Spatial Heterogeneity of Heavy Metals in Arid Oasis Soils and Its Irrigation Input–Soil Nutrient Coupling Mechanism
Abstract
1. Introduction
2. Study Area and Method
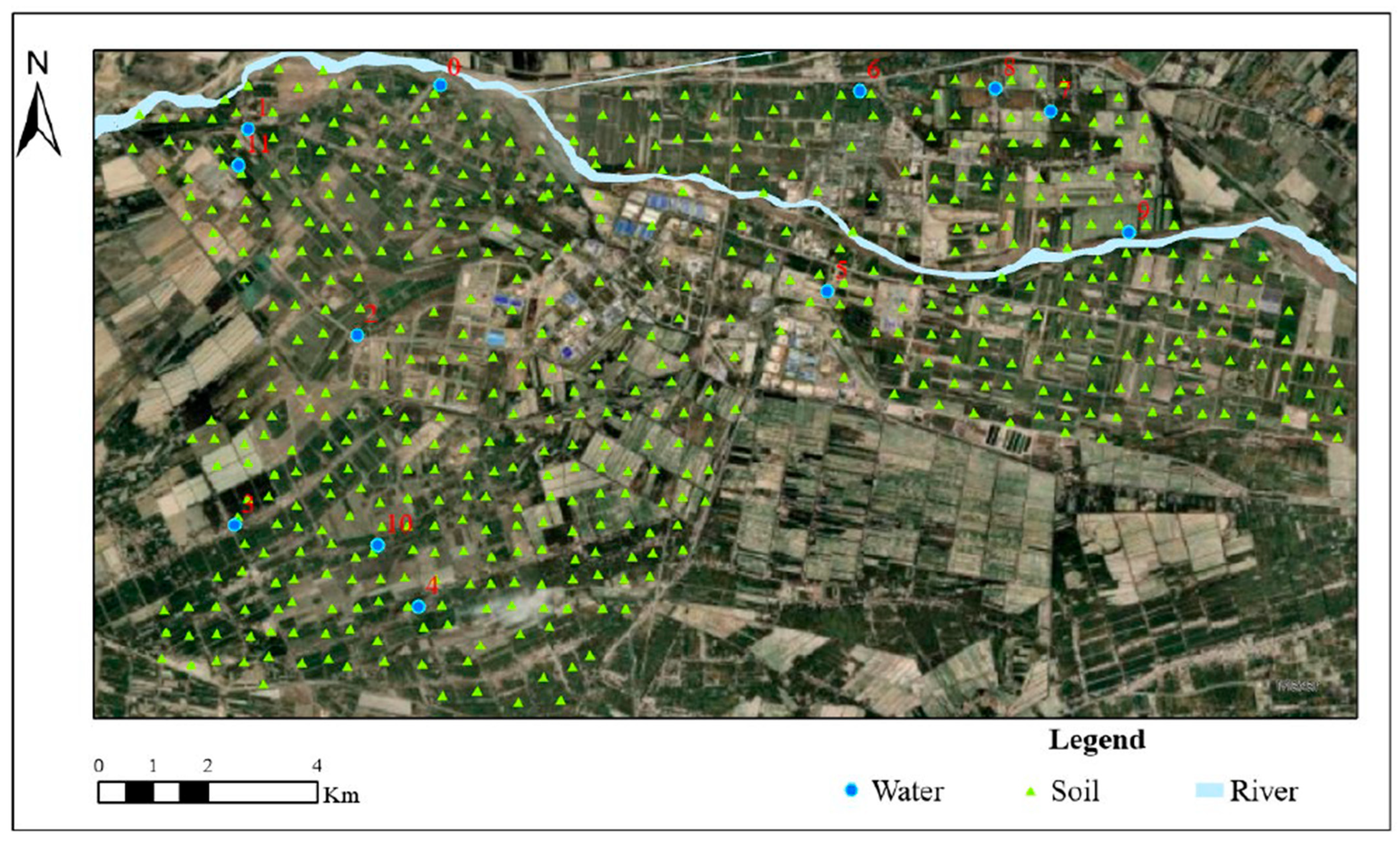
2.1. Study Area
2.2. Sample Collection
2.2.1. Surface Soil Sampling
2.2.2. Irrigation Water Sampling
2.3. Measurement Methods
2.4. Data Processing
3. Results and Analyses
3.1. Characterization of the Content of Irrigation Water by Indicator
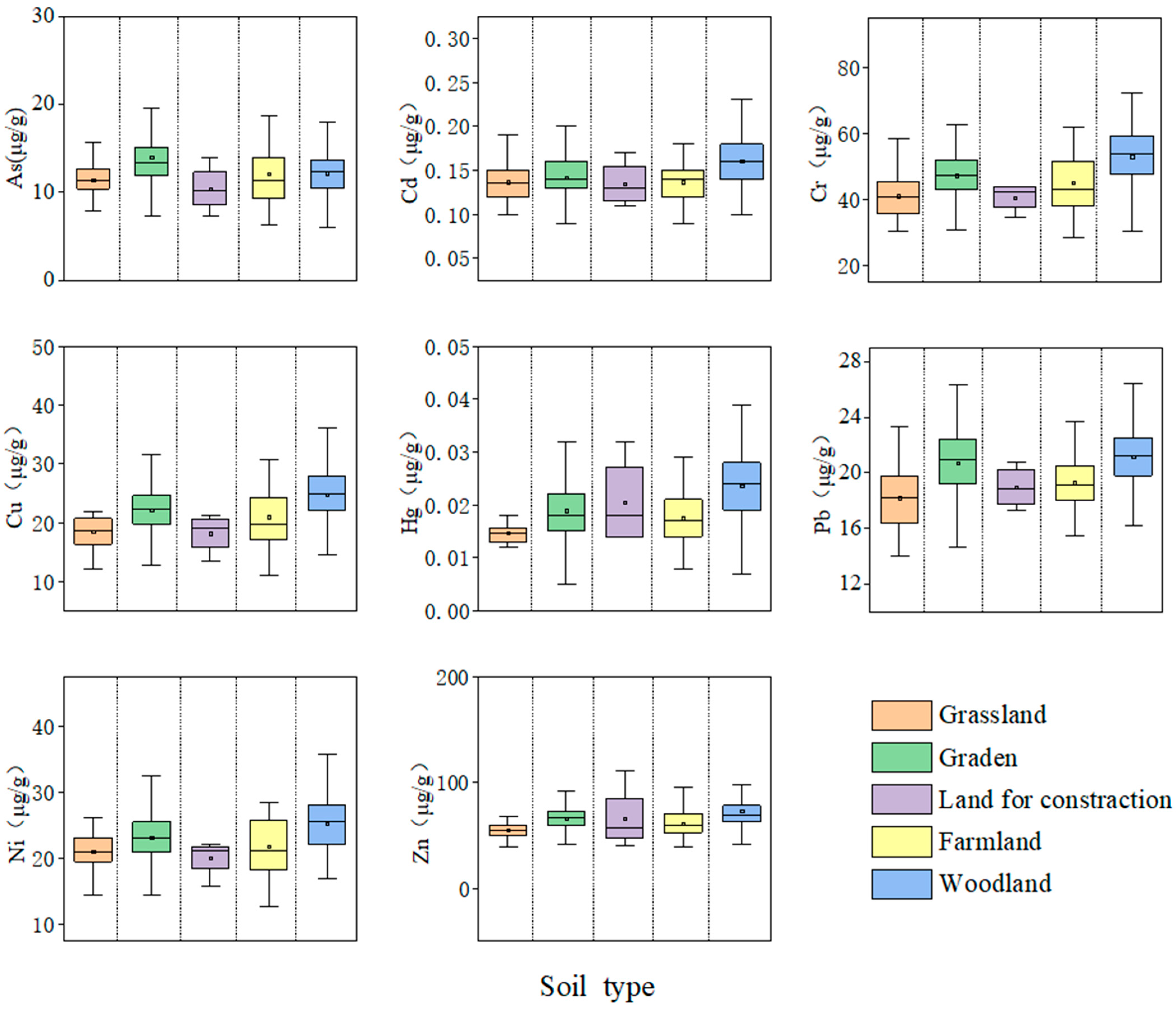
3.2. Characterization of Soil Heavy Metal Content
3.3. Matching Analysis of Spatial Interpolation Results of Soil Heavy Metals with Irrigation Water Sampling Points
3.4. Correlation Analysis Between Soil Heavy Metals and Soil Nutrients
4. Discussion
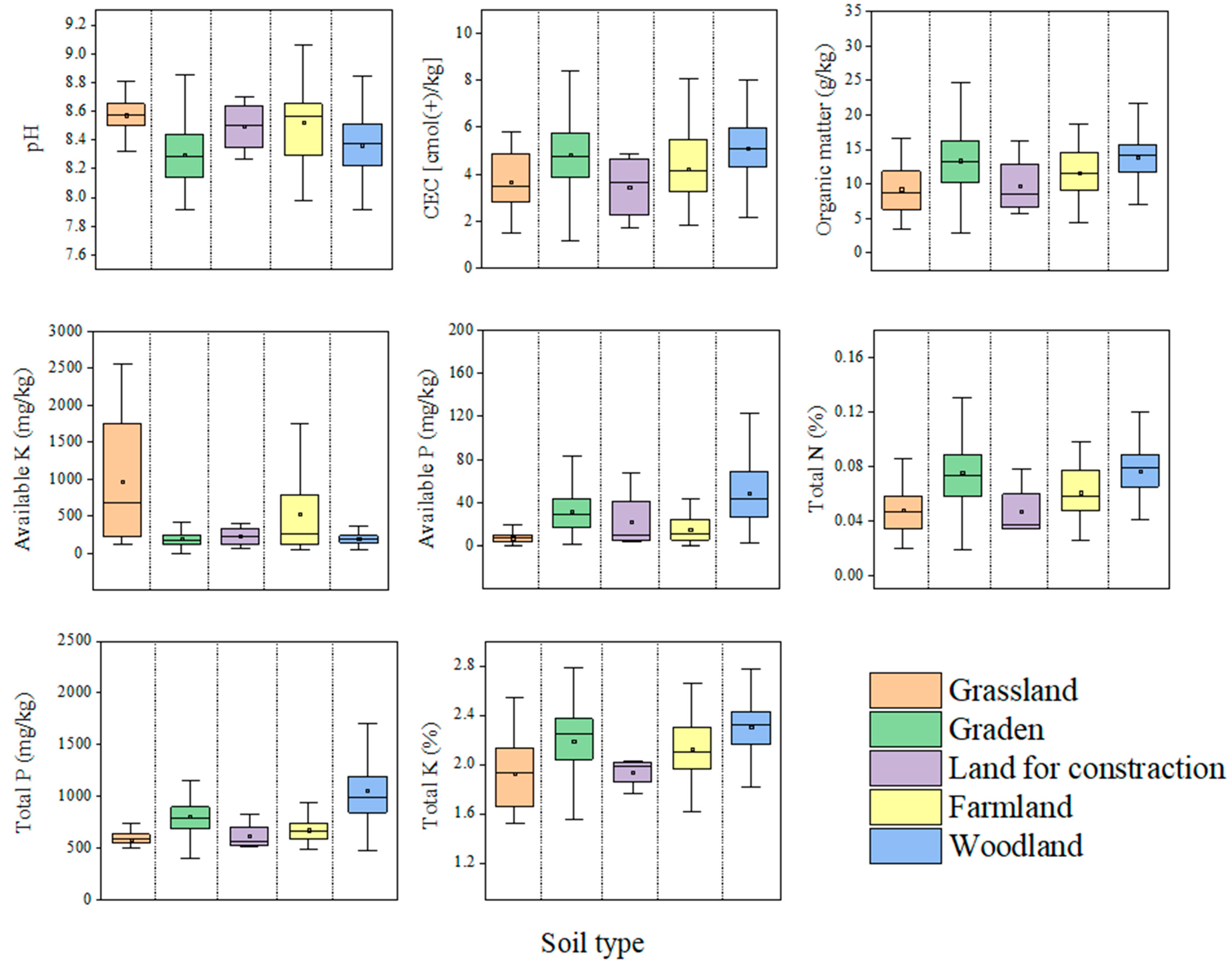
4.1. Characterization of Irrigation Water and Soil Physicochemical Property Contents
4.2. Analysis of Spatial Heterogeneity of Heavy Metals in Soil Around Irrigation Water
4.3. Correlation Analysis Between Heavy Metals and Physical and Chemical Properties of Soils of Different Land Use Types
5. Conclusions
5.1. Characterization of Soil Heavy Metal Content and Its Spatial Heterogeneity
5.2. Potential Driving Effects of Heavy Metals in Irrigation Water on Surrounding Soil Contamination
5.3. Interaction Mechanisms Between Soil Heavy Metals and Soil Physicochemical Properties
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, S.; Salehi, F. Comprehensive assessment of heavy metal (HMs) contamination and associated health risks in agricultural soils and groundwater proximal to industrial sites. Sci. Rep. 2025, 15, 7518. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Ali, M.U.; Xiao, P.; Zhao, P.; Wang, H.; Bibi, S. Heavy metals pollution from smelting activities: A threat to soil and groundwater. Ecotoxicol. Environ. Saf. 2024, 274, 116189. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Fu, Y.H.; Li, F.M.; Guo, S.H.; Zhao, M.Y. Cadmium concentration and its typical input and output fluxes in agricultural soil downstream of a heavy metal sewage irrigation area. J. Hazard. Mater. 2021, 412, 125203. [Google Scholar] [CrossRef]
- Abdulmanov, R.; Miftakhov, I.; Ishbulatov, M.; Ishbulatov, M.; Galeev, E.; Shafeeva, E. Comparison of the effectiveness of GIS-based interpolation methods for estimating the spatial distribution of agrochemical soil properties. Environ. Technol. Innov. 2021, 24, 101970. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C. Natural and human factors affect the distribution of soil heavy metal pollution: A review. Water Air Soil Pollut. 2020, 231, 350. [Google Scholar] [CrossRef]
- Bai, E.; Guo, W.; Zhang, H.; Tan, Y.; Li, X.Y.; Wei, Z.Y. Degradation mechanism of cultivated land and its protection technology in the central coal-grain overlapped area of China. J. Clean. Prod. 2024, 468, 143075. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Mohammed, S.A.S.; Almajed, A.; Al-Shamrani, M.A. Desorption of heavy metals from lime-stabilized arid-soils using different extractants. Int. J. Civ. Eng. 2020, 18, 449–461. [Google Scholar] [CrossRef]
- Peng, L.; Liu, P.; Feng, X.H.; Wang, Z.M.; Cheng, T.; Liang, Y.Z.; Lin, Z.; Shi, Z.Q. Kinetics of heavy metal adsorption and desorption in soil: Developing a unified model based on chemical speciation. Geochim. Cosmochim. Acta 2018, 224, 282–300. [Google Scholar] [CrossRef]
- Chirenje, T.; Ma, L.Q.; Chen, M.; Chen, M.; Zillioux, E.J. Comparison between background concentrations of arsenic in urban and non-urban areas of Florida. Adv. Environ. Res. 2003, 8, 137–146. [Google Scholar] [CrossRef]
- Khatun, J.; Intekhab, A.; Dhak, D. Effect of uncontrolled fertilization and heavy metal toxicity associated with arsenic (As), lead (Pb) and cadmium (Cd), and possible remediation. Toxicology 2022, 477, 153274. [Google Scholar] [CrossRef] [PubMed]
- GB/T 17141-1997; Soil Quality. Determination of Lead, Cadmium. Graphite Furnace Atomic Absorption Spectrophotometry; Chinese Standard: Beijing, China, 1997.
- Economou-Eliopoulos, M.; Megremi, I. Contamination of the soil–groundwater–crop system: Environmental risk and opportunities. Minerals 2021, 11, 775. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Theologou, E.; Bompoti, N.; Dermatas, D.; Panagiotakis, I. Occurrence, origin and transformation processes of geogenic chromium in soils and sediments. Curr. Pollut. Rep. 2016, 2, 224–235. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Hamon, R.E.; McLaren, R.G.; Speir, T.W.; Rogers, S.L. A bioavailability-based rationale for controlling metal and metalloid contamination of agricultural land in Australia and New Zealand. Soil Res. 2000, 38, 1037–1086. [Google Scholar] [CrossRef]
- Du, Y.J.; Fan, R.D.; Reddy, K.R.; Liu, S.Y.; Yang, Y.L. Impacts of presence of lead contamination in clayey soil–calcium bentonite cutoff wall backfills. Appl. Clay Sci. 2015, 108, 111–122. [Google Scholar] [CrossRef]
- Li, R.; Wu, H.; Ding, J.; Fu, W.M.; Gan, L.J.; Li, Y. Mercury pollution in vegetables, grains and soils from areas surrounding coal-fired power plants. Sci. Rep. 2017, 7, 46545. [Google Scholar] [CrossRef]
- Chaney, R.L.; Sterrett, S.B.; Mielke, H.W. The Potential for Heavy Metal Exposure from Urban Gardens and Soils. In Heavy Metals in Urban Gardens; University District Columbia Extension Service: Washington, DC, USA, 1984; pp. 37–84. [Google Scholar]
- Sharifi, S.A.; Zaeimdar, M.; Jozi, S.A.; Hejazi, R. Effects of soil, water and air pollution with heavy metal ions around lead and zinc mining and processing factories. Water Air Soil Pollut. 2023, 234, 760. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Hu, S.; Bai, Y.F. Effects of nitrogen enrichment on belowground communities in grassland: Relative role of soil nitrogen availability vs. soil acidification. Soil Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.P.; Yu, M.; Cao, N.N.; Yan, J.H. Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem. Geol. 2018, 501, 86–94. [Google Scholar] [CrossRef]
- Carmo, D.L.; Lima, L.B.; Silva, C.A. Soil fertility and electrical conductivity affected by organic waste rates and nutrient inputs. Rev. Bras. De Ciência Do Solo 2016, 40, e0150152. [Google Scholar] [CrossRef]
- Henderson, G.S. Soil organic matter: A link between forest management and productivity. Carbon Forms Funct. For. Soils 1995, 419–435. [Google Scholar] [CrossRef]
- Solomon, D.; Fritzsche, F.; Tekalign, M.; Lehmann, J.; Zech, W. Soil organic matter composition in the subhumid Ethiopian highlands as influenced by deforestation and agricultural management. Soil Sci. Soc. Am. J. 2002, 66, 68–82. [Google Scholar] [CrossRef]
- Nascimento, D.B.; Lopes, M.L.S.; Izidro, J.L.P.S. Nitrogen, phosphorus, and potassium cycling in pasture ecosystems. Ciência Anim. Bras. 2024, 25, e-76743. [Google Scholar] [CrossRef]
- Sorkau, E.; Boch, S.; Boeddinghaus, R.S.; Bonkowski, M.; Fischer, M.; Kandeler, E.; Klaus, V.H.; Kleinebecker, T.; Marhan, S.; Müller, J.; et al. The role of soil chemical properties, land use and plant diversity for microbial phosphorus in forest and grassland soils. J. Plant Nutr. Soil Sci. 2018, 181, 185–197. [Google Scholar] [CrossRef]
- Yang, P.; Mao, R.; Shao, H.; Gao, Y. The spatial variability of heavy metal distribution in the suburban farmland of Taihang Piedmont Plain, China. C. R. Biol. 2009, 332, 558–566. [Google Scholar] [CrossRef]
- Farella, N.; Lucotte, M.; Davidson, R.; Daigle, S. Mercury release from deforested soils triggered by base cation enrichment. Sci. Total Environ. 2006, 368, 19–29. [Google Scholar] [CrossRef]
- Xuerui Cao, X.; Xiaozi Wang, X.; Wenbin Tong, W.; Hanumanth, K. Distribution, availability and translocation of heavy metals in soil-oilseed rape (Brassica napus L.) system related to soil properties. Environ. Pollut. 2019, 252 Pt A, 733–741. [Google Scholar] [CrossRef]
- Jia, Y.; Guo, H.; Xi, B.; Jiang, Y.H.; Zhang, Z.; Yuan, R.X.; Yi, W.X.; Xue, X.L. Sources of groundwater salinity and potential impact on arsenic mobility in the western Hetao Basin, Inner Mongolia. Sci. Total Environ. 2017, 601, 691–702. [Google Scholar] [CrossRef]
- Suciu, N.A.; Vivo, R.D.; Rizzati, N.; Capri, E. Cd content in phosphate fertilizer: Which potential risk for the environment and human health? J. Curr. Opin. Environ. Sci. Health 2022, 30, 100392. [Google Scholar] [CrossRef]
- Tang, Y.; Shi, X.; Liu, Y.; Wang, T.; Zhang, D.; Wang, C. Effects of Potassium Fertilizer on Maize Growth and Heavy Metal Content in Heavy Metal Contaminated Soil. Int. J. Agric. Biol. 2020, 24, 1560–8530. [Google Scholar]
- Wyszkowski, M.; Brodowska, M.S. Content of trace elements in soil fertilized with potassium and nitrogen. Agriculture 2020, 10, 398. [Google Scholar] [CrossRef]
- Jalali, M.; Farahani, E.A.; Jalali, M. The impact of organic and inorganic fertilizers on availability and speciation of phosphorus and heavy metals in calcareous soils. Environ. Earth Sci. 2023, 82, 142. [Google Scholar] [CrossRef]
- Yu, L.; Lu, X.; He, Y.; Brookes, P.C.; Liao, H.; Xu, J.M. Combined biochar and nitrogen fertilizer reduces soil acidity and promotes nutrient use efficiency by soybean crop. J. Soils Sediments 2017, 17, 599–610. [Google Scholar] [CrossRef]
- Gao, J.; Han, H.; Gao, C.; Wang, Y.H.; Dong, B.; Xu, Z.X. Organic amendments for in situ immobilization of heavy metals in soil: A review. Chemosphere 2023, 335, 139088. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Bian, Y.; Miller, C.L.; Dong, W.; Jiang, X.; Liang, L. Mercury reduction and complexation by natural organic matter in anoxic environments. Proc. Natl. Acad. Sci. USA 2011, 108, 1479–1483. [Google Scholar] [CrossRef]
- Kwiatkowska-Malina, J. Functions of organic matter in polluted soils: The effect of organic amendments on phytoavailability of heavy metals. Appl. Soil Ecol. 2018, 123, 542–545. [Google Scholar] [CrossRef]
- Li, L.; Wu, H.; van Gestel, C.A.M.; Peijnenburg, W.J.G.M.; Allen, H.E. Soil acidification increases metal extractability and bioavailability in old orchard soils of Northeast Jiaodong Peninsula in China. Environ. Pollut. 2014, 188, 144–152. [Google Scholar] [CrossRef]



| Index | Assay Method | Index | Assay Method |
|---|---|---|---|
| Cu | ICP-MS | CEC | Ammonium acetate exchange method (pH 7.0) |
| Cr | ICP-OES | pH | Glass electrode method (soil/water = 1:2.5) |
| Ni | ICP-MS | B | ICP-MS |
| Zn | ICP-OES | Total N | Kjeldahl method |
| Pb | ICP-MS | Total P | Molybdenum antimony anti-spectrophotometry |
| Cd | ICP-MS | Total K | HF-HClO4 digestion-Flame photometry |
| Se | ICP-MS | Organic matter | Potassium dichromate oxidation-external heating method |
| As | ICP-MS | Available P | Olsen method (sodium bicarbonate extraction) |
| Hg | CVAAS | Available K | Ammonium acetate extraction-Flame photometry |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, C.; Wang, J.; Li, L.; He, J.; Zhao, F. Spatial Heterogeneity of Heavy Metals in Arid Oasis Soils and Its Irrigation Input–Soil Nutrient Coupling Mechanism. Sustainability 2025, 17, 7156. https://doi.org/10.3390/su17157156
Liu J, Li C, Wang J, Li L, He J, Zhao F. Spatial Heterogeneity of Heavy Metals in Arid Oasis Soils and Its Irrigation Input–Soil Nutrient Coupling Mechanism. Sustainability. 2025; 17(15):7156. https://doi.org/10.3390/su17157156
Chicago/Turabian StyleLiu, Jiang, Chongbo Li, Jing Wang, Liangliang Li, Junling He, and Funian Zhao. 2025. "Spatial Heterogeneity of Heavy Metals in Arid Oasis Soils and Its Irrigation Input–Soil Nutrient Coupling Mechanism" Sustainability 17, no. 15: 7156. https://doi.org/10.3390/su17157156
APA StyleLiu, J., Li, C., Wang, J., Li, L., He, J., & Zhao, F. (2025). Spatial Heterogeneity of Heavy Metals in Arid Oasis Soils and Its Irrigation Input–Soil Nutrient Coupling Mechanism. Sustainability, 17(15), 7156. https://doi.org/10.3390/su17157156






