Integrating Hydrogen into Power Systems: A Comprehensive Review
Abstract
1. Introduction
2. Technological Description and Mathematical Modeling of Electrolyzers and Fuel Cells
2.1. Electrochemical Model
2.2. Description of Latest Advances in Electrocatalyst Materials and Lower-Energy Anodic Reactions
2.3. Applied Models for Electrolyzers and Fuel Cells in Power System Planning and Economic Dispatch
3. Power to H2 Plants
4. Planning of Electrical Systems Considering Hydrogen Systems
4.1. Research Objectives and Key Findings
4.2. Planning Decisions and Optimization Frameworks
5. Participation of Hydrogen Systems in Electricity Markets and Ancillary Services
- Futures markets;
- Day-ahead market;
- Adjustment markets;
- Balancing market.
- Frequency Containment Reserve (FCR):
- –
- Also known as primary reserve, FCR is the first response to sudden frequency deviations caused by imbalances between generation and demand.
- –
- It is fully activated within seconds (typically within 30 s) and is distributed across multiple generators or flexible loads to autonomously stabilize the system frequency.
- –
- FCR operates locally based on frequency deviations without requiring intervention from the Transmission System Operator (TSO).
- Automatic Frequency Restoration Reserve (aFRR):
- –
- Also called secondary reserve, aFRR is automatically activated to restore the frequency to its nominal value and relieve FCR.
- –
- It typically responds within 30 s to a few minutes, adjusting the power output of generators or flexible loads based on control signals from the TSO.
- Manual Frequency Restoration Reserve (mFRR):
- –
- Also known as tertiary reserve, mFRR is manually activated by the TSO when additional power adjustments are required beyond what aFRR provides.
- –
- It is typically deployed within several minutes (up to 15 min) and is crucial for handling persistent imbalances in the system.
- –
- mFRR helps to restore the power system to its scheduled state and ensures sufficient reserves are available for new disturbances.
6. Modeling the Integration of Hydrogen Vehicles into Electrical Systems
7. Industrial Hydrogen Demand: Ammonia Production
8. Conclusions
- Future research is expected to extend catalyst lifespan, thereby decreasing dependence on critical metals and deepening the understanding of degradation mechanisms.
- Low-energy anodic reactions like UOR, AOR, and alcohol oxidation offer a promising alternative to traditional OER in hydrogen electrolysis, improving efficiency and enabling the conversion of waste into valuable products.
- P2G systems offer significant benefits for large-scale and long-term energy storage, integrating renewable energy sources like wind and solar. However, challenges such as the high capital costs of electrolyzers and the efficiency of hydrogen conversion processes need to be addressed to make P2G systems economically viable.
- Hydrogen storage and transportation are critical for the viability of P2G systems. Various storage methods, including compressed gas, liquid hydrogen, metal hydrides, and chemical storage, each have their own advantages and limitations. Efficient and cost-effective storage solutions are essential for large-scale hydrogen use.
- The integration of P2G systems with renewable energy and electrolyzer technologies is crucial for a low-carbon-energy future. While electrolyzers, especially PEM systems, are becoming more efficient, further improvements in cost and efficiency are necessary. Additionally, alternative methods like electrified steam methane reforming could enhance the efficiency and economics of hydrogen production.
- Joint planning of electric and hydrogen infrastructures enhances overall system performance as hydrogen mitigates renewable intermittency, enables long-duration storage, and facilitates sector coupling, contributing to greater flexibility and cost reductions.
- Hydrogen integration in energy systems is crucial for capacity expansion planning. The literature review contains numerous studies dealing with hydrogen production, storage, and transport, impacting sectors like transport, industry, and residential.
- Hydrogen supply chain planning models study future infrastructure needs under various carbon policy and demand scenarios, integrating power system capacity expansion studies.
- Integrated energy system planning models are able to optimize national-level electricity–hydrogen systems to meet energy demands while minimizing costs, highlighting the importance of coordinated planning.
- Electrolyzers are flexible assets capable of rapidly changing their power consumption levels, making them ideal candidates for providing ancillary services. They can bridge the gap between traditional frequency control mechanisms and the challenges posed by high penetration of renewable energy sources, addressing issues such as a decline in system inertia and larger frequency deviations.
- The flexibility of electrolyzers can significantly reduce the levelized cost of hydrogen. Investing in additional electrolysis capacity and hydrogen storage can unlock the full value of this flexibility, leading to lower production costs. Studies have shown that providing ancillary services, such as frequency control and voltage support, can enhance the profitability of electrolyzers.
- Technological advancements in electrolyzers, including the development of advanced catalysts and novel materials, have improved their efficiency and durability. The integration of electrolyzers into the global electricity market is expanding, with significant growth in production capacity and large-scale projects. Despite challenges such as high capital costs and the need for robust hydrogen infrastructure, the potential benefits, including reduced emissions and improved grid stability, are substantial.
- The number of FCEVs has increased in recent years and is expected to continue growing. However, this growth necessitates the rapid and extensive deployment of generation, transmission, distribution, and storage systems, particularly using photovoltaic panels and wind turbines.
- Hydrogen infrastructure complements the electrical grid and can be transported via pipelines or other means. Localized production of hydrogen at refueling stations can reduce transport costs. The number of operating hours is critical as investment costs, hydrogen production, and compression costs are directly linked to operational time.
- Several studies have indicated so far that green ammonia production can be economically viable, especially in regions with abundant renewable energy resources. For example, ammonia produced via PV–wind hybrids could be less expensive than ammonia produced from coal in China, and integrating solar and geothermal energy sources for ammonia production can be economically viable, with a payback period of under three years.
- Ammonia is increasingly recognized as a versatile energy vector due to its high hydrogen density and ease of storage and transportation. It can be used directly as a fuel in combustion engines and fuel cells, making it a promising candidate for applications in maritime transport and power generation. However, emissions from ammonia combustion require mitigation.
- Planning and operating ammonia plants, particularly for green ammonia production using renewable energy, involves complex decision-making. The optimal sizing of renewable power-to-ammonia systems and strategic location of production units can significantly reduce costs and improve efficiency. Models and methodologies have been proposed to address uncertainties in renewable energy generation and economic conditions. Additional research is needed to optimize the planning and operation of green ammonia plants.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Acronyms
| AC | Alternating current |
| ADRS | Active demand response service |
| AE | Alkaline electrolyzer |
| AEME | Anion exchange membrane electrolyzer |
| AFC | Alkaline fuel cell |
| AM | Adjustments market |
| AOR | Ammonia oxidation reaction |
| ASU | Air separation unit |
| aFRR | Average frequency restoration reserve |
| BM | Balancing market |
| CAPEX | Capital expenditures |
| CEP | Capacity expansion problem |
| DAM | Day-ahead market |
| DMFC | Direct methanol fuel cell |
| ESMR | Electrified steam methane reforming |
| EU | European Union |
| FC | Fuel cell |
| FCEV | Fuel cell electric vehicle |
| FCR | Frequency containment reserve |
| FM | Futures market |
| GSR | Gas steam reforming |
| HB | Haber–Bosch unit |
| HER | Hydrogen evolution reaction |
| IEC | International Electrotechnical Commission |
| IRA | Inflation Reduction Act |
| ISO | International Organization for Standardization |
| LCA | Life cycle assessment |
| LCOE | Levelized cost of electricity |
| LCOH | Levelized cost of hydrogen |
| LOHC | Liquid organic hydrogen carrier |
| MCFC | Molten carbonate fuel cell |
| mFRR | Manual frequency restoration reserve |
| MO | Market operator |
| OER | Oxygen evolution reaction |
| ORR | Oxygen reduction reaction |
| P2G | Power-to-gas |
| PAFC | Phosphoric acid fuel cell |
| PEM | Proton exchange membrane electrolyzer |
| PEMFC | Proton exchange membrane fuel cell |
| PPA | Power purchase agreement |
| PV | Photovoltaic |
| RHE | Reversible hydrogen electrode |
| SO | System operator |
| SOE | Solid oxide electrolyzer |
| SOFC | Solid oxide fuel cell |
| UHS | Underground hydrogen storage |
| UOR | Urea oxidation reaction |
Appendix A. Thermal and Mass Transport and Consumption/Production of Species Models for Electrolyzers and Fuel Cells
Appendix A.1. Thermal Model
Appendix A.2. Mass Transport and Consumption/Production of Species
Appendix B. Description of Table 3
- Column 1. Model. This column reports the name of the energy system planning model upon which the study developed in the article is based. If the study does not rely on a pre-existing model, it is indicated as “ad hoc”. Entries are
- REMix;
- PyPSA-Eur-Sec-30;
- PRIMES;
- Enertile;
- LIMES-EU;
- METIS;
- Balmorel;
- COMPETES;
- ad hoc.
- Column 2: Generation. This column provides the type of energy source underlying the electricity generation technologies considered as investment alternatives in the article. Entries are
- R: Renewable;
- C: Coal;
- N: Nuclear;
- G: Gas;
- H2: Hydrogen.
- Column 3: Grid. This column indicates whether the article considers the expansion of the electricity transmission grid among the planning decisions. Entries are
- y: considered;
- -: not considered.
- Column 4: Storage. This column identifies the technologies considered in the article for the expansion of electricity storage capacity. Technologies are classified under this category when the article specifically applies them in a manner such that the form of energy resulting from the conversion of electricity during storage is subsequently utilized for electricity generation. Entries are
- PH: Pumped-hydro;
- B: Batteries;
- CA: Compressed air;
- Th: Thermal;
- H2: Hydrogen;
- ns: not specified;
- -: not considered.
- Column 5: Prod. This column lists the technologies considered in the article for the planning of hydrogen production. Entries are
- E: Electrolysis;
- TW: Thermal water splitting;
- Bio: Biomass gasification;
- SMR: Steam methane reforming;
- -: not considered.
- Column 6: Trans. This column provides information regarding the hydrogen transportation technologies considered in the energy system planning proposed in the article. Entries are
- P: Pipelines;
- RF: Retrofitting gas pipelines;
- TR: Trucks;
- -: not considered.
- Column 7: Storage. This column presents the hydrogen storage technologies incorporated in the study as alternatives for system planning. Entries are
- TA: Tanks;
- GS: Geological storage;
- MTR: Mobile storage via trucks;
- PL: Pipeline linepack;
- ns: not specified;
- -: not considered.
- Column 8: Formulation. This column categorizes the formulation of the optimization problem on which the planning model of the article is based. The categorization considers both the continuous or discrete nature of the variables used to model the decisions and the linearity of the mathematical expressions that define the model. When the entry includes two acronyms, the first refers to the original formulation of the problem, and the second, shown in parentheses, refers to the simplified problem resulting from transformations performed to facilitate its resolution. Entries are
- LP: Linear programming;
- MILP: Mixed-integer linear programming;
- NLP: Non-linear programming;
- MINLP: Mixed-integer non-linear programming;
- MISOCP: Mixed-integer second-order cone programming.
- Column 9: Uncertainty. This column classifies the optimization problem addressed in the article according to whether it explicitly accounts for the uncertainty inherent in energy system planning and/or operation. Entries are
- D: Deterministic;
- S: Stochastic.
- Column 10: Stages. This column categorizes the optimization problem based on the number of planning stages considered in the article and explicitly represented in the problem formulation. A planning stage is defined as a time period during which planning decisions related to long-term investments can be made. Entries are
- SS: Single-stage;
- MS: Multi-stage.
References
- European Commission. Strategy 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1409650806265&uri=CELEX:52010DC0639 (accessed on 13 May 2025).
- European Commission. Clean Energy for All Europeans. 2019. Available online: https://op.europa.eu/en/publication-detail/-/publication/b4e46873-7528-11e9-9f05-01aa75ed71a1/language-en?WT.mc_id=Searchresult&WT.ria_c=null&WT.ria_f=3608&WT.ria_ev=search (accessed on 13 May 2025).
- European Commission. Powering a Climate-Neutral Economy: Commission Sets Out Plans for the Energy System of the Future and Clean Hydrogen. Available online: https://ec.europa.eu/commission/presscorner/api/files/document/print/en/ip_20_1259/IP_20_1259_EN.pdf (accessed on 13 May 2025).
- World Economic Forum. Green Hydrogen in China: A Roadmap for Progress. 2023. Available online: https://www.weforum.org/publications/green-hydrogen-in-china-a-roadmap-for-progress/ (accessed on 11 June 2025).
- Africa Energy Portal. Available online: https://africa-energy-portal.org/ (accessed on 28 May 2025).
- Hydrogen Council. The Africa Hydrogen Opportunity. Available online: https://hydrogencouncil.com/wp-content/uploads/2024/03/Hydrogen-Council-Africa-Hydrogen-Opportunity-.pdf (accessed on 28 May 2025).
- Global Hydrogen Hub. Global Hydrogen Hub (GH2). Available online: https://gh2.org (accessed on 28 May 2025).
- International Energy Agency. Global Hydrogen Review 2024: Latin America in Focus. Available online: https://www.iea.org/reports/global-hydrogen-review-2024/latin-america-in-focus (accessed on 28 May 2025).
- Babay, M.A.; Adar, M.; Chebak, A.; Mabrouki, M. Forecasting green hydrogen production: An assessment of renewable energy systems using deep learning and statistical methods. Fuel 2025, 381, 133496. [Google Scholar] [CrossRef]
- International Renewable Energy Agency. Geopolitics of the Energy Transformation The Hydrogen Factor 2022. Available online: https://www.spr.pe/wp-content/uploads/2022/02/irena-geopolitics-hydrogen-2022.pdf (accessed on 9 June 2025).
- RWE. NorthH2: A Green Hydrogen Hub in Northwest Europe. 2020. Available online: https://www.rwe.com/en/research-and-development/hydrogen-projects/north2/ (accessed on 18 August 2024).
- TKI Nieuw Gas. Overview of Hydrogen Projects in The Netherlands. Available online: https://topsectorenergie.nl/documents/81/TKI_Nieuw_Gas-Overview_Hydrogen_projects_in_the_Netherlands_versie_21_-_200801.pdf (accessed on 27 June 2025).
- Cozzolino, R.; Bella, G. A review of electrolyzer-based systems providing grid ancillary services: Current status, market, challenges and future directions. Front. Energy Res. 2024, 12, 1358333. [Google Scholar] [CrossRef]
- ISO 19880-1:2020; Gaseous Hydrogen—Fuelling Stations—Part 1: General Requirements. International Organization for Standardization: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/71940.html (accessed on 27 June 2025).
- IEC 62282; IEC 62282—Fuel Cell Technologies. International Electrotechnical Commission: Geneva, Switzerland, 2021. Available online: https://webstore.iec.ch/en/publication/59780 (accessed on 27 June 2025).
- Federal Ministry for Economic Affairs and Climate Action (BMWK). Standardization Roadmap Hydrogen Technologies. 2023. Available online: https://www.bundesregierung.de/breg-en/federal-cabinet/federal-minister-for-economic-affairs-and-climate-action-1988594 (accessed on 27 June 2025).
- Odenweller, A.; Ueckerdt, F. The green hydrogen ambition and implementation gap. Nat. Energy 2025, 10, 110–123. [Google Scholar] [CrossRef]
- Cheng, F.; Luo, H.; Jenkins, J.D.; Larson, E.D. Impacts of the Inflation Reduction Act on the Economics of Clean Hydrogen and Synthetic Liquid Fuels. Environ. Sci. Technol. 2023, 57, 15336–15347. [Google Scholar] [CrossRef]
- Wu, W.; Zhai, H.; Holubnyak, E. Technological evolution of large-scale blue hydrogen production toward the US Hydrogen Energy Earthshot. Nat. Commun. 2024, 15, 5684. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, H.; Sun, B.; Pan, F. Economic and environmental competitiveness of multiple hydrogen production pathways in China. Nat. Commun. 2025, 16, 4284. [Google Scholar] [CrossRef]
- Grubb, M.; Poncia, A.; Drummond, P.; Neuhoff, K.; Hourcade, J.C. Policy complementarity and the paradox of carbon pricing. Oxf. Rev. Econ. Policy 2023, 39, 711–730. [Google Scholar] [CrossRef]
- Chyong, C.K.; Italiani, E.; Kazantzis, N. Energy and climate policy implications on the deployment of low-carbon ammonia technologies. Nat. Commun. 2025, 16, 776. [Google Scholar] [CrossRef]
- Clean Hydrogen Partnership. Study on Accelerating the Deployment of Guarantees of Origin Schemes for Hydrogen. Available online: https://www.cde.ual.es/wp-content/uploads/2022/05/EG0122072ENN.en_.pdf (accessed on 7 June 2025).
- Mendicino, L.; Menniti, D.; Pinnarelli, A.; Sorrentino, N. Corporate power purchase agreement: Formulation of the related levelized cost of energy and its application to a real life case study. Appl. Energy 2019, 253, 113577. [Google Scholar] [CrossRef]
- Arellano, J.; Carrión, M. Electricity procurement of large consumers considering power-purchase agreements. Energy Rep. 2023, 9, 5384–5396. [Google Scholar] [CrossRef]
- European Commission. EU Innovation Fund. 2025. Available online: https://climate.ec.europa.eu/eu-action/funding-climate-action/innovation-fund_en (accessed on 6 June 2025).
- Chang, S.; Rajuli, M. An overview of pure hydrogen production via electrolysis and hydrolysis. Int. J. Hydrogen Energy 2024, 84, 521–538. [Google Scholar] [CrossRef]
- Emam, A.; Hamdan, M.; Nabah, B.; Elnajjar, E. A review on recent trends, challenges, and innovations in alkaline water electrolysis. Int. J. Hydrogen Energy 2024, 64, 599–625. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Shomope, I.; Al-Othman, A.; Alshraideh, H. Prediction of hydrogen production in proton exchange membrane water electrolysis via neural networks. Int. J. Thermofluids 2024, 24, 100849. [Google Scholar] [CrossRef]
- Clean Hydrogen Partnership. Clean Hydrogen JU 2022. Available online: https://www.clean-hydrogen.europa.eu/system/files/2022-02/Clean%20Hydrogen%20JU%20AWP%202022.pdf (accessed on 7 June 2025).
- Xu, Y.; Cai, S.; Chi, B.; Tu, Z. Technological limitations and recent developments in a solid oxide electrolyzer cell: A review. Int. J. Hydrogen Energy 2024, 50, 548–591. [Google Scholar] [CrossRef]
- Tucker, M. Progress in metal-supported solid oxide electrolysis cells: A review. Int. J. Hydrogen Energy 2020, 45, 24203–24218. [Google Scholar] [CrossRef]
- Horry, B.; Ozcan, H. Green hydrogen production by water electrolysis: Current status and challenges. Curr. Opin. Green Sustain. Chem. 2024, 47, 100932. [Google Scholar] [CrossRef]
- European Comision. Critical Raw Materials for Strategic Technologies and Sectors in the EU 2020. Available online: https://rmis.jrc.ec.europa.eu/uploads/CRMs_for_Strategic_Technologies_and_Sectors_in_the_EU_2020.pdf (accessed on 9 June 2025).
- Shaya, N.; Gloeser-Chahoud, S. A Review of Life Cycle Assessment (LCA) Studies for Hydrogen Production Technologies through Water Electrolysis: Recent Advances. Energies 2024, 17, 3968. [Google Scholar] [CrossRef]
- Hu, S.; Guo, B.; Ding, S.; Yang, F.; Dang, J.; Liu, B.; Gu, J.; Ma, J.; Ouyang, M. A Comprehensive Review of Alkaline Water Electrolysis Mathematical Modelling. Appl. Energy 2022, 327, 1–33. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Abdulrahman, G.A.Q. A Recent Comprehensive Review of Fuel Cells: History, Types and Applications. Int. J. Energy Res. 2024, 2024, 1–36. [Google Scholar] [CrossRef]
- Hajimolana, S.A.; Hussain, M.A.; Daud, W.M.A.A.W.; Soroush, M.; Shamiri, A. Mathematical Modelling of Solid Oxide Fuel Cells: A Review. Renew. Sustain. Energy Rev. 2011, 15, 1893–1917. [Google Scholar] [CrossRef]
- Raheli, E. Physics-Aware Operation of Power-to-X and Natural Gas Systems. Ph.D. Thesis, Technical University of Denmark, Kongens Lyngby, Denmark, 2023. [Google Scholar]
- Ulleberg, O. Modeling of Advanced Alkaline Electrolyzers: A System Simulation Approach. Int. J. Hydrogen Energy 2003, 28, 21–33. [Google Scholar] [CrossRef]
- Omran, A.; Lucchesi, A.; Smith, D.; Alaswad, A.; Amiri, A.; Wilberforce, T.; Sondré, J.R.; Olabi, A.G. Mathematical Model of a Proton-Exchange Membrane (PEM) Fuel Cell. Int. J. Thermofluids 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Marangio, F.; Santarelli, M.; Cali, M. Theoretical Model and Experimental Analysis of a High-Pressure PEM Water Electrolyser for Hydrogen Production. Int. J. Hydrogen Energy 2009, 34, 1143–1158. [Google Scholar] [CrossRef]
- Lebbal, M.E.; Lecoeuche, S. Identification and Monitoring of a PEM Electrolyser Based on Dynamical Modelling. Int. J. Hydrogen Energy 2009, 34, 5992–5999. [Google Scholar] [CrossRef]
- Asiaban, S.; Bozalakov, D.; Vandevelde, L. Development of a Dynamic Mathematical Model of PEM Electrolyser for Integration into Large-Scale Power Systems. Energy Convers. Manag. X 2024, 23, 100610. [Google Scholar] [CrossRef]
- Rahim, A.H.A.; Tijani, A.S.; Shukri, F.H.; Hanapi, S.; Sainan, K.I. Mathematical Modelling and Simulation Analysis of PEM Electrolyzer System for Hydrogen Production. In Proceedings of the 3rd IET International Conference on Clean Energy and Technology (CEAT) 2014, Kuching, Malaysia, 24–26 November 2014; pp. 1–7. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, Q.; Huang, Y.; Guan, J.; Wang, Y.; Yan, Y. Mathematical Modelling and Operation Parameters Analysis of Proton Exchange Membrane Fuel Cell. IOP Conf. Ser. Earth Environ. Sci. 2020, 467, 012071. [Google Scholar] [CrossRef]
- Janardhanan, V.M.; Deutschmann, O. Modeling of Solid-Oxide Fuel Cells. Z. Phys. Chem. 2007, 221, 443–478. [Google Scholar] [CrossRef]
- Harrison, K.W.; Hernández-Pacheco, E.; Mann, M.; Salehfar, H. Semi-Empirical Model for Determining PEM Electrolyzer Stack. J. Fuel Cell Sci. 2006, 3, 220–223. [Google Scholar] [CrossRef]
- García-Valverde, R.; Espinosa, N.; Urbina, A. Simple PEM Water Electrolyser Model and Experimental Validation. Int. J. Hydrogen Energy 2012, 37, 1927–1938. [Google Scholar] [CrossRef]
- Biaku, C.Y.; Dale, N.V.; Mann, M.D.; Salehfar, H.; Peters, A.J.; Han, T. A Semi-Empirical Study of the Temperature Dependence of the Anode Charge Transfer Coefficient of a 6 kW PEM Electrolyzer. Int. J. Hydrogen Energy 2008, 33, 4247–4254. [Google Scholar] [CrossRef]
- Hammoudi, M.; Henao, C.; Agbossou, K.; Dubé, Y.; Doumbia, M.L. New Multi-Physics Approach for Modelling and Design of Alkaline Electrolyzers. Int. J. Hydrogen Energy 2012, 37, 13895–13913. [Google Scholar] [CrossRef]
- Awasthi, A.; Scott, K.; Basu, S. Dynamic Modelling and Simulation of a Proton Exchange Membrane Electrolyzer for Hydrogen Production. Int. J. Hydrogen Energy 2011, 36, 14779–14786. [Google Scholar] [CrossRef]
- Abdin, Z.; Webb, C.J.; Gray, E.M. Modelling and Simulation of an Alkaline Electrolyser Cell. Energy 2017, 138, 316–331. [Google Scholar] [CrossRef]
- Yang, B.; Jafarian, M.; Freidoonimehr, N.; Arjomandi, M. Alkaline Membrane-Free Water Electrolyser for Liquid Hydrogen Production. Renew. Energy 2024, 233, 121172. [Google Scholar] [CrossRef]
- Maier, M.; Smith, K.; Dodwell, J.; Hinds, G.; Shearing, P.R.; Brett, D.J.L. Mass Transport in PEM Water Electrolysers: A Review. Int. J. Hydrogen Energy 2022, 47, 30–56. [Google Scholar] [CrossRef]
- Seyezhai, M.; Mathur, B.L. Mathematical modeling of proton exchange membrane fuel cell. Int. J. Comput. Appl. 2011, 20, 1–6. [Google Scholar] [CrossRef]
- Aouali, F.Z.; Becherif, M.; Ramadan, H.S.; Emziane, M.; Khellaf, A.; Mohammedi, K. Analytical modelling and experimental validation of proton exchange membrane electrolyser for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 1366–1374. [Google Scholar] [CrossRef]
- Sandeep, K.C.; Kamath, S.; Mistry, K.; Kumar, A.; Bhattacharya, S.K.; Bhanja, K.; Moran, S. Experimental studies and modeling of advanced alkaline water electrolyser with porous nickel electrodes for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 12094–12103. [Google Scholar] [CrossRef]
- Hug, W.; Bussmann, H.; Brinner, A. Intermitent operation and operation modeling of an alkaline electrolyzer. Int. J. Hydrogen Energy 1993, 18, 973–977. [Google Scholar] [CrossRef]
- Amores, E.; Rodríguez, J.; Carreras, C. Influence of operation parameters in the modeling of alkaline water electrolyzers for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 13063–13078. [Google Scholar] [CrossRef]
- Sánchez, M.; Amores, E.; Rodríguez, L.; Clemente-Jul, C. Semi-empirical model and experimental validation for the performance evaluation of a 15 kW alkaline water electrolyzer. Int. J. Hydrogen Energy 2018, 43, 20332–20345. [Google Scholar] [CrossRef]
- Sánchez, M.; Amores, E.; Abad, D.; Rodríguez, L.; Clemente-Jul, C. Aspen Plus model of an alkaline electrolysis system for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 3916–3929. [Google Scholar] [CrossRef]
- Srinivasan, S.; Ticianelli, E.A.; Derouin, C.R.; Redondo, A. Advances in solid polymer electrolyte fuel cell technology with low platinum loading electrodes. J. Power Sources 1988, 22, 359–375. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.M.; Srinivasan, S.; Chamberlin, C.E. Modeling of Proton Exchange Membrane Fuel Cell Performance with an Empirical Equation. J. Electrochem. Soc. 1995, 142, 2670. [Google Scholar] [CrossRef]
- Lee, J.H.; Lalk, T.R.; Appleby, A.J. Modeling electrochemical performance in large scale proton exchange membrane fuel cell stacks. J. Power Sources 1998, 70, 258–268. [Google Scholar] [CrossRef]
- Squadrito, G.; Maggio, G.; Passalacqua, E.; Lufrano, F.; Patti, A. An empirical equation for polymer electrolyte fuel cell (PEFC) behaviour. J. Appl. Electrochem. 1999, 29, 1449–1455. [Google Scholar] [CrossRef]
- Argyropoulos, P.; Scott, K.; Shukla, A.K.; Jackson, C. A semi-empirical model of the direct methanol fuel cell performance. Part I. Model development and verification. J. Power Sources 2003, 123, 190–199. [Google Scholar] [CrossRef]
- Pisani, L.; Murgia, G.; Valentini, M.; D’Aguanno, B. A new semi-empirical approach to performance curves of polymer electrolyte fuel cells. J. Power Sources 2002, 108, 192–203. [Google Scholar] [CrossRef]
- Vandenborre, H.; Leysen, R.; Vermeiren, P. Active Electrodes to Be Used in Advanced Alkaline-Water Electrolysis; Technical Report DE83900365; Centre d’etude d’energie Nucleare: Mol, Belgium, 1982. [Google Scholar]
- Janjua, M.; Le Roy, R. Electrocatalyst performance in industrial water electrolysers. Int. J. Hydrogen Energy 1985, 10, 11–19. [Google Scholar] [CrossRef]
- Kumar, N.; Aepuru, R.; Lee, S.; Park, S. Advances in catalysts for hydrogen production: A comprehensive review of materials and mechanisms. Nanomaterials 2025, 15, 256. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-Noble Metal-based Carbon Composites in Hydrogen-Evolution Reaction: Fundamentals to Applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef] [PubMed]
- Kitiphatpiboon, N.; Chen, M.; Feng, C.; Li, S.; Abudula, A.; Guan, G. Highly efficient electrocatalysts for seawater electrolysis under high current density: A critical review. MetalMat 2024, 1, 1–27. [Google Scholar] [CrossRef]
- Huang, F.; Yao, B.; Huang, Y.; Dong, Z. NiFe layered double hydroxide nanosheet arrays for efficient oxygen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2022, 47, 21725–21735. [Google Scholar] [CrossRef]
- Gupta, U.; Rao, C. Hydrogen generation by water splitting using MoS2 and other transition metal dichalcogenides. Nano Energy 2017, 41, 49–65. [Google Scholar] [CrossRef]
- Kim, S.; Lim, S.; Son, M.; Kim, T. Highly Active and Stable Bimetallic Ordered Catalysts for Oxygen Reduction Reaction Improvement in Polymer Exchange Membrane Fuel Cells. Appl. Surf. Sci. 2024, 656, 159620. [Google Scholar] [CrossRef]
- Fang, B.; Wanjala, B.; Yin, J.; Loukrakpam, R.; Luo, J.; Hu, X.; Last, J.; Zhong, C. Electrocatalytic performance of Pt-based trimetallic alloy nanoparticle catalysts in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2012, 37, 4627–4632. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Navlani-García, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Nitrogen-doped carbon materials as a promising platform toward the efficient catalysis for hydrogen generation. Appl. Catal. A Gen. 2019, 571, 25–41. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Z.; Liu, L.; Che, X.; Wang, J.; Zhu, Y.; Attfield, J.; Yang, M. Efficient and durable seawater electrolysis with a V2O3-protected catalyst. Sci. Adv. 2024, 10, 1–12. [Google Scholar] [CrossRef]
- Gong, Z.; Liu, J.; Yan, M.; Gong, H.; Ye, G.; Fei, H. Highly Durable and Efficient Seawater Electrolysis Enabled by Defective Graphene-Confined Nanoreactor. ACS Nano 2023, 17, 18372–18381. [Google Scholar] [CrossRef]
- Qiao, L.; Xi, C.; Li, C.; Zhang, K.; Li, Q.; Han, J.; Ding, Y. Self-Limited Formation of Nanoporous Nickel Heterostructure Catalyst for Electrochemical Hydrogen Production. Adv. Funct. Mater. 2024, 34, 2402286. [Google Scholar] [CrossRef]
- Du, J.; Chen, D.; Ding, Y.; Wang, L.; Li, F.; Sun, L. Highly stable and efficient oxygen evolution electrocatalyst based on Co oxides decorated with ultrafine Ru nanoclusters. Small 2023, 19, 2207611. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Biswas, R.; Sharma, R.; Burman, V.; Haldar, K. Access to carbon nanofiber composite hydrated cobalt phosphate nanostructure as an efficient catalyst for the hydrogen evolution reaction. Front. Chem. 2023, 11, 1129133. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Liu, X.; Zhao, W.; Liu, S.; Huang, X.; Zhao, Q. Tetra-coordinated W2S3 for efficient dual-pH hydrogen production. Angew. Chem. Int. Ed. 2024, 63, e202316306. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Shon, H.; Ni, B. Designing bifunctional catalysts for urea electrolysis: Progress and perspectives. Green Chem. 2024, 26, 631–654. [Google Scholar] [CrossRef]
- Wang, J.; Pan, Z.; Ge, H.; Zhao, H.; Xia, T.; Wang, B. Economic dispatch of integrated electricity-heat-hydrogen system considering hydrogen production by water electrolysis. Electronics 2023, 12, 4166. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, S.; Wang, P.; Jaroniec, M.; Zheng, Y.; Qiao, S. Urea catalytic oxidation for energy and environmental applications. Chem. Soc. Rev. 2024, 53, 637–702. [Google Scholar] [CrossRef]
- Putri, Y.; Syauqi, M.; Rahmawati, I.; Santoso, R.; Ivandini, T. Advancements in Ni-based catalysts for direct urea fuel cells: A comprehensive review. ChemElectroChem 2024, 11, e202300637. [Google Scholar] [CrossRef]
- Łuczak, J.; Lieder, M. Nickel-based catalysts for electrolytic decomposition of ammonia towards hydrogen production. Adv. Colloid Interface Sci. 2023, 319, 102949. [Google Scholar] [CrossRef]
- Wu, H.; Liu, X.; Ding, M. Dynamic economic dispatch of a microgrid: Mathematical models and solution algorithm. Electr. Power Energy Syst. 2014, 63, 336–346. [Google Scholar] [CrossRef]
- Maleki, A.; Pourfayaz, F.; Ahmadi, M.H. Design of a cost-effective wind/photovoltaic/hydrogen energy system for supplying a desalination unit by a heuristic approach. Sol. Energy 2016, 139, 666–675. [Google Scholar] [CrossRef]
- Wang, C.; Nehrir, M.H. Power management of a stand-alone wind/photovoltaic/fuel cell energy system. Renew. Energy 2008, 33, 1931–1942. [Google Scholar] [CrossRef]
- Matute, G.; Yusta, J.M.; Correas, L.C. Techno-economic modelling of water electrolysers in the range of several MW to provide grid services while generating hydrogen for different applications: A case study in Spain applied to mobility with FCEVs. Int. J. Hydrogen Energy 2019, 44, 17431–17442. [Google Scholar] [CrossRef]
- Matute, G.; Yusta, J.M.; Beyza, B.J.; Correas, L.C. Multi-state techno-economic model for optimal dispatch of grid connected hydrogen electrolysis systems operating under dynamic conditions. Int. J. Hydrogen Energy 2021, 46, 1449–1460. [Google Scholar] [CrossRef]
- Matute, G.; Yusta, J.M.; Beyza, B.J.; Monteiro, C. Optimal dispatch model for PV-electrolysis plants in self-consumption regime to produce green hydrogen: A Spanish case study. Int. J. Hydrogen Energy 2022, 47, 25202–25213. [Google Scholar] [CrossRef]
- Varela, C.; Mostafa, M.; Zondervan, E. Modeling alkaline water electrolysis for power-to-x applications: A scheduling approach. Int. J. Hydrogen Energy 2021, 46, 9303–9313. [Google Scholar] [CrossRef]
- Moazeni, S.; Miragha, A.H.; Defourny, B. A risk-averse stochastic dynamic programming approach to energy hub optimal dispatch. IEEE Trans. Power Syst. 2019, 34, 2169–2178. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Song, Y.; Xing, X.; Fu, C. Operation optimization of power to hydrogen and heat (P2HH) in ADN coordinated with the district heating network. IEEE Trans. Sustain. Energy 2019, 10, 1672–1683. [Google Scholar] [CrossRef]
- Cai, G.; Kong, L. Techno-economic Analysis of Wind Curtailment/Hydrogen Production/Fuel Cell Vehicle System with High Wind Penetration in China. CSEE J. Power Energy Syst. 2017, 3, 44–52. [Google Scholar] [CrossRef]
- Cau, G.; Cocco, D.; Petrollese, M.; Kaer, S.K.; Milan, C. Energy management strategy based on short-term generation scheduling for a renewable microgrid using a hydrogen storage system. Energy Convers. Manag. 2014, 87, 820–831. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Zhang, H.; Song, Y.; Chen, G.; Ding, L.; Liang, D. Optimal investment of electrolyzers and seasonal storages in hydrogen supply chains incorporated with renewable electric networks. IEEE Trans. Sustain. Energy 2020, 11, 1773–1784. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J.; Zhang, B.; Zhang, Z.; Mao, Z.; He, J. Low-carbon economic dispatch model of integrated energy system accounting for concentrating solar power and hydrogen-doped combustion. Sustainability 2024, 16, 4818. [Google Scholar] [CrossRef]
- Kong, L.; Li, L.; Cai, G.; Liu, C.; Ma, P.; Bian, Y.; Ma, T. Techno-economic analysis of hydrogen energy for renewable energy power smoothing. Int. J. Hydrogen Energy 2021, 46, 2847–2861. [Google Scholar] [CrossRef]
- Pan, G.; Gu, W.; Qiu, H.; Lu, Y.; Zhou, S.; Wu, Z. Bi-level mixed integer planning for electricity-hydrogen integrated energy system considering levelized cost of hydrogen. Appl. Energy 2020, 270, 115176. [Google Scholar] [CrossRef]
- Pavic, I.; Covic, N.; Pandzic, H. PV-battery-hydrogen plant: Cutting green hydrogen cost through multi-market positioning. Appl. Energy 2022, 328, 120103. [Google Scholar] [CrossRef]
- Gómez-Villarreal, H.; Cañas-Carretón, M.; Zárate-Miñano, R.; Carrión, M. Generation capacity expansion considering hydrogen power plants and energy storage systems. IEEE Access 2023, 11, 15525–15539. [Google Scholar] [CrossRef]
- Baumhof, M.T.; Raheli, E.; Johnsen, A.G.; Kazempour, J. Optimization of hybrid power plants: When is a detailed electrolyzer model necessary? In Proceedings of the IEEE Belgrade PowerTech, Belgrade, Serbia, 25–29 June 2023; pp. 1–10. [Google Scholar] [CrossRef]
- Raheli, E.; Werner, Y.; Kazempour, J. A conic model for electrolyzer scheduling. Comput. Chem. Eng. 2023, 179, 108450. [Google Scholar] [CrossRef]
- Virah-Sawmy, D.; Beck, F.J.; Sturmberg, B. Ignore variability, overestimate hydrogen production—Quantifying the effects of electrolyzer efficiency curves on hydrogen production from renewable energy sources. Int. J. Hydrogen Energy 2024, 72, 49–59. [Google Scholar] [CrossRef]
- Ferrara, A.; Jakubek, S.; Hametner, C. Energy management of heavy-duty fuel cell vehicles in real-world driving scenarios: Robust design of strategies to maximize the hydrogen economy and system lifetime. Energy Convers. Manag. 2021, 232, 113795. [Google Scholar] [CrossRef]
- International Energy Agency. Global Hydrogen Review 2023. Available online: https://iea.blob.core.windows.net/assets/ecdfc3bb-d212-4a4c-9ff7-6ce5b1e19cef/GlobalHydrogenReview2023.pdf (accessed on 7 June 2025).
- Hydrogen Council. Hydrogen Insights 2023. Available online: https://hydrogencouncil.com/wp-content/uploads/2023/05/Hydrogen-Insights-2023.pdf (accessed on 8 June 2025).
- Pires, F.; Romero-Cadaval, E.; Vinnikov, D.; Roasto, I.; Martins, J. Power converter interfaces for electrochemical energy storage systems—A review. Energy Convers. Manag. 2014, 86, 453–475. [Google Scholar] [CrossRef]
- Elmasides, C.; Kosmadakis, I.; Athanasiou, C. A comprehensive power management strategy for the effective sizing of a PV hybrid renewable energy system with battery and H2 storage. J. Energy Storage 2025, 106, 114790. [Google Scholar] [CrossRef]
- Bhayo, B.; Al-Kayiem, H.; Gilani, S.; Ismail, F. Power management optimization of hybrid solar photovoltaic-battery integrated with pumped-hydro-storage system for standalone electricity generation. Energy Convers. Manag. 2020, 215, 112942. [Google Scholar] [CrossRef]
- Gasanzade, F.; Witte, F.; Tuschy, I.; Bauer, S. Integration of geological compressed air energy storage into future energy supply systems dominated by renewable power sources. Energy Convers. Manag. 2023, 277, 116643. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Moarref, B.; Mostafa, S.; Modaresi, S. Optimal scheduling of power system integrated with hydrogen vehicles and flexible resources: A hybrid uncertainty management method. Int. J. Hydrogen Energy 2024, 100, 658–667. [Google Scholar] [CrossRef]
- Rodrigues, L.; Soares, T.; Rezende, I.; Fontoura, J.; Miranda, V. Virtual power plant optimal dispatch considering power-to-hydrogen systems. Int. J. Hydrogen Energy 2024, 68, 1019–1032. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Lu, J.; Yang, F.; Wu, T. Towards sustainable energy storage of new low-cost aluminum batteries from fundamental study to industrial applications. J. Power Sources 2025, 630, 236168. [Google Scholar] [CrossRef]
- Sadeq, A.; Homod, R.; Hussein, A.; Togun, H.; Mahmoodi, A.; Isleem, H.; Patil, A.; Moghadamm, A. Hydrogen energy systems: Technologies, trends, and future prospects. Sci. Total Environ. 2024, 20, 173622. [Google Scholar] [CrossRef]
- Mulky, L.; Srivastava, S.; Lakshmi, T.; Sandady, E.; Gour, S.; Thomas, N.; Priya, S.; Sudhakar, K. An overview of hydrogen storage technologies—Key challenges and opportunities. Mater. Chem. Phys. 2024, 325, 129710. [Google Scholar] [CrossRef]
- Kobayashi, C.; Tanabe, K. Electromagnetic-thermal-mass transport triple hybrid model for energy-efficient hydrogen release from metal particles by induction heating. Int. J. Hydrogen Energy 2024, 69, 918–926. [Google Scholar] [CrossRef]
- Kirichenko, O.; Kustov, L. Recent progress in development of supported palladium catalysts for dehydrogenation of heterocyclic liquid organic hydrogen carriers (LOHC). Int. J. Hydrogen Energy 2024, 88, 97–119. [Google Scholar] [CrossRef]
- Ali, M.; Khan, M.; Tuhin, R.; Kabir, M.; Azad, A.; Farrok, O. Hydrogen energy storage and transportation challenges: A review of recent advances. Hydrog. Energy Convers. Manag. 2024, 9, 255–287. [Google Scholar] [CrossRef]
- Khaing, M.; Yin, S. Lifecycle Management of Hydrogen Pipelines: Design, Maintenance, and Rehabilitation Strategies for Canada’s Clean Energy Transition. Energies 2025, 18, 240–266. [Google Scholar] [CrossRef]
- Revinova, S. Lazanyuk, I.; Gabrielyan, B.; Shahinyan, T.; Hakobyan, Y. Hydrogen in Energy Transition: The Problem of Economic Efficiency, Environmental Safety, and Technological Readiness of Transportation and Storage. Resources 2024, 13, 92–111. [Google Scholar] [CrossRef]
- Solomon, M. Heineken, W.; Scheffler, M.; Birth-Reichert, M. Cost Optimization of Compressed Hydrogen Gas Transport via Trucks and Pipelines. Energy Technol. 2023, 12, 2300785. [Google Scholar] [CrossRef]
- Escamilla, A.; Sánchez, D.; García-Rodríguez, L. Assessment of power-to-power renewable energy storage based on the smart integration of hydrogen and micro gas turbine technologies. Int. J. Hydrogen Energy 2022, 47, 17505–17525. [Google Scholar] [CrossRef]
- Arsalis, A.; Alexandrou, A.; Georghiou, G. Thermoeconomic modeling of a small-scale gas turbine-photovoltaic-electrolyzer combined-cooling-heating-and-power system for distributed energy applications. J. Clean. Prod. 2018, 188, 443–455. [Google Scholar] [CrossRef]
- Guandalini, G.; Campanari, S.; Romano, M. Power-to-gas plants and gas turbines for improved wind energy dispatchability: Energy and economic assessment. Appl. Energy 2015, 147, 117–130. [Google Scholar] [CrossRef]
- Schone, N.; Khairallah, J.; Heinz, B. Model-based techno-economic evaluation of power-to-hydrogen-to-power for the electrification of isolated African off-grid communities. Energy Sustain. Dev. 2022, 70, 592–608. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; Zhang, R.Z.; Zhang, J.R.; Ma, X.; Yang, W.W. Design optimization of a molten salt heated methane/steam reforming membrane reactor by universal design analysis and techno-economic assessment. Int. J. Hydrogen Energy 2024, 69, 1236–1245. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Bian, H.; Shen, M.; Lin, X. Energy, environment, and economic analyses on a novel hydrogen production method by electrified steam methane reforming with renewable energy accommodation. Energy Convers. Manag. 2022, 258, 115513. [Google Scholar] [CrossRef]
- Wustenhagen, R.; Wolsink, M.; Burer, M. Social acceptance of renewable energy innovation: An introduction to the concept. Energy Policy 2007, 35, 2683–2691. [Google Scholar] [CrossRef]
- González, J.; Rodríguez, M.; Suárez, P. Generation Capacity Expansion Considering Hydrogen Power Plants and Energy Storage Systems. Int. J. Hydrogen Energy 2023, 48, 5234–5248. [Google Scholar] [CrossRef]
- Conejo, A.J.; Baringo, L.; Kazempour, S.J.; Siddiqui, A.S. Investment in Electricity Generation and Transmission: Decision Making Under Uncertainty; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Birge, J.R.; Louveaux, F. Introduction to Stochastic Programming, 2nd ed.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Baringo, L.; Conejo, A.J. Transmission and Wind Power Investment. IEEE Trans. Power Syst. 2012, 27, 885–893. [Google Scholar] [CrossRef]
- Domínguez, R.; Conejo, A.J.; Carrión, M. Toward Fully Renewable Electric Energy Systems. IEEE Trans. Power Syst. 2015, 30, 316–326. [Google Scholar] [CrossRef]
- Bertsimas, D.; Sim, M. The Price of Robustness. Oper. Res. 2004, 52, 35–53. [Google Scholar] [CrossRef]
- Moreira, A.; Pozo, D.; Street, A.; Sauma, E. Reliable Renewable Generation and Transmission Expansion Planning: Co-Optimizing System’s Resources for Meeting Renewable Targets. IEEE Trans. Power Syst. 2017, 32, 3246–3257. [Google Scholar] [CrossRef]
- Gils, H.C.; Scholz, Y.; Pregger, T.; de Tena, D.L.; Heide, D. Integrated modelling of variable renewable energy-based power supply in Europe. Energy 2017, 123, 173–188. [Google Scholar] [CrossRef]
- Michalski, J.; Buenger, U.; Crotogino, F.; Donadei, S.; Schneider, G.S.; Pregger, T.; Cao, K.K.; Heide, D. Hydrogen generation by electrolysis and storage in salt caverns: Potentials, economics and systems aspects with regard to the German energy transition. Int. J. Hydrogen Energy 2017, 42, 13427–13443. [Google Scholar] [CrossRef]
- Won, W.; Kwon, H.; Han, J.H.; Kim, J. Design and operation of renewable energy sources based hydrogen supply system: Technology integration and optimization. Renew. Energy 2017, 103, 226–238. [Google Scholar] [CrossRef]
- Brown, T.; Schlachtberger, D.; Kies, A.; Schramm, S.; Greiner, M. Synergies of sector coupling and transmission reinforcement in a cost-optimised, highly renewable European energy system. Energy 2018, 160, 720–739. [Google Scholar] [CrossRef]
- Matsuo, Y.; Endo, S.; Nagatomi, Y.; Shibata, Y.; Komiyama, R.; Fujii, Y. A quantitative analysis of Japan’s optimal power generation mix in 2050 and the role of CO2-free hydrogen. Energy 2018, 165, 1200–1219. [Google Scholar] [CrossRef]
- Samsatli, S.; Samsatli, N.J. A multi-objective MILP model for the design and operation of future integrated multi-vector energy networks capturing detailed spatio-temporal dependencies. Appl. Energy 2018, 220, 893–920. [Google Scholar] [CrossRef]
- Evangelopoulou, S.; Vita, A.D.; Zazias, G.; Capros, P. Energy System Modelling of Carbon-Neutral Hydrogen as an Enabler of Sectoral Integration within a Decarbonization Pathway. Energies 2019, 12, 2551. [Google Scholar] [CrossRef]
- Müller, C.; Hoffrichter, A.; Wyrwoll, L.; Schmitt, C.; Trageser, M.; Kulms, T.; Beulertz, D.; Metzger, M.; Duckheim, M.; Huber, M.; et al. Modeling framework for planning and operation of multi-modal energy systems in the case of Germany. Appl. Energy 2019, 250, 1132–1146. [Google Scholar] [CrossRef]
- Han, S.; Kim, J. A multi-period MILP model for the investment and design planning of a national-level complex renewable energy supply system. Renew. Energy 2019, 141, 736–750. [Google Scholar] [CrossRef]
- Bødal, E.F.; Mallapragada, D.; Botterud, A.; Korpås, M. Decarbonization synergies from joint planning of electricity and hydrogen production: A Texas case study. Int. J. Hydrogen Energy 2020, 45, 32899–32915. [Google Scholar] [CrossRef]
- Cloete, S.; Hirth, L. Flexible power and hydrogen production: Finding synergy between CCS and variable renewables. Energy 2020, 192, 116671. [Google Scholar] [CrossRef]
- Lux, B.; Pfluger, B. A supply curve of electricity-based hydrogen in a decarbonized European energy system in 2050. Appl. Energy 2020, 269, 115011. [Google Scholar] [CrossRef]
- Moser, M.; Gils, H.C.; Pivaro, G. A sensitivity analysis on large-scale electrical energy storage requirements in Europe under consideration of innovative storage technologies. J. Clean. Prod. 2020, 269, 122261. [Google Scholar] [CrossRef]
- Gils, H.C.; Gardian, H.; Schmugge, J. Interaction of hydrogen infrastructures with other sector coupling options towards a zero-emission energy system in Germany. Renew. Energy 2021, 180, 140–156. [Google Scholar] [CrossRef]
- Husarek, D.; Schmugge, J.; Niessen, S. Hydrogen supply chain scenarios for the decarbonisation of a German multi-modal energy system. Int. J. Hydrogen Energy 2021, 46, 38008–38025. [Google Scholar] [CrossRef]
- He, G.; Mallapragada, D.S.; Bose, A.; Heuberger-Austin, C.F.; Gençer, E. Sector coupling via hydrogen to lower the cost of energy system decarbonization. Energy Environ. Sci. 2021, 14, 4635–4646. [Google Scholar] [CrossRef]
- Pietzcker, R.C.; Osorio, S.; Rodrigues, R. Tightening EU ETS targets in line with the European Green Deal: Impacts on the decarbonization of the EU power sector. Appl. Energy 2021, 293, 116914. [Google Scholar] [CrossRef]
- Sasanpour, S.; Cao, K.K.; Gils, H.C.; Jochem, P. Strategic policy targets and the contribution of hydrogen in a 100% renewable European power system. Energy Rep. 2021, 7, 4595–4608. [Google Scholar] [CrossRef]
- Arduin, I.; Andrey, C.; Bossmann, T. What energy infrastructure will be needed by 2050 in the EU to support 1.5 °C scenarios? F1000Research 2022, 11, 387. [Google Scholar] [CrossRef]
- Victoria, M.; Zeyen, E.; Brown, T. Speed of technological transformations required in Europe to achieve different climate goals. Joule 2022, 6, 1066–1086. [Google Scholar] [CrossRef]
- Schaffert, J.; Gils, H.C.; Fette, M.; Gardian, H.; Brandstaett, C.; Pregger, T.; Bruecken, N.; Tali, E.; Fiebrandt, M.; Albus, R.; et al. Integrating System and Operator Perspectives for the Evaluation of Power-to-Gas Plants in the Future German Energy System. Energies 2022, 15, 1174. [Google Scholar] [CrossRef]
- Gan, W.; Yan, M.; Yao, W.; Guo, J.; Fang, J.; Ai, X.; Wen, J. Multi-Network Coordinated Hydrogen Supply Infrastructure Planning for the Integration of Hydrogen Vehicles and Renewable Energy. IEEE Trans. Ind. Appl. 2022, 58, 2875–2886. [Google Scholar] [CrossRef]
- Wang, S.; Bo, R. Joint Planning of Electricity Transmission and Hydrogen Transportation Networks. IEEE Trans. Ind. Appl. 2022, 58, 2887–2897. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, P.; Li, Y.; Liu, J. Optimal Capacity Planning of Power to Hydrogen in Integrated Electricity-Hydrogen-Gas Energy Systems Considering Flexibility and Hydrogen Injection. Front. Energy Res. 2022, 10, 845637. [Google Scholar] [CrossRef]
- Martínez-Gordón, R.; Sánchez-Diéguez, M.; Fattahi, A.; Morales-España, G.; Sijm, J.; Faaij, A. Modelling a highly decarbonised North Sea energy system in 2050: A multinational approach. Adv. Appl. Energy 2022, 5, 100080. [Google Scholar] [CrossRef]
- Martínez-Gordón, R.; Gusatu, L.; Morales-España, G.; Sijm, J.; Faaij, A. Benefits of an integrated power and hydrogen offshore grid in a net-zero North Sea energy system. Adv. Appl. Energy 2022, 7, 100097. [Google Scholar] [CrossRef]
- Tao, Y.; Qiu, J.; Lai, S.; Sun, X. Coordinated Planning of Electricity and Hydrogen Networks with Hydrogen Supply Chain for Fuel Cell Electric Vehicles. IEEE Trans. Sustain. Energy 2023, 14, 1010–1023. [Google Scholar] [CrossRef]
- Morales-España, G.; Hernandez-Serna, R.; Tejada-Arango, D.A.; Weeda, M. Impact of large-scale hydrogen electrification and retrofitting of natural gas infrastructure on the European power system. Int. J. Electr. Power Energy Syst. 2024, 155, 109686. [Google Scholar] [CrossRef]
- Kountouris, I.; Bramstoft, R.; Madsen, T.; Gea-Bermudez, J.; Muenster, M.; Keles, D. A unified European hydrogen infrastructure planning to support the rapid scale-up of hydrogen production. Nat. Commun. 2024, 15, 5517. [Google Scholar] [CrossRef]
- da Silva, G.N.; Lantz, F.; Rochedo, P.R.R.; Szklo, A. Developing and applying the Hydrogen Economics and infRAstructure optimization model (HERA). Int. J. Hydrogen Energy 2024, 61, 1170–1186. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Qu, Z. Multi-stage collaborative planning of electricity-hydrogen-transportation coupling network considering carbon emission reduction. Electr. Power Syst. Res. 2024, 228, 110071. [Google Scholar] [CrossRef]
- Conejo, A.; Carrion, M.; Morales, J. Decision Making Under Uncertainty in Electricity Markets; Springer: New York, NY, USA, 2010. [Google Scholar]
- European Commission. Commission Regulation (EU) 2017/2195 of 23 Nov. 2017 Establishing a Guideline on Electricity Balancing. 2017. Available online: https://eur-lex.europa.eu/eli/reg/2017/2195/oj/eng (accessed on 8 March 2024).
- CNMV. Resolution of October 19, 2023, of the National Commission of Markets and Competition, Approving the New 7.5 Electrical Operation Procedure on the Active Demand Response Service (Spanish). Available online: https://www.boe.es/diario_boe/txt.php?id=BOE-A-2023-22497 (accessed on 8 March 2024).
- Arellano, J.; García-Cerezo, A.; Carrión, M. Optimal Participation in Manual Frequency Restoration Reserve-based Demand Response Programs for Large Consumers. IEEE Access 2025, 13, 59866–59885. [Google Scholar] [CrossRef]
- U.S. Department of Energy. 2024 Smart Grid System Report. 2024. Available online: https://www.energy.gov/sites/default/files/2024-02/2024%20Smart%20Grid%20System%20Report_untagged.pdf (accessed on 10 June 2025).
- Hale, E.; Bird, L.; Padmanabhan, R.; Volpi, C. Potential Roles for Demand Response in High-Growth Electric Systems with Increasing Shares of Renewable Generation. Technical Report NREL/TP-6A20-70630, National Renewable Energy Laboratory (NREL). 2018. Available online: https://docs.nrel.gov/docs/fy19osti/70630.pdf (accessed on 10 June 2025).
- IEEE Communications Society. IEEE SmartGridComm 2024 Tutorial on Distributed Control Strategies for DERs. 2024. Available online: https://sgc2024.ieee-smartgridcomm.org/sites/sgc2024.ieee-smartgridcomm.org/files/SmartGridComm2024_Tutorial_Distributed%20Control%20Strategies%20for%20Resilient%20Power%20Grid%20Operations.pdf (accessed on 10 June 2025).
- Springer Nature. Smart Grids and Sustainable Energy; Springer Nature: Berlin/Heidelberg, Germany, 2024; Available online: https://link.springer.com/journal/40866 (accessed on 27 June 2025).
- National Renewable Energy Laboratory. Renewable Energy-to-Grid Integration. 2023. Available online: https://www.nrel.gov/grid/renewable-energy-integration (accessed on 10 June 2025).
- Püschel-Løvengreen, S.; Dozein, M.G.; Low, S.; Mancarella, P. Separation event-constrained optimal power flow to enhance resilience in low-inertia power systems. Electric Power Systems Research 2020, 189, 106678. [Google Scholar] [CrossRef]
- Adrees, A.; Milanović, J.V.; Mancarella, P. Effect of inertia heterogeneity on frequency dynamics of low-inertia power systems. IET Gener. Transm. Distrib. 2019, 13, 2951–2958. [Google Scholar] [CrossRef]
- Dozein, M.G.; De Corato, A.M.; Mancarella, P. Virtual inertia response and frequency control ancillary services from hydrogen electrolyzers. IEEE Trans. Power Syst. 2022, 38, 2447–2459. [Google Scholar] [CrossRef]
- Liu, Y.; Mancarella, P.; Dozein, M.G.; Jalali, A.; Mancarella, P. Fast frequency response from utility-scale hydrogen electrolyzers. IEEE Trans. Sustain. Energy 2021, 12, 1707–1717. [Google Scholar] [CrossRef]
- Billimoria, F.; Mancarella, P.; Poudineh, R. Market and regulatory frameworks for operational security in decarbonizing electricity systems: From physics to economics. Oxford Open Energy 2022, 1, oiac007. [Google Scholar] [CrossRef]
- Guinot, B.; Montignac, F.; Champel, B.; Vannucci, D. Profitability of an Electrolysis Based Hydrogen Production Plant Providing Grid Balancing Services. Int. J. Hydrogen Energy 2015, 40, 8778–8787. [Google Scholar] [CrossRef]
- Luck, L.; Larscheid, P.; Maaz, A.; Moser, A. Economic Potential of Water Electrolysis within Future Electricity Markets. In Proceedings of the 2017 14th International Conference on the European Energy Market (EEM), Dresden, Germany, 6–9 June 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Mansilla, C.; Dautremont, S.; Shoai Tehrani, B.; Cotin, G.; Avril, S.; Burkhalter, E. Reducing the Hydrogen Production Cost by Operating Alkaline Electrolysis as a Discontinuous Process in the French Market Context. Int. J. Hydrogen Energy 2011, 36, 6407–6413. [Google Scholar] [CrossRef]
- Dadkhah, A.; Bozalakov, D.; De Kooning, J.D.M.; Vandevelde, L. On the Optimal Planning of a Hydrogen Refuelling Station Participating in the Electricity and Balancing Markets. Int. J. Hydrogen Energy 2021, 46, 1488–1500. [Google Scholar] [CrossRef]
- Samani, A.E.; D’Amicis, A.; De Kooning, J.D.; Bozalakov, D.; Silva, P.; Vandevelde, L. Grid Balancing with a Large-Scale Electrolyser Providing Primary Reserve. IET Renew. Power Gener. 2020, 14, 3070–3078. [Google Scholar] [CrossRef]
- Dadkhah, A.; Bozalakov, D.; De Kooning, J.D.M.; Vandevelde, L. Techno-Economic Analysis and Optimal Operation of a Hydrogen Refueling Station Providing Frequency Ancillary Services. IEEE Trans. Ind. Appl. 2022, 58, 5171–5183. [Google Scholar] [CrossRef]
- Rezaee Jordehi, A.; Mansouri, S.A.; Tostado-Véliz, M.; Carrión, M.; Hossain, M.J.; Jurado, F. A Risk-Averse Two-Stage Stochastic Model for Optimal Participation of Hydrogen Fuel Stations in Electricity Markets. Int. J. Hydrogen Energy 2024, 49, 188–201. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, W.; Li, H.; Liu, B.; Zhang, Z.; Wang, X. Multi-Stage Stochastic Programming Based Offering Strategy for Hydrogen Fueling Station in Joint Energy, Reserve Markets. Renew. Energy 2021, 180, 605–615. [Google Scholar] [CrossRef]
- Jain, R.; Nagasawa, K.; Veda, S.; Sprik, S. Integrated Grid ancillary services using electrolyzer-Based power-to-Gas systems with increasing renewable penetration. Adv. Electr. Eng. Electron. Energy 2023, 6, 100308. [Google Scholar] [CrossRef]
- Dalgas, T. The Value of Flexibility for Electrolyzers. 2022. Available online: https://energinet.dk/el/systemydelser/nyheder-om-systemydelser/2022/07/01/2022-07-01-flexibility-from-electrolysis/ (accessed on 10 June 2025).
- Johnsen, A.G.; Mitridati, L.; Zarrilli, D.; Kazempour, J. The Value of Ancillary Services for Electrolyzers. arXiv 2023, arXiv:2310.04321. [Google Scholar] [CrossRef]
- Varhegyi, G.; Nour, M. Advancing Fast Frequency Response Ancillary Services in Renewable-Heavy Grids: A Global Review of Energy Storage-Based Solutions and Market Dynamics. Energies 2024, 17, 100308. [Google Scholar] [CrossRef]
- Comisión Nacional de los Mercados y la Competencia (CNMC). Consulta Pública Sobre Ayudas al Autoconsumo Fotovoltaico. 2024. Available online: https://www.cnmc.es/ (accessed on 10 June 2025).
- National Renewable Energy Laboratory. Solar Energy in the United States: 2024 Annual Review. 2024. Available online: https://www.nrel.gov/solar/ (accessed on 10 June 2025).
- Baringo, L.; Carrión, M.; Domínguez, R. Electric Vehicles and Renewable Generation; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Echekki, T.; Lee, E. Turbulent Combustion Modeling: Advances, New Trends and Perspectives; Springer: New York, NY, USA, 2009. [Google Scholar]
- Sayer, M.; Ajanovic, A.; Haas, R. On the economics of a hydrogen bus fleet powered by a wind park – a case study for Austria. Int. J. Hydrogen Energy 2022, 47, 33153–33166. [Google Scholar] [CrossRef]
- Posso, F.; Pulido, A.; Acevedo-Paez, J.C. Towards the hydrogen economy: Estimation of green hydrogen production potential and the impact of its uses in Ecuador as a case study. Int. J. Hydrogen Energy 2023, 48, 11922–11942. [Google Scholar] [CrossRef]
- Camarillo, R. Hydrogen Cars: How They Work, Prices, Range, Refueling, Weaknesses, and Risks (Spanish). 2022. Available online: https://theconversation.com/coches-de-hidrogeno-como-funcionan-precios-autonomia-repostaje-debilidades-y-riesgos-193405 (accessed on 12 May 2025).
- Zhao, L.; Brouwer, J. Dynamic operation and feasibility study of a self-sustainable hydrogen fueling station using renewable energy sources. Int. J. Hydrogen Energy 2015, 40, 3822–3837. [Google Scholar] [CrossRef]
- (IRENA). Renewable Capacity Statistics 2020. 2020. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2020/Mar/IRENA_RE_Capacity_Statistics_2020.pdf (accessed on 13 May 2025).
- (IRENA). Global Renewables Report 2023. 2023. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2023/Jul/IRENA_Renewable_energy_statistics_2023.pdf (accessed on 13 May 2025).
- Caponi, R.; Monforti Ferrario, A.; Del Zotto, L.; Bocci, E. Hydrogen refueling stations and fuel cell buses: Four-year operational analysis under real-world conditions. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Gökçek, M.; Kale, C. Optimal design of a Hydrogen Refuelling Station (HRFS) powered by Hybrid Power System. Energy Convers. Manag. 2018, 161, 215–224. [Google Scholar] [CrossRef]
- de Santoli, L.; Lo Basso, G.; Bruschi, D. A small scale H2/NG production plant in Italy: Techno-economic feasibility analysis and costs associated with carbon avoidance. Int. J. Hydrogen Energy 2014, 39, 6497–6517. [Google Scholar] [CrossRef]
- Messaoudi, S.; Meziane, N. Messaoudi, D.; Settou, N.; Allouhi, A. Geographical, technical, economic, and environmental potential for wind to hydrogen production in Algeria: GIS-based approach. Int. J. Hydrogen Energy 2024, 50, 142–160. [Google Scholar] [CrossRef]
- Okonkwo, P.C. A case study on hydrogen refueling station techno-economic viability. Int. J. Hydrogen Energy 2024, 49, 736–746. [Google Scholar] [CrossRef]
- Das, H.; Tan, C.; Yatim, A.; Lau, K. Feasibility analysis of hybrid photovoltaic/battery/fuel cell energy system for an indigenous residence in East Malaysia. Renew. Sustain. Energy Rev. 2017, 76, 1332–1347. [Google Scholar] [CrossRef]
- Al-shara, A.; Sahin, A.; Ayar, T.; Yilbas, B. Techno-economic analysis and optimization of solar and wind energy systems for power generation and hydrogen production in Saudi Arabia. Renew. Sustain. Energy Rev. 2017, 69, 33–49. [Google Scholar] [CrossRef]
- Minutillo, M.; Perna, A.; Forcina, A.; Di Micco, S.; Jannelli, E. Analyzing the levelized cost of hydrogen in refueling stations with on-site hydrogen production via water electrolysis in the Italian scenario. Int. J. Hydrogen Energy 2021, 46, 13667–13677. [Google Scholar] [CrossRef]
- Nistor, S.; Dave, S.; Fan, Z.; Sooriyabandara, M. Technical and economic analysis of hydrogen refuelling. Appl. Energy 2016, 167, 211–220. [Google Scholar] [CrossRef]
- Popov, S.; Baldynov, O. The hydrogen energy infrastructure development in Japan. Green Energy Smarts 2018, 69, 02001. [Google Scholar] [CrossRef]
- Siyal, S.; Mentis, D.; Howells, M. Economic analysis of standalone wind-powered hydrogen refueling stations for road transport at selected sites in Sweden. Int. J. Hydrogen Energy 2015, 40, 9855–9865. [Google Scholar] [CrossRef]
- Okonkwo, P.; Islam, M.; Taura, U.; Barhoumi, E.; Mansir, I.; Das, B.; Sulaiman, M.; Agyekum, E.; Bahadur, I. A techno-economic analysis of renewable hybrid energy systems for hydrogen production at refueling stations. Int. J. Hydrogen Energy 2024, 78, 68–82. [Google Scholar] [CrossRef]
- Viktorsson, L.; Heinonen, J.; Skulason, J.; Unnthorsson, R. A step towards the hydrogen economy - a life cycle cost analysis of a hydrogen refueling station. Energies 2017, 10, 763. [Google Scholar] [CrossRef]
- Tang, O.; Rehme, J.; Cerin, P. Levelized cost of hydrogen for refueling stations with solar PV and wind in Sweden: On-grid or off-grid? Energy 2022, 241, 122906. [Google Scholar] [CrossRef]
- Bansal, S.; Zong, Y.; You, S.; Mihet-Popa, L.; Xiao, J. Technical and economic analysis of one-stop charging stations for battery and fuel cell EV with renewable energy sources. Energies 2020, 13, 2855. [Google Scholar] [CrossRef]
- Grüger, F.; Dylewski, L.; Robinius, M.; Stolten, D. Carsharing with fuel cell vehicles: Sizing hydrogen refueling stations based on refueling behavior. Appl. Energy 2018, 228, 1540–1549. [Google Scholar] [CrossRef]
- Dahbi, S.; Aziz, A.; Messaoudi, A.; Mazozi, I.; Kassmi, K.; Benazzi, N. Management of excess energy in a photovoltaic/grid system by production of clean hydrogen. Int. J. Hydrogen Energy 2018, 43, 5283–5299. [Google Scholar] [CrossRef]
- Tao, Y.; Qiu, J.; Lai, S.; Zhang, X.; Wang, G. Collaborative Planning for Electricity Distribution Network and Transportation System Considering Hydrogen Fuel Cell Vehicles. IEEE Trans. Transp. Electrif. 2020, 6, 1211–1225. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Yuan, G. Optimal scheduling of integrated energy systems including hydrogen electrolyzers, HFCVs, and electric vehicles. Energy Sci. Eng. 2023, 11, 3684–3694. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Y.; Dong, W.; Yang, Q.; Sun, S. Optimal energy management of multiple electricity-hydrogen integrated charging stations. Energy 2023, 262, 125624. [Google Scholar] [CrossRef]
- Andrews, J.; Shabani, B. Re-envisioning the role of hydrogen in a sustainable energy economy. Int. J. Hydrogen Energy 2012, 37, 1184–1203. [Google Scholar] [CrossRef]
- Zou, C.; Li, J.; Zhang, X.; Jin, X.; Xiong, B.; Yu, H.; Pan, S. Industrial status, technological progress, challenges, and prospects of hydrogen energy. Nat. Gas Ind. B 2022, 9, 427–447. [Google Scholar] [CrossRef]
- Cardoso, J.; Silva, V.; Rocha, R.; Hall, M.; Costa, M.; Eusébio, D. Ammonia as an Energy Vector: Current and Future Prospects for Low-Carbon Fuel Applications in Internal Combustion Engines. J. Clean. Prod. 2021, 296, 126562. [Google Scholar] [CrossRef]
- Society, T.R. Ammonia: Zero-Carbon Fertiliser, Fuel and Energy Store. 2020. Available online: https://royalsociety.org/-/media/policy/projects/green-ammonia/green-ammonia-policy-briefing.pdf (accessed on 20 August 2024).
- Smith, C.; Hill, A.; Torrente-Murciano, L. Current and Future Role of Haber–Bosch Ammonia in a Carbon-Free Energy Landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Flórez-Orrego, D.; de Oliveira Junior, S. Modeling and Optimization of an Industrial Ammonia Synthesis Unit: An Exergy Approach. Energy 2017, 137, 234–250. [Google Scholar] [CrossRef]
- Saygin, D.; Blanco, H.; Boshell, F.; Cordonnier, J.; Rouwenhorst, K.; Lathwal, P.; Gielen, D. Ammonia Production from Clean Hydrogen and the Implications for Global Natural Gas Demand. Sustainability 2023, 15, 1623. [Google Scholar] [CrossRef]
- Cinti, G.; Frattini, D.; Jannelli, E.; Desideri, U.; Bidini, G. Coupling Solid Oxide Electrolyser (SOE) and Ammonia Production Plant. Appl. Energy 2017, 192, 466–476. [Google Scholar] [CrossRef]
- Andersson, J.; Lundgren, J. Techno-economic Analysis of Ammonia Production via Integrated Biomass Gasification. Appl. Energy 2014, 130, 484–490. [Google Scholar] [CrossRef]
- Cesaro, Z.; Ives, M.; Nayak-Luke, R.; Mason, M.; Bañares-Alcántara, R. Ammonia to Power: Forecasting the Levelized Cost of Electricity from Green Ammonia in Large-Scale Power Plants. Appl. Energy 2021, 282, 116009. [Google Scholar] [CrossRef]
- Skribbe, S.; Liu, M.; Patel, S.; Rix, M.; Bensebaa, F.; Mak, L.; Wu, X.Y. The Levelized Cost of Carbon Abatement (LCCA) in Substituting Conventional Ammonia Production with Power-to-Ammonia for Fertilizer, Hydrogen and Export. Appl. Energy 2024, 373, 123859. [Google Scholar] [CrossRef]
- Fasihi, M.; Weiss, R.; Savolainen, J.; Breyer, C. Global Potential of Green Ammonia Based on Hybrid PV-Wind Power Plants. Appl. Energy 2021, 294, 116170. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Z.; Li, X.; Zhou, X. Investigation of a Novel Scheme Utilizing Solar and Geothermal Energies, Generating Power and Ammonia: Exergoeconomic and Exergoenvironmental Analyses and Cuckoo Search Optimization. Energy 2024, 298, 131344. [Google Scholar] [CrossRef]
- Cunanan, C.; Elorza Casas, C.; Yorke, M.; Fowler, M.; Wu, X.Y. Design and Analysis of an Offshore Wind Power to Ammonia Production System in Nova Scotia. Energies 2022, 15, 9558. [Google Scholar] [CrossRef]
- Hasan, M.; Mahlia, T.; Mofijur, M.; Rizwanul Fattah, I.; Handayani, F.; Ong, H.; Silitonga, A. A Comprehensive Review on the Recent Development of Ammonia as a Renewable Energy Carrier. Energies 2021, 14, 3732. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.; Nandiyanto, A. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Van Herle, J.; Maréchal, F.; Desideri, U. Techno-Economic Comparison of Green Ammonia Production Processes. Appl. Energy 2020, 259, 114135. [Google Scholar] [CrossRef]
- Wen, D.; Wei, X.; Bruneau, A.; Maroonian, A.; Maréchal, F.; Herle, J.V. Techno-economic analysis of ammonia to hydrogen and power pathways considering the emerging hydrogen purification and fuel cell technologies. Appl. Energy 2025, 390, 125871. [Google Scholar] [CrossRef]
- Sánchez, A.; Castellano, E.; Martín, M.; Vega, P. Evaluating Ammonia as Green Fuel for Power Generation: A Thermo-Chemical Perspective. Appl. Energy 2021, 293, 116956. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Y.; Zhou, X.; Wang, D.; Wei, Y.; Li, F. Co-Optimization of Decarbonized Operation of Coal-Fired Power Plants and Seasonal Storage Based on Green Ammonia Co-Firing. Appl. Energy 2023, 341, 121140. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Yang, D.; Yin, Z.; Lu, K.; Tan, D. A Comprehensive Assessment of the Environmental Impact and Combustion Efficiency of Using Ammonia/Hydrogen/Diesel Blends in a Diesel Engine. Energy 2024, 303, 131955. [Google Scholar] [CrossRef]
- Liu, H.; Ampah, J.; Zhao, Y.; Sun, X.; Xu, L.; Jiang, X.; Wang, S. A Perspective on the Overarching Role of Hydrogen, Ammonia, and Methanol Carbon-Neutral Fuels Towards Net Zero Emission in the Next Three Decades. Energies 2023, 16, 280. [Google Scholar] [CrossRef]
- Boero, A.; Kardux, K.; Kovaleva, M.; Salas, D.; Mooijer, J.; Mashruk, S.; Townsend, M.; Rouwenhorst, K.; Valera-Medina, A.; Ramirez, A. Environmental Life Cycle Assessment of Ammonia-Based Electricity. Energies 2021, 14, 6721. [Google Scholar] [CrossRef]
- Halim, I.; Zain, N.S.; Khoo, H.H. Assessing the feasibility of Ammonia utilization for Power generation: A techno-economic-environmental study. Appl. Energy 2025, 386, 125581. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, J.; Liu, F.; Li, J.; Zhao, Y.; Song, Y. Optimal Sizing and Pricing of Grid-Connected Renewable Power to Ammonia Systems Considering the Limited Flexibility of Ammonia Synthesis. IEEE Trans. Power Syst. 2024, 39, 3631–3648. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Heuser, P.; Heinrichs, H.U.; Xiao, J.; Liu, F. Co-Planning of Regional Wind Resources-Based Ammonia Industry and the Electric Network: A Case Study of Inner Mongolia. IEEE Trans. Power Syst. 2022, 37, 65–80. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, B.; Wu, Q.; Chung, C.Y.; Li, C.; Huang, S. Integrated Modelling and Enhanced Utilization of Power-to-Ammonia for High Renewable Penetrated Multi-Energy Systems. IEEE Trans. Power Syst. 2020, 35, 4769–4780. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review 2024. 2024. Available online: https://www.iea.org/reports/global-hydrogen-review-2024 (accessed on 20 August 2024).
- ISPT. Power to Ammonia, Feasibility Study for the Value Chains and Business Cases to Produce CO2-Free Ammonia Suitable for Various Market Applications. 2017. Available online: https://ispt.eu/media/DR-20-09-Power-to-Ammonia-2017-publication.pdf (accessed on 27 June 2025).
- Alcantara, R.B.; Dericks, G.; Fiaschetti, M.; Grunewald, P.; Lopez, J.M.; Tsang, A.; Yang, A.; Ye, L.; Zhao, S. Analysis of Islanded Ammonia-Based Energy Storage Systems; Technical report; University of Oxford: Oxford, UK, 2015; Available online: https://media.eng.ox.ac.uk/11082/ammonia-based_ess.pdf (accessed on 27 June 2025).
- Campion, N.; Nami, H.; Swisher, P.R.; Hendriksen, P.V.; Münster, M. Techno-economic assessment of green ammonia production with different wind and solar potentials. Renew. Sustain. Energy Rev. 2023, 173, 113057. [Google Scholar] [CrossRef]
- Osman, O.; Sgouridis, S.; Sleptchenko, A. Scaling the Production of Renewable Ammonia: A Techno-Economic Optimization Applied in Regions with High Insolation. J. Clean. Prod. 2020, 271, 121627. [Google Scholar] [CrossRef]
- Cameli, F.; Kourou, A.; Rosa, V.; Delikonstantis, E.; Galvita, V.; Van Geem, K.M.; Stefanidis, G.D. Conceptual process design and technoeconomic analysis of an e-ammonia plant: Green H2 and cryogenic air separation coupled with Haber-Bosch process. Int. J. Hydrogen Energy 2024, 49, 1416–1425. [Google Scholar] [CrossRef]
- Diéguez, P.M.; Ursúa, A.; Sanchis, P.; Sopena, C.; Guelbenzu, E.; Gandía, L.M. Thermal performance of a commercial alkaline water electrolyzer: Experimental study and mathematical modeling. Int. J. Hydrogen Energy 2008, 33, 7338–7354. [Google Scholar] [CrossRef]
- Rizwan, M.; Alstad, V.; Jäschke, J. Design considerations for industrial water electrolyzer plants. Int. J. Hydrogen Energy 2021, 46, 37120–37136. [Google Scholar] [CrossRef]
- Cammann, L.; Perera, A.; Alstad, V.; Jäschke, J. Design and operational analysis of an alkaline water electrolysis plant powered by wind energy. Int. J. Hydrogen Energy 2024, 93, 963–974. [Google Scholar] [CrossRef]
- Agbli, K.S.; Péra, M.C.; Hissel, D.; Rallières, O.; Turpin, C.; Doumbia, I. Multiphysics simulation of a PEM electrolyser: Energetic Macroscopic Representation approach. Int. J. Hydrogen Energy 2011, 36, 1382–1398. [Google Scholar] [CrossRef]
- Asensio, F.J.; Martín, J.I.S.; Zamora, I.; Saldaña, G.; Oñederra, O. Analysis of electrochemical and thermal models and modeling techniques for polymer electrolyte membrane fuel cells. Renew. Sustain. Energy Rev. 2019, 113, 109283. [Google Scholar] [CrossRef]
- Amphlett, J.C.; Mann, R.F.; Peppley, B.A.; Roberge, P.R.; Rodrigues, A. A model predicting transient responses of proton exchange membrane fuel cell. J. Power Sources 1996, 61, 183–188. [Google Scholar] [CrossRef]
- Ziogou, C.; Voutetakis, S.; Papadopoulou, S.; Georgiadis, M.C. Modeling, simulation and experimental validation of a PEM fuel cell system. Comput. Chem. Eng. 2011, 35, 1886–1900. [Google Scholar] [CrossRef]
- Barzegari, M.M.; Dardel, M.; Ramiar, A.; Alizadeh, E. An investigation of temperature effect on performance of dead-end cascade H2/O2 PEMFC stack with integrated humidifier and separator. Int. J. Hydrogen Energy 2016, 41, 3136–3146. [Google Scholar] [CrossRef]
- Ziogou, C.; Voutetakis, S.; Georgiadis, M.C.; Papadopoulou, S. Model predictive control (MPC) strategies for PEM fuel cell systems—A comparative experimental demonstration. Chem. Eng. Res. Des. 2018, 131, 656–670. [Google Scholar] [CrossRef]
- Vasu, G.; Tangirala, A.K. Control-orientated thermal model for proton-exchange membrane fuel cell systems. J. Power Sources 2008, 183, 98–108. [Google Scholar] [CrossRef]
- Shan, Y.; Choe, S.Y. A high dynamic PEM fuel cell model with temperature effects. J. Power Sources 2005, 145, 30–39. [Google Scholar] [CrossRef]
- Sankar, K.; Aguan, K.; Jana, A.K. A proton exchange membrane fuel cell with an airflow cooling system: Dynamics, validation and nonlinear control. Energy Convers. Manag. 2019, 183, 230–240. [Google Scholar] [CrossRef]
- Park, S.K.; Choe, S.Y. Dynamic modeling and analysis of a 20-cell PEM fuel cell stack considering temperature and two-phase effects. J. Power Sources 2008, 179, 660–672. [Google Scholar] [CrossRef]
- Sankar, K.; Jana, A.K. Nonlinear multivariable sliding mode control of a reversible PEM fuel cell integrated system. Energy Convers. Manag. 2018, 171, 541–565. [Google Scholar] [CrossRef]
- Xue, X.; Tang, J.; Smirnova, A.; England, R.; Sammes, N. System level lumped-parameter dynamic modeling of PEM fuel cell. J. Power Sources 2004, 133, 188–204. [Google Scholar] [CrossRef]
- Cheddie, D.; Munroe, N. Analytical correlations for intermediate temperature PEM fuel cells. J. Power Sources 2006, 160, 299–304. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, X.; Jiang, J.; Wu, B. An improved dynamic model considering effects of temperature and equivalent internal resistance for PEM fuel cell power modules. J. Power Sources 2006, 161, 1062–1068. [Google Scholar] [CrossRef]
- Sakas, G.; Ibáñez-Rioja, A.; Ruuskanen, V.; Kosonen, A.; Ahola, J.; Bergmann, O. Dynamic energy and mass balance model for an industrial alkaline water electrolyzer plant process. Int. J. Hydrogen Energy 2022, 47, 4325–4328. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.; Lee, K.S. One-dimensional dynamic modelling of a high pressure water electrolysis system for hydrogen production. Int. J. Hydrogen Energy 2013, 38, 2596–2609. [Google Scholar] [CrossRef]
- Sarrias-Mena, R.; Fernández-Ramírez, L.M.; García-Vázquez, C.A.; Jurado, F. Electrolyzer models for hydrogen production from wind energy systems. Int. J. Hydrogen Energy 2015, 40, 2927–2938. [Google Scholar] [CrossRef]
- Kimble, M.C.; White, R.E. A mathematical model of a hydrogen/oxygen alkaline fuel cell. J. Electrochem. Soc. 1991, 138, 3370–3382. [Google Scholar] [CrossRef]
- An, L.; Chai, Z.H.; Zeng, L.; Tan, P.; Zhao, T.S. Mathematical modeling of alkaline direct ethanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 14067–14075. [Google Scholar] [CrossRef]
- Ruy, R.S.; Gonzalez, E.R. Mathematical modeling of polymer electrolyte fuel cells. J. Power Sources 2005, 147, 32–45. [Google Scholar] [CrossRef]
- Lottin, O.; Antoine, B.; Colinart, T.; Didierjean, S.; Maranzana, G.; Moyne, C.; Ramousse, J. Modelling of the operation of Polymer Exchange Membrane Fuel Cells in the presence of electrodes flooding. Int. J. Therm. Sci. 2009, 48, 133–145. [Google Scholar] [CrossRef]
- Ramousse, J.; Deseure, J.; Lottin, O.; Diderjean, S.; Maillet, D. Modelling of heat, mass and charge transfer in a PEMFC single cell. J. Power Sources 2005, 145, 416–427. [Google Scholar] [CrossRef]
- Dannenberg, K.; Ekdunge, P.; Lindberhg, G. Mathematical model of the PEMFC. J. Appl. Electrochem. 2000, 30, 1377–1387. [Google Scholar] [CrossRef]
- Kakaç, S.; Pramuanjaroenkij, A.; Zhou, X.Y. A review of numerical modelling of solid oxide fuel cells. Int. J. Hydrogen Energy 2007, 32, 761–786. [Google Scholar] [CrossRef]
- Singh, D.; Lu, D.M.; Djilali, N. A two-dimensional analysis of mass transport in proton exchange membrane fuel cells. Int. J. Eng. Sci. 1999, 37, 431–452. [Google Scholar] [CrossRef]
- Maharluie, H.N.; Rahmani, M. Mathematical modeling of solid oxide fuel cell performance with indirect internal reforming and thermal interaction analysis. Energy Convers. Manag. 2024, 316, 118842. [Google Scholar] [CrossRef]
- Padullés, J.; Ault, G.W.; McDonald, J.R. An integrated SOFC plant dynamic model for power simulation. J. Power Sources 2000, 86, 495–500. [Google Scholar] [CrossRef]
- Liso, V.; Nielsen, M.P.; Koer, S.K.; Mortensen, H.H. Thermal modelling and temperature control of a PEM fuel cell system for forklift applications. Int. J. Hydrogen Energy 2014, 39, 8410–8420. [Google Scholar] [CrossRef]






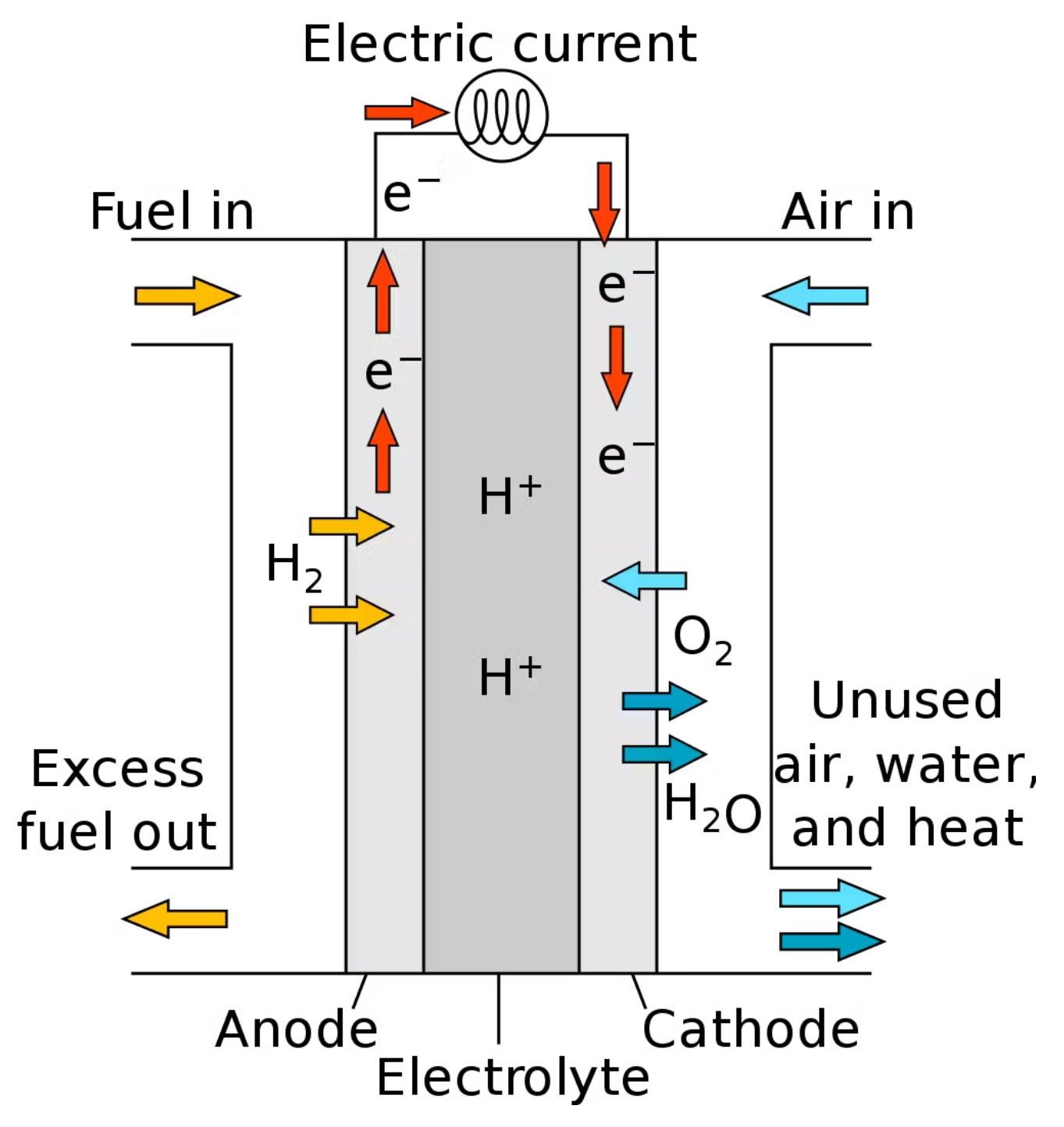


| Type | Electrolyte | Temp. (°C) | Efficiency (%) | Advantages and Disadvantages |
|---|---|---|---|---|
| Electrolyzers | ||||
| AE | KOH or NaOH | 60–80 | 60–70 | + Mature technology, low cost. |
| − Low current density, sensitive to impurities. | ||||
| PEM | Polymer membrane | 50–80 | 70–85 | + High efficiency, fast response. |
| − Expensive catalysts, requires pure water. | ||||
| SOE | Ceramic (YSZ) | 650–1000 | 85–90 | + High efficiency, can use steam. |
| − High temperature, material degradation. | ||||
| AEME | Anion exchange membrane | 50–80 | 65–75 | + Cheaper than PEM, no precious metals. |
| − Lower durability, still in development. | ||||
| Fuel Cells | ||||
| PEMFC | Proton exchange membrane | 50–100 | 40–60 | + Fast startup, compact design. |
| − Expensive catalysts, management issues. | ||||
| DMFC | Direct methanol | 60–130 | 30–40 | + Liquid fuel convenience, high energy density. |
| − Methanol crossover, low electrical efficiency. | ||||
| SOFC | Ceramic (YSZ) | 600–1000 | 50–65 | + High efficiency, fuel flexibility. |
| − High temp., slow startup. | ||||
| PAFC | Phosphoric acid | 150–200 | 40–50 | + Tolerant to impurities. |
| − Large size, slow startup. | ||||
| AFC | KOH | 60–90 | 45–55 | + High efficiency, low-cost catalysts. |
| − sensitivity, requires pure hydrogen. | ||||
| MCFC | Molten carbonate salts | 600–700 | 45–55 | + High efficiency, fuel flexibility. |
| − Corrosion issues, slow startup. | ||||
| Method | Key Parameters | Advantages | Disadvantages | Typical Applications |
|---|---|---|---|---|
|
Compressed Hydrogen |
|
|
| FCEVs |
|
Liquid Hydrogen |
|
|
| Space, large-scale |
|
Metal Hydrides |
|
|
| Stationary storage |
|
Chemical Storage (/LOHCs) |
|
|
| Long-distance |
|
Power to Methane |
|
|
| Gas grids/seasonal |
| Ref. | Model | Generation | Grid | Storage | Prod. | Trans. | Storage | Formulation | Uncertainty | Stages |
|---|---|---|---|---|---|---|---|---|---|---|
| [143] | REMix | R,C,N,G | y | PH,B | - | - | TA,GS | LP | D | SS |
| [144] | REMix | G | - | - | E | - | GS | LP | D | SS |
| [145] | ad hoc | R | - | - | E,TW,Bio | - | TA | MILP | D | SS |
| [146] | PyPSA-Eur-Sec-30 | R,G | y | B,H2 | E | - | TA | LP | D | SS |
| [147] | ad hoc | R,N,H2 | y | PH,B,H2 | E | - | TA | LP | D | SS |
| [148] | ad hoc | R,G,H2 | y | H2 | E,SMR | P | TA,GS | MILP | D | MS |
| [149] | PRIMES | R,C,N,G,H2 | - | PH,B,CA,H2 | E,SMR | - | - | NLP | D | MS |
| [151] | ad hoc | R | - | B | E,Bio | TR | TA | MILP | D | MS |
| [150] | ad hoc | R,C,G | - | PH,B,Th | E | - | ns | LP | D | MS |
| [152] | ad hoc | R,G,N,H2 | y | B,H2 | E,SMR | P | TA | LP | D | SS |
| [153] | ad hoc | R,C,G,H2 | - | B | E,SMR | - | - | LP | D | SS |
| [101] | ad hoc | - | - | - | E | - | TA | MILP | D | SS |
| [154] | Enertile | R,N,H2 | y | PH,H2 | E | - | ns | LP | D | SS |
| [155] | REMix | R,C,N,G | y | PH,B,CA,Th,H2 | E | - | GS | LP | D | SS |
| [104] | ad hoc | R,H2 | - | B | E | - | TA | MILP | S | SS |
| [156] | REMix | R,G | y | PH,B,Th | E | P,RF | TA,GS | LP | D | MS |
| [158] | ad hoc | - | - | - | E,SMR | P,TR | TA,TR,P | MILP(LP) | D | SS |
| [157] | ad hoc | R,G | y | B | E | P,RF,TR | TA | LP | D | SS |
| [159] | LIMES-EU | R,C,N,G,H2 | y | PH,B,H2 | E | - | ns | LP | D | MS |
| [160] | REMix | R,N,H2 | y | PH,B | E | P,M | TA,GS | LP | D | SS |
| [161] | METIS | G | y | PH,B | E,SMR | P,RF | GS | LP | D | SS |
| [164] | ad hoc | R | - | - | E | P | - | MISOCP | S | SS |
| [167] | ad hoc | R,G,N | y | - | E,SMR | P,RF | - | LP | D | SS |
| [168] | ad hoc | R,G | y | - | E | P,RF | GS | LP | D | SS |
| [163] | REMix | R,G | y | PH,B | E | P | GS | LP | D | SS |
| [162] | PyPSA-Eur-Sec-30 | R,C,N,G | y | B | E,SMR | P | TA,GS | LP | D | MS |
| [165] | ad hoc | - | y | - | E,SMR | P,TR | ns | MILP | D | SS |
| [166] | ad hoc | - | - | - | E | - | - | MINLP(MISOCP) | S | SS |
| [106] | ad hoc | R,G,H2 | - | PH,B | E | - | TA | MILP | S | SS |
| [169] | ad hoc | R | y | - | E | P,TR | TA,TR | MINLP(MILP) | D | MS |
| [171] | Balmorel | R,C,N,G | y | ns | E,SMR | P,RF | ns | LP | D | MS |
| [170] | COMPETES | R,C,N,G | y | PH,B,CA | E,SMR | P,RF | ns | LP | D | SS |
| [172] | ad hoc | R | - | B | E,SMR | P,TR | TA,P,TR | MILP | D | SS |
| [173] | ad hoc | R,H2 | - | H2 | E | P | TA | MINLP(MILP) | D | MS |
| Theme | References |
|---|---|
| FCEVs and grid integration | [164,213,214,227,228,229] |
| Energy system planning, including FCEVs | [211,212,220,221,222,223] |
| Techno-economic analysis of refueling stations | [209,210,224,225] |
| Renewable-based production with FCEVs | [204,226] |
| H2 as an energy carrier and sector coupling | [202,203,204,217] |
| Forecasting and adoption of FCEVs | [206,207,208] |
| Electrolyzer Modeling and Flexibility with FCEVs | [215,216,217,218,219] |
| Parameter | Approximate Value | Unit | References |
|---|---|---|---|
| Electrolytic conversion efficiency | 65–75 | % | [27,28,29,30,31,32,33,34,35,36] |
| Electricity consumed | 10–12 | MWh/ton NH3 | [258,259] |
| HB + ASU capital cost | 4192 | EUR/ton NH3/h | [260] |
| Annual total production cost | 415–840 | EUR/ton NH3 | [260,261] |
| CO2 footprint | 0.34–0.95 | ton CO2/ton NH3 | [262] |
| Plant lifetime | 20–30 | years | [260] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba, J.; Cañas-Carretón, M.; Carrión, M.; Hernández-Labrado, G.R.; Merino, C.; Muñoz, J.I.; Zárate-Miñano, R. Integrating Hydrogen into Power Systems: A Comprehensive Review. Sustainability 2025, 17, 6117. https://doi.org/10.3390/su17136117
Barba J, Cañas-Carretón M, Carrión M, Hernández-Labrado GR, Merino C, Muñoz JI, Zárate-Miñano R. Integrating Hydrogen into Power Systems: A Comprehensive Review. Sustainability. 2025; 17(13):6117. https://doi.org/10.3390/su17136117
Chicago/Turabian StyleBarba, Javier, Miguel Cañas-Carretón, Miguel Carrión, Gabriel R. Hernández-Labrado, Carlos Merino, José Ignacio Muñoz, and Rafael Zárate-Miñano. 2025. "Integrating Hydrogen into Power Systems: A Comprehensive Review" Sustainability 17, no. 13: 6117. https://doi.org/10.3390/su17136117
APA StyleBarba, J., Cañas-Carretón, M., Carrión, M., Hernández-Labrado, G. R., Merino, C., Muñoz, J. I., & Zárate-Miñano, R. (2025). Integrating Hydrogen into Power Systems: A Comprehensive Review. Sustainability, 17(13), 6117. https://doi.org/10.3390/su17136117







