Process Intensification of Anaerobic Digestion of Biowastes for Improved Biomethane Production: A Review
Abstract
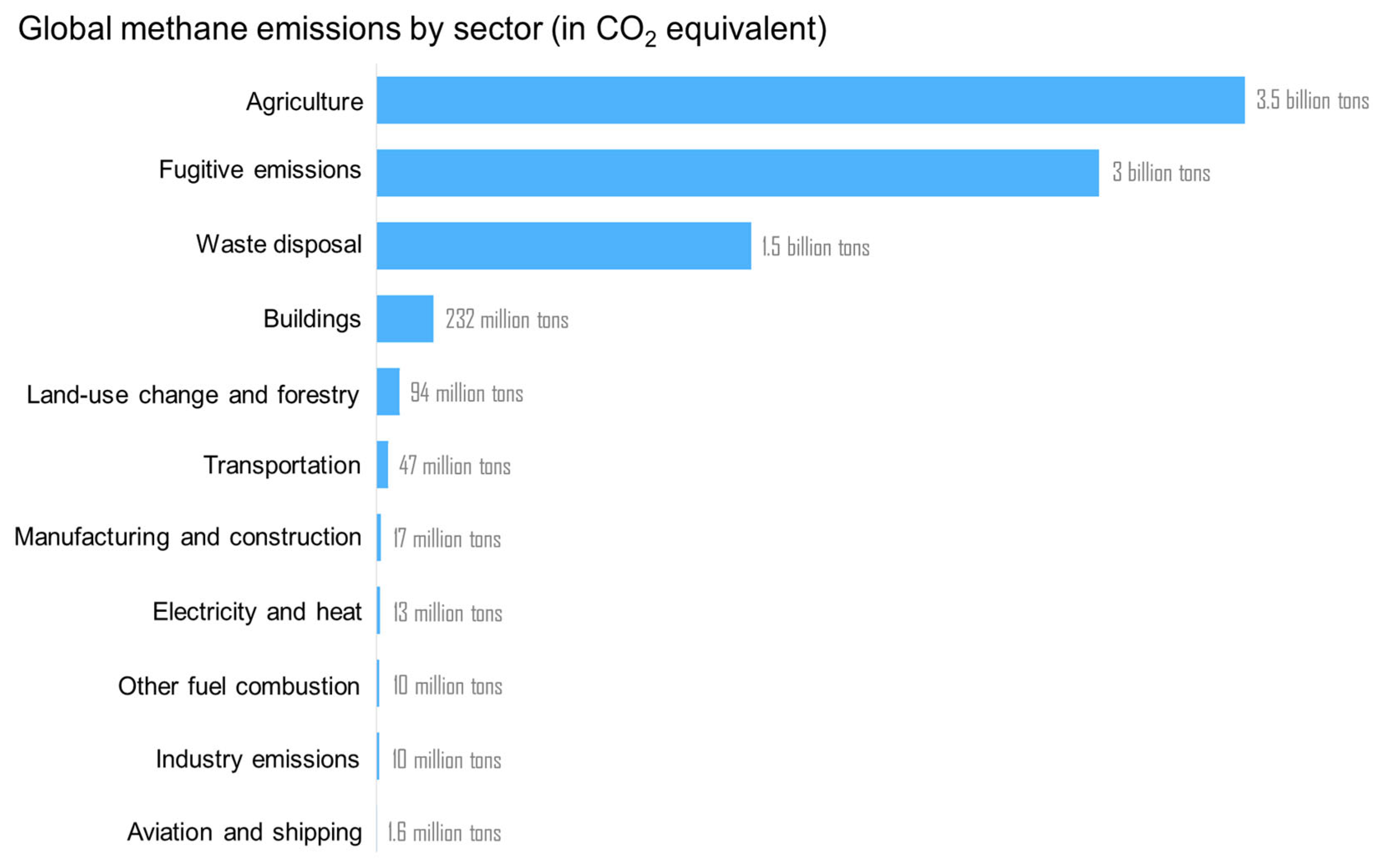
1. Introduction
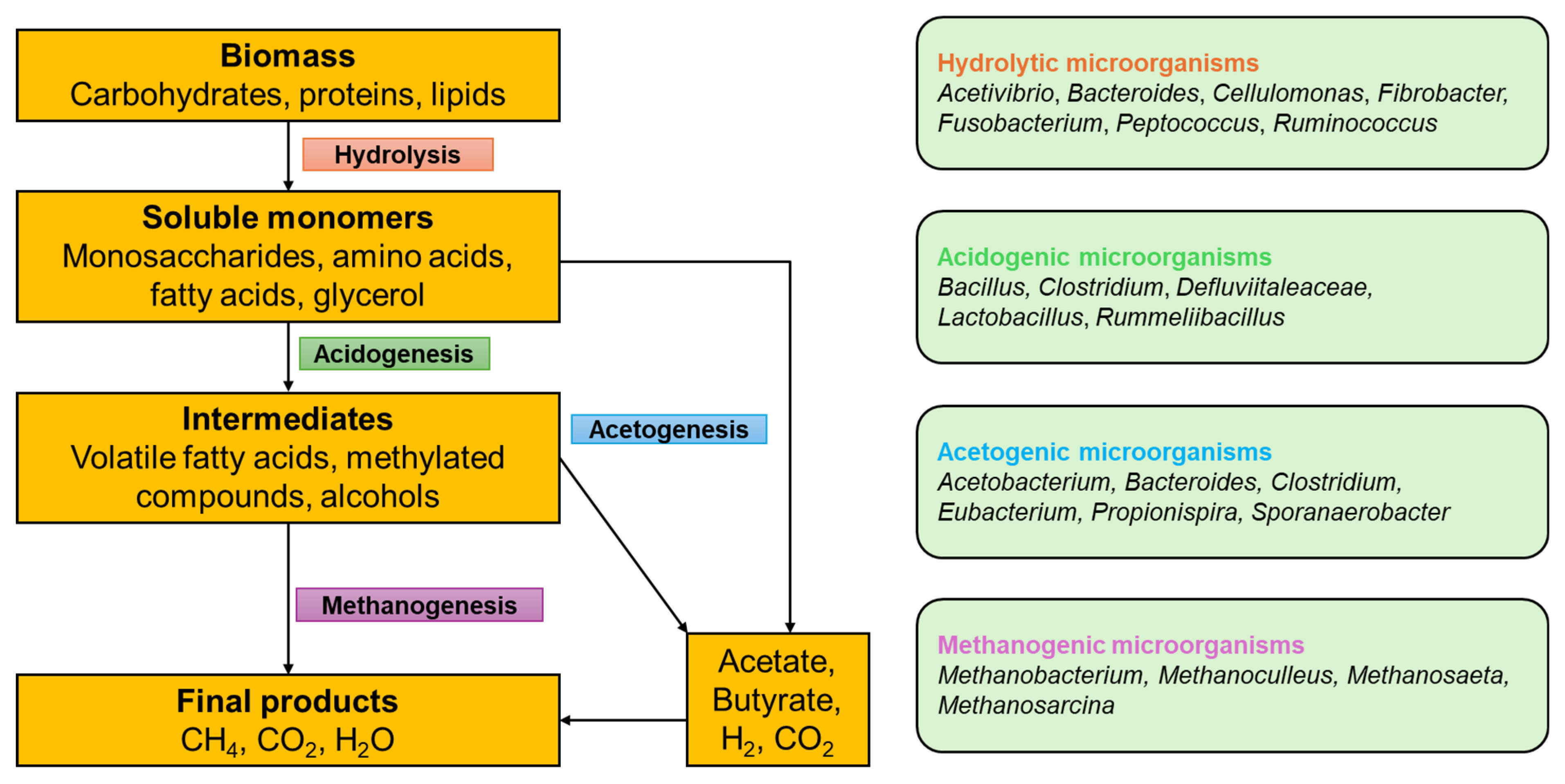
2. Anaerobic Digestion
3. Process Parameters Influencing Anaerobic Digestion
3.1. Temperature
3.2. Hydraulic Retention Time
3.3. pH and Volatile Fatty Acids
4. Pretreatment of Biomass
4.1. Extrusion
4.2. Microwave
4.3. Ultrasonication
4.4. Acids and Bases
4.5. Oxidizing Agents
4.6. Ionic Liquids
4.7. Biological Pretreatment
4.8. Integrated Pretreatment Methods
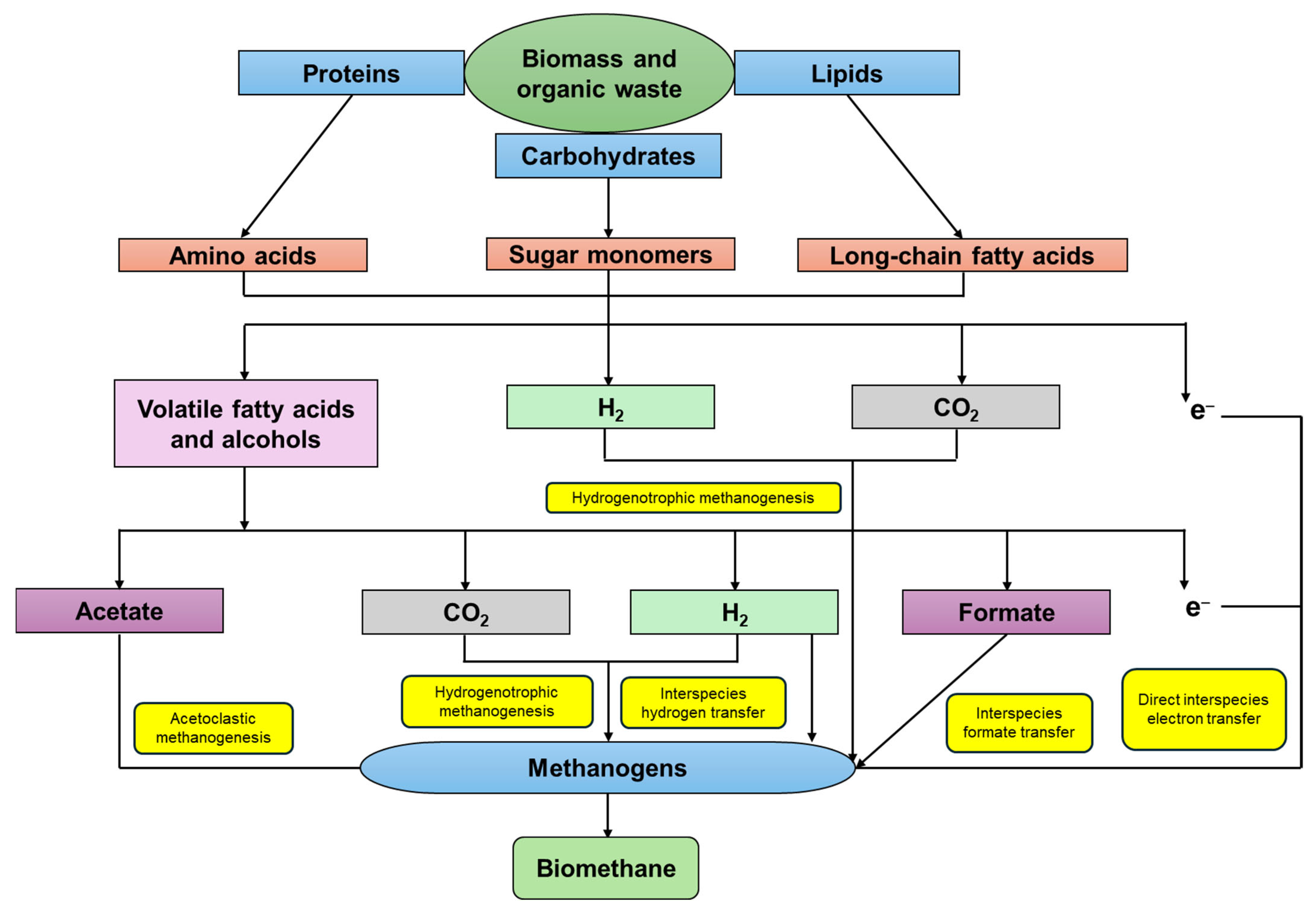
5. Direct Interspecies Electron Transfer in Anaerobic Digestion
6. Novel Additives for Anaerobic Digestion
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Statista. Globally-Averaged, Monthly Mean Atmospheric Methane (CH4) Abundance from 1990 to 2025. Available online: https://www.statista.com/statistics/1314344/atmospheric-concentration-of-ch4-historic-monthly/ (accessed on 10 June 2025).
- Our World in Data. Methane Emissions by Sector, World. 2021. Available online: https://ourworldindata.org/grapher/methane-emissions-by-sector?time=latest (accessed on 13 June 2025).
- Kiessé, T.S.; Corson, M.S.; Eugène, M.; Aubin, J.; Wilfart, A. Analysis of enteric methane emissions due to extreme variations in management practices of dairy-production systems. Agric. Syst. 2019, 173, 449–457. [Google Scholar] [CrossRef]
- Saha, M.K.; Mia, S.; Biswas, A.K.M.A.A.; Sattar, M.A.; Kader, M.A.; Jiang, Z. Potential methane emission reduction strategies from rice cultivation systems in Bangladesh: A critical synthesis with global meta-data. J. Environ. Manag. 2022, 310, 114755. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (USEPA). Understanding Global Warming Potentials. Available online: https://www.epa.gov/ (accessed on 10 June 2025).
- Nanda, S.; Azargohar, R.; Dalai, A.K.; Kozinski, J.A. An assessment on the sustainability of lignocellulosic biomass for biorefining. Renew. Sustain. Energy Rev. 2015, 50, 925–941. [Google Scholar] [CrossRef]
- Martín, M.; Taifouris, M.; Galán, G. Lignocellulosic biorefineries: A multiscale approach for resource exploitation. Bioresour. Technol. 2023, 385, 129397. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Berruti, F. Municipal solid waste management and landfilling technologies: A review. Environ. Chem. Lett. 2021, 19, 1433–1456. [Google Scholar] [CrossRef]
- Pattnaik, F.; Patra, B.R.; Nanda, S.; Mohanty, M.K.; Dalai, A.K.; Rawat, J. Drivers and barriers in the production and utilization of second-generation bioethanol in India. Recycling 2024, 9, 19. [Google Scholar] [CrossRef]
- Nanda, S.; Pattnaik, P.; Patra, B.R.; Kang, K.; Dalai, A.K. A Review of liquid and gaseous biofuels from advanced microbial fermentation processes. Fermentation 2023, 9, 813. [Google Scholar] [CrossRef]
- Farghali, M.; Osman, A.I.; Umetsu, K.; Rooney, D.W. Integration of biogas systems into a carbon zero and hydrogen economy: A review. Environ. Chem. Lett. 2022, 20, 2853–2927. [Google Scholar] [CrossRef]
- Ganjifar, A.; Karrabi, M.; Shahnavaz, B.; Dewil, R. A new concept for bioenergy yield in anaerobic digestion process: Highlighting current yield challenges. Fuel 2024, 375, 132558. [Google Scholar] [CrossRef]
- Talwar, P.; Agudelo, M.A.; Nanda, S. Pyrolysis process, reactors, products, and applications: A review. Energies 2025, 18, 2979. [Google Scholar] [CrossRef]
- Werkneh, A.A. Biogas impurities: Environmental and health implications, removal technologies and future perspectives. Heliyon 2022, 8, e10929. [Google Scholar] [CrossRef]
- Subbarao, P.M.V.; D’Silva, T.C.; Adlak, K.; Kumar, S.; Chandra, R.; Vijay, V.K. Anaerobic digestion as a sustainable technology for efficiently utilizing biomass in the context of carbon neutrality and circular economy. Environ. Res. 2023, 234, 116286. [Google Scholar] [CrossRef]
- Van Midden, C.; Harris, J.; Shaw, L.; Sizmur, T.; Pawlett, M. The impact of anaerobic digestate on soil life: A review. Appl. Soil Ecol. 2023, 191, 105066. [Google Scholar] [CrossRef]
- Pattnaik, F.; Tripathi, S.; Patra, B.R.; Nanda, S.; Kumar, V.; Dalai, A.K.; Naik, S. Catalytic conversion of lignocellulosic polysaccharides to commodity biochemicals: A review. Environ. Chem. Lett. 2021, 19, 4119–4136. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Kozinski, J.A. Butanol and ethanol production from lignocellulosic feedstock: Biomass pretreatment and bioconversion. Energy Sci. Eng. 2014, 2, 138–148. [Google Scholar] [CrossRef]
- Lomwongsopon, P.; Aramrueang, N. Mild Chemical pretreatment of cassava pulp for enhancing high-load anaerobic digestion. Bioresour. Technol. Rep. 2022, 17, 100896. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Wu, S.; He, J. Fungal pretreatment by Phanerochaete chrysosporium for enhancement of biogas production from corn stover silage. Appl. Biochem. Biotechnol. 2014, 174, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zheng, Y.; Li, Y. Fungal pretreatment of yard trimmings for enhancement of methane yield from solid-state anaerobic digestion. Bioresour. Technol. 2014, 156, 176–181. [Google Scholar] [CrossRef]
- Markt, R.; Prem, E.M.; Illmer, P.; Wagner, A.O.; Lackner, N.; Mutschlechner, M. Pre-Treatment with Trichoderma viride: Towards a better understanding of its consequences for anaerobic digestion. Environ. Microbiol. Rep. 2024, 16, e13281. [Google Scholar] [CrossRef]
- Ali, S.S.; Kornaros, M.; Manni, A.; Sun, J.; El-Shanshoury, A.E.R.; Kenawy, E.; Khalil, M.A. Enhanced anaerobic digestion performance by two artificially constructed microbial consortia capable of woody biomass degradation and chlorophenols detoxification. J. Hazard. Mater. 2020, 389, 122076. [Google Scholar] [CrossRef]
- Raut, M.P.; Pandhal, J.; Wright, P.C. Effective pretreatment of lignocellulosic co-substrates using barley straw-adapted microbial consortia to enhanced biomethanation by anaerobic digestion. Bioresour. Technol. 2021, 321, 124437. [Google Scholar] [CrossRef]
- Pazoki, M.; Dalaei, P. Alkaline and acid thermal hydrolysis of biological excess sludge in sequencing batch reactor. Desalin. Water Treat. 2016, 57, 23603–23609. [Google Scholar] [CrossRef]
- Jiménez-Castro, M.P.; Selene, L.; Zoffreo, A.; Timko, M.T.; Forster-Carneiro, T. Two-stage anaerobic digestion of orange peel without pre-treatment: Experimental evaluation and application to São Paulo State. J. Chem. Eng. 2020, 8, 104035. [Google Scholar] [CrossRef]
- Pang, H.; Qin, Q.; Jiao, Q.; He, J.; Pang, Z.; Wang, L. New insight into Na⁺-promoted microbial electrolysis cell towards hydrogen energy recovery: Feasibility and dual mechanism in salt-containing wastewater treatment. Chem. Eng. J. 2024, 496, 153623. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Chaudhary, D.K.; Dahal, R.H.; Trinh, N.H.; Kim, J.; Chang, S.W.; Hong, Y.; La, D.D.; Nguyen, X.C.; Ngo, H.H.; et al. Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Wongarmat, W.; Sittijunda, S.; Mamimin, C.; Reungsang, A. Acidogenic phase anaerobic digestion of pretreated sugarcane filter cake for co-digestion with biogas effluent to enhance the methane production. Fuel 2022, 310, 122466. [Google Scholar] [CrossRef]
- Kurade, B.H.; Jeon, B.H. Acetoclastic methanogenesis led by Methanosarcina in anaerobic co-digestion of fats, oil and grease for enhanced production of methane. Bioresour. Technol. 2018, 259, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Polman, E.M.N.; Gruter, G.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total. Environ. 2021, 753, 141953. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.E.; et al. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liu, P.; Khan, A.; Xiong, J.; Tian, F.; Li, X. A critical review on the interaction of substrate nutrient balance and microbial community structure and function in anaerobic co-digestion. Bioresour. Technol. 2018, 247, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.J.P.; Mishra, R.K.; Chinnam, S.; Binnal, P.; Dwivedi, N. A comprehensive study on anaerobic digestion of organic solid waste: A review on configurations, operating parameters, techno-economic analysis and current trends. Biotech. Notes 2024, 5, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Andr, P.; Teleken, J.G.; Meier, T.R.; Alves, H.J. Two-stage anaerobic digestion in agroindustrial waste treatment: A review. J. Environ. Manag. 2021, 281, 111854. [Google Scholar] [CrossRef] [PubMed]
- Cozannet, M.; Le Guellec, S.; Alain, K. A variety of substrates for methanogenesis. Case Stud. Chem. Environ. Eng. 2023, 8, 100533. [Google Scholar] [CrossRef]
- Marcoberardino, G.D.; Foresti, S.; Binotti, M.; Manzolini, G. Process intensification potentiality of a biogas membrane reformer for decentralized hydrogen production. Chem. Eng. Process. Process Intensif. 2018, 129, 131–141. [Google Scholar] [CrossRef]
- Dong, B.; Gao, P.; Zhang, D.; Chen, Y.; Dai, L.; Dai, X. A new process to improve short-chain fatty acids and bio-methane generation from waste activated sludge. J. Environ. Sci. 2016, 43, 159–168. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Yang, S.; Gong, H.; Ma, J.; Li, C.; Wang, K. Performance and microbial community evaluation of full-scale two-phase anaerobic digestion of waste activated sludge. Sci. Total Environ. 2022, 814, 152525. [Google Scholar] [CrossRef]
- Janesch, E.; Pereira, J.; Neubauer, P.; Junne, S. Phase separation in anaerobic digestion: A potential for easier process combination? Front. Chem. Eng. 2021, 3, 711971. [Google Scholar] [CrossRef]
- Chen, G.; Wu, W.; Xu, J.; Wang, Z. An anaerobic dynamic membrane bioreactor for enhancing sludge digestion: Impact of solids retention time on digestion efficacy. Bioresour. Technol. 2021, 329, 124864. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.C.; Ngo, H.H.; Sun, Q.; Yang, Y. Anaerobic dynamic membrane bioreactor (AnDMBR) for wastewater treatment: A review. Bioresour. Technol. 2018, 247, 1107–1118. [Google Scholar] [CrossRef]
- Cayetano, R.D.A.; Kim, G.B.; Park, J.H.; Kumar, G.; Kim, S.H. Waste activated sludge treatment in an anaerobic dynamic membrane bioreactor at varying hydraulic retention time: Performance monitoring and microbial community analysis. Int. J. Energy Res. 2020, 44, 12485–12495. [Google Scholar] [CrossRef]
- Park, J.; Cayetano, R.D.A.; Kwon, Y.; Kim, G.B.; Jo, Y.; Kim, S.H. Improved sludge anaerobic digestion capacity by dynamic membrane and alkaline-thermal pretreatment: Long-term continuous operation and techno-economic analysis. Chem. Eng. J. 2023, 474, 145735. [Google Scholar] [CrossRef]
- Park, J.; Kwon, Y.; Kim, G.B.; Jo, Y.; Park, S.; Yoon, Y.H.; Kim, S.H. Enhanced performance and economic feasibility of sewage sludge digestion using a two-stage anaerobic digestion with a dynamic membrane and alkaline-thermal pretreatment. Bioresour. Technol. 2025, 415, 131661. [Google Scholar] [CrossRef] [PubMed]
- Ozsefil, I.C.; Miraloglu, I.H.; Ozbayram, E.G.; Ince, B.; Ince, O. Bioaugmentation of anaerobic digesters with the enriched lignin-degrading microbial consortia through a metagenomic approach. Chemosphere 2024, 355, 141831. [Google Scholar] [CrossRef]
- Gállego-Bravo, A.K.; García-Mena, J.; Piña-Escobedo, A.; López-Jiménez, G.; Gutiérrez-Castillo, M.E.; Tovar-Gálvez, L.R. Monitoring of a microbial community during bioaugmentation with hydrogenotrophic methanogens to improve methane yield of an anaerobic digestion process. Biotechnol. Lett. 2023, 45, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Zhang, X.; Cheng, X.; Li, S.; Liang, X.; Tao, Y.; Li, Y. Impact of bioaugmentation on psychrophilic anaerobic digestion of corn straw. Bioresour. Technol. 2025, 417, 131886. [Google Scholar] [CrossRef]
- Perman, E.; Westerholm, M.; Liu, T.; Schnürer, A. Comparative study of high-solid anaerobic digestion at laboratory and industrial scale–process performance and microbial community structure. Energy Convers. Manag. 2024, 300, 117978. [Google Scholar] [CrossRef]
- Bona, D.; Beggio, G.; Weil, T.; Scholz, M.; Bertolini, S.; Grandi, L.; Pivato, A. Effects of woody biochar on dry thermophilic anaerobic digestion of organic fraction of municipal solid waste. J. Environ. Manag. 2020, 267, 110633. [Google Scholar] [CrossRef]
- Azizi, S.M.M.; Zakaria, B.S.; Haffiez, N.; Kumar, A.; Dhar, B.R. Pilot-Scale Investigation of conductive carbon cloth amendment for enhancing high-solids anaerobic digestion and mitigating antibiotic resistance. Bioresour. Technol. 2023, 385, 129411. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Wu, J.; Chen, C.; Ding, Y.; Liu, H.; Zhou, Y. Rethinking the biochar impact on the anaerobic digestion of food waste in bench-scale digester: Spatial distribution and biogas production. Bioresour. Technol. 2025, 420, 132115. [Google Scholar] [CrossRef]
- Ding, J.; Zhen, F.; Kong, X.; Hu, Y.; Zhang, Y.; Gong, L. Effect of biochar in modulating anaerobic digestion performance and microbial structure community of different inoculum sources. Fermentation 2024, 10, 151. [Google Scholar] [CrossRef]
- Zhang, L.; Lim, E.Y.; Loh, K.C.; Ok, Y.S.; Lee, J.T.; Shen, Y.; Tong, Y.W. Biochar enhanced thermophilic anaerobic digestion of food waste: Focusing on biochar particle size, microbial community analysis and pilot-scale application. Energy Convers. Manag. 2020, 209, 112654. [Google Scholar] [CrossRef]
- Sun, L.; Müller, B.; Westerholm, M.; Schnürer, A. Syntrophic acetate oxidation in industrial CSTR biogas digesters. J. Biotechnol. 2014, 171, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Global Methane Initiatives. Market Opportunities for Anaerobic Digestion of Livestock and Agro-Industrial Waste in India. In Business Models and Case Studies; Global Methane Initiatives: Washington, DC, USA, 2020. [Google Scholar]
- Tamborrino, A.; Catalano, F.; Leone, A.; Bianchi, B. A real case study of a full-scale anaerobic digestion plant powered by olive by-products. Foods 2021, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency (IEA). Biogas Production from Kitchen Wastes in Jinhua, China; Case Story; IEA Bioenergy: Paris, France, 2021; Volume 37, p. 12. [Google Scholar]
- International Energy Agency (IEA). Treatment of Pigment Wastewater and Generation of Natural Gas Standard Biomethane in Hangzhou, China; Case Story; IEA Bioenergy: Paris, France, 2021; Volume 37, p. 12. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Project Profile: Noblehurst Farms. Available online: https://www.epa.gov/agstar/project-profile-noblehurst-farms (accessed on 8 July 2025).
- International Energy Agency (IEA). Circular Economy System Integrating Biogas into Process to Produce High Quality Products from Recycled Paper; Case Story; IEA Bioenergy: Paris, France, 2021; Volume 37, p. 12. [Google Scholar]
- Akindolire, M.A.; Rama, H.; Roopnarain, A. Psychrophilic anaerobic digestion: A critical evaluation of microorganisms and enzymes to drive the process. Renew. Sustain. Energy Rev. 2022, 161, 112394. [Google Scholar] [CrossRef]
- Akindolire, M.A.; Ndaba, B.; Bello-Akinosho, M.; Rama, H.; Roopnarain, A. Bioprospecting bacteria from psychrophilic anaerobic digestate for potential plant growth-promoting attributes. Int. J. Microbiol. 2025, 23, 2208124. [Google Scholar] [CrossRef]
- Venkiteshwaran, K.; Bocher, B.; Maki, J.; Zitomer, D. Relating anaerobic digestion microbial community and process function. Microbiol. Insights 2016, 8, 37–44. [Google Scholar] [CrossRef]
- Wang, Z.; He, H.; Yan, J.; Xu, Z.; Yang, G.; Wang, H.; Zhao, Y.; Cui, Z.; Yuan, X. Influence of temperature fluctuations on anaerobic digestion: Optimum performance is achieved at 45 °C. Chem. Eng. J. 2024, 492, 152331. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, L.; Guo, W.; Zhang, W.; Sajjad, W.; Ilahi, N.; Usman, M.; Faisal, S.; Bahadur, A. Temperature drives microbial communities in anaerobic digestion during biogas production from food waste. Environ. Sci. Pollut. Res. 2024, 31, 53823–53838. [Google Scholar] [CrossRef]
- Hupfauf, S.; Plattner, P.; Otto, A.; Plattner, P.; Wagner, A.O.; Kaufmann, R.; Insam, H.; Podmirseg, S.M. Temperature shapes the microbiota in anaerobic digestion and drives efficiency to a maximum at 45 °C. Bioresour. Technol. 2018, 269, 309–318. [Google Scholar] [CrossRef]
- Elsayed, A.; Kakar, F.L.; Abdelrahman, A.M.; Ahmed, N.; AlSayed, A.; Zagloul, M.S.; Muller, C.; Bell, K.Y.; Santoro, D.; Norton, J.; et al. Enhancing anaerobic digestion Efficiency: A comprehensive review on innovative intensification technologies. Energy Convers. Manag. 2024, 320, 118979. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, K.; Wang, L.; Xu, R.; Lu, D.; Zhou, Y. Effect of sludge retention time on microbial succession and assembly in thermal hydrolysis pretreated sludge digesters: Deterministic versus stochastic processes. Water Res. 2022, 209, 117900. [Google Scholar] [CrossRef] [PubMed]
- Wandera, S.M.; Westerholm, M.; Qiao, W.; Yin, D.; Jiang, M.; Dong, R. The correlation of methanogenic communities’ dynamics and process performance of anaerobic digestion of thermal hydrolyzed sludge at short hydraulic retention times. Bioresour. Technol. 2019, 272, 180–187. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Ye, F.; Yang, F.; Lyu, Y.K. Determination of operational parameters for the first stage of continuous temperature-phased anaerobic digestion of oily food waste: Influent concentration, hydraulic retention time, pH control and temperature. J. Clean. Prod. 2024, 434, 139960. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R. Improvement of the anaerobic digestion of sewage sludge by co-digestion with wine vinasse and poultry manure: Effect of different hydraulic retention times. Fuel 2022, 321, 124104. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Wang, X. Effects of alkalinity sources on the stability of anaerobic digestion from food waste. Waste Manag. Res. 2015, 33, 1033–1040. [Google Scholar] [CrossRef]
- Wenjing, T.; Qin, J.; Liu, J.; Liu, F.; Gu, L. Effects of pine sawdust and shrimp shell biochar on anaerobic digestion under different acidification conditions. J. Environ. Chem. Eng. 2022, 10, 106581. [Google Scholar] [CrossRef]
- Patra, B.R.; Mukherjee, A.; Nanda, S.; Dalai, A.K. Biochar production, activation and adsorptive applications: A review. Environ. Chem. Lett. 2021, 19, 2237–2259. [Google Scholar] [CrossRef]
- Pezzolla, D.; Maria, F.D.; Zadra, C.; Massaccesi, L.; Sordi, A.; Gigliotti, G. Optimization of solid-state anaerobic digestion through the percolate recirculation. Biomass Bioenergy 2017, 96, 112–118. [Google Scholar] [CrossRef]
- Ketsub, N.; Whatmore, P.; Abbasabadi, M.; Doherty, W.O.; Kaparaju, P.; O’Hara, I.M.; Zhang, Z. Effects of pretreatment methods on biomethane production kinetics and microbial community by solid state anaerobic digestion of sugarcane trash. Bioresour. Technol. 2022, 352, 127112. [Google Scholar] [CrossRef]
- Zhai, N.N.; Zhang, T.; Yin, D.X.; Yang, G.; Wang, X.; Ren, G.; Feng, Y. Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure. Waste Manag. 2015, 38, 126–131. [Google Scholar] [CrossRef]
- Jiang, Q.; Zheng, Z.; Zhang, Y.; Zhang, X.; Liu, H. Key properties identification of biochar material in anaerobic digestion of sewage sludge for enhancement of methane production. J. Environ. Chem. Eng. 2023, 11, 109850. [Google Scholar] [CrossRef]
- Ni, Z.; Zhou, L.; Lin, Z.; Kuang, B.; Zhu, G.; Jia, J.; Wang, T. Iron-modified biochar boosts anaerobic digestion of sulfamethoxazole pharmaceutical wastewater: Performance and microbial mechanism. J. Hazard. Mater. 2023, 452, 131314. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, F.; Li, P.; Yang, F.; Pei, Z.; Yu, Q.; Zuo, X.; Liu, J. Effects of rice straw biochar on microbial community structure and metabolic function during anaerobic digestion. Sci. Rep. 2022, 12, 6971. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, J.S.; Kim, T.Y.; Kim, S.M.; Lee, B.H.; Jeon, C.H. Effect of lignocellulosic composition on biomass grindability for carbon-free power generation: Role of hemicellulose variation via pretreatment. J. Energy Inst. 2025, 119, 101982. [Google Scholar] [CrossRef]
- Lytras, G.; Koutroumanou, E.; Lyberatos, G. Anaerobic co-digestion of condensate produced from drying of household food waste and waste activated sludge. J. Environ. Chem. Eng. 2020, 8, 103947. [Google Scholar] [CrossRef]
- Arman, I.; Athar, M.; Arinjay, I.H.F. Optimization of microwave intensified slaughterhouse sludge pretreatment method to enhance anaerobic digestion process by response surface methodology. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, S.; Song, G.; Lu, C.; Liu, R.; Hu, C.; Qu, J. Ultrasonication-enhanced biogas production in anaerobic digestion of waste active sludge: A pilot scale investigation. Resour. Conserv. Recycl. 2023, 192, 106902. [Google Scholar] [CrossRef]
- Nanda, S.; Mohammad, J.; Reddy, S.N.; Kozinski, J.A.; Dalai, A.K. Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Convers. Biorefinery 2014, 4, 157–191. [Google Scholar] [CrossRef]
- Gomes, A.; Borges, A.; Peres, J.A.; Lucas, M.S. Bioenergy production from agro-industrial wastewater using advanced oxidation processes as pre-treatment. Catalysts 2023, 13, 1186. [Google Scholar] [CrossRef]
- Pérez-Pimienta, J.A.; Icaza-Herrera, J.P.A.; Méndoza-Pérez, J.A.; González-Álvarez, V.; Méndez-Acosta, H.O.; Arreola-Vargas, J. Mild reaction conditions induce high sugar yields during the pretreatment of Agave tequilana bagasse with 1-ethyl-3-methylimidazolium acetate. Bioresour. Technol. 2019, 275, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Rani, J.; Dhoble, A.S. Effect of fungal pretreatment by Pycnoporus sanguineus and Trichoderma longibrachiatum on the anaerobic digestion of rice straw. Bioresour. Technol. 2023, 387, 129503. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Y.; Jing, Y.; Manickam, S.; Chong, M.F.; Chong, S.; Tiong, T.J.; Lim, J.W.; Pan, G.T. Enzymatic Pretreatment to enhance anaerobic bioconversion of high strength wastewater to biogas: A review. Sci. Total Environ. 2020, 713, 136373. [Google Scholar] [CrossRef]
- Du, J.; Qian, Y.; Xi, Y.; Lü, X. Hydrothermal and alkaline thermal pretreatment at mild temperature in solid state for physicochemical properties and biogas production from anaerobic digestion of rice straw. Renew. Energy 2019, 139, 261–267. [Google Scholar] [CrossRef]
- Fasheun, D.O.; da Silva, A.S.A.; Teixeira, R.S.S.; Ferreira-Leitão, V.S. Enhancing methane production from cassava starch: The potential of extrusion pretreatment in single-stage and two-stage anaerobic digestion. Fuel 2024, 366, 131406. [Google Scholar] [CrossRef]
- Kong, X.; Xu, S.; Liu, J.; Li, H.; Zhao, K.; He, L. Enhancing anaerobic digestion of high-pressure extruded food waste by inoculum optimization. J. Environ. Manag. 2016, 166, 31–37. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, L.; Zhu, K.; Ma, J.; Li, A. Semi-continuous anaerobic digestion of extruded OFMSW: Process performance and energetics evaluation. Bioresour. Technol. 2018, 247, 103–115. [Google Scholar] [CrossRef]
- Yue, L.; Cheng, J.; Tang, S.; An, X.; Hua, J.; Dong, H.; Zhou, J. Ultrasound and microwave pretreatments promote methane production potential and energy conversion during anaerobic digestion of lipid and food wastes. Energy 2021, 228, 120525. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, M.; Lv, C.; Yue, P. The effect of microwave pretreatment on anaerobic co-digestion of sludge and food waste: Performance, kinetics and energy recovery. Environ. Res. 2020, 189, 109856. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Chaudhari, P.K.; Ghosh, P. Effect of microwave treatment on maximizing biogas yield for anaerobic co-digestion of fruit and vegetable waste and anaerobic sludge. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Xu, Y.; Hao, C.; Chen, Y.; Liu, C.; Xiao, Y. Ultrasonic pretreatment of cow dung for anaerobic digestion: Effect on methane production and microbial community. BioEnergy Res. 2024, 17, 660–668. [Google Scholar] [CrossRef]
- Zhao, K.; Song, G.; Lu, C.; Wang, J.; Liu, R.; Hu, C. Ultrasonication as anaerobic digestion pretreatment to improve sewage sludge methane production: Performance and microbial characterization. J. Environ. Sci. 2024, 146, 15–27. [Google Scholar] [CrossRef]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ning, Z.; Khalid, H.; Zhang, R.; Liu, G.; Chen, C. Enhancement of methane production from cotton stalk using different pretreatment techniques. Sci. Rep. 2018, 8, 3463. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhang, Y.; Liu, X.; Li, S.; Li, H.; Zhai, Y. Peracetic acid pretreatment improves biogas production from anaerobic digestion of sewage sludge by promoting organic matter release, conversion and affecting microbial community. J. Environ. Manag. 2024, 349, 119427. [Google Scholar] [CrossRef]
- Sarto, S.; Hildayati, R.; Syaichurrozi, I. Effect of chemical pretreatment using sulfuric acid on biogas production from water hyacinth and kinetics. Renew. Energy 2019, 132, 335–350. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R.; Khraisheh, M.A.M.; Shawaqfah, M. Enhancement of biogas production from agricultural wastes via pre-treatment with advanced oxidation processes. Fuel 2019, 253, 964–974. [Google Scholar] [CrossRef]
- Domínguez, D.E.; Ibañez López, M.E.; Mąkinia, J.; Fernández-Morales, F.J.; García Morales, J.L. Impact of nanoparticle addition and ozone pre-treatment on mesophilic methanogenesis in temperature-phased anaerobic digestion. Appl. Sci. 2024, 14, 9504. [Google Scholar] [CrossRef]
- Wang, D.; He, D.; Liu, X.; Xu, Q.; Yang, Q.; Li, X.; Liu, Y.; Wang, Q.; Ni, B.J.; Li, H. The underlying mechanism of calcium peroxide pretreatment enhancing methane production from anaerobic digestion of waste activated sludge. Water Res. 2019, 164, 114934. [Google Scholar] [CrossRef]
- Guo, H.; Tian, L.; Wang, Y.; Wang, Y.; Zheng, K.; Hou, J.; Zhao, Y.; Zhu, T.; Liu, Y. Enhanced anaerobic digestion of waste activated sludge with periodate-based pretreatment. Environ. Sci. Ecotechnol. 2023, 13, 100208. [Google Scholar] [CrossRef]
- Pérez-Pimienta, J.A.; García-López, R.M.; Méndez-Acosta, H.O.; González-Álvarez, V.; Simmons, B.A.; Méndoza-Pérez, J.A.; Arreola-Vargas, J. Ionic liquid-water mixtures enhance pretreatment and anaerobic digestion of Agave bagasse. Ind. Crops Prod. 2021, 171, 113924. [Google Scholar] [CrossRef]
- Li, W.; Xu, G. Enhancement of anaerobic digestion of grass by pretreatment with imidazolium-based ionic liquids. Environ. Technol. 2017, 38, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Marin-Batista, J.D.; Mohedano, A.F.; De la Rubia, M.A. Pretreatment of lignocellulosic biomass with 1-ethyl-3-methylimidazolium acetate for its eventual valorization by anaerobic digestion. Resources 2021, 10, 118. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, P.; Swagatika, S.; Ritesh, D. Microbial Pretreatment of lignocellulosic biomass for enhanced biomethanation and waste management. Biotech 2018, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, W.; Cai, F.; Liu, G.; Chen, C. Anaerobic and microaerobic pretreatment for improving methane production from paper waste in anaerobic digestion. Front. Microbiol. 2021, 12, 688290. [Google Scholar] [CrossRef]
- Cui, L.; Chen, J.; Fei, Q.; Ma, Y. The migration regularity and removal mechanism of antibiotic resistance genes during in situ enzymatic hydrolysis and anaerobic digestion of food waste. Bioresour. Technol. 2023, 385, 129388. [Google Scholar] [CrossRef]
- Demichelis, F.; Robotti, E.; Deorsola, F.A.; Cerruti, S.; Marengo, E.; Tommasi, T.; Fino, D. Technical feasibility and modeling of enzymatic pre-treatments of organic fraction of municipal solid waste to improve anaerobic digestion. J. Clean. Prod. 2024, 476, 143760. [Google Scholar] [CrossRef]
- Talwar, P.; Upadhyay, A.; Verma, N.; Singh, R.; Lindenberger, C.; Pareek, N.; Kovalev, A.A.; Zhuravleva, E.A.; Litti, Y.V.; Masakapalli, S.K.; et al. Utilization of agricultural residues for energy and resource recovery towards a sustainable environment. Environ. Sci. Pollut. Res. 2023, 31, 57354–57368. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Zhang, W.; Hong, Y.; Shen, G.; Wang, W.; Tang, H.; Zhang, S.; Pan, J.; Wang, W. Synergistic effect of two bacterial strains promoting anaerobic digestion of rice straw to produce methane. Environ. Res. 2024, 252, 118974. [Google Scholar] [CrossRef]
- Sahil, S.; Karvembu, P.; Kaur, R.; Katyal, P.; Phutela, U.G. Enhanced biogas production from rice straw through pretreatment with cellulase producing microbial consortium. Energy Nexus 2023, 12, 100246. [Google Scholar] [CrossRef]
- Lalak, J.; Kasprzycka, A.; Martyniak, D.; Tys, J. Effect of biological pretreatment of Agropyron elongatum “BAMAR” on biogas production by anaerobic digestion. Bioresour. Technol. 2016, 200, 194–200. [Google Scholar] [CrossRef]
- Hidalgo, D.; Garrote, L.; Castro, J.; Gómez, M. Influence of cavitation, pelleting, extrusion and torrefaction pretreatments on anaerobic biodegradability of barley straw and vine shoots. Chemosphere 2022, 289, 133165. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.M.; Biak, D.R.A.; Zubaidi, S.L. Microwave-assisted pyrolysis of biomass waste: A mini review. Processes 2020, 8, 1190. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, B.; Zhu, F.; Chen, Q.; Zhou, T.; Wang, Y. Study on the reduction of chlorine and heavy metals in municipal solid waste incineration fly ash by organic acid and microwave treatment and the variation of environmental risk of heavy metals. Sci. Total Environ. 2023, 870, 161929. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Dudek, M.; Dębowski, M.; Markowski, M.; Białobrzewski, I.; Zieliński, M. Influence of microwave thermohydrolysis on biomass digestion. Energies 2025, 18, 1370. [Google Scholar] [CrossRef]
- Nowicka, A.; Zieliński, M.; Dębowski, M.; Dudek, M. Progress in the production of biogas from maize silage after acid-heat pretreatment. Energies 2021, 14, 8018. [Google Scholar] [CrossRef]
- Manickam, S.; Boffito, D.C.; Flores, E.M.M.; Leveque, J.M.; Pflieger, R.; Pollet, B.G.; Ashokkumar, M. Ultrasonics and sonochemistry: Editors’ perspective. Ultrason. Sonochemistry 2023, 99, 106540. [Google Scholar] [CrossRef]
- Pasquier, J.; Paës, G.; Perré, P. Principal factors affecting the yield of dilute acid pretreatment of lignocellulosic biomass: A critical review. Bioresour. Technol. 2023, 369, 128439. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Wang, L.; Cai, L.Y.; Ma, Y.L. Study on inhibitors from acid pretreatment of corn stalk on ethanol fermentation by alcohol yeast. RSC Adv. 2020, 10, 38409–38415. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Du, M.; Yang, J.; Lu, Q.; Fu, Q.; He, D.; Zhao, J.; Wang, D. Peracetic acid promotes biohydrogen production from anaerobic dark fermentation of waste activated sludge. Sci. Total Environ. 2022, 844, 156991. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Kong, C.; Li, W.; Wang, J.; Zang, L. Methane production from wheat straw pretreated with CaO2/cellulase. RSC Adv. 2021, 11, 20541–20549. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, H.; Liang, Y.; Liang, Y.; Cao, Y.; Xiao, Y.; Ma, J. Degradation of bisphenol AF in water by periodate activation with FeS (Mackinawite) and the role of sulfur species in the generation of sulfate radicals. Chem. Eng. J. 2021, 407, 126738. [Google Scholar] [CrossRef]
- Padrino, B.; Lara-Serrano, M.; Morales-Delarosa, S.; Campos-Martín, J.M.; Fierro, J.L.; Martínez, F.; Melero, J.A.; Puyol, D. Resource recovery potential from lignocellulosic feedstock upon lysis with ionic liquids. Front. Bioeng. Biotechnol. 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, J.; Lee, D. Pretreatment of biomass using ionic liquids: Research updates. Renew. Energy 2017, 111, 77–84. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, K.; Zhang, Y.; Wang, M.; Yong, C.; Chen, L.; Qu, P.; Huang, H.; Sun, E.; Pan, M. Lignocellulose dissociation with biological pretreatment towards the biochemical platform: A review. Mater Today Bio 2022, 16, 100445. [Google Scholar] [CrossRef]
- Zhao, Z.T.; Ding, J.; Pang, J.W.; Bao, M.Y.; Luo, G.; Wang, B.Y.; Liu, B.F.; Zhang, L.Y.; Ren, N.Q.; Yang, S.S. Pretreatments of lignocellulosic biomass for biohydrogen biorefinery: Recent progress, techno-economic feasibility and prospectives. Crit. Rev. Environ. Sci. Technol. 2025, 55, 1070–1096. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, S.; Zhou, L.; Feng, L.; Li, Z.; Du, L.; Wei, Y. Improving the biogas potential of rice straw through microwave-assisted ammoniation pretreatment during anaerobic digestion. BioEnergy Res. 2022, 15, 1240–1250. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, J.; Xu, H.; Zhang, N.; Chen, Y.; Yang, J.; Wang, K.; Jiang, J. A Coupling strategy combined with acid-hydrothermal and novel DES pretreatment: Enhancing biomethane yield under solid-state anaerobic digestion and efficiently producing xylo-oligosaccharides and recovered lignin from poplar waste. Int. J. Biol. Macromol. 2024, 274, 133443. [Google Scholar] [CrossRef]
- Zou, X.; Yang, R.; Zhou, X.; Cao, G.; Zhu, R.; Ouyang, F. Effects of mixed alkali-thermal pretreatment on anaerobic digestion performance of waste activated sludge. J. Clean. Prod. 2020, 259, 120940. [Google Scholar] [CrossRef]
- Sousa, A.D.; Lima, A.; Clara, I.; Malveira, C.; Girão, B.H.A.; dos Santos, A.B. Effect of thermo-alkaline pretreatment and substrate inoculum ratio on methane production from dry and semi-dry anaerobic digestion of swine manure. Renew. Energy 2024, 231, 121015. [Google Scholar] [CrossRef]
- Maryam, A.; Badshah, M.; Sabeeh, M.; Jamal, S. Enhancing methane production from dewatered waste activated sludge through alkaline and photocatalytic pretreatment. Bioresour. Technol. 2021, 325, 124677. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Liu, X.; Xu, S.; Chen, M.; Yu, Q.; Xie, J. Combined hydrothermal pretreatment of agricultural and forestry wastes to enhance anaerobic digestion for methane production. Chem. Eng. J. 2024, 486, 150313. [Google Scholar] [CrossRef]
- Debowski, M.; Zielinski, M.; Nowicka, A.; Kazimierowicz, J. Influence of microwave-assisted chemical thermohydrolysis of lignocellulosic waste biomass on anaerobic digestion efficiency. Energies 2024, 17, 4207. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Y.; Chen, H. Enhancing methane production in anaerobic digestion of waste activated sludge by combined thermal hydrolysis and photocatalysis pretreatment. Bioresour. Technol. 2024, 411, 131353. [Google Scholar] [CrossRef]
- Abedi, S.; Khaleghi, M.; Naeimi, A. Enhancing biogas production using ultrasound-assisted thermal pretreatment technology for anaerobic co-digestion of sewage sludge and microalgae substrates. Adv. Environ. Technol. 2024, 10, 171–186. [Google Scholar] [CrossRef]
- Klein, M.; Neel, I.; Perkas, N.; Gedanken, A. Bioethanol production from Ficus religiosa leaves using microwave irradiation. J. Environ. Manag. 2016, 177, 20–25. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Optimization of microwave and NaOH pretreatments of wheat straw for enhancing biofuel yield. Energy Convers. Manag. 2019, 186, 82–92. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, H.; den Boer, E.; Wu, W.; Wang, Q.; Gao, M.; Vo, D.V.; Guo, M.; Xia, C. Effect of microwave/hydrothermal combined ionic liquid pretreatment on straw: Rumen anaerobic fermentation and enzyme hydrolysis. Environ. Res. 2022, 205, 112453. [Google Scholar] [CrossRef]
- Mahdavi, M.; Mirmohammadi, M.; Baghdadi, M.; Mahpishanian, S. Visible light photocatalytic degradation and pretreatment of lignin using magnetic graphitic carbon nitride for enhancing methane production in anaerobic digestion. Fuel 2022, 318, 123600. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, Q.; Wu, X.; Li, X.; Li, Q.; Wang, Y. Interspecies electron transfer in syntrophic methanogenic consortia: From cultures to bioreactors. Renew. Sustain. Energy Rev. 2016, 54, 1358–1367. [Google Scholar] [CrossRef]
- Cazier, E.A.; Trably, E.; Steyer, J.; Escudié, R. Reversibility of hydrolysis inhibition at high hydrogen partial pressure in dry anaerobic digestion processes fed with wheat straw and inoculated with anaerobic granular sludge. Waste Manag. 2019, 85, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Call, D.F. Electron transfer: Mechanisms and applications. Environ. Sci. Process. Impacts 2016, 18, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Wu, S.; Tan, Z.; Yang, C. Enhancing anaerobic digestion process with addition of conductive materials. Chemosphere 2021, 278, 130449. [Google Scholar] [CrossRef]
- Zhang, S.; Chang, J.; Liu, W.; Pan, Y.; Cui, K.; Chen, X.; Huang, X. A novel bioaugmentation strategy to accelerate methanogenesis via adding Geobacter sulfurreducens PCA in anaerobic digestion system. Sci. Total Environ. 2018, 642, 322–326. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Li, Y.; Dang, Y.; Zhu, T.; Quan, X. Potentially shifting from interspecies hydrogen transfer to direct interspecies electron transfer for syntrophic metabolism to resist acidic impact with conductive carbon cloth. Chem. Eng. J. 2017, 313, 10–18. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Z.; Zhang, Y. Using straw as a bio-ethanol source to promote anaerobic digestion of waste activated sludge. Bioresour. Technol. 2019, 286, 121388. [Google Scholar] [CrossRef]
- Lei, Y.; Wei, L.; Liu, T.; Xiao, Y.; Dang, Y.; Sun, D.; Holmes, D.E. Magnetite enhances anaerobic digestion and methanogenesis of fresh leachate from a municipal solid waste incineration plant. Chem. Eng. J. 2018, 348, 992–999. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Wang, C.; Xing, B.; Zhu, S.; Huang, J.; Zhu, L. Biochar facilitates rapid restoration of methanogenesis by enhancing direct interspecies electron transfer after high organic loading shock. Bioresour. Technol. 2021, 320, 124360. [Google Scholar] [CrossRef]
- Yang, S.; Wen, Q.; Chen, Z. Effect of KH2PO4-modified biochar on immobilization of Cr, Cu, Pb, Zn and As during anaerobic digestion of swine manure. Bioresour. Technol. 2021, 339, 125570. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, E.A.; Shekhurdina, S.V.; Laikova, A.; Kotova, I.B.; Loiko, N.G.; Popova, N.M.; Litti, Y.V. Enhanced thermophilic high-solids anaerobic digestion of organic fraction of municipal solid waste with spatial separation from conductive materials in a single reactor volume. J. Environ. Manag. 2024, 363, 121434. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tao, Z.; Yao, F.; Wu, B.; He, L.; Hou, K.; Yang, Q. Enhanced anaerobic co-digestion of waste activated sludge and food waste by sulfidated microscale zerovalent iron: Insights in direct interspecies electron transfer mechanism. Bioresour. Technol. 2020, 316, 123901. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Devi, P.; Patil, A.; Santana, J.C.; Bobowski, J.S.; Hallam, S.J.; Eskicioglu, C. Synthesis and evaluation of metal-impregnated carbon cloth supplementation to improve anaerobic digestion performance from municipal sludge. Chem. Eng. J. 2023, 471, 144164. [Google Scholar] [CrossRef]
- Başar, İ.A.; Eskicioglu, C.; Perendeci, N.A. Biochar and wood ash amended anaerobic digestion of hydrothermally pretreated lignocellulosic biomass for biorefinery applications. Waste Manag. 2022, 154, 350–360. [Google Scholar] [CrossRef]
- Zeynali, R.; Asadi, M.; Ankley, P.; Esser, M.; Brinkmann, M.; Soltan, J.; McPhedran, K. Sustainable enhancement of biogas production from a cold-region municipal wastewater anaerobic digestion process using optimized sludge-derived and commercial biochar additives. J. Clean. Prod. 2024, 478, 143948. [Google Scholar] [CrossRef]
- Li, A.; Zhang, B.; He, Z.W.; Tang, C.C.; Zhou, A.J.; Ren, Y.X.; Li, Z.; Wang, A.; Liu, W. Roles of quorum-sensing molecules in methane from anaerobic digestion aided by biochar. J. Environ. Manag. 2024, 366, 121867. [Google Scholar] [CrossRef]
- Jin, H.Y.; Yang, L.; Ren, Y.X.; Tang, C.C.; Zhou, A.J.; Liu, W.; Li, Z.; Wang, A.; He, Z.W. Insights into the roles and mechanisms of a green-prepared magnetic biochar in anaerobic digestion of waste activated sludge. Sci. Total Environ. 2023, 896, 165170. [Google Scholar] [CrossRef]
- Li, A.; Zhang, B.; Li, W.; Tang, C.C.; Zhou, A.J.; Ren, Y.X.; Li, Z.; Liu, W.; He, Z.W. Quorum-sensing molecules regulate biochar-assisted anaerobic digestion system for methane production: Single-stage vs two-stage digestion. Renew. Energy 2024, 235, 121367. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Ao, Z.; Li, Y.; Zhao, Z.; Zhang, Y. Boosting conversion of waste activated sludge to methane during anaerobic digestion via facilitating direct interspecies electron transfer with glycerol. Renew. Energy 2024, 233, 121176. [Google Scholar] [CrossRef]
- Li, J.; Wu, S.; Zhang, W.; Pan, B.; Hua, M. Enhanced anaerobic digestion for energy recovery from brewery wastewater employing nano zero-valent iron loaded biochar prepared by residual sludge. Chem. Eng. J. 2024, 499, 156466. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, M.; Yan, S.; Yong, X.; Zhang, X.; Awasthi, M.K.; Xi, Y.; Zhou, J. Effects of hydrochar and biogas slurry reflux on methane production by mixed anaerobic digestion of cow manure and corn straw. Chemosphere 2023, 310, 136876. [Google Scholar] [CrossRef] [PubMed]
- Liakos, D.; Altiparmaki, G.; Kalampokidis, A.; Lekkas, D.F.; Vakalis, S. The role of hydrochar on the production of biogas and volatile fatty acids during anaerobic digestion of cheese whey wastewater. Sustain. Chem. Pharm. 2023, 35, 101153. [Google Scholar] [CrossRef]
- Ren, S.; Tong, Z.; Yong, X.; Xi, Y.; Liu, F.; Zhou, J. The new strategies of using nitrogen and iron modified hydrochar to enhance methane production during co-anaerobic digestion of cow manure and corn straw. J. Environ. Chem. Eng. 2024, 12, 114127. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, C.; Sun, M.; Usman, M.; Cui, Y.; Zhang, S.; Ni, B.; Luo, G. Syntrophic propionate degradation in anaerobic digestion facilitated by hydrochar: Microbial insights as revealed by genome-centric metatranscriptomics. Environ. Res. 2024, 261, 119717. [Google Scholar] [CrossRef] [PubMed]
- Bhujbal, S.K.; Joshi, A.; Ghosh, P.; Kumar, V. Investigating role of corn stover biochar supplementation on continuous pilot scale anaerobic digestion: Performance and microbial community dynamics. Bioresour. Technol. 2025, 416, 131767. [Google Scholar] [CrossRef]
- Feng, L.; Mu, H.; Zhao, L.; He, S.; Liu, Y.; Gao, Z.; Hu, T.; Zhao, Q.; Wei, L. Enhancement of biogas production from sludge anaerobic digestion via supplementing magnetic co-pyrolysis biochar: Dosage response and syntrophic metabolism. Environ. Funct. Mater. 2024, 2, 201–212. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Ma, C.; White, J.C.; Zhao, Z.; Duan, C.; Xing, B. Carbon nanomaterials induce residue degradation and increase methane production from livestock manure in an anaerobic digestion system. J. Clean. Prod. 2019, 240, 118257. [Google Scholar] [CrossRef]
- Chan, S.; Nishi, K.; Koyama, M.; Matsuyama, T.; Ida, J. Advanced anaerobic digestion by co-immobilization of anaerobic microbes and conductive particles in hydrogel for enhanced methane production performance. Biochem. Eng. J. 2025, 213, 109563. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Ye, M.; Zha, X.; He, R. Effects of Fe-modified digestate hydrochar at different hydrothermal temperatures on anaerobic digestion of swine manure. Bioresour. Technol. 2024, 395, 130393. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Ren, Z.; Zuo, R.; Zhang, T.; Li, Y.; Wang, Y.; Liu, Z.; Sun, Z.; Han, Y.; et al. Tree-based machine learning model for visualizing complex relationships between biochar properties and anaerobic digestion. Bioresour. Technol. 2023, 374, 128746. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, F.; Denayer, J.F.M.; Mohammadi, A.; Khoshnevisan, B.; Karimi, K. A Critical review on pretreatment and detoxification techniques required for biofuel production from the organic fraction of municipal solid waste. Bioresour. Technol. 2023, 368, 128316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, C.; Ren, H.Y.; Wu, J.T.; Meng, J.; Nan, J.; Ren, N.Q. Feasibility of enhancing hydrogen production from cornstalk hydrolysate anaerobic fermentation by RCPH-biochar. Bioresour. Technol. 2020, 297, 122505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Z.; Ren, H.Y.; Chen, C.; Nan, J.; Cao, G.L.; Ren, N.Q. Residue cornstalk derived biochar promotes direct bio-hydrogen production from anaerobic fermentation of cornstalk. Bioresour. Technol. 2021, 320, 124338. [Google Scholar] [CrossRef]
- Saif, I.; Thakur, N.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Li, X. Biochar assisted anaerobic digestion for biomethane production: Microbial symbiosis and electron transfer. J. Environ. Chem. Eng. 2022, 10, 107960. [Google Scholar] [CrossRef]
- Devi, P.; Eskicioglu, C. Effects of biochar on anaerobic digestion: A review. Environ. Chem. Lett. 2024, 22, 2845–2886. [Google Scholar] [CrossRef]
- Kundu, R.; Kunnoth, B.; Pilli, S.; Pollisetty, V.R.; Tyagi, R.D. Biochar symbiosis in anaerobic digestion to enhance biogas production: A comprehensive review. J. Environ. Manag. 2023, 344, 118743. [Google Scholar] [CrossRef]
- Lou, T.; Yin, Y.; Wang, J. Recent advances in effect of biochar on fermentative hydrogen production: Performance and mechanisms. Int. J. Hydrogen Energy 2024, 57, 315–327. [Google Scholar] [CrossRef]
- He, P.; Zhang, H.; Duan, H.; Shao, L.; Lü, F. Continuity of biochar-associated biofilm in anaerobic digestion. Chem. Eng. J. 2020, 390, 124605. [Google Scholar] [CrossRef]
- Ngo, T.; Ranlaul, K.; Ball, A.S. Enhanced methane production during the anaerobic digestion of chicken manure through the addition of pristine and recovered biochar. Clean. Waste Syst. 2024, 7, 100126. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; He, X.; Liang, D.; Liu, X.; He, C.; Jiao, Y. Investigation into the effects of different recycled magnetic additives on anaerobic co-digestion of sludge and straw. Fuel 2024, 358, 130245. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Y.; Yao, J.; Zheng, X.; Wandera, S.M.; Dong, R.; Li, Y.Y.; Qiao, W. The materials flow and membrane filtration performance in treating the organic fraction of municipal solid waste leachate by a high solid type of submerged anaerobic membrane bioreactor. Bioresour. Technol. 2021, 329, 124927. [Google Scholar] [CrossRef]
- Ma, G.; Chen, Y.; Ndegwa, P. Association between methane yield and microbiota abundance in the anaerobic digestion process: A meta-regression. Renew. Sustain. Energy Rev. 2021, 135, 110212. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Zhan, J.; Zhang, Y.; Zhao, Z.; Zhao, Z. Combining metal-microbe and microbe-microbe dual direct electron transfer on Fe(0)-cathode of bio-electrochemical system to enhance anaerobic digestion of cellulose wastewater. Chin. Chem. Lett. 2022, 33, 3106–3112. [Google Scholar] [CrossRef]
| Name and Location | Feedstock | Digester Type | Biogas Production Potential | Project Start Year | Capacity | Primary Use | Reference |
|---|---|---|---|---|---|---|---|
| ARC Bio Fuel Private Limited (India) | Cow and poultry manure | Continuous stirred tank reactor | 5000 m3/day | 2016 | - | Bio-CNG | Global Methane Initiatives [57] |
| Frantoio Oleario Domenico Cassese (Italy) | Olive oil byproduct | Two-stage bioreactor | Biogas to generate 100 kW of electricity | - | 400 m3 | Combined heat and power | Tamborrino et al. [58] |
| Govind Godham Gaushala (India) | Cattle manure | Floating drum | 150 m3/tank | 2014 | - | Cooking and electricity generation | Global Methane Initiatives [57] |
| Jinhua Kitchen Waste (China) | Kitchen waste | Continuous stirred tank reactor | 3944 m3/day | 2016 | 1200 m3 | Electricity generation | IEA [59] |
| Kern Cluster (USA) | Manure and food waste | Covered lagoons | 5 million diesel gallons equivalents | 2013 | - | Electricity generation, injection into natural gas pipelines, and Bio-CNG | Global Methane Initiatives [57] |
| Lily Group (China) | Pigment wastewater | Up-flow anaerobic sludge bed | 2.7 million m3/year | 2021 | 1500 m3 per reactor | Injected into natural gas pipelines | IEA [60] |
| Noblehurst Farms (USA) | Manure, food waste, whey, and process water | Mixed bioreactor | 432,000 ft3/day | 2015 | 1,336,710 gallons | Combined heat and power | USEPA [61] |
| W. Hamburger Facility (Austria) | Wastewater | Up-flow anaerobic sludge blanket | 17,600 Nm3/day | 2016 | 3500 m3 | Combined heat and power | IEA [62] |
| Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Physical pretreatment methods | |||
| Size reduction |
|
| Kim et al. [83] |
| Drying |
|
| Lytras et al. [84] |
| Microwave |
|
| Arman et al. [85] |
| Ultrasonication |
|
| Zhao et al. [86] |
| Chemical pretreatment methods | |||
| Acid and alkali |
|
| Nanda et al. [87] |
| Oxidizing agents |
|
| Gomes and Lucas [88] |
| Ionic liquids |
|
| Pérez-Pimienta et al. [89] |
| Biological pretreatment technologies | |||
| Bacterial or fungal pretreatment |
|
| Rani and Dhoble [90] |
| Enzymatic saccharification |
|
| Xiu et al. [91] |
| Integrated pretreatment methods | |||
| Physical, chemical, and biological pretreatment |
|
| Du et al. [92] |
| Feedstock | Reaction Conditions | Main Observations | Reference |
|---|---|---|---|
| Pretreatment: Extrusion | |||
| Cassava starch and sugarcane bagasse |
|
| Fasheun et al. [93] |
| Food waste |
|
| Kong et al. [94] |
| Organic fraction of municipal solid waste |
|
| Mu et al. [95] |
| Pretreatment: Microwave | |||
| Food and lipid waste |
|
| Yue et al. [96] |
| Food waste |
|
| Liu et al. [97] |
| Fruit and vegetable waste |
|
| Agrawal et al. [98] |
| Slaughterhouse sludge |
|
| Arman et al. [85] |
| Pretreatment: Ultrasonication | |||
| Cow dung |
|
| Xu et al. [99] |
| Sewage sludge |
|
| Zhao et al. [100] |
| Waste active sludge |
|
| Zhao et al. [86] |
| Feedstock | Reaction Conditions | Main Observations | Reference |
|---|---|---|---|
| Pretreatment: Acids and bases | |||
| Cassava pulp |
|
| Lomwongsopon and Aramrueang [19] |
| Cotton stalk |
|
| Zhang et al. [102] |
| Sludge |
|
| Ren et al. [103] |
| Water hyacinth |
|
| Sarto et al. [104] |
| Pretreatment: Oxidizing agents | |||
| Agricultural residues |
|
| Almomani et al. [105] |
| Digestate |
|
| Domínguez et al. [106] |
| Waste-activated sludge |
|
| Wang et al. [107] |
| Waste-activated sludge |
|
| Guo et al. [108] |
| Pretreatment: Ionic liquids | |||
| Agave bagasse |
|
| Pérez-Pimienta et al. [109] |
| Grass (Axonopus compressus) |
|
| Li and Xu [110] |
| Wheat straw, barley straw, and grape stem |
|
| Marin-batista et al. [111] |
| Feedstock | Reaction Conditions | Main Observations | Reference |
|---|---|---|---|
| Barley straw and natural meadow hay straw |
|
| Raut et al. [24] |
| Corrugated board and tissue paper |
|
| Song et al. [113] |
| Food waste |
|
| Cui et al. [114] |
| Organic fraction of municipal solid waste |
|
| Demichelis et al. [115] |
| Pearl millet, wheat, and rice straw |
|
| Talwar et al. [116] |
| Rice straw |
|
| Wang et al. [117] |
| Rice straw |
|
| Rani and Dhoble [90] |
| Rice straw |
|
| Sahil et al. [118] |
| Tall wheat grass (Agropyron elongatum) |
|
| Lalak et al. [119] |
| Pretreatment | Reaction Conditions | Main Observations | Reference |
|---|---|---|---|
| Acid-hydrothermal and deep eutectic solvents |
|
| Xie et al. [137] |
| Alkali and thermal pretreatment |
|
| Zou et al. [138] |
| Alkali and thermal pretreatment |
|
| Sousa et al. [139] |
| Alkaline and photocatalytic pretreatment |
|
| Maryam et al. [140] |
| Hydrothermal pretreatment and co-hydrothermal pretreatment |
|
| Zou et al. [141] |
| Microwave-assisted ammonization |
|
| Liu et al. [136] |
| Microwave-assisted chemical thermohydrolysis |
|
| Debowski et al. [142] |
| Thermal hydrolysis and photocatalysis |
|
| Chen et al. [143] |
| Ultrasound-assisted thermal pretreatment |
|
| Abedi et al. [144] |
| Feedstock | Additive Properties | Gas Yield | Reference |
|---|---|---|---|
| Activated sludge |
| Nearly 70% increase in biomethane yield | Zeynali et al. [164] |
| Activated sludge |
| Nearly 52% increase in biomethane yield | Li et al. [165] |
| Activated sludge |
| Nearly 22% increase in biomethane yield | Jin et al. [166] |
| Activated sludge |
| Nearly 42% increase in biomethane yield (135 mL/g) | Li et al. [167] |
| Activated sludge |
| Nearly 1.3-fold increase in biomethane yield | Wang et al. [168] |
| Brewing wastewater and sludge |
| Nearly 1.4-fold increase in biomethane yield (212 mL) | Li et al. [169] |
| Cattle manure and corn straw |
| Nearly 34% increase in biomethane yield | Yang et al. [170] |
| Cheese whey wastewater and wine sludge |
| Biomethane yield of 130–140 mL/g VS | Liakos et al. [171] |
| Cow manure and corn straw |
| Nearly 36% increase in biomethane yield | Ren et al. [172] |
| Propionate |
| Nearly 57% increase in biomethane yield | Shi et al. [173] |
| Rice straw |
| Nearly 37% increase in biomethane yield of 230 L/kg VS | Bhujbal et al. [174] |
| Sewage sludge |
| Biomethane yield of 144 mL/g VS | Feng et al. [175] |
| Sheep manure |
| Nearly 34% increase in biomethane yield | Hao et al. [176] |
| Sludge |
| Nearly 1.3 times increase in biomethane yield | Chan et al. [177] |
| Swine manure |
| Biogas yield of 265 mL/g TS | Shen et al. [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahil, S.; Nanda, S. Process Intensification of Anaerobic Digestion of Biowastes for Improved Biomethane Production: A Review. Sustainability 2025, 17, 6553. https://doi.org/10.3390/su17146553
Sahil S, Nanda S. Process Intensification of Anaerobic Digestion of Biowastes for Improved Biomethane Production: A Review. Sustainability. 2025; 17(14):6553. https://doi.org/10.3390/su17146553
Chicago/Turabian StyleSahil, Sahil, and Sonil Nanda. 2025. "Process Intensification of Anaerobic Digestion of Biowastes for Improved Biomethane Production: A Review" Sustainability 17, no. 14: 6553. https://doi.org/10.3390/su17146553
APA StyleSahil, S., & Nanda, S. (2025). Process Intensification of Anaerobic Digestion of Biowastes for Improved Biomethane Production: A Review. Sustainability, 17(14), 6553. https://doi.org/10.3390/su17146553







