Plant Diversity of Concessions Held by Catholic Religious Groups in Three Cities of the Democratic Republic of the Congo
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Plant Composition and Biological Spectrum of Catholic Religious Groups’ Concessions and Area Effects
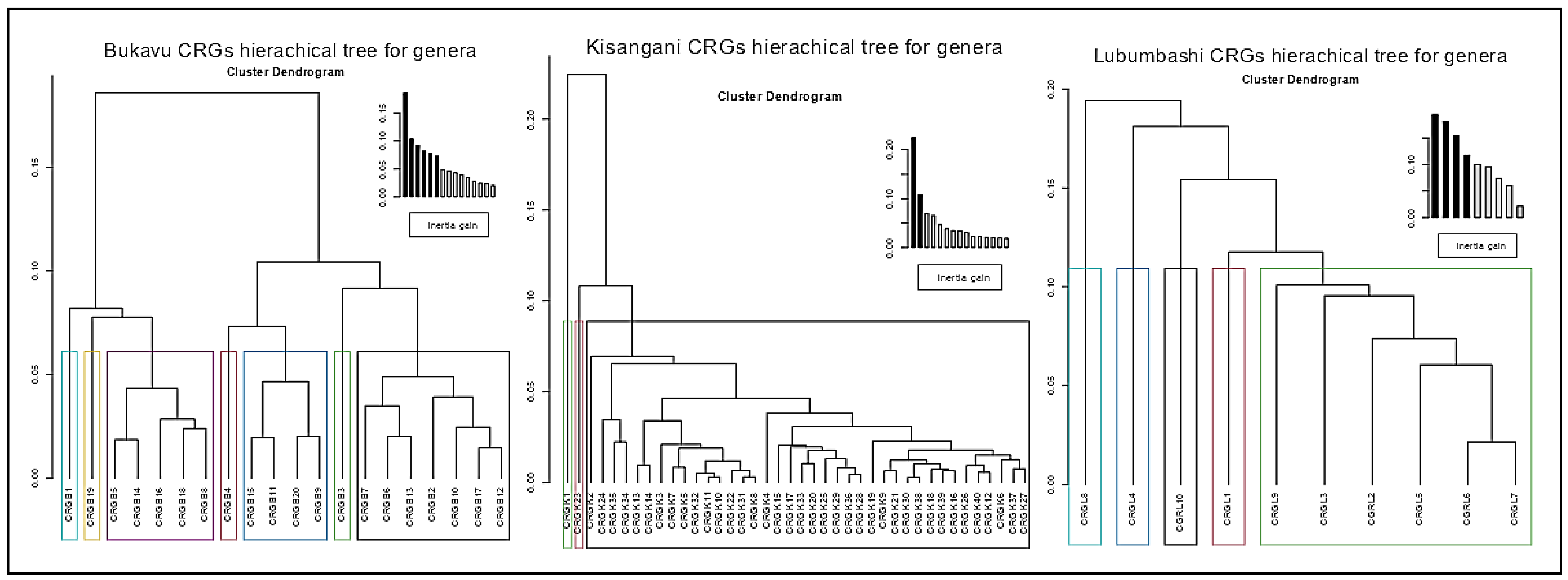
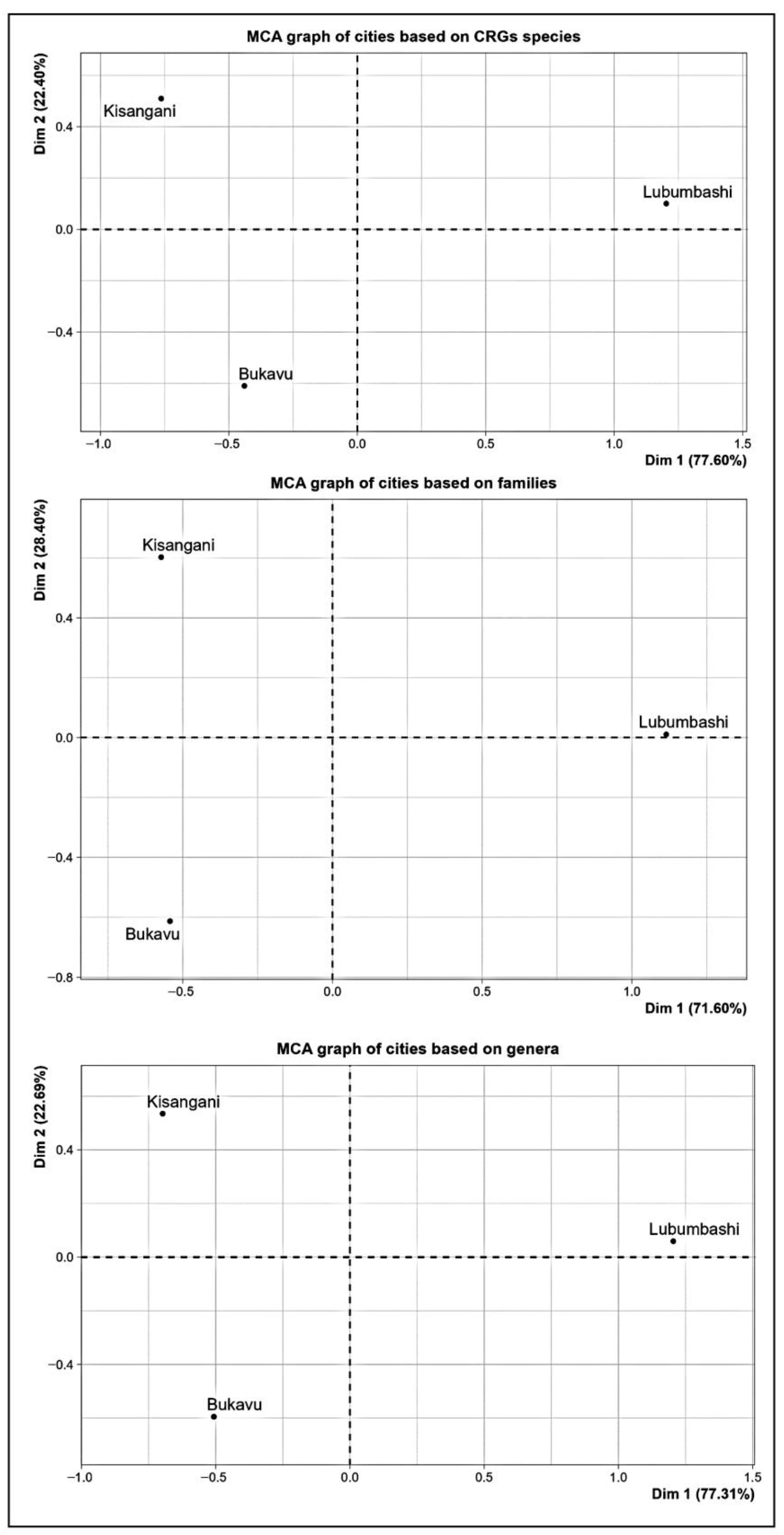
3.2. Comparative Plant Composition Between CRGs and Cities
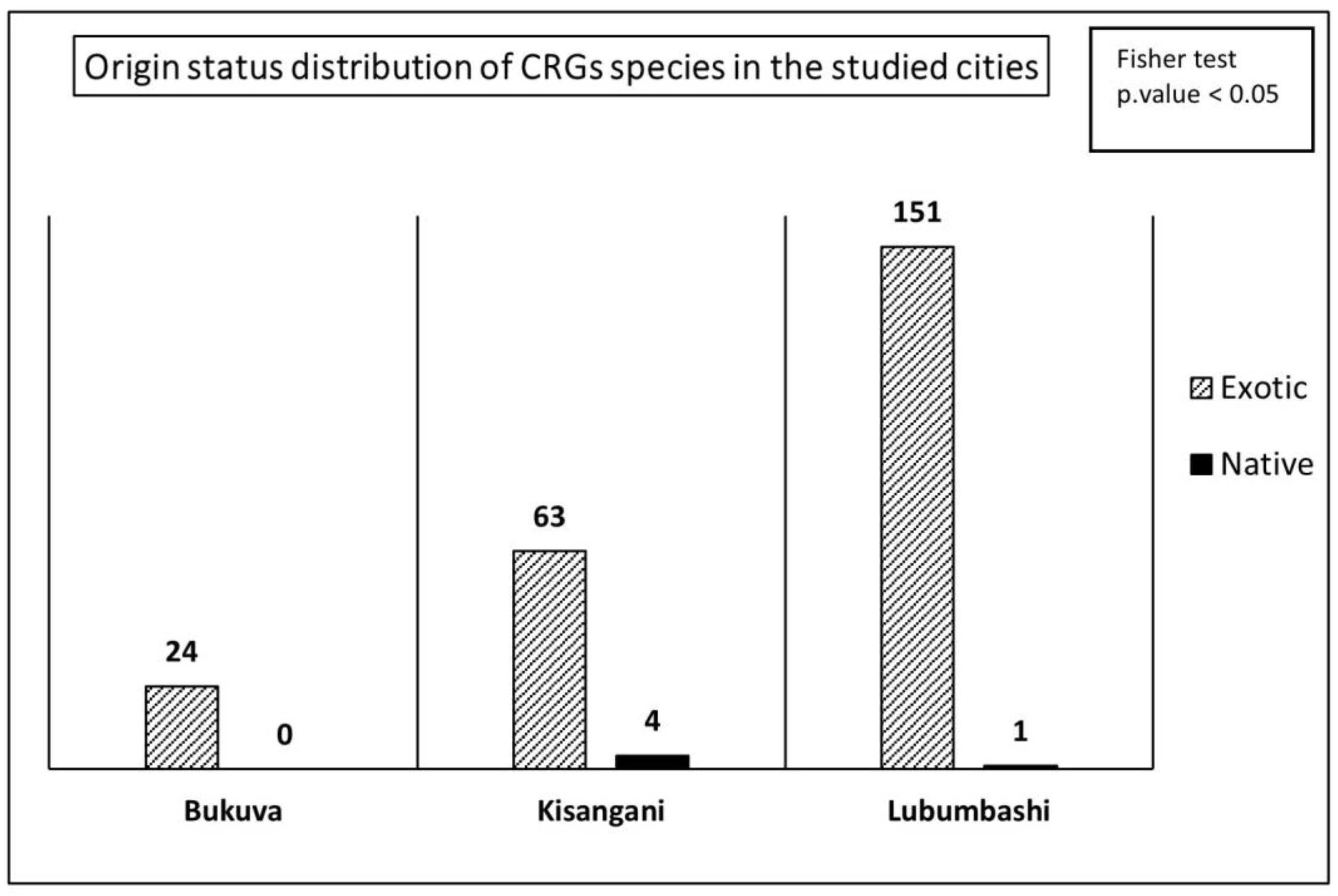
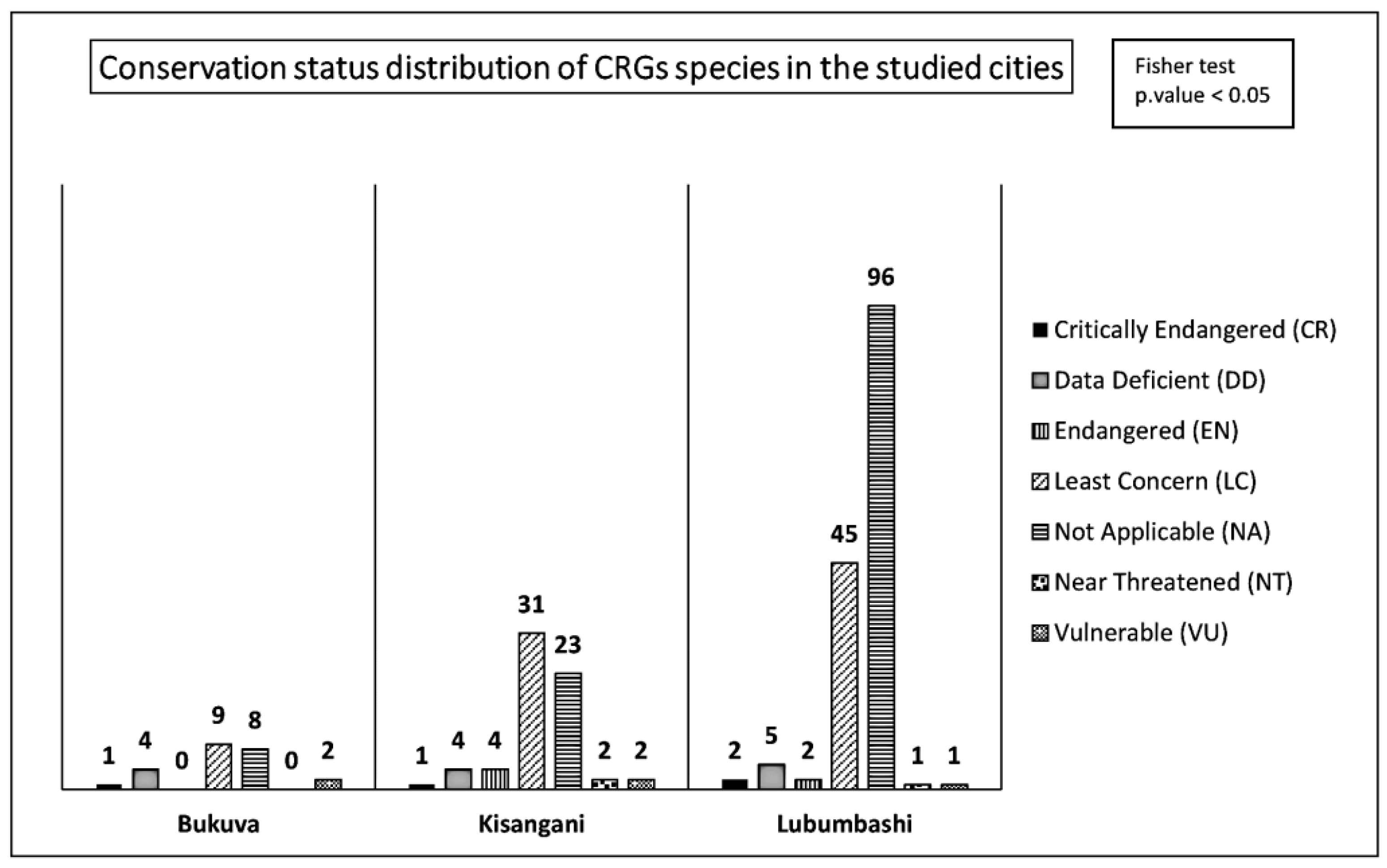
3.3. Species Origin and Conservation Status in CRGs
4. Discussion
4.1. Methodological Considerations
4.2. Intra- and Inter-City Floristic Variation in CRGs
4.3. Phytobiodiverse Significance of CRGs
4.4. Implications for Urban Biodiversity Management and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| CRG’s Code | CRG’s Name | Area (ha) |
|---|---|---|
| Lubumbashi | ||
| CRGL1 | Convent of Saint Paul Parish | 0.11 |
| CRGL2 | Theological Institute—Chaplains of Work | 0.32 |
| CRGL3 | Tabora University Cultural Center | 0.17 |
| CRGL4 | Theologicum | 1.25 |
| CRGL5 | Provincial House of the Franciscans | 0.2 |
| CRGL6 | Tertiary Capuchin Sisters—Nazareth Homes | 0.15 |
| CRGL7 | Scholasticate—Chaplains of Work | 0.15 |
| CRGL8 | Laura House | 8.32 |
| CRGL9 | Carmelite Sisters | 0.58 |
| CRGL10 | Mercedarian Missionaries | 0.19 |
| Bukavu | ||
| CRGB1 | Bukavu Amani Center | 0.51 |
| CRGB2 | Kasongo Procuracy | 6.59 |
| CRGB3 | The Corniche | 0.24 |
| CRGB4 | Xaverian Sisters | 5.07 |
| CRGB5 | Missionaries of Africa | 0.41 |
| CRGB6 | Cirezi High School | 0.52 |
| CRGB7 | Cathedral of Our Lady of Bukavu | 1.05 |
| CRGB8 | Solidarity | 2.89 |
| CRGB9 | Saint Joseph Sisters | 1.09 |
| CRGB10 | Father Vavassori Health Center | 3.85 |
| CRGB11 | Saint John the Baptist Parish—Cahi | 2.37 |
| CRGB12 | Antonella School | 0.97 |
| CRGB13 | Holy Family Parish of Bagira | 0.06 |
| CRGB14 | Nyakavogo High School | 5.13 |
| CRGB15 | Nyakavogo Primary School | 2.15 |
| CRGB16 | Catholic University of Bukavu Bugabo | 1.29 |
| CRGB17 | Saint Francis Xavier Parish—Kadutu | 0.65 |
| CRGB18 | Fundi Maendeleo Technical Institute | 14.38 |
| CRGB19 | Wima High School | 19.71 |
| CRGB20 | General Economat | 5.64 |
| Kisangani | ||
| CRGK1 | Kisangani Little Seminary of Mandombe | 3 |
| CRGK2 | Saint Peter Parish | 3 |
| CRGK3 | Saint Albert Chapel | 2 |
| CRGK4 | Saint Martha Parish | 8 |
| CRGK5 | Cathedral of Our Lady of the Most Holy Rosary | 8 |
| CRGK6 | Father Dehonus Scholasticate | 8 |
| CRGK7 | Simama Center | 3 |
| CRGK8 | Servant Sisters of Jesus | 10 |
| CRGK9 | Sisters of the Holy Family Mediatrix | 1 |
| CRGK10 | Augustinian Sisters | 1 |
| CRGK11 | Pastoral House of the Sacred Heart | 10 |
| CRGK12 | Convent of the Priests of Mont Fortaint | 3 |
| CRGK13 | Bel Vedere | 25 |
| CRGK14 | Saint Gabriel Parish | 4 |
| CRGK15 | Convent of the Priests of the Sacred Heart | 2 |
| CRGK16 | Sisters of Jesus Educator Station Kis-Bondo | 2 |
| CRGK17 | Canonical Sisters | 3 |
| CRGK18 | Sisters Novitiate Holy Family | 3 |
| CRGK19 | Saint Camille Parish | 0.4 |
| CRGK20 | Josephites of Kinzambi | 0.49 |
| CRGK21 | Sisters Holy Family Artisan | 0.15 |
| CRGK22 | Marist Brothers | 2 |
| CRGK23 | Formation House Scholasticate | 2 |
| CRGK24 | Saint Augustine Major Seminary | 1 |
| CRGK25 | Saint Lawrence Parish | 4 |
| CRGK26 | Deo Soli/Scholasticate | 0.25 |
| CRGK27 | Daughters of Wisdom | 0.08 |
| CRGK28 | Sisters Immaculate Conception | 7 |
| CRGK29 | Sisters Saint Joseph House | 0.32 |
| CRGK30 | Saint John Parish | 2.5 |
| CRGK31 | Blessed Isidore Bakanja Parish | 0.49 |
| CRGK32 | Blessed Anuarité Parish | 2 |
| CRGK33 | Deo Soli/Scholasticate 7th Plateau | 0.25 |
| CRGK34 | Comboni House | 0.49 |
| CRGK35 | Technical High School Mapendano | 7 |
| CRGK36 | The Moinnaux | 4 |
| CRGK37 | Mary Queen of Peace | 49 |
| CRGK38 | Christ the King Parish | 4 |
| CRGK39 | Saint Ignatius Parish | 3 |
| CRGK40 | Saint Joseph Artisan Parish | 20 |
| No. | Scientific Name | Family | Conservation Status | Life Form | Origin Status | Bukavu | Kisangani | Lubumbashi |
|---|---|---|---|---|---|---|---|---|
| 1 | Acacia auriculiformis A. Cunn. ex Benth., 1842 | Fabaceae | LC | Ph | Ex | − | + | − |
| 2 | Acacia nilotica (L.) Willd. ex Delile | Fabaceae | LC | Ph | Ex | − | − | − |
| 3 | Acalypha wilkesiana Müll. Arg., 1866 | Euphorbiaceae | NA | Ph | Ex | − | − | + |
| 4 | Agave americana L. | Asparagaceae | LC | Ch | Ex | − | − | + |
| 5 | Agave attenuata Salm-Dyck, 1834 | Asparagaceae | NA | Ch | Ex | − | − | + |
| 6 | Aglaonema commutatum Schott, 1856 | Araceae | NA | Hem | Ex | − | − | + |
| 7 | Albizia chinensis (Osbeck) Merr., 1916 | Fabaceae | NA | Ph | Ex | − | + | − |
| 8 | Albizia gummifera (J. F. Gmel.) C. A. Sm., 1930 | Mimosaceae | LC | Ph | Ex | − | + | − |
| 9 | Albizia julibrissin Durazz., 1772 | Fabaceae | NA | Ph | Ex | − | + | − |
| 10 | Alocasia macrorrhizos (L.) G. Don, 1839 | Araceae | NA | Ge | Ex | − | − | + |
| 11 | Aloe arborescens Mill., 1768 | Asphodelaceae | LC | Ge | Ex | − | − | + |
| 12 | Aloe vera (L.) Burm. f., 1768 | Asphodelaceae | NA | Ge | Ex | − | − | + |
| 13 | Alternanthera brasiliana (L.) Kuntze, 1891 | Amaranthaceae | NA | Ph | Ex | − | − | + |
| 14 | Amaranthus hybridus L., 1753 | Amaranthaceae | NA | Ch | Ex | − | − | + |
| 15 | Annona muricata L., 1753 | Annonaceae | LC | Ph | Ex | − | − | + |
| 16 | Annona senegalensis Pers., 1806 | Annonaceae | LC | Ph | Ex | − | + | − |
| 17 | Anonidium mannii (Oliv.) Engler & Diels, 1901 | Annonaceae | LC | Ph | Ex | − | + | − |
| 18 | Anthocleista schweinfurthii Gilg, 1893 | Loganiaceae | LC | Ph | Ex | − | + | − |
| 19 | Antigonon leptopus Hook. & Arn., 1838 | Polygonaceae | NA | Ch | Ex | − | − | + |
| 20 | Araucaria cunninghamii Aiton ex D. Don, 1837 | Araucariaceae | LC | Ph | Ex | − | − | + |
| 21 | Archontophoenix alexandrae H. Wendl. & Drude, 1875 | Arecaceae | LC | Ph | Ex | − | − | + |
| 22 | Aristaloe aristata Adrian Hardy Haworth, 1825 | Xanthorrhoeaceae | NA | Ge | Ex | − | − | + |
| 23 | Artocarpus altilis (Parkinson) Fosberg, 1941 | Moraceae | NA | Ph | Ex | − | + | − |
| 24 | Artocarpus camansi Blanco, 1837 | Moraceae | NT | Ph | Ex | − | + | − |
| 25 | Artocarpus heterophyllus Lam., 1789 | Moraceae | NA | Ph | Ex | − | − | + |
| 26 | Aspidistra elatior Blume, 1834 | Asparagaceae | NA | Ph | Ex | − | − | + |
| 27 | Asplenium nidus L., 1753 | Aspleniaceae | NA | Hem | Ex | − | − | + |
| 28 | Autranella congolensis (De Wild.) A. Chev. | Sapotaceae | EN | Ph | NA | − | + | − |
| 29 | Averrhoa carambola L., 1753 | Oxalidaceae | DD | Ph | Ex | − | + | − |
| 30 | Bambusa vulgaris Schrad. ex J. C. Wendl., 1810 | Poaceae | NA | Ph | Ex | + | − | + |
| 31 | Bauhinia variegata Carl Von Linne, 1753 | Fabaceae | LC | Ph | Ex | − | − | + |
| 32 | Begonia rex Jules Antoine Adolph Henri Putzeys, 1856 | Begoniaceae | NA | Epi | Ex | − | − | + |
| 33 | Bellucia pentamera Naudin | Melastomataceae | LC | Ph | Ex | − | + | − |
| 34 | Borassus flabellifer L., 1977 | Arecaceae | NA | Ph | Ex | − | − | + |
| 35 | Bougainvillea glabra Philibert Commerson, 1760 | Nyctaginaceae | LC | Ph | Ex | − | − | + |
| 36 | Breynia disticha J. R. Forst. & G. Forst., 1775 | Euphorbiaceae | NA | Ph | Ex | − | − | + |
| 37 | Caladium bicolor (Aiton) Vent., 1801 | Araceae | NA | Ch | Ex | − | − | + |
| 38 | Callistemon citrinus (Curtis) Skeels, 1913 | Myrtaceae | NA | Ph | Ex | − | − | + |
| 39 | Callistemon viminalis (Sol. ex Gaertn.) G. Don, 1830 | Myrtaceae | NA | Ph | Ex | − | − | + |
| 40 | Cananga odorata Albert Schwenger, 1860 | Annonaceae | LC | Ph | Ex | − | + | − |
| 41 | Canna indica L., 1753 | Cannaceae | NA | Ph | Ex | − | − | + |
| 42 | Carica papaya L., 1753 | Caricaceae | DD | Ph | Ex | + | + | + |
| 43 | Cascabela thevetia (Pers.) K. Schum,1895 | Apocynaceae | LC | Ph | Ex | − | − | + |
| 44 | Casimiroa edulis La Llave & Lex, 1825 | Rutaceae | LC | Ph | Ex | − | − | + |
| 45 | Cassia siamea (Lam.) H. S. Irwin & Barneby, 1982 | Fabaceae | LC | Ph | Ex | − | + | − |
| 46 | Catharanthus roseus (L.) G. Don, 1837 | Apocynaceae | NA | Ph | Ex | − | − | + |
| 47 | Celosia cristata L., 1753 | Amaranthaceae | LC | Ph | Ex | − | − | + |
| 48 | Cestrum nocturnum L., 1753 | Solanaceae | LC | Ph | Ex | − | − | + |
| 49 | Chamaedorea cataractarum Mart., 1849 | Arecaceae | NA | Ph | Ex | − | − | + |
| 50 | Chamaerops humilis L., 1753 | Arecaceae | LC | Ph | Ex | − | − | + |
| 51 | Chelidonium majus L., 1753 | Papaveraceae | NA | Ph | Ex | − | − | + |
| 52 | Chlorophytum comosum Jacques, 1862 | Asparagaceae | NA | Hem | Ex | − | − | + |
| 53 | Citrus aurantium L., 1753 | Rutaceae | NA | Ph | Ex | − | − | + |
| 54 | Citrus limon (L.) Osbeck, 1765 | Rutaceae | NA | Ph | Ex | + | + | + |
| 55 | Citrus maxima (Burm.) Merrill, 1917 | Rutaceae | NA | Ph | Ex | + | + | − |
| 56 | Citrus reticulata Blanco, 1837 | Rutaceae | NA | Ph | Ex | − | + | − |
| 57 | Citrus sinensis (L.) Osbeck, 1765 | Rutaceae | NA | Ph | Ex | + | + | − |
| 58 | Clerodendrum thomsoniae Balf., 1862 | Lamiaceae | NA | Ph | Ex | − | − | + |
| 59 | Clivia miniata William J. Burchell en 1815 | Amaryllidaceae | NA | Ph | Ex | − | − | + |
| 60 | Cocos nucifera L., 1753 | Arecaceae | NA | Ph | Ex | − | + | − |
| 61 | Codiaeum variegatum (L.) Rumph. ex A. Juss., 1824 | Euphorbiaceae | LC | Ph | Ex | − | − | + |
| 62 | Coffea arabica L., 1753 | Rubiaceae | EN | Ph | Ex | − | + | + |
| 63 | Cola acuminata (P. Beauv.) Schott & Endl., 1832 | Malvaceae | LC | Ph | Ex | − | + | − |
| 64 | Coleus amboinicus Lour., 1790 | Lamiaceae | NA | Ph | Ex | − | − | + |
| 65 | Coleus scutellarioides (L.) Benth., 1830 | Lamiaceae | NA | Ph | Ex | − | − | + |
| 66 | Colocasia esculenta (L.) Schott, 1832 | Araceae | LC | Ph | Ex | − | − | + |
| 67 | Cordyline fruticosa (L.) A. Chev., 1919 | Asparagaceae | LC | Ph | Ex | − | − | + |
| 68 | Cornus drummondii C. A. Mey., 1845 | Cornaceae | LC | Ph | Ex | − | − | + |
| 69 | Cupaniopsis anacardioides (A. Rich.) Radlk., 1879 | Sapindaceae | LC | Ph | Ex | − | − | + |
| 70 | Cuphea hyssopifolia Kunth, 1823 | Lythraceae | NA | Ph | Ex | − | − | + |
| 71 | Cupressus macrocarpa Hartw., 1847 | Cyperaceae | NA | Ph | Ex | − | − | + |
| 72 | Cycas revoluta Carl Peter Thunberg, 1782 | Cycadaceae | NA | Ph | Ex | − | − | + |
| 73 | Cyperus alternifolius Carl von Linné, 1767 | Cyperaceae | NA | Ph | Ex | − | − | + |
| 74 | Cyperus esculentus L., 1753 | Cyperaceae | NA | Ph | Ex | − | − | + |
| 75 | Cyperus papyrus Linné, 1753 | Cyperaceae | NA | Ge | Ex | − | − | + |
| 76 | Dacryodes edulis [G.Don] H. J. Lam, 1832 | Burseraceae | NA | Ph | Ex | − | + | − |
| 77 | Dianella ensifolia (L.) Redouté, 1802 | Asphodelaceae | NA | Ph | Ex | − | − | + |
| 78 | Dieffenbachia seguine (Jacq.) Schott, 1829 | Araceae | NA | Ph | Ex | − | − | + |
| 79 | Dillenia indica (L.), 1753 | Dilleniaceae | LC | Ph | Ex | − | − | + |
| 80 | Dodonaea viscosa Jacq., 1760 | Sapindaceae | LC | Ph | Ex | − | − | + |
| 81 | Dracaena fragrans (L.) Ker Gawl., 1808 | Asparagaceae | LC | Ph | Ex | − | − | + |
| 82 | Dracaena reflexa Lam., 1786 | Asparagaceae | NA | Ph | Ex | − | − | + |
| 83 | Duranta erecta L., 1753 | Verbenaceae | LC | Ph | Ex | − | − | + |
| 84 | Dypsis lutescens (H. Wendl.) Beentje & J. Dransf., 1995 | Arecaceae | NT | Ph | Ex | − | − | + |
| 85 | Elaeis guineensis Jacq., 1763 | Arecaceae | LC | Ph | Ex | + | + | + |
| 86 | Entandrophragma candollei Harms, 1896 | Meliaceae | VU | Ph | Ex | − | + | − |
| 87 | Epipremnum aureum (Linden & André) Bunting, 1964 | Araceae | NA | Ch | Ex | − | − | + |
| 88 | Erythrina abyssinica Lam. ex DC., 1825 | Fabaceae | NA | Ph | Ex | + | − | − |
| 89 | Eucalyptus globulus Labill., 1800 | Myrtaceae | LC | Ph | Ex | + | − | − |
| 90 | Eucharis amazonica Linden ex Planch., 1857 | Liliaceae | NA | Ge | Ex | − | − | + |
| 91 | Euphorbia cotinifolia L., 1753 | Euphorbiaceae | NA | Ph | Ex | − | − | + |
| 92 | Euphorbia resinifera O. Berg, 1863 | Euphorbiaceae | NA | Ph | Ex | − | − | + |
| 93 | Euphorbia royleana E. Ursch et J. D. Léandri, 1954 | Euphorbiaceae | NA | Ph | Ex | − | − | + |
| 94 | Ficus benjamina L., 1767 | Moraceae | LC | Ph | Ex | − | − | + |
| 95 | Ficus mucuso Welw. ex Ficalho, 1884 | Moraceae | LC | Ph | Ex | − | + | − |
| 96 | Ficus vallis-choudae Delile, 1843 | Marantaceae | NA | Ph | Ex | − | + | − |
| 97 | Fragaria vesca L., 1753 | Rosaceae | LC | Ch | Ex | − | − | + |
| 98 | Goeppertia makoyana (É.Morren) Borchs. & S. Suárez, 2012 | Marantaceae | NA | Ch | Ex | − | − | + |
| 99 | Goeppertia zebrina (Sims) Nees, 1831 | Marantaceae | NA | Ph | Ex | − | − | + |
| 100 | Graptophyllum balansae Heine, 1976 | Acanthaceae | NA | Ph | Ex | − | − | + |
| 101 | Grevillea robusta A.Cunn. ex R. Br., 1830 | Proteaceae | LC | Ph | Ex | + | + | − |
| 102 | Harungana madagascariensis Lam. ex Poir., 1804 | Hypericaceae | LC | Ph | Ex | − | + | − |
| 103 | Hemerocallis fulva (L.) L., 1762 | Asphodelaceae | NA | Ph | Ex | − | − | + |
| 104 | Hevea brasiliensis (Willd. ex A. Juss.) Mull. Arg., 1865 | Euphorbiaceae | LC | Ph | Ex | − | + | − |
| 105 | Hibiscus rosa-sinensis L., 1753 | Malvaceae | NA | Ph | Ex | − | − | + |
| 106 | Hibiscus tiliaceus L., 1753 | Malvaceae | NA | Ph | Ex | − | + | − |
| 107 | Hydrocotyle verticillata Thunb., 1798 | Araliaceae | LC | Ge | Ex | − | − | + |
| 108 | Hymenocallis littoralis (Jacq.) Salisb., 1812 | Amaryllidaceae | NA | Ph | Ex | − | − | + |
| 109 | Hyophorbe lagenicaulis (L. H. Bailey) H. E. Moore, 1976 | Arecaceae | CR | Ph | Ex | − | − | + |
| 110 | Ipomoea indica (Burm.) Merr., 1917 | Convolvulaceae | DD | Ph | Ex | − | − | + |
| 111 | Iresine diffusa Humb. & Bonpl. ex Willd., 1806 | Amaranthaceae | NA | Ph | Ex | − | − | + |
| 112 | Iris pseudacorus L., 1753 | Iridaceae | LC | Ph | Ex | − | − | + |
| 113 | Jacaranda mimosifolia D. Don, 1822 | Bignoniaceae | VU | Ph | Ex | + | − | − |
| 114 | Kalanchoe daigremontiana Raym.-Hamet & H. Perrier, 1914 | Crassulaceae | EN | Ph | Ex | − | − | + |
| 115 | Lagerstroemia indica L., 1759 | Lythraceae | LC | Ph | Ex | − | − | + |
| 116 | Lannea discolor (Sond.) Engl., | Anacardiaceae | LC | Ph | Ex | + | − | − |
| 117 | Lantana camara L., 1753 s.s. | Verbenaceae | NA | Ph | Ex | − | − | + |
| 118 | Lavandula angustifolia Mill., 1768 | Lamiaceae | LC | Ph | Ex | − | − | + |
| 119 | Leucaena leucocephala (Lam.) De Wit, 1961 | Fabaceae | CR | Ph | Ex | + | + | + |
| 120 | Leucanthemum maximum (Ramond) DC., 1837 | Asteraceae | NA | Ph | Ex | − | − | + |
| 121 | Ligustrum sinense Lour., 1790 | Oleaceae | NA | Ph | Ex | − | − | + |
| 122 | Liriope muscari (Decne.) L. H. Bailey, 1929 | Asparagaceae | NA | Ge | Ex | − | − | + |
| 123 | Livistona chinensis (Jacq.) R. Br. ex Mart., 1838 | Arecaceae | NA | Ph | Ex | − | − | + |
| 124 | Malus domestica (Suckow) Borkh., 1803 | Rosaceae | NA | Ph | Ex | + | − | − |
| 125 | Malvaviscus arboreus Cav., 1787 | Malvaceae | LC | Ph | Ex | − | − | + |
| 126 | Mangifera indica L., 1753 | Anacardiaceae | DD | Ph | Ex | + | + | + |
| 127 | Manihot esculenta Crantz, 1766 | Euphorbiaceae | DD | Ph | Ex | − | − | + |
| 128 | Markhamia lutea (Benth.) K. Schum. | Bignoniaceae | LC | Ph | Ex | + | − | − |
| 129 | Melissa officinalis L., 1753 | Lamiaceae | LC | Ph | Ex | − | − | + |
| 130 | Milicia excelsa (Welw.) C. C. Berg, 1982 | Moraceae | NT | Ph | Ex | − | + | − |
| 131 | Millettia laurentii De Wild | Fabaceae | EN | Ph | Ex | − | + | − |
| 132 | Millettia novo-guineensis Kaneh. & Hatus. | Fabaceae | NA | Ph | Ex | − | + | − |
| 133 | Monstera deliciosa, Liebn., 1849 | Araceae | NA | Ph | Ex | − | − | + |
| 134 | Moringa oleifera Lam. | Moringaceae | LC | Ph | Ex | + | + | − |
| 135 | Morus alba L., 1753 | Moraceae | NA | Ph | Ex | − | − | + |
| 136 | Musa acuminata Colla, 1820 | Musaceae | LC | Ph | Ex | − | + | + |
| 137 | Musa basjoo Siebold ex Iinuma, 1830 | Musaceae | LC | Ph | Ex | − | − | + |
| 138 | Musanga cecropioides R. Br. ex Tedlie, 1819 | Urticaceae | LC | Ph | Ex | − | + | − |
| 139 | Myrianthus arboreus P. Beauv., 1804–1805 | Cecropiaceae | LC | Ph | Na | − | + | − |
| 140 | Nephrolepis cordifolia (L.) C. Presl, 1836 | Nephrolepidaceae | NA | Ph | Ex | − | − | + |
| 141 | Nephrolepis exaltata (L.) Schott, 1834 | Nephrolepidaceae | LC | Ph | Ex | − | − | + |
| 142 | Nerium oleander L., 1753 | Apocynaceae | LC | Ph | Ex | − | − | + |
| 143 | Newbouldia laevis (P. Beauv.) Seem. | Bignoniaceae | LC | Ph | Ex | − | + | − |
| 144 | Olea europaea L., 1753 | Oleaceae | DD | Ph | Ex | + | − | − |
| 145 | Oxalis griffithii Edgew. & Hook.f. | Oxalidaceae | NA | Ch | Ex | − | − | + |
| 146 | Passiflora edulis Sims, 1818 | Passifloraceae | NA | Ph | Ex | − | − | + |
| 147 | Peltandra virginica (Linnaeus) Schott & Endlicher | Araceae | NA | Ph | Ex | − | − | + |
| 148 | Peperomia obtusifolia (L.) A. Dietr., 1831 | Piperaceae | NA | Ch | Ex | − | − | + |
| 149 | Persea americana Mill., 1768 | Lauraceae | NA | Ph | Ex | + | + | + |
| 150 | Persicaria microcephala Seikei Zusetsu, 1804 | Polygonaceae | NA | Ph | Ex | − | − | + |
| 151 | Petersianthus macrocarpus (P. Beauv.) Liben | Lecythidaceae | LC | Ph | Ex | − | + | − |
| 152 | Petunia sp Wijsman, 1990 | Solanaceae | NA | Ph | Ex | − | − | + |
| 153 | Phoenix canariensis Chabaud, 1882 | Arecaceae | LC | Ph | Ex | − | − | + |
| 154 | Phyllostachys viridiglaucescens (Carrière) Rivière & C. Rivière, 1878 | Poaceae | NA | Ph | Ex | − | + | − |
| 155 | Pinellia pedatisecta Schott | Araceae | NA | Ph | Ex | − | − | + |
| 156 | Pinus patula Schltdl. & Cham., 1831 | Pinaceae | VU | Ph | Ex | + | − | − |
| 157 | Pittosporum tobira (Murray) W. T. Aiton | Pittosporaceae | NA | Ph | Ex | − | − | + |
| 158 | Plumeria rubra L., 1753 | Apocynaceae | LC | Ph | Ex | − | + | + |
| 159 | Polyscias scutellaria (Burm.f.) Fosberg, 1948 | Araliaceae | NA | Ph | Ex | − | − | + |
| 160 | Prunus caroliniana (Mill.) Aiton | Rosaceae | LC | Ph | Ex | − | − | + |
| 161 | Prunus domestica L., 1753 | Rosaceae | DD | Ph | Ex | + | + | − |
| 162 | Pseudospondias microcarpa (A. Rich.) Engl., 1883 | Anacardiaceae | LC | Ph | Ex | − | + | − |
| 163 | Psidium guajava L., 1753 | Myrtaceae | LC | Ph | Ex | + | + | + |
| 164 | Pteris vittata L., 1753 | Pteridaceae | LC | Ph | Ex | − | − | + |
| 165 | Pycnanthus angolensis (Welw.) Warb. Notizbl. Königl. Bot. Gart, 1895 | Myristicaceae | LC | Ph | Na | − | + | − |
| 166 | Ravenala madagascariensis Sonn., 1782 | Strelitziaceae | LC | Ph | Ex | − | − | − |
| 167 | Ribes aureum Pursh, 1813 | Grossulariaceae | NA | Ph | Ex | − | + | − |
| 168 | Ricinodendron heudelotii (Baill.) Pierre ex Heckel, 1898 | Euphorbiaceae | LC | Ph | Na | − | − | + |
| 169 | Rosa multiflora Thunb., 1784 | Rosaceae | NA | Ph | Ex | − | − | + |
| 170 | Rosa chinensis Jacq., 1768 | Rosaceae | NA | Ph | Ex | − | − | + |
| 171 | Roystonea regia (Kunth) O. F. Cook, 1900 | Arecaceae | LC | Ph | Ex | − | + | − |
| 172 | Rudbeckia laciniata L., 1753 | Asteraceae | NA | Ph | Ex | − | − | + |
| 173 | Ruellia simplex C. Wright, 1870 | Acanthaceae | NA | Ph | Ex | − | − | + |
| 174 | Sabal palmetto (Walter) Lodd. ex Schult. & Schult.f., 1830 | Arecaceae | NA | Ph | Ex | − | − | + |
| 175 | Saccharum officinarum L., 1753 | Poaceae | NA | Ph | Ex | − | − | + |
| 176 | Saintpaulia ionantha Rubra, 1896 | Gesneriaceae | VU | Ge | Ex | − | − | + |
| 177 | Salix alba L., 1753 | Salicaceae | LC | Ph | Ex | − | − | + |
| 178 | Sambucus canadensis L., 1753 | Adoxaceae | NA | Ph | Ex | − | − | + |
| 179 | Sanchezia speciosa Leonard, 1926 | Acanthaceae | NA | Ph | Ex | − | − | + |
| 180 | Sansevieria trifasciata Prain 1903 | Asparagaceae | NA | Ge | Ex | − | − | + |
| 181 | Schefflera arboricola (Hayata) Merr. | Araliaceae | NA | Ph | Ex | − | − | + |
| 182 | Senna occidentalis (L.) Link, 1829 | Fabaceae | LC | Ph | Ex | − | + | − |
| 183 | Senna siamea (Lam.) H. S. Irwin & Barneby, 1982 | Fabaceae | LC | Ph | Ex | + | − | − |
| 184 | Spathiphyllum wallisii Regel, 1877 | Araceae | NA | Ph | Ex | − | + | − |
| 185 | Spathodea campanulata P. Beauv., 1805 | Bignoniaceae | LC | Ph | Ex | − | − | + |
| 186 | Sphagneticola trilobata (L.) Pruski, 1996 | Asteraceae | NA | Ch | Ex | + | − | − |
| 187 | Spondias dulcis Parkinson, 1773 | Anacardiaceae | NA | Ph | Ex | − | − | + |
| 188 | Spondias mombin L., 1753 | Anacardiaceae | LC | Ph | Ex | − | + | − |
| 189 | Strelitzia reginae Banks, 1788 | Strelitziaceae | NA | Ph | Ex | − | + | − |
| 190 | Syagrus romanzoffiana (Cham.) Glassman, 1968 | Arecaceae | NA | Ph | Ex | − | − | + |
| 191 | Symphyotrichum novi-belgii (L.) G. L. Nesom, 1995 | Asteraceae | NA | Ph | Ex | − | − | + |
| 192 | Symphyotrichum salignum (Willd.) G.L.Nesom, 1995 | Asteraceae | NA | Ph | Ex | − | − | + |
| 193 | Syngonium podophyllum Schott, 1851 | Araceae | NA | Ph | Ex | − | − | + |
| 194 | Syzygium cumini (L.) Skeels, 1912 | Lamiaceae | NA | Ph | Ex | − | − | + |
| 195 | Syzygium jambos (L.) Alston, 1931 | Lamiaceae | NA | Ph | Ex | − | + | − |
| 196 | Syzygium manii (King) N. P. Balakrishnan | Lamiaceae | NA | Ph | Ex | − | + | − |
| 197 | Tabernaemontana divaricata (L.) R. Br. ex Roem. & Schult., 1819 | Apocynaceae | NA | Hem | Ex | − | − | + |
| 198 | Tagetes erecta L., 1753 | Asteraceae | NA | Ch | Ex | − | − | + |
| 199 | Tectona grandis L.f., 1782 | Lamiaceae | EN | Ph | Ex | − | + | − |
| 200 | Terminalia catappa L., 1767 | Combretaceae | LC | Ph | Ex | + | + | + |
| 201 | Terminalia ivorensis A. Chev., 1909 | Combretaceae | VU | Ph | Ex | − | + | − |
| 202 | Terminalia superba Engl. & Diels, 1899 | Combretaceae | NA | Ph | Ex | − | + | − |
| 203 | Theobroma cacao L., 1753 | Malvaceae | NA | Ph | Ex | − | + | − |
| 204 | Thyrsacanthus tubaeformis (Bertol.) Nees, 1847 | Acanthaceae | NA | Ph | Ex | − | − | + |
| 205 | Tithonia diversifolia (Hemsl.) A. Gray, 1883 | Asteraceae | NA | Ph | Ex | − | − | + |
| 206 | Tradescantia fluminensis Vell., 1829 | Commelinaceae | NA | Ch | Ex | − | − | + |
| 207 | Tradescantia pallida (Rose) D. R. Hunt, 1976 | Commelinaceae | NA | Ch | Ex | − | − | + |
| 208 | Tradescantia zebrina hort. ex Bosse, 1849 | Commelinaceae | NA | Ph | Ex | − | − | + |
| 209 | Treculia africana Decne. ex Trécul | Moraceae | LC | Ph | Ex | − | + | − |
| 210 | Uapaca esculenta A. Chev. ex Aubrév. & Leandri | Phyllanthaceae | LC | Ph | Ex | − | + | − |
| 211 | Umbellularia californica (Hook. & Arn.) Nutt., 1842 | Lauraceae | LC | Ph | Ex | − | − | + |
| 212 | Vachellia karroo (Hayne) Banfi & Galasso | Fabaceae | LC | Ph | Ex | − | + | − |
| 213 | Vernonia amygdalina Delile | Asteraceae | NA | Ph | Na | − | + | − |
| 214 | Vitex trifolia L., 1753 | Lamiaceae | NA | Ph | Ex | − | + | − |
| 215 | Volkameria inermis L., 1753 | Lamiaceae | NA | Ph | Ex | − | − | + |
| 216 | Yucca gigantea Lem., 1859 | Asparagaceae | DD | Ph | Ex | − | − | + |
| 217 | Zamioculcas zamiifolia (Lodd.) Engl., 1905 | Araceae | NA | Ph | Ex | − | − | + |
| 218 | Zantedeschia aethiopica (L.) Spreng., 1826 | Araceae | LC | Ph | Ex | − | − | + |
| 219 | Zephyranthes longifolia Hemsl. | Amaryllidaceae | NA | Ph | Ex | − | − | + |
| 220 | Zinnia elegans Jacq., 1792 | Asteraceae | NA | Ph | Ex | − | − | + |
References
- Bogaert, J.; Vranken, I.; André, M. Anthropogenic Effects in Landscapes: Historical Context and Spatial Pattern. In Biocultural Landscapes; Hong, S.-K., Bogaert, J., Min, Q., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 89–112. ISBN 978-94-017-8940-0. [Google Scholar]
- Muteya, H.K.; Nghonda, D.-D.N.; Malaisse, F.; Waselin, S.; Sambiéni, K.R.; Kaleba, S.C.; Kankumbi, F.M.; Bastin, J.-F.; Bogaert, J.; Sikuzani, Y.U. Quantification and Simulation of Landscape Anthropization around the Mining Agglomerations of Southeastern Katanga (DR Congo) between 1979 and 2090. Land 2022, 11, 850. [Google Scholar] [CrossRef]
- Andre, M.; Mahy, G.; Lejeune, P.; Bogaert, J. Vers Une Synthèse de La Conception et Une Définition Des Zones Dans Le Gradient Urbain-Rural. Biotechnol. Agron. Société Environ. 2014, 18, 61–74. [Google Scholar]
- Gutu Sakketa, T. Urbanisation and Rural Development in Sub-Saharan Africa: A Review of Pathways and Impacts. Res. Glob. 2023, 6, 100133. [Google Scholar] [CrossRef]
- Suarez-Rubio, M.; Bates, P.J.J.; Aung, T.; Hlaing, N.M.; Oo, S.S.L.; Htun, Y.K.Z.; Mar, S.M.O.; Myint, A.; Wai, T.L.L.; Mo, P.M.; et al. Bird Diversity along an Urban to Rural Gradient in Large Tropical Cities Peaks in Mid-Level Urbanization. PeerJ 2023, 11, e16098. [Google Scholar] [CrossRef]
- World Urbanization Prospects The 2018 Revision. Available online: https://www.un.org/en/desa/2018-revision-world-urbanization-prospects (accessed on 4 October 2024).
- Damon, J. Peuplement, migrations, urbanisation: Où va la population mondiale? Popul. Avenir 2016, 728, 4–7. [Google Scholar] [CrossRef]
- Guo, H.; Qiao, W.; Liu, J. Dynamic Feedback Analysis of Influencing Factors of Existing Building Energy-Saving Renovation Market Based on System Dynamics in China. Sustainability 2019, 11, 273. [Google Scholar] [CrossRef]
- Bogaert, J.; Biloso, A.; Vranken, I.; Andre, M. Peri-Urban Dynamics: Landscape Ecology Perspectives. In Territoires Périurbains: Développement, Enjeux et Perspectives dans les Pays du Sud; Presses Agronomiques de Gembloux: Gembloux, Belgium, 2015; pp. 63–73. ISBN 978-2-87016-136-4. [Google Scholar]
- Huang, H.; Zhuo, L.; Li, Z.; Ji, X.; Wu, P. Effects of Multidimensional Urbanisation on Water Footprint Self-Sufficiency of Staple Crops in China. J. Hydrol. 2023, 618, 129275. [Google Scholar] [CrossRef]
- Power, A.L.; Tennant, R.K.; Jones, R.T.; Tang, Y.; Du, J.; Worsley, A.T.; Love, J. Monitoring Impacts of Urbanisation and Industrialisation on Air Quality in the Anthropocene Using Urban Pond Sediments. Front. Earth Sci. 2018, 6, 131. [Google Scholar] [CrossRef]
- Useni Sikuzani, Y.; Malaisse, F.; Cabala Kaleba, S.; Kalumba Mwanke, A.; Yamba, A.M.; Nkuku Khonde, C.; Bogaert, J.; Munyemba Kankumbi, F. Tree Diversity and Structure on Green Space of Urban and Peri-Urban Zones: The Case of Lubumbashi City in the Democratic Republic of Congo. Urban For. Urban Green. 2019, 41, 67–74. [Google Scholar] [CrossRef]
- Abdulai, I.; Osumanu, I. How Urbanisation Shapes Availability of Provisioning Ecosystem Services in Peri-Urban Ghana. Int. J. Urban Sustain. Dev. 2023, 15, 282–298. [Google Scholar] [CrossRef]
- Kraemer, R.; Kabisch, N. Parks Under Stress: Air Temperature Regulation of Urban Green Spaces Under Conditions of Drought and Summer Heat. Front. Environ. Sci. 2022, 10, 849965. [Google Scholar] [CrossRef]
- Li, G.; Fang, C.; Li, Y.; Wang, Z.; Sun, S.; He, S.; Qi, W.; Bao, C.; Ma, H.; Fan, Y.; et al. Global Impacts of Future Urban Expansion on Terrestrial Vertebrate Diversity. Nat. Commun. 2022, 13, 1628. [Google Scholar] [CrossRef] [PubMed]
- Threlfall, C.; Mata, L.; Mackie, J.; Hahs, A.; Stork, N.; Williams, N.; Livesley, S. Increasing Biodiversity in Urban Green Spaces through Simple Vegetation Interventions. J. Appl. Ecol. 2017, 54, 1874–1883. [Google Scholar] [CrossRef]
- Carter, E.J. L’avenir de La Foresterie Urbaine dans les Pays en Développement: Un Document de Réflexion. Available online: https://www.fao.org/4/t1680f/t1680f00.htm (accessed on 4 October 2024).
- Aram, F.; Higueras Garcia, E.; Solgi, E.; Mansournia, S. Urban Green Space Cooling Effect in Cities. Heliyon 2019, 5, 1339. [Google Scholar] [CrossRef]
- Wolch, J.R.; Byrne, J.; Newell, J.P. Urban Green Space, Public Health, and Environmental Justice: The Challenge of Making Cities ‘Just Green Enough’. Landsc. Urban Plan. 2014, 125, 234–244. [Google Scholar] [CrossRef]
- Kothencz, G.; Kolcsár, R.; Cabrera-Barona, P.; Szilassi, P. Urban Green Space Perception and Its Contribution to Well-Being. Int. J. Environ. Res. Public Health 2017, 14, 766. [Google Scholar] [CrossRef]
- Kondo, M.C.; Fluehr, J.M.; McKeon, T.; Branas, C.C. Urban Green Space and Its Impact on Human Health. Int. J. Environ. Res. Public Health 2018, 15, 445. [Google Scholar] [CrossRef]
- Yilma, G.; Derero, A. Carbon Stock and Woody Species Diversity Patterns in Church Forests along Church Age Gradient in Addis Ababa, Ethiopia. Urban Ecosyst. 2020, 23, 971–983. [Google Scholar] [CrossRef]
- Neal, S.; Bennett, K.; Jones, H.; Cochrane, A.; Mohan, G. Multiculture and Public Parks: Researching Super-Diversity and Attachment in Public Green Space. Popul. Space Place 2015, 21, 463–475. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, F.; Che, Y. Public Green Spaces and Human Wellbeing: Mapping the Spatial Inequity and Mismatching Status of Public Green Space in the Central City of Shanghai. Urban For. Urban Green. 2017, 27, 59–68. [Google Scholar] [CrossRef]
- You, H. Characterizing the Inequalities in Urban Public Green Space Provision in Shenzhen, China. Habitat Int. 2016, 56, 176–180. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Ma, X. Evaluation of the Accessible Urban Public Green Space at the Community-Scale with the Consideration of Temporal Accessibility and Quality. Ecol. Indic. 2021, 131, 108231. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Z.; Webster, C. Estimating the Mediate Effect of Privately Green Space on the Relationship between Urban Public Green Space and Property Value: Evidence from Shanghai, China. Land Use Policy 2016, 54, 439–447. [Google Scholar] [CrossRef]
- Poortinga, W.; Bird, N.; Hallingberg, B.; Phillips, R.; Williams, D. The Role of Perceived Public and Private Green Space in Subjective Health and Wellbeing during and after the First Peak of the COVID-19 Outbreak. Landsc. Urban Plan. 2021, 211, 104092. [Google Scholar] [CrossRef]
- Hanson, H.I.; Eckberg, E.; Widenberg, M.; Alkan Olsson, J. Gardens’ Contribution to People and Urban Green Space. Urban For. Urban Green. 2021, 63, 127198. [Google Scholar] [CrossRef]
- Hutt-Taylor, K. Assessing Urban Tree Taxonomic Diversity, Composition and Structure Across Public and Private Green Space Types: A Community-Based Tree Inventory. Master’s Thesis, Concordia University, Montreal, QC, Canada, 2021. [Google Scholar]
- Useni Sikuzani, Y.U.; Kalonda, B.K.; Mukenza, M.M.; Mleci, J.Y.; Kalenga, A.M.; Malaisse, F.; Bogaert, J. Exploring Floristic Diversity, Propagation Patterns and Plant Functions in Domestic Gardens Across Urban Planning Gradient in Lubumbashi, DR Congo. Ecologies 2024, 5, 512–537. [Google Scholar] [CrossRef]
- Lubbe, C.S.; Siebert, S.J.; Cilliers, S.S. Floristic Analysis of Domestic Gardens in the Tlokwe City Municipality, South Africa. Bothalia 2011, 41, 351–361. [Google Scholar] [CrossRef]
- Al-Kofahi, S.D.; Al-Kafawin, A.M.; Al-Gharaibeh, M.M. Investigating Domestic Gardens Landscape Plant Diversity, Implications for Valuable Plant Species Conservation. Environ. Dev. Sustain. 2023, 26, 21259–21279. [Google Scholar] [CrossRef]
- Useni Sikuzani, Y.; Mpibwe Kalenga, A.; Yona Mleci, J.; N’Tambwe Nghonda, D.; Malaisse, F.; Bogaert, J. Assessment of Street Tree Diversity, Structure and Protection in Planned and Unplanned Neighborhoods of Lubumbashi City (DR Congo). Sustainability 2022, 14, 3830. [Google Scholar] [CrossRef]
- Musanganya, D. La Matrice Intellectuelle du Catholicisme Social Face à l’Etat Faible au Congo ( RDC) Entre 1990 et 2018. Ph.D. Thesis, Université Paris-Est, Paris, France, 2023. [Google Scholar]
- André, G.; Poncelet, M. Héritage Colonial et Appropriation Du Pouvoir d’éduquer. Approche Socio-Historique Du Champ de l’éducation Primaire En RDC. Cah. Rech. léducation Savoirs 2013, 2, 271. [Google Scholar]
- Kasangana, A.C. L’église Catholique et le Congo «Belge»: Approche Historico-Juridique des Relations Institutionnelles (1885–1960). Ph.D. Thesis, Université Paris-Saclay, Paris, France, 2022. [Google Scholar]
- Hounto, G.; Tente, B.; Yabi, F.; Yabi, I. Diversité et Connaissance Ethnobotanique Des Espèces Végétales de La Forêt Sacrée de Badjamè et Zones Connexes Au Sud-Ouest Du Benin. Rev. Sci. Tech. For. Environ. Bassin Congo 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Kaczyńska, M. The Church Garden as an Element Improving the Quality of City Life—A Case Study in Warsaw. Urban For. Urban Green. 2020, 54, 126765. [Google Scholar] [CrossRef]
- Statistics of the Catholic Church in the Democratic Republic of the Congo and in South Sudan as of 31 December 2021 (from the Central Office for Church Statistics). Available online: https://press.vatican.va/content/salastampa/en/bollettino/pubblico/2023/01/24/230124d.html (accessed on 4 October 2024).
- Subbotina, N. The Sustainable Development Goals and Catholic Social Teaching Perspective on Biodiversity in the Context of Protection of Forest Ecosystems in the Ukrainian Carpathians. Analecta UCU Ser. Theol. 2022, 9, 183–199. [Google Scholar] [CrossRef]
- Frascaroli, F. Catholicism and Conservation: The Potential of Sacred Natural Sites for Biodiversity Management in Central Italy. Hum. Ecol. 2013, 41, 587–601. [Google Scholar] [CrossRef]
- Jerie, S. The Role of the Church in Sustainable Environmental Management in Zimbabwe: A Case Study of the Bulawayo Archdiocese of the Roman Catholic Church. J. Sustain. Dev. Afr. 2010, 12, 217–226. [Google Scholar]
- Hill, J.L.; Curran, P.J.; Foody, G.M. The Effect of Sampling on the Species-Area Curve. Glob. Ecol. Biogeogr. Lett. 1994, 4, 97. [Google Scholar] [CrossRef]
- Cain, S.A. The Species-Area Curve. Am. Midl. Nat. 1938, 19, 573–581. [Google Scholar] [CrossRef]
- Savard, J.-P.L.; Clergeau, P.; Mennechez, G. Biodiversity Concepts and Urban Ecosystems. Landsc. Urban Plan. 2000, 48, 131–142. [Google Scholar] [CrossRef]
- Les Jardins Comme Moyens d’Existence. Available online: https://www.fao.org/4/y5112f/y5112f00.htm (accessed on 4 October 2024).
- Tremblay, M.-H.; Simard, M. Les effets de proximité dans l’appropriation collective d’un grand parc paysager à saguenay. Vertigo 2011, 11. [Google Scholar] [CrossRef]
- Gueymard, S. Facteurs environnementaux de proximité et choix résidentiels. Dév. Durable Territ. Écon. Géogr. Polit. Droit Sociol. 2006, 7. [Google Scholar] [CrossRef]
- Sakhraoui, N.; Metallaoui, S.; Chefrour, A.; Hadef, A. La flore exotique potentiellement envahissante d’Algérie: Première description des espèces cultivées en pépinières et dans les jardins. Biotechnol. Agron. Soc. Environ. 2019, 23, 63–73. [Google Scholar] [CrossRef]
- World Population Review, DR Congo Cities by Population. 2025. Available online: https://worldpopulationreview.com/cities/dr-congo (accessed on 16 July 2025).
- L’insalubrité Publique et la Santé Environnementale Dans le District Sanitaire de Bukavu—ISDR BUKAVUISDR BUKAVU. Available online: https://isdrbukavu.ac.cd/produit/linsalubrite-publique-et-la-sante-environnementale-dans-le-district-sanitaire-de-bukavu/ (accessed on 4 October 2024).
- United Nations Conférence des Nations Unies sur le Logement et le Développement Urbain Durable: Habitat III|Nations Unies. Available online: https://www.un.org/fr/conferences/habitat/quito2016 (accessed on 4 October 2024).
- Vwima Ngezirabona, S.; Mastaki, J.-L.; Lebailly, P. Le rôle du commerce frontalier des produits alimentaires avec le Rwanda dans l’approvisionnement des ménages de la ville de Bukavu (province du Sud-Kivu). In Les Cahiers de l’Association Tiers-Monde n° 28-2013: XXVIIIes Journées sur le Développement “Mobilités Internationales, Déséquilibres et Développement: Vers un Développement Durable et une Mondialisation Décarbonée?”; Brot, J., Ed.; Association Tiers-Monde: Paris, France, 2013. [Google Scholar]
- Balasha, A.M.; Murhula, B.B.; Munahua, D.M. Yard Farming in the City of Lubumbashi: Resident Perceptions of Home Gardens in Their Community. J. City Dev. 2019, 1, 46–53. [Google Scholar]
- Sêdami, A.B.; Naéssé, A.V.; Julien, D.; Firmin, A.D. Practice of Home Gardens (HG) in the Suburban Area between Cotonou and Ouidah in Southern Benin. J. Biodivers. Environ. Sci. 2016, 9, 29–38. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; The Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- IUCN. Guidelines for Species Conservation Planning: Version 1.0; IUCN: Cambridge, UK, 2017; ISBN 978-2-8317-1877-4. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. Available online: http://www.rstudio.com/ (accessed on 4 October 2024).
- Logan, M. Biostatistical Design and Analysis Using R: A Practical Guide, 1st ed.; Wiley: New York, NY, USA, 2010; ISBN 978-1-4051-9008-4. [Google Scholar]
- Pauline Vaissie, Astrid Monge, Francois Husson Factoshiny: Perform Factorial Analysis from “FactoMineR” with a Shiny Application 2015, 2.6. Available online: https://cran-e.com/package/Factoshiny (accessed on 4 October 2024).
- Magurran, A.E. Measuring Biological Diversity; John Wiley & Sons: New York, NY, USA, 2013; ISBN 978-1-118-68792-5. [Google Scholar]
- Subba, L.; Pala, N.; Shukla, G.; Chakravarty, S. Inventory of Flora in Home Gardens of Sub-Humid Tropical Landscapes, West Bengal, India. Int. J. Usufructs Manag. 2016, 7, 47–54. [Google Scholar]
- Regassa, R. Useful Plant Species Diversity in Homegardens and Its Contribution to Household Food Security in Hawassa City, Ethiopia. Afr. J. Plant Sci. 2016, 10, 211–233. [Google Scholar] [CrossRef]
- Albuquerque, U.P.; Andrade, L.H.C.; Caballero, J. Structure and Floristics of Homegardens in Northeastern Brazil. J. Arid Environ. 2005, 62, 491–506. [Google Scholar] [CrossRef]
- Samus, A.; Freeman, C.; Dickinson, K.; van Heezik, Y. An Examination of the Factors Influencing Engagement in Gardening Practices That Support Biodiversity Using the Theory of Planned Behavior. Biol. Conserv. 2023, 286, 110252. [Google Scholar] [CrossRef]
- Lemessa, D.; Legesse, A. Non-Crop and Crop Plant Diversity and Determinants in Homegardens of Abay Chomen District, Western Ethiopia. Biodivers. Int. J. 2018, 2, 433–439. [Google Scholar] [CrossRef]
- Voelcker, B. Climate, Capital, and Colonialism: A Congolese Perspective. J. Clim. Resil. Justice 2023, 1, 55–65. [Google Scholar] [CrossRef]
- Kabir, E.; Webb, E.L. Can Homegardens Conserve Biodiversity in Bangladesh? Biotropica 2008, 40, 95–103. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental Heterogeneity as a Universal Driver of Species Richness across Taxa, Biomes and Spatial Scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Idohou, R.; Fandohan, A.; Salako, V.; Kassa, B.; Gbedomon, R.; Yédomonhan, H.; Glele Kakaï, R.L.; Assogbadjo, A. Biodiversity Conservation in Home Gardens: Traditional Knowledge, Use Patterns and Implications for Management. Int. J. Biodivers. Sci. Manag. 2014, 10, 89–100. [Google Scholar] [CrossRef]
- Lepczyk, C.; Aronson, M.; Evans, K.; Goddard, M.; Lerman, S.; MacIvor, J.S. Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. BioScience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Souto, T.; Ticktin, T. Understanding Interrelationships Among Predictors (Age, Gender, and Origin) of Local Ecological Knowledge1. Econ. Bot. 2012, 66, 149–164. [Google Scholar] [CrossRef]
- Kumar, B.M.; Nair, P.K.R. The Enigma of Tropical Homegardens. Agrofor. Syst. 2004, 61, 135–152. [Google Scholar] [CrossRef]
- Joscha, B.; Veith, M.; Hochkirch, A. Biodiversity in Cities Needs Space: A Meta-Analysis of Factors Determining Intra-Urban Biodiversity Variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef]
- Scheiner, S.M.; Chiarucci, A.; Fox, G.A.; Helmus, M.R.; McGlinn, D.J.; Willig, M.R. The Underpinnings of the Relationship of Species Richness with Space and Time. Ecol. Monogr. 2011, 81, 195–213. [Google Scholar] [CrossRef]
- Magurran, A. Measuring Biological Diversity; Blackwell Science Ltd.: Oxford, UK, 2004; ISBN 978-0-632-05633-0. [Google Scholar]
- Rodrigues, P.M.S.; Schaefer, C.E.G.R.; de Oliveira Silva, J.; Ferreira Júnior, W.G.; dos Santos, R.M.; Neri, A.V. The Influence of Soil on Vegetation Structure and Plant Diversity in Different Tropical Savannic and Forest Habitats. J. Plant Ecol. 2016, 11, 226–236. [Google Scholar] [CrossRef]
- Sewagegn, G.B.; Abate, D.F.; Girma, Y.G. Woody Species Diversity and Carbon Stock of Church Forests along Age Gradient in Dangila District, Awi-Zone, Ethiopia. Heliyon 2022, 8, e10491. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Clark, E.J.; Frost, G.A.; Randell, T.E.; Pitchford, J.W.; Hill, J.K. Changes in Species Diversity Following Habitat Disturbance Are Dependent on Spatial Scale: Theoretical and Empirical Evidence. J. Appl. Ecol. 2008, 45, 1531–1539. [Google Scholar] [CrossRef]
- Bentsi-Enchill, F.; Damptey, F.G.; Pappoe, A.N.M.; Ekumah, B.; Akotoye, H.K. Impact of Anthropogenic Disturbance on Tree Species Diversity, Vegetation Structure and Carbon Storage Potential in an Upland Evergreen Forest of Ghana, West Africa. Trees For. People 2022, 8, 100238. [Google Scholar] [CrossRef]
- Niklas, K.J. Life Forms, Plants. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 2160–2167. ISBN 978-0-08-045405-4. [Google Scholar]
- Nguinambaye, M.; Nana, R.; Mbayngone, E.; Djinet, A.; Badiel, B.; Tamini, Z. Distribution et Usages des Ampelocissus Dans la Zone de Donia Au Sud Du Tchad. Int. J. Biol. Chem. Sci. 2015, 9, 186. [Google Scholar] [CrossRef]
- Useni Sikuzani, Y.; Malaisse, F.; Yona, J.M.; Mwamba, T.M.; Bogaert, J. Diversity, Use and Management of Household-Located Fruit Trees in Two Rapidly Developing Towns in Southeastern D.R. Congo. Urban For. Urban Green. 2021, 63, 127220. [Google Scholar] [CrossRef]
- Vijayakumari, J.; Prabha, V.S.; Rubi, J.; Raj, T.L.; Rayan, S. Floristic Diversity Assessment of Home Garden in Palayamkottai Region of Tirunelveli District, Tamil Nadu a Means of Sustainable Biodiversity Conservation. Int. J. Trend Sci. Res. Dev. 2019, 3, 1484–1491. [Google Scholar] [CrossRef]
- Andersson, E.; Tengö, M.; McPhearson, T.; Kremer, P. Cultural Ecosystem Services as a Gateway for Improving Urban Sustainability. Ecosyst. Serv. 2015, 12, 165–168. [Google Scholar] [CrossRef]
- Bennett, A. Linkages in the Landscape; The Role of Corridors and Connectivity in Wildlife Conservation; IUCN: Gland, Switzerland, 2003. [Google Scholar]
- Battisti, C. Ecological Networks as Planning Tools for African Fragmented Landscapes: Overcoming Weaknesses for an Effective Connectivity conservation. Afr. J. Ecol. 2024, 62, e13186. [Google Scholar] [CrossRef]
- Savary, P.; Lessard, J.-P.; Peres-Neto, P.R. Heterogeneous Dispersal Networks to Improve Biodiversity Science. Trends Ecol. Evol. 2024, 39, 229–238. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, S.; Hobbs, R. A Framework for Conceptualizing Human Effects on Landscapes and Its Relevance to Management and Research Models. Conserv. Biol. 1999, 13, 1282–1292. [Google Scholar] [CrossRef]
- Battisti, C.; Frank, B.; Fanelli, G. Children as Drivers of Change: The Operational Support of Young Generations to Conservation Practices. Environ. Pract. 2018, 20, 129–135. [Google Scholar] [CrossRef]
- DeGeorges, P.A.; Reilly, B.K. The Realities of Community Based Natural Resource Management and Biodiversity Conservation in Sub-Saharan Africa. Sustainability 2009, 1, 734–788. [Google Scholar] [CrossRef]
- Battisti, C.; Cerfolli, F. From Citizen Science to Citizen Management: Suggestions for a Pervasive Fine-Grained and Operational Approach to Biodiversity Conservation. Isr. J. Ecol. Evol. 2021, 68, 8–12. [Google Scholar] [CrossRef]
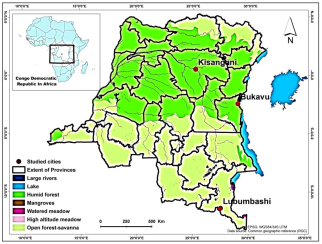
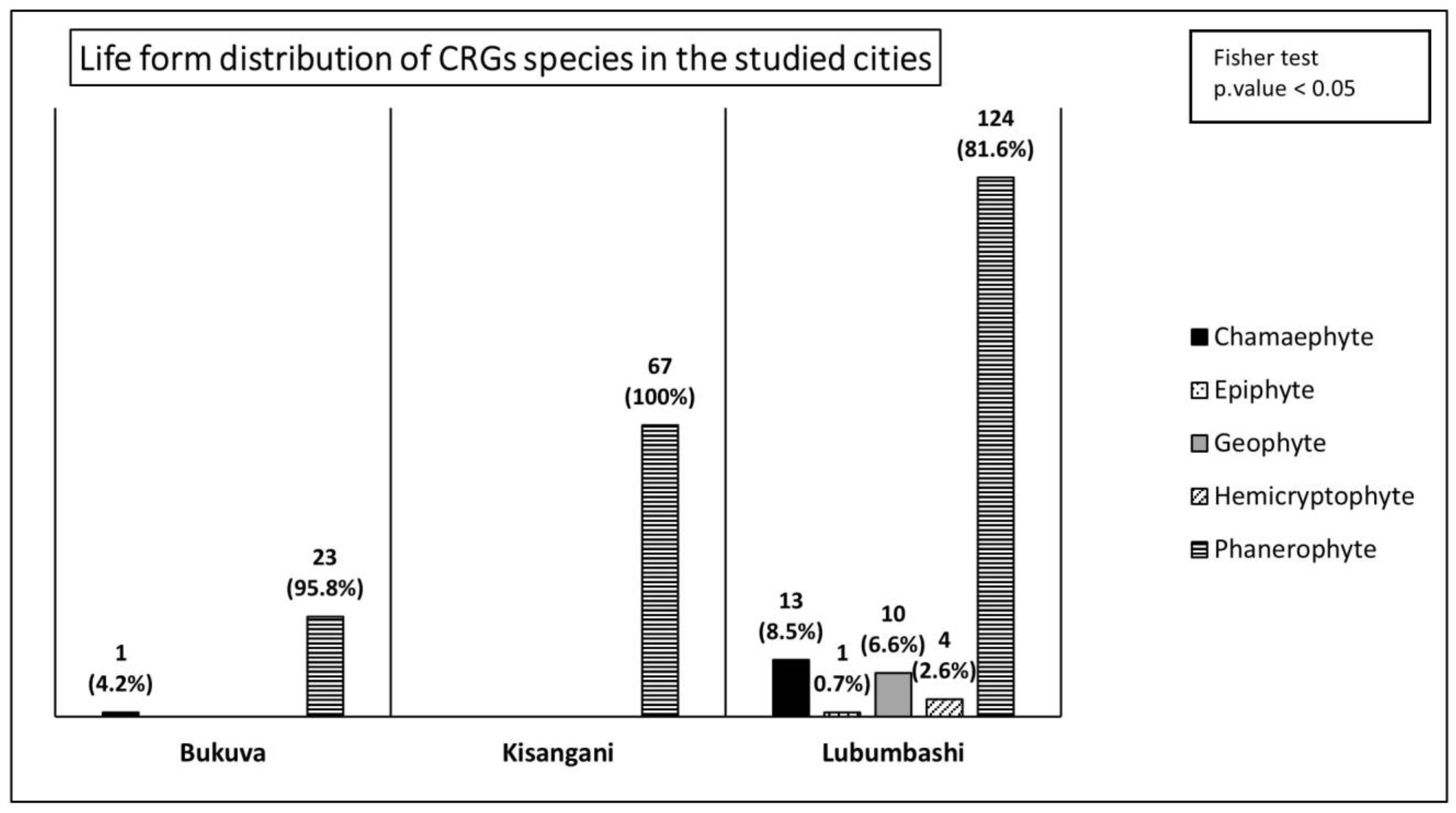
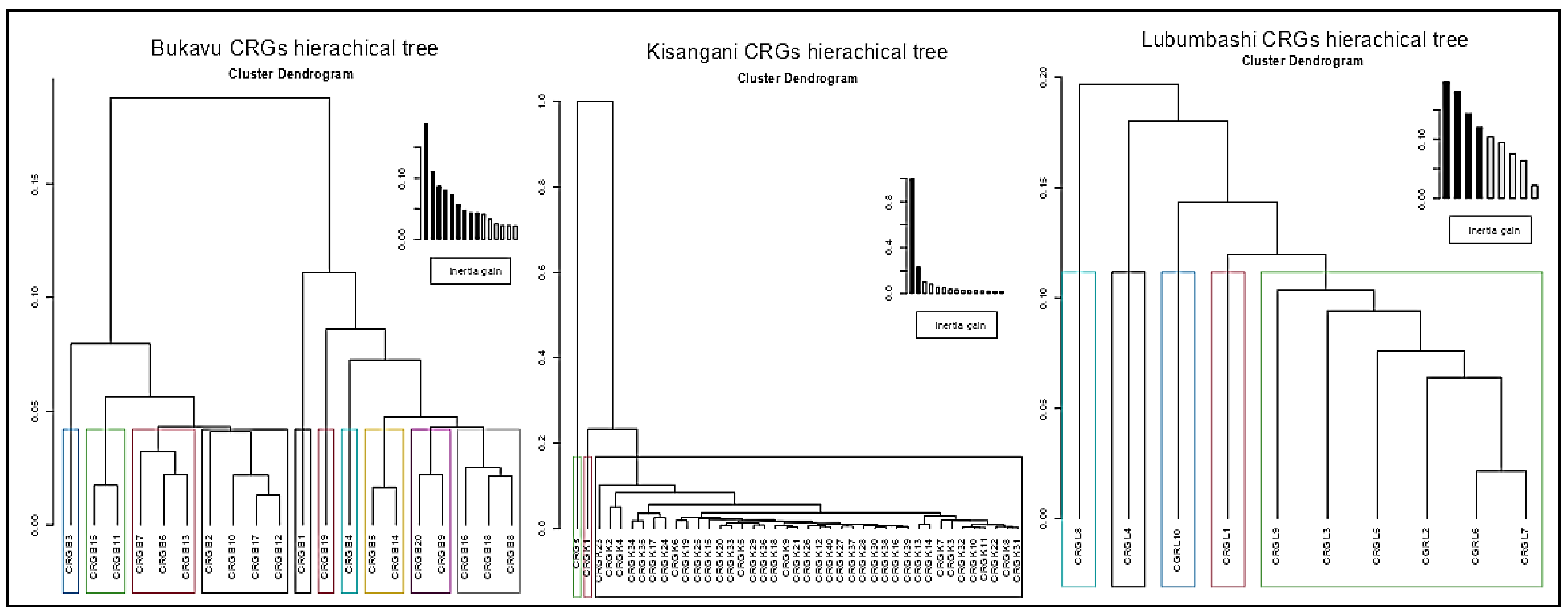
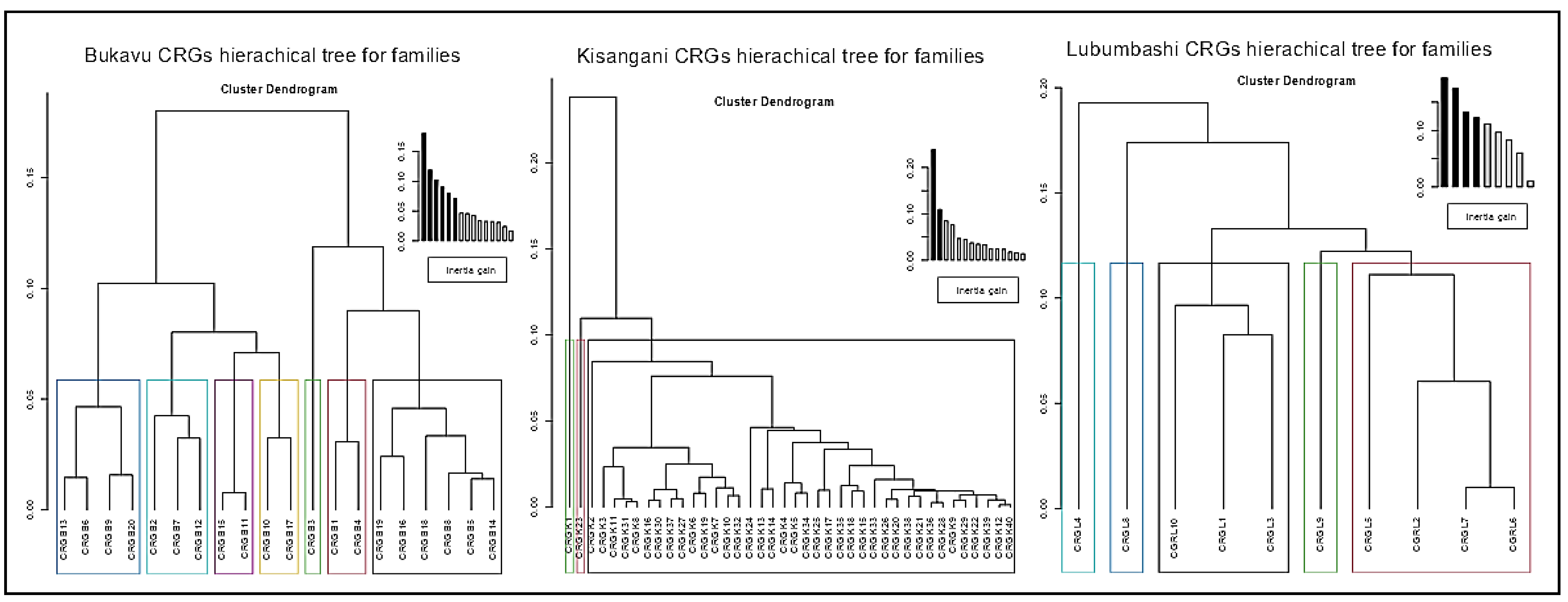
| City (Old Name) | Bukavu (Costermansville) | Kisangani (Stanleyville) | Lubumbashi (Elisabethville) |
|---|---|---|---|
| Coordinates | 2°30′55″ S, 28°50′42″ E | 0°31′ N, 25°11′ E | 11°61′55″ S, 27°48′61″ E |
| Area (km2) | 60 | 1910 | 747 |
| Population (2025) | 1,369,430 | 1,546,690 | 3,061,340 |
| Altitude (m) | Mean: 1654 Min: 1422 Max: 2190 | Mean: 415 Min: 378 Max: 503 | Mean: 1259 Min: 1167 Max: 1411 |
| Climate Type | Tropical montane (BWh); dry season: May–August; rainy: September–April | Equatorial humid (Af); two rainy seasons; dry: Jan, July–August | Subtropical dry winter (CW6); rainy: November; dry: May–September |
| Rainfall (mm/year) | 1500–2200 | 1500–2000 | ~1200 |
| Temperature (°C) | 20.5 | 25 | 20 |
| Dominant Soil | Andosols (volcanic, clayey, permeable) | Ferralsols (sandy-clay soils) | Ferralsols (young, red ferrallitic soils) |
| Vegetation Type | Montane forest | Dense rainforest | Miombo woodland |
| Cities | Specific Richness | Number of Families | Number of Genera | Area (ha) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Total | Mean | SD | Total | Mean | SD | Total | Mean | SD | Total | |
| Bukavu (n = 20) | 9.2 a | 4.7 | 24 | 6.9 a | 3.0 | 15 | 8.7 a | 4.5 | 22 | 3.7 a | 5.0 | 74.6 |
| Kisangani (n = 40) | 12.1 a | 8.3 | 72 | 9.2 a | 5.1 | 36 | 11.5 a | 7.3 | 56 | 5.2 a | 8.7 | 209.4 |
| Lubumbashi (n =10) | 24.1 b | 10.8 | 152 | 17.9 b | 7.5 | 60 | 23.7 b | 10.6 | 137 | 1.1 b | 2.5 | 11.4 |
| Cities | Taxa | Parameters |
| Family | Relative Dominance | |
| Bukavu (Rs = 24) | Bignoniaceae | 12.5% |
| Fabaceae | 12.5% | |
| Rutaceae | 12.5% | |
| Anacardiaceae | 8.3% | |
| Myrtaceae | 8.3% | |
| Rosaceae | 8.3% | |
| All others * | 4.2% | |
| Kisangani (Rs = 67) | Fabaceae | 15.3% |
| Moraceae | 9.7% | |
| Anacardiaceae | 5.6% | |
| Myrtaceae | 5.6% | |
| Rutaceae | 5.6% | |
| Lubumbashi (Rs = 152) | Araceae | 8.6% |
| Arecaceae | 7.2% | |
| Asparagaceae | 6.6% | |
| Genus | Dominance relative | |
| Bukavu (Rs = 24) | Citrus | 12.5% |
| All others * | 4.2% | |
| Kisangani (Rs = 67) | Acacia | 5.6% |
| Citrus | 5.6% | |
| Albizia | 4.2% | |
| Ficus | 4.2% | |
| Terminalia | 4.2% | |
| Lubumbashi (Rs = 152) | Cyperus | 2.0% |
| Euphorbia | 2.0% | |
| Tradescantia | 2.0% | |
| All others * | 1.3% |
| Cities | Taxa | Parameters |
|---|---|---|
| Species | Relative Frequence | |
| Bukavu (n = 20) | Pinus patula Schltdl. & Cham., 1831 | 75.0% |
| Eucalyptus globulus Labill., 1800 | 70.0% | |
| Citrus limon (L.) Osbeck, 1765 | 65.0% | |
| Psidium guajava L., 1753 | 65.0% | |
| Mangifera indica L., 1753 | 60.0% | |
| Markhamia lutea (Benth.) K. Schum. | 60.0% | |
| Persea americana Mill., 1768 | 60.0% | |
| Kisangani (n = 40) | Persea americana Mill., 1768 | 82.5% |
| Elaeis guineensis Jacq., 1763 | 75.0% | |
| Mangifera indica L., 1753 | 67.5% | |
| Lubumbashi (n = 10) | Cordyline fruticosa (L.) A. Chev., 1919 | 80.0% |
| Musa acuminata Colla, 1820 | 60.0% | |
| Acalypha wilkesiana Müll. Arg., 1866 | 50.0% | |
| Carica papaya L., 1753 | 50.0% | |
| Citrus limon (L.) Osbeck, 1765 | 50.0% | |
| Codiaeum variegatum (L.) Rumph. ex A. Juss., 1824 | 50.0% | |
| Families | Relative Frequence | |
| Bukavu (n = 20) | Myrtaceae | 90.0% |
| Anacardiaceae | 75.0% | |
| Bignoniaceae | 75.0% | |
| Pinaceae | 75.0% | |
| Rutaceae | 65.0% | |
| Kisangani (n =40) | Arecaceae | 85.0% |
| Lauraceae | 82.5% | |
| Fabaceae | 77.5% | |
| Lubumbashi | Asparagaceae | 100.0% |
| Arecaceae | 90.0% | |
| Euphorbiaceae | 90.0% | |
| Lamiaceae | 80.0% | |
| Genera | Relative Frequence | |
| Bukavu (n = 20) | Pinus | 68.2% |
| Eucalyptus | 63.6% | |
| Citrus | 59.1% | |
| Psidium | 59.1% | |
| Kisangani (n =40) | Persea | 82.5% |
| Elaeis | 75.0% | |
| Mangirefa | 67.5% | |
| Lubumbashi (n = 10) | Cordyline | 80.0% |
| Citrus | 60.0% | |
| Musa | 60.0% | |
| Acalypha | 50.0% | |
| Carica | 50.0% | |
| Codiaeum | 50.0% | |
| Dracaena | 50.0% | |
| Tradescantia | 50.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukubu Pika, L.; Mugisho Mukotanyi, S.; Pyame Onyo, D.; Ndele, A.B.; Mobunda Tiko, J.; Bwazani Balandi, J.; Sambieni, K.R.; Meniko To Hulu, J.P.; Bastin, J.-F.; Meersmans, J.; et al. Plant Diversity of Concessions Held by Catholic Religious Groups in Three Cities of the Democratic Republic of the Congo. Sustainability 2025, 17, 6732. https://doi.org/10.3390/su17156732
Mukubu Pika L, Mugisho Mukotanyi S, Pyame Onyo D, Ndele AB, Mobunda Tiko J, Bwazani Balandi J, Sambieni KR, Meniko To Hulu JP, Bastin J-F, Meersmans J, et al. Plant Diversity of Concessions Held by Catholic Religious Groups in Three Cities of the Democratic Republic of the Congo. Sustainability. 2025; 17(15):6732. https://doi.org/10.3390/su17156732
Chicago/Turabian StyleMukubu Pika, Léa, Serge Mugisho Mukotanyi, David Pyame Onyo, Aloïse Bitagirwa Ndele, Joël Mobunda Tiko, Julien Bwazani Balandi, Kouagou Raoul Sambieni, Jean Pierre Meniko To Hulu, Jean-François Bastin, Jeroen Meersmans, and et al. 2025. "Plant Diversity of Concessions Held by Catholic Religious Groups in Three Cities of the Democratic Republic of the Congo" Sustainability 17, no. 15: 6732. https://doi.org/10.3390/su17156732
APA StyleMukubu Pika, L., Mugisho Mukotanyi, S., Pyame Onyo, D., Ndele, A. B., Mobunda Tiko, J., Bwazani Balandi, J., Sambieni, K. R., Meniko To Hulu, J. P., Bastin, J.-F., Meersmans, J., Useni Sikuzani, Y., & Bogaert, J. (2025). Plant Diversity of Concessions Held by Catholic Religious Groups in Three Cities of the Democratic Republic of the Congo. Sustainability, 17(15), 6732. https://doi.org/10.3390/su17156732







