Sustainable Lightweight Aggregates from Diatomite Residue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
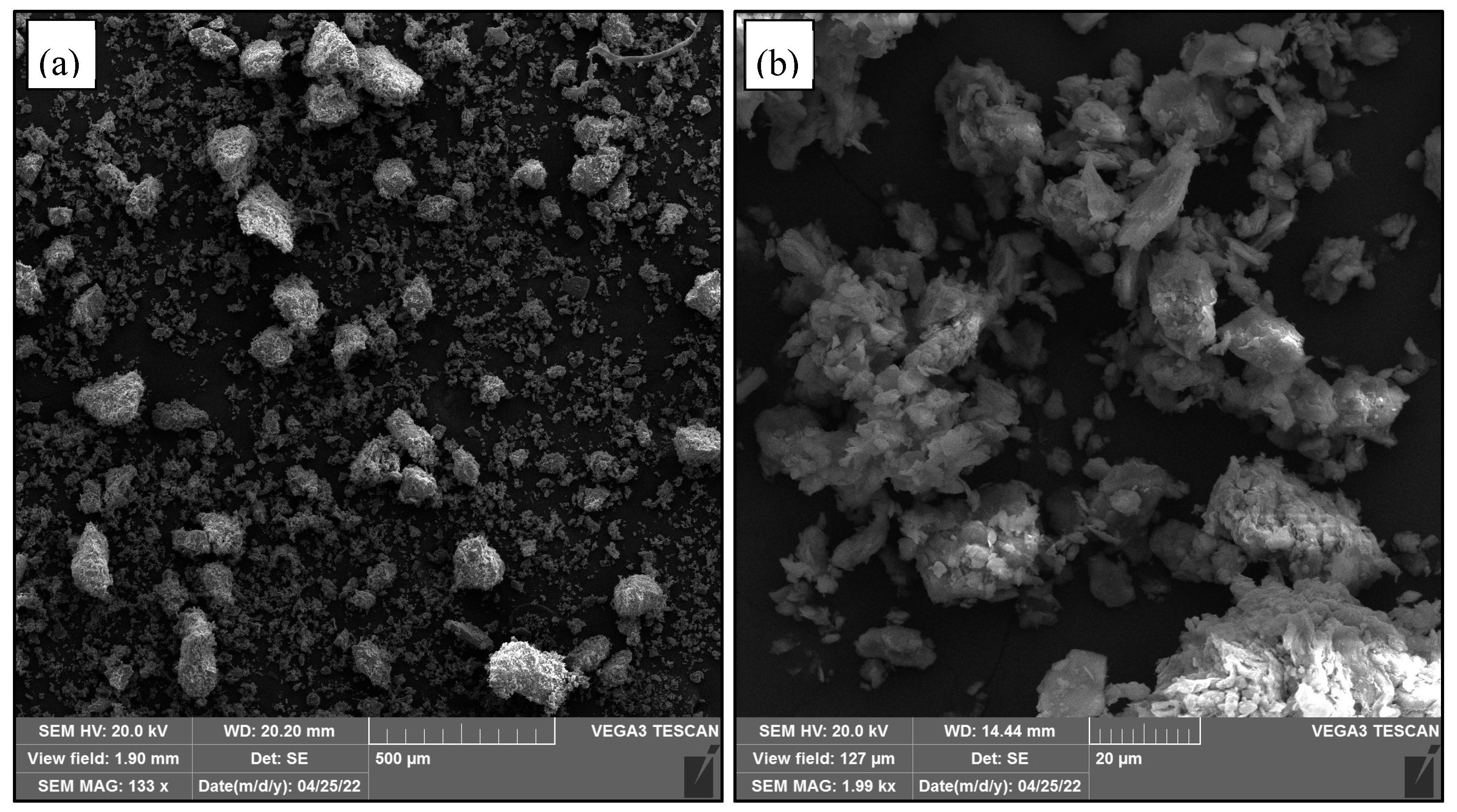
Characterisation of Raw Materials
2.2. Methods
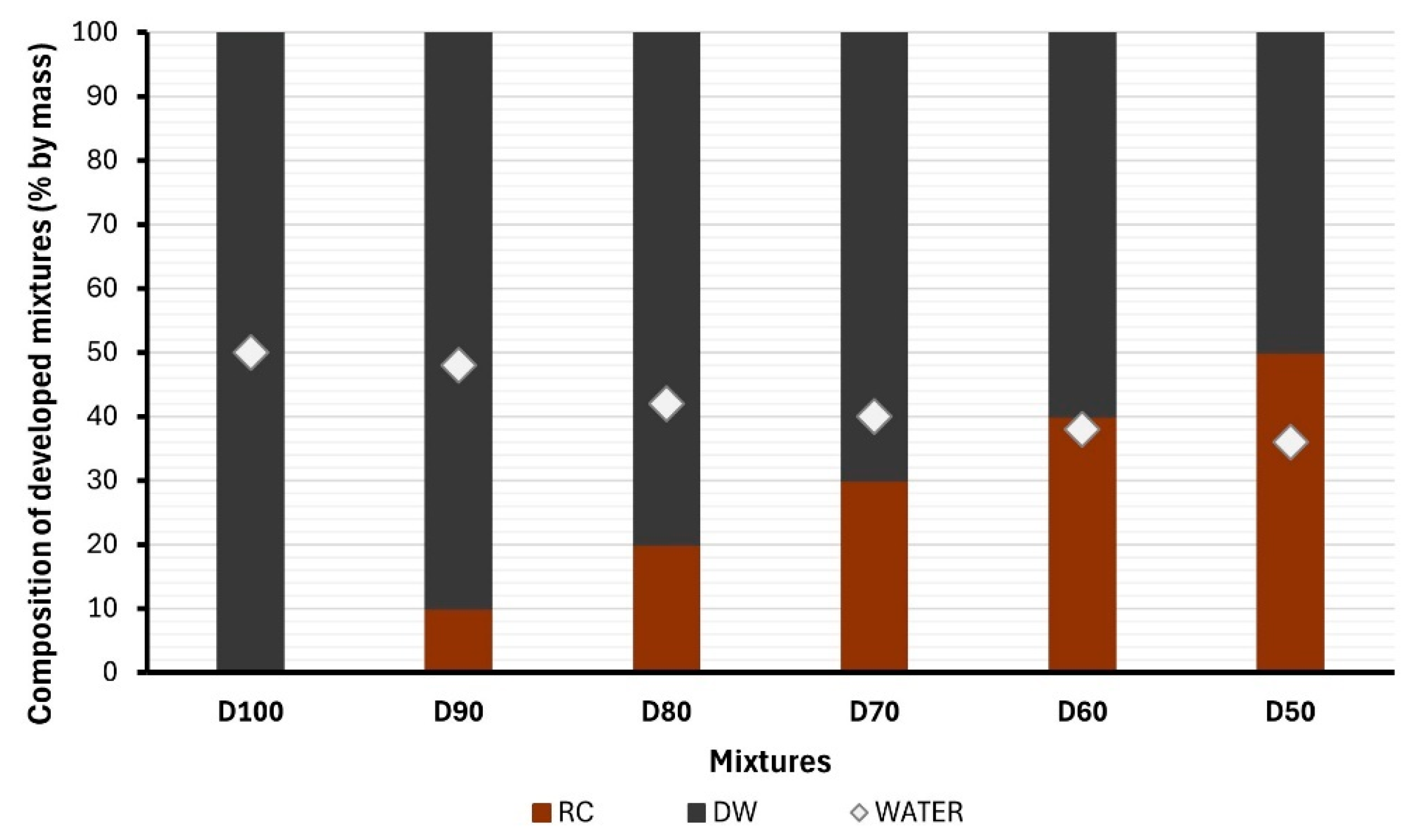
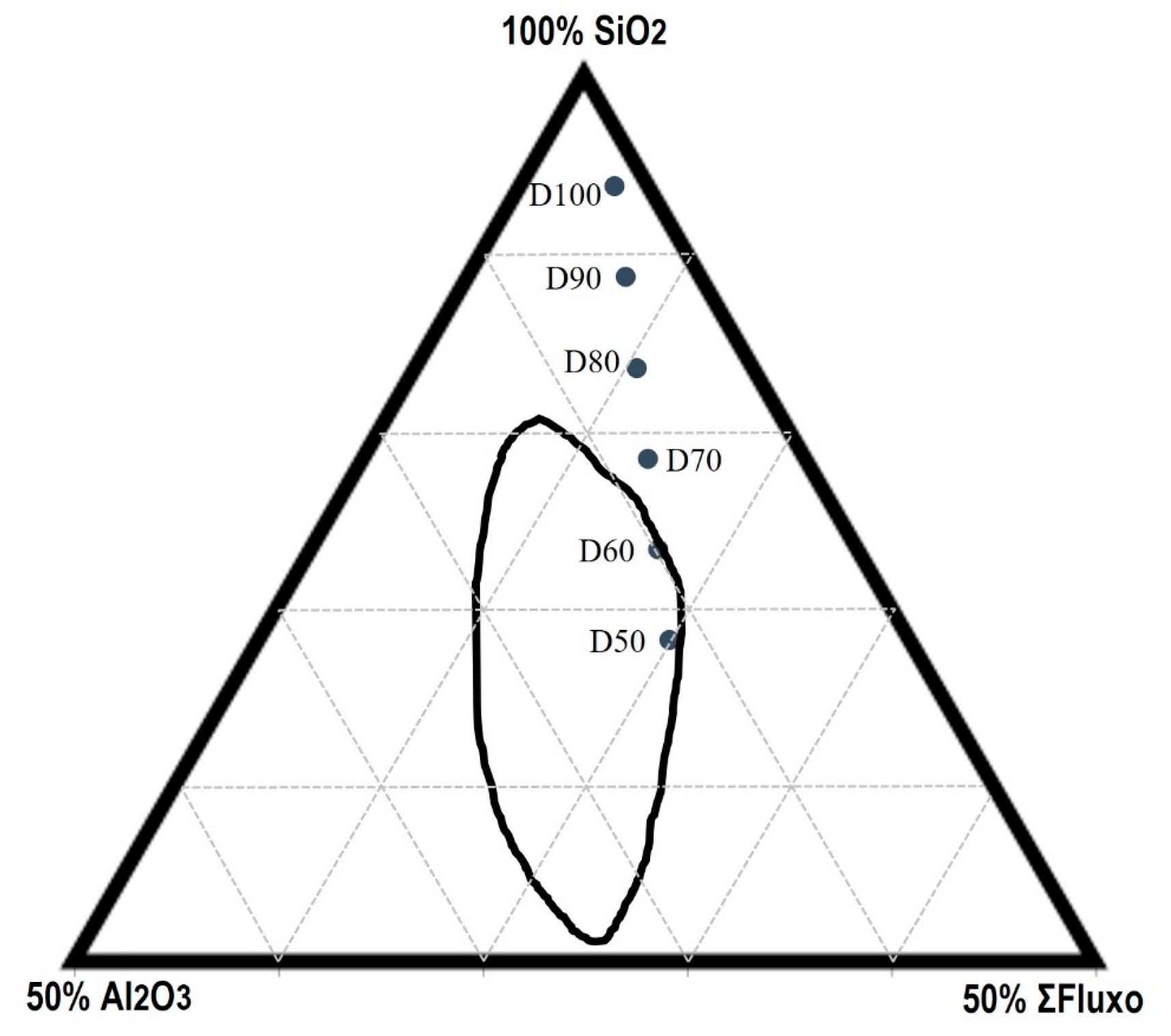
2.2.1. Mixtures Composition
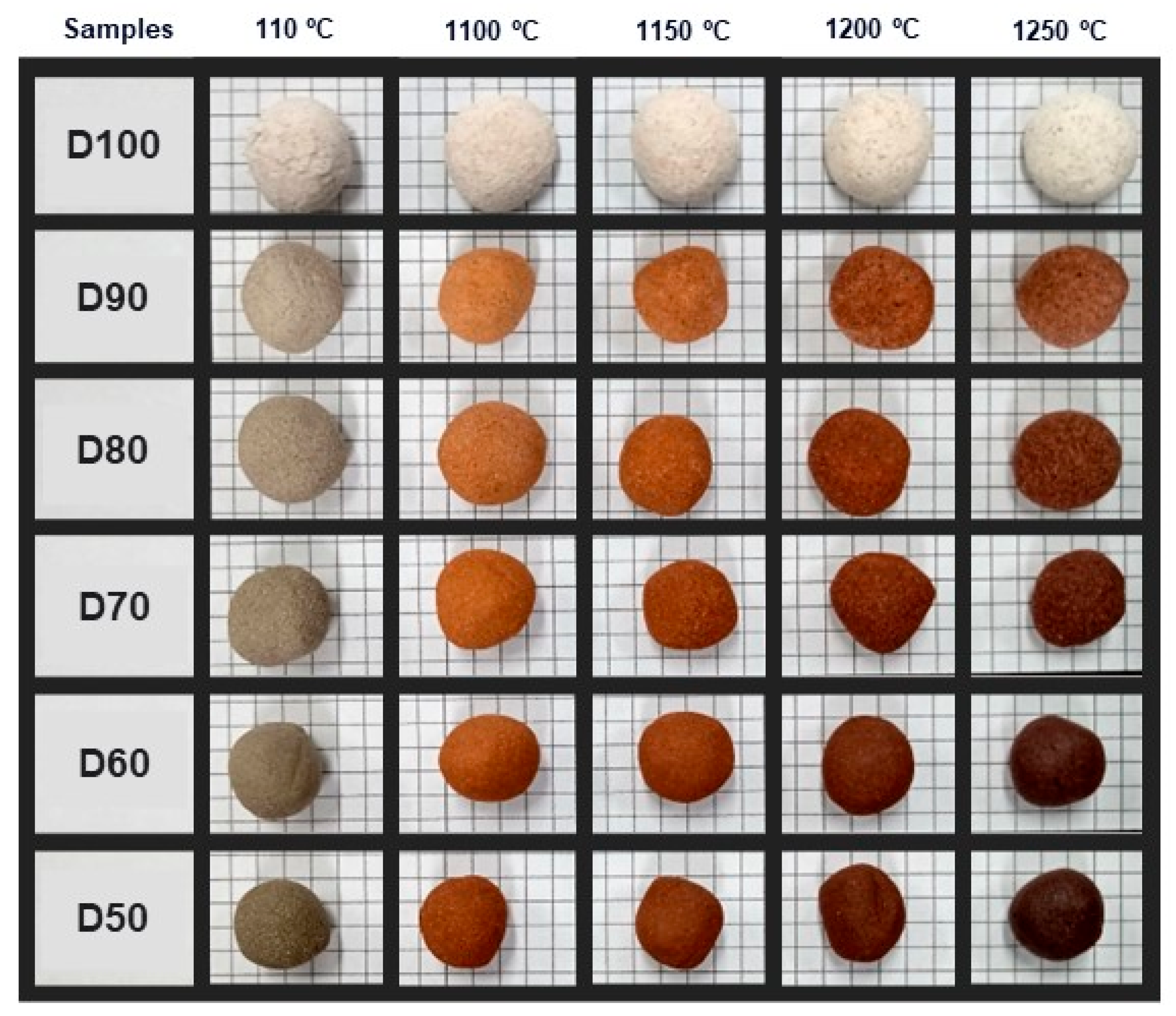
2.2.2. Manufacture of LWA
2.2.3. Characterisation of Lightweight Aggregates
2.2.4. Commercial Potential of the Formulations
3. Results and Discussion
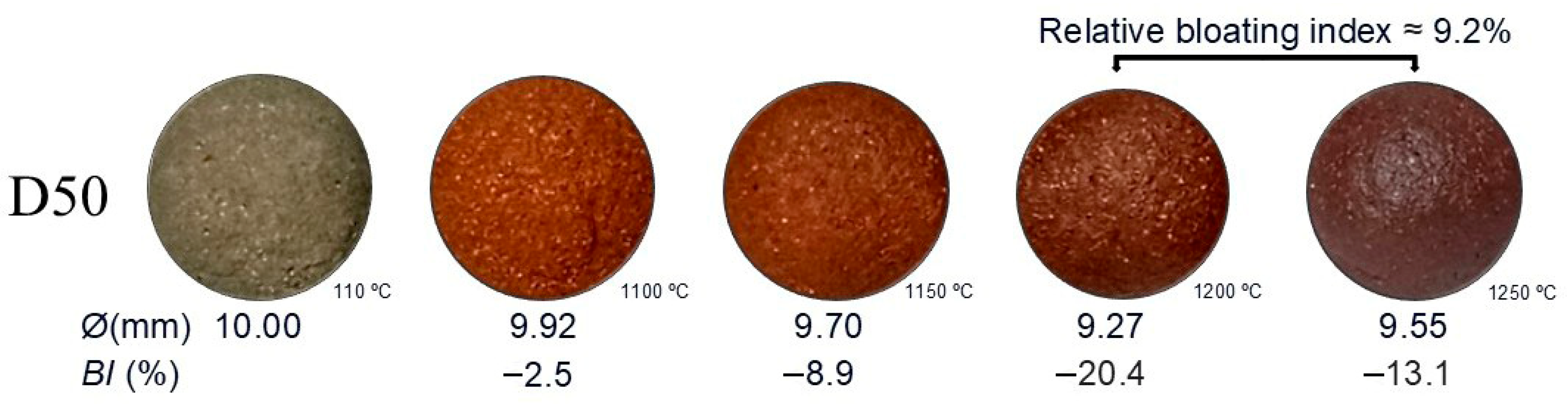
3.1. Bloating Index (BI)
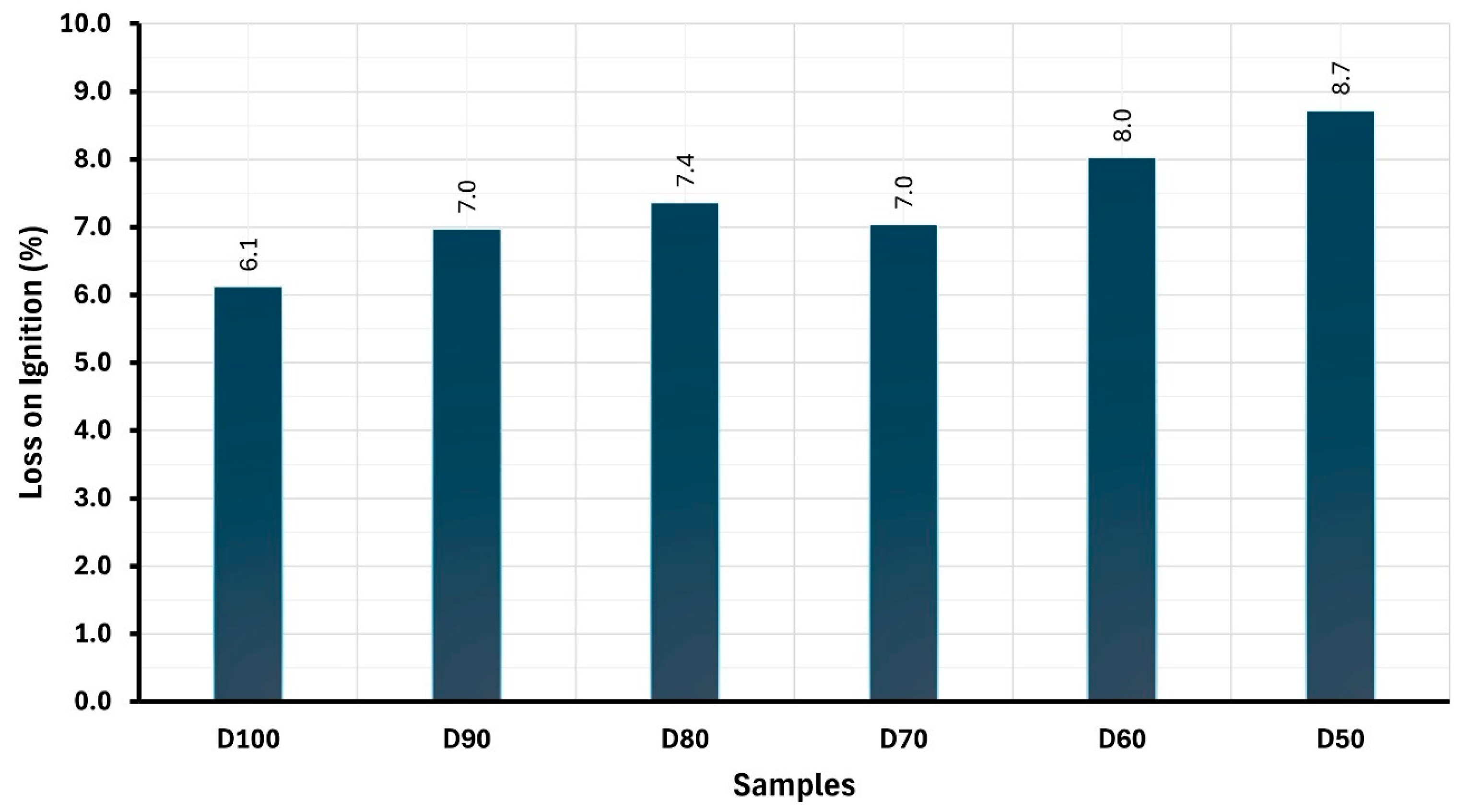
3.2. Loss of Ignition (LOI)
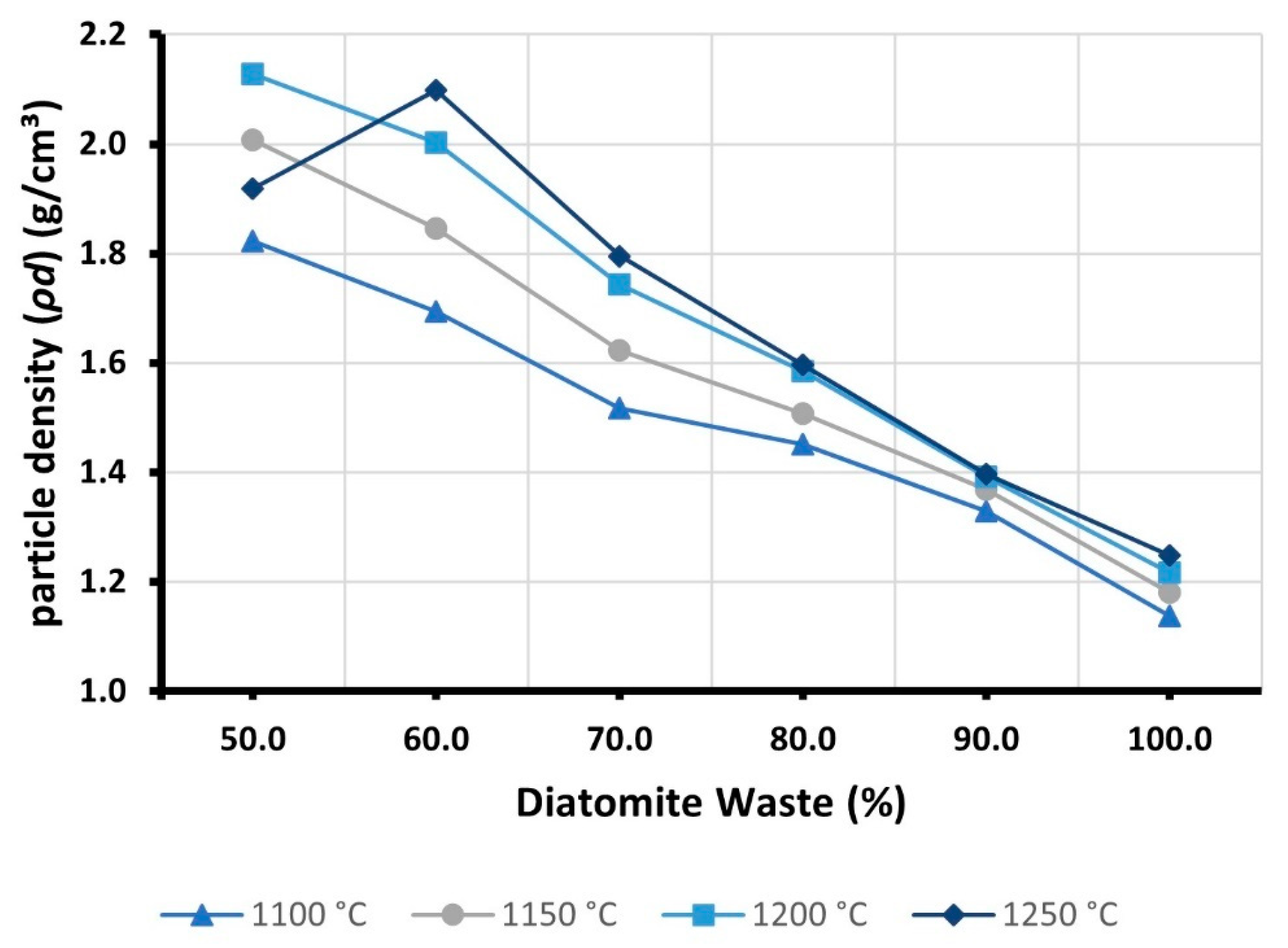
3.3. Density (ρd)
3.4. Closed Porosity
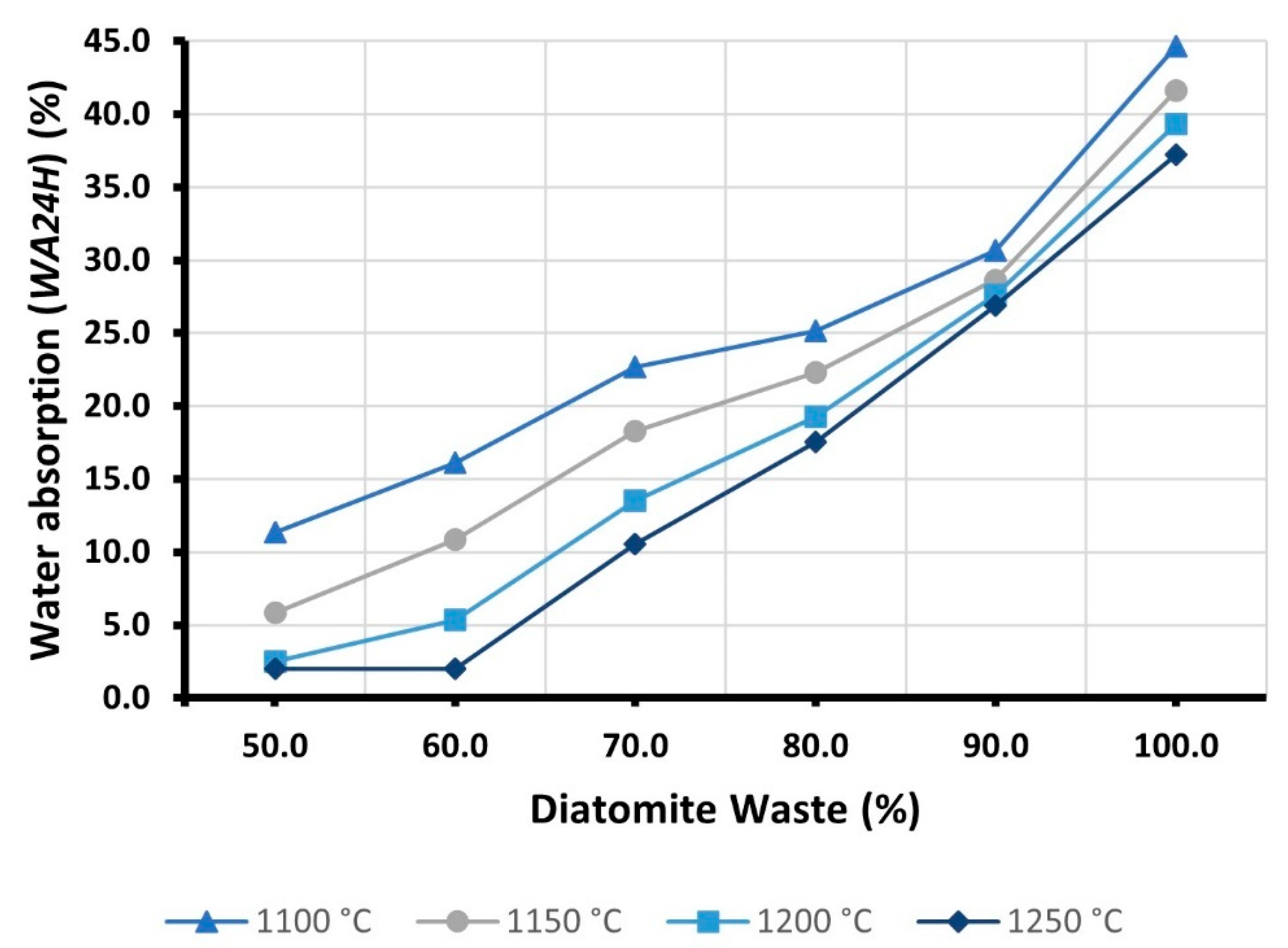
3.5. Water Absorption (WA24H)
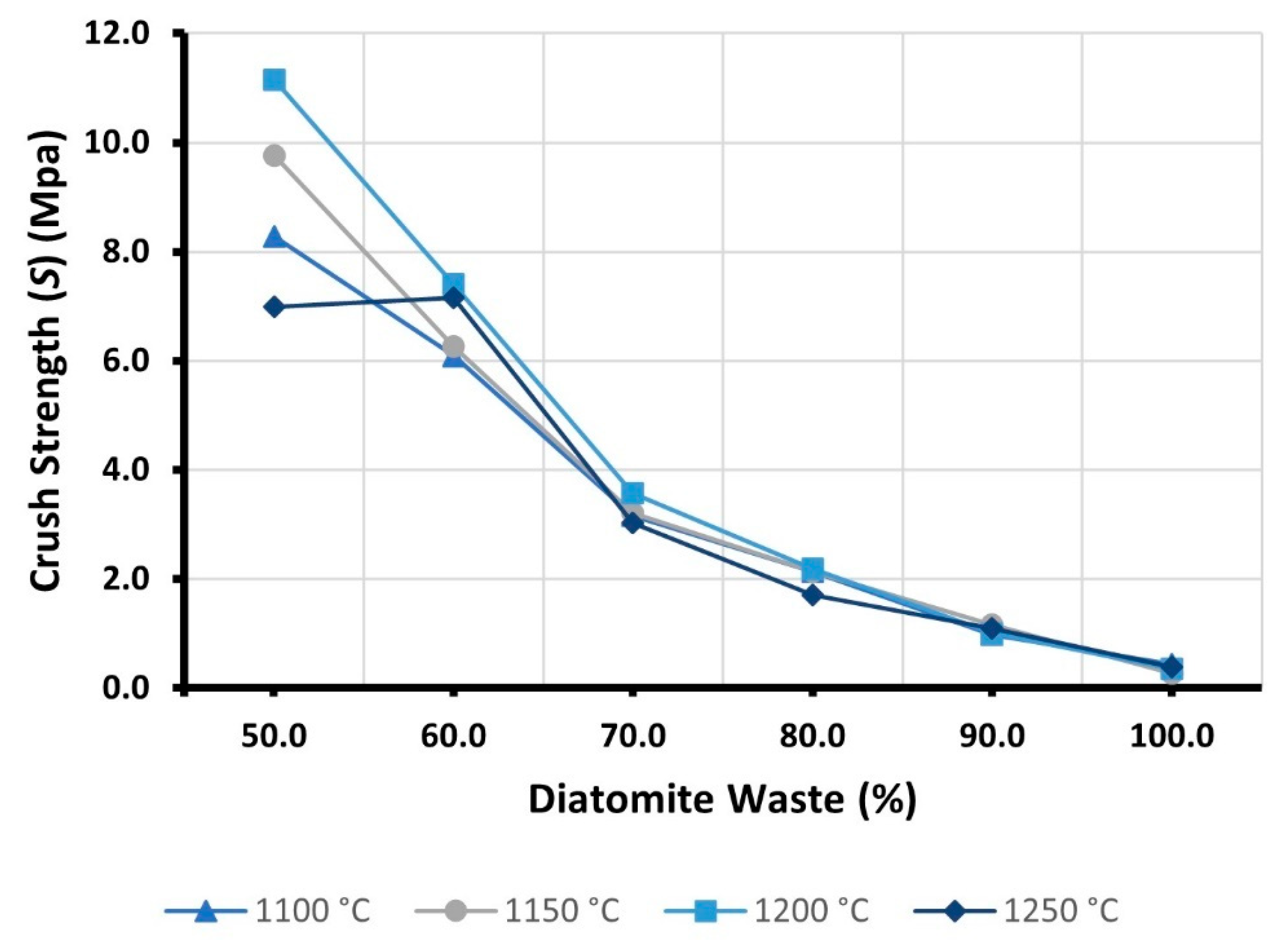
3.6. Crushing Strength (S)
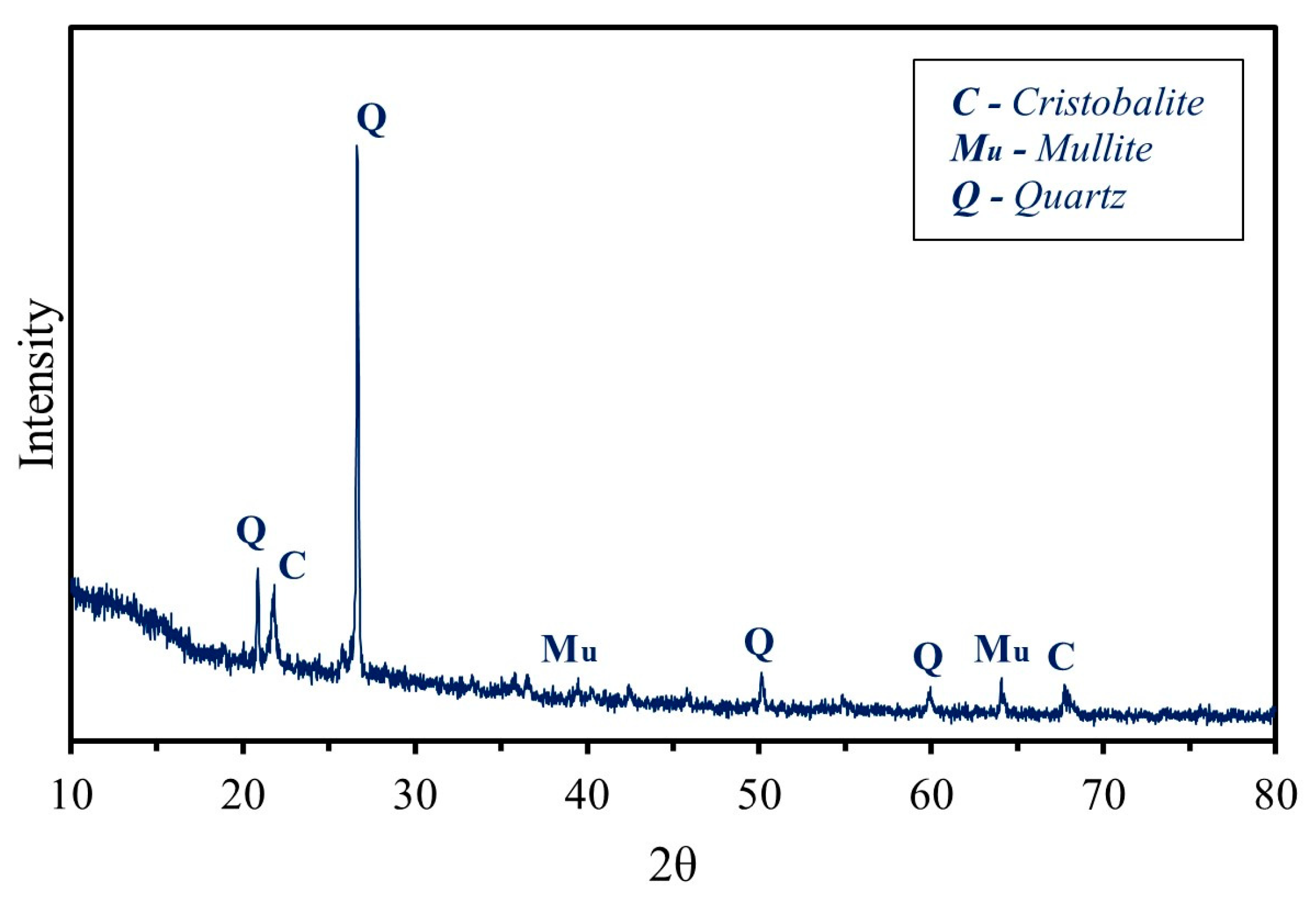
3.7. Mineralogical Composition
3.8. Microstructure
3.9. Commercial Potential of the Formulations
4. Conclusions
- To manufacture LWAs with good physical and mechanical properties, it is recommended that the addition of DW to the mixtures be limited to levels of less than 90%. In samples richer in diatomite residue, the effect of DW on the physical and mechanical characteristics of LWAs is more significant than the influence of the firing method adopted. Regardless of the sintering temperature applied, all the samples developed with 100% DW had a crushing strength of less than 1.0 MPa, preventing these specimens from being recommended for any commercial application.
- The high porosity of DW was found to affect the particle density of LWAs. Typically, the partial replacement of RC with DW resulted in a gradual reduction of ρd. On the other hand, the addition of DW also increased open porosity, negatively affecting the strength and water absorption of the samples. At 1250 °C, when the DW content was increased from 50 to 100 percent, the particle density decreased by almost 35 percent. However, S was reduced by nearly 95%, while WA24H was increased by more than 1200%.
- In many cases, the LWAs manufactured had physical and mechanical properties higher than the values required by national and international standards, as well as the results found in commercial LWAs. In total, 16 of the 24 specimens manufactured met the criteria for particle density, crushing strength, and water absorption indices required for using LWAs in commercial applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Equations Used for the Characterisation of Lightweight Aggregates
Appendix A.1. Bloating Index
Appendix A.2. Actual Density and Porosity
Appendix A.3. Crushing Strength
Appendix B. Relative Bloating Index of the Other Mixtures
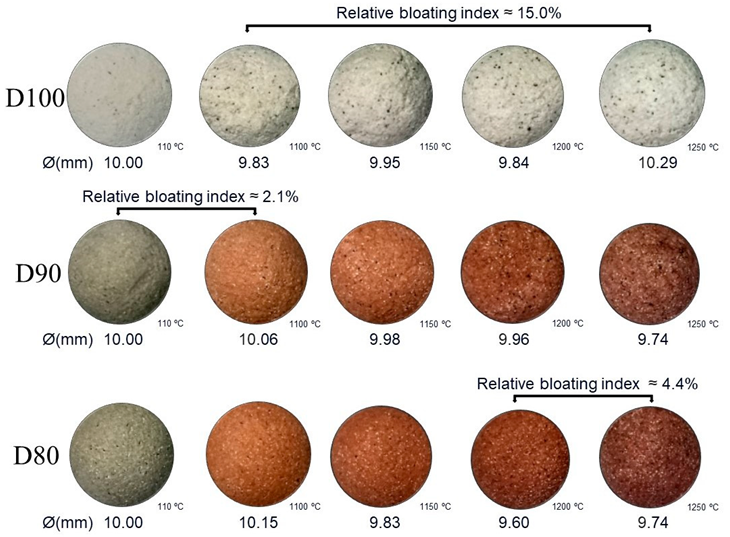
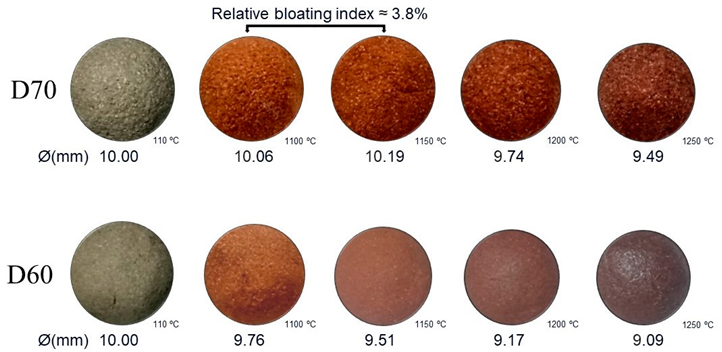
Appendix C. Real Density and Dry Density of the Mixtures
| Samples | Real Density | Dry Density (g/cm3) | ||||||
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| D100 | 2.48 | 2.47 | 2.47 | 2.46 | 2.31 | 2.32 | 2.34 | 2.33 |
| D90 | 2.49 | 2.49 | 2.49 | 2.47 | 2.24 | 2.25 | 2.26 | 2.24 |
| D80 | 2.50 | 2.51 | 2.50 | 2.49 | 2.29 | 2.27 | 2.28 | 2.22 |
| D70 | 2.51 | 2.53 | 2.52 | 2.50 | 2.31 | 2.31 | 2.28 | 2.21 |
| D60 | 2.52 | 2.55 | 2.53 | 2.52 | 2.33 | 2.31 | 2.24 | 2.19 |
| D50 | 2.53 | 2.56 | 2.55 | 2.53 | 2.30 | 2.27 | 2.25 | 2.00 |
Appendix D. Total Porosity (pt) and Open Porosity (po) of Mixtures
| Samples | Total Porosity (%) | Open Porosity (%) | ||||||
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| D100 | 54.09 | 52.26 | 50.71 | 49.18 | 50.81 | 49.15 | 47.92 | 46.43 |
| D90 | 46.56 | 45.06 | 43.99 | 43.49 | 40.76 | 39.21 | 38.51 | 37.51 |
| D80 | 41.91 | 39.91 | 36.66 | 35.78 | 36.50 | 33.62 | 30.57 | 28.06 |
| D70 | 39.57 | 35.77 | 30.71 | 28.26 | 34.43 | 29.68 | 23.58 | 18.94 |
| D60 | 32.82 | 27.50 | 20.91 | 16.69 | 27.27 | 20.04 | 10.70 | 4.25 |
| D50 | 28.00 | 21.67 | 16.44 | 24.32 | 20.69 | 11.70 | 5.32 | 3.88 |
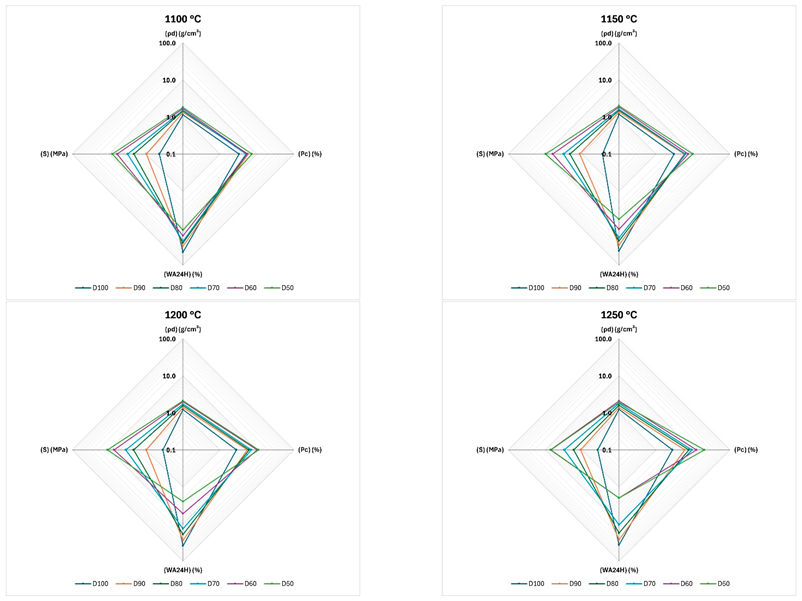
Appendix E. Radar Charts of Normalised Physical and Mechanical Properties at Each Sintering Temperature
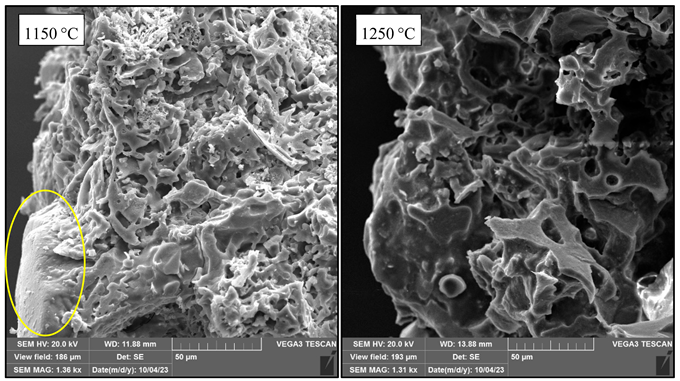
Appendix F. Micrographs of D70 at 1150 °C and 1250 °C Highlighting the Evolution of Diatomite Particles
Appendix G. Workflow Diagram of the Experimental Procedures and Key Outcomes
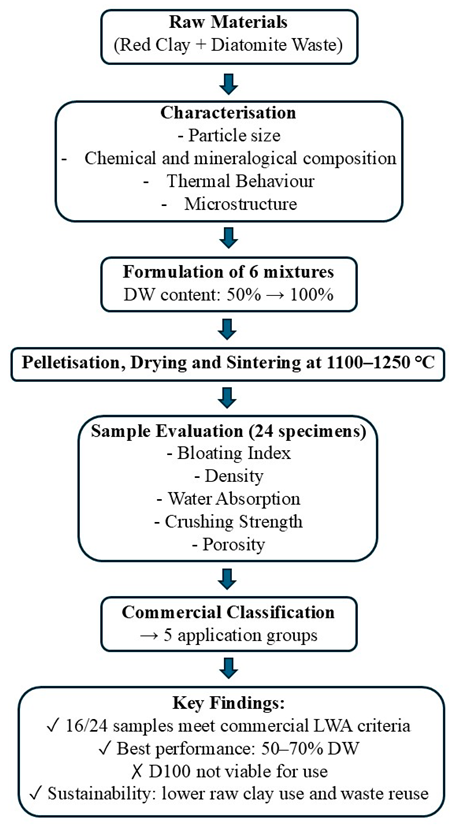
References
- NBR NM 35; Lightweight Aggregates for Structural Concrete—Specifications. ABNT: Rio de Janeiro, Brazil, 1995.
- NBR 9935; Aggregates—Terminology. ABNT: Rio de Janeiro, Brazil, 2011.
- Chandra, S.; Berntsson, L. Lightweight Aggregate Concrete; Elsevier: Amsterdam, The Netherlands, 2002; ISBN 081551820X. [Google Scholar]
- Dondi, M.; Cappelletti, P.; D’Amore, M.; de Gennaro, R.; Graziano, S.F.; Langella, A.; Raimondo, M.; Zanelli, C. Lightweight Aggregates from Waste Materials: Reappraisal of Expansion Behavior and Prediction Schemes for Bloating. Constr. Build. Mater. 2016, 127, 394–409. [Google Scholar] [CrossRef]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M. Production of Lightweight Aggregates from Mining and Industrial Wastes. J. Environ. Manag. 2009, 90, 2801–2812. [Google Scholar] [CrossRef] [PubMed]
- Ducman, V.; Mladenovič, A.; Šuput, J.S. Lightweight Aggregate Based on Waste Glass and Its Alkali–Silica Reactivity. Cem. Concr. Res. 2002, 32, 223–226. [Google Scholar] [CrossRef]
- Rossignolo, J.A. Structural Lightweight Concrete: Production, Properties, Microstructure and Applications; PINI: São Paulo, Brazil, 2009; ISBN 9788572662208. [Google Scholar]
- Chung, S.-Y.; Sikora, P.; Kim, D.J.; El Madawy, M.E.; Abd Elrahman, M. Effect of Different Expanded Aggregates on Durability-Related Characteristics of Lightweight Aggregate Concrete. Mater. Charact. 2021, 173, 110907. [Google Scholar] [CrossRef]
- Lau, P.C.; Teo, D.C.L.; Mannan, M.A. Mechanical, Durability and Microstructure Properties of Lightweight Concrete Using Aggregate Made from Lime-Treated Sewage Sludge and Palm Oil Fuel Ash. Constr. Build. Mater. 2018, 176, 24–34. [Google Scholar] [CrossRef]
- de Souza, M.M. Development of Lightweight Aggregates from Scheelite Waste, Sewage Sludge and Rice Husk Ash. Master’s Dissertation, Federal University of Rio Grande do Norte, Natal, Brasil, 2019. [Google Scholar]
- EN 13055-1; Lightweight Aggregates-Part 1: Lightweight Aggregates for Concrete, Mortar and Grout. British Standards Institution: London, UK, 2002; pp. 1–40.
- Cheeseman, C.R.; Makinde, A.; Bethanis, S. Properties of Lightweight Aggregate Produced by Rapid Sintering of Incinerator Bottom Ash. Resour. Conserv. Recycl. 2005, 43, 147–162. [Google Scholar] [CrossRef]
- Ayati, B.; Ferrándiz-Mas, V.; Newport, D.; Cheeseman, C. Use of Clay in the Manufacture of Lightweight Aggregate. Constr. Build. Mater. 2018, 162, 124–131. [Google Scholar] [CrossRef]
- Quina, M.J.; Almeida, M.A.; Santos, R.; Bordado, J.M.; Quinta-Ferreira, R.M. Compatibility Analysis of Municipal Solid Waste Incineration Residues and Clay for Producing Lightweight Aggregates. Appl. Clay Sci. 2014, 102, 71–80. [Google Scholar] [CrossRef]
- Soltan, A.M.M.; Kahl, W.-A.; Abd EL-Raoof, F.; Abdel-Hamid El-Kaliouby, B.; Abdel-Kader Serry, M.; Abdel-Kader, N.A. Lightweight Aggregates from Mixtures of Granite Wastes with Clay. J. Clean. Prod. 2016, 117, 139–149. [Google Scholar] [CrossRef]
- Świerczek, L.; Cieślik, B.M.; Konieczka, P. The Potential of Raw Sewage Sludge in Construction Industry—A Review. J. Clean. Prod. 2018, 200, 342–356. [Google Scholar] [CrossRef]
- Lyra, G.P.; dos Santos, V.; De Santis, B.C.; Rivaben, R.R.; Fischer, C.; Pallone, E.M.d.J.A.; Rossignolo, J.A. Reuse of Sugarcane Bagasse Ash to Produce a Lightweight Aggregate Using Microwave Oven Sintering. Constr. Build. Mater. 2019, 222, 222–228. [Google Scholar] [CrossRef]
- Souza, M.M.; Anjos, M.A.S.; Sá, M.V.V.A. Using Scheelite Residue and Rice Husk Ash to Manufacture Lightweight Aggregates. Constr. Build. Mater. 2021, 270, 121845. [Google Scholar] [CrossRef]
- Eom, J.Y.; Yang, S.J.; Lee, M.J.; Yang, Y.R.; Wie, Y.M.; Lee, K.G.; Lee, K.H. Recycling Fly Ash into Lightweight Aggregate: Life Cycle Assessment and Economic Evaluation of Waste Disposal. Sustainability 2024, 16, 9271. [Google Scholar] [CrossRef]
- Kwek, S.Y.; Awang, H. Utilisation of Recycled Silt from Water Treatment and Palm Oil Fuel Ash as Geopolymer Artificial Lightweight Aggregate. Sustainability 2021, 13, 6091. [Google Scholar] [CrossRef]
- Lim, Y.C.; Chen, C.-F.; Chen, C.-W.; Dong, C.-D. Valorization of Dredged Harbor Sediments through Lightweight Aggregate Production: Application of Waste Oyster Shells. Sustainability 2023, 15, 5466. [Google Scholar] [CrossRef]
- Mendonça de Souza, M.; Barbosa, N.P.; dos Anjos, M.A.S.; Aguiar, J.G.C.; da Silva Neto, J.A.; Pederneiras, C.M. Recycling Red Ceramic Waste as a Raw Material for Lightweight Aggregates. Appl. Sci. 2025, 15, 5729. [Google Scholar] [CrossRef]
- Sousa, J.T.F.d.; Anjos, M.A.S.d.; Neto, J.A.d.S.; Farias, E.C.d.; Branco, F.G.; Maia Pederneiras, C. Self-Compacting Concrete with Artificial Lightweight Aggregates from Sugarcane Ash and Calcined Scheelite Mining Waste. Appl. Sci. 2025, 15, 452. [Google Scholar] [CrossRef]
- da Silva Neto, J.A.; dos Anjos, M.A.S.; Dutra, R.P.S.; Mendonça de Souza, M.; Pederneiras, C.M. Development of Sustainable Artificial Lightweight Aggregates with Binary Mixtures of Waste Rich in Aluminosilicate and Carbonate in Kaolinitic Clay. Sustainability 2025, 17, 2017. [Google Scholar] [CrossRef]
- Singh, N.; Raza, J.; Colangelo, F.; Farina, I. Advancements in Lightweight Artificial Aggregates: Typologies, Compositions, Applications, and Prospects for the Future. Sustainability 2024, 16, 9329. [Google Scholar] [CrossRef]
- Muñoz-Vélez, M.F.; Salazar-Serna, K.; Escobar-Torres, D.; Rojas-Manzano, M.A.; Gómez-Gómez, A.; Maury-Ramírez, A. Circular Economy: Adding Value to the Post-Industrial Waste through the Transformation of Aluminum Dross for Cement Matrix Applications. Sustainability 2023, 15, 13952. [Google Scholar] [CrossRef]
- Rezende, D.A.; Fumagalli, L.A.W.; Bartling, H.; Okon, G.U.; Gallego, A.R. Sustainable City Strategies for Strategic Digital City Project in the Sustainable Development Goals (SDGs) Context. Land 2025, 14, 1195. [Google Scholar] [CrossRef]
- Javed, M.H.; Ahmad, A.; Rehan, M.; Farooq, M.; Farhan, M.; Raza, M.A.; Nizami, A.-S. Advancing Circular Economy Through Optimized Construction and Demolition Waste Management Under Life Cycle Approach. Sustainability 2025, 17, 4882. [Google Scholar] [CrossRef]
- Krellenberg, K.; Bergsträßer, H.; Bykova, D.; Kress, N.; Tyndall, K. Urban Sustainability Strategies Guided by the SDGs—A Tale of Four Cities. Sustainability 2019, 11, 1116. [Google Scholar] [CrossRef]
- Schiavina, M.; Melchiorri, M.; Corbane, C.; Florczyk, A.J.; Freire, S.; Pesaresi, M.; Kemper, T. Multi-Scale Estimation of Land Use Efficiency (SDG 11.3. 1) across 25 Years Using Global Open and Free Data. Sustainability 2019, 11, 5674. [Google Scholar] [CrossRef]
- Uslu, İ.; Uysal, O.; Aktaş, C.; Chang, B.; Yaman, İ.Ö. Dematerialization of Concrete: Meta-Analysis of Lightweight Expanded Clay Concrete for Compressive Strength. Sustainability 2024, 16, 6346. [Google Scholar] [CrossRef]
- Sosa Lucio, M.D.; Oh, E.-J.; Ha, J.-H.; Lee, J.; Lee, H.-J.; Song, I.-H. Effects of Processing Conditions on the Properties of Porous Diatomite Granules Prepared by Sodium Alginate Gelation. Appl. Sci. 2023, 13, 9474. [Google Scholar] [CrossRef]
- Stefanou, E.; Kantiranis, N.; Chatzicharalambous, K.; Mytiglaki, C.; Stamatakis, M.; Georgiadis, G. Diatomaceous Silica in Environmental Applications: A Case Study from the Lacustrine Deposit of Limnos Island, Aegean Sea, Greece. Minerals 2022, 12, 523. [Google Scholar] [CrossRef]
- Przybek, A. Assessment of Physico-Chemical Behavior and Sorptivity—Diatomaceous Earth as Support for Paraffinic Phase-Change Materials. Materials 2024, 17, 4691. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, N.; Yilmaz, A. Use of Diatomite as Partial Replacement for Portland Cement in Cement Mortars. Constr. Build. Mater. 2009, 23, 284–288. [Google Scholar] [CrossRef]
- Sriram, G.; Kigga, M.; Uthappa, U.T.; Rego, R.M.; Thendral, V.; Kumeria, T.; Jung, H.-Y.; Kurkuri, M.D. Naturally Available Diatomite and Their Surface Modification for the Removal of Hazardous Dye and Metal Ions: A Review. Adv. Colloid Interface Sci. 2020, 282, 102198. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, T.; Park, S.-J.; Kim, Y.-H. Reinforcing Effects of Gypsum Composite with Basalt Fiber and Diatomite for Improvement of High-Temperature Endurance. Constr. Build. Mater. 2022, 325, 126762. [Google Scholar] [CrossRef]
- Li, S.; Bao, C.; Ma, H.; Dong, W.; Song, S.; Wang, Q.; Liu, R.; Wu, P. Fabrication and Properties of Diatomite Ceramics with Hierarchical Pores Based on Direct Stereolithography. Ceram. Int. 2022, 48, 6266–6276. [Google Scholar] [CrossRef]
- Galán-Arboledas, R.J.; Cotes-Palomino, M.T.; Bueno, S.; Martínez-García, C. Evaluation of Spent Diatomite Incorporation in Clay Based Materials for Lightweight Bricks Processing. Constr. Build. Mater. 2017, 144, 327–337. [Google Scholar] [CrossRef]
- Mei, W.Y.; Hui, T.H. Regeneration of the Brewing Spent Diatomite. Adv. Mat. Res. 2014, 998–999, 1425–1428. [Google Scholar] [CrossRef]
- Gong, X.; Tian, W.; Wang, L.; Bai, J.; Qiao, K.; Zhao, J. Biological Regeneration of Brewery Spent Diatomite and Its Reuse in Basic Dye and Chromium (III) Ions Removal. Process Saf. Environ. Prot. 2019, 128, 353–361. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Wang, J.; Yu, G.; Liu, J.; Du, Y.; Li, Y.; Li, H. Controlled Synthesis of Zeolite Adsorbent from Low-Grade Diatomite: A Case Study of Self-Assembled Sodalite Microspheres. J. Environ. Sci. 2020, 91, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, X.; Zhang, G.; Zheng, S.; Frost, R.L. A Novel Method for Purification of Low Grade Diatomite Powders in Centrifugal Fields. Int. J. Miner. Process 2013, 125, 18–26. [Google Scholar] [CrossRef]
- Man, J.; Gao, W.; Yan, S.; Liu, G.; Hao, H. Preparation of Porous Brick from Diatomite and Sugar Filter Mud at Lower Temperature. Constr. Build. Mater. 2017, 156, 1035–1042. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Z.; Zheng, S. Physical Purification of Diatomite Based on Laminar-Flow Centrifugal Separation. Physicochem. Probl. Miner. Process. 2014, 50, 705–718. [Google Scholar]
- Font, A.; Soriano, L.; Reig, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Payá, J. Use of Residual Diatomaceous Earth as a Silica Source in Geopolymer Production. Mater. Lett. 2018, 223, 10–13. [Google Scholar] [CrossRef]
- Costa, R.F.; Ferreira, A.D.; Silva Junior, J.J.d.; Barbosa, P.M.A.; Bandeira, D.J.A.; Rezende, M.L.d.S.; Nascimento, J.J.d.S. Drying of Clay/Diatomite Hybrid Ceramic Plates: An Experimental Study. Res. Soc. Dev. 2021, 10, e13710817174. [Google Scholar] [CrossRef]
- Hao, L.; Gao, W.; Yan, S.; Niu, M.; Liu, G.; Hao, H. Preparation and Characterization of Porous Ceramics with Low-Grade Diatomite and Oyster Shell. Mater. Chem. Phys. 2019, 235, 121741. [Google Scholar] [CrossRef]
- Letelier, V.; Tarela, E.; Muñoz, P.; Moriconi, G. Assessment of the Mechanical Properties of a Concrete Made by Reusing Both: Brewery Spent Diatomite and Recycled Aggregates. Constr. Build. Mater. 2016, 114, 492–498. [Google Scholar] [CrossRef]
- Souza, M.M.; Anjos, M.A.S.; Sá, M.V.V.A.; Souza, N.S.L. Developing and Classifying Lightweight Aggregates from Sewage Sludge and Rice Husk Ash. Case Stud. Constr. Mater. 2020, 12, e00340. [Google Scholar] [CrossRef]
- Cougny, G. Spécifications Sur Les Matières Premières Argileuses Pour La Fabrication de Granulats Légers Expanses. Bull. Int. Assoc. Eng. Geol. 1990, 41, 47–55. [Google Scholar] [CrossRef]
- Mun, K.J. Development and Tests of Lightweight Aggregate Using Sewage Sludge for Nonstructural Concrete. Constr. Build. Mater. 2007, 21, 1583–1588. [Google Scholar] [CrossRef]
- RILEY, C.M. Relation of Chemical Properties to the Bloating of Clays. J. Am. Ceram. Soc. 1951, 34, 121–128. [Google Scholar] [CrossRef]
- Lau, P.C.; Teo, D.C.L.; Mannan, M.A. Characteristics of Lightweight Aggregate Produced from Lime-Treated Sewage Sludge and Palm Oil Fuel Ash. Constr. Build. Mater. 2017, 152, 558–567. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, K.G.; Lee, Y.S.; Wie, Y.M. Manufacturing and Application of Artificial Lightweight Aggregate from Water Treatment Sludge. J. Clean. Prod. 2021, 307, 127260. [Google Scholar] [CrossRef]
- Costa, J.E.B. Hzsm-5 Zeolites Synthesised from Alternative Sources of Silica and Aluminium for Deoxygenation of Coconut Fibre Pyrolysis Products. Ph.D. Thesis, Federal University of Rio Grande do Norte, Natal, Brazil, 2017. [Google Scholar]
- Li, B.; Ling, T.-C.; Qu, L.; Wang, Y. Effects of a Two-Step Heating Process on the Properties of Lightweight Aggregate Prepared with Sewage Sludge and Saline Clay. Constr. Build. Mater. 2016, 114, 119–126. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Wang, W.; Guo, J.; Zhang, L.; Zhang, H. Effect of SiO2 and Al2O3 on Characteristics of Lightweight Aggregate Made from Sewage Sludge and River Sediment. Ceram. Int. 2018, 44, 4313–4319. [Google Scholar] [CrossRef]
- Santana Leal de Souza, N. Desenvolvimento de Agregados Leves a Partir de Resíduos Industriais e Matérias-Primas Locais (Nordeste/Brasil). Master’s Dissertation, Federal University of Rio Grande do Norte, Natal, Brazil, 2019. [Google Scholar]
- Pei, J.; Pan, X.; Qi, Y.; Yu, H.; Tu, G. Preparation and Characterization of Ultra-Lightweight Ceramsite Using Non-Expanded Clay and Waste Sawdust. Constr. Build. Mater. 2022, 346, 128410. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, S.-Y. Application of Water Treatment Sludge in the Manufacturing of Lightweight Aggregate. Constr. Build. Mater. 2013, 43, 174–183. [Google Scholar] [CrossRef]
- NBR 16917; Coarse Aggregate—Determination of Density and Water Absorption. ABNT: Rio de Janeiro, Brazil, 2021.
- ME 093; Soils—Determination of Real Density. DNER: Rio de Janeiro, Brazil, 1994.
- Moreno-Maroto, J.M.; Cobo-Ceacero, C.J.; Uceda-Rodríguez, M.; Cotes-Palomino, T.; Martínez García, C.; Alonso-Azcárate, J. Unraveling the Expansion Mechanism in Lightweight Aggregates: Demonstrating That Bloating Barely Requires Gas. Constr. Build. Mater. 2020, 247, 118583. [Google Scholar] [CrossRef]
- Cobo-Ceacero, C.J.; Moreno-Maroto, J.M.; Guerrero-Martínez, M.; Uceda-Rodríguez, M.; López, A.B.; Martínez García, C.; Cotes-Palomino, T. Effect of the Addition of Organic Wastes (Cork Powder, Nut Shell, Coffee Grounds and Paper Sludge) in Clays to Obtain Expanded Lightweight Aggregates. Boletín Soc. Esp. Cerámica Vidr. 2023, 62, 88–105. [Google Scholar] [CrossRef]
- Li, Y.; Wu, D.; Zhang, J.; Chang, L.; Wu, D.; Fang, Z.; Shi, Y. Measurement and Statistics of Single Pellet Mechanical Strength of Differently Shaped Catalysts. Powder Technol. 2000, 113, 176–184. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Mueller, A.; Nguyen, V.T.; Nguyen, C.T. Development and Characterization of Lightweight Aggregate Recycled from Construction and Demolition Waste Mixed with Other Industrial By-Products. Constr. Build. Mater. 2021, 313, 125472. [Google Scholar] [CrossRef]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M.; Barrenechea, J.F.; Luque, F.J. Microstructure and Mineralogy of Lightweight Aggregates Manufactured from Mining and Industrial Wastes. Constr. Build. Mater. 2011, 25, 3591–3602. [Google Scholar] [CrossRef]
- Chuang, K.-H.; Lu, C.-H.; Chen, J.-C.; Wey, M.-Y. Reuse of Bottom Ash and Fly Ash from Mechanical-Bed and Fluidized-Bed Municipal Incinerators in Manufacturing Lightweight Aggregates. Ceram. Int. 2018, 44, 12691–12696. [Google Scholar] [CrossRef]
- Lynn, C.J.; Dhir, R.K.; Ghataora, G.S.; West, R.P. Sewage Sludge Ash Characteristics and Potential for Use in Concrete. Constr. Build. Mater. 2015, 98, 767–779. [Google Scholar] [CrossRef]
- Ke, Y.; Beaucour, A.L.; Ortola, S.; Dumontet, H.; Cabrillac, R. Influence of Volume Fraction and Characteristics of Lightweight Aggregates on the Mechanical Properties of Concrete. Constr. Build. Mater. 2009, 23, 2821–2828. [Google Scholar] [CrossRef]
- Molinari, C.; Zanelli, C.; Guarini, G.; Dondi, M. Bloating Mechanism in Lightweight Aggregates: Effect of Processing Variables and Properties of the Vitreous Phase. Constr. Build. Mater. 2020, 261, 119980. [Google Scholar] [CrossRef]
- Huang, S.-C.; Chang, F.-C.; Lo, S.-L.; Lee, M.-Y.; Wang, C.-F.; Lin, J.-D. Production of Lightweight Aggregates from Mining Residues, Heavy Metal Sludge, and Incinerator Fly Ash. J. Hazard. Mater. 2007, 144, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Tuan, B.L.A.; Hwang, C.-L.; Lin, K.-L.; Chen, Y.-Y.; Young, M.-P. Development of Lightweight Aggregate from Sewage Sludge and Waste Glass Powder for Concrete. Constr. Build. Mater. 2013, 47, 334–339. [Google Scholar] [CrossRef]
- Cinexpan, Expanded Clay. Technical Data Sheet Type 1506. Available online: https://www.cinexpan.com.br/pdf/ficha-tecnica-tipo-1506.pdf (accessed on 24 June 2024).
- Bernhardt, M.; Justnes, H.; Tellesbø, H.; Wiik, K. The Effect of Additives on the Properties of Lightweight Aggregates Produced from Clay. Cem. Concr. Compos. 2014, 53, 233–238. [Google Scholar] [CrossRef]
- Loutou, M.; Hajjaji, M. Clayey Wastes-Based Lightweight Aggregates: Heating Transformations and Physical/Mechanical Properties. Appl. Clay Sci. 2017, 150, 56–62. [Google Scholar] [CrossRef]
- Ren, Y.; Ren, Q.; Huo, Z.; Wu, X.; Zheng, J.; Hai, O. Preparation of Glass Shell Fly Ash-Clay Based Lightweight Aggregate with Low Water Absorption by Using Sodium Carbonate Solution as Binder. Mater. Chem. Phys. 2020, 256, 123606. [Google Scholar] [CrossRef]
- Piszcz-Karaś, K.; Klein, M.; Hupka, J.; Łuczak, J. Utilization of Shale Cuttings in Production of Lightweight Aggregates. J. Environ. Manag. 2019, 231, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Franus, M.; Panek, R.; Madej, J.; Franus, W. The Properties of Fly Ash Derived Lightweight Aggregates Obtained Using Microwave Radiation. Constr. Build. Mater. 2019, 227, 116677. [Google Scholar] [CrossRef]
- da Silva Neto, J.A.; dos Anjos, M.A.S.; Dutra, R.P.S.; Mendonça de Souza, M.; Pederneiras, C.M. Optimization via Taguchi of Artificial Lightweight Aggregates Obtained from Kaolinite Clay and Ceramic Waste: Development and Industrial Applications. Buildings 2025, 15, 2003. [Google Scholar] [CrossRef]

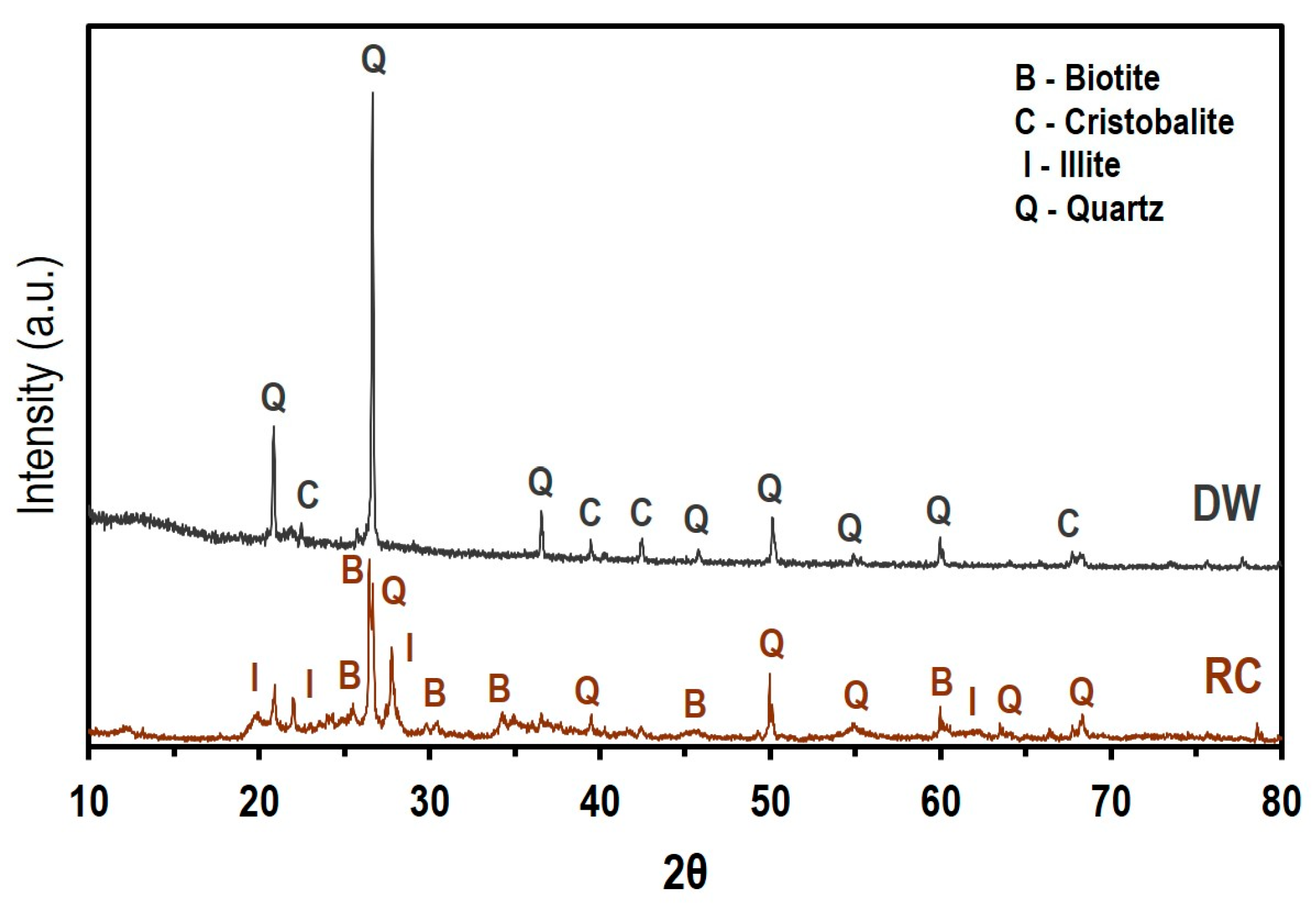
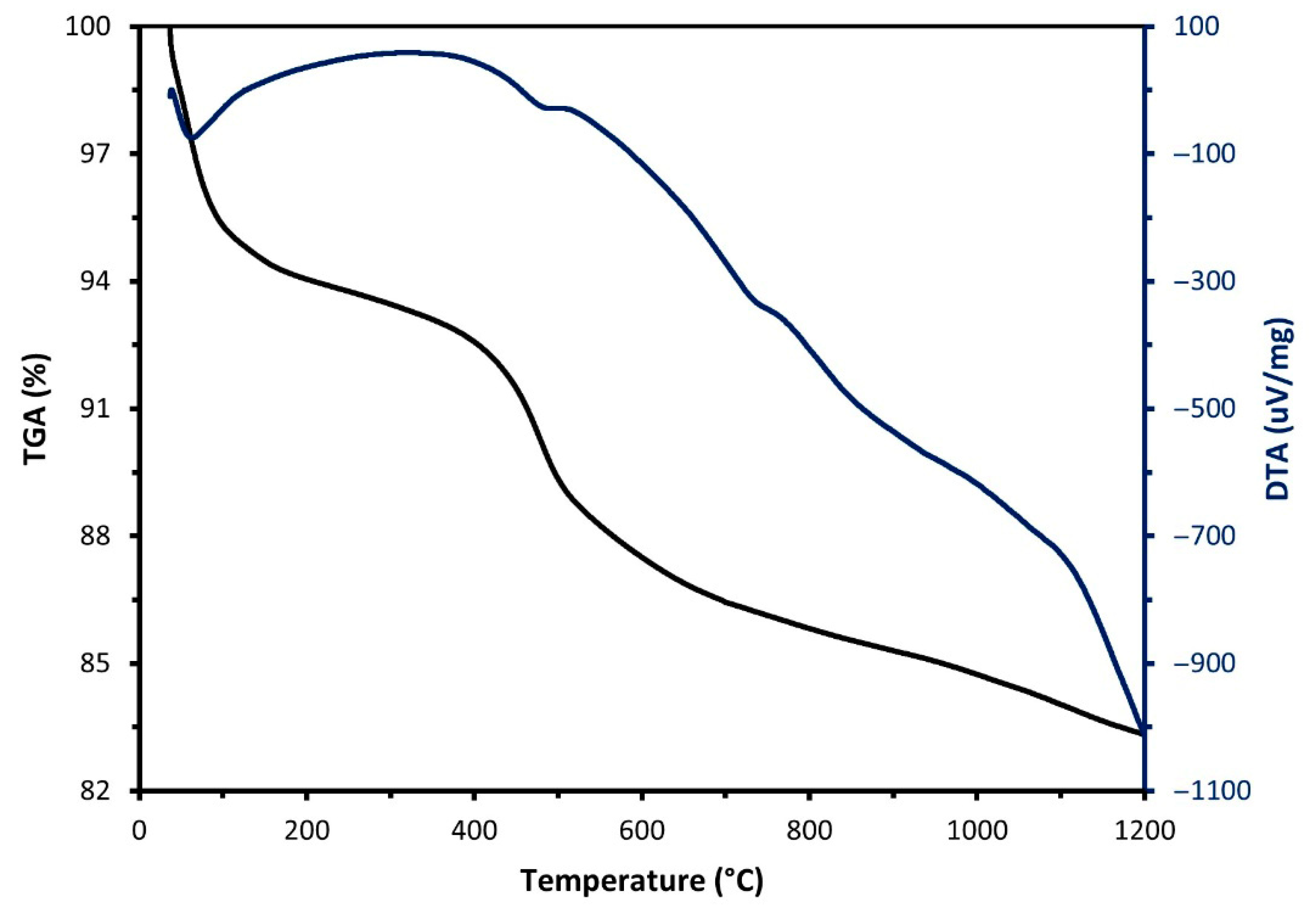
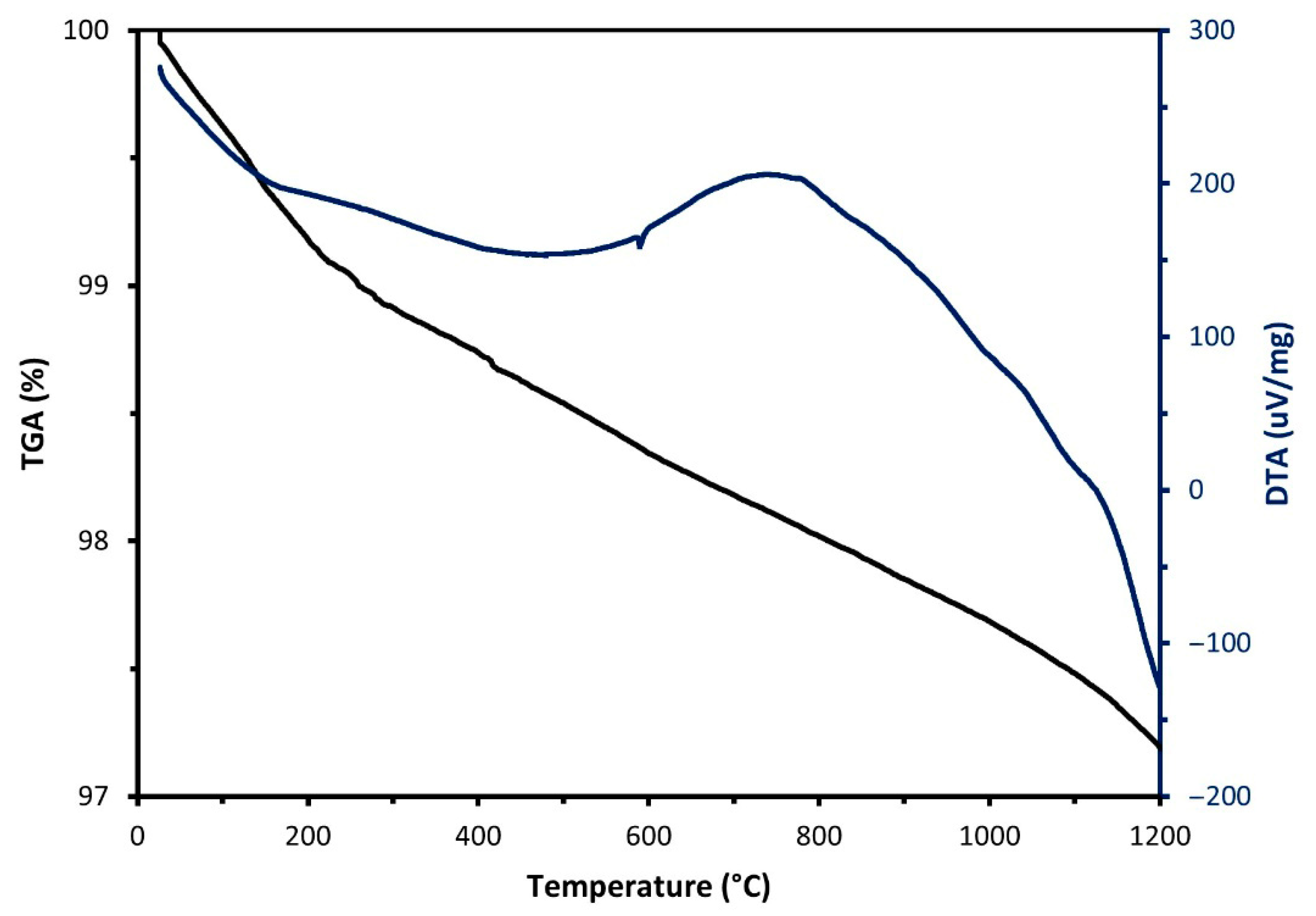



| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | Others | LOI (%) |
|---|---|---|---|---|---|---|---|---|---|
| DW | 90.54 | 1.61 | 2.81 | 1.12 | 0.00 | 0.00 | 0.43 | 3.49 | 0.91% |
| RC | 41.45 | 21.02 | 27.99 | 1.17 | 2.09 | 0.00 | 2.96 | 3.33 | 8.6% |
| Samples | Bloating Index (BI) (%) | |||
|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| D100 | −5.2 | −1.4 | −5.1 | 0.3 |
| D90 | 2.1 | −0.4 | −0.6 | −7.5 |
| D80 | 4.5 | −4.9 | −11.5 | −7.9 |
| D70 | 1.5 | 5.7 | −7.5 | −15.3 |
| D60 | −6.9 | −14.0 | −22.7 | −24.9 |
| D50 | −2.5 | −8.9 | −20.4 | −13.1 |
| Samples | Particle Density (pd) (g/cm3) | |||
|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| D100 | 1.14 | 1.18 | 1.22 | 1.25 |
| D90 | 1.33 | 1.37 | 1.39 | 1.40 |
| D80 | 1.45 | 1.51 | 1.58 | 1.60 |
| D70 | 1.52 | 1.62 | 1.74 | 1.80 |
| D60 | 1.69 | 1.85 | 2.00 | 2.10 |
| D50 | 1.82 | 2.00 | 2.13 | 1.92 |
| Samples | Closed Porosity (Pc) (%) | |||
|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| D100 | 3.3 | 3.1 | 2.8 | 2.8 |
| D90 | 5.8 | 5.8 | 5.5 | 6.0 |
| D80 | 5.4 | 6.3 | 6.1 | 7.7 |
| D70 | 5.1 | 6.1 | 7.1 | 9.3 |
| D60 | 5.5 | 7.5 | 10.2 | 12.4 |
| D50 | 7.3 | 10.0 | 11.1 | 20.4 |
| Samples | WA24H (%) | |||
|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| D100 | 44.7 | 41.6 | 39.4 | 37.2 |
| D90 | 30.7 | 28.6 | 27.7 | 26.9 |
| D80 | 25.1 | 22.3 | 19.3 | 17.6 |
| D70 | 22.7 | 18.3 | 13.5 | 10.6 |
| D60 | 16.1 | 10.9 | 5.3 | 2.0 |
| D50 | 11.4 | 5.8 | 2.5 | 2.0 |
| Samples | Crushing Strength (S) (MPa) | |||
|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| D100 | 0.44 | 0.28 | 0.35 | 0.38 |
| D90 | 0.98 | 1.16 | 1.00 | 1.10 |
| D80 | 2.13 | 2.13 | 2.19 | 1.70 |
| D70 | 3.17 | 3.20 | 3.57 | 3.03 |
| D60 | 6.09 | 6.27 | 7.41 | 7.15 |
| D50 | 8.27 | 9.76 | 11.14 | 6.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, M.M.d.; Barbosa, N.P.; Anjos, M.A.S.d.; Farias, E.C.d.; Aguiar, J.G.C.; Neto, J.A.d.S.; Pederneiras, C.M. Sustainable Lightweight Aggregates from Diatomite Residue. Sustainability 2025, 17, 6508. https://doi.org/10.3390/su17146508
Souza MMd, Barbosa NP, Anjos MASd, Farias ECd, Aguiar JGC, Neto JAdS, Pederneiras CM. Sustainable Lightweight Aggregates from Diatomite Residue. Sustainability. 2025; 17(14):6508. https://doi.org/10.3390/su17146508
Chicago/Turabian StyleSouza, Maelson Mendonça de, Normando Perazzo Barbosa, Marcos Alyssandro Soares dos Anjos, Evilane Cássia de Farias, João Gabriel Cruz Aguiar, José Anselmo da Silva Neto, and Cinthia Maia Pederneiras. 2025. "Sustainable Lightweight Aggregates from Diatomite Residue" Sustainability 17, no. 14: 6508. https://doi.org/10.3390/su17146508
APA StyleSouza, M. M. d., Barbosa, N. P., Anjos, M. A. S. d., Farias, E. C. d., Aguiar, J. G. C., Neto, J. A. d. S., & Pederneiras, C. M. (2025). Sustainable Lightweight Aggregates from Diatomite Residue. Sustainability, 17(14), 6508. https://doi.org/10.3390/su17146508







