Chemical and Biochemical Properties of Common Nettle (Urtica dioica L.) Depending on Various Nitrogen Fertilization Doses in Crop Production
Abstract
1. Introduction
2. Materials and Methods
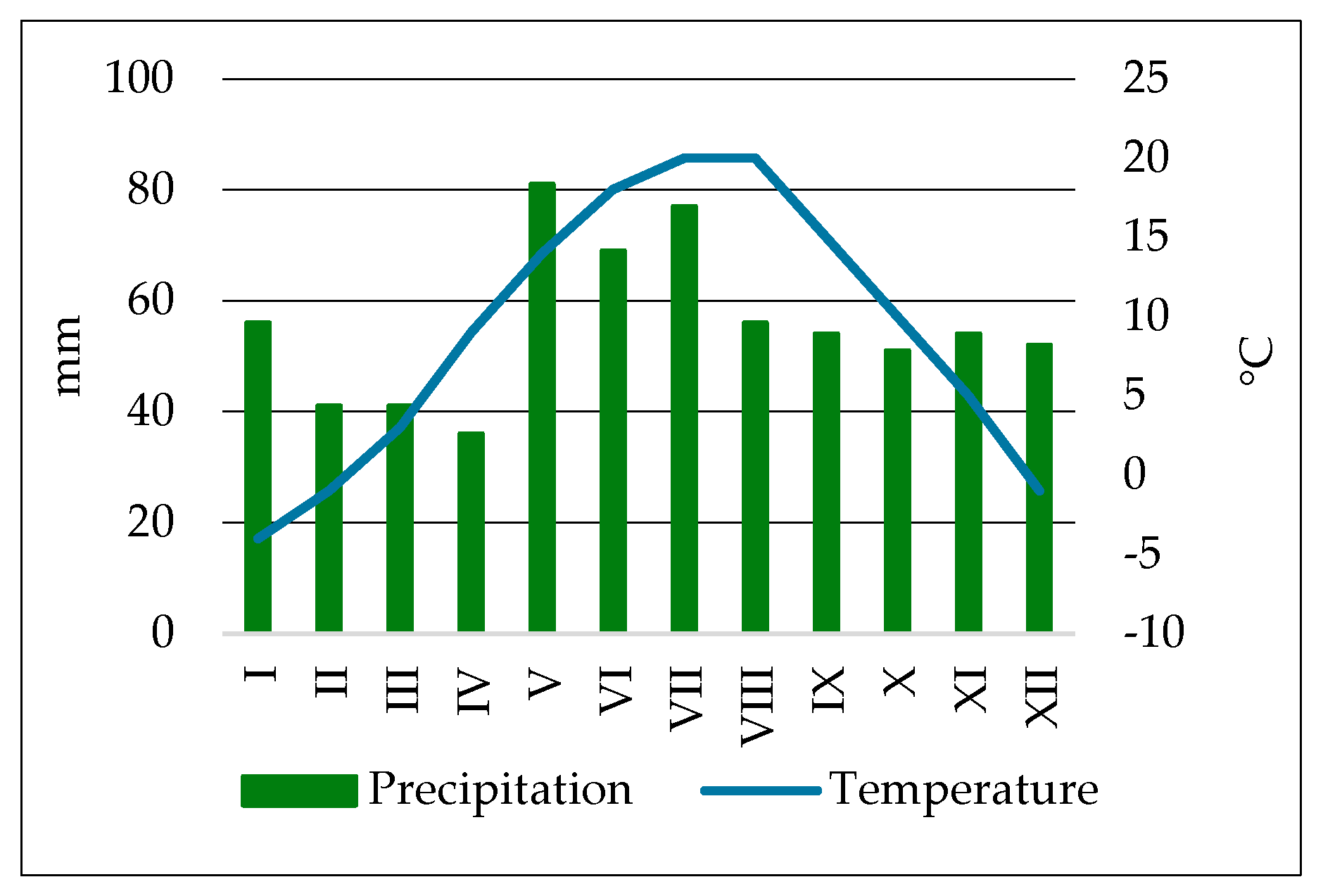
2.1. Soil Sampling Location
2.2. Method
2.2.1. Soil Analysis
- -
- Physicochemical properties were determined in samples of disturbed soil, taken from a horizon of 0–25 cm. The soil was air-dried, sieved through a 2 mm mesh, and granulometric composition determined by laser diffraction using a Mastersizer MS 2000 analyser (Malvern Panalytical, Worcestershire, UK)
- -
- pH was measured potentiometrically in 1 M KCl extract [33]
- -
- Total organic carbon (TOC) and total nitrogen (TN) were determined with the Vario Max CN analyser (Elementar, Hanau in Germany).
2.2.2. Plant Analysis
- -
- The contents of Zn, Cu, Mn, and Fe in the extract were determined by flame absorption spectroscopy using a Solaar S4 apparatus (Thermo Elementar, Germany).
- -
- The contents of chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoids were determined according to Lichtenthaler [34] as well as Lichtenthaler and Buschmann [35]. The contents of chlorophyll a, chlorophyll b, and carotenoids were determined using a spectrophotometer at wavelengths (λmax) of 645 nm, 662 nm, and 470 nm, respectively. The content of plant pigments was calculated in milligrams per gram of fresh weight of the sample (mg g−1 f.w.). The contents of Chl a and Chl b were used to determine the ratio of these pigments (Chl a/b, which is an indicator of leaf health) and the total chlorophyll content (a + b).
- -
- The content of ascorbic acid (AAC) was determined by titration in an acidic medium with a solution of the standard dye 2,6-dibromophenolindophenol until a pink colour was obtained for 30 s [36].
- -
- Antioxidant activity (AA) was determined using the 2,2-diphenyl−1-picrylhydrazyl free radical (DPPH) method according to Zeipin et al. [37]. To 1 g of nettle leaves, 10 mL of methanol was added. After adding the methanolic DPPH solution, the spectrophotometric measurement was performed at a wavelength of 515 nm. Antioxidant activity (%) was calculated by the following equation:where Ass—absorbance of the test sample; A0—absorbance of the control DPPH solution with 0.3 mL of methanol.
2.3. Statistics
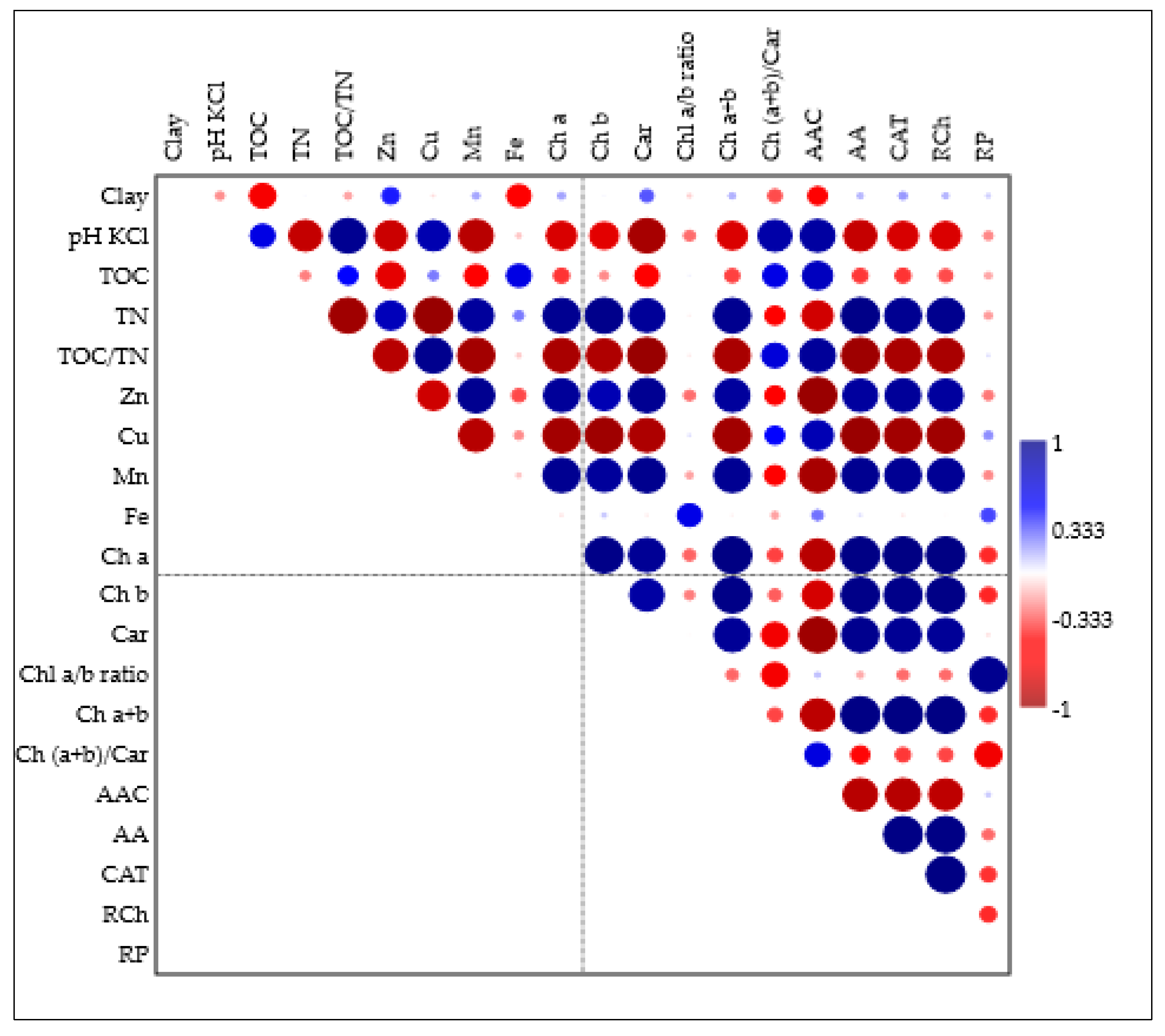
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Al-Ghamdi, A.A.; Khan, A.; Zeeshan, M.; Elshikh, M.S.; Abbasi, A.M.; Zhou, X.-B. Nitrogen fertilizer modulates plant growth, chlorophyll pigments and enzymatic activities under different irrigation regimes. Agronomy 2022, 12, 845. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Dębska, B.; Lamparski, R.; Michalska, A.; Pobereżny, J.; Wszelaczyńska, E.; Bartkowiak, A.; Szczepanek, M.; Banach-Szott, M.; Knapowski, T. Influence of plant growth retardants and nitrogen doses on the content of plant secondary metabolites in wheat, the presence of pests, and soil quality parameters. Agriculture 2023, 13, 1121. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Dębska, B.; Majcherczak, E.; Michalska, A. Mineral components, organic matter quality and soil enzymatic activity under the influence of differentiated farmyard manure and nitrogen fertilisation. Minerals 2024, 14, 645. [Google Scholar] [CrossRef]
- Zhao, L.-S.; Li, K.; Wang, Q.-M.; Song, X.-Y.; Su, H.-N.; Xie, B.-B.; Zhang, X.-Y.; Huang, F.; Chen, X.-L.; Zhou, B.-C.; et al. Nitrogen starvation impacts the photosynthetic performance of Porphyridium cruentum as revealed by chlorophyll a fluorescence. Sci. Rep. 2017, 7, 8542. [Google Scholar] [CrossRef]
- Yañez-Mansilla, E.; Cartes, P.; Reyes-Díaz, M.; Ribera-Fonseca, A.; Rengel, Z.; Alberdi, M. Leaf nitrogen thresholds ensuring high antioxidant features of Vaccinium corymbosum cultivars. J. Soil Sci. Plant Nutr. 2015, 15, 574–586. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, S.; Yang, Q.; Chen, S.; Qi, C.; Liu, X.; Liang, J.; Wang, H. Nitrogen application alleviates impairments for Jatropha curcas L. seedling growth under salinity stress by regulating photosynthesis and antioxidant enzyme activity. Agronomy 2023, 13, 1749. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhu, G.; Zhou, G.; Song, X.; Hussein Ibrahim, M.E.; Ibrahim Salih, E.G. Effect of N on growth, antioxidant capacity, and chlorophyll content of sorghum. Agronomy 2022, 12, 501. [Google Scholar] [CrossRef]
- Đurović, S.; Micić, D.; Šorgić, S.; Popov, S.; Gašić, U.; Tosti, T.; Kostić, M.; Smyatskaya, Y.A.; Blagojević, S.; Zeković, Z. Recovery of polyphenolic compounds and vitamins from the stinging nettle leaves: Thermal and behavior and biological activity of obtained extracts. Molecules 2023, 28, 2278. [Google Scholar] [CrossRef]
- Kukrić, Z.Z.; Topalić-Trivunović, N.L.; Kukavica, M.B.; Matoš, S.B.; Pavičić, S.S.; Boroja, M.M.; Savic, A.V. Characterization of antioxidant and antimicrobial activities of nettle leaves (Urtica dioica L.). Acta Period. Technol. 2012, 43, 257–272. [Google Scholar] [CrossRef]
- Kwiecien, M.; Winiarska-Mieczan, A. Effect of addition of herbs on body weight and assessment of physical and chemical alterations in the tibia bones of broiler chickens. J. Elem. 2009, 14, 705–715. [Google Scholar] [CrossRef]
- Rayburn, K.; Fleischbein, E.; Song, J.; Allen, B.; Kundert, M.; Leiter, C.; Bush, T. Stinging nettle cream for osteoarthritis. Altern. Ther. Health Med. 2009, 15, 60–61. [Google Scholar] [PubMed]
- Paulauskienne, A.; Taraseviciene, Ž.; Laukagalis, V. Influence of harvesting time on the chemical composition of wild stinging nettle (Urtica dioica L.). Plants 2021, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Szewczuk, C.; Mazur, M. Effect of different rates of nitrogen fertilisers on chemical composition of stinging nettle (Urlica dioica L.) plants harvested at three development stages. Part II. Contents of minerals. Acta Sci. Pol. Agric. 2004, 3, 239–248. [Google Scholar]
- Rodman, S.; Zutic, I.; Fabek, S.; Žlabur, J.Š.; Benko, B.; Toth, N.; Coga, L. Influence of nitrogen fertilization on chemical composition of cultivated nettle. Emir. J. Food Agric. 2015, 27, 889–896. [Google Scholar] [CrossRef]
- Rodman, S.; Zutic, I.; Coga, L.; Fabek, S.; Benko, B.; Toth, N. Yield and mineral Content of stinging nettle as affected by nitrodgen fertilization. J. Agr. Sci. Techol. 2016, 18, 1117–1128. [Google Scholar]
- Adamczyk, M.; Grabarczyk, M. Communication—Application of the adsorptive stripping voltammetry to the determination of Ti (IV) content in extracts of stinging nettle leaves. J. Electrochem. Soc. 2021, 168, 016505. [Google Scholar] [CrossRef]
- Domagała, B.; Gospodarek, J. Olfactometric response of selected crop pests to the scent of common nettle (Urtica dioica L.) and the influence of aqueous plant extracts on their behavior. Prog. Plant Prot. 2024, 64, 43–52. [Google Scholar] [CrossRef]
- Tumolo, T.; Lanfer-Marquez, U.M. Copper chlorophyllin: A food colorant with bioactive properties? Food Res. Int. 2012, 46, 451–459. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion on the re-evaluation of chlorophylls (E 140(i)) as food additives. EFSA J. 2015, 13, 4089. [Google Scholar] [CrossRef]
- Selzer, L.J.; Busso, C.A. Pigments and photosynthesis of understory grasses: Light irradiance and soil moisture effects. Russ. J. Plant Physiol. 2016, 63, 224–234. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Rebolloso-Fuentes, M.M.; Torija Isasa, M.E. Fatty acids and carotenoids from Stinging Nettle (Urtica dioica L.). J. Food Compos. Anal. 2003, 16, 111–119. [Google Scholar] [CrossRef]
- Kosolapov, V.M.; Cherniavskih, V.I.; Zarudny, V.A.; Mazur, K.; Konieczna, A.; Tseiko, L.; Dumacheva, E.V.; Dumachev, D.V. Observations on the productivity of breeding specimens of Urtica dioica L. from European russian ecotopes in comparison with the breeding variety under field crop conditions. Agronomy 2021, 12, 76. [Google Scholar] [CrossRef]
- Załęcki, R.; Kordana, S.; Kucharski, W.; Kozłowski, J. Pokrzywa zwyczajna (Urtica dioica L.); Instrukcja Uprawy: Poznań, Poland, 1997. (In Polish) [Google Scholar]
- Vogl, C.R.; Hartl, A. Production and Processing of Organically Grown Fiber Nettle (Urtica dioica L.) and Its Potential Use in the Natural Textile Industry: A Review. Am. J. Altern. Agric. 2003, 18, 119–128. [Google Scholar] [CrossRef]
- Grevsen, K.; Frette, X.C.; Christensen, L.P. Concentration and composition of flavonol glycosides and phenolic acids in aerial parts of stinging nettle (Urtica dioica L.) are affected by nitrogen fertilization and by harvest time. Europ. J. Hort. Sci. 2008, 73, 20–22. [Google Scholar] [CrossRef]
- Weiß, F. Effects of varied nitrogen fertilization and cutting treatments on the development and yield components of cultivated stinging nettles. Acta Hortic. 1993, 331, 137–144. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Dominquez-Perles, R.; Moreno, D.A.; Muries, B.; Alcaraz-Lόpez, C.; Bastías, E.; García-Viguera, C.; Carvajal, M. Minerals in plant food: Effect of agricultural practices and role in human health. A review. Agron. Sustain. Dev. 2010, 30, 295–309. [Google Scholar] [CrossRef]
- Wołoszczak, E.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z.; Nawirska-Olszańska, A. The effect of nitrogen form and dose on yield, chemical composition and antioxidant activity of stinging nettle (Urtica dioica L.). Herba Pol. 2009, 55, 84–93. [Google Scholar]
- Biczak, R.; Pawłowska, B.; Pilis, W.; Szczegielniak, J.; Wróbel, J.; Telesiński, A. Phytotoxicity and Effect of Ionic Liquids on Antioxidant Parameters in Spring Barley Seedlings: The Impact of Exposure Time. Processes 2020, 8, 1175. [Google Scholar] [CrossRef]
- Sharma, I.; Ahmad, P. Catalase: A versatile antioxidant in plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; Chapter 4; pp. 131–148. [Google Scholar] [CrossRef]
- Kondracki, J. Geografia Regionalna Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2012; ISBN 9788301160227. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. In World Soil Resources Reports No. 106; FAO: Rome, Italy.
- PN-ISO 10390; Chemical and Agricultural Analysis: Determining Soil pH. Polish Standards Committee: Warszawa, Poland, 1997.
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- PN A-04019:1998; Food Products—Determination of Vitamin C. Polish Committee for Standardization: Warsaw, Poland, 1998.
- Zeipina, S.; Alsina, I.; Lepse, L.; Dūma, M. Antioxidant activity in nettle (Urtica dioica L.) and garden orache (Atriplex hortensis L.) leaves during vegetation period. Chem. Technol. 2015, 66, 29–33. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Orwin, K.H.; Wardle, D.A. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biol. Biochem. 2004, 36, 1907–1912. [Google Scholar] [CrossRef]
- Chaer, G.; Fernandes, M.; Myrold, D.; Bottomley, P. Comparative resistance and resilience of soil microbial communities and enzyme activities in adjacent native forest and agricultural soils. Microb. Ecol. 2009, 58, 414–424. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. Past: Paleontological statistics software package for educaton and data anlysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- USDA. Keys to Soil Taxonomy, 10th ed.; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2006; pp. 1–332.
- Ali, S.; Liu, K.; Ahmed, W.; Jing, H.; Qaswar, M.; Kofi Anthonio, C.; Maitlo, A.A.; Lu, Z.; Liu, L.; Zhang, H. Nitrogen mineralization, soil microbial biomass and extracellular enzyme activities regulated by long-term N fertilizer inputs: A comparison study from upland and paddy soils in a red soil region of China. Agronomy 2021, 11, 2057. [Google Scholar] [CrossRef]
- Shonte, T.T.; Duodu, K.G.; de Kock, H.L. Effect of drying methods on chemical composition and antioxidant activity of underutilized stinging nette leaves. Heliyon 2020, 6, e03938. [Google Scholar] [CrossRef]
- Tack, F.M.; Verloo, M.G. Metal contents in stinging nettle (Urtica dioica L.) as affected by soil characteristics. Sci. Total Environ. 1996, 192, 31–39. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Lόpez-Pérez, L.; Hernández, M.; Lόpez-Berenguer, C.; FernándezGarcía, N.; Carvajal, M. Agricultural practices for enhanced human health. Phytochem. Rev. 2008, 7, 251–260. [Google Scholar] [CrossRef]
- Rutto, L.K.; Ansari, M.S.; Brandt, M. Biomass yield and dry matter partitioning in greenhouse-grown stinging nettle under different fertilization regimes. HortTechnology 2012, 22, 751–756. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Kacprzak, M.; Grobelak, A.; Placek, A. The influence of selected soil parameters on the mobility of heavy metals in soils. Environ. Prot. Eng. 2012, 15, 81–92. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: London, UK; New York, NY, USA, 2011; ISBN 978-1-4200-9368-1. [Google Scholar] [CrossRef]
- Kowol, J.; Kwapuliński, J.; Brodziak-Dopierała, B.; Paukszto, A.; Bogunia, M.; Rochel, R.; Ahnert, B. Influence of a transboundart emission on bioavailability of metals of stinging nettle from soil. Pol. J. Environ. Stud. 2011, 20, 115–124. [Google Scholar]
- Boshoff, M.; De Jonge, M.; Scheifler, R.; Brrvoets, L. Predicting As, Cd, Cu, Pb and Zn levels in grasses (Agrostis sp. and Poa sp.) and stinging nettle (Urlica dioica) applying soil-plant transfer models. Sci. Total Environ. 2014, 493, 862–871. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: A comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Feng, Y.; Wang, X.; Li, J.; Xu, Z.; Phonenasay, S.; Lou, Q.; Han, Z.; Lu, W. Effects of nitrogen application rate on the photosynthetic pigment, leaf fluorescence characteristics, and yield of indica hybrid rice and their interrelations. Sci. Rep. 2021, 11, 7485. [Google Scholar] [CrossRef]
- Sonobe, R.; Yamashita, H.; Mihara, H.; Morita, A.; Ikka, T. Estimation of leaf chlorophyll a, b and carotenoid contents and their ratios using hyperspectral reflectance. Remote Sens. 2020, 12, 3265. [Google Scholar] [CrossRef]
- Aziz, A.; Akram, N.A.; Ashraf, M. Influence of natural and synthetic vitamin C (ascorbic acid) on primary and secondary metabolites and associated metabolism in quinoa (Chenopodium quinoa Willd.) plants under water deficit regimes. Plant Physiol. Biochem. 2018, 123, 192–203. [Google Scholar] [CrossRef]
- Rajasree, G.; Pillai, G.R. Effect of nitrogen nutrition on fruit quality and shelf life of cucurbitaceous vegetable bitter gourd. J. Plant Nutr. 2012, 35, 1139–1153. [Google Scholar] [CrossRef]
- Caballero, B.; Finglas, P.M.; Toldrá, F. Antioxidant activity of foods. In Encyclopedia of Food and Health; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Bartkowiak, A.; Lemanowicz, J.; Rydlewska, M.; Sowiński, P. The Impact of proximity to road traffic on heavy metal accumulation and enzyme activity in urban soils and dandelion. Sustainability 2024, 16, 812. [Google Scholar] [CrossRef]
- Noor, H.; Ding, P.; Ren, A.; Sun, M.; Gao, Z. Effects of nitrogen fertilizer on photosynthetic characteristics and yield. Agronomy 2023, 13, 1550. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, S.; Kamran, M.; Yang, X.N.; Hou, F.J.; Yang, B.P.; Ding, R.X.; Liu, T.; Han, Q.F. Uniconazole and nitrogen fertilization trigger photosynthesis and chlorophyll fluorescence, and delay leaf senescence in maize at a high population density. Photosynthetica 2021, 59, 192–202. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A. Soil enzyme activity response under the amendment of different types of biochar. Agronomy 2022, 12, 569. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A.; Tobiasova, E. The application of biochar from waste biomass to improve soil fertility and soil enzyme activity and increase carbon sequestration. Energies 2023, 16, 380. [Google Scholar] [CrossRef]
- Eskandari, H. The importance of iron (Fe) in plant products and mechanism of its uptake by plants. J. Appl. Environ. Biol. Sci. 2011, 1, 448–452. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Nutrient Management: An Introduction to Nutrient Management, 8th ed.; Pearson: Upper Saddle River, NJ, USA, 2014; 505p. [Google Scholar]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and pharmacological importance of stinging nettle (Urtica dioica L.): A review. Heliyon 2022, 8, e09717. [Google Scholar] [CrossRef]
- Feszterova, M.; Kowalska, M.; Misiakova, M. Stability of vitamin C content in plant and vegetable juices under different storing conditions. Appl. Sci. 2023, 13, 10640. [Google Scholar] [CrossRef]
- Grzyś, E. The role and importance of micronutriens in plants nutrition. Adv. Agric. Sci. 2004, 502, 89–99. [Google Scholar]
- Ishfaq, M.; Wang, Y.; Xu, J.; Hassan, M.; Yuan, H.; Liu, L.; He, B.; Ejaz, I.; White, F.; Cakmak, I.; et al. Improvement of nutritional quality of food crops with fertilizer: A global meta-analysis. Agron. Sustain. Dev. 2023, 43, 7. [Google Scholar] [CrossRef]
- Mishra, B.K.; Rastogi, A.; Shukla, S. Regulatory role of mineral elements in the metabolism of medicinal plants. In Medicinal and Aromatic Plant Science and Biotechnology; Global Science Books Publications: Singapore, 2012; Volume 6, pp. 1–23. [Google Scholar]
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Quality evaluation of light-and dark-colored Hungarian honeys, focusing on botanical origin, antioxidant capacity and mineral content. Molecules 2021, 26, 2825. [Google Scholar] [CrossRef]
- Batool, M.; Nadeem, M.; Imran, M.; Gulzar, N.; Shahid, M.Q.; Shahbaz, M.; Ajmal, M.; Khan, I.T. Impact of vitamin E and selenium on antioxidant capacity and lipid oxidation of cheddar cheese in accelerated ripening. Lipids Health Dis. 2018, 17, 79. [Google Scholar] [CrossRef]
- Hassan, F.; Mobarez, S.; Mohamed, M.; Attia, Y.; Mekawy, A.; Mahrose, K. Zinc and/or selenium enriched spirulina as antioxidants in growing rabbit diets to alleviate the deleterious impacts of heat stress during summer season. Animals 2021, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Signorella, S.; Palopoli, C.; Daier, V.; Ledesma, G. Manganese and Catalases. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
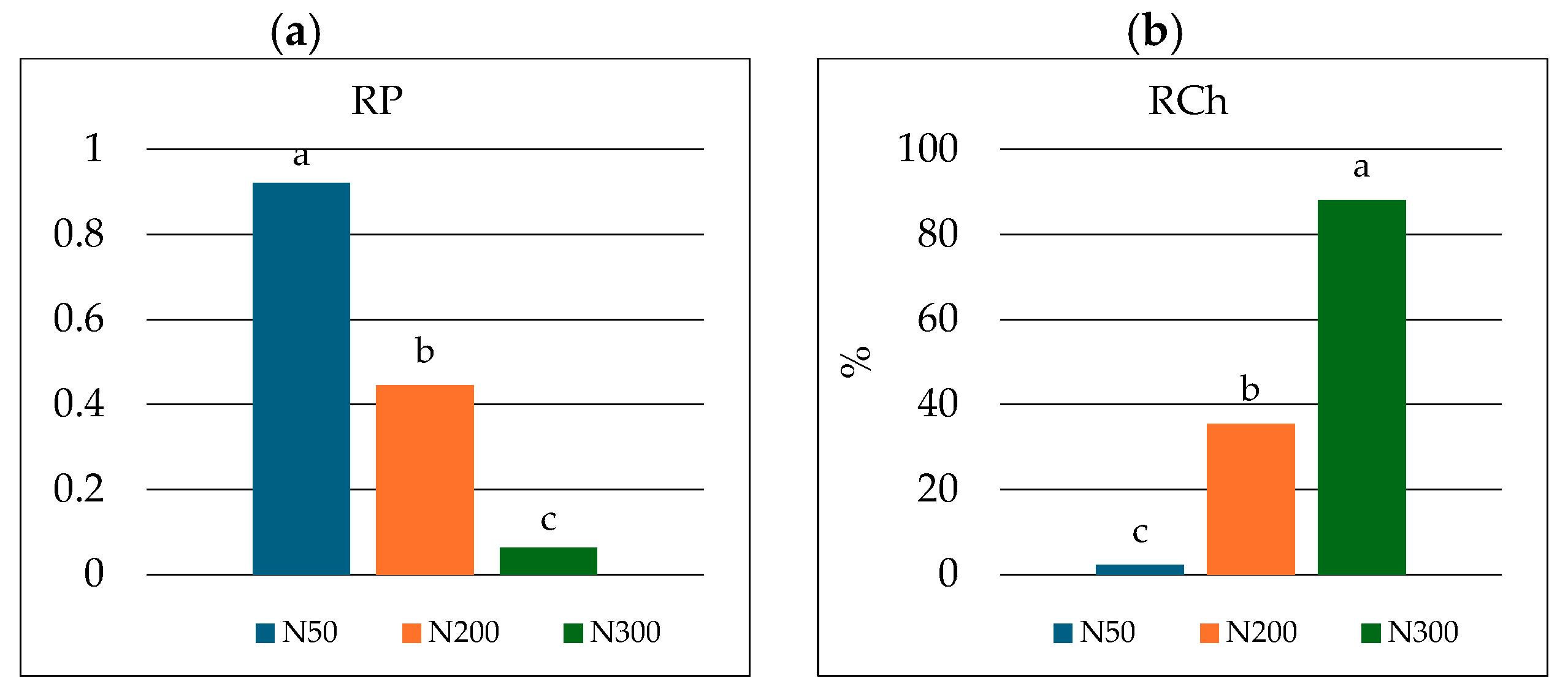
| Dose Nitrogen kg N ha −1 | Sand % | Silt % | Clay % | pH 1M KCl | TOC g kg−1 | TN g kg−1 |
|---|---|---|---|---|---|---|
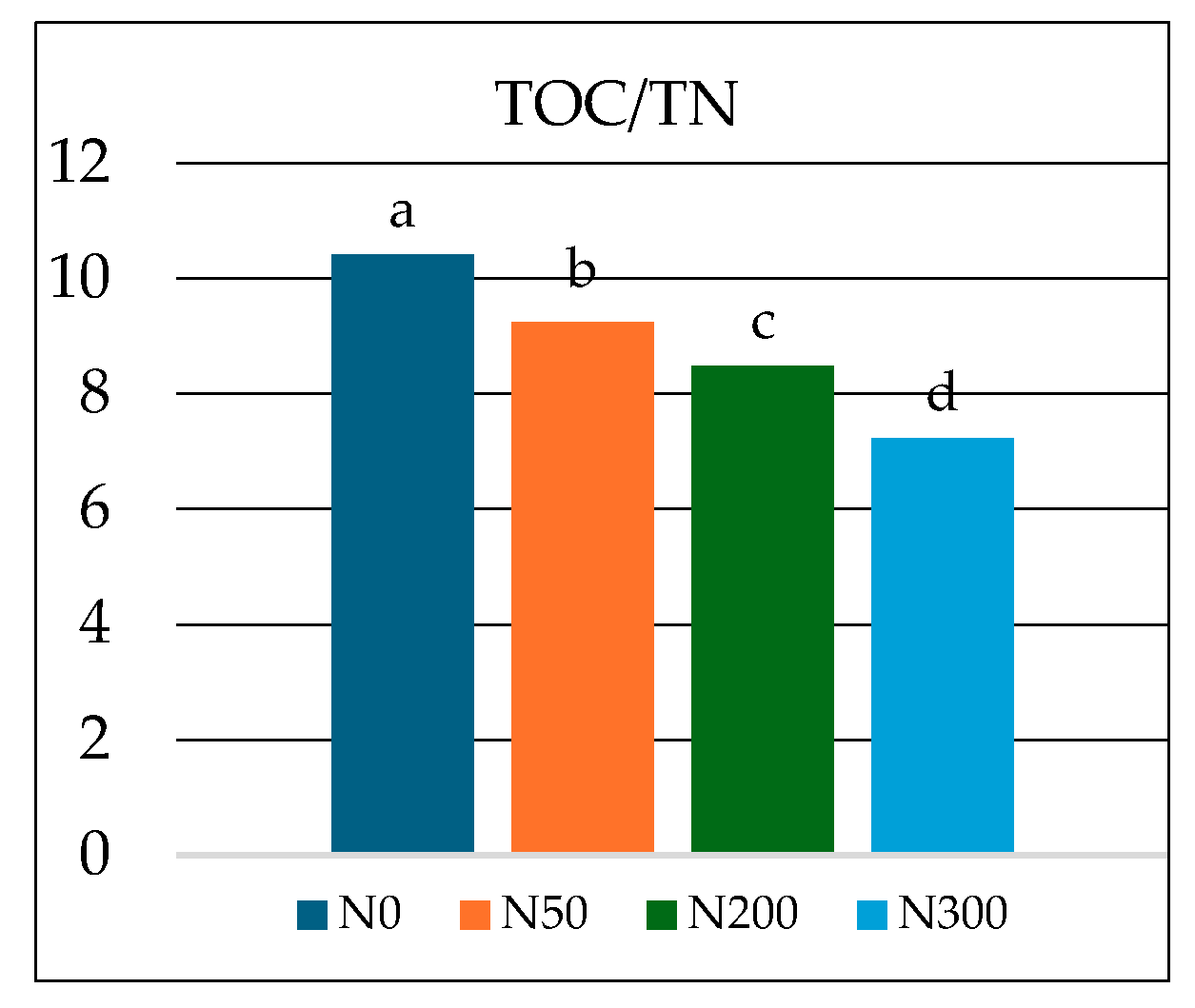
| * N0 | 55.66 ± 5.31 | 38.24 ± 4.40 | 6.10 ± 0.16 | 6.63 ± 0.14 | 12.46 a ± 0.03 | 1.20 c ± 0.02 |
| N50 | 53.82 ± 4.50 | 40.05 ± 3.92 | 6.13 ± 0.12 | 5.88 ± 0.22 | 12.30 ab ± 0.12 | 1.33 b ± 0.01 |
| N200 | 55.04 ± 5.66 | 38.68 ± 5.23 | 6.28 ± 0.12 | 5.61 ± 0.18 | 11.66 c ± 0.17 | 1.38 b ± 0.02 |
| N300 | 55.84 ± 4.82 | 37.99 ± 4.80 | 6.17 ± 0.07 | 5.44 ± 0.10 | 12.17 b ± 0.08 | 1.69 a ± 0.04 |
| Nitrogen Dose kg N ha−1 | Zn | Cu | Mn | Fe |
|---|---|---|---|---|
| mg kg−1 | ||||
| * N0 | 20.27 b ± 2.15 | 6.69 a ± 1.05 | 376.2 c ± 3.34 | 305.2 ab ± 9.70 |
| N50 | 20.55 b ± 3.09 | 6.08 ab ± 1.99 | 417.5 bc ± 19.26 | 368.8 a ± 10.15 |
| N200 | 24.98 a ± 1.28 | 5.75 b ± 2.17 | 553.6 ab ± 8.84 | 265.5 b ± 3.75 |
| N300 | 25.48 a ± 2.42 | 4.22 c ± 1.44 | 634.5 a ± 11.92 | 340.6 a ± 21.29 |
| Nitrogen Dose kg N ha −1 | Chl a mg g−1 FW | Chl b mg g−1 FW | Car mg g−1 FW |
|---|---|---|---|
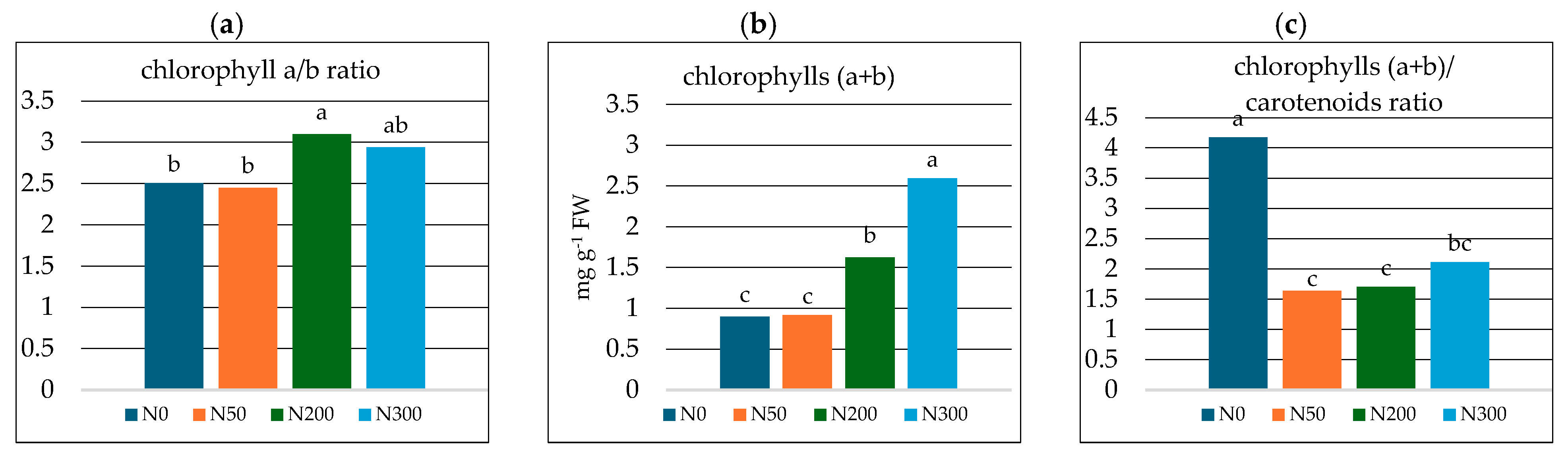
| * N0 | 0.641 c ± 0.021 | 0.256 c ± 0.022 | 0.215 d ± 0.015 |
| N50 | 0.651 c ± 0.019 | 0.266 c ± 0.021 | 0.561 c ± 0.055 |
| N200 | 1.227 b ± 0.063 | 0.396 b ± 0.035 | 0.952 b ± 0.074 |
| N300 | 1.936 a ± 0.088 | 0.659 a ± 0.070 | 1.231 a ± 0.086 |
| Nitrogen Dose kg N ha −1 | AAC mg 100 g−1 FW | AA % | CAT mg H2O2 kg−1 h−1 |
|---|---|---|---|
| * N0 | 115.0 a ± 1.53 | 54.79 c ± 1.17 | 15.87 c ± 0.846 |
| N50 | 87.4 b ± 1.11 | 58.12 c ± 1.53 | 16.22 c ± 0.952 |
| N200 | 12.3 c ± 0.863 | 67.56 b ± 1.89 | 21.96 b ± 1.09 |
| N300 | 8.7 c ± 0.258 | 82.35 a ± 2.56 | 29.85 a ± 1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemanowicz, J.; Bartkowiak, A. Chemical and Biochemical Properties of Common Nettle (Urtica dioica L.) Depending on Various Nitrogen Fertilization Doses in Crop Production. Sustainability 2025, 17, 6394. https://doi.org/10.3390/su17146394
Lemanowicz J, Bartkowiak A. Chemical and Biochemical Properties of Common Nettle (Urtica dioica L.) Depending on Various Nitrogen Fertilization Doses in Crop Production. Sustainability. 2025; 17(14):6394. https://doi.org/10.3390/su17146394
Chicago/Turabian StyleLemanowicz, Joanna, and Agata Bartkowiak. 2025. "Chemical and Biochemical Properties of Common Nettle (Urtica dioica L.) Depending on Various Nitrogen Fertilization Doses in Crop Production" Sustainability 17, no. 14: 6394. https://doi.org/10.3390/su17146394
APA StyleLemanowicz, J., & Bartkowiak, A. (2025). Chemical and Biochemical Properties of Common Nettle (Urtica dioica L.) Depending on Various Nitrogen Fertilization Doses in Crop Production. Sustainability, 17(14), 6394. https://doi.org/10.3390/su17146394







