Abstract
Permeate Gap Membrane Distillation (PGMD) is an emerging desalination technology that offers a promising alternative for freshwater production, particularly in energy-efficient and sustainable applications. This review provides a comprehensive analysis of PGMD, covering its fundamental principles, heat and mass transfer mechanisms, and key challenges such as temperature and concentration polarization. Various optimisation strategies, including Response Surface Morphology (RSM), Differential Evolution techniques, and Computational Fluid Dynamics (CFD) modelling, are explored to enhance PGMD performance. The study further discusses the latest advancements in system design, highlighting optimal configurations and the integration of PGMD with renewable energy sources. Factors influencing PGMD performance, such as operational parameters (flow rates, temperature, and feed concentration) and physical parameters (gap width, membrane properties, and cooling plate conductivity), are systematically analysed. Additionally, the techno-economic feasibility of PGMD for large-scale freshwater production is evaluated, with a focus on cost reduction strategies, energy efficiency, and hybrid system innovations. Finally, this review outlines the current limitations and future research directions for PGMD, emphasising novel system modifications, improved heat recovery techniques, and potential industrial applications. By consolidating recent advancements and identifying key challenges, this paper aims to guide future research and facilitate the broader adoption of PGMD in sustainable desalination and water purification processes.
1. Introduction
Overcoming the shortage of pure water is one of the crucial issues in our current world. Between the mid-1990s and mid-2000s, 1.8 to 2.9 billion people experienced severe water scarcity for at least 4 to 6 months per year, half a billion faced severe water scarcity all year round, and four billion people faced severe water scarcity for at least part of the year [1]. The projection shows that by the year 2050, water scarcity will affect around 2.7 to 3.2 billion people [2,3] as the world population reaches nine billion [4], accompanied by a 20–30% increase in water demand [5]. Extreme weather events like bushfires, floods, landslides, and droughts could be another reason behind the displacement of around 200 million people seeking fresh and clean water by 2050 [6]. This escalating water crisis poses a significant challenge to sustainable development worldwide [6]. The unequal distribution of freshwater resources and population density further complicates the problem [7]. While Earth’s total water volume is approximately 1.4 × 1018 m3 [8], covering 71% of the Earth’s surface [9], approximately 96.5% of water is in the ocean, leaving only 3.5% as freshwater [8]. The distribution of global water reserves is illustrated in Figure 1. Socio-economic development and population growth have increased water demand [10] and are exacerbated by climate change. With limited freshwater accessibility and rising human activities, meeting this demand appears increasingly difficult. As traditional freshwater sources become increasingly strained, solutions such as desalination, extracting fresh water from the sea, or brackish water beyond the hydrological water cycle have emerged as potential alternatives, particularly in coastal areas, where one-third of the population lives [11].
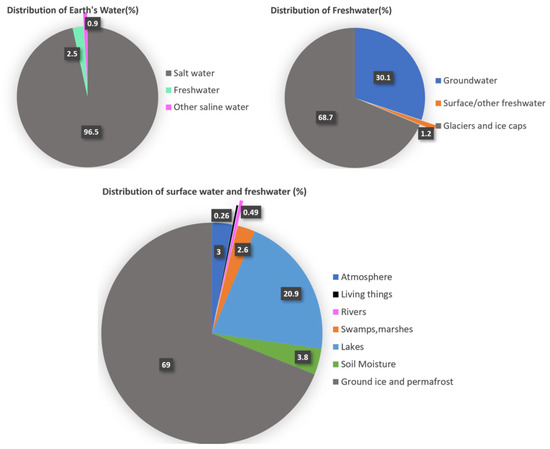
Figure 1.
Global water reserve(Numbers are rounded) [12].
1.1. Overview of Desalination Technologies
As of 2022, over 21,000 desalination plants produce nearly 110 million m3 of fresh water daily, benefiting more than 300 million people [13]. Figure 2 shows global trends in population growth, water consumption, and the number of desalination plants in operation. The popularity and necessity of desalination plants have resulted in the development of various technologies in desalination, which are mainly categorized into thermal- and membrane-based processes [14].
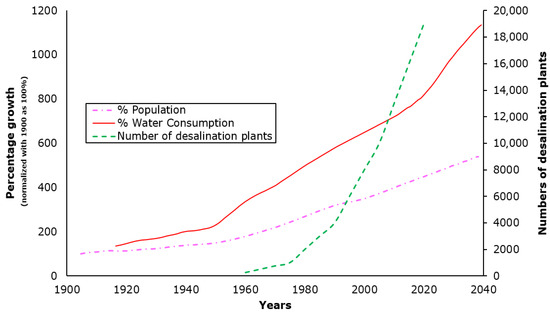
Figure 2.
Global trend on Population, water consumption, and desalination plants [5,15].
To better understand the mechanisms involved in desalination, Figure 3a illustrates the classification of desalination based on the mechanism of operation. The saturation pressure, osmotic pressure, and ion exchange are the driving forces, and how these driving forces play a role in desalination for different processes is shown in Figure 3b–d. Although all the processes produce potable water, the fundamental mechanism and type of energy consumed are the main differences. Understanding these principles is crucial in designing and optimising water treatment processes. The saturation point process relies on thermal energy for its operation. The saturation point represents the point of phase change with respect to operating pressure and temperature. The produced vapor as a result of thermal energy input is trapped and condensed to produce fresh water, leaving behind high-concentration brine. In short, the enthalpy of vaporisation (i.e., latent heat, ) must be overcome, which involves breaking the intermolecular forces between water molecules to transition from liquid to vapour (enthalpy of vaporisation). The high energy requirement for this phase change makes a significant factor in energy consumption. Multi-stage flash (MSF) and multi-effect distillation (MED) are popular desalination technologies that rely on the saturation point process [16].
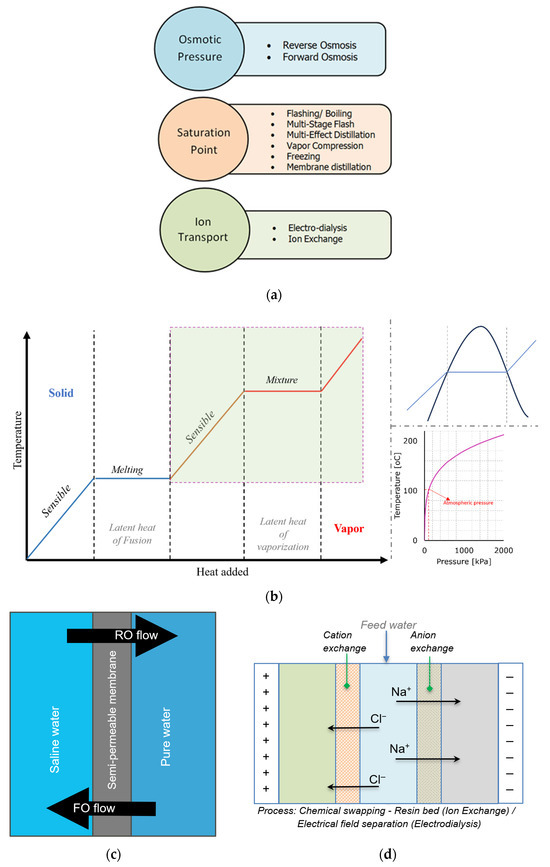
Figure 3.
Schematic diagram for saturation point process, osmotic pressure, and ion-exchange. (a) Classification of desalination based on the driving forces. (b) Saturation Point. (c) Osmotic Pressure. (d) Ion exchange.
On the other hand, the osmotic pressure process uses mechanical or electrical energy to operate. The osmotic pressure is related to the quantity of ions dissolved in the saltwater. It strongly influences the tendency of water to pass through the semipermeable membrane to separate fresh water from seawater. The separation of freshwater is based on differences in size, solubility, or other solute properties. The energy consideration here is related to the minimum work required to overcome osmotic pressure, derived from the Gibbs free energy of separation, ΔGsep, and the Gibbs free energy of mixing, ΔGmix. The driving force of water transport is the gradient in the applied pressure, balanced by frictional forces due to water-membrane and ion-water [17], while the ion-transport mechanism completely depends on the electrical field (electro-dialysis) or chemical (ion-exchange) energy. Here, the salt ions are displaced from the solution, passing through the cation-exchange and anion-exchange system. These systems can be resin beds in case of the ion-exchange process to assist chemical swapping or membranes in case of electrodialysis to separate electrically charged ions. The cation and anion migrate towards the cation and anion exchange system, which dilutes the feed water, resulting in an increase in concentration of water on the other sides of the resin/membrane [18].
Similar to the increasing demand for fresh water, the demand for energy is also increasing. Desalinating seawater can be energy-intensive if not properly optimised. Over the last two decades, researchers have focused their efforts on developing desalination technologies that are energy efficient. Table 1 shows the operating conditions and performance of the most common thermal and membrane-based technologies. Thermal-based processes (i.e., saturation point processes) are well known for their ability to handle high feed water salinities. However, they consume a large amount of energy, and to make them energy efficient, they must be cascaded in multiple stages. In contrast, membrane-based separation technology, such as reverse osmosis (RO), has low energy consumption but cannot treat highly saline (above 60,000 ppm) water, and produces large volumes of rejected brine, which poses an environmental risk. While the ion exchange process has similar energy efficiency as RO, it cannot handle feed water above 5000 ppm salinity. To overcome the above limitations, a thermally driven membrane-based desalination technology was developed by Bodell in 1963. The membrane distillation/desalination (MD) technology can, in theory, handle feed water close to saturated salinities and operate at low temperatures of around 60–90 °C, making it compatible with waste heat from various industries and renewable energy sources [19,20]. Utilising the waste heat of industries on the one hand can increase the efficiency of the industries, while on the other hand, it can reduce thermal pollution, for example, hot water discharge to water bodies. The technology can also utilise low-grade heat sources like geothermal and solar energy [21]. Similarly, unlike other desalination methods that are capable of treating high-salinity water, MD offers the advantage of achieving a higher water recovery ratio of 60–80%. Since the technology is in a developing phase, there are mentions of complexity, limitations on scalability, and relatively lower flux as limitations. In the following section, the development of MD-based desalination systems will be discussed.

Table 1.
Comparison between saturation point, osmotic pressure, and ion-exchange processes [22,23,24,25,26,27,28,29,30,31,32,33].
1.2. Membrane Distillation (MD) for Desalination
Membrane Distillation was first patented by Bodell in 1963, employing silicon rubber membranes for the vapour diffusion of saline water, and in 1967, the first journal paper on MD was published in “Industrial & Engineering Chemistry Process Design Development” [34]. However, MD received attention only in the early 1980s when membranes such as Gore-Tex Membrane (expanded polytetrafluoroethylene, PTFE, porous membrane supplied by Gore & Associated Co., Elkton, MD, USA) and modules with better characteristics became available [35].
Membrane distillation has a semipermeable membrane separating a feed and permeate stream of water. The feed stream is in direct contact with the membrane surface, where the hydrophobic properties and the vapour pressure differences facilitate the selective transport of water vapour across the membrane while preventing liquid water from passing through. The vapour on the permeate side becomes condensed and is collected as distilled water [18,36]. Based on the collection mode on the permeate side, MD has four basic configurations: Direct Contact Membrane Distillation (DCMD), Air/Permeate Gap Membrane Distillation (AGMD/PGMD), Sweeping Gas Membrane Distillation (SGMD), and Vacuum Membrane Distillation (VMD) [37]. Figure 4 shows the configurations and mechanisms of these processes.
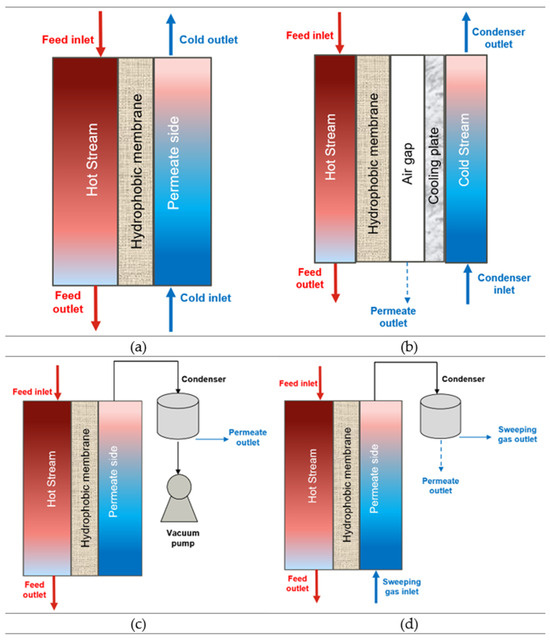
Figure 4.
Types of Membrane Distillation process: (a) DCMD, (b) AGMD/PGMD, (c) VMD, and (d) SGMD.
The performance of membrane distillation (MD) systems, particularly in terms of flux and energy consumption (SEC), is influenced by several key factors:
- Feed Temperature: Higher feed temperatures increase the vapour pressure difference across the membrane. This means raising the feed temperature enhances the flux production for all the modules. However, higher temperatures can also increase energy consumption [38].
- Condenser Temperature: Lower condenser temperatures create a higher driving force for vapour transport, improving flux [39].
- Flow Rates: Increased feed and condenser flow rates enhance the heat and mass transfer coefficients by transitioning the laminar flow rate into the turbulent regime, improving flux while minimizing temperature polarization [40].
- Membrane Characteristics: Membrane porosity, pore size, hydrophobicity, and thermal conductivity directly affect vapour transport efficiency and resistance to fouling or wetting [41].
- Gap Width: In configurations like AGMD/PGMD, the width of the permeate gap influences the heat recovery and temperature distribution, impacting both flux and SEC.
- Thermal Conductivity of Materials: High-conductivity materials in cooling plates or membranes improve heat recovery, reducing thermal losses and energy requirements.
1.3. Permeate Gap Membrane Distillation (PGMD)
The permeate gap membrane distillation (PGMD) improves upon conventional MD configurations such as DCMD and AGMD through its unique design, which incorporates a permeate gap between the membrane and the condensation surface. This gap is filled with water, which serves as a medium for latent heat recovery and reduces thermal losses [42]. Table 2 shows the key advantages and limitations of PGMD over DCMD and AGMD, including the following:

Table 2.
Comparative performance of MD configurations.
- Improved Heat Recovery: The water gap in PGMD facilitates effective internal heat recovery, reducing specific thermal energy consumption.
- Enhanced Thermal Efficiency: The water inside the permeate gap reduces the heat loss, lowering mass transfer resistance.
- Design Flexibility: PGMD allows for innovative gap modifications, such as propellers or gap circulation, further enhancing heat and mass transfer.
DCMD had always been the researchers’ first choice for experimental studies. As of today, DCMD has gained significant attention from technical reviewers, as evidenced by numerous reviews dedicated to DCMD [50,51,52,53,54,55], along with AGMD [56,57]. However, PGMD, an innovative and promising variant of membrane distillation, remains relatively underexplored. Despite its potential advantages, such as higher thermal efficiency, improved heat recovery, and suitability for coupling with renewable energy systems, limited studies and reviews have been conducted on PGMD. This lack of consolidated knowledge challenges its border adoption and optimisation.
Therefore, it is imperative to bridge this gap by accumulating the existing research on PGMD. A comprehensive review focusing on PGMD can provide valuable insights into its unique design features, operational parameters, modelling techniques, experimental studies, membrane materials, and potential applications. Such a review study will guide future research directions and drive technological innovations in PGMD. Moreover, by exploring its compatibility with renewable energy integration, this review can contribute to advancing sustainable desalination. This paper aims to address these gaps by offering a detailed analysis of PGMD, thereby fostering a deeper understanding of its capabilities and paving the way for its broader implementation in both academic research and industrial applications.
2. Fundamentals of Permeate Gap Membrane Distillation
2.1. Mechanism of Freshwater Production in PGMD
The permeate gap membrane distillation (PGMD) configuration is alternatively referred to as water gap membrane distillation (WGMD) and liquid gap membrane distillation (LGMD). In PGMD configuration, the hydrophobic membrane is in direct contact with the feed solution and the permeate on the other side. The cooling plate is situated between the permeate and the condenser channel. The condenser could be any other liquid. Figure 5 shows the PGMD construction with a gap between the membrane and condenser plate filled with permeate (freshwater). Due to the hydrophobic nature of the membrane, the selective transport mechanism enables the separation of water and salt. The vapour difference in the thermal gradient across the membrane drives the transfer of vapour mass from the feed to the permeate side. As shown in Figure 5, the feed side’s bulk temperature decreases to at the membrane water interface on the feed side. The evaporated water vapour from the hot stream (feed side) passes across the hydrophobic membrane towards the permeate side and is condensed at the permeate temperature of Tp. The temperature of the condenser (Tc,c) on the other side of the cooling plate is less than that of the permeate temperature Tp. The difference in temperature creates a vapour pressure difference between the feed side of the membrane and permeate side of the membrane , which is the driving force for the operation of PGMD for heat and mass transfer. The following section explains the heat and mass transfer modelling for PGMD.
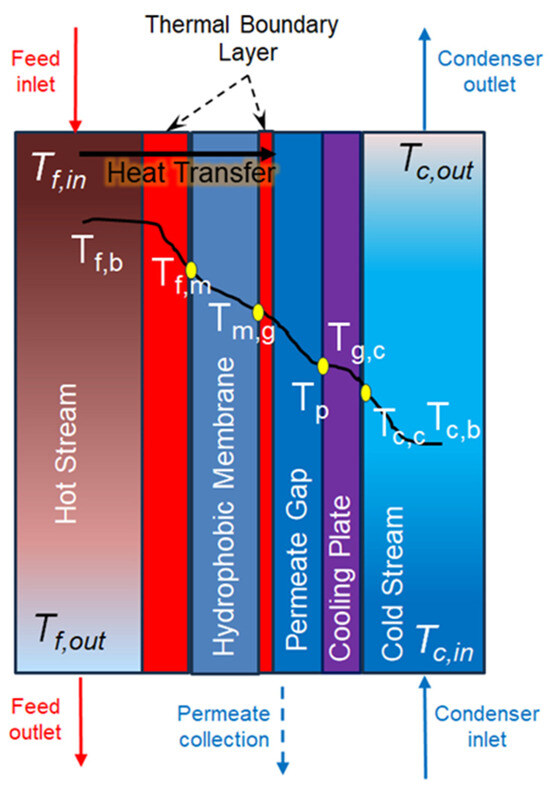
Figure 5.
Temperature variation inside the PGMD flat sheet module.
2.2. Heat and Mass Transfer in PGMD
In PGMD, the vapour from the feed side is transferred through diffusion, allowing vapour molecules to move across the membrane through pores and condense at the cooled surface. Additionally, heat transfer is affected by both conduction and mass transfer. Some heat is lost through conduction across the membrane material, while latent heat is carried with the vapor as it moves through the membrane and condenses on the permeate side. The interplay between these heat and mass transfer processes determines the overall efficiency of the system.
2.2.1. Heat and Mass Transfer Modelling
Heat transfer: Heat transfer occurs in PGMD by the following mechanism [58]:
- (a)
- Convection heat transfer from the feed bulk to the membrane surface,
- (b)
- Pure conduction heat transfer through the solid portion of the membrane, and heat transfer associated with evaporative vapour flux passing through the membrane.
- (c)
- On the permeate side, the heat transfer is due to pure conduction in the case of a stagnant permeate gap. However, in most cases, natural convection is considered.
- (d)
- Conduction heat transfer passes passing through the cooling plate.
- (e)
- Convective heat transfer passes passing from the cooling plate to the condenser water.
The rate of heat transfer within the PGMD module is a steady-state process [59]
The coefficient of heat transfer of the feed () and condenser () stream depends upon the Nusselt number as follows [60]:
In permeate, the effective thermal conductivity of the water is determined by the following [58]:
In the permeate, heat transfer within the boundary layers is initially dominated by conduction. However, natural convection can be surpassed due to the temperature gradient across the boundary layer, as characterized by the Rayleigh number () [61]. For < 2000, natural convection inside the permeate gap is neglected. Conversely, > 3 × 103, a turbulent regime is anticipated to form within the permeate gap, and the exponent of () is (1/4). For effective thermal conductivity () of the permeate gap while considering natural convention, Alawad et al. [58] modified the Nusselt number of water inside the gap by replacing (1/4) with (1/5), considering the Rayleigh number to be > 2 × 108 for a permeate gap width bigger than 32 mm at 75 °C.
For the novel PGMD, Lawal et al. installed an impeller within the permeate. The modification introduced three more heat transfer mechanisms: (1) convective heat transfer between the membrane and impeller, (2) convective heat transfer between the impeller and cooling plate, and (3) conductive heat transfer through the impeller blade [62].
Mass transfer: Knudsen diffusion, Knudsen-molecular diffusion, molecular or ordinary diffusion, and viscous or Poiseuille flow are the mechanisms responsible for the vapour transport through the pores of the membrane in the Dusty Gas (DG) Model. In the DG Model, the average pore size is considered for predicting the mass transfer of volatile water molecules, assuming an average temperature across the membrane. The following steps govern water vapour transport across the membrane in the PGMD system, facilitating the separation of solutes.
- (a)
- Vaporization: Initially, water transitions from the liquid phase to the vapour phase. This phase transformation occurs at the interface where the feed solution contacts the membrane surface.
- (b)
- Membrane passage: Water vapour diffuses through the membrane pores because of vapour pressure differences across the membrane. The vapour pressure on either side of the membrane, as well as within the membrane pores, is determined by the following Antoine equation [63]:
The water vapour pressure inside the pore is as follows:
The mean temperature of the membrane is as follows:
The mass transfer through the membrane is influenced by the difference in vapour pressure across the membrane and the salinity of the feed solution. Salinity is correlated in the following equation, where is the molar fraction of NaCl and is the molar fraction of water in the solution [64].
Since MD processes are operated under atmospheric conditions, ≈105 Pa [65], the total pore pressure is assumed to remain the same. The air pressure in membrane pores is the difference between total and vapour pressure in the pores [66]
In PGMD, Knudsen diffusion and molecular diffusion mechanisms are responsible for mass transfer through membrane pores. The resistances associated with Knudsen and molecular diffusion [58,59], along with total resistance, are expressed as follows [58]:
Here, represents the ratio of Knudsen to Molecular diffusion, is the diffusion coefficient, and is the total pressure inside the membrane pore (water and air). For air/water, the product (Pa m2/s) is calculated from the following equations [67]:
The Knudsen number () is given by the following:
The mean free path, , of vapour molecules is defined as below [68]:
where, is the Boltzmann constant (1.381 × 10−23 J/K and is the collision diameter () of water molecules.
- (c)
- Condensation: Finally, the water vapour condenses back into the liquid phase. The condensation occurs at the interface of the cooling plate within the permeate channel [69]. The distillation flux (), is postulated to be directly proportional to the vapour pressure disparity across the membrane and is calculated as follows [42,46,69,70]:
Here, depends on membrane properties and gas conditions within the membrane pores [70]. To determine accurate experimental values for temperatures and presents challenges and a practical approach is to use bulk temperature.
2.2.2. Temperature and Concentration Polarisation
Temperature polarisation is when the temperature at the membrane interface differs from the bulk feed solution [46]. During evaporation, latent energy is released with the vapour, resulting in a temperature decline at the membrane water interface () compared to the bulk feed solution , creating a thermal gradient layer [71] at the region near the membrane surface where the heat transfer occurs principally through conduction. The developed thermal boundary layer affects temperature gradients, which in turn influence the intensity of temperature polarisation [72]. A thinner thermal boundary layer on the feed side favours heat transfer, increasing the interfacial temperature differences, and amplifies the vapour pressure gradient, thereby improving the mass transfer rate. As per Antoine’s equation, temperature and vapour pressure have an exponential relation during phase change transitions, such as evaporation [73,74]. A decrease in the interfacial temperature reduces vapour pressure, diminishing the driving force for the mass transfer across the membrane [75]. Temperature Polarisation Coefficient serves as an indicator for evaluating temperature polarisation. It can be computed from the temperature difference between the values of two sides of the membrane surface to the bulk. In membrane distillation, spacers are often used as a turbulent promotor to decrease the phenomenon of temperature polarisation. Mathematically, TPC is expressed as follows [76,77]:
Gao et al. [78] observed that the TPC was affected by factors such as hollow fibre packing density and feed velocity. When the feed velocity was increased from 0.23 to 0.81 m/s (still within the laminar flow regime), the thermal boundary layer was reduced, decreasing temperature polarisation and enhancing mass transfer. The TPC value observed was in the range of 0.2–0.5.
At a stable state, within membrane distillation, the convective movement of solutes towards the membrane surface is precisely counteracted by the diffusive flux of solutes moving away from the membrane surface towards the bulk. This state of equilibrium establishes a stable and consistent environment in the membrane distillation process. However, solute particles become deposited onto the membrane surface over time, gradually increasing their concentration at the membrane interface. This concentration builds up results in a diffusive flow of solutes from the high concentration region (membrane interface) to the low concentration region (bulk feed), a phenomenon known as concentration polarisation [46,79]. The concentration polarisation coefficient evaluates the concentration polarization as follows [46,58,77,80]:
The Nernst film model is used to find salt concentration on hydrophobic surfaces as follows [77]:
Increasing solute concentration on the feed side reduces the feed side vapour pressure, thereby reducing the vapour pressure gradient and mass transfer coefficient [81]. Operating at feed temperatures in the range of (50–90) °C, flow rates of 1.5 L/min, 3 L/min, and 6 L/min, Alawad et al. [58] concluded that the CPC increased with rising feed temperature but decreased with higher feed flow rates. The CP was maximum around 1.04 at a feed temperature of 90 °C and a feed flow rate of 1.5 L/min. Increasing the feed flow facilitated better mixing within the concentration boundary layer, mitigating CP.
In summary, temperature and concentration polarization significantly impact membrane distillation performance by reducing the driving force for mass transfer. Temperature polarization occurs due to the development of a thermal boundary layer at the membrane interface, reducing the interfacial temperature and vapour pressure difference. Similarly, concentration polarization arises from solute accumulation at the membrane surface, which decreases vapour pressure and impairs mass transfer. Both effects can be mitigated by employing strategies such as increased feed velocity, optimised spacer design, and enhanced mixing. Understanding and controlling these phenomena is crucial for improving the efficiency and scalability of membrane distillation processes.
2.3. Optimisation Strategies for PGMD
Different literatures have used different optimisation techniques to find the optimum values of the operating parameters such as inlet feed temperature, feed flow rate, condenser inlet temperature, condenser flow rate, and the water gap thickness for PGMD.
2.3.1. Response Surface Morphology (RSM)
Response Surface Morphology (RSM) is a mathematical and statistical tool aimed at optimising and analysing the responses influenced by various qualitative variables. At first, an experimental plan is designed to obtain a response value. It then establishes a mathematical model aligned with the obtained data. The primary objective is to forecast the values of the qualitative variables that lead to optimal responses. The predicted value (denoted by R) is determined by the quantitative levels of operating variables (Φ1, Φ2,… Φk), indicating a functional relationship denoted as follows [82]:
R = f(ϕ1, ϕ2, ……, ϕk)
Equation (17) function can take various forms, such as linear, factorial, or higher-order expressions. Generally, the first-order model is suitable for a linear relationship and is expressed as follows:
R = C0 + C1. Φ1 + C2. Φ2 +……..+ Ck. ϕk
On the other hand, a second-order model can be expressed as follows:
R = C0 + C1. Φ1 + C2. Φ2 + C11. Φ211 + C22. Φ222 + C12. Φ1. Φ2
Ruiz-Aguirre et al. [82] proposed the RSM method to optimise the flux and STEC by changing the feed flow rate, condenser temperature, and inlet temperature in the RSM model. The developed model was statistically evaluated by the analysis of variance (ANOVA), concluding that the RSM model is suitable for predicting system flux and specific energy consumption in the PGMD module.
2.3.2. Differential Evolution Techniques
Conventional configurations like AGMD, DCMD, SGMD, and VMD have been extensively studied to optimise operation parameters to enhance flux or reduce energy consumption and production costs. In the case of PGMD, Alawad et al. [83] employed the differential evolution (DE) technique to determine the optimum values for operational parameters. DE is an optimisation technique based on population dynamics and is characterized by simplicity, speed, and robustness. It utilised real-valued coding for floating-point numbers, with key parameters in DE including the crossover constant (CR), population size (NP), and scaling factor (F), all of which can be easily adjusted. The algorithm generates candidate solutions (denoted as ) to form a population () for each generation, where each candidate solution comprises a specific set of variables (xnj), corresponding to the problem’s dimension. The structure is expressed as follows [84]:
: generation, : population size.
: problem dimension.
Alawad et al. [83] used a one-dimensional heat and mass transfer model to optimise flux, specific energy consumption, and unit production cost. At a feed flow rate of 3.246 L/min, the inlet temperature of 90 °C, the condenser inlet temperature of 5.014 °C, condenser temperature flow rate of 4.875 L/min, and gap thickness of thickness 2 mm, the maximum flux was 136 kg/m2h, minimum water production cost was 7 $/m3 and lowest specific thermal energy consumption was 876 kWh/m3.
Hariri et al. [85] advanced PGMD optimisation by integrating Artificial Neural Network (ANN) with DE in the Permeate Gap Membrane Distillation with Circulating Gap Water (C-PGMD) system. By comparing two heat sources, waste-heat and electrical sources, excluding gap thickness, unlike in the conventional PGMD system. For the waste heat source, optimal performance was achieved with 12 stages, a feed temperature of 90 °C, a 4.8 L/min feed flow rate, and gap circulation rate per stage, and a condenser flow rate of 4.64 L/min, resulting in a flux of 555 L/m2h (@20C 553.89 kg/m2h) and an STEC of 592 kWh/m3. For electrical heat source, the optimal configuration also involved 12 stages and a feed temperature of 90 °C but with a feed flow rate of 4.6 L/min per stage, and 5 L/min condenser flow rate and gap circulation rate per stage, achieving a flux of 544 L/m2h (@20C 542.912 kg/m2h) and an STEC of 591 kWh/m3.
In real-world applications, the optimisation of PGMD faces challenges due to fluctuations in operating parameters, making it impractical to maintain optimum conditions consistently. Advanced techniques like Response Surface Morphology (RSM) and Differential Evolution (DE) could address this by identifying robust operating ranges rather than fixed points. RSM aids in predicting system behaviour under varying conditions, while DE adapts to parameter variability to maximize performance. These methods improve flux, energy efficiency, and cost-effectiveness and enhance system resilience, ensuring reliable operation despite real-world inconsistencies in feed temperature, flow rates, and other variables. This adaptability is critical for practical desalination systems.
2.3.3. Computational Fluid Dynamics (CFD) Modelling
CFD analysis is an advanced and realistic modelling method for a more detailed study of fluid dynamics and thermal and mass transfer phenomena. For membrane distillation, it involves wake flow around spacer and turbulence created by it, selective permeability of vapour through transmembrane boundary, loss of latent heat energy due to mass transfer, porous flow and shear stress at the pores, direct contact condensation in permeate channel, release of latent heat and subsequent rise in temperature of the condenser fluid, along with temperature polarization and concentration polarization. These phenomena, together with basic fluid flow principles on mass, moment, and energy, resolve the thermal-fluid behaviour in the system. There is limited CFD analysis in PGMD; here, we review those works. Birgi et al. [86] developed an experimentally verified 3D computational model of PGMD in Star-CCM+ to identify the influencing factors for their system’s permeate flux and thermal efficiency. The model has a 16 cm × 12 cm membrane surface area and a depth of 4 mm. The k-E turbulence model was used for both feed and permeate channels. The factorial analysis design was used to identify the membrane distillation coefficient as the most influential parameter for the unit’s performance. Figure 6 shows the information on the computational model and its relevance.
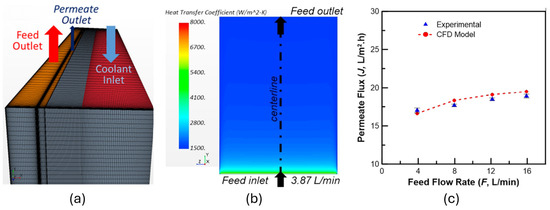
Figure 6.
Computational model and its relevance [86]. (a) Computational model. (b) HT coefficient contour. (c) Relevance of the model.
Elbessomy et al. [87] developed an experimentally verified 2D axisymmetric fully coupled model involving concentrated solution, membrane, and condenser channel for hollow fibre water gap membrane distillation in COMSOL Multiphysics software. The computation was performed assuming laminar flow in the feed and condenser channels, stagnant water in the gap, uniform fibre porosity, negligible heat loss, and ignoring the wetting and fouling factor of the module. The study showed the possibility of freshwater production (1 kg/m2 h−1) at ultra-low temperatures, i.e., 25 °C at feed and 5 °C at condenser. Figure 7 shows the computation model temperature polarization at the feed channel and the flux and productivity of the unit at the lower range of feed temperatures. This strongly indicates the feasibility of using WGMD in low-grade waste heat recovery. In another study by Elbessomy et. al. [88] on 3D helical fibre configuration using COMSOL, similar assumptions were made to perform a detailed study of the helical configuration of the membrane, with a feed temperature of 70 °C and 50 turns of single helix, 25.3 m3 of freshwater per unit per day. With three stages of such a unit, the GOR increases by 117.6%.
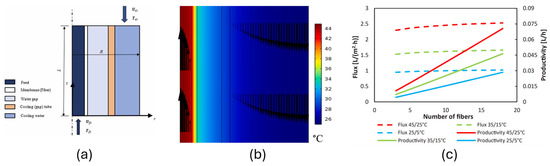
Figure 7.
Computational model of temperature polarization at the feed channel and the flux [87]. (a) Computational model. (b) Velocity contour. (c) Feasibility of WGMD at low temperature range.
3. Design, Development, and Testing of PGMD Systems
3.1. Optimal System Configuration for PGMD
Configuration of multi-stage and pure water flow in the gap could be analysed and provide a more comprehensive understanding of the PGMD system’s potential, limitations, and optimal configuration for practical implementation and commercialization, aiding future researchers in developing suitable rigs for application. Figure 8 shows the configuration for a multi-stage permeate gap MD system. Gao et al. [89] investigated the impact of a hollow fibre multi-stage PGMD process on energy efficiency using Gain Output Ratio (GOR) as an indicator. They observed that the increasing stage increased the GOR, rising from 0.12 (single-stage) to 2.4 (20-stage). A GOR above 1 was observed in systems with more than nine stages, indicating that a multi-stage process can conserve more thermal energy with internal heat recovery. Alawad et al. [64] used a theoretical model to compare a solar power multi-stage system with an electric heater-based multi-stage system, as previously analysed by Alawad et al. [83]. Integrating the solar power system boasts roughly 107% improvement in GOR. In the single stage, the highest daily production of 11.7 L/day was achieved in June, while the lowest, 10.3 L/day, was produced in January in Dhahran City, Saudi Arabia. This production was increased by 52% with the three-stage system. When eight stages were connected with three solar collectors, the productivity increased by 170–200%, and GOR improved by 55% compared to the single stage with one collector, achieving a flux of 70 L/day [64].
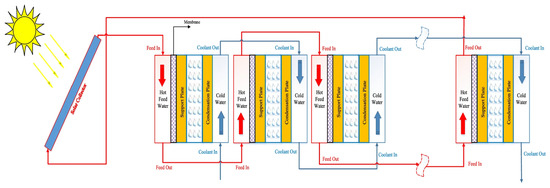
Figure 8.
Multi-stage permeate gap MD system-series stages connections [64].
Cipollina et al. [90] developed a multi-stage lab prototype to scale it up into a larger pilot system for small, remote communities using solar thermal energy. They observed that the GOR increased 20-fold when the system was expanded from a single stage to nine stages, operating at a feed flow rate of 0.5 L/min and a hot feed temperature of 80 °C, with a total membrane area of 2.28 m2. Khalifa and Alawad [39] investigate the multi-stage (3 stages) AGMD and WGMD viz series, parallel, and mixed. Additionally, specific energy consumption and distillate productivity for single- and multi-stage systems were compared. The single-stage WGMD had a permeate flux approximately 2 to 2.5 times greater than the single-stage AGMD system. While the parallel flow arrangement of the three-stage yields higher permeate flux, ranging from 23.8 kg/m2h to 30.3 kg/m2h, compared to the series flow arrangement, from 21.9 kg/m2h to 27.8 kg/m2h of WGMD. The orientation of water flow within the gap channels of the PGMD system plays a critical role in determining its operational performance. Swaminathan et al. [91] investigate the impact of water flow configuration in the gap, counter-current flow, parallel flow, and perpendicular (crossflow) in PGMD and CGMD (Conductive Gap MD). The CGMD system includes a conductive plate within the gap of the PGMD system. In counter-current flow, the pure water flows opposite to the condenser fluid across the condensing plate. Since the water exiting the cold stream in counter-current configuration retains a higher temperature, less preheating is required, resulting in a higher GOR than other configurations in both PGMD and CGMD. For PGMD, counter-current flow achieved a flux of 5.8 L/m2h (@20C 5.7884 kg/m2h). Multi-stage PGMD processes often optimise internal heat recovery, improving energy efficiency at specific stages when compared to single-stage processes.
The challenge of low permeate flux in MD processes, compared to conventional desalination methods like reverse osmosis (RO), can be mitigated by employing multi-stage designs.
3.2. Integration of PGMD with Sustainable Energy Sources
Recently, a concerted effort has been made to integrate MD with renewable energy sources. The goal is to minimize reliance on conventional fossil fuels for thermal energy generation in MD, thereby reducing its environmental impact. Solar energy is a significant renewable resource harnessed for thermal energy production in MD systems. Notably, studies have explored solar-driven membrane distillation (SDMD) and the combination of MD with solar-generated steam (SGSP) as sustainable approaches, leveraging environmentally friendly solar power. While existing studies predominantly focused on DCMD, VMD, and AGMD configuration of MD [92,93,94,95], limited studies have explored the application of solar energy in PGMD.
Alquraish et al. [96] established a PGMD solar pilot project in Kairouan, Tunisia, to study its performance under local climatic conditions. The system operated with feed flow rates ranging from 200 to 600 L/h, subject to fluctuating solar radiation from 7:00 AM to 5:00 PM. The unit utilised a solar collector with an area of 6 m2 and no thermal storage tank, achieving a daily production of approximately 15.92 L/m2h (@20C 15.88 kg/m2h) when solar radiation peaked at 978.27 W/m2. The specific thermal energy consumption (STEC) varied between 90 and 310 kWh/m3. For a practical approach, Cipollina et al. [90] proposed a solar-powered PGMD prototype for rooftop installation at the University of Palermo Chemical Engineering Department. Under operating conditions of an inlet temperature of 80 °C, a membrane area of 2.28 m2, a solar collector area of 11 m2, and a thermal storage tank volume of 1.3 m3, the PGMD system achieved a permeate flux of 12 L/m2·h, which is 20% higher than that of the AGMD system. However, specific details regarding solar power installation were not provided in the study. PGMD configuration can be integrated with low or medium-grade waste heat sources ranging from 25–80 °C. Elbessomy et al. [87] designed a compact hollow fibre water gap membrane distillation module with a shell diameter of 50 mm, 91 fibres, 100 mm effective module length, 1.62 m/s feed in velocity, and 0.0652 m/s condenser velocity, integrated with ultra-low grade waste heat sources. At a feed-in temperature of 25 °C and a 5 °C coolant temperature, the specific product rate was 4.8 , while at 80 °C of feed in temperature and 25 °C coolant in temperature the specific product rate was 76.2 . The specific product rate is the amount of freshwater produced per day per cubic meter of the module volume. Figure 9 shows the PGMD powered by waste heat.
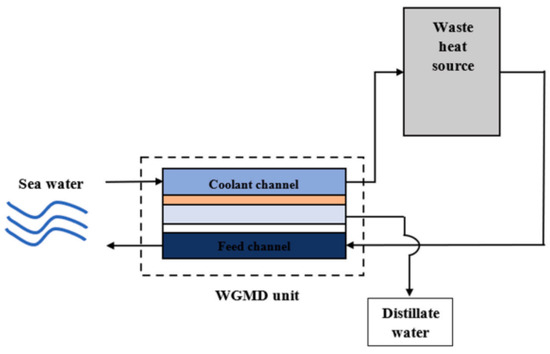
Figure 9.
PGMD powered by waste heat [87].
Although lab-scale experiments have shown encouraging results, scaling up to pilot and real-world applications often yields differing outcomes. Zaragoza et al. [97] used two different test-bed solar thermal fields at Plataforma Solar de Almeria in SE Spain to evaluate different commercial and pre-commercial MD prototypes. These facilities included custom flat-plate collectors (Solaris CP1 Nova) with a nominal thermal power of 7 kW, operating at approximately 90 °C using water as the heat transfer medium. The systems were connected to thermal storage units to maintain a steady-state heat supply when needed. Saline water temperature was regulated using a cooler capable of handling saline solutions, and the feed was heated using solar thermal energy via a heat exchanger before being reintroduced into the MD modules. Distillate flux improved with higher feed flow rates; however, flux values in pilot-scale systems were significantly lower than those observed in lab-scale modules. The concentration factor or rejection ratio (RR) was below 10% across most modules, with the exception of the WTS-40A module, which exhibited performance comparable to larger-scale desalination technologies like reverse osmosis. The distillate quality was high, achieving a feed conductivity reduction of up to four orders of magnitude. However, specific thermal energy consumption (STEC) varied widely among the modules. Flat-sheet membrane-based SC and M33 modules exhibited high STEC, indicating suboptimal heat recovery. In contrast, the spiral-wound Oryx 150 LGMD module achieved the lowest STEC, approximately 210 kWh/m3. Additionally, connecting PT5 modules in series reduced STEC by a factor of three compared to standalone operation, highlighting the potential of spiral-wound and multi-stage modules for improved energy efficiency. The prototypes used for the experiments are listed below in Table 3.

Table 3.
Tested modules by different manufacturers and configurations, Zaragoza et al. [97].
A pilot-scale solar-powered C-PGMD unit located in Dhahran city, Saudi Arabia, Hariri et al. [30] set up 30 C-PGMD stages with an 8 mm gap width. The system operated with a feed flow rate of 5.6 L/min, a condenser flow rate of 4.6 L/min, an inlet temperature of 70 °C, and a permeate circulation rate of 2.3 L/min per stage in three heating scenarios: (a) electric heating, (b) solar thermal heating, and (c) a hybrid solar-electric approach. The solar-only configuration required at least 25 flat-plate collectors to maintain optimal operating temperatures. The hybrid system demonstrated the highest daily water production, reaching 511 L/day with 40 flat-plate collectors, outperforming both solar-only (220 L/day) and electric-only (445 L/day) scenarios.
The combination of PGMD with renewable energy sources, particularly solar power systems, is increasingly being investigated to lessen reliance on fossil fuels and mitigate environmental impact.
Studies demonstrate the potential of solar-driven PGMD (SDMD) and solar-powered PGMD prototypes with improved energy efficiency and freshwater production. However, scalability challenges remain, and real-world pilot results show differing performance compared to lab-scale models. Hybrid solar-electric configurations, such as the C-PGMD unit in Dhahran, offer significant improvements in daily production. Additionally, the use of low-grade waste heat sources further enhances PGMD’s feasibility for sustainable desalination in remote areas, despite limited commercial applications.
4. Factors Influencing PGMD Performance
4.1. Operational Factors
4.1.1. Feed and Condenser Flow Rate
Alquraish et al. [96] demonstrated that increasing the feed flow rate from 200 L/h to 600 L/h led to a significant enhancement in distillation production, with flux increasing by 43.33% (from 4.5 kg/m2h to 9 kg/m2h), and a 62.63% (from 5 kg/m2h to 11.5 kg/m2h) across two experimental setups. Similarly, Cheng et al. [98] tested the effect of flow rate on the flux in AGMD and PGMD, concluding that PGMD exhibited a larger flux. Doubling the flow rate from 12 L/h to 24 L/h resulted in a 13.25% increase in flux (from 6 kg/m2h to 8 kg/m2h) in the PGMD system. Alawad et al. [58] examined the relationship between feed flow rate and temperature polarization. In the laminar flow at lower rates (1.5–3 L/min), TP has minimal effect, with a TPC value of 0.008. However, at flow rates exceeding 3L/min, where turbulence increased, TP becomes more significant, reducing the TPC value to 0.004. Despite the benefits of higher feed flow rates in enhancing flux, increased energy consumption and a decline in GOR were observed, as depicted in Figure 10. For instance, increasing the feed flow rate (1.5–6) L/min at 90 °C reduced the GOR from 1.3 to 0.5. Gao et al. [42] investigated the effects of different feed velocities (0.28–0.81) m/s at 70 °C feed inlet temperature on PGMD, DCMD, and SGMD. Flux improved across all configurations with higher feed velocity, but for similar flux, PGMD exhibited superior energy efficiency with lower thermal energy consumption compared to DCMD and SGMD. Based on CFD analysis by Elbessomy et al. [88], it was revealed that increasing inlet velocity (0.29–1.45 m/s) in a hollow-fibre water gap membrane distillation (HF-WGMD) module reduced salt concentration at the membrane interface from 65,401 ppm to 58,945 ppm and elevated the average temperature at the feed–membrane interface from 51.9 °C to 64.2 °C. Khalifa et al. [99] experimentally showed that turbulent flow was more pronounced at flow rates above 3 L/min in an 8 mm gap width compared to a 16 mm gap width. Beyond a feed flow rate of 5 L/min, flux improvements diminished, suggesting an optimal flow rate for balancing efficiency, energy consumption, and adherence to liquid entry pressure (LEP) constraints.
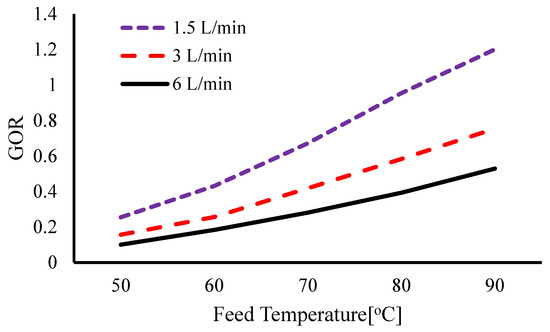
Figure 10.
Combined impact of feed temperature/flow rate on GOR [58].
For condenser flow rates between 1 L/min and 4 L/min, Alawad et al. [58] reported that flux was significantly impacted only at flow rates below 1 L/min, while 2 L/min was sufficient for effective heat transfer. Khalifa et al. [100] found negligible flux differences when increasing the condenser flow rate from 2 L/min to 4 L/min at a feed flow rate of 1.5 L/min and a feed temperature of 80 °C.
4.1.2. Feed and Condenser Temperature
Aguirre et al. [82] developed a predictive model correlating flux and STEC with inlet and condenser temperature. With a thermal power supply of 7 kW at 90 °C, increasing the feed temperature from 60 °C to 90 °C nearly doubled the flux while reducing STEC by 30%. At an evaporator inlet temperature of 80 °C and a condenser inlet temperature of 20 °C, the maximum flux was 2.66 L/m2h (@20C 2.65 kg/m2h) with a minimum STEC of 255.8 kWh/m3. Alawad et al. [58] observed an exponential increase in the flux by 410% when the feed temperature rose from 50 °C to 90 °C while the condenser temperature remained at 25 °C. Lowering the condenser temperature from 25 °C to 5 °C enhanced flux by 65% at 50 °C feed temperature, as depicted in Figure 11.
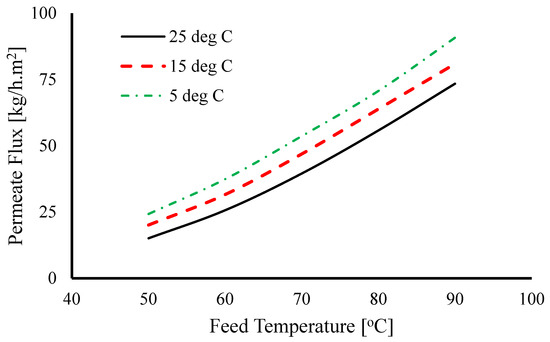
Figure 11.
Effect of feed temperature on permeate flux at different condenser temperatures [58].
In the study of Cipollina et al. [90], for the configurations PGMD, AGMD, and partial Vacuum Gap MD, the permeate flux increased with increasing temperature from 50 °C to 80 °C. However, PGMD performed superiorly to AGMD and partial VGMD. Due to enhanced energy recovery within the module and lower mass transfer resistance compared to the air gap in AGMD, the PGMD module achieved the highest flux. At the gap, the temperature on the permeate side of the membrane is lower compared to AGMD. The temperature differential enhances the vapour driving force. Furthermore, the increase in permeate flux observed in PGMD was associated with the proximity between the liquid/vapor interfaces on both sides of the hydrophobic thin top layer. At a feed temperature of 80 °C and a cold inlet temperature around 17 °C to 20 °C, the permeate flux obtained was 14–15 L/m2h (@20C 13.972–14.97 kg/m2h) and 10–12 L/m2h (@20C 9.98–11.97 kg/m2h) for PGMD and AGMD, respectively. Essalhi et al. [77] investigated PGMD using ethylene glycol as the condenser liquid and found higher flux rates at higher feed temperatures. The study also demonstrated a direct relation between feed temperature and duration to fill the permeate gap. As shown in Figure 12, at a lower feed temperature of 35 °C, the time taken to fill the permeate gap is much longer (2800 s), whereas at 80 °C, the duration decreased to around 200 s. Similarly, Francis et al. [61] showed for all the configuration, WGMD, AGMD, sand gap MD, and sponge (polyurethane) gap MD, at condenser temperature of 20 °C and flow rate of 1.5 L/min, the highest fluxes were achieved at feed temperature of 80 °C 20 kg/m2h, 6 kg/m2h, 11 kg/m2h, and 5 kg/m2h respectively. Increasing the inlet temperature from 40 °C to 80 °C, the flux was increased by 400% for sponge MD, 500% for AGMD, 450% for sand gap MD, and 566% for WGMD, showing the dominant effect of inlet temperature for WGMD.
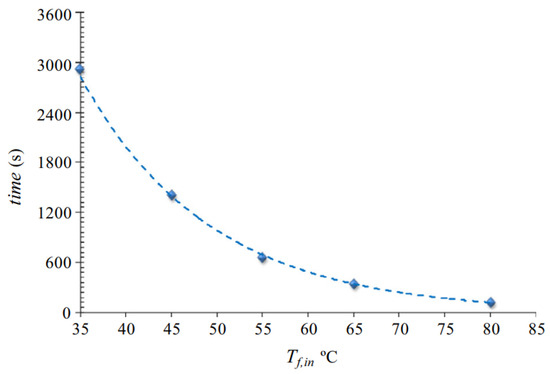
Figure 12.
Necessary time required to fill the permeate gap with the produced water as a function of the applied feed inlet temperature [77].
The effect of inlet temperature was examined in a comparison between DCMD, SGMD, and PGMD by Gao et al. [42]. All the modes were coherent in the increase in flux with the increasing temperature (40–70°C). While PGMD demonstrated the largest relative increase in flux with temperature (an 800% improvement compared to DCMD’s 266%), DCMD still maintained the highest absolute flux values, 30% higher flux, particularly at elevated feed temperatures. This suggests that temperature has a more pronounced effect on driving force in PGMD, but DCMD remains more productive in terms of total permeate output at higher operating temperatures. PGMD, DCMD, and SGMD have different trends in the global mass transfer coefficient. In PGMD, it increases initially from 40 °C to 60 °C, then decreases above 60 °C. At lower temperatures, increased transverse vapour flux breaks down the laminar boundary layer, enhancing heat transfer and reducing TPC, but at higher temperatures, temperature polarization becomes more severe, increasing TPC. In DCMD and SGMD, global coefficients decreased slightly with increasing temperature, indicating more severe temperature polarization at higher temperatures.
In a CFD of double helical fibre configuration with 50 turns, Elbessomy et al. [88] examined the feed inlet temperature in water vapour transportation. Increasing feed temperature from 40 °C to 70 °C increased the average vapour concentration difference across the membrane from 0.763 mol/m3 to 3.709 mol/m3 for a 50-turn double helical fibre module. Higher feed temperatures reduced STEC. The best STEC of 3.9 kWh/kg was achieved at 70 °C feed temperature with 50 single helical turns. Increasing condenser velocity to 0.21 m/s (initial velocity 0.0031 m/s) in a single fibre module reduced the average water gap temperature from 56.4 °C to 33.2 °C.
4.1.3. Feed Concentration
Alawad et al. [58] explore a solution concentration of various ranges, including pure and saline water (60,000 mg/L), across three distinct feed temperatures: 50 °C, 70 °C, and 90 °C. The reduction in flux with increasing feed concentration was more significant at lower temperatures, approximately 65% at 50 °C, whereas 40% at 70 °C, and the lowest of 30% at 90 °C, recommending using the highest feed temperature while treating high saline water. However, increasing feed temperature increases the concentration polarization coefficient, ranging from 1.012–1.04 from 50 °C to 90 °C, and continuous operation results in salt deposition on the membrane surface.
Using porous composite hydrophobic/hydrophilic membranes in PGMD and AGMD, Essalhi et al. [77] employed two feed solutions containing NaCl (at concentrations of 12,000 mg/L and 30,000 mg/L). The flux decreased as the concentration increased for both configurations while maintaining high salt rejection between 99.61% and 99.81%. The concentration polarization coefficient slightly increased with decreasing feed concentration for both PGMD and AGMD, suggesting an increase in feed flow rate to establish turbulent flow instead of laminar flow, ensuring the salt concentration at the membrane surface is closer to that of the bulk feed solution Figure 13.
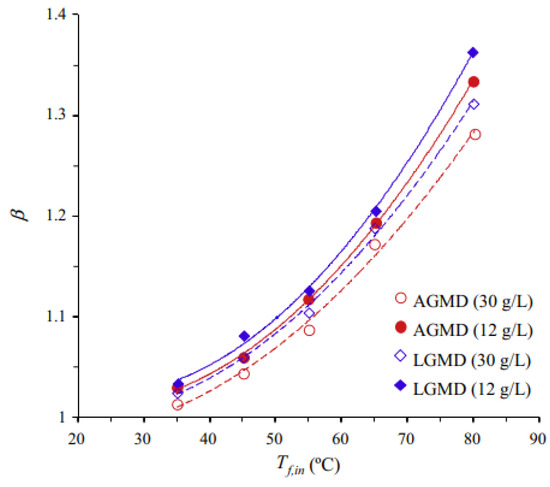
Figure 13.
CPC in AGMD and LGMD at different feed temperatures and NaCl concentrations [77].
Khalifa et al. [99] investigate the impact of gap circulation and cooling in the condenser channel on feed concentration using NaCl solutions of 150, 15,000, and 35,000 mg/L in various water sources: tap water, brackish water, and seawater. As feed concentration increased, vapour mass flux decreased due to reduced vapour pressure caused by salinity and membrane concentration polarization. This trend was consistent with and without gap circulation and cooling. The quality of the produced distilled water was within the range of 3–10 mg/L, with a 99.95% salt rejection rate even at a high feed concentration of 35,000 mg/L. While increasing feed concentration from 0 g/kg to 300 g/kg at 1 L/min feed flow rate, the flux decreased from 7 kg/m2h to 5 kg/m2h at 130 g/kg, 2 kg/m2h at 300 g/kg in the study carried by Mahmoudi et al. [49]. While most of the experiments are focused on different feed concentrations, Elsheniti et al. [101] investigated the treatment of high saline brine rejected from the RO system, ranging from 70,000 to 233,333 ppm, integrating with DCMD and PGMD configurations. While in such a high salinity, DCMD was superior in terms of flux production, the integration of PGMD exhibited a thermal advantage, achieving an 8.2% reduction in cumulative STEC compared to DCMD.
PGMD performance is influenced by feed and condenser flow rates, with higher flow rates, the flux is enhanced, but it increases energy consumption. Increasing feed temperature boosts flux, and a similar effect is observed when the condenser temperature is reduced. Feed concentration impacts flux; with higher concentrations, the performance degrades, particularly at lower temperatures, though higher temperatures help mitigate this effect.
4.2. Physical Factors
4.2.1. Gap Width
Alawad et al. [58] conducted simulations to examine the impact of varying water gap widths (1, 2, 4, 8, 16, and 32 mm) on vapour flux under different operating conditions. As the gap thickness increases from 1 mm to 32 mm, the vapour flux decreases Figure 14. A reduction in gap thickness from 32 mm to 1 mm resulted in a significant rise in vapour flux. At a feed temperature of 90 °C, the flux rose by 76% (from 64 kg/m2h to 119 kg/m2h), while at 50 °C, the flux increased by 108% (from 15 kg/m2h to 32 kg/m2h). However, narrowing the gap width from 32 mm to 2 mm yielded only a 20% improvement at 50 °C. For narrower gaps, such as 1 mm, heat transfer is primarily through conduction, while in wider gaps (8, 12, and 16 mm), natural convection plays a role, though its impact on flux remains limited. Similarly, at the gap widths of 9 mm and 13 mm, Francis et al. [61] could not find any significant difference in the flux production. Khalifa et al. [99] investigate the effect of 8 mm, 16 mm, and 32 mm of gap width on flux production with temperatures ranging from 50 °C to 80 °C. At 60 °C, the flux increased from 80 kg/m2h to 108 kg/m2h as the gap width expanded from 8 mm to 16 mm, but it slightly declined to 102 kg/m2h at 32 mm. At 80 °C, the flux increased from 164 kg/m2h to 201 kg/m2h between 8 mm and 16 mm, followed by a reduction to 189 kg/m2h at 32 mm. These findings highlight the importance of optimising gap width to maximize vapour flux while balancing heat transfer mechanisms.
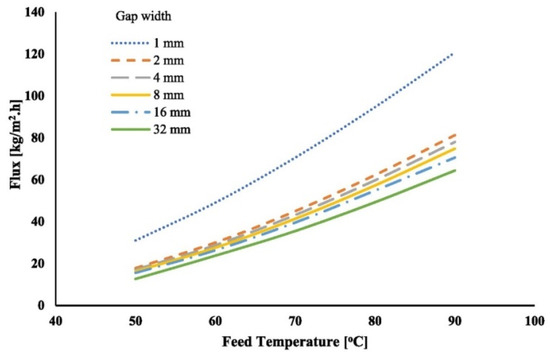
Figure 14.
Effect of feed temperature on permeate flux at different gap thicknesses [58].
4.2.2. Membrane Materials and Other Properties
The membrane serves as a critical physical barrier in the MD process. Hydrophobic microfiltration membranes are commonly employed in MD applications [102]. Two primary configurations dominate: hollow fibre membranes, typically fabricated from materials like polyvinylidene fluoride (PVDF), polypropylene (PP), and PVDF-PTFE composite [103,104], and flat sheet membranes, which are predominantly made from PP, PTFE, and PVDF [37].
The hydrophobic nature of these membranes ensures that water does not spread across their surfaces, leading to reduced contact and the formation of spherical water droplets due to their low surface energy [105]. This “water-repellent” property prevents pore wetting [106]. However, membrane wettability may occur if the hydrostatic pressure surpasses the liquid entry pressure (LEP). Maintaining operating pressures below the LEP, typically ranging from 0.5 to 3.5 bar for hydrophobic membranes, is essential to prevent feed solution infiltration [107]. LEP depends on factors such as the solution’s surface tension (), contact angle (), the size and shape of the membrane pores () as given by Laplace–Young equation. The concentration of the feed, and the presence of organic solutes and surfactants mater may not be explained in the Laplace–Young equation, but it has impact on and of membrane [108] as follows:
To ensure optimal permeate flux and prevent the wetting of membrane pores, the membrane requires specific attributes, including effective hydrophobicity [109], high porosity, desirable thermal stability, and excellent chemical stability [110]. Poor hydrophobicity can lead to pore wetting, diminishing rejection rates, and adversely affecting performance and lifespan [111]. Membrane design must also align with specific contamination challenges [112]. Khalifa et al. [113] evaluated PTFE and PVDF membranes with a 0.45 µm pore size in both PGMD and AGMD systems under various operating conditions. Results showed that PTFE membranes yielded higher permeate flux compared to PVDF membranes under identical conditions, attributed to PTFE’s superior hydrophobicity, higher porosity, thinner structure, and distinct thermal properties. The influence of thermal characteristics was particularly pronounced in PGMD systems. For example, at a feed temperature of 50 °C, PTFE membranes exhibited 160% higher flux than PVDF membranes, though this difference diminished to 7% at 90 °C [113].
PTFE Membrane
Polytetrafluoroethylene (PTFE) is widely recognized for its outstanding hydrophobicity, excellent thermal stability, and resistance to chemical degradation, making it a leading choice for MD applications. However, the manufacturing processes for PTFE membranes, such as extrusion, sintering, and stretching, present challenges that increase production complexity [34].
Exploring MD for off-grid water production, Cipollina et al. [90] developed a pilot MD unit using a commercial PTFE membrane (Gore™ Microfiltration Media GMM-203) supported by PP. Performance was evaluated across various MD configurations, including PGMD, AGMD, and VMD. The PGMD configuration outperformed others and was subsequently used for predictive analysis. The performance of a multi-stage system was observed by varying membrane area A1 = 0.042 m2, A2 = 0.084 m2, and A3 = 0.021 m2, changing the operating parameters such as feed inlet temperature and feed flow rate across three different module designs: a three-stage, six-stage, and nine-stage module. The distillation flux exhibited an increase of 140% when the feed flow rate was increased (1–4 L/min) or when the membrane surface was reduced (A3), either by decreasing the number of stages (stage 1 has the highest) or the individual membrane area at 80 °C. In both scenarios, the increase was associated with enhancing the transmembrane temperature differences while also reducing thermal integration within the system. Francis et al. [61] studied two PTFE membranes (M1 and M2) with similar active layers but different physical properties Table 4 within different modules: deionized water, polypropylene mesh, sand, and polyurethane (sponge). The M1 membrane achieved twice the flux (20.45 kg/m2h) compared to M2 (11.2 kg/m2h) at 80 °C feed and 20 °C condenser temperatures, attributed to its lower thickness and higher contact angle Figure 15.
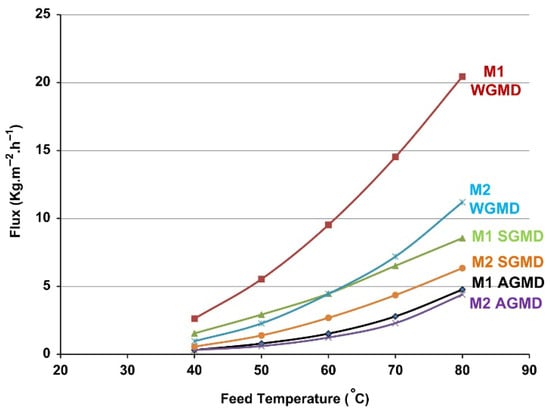
Figure 15.
Flux comparison between M1 and M2 membranes during the AGMD, sand gap MD, and WGMD in 13 mm gap width [61].
PVDF Membrane
Flat sheet [61,77,80,90,100] and spiral wound [114,115,116] PVDF membranes are widely used in MD research. Since hollow fibre membrane has a larger specific area without any supporting plate, Gao et al. [42] used Polyvinylidene Fluoride (PVDF) hollow fibre membrane manufactured by a Non-Solvent Induced Phase Separation (NIPS) process in Tianjin Polytechnic University with a 49 m2/m3 membrane packing density and eight fibers within the module for a comparative study between PGMD, DCMD, and SGMD under different operating conditions. At 70 °C and 0.81 m/s feed velocity, DCMD exhibited 30% higher flux than PGMD. PGMD demonstrates lower STEC at equivalent flux, favouring PGMD for combined flux and energy efficiency. Different hydrodynamic behaviours arise from variations in fibre packing density. Gao et al. [78] analysed the performance of five PGMD modules with varying parameters, such as the number of channels, hollow fibres, and cooling plate materials. A module with HDPE cooling plates, eight gap channels, and a membrane area of 0.0102 m2 achieved a flux of 9 L/m2h. Increasing hollow fibre packing density reduced the global mass transfer coefficient due to increased resistance. In an investigation aimed at examining the performance of hybrid PVDF membranes for WGMD and AGMD operation, five different wt% PVDF/PMMA (poly methyl methacrylate) were fabricated by Lin et al. [117] by blending PVDF with PMMA (wt%) in DMSO (dimethyl sulfoxide) as a casting solution, viz, MA-0, MA-2, MA-4, MA-6, and MA-8. MA-8 with higher PMMA produced the highest permeate flux of 19.12 L/m2h in WGMD at a hot feed flow rate of 0.6 L/min at 60 ± 0.2 °C. When compared with the performance of DCMD, though DCMD has the highest flux of 22.36 L/m2h (@20C, 22.31 kg/m2h), the GOR is lower compared to WGMD. Thus, the result concluded that the PVDF/PMMA composite membrane not only changed the surface morphology by increasing contact angle, porosity, and energy efficiency with an increase in PMMA wt% but also reduced the cost of manufacturing membrane in the MD system.
Other Membrane Materials
Recent developments in composite membranes have introduced hydrophobic–hydrophilic designs to minimize mass transfer resistance and reduce conductive heat loss. These membranes typically feature a thin hydrophobic layer atop a hydrophilic sub-layer [118,119,120,121]. Essalhi et al. [77] conducted a comparative analysis of PGMD and AGMD configurations using a bi-layered porous composite membrane with hydrophobic/hydrophilic properties fabricated via the phase inversion technique. The membrane incorporated a fluorinated surface-modifying macromolecule (SMM) with polyetherimide (PEI). Although PGMD exhibited higher mass transfer resistance, its enhanced heat transfer coefficient led to a marginally improved permeate flux (2.2–6.5%) and a salt rejection rate of 99.61%. Cheng et al. [98] evaluated PP hollow fibre membranes in PGMD and AGMD, reporting a 7.9% higher flux and 59.82% improvement in GOR for PGMD. Both configurations achieved over 99.8% salt rejection.

Table 4.
Summary of membrane characteristics used in PGMD.
Table 4.
Summary of membrane characteristics used in PGMD.
| Porosity (%) | Average Pore Size (μm) | Contact Angle (°C) | Thickness (μm) | Surface Area (m2) | References |
|---|---|---|---|---|---|
| PTFE | |||||
| - | 0.22 | - | 140–200 | 0.12 | [49] |
| 80 | 0.45 | 139 | 154 | 3.081 × 10−3 | [62] |
| 80 | 0.05 | - | 70 | 10 | [82] |
| - | 0.24 | 160 | 100 | 0.005 | [61] |
| - | 0.26 | 140 | 170 | 0.005 | [61] |
| 80 | 0.2 | - | 240 | 0.042 | [90] |
| 80 | 0.45 | - | 154 | 3.081 × 10−3 | [122] |
| 80 | 379 ± 8 nm | 140 | 153.9 ± 13.6 | 0.00724 | [100] |
| 80 | 0.45 | 139 | 154 ± 14 | 0.0066 | [123] |
| 0.22 | 100 | 140–200 | 0.12 | [124] | |
| PVDF | |||||
| 75 | 0.22 | 100 | 125 | 0.12 | [124] |
| 81.7 | 0.15 | ID = 102.8 OD = 96.4 | 180 | 0.0124 | [42] |
| 80 | 0.2 | - | 200 | 72 | [125] |
| Other membrane materials | |||||
| 80 | 0.046 | 94.8 ± 0.5 | 64.7 ± 6.3 | 5.53 × 10−3 | [77] |
| 68 | 0.2 | 110 | - | 0.1691 | [98] |
Other Membrane-Related Issues—Membrane Fouling
Membrane fouling, which refers to the deposition of organic and inorganic particles on the hydrophobic side of the membrane surface and pores, along with membrane wetting, represents a foremost challenge for MD-based systems’ long-term performance and sustainability [126,127,128,129]. These issues impede operational efficiency by blocking membrane pores and increasing the risk of wetting [130,131], leading to reduced permeate flux, higher energy consumption, and shorter membrane lifespan [132,133]. Table 5 outlines the mechanisms and effects of membrane fouling.
Several antifouling techniques have been proposed to combat fouling, including enhancing feed flow rates, employing hydraulic cleaning techniques, minimizing surface roughness [134], altering the membrane water-repelling properties through approaches such as magnetic water treatment, and modifying the membrane’s surface charge, which influences fouling behaviour [135,136]. Additionally, graphene-derived coatings have shown promise in enhancing both permeate flux and antifouling properties by improving surface characteristics, such as roughness and hydrophobicity [137,138,139].
While fouling studies are predominantly conducted using DCMD systems, further research is needed to understand fouling behaviour in PGMD configurations, where the fouling mechanism may differ due to the unique flow dynamics and thermal conditions.

Table 5.
Mechanism and observation of membrane fouling [128,140].
Table 5.
Mechanism and observation of membrane fouling [128,140].
| Fouling | Mechanisms | Impact | Reference Image |
|---|---|---|---|
| Organic |
|
|  |
| Inorganic |
|
|  |
4.2.3. Cooling Plate and Gap Thermal Conductivity
In addition to fouling, another critical factor affecting MD systems’ efficiency is the thermal management within the system. In the PGMD configuration, the vapour condenses on the cooling plate, which is positioned between the permeate and condenser channels. Specifically, the properties of the cooling plate and the gap thermal conductivity play a crucial role in the overall heat and mass transfer phenomena. The thermal conductivity of these components directly influences the flux and energy consumption in the system. Lawal et al. [141] investigated the effect of cooling plate materials on PGMD systems, particularly for hollow fibre modules. Table 6 provides detailed information on the relationship between flux, thermal conductivity, and gained output ratio (GOR) for various cooling plate materials. A mathematical model developed by Gao et al. [89] highlighted that increasing the thermal conductivity of the cooling plate from 0.1 W/mK to 5 W/mK resulted in a 14% enhancement in flux and a 17% reduction in specific thermal energy consumption (STEC). However, beyond 5 W/mK, further increases in thermal conductivity had negligible effects on both flux and STEC.

Table 6.
Flux and GOR with different cooling plates’ thermal conductivity [141].
The influence of thermal conductivity of the gap channel was studied by Memon et al. [142] in water gap membrane distillation (WGMD) and material gap membrane distillation (MGMD). For MGMD, gap materials with varying thermal conductivities, such as high-conductivity graphite and low-conductivity materials like silica gel and zeolite, were analysed. The graphite-filled MGMD configuration outperformed WGMD, exhibiting 11–22% higher permeate flux across a feed temperature range of 40 °C to 70 °C. Conversely, MGMD with silica gel or zeolite resulted in a 17–27% lower permeate flux than WGMD.
5. Feasibility of PGMD for Commercial Freshwater Production
5.1. Techno-Economic Analysis of PGMD Systems
Production cost is one of the most crucial aspects of freshwater production from desalination systems. However, limited information is accessible regarding the cost analysis of membrane distillation [34]. The limited availability of cost estimation data for MD is attributed to the predominant focus on small-scale, with limited implementation on a commercial scale. This has led to fluctuations in capital investment costs, covering modules and membranes [143]. A commonly referenced cost analysis model is the one proposed by Al-Obaidani et al., which calculates the annualized capital and operational expenses to estimate the cost of water production in MD systems. The annual capital cost includes equipment acquisition and installation, as well as the operational costs of running system components. On the other hand, operating expenses encompass membrane replacement, routine maintenance, energy consumption, labour, and administrative management [144]. Higher freshwater production rates and reduced energy inputs are crucial to minimizing water production costs. However, MD systems face challenges in achieving economic viability, particularly in remote or isolated regions where capital and operational costs are higher. One of the major barriers to MD adoption is the relatively low permeate flux compared to conventional desalination technologies, such as reverse osmosis (RO). While efforts to improve performance and reduce energy consumption are ongoing, achieving economic feasibility remains a critical challenge. To address the issue of low permeate flux, multi-stage MD systems have been proposed as a potential solution to enhance both efficiency and productivity. Table 7 shows the flux and the production cost of freshwater from PGMD with different energy sources.

Table 7.
Techno-economic performance of PGMD.
5.1.1. Cost Analysis of Multi-Stage PGMD Systems
Alawad et al. [144] investigated the impact of inlet temperature along with stage number in an insulated and non-insulated multi-stage PGMD system by modelling. The freshwater production expenses at a 2.3 L/min inlet feed flow rate, 2.3 L/min condenser flow rate, 150 ppm salinity, 4 mm gap thickness, 20 years lifetime, 90 °C inlet feed temperature, and 20 °C condenser inlet temperature are given in Figure 16. The monthly replacement of the membrane led to a major increase of 72% in production expenses. Extending the system’s lifespan from 9 to 30 years led to a substantial 83% decrease in the overall production expenses of freshwater.
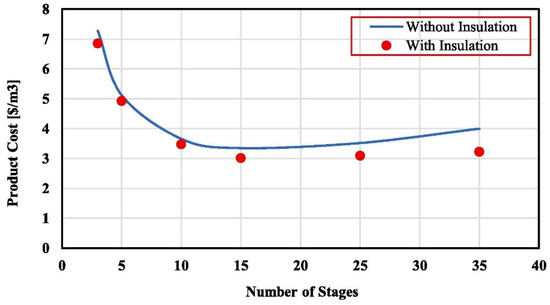
Figure 16.
Multi-stage system production cost [144].
Additionally, incorporating propellers within the gap to enhance heat and mass transfer efficiency in the proposed P-WGMD system significantly reduced freshwater production costs. According to Alawad et al. [122], this modification lowered production costs by 17.35% to 45.18%, decreasing the cost range from US $9.97–US $21.11/m3 to US $8.24–US $11.57/m3 compared to systems without a propeller.
5.1.2. Impact of Energy Sources on Water Production Costs
To achieve cost-effective water production in MD processes, reducing energy consumption emerges as a critical objective. Consequently, energy usage becomes a pivotal consideration when implementing MD for freshwater production. With the aim of comparing different types of energy input, Vias et al. [145] evaluated the cost of water production using various energy sources, including low-grade heat, solar energy, fossil fuels, and grid electricity. For PGMD systems, production costs ranged from €1.56 to €7.53/m3 (In May 2025 US $3.27 to 15.77/m3), with the lowest costs associated with utilising waste heat as an energy source. Comparatively, air-gap MD (AGMD) systems exhibited production costs ranging from €2.38 to €9.60/m3 (In May 2025 US $4.98 to 20.10/m3).
Cooling methods also play a critical role in energy efficiency. Hariri et al. [30] revealed that utilising ambient water for cooling instead of a water chiller reduced energy consumption significantly. For example, the energy consumption for cooling with a chiller was approximately 1500 kWh/m3, nearly twice the 740 kWh/m3 required when ambient water was used. Consequently, the water production cost decreased by 35%, from $0.068/L to $0.044/L (US $68/m3 to US $44/m3), with a single solar flat plate collector (FPC) and 13%, from $0.046/L to $0.038/L (US $46/m3 to US $38/m3), with two solar FPCs.
5.1.3. Optimisation Techniques for Cost Reduction
Optimisation techniques are also used to optimise water production costs for the MD system. Hariri et al. [85] employed ANN and DE models to predict and optimise the freshwater production cost in two scenarios of a multi-stage circulating PGMD system. In the first condition, the heat was supplied by waste heat, and in the second, it was supplied by an electrical source. The lowest production cost was achieved with 12 stages in the waste-heat-powered system, reducing costs by 25%, from US $2.35/m3 to US $1.80/m3. For the electrically powered system, costs decreased by 1.7% between one and 12 stages.
5.1.4. Comparative Cost Analysis of Solar-Powered MD Systems
The cost of unit water production for a pilot-scale solar power C-PGMD unit was investigated by Hariri et al. [30] for three scenarios based on heating modes (electric heating, solar heating, and hybrid solar-electric heating) on the assumption and estimation states. Solar power C-PGMD system had the lowest production cost of 0.016 $/L (US $16/m3) with 40 Flat Plate Collectors (FPCs) producing 6 L/Day/m2, outperforming other solar MD configurations such as SGMD, DCMD, and AGMD.
While multi-stage systems improve permeate flux, further modifications are necessary to optimise the efficiency of MD systems. These modifications focus on reducing energy consumption and enhancing overall system performance.
5.2. Enhancements and Innovations for Commercial Viability
5.2.1. System Modifications for Improved Performance
To address the limitation of traditional PGMD, several innovative modifications have been explored to improve its performance metrics. Lawal et al. [62] introduced an advanced membrane distillation system, i-PGMD, which incorporates an aluminium impeller within the water gap. The impeller, with a thickness of 0.6 mm and a radius of 30 mm, was designed to enhance both heat and mass transfer. Operating at 1100 revolutions per minute (rpm), the i-PGMD system demonstrated notable improvements over the conventional PGMD system. Specifically, the modified system achieved a 12% increase in system-specific thermal energy consumption (STEC), a 151% enhancement in permeate flux, and a 14% improvement in the gain output ratio (GOR). Maximum flux values of 181.08 kg/m2·h and 94.04 kg/m2·h, along with GOR values of 0.59 and 0.51, were recorded for i-PGMD and PGMD, respectively. Additionally, the minimum STEC values were 1129.27 kWh/m3 and 1302.60 kWh/m3. These improvements were primarily attributed to reduced resistance to heat and mass transfer within the gap. Similarly, Lawal et al. [146] conducted a comparative investigation between conventional AGMD and WGMD, alongside their modified configuration, M-AGMD and M-WGMD. The modifications involved the integration of an impeller within the gap chamber to enhance internal mixing and heat transfer. Among the four configurations, M-WGMD demonstrated the highest permeate flux, followed sequentially by WGMD, M-AGMD, and AGMD. The impeller angle exerted a greater influence on performance than the blade material. An impeller angle of 45° was identified as the most effective configuration. In terms of blade material, aluminium exhibited higher performance than plastic. However, the angle of installation remained the dominant factor affecting flux. Though M-WGMD had higher flux of 128 kg/m2·h, M-AGMD has higher GOR of 0.8696 due difference in their thermal conductivity and heat transfer behaviour within the gap.
In another study, Alawad et al. [122] explored integrating a propeller system within the water gap to enhance the system’s performance. The propeller, fabricated from aluminium with a thickness of 0.6 mm and a diameter of 60 mm, was positioned 2 mm from the membrane surface and operated using a 12 V DC motor at a rotational speed of 700 rpm. The study systematically investigated the influence of critical design parameters, including the propeller’s material, diameter, thickness, and position within the gap. The findings revealed that the propeller’s material and thermal conductivity were pivotal in improving performance (as shown in Figure 17). Increasing the thermal conductivity of the propeller material from 0.5 W/m·K to 700 W/m·K significantly reduced the overall heat transfer resistance, lowering it from 0.00111 m2·K/W to 0.00013 m2·K/W. Conversely, the thickness of the propeller material exhibited minimal influence on system performance, mainly when using materials with high thermal conductivity. The study also assessed the impact of water gap thickness on system behaviour. A gradual increase in gap thickness from 5 mm to 30 mm resulted in a minor reduction in permeate flux from 117.15 kg/m2·h to 114 kg/m2·h, corresponding to a decrease of approximately 2.7%. Additionally, the STEC showed a slight increase from 1141 kWh/m3 to 1143 kWh/m3. The observed reduction in flux with wider gaps was attributed to elevated temperatures within the gap, which impeded mass transfer and condensation processes. Furthermore, wider gaps diminished the effectiveness of turbulence and mixing induced by the propeller, reducing heat and mass transfer efficiencies. The slight increase in STEC was associated with lower productivity as the gap width expanded. Under comparable operating conditions, the propeller-enhanced water gap membrane distillation (P-WGMD) system demonstrated significantly better performance than the conventional WGMD system. The P-WGMD system achieved a maximum distillate flux of 322 kg/m2·h and substantially reduced thermal resistance within the gap. These advancements contributed to lower freshwater production costs, ranging from $8.24 to $11.57 per cubic meter, compared to the baseline WGMD system, which incurred costs between $9.97 and $21.11 per cubic meter.
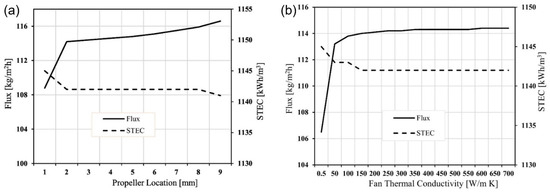
Figure 17.
Influence of (a) propeller location and (b) fan thermal conductivity on flux and STEC [122].
5.2.2. Innovations in Gap Circulation and Heat Recovery
To increase the productivity of the basic PGMD, Khalifa et al. [123] introduced a modified PGMD system incorporating water circulation within the gap to enhance productivity by increasing the heat and mass transfer coefficients, achieving an average improvement of 90%. In this design, distilled water was extracted from the lower section of the gap and reintroduced at the top through nozzles, creating high-velocity jets that facilitated enhanced mixing within the distillate channel. This circulation system promoted uniform temperature distribution throughout the gap, as shown in Figure 18a, and improved thermal performance by enhancing interaction between the water and the heat and mass transfer surfaces, namely the membrane and cooling plate. Two nozzles were strategically placed perpendicular to these surfaces, ensuring efficient heat exchange and uniform load distribution across the gap. The introduction of gap water circulation significantly improved energy efficiency compared to conventional WGMD systems without circulation. SEC reductions of 15% were reported at a feed temperature of 50 °C, increasing to 25% at 90 °C. Additionally, GOR demonstrated 5–22% enhancements due to the improved thermal and mass transfer performance. In a subsequent investigation, Khalifa et al. [99] incorporated a heat exchanger into the system, positioned between the condenser channel and the gap circulation loop. For feed temperatures ranging from 60 °C to 90 °C, the inclusion of gap circulation alone increased flux by approximately 60% compared to the standard PGMD module. When coupled with the heat exchanger, the system achieved a flux increase of nearly 90% relative to the conventional PGMD design. Despite these improvements, the traditional PGMD system exhibited lower STEC compared to the modified systems. To optimise both energy efficiency and productivity, the recommended operating temperature range for the enhanced system was determined to be between 70 °C and 80 °C. These modifications demonstrate significant potential for improving the performance and energy efficiency of PGMD technology.
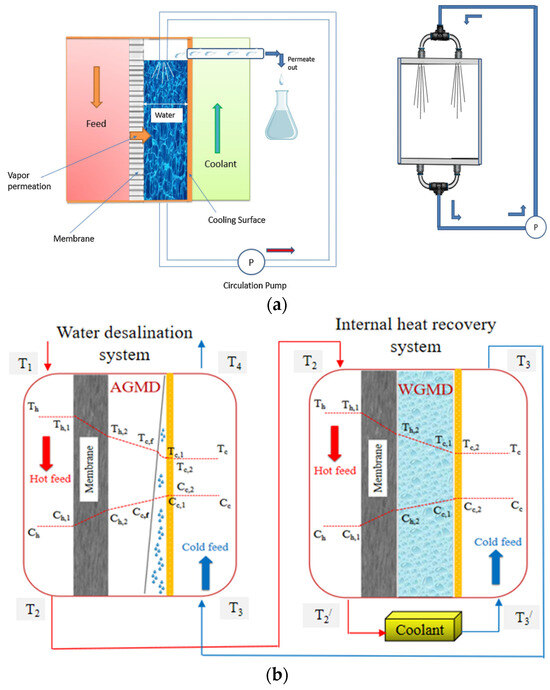
Figure 18.
Modified PGMD modules. (a) PGMD Module with Gap circulation and circulation/Injection cycle [123]. (b) AG-WGMD [147].
5.2.3. Hybrid Designs for Internal Heat Recovery
PGMD has advantages over other MD systems as it can be used as an internal heat recovery system. Zeid et al. [147] proposed a novel AG-WGMD system, which integrates a WGMD module and an AGMD module in series. In this configuration, the hot and cold feeds of the AGMD and WGMD modules are circulated in a counter-current flow. By utilising the internal heat recovery capabilities of the WGMD, the cold feed entering the AGMD is preheated, raising its inlet temperature (T3). Within the AGMD module, the cold feed (T3) absorbs additional heat from the hot feed, increasing its temperature to T4, which is further utilised to increase the inlet temperature of the hot feed (T1). The rise in temperature T3 and T4 subsequently widens the temperature difference across the membrane, reducing the temperature–concentration boundary layers at the side of the hot feed in the AGMD, as illustrated in Figure 18b. The performance of the AG-WGMD system compared with that of the standalone AGMD system under varying scenarios. At a feed flow rate of 18 L/h with an inlet temperature of 80 °C, salt concentration of 5000 mg/L, and a condenser temperature of 20 °C, the AG-WGMD system achieved a maximum flux of 5.6 kg/m2·h, which was 30.91% higher than that of the standalone AGMD system. This enhancement in performance demonstrates the efficacy of the integrated heat recovery system in boosting the flux and improving overall system efficiency.
5.2.4. Alternative Applications of PGMD
Membrane distillation (MD) has advanced beyond its traditional role in seawater desalination and now serves a broad range of applications, including wastewater treatment, recovery of valuable substances such as mineral acids, fruit juices, sugars, alcohol [148,149,150], removal of molecular contaminants like ammonia, aromatic compounds, trichloroethane, halogenated VOCs [151,152,153], and the extraction of boron and arsenic from aqueous solutions [53]. Over time, PGMD technology has also demonstrated its adaptability for diverse applications beyond desalination.
Unlike conventional membrane distillation systems, LGMD introduces a condensing wall within the membrane module. This structural innovation separates the condenser, vapour, and permeate compartments, thereby preventing direct membrane exposure and minimizing risks of membrane damage or contamination of the refrigerant. While LGMD cannot entirely eliminate corrosion concerns, it significantly mitigates risks associated with catastrophic failures, thus enhancing operational safety.
Yue et al. [154] developed an innovative system combining LGMD with a heat pump that employs low boiling-point fluids (e.g., R123, R600, R152a) and high boiling-point fluids (e.g., toluene, hexane, octane) to concentrate sulfuric acid solutions with molar fractions between 0.1 and 0.5, operating at feed temperatures ranging from 40 °C to 90 °C. This configuration achieved a water production Gain Output Ratio (GOR) approximately 33 times greater than conventional systems, with high-boiling-point fluids consistently producing superior GOR values compared to low-boiling-point alternatives.
Natural organic matter, such as humic acid, is prevalent in water sources and seawater, originating from the degradation of plant and animal matter [155,156,157]. These organic substances play a significant role in membrane fouling during water treatment processes. Humic acids and other organic pollutants form layers on membrane surfaces, diminishing permeate flux and potentially damaging the membrane [158,159]. Vias et al. [160] studied the diffusion behaviour of humic substances in two MD configurations—AGMD and WGMD—under varying conditions, including feed temperatures, flow rates, and membrane materials (e.g., hydrophobic PTFE, hydrophobic polyethylene, omniphobic polyethylene, and hydrophilic polyurethane-coated PTFE). Electrical conductivity rejection consistently exceeded 99%, whereas humic acid rejection was 29.17% higher in AGMD than WGMD for synthetic saline water with HA at 70 °C feed temperature, and 18 °C condenser temperature. In WGMD, the permeate side is in direct contact with liquid, allowing HA to pass through the membrane to dissolve directly into the permeate water. Among the tested membranes, omniphobic membranes demonstrated superior humic acid rejection, suggesting their potential efficacy for rejecting other organic contaminants with similar properties.
Mahmoudi et al. [124] integrated PGMD technology with a combined water and power generation (CWP) process, enabling simultaneous freshwater and energy production. In this system, the permeate line includes a restriction mechanism that regulates flow to generate static pressure, which can be utilised for energy conversion. Two experimental setups were proposed:
- (a)
- Using PGMD as a water pump: The permeate produced by the PGMD was directed to specific height levels, effectively utilising it as a water pump.
- (b)
- Using PGMD as an air compressor: The permeate was compressed into a sealed, pressurised tank.
Using a PTFE membrane, the system achieved a hydraulic pressure of 0.55 bar, permeate flux of 4 kg/m2·h, power density of 0.06 W/m2, and specific energy production (SEP) of 0.015 kWh/m3. When a PVDF membrane was used, the performance improved to 1.1 bar hydraulic pressure, 12.5 kg/m2h permeate flux, 0.4 W/m2 power density, and 0.03 kWh/m3 SEP.
In summary, the commercial feasibility of PGMD technology for freshwater production is determined by a combination of factors, including energy sources, feed temperature, and system modifications. Among these, utilising waste heat emerges as the most cost-effective energy option, making it particularly advantageous for reducing operational expenses. Furthermore, advancements such as multi-stage designs, propellers, and gap circulation have demonstrated their ability to significantly enhance mass and heat transfer, leading to lower freshwater production costs and improved system efficiency. For example, optimising propeller position and diameter boosts flux and contributes to substantial energy savings. Beyond its potential in freshwater production, PGMD technology showcases remarkable versatility, with applications ranging from wastewater treatment and valuable component recovery to power generation. These additional applications further underscore its commercial viability. However, despite these promising developments, challenges such as energy consumption and system optimisation persist. Addressing these issues through continued research and technological innovation will be critical in unlocking the full potential of PGMD systems for wider use in desalination and beyond.
6. Conclusions and Future Outlook
The application of MD technology for freshwater production is emerging as a sustainable desalination technology. It bridges the gap between thermal-driven and membrane-driven processes and their scope by integrating renewable or waste energy sources. In particular, utilising waste heat has been identified as a cost-effective energy source, reducing operational expenses and enhancing sustainability. The maturity of this technology over the years involves innovations in the design, modelling, testing, and application of MD for better performance. Advancements such as propeller-assisted designs, optimised gap configurations, and hybrid module designs have significantly improved system efficiency and lowered freshwater production costs. One such design is PGMD, which was designed to mitigate thermal losses by optimising the vapour pressure gradient, which is crucial for efficient mass transfer.
- It was suggested to use a multi-stage PGMD process as the GOR of the system increases with an increase in the number of stages compared to a single stage, and using solar energy as a heating process is even more effective than using an electric heater. Additionally, leveraging waste heat in multi-stage PGMD systems could further reduce costs, emphasising the need for continued research into combining waste heat with solar energy integration.
- Even though we can recover latent heat in PGMD, the distilled flux obtained from a bench-top lab scale is often more than that of a pilot-scale solar integrated system. Therefore, more pilot-scale solar-integrated experiments are needed before commercial application.
- Experimental studies have identified key operational parameters that influence PGMD performance, including feed temperature, condenser temperature, feed flow rates, condenser flow rate, and gap width. The flux increases with an increase in feed temperature and a decrease in condenser temperature. The increased feed and condenser flow rates increase distilled flux; this is also the case for the gap width at certain widths. Increasing the thermal conductivity of the cooling plate increases the effective heat-exchanging capacity between the cooling plate and the permeate. Therefore, a material with high thermal conductivity should be considered while designing the module. These operational factors are pivotal in optimising the system’s efficiency and ensuring high salt rejection rates. The impact of these factors is well-studied on a bench-top lab scale.
- Replacement of the membrane due to fouling increases operational and maintenance costs and freshwater production costs. Limited studies have been conducted on the manufacturing techniques of the antifouling membrane used in PGMD. Membrane durability strongly influences the economic viability of PGMD, and addressing fouling through improved materials and manufacturing techniques remains a priority.
- A water gap in PGMD has advantages over other MD configurations as it provides an opportunity to utilise and modify the module within the gap. Innovations such as adding propellers, impeller-assisted PGMD, gap circulation, and multi-stage configurations are some of the techniques to enhance mass and heat transfer in PGMD and decrease STEC. Gap circulation has effectively promoted uniform temperature distribution, improving heat recovery and energy efficiency. Combining internal heat recovery systems in hybrid PGMD modules, such as the air gap–water gap (AG-WG) design, has expanded the operational flexibility of PGMD and further enhanced distillate flux.
- The feasibility of PGMD to combine water and power production (CWP) systems further enhances its sustainability credentials, aligning with global efforts to reduce carbon emissions and energy consumption in water treatment processes.
Future research directions are identified, focusing on improving membrane materials, optimising system configurations, and scaling up PGMD technology for commercial applications. The review emphasises the need for continued innovation in modelling techniques and gap channels to accurately capture the complex interactions within PGMD systems and improve system efficiency. Over time, the water at the permeate gap becomes stagnant, decreasing heat and mass transfer efficiency. There has been research going on to improve the heat and mass transfer efficiency of the permeate gap, and further research in this area remains essential to optimize system performance. The presence of water in the permeate gap facilitates the direct diffusion of organic matter, such as humic acid, into the permeate stream. Additional research can be conducted to analyse the membrane wetting and fouling due to organic matter, phenomena in PGMD, by long-term testing. By bridging the gap between theoretical research and practical implementation, this review contributes to advancing PGMD as an effective economic solution for freshwater and energy production. By integrating solar energy and waste heat in scalable systems, PGMD technology can align more closely with global sustainability goals, providing an effective and economical solution for freshwater production.
Funding
This research received no external funding.
Conflicts of Interest
There are no conflicts of interest.
Nomenclature
| Area of the condenser channel | m2 | |
| Area of membrane at the feed side | m2 | |
| Area of Water permeation | m2 | |
| Gap area | m | |
| Area of the cooling plate | m2 | |
| Membrane mass transfer resistance | kg/m2sPa | |
| Knudsen diffusion resistance | - | |
| Molecular diffusion resistance | - | |
| Membrane surface salt concentration | kg/m3 | |
| Salt concentration on the bulk feed side | kg/m3 | |
| Feed side hydraulic diameter | m | |
| Condenser side hydraulic diameter | m | |
| Diameter of the membrane pore | m | |
| Coefficient of diffusion | m2/s | |
| Coefficient of heat transfer on the feed side | W/m2K | |
| Coefficient of heat transfer on the condenser side | W/m2K | |
| Vapor mass flux | kg/m2h | |
| Permeate gap thermal conductivity | W/mK | |
| Effective thermal conductivity of the membrane | W/mK | |
| Cooling plate thermal conductivity | W/mK | |
| Feed thermal conductivity | W/mK | |
| Condenser thermal conductivity | W/mK | |
| Knudsen number | - | |
| Boltzmann constant | J/K | |
| mass transfer coefficient | - | |
| L | Length of module | m |
| Universal gas constant | J/mol K | |
| Rayleigh number | - | |
| Molecular weight of water | kg/mol | |
| Nusselt number of water at the permeate gap | - | |
| Nusselt number of water at the feed side | - | |
| Nusselt number of water in the cold stream | - | |
| Vapour pressure at | K | |
| Vapour pressure at | K | |
| Air pressure inside the pore | Pa | |
| Mean pressure within the membrane pore | Pa | |
| Total pore pressure | Pa | |
| Vapor pressure of water inside the pores | Pa | |
| Prandtl number of water at the permeate gap | - | |
| Feed side membrane surface temperature | K | |
| Membrane surface temperature at the permeate gap | K | |
| Mean temperature of the membrane | K | |
| Temperature at the permeate gap | K | |
| Temperature of the cooling plate at the permeate gap | K | |
| Temperature of the cooling plate at the condenser side | K | |
| Bulk condenser temperature | K | |
| Bulk feed temperature | K | |
| Molar fraction of water | - | |
| Molar fraction of NaCl | - | |
| Mean free path | m | |
| Porosity of the membrane | - | |
| Membrane thickness | m | |
| Gap thickness | m | |
| Thickness of the cooling plate | m | |
| Density of feed solution | kg/m3 | |
| Rate of heat transfer on the feed side | W/m2 | |
| Rate of heat transfer in the membrane | W/m2 | |
| Rate of heat transfer in the permeate gap | W/m2 | |
| Rate of heat transfer in the cooling plate | W/m2 | |
| Rate of heat transfer in the condenser stream | W/m2 | |
| Vapor pressure difference across the membrane | Pa | |
| γ | Surface tension | N/m |
| θ | Contact angle | ° |
| Pore radius | m |
Abbreviations
| ANN | Artificial Neural Network |
| (AG-WG) MD | Air Gap–Water Gap Membrane Distillation |
| B | Billion |
| BWRO | Brackish Water Reverse Osmosis |
| CPC | Concentration Polarization Coefficient |
| CR | Crossover Constant |
| CGMD | Conductive Gap Membrane Distillation |
| C-PGMD | PGMD with Circulating Gap Water |
| DE | Differential Evolution |
| DCMD | Direct Contact Membrane Distillation |
| ED | Electrodialysis |
| FPC | Flat Plate Collector |
| GOR | Gain Output Ratio |
| HA | Humic Acid |
| HDPE | High-Density Polyethylene |
| i-PGMD | PGMD with Impeller |
| LGMD | Liquid Gap Membrane Distillation |
| LEP | Liquid Entry Pressure |
| MD | Membrane Distillation |
| MED | Multi-Effect Distillation |
| MSF | Multi-Stage Flash |
| MGMD | Material Gap Membrane Distillation |
| M-AGMD | Modified Air Gap Membrane Distillation |
| M-WGMD | Modified Water Gap Membrane Distillation |
| PGMD | Permeate Gap Membrane Distillation |
| RMS | Response Surface Morphology |
| RO | Reverse Osmosis |
| SEC | Specific Energy Consumption |
| SGMD | Sweep Gas Membrane Distillation |
| STEC | Specific Thermal Energy Consumption |
| SWRO | Seawater Reverse Osmosis |
| VGMD | Partial Vacuum Gap Membrane Distillation |
| TPC | Temperature Polarization Coefficient |
| VMD | Vacuum Membrane Distillation |
| WGMD | Water Gap Membrane Distillation |
References
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. World Water Assessment Programme, in The United Nations World Water Development Report 2018. Available online: https://www.unwater.org/publications/world-water-development-report-2018/ (accessed on 12 December 2024).
- Kumar, P.; Date, A.; Mahmood, N.; Kumar Das, R.; Shabani, B. Freshwater supply for hydrogen production: An underestimated challenge. Int. J. Hydrogen Energy 2024, 78, 202–217. [Google Scholar] [CrossRef]
- OECD. OECD Environmental Outlook to 2050, Chapter: C.C.; OECD: Paris, France, 2012. [Google Scholar]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Forum, W.E. The Global Risk Report 2020. Available online: https://www3.weforum.org/docs/WEF_Global_Risk_Report_2020.pdf (accessed on 12 November 2024).
- Djuma, H.; Bruggeman, A.; Eliades, M.; Lange, M. Non-conventional water resources research in semi-arid countries of the Middle East. Desalination Water Treat. 2016, 57, 2290–2303. [Google Scholar]
- Oki, T.; Entekhabi, D.; Harrold, T.I. The global water cycle. Glob. Energy Water Cycles 1999, 10, 27. [Google Scholar]
- USGS. How Much Water is There on Earth? Water Science School. Available online: https://www.usgs.gov/special-topics/water-science-school/science/how-much-water-there-earth?qt-science_center_objects=0#qt-science_center_objects (accessed on 1 December 2024).
- Programme, U.W.W.A.; Engin, K.; Richard, C. The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water; Facts, Figures and Action Examples; United Nations Educational, Scientific and Cultural Organisation: Paris, France, 2023; Available online: https://policycommons.net/artifacts/10611945/the-united-nations-world-water-development-report-2023/ (accessed on 4 January 2025).
- Reimann, L.; Vafeidis, A.T.; Honsel, L.E. Population development as a driver of coastal risk: Current trends and future pathways. Camb. Prism. Coast. Futures 2023, 1, e14. [Google Scholar] [CrossRef]
- Igor, S. World Fresh Water Resources, Water in Crisis: A Guide to the World’s; Oxford University Press, Inc.: Oxford, UK, 1993. [Google Scholar]
- Marc-Antoine Eyl-Mazzega, É.C. The Geopolitics of Seawater Desalination; Études de l’Ifri, Ifri: Paris, France, 2022. [Google Scholar]
- Ahmad, Z.; Shukla, A.K.; Singh, V.; Sharma, M.; Kumar, P. Thermodynamic analysis of solar powered trigeneration arrangement for cooling, power and drinking water generation. Songklanakarin J. Sci. Technol. 2022, 44, 1419–1426. [Google Scholar]
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S.-m. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Ghalavand, Y.; Hatamipour, M.S.; Rahimi, A. A review on energy consumption of desalination processes. Desalination Water Treat. 2015, 54, 1526–1541. [Google Scholar] [CrossRef]
- Lu, X.; Elimelech, M. Fabrication of desalination membranes by interfacial polymerization: History, current efforts, and future directions. Chem. Soc. Rev. 2021, 50, 6290–6307. [Google Scholar]
- Charcosset, C. A review of membrane processes and renewable energies for desalination. Desalination 2009, 245, 214–231. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Xu, Y.; Zhuo, Y.; Lu, G. Sustainable thermal-based desalination with low-cost energy resources and low-carbon footprints. Desalination 2021, 520, 115371. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, O. Thermodynamic analysis of solid oxide fuel cell-gas turbine-organic Rankine cycle combined system. Int. J. Mater. Sci. Mech. Eng. 2019, 6, 98–102. [Google Scholar]
- Moreira, V.R.; Raad, J.V.; Lazarini, J.X.; Santos, L.V.S.; Amaral, M.C.S. Recent progress in membrane distillation configurations powered by renewable energy sources and waste heat. J. Water Process Eng. 2023, 53, 103816. [Google Scholar] [CrossRef]
- Shahzad, M.W.; Burhan, M.; Ang, L.; Ng, K.C. Energy-water-environment nexus underpinning future desalination sustainability. Desalination 2017, 413, 52–64. [Google Scholar] [CrossRef]
- Nassrullah, H.; Anis, S.F.; Hashaikeh, R.; Hilal, N. Energy for desalination: A state-of-the-art review. Desalination 2020, 491, 114569. [Google Scholar] [CrossRef]
- Rio Carrillo, A.M.; Frei, C. Water: A key resource in energy production. Energy Policy 2009, 37, 4303–4312. [Google Scholar] [CrossRef]
- Gude, V.G. Desalination and sustainability—An appraisal and current perspective. Water Res. 2016, 89, 87–106. [Google Scholar] [CrossRef]
- Mayor, B. Growth patterns in mature desalination technologies and analogies with the energy field. Desalination 2019, 457, 75–84. [Google Scholar]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar]
- Faigon, M. Success behind advanced SWRO desalination plant. Filtr. Sep. 2016, 53, 29–31. [Google Scholar]
- Schunke, A.J.; Hernandez Herrera, G.A.; Padhye, L.; Berry, T.-A. Energy recovery in SWRO desalination: Current status and new possibilities. Front. Sustain. Cities 2020, 2, 9. [Google Scholar]
- Hafiz Al Hariri, A.; Khalifa, A.E.; Alawad, S.M. Techno-economic analysis of solar-powered membrane distillation system with circulated permeate gap. Sol. Energy 2024, 267, 112243. [Google Scholar] [CrossRef]
- Ankoliya, D.; Mudgal, A.; Sinha, M.K.; Patel, V.; Patel, J. Techno-economic analysis of a hybrid electrodialysis–batch reverse osmosis process for brackish water desalination. AQUA—Water Infrastruct. Ecosyst. Soc. 2023, 72, 593–607. [Google Scholar]
- Biswas, W.K.; Yek, P. Improving the carbon footprint of water treatment with renewable energy: A Western Australian case study. Renew. Wind. Water Sol. 2016, 3, 14. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, O. Investigations on integrating SOFC with gas turbine for performance enhancement and sustainable energy. J. Energy Environ. Sustain. 2019, 7, 42–46. [Google Scholar]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef]
- Esato, K.; Eiseman, B. Experimental evaluation of Gore-Tex membrane oxygenator. J. Thorac. Cardiovasc. Surg. 1975, 69, 690–697. [Google Scholar] [CrossRef]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation using low-grade energy for desalination: A review. J. Environ. Chem. Eng. 2021, 9, 105818. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.-D.; Duke, M.; Gomez, J.; Gray, S. Advances in Membrane Distillation for Water Desalination and Purification Applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A.G. Heat transport and membrane distillation coefficients in direct contact membrane distillation. J. Membr. Sci. 2003, 212, 177–193. [Google Scholar]
- Khalifa, A.E.; Alawad, S.M. Air gap and water gap multistage membrane distillation for water desalination. Desalination 2018, 437, 175–183. [Google Scholar] [CrossRef]
- Macossay, J.; Marruffo, A.; Rincon, R.; Eubanks, T.; Kuang, A. Effect of needle diameter on nanofiber diameter and thermal properties of electrospun poly (methyl methacrylate). Polym. Adv. Technol. 2007, 18, 180–183. [Google Scholar]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar]
- Gao, L.; Zhang, J.; Gray, S.; Li, J.-D. Experimental study of hollow fiber permeate gap membrane distillation and its performance comparison with DCMD and SGMD. Sep. Purif. Technol. 2017, 188, 11–23. [Google Scholar] [CrossRef]
- Dow, N.; Gray, S.; Li, J.-d.; Zhang, J.; Ostarcevic, E.; Liubinas, A.; Atherton, P.; Roeszler, G.; Gibbs, A.; Duke, M. Pilot trial of membrane distillation driven by low grade waste heat: Membrane fouling and energy assessment. Desalination 2016, 391, 30–42. [Google Scholar] [CrossRef]
- Zakrzewska-Trznadel, G.; Harasimowicz, M.; Chmielewski, A.G. Concentration of radioactive components in liquid low-level radioactive waste by membrane distillation. J. Membr. Sci. 1999, 163, 257–264. [Google Scholar]
- Duong, H.C.; Cooper, P.; Nelemans, B.; Cath, T.Y.; Nghiem, L.D. Evaluating energy consumption of air gap membrane distillation for seawater desalination at pilot scale level. Sep. Purif. Technol. 2016, 166, 55–62. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Banat, F.; Jwaied, N.; Rommel, M.; Koschikowski, J.; Wieghaus, M. Desalination by a “compact SMADES” autonomous solarpowered membrane distillation unit. Desalination 2007, 217, 29–37. [Google Scholar] [CrossRef]
- Raluy, R.G.; Schwantes, R.; Subiela, V.J.; Peñate, B.; Melián, G.; Betancort, J.R. Operational experience of a solar membrane distillation demonstration plant in Pozo Izquierdo-Gran Canaria Island (Spain). Desalination 2012, 290, 1–13. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Moazami Goodarzi, G.; Dehghani, S.; Akbarzadeh, A. Experimental and theoretical study of a lab scale permeate gap membrane distillation setup for desalination. Desalination 2017, 419, 197–210. [Google Scholar] [CrossRef]
- Roslan, S.N.A.B.; Alias, N.H.; Othman, N.H.; Aziz, M.H.A.; Marpani, F.; Mat-Shayuti, M.S.; Shahruddin, M.Z.; Djokokusworo, T. Application of response surface methodology for modelling of direct contact membrane distillation system: A review. Desalination Water Treat. 2022, 257, 169–184. [Google Scholar] [CrossRef]
- Ferreira, R.K.M.; Ramlow, H.; Marangoni, C.; Machado, R.A.F. A review on the manufacturing techniques of porous hydrophobic ceramic membranes applied to direct contact membrane distillation. Adv. Appl. Ceram. 2021, 120, 336–357. [Google Scholar] [CrossRef]
- Foureaux, A.F.S.; Moreira, V.R.; Lebron, Y.A.R.; Santos, L.V.S.; Amaral, M.C.S. Direct contact membrane distillation as an alternative to the conventional methods for value-added compounds recovery from acidic effluents: A review. Sep. Purif. Technol. 2020, 236, 116251. [Google Scholar] [CrossRef]
- Ashoor, B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review. Desalination 2016, 398, 222–246. [Google Scholar]
- Hitsov, I.; Eykens, L.; De Schepper, W.; De Sitter, K.; Dotremont, C.; Nopens, I. Full-scale direct contact membrane distillation (DCMD) model including membrane compaction effects. J. Membr. Sci. 2017, 524, 245–256. [Google Scholar]
- Ullah, R.; Khraisheh, M.; Esteves, R.J.; McLeskey, J.T., Jr.; AlGhouti, M.; Gad-el-Hak, M.; Tafreshi, H.V. Energy efficiency of direct contact membrane distillation. Desalination 2018, 433, 56–67. [Google Scholar]
- Al-Zoubi, H.; Al-Amri, F.; Khalifa, A.E.; Al-Zoubi, A.; Abid, M.; Younis, E.; Mallick, T.K. A comprehensive review of air gap membrane distillation process. Desalination Water Treat. 2018, 110, 27–64. [Google Scholar] [CrossRef]
- Shahu, V.T.; Thombre, S.B. Air gap membrane distillation: A review. J. Renew. Sustain. Energy 2019, 11, 045901. [Google Scholar] [CrossRef]
- Alawad, S.M.; Khalifa, A.E. Analysis of water gap membrane distillation process for water desalination. Desalination 2019, 470, 114088. [Google Scholar] [CrossRef]
- Im, B.-G.; Lee, J.-G.; Kim, Y.-D.; Kim, W.-S. Theoretical modeling and simulation of AGMD and LGMD desalination processes using a composite membrane. J. Membr. Sci. 2018, 565, 14–24. [Google Scholar] [CrossRef]
- Chen, T.-C.; Ho, C.-D.; Yeh, H.-M. Theoretical modeling and experimental analysis of direct contact membrane distillation. J. Membr. Sci. 2009, 330, 279–287. [Google Scholar] [CrossRef]
- Francis, L.; Ghaffour, N.; Alsaadi, A.A.; Amy, G.L. Material gap membrane distillation: A new design for water vapor flux enhancement. J. Membr. Sci. 2013, 448, 240–247. [Google Scholar] [CrossRef]
- Lawal, D.U. Performance enhancement of permeate gap membrane distillation system augmented with impeller. Sustain. Energy Technol. Assess. 2022, 54, 102792. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Membrane Distillation; Elsevier: Amsterdam, The Netherlands, 2011; Volume 41. [Google Scholar]
- Alawad, S.M.; Khalifa, A.E.; Al Hariri, A.H. Theoretical Investigation into the Dynamic Performance of a Solar-Powered Multistage Water Gap Membrane Distillation System. Arab. J. Sci. Eng. 2023, 48, 12499–12511. [Google Scholar] [CrossRef]
- Khayet, M.; Godino, M.P.; Mengual, J.I. Modelling Transport Mechanism Through A Porous Partition. J. Non-Equilib. Thermodyn. 2001, 26, 1–14. [Google Scholar] [CrossRef]
- Khalifa, A.; Lawal, D.; Antar, M.; Khayet, M. Experimental and theoretical investigation on water desalination using air gap membrane distillation. Desalination 2015, 376, 94–108. [Google Scholar] [CrossRef]
- Khayet, M.; Velázquez, A.; Mengual, J.I. Modelling mass transport through a porous partition: Effect of pore size distribution. J. Non-Equilib. Thermodyn. 2004, 29, 279–299. [Google Scholar]
- Li, N.N.; Fane, A.G.; Ho, W.W.; Matsuura, T. Advanced Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Zhang, J.; Dow, N.; Duke, M.; Ostarcevic, E.; Li, J.-D.; Gray, S. Identification of material and physical features of membrane distillation membranes for high performance desalination. J. Membr. Sci. 2010, 349, 295–303. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.-D.; Duke, M.; Xie, Z.; Gray, S. Performance of asymmetric hollow fibre membranes in membrane distillation under various configurations and vacuum enhancement. J. Membr. Sci. 2010, 362, 517–528. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Khraisheh, M.A.M.M.; Fard, A.K.; Benyahia, F.; Adham, S. A predictive model for the assessment of the temperature polarization effect in direct contact membrane distillation desalination of high salinity feed. Desalination 2014, 341, 38–49. [Google Scholar] [CrossRef]
- Martínez-Díez, L.; Vázquez-González, M.I. Temperature and concentration polarization in membrane distillation of aqueous salt solutions. J. Membr. Sci. 1999, 156, 265–273. [Google Scholar] [CrossRef]
- Srisurichan, S.; Jiraratananon, R.; Fane, A.G. Mass transfer mechanisms and transport resistances in direct contact membrane distillation process. J. Membr. Sci. 2006, 277, 186–194. [Google Scholar] [CrossRef]
- Yun, Y.; Ma, R.; Zhang, W.; Fane, A.G.; Li, J. Direct contact membrane distillation mechanism for high concentration NaCl solutions. Desalination 2006, 188, 251–262. [Google Scholar] [CrossRef]
- Anvari, A.; Azimi Yancheshme, A.; Kekre, K.M.; Ronen, A. State-of-the-art methods for overcoming temperature polarization in membrane distillation process: A review. J. Membr. Sci. 2020, 616, 118413. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.-D.; Gray, S. Effect of applied pressure on performance of PTFE membrane in DCMD. J. Membr. Sci. 2011, 369, 514–525. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Application of a porous composite hydrophobic/hydrophilic membrane in desalination by air gap and liquid gap membrane distillation: A comparative study. Sep. Purif. Technol. 2014, 133, 176–186. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, J.; Gray, S.; Li, J.-D. Influence of PGMD module design on the water productivity and energy efficiency in desalination. Desalination 2019, 452, 29–39. [Google Scholar] [CrossRef]
- Curcio, E.; Drioli, E. Membrane Distillation and Related Operations—A Review. Sep. Purif. Rev. 2005, 34, 35–86. [Google Scholar] [CrossRef]
- Ugrozov, V.V.; Elkina, I.B.; Nikulin, V.N.; Kataeva, L.I. Theoretical and experimental research of liquid-gap membrane distillation process in membrane module. Desalination 2003, 157, 325–331. [Google Scholar] [CrossRef]
- Suleman, M.; Asif, M.; Jamal, S.A. Temperature and concentration polarization in membrane distillation: A technical review. Desalin. Water Treat 2021, 229, 52–68. [Google Scholar]
- Ruiz-Aguirre, A.; Andrés-Mañas, J.A.; Fernández-Sevilla, J.M.; Zaragoza, G. Modeling and optimization of a commercial permeate gap spiral wound membrane distillation module for seawater desalination. Desalination 2017, 419, 160–168. [Google Scholar] [CrossRef]
- Alawad, S.M.; Khalifa, A.E.; Abido, M.A.; Antar, M.A. Differential evolution optimization of water gap membrane distillation process for water desalination. Sep. Purif. Technol. 2021, 270, 118765. [Google Scholar] [CrossRef]
- Abido, M.A.; Al-Ali, N.A. Multi-Objective Optimal Power Flow Using Differential Evolution. Arab. J. Sci. Eng. 2012, 37, 991–1005. [Google Scholar] [CrossRef]
- Hariri, A.H.A.; Khalifa, A.E.; Talha, M.; Awda, Y.; Hasan, A.; Alawad, S.M. Artificial neural network and differential evolution optimization of a circulated permeate gap membrane distillation unit. Sep. Purif. Technol. 2024, 338, 126517. [Google Scholar] [CrossRef]
- Yazgan-Birgi, P.; Hassan Ali, M.I.; Swaminathan, J.; Lienhard, J.H.; Arafat, H.A. Computational fluid dynamics modeling for performance assessment of permeate gap membrane distillation. J. Membr. Sci. 2018, 568, 55–66. [Google Scholar] [CrossRef]
- Elbessomy, M.O.; Elsamni, O.A.; Elsheniti, M.B.; Elsherbiny, S.M. Optimum configurations of a compact hollow-fiber water gap membrane distillation module for ultra-low waste heat applications. Chem. Eng. Res. Des. 2023, 195, 218–234. [Google Scholar] [CrossRef]
- Elbessomy, M.O.; Elsheniti, M.B.; Elsherbiny, S.M.; Rezk, A.; Elsamni, O.A. Productivity and Thermal Performance Enhancements of Hollow Fiber Water Gap Membrane Distillation Modules Using Helical Fiber Configuration: 3D Computational Fluid Dynamics Modeling. Membranes 2023, 13, 843. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, J.; Gray, S.; Li, J.-D. Modelling mass and heat transfers of Permeate Gap Membrane Distillation using hollow fibre membrane. Desalination 2019, 467, 196–209. [Google Scholar] [CrossRef]
- Cipollina, A.; Di Sparti, M.G.; Tamburini, A.; Micale, G. Development of a Membrane Distillation module for solar energy seawater desalination. Chem. Eng. Res. Des. 2012, 90, 2101–2121. [Google Scholar] [CrossRef]
- Swaminathan, J.; Chung, H.W.; Warsinger, D.M.; AlMarzooqi, F.A.; Arafat, H.A.; Lienhard V, J.H. Energy efficiency of permeate gap and novel conductive gap membrane distillation. J. Membr. Sci. 2016, 502, 171–178. [Google Scholar] [CrossRef]
- Saleh, A.; Qudeiri, J.A.; Al-Nimr, M.A. Performance investigation of a salt gradient solar pond coupled with desalination facility near the Dead Sea. Energy 2011, 36, 922–931. [Google Scholar] [CrossRef]
- Pal, P.; Manna, A.K. Removal of arsenic from contaminated groundwater by solar-driven membrane distillation using three different commercial membranes. Water Res. 2010, 44, 5750–5760. [Google Scholar] [CrossRef]
- Suárez, F.; Tyler, S.W.; Childress, A.E. A theoretical study of a direct contact membrane distillation system coupled to a salt-gradient solar pond for terminal lakes reclamation. Water Res. 2010, 44, 4601–4615. [Google Scholar] [CrossRef]
- Summers, E.K.; Arafat, H.A.; Lienhard, J.H. Energy efficiency comparison of single-stage membrane distillation (MD) desalination cycles in different configurations. Desalination 2012, 290, 54–66. [Google Scholar] [CrossRef]
- Alquraish, M.M.; Mejbri, S.; Abuhasel, K.A.; Zhani, K. Experimental Investigation of a Pilot Solar-Assisted Permeate Gap Membrane Distillation. Membranes 2021, 11, 336. [Google Scholar] [CrossRef]
- Zaragoza, G.; Ruiz-Aguirre, A.; Guillén-Burrieza, E. Efficiency in the use of solar thermal energy of small membrane desalination systems for decentralized water production. Appl. Energy 2014, 130, 491–499. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, Y.; Li, P.; Li, W.; Wang, F. Comparative study of air gap and permeate gap membrane distillation using internal heat recovery hollow fiber membrane module. Desalination 2018, 426, 42–49. [Google Scholar] [CrossRef]
- Khalifa, A.E. Membrane Distillation Desalination System With Gap Circulation and Cooling Using a Built-in Heat Exchanger. J. Energy Resour. Technol. 2020, 143. [Google Scholar] [CrossRef]
- Khalifa, A.E. Water and air gap membrane distillation for water desalination—An experimental comparative study. Sep. Purif. Technol. 2015, 141, 276–284. [Google Scholar] [CrossRef]
- Elsheniti, M.B.; Elbessomy, M.O.; Rezk, A.; Fouly, A.; Elsherbiny, S.M.; Elsamni, O.A. Comparative analyses of transient batch operations of hollow-fiber DCMD and WGMD desalination systems at high salinity levels. Desalination 2025, 600, 118492. [Google Scholar] [CrossRef]
- Burgoyne, A.; Vahdati, M. Direct contact membrane distillation. Sep. Sci. Technol. 2000, 35, 1257–1284. [Google Scholar]
- Song, L.; Li, B.; Sirkar, K.K.; Gilron, J.L. Direct Contact Membrane Distillation-Based Desalination: Novel Membranes, Devices, Larger-Scale Studies, and a Model. Ind. Eng. Chem. Res. 2007, 46, 2307–2323. [Google Scholar] [CrossRef]
- Teoh, M.M.; Chung, T.-S. Membrane distillation with hydrophobic macrovoid-free PVDF–PTFE hollow fiber membranes. Sep. Purif. Technol. 2009, 66, 229–236. [Google Scholar] [CrossRef]
- Picard, C.; Larbot, A.; Tronel-Peyroz, E.; Berjoan, R. Characterisation of hydrophilic ceramic membranes modified by fluoroalkylsilanes into hydrophobic membranes. Solid State Sci. 2004, 6, 605–612. [Google Scholar] [CrossRef]
- Schondelmaier, D.; Cramm, S.; Klingeler, R.; Morenzin, J.; Zilkens, C.; Eberhardt, W. Orientation and Self-Assembly of Hydrophobic Fluoroalkylsilanes. Langmuir 2002, 18, 6242–6245. [Google Scholar] [CrossRef]
- Samadi, A.; Ni, T.; Fontananova, E.; Tang, G.; Shon, H.; Zhao, S. Engineering antiwetting hydrophobic surfaces for membrane distillation: A review. Desalination 2023, 563, 116722. [Google Scholar] [CrossRef]
- Rácz, G.; Kerker, S.; Kovács, Z.; Vatai, G.; Ebrahimi, M.; Czermak, P. Theoretical and experimental approaches of liquid entry pressure determination in membrane distillation processes. Period. Polytech. Chem. Eng. 2014, 58, 81–91. [Google Scholar]
- Ghorabi, S.; Ashtiani, F.Z.; Karimi, M.; Fouladitajar, A.; Yousefi, B.; Dorkalam, F. Development of a novel dual-bioinspired method for synthesis of a hydrophobic/hydrophilic polyethersulfone coated membrane for membrane distillation. Desalination 2021, 517, 115242. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Faki, B.; Luque-Alled, J.M.; Alberto, M.; Vijayaraghavan, A.; Holmes, S.M.; Szekely, G.; Badawy, M.I.; Shokri, N.; et al. Flux-enhanced PVDF mixed matrix membranes incorporating APTS-functionalized graphene oxide for membrane distillation. J. Membr. Sci. 2018, 554, 309–323. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, Z.; Wei, Y.; Zhang, Y.; Yuan, Y.; Wang, J. Preparation of PVDF-CTFE hydrophobic membranes for MD application: Effect of LiCl-based mixed additives. J. Membr. Sci. 2016, 506, 71–85. [Google Scholar] [CrossRef]
- Zhang, J.; Gray, S.; Li, J.-D. Modelling heat and mass transfers in DCMD using compressible membranes. J. Membr. Sci. 2012, 387-388, 7–16. [Google Scholar] [CrossRef]
- Khalifa, A.E. Performances of air gap and water gap MD desalination modules. Water Pract. Technol. 2018, 13, 200–209. [Google Scholar] [CrossRef]
- Winter, D.; Koschikowski, J.; Wieghaus, M. Desalination using membrane distillation: Experimental studies on full scale spiral wound modules. J. Membr. Sci. 2011, 375, 104–112. [Google Scholar] [CrossRef]
- Winter, D.; Koschikowski, J.; Ripperger, S. Desalination using membrane distillation: Flux enhancement by feed water deaeration on spiral-wound modules. J. Membr. Sci. 2012, 423–424, 215–224. [Google Scholar] [CrossRef]
- Ruiz-Aguirre, A.; Andrés-Mañas, J.A.; Fernández-Sevilla, J.M.; Zaragoza, G. Comparative characterization of three commercial spiral-wound membrane distillation modules. Desalination Water Treat. 2017, 61, 152–159. [Google Scholar] [CrossRef]
- Lin, Y.-X.; Liou, Y.-K.; Lee, S.L.; Chen, S.-Y.; Tao, F.-T.; Cheng, T.-W.; Tung, K.-L. Preparation of PVDF/PMMA composite membrane with green solvent for seawater desalination by gap membrane distillation. J. Membr. Sci. 2023, 679, 121676. [Google Scholar] [CrossRef]
- Khayet, M.; Mengual, J.I.; Matsuura, T. Porous hydrophobic/hydrophilic composite membranes: Application in desalination using direct contact membrane distillation. J. Membr. Sci. 2005, 252, 101–113. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T.; Mengual, J.I. Porous hydrophobic/hydrophilic composite membranes: Estimation of the hydrophobic-layer thickness. J. Membr. Sci. 2005, 266, 68–79. [Google Scholar] [CrossRef]
- Qtaishat, M.; Khayet, M.; Matsuura, T. Novel porous composite hydrophobic/hydrophilic polysulfone membranes for desalination by direct contact membrane distillation. J. Membr. Sci. 2009, 341, 139–148. [Google Scholar] [CrossRef]
- Qtaishat, M.; Rana, D.; Khayet, M.; Matsuura, T. Preparation and characterization of novel hydrophobic/hydrophilic polyetherimide composite membranes for desalination by direct contact membrane distillation. J. Membr. Sci. 2009, 327, 264–273. [Google Scholar] [CrossRef]
- Alawad, S.M.; Lawal, D.U.; Khalifa, A.E.; Aljundi, I.H.; Antar, M.A.; Baroud, T.N. Analysis of water gap membrane distillation process with an internal gap circulation propeller. Desalination 2023, 551, 116379. [Google Scholar] [CrossRef]
- Khalifa, A.E. Flux enhanced water gap membrane distillation process-circulation of gap water. Sep. Purif. Technol. 2020, 231, 115938. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Date, A.; Akbarzadeh, A. Further investigation of simultaneous fresh water production and power generation concept by permeate gap membrane distillation system. J. Membr. Sci. 2019, 572, 230–245. [Google Scholar] [CrossRef]
- Swaminathan, J.; Chung, H.W.; Warsinger, D.M.; Lienhard V, J.H. Membrane distillation model based on heat exchanger theory and configuration comparison. Appl. Energy 2016, 184, 491–505. [Google Scholar] [CrossRef]
- Shalaby, S.M.; Kabeel, A.E.; Abosheiasha, H.F.; Elfakharany, M.K.; El-Bialy, E.; Shama, A.; Vidic, R.D. Membrane distillation driven by solar energy: A review. J. Clean. Prod. 2022, 366, 132949. [Google Scholar] [CrossRef]
- Curcio, E.; Ji, X.; Di Profio, G.; Sulaiman, A.O.; Fontananova, E.; Drioli, E. Membrane distillation operated at high seawater concentration factors: Role of the membrane on CaCO3 scaling in presence of humic acid. J. Membr. Sci. 2010, 346, 263–269. [Google Scholar] [CrossRef]
- Gryta, M. Fouling in direct contact membrane distillation process. J. Membr. Sci. 2008, 325, 383–394. [Google Scholar]
- Gryta, M. Effect of iron oxides scaling on the MD process performance. Desalination 2007, 216, 88–102. [Google Scholar] [CrossRef]
- Karakulski, K.; Gryta, M.; Morawski, A. Membrane processes used for potable water quality improvement. Desalination 2002, 145, 315–319. [Google Scholar]
- Gryta, M. Concentration of NaCl solution by membrane distillation integrated with crystallization. Sep. Sci. Technol. 2002, 37, 3535–3558. [Google Scholar]
- Wang, P.; Chung, T.-S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Mundhenk, N.; Huttenloch, P.; Kohl, T.; Steger, H.; Zorn, R. Metal corrosion in geothermal brine environments of the Upper Rhine graben—Laboratory and on-site studies. Geothermics 2013, 46, 14–21. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, R. Novel membrane surface modification to enhance anti-oil fouling property for membrane distillation application. J. Membr. Sci. 2013, 447, 26–35. [Google Scholar]
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2005, 171, 111–131. [Google Scholar] [CrossRef]
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic modification of TiO2 nanocomposite PVDF membranes for applications in membrane distillation. J. Membr. Sci. 2012, 415–416, 850–863. [Google Scholar] [CrossRef]
- Seraj, S.; Mohammadi, T.; Tofighy, M.A. Graphene-based membranes for membrane distillation applications: A review. J. Environ. Chem. Eng. 2022, 10, 107974. [Google Scholar] [CrossRef]
- Bhadra, M.; Roy, S.; Mitra, S. Desalination across a graphene oxide membrane via direct contact membrane distillation. Desalination 2016, 378, 37–43. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.; Xu, Z.; Liu, Y.; Cheng, F. Simultaneous anti-fouling and flux-enhanced membrane distillation via incorporating graphene oxide on PTFE membrane for coking wastewater treatment. Appl. Surf. Sci. 2020, 531, 147349. [Google Scholar]
- Naidu, G.; Jeong, S.; Kim, S.-J.; Kim, I.S.; Vigneswaran, S. Organic fouling behavior in direct contact membrane distillation. Desalination 2014, 347, 230–239. [Google Scholar]
- Lawal, D.U.; Abdulazeez, I.; Alsalhy, Q.F.; Usman, J.; Abba, S.I.; Mansir, I.B.; Sathyamurthy, R.; Kaleekkal, N.J.; Imteyaz, B. Experimental Investigation of a Plate–Frame Water Gap Membrane Distillation System for Seawater Desalination. Membranes 2023, 13, 804. [Google Scholar] [CrossRef] [PubMed]
- Memon, S.; Im, B.-G.; Lee, H.-S.; Kim, Y.-D. Comprehensive experimental and theoretical studies on material-gap and water-gap membrane distillation using composite membranes. J. Membr. Sci. 2023, 666, 121108. [Google Scholar] [CrossRef]
- Khayet, M. Solar desalination by membrane distillation: Dispersion in energy consumption analysis and water production costs (a review). Desalination 2013, 308, 89–101. [Google Scholar] [CrossRef]
- Alawad, S.M.; Khalifa, A.E.; Antar, M.A. Performance analysis of multistage water gap membrane distillation system with economic evaluation. Appl. Therm. Eng. 2021, 184, 116297. [Google Scholar] [CrossRef]
- Amaya-Vías, D.; López-Ramírez, J.A. Techno-Economic Assessment of Air and Water Gap Membrane Distillation for Seawater Desalination under Different Heat Source Scenarios. Water 2019, 11, 2117. [Google Scholar] [CrossRef]
- Lawal, D.U.; Alawad, S.; Usman, J.; Abba, S.I.; Sekar, S.; Mansir, I.B.; Baroud, T.; Alsalhy, Q.F.; Yassin, M.A.; Shah, S.M.H.; et al. Performance assessment of a modified air-gap and water-gap membrane distillation systems. Desalination Water Treat. 2024, 317, 100303. [Google Scholar] [CrossRef]
- Abu-Zeid, M.A.E.-R.; Lu, X.; Zhang, S. Enhancement of the air gap membrane distillation system performance by using the water gap module. Water Supply 2020, 20, 2884–2902. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Banat, F.; Qtaishat, M.; Al-Khateeb, M. Concentration of sucrose solutions via vacuum membrane distillation. Desalination 2006, 195, 60–68. [Google Scholar] [CrossRef]
- Mohammadi, T.; Bakhteyari, O. Concentration of l-lysine monohydrochloride (l-lysine–HCl) syrup using vacuum membrane distillation. Desalination 2006, 200, 591–594. [Google Scholar] [CrossRef]
- Elkina, I.B.; Gilman, A.B.; Ugrozov, V.V.; Volkov, V.V. Separation of Mineral Acid Solutions by Membrane Distillation and Thermopervaporation through Porous and Nonporous Membranes. Ind. Eng. Chem. Res. 2013, 52, 8856–8863. [Google Scholar] [CrossRef]
- Wu, B.; Tan, X.; Li, K.; Teo, W.K. Removal of 1,1,1-trichloroethane from water using a polyvinylidene fluoride hollow fiber membrane module: Vacuum membrane distillation operation. Sep. Purif. Technol. 2006, 52, 301–309. [Google Scholar] [CrossRef]
- Gryta, M. Concentration of aqueous solutions of caprolaclam by membrane distillation. Polimery 2022, 51, 665–670. [Google Scholar]
- Wu, B.; Tan, X.; Teo, W.K.; Li, K. Removal of Benzene/Toluene from Water by Vacuum Membrane Distillation in a PVDF Hollow Fiber Membrane Module. Sep. Sci. Technol. 2005, 40, 2679–2695. [Google Scholar] [CrossRef]
- Yue, C.; Peng, Y.; Chen, L.; Schaefer, L.A. Thermal analysis of a heat pump-based liquid gap membrane distillation H2SO4 system. Chem. Eng. Process. Process Intensif. 2021, 167, 108509. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Naidu, G.; Jeong, S.; Vigneswaran, S. Interaction of humic substances on fouling in membrane distillation for seawater desalination. Chem. Eng. J. 2015, 262, 946–957. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Schlenger, P.; García-Valverde, M. A comprehensive structural evaluation of humic substances using several fluorescence techniques before and after ozonation. Part I: Structural characterization of humic substances. Sci. Total Environ. 2014, 476–477, 718–730. [Google Scholar] [CrossRef]
- Chowdhury, S.; Khan, N.; Kim, G.H.; Harris, J.; Longhurst, P.; Bolan, N.S. Chapter 22—Zeolite for Nutrient Stripping From Farm Effluents. In Environmental Materials and Waste; Prasad, M.N.V., Shih, K., Eds.; Academic Press: New York, NY, USA, 2016; pp. 569–589. [Google Scholar]
- Bond, T.; Goslan, E.H.; Parsons, S.A.; Jefferson, B. A critical review of trihalomethane and haloacetic acid formation from natural organic matter surrogates. Environ. Technol. Rev. 2012, 1, 93–113. [Google Scholar] [CrossRef]
- Amaya-Vías, D.; López-Ramírez, J.A.; Gray, S.; Zhang, J.; Duke, M. Diffusion behavior of humic acid during desalination with air gap and water gap membrane distillation. Water Res. 2019, 158, 182–192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).