Abstract
The EU’s Circular Economy Action Plan promotes the use of organic waste as fertilizer, thus allowing the recycling of nutrients in the agricultural system. Research on the agronomic reuse of composted substrates previously employed for mushroom cultivation remains limited, despite their rich content of plant residues and fungal biomass, which could be repurposed as soil amendments under suitable conditions. This study evaluated the agronomic potential of spent mushroom substrates from Agaricus bisporus and Pleurotus ostreatus, including recomposted A. bisporus residues. A range of analytical procedures was employed to assess their suitability for soil improvement and the formation of humic-like substances, including physical, chemical, microbiological, phytotoxicity, and pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) analyses. The spent Pleurotus substrate exhibited low nutrient content (1.1% N, negligible P, 0.9% K), but high water retention (820 kg water Mg−1) and 48% organic carbon (OC), indicating its potential as a soil amendment or seedling substrate. In contrast, spent and composted Agaricus substrates showed moderate nutrient content (1.8–2.7% N; 0.8–0.7% P and 1.3–1.8% K), appropriate C/N ratios (10–15), and sufficient OC levels (24–30%), supporting their use as fertilizers. However, elevated salinity levels (18–23 dS m−1) may restrict their application for salt-sensitive crops. No significant phytotoxic effects on seed germination were observed, and microbiological analyses confirmed the absence of Salmonella spp. in the three substrates. Py-GC/MS revealed a humic acid-like fraction comprising altered lignin structures enriched with lipid and nitrogen compounds. Overall, the studied materials demonstrate promising agronomic value and the capacity to contribute to long-term soil carbon storage.
1. Introduction
The recycling of waste to obtain organic amendments allows the recovery of nutrients, thus favoring sustainable agricultural production and a circular economy. In accordance with the zero-waste concept, various technologies can be used to recover nutrients from waste [1]. The intensification of agriculture has brought about a decline in soil organic matter (SOM), thereby affecting soil fertility [2]. In this sense, OC—in addition to the macronutrients, N, P, and K—is of paramount importance for preserving soil fertility through SOM maintenance [3]. In this context, the nutrient recycling potential of waste can contribute to the effective management of agricultural soils. Organic fertilizers come from numerous agricultural and industrial activities [4]. To assess the value of organic wastes in agriculture, it is first necessary to conduct a complete analytical characterization [5]. In this sense, the mineral nutrient content of the waste can indicate its potential to supply nutrients to plants, complementing or in some cases replacing mineral fertilization [6].
Spent mushroom substrate (SMS) is a by-product of the mushroom industry. The production of edible fungi has increased in the last decade and is projected to continue growing for the period 2018–2026 due to increased population and changes in dietary habits. Agaricus spp. (30%) and Pleurotus spp. (27%) account for half the world’s mushroom production [7]. In 2017, global mushroom production reached around 10.2 million tons, with Spain as the fifth in the world producer ranking (160 thousand tons per year; [8], with around 19% of its production concentrated in two regions, viz, Castilla-La Mancha and La Rioja). The cultivation of mushrooms results in the generation of spent mushroom substrate (SMS) following approximately three to four cycles of fructification. Given that the production of one kilogram of mushrooms requires approximately five kilograms of SMS [9], resulting in the accumulation of substantial amounts of waste within the production areas. In this context, and as required by the European Circular Economy Strategy [10], SMS should be managed to obtain new resources [11]. A recent review by Steward–Wade [12] on the efficacy of organic waste derived from container plant production as an amendment concluded that each specific type of waste should be assessed on the basis of its characteristics, drawbacks, and suitability.
The SMS can be used as a fertilizer for plant production, soil amendment, and/or as a substrate for seedling cultivation (i.e., container medium). Its application can be made directly (i.e., as fresh material after the spent substrate is moved from the production site) or after an aerobic composting process. In addition, the use of SMS in a new cycle of mushroom production is a viable option that should be considered.
The SMS contains available macro- and micronutrients [13,14]. However, its composition must be previously evaluated to establish which crop is most suitable. Furthermore, it is a rich source of organic matter (OM), which can improve soil properties [15], depending on the quality of the product and the rate applied. The content of OM in SMSs can range from 40% to 60% [16]. Its use as a soil conditioner could prevent soil degradation, increasing carbon sequestration [17]. A further aspect of great interest when evaluating organic leftovers is their potential to become organic substances resistant to biodegradation and with active colloidal properties, as in the case of the soil humic acids (HAs). It has been determined that not all residual biomass possesses this capacity; this is largely dependent on the presence of precursor substances, such as polyphenolic biopolymers derived from plants. On the other hand, the predominance of carbohydrates and other aliphatic substances has been shown to determine the rapid biodegradation of OM without subsequent accumulation in soil [18,19]. A study comparing the use of SMS with the use of peat for the production of Brassica oleracea proposed that up to 50% of peat could be replaced by SMS [20].
Previous studies have explored the agronomic reuse of spent mushroom substrates (SMS), highlighting both their potential and limitations. Composting SMS has been shown to enhance its stability and reduce phytotoxicity, making it suitable as a soil amendment or organic fertilizer in various cropping systems. For instance, it has been demonstrated that composted SMS improves soil pH, OM content, and root development in degraded soils [21] However, other authors have reported that SMS may present challenges such as elevated salinity, biological instability, or the presence of residual contaminants, which require pre-treatment or blending with other materials to ensure safe application [22,23]. Moreover, the humification potential of SMS-derived composts has been linked to their lignocellulosic composition and microbial activity during processing, which influences their capacity to contribute to long-term soil carbon storage [24]. In this context, our study builds on these findings by evaluating the physicochemical, biological, and humic acid characteristics of SMS from Agaricus and Pleurotus spp., with a focus on their suitability for soil amendment and carbon sequestration.
The composting of SMS is an aerobic process that is carried out in piles for about three months, with turnings to enhance aeration and maintain adequate humidity. Composting is a process that yields a final product that is both stable and homogeneous in composition, exhibiting a distinct profile from that of the substrate from which it was derived. The efficiency of the process is determined by the initial composition of the substrates [25].
The amendment with OM is of particular pertinence to Mediterranean soils, which are often characterized by low levels of OC (approximately 75% of the soils contain < 2% OC) [26]. In Spain, half the soils, including arable land, contain less than 1% OC. The “4 per 1000” initiative promoted in the Paris Climate Summit (COP 21) seeks to implement agricultural practices adapted to local conditions, with the aim of increasing the amount of soil OC by 0.4% per year as a climate change mitigation strategy [27]. In this sense, the utilization of organic residues with a high OM content—such as agricultural residues—as fertilizers, amendments, and/or substrates for seedlings would provide a sustainable practice.
This approach aligns with broader EU climate and agricultural strategies, including the Common Agricultural Policy (CAP) [28] and the Circular Economy Action Plan (CEAP) [29], which promote sustainable soil management, carbon farming, and the valorization of organic residues. The CAP 2023–2027 [30] encourages practices that enhance soil OC through eco-schemes and agri-environmental measures, while CEAP supports the reuse of bio-based materials to reduce resource pressure and foster regenerative agriculture. Therefore, our hypothesis is based on the fact that the use of spent mushroom substrates as soil amendments contributes not only to local soil rehabilitation, improving their resilience, but also to EU-wide goals for climate neutrality, resource efficiency, and circularity.
In order to ascertain the suitability of organic residues as fertilizers, soil amendments, and/or seedling substrates, it is necessary to evaluate various analytical characteristics of these materials, including available nutrients, OC content, and C/N ratio. In addition to these characteristics, information on the forms of nutrients available to crops, particularly N and P, is relevant to evaluate the efficient use of such organic residues in agriculture, i.e., the capacity of these residues to improve nutrient cycling in the agricultural system.
The physical properties of organic residues, including bulk density and porosity, are significant factors to be considered when assessing the potential of these materials as soil amendments or seedling substrates [31]. Furthermore, in order to avoid phytotoxicity, it is imperative to give due consideration to the maturity of organic residues when they are to be used in agricultural soils [32]. The presence of harmful compounds in the residues that are applied fresh or after an inadequate composting process can lead to phytotoxicity [33]. In this context, the phytotoxicity test [34] is useful when studying the potential of organic waste products as amendments for agricultural soil. Furthermore, pathogen detection is also necessary to ensure the safety of organic residues, especially when used as a substrate [35].
The utilization of SMSs as fertilizers or amendments for agricultural soils, and as seedling substrates, is contingent upon their physical, chemical, phytotoxic, and microbiological characteristics. Furthermore, the composting process has been shown to result in the formation of more stable materials, enhanced nutrient availability, and alterations in carbon fractions [36] and prevents the presence of pathogens [37].
While the use of SMS offers notable agronomic advantages, some studies have reported phytotoxic effects associated with the application of fresh, untreated SMS. These adverse effects are primarily linked to the presence of soluble salts, organic acids, and phenolic compounds that can hinder seed germination and early plant development. Pre-treatment methods such as composting or superheated steam torrefaction have proven effective in mitigating these compounds, significantly improving germination rates and the safety of SMS as a soil amendment [38].
Concerning regulatory aspects, in the European context, the agronomic use of SMS is subject to regulatory frameworks including the Nitrates Directive [39], which governs the application of nitrogen-rich organic materials to prevent groundwater pollution. Additionally, Regulation (EU) 2019/1009 [35] concerning fertilizing products stipulates specific safety, traceability, and labelling requirements for recycled organic inputs.
As regards recommended usage practices, the use of aged or composted SMS is widely recommended to minimize phytotoxicity and salinity-related risks. The SMS application should be avoided in salt-sensitive crops such as lettuce and strawberry unless pre-analysis of electrical conductivity and nutrient content is performed. Moreover, recent studies suggest diversified applications—including its incorporation into seedling substrates, use as mulch in turf establishment, and transformation into liquid biofertilizers via anaerobic fermentation—highlighting the material’s versatility in sustainable farming systems [40,41].
Conventionally, the potential adverse characteristics of raw organic substrates applied directly to soil have been mitigated through the implementation of composting treatments, which can vary in duration. However, it should be noted that this is a relatively expensive practice and must be considered from an economic viability perspective. Although composting SMS entails operational costs related to labor, energy, and infrastructure, several studies suggest that its agronomic and environmental benefits may outweigh these expenses under appropriate conditions. Iglesias et al. (2025) [41] conducted a life cycle costing (LCC) analysis across three European regions and found that composted SMS, when used as a soil improver, offers substantial environmental advantages and competitive economic performance compared to synthetic fertilizers. Moreover, Martín et al. (2023) [42] emphasized that valorizing SMS through composting or pelletization not only reduces disposal costs but also generates marketable products, aligning with circular economy principles. While economic feasibility varies by region and substrate type, these findings support the practical adoption of composted SMS in sustainable agriculture.
Assuming the above considerations, the objective of this study is to evaluate the agronomic potential of three SMSs as fertilizers, amendments, and/or seedling substrates. This study also examined their residual phytotoxicity, physical, chemical, and microbiological characteristics, and their contribution to the supply of humic substances to the soil, with a view to enhancing soil sustainability.
2. Materials and Methods
2.1. The Origin of Spent Mushroom Substrates
The SMSs of Agaricus bisporus (SMS-A) and Pleurotus sp. (SMS-P) were provided by the “Centro Tecnológico de Investigación del Champiñón” in La Rioja (Spain). Their composition is mainly dependent on the original raw materials used and the mycelia remains of the fungi after harvest.
To prepare the substrate for Agaricus growing, wheat straw, laying hen manure, gypsum, and urea are added, mixed, and watered. The mixture is then left to ferment and turned for 17–20 days until it reaches 76% humidity and 80 °C in aerobic conditions. The substrate is then pasteurized and mixed with the mycelium. The fungus colonizes the substrate to fructify, and a layer of calcium carbonate is applied to the mushroom beds. After the mushroom production decay (3–4 fructifications), the substrate SMS-A is produced. In the case of Pleurotus, the substrate, which contains wheat straw, is mixed with gypsum and watered in aerobic conditions until it reaches 65–70% humidity. It is then pasteurized, and the Pleurotus mycelium is added. After Pleurotus production has depleted, the SMS-P substrate is produced.
The SMS-A composting was performed on open land for 8 weeks under controlled temperature and humidity to produce a SMS compost (SMS-CO). During this process, the mycelium is removed. In both cases, the procedure for preparing the substrates, the origin, and the mixing composition of the materials were the same. The SMS composition was similar between batches over time, which makes the product relatively stable and homogeneous. However, in order to ensure the representativeness of the results, six replicates of each substrate were used for the corresponding analyses.
2.2. Chemical Analyses
The following chemical characteristics were evaluated: pH and electrical conductivity (EC) in saturated paste and in 1:2.5 w/v; dry matter (DM) after drying at 105 °C; and OM by calcination at 580 °C. The OC was calculated using the Van Bemmelen factor, which considers that 58% of OM is OC. Available cations (K+, Ca2+, Mg2+, and Na+) were extracted with ammonium acetate and determined by atomic absorption [43]. Total N was determined by the Kjeldahl method, and NH4+-N, NO3−-N, and ureic N by [44]. Organic N was calculated from the difference between total N and mineral N. Total P was extracted using sulfuric and nitric acids [45], and soluble P was measured in water and in ammonium citrate [44]. All determinations were carried out on fresh samples except organic C and cation content, which were based on dry samples.
2.3. Organic C and Its Fractions (Humic and Fulvic Acids)
Total oxidizable OC was determined by potassium dichromate oxidation [46]. Fractionation of OM was carried out following the methodology described in [47]. Total humic extract (THE) was obtained by successive extractions with sodium pyrophosphate and sodium hydroxide. The humic acids (HAs) were then precipitated with HCl. The carbon content in THE (C-THE) and HA (C-HA) fractions was determined by oxidation with potassium dichromate [46]. The C from fulvic acids (C-FAs) was determined as the difference between THE and HAs.
Soluble C in soil was extracted in water (Cw; 1:10; substrate:distilled water), and samples were shaken for 1 h and centrifuged at 10,000 rpm [48]. After filtration, the supernatant was evaporated in a water bath, and the C content in the residue was determined by oxidation with potassium dichromate [46]. The polymerization and humification indexes used were considered sensitive for monitoring humification and as indicators of the degree of maturity of organic materials.
The degree of polymerization (DP), humification ratio (HR), and humification index (HI) were defined as in [49] and are described by the equations [Equations (1) and (2)] and as in [50] in [Equation (3)], as follows:
where C-FA corresponds to C content in FAs, and C-HA to C content in HAs. C-THE is the sum of C-FAs and C-HAs, and TOCx is total OC.
2.4. Physical Characteristics: Water Retention, Bulk Density, Real Density, and Total Porosity
Bulk density (BD) was recorded in an undisturbed substrate as the relationship between dry weight and the volume that it occupies, and was measured using a graduated cylinder. Real density (RD, particle density) is measured as the mass of the solid material and the volume it occupies, excluding space, using the pycnometer principle (i.e., the solid matter is equal to the water volume occupied). Both densities were determined in fresh samples and were considered in relation to DM (105 °C) expressed in kg DM m−3 [51].
The ratio between the two densities allows the calculation of the total porosity (ɛ) of the organic residue [Equation (4)], as follows:
Soil water retention was measured by saturating the substrate with distilled water (24 h) until all porous space was occupied by water. The water was then drained by gravity for 24–48 h until the flow ceased. The difference in weight between a substrate after draining and the sample substrate dried in an oven (at 105 °C) was considered the total water retention of the substrate. Total water retention included substrate humidity (i.e., constitutive water). The latter value was subtracted to obtain water retention in fresh substrate samples. The water content of fresh substrate samples was obtained in an oven (at 105 °C; DM). All physical determinations were performed on a fresh sample.
2.5. Phytotoxicity Test and Pathogen Analysis
The phytotoxicity test was conducted as in Zucconi et al. (1985) [34] using garden cress seeds (Lepidium sativum). In brief, 20 cress seeds were placed in a Petri dish moistened with 6 mL of distilled water (control) or a substrate extract in hot water (60 °C). Experiments were carried out in triplicate. After two days of seed incubation in the dark at 25–26 °C, the percentage of germination and the length of the roots of the seedlings were measured, and the germination index (GI) was calculated as follows [Equation (5)]:
where GS is the number of seeds germinated in the substrate treatment, GC is the number of germinated seeds in the control treatment (with distilled water), LS is the root length in the substrate treatment, and LC is the root length in the control treatment.
To evaluate the presence of pathogens, Escherichia coli (most probable number; MPN) [52] and Salmonella spp. [53] were determined.
Both determinations were carried out using a fresh sample.
2.6. Pyrolysis-Gas Chromatography–Mass Spectrometry (Py-GC/MS)
This technique was used to characterize the HA fraction, which is considered to be the most active compost constituent from an agrobiological perspective. The aim was to explore the extent to which SMS composting determines the formation of a material similar to the HAs obtained from the soil.
Analytical pyrolysis was performed as described in [54]. In brief, a microfurnace pyrolyser (Frontier Laboratories, model 2020i;Fukushima, Japan) coupled online with a GC/MS system, Agilent 6890N (Technologies, Palo Alto, CA, USA), was used. Samples of ca. 10 mg were placed in small crucible capsules and introduced into a pre-heated micro-furnace at 400 °C for 1 min. The pyrolysis compounds were separated in the gas chromatograph, equipped with a low-polar fused silica (5% phenylmethylpolysiloxane) capillary column, Agilent J&W HP–5 ms Ultra Inert (30 m × 250 μm × 0.25 μm film thickness). The chromatograph oven was set to 50 °C for 1 min, then raised to 100 °C at a rate of 30 °C min−1, from 100 °C to 300 °C at 10 °C min−1, and kept at 300 °C for 10 min at a rate of 20 °C min−1. Helium at a constant flow of 1 mL min−1 was used as carrier gas. The compounds were identified from their electron impact mass spectra at 70 eV (40 to 450 amu) in an Agilent 5973 (Technologies, Palo Alto, CA, USA) mass selective detector operated in scan mode. The identification of the compounds was carried out by fragmentometry, also extracting single-ion traces for homologous series, and searching in digital mass spectral NIST (Gathersburg, MD, USA) and Wiley (Weinheim, Germany) libraries.
2.7. Statistical Analysis
Duncan’s test was performed to determine significant differences (p < 0.05) between treatments in a multivariate Analysis of Variance (ANOVA). The statistical analysis was carried out using SAS software version 9.3 [55].
3. Results
3.1. Physical, Chemical, and Biological Characteristics of the Substrate
The pH and EC values of SMS-CO were similar to those obtained for SMS-A; the two substrates showed higher values for these variables than in SMS-P (Table 1).

Table 1.
Physico-chemical characterization of spent mushroom substrates.
The OM content of SMS-CO was lower than that of SMS-A and SMC-P, the latter showing the highest OM values. OM losses could be attributed to substrate biodegradation during the composting process (Table 1).
The mean values of total N, TOCx, and C/N in SMS-A and SMS-P were within the ranges reported elsewhere for these types of substrates [56,57].
The highest nutrient contents were observed in SMS-A. In this regard, SMS-A and SMS-CO showed a mineral N content of 19% and 16% in relation to total N, respectively. These values are attributable to the presence of laying hen manure (Table 2).

Table 2.
Nutrient content (mean values) and microbiological analyses of spent mushroom substrates.
In the case of SMS-CO, the composting process transformed part of the organic N into inorganic N. Moreover, mineralization (formation of NO3−-N) and N losses occurred during composting, thus reducing fertilization value. Consequently, lower values of total N were found in SMS-CO than in SMS-A (Table 2).
The negligible concentration of P in SMS-P is attributed to the composition of this substrate. In SMS-A and SMS-CO, the total P content was similar (0.77 and 0.66%, respectively) due to the presence of laying hen manure in their composition. After composting, the soluble P/total P ratio decreased (Table 2). Regarding available cations (K+, Na+, Ca2+, and Mg2+), SMS-P showed lower values than the other two substrates. This is attributable to the addition of laying hen manure and calcium carbonate on the SMS-A (and SMS-CO) substrates.
During composting, a slight reduction of available cations was observed in relation to the non-composted substrate (SMS-A). The microbiological analyses indicated the absence of Salmonella in all three substrates; however, E. coli was detected in SMS-A and SMS-P (Table 2). The TOCx content was as follows: SMS-P > SMS-A > SMS-CO (Table 3). The fresh SMSs (SMS-A and SMS-P) showed higher water-soluble C content, being around 4.5–5 times greater than the composted substrate (Table 3). Comparison of SMS-A and SMS-CO revealed a significant decrease in TOCx, Cw, C-THE, C-HA, and C-FA, attributable to the composting process (Figure 1).

Table 3.
Carbon fractions: total carbon (TOCx), soluble C (Cw), C from total humic extract (C-THE), C from humic acids (C-HAs), and C from fulvic acids (C-FAs).
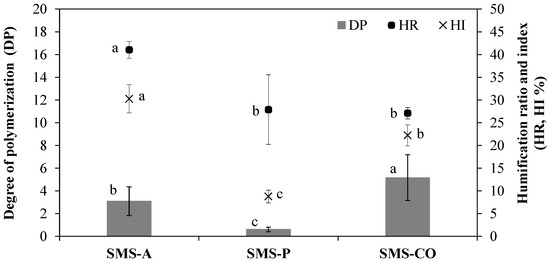
Figure 1.
Mean values of degree of polymerization (DP), humification ratio (HR), and humification index (HI) for spent mushroom substrates (SMS-A, SMS-P, and SMS-CO). Bars indicate standard deviation (n = 6). Different letters in each parameter show significant differences (p < 0.05).
The physical properties, namely bulk density or particle density, water retention, and porosity, of the three SMSs indicated that they were all suitable for use as substrates (i.e., for seedling growth or container medium) (Table 4).

Table 4.
Physical properties of the three substrates.
The C-THE values in fresh substrates were similar (around 12% C/DM), being between 1.7 and 1.9 times higher than that observed in SMS-CO. The ratio between HA and FA differed between the substrates. In this regard, while 74% of C-THE was present as C-HA in SMS-A, this figure was only 37% in SMS-P (Table 3).
Indeed, SMS-P and SMS-CO showed a stimulatory effect, causing a significant increase in radicle length compared to the control (Table 5).

Table 5.
Lepidium sativum seed germination and radicle length.
The phytotoxicity test ruled out any risk of the three substrates, as no differences were observed in the GI or radicle length ratio (Figure 2).
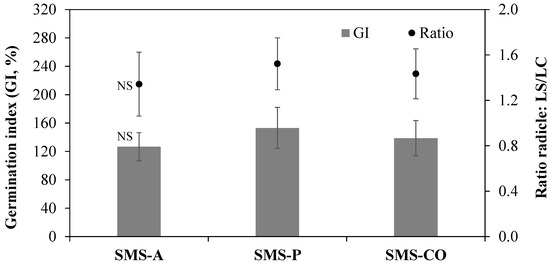
Figure 2.
Mean values (n = 3 ± standard deviation) of germination index and ratio between radicle length of substrate treatment (LS) and control (LC). NS indicates no significant differences between treatments, p > 0.05.
3.2. Analysis by Pyrolysis-Gas Chromatography
The pyrolysis compounds released from the HA-like fraction isolated from the SMSs samples are illustrated in Figure 3. In all samples, the predominance of lignin-derived units was found, consisting mainly of methoxyphenols, including guaiacil (G)-lignin units (guaiacol and its methyl-, ethyl-, vinyl-, propenyl- and aceto-derivatives) as well as the corresponding syringyl (S) equivalents. Vinylphenol (typical of grass lignins) was also found in the samples. On the other hand, the accumulation of non-methoxylated aromatic compounds (alkylbenzenes, alkylphenols, and alkylnaphthalenes, labelled in Figure 3 as “aromatics of non-specific origin” has been considered, in the case of SOM, an indicator of advanced stages of humification [58], which are recognized by the microbial demethoxylation of lignin. The HA-like fractions also released several carbohydrate-derived compounds (methylfuran, furanone, furaldehydes, and cyclic ketones) as well as N-containing compounds, including pyrroles, pyridines, and indoles from chitin, peptides, and proteins.
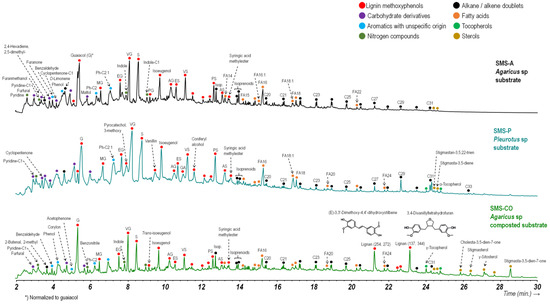
Figure 3.
Chromatographic separation of compounds released by analytical pyrolysis from the SMSs humic acid-type fraction. Peak heights were normalized to guaiacol (G). A major series of compounds with similar origin is marked with colored circles, and major peaks are labeled. In the case of lignin-derived compounds (mainly 4-H-, methyl-, ethyl-, vinyl-, propenyl-, and aceto- derivatives of guaiacol and syringol), the following acronyms are used: guaiacol (G), methylguaiacol (MG), ethylguaiacol (EG), vinylguaiacol (VG), propenylguaiacol (PG), acetoguaiacone (AG), syringol (S), methylsyringol (MS), ethylsyringol (ES), vinylsyringol (VS), propenylsyringol (PS), and acetosyringone (AS).
Additional major compounds that were detected consisted of n-alkanes, n-alkenes, and alkanoic acids (fatty acids), which in most cases can be valuable surrogates to the extent that microbially derived lipids have been incorporated into substrate-derived lipid assemblages [59]. A detail to indicate is the presence—only in the recomposted Agaricus compost sample—of major compounds consisting of lignans (i.e., secondary metabolites consisting of two C6–C3 units joined by β–β′ bonds) which could be interpreted as indicators of lignin that is more easily depolymerizable after composting treatment.
4. Discussion
4.1. Physical and Chemical Characteristics of the Substrates
The low bulk and real density values exhibited by SMS-P can be attributed to the presence of poorly decomposed wheat straw. Furthermore, the maximum water-holding capacity and porosity of SMS-P are advantageous physical properties for use as a substrate (Table 4). Although there are no universally accepted standards values for the physical properties of substrates [31], the evaluated parameters showed adequate properties for their use as soil amendments or seedling substrates.
The high salinity content of substrates SMS-A and SMS-CO (ranging from 18 to 22 dS m−1) is generally the main limiting factor for their reuse. This range can inhibit the germination of seeds in salt-sensitive horticultural species, thereby limiting the use of these substrates [60,61] or when they are reused for Agaricus production [62,63]. Previous studies have demonstrated that salinity in composts and spent substrates can be effectively reduced through water washing or by blending with low-salinity materials [64,65], improving their suitability for use in salt-sensitive cropping, thereby increasing the number of reuses. In contrast, SMS-P showed an EC value 5 times lower than SMS-A and SMS-CO (Table 1).
The SMS-CO showed a higher DM and lower OM content than SMS-A, attributable to changes during composting [66]. This process reduces the percentage of moisture (around 50%) compared to fresh substrate (SMS-CO vs. SMS-A), thereby lowering transport costs (i.e., double DM and nutrients per unit volume).
The high OM content of the three substrates (>40%; Table 1) gives them a good aptitude to increase C in the soil. The OM values agree with those obtained by [67] and are attributed to the lignocellulosic nature (high C content) of SMS-P, which comprised cereal straw that had not yet decomposed. The mean values of total N, TOCx, and C/N in SMS-A and SMS-P were within the ranges for these types of materials.
The C/N ratio indicates the decomposition of organic residues by microorganisms, which use the C of these residues to grow and provide mineral N for crops. The optimum ratio for N release by mineralization is 12–15 (i.e., requires 1 unit of available N for every 12–15 units of available C from OM). The comparatively high C/N ratio observed in SMS-P therefore implies that C exceeds the amount of N in the soil, and microorganisms use the N from the soil, thus limiting its supply to the plant. This latter process, known as N immobilization, can result in N deficiencies for crops [68]. From this point of view, SMS-P would not be a suitable source of N for crop production. If this substrate were used alone, the addition of an N source would be required for crop development. Alternatively, it could be used to obtain a mixed formulation with other substrates or fertilizers.
The nitrate content in SMS-A and SMS-P was under detection limits. However, the presence of nitrate in SMS-CO suggested the mineralization of N by reduction to ammonium during composting (SMS-A vs. SMS-CO, Table 2), according to results obtained by other authors [36,69]. Application of the evaluated substrates as sources of N, according to the rates permitted by the EU Nitrates Directive (rate 170 kg total-N ha−1 yr−1 in vulnerable nitrate zones [39], would be equivalent to applied rates of 6.3 Mg DM ha−1 for SMS-A, 15.5 Mg DM ha−1 for SMS-P, and 9.4 Mg DM ha−1 for SMS-CO. Application rates between 135 and 145 kg organic N ha−1 (80–85% of total N applied) lead to high residual N effects and low mineral N application), which can be sufficient for crop establishment with soil N content and the consequent savings in pre-sowing fertilization in SMS-A and SMS-CO (20–26 kg N ha−1). And at this rate, annually the addition of OM will be 3.6 Mg OM ha−1, 11.2 Mg OM ha−1, and 4.1 Mg OM ha−1 for SMS-A, SMS-P, and SMS-CO, respectively, the contribution of SMS-P in OC or OM being 2.6 to 3.1 times compared to SMS-CO and SMS-A, respectively.
4.2. Assessment of the Environmental Safety of the Substrates: Microbial Analysis and Phytotoxicity Testing
The microbiological analyses indicated the lack of Salmonella in the three substrates, while the presence of E. coli in SMS-A was associated with the laying hen manure in its composition and with ineffective pasteurization. This pathogen was not observed in SMS-CO because of the composting process. The presence of E. coli in SMS-A and SMS-P, which was not anticipated (i.e., pasteurization), may be linked with cross-contamination during storage at the mushroom production facility. Such cross-contamination is a limiting factor for the commercialization of substrates and should be controlled in order to guarantee the safe application of these types of products [11].
On the other hand, it is essential to evaluate the phytotoxicity of substrates, since fresh immature organic waste may contain components that could inhibit plant growth, such as soluble inorganic compounds [70] as well as unstable organic substances, that could adversely affect the dynamics of soil OM [34]. The slow transformation of lignocellulosic compounds can result in phytotoxic by-products, even after composting [71]. As demonstrated in Table 5, our findings indicate that none of the substrates exerted any deleterious effects on seed germination or on the length of radicles. It is possible to interpret these results as indirect evidence that the substrates used do not pose significant environmental risks. The absence of phytotoxic effects suggests that composted residues are free from allelopathic compounds, antibiotic metabolites derived from fungal activity, or other xenobiotic substances such as pesticide residues. Indeed, the absence of inhibition in germination and root elongation, coupled with the sanitary results (the absence of Salmonella spp. and E. coli MPN values), supports the hypothesis that the materials are microbiologically safe and chemically stable. Previous studies have demonstrated that proper composting can significantly reduce microbial risks and degrade organic pollutants, including pesticide residues, through microbial activity and thermophilic phases [72,73].
It is worth mentioning that while the impact of the water-soluble extract of the substrates on seed germination was not significant, notable increases in rootlet length were observed. This suggests that soluble compounds present in the substrates have a stimulatory effect on plant metabolism or growth. Such effects are well documented in the literature, particularly in relation to humic substances and their soil- or compost-derived precursors. These compounds are known to release auxin-like molecules that promote root development and overall plant vigor [72,73].
Although none of the evaluated SMSs showed phytotoxicity (Figure 2), we recommend that the phytotoxicity of the substrate be evaluated in relation to the amount of substrate to be used and the crop involved. The characteristics determined suggest that the analyzed substrates allow for their easy handling in the field and a reduction in transport costs, although the composting process itself is expensive.
4.3. Analysis of Soil Organic Matter Fractions and Molecular Characterization of the Humic Acid-Type Substances by Py-GC/MS
The OC, especially Cw, is the primary energy source for microbial biomass, and it affects the microbial activity and population in the soil [74,75].
The DP (degree of polymerization) value in SMS-P was markedly lower (0.6) than in SMS-A and SMS-CO (3 and 5, respectively; Figure 2), which is consistent with the high C-FA fraction of SMS-P (Table 3). This indicates the presence of “relatively poorly evolved” OM (i.e., large wheat straw fragments were visually observed). Depending on their chemical structure, compounds can be classified into several categories on the basis of their decomposition rate [68]: rapid (e.g., sugars, starches, and proteins), slow (e.g., cellulose, fats, waxes, and resins), or very slow (e.g., lignin).
In SMS-P, the presence of wheat straw (hemicellulose, cellulose, and lignin) led to a slow rate of decomposition [76]. This observation is consistent with its high C/N ratio (40–45; Table 1) and a low HI, which indicates the low maturity of this substrate (Figure 1). In contrast, in the case of SMS-A and SMS-CO, a higher HI and a C-HA content greater than that of C-FA indicated the presence of humified or evolved OM, an observation consistent with their C/N ratios (11–15) (Table 1).
The Py-GC/MS analysis of HA-type fractions in the different residue types shows a similar composition, but fundamental quantitative differences between the samples.
A conspicuous pattern of methoxyphenols (11 phenol index) is observed, suggesting the presence of lignin in the early stages of microbial transformation [59]. This corresponds to the low ratios between guaiacol and syringol and, in general, between all syringyl-type methoxyphenols with respect to guaiacyl-type ones, although it is lower in the case of the recomposted Agaricus substrate.
In fact, guaiacyl-type lignins are considered to have a higher degree of internal cross-linking than syringyl-type lignins and are consequently more resistant to biodegradation. For this reason, the progressive decrease in the syringyl-to-guaiacyl ratio has been reported as an indicator of advanced humification. i.e., the selective biodegradation of comparatively labile lignin moieties, i.e., with a higher syringyl (methoxyl) content (S-type) [77].
The presence of lignans as major products in this recomposted sample could also be interpreted as evidence of a more profound alteration of the lignin in this substrate. This would result in the formation of high-molecular-weight oligomeric products that are more easily released under pyrolysis conditions.
The other main aromatic compounds are the non-methoxylated aromatics, such as phenols and alkylbenzenes. These are comparatively frequent in the recomposted Agaricus compost, suggesting advanced transformation stages, with the conversion of lignin into polyphenolic macromolecular substances that are more similar to those present in SOM [78].
In this sense, it is also worth noting the presence of very low proportions of polycyclic aromatic compounds with 2 or 3 rings (naphthalenes, phenanthrenes, etc.), which are relatively frequent in soil HAs. This confirms that a similar degree of maturity has not been reached, as well as indicating the absence of pyrogenic material in the samples under study [79].
Finally, the greater number and abundance of steroids in the recomposted substrate are consistent with previous studies [80], indicating that lipids, steroids, and N-compounds were relatively enriched over time due to their more difficult degradation.
Other significant differences between the samples are found in the patterns of N-compounds, which tend to predominate in the recomposted sample (mainly pyridines, benzonitriles, and indoles), suggesting incorporation of stable N-compounds in HA-type fractions.
Regarding the alkyl series, all the samples exhibit well-defined, well-marked homologous series formed by fatty acids with a particular abundance of palmitic acid (C16) and stearic acid (C18). There were no notable variations in the fatty acid series between the substrates, although they were found in lower proportions in the recomposted sample. Among the unsaturated fatty acids, oleic acid (C18:1) predominates, particularly in the composted Agaricus substrate. This indicates a comparatively lower degree of transformation, given that unsaturated fatty acids are more reactive and easily biodegradable than their saturated counterparts (the C18:1/C18 ratio is greater than 1 in this substrate). Regarding hydrocarbons, all samples exhibit a typical series consisting of alkane and olefin doublets in the range between C17 and C31. The alkane series shows a predominance of molecules with an odd number of carbon atoms, indicating an origin inherited from the epicuticular lipids of vascular plants [81].
The Agaricus compost, which consistently exhibits characteristics suggesting comparatively low transformation, and showed the highest proportions of long-chain alkanes (C27 to C31). In contrast, these compounds appear to have degraded in the other samples, representing minor pyrolytic products. This suggests microbial degradation and/or the concomitant concentration of the main lignin degradation products.
The comparatively lower degree of transformation of the Pleurotus substrate coincides with the highest proportions of C6–C3 lignin-derived products, such as isoeugenol and PS, in accordance with typical microbial utilization patterns of lignin, i.e., demethoxylation, and cleavage of C3 side chains.
In general, the molecular composition of the HA type fractions of the three types of substrate exhibits typical patterns of weakly transformed lignin. They contain some residual carbohydrate content and incorporate alkyl products mainly inherited from the initial material.
The lowest degree of transformation corresponds to the Pleurotus substrate, in which lignin is poorly transformed, resulting in a high S/G ratio and a high proportion of C6–C3 phenolic units. This includes preserved long-chain alkyl compounds and a comparatively high proportion of unsaturated fatty acids. In contrast, the highest degree of transformation is observed in the recomposted Agaricus substrate, where the greatest demethoxylation occurs alongside the accumulation of non-methoxylated aromatic products. This substrate has undergone the most intense accumulation of recalcitrant compounds inherited from the vegetation, including steroids, lignans, and N-products.
4.4. The Potential of SMS Humic Substances to Transform into Long-Term Stable Forms of Organic Matter in the Soil
Overall, the data obtained from the molecular characterization of OM support previous studies’ findings showing that the composting process does not lead to the formation of humic substances that are sufficiently similar to those found in soil, even when carried out with significant weight loss. When the composting substrate consists of lignocellulosic material, the lignins undergo classic demethoxylation and carboxylation transformations, incorporating peptidic and lipid compounds derived from microbial metabolism. The end result is a type of oxidized, high-molecular-weight lignoprotein [82].
In any event, the composting process does not result in the formation of humic-like substances as those found in soil, even under accelerated aerobic and thermophilic conditions. This would require a significant mineral substrate to enable the selective preservation of particular organic components and the fixation or insolubilization of products within the soil matrix. These products would then be incorporated into humic macromolecules. Alternating phases of humidity and desiccation favor structural condensation and the formation of free and stable radicals that give HAs their dark color. In these advanced stages of transformation, humic-type macromolecules exhibit a high degree of condensation and a chaotic, disordered structure, which makes them difficult to degrade by soil enzymes [83]. The results of this study suggest that the substrates in question have already accumulated heavily transformed lignin as a precursor of soil HAs. Depending on climatic conditions and the mineral substrate, this material may evolve more or less efficiently towards the formation of recalcitrant humic substances that are resistant to long-term degradation.
5. Conclusions
Spent mushroom substrates (SMS) show agronomic potential with distinct applications. SMS-P (Pleurotus) is unsuitable as a nitrogen source due to low nutrient content and high C/N ratio, but its favorable physical properties and low salinity make it viable as a growth medium when supplemented with N. SMS-A and SMS-CO (Agaricus) are effective fertilizers and organic amendments thanks to their nutrient profile and humic content, though their high salinity limits use with salt-sensitive crops. None of the substrates showed phytotoxicity. Additionally, the humic-like fractions from composted SMS exhibit molecular characteristics that support their role in soil carbon enrichment. Overall, SMS can aid nutrient recycling and foster soil health and resilience.
Author Contributions
Conceptualization, M.C.L. and M.R.Y.; methodology, M.C.L., M.R.Y., G.A. and J.A.G.-P.; software, M.C.L. and M.R.Y.; validation, M.C.L., M.R.Y., G.A. and J.A.G.-P.; formal analysis, M.C.L. and M.R.Y.; investigation, M.C.L., M.R.Y., G.A. and J.A.G.-P.; resources, M.C.L. and J.A.G.-P.; data curation, M.C.L.; writing—original draft preparation, M.R.Y., M.C.L. and G.A.; writing—reviewing and editing, M.C.L., M.R.Y. and G.A.; visualization, M.C.L., M.R.Y., G.A. and J.A.G.-P.; supervision, M.C.L., M.R.Y. and G.A.; project administration, M.C.L.; funding acquisition, M.C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant RTA-2015-00060-C04-04 funded by MICIU/AEI/10.13039/501100011033 and “ERDF A way of making Europe” and by IMIDRA (grant FP21-NMO). M.R. Yagüe was supported by grant DOC 2015-021 funded by MICIU/AEI/10.13039/501100011033 “ESF Investing in your future”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors thank the technical staff of the soil laboratory of the Agro-environmental Research Area (IMIDRA) for analytical support and the Centro Tecnológico de Investigación del Champiñón de La Rioja (CTICH) for providing the substrates.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BD | Bulk density |
| Cw | Carbon soluble in water |
| DM | Dry matter |
| DP | Degree of polymerization |
| EC | Electrical conductivity |
| FA | Fulvic acids |
| FS | Fresh sample |
| GI | Germination index |
| HA | Humic acid |
| HI | Humification index |
| HR | Humification ratio |
| OC | Organic carbon |
| OM | Organic matter |
| RD | Particle density |
| Py-GC/MS | pyrolysis-gas chromatography/mass spectrometry |
| SMS-A | Spent mushroom substrate of Agaricus bisporus |
| SMS-CO | Spent mushroom substrate compost |
| SMS-P | Spent mushroom substrate of Pleurotus ostreatus |
| SOM | Soil organic matter |
| THE | Total humic extract |
| TOCx | Total OC by oxidation |
References
- Ahmed, M.; Ahmad, S.; Fayyaz-ul-Hassan; Qadir, G.; Hayat, R.; Shaheen, F.A.; Raza, M.A. Innovative processes and technologies for nutrient recovery from wastes: A Comprehensive Review. Sustainability 2019, 11, 4938. [Google Scholar] [CrossRef]
- Steinmann, T.; Welp, G.; Wolf, A.; Holbeck, B.; Große-Rüschkampm, T.; Amelung, W. Repeated monitoring of organic carbon stocks after eight years reveals carbon losses from intensively managed agricultural soils in Western Germany. J. Plant Nutr. Soil Sci. 2016, 179, 355–366. [Google Scholar] [CrossRef]
- Verger, Y.; Petit, C.; Barles, S.; Billen, G.; Garnier, J.; Esculier, F.; Maugis, P. A N, P, C, and water flows metabolism study in a peri-urban territory in France: The case study of the Saclay plateau. Resour. Conserv. Recycl. 2018, 137, 200–213. [Google Scholar] [CrossRef]
- Ramachandra, T.V.; Bharath, H.A.; Kulkarni, G.; Han, S.S. Municipal solid waste: Generation composition and GHG emissions in Bangalore, India. Renew. Sustain. Energy Rev. 2018, 82, 1122–1136. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Shi, D.; Liu, G.; Zhao, Y.; Simaoka, T. Environmental challenges impeding the composting of biodegradable municipal solid waste: A critical review. Resour. Conserv. Recycl. 2017, 122, 51–65. [Google Scholar] [CrossRef]
- Xun, W.; Xiong, W.; Huang, T.; Ran, W.; Li, D.; Shen, Q.; Li, Q.; Zhang, R. Swine manure and quicktime have different impacts on chemical properties and composition of bacterial communities of and acidic soil. Appl. Soil Ecol. 2016, 100, 34–44. [Google Scholar] [CrossRef]
- Industry Report. Global Mushroom Market Size, Market Share, Application Analysis, Regional Outlook, Growth Trends, Key Players, Competitive Strategies 2018 to 2026. PR Newswire. 2018. Available online: https://www.prnewswire.com/news-releases/global-mushroom-market-2018-2026-the-market-is-expected-to-grow-at-a-cagr-of-7-9-300712332.html (accessed on 4 August 2025).
- FAOstat. 2019. Available online: https://www.fao.org/statistics/en (accessed on 19 June 2025).
- Finney, K.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. The Reuse of Spent Mushroom Compost and Coal Tailings for Energy Recovery: Comparison of Thermal Treatment Technologies. Bioresour. Technol. 2009, 100, 310–315. [Google Scholar] [CrossRef]
- Grimm, D.; Wösten, H.A.B. Mushroom in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef]
- Zied, D.C.; Sánchez, J.E.; Noble, R.; Pardo-Giménez, A. Use of spent mushroom substrate in new mushroom crops to promote the transition towards a circular economy. Agronomy 2020, 10, 1239. [Google Scholar] [CrossRef]
- Stewart-Wade, S.M. Efficiency of organic amendments used in containerized plant production: Part 1–Compost-based amendments. Sci. Hortic. 2020, 266, 108856. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Bustamante, M.A.; Moral, R.; Moreno-Caselles, J. Relations between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma 2012, 173–174, 152–161. [Google Scholar] [CrossRef]
- Hackett, R. Spent mushroom compost as a nitrogen source for spring barley. Nutr Cycl. Agroecosyst. 2015, 102, 253–263. [Google Scholar] [CrossRef]
- Gümüs, I.; Seker, C. Effects of spent mushroom compost applications on the physicochemical properties of a degraded soil. Solid Earth 2017, 8, 1153–1160. [Google Scholar] [CrossRef]
- Ma, X.; Yan, S.; Wang, M. Spent mushroom substrate: A review on present and future of green applications. J. Environ. Manag. 2025, 373, 123970. [Google Scholar] [CrossRef]
- Becher, M.; Banach-Szott, M.; Godlewska, A. Organic Matter Properties of Spent Button Mushroom Substrate in the Context of Soil Organic Matter Reproduction. Agronomy 2021, 11, 204. [Google Scholar] [CrossRef]
- Almendros, G.; Dorado, J.; González-Vila, F.J.; Blanco, M.J.; Lankes, U. 13C NMR assessment of decomposition patterns during composting of forest and shrub biomass. Soil Biol. Biochem. 2000, 32, 793–804. [Google Scholar] [CrossRef]
- Kadiri, M.; Mustapha, Y. The use of spent mushroom substrate of Lentinus subnudus (Berk) as a soil conditioner for vegetables. Bayero J. Pure Appl. Sci. 2010, 3, 16–19. [Google Scholar]
- Sendi, H.; Mohamed, M.T.M.; Anwar, M.P.; Saud, H.M. Spent mushroom waste as a media replacement for peat moss in Kai-Lan (Brassica oleracea var. Alboglabra) production. Sci. World J. 2013, 2013, 25862. [Google Scholar] [CrossRef]
- López, R.; Antelo, J.; Silva, A.C.; Bento, F.; Fiol, S. Factors that affect physicochemical and acid-base properties of compost and its potential use as a soil amendment. J. Environ. Manag. 2021, 300, 113702. [Google Scholar] [CrossRef]
- Garau, M.; Pinna, M.V.; Nieddu, M.; Castaldi, P.; Garau, G. Mixing compost and biochar can enhance the chemical and biological recovery of soils contaminated by potentially toxic elements. Plants 2024, 13, 284. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, Y.; Li, Z.; Huang, B.; Luo, N.; Liu, C.; Zeng, G. Compost as a soil amendment to remediate heavy metal-contaminated agricultural soil: Mechanisms, efficacy, problems, and strategies. Water Air Soil Pollut. 2016, 227, 359. [Google Scholar] [CrossRef]
- Maffia, A.; Marra, F.; Canino, F.; Battaglia, S.; Mallamaci, C.; Oliva, M.; Muscolo, A. Humic substances from waste-based fertilizers for improved soil fertility. Agronomy 2024, 14, 2657. [Google Scholar] [CrossRef]
- Straatsma, G.; Gerrits, J.P.G.; Thissen, J.T.N.M.; Amsing, J.G.M.; Loeffen, H.; Van Griensven, L.J.L.D. Adjustment of the composting process for mushroom cultivation based on initial substrate composition. Bioresour. Technol. 2000, 72, 67–74. [Google Scholar] [CrossRef]
- Zdruli, P.; Jones, R.J.A.; Montanarella, L. Organic Matter in the Soils of Southern Europe; European Soil Bureau Technical Report, EUR 21083 EN; Office for Official Publications of the European Communities: Luxembourg, 2004; 16p. [Google Scholar]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.S.; Das, D.S.; Field, D.J.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- European Commission. Circular Economy Action Plan: For a Cleaner and More Competitive Europe; European Commission: Brussels, Belgium, 2020; Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 26 July 2025).
- European Commission. The Common Agricultural Policy 2023–2027: Key Policy Objectives; European Commission: Brussels, Belgium, 2023; Available online: https://agriculture.ec.europa.eu/common-agricultural-policy/cap-overview/cap-glance/key-policy-objectives-cap-2023-27_en (accessed on 26 July 2025).
- European Commission. The “4 per 1000” Initiative: Soils for Food Security and Climate; European Commission: Brussels, Belgium, 2023; Available online: https://4p1000.org/?lang=en (accessed on 26 July 2025).
- Bilderback, T.E.; Warren, S.L.; Owen, J.S.; Albano, J.P. Healthy substrates need physical too! HortTechnology 2005, 15, 747–751. [Google Scholar] [CrossRef]
- Acosta, Y.; Paolini, J.; Benítez, E. Indice de humificación y prueba de fitotoxicidad en residuos orgánicos de uso agrícola potencia. Rev. Fac. Agron. 2004, 21, 185–194. [Google Scholar]
- Senesi, N. Composted materials as organic fertilizers. Sci. Total Environ. 1989, 81/82, 521–542. [Google Scholar] [CrossRef]
- Zucconi, F.; Monaco, A.; Forte, M. Phytotoxins during the stabilization of organic matter. In Composting of Agricultural and Other Wastes; Gasser, J.K.R., Ed.; Elsevier Applied Science Publication: New York, NY, USA, 1985; pp. 73–86. [Google Scholar]
- EU 2019/1009. Regulation 2019/1009 of the European Parliament and the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilizing Products and Amending Regulations (EC) No.1069/2009 and (EC) No. 1107/2009 and Repealing Regulation (EC) No. 2003/2003; European Union: Brussels, Belgium, 2019.
- Ma, Y.; Liu, L.; Zhou, X.; Tian, T.; Xu, S.; Li, D.; Li, C.; Li, Y. Optimizing straw-rotting cultivation for sustainable edible mushroom production: Composting spent mushroom substrate with straw additions. J. Fungi 2023, 9, 925. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Doyle, M.P.; Erickson, M.C.; Jiang, X.; Millner, P.; Sharma, M. Composting to inactivate foodborne pathogens for crop soil application: A review. J. Food Prot. 2018, 81, 1821–1837. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Yang, R.; Lin, W.; Wang, H.; Kang, Y.; Qi, Z.; Zhou, W. Reducing the phytotoxicity of spent mushroom substrate (SMS) for sustainable growing media by superheated steam torrefaction: Effects of temperature and residence time. Biomass Convers. Biorefin. 2025, 15, 4733–4744. [Google Scholar] [CrossRef]
- European Union. Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources; European Union: Brussels, Belgium, 1991; Available online: https://eur-lex.europa.eu/eli/dir/1991/676/oj/eng (accessed on 4 August 2025).
- Chamoli, V.; Singh, K.; Kaushik, G.; Pant, A. The usage of spent mushroom substrate. Int. J. Plant Pathol. Microbiol. 2023, 3, 48–50. [Google Scholar]
- Iglesias, H.; Paredes Ortiz, A.; Soriano Disla, J.M.; Lara-Guillén, A.J. Environmental and Economic Life Cycle Impacts of Using Spent Mushroom Substrate as a Soil Improver. Environments 2025, 12, 31. [Google Scholar] [CrossRef]
- Martín, C.; Zervakis, G.I.; Xiong, S.; Koutrotsios, G.; Strætkvern, K.O. Spent substrate from mushroom cultivation: Exploitation potential toward various applications and value-added products. Bioengineered 2023, 14, 2252138. [Google Scholar] [CrossRef] [PubMed]
- Bower, C.A.; Reitemeier, R.F.; Fireman, M. Exchange cation analysis of saline and alkali soils. Soil Sci. 1952, 73, 251–261. [Google Scholar] [CrossRef]
- AOAC—Association Official Analytical Chemists. Official Methods of Analysis of Association of Official Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2010. [Google Scholar]
- Ministerio de Agricultura, Pesca y Alimentación. Orden del 17 de septiembre de 1981 por la que se establecen métodos oficiales de análisis de aceites y grasas, aguas, carnes y productos cárnicos, fertilizantes, productos fitosanitarios, leche y productos lácteos, piensos y sus primeras materias, productos orgánicos fertilizantes, plantas, suelos, productos derivados de la uva y similares y toma de muestras. BOE 1981, 246, 24003–24034. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic carbon matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Dabin, B. Étude d’une méthode d’extraction de la matière humique du sol. Sci. Sol 1971, 1, 47–63. [Google Scholar]
- Chantigny, M.H.; Angers, D.A.; Kaiser, K.; Kalbitz, K. Extraction and characterization of dissolves organic matter. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Roig, A.; Lax, A.; Cegarra, J.; Costa, F.; Hérnandez, M.T. Cation-exchange capacity as a parameter for measuring the humification degree of manures. Soil Sci. 1988, 146, 311–316. [Google Scholar] [CrossRef]
- Roletto, E.; Barberis, R.; Consiglio, M.; Jodice, R. Chemical parameters for evaluating compost maturity. Biocycle 1985, 26, 46–47. [Google Scholar]
- Niedziela, C.E.; Nelson, P.V. A rapid method for determining physical properties of undisturbed substrate. HortScience 1982, 27, 1279–1280. [Google Scholar] [CrossRef]
- ISO 7251:2005; Microbiology of Food and Animal Feeding Stuffs Horizontal Method for the Detection and Enumeration of Presumptive Escherichia coli—Most Probable Number Technique. International Organization for Standardization (ISO): Geneva, Switzerland, 2005.
- UNE-EN ISO 6579-1:2017; Microbiología de la cadena alimentaria. Método horizontal para la detección, enumeración y serotipado de Salmonella. Parte 1: Detección de Salmonella spp. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Jiménez-Morillo, N.T.; Cabrita, M.J.; Dias, C.B.; González-Vila, F.J.; González-Pérez, J.A. Pyrolysis-compound-specific hydrogen isotope analysis (δ2H Py-CSIA) of Mediterranean olive oils. Food Control 2020, 110, 107023. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT Software, Version B8.2; SAS Institute: Cary, NC, USA, 1999. [Google Scholar]
- Almendros, G. An analysis of some wheat straw humification factors and their bearing on the response to compost of soil and plant. Sci. Total Environ. 1989, 81/82, 569–578. [Google Scholar] [CrossRef]
- Tinoco, P.; Almendros, G.; González-Vila, F.J.; Lankes, U.; Lüdemann, H.-D. Analysis of carbon and nitrogen forms in soil fractions after addition of 15N-compost by 13C and 15N nuclear magnetic resonance. J. Agric. Food Chem. 2004, 52, 5412–5417. [Google Scholar] [CrossRef]
- Dorado, J.; Almendros, G.; González-Vila, F.J. Response of humic acid structure to soil tillage management as revealed by analytical pyrolysis. J. Anal. Appl. Pyrolysis 2016, 117, 56–63. [Google Scholar] [CrossRef]
- Jiménez-González, M.A.; Álvarez, A.M.; Carral, P.; González-Vila, F.J.; Almendros, G. The diversity of methoxyphenols released by pyrolysis-gas chromatography as predictor of soil carbon storage. J. Chromatogr. A 2017, 1508, 130–137. [Google Scholar] [CrossRef]
- Yagüe, M.R.; Lobo, M.C. Reuse of the spent mushroom substrate in a vegetable seedbed. Inf. Tec. Econ. Agrar. 2021, 117, 347–359. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Pérez-Murcina, M.D.; Bustamante, M.A.; Moral, R. Spent mushroom substsrates as component of growing media for gemination and growth of horticultural plants. Bioresour. Technol. 2009, 100, 4227–4232. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Pardo-González, J.E. Evaluation of casing material made from spent mushroom substrate and coconut fibre pit for use in production of Agaricus biporus (Lage) Imbach. Span. J. Agric. Res. 2008, 6, 683–690. [Google Scholar] [CrossRef]
- Vieira Junior, W.; Caitano, C.; Alves, L.; Teixeira, P.A.; Noble, R.; Pardo, J.; Zied, D. From waste to resource: Sustainable reuse of spent shiitake mushroom substrate in subsequent production cycles. Int. Biodeterior. Biodegrad. 2025, 200, 106034. [Google Scholar] [CrossRef]
- Illera-Vives, M.; López-Mosquera, M.E.; Salas-Sanjuan, M.C.; López-Fabal, A. Leaching techniques for saline wastes composts used as growing media in organic agriculture: Assessment and modelling. Environ. Sci. Pollut. Res. 2015, 22, 6854–6863. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Gomis, M.P.; Pérez-Murcia, M.D.; Gangi, D.; Ceglie, F.G.; Paredes, C.; Pérez-Espinosa, A.; Bernal, M.P.; Moral, R. Use of livestock waste composts as nursery growing media: Effect of a washing pre-treatment. Sci. Hortic. 2021, 281, 109954. [Google Scholar] [CrossRef]
- Chefetz, B.; Van Heemst, J.D.H.; Chen, Y.; Romaine, C.P.; Chorover, J.; Rosario, R.; Mingxin, G.; Hatcher, P.G. Organic matter transformation during weathering process of spent mushroom substrate. J. Environ. Qual. 2000, 29, 592–602. [Google Scholar] [CrossRef]
- Paredes, C.; Medina, E.; Moral, E.; Pérez-Murcia, M.D.; Moreno-Caselles, J.; Bustamante, M.A.; Cecilia, J.A. Characterization of different organic matter fractions of spent mushroom substrate. Commun. Soil Sci. Plant Anal. 2009, 40, 150–161. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production; FAO Soils Bulletin 80; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005. [Google Scholar]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Ciavatta, C.; Govi, M.; Sequi, P. Characterization of organic matter in compost produced with municipal solid wastes: An Italian approach. Compost Sci. Util. 1993, 1, 75–81. [Google Scholar] [CrossRef]
- Gallardo-Lara, F.; Nogales, R. Effect of the application of town refuse compost on the soil-plant system: A review. Biol. Wastes 1987, 19, 35–62. [Google Scholar] [CrossRef]
- Nabi, F.; Sarfaraz, A.; Kama, R.; Kanwal, R.; Li, H. Structure-based function of humic acid in abiotic stress alleviation in plants: A review. Plants 2025, 14, 1916. [Google Scholar] [CrossRef]
- Gowthamchand, N.J.; Sujaina, M. A review on humic substances: Their significance in agriculture as stimulations of plant physiological growth and development. Pharma Innov. J. 2023, SP-12, 177–184. [Google Scholar]
- Haynes, R.J. Labile organic matter as an indicator of organic matter quality in arable and pastoral soils in New Zealand. Soil Biol. Biochem. 2000, 32, 211–219. [Google Scholar] [CrossRef]
- Hofman, J.; Bezchlebova, J.; Dusek, L.; Dolezal, L.; Holoubek, I.; Andel, P.; Ansorgova, A.; Maly, S. Novel approach to monitoring of the soil biological quality. Environ. Int. 2003, 28, 771–778. [Google Scholar] [CrossRef]
- Summerell, B.A.; Burgess, L.W. Decomposition and chemical-composition of cereal straw. Soil Biol. Biochem. 1989, 21, 551–559. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions. Walter de Gruyter: Berlin, Germany, 1984. [Google Scholar]
- Schulten, H.R.; Schnitzer, M. A state of the art structural concept for humic substances. Naturwissenschaften 1993, 80, 29–30. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; Almendros, G.; de la Rosa, J.M.; González-Vila, F.J. Appraisal of polycyclic aromatic hydrocarbons (PAHs) in environmental matrices by analytical pyrolysis (Py-GC/MS). J. Anal. Appl. Pyrolysis 2014, 109, 1–8. [Google Scholar] [CrossRef]
- Leinweber, P.; Jordan, E.; Schulten, H.-R. Molecular characterization of soil organic matter in Pleistocene moraines from the Bolivian Andes. Geoderma 1996, 72, 133–148. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Mazurek, M.A. Organic matter of the troposphere-II. Natural background of biogenic lipid matter in aerosols over the rural western United States, Atmos. Environ. 1982, 16, 2139–2159. [Google Scholar]
- Almendros, G.; Lobo, M.C.; Polo, A.; Dorado, E. Naturaleza y propiedades de la materia orgánica en dos tipos de compost de paja de trigo. Anal. Edafol. Agrobiol. 1984, 42, 2083–2093. [Google Scholar]
- Almendros, G.; González-Pérez, J.A. Soil organic carbon sequestration mechanisms and the molecular composition of soil organic matter—A review. Sustainability 2025, 17, 6689. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).