Engineered Ceramic Composites from Electrolytic Manganese Residue and Fly Ash: Fabrication Optimization and Additive Modification Mechanisms
Abstract
1. Introduction
2. Material and Methods
2.1. Raw Materials and Additives
2.2. Material Preparation Methods
2.3. Characterization of Material Methods
3. Results and Discussion
3.1. Effect of EMR/FA Ratios on the Properties of Ceramic Materials
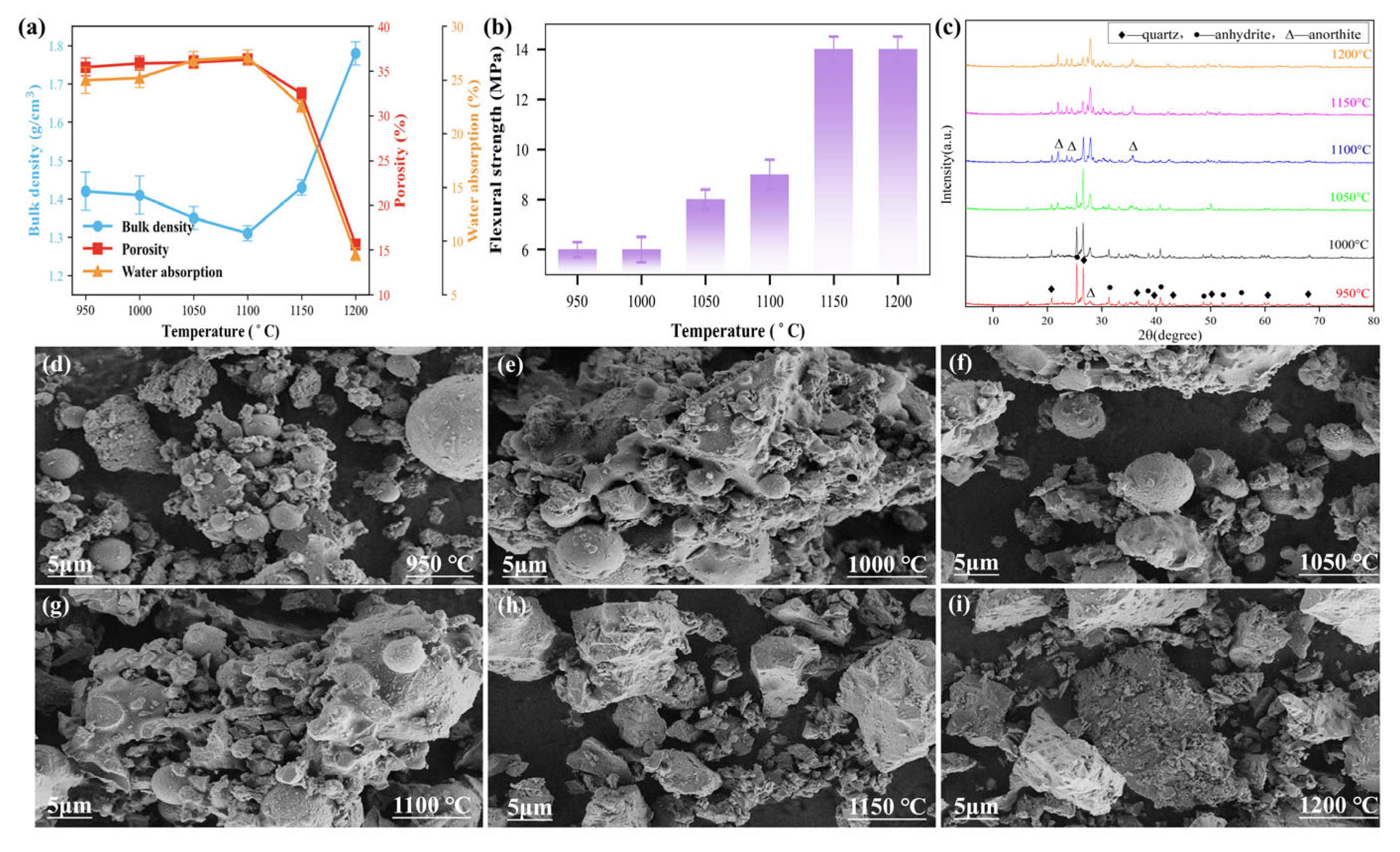
3.2. Effect of Sintering Temperature on the Properties of Ceramic Materials
3.3. Effects of Different Additives on Ceramic Material Properties
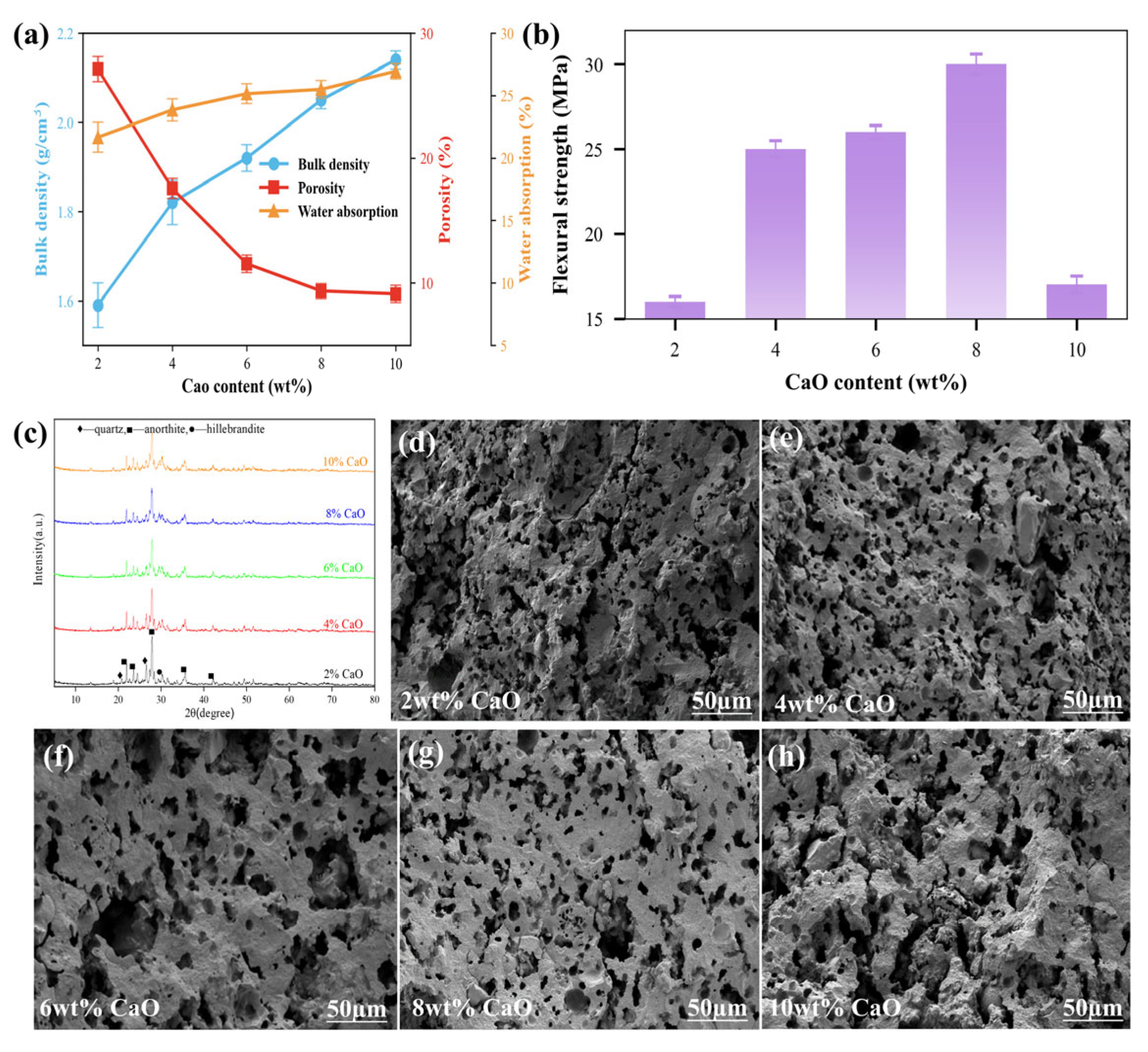
3.3.1. CaO
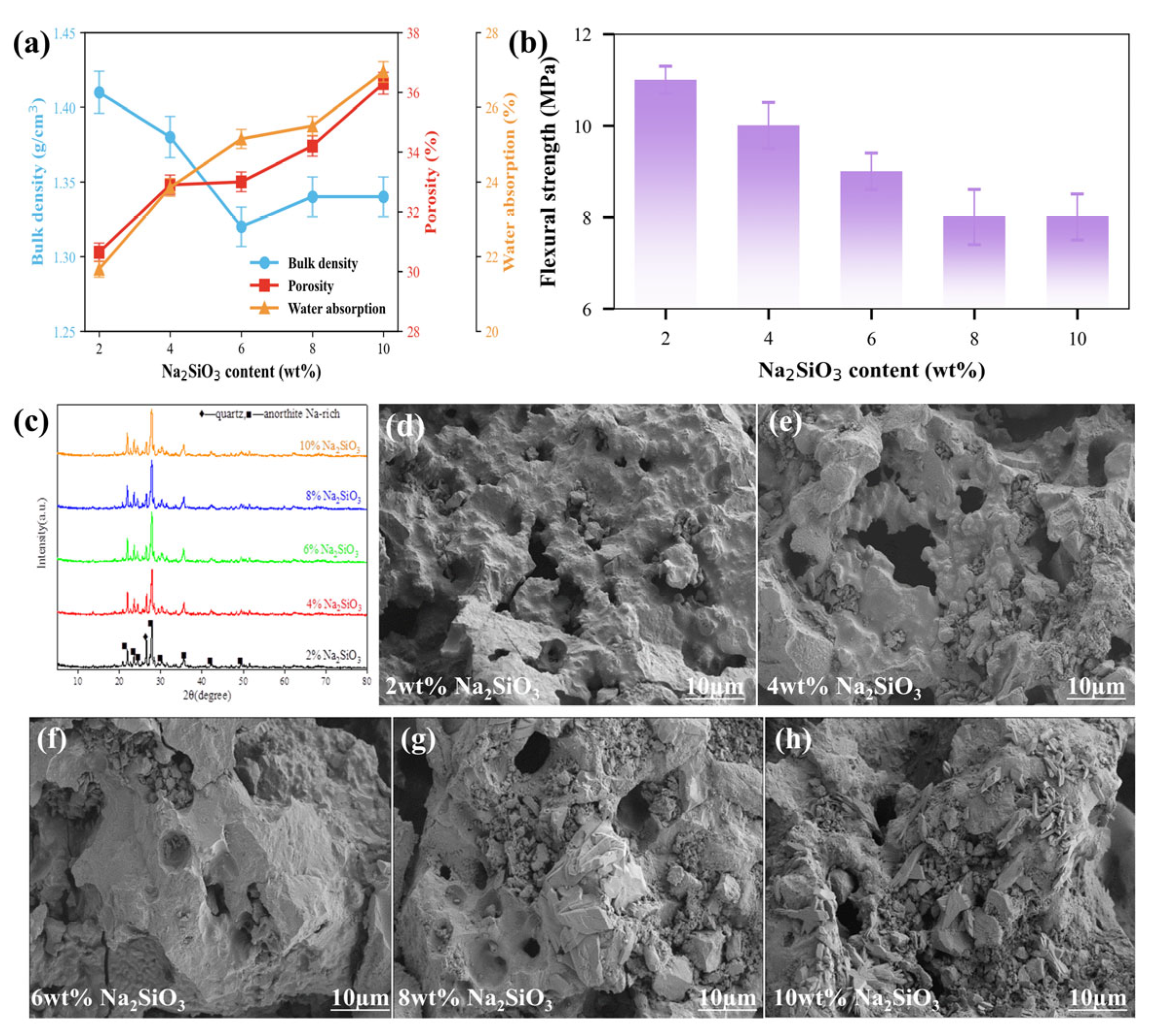
3.3.2. Na2SiO3
3.3.3. ZrO2
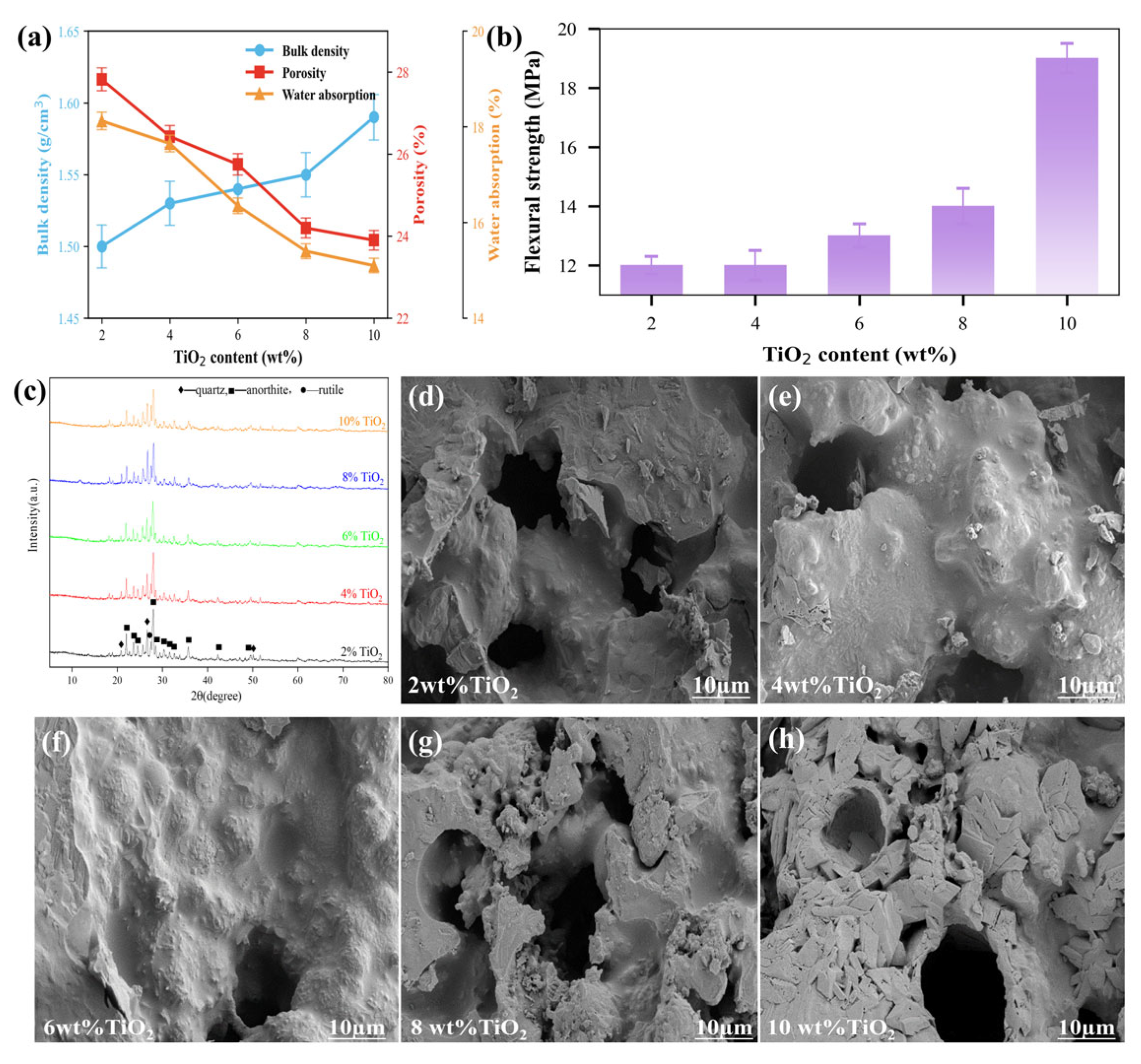
3.3.4. TiO2
3.4. Heavy Metal Leaching Toxicity Analysis
3.5. Thermal Shock Resistance and Thermal Expansion Behavior
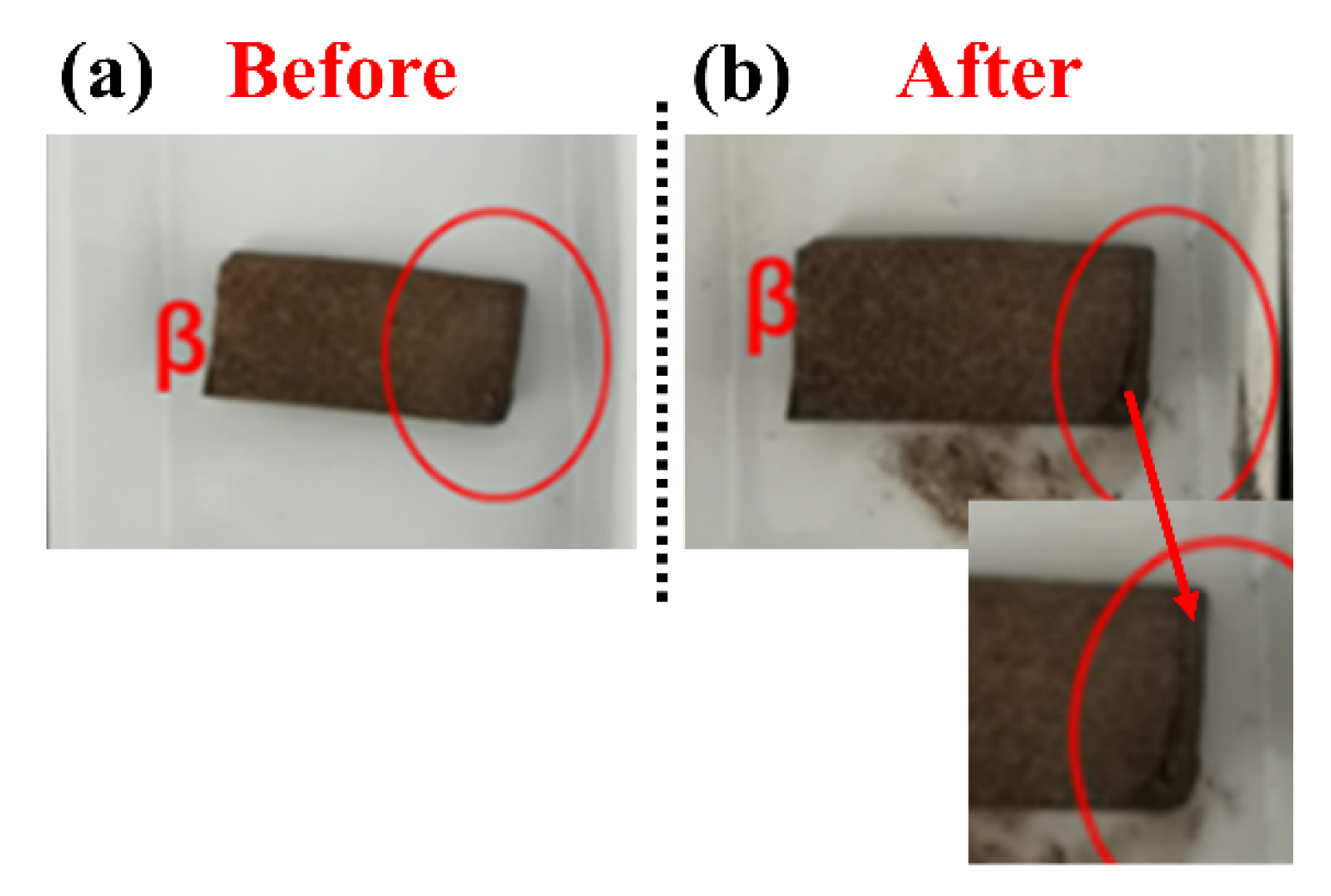
3.5.1. Thermal Shock Resistance
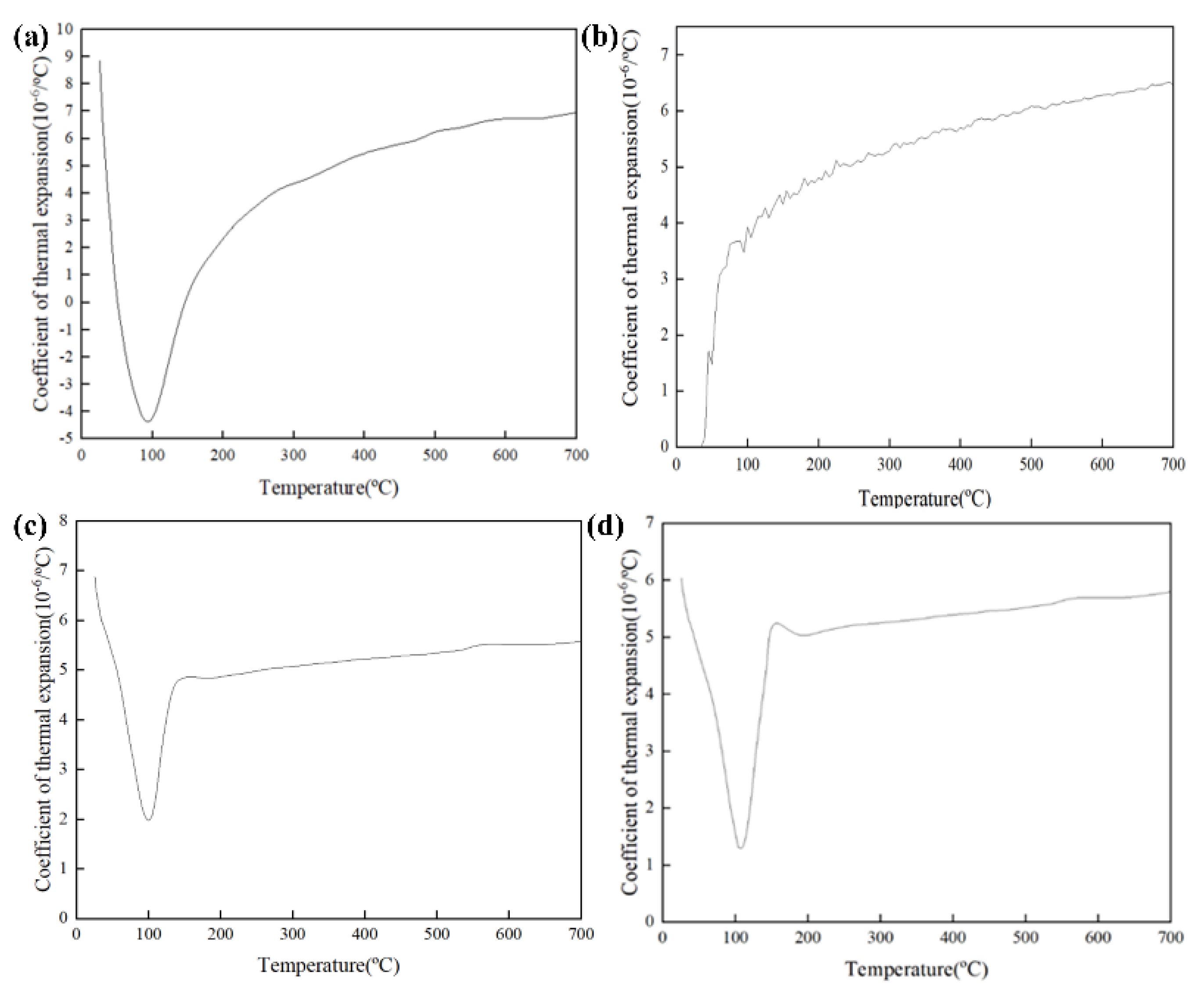
3.5.2. Thermal Expansion Behavior
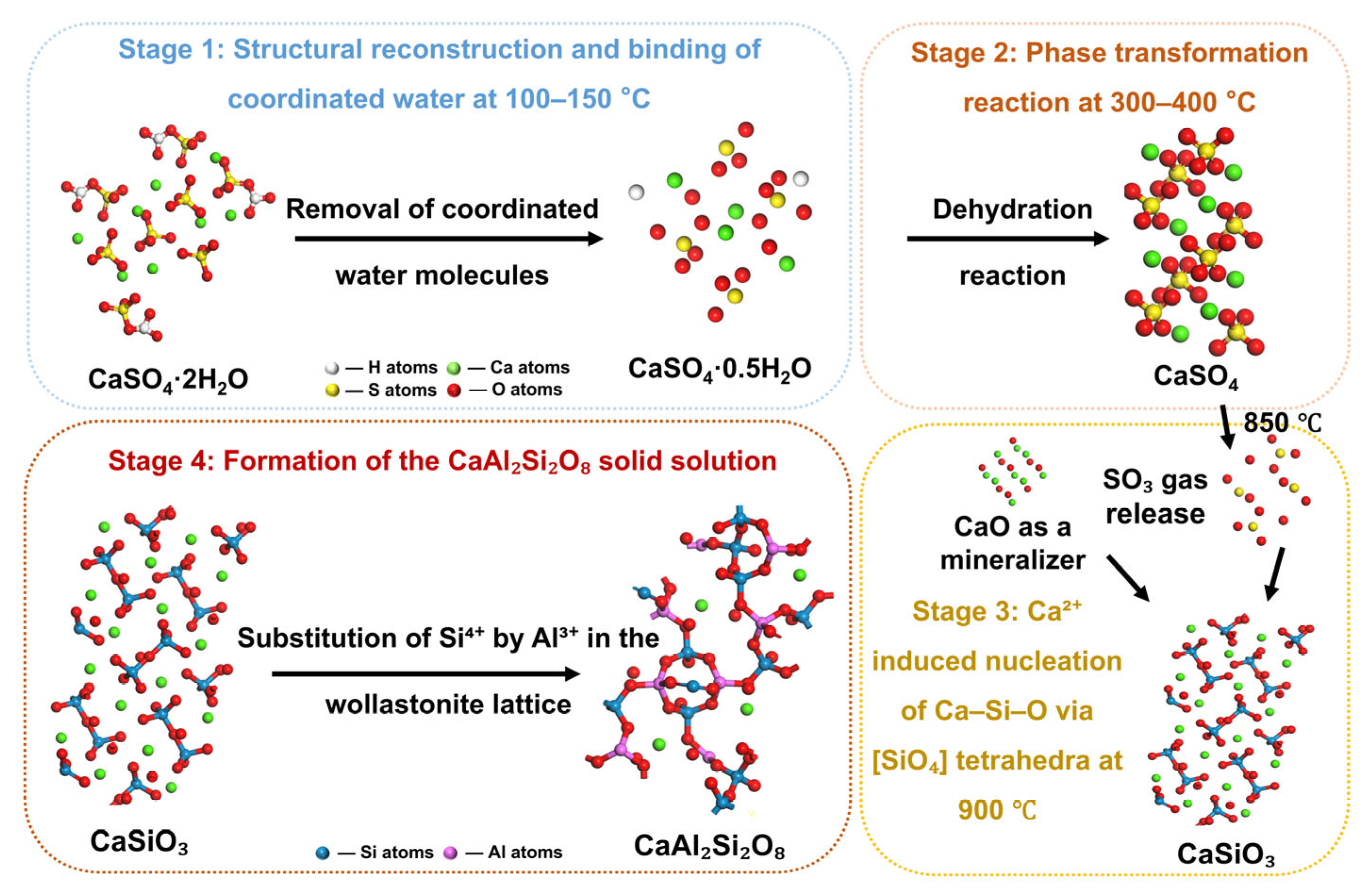
3.6. Mechanisms of Reaction Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashish, S.; Pankaj Kumar, D.; Abdul Wahab, H.; Mohammad, Y.; Hesam, K.; Shreeshivadasan, C. Challenges and opportunities of utilizing municipal solid waste as alternative building materials for sustainable development goals: A review. Sustain. Chem. Pharm. 2022, 27, 100706. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakajima, L. Sustainable development goals for advanced materials provided by industrial wastes and biomass sources. Curr. Opin. Green Sustain. Chem. 2021, 28, 100439. [Google Scholar] [CrossRef]
- Liu, Z.; Adams, M.; Cote, R.P.; Geng, Y.; Li, Y. Comparative study on the pathways of industrial parks towards sustainable development between China and Canada. Resour. Conserv. Recycl. 2018, 128, 417–425. [Google Scholar] [CrossRef]
- Su, L.; Wu, S.; Fu, G.; Zhu, W.; Zhang, X.; Liang, B. Creep characterisation and microstructural analysis of municipal solid waste incineration fly ash geopolymer backfill. Sci. Rep. 2024, 14, 29828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.F.; Wang, Z.Q.; Tao, Y.L.; Ju, L.Y.; Yang, H. Macro–micro investigation on stabilization sludge as subgrade filler by the ternary blending of steel slag and fly ash and calcium carbide residue. J. Clean. Prod. 2024, 447, 141496. [Google Scholar] [CrossRef]
- Fu, Y.; Qiao, H.; Feng, Q.; Chen, K.; Li, Y.; Xue, C.; Zhang, Y. Review of new methods for resource utilisation of electrolytic manganese residue and its application in building materials. Constr. Build. Mater. 2023, 401, 132901. [Google Scholar] [CrossRef]
- He, S.; Jiang, D.; Hong, M.; Liu, Z. Hazard-free treatment and resource utilisation of electrolytic manganese residue: A review. J. Clean. Prod. 2021, 306, 127224. [Google Scholar] [CrossRef]
- He, D.; Shu, J.; Wang, R.; Chen, M.; Wang, R.; Gao, Y.; Liu, R.; Liu, Z.; Xu, Z.; Tan, D.; et al. A critical review on approaches for electrolytic manganese residue treatment and disposal technology: Reduction, pretreatment, and reuse. J. Hazard. Mater. 2021, 418, 126235. [Google Scholar] [CrossRef]
- Li, J.; Sun, P.; Li, J.; Lv, Y.; Ye, H.; Shao, L.; Du, D. Synthesis of electrolytic manganese residue-fly ash based geopolymers with high compressive strength. Constr. Build. Mater. 2020, 248, 118489. [Google Scholar] [CrossRef]
- Su, H.; Zhou, W.; Lyu, X.; Liu, X.; Gao, W.; Li, C.; Li, S. Remediation treatment and resource utilization trends of electrolytic manganese residue. Miner. Eng. 2023, 202, 108264. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, G.; Qu, B.; Gong, C. Characterization of a fly ash-based hybrid well cement under different temperature curing conditions for natural gas hydrate drilling. Constr. Build. Mater. 2024, 445, 137874. [Google Scholar] [CrossRef]
- Jiang, Z.; He, G.; Duan, Y.; Jiang, Y.; Lin, Y.; Zhu, Y.; Wang, J. Contrasting effects of various factors upon the properties of foam ceramics and the mechanisms of crystalline phase reconstruction and microstructure regulation. Ceram. Int. 2024, 50, 21645–21657. [Google Scholar] [CrossRef]
- Bao, H.; Zheng, Z.; Xu, G.; Li, R.; Wang, Q.; Saafi, M.; Ye, J. Performance and mechanism of sand stabilization via microbial-induced CaCO3 precipitation using phosphogypsum. J. Clean. Prod. 2024, 468, 142999. [Google Scholar] [CrossRef]
- Venkatesan, G.; Alengaram, U.J.; Ibrahim, S.; Ibrahim, M.S.I. Effect of Fly Ash characteristics, sodium-based alkaline activators, and process variables on the compressive strength of siliceous Fly Ash geopolymers with microstructural properties: A comprehensive review. Constr. Build. Mater. 2024, 437, 136808. [Google Scholar] [CrossRef]
- Xu, W.; Tang, Z.; Xie, Y.; Long, G.; Zhu, H.; Kai, M.; Peng, L.; Wang, L.; Zaland, S. Effect of synthesis parameters on the alkali activation reaction degree and the relationship between reaction degree and microstructure of fly ash-based geopolymers. J. Build. Eng. 2024, 93, 109874. [Google Scholar] [CrossRef]
- Xiao, Y.; Cheng, D.; Li, G.; Yin, R.; Li, P.; Gao, Z. Preparation of MgO ceramics by low temperature sintering with MgF2 and Al2O3 as sintering additives. J. Electroceramics 2025, 54, 1–12. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Zhang, Y.; Zhang, X.; Guo, J.; Fu, Q.; Wu, L. High-temperature ablation resistance prediction of ceramic coatings using machine learning. J. Am. Ceram. Soc. 2024, 108, 20136. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, G.; Guan, X.; Yan, K.; Li, H.; Meng, Z.; Zhang, W.; Li, Z. An innovative approach to prepare calcium oxide from calcium carbide slag based on sequential mineral transformation. ACS Sustain. Chem. Eng. 2024, 1, 1279–1290. [Google Scholar] [CrossRef]
- Tian, X.K.; Lin, S.C.; Yan, J.; Zhao, C.Y. Sintering mechanism of calcium oxide/calcium carbonate during thermochemical heat storage process. Chem. Eng. J. 2021, 428, 131229. [Google Scholar] [CrossRef]
- Ali, H.; Gepreel, M.A.-H. Limitations and advancements of Sodium Silicate inorganic sand binders; A review. Int. J. Mater. Technol. Innov. 2022, 2, 47–54. [Google Scholar] [CrossRef]
- He, F.; Wang, X.; Wu, D. Phase-change characteristics and thermal performance of form-stable n-alkanes/silica composite phase change materials fabricated by sodium silicate precursor. Renew. Energy 2015, 74, 689–698. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, R.; Wang, B. Novel self-reinforcing ZrO2–SiO2 aerogels with high mechanical strength and ultralow thermal conductivity. Ceram. Int. 2018, 44, 15440–15445. [Google Scholar] [CrossRef]
- Srinivas, J.; Karthikeyan, K.R.; Senthil, T.; Yesuraj, K.; Aultrin, K.J. Characterization of Mechanical and Viscoelastic Properties of Ceramic Nanoparticle-Reinforced Polymer Composites. J. Polym. Compos. 2024, 13, 2321–2810. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X. Titanium dioxide nanomaterials: Self-structural modifications. Chem. Rev. 2014, 114, 9890–9918. [Google Scholar] [CrossRef] [PubMed]
- Sadia, S.I.; Shishir, M.K.H.; Ahmed, S.; Aidid, A.R.; Islam, M.M.; Rana, M.M.; Al-Reza, S.M.; Alam, M.A. Crystallographic biography on nanocrystalline phase of polymorphs titanium dioxide (TiO2): A perspective static review. S. Afr. J. Chem. Eng. 2024, 50, 51–64. [Google Scholar] [CrossRef]
- Ji, R.; Liu, Y.; Zhang, Y.; Wang, F. Machining performance of silicon carbide ceramic in end electric discharge milling. Int. J. Refract. Met. Hard Mater. 2010, 29, 117–122. [Google Scholar] [CrossRef]
- Ji, R.; Liu, Y.; Zhang, Y.; Cai, B.; Ma, J.; Li, X. Influence of dielectric and machining parameters on the process performance for electric discharge milling of SiC ceramic. Int. J. Adv. Manuf. Technol. 2011, 59, 127–136. [Google Scholar] [CrossRef]
- Ji, R.; Zhao, Q.; Zhao, L.; Liu, Y.; Jin, H.; Wang, L.; Wu, L.; Xu, Z. Study on high wear resistance surface texture of electrical discharge machining based on a new water-in-oil working fluid. Tribol. Int. 2023, 180, 108218. [Google Scholar] [CrossRef]
- Sha, F.; Dong, Y.; Gu, S.; Fan, X.; Xiao, W. Study on novel alkali-activated cementitious grout for scour control of offshore foundation. Geomech. Energy Environ. 2025, 42, 100663. [Google Scholar] [CrossRef]
- Liu, B.; Sun, J.; Guo, L.; Shi, H.; Feng, G.; Feldmann, L.; Yin, X.; Riedel, R.; Fu, Q.; Li, H. Materials design of silicon based ceramic coatings for high temperature oxidation protection. Mater. Sci. Eng. R Rep. 2025, 163, 100936. [Google Scholar] [CrossRef]
- Amorós, J.L.; Blasco, E.; Feliu, C.; Moreno, A. Effect of kaolin addition on the sinter-crystallisation kinetics of compacts of a crystallising frit. J. Non-Cryst. Solids 2022, 596, 121864. [Google Scholar] [CrossRef]
- HJ/T 299-2007; Identification Standards for Hazardous Wastes—Identification for Extraction Toxicity. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2007.
- JC/T 626–2006; Common Brick. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2006.
- Andrini, L.; Gauna, M.R.; Conconi, M.S.; Suarez, G.; Requejo, F.G.; Aglietti, E.F.; Rendtorff, N.M. Extended and local structural description of a kaolinitic clay, its fired ceramics and intermediates: An XRD and XANES analysis. Appl. Clay Sci. 2016, 124, 39–45. [Google Scholar] [CrossRef]
- Yang, H.L.; Li, Z.S.; Ding, Y.D.; Ge, Q.Q.; Shi, Y.J.; Jiang, L. Effect of Silicon Source (Fly Ash, Silica Dust, Gangue) on the Preparation of Porous Mullite Ceramics from Aluminum Dross. Materials 2022, 15, 7212. [Google Scholar] [CrossRef] [PubMed]
- Ding, C. Preparation and thermal shocks of mullite-based porous ceramic materials using the reaction sintering method. Int. J. Appl. Ceram. Technol. 2025, 22, e15091. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Ke, X.; Jiao, X.; Li, R.; Shi, C. Geopolymer synthesized from electrolytic manganese residue and lead-zinc smelting slag: Compressive strength and heavy metal immobilization. Cem. Concr. Compos. 2022, 134, 104806. [Google Scholar] [CrossRef]
- Jiang, J.; Luo, H.; Wang, S.; Ou, X.; Su, J.; Lyu, Z.; Chen, J.; Wei, D. Synthesis of tailing slurry-based geopolymers for the highly efficient immobilization of heavy metals: Behavior and mechanism. Appl. Clay Sci. 2023, 247, 107199. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Navrotsky, A.; Green, D.J. Experimental Approaches to the Thermodynamics of Ceramics Above 1500 °C. J. Am. Ceram. Soc. 2012, 95, 1463–1482. [Google Scholar] [CrossRef]
- Heimann, R.B.; Maggetti, M. The struggle between thermodynamics and kinetics: Phase evolution of ancient and historical ceramics. GeoScienceWorld 2019, 20, 130. [Google Scholar]
- Wang, Z.; Lyu, X.; Yao, G.; Wu, P.; Wang, J.; Wei, J. Preparation of Ca–Si–Al–Mg porous ceramics by Co-operation of Ca&Mg-contained soda residue and altered rock gold tailings. J. Clean. Prod. 2020, 262, 121345. [Google Scholar]
- Megaw, H.D.; Kempster, C.J.E.; Radoslovich, E.W. The structure of anorthite, CaAl2Si2O8. II. Description and discussion. Acta Crystallogr. 1962, 15, 1017–1035. [Google Scholar] [CrossRef]
- Kushiro, I.; Yoder, H.S., Jr. Anorthite—Forsterite and Anorthite—Enstatite Reactions and their bearing on the Basalt—Eclogite Transformation. J. Petrol. 1966, 7, 337–362. [Google Scholar] [CrossRef]
- Cengizler, H.; Koç, M.; Şan, O. Production of ceramic glass foam of low thermal conductivity by a simple method entirely from fly ash. Ceram. Int. 2021, 47, 28460–28470. [Google Scholar] [CrossRef]
- Goga, F.; Dudric, R.; Cormos, C.; Imre, F.; Bizo, L.; Misca, R. Fly ash from thermal power plant, raw material for glass-ceramic. Environ. Eng. Manag. J. 2013, 12, 337–342. [Google Scholar] [CrossRef]
- Wons, W.; Rzepa, K.; Reben, M.; Murzyn, P.; Sitarz, M.; Olejniczak, Z. Effect of thermal processing on the structural characteristics of fly ashes. J. Mol. Struct. 2018, 1165, 299–304. [Google Scholar] [CrossRef]
- Wang, C.; Yan, W.; Chen, Q.; Wang, X. Microstructures and properties of microporous MgO-MgAl2O4 refractory aggregates from Mg(OH)2 powder and α-Al2O3 micro-powder. Constr. Build. Mater. 2024, 426, 136144. [Google Scholar] [CrossRef]
- Tu, Y.; Zhang, Y.; Su, Z.; Jiang, T. Mineralization mechanism of limonitic laterite sinter under different fuel dosage: Effect of FeO. Powder Technol. 2022, 398, 117064. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Wang, Z.; Anthony, E.J. The kinetics and pore structure of sorbents during the simultaneous calcination/sulfation of limestone in CFB. Fuel 2017, 208, 203–213. [Google Scholar] [CrossRef]
- Kucharczyk, S.; Sitarz, M.; Zajac, M.; Deja, J. The effect of CaO/SiO2 molar ratio of CaO-Al2O3-SiO2 glasses on their structure and reactivity in alkali activated system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 194, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Duan, Y.; Li, M.; Wang, P.; Pang, B.; Xu, H.; Hou, D. Unveiling the dissolution mechanism of calcium ions from CSH substrates in Na2SO4 solution: Effects of Ca/Si ratio. Appl. Surf. Sci. 2025, 680, 161443. [Google Scholar] [CrossRef]
- Maruyama, I.; Kontani, O.; Takizawa, M.; Sawada, S.; Ishikawao, S.; Yasukouchi, J.; Sato, O.; Etoh, J.; Igari, T. Development of soundness assessment procedure for concrete members affected by neutron and gamma-ray irradiation. J. Adv. Concr. Technol. 2017, 15, 440–523. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sheikh, M.A. Synthesis of sodium silicate and its application of dielectric relaxation spectroscopy—A report. J. Micromechanics Mol. Phys. 2024, 9, 15–19. [Google Scholar] [CrossRef]
- Manurung, R.; Hasibuan, R.; Siregar, A.G.A. Preparation and characterization of lithium, sodium, and potassium silicate from palm leaf as a potential solid base catalyst in developed biodiesel production. Case Stud. Chem. Environ. Eng. 2024, 9, 100543. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Chakraborty, S. Spectroscopic approach to understanding complex impedance in sodium silicate. Phys. Open 2025, 23, 100255. [Google Scholar] [CrossRef]
- Sengupta, P.; Bhattacharjee, A.; Maiti, H.S. Zirconia: A Unique Multifunctional Ceramic Material. Trans. Indian Inst. Met. 2019, 72, 1981–1998. [Google Scholar] [CrossRef]
- Zheng, W.; Cui, J.; Sheng, L.; Chao, H.; Peng, Z.; Shen, C. Effect of complex nucleation agents on preparation and crystallization of CaO-MgO-Al2O3-SiO2 glass-ceramics for float process. J. Non-Cryst. Solids 2016, 450, 6–11. [Google Scholar] [CrossRef]
- Celtek, M.; Sengul, S. Thermodynamic and dynamical properties and structural evolution of binary Zr80Pt20 metallic liquids and glasses: Molecular dynamics simulations. J. Non-Cryst. Solids 2018, 498, 32–41. [Google Scholar] [CrossRef]
- Sathiyakumar, M.; Gnanam, F.D. Influence of MnO and TiO2 additives on density, microstructure and mechanical properties of Al2O3. Ceram. Int. 2002, 28, 195–200. [Google Scholar] [CrossRef]
- Sun, M.; Zheng, X.; Liu, X.; Wang, J.; Han, B.; Wu, S.; Zhao, R.; Zhu, Z.; Shen, S.; Liu, S. Effects of TiO2 nanobelts addition on microstructure and physical properties of vitrified bonds. Ceram. Int. 2024, 50, 45125–45132. [Google Scholar] [CrossRef]
- Maeda, K.; Sera, Y.; Yasumori, A. Effect of molybdenum and titanium oxides on mechanical and thermal properties of cordierite–enstatite glass-ceramics. J. Non-Cryst. Solids 2016, 434, 13–22. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, G.; Dong, J.; Hou, J.; He, G. Damping behavior and energy absorption capability of porous magnesium. J. Alloys Compd. 2016, 680, 522–530. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Tian, X.; Chen, X. Superior CO2 uptake and enhanced compressive strength for carbonation curing of cement-based materials via flue gas. Constr. Build. Mater. 2022, 346, 128364. [Google Scholar] [CrossRef]
- Shao, F.; Zhao, H.; Ni, J.; Zhuang, Y.; Sheng, J.; Yang, J.; Zhong, X.; Tao, S. Corrosion behavior and mechanical properties of plasma sprayed Al2O3-aluminum bronze and Ca2SiO4-aluminum bronze coatings. Mater. Chem. Phys. 2023, 311, 128579. [Google Scholar] [CrossRef]
- 66. GB/T 3810.9–2016; Test Methods for Ceramic Tiles—Part 9: Resistance to Thermal Shock. Standardization Administration of China: Beijing, China, 2016.
- Belmonte, M. Advanced ceramic materials for high temperature applications. Adv. Eng. Mater. 2006, 8, 693–703. [Google Scholar] [CrossRef]
- Justin, J.; Jankowiak, A. Ultra High Temperature Ceramics: Densification, Properties and Thermal Stability. Aerosp. Lab. 2011, 3, 1–11. [Google Scholar]
- Li, K.; Wang, D.; Chen, H.; Guo, L. Normalized evaluation of thermal shock resistance for ceramic materials. J. Adv. Ceram. 2014, 3, 250–258. [Google Scholar] [CrossRef]
- Dove, M.T.; Fang, H. Negative thermal expansion and associated anomalous physical properties: Review of the lattice dynamics theoretical foundation. Rep. Prog. Phys. 2016, 79, 066503. [Google Scholar] [CrossRef]
- Şener, S.; Bilgen, S.; Özbayoğlu, G. Effect of heat treatment on grindabilities of celestite and gypsum and separation of heated mixture by differential grinding. Miner. Eng. 2004, 17, 473–475. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Calcium sulphate hemihydrate hydration leading to gypsum crystallization. Prog. Cryst. Growth Charact. Mater. 2007, 53, 57–77. [Google Scholar] [CrossRef]
- Gurgul, S.J.; Seng, G.; Williams, G.R. A kinetic and mechanistic study into the transformation of calcium sulfate hemihydrate to dihydrate. J. Synchrotron Radiat. 2019, 26, 774–784. [Google Scholar] [CrossRef]
- Pankrushina, E.A.; Votyakov, S.L.; Shchapova, Y.V. Statistical approaches in the analysis of in situ thermo-Raman spectroscopic data for gypsum as a basis for studying dehydration and phase transformations in crystalline hydrates. J. Raman Spectrosc. 2021, 52, 877–889. [Google Scholar] [CrossRef]
- Tian, H.; Guo, Q.; Chang, J. Investigation into Decomposition Behavior of CaSO4 in Chemical-Looping Combustion. Energy Fuels 2008, 22, 3915–3921. [Google Scholar] [CrossRef]
- Sheng, S.U.; Tao, L.I.U.; Yi, W.A.N.G.; Song, H.U. Study on CO2 absorption-mineralization characteristics of mixed amine solution coupled with CaO and key influencing factors in mineralization process. J. Fuel Chem. Technol. 2022, 50, 1371–1380. [Google Scholar]
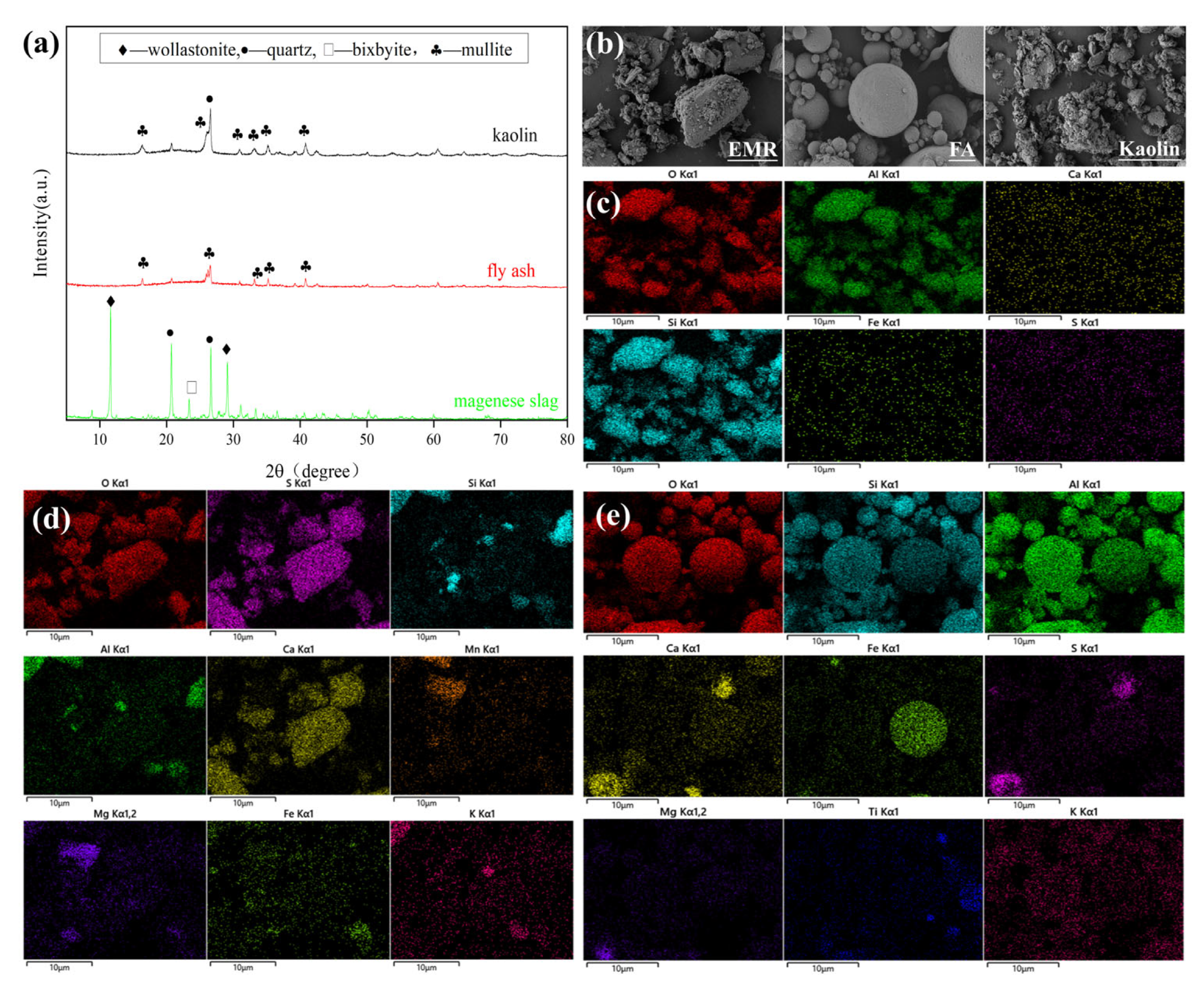




| Content (%) | SiO2 | Al2O3 | SO3 | CaO | Fe2O3 |
|---|---|---|---|---|---|
| EMR | 24.26 | 6.21 | 38.01 | 18.32 | 3.12 |
| FA | 50.45 | 31.17 | 1.54 | 3.99 | 5.53 |
| Kaolin | 55.05 | 41.67 | 0.02 | 0.32 | 0.69 |
| MnO | MgO | Na2O | K2O | TiO2 | |
| EMR | 4.35 | 1.78 | 1.15 | 1.03 | 0.48 |
| FA | 0.06 | 0.78 | 1.18 | 2.68 | 1.49 |
| Kaolin | - | - | 0.38 | 0.26 | 1.00 |
| Number | EMR (g) | FA (g) | Kaolin (g) |
|---|---|---|---|
| 1 | 85 | 0 | 15 |
| 2 | 60 | 25 | 15 |
| 3 | 50 | 35 | 15 |
| 4 | 45 | 40 | 15 |
| 5 | 40 | 45 | 15 |
| 6 | 35 | 50 | 15 |
| 7 | 25 | 60 | 15 |
| 8 | 0 | 85 | 15 |
| As | Cr | Cu | Pb | Hg | Cd | Mn | |
|---|---|---|---|---|---|---|---|
| EMR | 2.74 | 0.25 | 0.41 | 0.49 | 0.026 | 0.034 | 189.29 |
| FA | 0.11 | 0.3 | 0.12 | 0.08 | 0.017 | 0.002 | 8 |
| 8 wt% CaO | 0.59 | 0.24 | 0.14 | 0.14 | 0.023 | 0.005 | 38.4 |
| Total Fixation Efficiency (%) | 80.14 | 56.36 | 73.58 | 75.44 | 46.51 | 86.11 | 80.54 |
| 2 wt% Na2SiO3 | 0.42 | 0.20 | 0.2 | 0.13 | 0.034 | 0.005 | 38.9 |
| Total Fixation Efficiency (%) | 85.26 | 63.64 | 62.26 | 77.19 | 20.93 | 86.11 | 80.28 |
| 10 wt% ZrO2 | 0.38 | 0.23 | 0.22 | 0.09 | 0.0263 | 0.01 | 38.6 |
| Total Fixation Efficiency (%) | 86.67 | 58.18 | 58.49 | 84.21 | 38.84 | 72.33 | 80.44 |
| 10 wt% TiO2 | 0.19 | 0.18 | 0.15 | 0.02 | 0.0245 | 0 | 30.1 |
| Total Fixation Efficiency (%) | 93.33 | 67.27 | 71.70 | 96.49 | 43.02 | 100 | 84.74 |
| 8 wt% CaO | 2 wt% Na2SiO3 | 10 wt% ZrO2 | 10 wt% TiO2 | |
|---|---|---|---|---|
| Code | α | β | γ | δ |
| Sample | Length Before (cm) | Length After (cm) | Shrinkage (%) |
|---|---|---|---|
| α | 5.31 | 5.18 | 2.45 |
| γ | 5.79 | 5.09 | 12.09 |
| β | 4.39 | 3.75 | 14.58 |
| δ | 5.42 | 4.89 | 9.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Li, S.; Li, Z.; Zhang, D.; An, G.; Shi, X.; Sun, X.; Li, K. Engineered Ceramic Composites from Electrolytic Manganese Residue and Fly Ash: Fabrication Optimization and Additive Modification Mechanisms. Sustainability 2025, 17, 6647. https://doi.org/10.3390/su17146647
He Z, Li S, Li Z, Zhang D, An G, Shi X, Sun X, Li K. Engineered Ceramic Composites from Electrolytic Manganese Residue and Fly Ash: Fabrication Optimization and Additive Modification Mechanisms. Sustainability. 2025; 17(14):6647. https://doi.org/10.3390/su17146647
Chicago/Turabian StyleHe, Zhaohui, Shuangna Li, Zhaorui Li, Di Zhang, Guangdong An, Xin Shi, Xin Sun, and Kai Li. 2025. "Engineered Ceramic Composites from Electrolytic Manganese Residue and Fly Ash: Fabrication Optimization and Additive Modification Mechanisms" Sustainability 17, no. 14: 6647. https://doi.org/10.3390/su17146647
APA StyleHe, Z., Li, S., Li, Z., Zhang, D., An, G., Shi, X., Sun, X., & Li, K. (2025). Engineered Ceramic Composites from Electrolytic Manganese Residue and Fly Ash: Fabrication Optimization and Additive Modification Mechanisms. Sustainability, 17(14), 6647. https://doi.org/10.3390/su17146647




