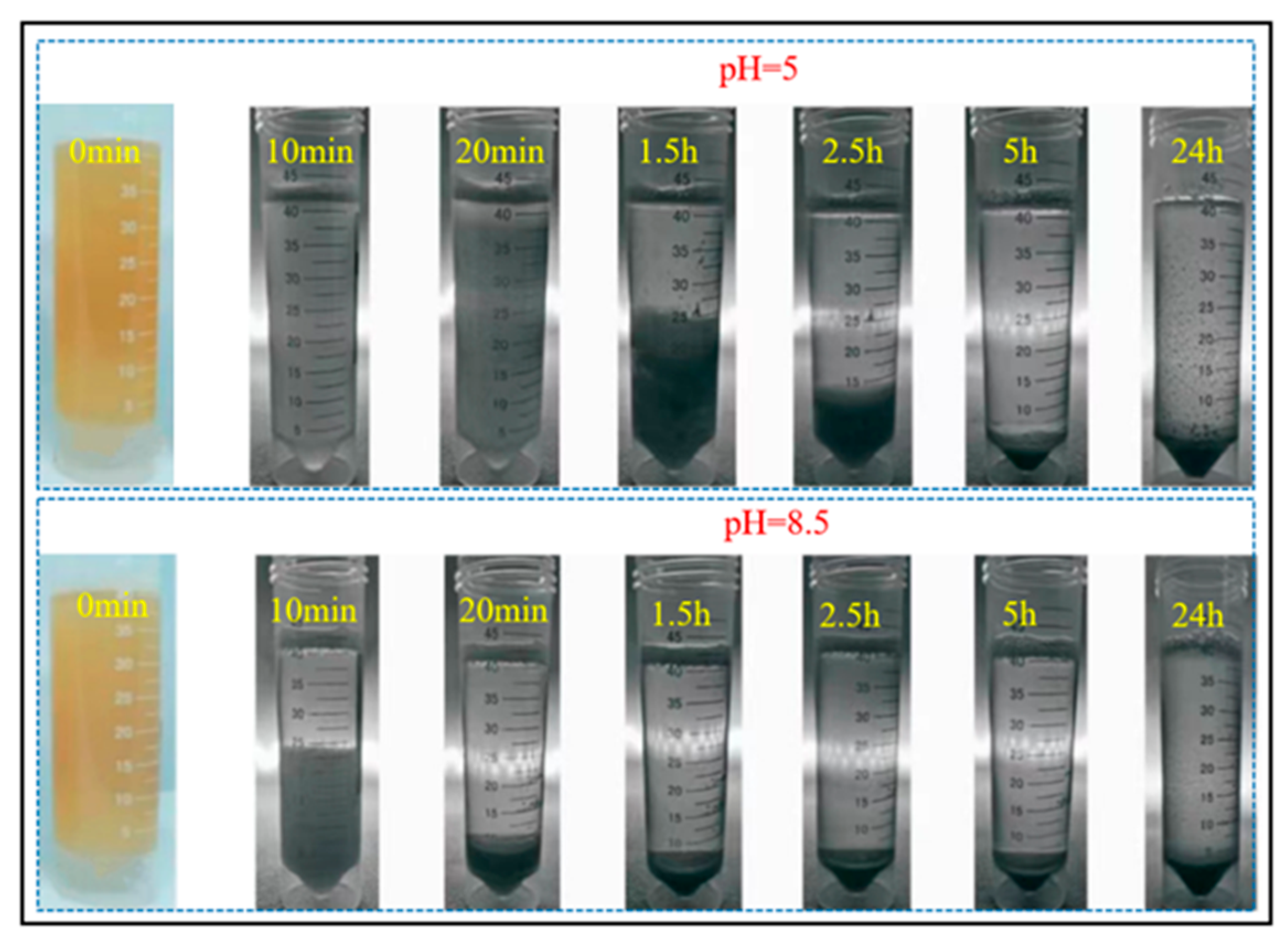
6.2. One-Phase Low-pH Injection Method
6.3. Auxiliary Additives
7. Discussion and Suggestions for Future Research
- (1)
- MICP technology based on multiple reaction pathways, such as urea hydrolysis, denitrification, iron reduction, and sulfate reduction, can develop adaptable process protocols tailored to geological environment conditions. These pathways enable targeted mineralization control in complex engineering scenarios by selecting appropriate reaction pathways, directionally acclimating functional microbial communities, and regulating the reaction environment. To address the attenuation of microbial activity in extreme environments, self-healing technology use microcapsules to encapsulate bacteria and spores, thereby extending the survival period of microorganisms. Facing the complex environmental governance demands of mines, the innovative development of MICP will focus on three cutting-edge directions. The first direction is (1) extreme environment solid waste solidification, where the development of acid-resistant, high-temperature-resistant, and anaerobic engineered strains [149], and directional acclimation of mutant strains that can utilize specific heavy metal ions (Cu2+, Zn2+) as cofactors, are essential to achieve in situ storage of mine tailings, wastewater, and geothermal reservoir pollutants. The second is (2) the use of solidified waste materials as sustainable materials to construct a microbial mineralization system, with red mud, fly ash, and concrete waste powder, among others, serving as raw materials. Finally, the third direction is the (3) establishment of long-term safety and stability prediction models for mineralized products and the development of urea-free mineralization pathways under special conditions [150], thereby promoting the advancement of MICP technology toward precision and low-carbon goals.
- (2)
- Auxiliary additives significantly enhance the solidification performance of pollutants. For instance, the skeletal structure of activated carbon not only optimizes the seepage pathways through its pore-filling effect but also provides additional microbial adsorption sites, thereby promoting the uniform distribution of CaCO3 within the waste matrix. However, the geometric characteristics, dosages, and surface of additives such as activated carbon and reactive magnesium oxide must be carefully matched with the pore structure of the target waste to avoid issues such as stress concentration and impeded microbial migration. The current technical bottleneck lies in the efficient remediation of fine-grained tailings. Breakthrough directions focus on the development of nanoscale auxiliary additives, such as functionalized nanofibers and mesoporous silica particles [151], which regulate the distribution gradient of the solution in the fine-grained pore network and induce directional mineralization [152]. These materials will utilize the multi-scale synergistic effects of microorganisms, CaCO3, and auxiliary additives in fine-grained tailings, providing new ideas for the stabilization of high-plasticity clay. Additionally, there is a lack of experimental research on MICP technology in the field of underground deep pollution barriers. Future research should prioritize the development of slow-release cementation solution formulations to extend the effective temporal window for mineralization reactions, thereby providing robust technical support for the long-term in situ immobilization of deep waste materials.
- (3)
- To address the issue of uneven CaCO3 distribution caused by the low permeability characteristics of fine-grained tailings and clay slopes, researchers have proposed a one-phase low-pH injection method. Such a technique inhibits premature CaCO3 flocculation and deposition by controlling the pH conditions, extends the diffusion range of the solution, and generates CaCO3 after the solution has seeped uniformly, thereby shifting the mineralization reaction zone from surface deposition to deep-layer uniform distribution. In contrast, in the coarse-grained tailings system, the large-pore structures reduce bacterial adsorption, thereby diminishing the effectiveness of tailings solidification and heavy metal immobilization. The current solution optimizes the bacterial attachment interface by introducing auxiliary additives such as activated carbon and activated magnesium oxide, thereby reducing CaCO3 loss. Future research should concentrate on elucidating the multi-scale dynamic evolution mechanisms, combining CT scanning [153,154], numerical simulation [155], and microfluidic technology to reveal [156], from a microscopic perspective, microbial behavior and crystal growth laws in waste material pores. Such a breakthrough will lay a theoretical foundation for the precise regulation of biomineralization in complex environments.
- (4)
- To break through the bottleneck of mineralization reaction differences faced by MICP technology in engineering applications, it is necessary to establish a multi-angled collaborative monitoring system to analyze the distribution characteristics of CaCO3 in real time [157]. Based on the current research status, in situ real-time monitoring systems integrated with deep learning have the greatest potential for breakthrough in this field. Among these monitoring methods, approaches such as conductivity, biosensors, and sound waves have shown preliminary progress in monitoring the mineralization process. This system is expected to effectively address the challenge of achieving a balance between mineralization efficiency and environmental adaptability under complex environmental conditions. At the same time, technology transformation should advance in parallel with the standardization process, establishing a unified mineralization evaluation standard, including core performance indicators such as the safety assessment of bacterial strains, the uniformity of CaCO3 distribution, and the recycling standards of waste. This will promote the transition of MICP technology from laboratory research to standardized engineering practice, providing full-cycle technical support for waste solidification and resource utilization.
8. Conclusions
- (1)
- As a green biological reinforcement method, MICP technology has demonstrated unique environmental advantages and controllable cementation effects in the field of mining area remediation. Such a technique precisely regulates the generation of CaCO3 precipitates through microbial metabolic activities and chemical reactions such as urea hydrolysis, denitrification, and sulfate reduction, and achieves soil particle solidification through three methods: surface coating, pore filling, and particle bridging. These methods effectively enhance the strength and stability of tailings dams and slopes and prevent the migration of heavy metal ions. At present, MICP technology has made remarkable progress in laboratory research and field research.
- (2)
- Waste solidification and slope-reinforcement performance are jointly influenced by the intrinsic properties of soil and external environmental factors. Among these, solid waste characteristics, bacterial solution, cementation solution, grouting method, temperature, and pH are the key determinants of solidification effectiveness. The particle size distribution and pore structure of the soil directly affect microbial adsorption and migration pathways, thereby influencing the uniformity of CaCO3 distribution within geotechnical materials. The bacterial and cementation solution are essential components of the MICP reaction. The type of bacteria and calcium source jointly determine the morphology of CaCO3 crystals, while the activity of urease and the concentration of cementation solution jointly determine the mineralization reaction rate and the spatial distribution of CaCO3. The grouting method directly affects the spatial distribution of CaCO3 by regulating the seepage path of the solution, ultimately determining the macroscopic mechanical and permeation properties of the solidified body. Temperature and pH mainly regulate the solidification process by affecting bacterial activity, urease activity, and MICP reaction rate.
- (3)
- MICP performance in waste solidification has been significantly enhanced through three key improvement methods. The first involves self-healing technology, which utilizes microcapsules to encapsulate dormant bacteria, enabling targeted, crack-triggered microbial repair. The second is the one-phase low-pH injection method, which creates an acidic environment to delay CaCO3 precipitation and prevent surface blockage. Concurrently, auxiliary additives optimize waste material spatial structure, provide more microbial attachment sites, and significantly enhance the mechanical properties and stability of bio-solidified materials. Through the synergistic effect of biochemical regulation and material design, these improvement methods promote the development of MICP technology towards self-healing scenarios, deep solidification, and engineering applications in extreme environments.
- (4)
- Although MICP technology has achieved remarkable progress in the research of mine waste solidification and resource utilization, its engineering promotion still faces many challenges. Future research urgently needs to focus on developing urea-free mineralization pathways, screening low-environmental-sensitivity strains, researching and developing nanoscale auxiliary additives, constructing multi-scale dynamic models, establishing in situ real-time monitoring systems integrated with deep learning, and setting systematic mineralization evaluation standard system. These breakthroughs will provide a biological solution that combines engineering reliability, environmental compatibility, and economic feasibility for global waste management.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MICP | microbially induced carbonate precipitation |
| CO2 | carbon dioxide |
| CaCO3 | calcium carbonate |
| UCS | uniaxial compressive strength |
| SEM | scanning electron microscope |
| XRD | X-ray diffraction |
| MIC | minimum inhibitory concentration |
References
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Yang, L.; Jia, H.; Wu, A.; Jiao, H.; Chen, X.; Kou, Y.; Dong, M. Particle aggregation and breakage kinetics in cemented paste backfill. Int. J. Min. Met. Mater. 2024, 31, 1965–1974. [Google Scholar] [CrossRef]
- Qiao, L.; Liu, X.; Zhang, S.; Zhang, L.; Li, X.; Hu, X.; Zhao, Q.; Wang, Q.; Yu, C. Distribution of the microbial community and antibiotic resistance genes in farmland surrounding gold tailings: A metagenomics approach. Sci. Total Environ. 2021, 779, 146502. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, M.; Li, B.; Dong, Y. Mechanisms, application advances and future perspectives of microbial-induced heavy metal precipitation: A review. Int. Biodeter. Biodegr. 2023, 178, 105544. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Kumar, A.; Song, H.; Mishra, S.; Zhang, W.; Zhang, Y.; Zhang, Q.; Yu, Z. Application of microbial-induced carbonate precipitation (MICP) techniques to remove heavy metal in the natural environment: A critical review. Chemosphere 2023, 318, 137894. [Google Scholar] [CrossRef]
- Lin, W.; Lin, W.; Cheng, X.; Chen, G.; Ersan, Y.C. Microbially induced desaturation and carbonate precipitation through denitrification: A review. Appl. Sci. 2021, 11, 7842. [Google Scholar] [CrossRef]
- Lu, T.; Wei, Z.; El Naggar, M.H.; Wang, W.; Yang, Y.; Tian, X.; Guo, H. Effect of chemical environment on copper tailings reinforced by microbially induced carbonate precipitation. Constr. Build. Mater. 2023, 400, 132894. [Google Scholar] [CrossRef]
- Taharia, M.; Dey, D.; Das, K.; Sukul, U.; Chen, J.; Banerjee, P.; Dey, G.; Sharma, R.K.; Lin, P.; Chen, C. Microbial induced carbonate precipitation for remediation of heavy metals, ions and radioactive elements: A comprehensive exploration of prospective applications in water and soil treatment. Ecotoxicol. Environ. Saf. 2024, 271, 115990. [Google Scholar] [CrossRef]
- Zúñiga-Barra, H.; Toledo-Alarcón, J.; Torres-Aravena, Á.; Jorquera, L.; Rivas, M.; Gutiérrez, L.; Jeison, D. Improving the sustainable management of mining tailings through microbially induced calcite precipitation: A review. Miner. Eng. 2022, 189, 107855. [Google Scholar] [CrossRef]
- Li, X.; Huang, F.; Sun, Q.; Ling, H.; Liu, J.; An, Y.; Liu, L. Analysis of limestone mine dust curing based on microbially induced calcium carbonate precipitation and its mechanism. J. Environ. Chem. Eng. 2024, 12, 114041. [Google Scholar] [CrossRef]
- Zhou, G.; Lv, Y.; Li, L.; Li, S.; Zhang, X.; Liu, Y. Analysis of coal dust consolidation performance and mechanism based on in-situ screening of high urease-producing bacteria. J. Environ. Chem. Eng. 2024, 12, 112030. [Google Scholar] [CrossRef]
- Sun, X.; Xiang, J.; Xiong, B.; Kong, X.; Qiu, J. Combined biological and cement solidification of lead-zinc tailings for backfill preparation and its environmental effects. Constr. Build. Mater. 2024, 420, 135601. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Guan, X. Multitechnique investigation of concrete with coal gangue. Constr. Build. Mater. 2021, 301, 124114. [Google Scholar] [CrossRef]
- Guan, X.; Liu, Y.; Zhang, X.; Hou, X.; Yang, H. Enhancement mechanism of microbial mineralization modified coal gangue aggregate properties. Constr. Build. Mater. 2024, 440, 137477. [Google Scholar] [CrossRef]
- Huang, W.; Chen, Z.; Liu, H.; Wang, H.; Wei, Z. Microbial induced carbonate precipitation for cadmium removal in flue gas from sludge incineration. J. Environ. Chem. Eng. 2024, 12, 112573. [Google Scholar] [CrossRef]
- Que, Y.; Zhang, H.; Zhu, T.; Leung, A.K.; Lu, D.; Jiang, Z. Amending foamed lightweight soil with tailings sand for embankment applications: Physical properties, durability, and microstructure. Constr. Build. Mater. 2022, 350, 128912. [Google Scholar] [CrossRef]
- Zhuang, D.; Chen, S.; Li, J.; Han, S.; Guo, Y. Recycled coarse aggregate from waste concrete strengthened by microbially induced calcium carbonate precipitation. Environ. Technol. Innov. 2025, 37, 103981. [Google Scholar] [CrossRef]
- Liu, Y.; Ali, A.; Su, J.F.; Li, K.; Hu, R.; Wang, Z. Microbial-induced calcium carbonate precipitation: Influencing factors, nucleation pathways, and application in waste water remediation. Sci. Total. Environ. 2023, 860, 160439. [Google Scholar] [CrossRef]
- Qin, J.; Cao, H.; Xu, Y.; He, F.; Zhang, F.; Wang, W. Efficient removal of Cr (iii) by microbially induced calcium carbonate precipitation. Rsc. Adv. 2025, 15, 2840–2849. [Google Scholar] [CrossRef]
- DeJong, J.T.; Kavazanjian, E. Bio-mediated and Bio-inspired Geotechnics. In Geotechnical Fundamentals for Addressing New World Challenges; Springer: Cham, Switzerland, 2019; pp. 193–207. [Google Scholar] [CrossRef]
- Behzadipour, H.; Sadrekarimi, S. Effects of microbially induced calcite precipitation on static liquefaction behavior of a gold tailings sand. Biogeotechnics 2024, 2, 100097. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wang, H.; Chen, R.; Wu, L. Bio-cementation for the mitigation of surface erosion in loess slopes based on simulation experiment. J. Soils Sediments 2022, 22, 1804–1818. [Google Scholar] [CrossRef]
- Duan, Y.; Yuan, Q.; Yu, C.; Zheng, C. A large-scale study on solidification of gold tailings based on microbially induced carbonate precipitation (MICP). Biogeotechnics 2025, 3, 100164. [Google Scholar] [CrossRef]
- Dagliya, M.; Satyam, N.; Garg, A. Optimization of growth medium for microbially induced calcium carbonate precipitation (MICP) treatment of desert sand. J. Arid. Land 2023, 15, 797–811. [Google Scholar] [CrossRef]
- Aytekin, B.; Mardani, A.; Yazıcı, Ş. State-of-art review of bacteria-based self-healing concrete: Biomineralization process, crack healing, and mechanical properties. Constr. Build. Mater. 2023, 378, 131198. [Google Scholar] [CrossRef]
- Wang, W.; Wang, T.; Sun, Z.; Bo, Y.; Zou, C.; Wang, Z.; Zheng, C. Solidification treatment of rare earth tailings by a renewable biological cementation method. Process Saf. Environ. Prot. 2023, 179, 585–592. [Google Scholar] [CrossRef]
- Song, H.; Wang, B.; Yu, Z.; Kumar, A.; Wei, S.; An, J. Multifunctional bacterium induced carbonate precipitation with low nitrogen effectively remediates cadmium polluted water. J. Water Process Eng. 2024, 67, 106204. [Google Scholar] [CrossRef]
- Niu, Q.; Li, C.; Liu, Z.; Li, Y.; Meng, S.; He, X.; Liu, X.; Wang, W.; He, M.; Yang, X.; et al. Solidification of uranium mill tailings by MBS-MICP and environmental implications. Nucl. Eng. Technol. 2022, 54, 3631–3640. [Google Scholar] [CrossRef]
- Song, Y.; Liu, W.; Li, J.; Chen, Y.; Cheng, J.; Zhang, J.; Zheng, J. Mechanical properties and stability of zinc-contaminated red clay cured by MICP synergistically activated MgO. Biogeotechnics 2024, 100132. [Google Scholar] [CrossRef]
- Chen, Q.; Yuan, X.; Wu, A.; Liu, Y. Enhancing CO2 mitigation potential and mechanical properties of shotcrete in underground mining utilizing microbially induced calcium carbonate precipitation. Int. J. Min. Sci. Technol. 2024, 34, 1643–1653. [Google Scholar] [CrossRef]
- Liu, B.; Xie, Y.; Tang, C.; Pan, X.; Jiang, N.; Singh, D.N.; Cheng, Y.; Shi, B. Bio-mediated method for improving surface erosion resistance of clayey soils. Eng. Geol. 2021, 293, 106295. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Murdoch, WA, Australia, 2004. Available online: https://researchportal.murdoch.edu.au/esploro/outputs/doctoral/Microbial-CaCO3-precipitation-for-the-production/991005540291407891 (accessed on 25 June 2024).
- Jin, C.; Feng, Q.; Wang, Q. Experimental Study on Tailings Reconstructed Backfill Based on Facultative Anaerobic Bacteria MICP Technology. J. Northeast. Univ. (Nat. Sci.) 2022, 43, 1322–1328. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, L.; Dong, Y.; Gao, Z. Comparative study on the effect of SRB and Sporosarcina pasteurii on the MICP cementation and solidification of lead–zinc tailings. Chem. Eng. J. 2024, 495, 153446. [Google Scholar] [CrossRef]
- Bachmeier, K.L.; Williams, A.E.; Warmington, J.R.; Bang, S.S. Urease activity in microbiologically-induced calcite precipitation. J. Biotechnol. 2002, 93, 171–181. [Google Scholar] [CrossRef]
- Hamdan, N.; Kavazanjian, E., Jr.; Rittmann, B.E.; Karatas, I. Carbonate mineral precipitation for soil improvement through microbial denitrification. Geomicrobiol. J. 2017, 34, 139–146. [Google Scholar] [CrossRef]
- Abdolvand, Y.; Sadeghiamirshahidi, M.; Keenum, I. Denitrification processes, inhibitors, and their implications in ground improvement. Biogeotechnics 2025, 100176. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Wang, Y.; Wang, J.; Cao, J.; Zhang, G. Improved methods, properties, applications and prospects of microbial induced carbonate precipitation (MICP) treated soil: A review. Biogeotechnics 2025, 3, 100123. [Google Scholar] [CrossRef]
- He, Z.; Xu, Y.; Wang, W.; Yang, X.; Jin, Z.; Zhang, D.; Pan, X. Synergistic mechanism and application of microbially induced carbonate precipitation (MICP) and inorganic additives for passivation of heavy metals in copper-nickel tailings. Chemosphere 2023, 311, 136981. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, Z.; Di, J.; Wang, D.; Yang, Z.; Wang, Y.; Guo, X.; Li, K. Experimental study on solidification and remediation of lead–zinc tailings based on microbially induced calcium carbonate precipitation (MICP). Constr. Build. Mater. 2023, 369, 130611. [Google Scholar] [CrossRef]
- Wang, L.; Yao, J.; Liu, X.; Liu, J.; Ma, Z.; Chen, X.; Cao, C.; Li, R.; Jiang, J. Ureolytic Nocardia tenerifensis-driven carbonate precipitation for enhanced La3+ adsorption and immobilization. J. Clean. Prod. 2024, 482, 144193. [Google Scholar] [CrossRef]
- Li, S.; Wu, S.; Wang, S.; Liu, G.; Zhan, Y.; Tong, J.; Zhou, K.; Xie, H. High-efficiency removal of rare earth elements from acid mine drainage by microbially induced carbonate precipitation process. J. Water. Process. Eng. 2025, 71, 107134. [Google Scholar] [CrossRef]
- Wang, W.; Zou, C.; Wang, T.; Wang, B.; Zhu, M.; Zhang, W.; Zhao, L.; Wang, Z.; Wang, Z. Two factors facilitate the cost-effective and harmless cementation of rare earth slags through MICP technology: Carbonic anhydrase bacteria and endogenous calcium ions. J. Environ. Chem. Eng. 2024, 12, 114434. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Yue, C.; Si, Y. Biogenic calcium improved Cd2+ and Pb2+ immobilization in soil using the ureolytic bacteria Bacillus pasteurii. Sci. Total Environ. 2024, 921, 171060. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Wang, X.; Ai, S.; Meng, X.; Liu, Z.; Yang, F.; Cheng, K. Synergistic enhancement of cadmium immobilization and soil fertility through biochar and artificial humic acid-assisted microbial-induced calcium carbonate precipitation. J. Hazard. Mater. 2024, 476, 135140. [Google Scholar] [CrossRef]
- Ji, G.; Huan, C.; Zeng, Y.; Lyu, Q.; Du, Y.; Liu, Y.; Xu, L.; He, Y.; Tian, X.; Yan, Z. Microbiologically induced calcite precipitation (MICP) in situ remediated heavy metal contamination in sludge nutrient soil. J. Hazard. Mater. 2024, 473, 134600. [Google Scholar] [CrossRef]
- Cuaxinque-Flores, G.; Talavera-Mendoza, O.; Aguirre-Noyola, J.L.; Hernández-Flores, G.; Martínez-Miranda, V.; Rosas-Guerrero, V.; Martínez-Romero, E. Molecular and geochemical basis of microbially induced carbonate precipitation for treating acid mine drainage: The case of a novel Sporosarcina genomospecies from mine tailings. J. Hazard. Mater. 2024, 476, 135005. [Google Scholar] [CrossRef]
- Sujiritha, P.B.; Vikash, V.L.; Ponesakki, G.; Ayyadurai, N.; Kamini, N.R. Microbially induced carbonate precipitation with Arthrobacter creatinolyticus: An eco-friendly strategy for mitigation of chromium contamination. J. Environ. Manag. 2024, 365, 121300. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, C.; Jiang, N.; Pan, X.; Liu, B.; Wang, Y.; Shi, B. Microbial-induced carbonate precipitation (MICP) technology: A review on the fundamentals and engineering applications. Environ. Earth. Sci. 2023, 82, 229. [Google Scholar] [CrossRef]
- Liu, M.; Hu, H.; Islam, M.S.; Rao, X.; Zhu, J.; Fang, L.; Fu, Q. How to balance cadmium immobilization and greenhouse gas emissions when using microbial induced-calcite precipitation (MICP): Regulate the N/Ca molar ratio and dosage. J. Environ. Chem. Eng. 2025, 13, 116351. [Google Scholar] [CrossRef]
- Chen, J.; Zou, C.; Wang, W.; Zheng, C.; Jiang, Q.; Wang, Z. Remediation of heavy metal contaminated soil in mining areas with vaterite-type biological calcium carbonate. Process Saf. Environ. Prot. 2024, 192, 649–659. [Google Scholar] [CrossRef]
- Feng, J.; Dang, W.; Gao, Q.; Jiang, K.; Zhu, S.; Ni, J.; Zhang, J.; Lu, P.; Wei, W.; Song, H.; et al. Urea-hydrolyzing Bacillus pasteurii microbially induced calcite precipitation for Ca2+ removal and enhanced anaerobic granular sludge activity in high-calcium wastewater. J. Water Process Eng. 2024, 68, 106545. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Su, J.; Li, X.; Wen, G.; Li, X. Simultaneous removal of calcium, phosphorus, and bisphenol A from industrial wastewater by Stutzerimonas sp. ZW5 via microbially induced calcium precipitation (MICP): Kinetics, mechanism, and stress response. J. Hazard. Mater. 2024, 473, 134700. [Google Scholar] [CrossRef]
- Xue, Z.; Cheng, W.; Wang, L.; Qin, P.; Zhang, B. Revealing degradation and enhancement mechanisms affecting copper (Cu) immobilization using microbial-induced carbonate precipitation (MICP). J. Environ. Chem. Eng. 2022, 10, 108479. [Google Scholar] [CrossRef]
- Song, H.; Kumar, A.; Ding, Y.; Wang, J.; Zhang, Y. Removal of Cd2+ from wastewater by microorganism induced carbonate precipitation (MICP): An economic bioremediation approach. Sep. Purif. Technol. 2022, 297, 121540. [Google Scholar] [CrossRef]
- Song, H.; Sha, J.; Wei, S.; An, J. Low nitrogen MICP remediation of Pb contaminated water by multifunctional microbiome UN-1. Environ. Technol. Innov. 2025, 38, 104097. [Google Scholar] [CrossRef]
- Chen, W.; Chen, L.; Yin, S. Effect of bacteria on cemented fly-ash backfill: Mechanical strength, hydration mechanism and leaching behavior. Constr. Build. Mater. 2025, 472, 140791. [Google Scholar] [CrossRef]
- Dhriyan, S.S.; Verma, A.K.; Prasad, A. Evaluating the potential of bio-cementing pond ash using Microbially Induced Calcite Precipitation (MICP). Constr. Build. Mater. 2025, 467, 140232. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, Y.; Wang, Y.; Zheng, L.; Zhang, Y.; Li, L.; Sun, B.; Li, S.; Zhu, Y. Study on MICP dust suppression technology in open pit coal mine: Preparation and mechanism of microbial dust suppression material. J. Environ. Manag. 2023, 343, 118181. [Google Scholar] [CrossRef]
- Holeček, P.; Kliková, K.; Koňáková, D.; Stiborová, H.; Nežerka, V. Ureolytic bacteria-assisted recycling of waste concrete fines. Powder Technol. 2024, 434, 119310. [Google Scholar] [CrossRef]
- Raheem, L.S.; Khadim, H.J. Microbial-induced carbonate precipitation using eggshells and scallop shells as recycled materials. Case Stud. Chem. Environ. Eng. 2024, 10, 100867. [Google Scholar] [CrossRef]
- Kliková, K.; Holeček, P.; Koňáková, D.; Stiborová, H.; Nežerka, V. Exploiting Bacillus pseudofirmus and Bacillus cohnii to promote CaCO3 and AFt phase formation for stabilizing waste concrete fines. Cem. Concr. Compos. 2025, 155, 105839. [Google Scholar] [CrossRef]
- Cui, J.; Xie, S.; Jia, G.; Yan, Y.; Liu, W.; Li, Z. Utilizing microbial-induced carbonate precipitation technology and carbonation for recycling municipal solid waste incineration fly ash in the production of bricks. Constr. Build. Mater. 2024, 420, 135458. [Google Scholar] [CrossRef]
- Bhukya, P.K.; Adla, N.; Arnepalli, D.N. Numerical optimisation of microbially induced calcite precipitation (MICP) injection strategies for sealing the aquifer’s leakage paths for CO2 geosequestration application. Adv. Water Resour. 2024, 193, 104800. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Su, J.; Li, X.; Zhang, Q. Multiple effects of microbially induced calcium precipitation on bacteria under different molar volumes of organic pollutants. J. Environ. Manag. 2024, 370, 122591. [Google Scholar] [CrossRef]
- Kirkland, C.M.; Thane, A.; Hiebert, R.; Hyatt, R.; Kirksey, J.; Cunningham, A.B.; Gerlach, R.; Spangler, L.; Phillips, A.J. Addressing wellbore integrity and thief zone permeability using microbially-induced calcium carbonate precipitation (MICP): A field demonstration. J. Pet. Sci. Eng. 2020, 190, 107060. [Google Scholar] [CrossRef]
- Cao, H.; Gao, G.; Rao, L.; Zhang, Y.; Sun, Z.; Zhang, J.; Wang, T.; Ding, G.; Zhao, H. Microbially induced calcium carbonate precipitation to combat desertification: A field application experiment. J. Clean. Prod. 2024, 468, 143085. [Google Scholar] [CrossRef]
- Liu, B.; Tang, C.; Pan, X.; Cheng, Q.; Xu, J.; Lv, C. Mitigating rainfall induced soil erosion through bio-approach: From laboratory test to field trail. Eng. Geol. 2025, 344, 107842. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Wang, L.; Zhu, Y.; Rui, S. Field implementation to resist coastal erosion of sandy slope by eco-friendly methods. Coast. Eng. 2024, 189, 104489. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Wang, H.; Miao, L.; Cao, Z.; Wu, L. Bio-cementation for tidal erosion resistance improvement of foreshore slopes based on microbially induced magnesium and calcium precipitation. J. Rock Mech. Geotech. Eng. 2024, 16, 1696–1708. [Google Scholar] [CrossRef]
- Ghasemi, P.; Montoya, B.M. Field implementation of microbially induced calcium carbonate precipitation for surface erosion reduction of a coastal plain sandy slope. J. Geotech. Geoenviron. Eng. 2022, 148, 04022071. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, L.; Dong, Y.; Liu, X.; Wang, X. Analysis of unconfined compressive strength and environmental impact of MICP-treated lead-zinc tailings sand instead of sand as embankment material. Sci. Total. Environ. 2024, 931, 172809. [Google Scholar] [CrossRef]
- Tan, H.; Chen, F.; He, J.; Chen, J. Effect of soil particle size on the reaction rate of microbial-induced calcium precipitation. J. Harbin Eng. Univ. 2019, 40, 1884–1889. [Google Scholar] [CrossRef]
- Xu, P.; Wen, Z.; Yang, S.; Liu, Z.; Leng, M.; Peng, J. Experimental Investigation in MICP Solidified Sands of Different Particle Sizes. Geol. J. China Univ. 2021, 27, 738–745. [Google Scholar] [CrossRef]
- Yin, L.; Tang, C.; Xie, Y.; Lu, C.; Jiang, N.; Shi, B. Factors affecting improvement in engineering properties of geomaterials by microbial-induced calcite precipitation. Rock Soil Mech. 2019, 40, 2525–2546. [Google Scholar] [CrossRef]
- Tang, C.; Yin, L.; Jiang, N.; Zhu, C.; Zeng, H.; Li, H.; Shi, B. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 2020, 79, 94. [Google Scholar] [CrossRef]
- De Oliveira, D.; Horn, E.; Randall, D.G. Copper mine tailings valorization using microbial induced calcium carbonate precipitation. J. Environ. Manag. 2021, 298, 113440. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Cord-Ruwisch, R.; Shahin, M.A. Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can. Geotech. J. 2013, 50, 81–90. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, Y.; Shao, G. Experimental study on improving the effect of microorganisms in solidifying fine-grained soil by red mud. Soils Found. 2025, 65, 101562. [Google Scholar] [CrossRef]
- Saricicek, Y.E.; Gurbanov, R.; Pekcan, O.; Gozen, A.G. Comparison of microbially induced calcium carbonate precipitation eligibility using Sporosarcina pasteurii and Bacillus licheniformis on two different sands. Geomicrobiol. J. 2019, 36, 42–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Cheng, X. Influences of calcium sources on microbially induced carbonate precipitation in porous media. Mater. Res. Innov. 2014, 18, S2-79–S2-84. [Google Scholar] [CrossRef]
- Zhang, X.; Zhan, Q.; Su, Y.; Qian, C.; Shao, J.; Wang, Z. Scale preparation of core–shell bacterial healing agent for concrete and evaluation of shell protection and core mineralization ability. Constr. Build. Mater. 2023, 406, 133366. [Google Scholar] [CrossRef]
- Lai, H.; Ding, X.; Cui, M.; Zheng, J.; Chu, J.; Chen, Z. Factors affecting the effectiveness of biocementation of soil. Biogeotechnics 2024, 2, 100087. [Google Scholar] [CrossRef]
- Bian, Z.; Dong, W.; Li, X.; Song, Y.; Huang, H.; Hong, K.; Hu, K. Enrichment of Terbium (III) under synergistic effect of biosorption and biomineralization by Bacillus sp. DW015 and Sporosarcina pasteurii. Microbiol. Spectr. 2024, 12, e00760-24. [Google Scholar] [CrossRef]
- Clarà Saracho, A.; Haigh, S.K.; Hata, T.; Soga, K.; Farsang, S.; Redfern, S.A.; Marek, E. Characterisation of CaCO3 phases during strain-specific ureolytic precipitation. Sci. Rep. 2020, 10, 10168. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; He, J. A highly effective strain screened from soil and applied in cementing fine sand based on MICP-bonding technology. J. Biotechnol. 2022, 350, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Dong, W.; Ning, Z.; Song, Y.; Hu, K. Recovery of terbium by Lysinibacillus sp. DW018 isolated from ionic rare earth tailings based on microbial induced calcium carbonate precipitation. Front. Microbiol. 2024, 15, 1416731. [Google Scholar] [CrossRef]
- Fouladi, A.S.; Arulrajah, A.; Chu, J.; Horpibulsuk, S. Application of Microbially Induced Calcite Precipitation (MICP) technology in construction materials: A comprehensive review of waste stream contributions. Constr. Build. Mater. 2023, 388, 131546. [Google Scholar] [CrossRef]
- Liang, S.; Fang, C.; Niu, J.; Zeng, W. Research on effect of microbial induced calcite precipitation adopting different calcium sources on cemente granite residual soil. Ind. Constr. 2019, 49, 102–107. [Google Scholar] [CrossRef]
- Liang, S.; Lin, J.; Niu, J.; Feng, D.; Gong, X.; Luo, Q. An Experimental Study of Oyster Shell as Calcium Source for Microbial Solidification. J. Guangdong Univ. Technol. 2020, 37, 48–52. [Google Scholar] [CrossRef]
- Yang, S.; Peng, J.; Wen, Z.; Liu, Z.; Leng, M.; Xu, P. Application of concentrated seawater as calcium source solution in sand reinforcement using MICP. Rock Soil Mech. 2021, 42, 746–754. [Google Scholar] [CrossRef]
- Lin, W.; Gao, Y.; Lin, W.; Zhuo, Z.; Wu, W.; Cheng, X. Seawater-based bio-cementation of natural sea sand via microbially induced carbonate precipitation. Environ. Technol. Innov. 2023, 29, 103010. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Xiao, Y.; Chu, J.; Xiao, P.; Wang, Y. Biocementation of calcareous sand using soluble calcium derived from calcareous sand. Bull. Eng. Geol. Environ. 2018, 77, 1781–1791. [Google Scholar] [CrossRef]
- Song, Z.; Wu, C.; Shen, D.; He, M.; Zhang, F. Microbially induced carbonate precipitation under high temperature and high pressure: Implications for geological CO2 storage. J. Rock Mech. Geotech. Eng. 2025, 17, 3872–3882. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, C.; Pan, X.; Liu, B.; Xie, Y.; Cheng, Q.; Shi, B. Application of microbial induced carbonate precipitation for loess surface erosion control. Eng. Geol. 2021, 294, 106387. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, X.; Guan, W.; Liu, Y.; Xia, X.; Yuan, J.; Chen, X. Mineralization performance and crystal characteristics of microbial induced carbonate precipitation in lead–zinc tailings under multiple factors. Constr. Build. Mater. 2023, 403, 133081. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Y.; Zhang, X.; Sarajpoor, S.; Zhang, S.; Yao, X. Experimental study on permeability and strength characteristics of MICP-treated calcareous sand. Biogeotechnics 2023, 1, 100034. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Bi, J.; Zhao, Y.; Li, Y.; Zhong, X.; Zheng, K. Influences of calcium and magnesium sources on microbially modified strongly weathered phyllite filler. Constr. Build. Mater. 2024, 416, 135118. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Cheng, X. Role of calcium sources in the strength and microstructure of microbial mortar. Constr. Build. Mater. 2015, 77, 160–167. [Google Scholar] [CrossRef]
- Liufu, Z.; Yuan, J.; Shan, Y.; Cui, J.; Tong, H.; Zhao, J. Effect of particle size and gradation on compressive strength of MICP-treated calcareous sand. Appl. Ocean. Res. 2023, 140, 103723. [Google Scholar] [CrossRef]
- Wang, J.; Xu, N.; Wang, H.; Zhou, R.; Shi, Y.; Pan, W.; Huang, X.; Long, Y. Laboratory experiment and micro-mechanism analysis on the influence of particle size and distribution on MICP enhanced soil. Constr. Build. Mater. 2025, 471, 140679. [Google Scholar] [CrossRef]
- Yuan, X.; Zhu, T.; Wang, Q.; Chen, H.; Lin, S.; Wang, X.; Xu, X. An experimental investigation of dispersive soils treated by microbially induced calcium carbonate precipitation (MICP). Constr. Build. Mater. 2024, 446, 137941. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Zomorodian, S.M.A.; Niazi, A.; O’Kelly, B.C. Improving sand with microbial-induced carbonate precipitation. Proc. Inst. Civ. Eng. Ground Improv. 2015, 168, 217–230. [Google Scholar] [CrossRef]
- Whiffin, V.S.; Van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Qi, M.; Ge, S.; Dadda, A. Micro-macro investigation on bio-cemented sand under different grouting saturation: An effective enhancement method. Geomech. Energy Environ. 2024, 37, 100530. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, R.; Xu, Y.; Zheng, J. Immobilization of Cd2+ in an aqueous environment using a two-step microbial-induced carbonate precipitation method. J. Environ. Manag. 2024, 351, 119868. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Bio-cementation of sandy soil using microbially induced carbonate precipitation for marine environments. Geotechnique 2014, 64, 1010–1013. [Google Scholar] [CrossRef]
- Cheshomi, A.; Mansouri, S. Study the grain size and infiltration method effects for sand soil improvement using the microbial method. Geomicrobiol. J. 2020, 37, 355–365. [Google Scholar] [CrossRef]
- Harkes, M.P.; Van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M.C. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Peng, L.; Chen, X.; Qi, J.; Zhu, T. Study on strength characteristics and strengthening mechanism of microbial reinforced silt. Mater. Rep. 2024, 38, 99–105. [Google Scholar] [CrossRef]
- Lin, W.; Cheng, X.; You, S.; Wang, H.; Xu, X.; Guo, H.; Li, M. Experimental Study on Reinforcement of Desert Aeolian Sand by Micp Technology. Eng. Mech. 2023, 11, 1–13. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Chen, Y.; Liu, L.; Li, W.; Han, Y. Uniformity of Microbial Injection for Reinforcing Saturated Calcareous Sand: A Multi-Test Approach. Biogeotechnics 2024, 3, 100105. [Google Scholar] [CrossRef]
- Yu, X.; Yang, H. One-phase MICP and two-phase MISP composite cementation. Constr. Build. Mater. 2023, 409, 133724. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, C.; Liu, B.; Cheng, Q.; Yin, L.; Jiang, N.; Shi, J. Water stability improvement of clayey soil based on microbial induced calcite precipitation. J. Zhejiang Univ. (Eng. Sci.) 2019, 53, 1438–1447. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, C.; Liu, B.; Pan, X.; Wang, D.; Lu, C.; Li, H. Experimental Sthdy on Microbial Solidified Sand Based on Calcium Source Extracted from Limestone Powder. Geol. J. China Univ. 2021, 27, 746–753. [Google Scholar] [CrossRef]
- Yasuhara, H.; Neupane, D.; Hayashi, K.; Okamura, M. Experiments and predictions of physical properties of sand cemented by enzymatically-induced carbonate precipitation. Soils Found. 2012, 52, 539–549. [Google Scholar] [CrossRef]
- Oliveira, P.J.V.; Freitas, L.D.; Carmona, J.P. Effect of soil type on the enzymatic calcium carbonate precipitation process used for soil improvement. J. Mater. Civ. Eng. 2017, 29, 04016263. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Mujah, D. Influence of key environmental conditions on microbially induced cementation for soil stabilization. J. Geotech. Geoenviron. Eng. 2017, 143, 04016083. [Google Scholar] [CrossRef]
- Peng, J.; He, X.; Liu, Z.; Feng, Q.; He, J. Experimental research on influence of low temperature on MICP-treated soil. Chin. J. Geotech. Eng. 2016, 38, 1769–1774. [Google Scholar] [CrossRef]
- Ali, A.; Li, M.; Su, J.; Li, Y.; Wang, Z.; Bai, Y.; Ali, E.F.; Shaheen, S.M. Brevundimonas diminuta isolated from mines polluted soil immobilized cadmium (Cd2+) and zinc (Zn2+) through calcium carbonate precipitation: Microscopic and spectroscopic investigations. Sci. Total. Environ. 2022, 813, 152668. [Google Scholar] [CrossRef]
- Peng, J.; Feng, Q.; Sun, Y. Influences of temperatures on MICP-treated soils. Chin. J. Geotech. Eng. 2018, 40, 1048–1055. [Google Scholar] [CrossRef]
- Zehner, J.; Røyne, A.; Wentzel, A.; Sikorski, P. Microbial-induced calcium carbonate precipitation: An experimental toolbox for in situ and real time investigation of micro-scale pH evolution. RSC Adv. 2020, 10, 20485–20493. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, J.; Youn, H. Effect of temperature, pH, and reaction duration on microbially induced calcite precipitation. Appl. Sci. 2018, 8, 1277. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. New insights into the role of pH and aeration in the bacterial production of calcium carbonate (CaCO3). Appl. Microbiol. Biotechnol. 2017, 101, 3131–3142. [Google Scholar] [CrossRef]
- Chen, B.; Sun, W.; Sun, X.; Cui, C.; Lai, J.; Wang, Y.; Feng, J. Crack sealing evaluation of self-healing mortar with Sporosarcina pasteurii: Influence of bacterial concentration and air-entraining agent. Process Biochem. 2021, 107, 100–111. [Google Scholar] [CrossRef]
- Xue, Z.; Cheng, W.; Wang, L.; Xie, Y.; Qin, P. Effect of a harsh circular environment on self-healing microbial-induced calcium carbonate materials for preventing Pb2+ migration. Environ. Technol. Innov. 2023, 32, 103380. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Wang, Z.; Yao, W. Use of recycled concrete aggregates as carriers for self-healing of concrete cracks by bacteria with high urease activity. Constr. Build. Mater. 2022, 337, 127581. [Google Scholar] [CrossRef]
- Shivanshi, S.; Chakraborti, G.; Upadhyaya, K.S.; Kannan, N. A study on bacterial self-healing concrete encapsulated in lightweight expanded clay aggregates. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Son, Y.; Min, J.; Jang, I.; Yi, C.; Park, W. Development of a novel compressed tablet-based bacterial agent for self-healing cementitious material. Cem. Concr. Compos. 2022, 129, 104514. [Google Scholar] [CrossRef]
- Du, W.; Qian, C.; Xie, Y. Demonstration application of microbial self-healing concrete in sidewall of underground engineering: A case study. J. Build. Eng. 2023, 63, 105512. [Google Scholar] [CrossRef]
- Jiang, L.; Han, Q.; Wang, W.; Zhang, Y.; Lu, W.; Li, Z. A sugar-coated microbial agent for self-healing cracks in concrete. J. Build. Eng. 2023, 66, 105890. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, C.; Liu, B.; Zhang, T.; Cheng, Q.; Shi, B. Mechanical behavior of clayey soil treated by new one-phase MICP technique. J. Eng. Geol. 2020, 28, 306–316. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech. 2019, 14, 615–626. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Guan, D.; Zhou, Y.; Cheng, L.; Zheng, J. Influence of different bacterial grouting strategies on MICP one-phase injection method. J. Hohai Univ. (Nat. Sci.) 2020, 48, 222–230. [Google Scholar] [CrossRef]
- Lai, Y.; Yu, J.; Liu, S.; Liu, J.; Wang, R.; Dong, B. Experimental study to improve the mechanical properties of iron tailings sand by using MICP at low pH. Constr. Build. Mater. 2021, 273, 121729. [Google Scholar] [CrossRef]
- Chen, S.; Kang, B.; Zha, F.; Chen, X. Synergistic solidification of heavy metal tailings by polyethylene glycol (PEG) and microorganisms. Environ. Technol. Innov. 2024, 36, 103788. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, B.; Zheng, H.; Tian, Y.; Wu, L. Study on the microstructure and mechanical properties of uranium tailings reinforced by MICP under the action of activated carbon. Sep. Purif. Technol. 2025, 367, 132947. [Google Scholar] [CrossRef]
- Shu, Y.; Song, Y.; Fang, H.; Wang, D.; Lu, W.; Zhao, C.; Chen, L.; Song, X. Enhancing microbial-induced carbonate precipitation (MICP) sand consolidation with alkali-treated jute fibers. Powder Technol. 2024, 441, 119845. [Google Scholar] [CrossRef]
- Duan, Y.; Niu, L.; Li, B.; He, Y.; Xu, X.; Yu, C.; Wang, Z.; Xiao, C.; Zheng, C. Montmorillonite-coupled microbially induced carbonate precipitation (MICP) enhanced contaminant removal and carbon capture in cyanide tailings. J. Environ. Chem. Eng. 2024, 12, 113498. [Google Scholar] [CrossRef]
- Zhang, J.; Su, P.; Wen, K.; Li, Y.; Li, L. Mechanical performance and environmental effect of coal fly ash on MICP-induced soil improvement. KSCE J. Civ. Eng. 2020, 24, 3189–3201. [Google Scholar] [CrossRef]
- Choi, S.G.; Wang, K.; Chu, J. Properties of biocemented, fiber reinforced sand. Constr. Build. Mater. 2016, 120, 623–629. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Y.; Chen, R.; Liu, H.; Yao, X. Effect of incorporating discarded facial mask fiber on mechanical properties of MICP–treated sand. Constr. Build. Mater. 2023, 395, 132299. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Wang, H.; Ruan, F. Characteristics of cadmium in contaminated soil immobilized by biochar enhanced microbially induced calcite precipitation. Constr. Build. Mater. 2024, 449, 138531. [Google Scholar] [CrossRef]
- Iamchaturapatr, J.; Piriyakul, K.; Petcherdchoo, A. Characteristics of sandy soil treated using EICP-based urease enzymatic acceleration method and natural hemp fibers. Case. Stud. Constr. Mat. 2022, 16, e00871. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, J.; Luo, Y.; Qi, X.; Hussain, J.; Jiang, G. Innovative approach to enhancing phosphogypsum mechanical properties and stabilizing/solidifying contaminants. Sustain. Chem. Pharm. 2024, 41, 101712. [Google Scholar] [CrossRef]
- Duan, C.; Yu, X.; Yao, X.; Zhu, J.; Li, G. Coupling reinforcement of uranium tailings via Klebsiella-induced calcium carbonate precipitation and waterborne polyurethane. Constr. Build. Mater. 2023, 400, 132641. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Kaur, G.; Dhami, N.K.; Goyal, S.; Mukherjee, A.; Reddy, M.S. Utilization of carbon dioxide as an alternative to urea in biocementation. Constr. Build. Mater. 2016, 123, 527–533. [Google Scholar] [CrossRef]
- Ghalandarzadeh, S.; Courcelles, B.; Boudreault, R.; Arenson, L.U.; Maghoul, P. Effect of freeze-thaw cycles on engineering properties of nano-SiO2 enhanced microbially induced calcium carbonate precipitation in kaolinite clay. Cold Reg. Sci. Technol. 2025, 234, 104459. [Google Scholar] [CrossRef]
- Xu, F.; Wang, D.; Xu, X.; Xiao, Z. Strengthening effect of nano-SiO2 on microbial induced carbonate precipitation (MICP) solidified sediment: Macro- and micro-analysis. Geomech. Energy Environ. 2024, 38, 100555. [Google Scholar] [CrossRef]
- Wu, Y.; Tahmasebi, P.; Lin, C.; Zahid, M.A.; Dong, C.; Golab, A.N.; Ren, L. A comprehensive study on geometric, topological and fractal characterizations of pore systems in low-permeability reservoirs based on SEM, MICP, NMR, and X-ray CT experiments. Mar. Pet. Geol. 2019, 103, 12–28. [Google Scholar] [CrossRef]
- Jiao, H.; Yang, W.; Ruan, Z.; Yu, J.; Liu, J.; Yang, Y. Microscale mechanism of tailing thickening in metal mines. Int. J. Miner. Metall. Mater. 2023, 30, 1538–1547. [Google Scholar] [CrossRef]
- Jiang, Q.; Huang, M.; Xu, K.; Cui, M.; Li, S.; Jin, G. Statistical damage constitutive model of MICP-treated specimens based on lognormal distribution. Acta Geotech. 2025, 20, 1759–1775. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y. The impact of Microbially Induced Calcite Precipitation (MICP) on sand internal erosion resistance: A microfluidic study. Transp. Geotech. 2024, 49, 101404. [Google Scholar] [CrossRef]
- Feng, D.; Chu, Y.; Feng, L.; Wang, L.; Huang, Y. Pore-Scale Modeling of the MICP process by using a coupled FEM-LBM-CA Model: With a focus on the heterogeneity of the pore structures. Comput. Geotech. 2024, 172, 106414. [Google Scholar] [CrossRef]








| Parameters | Detection Limit | Control (mg/L) | T1 (AMD–Urea Sporosarcina sp. UB5) | Removal Rate (%) |
|---|---|---|---|---|
| Ag | 0.025 | 0.038 ± 0.002 | <0.025 | 100 |
| Al | 0.025 | 19.166 ± 1.144 | 0.222 ± 0.007 | 99 |
| As | 0.01 | 0.163 ± 0.018 | <0.01 | 100 |
| Ca | 0.05 | 1546.222 ± 76.036 | 352.500 ± 10.135 | 77 |
| Cd | 0.005 | 1.236 ± 0.032 | 0.030 ± 0.001 | 98 |
| Co | 0.025 | 0.034 ± 0.00 | <0.025 | 100 |
| Cr | 0.025 | <0.025 | Non detected | - |
| Cu | 0.025 | 1.917 ± 0.083 | 0.300 ± 0.005 | 84 |
| Fe | 0.025 | 148.056 ± 5.245 | 0.078 ± 0.035 | 99 |
| Zn | 0.025 | 90.200 ± 8.479 | 0.848 ± 0.007 | 99 |
| Mn | 0.025 | 13.770 ± 0.431 | 4.560 ± 0.0094 | 67 |
| Pb | 0.025 | 0.042 ± 0.005 | Non detected | 100 |
| Bacteria | Test Sample | Solidification Effect | Reference |
|---|---|---|---|
| Bacillus sp. DW015 | 400 μmol/L Tb (III) Wastewater | The adsorption efficiency of Tb is above 90%. | [86] |
| Sporosarcina pasteurii | The adsorption efficiency of Tb is above 59.7%. | ||
| Lysinibacillus sp. DW018. | 400 μmol/L Tb (III) Wastewater | The adsorption efficiency of Tb is above 98%. | [89] |
| XR1 | Fine sandy soil | The peak strength of the solidified sand reached 887 kPa. | [88] |
| Sporosarcina pasteurii | The peak strength of the solidified sand reached 504 kPa. | ||
| Sulfate-reducing bacteria | Lead–zinc tailings sand | The maximum UCS of sample reached 0.22 MPa. It has a better effect in fixing SO42− in the tailings. | [36] |
| Sporosarcina pasteurii | The maximum UCS of sample reached 0.95 MPa. |
| Auxiliary Additives | Content (%) | Bacteria | Waste | Performance |
|---|---|---|---|---|
| Montmorillonite | 1, 3, 5, 7, 9 | Bacillus pasteurii [141] | Cyanide tailings | Cr, Cu and Pb leaching concentrations were reduced by 87.18, 60.56, and 88.79%, respectively. |
| Activated MgO | 1, 2, 5, 10 | Sporosarcina pasteurii [30] | Zinc ions disrupted soil | This treatment resulted in a UCS of 1.196 MPa and a Zn2+ leaching concentration of 0.1414 mg/L. |
| Coal fly ash | 3, 6, 9 | Sporosarcina pasteurii [142] | Ottawa silica sands | The peak deviator stress increased by 144%, 154%, and 115% when the additions of coal fly ash were 3%, 6%, and 9%, respectively. |
| PVA fibers | 0.8 | Freeze-dried Bacillus Sp. [143] | Ottawa silica sand | UCS increased by 138%, STS increased by 186%, permeability decreased by 126%, and brittleness decreased by half. |
| Discarded facial mask fiber | 0.2 | Sporosarcina pasteurii [144] | ISO standard sand | MICP treatment improved the UCS and reduced the water weakening of sand columns. |
| Biochar | 2, 3, 4, 5 | Sporosarcina pasteurii [145] | Contaminated soil | The addition of biochar enhanced the efficiency of Cd2+ immobilization through MICP, resulting in the UCS of the samples being 3.06 times that of the untreated samples. |
| Natural hemp fibers | 2.5 | Urease producing bacteria [146] | Natural sand | The treatment improves the strength, cohesion and internal friction angle of the sand. |
| Reactive magnesium oxide cement | 20 | Bacillus cereus [147] | Phosphogypsum | The UCS was able to achieve 3.2 MPa and the permeability coefficient was reduced by two orders of magnitude. |
| Fly ash | 5 | |||
| Waterborne polyurethane | 5, 10, 15, 20 | Bacterial strain Klebsiella [148] | Uranium tailings | The peak deviatoric stress of the improved specimens increased by 45.7% and the elastic modulus increased by 231.3%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hu, K.; Pan, M.; Dong, W.; Wang, X.; Zhu, X. Research and Application of Green Technology Based on Microbially Induced Carbonate Precipitation (MICP) in Mining: A Review. Sustainability 2025, 17, 7587. https://doi.org/10.3390/su17177587
Liu Y, Hu K, Pan M, Dong W, Wang X, Zhu X. Research and Application of Green Technology Based on Microbially Induced Carbonate Precipitation (MICP) in Mining: A Review. Sustainability. 2025; 17(17):7587. https://doi.org/10.3390/su17177587
Chicago/Turabian StyleLiu, Yuzhou, Kaijian Hu, Meilan Pan, Wei Dong, Xiaojun Wang, and Xingyu Zhu. 2025. "Research and Application of Green Technology Based on Microbially Induced Carbonate Precipitation (MICP) in Mining: A Review" Sustainability 17, no. 17: 7587. https://doi.org/10.3390/su17177587
APA StyleLiu, Y., Hu, K., Pan, M., Dong, W., Wang, X., & Zhu, X. (2025). Research and Application of Green Technology Based on Microbially Induced Carbonate Precipitation (MICP) in Mining: A Review. Sustainability, 17(17), 7587. https://doi.org/10.3390/su17177587