Sustainable Remediation Strategies and Technologies of Per- and Polyfluoroalkyl Substances (PFAS)-Contaminated Soils: A Critical Review
Abstract
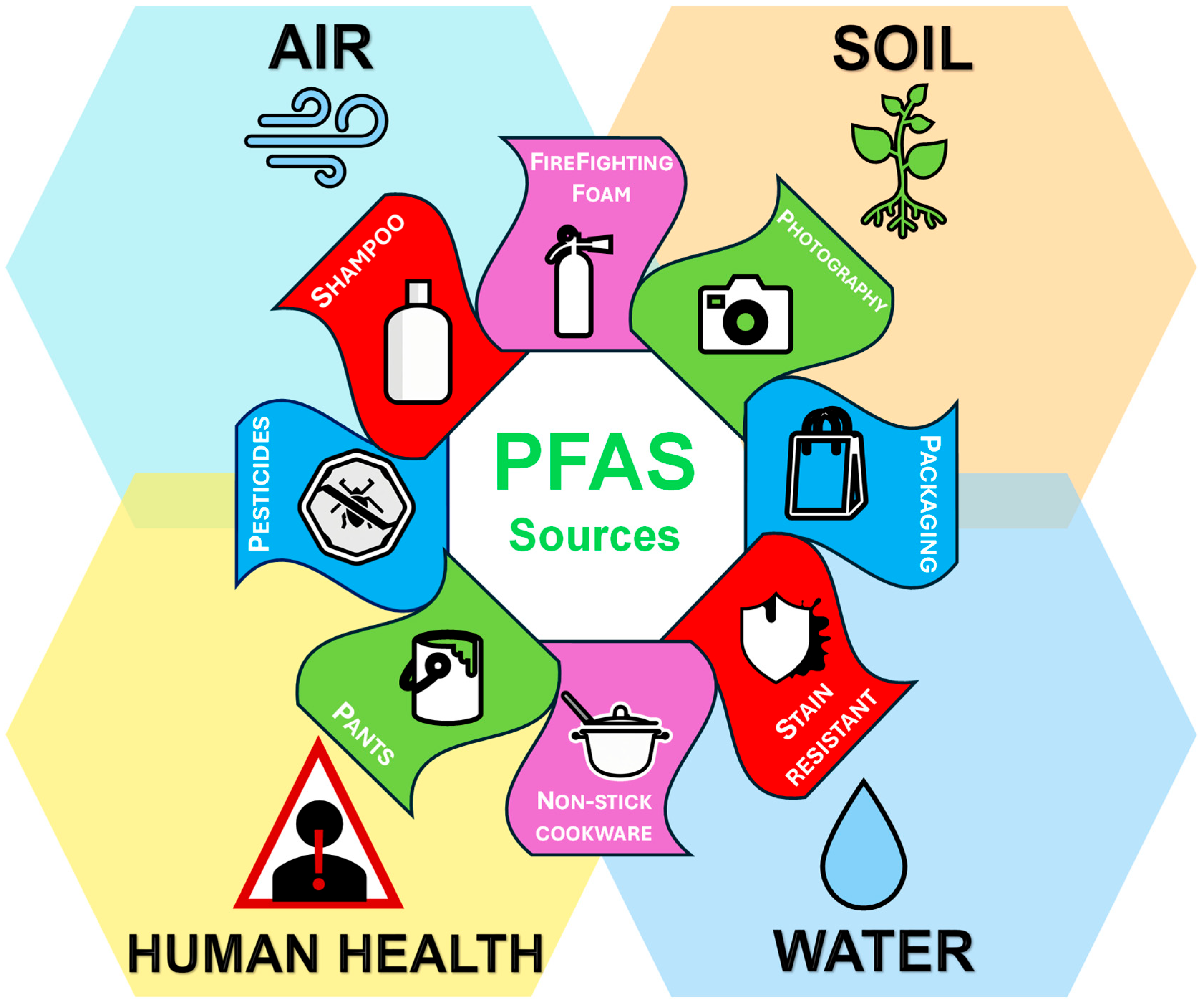
1. Introduction
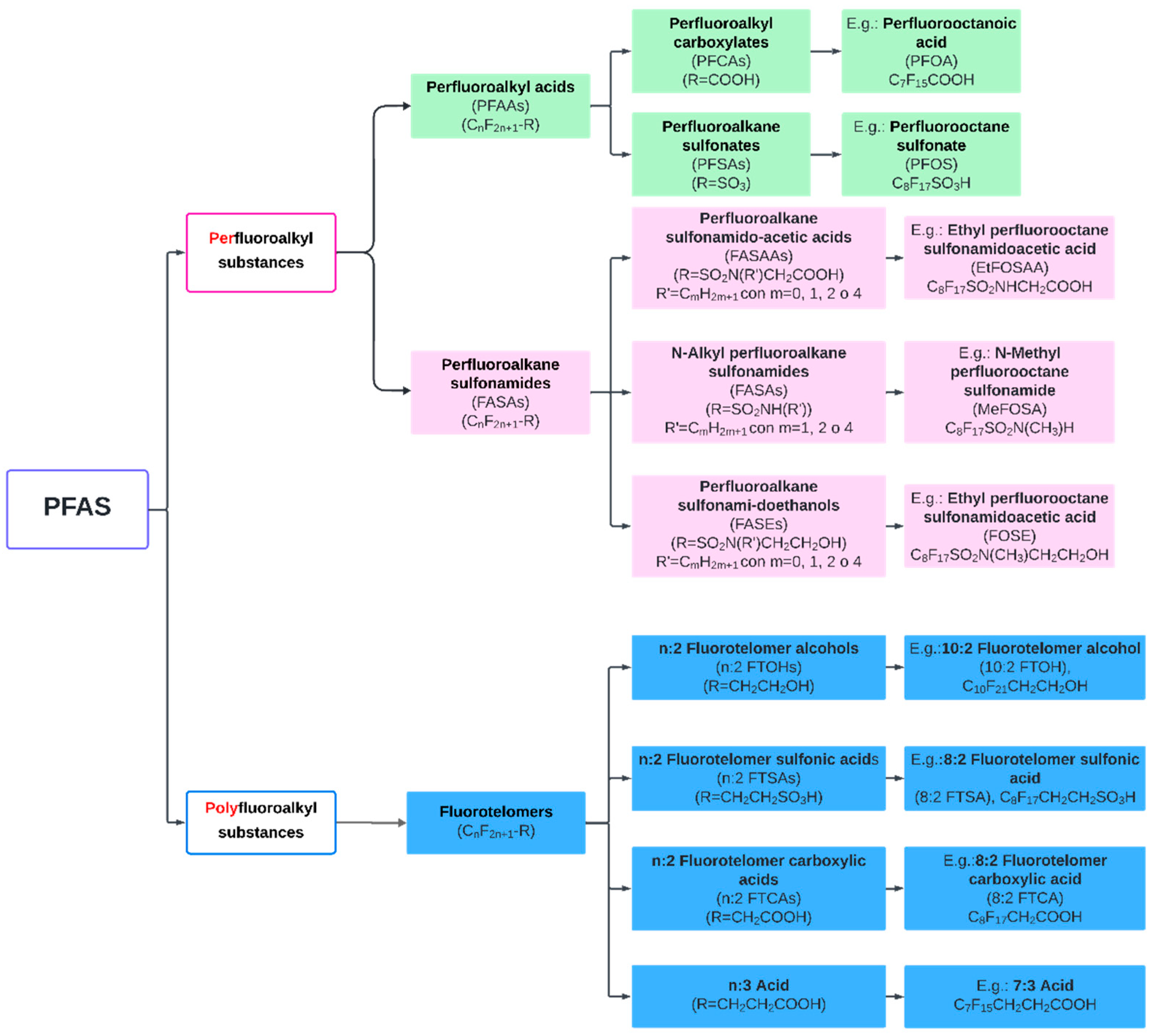
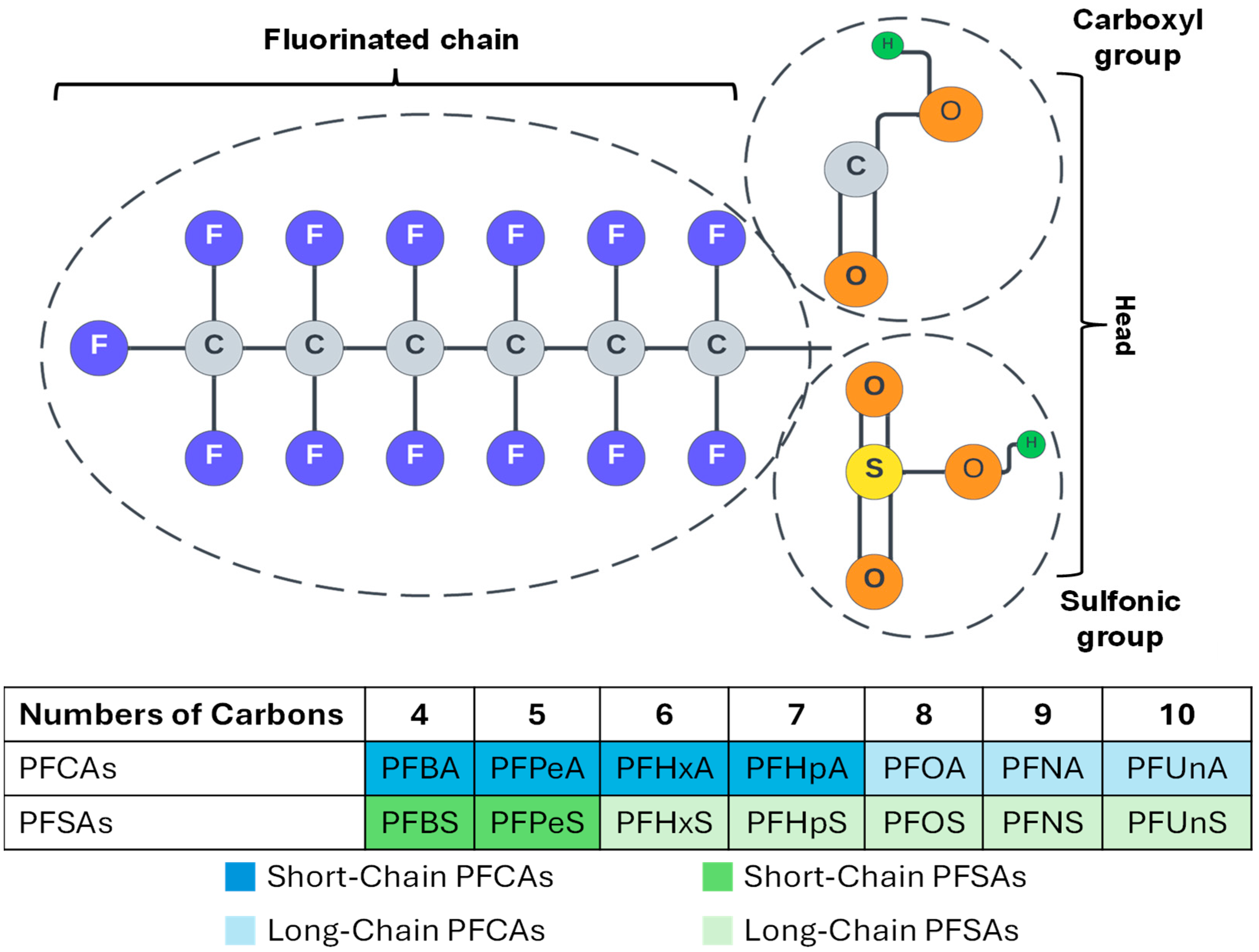
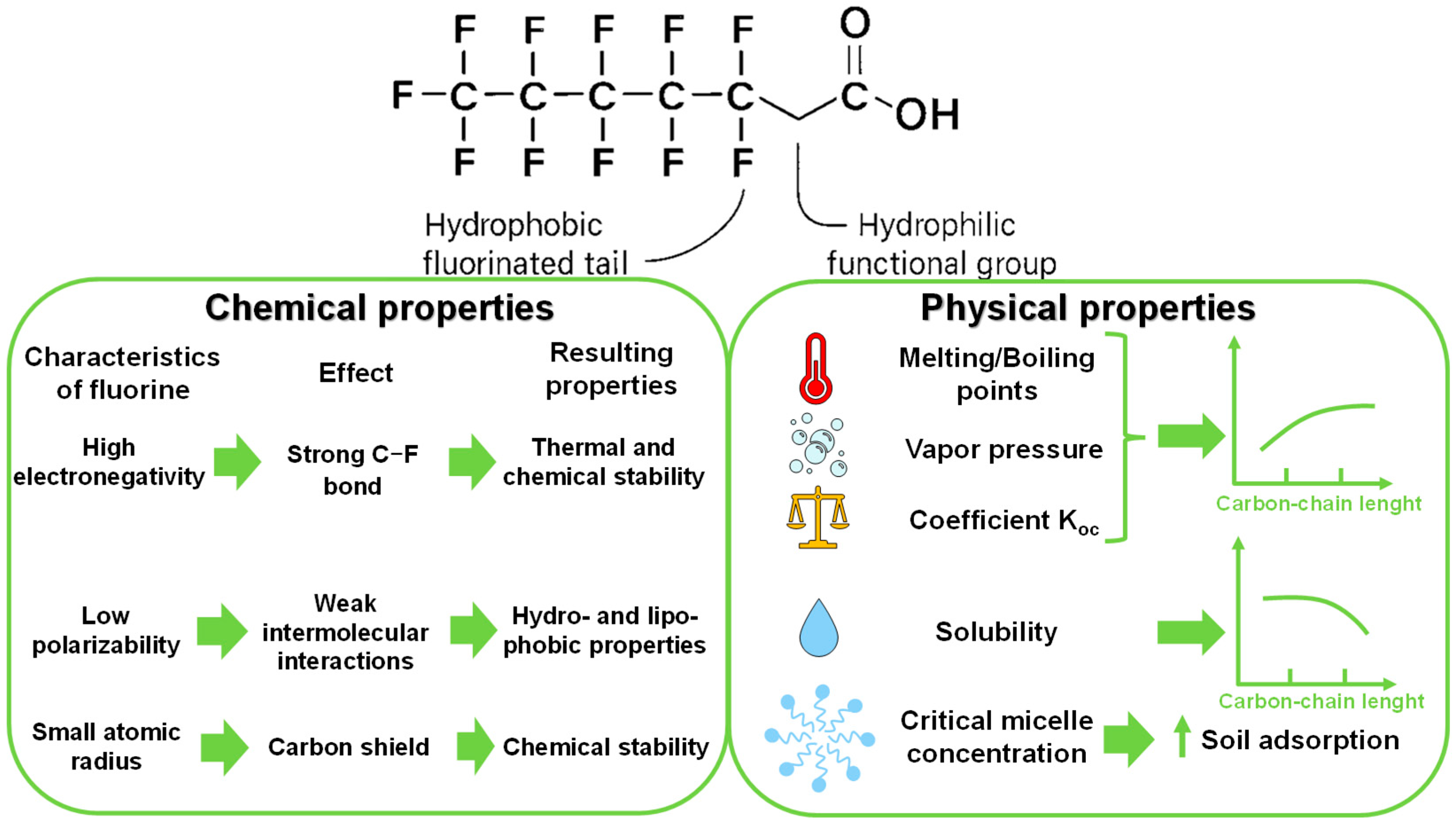
2. PFAS Characterization and Behavior in Soil
3. PFAS Remediation Technologies
3.1. PFAS Immobilization Technologies
3.1.1. Sorption
3.1.2. Stabilization/Solidification (S/S)
3.2. PFAS Mobilization Technologies
3.2.1. Soil Washing/Flushing
3.2.2. Phytoremediation
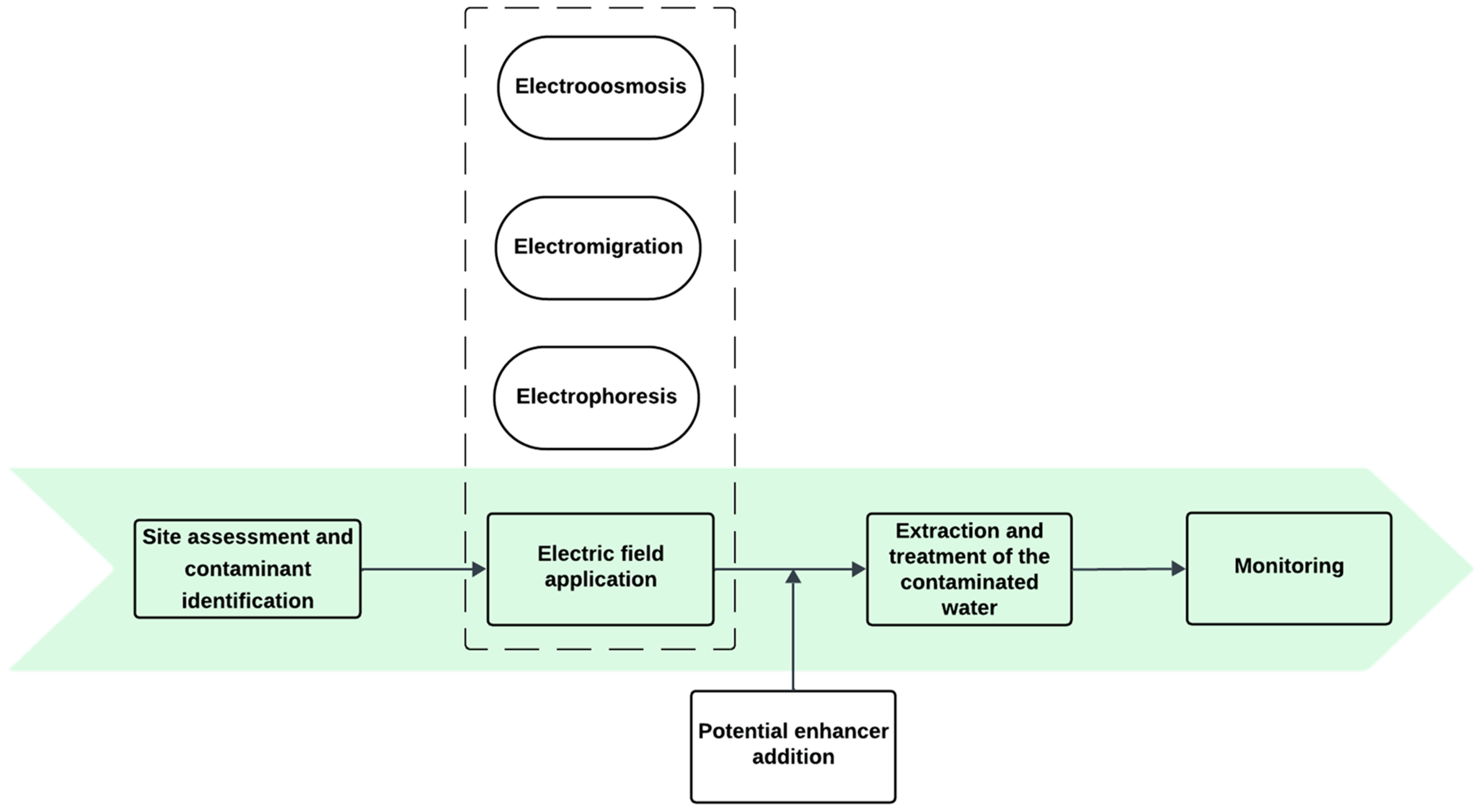
3.2.3. Electrokinetic (EK) Remediation
3.3. PFAS Destruction Technologies
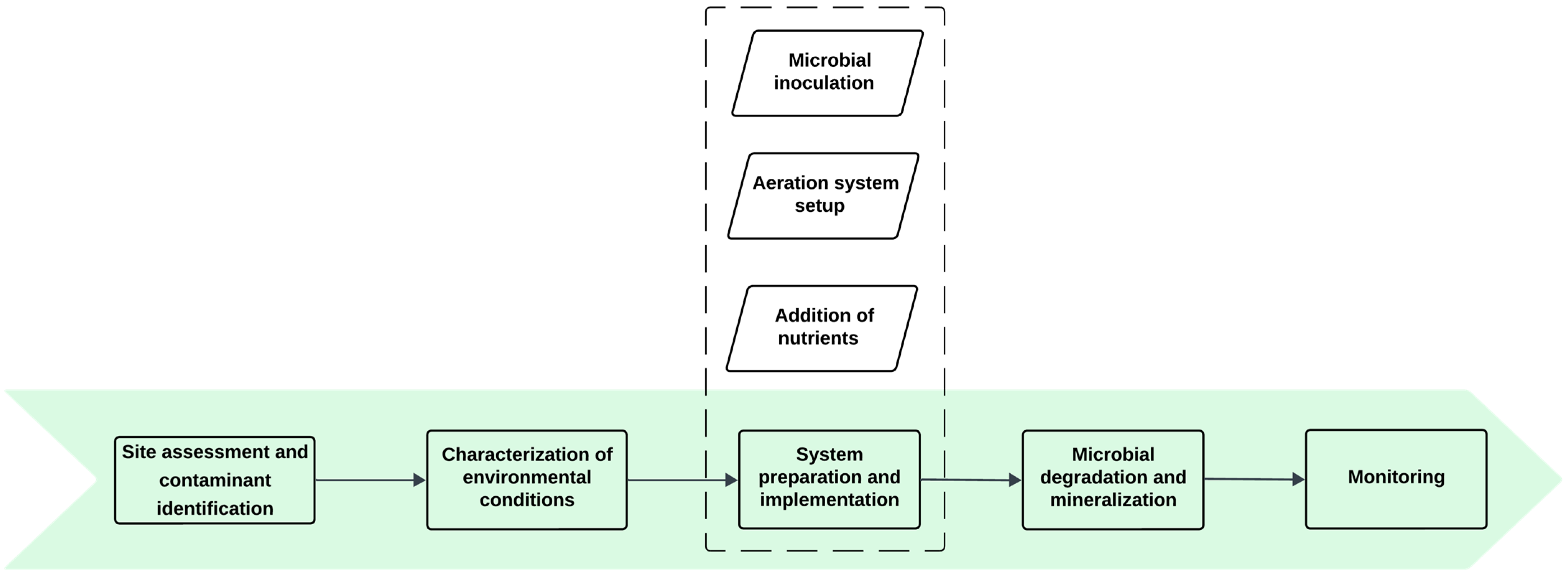
3.3.1. Bioremediation
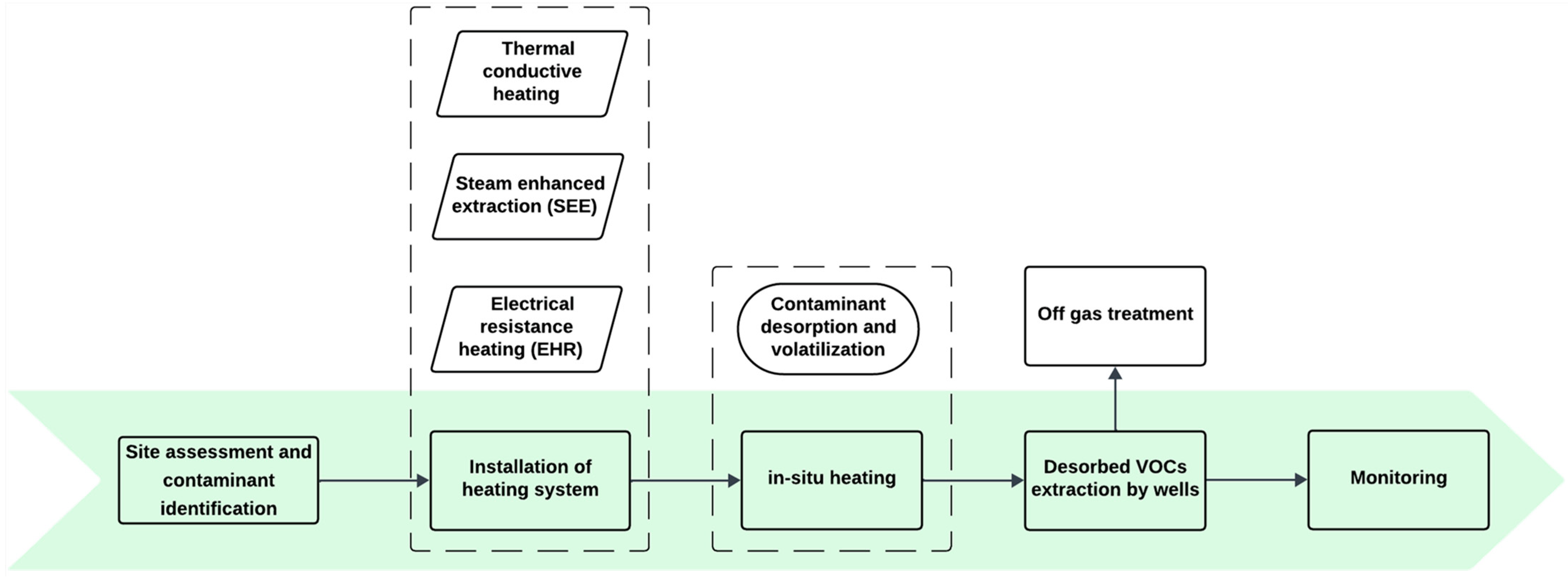
3.3.2. Thermal Treatment
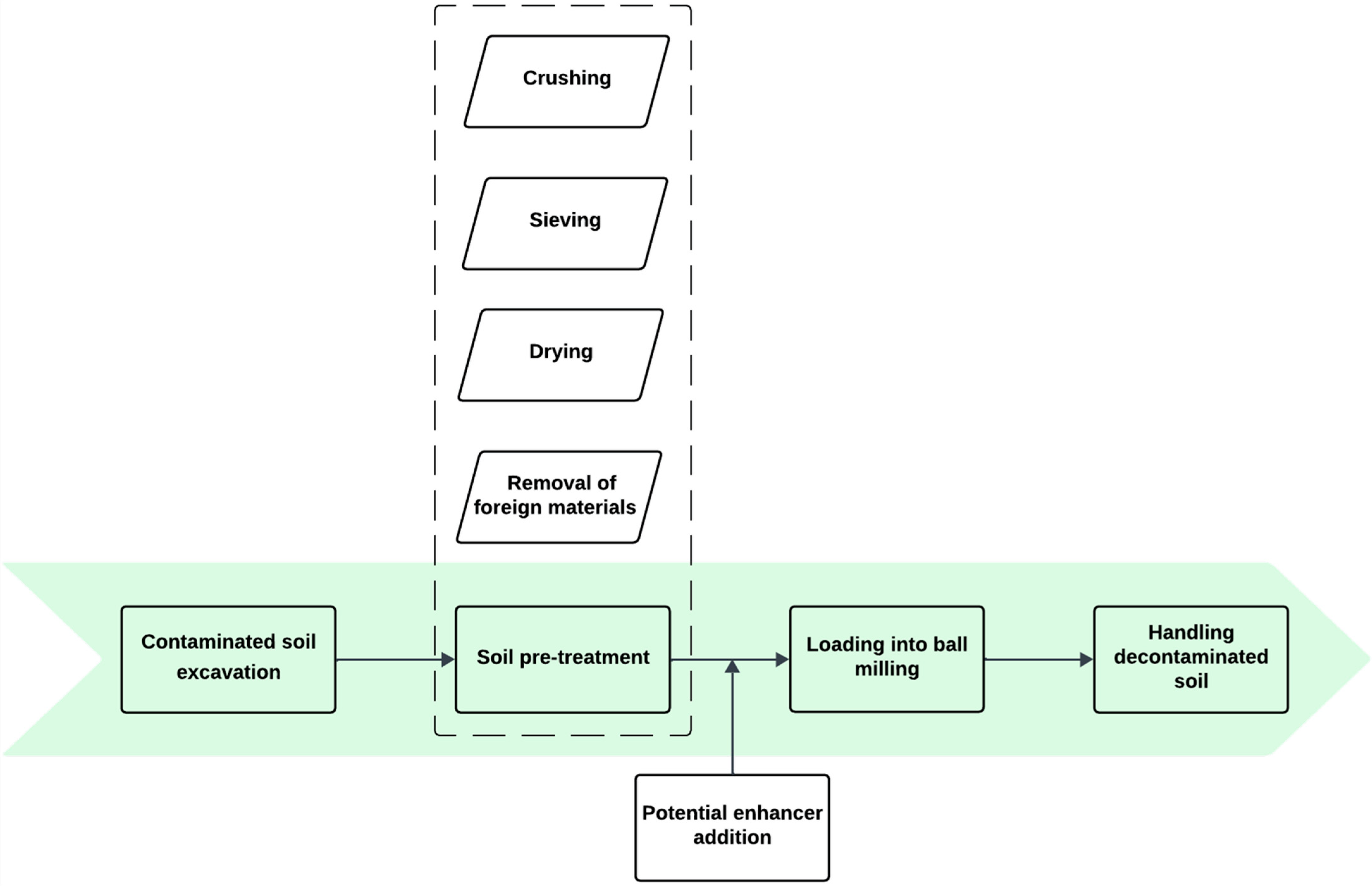
3.3.3. Ball Milling
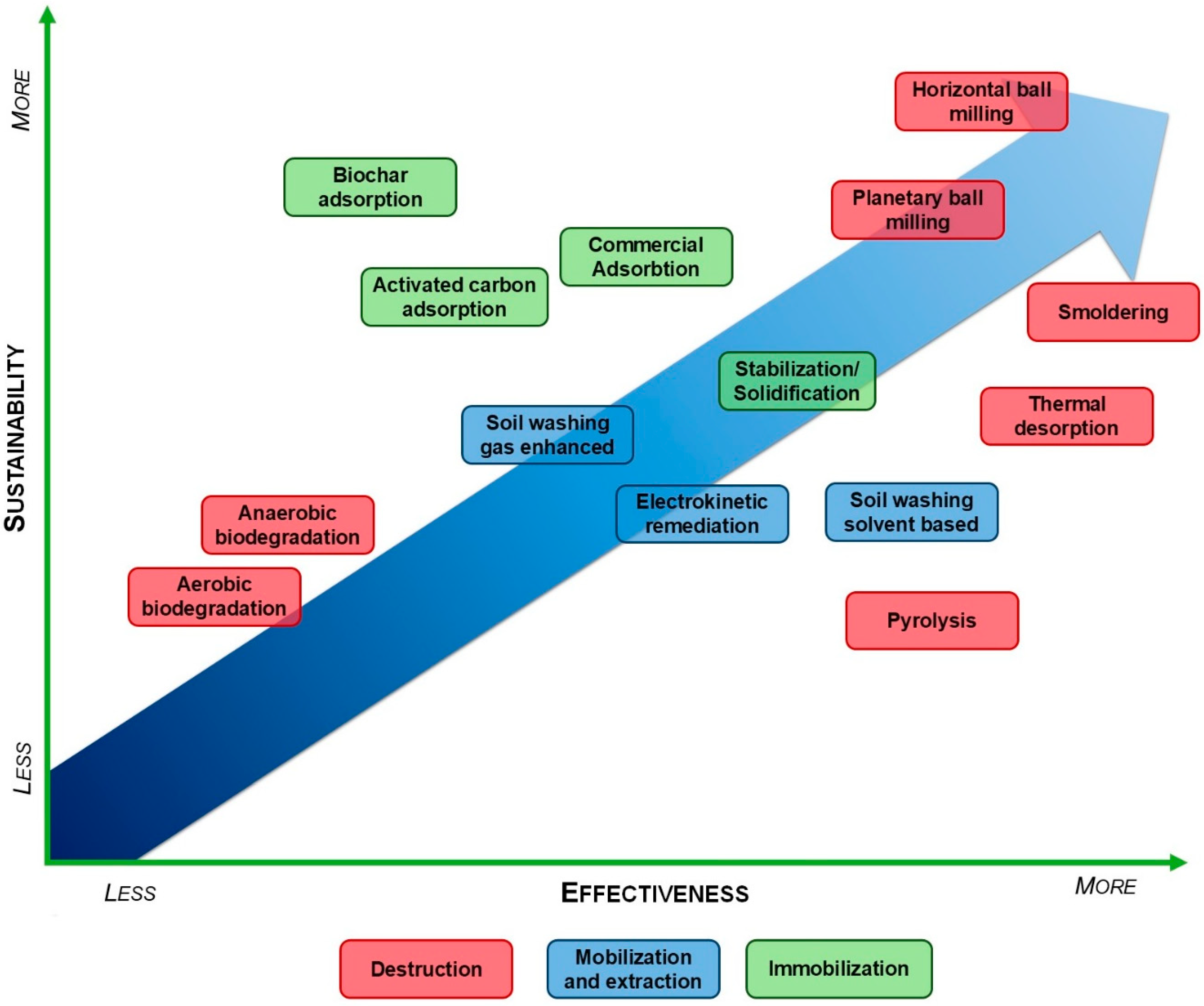
4. PFAS Soil Remediation Technologies: Effectiveness, Sustainability, and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Scheringer, M.; Wang, Z. The High Persistence of PFAS Is Sufficient for Their Management as a Chemical Class. Environ. Sci. Process. Impacts 2020, 22, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, A.; Maizel, A.C.; Kulkarni, P.R.; Adamson, D.T.; Kornuc, J.J.; Higgins, C.P. Enhanced Extraction of AFFF-Associated PFASs from Source Zone Soils. Environ. Sci. Technol. 2020, 54, 4952–4962. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, T.; Jones, R.; Kastury, F.; Juhasz, A.L. Soil Amendments Reduce PFAS Bioaccumulation in Eisenia Fetida Following Exposure to AFFF-Impacted Soil. Environ. Pollut. 2024, 358, 124489. [Google Scholar] [CrossRef] [PubMed]
- Tansel, B. PFAS Risk Propagation Terminology in Spatial and Temporal Scales: Risk Intensification, Risk Attenuation, and Risk Amplification. Sci. Total Environ. 2022, 835, 155503. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Sarkar, B.; Yan, Y.; Li, Q.; Wijesekara, H.; Kannan, K.; Tsang, D.C.W.; Schauerte, M.; Bosch, J.; Noll, H.; et al. Remediation of Poly- and Perfluoroalkyl Substances (PFAS) Contaminated Soils—To Mobilize or to Immobilize or to Degrade? J. Hazard. Mater. 2021, 401, 123892. [Google Scholar] [CrossRef] [PubMed]
- Abunada, Z.; Alazaiza, M.Y.D.; Bashir, M.J.K. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water 2020, 12, 3590. [Google Scholar] [CrossRef]
- Dhore, R.; Murthy, G.S. Per/Polyfluoroalkyl Substances Production, Applications and Environmental Impacts. Bioresour. Technol. 2021, 341, 125808. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fang, Y.; Cui, X.; Zhou, D. Effects of Trace PFOA on Microbial Community and Metabolisms: Microbial Selectivity, Regulations and Risks. Water Res. 2022, 226, 119273. [Google Scholar] [CrossRef] [PubMed]
- Lesmeister, L.; Lange, F.T.; Breuer, J.; Biegel-Engler, A.; Giese, E.; Scheurer, M. Extending the Knowledge about PFAS Bioaccumulation Factors for Agricultural Plants—A Review. Sci. Total Environ. 2021, 766, 142640. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Sun, H.; Song, M.; Jiang, L.; Li, Y.; Lu, W.; Ying, G.-G.; Luo, C.; Zhang, G. Per- and Polyfluoroalkyl Substances (PFAS) in the Soil–Plant System: Sorption, Root Uptake, and Translocation. Environ. Int. 2021, 156, 106642. [Google Scholar] [CrossRef] [PubMed]
- Adu, O.; Ma, X.; Sharma, V.K. Bioavailability, Phytotoxicity and Plant Uptake of per-and Polyfluoroalkyl Substances (PFAS): A Review. J. Hazard. Mater. 2023, 447, 130805. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsdóttir, O.; Abdallah, M.A.-E.; Harrad, S. Dermal Bioaccessibility of Perfluoroalkyl Substances from Household Dust; Influence of Topically Applied Cosmetics. Environ. Res. 2023, 238, 117093. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ishaq, Z.; He, C.; Banks, A.P.W.; Bräunig, J.; Thai, P.K.; Jayarathne, A.; Mueller, J.F.; Wang, X. Per- and Polyfluoroalkyl Substances (PFAS) in Floor Dust from Different Indoor Environments in Australia: Levels, Variation, and Human Exposure Risks. Chemosphere 2024, 366, 143372. [Google Scholar] [CrossRef] [PubMed]
- Rayner, J.L.; Slee, D.; Falvey, S.; Kookana, R.; Bekele, E.; Stevenson, G.; Lee, A.; Davis, G.B. Laboratory Batch Representation of PFAS Leaching from Aged Field Soils: Intercomparison across New and Standard Approaches. Sci. Total Environ. 2022, 838, 156562. [Google Scholar] [CrossRef] [PubMed]
- Berg, V.; Nøst, T.H.; Hansen, S.; Elverland, A.; Veyhe, A.-S.; Jorde, R.; Odland, J.Ø.; Sandanger, T.M. Assessing the Relationship between Perfluoroalkyl Substances, Thyroid Hormones and Binding Proteins in Pregnant Women; a Longitudinal Mixed Effects Approach. Environ. Int. 2015, 77, 63–69. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2020, 40, 631–657. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2020, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ng, C. Absorption, Distribution, and Toxicity of per- and Polyfluoroalkyl Substances (PFAS) in the Brain: A Review. Environ. Sci. Process. Impacts 2021, 23, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Bayode, A.A.; Emmanuel, S.S.; Akinyemi, A.O.; Ore, O.T.; Akpotu, S.O.; Koko, D.T.; Momodu, D.E.; López-Maldonado, E.A. Innovative Techniques for Combating a Common Enemy Forever Chemicals: A Comprehensive Approach to Mitigating per- and Polyfluoroalkyl Substances (PFAS) Contamination. Environ. Res. 2024, 261, 119719. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of Poly- and Perfluoroalkyl Substances (PFAS) from Water by Adsorption: Role of PFAS Chain Length, Effect of Organic Matter and Challenges in Adsorbent Regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef] [PubMed]
- Greger, M.; Landberg, T. Removal of PFAS from Water by Aquatic Plants. J. Environ. Manag. 2024, 351, 119895. [Google Scholar] [CrossRef] [PubMed]
- Wanninayake, D.M. Comparison of Currently Available PFAS Remediation Technologies in Water: A Review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef] [PubMed]
- Boyer, T.H.; Fang, Y.; Ellis, A.; Dietz, R.; Choi, Y.J.; Schaefer, C.E.; Higgins, C.P.; Strathmann, T.J. Anion Exchange Resin Removal of Per- and Polyfluoroalkyl Substances (PFAS) from Impacted Water: A Critical Review. Water Res. 2021, 200, 117244. [Google Scholar] [CrossRef] [PubMed]
- Amen, R.; Ibrahim, A.; Shafqat, W.; Hassan, E.B. A Critical Review on PFAS Removal from Water: Removal Mechanism and Future Challenges. Sustainability 2023, 15, 16173. [Google Scholar] [CrossRef]
- Sleep, J.A.; Juhasz, A.L. A Review of Immobilisation-Based Remediation of Per- and Poly-Fluoroalkyl Substances (PFAS) in Soils. Curr. Pollut. Rep. 2021, 7, 524–539. [Google Scholar] [CrossRef]
- Abou-Khalil, C.; Sarkar, D.; Braykaa, P.; Boufadel, M.C. Mobilization of Per- and Polyfluoroalkyl Substances (PFAS) in Soils: A Review. Curr. Pollut. Rep. 2022, 8, 422–444. [Google Scholar] [CrossRef]
- Shahsavari, E.; Rouch, D.; Khudur, L.S.; Thomas, D.; Aburto-Medina, A.; Ball, A.S. Challenges and Current Status of the Biological Treatment of PFAS-Contaminated Soils. Front. Bioeng. Biotechnol. 2021, 8, 602040. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-G.; Birch, Q.T.; Nadagouda, M.N.; Dionysiou, D.D. Advanced Destruction Technologies for PFAS in Soils: Progress and Challenges. Curr. Opin. Environ. Sci. Health 2023, 33, 100459. [Google Scholar] [CrossRef]
- Longendyke, G.K.; Katel, S.; Wang, Y. PFAS Fate and Destruction Mechanisms during Thermal Treatment: A Comprehensive Review. Environ. Sci. Process. Impacts 2022, 24, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Uriakhil, M.A.; Sidnell, T.; De Castro Fernández, A.; Lee, J.; Ross, I.; Bussemaker, M. Per- and Poly-Fluoroalkyl Substance Remediation from Soil and Sorbents: A Review of Adsorption Behaviour and Ultrasonic Treatment. Chemosphere 2021, 282, 131025. [Google Scholar] [CrossRef] [PubMed]
- Mahinroosta, R.; Senevirathna, L. A Review of the Emerging Treatment Technologies for PFAS Contaminated Soils. J. Environ. Manag. 2020, 255, 109896. [Google Scholar] [CrossRef] [PubMed]
- Gaines, L.G.T.; Sinclair, G.; Williams, A.J. A Proposed Approach to Defining Per- and Polyfluoroalkyl Substances (PFAS) Based on Molecular Structure and Formula. Integr. Environ. Assess. Manag. 2023, 19, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- Reinikainen, J.; Bouhoulle, E.; Sorvari, J. Inconsistencies in the EU Regulatory Risk Assessment of PFAS Call for Readjustment. Environ. Int. 2024, 186, 108614. [Google Scholar] [CrossRef] [PubMed]
- Interstate Technology; Regulatory Council (ITRC). PFAS Technical and Regulatory Guidance Document; Interstate Technology & Regulatory Council (ITRC): Washington, DC, WA, USA, 2023. [Google Scholar]
- Balgooyen, S.; Remucal, C.K. Impacts of Environmental and Engineered Processes on the PFAS Fingerprint of Fluorotelomer-Based AFFF. Environ. Sci. Technol. 2023, 57, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Gong, T.; Wu, Y.; Chen, G. Cutting-Edge Technologies and Relevant Reaction Mechanism Difference in Treatment of Long- and Short-Chain per- and Polyfluoroalkyl Substances: A Review. Chemosphere 2024, 354, 141692. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.A.; Hungerbühler, K. Bioaccumulation of Perfluorinated Alkyl Acids: Observations and Models. Environ. Sci. Technol. 2014, 48, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Huang, J.; Luo, F.; Fang, Z.; Yu, D.; Zhang, X.; Wang, D.; Xing, M.; Luo, J. Understanding the Selective Removal of Perfluoroalkyl and Polyfluoroalkyl Substances via Fluorine–Fluorine Interactions: A Critical Review. Environ. Sci. Technol. 2024, 58, 16669–16689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, J.-J.; Lyu, X.-J.; Yin, H.; Sheng, G.-P. Complete Mineralization of Perfluorooctanoic Acid (PFOA) by γ-Irradiation in Aqueous Solution. Sci. Rep. 2014, 4, 7418. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.C.E.; Wanninayake, D.; Chen, D.; Nguyen, N.-T.; Li, Q. Physicochemical Properties and Interactions of Perfluoroalkyl Substances (PFAS)—Challenges and Opportunities in Sensing and Remediation. Sci. Total Environ. 2023, 905, 166764. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, A.; Mudlaff, M.; Gorb, L.; Bulawska, N.; Zdybel, S.; Bakker, M.; Peijnenburg, W.; Puzyn, T. Expanding the Applicability Domain of QSPRs for Predicting Water Solubility and Vapor Pressure of PFAS. Chemosphere 2023, 340, 139965. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.F.; Peldszus, S.; Anderson, W.B. Behaviour and Fate of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Drinking Water Treatment: A Review. Water Res. 2014, 50, 318–340. [Google Scholar] [CrossRef] [PubMed]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel Treatment Technologies for PFAS Compounds: A Critical Review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L. Polyfluoroalkyl Compounds in the Aquatic Environment: A Review of Their Occurrence and Fate. J. Environ. Monit. 2011, 13, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Dalahmeh, S.; Tirgani, S.; Komakech, A.J.; Niwagaba, C.B.; Ahrens, L. Per- and Polyfluoroalkyl Substances (PFASs) in Water, Soil and Plants in Wetlands and Agricultural Areas in Kampala, Uganda. Sci. Total Environ. 2018, 631–632, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.P.; Luthy, R.G. Sorption of Perfluorinated Surfactants on Sediments. Environ. Sci. Technol. 2006, 40, 7251–7256. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Xie, Z.; Ebinghaus, R. Distribution of Perfluoroalkyl Compounds in Seawater from Northern Europe, Atlantic Ocean, and Southern Ocean. Chemosphere 2010, 78, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption Behavior and Mechanism of Perfluorinated Compounds on Various Adsorbents—A Review. J. Hazard. Mater. 2014, 274, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shih, K.; Ma, R.; Li, X. Influence of Cations on the Partition Behavior of Perfluoroheptanoate (PFHpA) and Perfluorohexanesulfonate (PFHxS) on Wastewater Sludge. Chemosphere 2015, 131, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Reinhard, M.; Nguyen, V.T.; Gin, K.Y.-H. Reversible and Irreversible Sorption of Perfluorinated Compounds (PFCs) by Sediments of an Urban Reservoir. Chemosphere 2016, 144, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Milinovic, J.; Lacorte, S.; Rigol, A.; Vidal, M. Sorption of Perfluoroalkyl Substances in Sewage Sludge. Environ. Sci. Pollut. Res. 2016, 23, 8339–8348. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fang, X.; Zhou, Z.; Liao, X.; Zou, J.; Yuan, B.; Sun, W. Adsorption of Perfluorinated Acids onto Soils: Kinetics, Isotherms, and Influences of Soil Properties. Sci. Total Environ. 2019, 649, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Guelfo, J.L.; Higgins, C.P. Subsurface Transport Potential of Perfluoroalkyl Acids at Aqueous Film-Forming Foam (AFFF)-Impacted Sites. Environ. Sci. Technol. 2013, 47, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Qu, B.; Guan, Y.; Jiang, J.; Chen, X. Influence of Salinity and Temperature on Uptake of Perfluorinated Carboxylic Acids (PFCAs) by Hydroponically Grown Wheat (Triticum aestivum L.). SpringerPlus 2016, 5, 541. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Xie, Z.; Zhang, Y.; Liu, X.; Xie, S.; Huang, J.; Yu, L. Perfluoroalkyl Substances (PFASs) Influence the Structure and Function of Soil Bacterial Community: Greenhouse Experiment. Sci. Total Environ. 2018, 642, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yang, G.; Lehmann, A.; Riedel, S.; Rillig, M.C. Effects of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) on Soil Structure and Function. Soil Ecol. Lett. 2023, 5, 108–117. [Google Scholar] [CrossRef]
- Wang, Y.; Munir, U.; Huang, Q. Occurrence of Per- and Polyfluoroalkyl Substances (PFAS) in Soil: Sources, Fate, and Remediation. Soil Environ. Health 2023, 1, 100004. [Google Scholar] [CrossRef]
- Askeland, M.; Clarke, B.O.; Cheema, S.A.; Mendez, A.; Gasco, G.; Paz-Ferreiro, J. Biochar Sorption of PFOS, PFOA, PFHxS and PFHxA in Two Soils with Contrasting Texture. Chemosphere 2020, 249, 126072. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, S.; Centner, M.; McLaughlin, M.J. Durability of Sorption of Per- and Polyfluorinated Alkyl Substances in Soils Immobilised Using Common Adsorbents: 1. Effects of Perturbations in pH. Sci. Total Environ. 2021, 766, 144857. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, S.; McLaughlin, M.J. Durability of Sorption of Per- and Polyfluorinated Alkyl Substances in Soils Immobilized Using Common Adsorbents: 2. Effects of Repeated Leaching, Temperature Extremes, Ionic Strength and Competing Ions. Sci. Total Environ. 2021, 766, 144718. [Google Scholar] [CrossRef] [PubMed]
- Sorengard, M.; Kleja, D.B.; Ahrens, L. Stabilization of Per- and Polyfluoroalkyl Substances (PFASs) with Colloidal Activated Carbon (PlumeStop®) as a Function of Soil Clay and Organic Matter Content. J. Environ. Manag. 2019, 249, 109345. [Google Scholar] [CrossRef] [PubMed]
- Sörengård, M.; Gago-Ferrero, P.; Kleja, D.B.; Ahrens, L. Laboratory-Scale and Pilot-Scale Stabilization and Solidification (S/S) Remediation of Soil Contaminated with per- and Polyfluoroalkyl Substances (PFASs). J. Hazard. Mater. 2021, 402, 123453. [Google Scholar] [CrossRef] [PubMed]
- Grimison, C.; Knight, E.R.; Nguyen, T.M.H.; Nagle, N.; Kabiri, S.; Bräunig, J.; Navarro, D.A.; Kookana, R.S.; Higgins, C.P.; McLaughlin, M.J.; et al. The Efficacy of Soil Washing for the Remediation of Per- and Poly-Fluoroalkyl Substances (PFASs) in the Field. J. Hazard. Mater. 2023, 445, 130441. [Google Scholar] [CrossRef] [PubMed]
- Høisæter, Å.; Arp, H.P.H.; Slinde, G.; Knutsen, H.; Hale, S.E.; Breedveld, G.D.; Hansen, M.C. Excavated vs Novel in Situ Soil Washing as a Remediation Strategy for Sandy Soils Impacted with Per- and Polyfluoroalkyl Substances from Aqueous Film Forming Foams. Sci. Total Environ. 2021, 794, 148763. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tanaka, S.; Kitaji, Y.; Hashikomi, S.; Xu, Y.; Ikeo, T. Remediation of Per- and Polyfluoroalkyl Substances (PFAS) Contaminated Soil via Soil Washing with Various Water-Organic Solvents. J. Hazard. Mater. 2024, 480, 135943. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yan, Z.; Qian, S.; Xiong, T.; Grieger, K.D.; Wang, X.; Liu, C.; Zhi, Y. Phytoextraction of Per- and Polyfluoroalkyl Substances (PFAS) by Weeds: Effect of PFAS Physicochemical Properties and Plant Physiological Traits. J. Hazard. Mater. 2023, 454, 131492. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, Y.; Zhu, Y.; Sun, Y.; Ma, J.; Wang, L. Enhanced Electrokinetic Remediation by Magnetic Induction for the Treatment of Co-Contaminated Soil. J. Hazard. Mater. 2023, 452, 131264. [Google Scholar] [CrossRef] [PubMed]
- Niarchos, G.; Sörengård, M.; Fagerlund, F.; Ahrens, L. Electrokinetic Remediation for Removal of Per- and Polyfluoroalkyl Substances (PFASs) from Contaminated Soil. Chemosphere 2022, 291, 133041. [Google Scholar] [CrossRef] [PubMed]
- Ganbat, N.; Altaee, A.; Zhou, J.L.; Lockwood, T.; Al-Juboori, R.A.; Hamdi, F.M.; Karbassiyazdi, E.; Samal, A.K.; Hawari, A.; Khabbaz, H. Investigation of the Effect of Surfactant on the Electrokinetic Treatment of PFOA Contaminated Soil. Environ. Technol. Innov. 2022, 28, 102938. [Google Scholar] [CrossRef]
- Huang, S.; Jaffé, P.R. Defluorination of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Acidimicrobium Sp. Strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef] [PubMed]
- Lorah, M.M.; He, K.; Blaney, L.; Akob, D.M.; Harris, C.; Tokranov, A.; Hopkins, Z.; Shedd, B.P. Anaerobic Biodegradation of Perfluorooctane Sulfonate (PFOS) and Microbial Community Composition in Soil Amended with a Dechlorinating Culture and Chlorinated Solvents. Sci. Total Environ. 2024, 932, 172996. [Google Scholar] [CrossRef] [PubMed]
- Alinezhad, A.; Challa Sasi, P.; Zhang, P.; Yao, B.; Kubátová, A.; Golovko, S.A.; Golovko, M.Y.; Xiao, F. An Investigation of Thermal Air Degradation and Pyrolysis of Per- and Polyfluoroalkyl Substances and Aqueous Film-Forming Foams in Soil. ACS EST Eng. 2022, 2, 198–209. [Google Scholar] [CrossRef]
- Rassaei, F. Sustainable Per- and Polyfluoroalkyl Substances Contaminated Soil Remediation: Evaluating the Potential of Thermal Desorption. Environ. Prog. Sustain. Energy 2024, 43, e14251. [Google Scholar] [CrossRef]
- Turner, L.P.; Kueper, B.H.; Jaansalu, K.M.; Patch, D.J.; Battye, N.; El-Sharnouby, O.; Mumford, K.G.; Weber, K.P. Mechanochemical Remediation of Perfluorooctanesulfonic Acid (PFOS) and Perfluorooctanoic Acid (PFOA) Amended Sand and Aqueous Film-Forming Foam (AFFF) Impacted Soil by Planetary Ball Milling. Sci. Total Environ. 2021, 765, 142722. [Google Scholar] [CrossRef] [PubMed]
- Battye, N.J.; Patch, D.J.; Roberts, D.M.D.; O’Connor, N.M.; Turner, L.P.; Kueper, B.H.; Hulley, M.E.; Weber, K.P. Use of a Horizontal Ball Mill to Remediate Per- and Polyfluoroalkyl Substances in Soil. Sci. Total Environ. 2022, 835, 155506. [Google Scholar] [CrossRef] [PubMed]
- Bräunig, J.; Baduel, C.; Barnes, C.M.; Mueller, J.F. Sorbent Assisted Immobilisation of Perfluoroalkyl Acids in Soils—Effect on Leaching and Bioavailability. J. Hazard. Mater. 2021, 412, 125171. [Google Scholar] [CrossRef] [PubMed]
- Hearon, S.E.; Orr, A.A.; Moyer, H.; Wang, M.; Tamamis, P.; Phillips, T.D. Montmorillonite Clay-Based Sorbents Decrease the Bioavailability of per- and Polyfluoroalkyl Substances (PFAS) from Soil and Their Translocation to Plants. Environ. Res. 2022, 205, 112433. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.E.; Arp, H.P.H.; Slinde, G.A.; Wade, E.J.; Bjørseth, K.; Breedveld, G.D.; Straith, B.F.; Moe, K.G.; Jartun, M.; Høisæter, Å. Sorbent Amendment as a Remediation Strategy to Reduce PFAS Mobility and Leaching in a Contaminated Sandy Soil from a Norwegian Firefighting Training Facility. Chemosphere 2017, 171, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sørmo, E.; Silvani, L.; Bjerkli, N.; Hagemann, N.; Zimmerman, A.R.; Hale, S.E.; Hansen, C.B.; Hartnik, T.; Cornelissen, G. Stabilization of PFAS-Contaminated Soil with Activated Biochar. Sci. Total Environ. 2021, 763, 144034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cornelissen, G.; Silvani, L.; Zivanovic, V.; Smebye, A.B.; Sørmo, E.; Thune, G.; Okkenhaug, G. Industrial Byproducts for the Soil Stabilization of Trace Elements and Per- and Polyfluorinated Alkyl Substances (PFASs). Sci. Total Environ. 2022, 820, 153188. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.H.; Zuverza-Mena, N.; Dimkpa, C.O.; Nason, S.L.; Thomas, S.; White, J.C. PFAS Remediation in Soil: An Evaluation of Carbon-Based Materials for Contaminant Sequestration. Environ. Pollut. 2024, 344, 123335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liang, Y. Performance of Different Sorbents toward Stabilizing Per- and Polyfluoroalkyl Substances (PFAS) in Soil. Environ. Adv. 2022, 8, 100217. [Google Scholar] [CrossRef]
- Barth, E.; McKernan, J.; Bless, D.; Dasu, K. Investigation of an Immobilization Process for PFAS Contaminated Soils. J. Environ. Manag. 2021, 296, 113069. [Google Scholar] [CrossRef] [PubMed]
- Sörengård, M.; Kleja, D.B.; Ahrens, L. Stabilization and Solidification Remediation of Soil Contaminated with Poly- and Perfluoroalkyl Substances (PFASs). J. Hazard. Mater. 2019, 367, 639–646. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.T.; Anderson, R.H.; Lang, J.R.; Liles, D.; Matteson, K.; Olechiw, T. Field-Scale Demonstration of PFAS Leachability Following In Situ Soil Stabilization. ACS Omega 2022, 7, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Ciabattoni, E.; Piubellini, S.; Sezenna, E.; Saponaro, S. Trattamenti E Gestione Di Terreni Contaminati Da Pfas Non Polimerici. Ing. DellAmbiente 2025, 12, 38–52. [Google Scholar] [CrossRef]
- Senevirathna, S.T.M.L.D.; Mahinroosta, R.; Li, M.; KrishnaPillai, K. In Situ Soil Flushing to Remediate Confined Soil Contaminated with PFOS- an Innovative Solution for Emerging Environmental Issue. Chemosphere 2021, 262, 127606. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Chaudhary, A.; Hanna, K. Efficient PFAS Removal from Contaminated Soils through Combined Washing and Adsorption in Soil Effluents. J. Hazard. Mater. 2024, 476, 135118. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Dorian, B.; Gao, L.; Xie, Z.; Cran, M.; Muthukumaran, S.; Sidiroglou, F.; Gray, S.; Zhang, J. Remediation of Poly-and Perfluoroalkyl Substances (PFAS) Contaminated Soil Using Gas Fractionation Enhanced Technology. Sci. Total Environ. 2022, 827, 154310. [Google Scholar] [CrossRef] [PubMed]
- Trellu, C.; Mousset, E.; Pechaud, Y.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Removal of Hydrophobic Organic Pollutants from Soil Washing/Flushing Solutions: A Critical Review. J. Hazard. Mater. 2016, 306, 149–174. [Google Scholar] [CrossRef] [PubMed]
- Ishtaweera, P.; Baker, G.A. Progress in the Application of Ionic Liquids and Deep Eutectic Solvents for the Separation and Quantification of Per- and Polyfluoroalkyl Substances. J. Hazard. Mater. 2024, 465, 132959. [Google Scholar] [CrossRef] [PubMed]
- Quinnan, J.; Morrell, C.; Nagle, N.; Maynard, K.G. Ex Situ Soil Washing to Remove PFAS Adsorbed to Soils from Source Zones. Remediat. J. 2022, 32, 151–166. [Google Scholar] [CrossRef]
- Huff, D.K.; Morris, L.A.; Sutter, L.; Costanza, J.; Pennell, K.D. Accumulation of Six PFAS Compounds by Woody and Herbaceous Plants: Potential for Phytoextraction. Int. J. Phytoremediat. 2020, 22, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Nassazzi, W.; Wu, T.-C.; Jass, J.; Lai, F.Y.; Ahrens, L. Phytoextraction of Per- and Polyfluoroalkyl Substances (PFAS) and the Influence of Supplements on the Performance of Short–Rotation Crops. Environ. Pollut. 2023, 333, 122038. [Google Scholar] [CrossRef] [PubMed]
- Nason, S.L.; Thomas, S.; Stanley, C.; Silliboy, R.; Blumenthal, M.; Zhang, W.; Liang, Y.; Jones, J.P.; Zuverza-Mena, N.; White, J.C.; et al. A Comprehensive Trial on PFAS Remediation: Hemp Phytoextraction and PFAS Degradation in Harvested Plants. Environ. Sci. Adv. 2024, 3, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.; Heyn, J.; Thiele, H.; Hüther, J.; Failing, K.; Georgii, S.; Brunn, H. Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from Soil to Plants. Arch. Environ. Contam. Toxicol. 2009, 57, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Blaine, A.C.; Rich, C.D.; Sedlacko, E.M.; Hyland, K.C.; Stushnoff, C.; Dickenson, E.R.V.; Higgins, C.P. Perfluoroalkyl Acid Uptake in Lettuce (Lactuca sativa) and Strawberry (Fragaria ananassa) Irrigated with Reclaimed Water. Environ. Sci. Technol. 2014, 48, 14361–14368. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Lei, M.; Chen, T. Cost–Benefit Calculation of Phytoremediation Technology for Heavy-Metal-Contaminated Soil. Sci. Total Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Abou-Khalil, C.; Kewalramani, J.; Zhang, Z.; Sarkar, D.; Abrams, S.; Boufadel, M.C. Effect of Clay Content on the Mobilization Efficiency of Per- and Polyfluoroalkyl Substances (PFAS) from Soils by Electrokinetics and Hydraulic Flushing. Environ. Pollut. 2023, 322, 121160. [Google Scholar] [CrossRef] [PubMed]
- Dhulia, A.; Abou-Khalil, C.; Kewalramani, J.; Sarkar, D.; Boufadel, M.C. Mobilization of Per- and Poly-Fluoroalkyl Substances (PFAS) in Soils with Different Organic Matter Contents. Chemosphere 2024, 361, 142503. [Google Scholar] [CrossRef] [PubMed]
- Ganbat, N.; Hamdi, F.M.; Ibrar, I.; Altaee, A.; Alsaka, L.; Samal, A.K.; Zhou, J.; Hawari, A.H. Iron Slag Permeable Reactive Barrier for PFOA Removal by the Electrokinetic Process. J. Hazard. Mater. 2023, 460, 132360. [Google Scholar] [CrossRef] [PubMed]
- Ganbat, N.; Altaee, A.; Hamdi, F.M.; Zhou, J.; Chowdhury, M.H.; Zaidi, S.J.; Samal, A.K.; Almalki, R.; Tapas, M.J. PFOA Remediation from Kaolinite Soil by Electrokinetic Process Coupled with Activated Carbon/Iron Coated Activated Carbon—Permeable Reactive Barrier. J. Contam. Hydrol. 2024, 267, 104425. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.; Reddy, V.N.; Sunandini, V.; Hemalatha, K. Cost Estimation of Electrokinetic Soil Remediation for Removal of Six Toxic Metals from Contaminated Soil. Nat. Environ. Pollut. Technol. 2020, 19, 1899–1904. [Google Scholar] [CrossRef]
- Beškoski, V.P.; Yamamoto, A.; Nakano, T.; Yamamoto, K.; Matsumura, C.; Motegi, M.; Beškoski, L.S.; Inui, H. Defluorination of Perfluoroalkyl Acids Is Followed by Production of Monofluorinated Fatty Acids. Sci. Total Environ. 2018, 636, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.G.; Lim, H.-J.; Na, S.-H.; Choi, B.-I.; Shin, D.-S.; Chung, S.-Y. Biodegradation of Perfluorooctanesulfonate (PFOS) as an Emerging Contaminant. Chemosphere 2014, 109, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.B.; Chai, L.Y.; Xie, Y.; Peng, Q.J.; Peng, Q.Z. Isolation, Identification, and Degradation Performance of a PFOA-Degrading Strain. Genet. Mol. Res. 2016, 15, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Chetverikov, S.P.; Sharipov, D.A.; Korshunova, T.Y.; Loginov, O.N. Degradation of Perfluorooctanyl Sulfonate by Strain Pseudomonas Plecoglossicida 2.4-D. Appl. Biochem. Microbiol. 2017, 53, 533–538. [Google Scholar] [CrossRef]
- Luo, Q.; Liang, S.; Huang, Q. Laccase Induced Degradation of Perfluorooctanoic Acid in a Soil Slurry. J. Hazard. Mater. 2018, 359, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.S.-C.; Szostek, B.; DeRito, C.M.; Madsen, E.L. Investigating the Biodegradability of Perfluorooctanoic Acid. Chemosphere 2010, 80, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Herrera, V.; Field, J.A.; Luna-Velasco, A.; Sierra-Alvarez, R. Microbial Toxicity and Biodegradability of Perfluorooctane Sulfonate (PFOS) and Shorter Chain Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs). Environ. Sci. Process. Impacts 2016, 18, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Coon, C.M.; Doherty, M.E.; McHugh, E.A.; Warner, M.C.; Walters, C.L.; Orahood, O.M.; Loesch, A.E.; Hatfield, D.C.; Sitko, J.C.; et al. Engineering and Characterization of Dehalogenase Enzymes from Delftia Acidovorans in Bioremediation of Perfluorinated Compounds. Synth. Syst. Biotechnol. 2022, 7, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Al Amin, M.; Luo, Y.; Nolan, A.; Mallavarapu, M.; Naidu, R.; Fang, C. Thermal Kinetics of PFAS and Precursors in Soil: Experiment and Surface Simulation in Temperature-Time Plane. Chemosphere 2023, 318, 138012. [Google Scholar] [CrossRef] [PubMed]
- Sörengård, M.; Lindh, A.-S.; Ahrens, L. Thermal Desorption as a High Removal Remediation Technique for Soils Contaminated with Per- and Polyfluoroalkyl Substances (PFASs). PLoS ONE 2020, 15, e0234476. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, A.L.; Brown, J.K.; Patch, D.J.; Major, D.; Weber, K.P.; Gerhard, J.I. Remediation of PFAS-Contaminated Soil and Granular Activated Carbon by Smoldering Combustion. Environ. Sci. Technol. 2020, 54, 12631–12640. [Google Scholar] [CrossRef] [PubMed]
- Stallings, P.; Ramsay, B.; Rickabaugh, T.; Crownover, E.; Heron, G.; Stauch, L.; Pennell, K.; Woodcock, M. Ex Situ Thermal Treatment of PFAS-Impacted Soils; ER20-5198; Office of the Deputy Assistant Secretary of Defense (Energy Resilience and Optimization): Washington, DC, WA, USA, 31 October 2023. [Google Scholar]
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Kochunarayanan, P.T.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A Review of Emerging Technologies for Remediation of PFASs. Remediat. J. 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Turner, L.P.; Kueper, B.H.; Patch, D.J.; Weber, K.P. Elucidating the Relationship between PFOA and PFOS Destruction, Particle Size and Electron Generation in Amended Media Commonly Found in Soils. Sci. Total Environ. 2023, 888, 164188. [Google Scholar] [CrossRef] [PubMed]
- Battye, N.; Patch, D.; Koch, I.; Monteith, R.; Roberts, D.; O’Connor, N.; Kueper, B.; Hulley, M.; Weber, K. Mechanochemical Degradation of Per- and Polyfluoroalkyl Substances in Soil Using an Industrial-Scale Horizontal Ball Mill with Comparisons of Key Operational Metrics. Sci. Total Environ. 2024, 928, 172274. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, G.; Huang, J.; Yu, G. A Mini-Review on Mechanochemical Treatment of Contaminated Soil: From Laboratory to Large-Scale. Crit. Rev. Environ. Sci. Technol. 2018, 48, 723–771. [Google Scholar] [CrossRef]
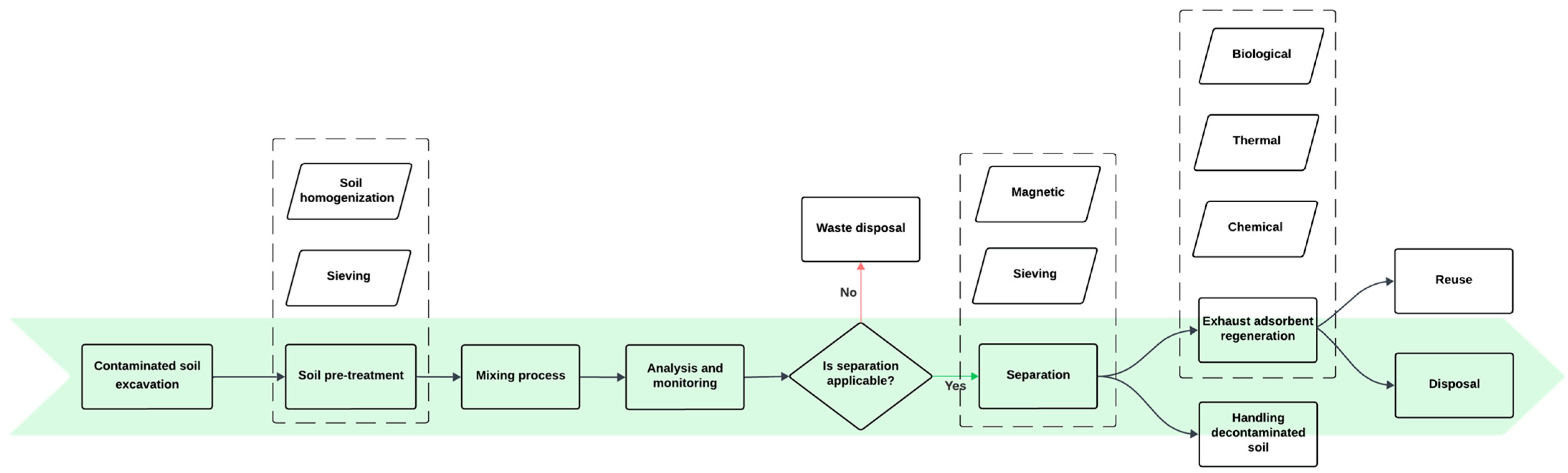
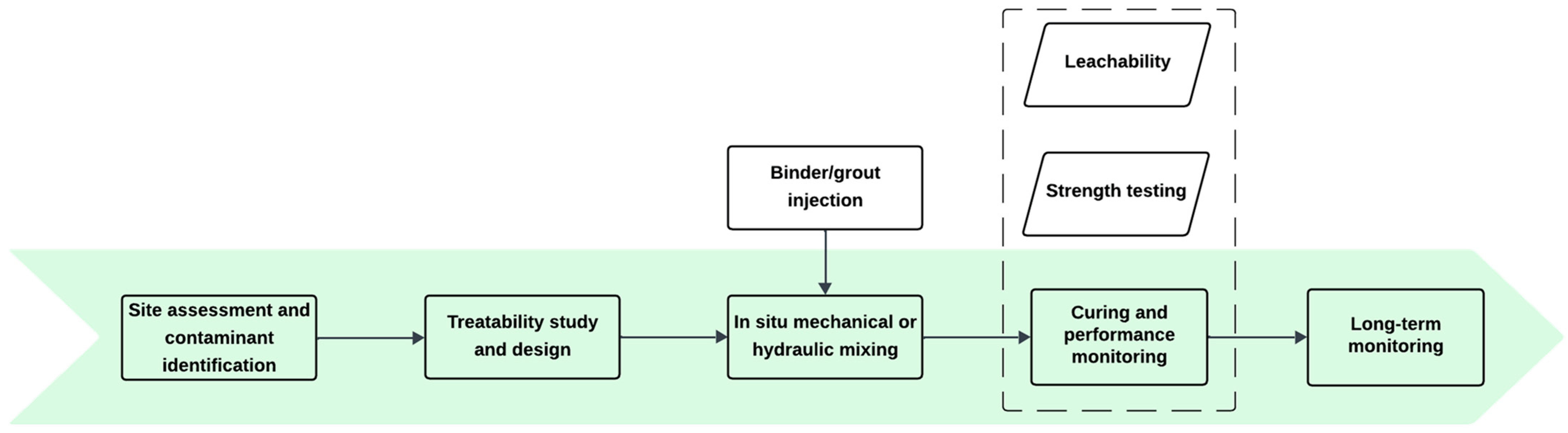
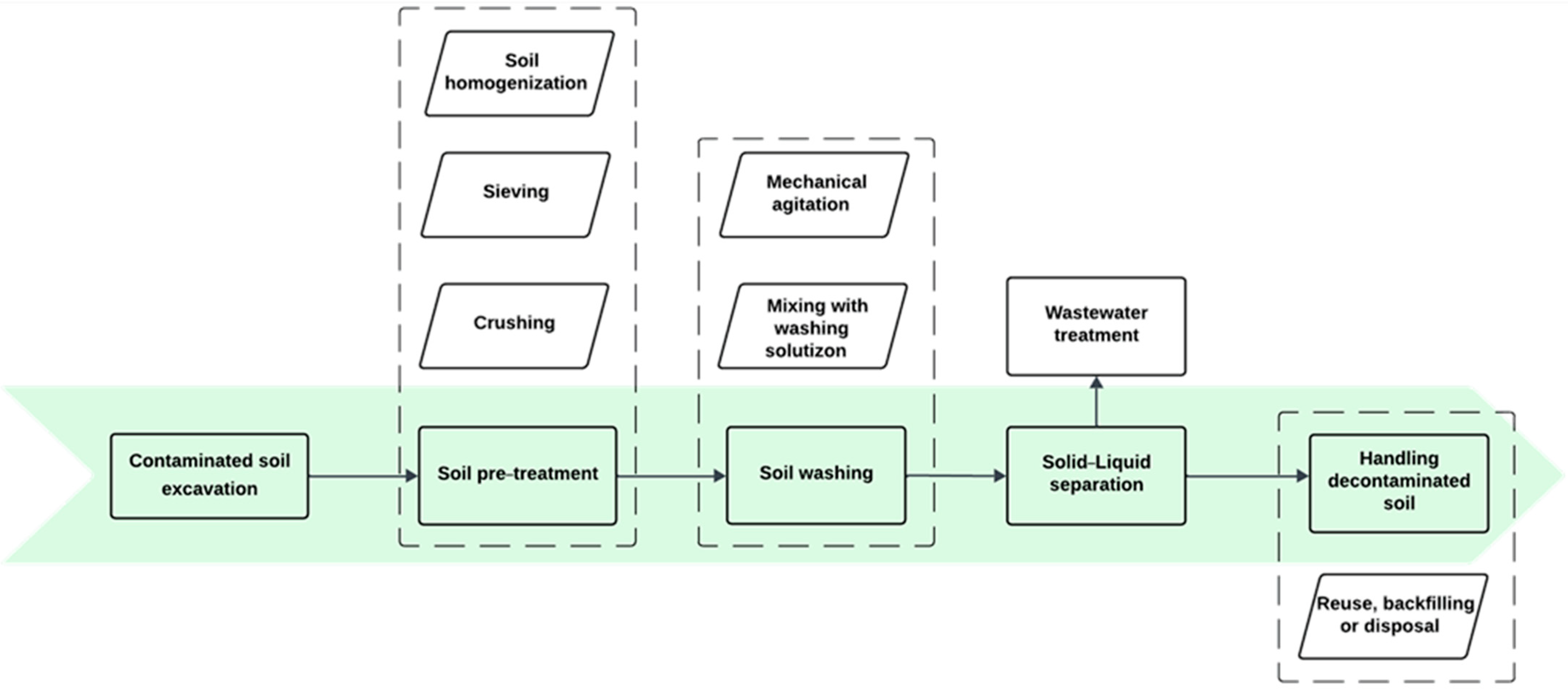
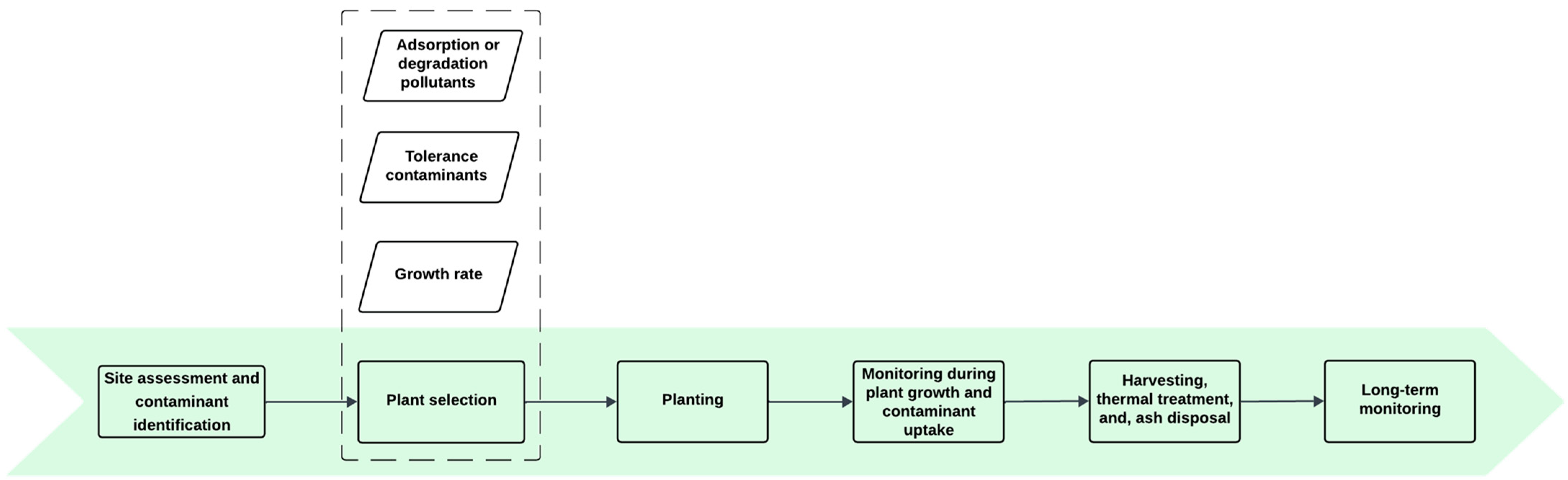
| Soil Type | PFAS (C-mg/kg) | Experimental Conditions | Results (%) | Ref. |
|---|---|---|---|---|
| Loamy sand | PFOA, PFOS, PFHxS and PFHxA (C: 10) | Bench scale: Pine-biochar (5% w/w) | 88.7 (PFOS removal) 40.5 (PFOA removal) 46.2 (PFHxS removal) 8.9 (PFHxA removal) | [58] |
| Sandy clay loam | 66.3 (PFOS removal) 23.2 (PFOA removal) 9.6 (PFHxS removal) 13.4 (PFHxA removal) | |||
| Loamy sand | ∑29 PFAS (C: 30–31,730) | Bench scale: RB or Pul Fsorb 400 (1–5% w/w; pH = 5.1 or =9.3) | >99 (PFAS leaching reduction; RB) ~97 (pH 5.1)–>99 (pH 9.3) (PFAS leaching reduction; Pul Fsorb 400) | [59] |
| Sandy | Σ29 PFAS (C: 0.0004–0.67) | Bench scale: RB or GAC (1–5% w/w; pH = 5.1 or =9.3) | >99 (PFAS leaching reduction) ~99.9 (pH 5.1) and ~99 (pH 9.1) (PFAS leaching reduction; RB) | |
| Loamy sand | ∑20 PFAS (C: 37,200) | Bench scale: RB (5% w/w; OP, HA) | >99 (Most PFAS leaching reduction) 92 (PFBA leaching reduction) | [60] |
| Loamy sand | ∑12 PFAS (C: 14,000) | Bench scale: RB (15–30% w/w) or RB+ (15%) | 99.99 (PFOS removal; 30% RB) 99.92 (PFHxS removal; 15% RB) | [76] |
| Sandy | ∑12 PFAS (C: 2400) | 99.91 PFOS removal (25% RB) 99.30 PFHxS removal (25% RB) | ||
| - | PFOA (C: 1000) | Bench scale: Carnitine or choline (2% w/w) | 58.0 (carnitine) and 57.9 (choline) (bioavailability reduction) | [77] |
| PFOS (C: 1000) | Bench scale: Carnitine or choline (2% w/w) | 77.5 (carnitine) and 79.4 (choline) (bioavailability reduction) | ||
| Sandy | ∑11 PFAS (C: 6.4–2510) | Bench scale: PAC, Mts and compost (3% w/w) | 28 and 34 (PFAS leaching reduction; compost soil) 28 and 40 (PFAS leaching reduction; montmorillonite) | [78] |
| Loamy sand | ∑6 PFAS (C: 2.4–1000 | Bench scale: Activated biochar (0.5–5% w/w) | 0–60 (PFAS leaching reduction; w/w < 5%) 23–100 (PFAS leaching reduction; w/w = 5%) | [79] |
| Sandy clay loam | ∑6 PFAS (C: 7.7–3400) | >90 (Most PFAS) and >57 (PFBA) (leaching reduction; w/w: 0.5%) >98 (Most PFAS) and >79 (PFBA) (leaching reduction; w/w: 1–5%) | ||
| Peat | ∑9 PFAS (C: 1200) | Bench scale: Fe-char amendment (1–20% w/w) | ~0 (PFAS leaching reduction) | [80] |
| - | ∑28 PFAS (C: 3093–32,780) | Field-scale: RB100 and RB300 (1–10% w/w) | ~85–99.4 (PFAS leaching reduction; RB100; w/w: 1–10%) >16% (PFAS bioavailability reduction; RB100; w/w: 5%) >14% (PFAS bioavailability reduction; RB300; w/w: 5%) | [81] |
| Soil Type | PFAS (C-mg/kg) | Experimental Conditions | Results (%) | Ref. |
|---|---|---|---|---|
| Clay | Σ30 PFAS | Pilot-scale: PC–FA–GGBS (15%) + GAC (0.2%) (6-year rains simulation) | 28 to 4.5 (PFHxS) 0 to 50 (PFPeA) >97 (PFHxA, PFOA, PFHxS, and PFOS) | [62] |
| Sand | Σ24 PFAS (C: 7–2282) | Bench-scale: PC + GAC, modified clay and AC–clay blend | 87.1–99.9 (GAC) | [83] |
| Sandy Clay Loam | Σ24 PFAS (C: 6–13,676) | 95.4–99.9 (GAC) | ||
| Loamy sand | Σ14 PFAS (C: 200–1500) | Bench scale: binders (PC, FA, and GGBS; ratio 1:1:2) + additives (PAC, RB, bentonite, calcium chloride, hydrotalcite, chitosan, zeolite) | >90 (Short-chain PFAS; PAC and RB) 99– 99.9 (Long-chain PFAS; PAC and RB) ~0 (Bentonite, Idroalcite, Chitosano, Zeolite) 50 to 38 (PFOS) 28 to 4.5 (PFHxS) | [84] |
| Sandy Clay Loam | Σ24 PFAS (C: 6–13,676) | Field-scale: PC (5–15%) + RB or FS (5–10%) | >99% (PFASs) | [85] |
| Soil Type | PFAS (C-mg/kg) | Experimental Conditions | Results (%) | Ref. |
|---|---|---|---|---|
| Clay | Σ30 PFAS (C: 0.4–2666.5) | Pilot-scale: Soil washing | 94.4–97.1 (PFCA short-chain) 85.7–94.9 (PFSA short-chain) 13.6–51.4 (PFSA long-chain) | [63] |
| Sand | PFOS (C > 751) PFOS (C > 1291) | Field-scale: soil washing (L/S: 5 L/kg) Field-scale: soil flushing (L/S: 15.3 L/kg) | 11–73 23–62 | [64] |
| Sandy loamy silt | Σ35 PFAS (C: 56.1) | Bench-scale: Soil washing + MeOH (5–80%), EtOH (5–80%) or ACN (80%) (L/S = 528) | 78 (PFOA)–24 (PFOS) (water) 96 (PFOA)–35 (PFOS) (5% EtOH) 75 (PFOA)–37 (PFOS) (5% MeOH) 86 (PFOA)–59 (PFOS) (80% EtOH) 93 (PFOA)–53 (PFOS) (80% MeOH) 100 (PFOA)–80 (PFOS) (80% ACN) | [65] |
| Sand | PFOS (C: 1000) | Bench scale: soil flushing + EtOH (50%) | >98 (PFOS) | [87] |
| Silty clay loam | GenX, PFOA, PFOS, PFDA, 6:2 FTAB (C:200 each) | Bench-scale: soil washing + MeOH (1–50%) + EtOH (1–50%) + HPCD (1–10 mg/g) | 92–98 (PFAS; MeOH 50% + HPCD 10 mg/g) 51 (HPCD 1 mg/g))–99 (HPCD 10 mg/g) (PFOS) | [88] |
| Clay | Σ23 PFAS (C: 24,230) | Bench scale: soil washing + O2 (W:S = 2.0–2.5; t = 5–10 min) | 58.1 (PFOS; Ozonated oxygen) 72.9 (PFHxS; Ozonated oxygen) 54.7 (PFOA; Ozonated oxygen) | [89] |
| Soil Type | PFAS (C-mg/kg) | Experimental Conditions | Results (%) | Ref. |
|---|---|---|---|---|
| - | Σ11 PFAS (C: 16.65–297.52) | Greenhouse: Juncus effusus | 39.2 μg/kg (Shoots) 16.8 μg/kg (Roots) | [66] |
| - | Σ6 PFAS | Greenhouse: Eight herbaceous and seven woody plant species | 1–38,121 μg/kg (PFAS, Herbaceous) 41–35,975 μg/kg (PFAS, Foliage woody’s plant) | [93] |
| - | Σ14 PFAS (C: 1500, each) | Greenhouse: Sunflower, mustard, and hemp | 3–11,000 μg/kg (Sunflowers) 5–14,000 μg/kg (Mustards) 70–960 μg/kg (Hemp) | [94] |
| - | Σ20 PFAS (C: 0.75–22) | Field-scale: H51, Hlesia, Hlianato, and ChinMa | 1.4 mg (PFAS, Leaves) | [95] |
| Silty | PFOA/PFOS (C:250–50,000) | Pilot-scale: Corn, oat, potatoes, wheat, and perennial ryegrass cultivation | 15,500 (PFOA)–7900 μg/kg (PFOS) (Corn, Straw) 440 μg/kg (PFOA, Corn, Ears) 217,000 (PFOA)–41,400 μg/kg (PFOS) (Oat, Straw) 1480 μg/kg (PFOA, Oat, Grains) 52 (PFOA)–34 μg/kg (PFOS) (Potatoes, Tuber) | [96] |
| Sand | Σ9 PFAS (C: 3.3–25,100) | Greenhouse: Lettuce cultivation (0.4% OC) | 25,000 μg/kg (PFBA, Leaves) | [97] |
| Greenhouse: Strawberry cultivation (0.4% OC) | >10,000 μg/kg (PFBA and PFPeA, Fruits) 5450 μg/kg (PFHxA, Roots) |
| Soil Type | PFAS (C-mg/kg) | Experimental Conditions | Results (%) | Ref. |
|---|---|---|---|---|
| Kaolin clay | PFOA (100,000) | Bench-scale + SDS, NaC or TW80 (5%) (I: 10–20 mA; t: 7–14 days) | 14.5–75.7 (PFOA) | [69] |
| Loamy sand | PFOA and PFOS (10,000) | Bench-scale (∆V: 30 V; t: 14 days; ∆CC: 5–25%) | 75–85 (PFOA) 20–30 (PFOS) | [99] |
| Bench-scale (∆V: 30 V; t: 14 days; ∆CC: 50–75%) | 95–100 (PFOA) 60–80 (PFOS) | |||
| Loamy sand | PFOA (10,000) | Bench-scale (∆V: 30 V; t: 14 days; ∆OM: 5–75%) | 70–80 (PFOA) >60 (PFOS) | [100] |
| Kaolin clay | PFOA (10,000) | Bench-scale + NaC + PRB (iron slag/AC) (P: middle; t: 2–3 weeks) | 33 (No PRB) 75 (PRB 50/50) 79 (PRB 70/30) 63.9 (PRB 50/50) | [101] |
| Kaolin clay | PFOA (10,000) | Bench-scale + NaC + PRB (AC, FeAC) (P: middle, anode; t: 14 days) | 59.55 (PRB: FeAC; P: middle) 52.35 (PRB: AC; P: middle) 40.37 (PRB: ReAC; P: middle) 21.96 (PRB: FeAC; P: anode) 20.62 (PRB: ReFeAC; P: middle) | [102] |
| Soil Type | PFAS (C-mg/kg) | Experimental Conditions | Results (%) | Ref. |
|---|---|---|---|---|
| - | PFOS and PFOA | Aerobic treatment + A6 (t: 100 days) | 35 (PFOS) 63 (PFOA) | [70] |
| Silty sand | Σ15 PFAS (C: 1.2–59) | Aerobic treatment + CB and YM (t: 28 days) | 46 (PFOS, CB) 16 (PFOA, CB) 69 (PFOS, YM) 36 (PFOA, YM) | [104] |
| - | PFOS | Aerobic treatment + HJ4 (t: 48 h) | 67 | [105] |
| Silty sand | PFOA | Aerobic treatment + PP (t: 72 h) | 32.4 (PFOA 500 mg/L) 12.6 (PFOA 1000 mg/L) | [106] |
| Aerobic treatment + PP (t: 72 h) + glucose | 48.1 | |||
| - | PFOS | Aerobic treatment + PP 2.4-D (t: 6 days) | 75 | [107] |
| Silty sand | PFOA (C: 500) | EOH + laccase (PO and PS; d: 60 U) + mediator (soybean-meal) | 35 (PO) 40 (PS) | [108] |
| EOH + laccase (PO and PS; d: 20/4 wk) + mediator (soybean-meal) | 29 (PO) 35 (PS) | |||
| Silt loam | PFOA | Anaerobic treatment + mmc (substrates: acetate, lactate, ethanol; t: 259 days) | ~0 | [109] |
| - | PFOS | MD (aerobic and anaerobic; t: 177 weeks) | 0 | [110] |
| Soil Type | PFAS (C-μg/kg) | Treatment (Conditions) | Degradation (%) | Ref. |
|---|---|---|---|---|
| Clay loam | Σ18 PAFS | Bench-scale: Thermal desorption (air; N2; T: 125–500 °C; t: 30 min) | > 99 (PFAS; T: 400 °C) 60–71 (PFSA; T: 400 °C) 99.8 (HFPO-DA; T: 300 °C) | [72] |
| Kaolinite clay | > 99 (all PFAS; T: >400 °C) 60–71 (PFSA; T: 400 °C) 99.8 (HFPO-DA; T: 300 °C) | |||
| Calcareous soil | Σ14 PAFS (700) | Bench-scale: Thermal desorption (T: 150, 550 °C) | 29-36.3 (all PFAS; T: 150°C) 96.4–97.5 (all PFAS; T: 550°C) 97.27 (PFBS; T: 550°C) 32.75 (PFNA; T:150°C) | [73] |
| - | Σ6 PAFS | Bench-scale: Thermal desorption (T: 100-400 °C; t: 0-600 s) | 100 (PFAS; T: 300 °C; t > 300 s) ~99 (PFOS; T: 400 °C; t: 60 s) 23 (PFAS; T: 100 °C, t: 600 s) | [112] |
| Clay | Σ9 PAFS (4000) | Bench-scale: Thermal desorption (T: 350, 450, 550 °C, t: 15–75 min) | >99 (PFCA/FOSA; T: 350 °C) 51–66 (PFSA; T: 350 °C) >99 (PFAS; T: 450 °C) | [113] |
| Loamy sand soil | >97 (PFPeA; T: 550 °C) 71–93 (PFCA; T: 550 °C) 99 (all PFSA; T: 450 °C) | |||
| Silty Clay | Σ9 PAFS (25) | Bench-scale: Thermal desorption (T: 350, 450, 550 °C, t: 15–75 min) | 43 (all PFAS; T: 350 °C) | |
| Sand | Σ6 PAFS (3000–5000) | Bench-scale: Smoldering combustion + GAC (w: 50 g/kg sand; air > 5 cm/s; T > 900 °C) | >98.9 (all PFAS; 16–17% defluorination) | [114] |
| Σ6 PAFS (3000–5000) | Bench scale: Smoldering combustion + GAC (w: 15 g/kg sand; air > 5 cm/s; T = 642 °C) | >98.9 (all PFAS; no defluorination) | ||
| Σ3 PAFS (200) | Benche scale: Smoldering combustion + GAC (w: 51 g/kg sand; air > 5 cm/s; T > 1000 °C) | >99.9 (all PFAS; high defluorination) | ||
| PFOS, PFOA (0.6–250) | Field-scale: FH ex situ stockpiles (T: 350, 400 °C) | >95.3 (Average) | [115] |
| Soil Type | PFAS (C-μg/kg) | Treatment (Conditions) | Degradation (%) | Ref. |
|---|---|---|---|---|
| Sand (dry) | PFOS and PFOA | Bench-scale: PBM with/without KOH (w: 15 g; t: 15 min–4 h) | 70–98 (PFOS, w/o KOH) | [74] |
| 82–96 (PFOA, KOH) | ||||
| Bench-scale: PBM with/without KOH (w: 40 g; t: 15 min–4 h) | 90–99 (PFOS, w/o KOH) | |||
| Bench-scale: HBM without/with KOH (V: 25 L, t: 3 h) | 94–99 (PFOA, KOH) | |||
| Sand (saturated) | Bench-scale: PBM with/without KOH (w: 15 g; t: 15 min–4 h) | 29–38 (PFOA, w/o KOH) | ||
| Bench-scale: HBM without/with KOH (V: 25 L, t: 3 h) | 74–83 (PFOA, KOH) | |||
| Bench-scale: PBM with/without KOH (w: 40 g; t: 15 min–4 h) | 97–99 (PFOS, w/o KOH) | |||
| 88–92 (PFOA, KOH) | ||||
| NSS | PFOS (6.1) and 6:2 FTSA (2.4) | Bench-scale: HBM without/with KOH (V: 1 L, t: 3 h) | 19 (w/o KOH), 43 (KOH) (PFOS; t: 3 h) | [75] |
| 97 (w/o KOH), >88 (KOH) (6:2 FTSA) | ||||
| Sand | PFOS and 6:2 FTSA (~1.4 each) | Bench-scale: HBM without/with KOH (V: 1 L, t: 3 h) | 15 (w/o KOH), 69 (KOH) (PFOS) | |
| Bench-scale: HBM without/with KOH (V: 25 L, t: 3 h) | 67 (w/o KOH), 61 (KOH) (6:2 FTSA) | |||
| Clay | PFOS and 6:2 FTSA (~0.6 each) | Bench-scale: HBM without/with KOH (V: 1 L, t: 3 h) | 5 (w/o KOH), 81 (KOH) (PFOS) | |
| Bench-scale: HBM without/with KOH (V: 25 L, t: 3 h) | 12 (w/o KOH), 85 (KOH) (6:2 FTSA) | |||
| Silica sand NSS Calcite Marble | PFOS (18.3) and PFOA (16.1) PFOS (12.7) and PFOA (18) PFOS (17.8) and PFOA (19) PFOS (20.2) and PFOA (25.5) | Bench-scale: PBM (t: 4 h) | 99 (PFOS), 99 (PFOA) | [117] |
| 95 (PFOS), 96 (PFOA) | ||||
| 91 (PFOS), 93 (PFOA) | ||||
| 83 (PFOS), 92 (PFOA) | ||||
| NSS | 6:2 FTS (~3.5), PFOS and PFOA (~5) | Pilot-scale: HBM without/with KOH (V: 267 L; t: 10–120 min) | ~0 (6:2 FTS), 69 (PFOS), 70 (PFOA) (w/o KOH) | [118] |
| 97 (6:2 FTS), 70 (PFOS), 74 (PFOA) (KOH) | ||||
| Silty sand | 6:2 FTS, PFOS and 8:2 FTS (2.6–5.6) | Pilot-scale: HBM without/with KOH (V: 267 L; t: 10–120 min) | 46 (6:2 FTS), 50 (PFOS), 55 (PFOA) (w/o KOH) | |
| 46 (6:2 FTS), 31 (PFOS), 33 (PFOA) (KOH) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napoli, R.; Fazzino, F.; Vagliasindi, F.G.A.; Falciglia, P.P. Sustainable Remediation Strategies and Technologies of Per- and Polyfluoroalkyl Substances (PFAS)-Contaminated Soils: A Critical Review. Sustainability 2025, 17, 6635. https://doi.org/10.3390/su17146635
Napoli R, Fazzino F, Vagliasindi FGA, Falciglia PP. Sustainable Remediation Strategies and Technologies of Per- and Polyfluoroalkyl Substances (PFAS)-Contaminated Soils: A Critical Review. Sustainability. 2025; 17(14):6635. https://doi.org/10.3390/su17146635
Chicago/Turabian StyleNapoli, Rosario, Filippo Fazzino, Federico G. A. Vagliasindi, and Pietro P. Falciglia. 2025. "Sustainable Remediation Strategies and Technologies of Per- and Polyfluoroalkyl Substances (PFAS)-Contaminated Soils: A Critical Review" Sustainability 17, no. 14: 6635. https://doi.org/10.3390/su17146635
APA StyleNapoli, R., Fazzino, F., Vagliasindi, F. G. A., & Falciglia, P. P. (2025). Sustainable Remediation Strategies and Technologies of Per- and Polyfluoroalkyl Substances (PFAS)-Contaminated Soils: A Critical Review. Sustainability, 17(14), 6635. https://doi.org/10.3390/su17146635






