Microwave-Assisted Pyrolysis of Polyethylene and Polypropylene from End-of-Life Vehicles: Hydrogen Production and Energy Valorization
Abstract
1. Introduction
- Ordinary plastics (PE, PP) have dielectric properties that do not allow them to convert microwave energy into heat (loss tangent = 0.001–0.002). For this reason, it is mandatory to use compounds that absorb microwave energy very well and allow the heating of the reaction mixture, such as silicon carbide (tan δ = 0.25−0.37), activated carbon (tan δ = 0.31−0.9), or ferric oxide (tan δ = 0.199) [37];
- The depth of penetration of microwaves in the mixture of materials subjected to pyrolysis is limited to a few tens of cm. For this reason, pyrolysis reactors are small: laboratory-scale reactors are typically made of quartz while pilot-scale reactors operate continuously but with relatively low flow rates—around 10 kg/h [36].
2. Materials and Methods
2.1. Feedstock
2.2. Catalyst Preparation
2.3. Characterization Techniques
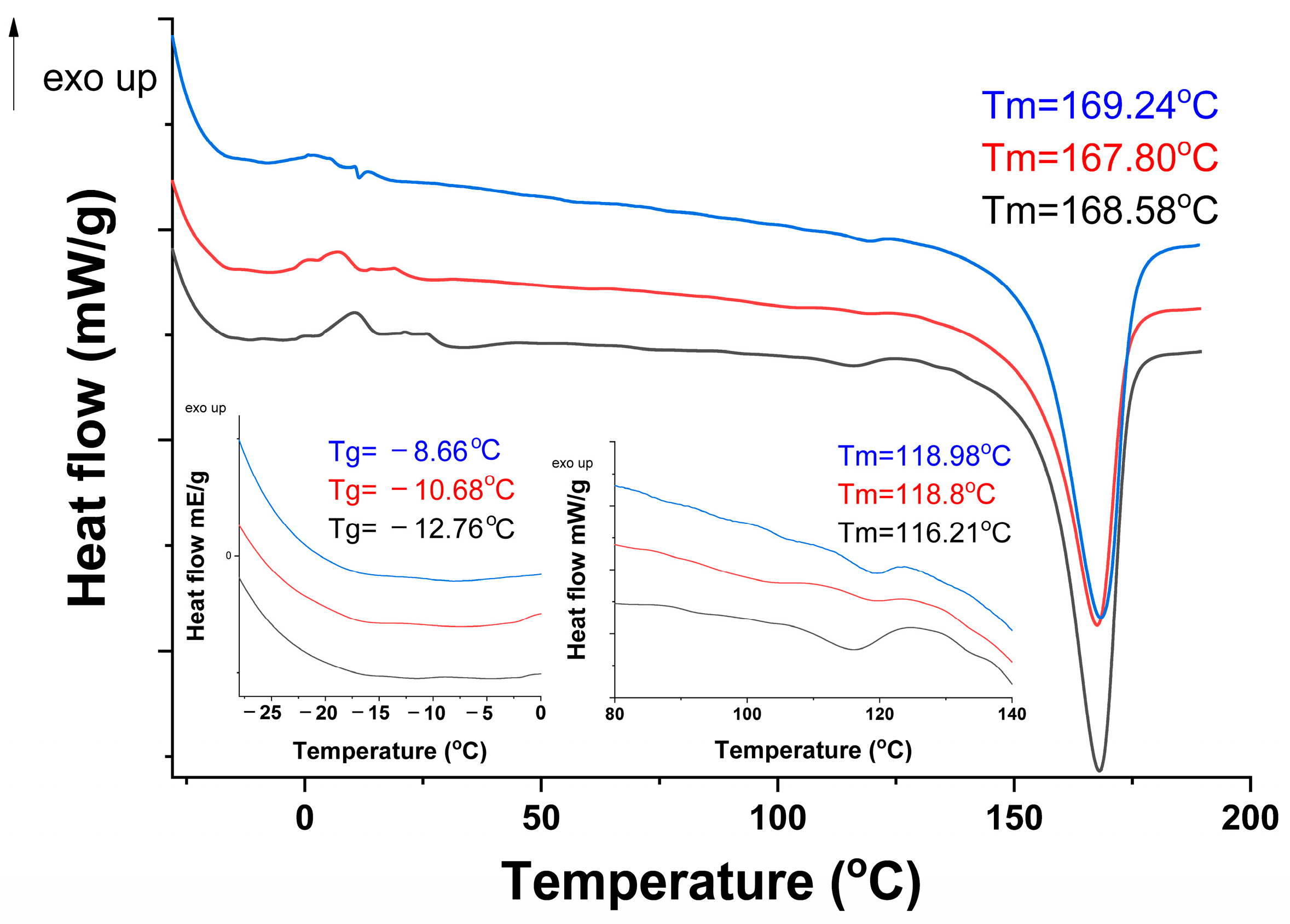
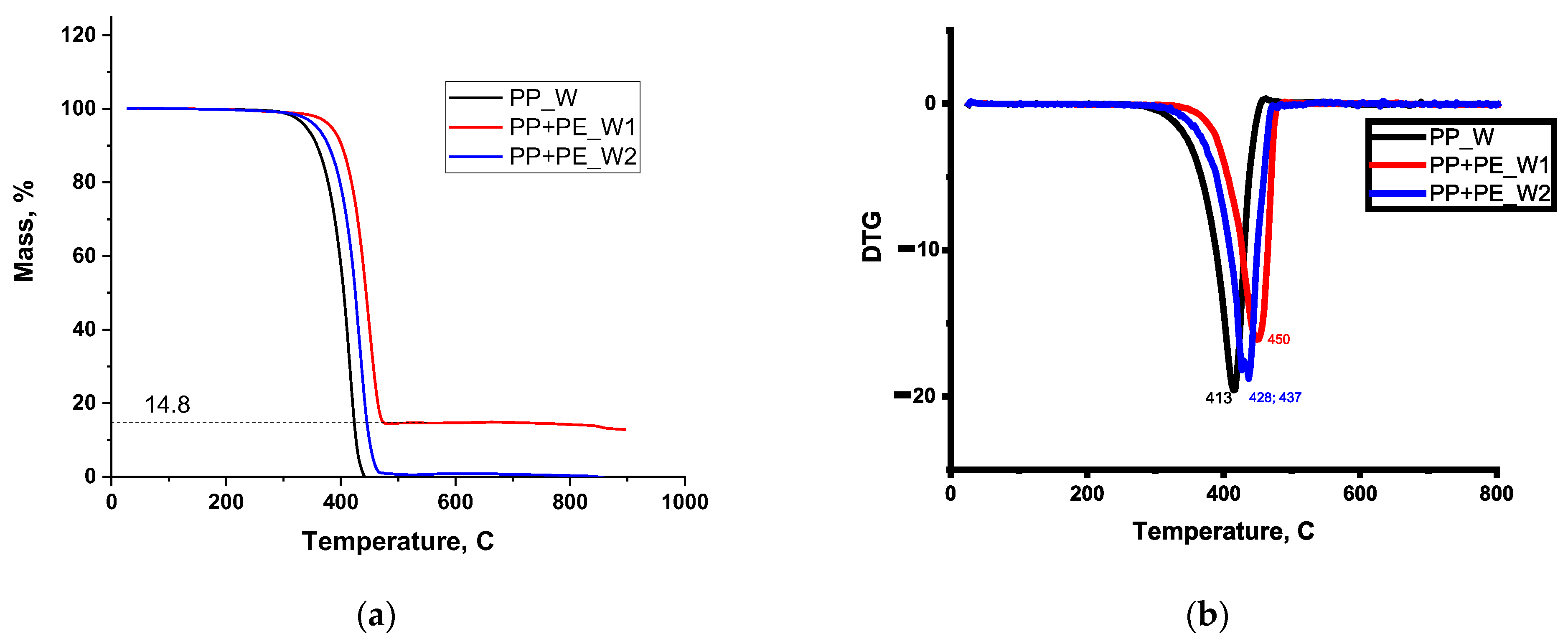
2.3.1. Analysis of Raw Materials (FTIR/DSC/XRD/TGA-DTG)
2.3.2. Active Carbon Analysis
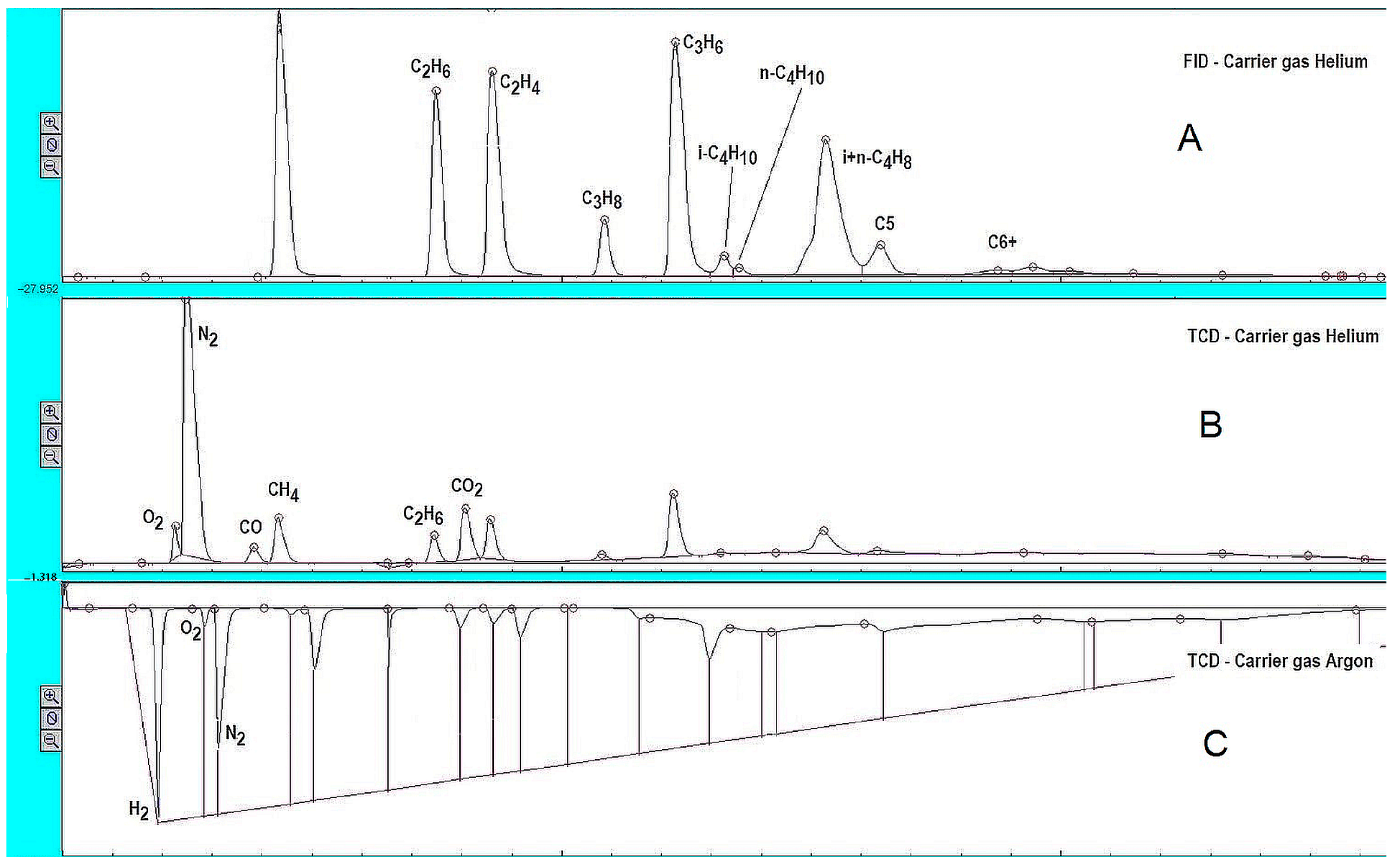
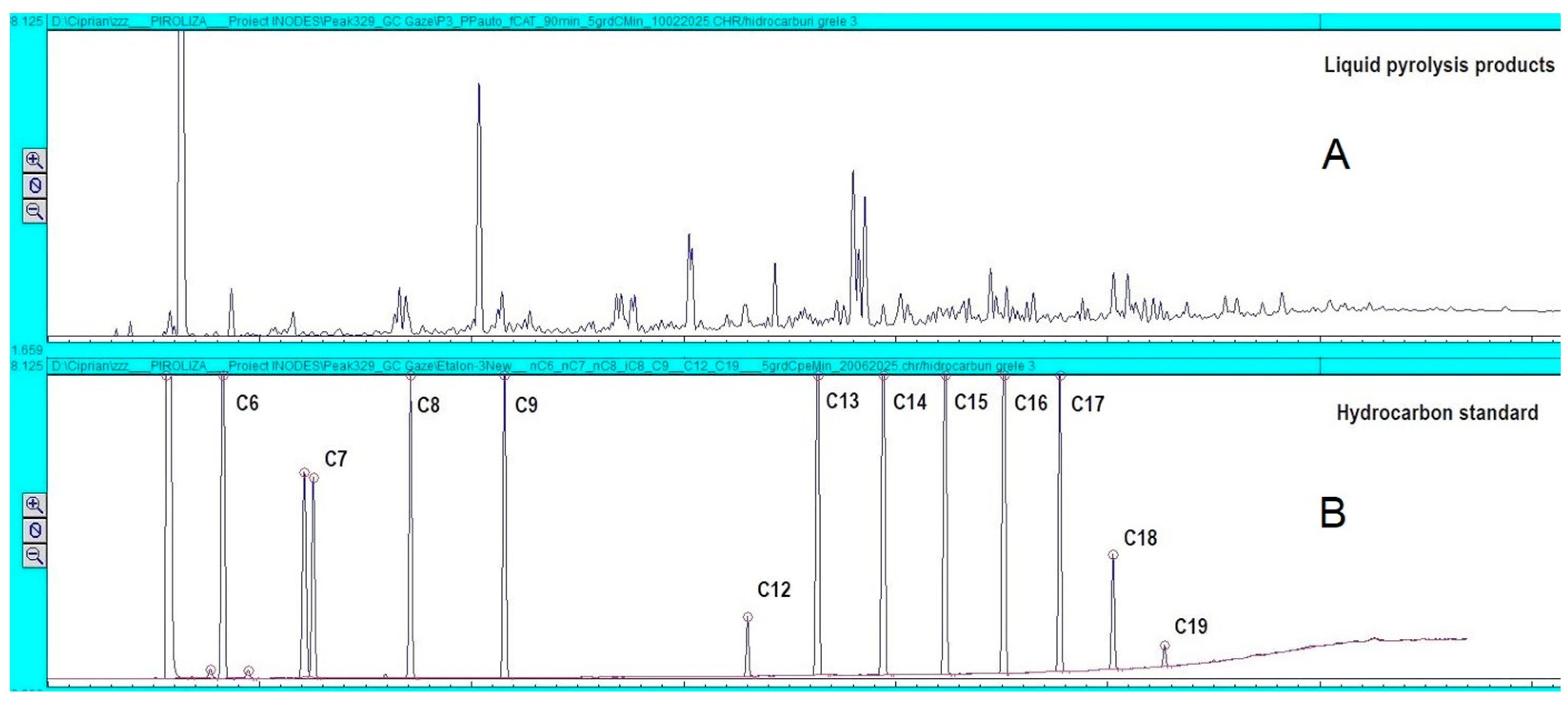
2.3.3. GC Analysis of the Liquid and Gaseous Products Obtained
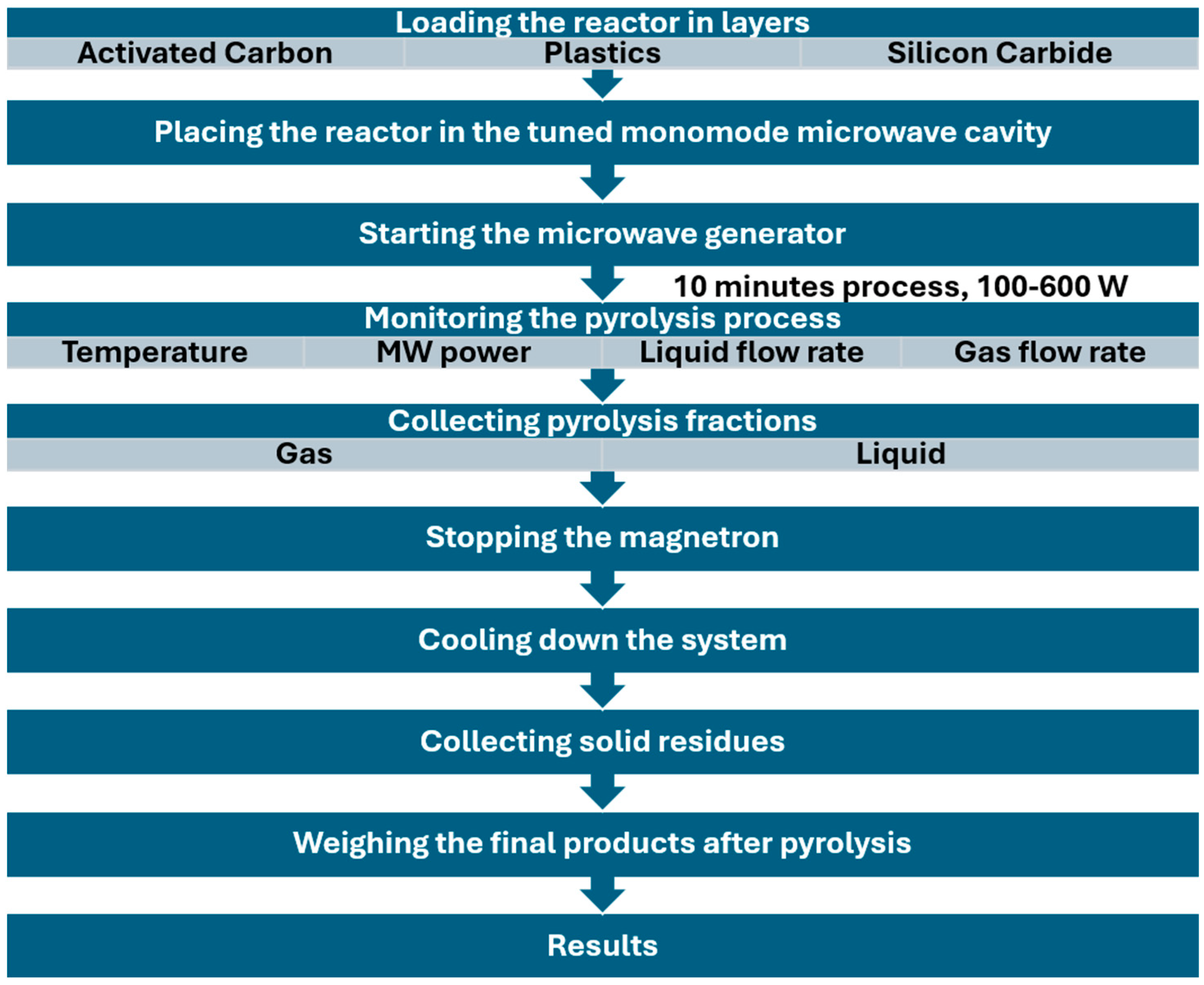
2.4. Microwave Pyrolysis Setup
2.5. Product Yield
2.6. Calorific Value of Gases
3. Results and Discussion
- -
- FT-IR/DSC/XRD analysis to establish the composition of plastic waste;
- -
- TGA/DTG analysis to establish the behavior at thermal decomposition.
3.1. FTIR/DSC/XRD/TGA Analysis of Raw Materials
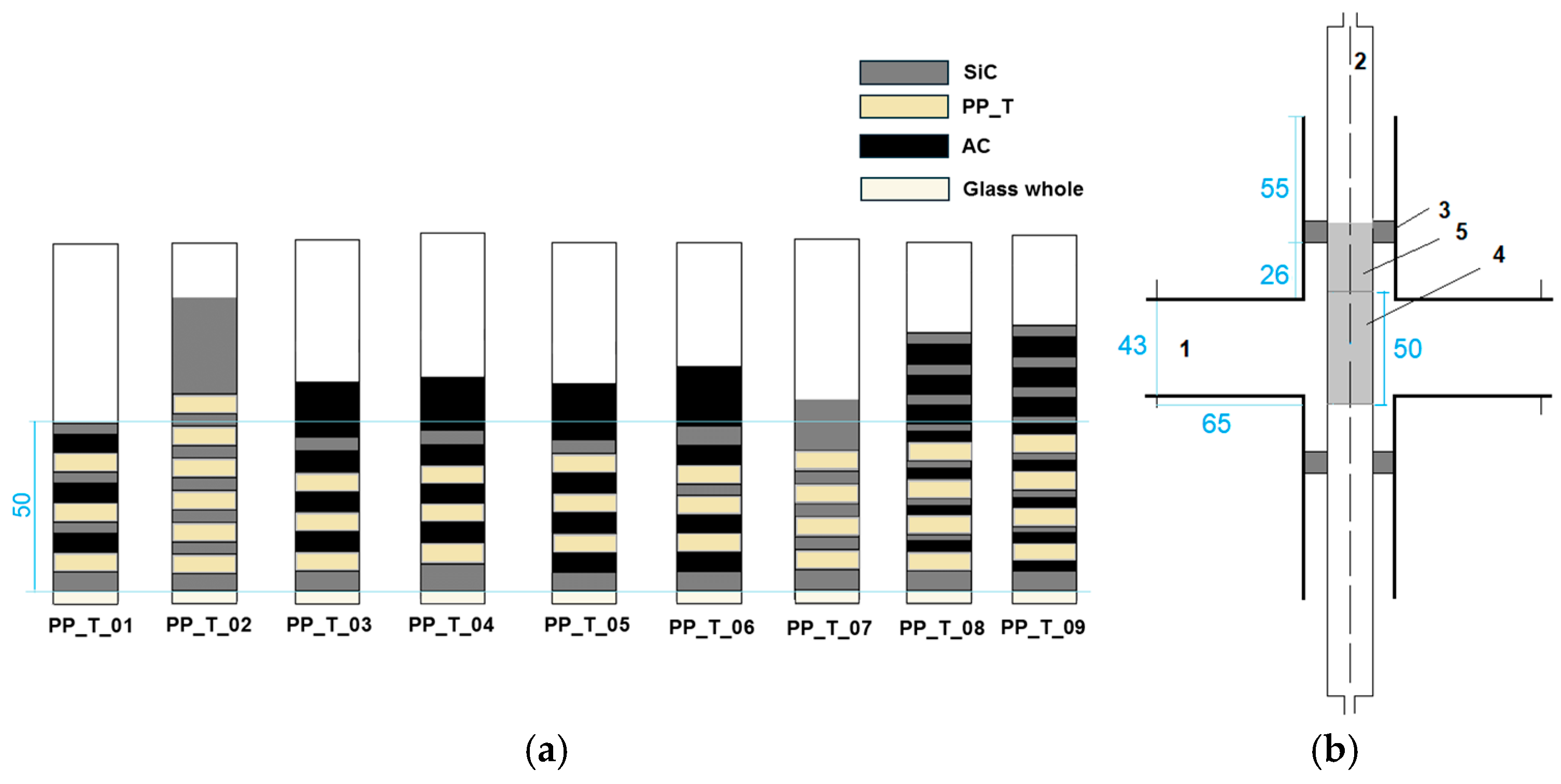
3.2. Experimental Configurations and Product Yields
3.3. Influence of Plastic Type
3.4. Influence of MW Heating and AC
3.5. Reuse of CA in Pyrolysis
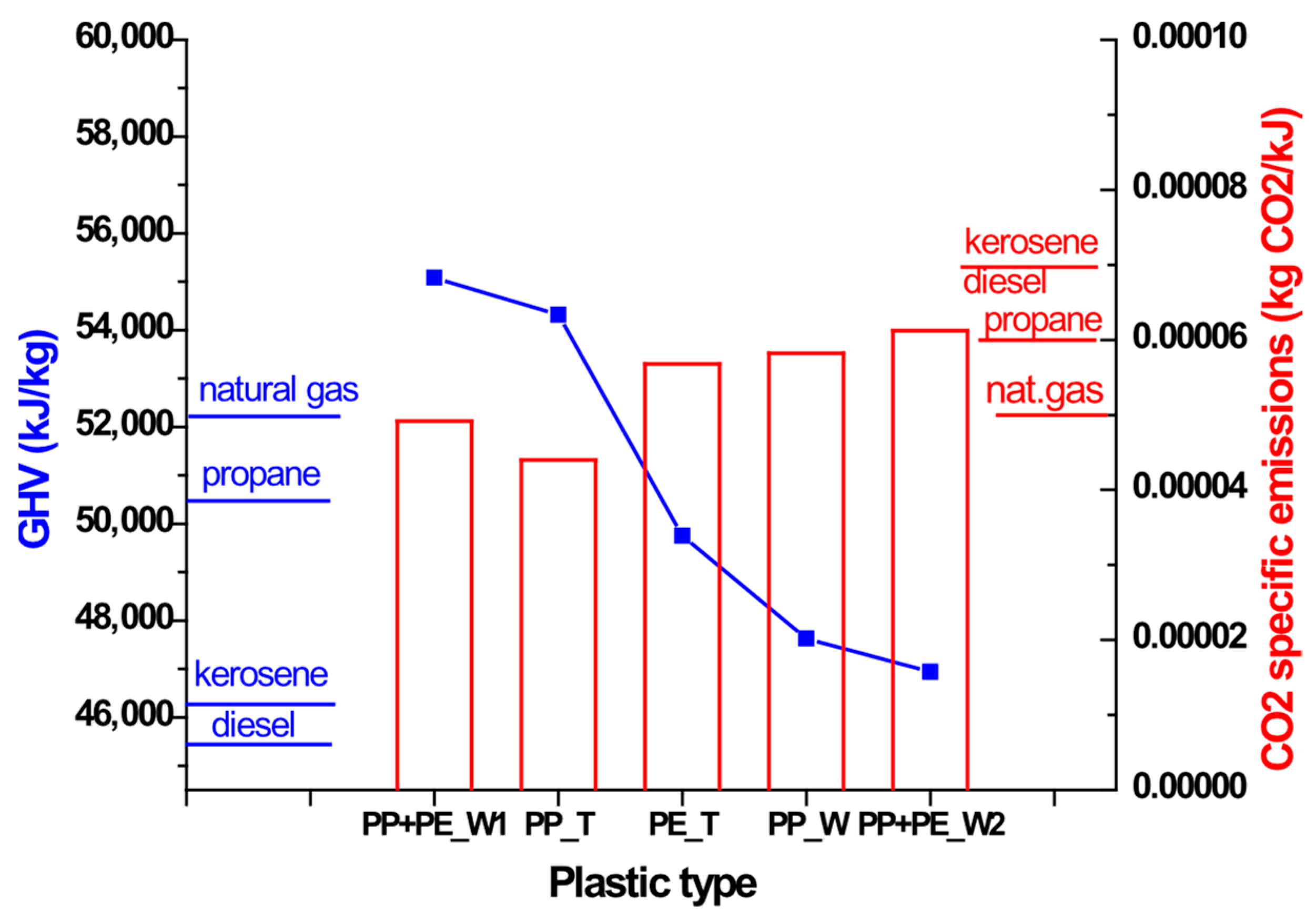
3.6. Calorific Power of Gases
3.6.1. Analysis of the Gaseous Mixture (Vol.%)
3.6.2. Analysis of the Gaseous Mixture (wt.%)
3.7. Liquid Fraction
3.8. Energy Distribution: Gas and Liquid Fractions
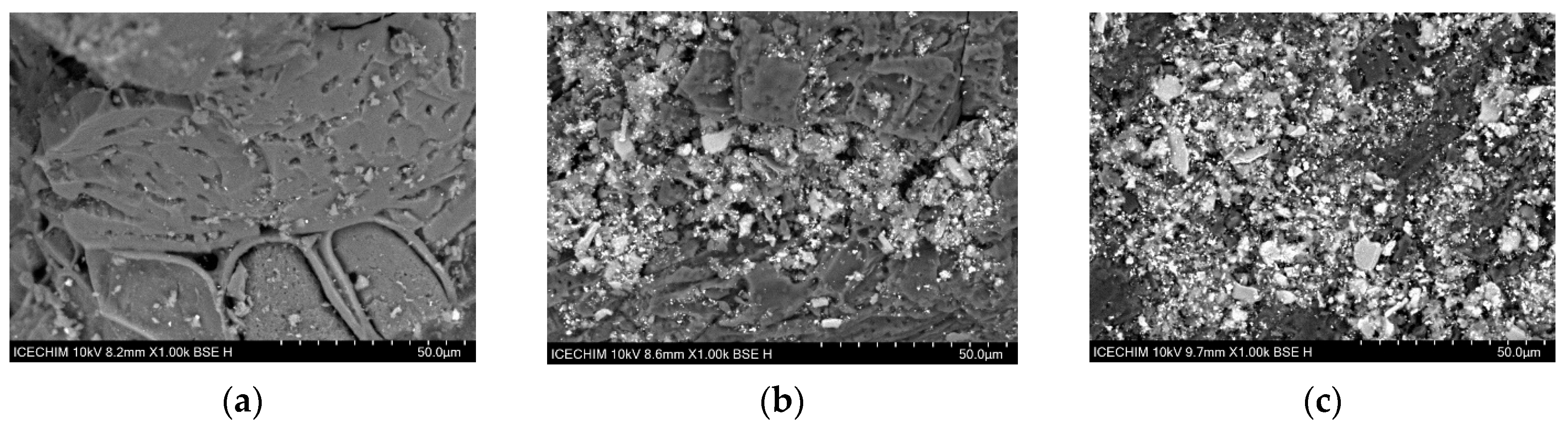
3.9. Morphological and Textural Changes in Activated Carbon and Silicon Carbide
4. Limitations and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PP | Polypropylene |
| PE | Polyethylene |
| PS | Polystyrene |
| ELV | End-of-life vehicle |
| PVC | Polyvinyl chloride |
| PET | Polyethylene terephthalate |
| PA | Polyamide |
| PC | Polycarbonate |
| PU | Polyurethane |
| LDPE | Low-density polyethylene |
| PMMA | Polymethyl methacrylate |
| MW | Microwave |
| MAP | Microwave-assisted pyrolysis |
| SiC | Silicon carbide |
| AC | Activated carbon |
| GC | Gas Chromatography |
| FTIR | Fourier-transform infrared spectroscopy |
| XRD | X-ray diffraction |
| MS | Mass spectrometry |
| BSE | Backscattered electron |
| FID | Flame ionization detector |
| TCD | Thermal conductivity detector |
| TGA | Thermogravimetric analysis |
| IR | Infrared |
| DTG | Derivative thermogravimetry |
| DSC | Differential scanning calorimetry |
| GHV | Gross Heating Value (or Higher Heating Value) |
| SEM | Scanning Electron Microscopy |
| BET | Brunauer–Emmett–Teller (surface area analysis method) |
| BJH | Barrett–Joyner–Halenda (desorption branch of the isotherm) |
References
- European Commission/Environment/Circular Economy—A New Circular Economy Action Plan. For a Cleaner and More Competitive Europe. 2020. Available online: https://ec.europa.eu/environment/circular-economy/index_en.htm (accessed on 15 February 2025).
- Davidson, M.G.; Furlong, R.A.; McManus, M.C. Developments in the life cycle assessment of chemical recycling of plastic waste—A review. J. Clean. Prod. 2021, 293, 126163. [Google Scholar] [CrossRef]
- Schwarz, A.E.; Ligthart, T.N.; Godoi Bizarro, D.; De Wild, P.; Vreugdenhil, B.; van Harmelen, T. Plastic recycling in a circular economy; determining environmental performance through an LCA matrix model approach. Waste Manag. 2021, 121, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Rovinaru, F.I.; Rovinaru, M.D.; Rus, A.V. The Economic and Ecological Impacts of Dismantling End-of-Life Vehicles in Romania. Sustainability 2019, 11, 6446. [Google Scholar] [CrossRef]
- Barbeş, L.; Manea, L.C.; Manea, A.T.; Ionaşcu, S.L. Advancements in Automotive Waste Management. In Proceedings of the CONAT 2024 International Congress of Automotive and Transport Engineering, Brasov, Romania, 6–8 November 2024; Springer Nature: Cham, Switzerland, 2025. [Google Scholar]
- Institute for Electrotechnical Research and Design. Development of a Methodology for Monitoring the Achievement of Targets Regarding the Reuse, Recycling, and Recovery of End-of-Life Vehicles Internal Report; CALIST Contract No. 5798/13.11.2006; Institute for Electrotechnical Research and Design: Bucharest, Romania, 2006. (In Romanian) [Google Scholar]
- Pischinger, S.; Seiffert, U. Vieweg Handbuch Kraftfahrzeugtechnik, 9th ed.; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 978-3-658-25556-5. [Google Scholar]
- Cardamone, G.F.; Ardolino, F.; Arena, U. Can plastics from end-of-life vehicles be managed in a sustainable way? Sustain. Prod. Consum. 2022, 29, 115–127. [Google Scholar] [CrossRef]
- Ortego, A.; Russo, S.; Iglesias-Émbil, M.; Valero, A.; Magdalena, R. Exergy Assessment of Plastic Car Parts. Vehicles 2023, 5, 1211–1226. [Google Scholar] [CrossRef]
- Jeswani, H.; Kruger, C.; Russ, M.; Horlacher, M.; Antony, F.; Hann, S.; Azapagic, A. Life cycle environmental impacts of chemical recycling via pyrolysis of mixed plastic waste in comparison with mechanical recycling and energy recovery. Sci. Total Environ. 2021, 769, 144483. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Nakatani, J. Implications of chemical recycling of plastic waste for climate change impacts: A critical review. Sustain. Prod. Consum. 2024, 48, 301–323. [Google Scholar] [CrossRef]
- Das, S.; Liang, C.; Dunn, J.B. Plastics to fuel or plastics: Life cycle assessment-based evaluation of different options for pyrolysis at end-of-life. Waste Manag. 2022, 153, 81–88. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Senthil Kumar, P.; Rangasamy, G.; Praveen Perumal, L.; Sanaulla, S.; Nilavendhan, S.; Manivasagan, V.; Saranya, K. A critical review on pyrolysis method as sustainable conversion of waste plastics into fuels. Fuel 2023, 337, 126890. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Schaller, D.; Khan, M.M.K.; Hasan, M.M.; Hazrat, M.A. Transport fuel from waste plastics pyrolysis—A review on technologies, challenges opportunities. Energy Convers. Manag. 2022, 258, 115451. [Google Scholar] [CrossRef]
- Kulas, D.G.; Zolghadr, A.; Chaudhari, U.S.; Shonnard, D.R. Economic and environmental analysis of plastics pyrolysis after secondary sortation of mixed plastic waste. J. Clean. Prod. 2023, 384, 135542. [Google Scholar] [CrossRef]
- Hasan, M.M.; Haque, R.; Jahirul, M.I.; Rasul, M.G. Pyrolysis of plastic waste for sustainable energy Recovery: Technological advancements and environmental impacts. Energy Convers. Manag. 2025, 326, 119511. [Google Scholar] [CrossRef]
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, P.; Amadei, A.M.; Klenert, D.; Nessi, S.; Tonini, D.; Tosches, D.; Ardente, F.; Saveyn, H. Environmental and Economic Assessment of Plastic Waste Recycling; EUR 31423 EN; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics waste management: A review of pyrolysis technology. Clean Technol. Recycl. 2021, 1, 50–69. [Google Scholar] [CrossRef]
- Venturelli, M.; Falletta, E.; Pirola, C.; Ferrari, F.; Milani, M.; Montorsi, L. Experimental evaluation of the pyrolysis of plastic residues and waste tires. Appl. Energy 2022, 323, 119583. [Google Scholar] [CrossRef]
- van Eijk, F.; Beuken, E.; Sederel, W.; Groen, J.; Perez, M.; Locati, G. Chemical Recycling in Circular Perspective. From Vision to Action: How Chemical Recycling Steers the Transition Towards a Circular and Carbon Neutral Chemical Industry. 2023. Available online: https://circulareconomy.europa.eu/platform/sites/default/files/2023-08/Chemical%20Recycling%20in%20Circular%20Perspective.pdf (accessed on 2 June 2025).
- Munir, D.; Irfan, M.F.; Usman, M.R. Hydrocracking of virgin and waste plastics: A detailed review. Renew. Sustain. Energy Rev. 2018, 90, 490–515. [Google Scholar] [CrossRef]
- Munir, D.; Amer, H.; Aslam, R.; Bououdina, M.; Usman, M.R. Composite zeolite beta catalysts for catalytic hydrocracking of plastic waste to liquid fuels. Mater. Renew. Sustain. Energy 2020, 9, 9. [Google Scholar] [CrossRef]
- Kunwar, B.; Moser, B.R.; Chandrasekaran, S.R.; Rajagopalan, N.; Sharma, B.K. Catalytic and thermal depolymerization of low value post-consumer high density polyethylene plastic. Energy 2016, 111, 884–892. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, S. Pyrolytic degradation of polyethylene in autoclave under high pressure to obtain fuel. J. Anal. Appl. Pyrolysis 2017, 124, 298–302. [Google Scholar] [CrossRef]
- Brems, A.; Dewil, R.; Baeyens, J.; Zhang, R. Gasification of plastic waste as waste-to-energy or waste-to-syngas recovery route. Nat. Sci. 2013, 5, 695–704. [Google Scholar] [CrossRef]
- Calinescu, I.; Psenovschi, G.; Cojocaru, M.; Chisega-Negrila, C.G.; Albulescu, C.; Brebu, M.; Trifan, A.; Ignat, N.D.; Chipurici, P. Catalytic Pyrolysis of Low-Density Polyethylene Waste. Sustainability 2024, 16, 6788. [Google Scholar] [CrossRef]
- Punkkinen, H.; Oasmaa, A.; Luntama, J.L.; Nieminen, M.; Laine-Ylijok, J. Thermal Conversion of Plastic Containing Waste: A Review; Research Report No D4.1-22; VTT’s Research Information Portal: Espoo, Finland, 2017. [Google Scholar]
- Wang, X.; Cheng, H.; Ye, G.; Fan, J.; Yao, F.; Wang, Y.; Jiao, Y.; Zhu, W.; Huang, H.; Ye, D. Key factors and primary modification methods of activated carbon and their application in adsorption of carbon-based gases: A review. Chemosphere 2022, 287, 131995. [Google Scholar] [CrossRef]
- Sun, K.; Huang, Q.; Meng, X.; Chi, Y.; Yan, J. Catalytic pyrolysis of waste polyethylene into aromatics by H3PO4-activated carbon. Energy Fuels 2018, 32, 9772–9781. [Google Scholar] [CrossRef]
- González, Y.S.; Costa, C.; Márquez, M.C.; Ramos, P. Thermal and catalytic degradation of polyethylene wastes in the presence of silica gel, 5A molecular sieve and activated carbon. J. Hazard. Mater. 2011, 187, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, D.; Lei, H.; Villota, E.; Ruan, R. Jet fuel production from waste plastics via catalytic pyrolysis with activated carbons. Appl. Energy 2019, 251, 113337. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, B.; He, M.; Zhang, Z.; Tang, C.; Zhao, J.; Wu, H.; Fan, Y.; Ma, W. Microwave-coupled recycling of plastic waste into hydrogen and carbon nanotubes over economical iron-based catalyst. Int. J. Hydrogen Energy 2025, 115, 24–36. [Google Scholar] [CrossRef]
- Hu, X.; Ma, D.; Zhang, G.; Ling, M.; Hu, Q.; Liang, K.; Lu, J.; Zheng, Y. Microwave-assisted pyrolysis of waste plastics for their resource reuse: A technical review. Carbon Resour. Convers. 2023, 6, 215–228. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, M.; Zhou, T.; Yang, J.; Yang, Y.; Liu, T.; Dai, S.; Huang, Q.; Cen, Q.; Xiao, P.; et al. A review on liquid fuel produced from microwave-assisted pyrolysis of plastic waste. Process Saf. Environ. Prot. 2024, 187, 833–844. [Google Scholar] [CrossRef]
- Zhou, N.; Dai, L.; Lv, Y.; Li, H.; Deng, W.; Guo, F.; Chen, P.; Lei, H.; Ruan, R. Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production. Chem. Eng. J. 2021, 418, 129412. [Google Scholar] [CrossRef]
- Islam, K.M.O.; Ahmad, N.; Ummer, A.C.; Ahmed, U.; Siddiqui, M.N.; Millan, M.; Abdul Jameel, A.G. Microwave-Assisted pyrolysis of waste plastics: A comprehensive review on process parameters, catalysts, and future prospects. Results Eng. 2025, 26, 105571. [Google Scholar] [CrossRef]
- Putra, P.H.M.; Rozali, S.; Patah, M.F.A.; Idris, A. A review of microwave pyrolysis as a sustainable plastic waste management technique. J. Environ. Manag. 2022, 303, 114240. [Google Scholar] [CrossRef]
- Guo, G.; Fan, K.; Guo, Z.; Guo, W. Pyrolysis behavior of automotive polypropylene plastics: ReaxFF molecular dynamics study on the co-pyrolysis of polypropylene and EPDM/POE. Energy 2023, 280, 128202. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]
- Kevin Ejiogu, I.; Ibeneme, U.; Olukemi Aiyejagbara, M.; Oyemachi, D. Pyrolysis of Waste Plastics as an Effective Tool in Sustainable Environmental Waste Management. Mod. Chem. 2021, 9, 33–39. [Google Scholar] [CrossRef]
- Zhou, X.-L.; He, P.-J.; Peng, W.; Lü, F.; Shao, L.-M.; Zhang, H. From plastics to methane and carbon spheres: The evolution of pyrolysis products during pyrolysis under autogenic atmosphere. J. Anal. Appl. Pyrolysis 2022, 161, 105421. [Google Scholar] [CrossRef]
- The Engineering ToolBox. Heating Values of Fuel Gases. Available online: https://www.engineeringtoolbox.com/heating-values-fuel-gases-d_823.html (accessed on 15 May 2025).
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 53, 73–75. ISBN 978-0-470-09307-8. [Google Scholar]
- Barbes, L.; Stihi, C.; Radulescu, C. ATR-FTIR spectrometry characterisation of polymeric materials. Rom. Rep. Phys. 2014, 66, 765–777. [Google Scholar]
- Dai, S.; Luo, C.; Zhang, C.; Wang, H.; Zhang, Y.; Yuan, L. Determination of small-amount polypropylene in imported recycled polyethylene/polypropylene blends by Fourier transform infrared spectroscopy. EDP Sci. 2021, 261, 02067. [Google Scholar] [CrossRef]
- Bashirgonbadi, A.; Ureel, Y.; Delva, L.; Fiorio, R.; Van Geem, K.M.; Ragaert, K. Accurate determination of polyethylene (PE) and polypropylene (PP) content in polyolefin blends using machine learning-assisted differential scanning calorimetry (DSC) analysis. Polym. Test. 2024, 131, 108353. [Google Scholar] [CrossRef]
- Ding, K.; Liu, S.; Huang, Y.; Liu, S.; Zhou, N.; Peng, P.; Wang, Y.; Chen, P.; Ruan, R. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers. Manag. 2019, 196, 1316–1325. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Cui, L.; Liu, Y.; Li, B.; Liu, W. Microwave-assisted pyrolysis of polypropylene plastic for liquid oil production. J. Clean. Prod. 2023, 411, 137303. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Cui, L.; Xiong, Q.; Mostafa, E. Microwave-assisted fluidized bed reactor pyrolysis of polypropylene plastic for pyrolysis gas production towards a sustainable development. Appl. Energy 2023, 342, 121099. [Google Scholar] [CrossRef]
- Carbon Dioxide Emissions Coefficients. Available online: https://www.eia.gov/environment/emissions/co2_vol_mass.php (accessed on 14 May 2025).




| Process | Target Plastic | Temperature Range | Main Outputs | References |
|---|---|---|---|---|
| Mechanical recycling | PE, PP, PS, PET | 20–250 °C | Pellets, flakes | [17,18] |
| Chemical recycling | Mixed plastic waste | - | Feedstock for the chemical industry | [19] |
| Solvent purification | PS, PE, PP, PVC | 100–250 °C | Purified polymers, monomers | [17,18] |
| Chemical depolymerization | PA, PC, PU, PET | 200–300 °C | Monomers (e.g., terephthalic acid, ethylene glycol) | [20] |
| Hydro-conversion | Mixed plastics | 300–500 °C | Synthetic fuels | [21,22] |
| Thermal depolymerization | PMMA, PS | 450–600 °C | Monomers (e.g., styrene) | [18,23] |
| Gasification | Mixed plastic waste | 700–1200 °C | Syngas (CO + H2), slag | [24,25,26] |
| Pyrolysis | Mixed plastic waste, PE, PP, PS | 400–800 °C | Liquid fuels, syngas, and char | [16,17,27,28] |
| Thermo-catalytic degradation | PE, PP, PS, | 400–600 °C | Lower-molecular hydrocarbons, aromatics | [22,27] |
| Parameter | Value |
|---|---|
| Microwave frequency | 2.45 GHz |
| Maximum MW power | 600 W |
| Cavity type | Monomode |
| Reactor material | Quartz |
| Component | Gross Heating Value, kJ/m3 | CO2 Emissions (L/L) |
|---|---|---|
| H2 | 12.109 | 0 |
| CO | 12.035 | 1 |
| CH4 | 37.669 | 1 |
| C2H6 | 66.433 | 2 |
| CO2 | 0.000 | 0 |
| C2H4 | 60.769 | 2 |
| C3H8 | 95.830 | 3 |
| C3H6 | 87.037 | 3 |
| i-C4H10 | 120.160 | 4 |
| n-C4H10 | 120.160 | 4 |
| 1-C4H8 | 114.646 | 4 |
| 2-C4H8 | 114.646 | 4 |
| C5 | 148.328 | 5 |
| C6 | 173.888 | 6 |
| Sample | Absorption Bands (cm−1) | Assignments | References |
|---|---|---|---|
| PE_T | 2915 (vs) 2847 (s) 1465 (m) 719 (m) | −CH2– (asy) C–H stretch –CH2– (sy) C–H stretch –CH2– scissoring vibration –CH2–rocking vibration | [44] |
| PP_T PP_W | 2954 (m) 2917 (s) 2844 (w) 1455; 1456 (m) 1376 (m-s) | –CH3(asy) C–H stretch –CH2– (asy) C–H stretch –CH2– (sy) C–H stretch –CH2–scissoring vibration -CH3 umbrella bending mode | [45] |
| PE+PP-1 PE+PP-2 | 2954 (m) 2915 (s) 2847 (w) 1459; 1455 (m) 1376 (m-s) 1165 (m-s) | –CH3(asy) C–H stretch –CH2– (asy) C–H stretch –CH2– (sy) C–H stretch –CH2–scissoring vibration –CH3 umbrella bending mode –CH3 symmetric deformation and C–C backbone | [46] |
| Configuration | Amounts (g) | Content (%) | Conversion (%) | H2 (vol.%) | |||
|---|---|---|---|---|---|---|---|
| Feedstock | AC | SiC | SiC | AC | |||
| PP_T_01 | 3 | 3 | 5 | 45.45 | 27.27 | 55 | 7.25 |
| PP_T_02 | 6 | 0 | 15 | 71.43 | 0.00 | 66 | 16.19 |
| PP_T_03 | 3 | 6 | 4 | 30.77 | 46.15 | 62.3 | 19.75 |
| PP_T_04 | 3 | 6 | 5 | 35.71 | 42.86 | 9.5 | 10.41 |
| PP_T_05 | 3 | 6 | 4 | 30.77 | 46.15 | 40.3 | 19.27 |
| PP_T_06 | 3 | 6 | 5 | 35.71 | 42.86 | 57 | 25.37 |
| PP_T_07 | 4 | 0 | 8 | 66.67 | 0.00 | 93.50 | 22.74 |
| PP_T_08 | 4 | 5 | 7 | 43.75 | 31.25 | 92.75 | 13.87 |
| PP_T_09 | 4 | 5.5 | 8 | 45.71 | 31.43 | 90.3 | 45.23 |
| Exp. | Results (wt.%) | Conversion (%) | H2 Efficiency (g/g) | H2 Yield (mmol/g Plastic) | |||
|---|---|---|---|---|---|---|---|
| Unreacted | Solid | Liquid | Gas | ||||
| PE_T | 7 ± 0.15 | 12.1 ± 0.6 | 9.95 ± 1.1 | 77.9 ± 1.5 | 93.0 ± 0.4 | 20.03 ± 1.6 | 14.3 ± 0.6 |
| PP_T | 9.75 ± 0.3 | 6.65 ± 1.2 | 11.91 ± 1.3 | 81.44 ± 1.5 | 90.3 ± 0.3 | 44.81 ± 1.8 | 32.0 ± 0.3 |
| PP_W | 5 ± 0.05 | 7.72 ± 0.7 | 14.39 ± 1.5 | 77.89 ± 1.8 | 95.0 ± 0.2 | 27.5 ± 3.5 | 19.7 ± 2.4 |
| PP+PE_W1 | 1 ± 0.2 | 22.32 ± 2.1 | 11.3 ± 1.2 | 66.7 ± 1.7 | 99.0 ± 0.5 | 56.4 ± 2.1 | 34.4 ± 0.4 |
| PP+PE_W2 | 3.17 ± 0.7 | 4.48 ± 0.03 | 7.08 ± 2.0 | 87.08 ± 1.0 | 96.8 ± 0.6 | 8.66 ± 0.1 | 6.2 ± 0.1 |
| PP_W_conv_1 | 3.6 ± 0.1 | 3.46 ± 0.5 | 6.92 ± 0.8 | 89.62 ± 1.9 | 96.3 ± 0.3 | 6.68 ± 0.3 | 4.77 ± 1.4 |
| PP_W_conv_2 | 0.2 ± 0.05 | 0.1 ± 0.02 | 13.3 ± 0.7 | 85.6 ± 2.1 | 99.0 ± 0.2 | 3.64 ± 0.4 | 2.6 ± 1.2 |
| PP_W2 | 16.5 ± 0.8 | 4.4 ± 0.6 | 10.4 ± 0.7 | 85.2 ± 1.8 | 83.3 ± 0.5 | 37.2 ± 0.5 | 26.5 ± 0.8 |
| References | Feed | Microwave Oven and Settings | Temp. | Product Distribution |
|---|---|---|---|---|
| [48] | LDPE | 6 microwave ovens with power of 1800 W and frequency of 2.45 GHz were used. 1 L round-bottom flask was used as a reactor vessel. SiC absorbent. Microwave power used 150–200 W. HY catalyst | 450 °C | Oil: 34% Gas: 38% Coke: 28% |
| 500 °C | Oil: 57% Gas: 42% Coke: 3% | |||
| 550 °C | Oil: 54% Gas: 44% Coke: 2% | |||
| 600 °C | Oil: 49% Gas: 50% Coke: 1% | |||
| [49] | PP | Microwave power: 0–1000 W, Frequency: 2.45 GHz, 100 mL Quartz reactor used as vessel, Absorbent SiC: 2–3 mm, Microwave power used 600 W | 350 °C | Oil: 3% Gas: 8% Residue: 89% |
| 450 °C | Oil: 79% Gas: 14% Residue: 7% | |||
| 550 °C | Oil: 66% Gas: 34% Residue: 0% | |||
| [50] | PP | Microwave fluidizing bed reactor was used. Microwave oven power range: 200–1000 W, Microwave frequency: 2.45 GHz, Absorbent SiC: 40 g, Microwave power used 800 W, Fluidizing velocity: 2.3 × 10–3 m/s | 700 °C | Wax: 35% Gas: 65% |
| 900 °C | Wax: 24% Gas: 76% | |||
| 1000 °C | Wax: 34% Gas: 66% | |||
| 1100 °C | Wax: 39% Gas: 61% | |||
| This paper | PE PP PP+PE | Quartz reactor in monomode applicator; 2.45 GHz; 600 W max power | 500–700 °C 500–700 °C 500–700 °C | Oil: 9.9% Gas: 77.9% Residue: 12.1% Unreacted: 7% |
| Oil: 10.4–14.4% Gas: 77.9–85.2% Residue: 4.4–7.7% Unreacted: 5–16.5% | ||||
| Oil: 7.1–11.3% Gas: 66.7–87–1 Residue: 4.5–22% Unreacted: 1–3.2% |
| Exp. | Concentration (vol.%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CH4 | C2H6 | CO2 | C2H4 | C3H8 | C3H6 | i-C4H10 | n-C4H10 | 1-C4H8 | 2-C4H8 | C5 | C6+ | |
| PE_T | 36.63 | 2.11 | 22.76 | 5.42 | 0.71 | 18.39 | 1.94 | 3.93 | 0.00 | 1.00 | 2.04 | 1.92 | 0.57 | 2.59 |
| PP_T | 45.23 | 1.04 | 43.28 | 8.61 | 1.51 | 0.03 | 0.03 | 0.15 | 0.01 | 0.00 | 0.00 | 0.07 | 0.02 | 0.02 |
| PP_W | 46.50 | 0.78 | 16.52 | 4.25 | 4.09 | 15.53 | 1.89 | 5.41 | 0.22 | 0.15 | 0.59 | 2.06 | 0.48 | 1.52 |
| PP + PE_W1 | 75.35 | 0.16 | 0.29 | 7.07 | 0.35 | 0.68 | 1.21 | 3.35 | 0.36 | 0.29 | 0.30 | 2.68 | 1.51 | 6.41 |
| PP + PE_W2 | 20.05 | 0.68 | 23.32 | 8.25 | 2.50 | 19.64 | 1.79 | 7.96 | 0.95 | 0.58 | 1.16 | 4.92 | 2.25 | 5.93 |
| PP_W_conv_1 | 16.51 | 0.35 | 26.62 | 9.65 | 3.24 | 13.34 | 2.31 | 7.65 | 1.38 | 1.48 | 1.11 | 5.90 | 3.29 | 7.16 |
| PP_W_conv_2 | 9.32 | 0.00 | 30.00 | 10.69 | 0.00 | 21.00 | 2.16 | 9.87 | 0.30 | 0.43 | 1.38 | 6.55 | 1.69 | 6.61 |
| PP_W2 | 46.16 | 2.37 | 27.07 | 2.33 | 0.54 | 14.32 | 1.2 | 2.43 | 0.16 | 0.07 | 0.31 | 1.52 | 0.37 | 1.15 |
| Exp. | GHVmixture | CO2 Emissions | CO2 Specific Emissions | |
|---|---|---|---|---|
| kJ/m3 | kJ/kg | L CO2/L Gas Mixture | kg CO2/kJ | |
| PE_T | 44,398.25 | 49,750.55 | 1.28 | 5.68 × 10−5 |
| PP_T | 27,959.21 | 54,320.32 | 0.63 | 4.40 × 10−5 |
| PP_W | 37,577.78 | 47,625.47 | 1.36 | 5.82 × 10−5 |
| PP + PE_W1 | 36,016.20 | 55,087.35 | 0.90 | 4.92 × 10−5 |
| PP + PE_W2 | 59,826.72 | 46,941.68 | 1.86 | 6.12 × 10−5 |
| PP_W_conv_01 | 64,272.68 | 46,313.50 | 2.02 | 6.17 × 10−5 |
| PP_W_conv_02 | 66,926.83 | 48,457.28 | 2.12 | 6.23 × 10−5 |
| PP_W2 | 34,509.63 | 52,137.78 | 0.91 | 5.16 × 10−5 |
| Fuel | GHV | CO2 Emissions | |
|---|---|---|---|
| kJ/m3 | kJ/kg | kg CO2/kJ | |
| propane | 99,000 | 50,400 | 5.96 × 10−5 |
| kerosene | 37,400 | 46,200 | 6.94 × 10−5 |
| diesel | 38,578 | 45,600 | 7.03 × 10−5 |
| natural gas | 37,285 | 52,200 | 5.01× 10−5 |
| Exp. | C4–C9 (wt. %) | C10–C13 (wt. %) | C14–C19 (wt. %) | C20+ (wt. %) |
|---|---|---|---|---|
| PP_T | 66.7 ± 5 | 27.5 ± 7 | 4.3 ± 2 | 1.45 ± 1 |
| PP_W | 50.7 ± 7 | 41.7 ± 8 | 6 ± 0.3 | 1.5 ± 1.2 |
| PP + PE_W1 | 73.5 ± 6 | 15.7 ± 3 | 5.7 ± 2 | 5.1 ± 2 |
| PP + PE_W2 | 36.4 ± 12 | 35 ± 8 | 11.6 ± 7 | 16.4 ± 13 |
| PP_W_conv_01 | 20.4 ± 4 | 56.2 ± 8 | 9.9 ± 1.1 | 13.5 ± 1.5 |
| PP_W_conv_02 | 35.9 ± 6 | 38.8 ± 6.5 | 18.3 ± 3 | 6.9 ± 2 |
| PP_W2 | 40.8 ± 9 | 42.5 ± 8 | 10.2 ± 4 | 6.5 ± 3 |
| Exp. | GHV | ||
|---|---|---|---|
| Gas | Liquid | Gas/Liquid | |
| PE_T | 38,755.68 | 4557.1 | 8.50 |
| PP_T | 44,238.47 | 5450.2 | 8.12 |
| PP_W | 37,095.48 | 5221.2 | 7.10 |
| PP + PE_W1 | 36,836.91 | 4950.9 | 7.44 |
| PP + PE_W2 | 40,876.82 | 3893.0 | 10.50 |
| PP_W2 | 44,421.39 | 4763.2 | 9.33 |
| Exp. | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| control | 825.89 | 0.45 | 3.87 |
| MW | 604.52 | 0.34 | 3.80 |
| conventional | 738.17 | 0.4 | 3.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psenovschi, G.; Calinescu, I.; Fiti, A.; Chisega-Negrila, C.-G.; Ionascu, S.-L.; Barbes, L. Microwave-Assisted Pyrolysis of Polyethylene and Polypropylene from End-of-Life Vehicles: Hydrogen Production and Energy Valorization. Sustainability 2025, 17, 6196. https://doi.org/10.3390/su17136196
Psenovschi G, Calinescu I, Fiti A, Chisega-Negrila C-G, Ionascu S-L, Barbes L. Microwave-Assisted Pyrolysis of Polyethylene and Polypropylene from End-of-Life Vehicles: Hydrogen Production and Energy Valorization. Sustainability. 2025; 17(13):6196. https://doi.org/10.3390/su17136196
Chicago/Turabian StylePsenovschi, Grigore, Ioan Calinescu, Alexandru Fiti, Ciprian-Gabriel Chisega-Negrila, Sorin-Lucian Ionascu, and Lucica Barbes. 2025. "Microwave-Assisted Pyrolysis of Polyethylene and Polypropylene from End-of-Life Vehicles: Hydrogen Production and Energy Valorization" Sustainability 17, no. 13: 6196. https://doi.org/10.3390/su17136196
APA StylePsenovschi, G., Calinescu, I., Fiti, A., Chisega-Negrila, C.-G., Ionascu, S.-L., & Barbes, L. (2025). Microwave-Assisted Pyrolysis of Polyethylene and Polypropylene from End-of-Life Vehicles: Hydrogen Production and Energy Valorization. Sustainability, 17(13), 6196. https://doi.org/10.3390/su17136196







