Diversity and Metacommunity Structure of Aquatic Macrophytes: A Study in Mediterranean Mountain Wetlands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Environmental and Spatial Variables
2.3. Data Analysis

3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpenter, S.R.; Lodge, D.M. Effects of submersed macrophytes on ecosystem processes. Aquat. Bot. 1986, 26, 341–370. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Strzałek, M.; Kufel, L.; Apolinarska, K.; Becher, M.; Biardzka, E.; Brzozowski, M.; Kiełczewski, R.; Kowalewski, G.; Pukacz, A.; Woszczyk, M.; et al. Recycling and deposition of inorganic carbon from calcium carbonate encrustations of charophytes. Limnol. Oceanogr. 2024, 69, 279–289. [Google Scholar] [CrossRef]
- Blindow, I. Distribution of charophytes along the Swedish coast in relation to salinity and eutrophication. Int. Rev. Hydrobiol. 2000, 85, 707–717. [Google Scholar] [CrossRef]
- Thomaz, S.M.; da Cunha, E.R. The role of macrophytes in habitat structuring in aquatic ecosystems: Methods of measurements, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnol. Brasil. 2010, 22, 218–236. [Google Scholar] [CrossRef]
- Kolada, A. Charophyte variation in sensitivity to eutrophication affects their potential for the trophic and ecological status indication. Knowl. Manag. Aquat. Ecosyst. 2021, 422, 30. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jeong, K.S.; Kim, S.K.; La, G.H.; Chang, K.H.; Joo, G.J. Role of macrophytes as microhabitats for zooplankton community in lentic freshwater ecosystems of South Korea. Ecol. Inform. 2014, 24, 177–185. [Google Scholar] [CrossRef]
- Humphries, P. Aquatic macrophytes, macroinvertebrates associations and water levels in a lowland Tasmanian river. Hydrobiologia 1996, 321, 219–233. [Google Scholar] [CrossRef]
- Rezende, R.S.; Monçao, F.S.; Gonçalvez, J.F., Jr.; dos Santos, A.M. Macroinvertebrate associated with macrophyte beds in a Cerrado stream. Limnetica 2019, 38, 639–652. [Google Scholar] [CrossRef]
- Sánchez-Botero, J.I.; Leitao, R.P.; Caramaschi, E.P.; Garcez, D.S. The aquatic macrophytes as refuge, nursery and feeding habitats for freshwater fish from Cabiúnas Laggon, Restinga de Jurubatiba National Park, Rio de Janeiro, Brazil. Acta Limnol. Bras. 2007, 19, 143–153. [Google Scholar]
- Engelhardt, K.A.M.; Ritchie, M.E. Effects of macrophyte species richness on wetland ecosystem functioning and services. Nature 2001, 411, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Romanchuk, L.; Fedonyuk, T.; Pazych, V.; Fedonyuk, R.; Khant, G.; Petruk, A. Assessment of the stability of aquatic ecosystems development on the basis of indicators of the macrophytes fluctuating asymmetry. East. Eur. J. Entero. Technol. 2018, 4, 54–61. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, J.; Su, H.; Chen, J.; Rao, Q.; Yang, J.; Chou, Q.; Wang, L.; Deng, X.; Xie, P. Eutrophication decreases ecological resilience by reducing species diversity and altering functional traits of submerged macrophytes. Glob. Change Biol. 2023, 29, 5000–5013. [Google Scholar] [CrossRef]
- Bita-Nicolae, C. Distribution and conservation status of the mountain wetlands in the Romanian Carpathians. Sustainability 2022, 14, 16672. [Google Scholar] [CrossRef]
- Onaindia, M.; Amezaga, I.; Garcisu, C.; García-Bikuña, B. Aquatic macrophytes as biological indicators of environmental conditions of rivers in north-eastern Spain. Ann. Limnol. Int. J. Limnol. 2005, 41, 175–182. [Google Scholar] [CrossRef]
- Alahuhta, J.; Heino, J. Spatial extent, regional specificity and metacommunity structuring in lake macrophytes. J. Biogeogr. 2013, 40, 1572–1582. [Google Scholar] [CrossRef]
- Alahuhta, J.; Lindholm, M.; Bove, C.P.; Chappuis, E.; Clayton, J.; de Winton, M.; Feldmann, T.; Ecke, F.; Gacia, E.; Grillas, P.; et al. Global patterns in the metacommunity structuring of lake macrophytes: Regional variations and driving factors. Oecologia 2018, 188, 1167–1182. [Google Scholar] [CrossRef]
- García-Girón, J.; Wilkes, M.; Fernández-Aláez, M.; Fernández-Aláez, C. Processes structuring macrophyte metacommunities in Mediterranean ponds: Combining novel methods to disentangle the role of dispersal limitation, species sorting and spatial scales. J. Biogeogr. 2019, 46, 646–656. [Google Scholar] [CrossRef]
- García-Girón, J.; Heino, J.; García-Criado, F.; Fernández-Aláez, C.; Alahuhta, J. Biotic interactions hold the key to understanding metacommunity organization. Ecography 2020, 43, 1180–1190. [Google Scholar] [CrossRef]
- Lesiv, M.S.; Polishchuk, A.I.; Antonyak, H.L. Aquatic macrophytes: Ecological features and functions. Biol. Stud. 2020, 14, 79–94. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Presley, S.J.; Higgins, C.L.; Willig, M.R. A comprehensive framework for the evaluation of metacommunity structure. Oikos 2010, 119, 908–917. [Google Scholar] [CrossRef]
- Pérez-Martínez, C.; Rühland, K.M.; Smol, J.P.; Jones, V.J.; Conde-Porcuna, J.M. Long-term ecological changes in Mediterranean mountain lakes linked to recent climate change and Saharan dust deposition revealed by diatom analyses. Sci. Total Environ. 2020, 727, 138519. [Google Scholar] [CrossRef]
- Godoy, V.; Calero, M.; González-Olalla, J.M.; Martín-Lara, M.A.; Olea, N.; Ruiz-Gutiérrez, A.; Villar-Argáiz, M. The human connection: First evidence of microplastics in remote high mountain lakes of Sierra Nevada, Spain. Environ. Pollut. 2022, 311, 119922. [Google Scholar] [CrossRef]
- Cirujano, S.; Cambra, J.; Sánchez Castillo, P.M.; Meco, A.; Flor Arnau, N. Flora ibérica. Algas continentales. In Carófitos (Characeae), 1st ed.; Real Jardín Botánico, CSIC: Madrid, Spain, 2008. [Google Scholar]
- Cirujano Bracamonte, S.; Meco Molina, A.; García Murillo, P.; Chirino Argenta, M. Flora acuática Española. In Hidrófitos vasculares, 1st ed.; Real Jardín Botánico, CSIC: Madrid, Spain, 2014. [Google Scholar]
- Schubert, H.; Blindow, I.; Nat, E.; Korsch, H.; Gregor, T.; Denys, L.; Stewart, N.; van de Weyer, K.; Romanov, R.; Casanova, M.T. (Eds.) Charophytes of Europe, 1st ed.; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Hammer, Ø.; Harper, D.; Ryan, P. Paleontological statistics software: Package for educational and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Blanco, S.; Olenici, A.; Ortega, F.; Jiménez-Gómez, F.; Guerrero, F. Identifying environmental drivers of benthic diatom diversity: The case of Mediterranean mountain ponds. PeerJ 2019, 8, e8825. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; American Public Health Association (APHA): Washington, DC, USA; American Water Works Association (AWWA): Washington, DC, USA; Water Environment Federation (WEF): Washington, DC, USA, 2012. [Google Scholar]
- Dray, S.; Legendre, P.; Peres-Neto, P.R. Spatial modeling: A comprehensive framework for principal coordinate analysis of neighbor matrices (PCNM). Ecol. Mod. 2006, 196, 483–493. [Google Scholar] [CrossRef]
- McIntire, E.J.B.; Fajardo, A. Beyond description: The active and effective way to infer processes from spatial patterns. Ecology 2009, 90, 46–56. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011. [Google Scholar]
- Dray, S.; Pélissier, R.; Couteron, P.; Fortin, M.J.; Legendre, P.; Peres-Neto, P.R.; Bellier, E.; Bivand, R.; Blanchet, F.G.; De Cáceres, M.; et al. Community ecology in the age of multivariate multiscale spatial analysis. Ecol. Monogr. 2012, 82, 257–275. [Google Scholar] [CrossRef]
- Bellier, E.; Monestiez, P.; Durbec, J.P.; Candau, J.N. Identifiying spatial relationships at multiple scales: Principal coordinates of neighbour matrices (PCNM) and geostatistical approaches. Ecography 2007, 30, 385–399. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P.; Avois-Jacquet, C.; Tuomisto, H. Dissecting the spatial structure of ecological data at multiple scales. Ecology 2004, 85, 1826–1832. [Google Scholar] [CrossRef]
- Castillo-Escrivà, A.; Valls, L.; Rochera, C.; Camacho, A.; Mesquita-Joanes, F. Metacommunity dynamics of Ostracoda in temporary lakes: Overall strong niche effects except at the onset of the flooding period. Limnologica 2017, 62, 104–110. [Google Scholar] [CrossRef]
- Dray, S.; Bauman, D.; Blanchet, G.; Borcard, D.; Clappe, S.; Guénard, G.; Jombart, T.; Larocque, G.; Legendre, P.; Madi, N.; et al. R Package, Version 0.3-20. Adespatial: Multivariate Multiscale Spatial Analysis. 2022. Available online: https://cran.r-project.org/web/packages/adespatial/index.html (accessed on 1 July 2025).
- Leibold, M.A.; Mikkelson, G.M. Coherence, species turnover, and boundary clumping: Elements of meta-community structure. Oikos 2002, 97, 237–250. [Google Scholar] [CrossRef]
- Legendre, P.; Borcard, D.; Roberts, D.W. Variation partitioning involving orthogonal spatial eigenfunction submodels. Ecology 2012, 93, 1234–1240. [Google Scholar] [CrossRef]
- Gascón, S.; Arranz, I.; Cañedo-Argüelles, M.; Nebra, A.; Ruhí, A.; Rieradevall, M.; Caiola, N.; Sala, J.; Ibáñez, C.; Quintana, X.D.; et al. Environmental filtering determines metacommunity structure in wetland microcrustaceans. Oecologia 2016, 181, 193–205. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and evenness: Unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Dallas, T.; Presley, S.J. Relative importance of host environment, transmission potential and host phylogeny to the structure of parasite metacommunities. Oikos 2014, 123, 866–874. [Google Scholar] [CrossRef]
- Grönroos, M.; Heino, J.; Siqueira, T.; Landeiro, V.L.; Kotanen, J.; Bini, L.M. Metacommunity structuring in stream networks: Roles of dispersal mode, distance type, and regional environmental context. Ecol. Evol. 2013, 3, 4473–4487. [Google Scholar] [CrossRef]
- Göthe, E.; Baattrup-Pedersen, A.; Wiberg-Larsen, P.; Graeber, D.; Kristensen, E.A.; Friberg, N. Environmental and spatial controls of taxonomic versus trait composition of stream biota. Freshw. Biol. 2016, 62, 397–413. [Google Scholar] [CrossRef]
- Tolonen, K.T.; Vilmi, A.; Karjalainen, S.M.; Hellsten, S.; Sutela, T.; Heino, J. Ignoring spatial effects results in inadequate models for variation in littoral macroinvertebrate diversity. Oikos 2016, 126, 852–862. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward selection of explanatory variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 July 2025).
- Morisita, M. Composition of the I-index. Res. Popul. Ecol. 1971, 13, 1–27. [Google Scholar] [CrossRef]
- Vellend, M. Conceptual synthesis in community ecology. Quart. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Henriques-Silva, R.; Lindo, Z.; Peres-Neto, P.R. A community of metacommunities: Exploring patterns in species distribution across large geographical areas. Ecology 2013, 94, 627–639. [Google Scholar] [CrossRef]
- Alves, A.T.; Petsch, D.K.; Barros, F. Drivers of benthic metacommunity structure along tropical estuaries. Sci. Rep. 2020, 10, 1739. [Google Scholar] [CrossRef]
- Gilbert, J.D.; Márquez, F.J.; Guerrero, F. Assessing the zooplankton metacommunity (Branchiopoda and Copepoda) from Mediterranean wetlands in agricultural landscapes. Diversity 2023, 15, 362. [Google Scholar] [CrossRef]
- Ricotta, C. On beta diversity decomposition: Trouble shared is not trouble halved. Ecology 2010, 91, 1981–1983. [Google Scholar] [CrossRef]
- Williams-Subiza, E.A.; Brand, C.; Miserendino, M.L. Metacommunity structure analysis reveals nested patterns in deconstructed macroinvertebrates assemblages. Ecol. Entomol. 2020, 45, 1284–1295. [Google Scholar] [CrossRef]
- Heino, J.; Nokela, T.; Soininen, J.; Tolkkinen, M.; Virtanen, L.; Virtanen, R. Elements of metacommunity structure and community environment relationships in stream organisms. Freshw. Biol. 2015, 60, 973–988. [Google Scholar] [CrossRef]
- Heino, J.; Soininen, J.; Alahuhta, J.; Lappalainen, J.; Virtanen, R. A comparative analysis of metacommunity types in the freshwater realm. Ecol. Evol. 2015, 5, 1525–1537. [Google Scholar] [CrossRef]
- Valanko, S.; Heino, J.; Westerbom, M.; Viitasalo, M.; Norkko, A. Complex metacommunity structure for benthic invertebrates in a low diversity coastal system. Ecol. Evol. 2015, 5, 5203–5215. [Google Scholar] [CrossRef]
- García-Girón, J.; Heino, J.; Baastrup-Spohr, L.; Clayton, J.; de Winton, M.; Feldmann, T.; Fernández-Aláez, C.; Ecke, F.; Hoyer, M.V.; Kolada, A.; et al. Elements of lake macrophyte metacommunity structure. Global variation and community-environment relationships. Limnol. Oceanogr. 2020, 65, 2883–2895. [Google Scholar] [CrossRef]
- Tierno-Cinque, A.; Tierno de Figueroa, J.M.; Luzón-Ortega, J.M.; López-Rodríguez, J.M. Analysis of the elements of metacommunity structure in a Mediterranean basin: Implications in the framework of global change. Aquat. Sci. 2025, 87, 41. [Google Scholar] [CrossRef]
- Patterson, B.D.; Atmar, W. Nested subsets and the structure of insular mammalian faunas and archipelagos. Biol. J. Lin. Soc. 1986, 28, 65–82. [Google Scholar] [CrossRef]
- Andrew, S.M.; Totland, Ø.; Moe, S.R. Spatial variation in plant species richness and diversity along human disturbance and environmental gradients in a tropical wetland. Wetl. Ecol. Manage. 2015, 23, 395–404. [Google Scholar] [CrossRef]
- Datry, T.; Corti, R.; Heino, J.; Hugueny, B.; Rolls, R.J.; Ruhí, A. Habitat fragmentation and metapopulation, metacommunity, and metaecosystem dynamic in intermittent rivers and ephemeral streams. In Intermittent Rivers and Ephemeral Streams Ecology and Management, 1st ed.; Datry, T., Bonada, N., Boulton, A., Eds.; Academic Press: London, UK, 2017; pp. 377–403. [Google Scholar]
- Bender, M.G.; Leprieur, F.; Mouillot, D.; Kulbicki, M.; Parravicini, V.; Pie, M.R.; Barneche, D.R.; Oliviera-Santos, L.G.R.; Floeter, S.R. Isolation drives taxonomic and functional nestedness in tropical reef fish faunas. Ecography 2017, 40, 425–435. [Google Scholar] [CrossRef]
- Herkül, K.; Torn, K.; Möller, T. The environmental niche separation between charophytes and angiosperms in the northern Baltic Sea. Bot. Lett. 2018, 165, 115–127. [Google Scholar] [CrossRef]
- Nathan, R.; Schurr, F.M.; Spiegel, O.; Steinitz, O.; Trakhtenbrot, A.; Tsoar, A. Mechanisms of long-distance seed dispersal. Trends Ecol. Evol. 2008, 23, 638–647. [Google Scholar] [CrossRef]
- Nathan, R.; Muller-Landau, H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 2000, 15, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Hanski, I. Metapopulation dynamics. Nature 1998, 396, 41–49. [Google Scholar] [CrossRef]
- Parra, G.; Guerrero, F.; Armengol, J.; Brendonck, L.; Brucet, S.; Finlayson, C.M.; Gomes-Barbosa, L.; Grillas, P.; Jeppesen, E.; Ortega, F.; et al. The future of temporary wetlands in drylands under global change. Inland Waters 2021, 11, 445–456. [Google Scholar] [CrossRef]
- Connor, E.F.; McCoy, E.D. The statistics and biology of the species-area relationship. Am. Nat. 1979, 113, 791–833. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. An equilibrium theory of insular zoogeography. Evolution 1963, 17, 373–387. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Lönnell, N.; Hylander, K.; Jonsson, B.G.; Sundberg, S. The fate of the missing spores—Patterns of realized dispersal beyond the closest vicinity of a sporulating moss. PLoS ONE 2012, 7, e41987. [Google Scholar] [CrossRef]
- Baastrup-Spohr, L.; Iversen, L.L.; Borum, J.; Sand-Jensen, K. Niche specialization and functional traits regulate the rarity of charophytes in the Nordic countries. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 609–621. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Pedersen, N.L.; Thorsgaard, I.; Moeslund, B.; Borum, J.; Brodersen, K.P. 100 years of vegetation decline and recovery in Lake Fure, Denmark. J. Ecol. 2008, 96, 260–271. [Google Scholar] [CrossRef]
- Guerrero, F. Advances in mountain and Mediterranean wetlands conservation. Water 2021, 13, 1953. [Google Scholar] [CrossRef]
- Bernardo-Madrid, R.; González-Suárez, M.; Rosvall, M.; Rueda, M.; Revilla, E.; Carrete, M.; Tella, J.L.; Astigarraga, J.; Calatayud, J. A general rule on the organization of biodiversity in Earth’s biogeographical regions. Nat. Ecol. Evol. 2025. [Google Scholar] [CrossRef]
- Fornell Muñoz, A.; Guerrero, F. Mediterranean basin wetlands as a vertebral axis of the territory: Relationships with Roman roads and contemporary livestock trails. Nat. Cult. 2019, 14, 61–78. [Google Scholar] [CrossRef]
- Arnanz, C.; Arcolo, P.; Amador, P.; Azcárate, F.M.; Llusia, D.; Hevia, V. Exploring the role of a Mediterranean transhumance drove road as shelter for amphibian breeding. Anthropocene 2025, 50, 100469. [Google Scholar] [CrossRef]
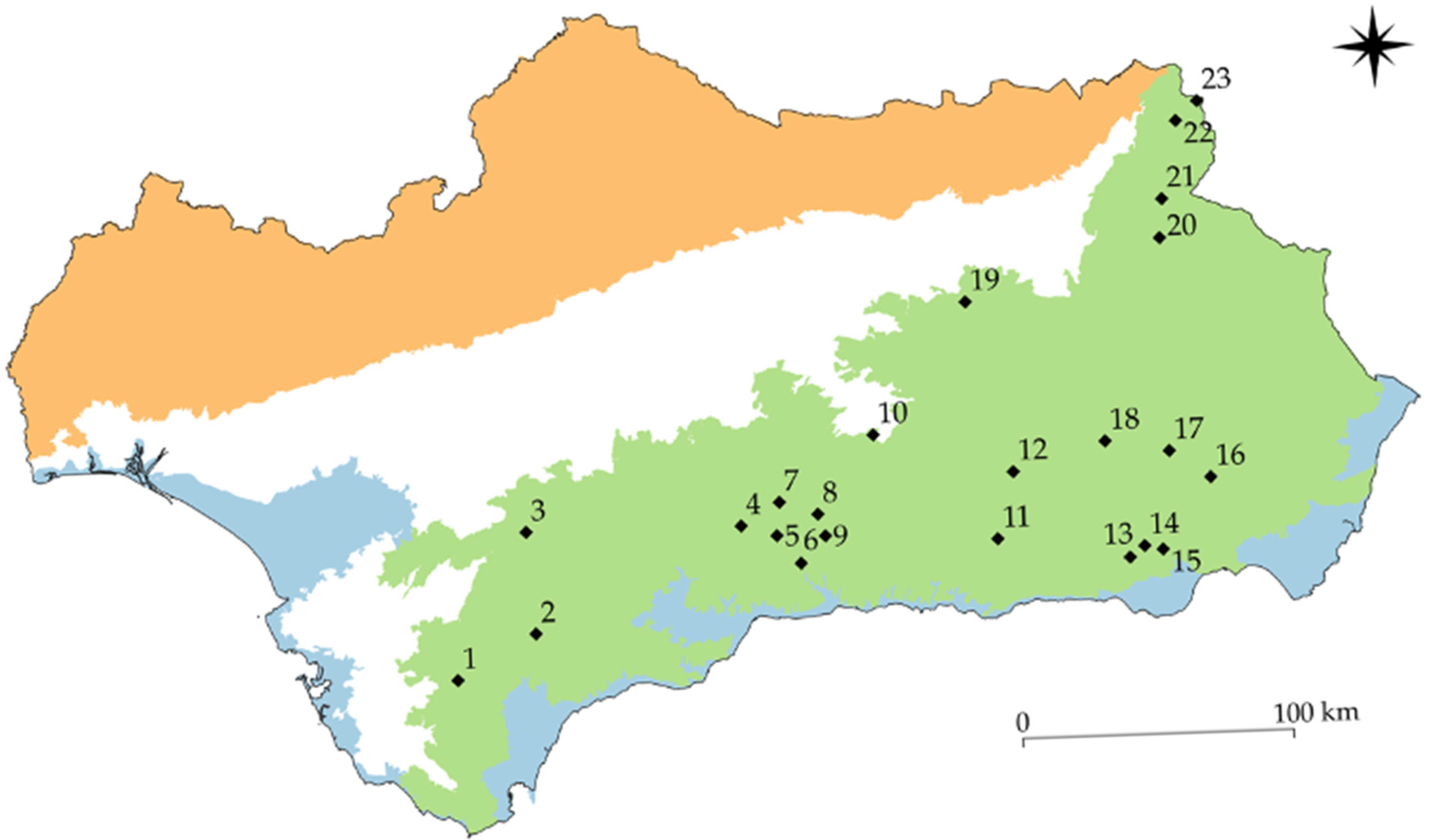

| Ponds |
EC (mS cm−1) |
Turbidity (NTU) |
D (m) |
WS (m2) |
Alt (m) | TS | Hydroperiod |
TP (µg L−1) |
TN (mg L−1) | Pond Isolation |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.21 | 6.94 | 1.5 | 2026.4 | 538 | S | T | 147.23 | 2.01 | 0.75/0.90/1.00 |
| 2 | 0.58 | 3.58 | 3 | 380.65 | 970 | L | P | 62.73 | 0.32 | 0.49/0.64/-- |
| 3 | 0.62 | 45.33 | 2 | 17,908.9 | 406 | T | T | 391.87 | 4.85 | 0.36/0.47/-- |
| 4 | 0.86 | 137 | 3 | 59,161.22 | 729 | T | T | 263.55 | 2.31 | 0.67/0.72/0.33 |
| 5 | 0.33 | 3.90 | 0.75 | 913.68 | 1162 | L | T | 19.95 | 0.57 | 1.00/1.00/-- |
| 6 | 0.94 | 3.76 | 2.5 | 551.17 | 387 | T | T | 16.30 | 0.37 | 0.87/--/0.38 |
| 7 | 10.4 | 1.93 | 5 | 67,113.49 | 794 | T | P | 25.69 | 1.82 | 0.91/0.99/0.38 |
| 8 | 0.15 | 2.36 | 1 | 2336.88 | 1347 | L | T | 22.56 | 0.84 | 0.85/0.89/-- |
| 9 | 0.34 | 8.17 | 2.5 | 5752.28 | 896 | L | P | 2428.83 | 3.08 | 0.78/0.78/0.27 |
| 10 | 0.29 | 3.51 | 0.4 | 17.34 | 1317 | L | P | 80.46 | 2.57 | 0.23/0.27/-- |
| 11 | 0.11 | 5.46 | 1.5 | 1197.69 | 1775 | S | P | 53.34 | 0.48 | 0.13/0.15/-- |
| 12 | 0.03 | 1.63 | 0.2 | 22.74 | 2068 | S | T | 77.59 | 0.45 | 0.15/0.19/-- |
| 13 | 0.12 | 23.27 | 2.5 | 4787.22 | 1830 | L | T | 110.71 | 1.01 | 0.58/0.68/0.25 |
| 14 | 0.13 | 61 | 1.5 | 4084.82 | 1713 | L | T | 175.40 | 1.55 | 0.68/0.79/0.30 |
| 15 | 0.28 | 67 | 2.5 | 1513.04 | 1340 | L | P | 45.51 | 0.52 | 0.67/0.78/0.29 |
| 16 | 0.18 | 72 | 0.5 | 180.06 | 1050 | S | T | 712.67 | 8.82 | 0.42/0.48/0.16 |
| 17 | 0.07 | 3.73 | 0.6 | 51.81 | 1892 | S | P | 37.17 | 1.2 | 0.43/0.49/0.17 |
| 18 | 1.56 | 2.60 | 1.25 | 283.71 | 1275 | L | P | 13.17 | 0.73 | 0.30/0.34/0.10 |
| 19 | 0.31 | 1.54 | 2 | 265.15 | 1340 | L | P | 12.13 | 0.26 | 0.00/0.00/0.00 |
| 20 | 0.28 | >1000 | 0.5 | 959.03 | 1967 | L | T | 3310.38 | 28.54 | 0.30/0.33/-- |
| 21 | 0.42 | 41.27 | 2 | 229.71 | 1661 | L | P | 104.46 | 1.94 | 0.47/0.54/0.09 |
| 22 | 0.35 | 8.09 | 2 | 4669.39 | 1264 | L | T | 110.45 | 4.02 | 0.83/0.95/0.28 |
| 23 | 0.25 | 6.50 | 2.75 | 4406.9 | 1288 | L | T | 14.21 | 0.54 | 0.81/0.93/0.30 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Charophytes | |||||||||||||||||||||||
| Chara connivens | x | x | |||||||||||||||||||||
| Chara curta | x | ||||||||||||||||||||||
| Chara globularis | x | x | x | x | x | x | x | ||||||||||||||||
| Chara vulgaris | x | x | x | x | x | x | |||||||||||||||||
| Nitella flexilis | x | ||||||||||||||||||||||
| Nitella syncarpa | x | ||||||||||||||||||||||
| Nitella translucens | x | ||||||||||||||||||||||
| Sphaerochara prolifera | x | x | x | ||||||||||||||||||||
| Tolypella glomerata | x | ||||||||||||||||||||||
| Tolypella hispanica | x | x | |||||||||||||||||||||
| Vascular plants | |||||||||||||||||||||||
| Baldellia ranunculoides | x | x | |||||||||||||||||||||
| Callitriche lusitanica | x | ||||||||||||||||||||||
| Callitriche stagnalis | x | x | |||||||||||||||||||||
| Dasmasomium polispermum | x | x | |||||||||||||||||||||
| Myriophylum alterniflorum | x | ||||||||||||||||||||||
| Myriophylum spicatum | x | x | |||||||||||||||||||||
| Polypogum amphibium | x | ||||||||||||||||||||||
| Potamogeton berchtoldii | x | ||||||||||||||||||||||
| Potamogeton pectinatus | x | ||||||||||||||||||||||
| Potamogeton polygonifolius | x | ||||||||||||||||||||||
| Potamogeton pusillus | x | ||||||||||||||||||||||
| Rannunculus hederaceus | x | ||||||||||||||||||||||
| Rannunculus trychophylus | x | x | x | x | x | x | |||||||||||||||||
| Rannunculus peltatus peltatus | x | x | x | x | x | x | x | x | |||||||||||||||
| Zannichellia contorta | x | ||||||||||||||||||||||
| Zanichellia pedunculata | x | ||||||||||||||||||||||
| Zanichellia peltata | x | ||||||||||||||||||||||
| Zannichellia palustris | x | x |
| Total | Seeds | Spores | ||
|---|---|---|---|---|
| Coherence | embAbs | 57 | 6 | 18 |
| Coh Z | −9.84 | −5.83 | −3.72 | |
| p | <0.001 | <0.001 | 0.002 | |
| Sim mean | 275 | 112 | 45 | |
| Sim sd | 22 | 18 | 8 | |
| Turnover | Turnover | 1801 | 467 | 203 |
| Tur Z | −0.52 | −0.65 | −0.92 | |
| p | 0.610 | 0.517 | 0.367 | |
| Sim mean | 1845 | 480 | 236 | |
| Sim sd | 85 | 21 | 37 | |
| Boundary clumping | Index | 1.50 | 1.17 | 0.87 |
| p | 0.002 | 0.175 | 0.384 | |
| df | 20 | 19 | 12 |
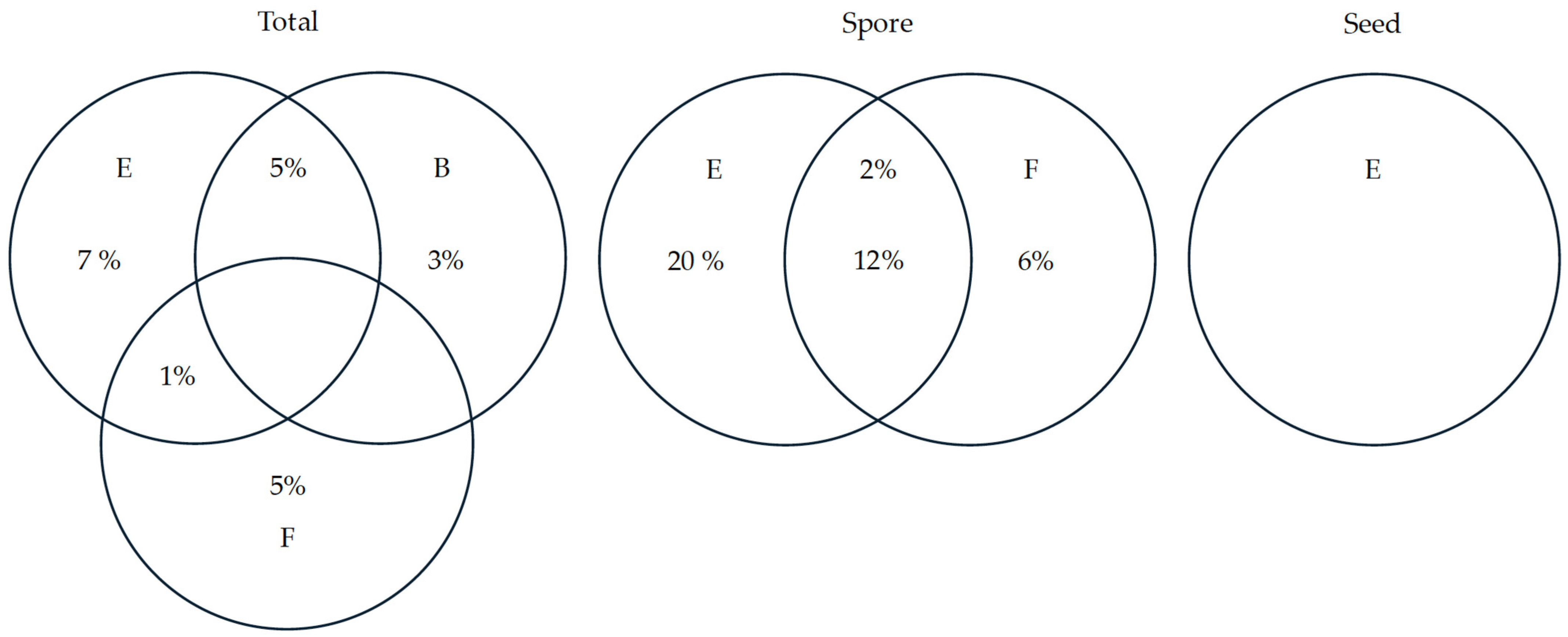
| Fraction | df | Adj. R2 | Variables | |
|---|---|---|---|---|
| Total | E | 2 | 0.13 | Hydroperiod and size |
| B | 1 | 0.08 | MEM1 and MEM2 | |
| F | 1 | 0.06 | MEM20 | |
| E ∩ B | 4 | 0.05 | Hydroperiod, size, MEM1 and ME2 | |
| E ∩ F Residuals | 3 -- | 0.01 0.67 | Hydroperiod, size and MEM20 -- | |
| Spore | E | 3 | 0.32 | Hydroperiod, isolation and size |
| F | 2 | 0.18 | MEM3 and MEM12 | |
| E ∩ F Residuals | 5 -- | 0.12 0.38 | Hydroperiod, isolation, size, MEM3 and MEM12 -- | |
| Seed | E Residuals | -- -- | -- -- | Hydroperiod, substrate and elevation -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero, F.; Ortega, F.; García-Rodríguez, G.; Gilbert, J.D. Diversity and Metacommunity Structure of Aquatic Macrophytes: A Study in Mediterranean Mountain Wetlands. Sustainability 2025, 17, 6103. https://doi.org/10.3390/su17136103
Guerrero F, Ortega F, García-Rodríguez G, Gilbert JD. Diversity and Metacommunity Structure of Aquatic Macrophytes: A Study in Mediterranean Mountain Wetlands. Sustainability. 2025; 17(13):6103. https://doi.org/10.3390/su17136103
Chicago/Turabian StyleGuerrero, Francisco, Fernando Ortega, Gema García-Rodríguez, and Juan Diego Gilbert. 2025. "Diversity and Metacommunity Structure of Aquatic Macrophytes: A Study in Mediterranean Mountain Wetlands" Sustainability 17, no. 13: 6103. https://doi.org/10.3390/su17136103
APA StyleGuerrero, F., Ortega, F., García-Rodríguez, G., & Gilbert, J. D. (2025). Diversity and Metacommunity Structure of Aquatic Macrophytes: A Study in Mediterranean Mountain Wetlands. Sustainability, 17(13), 6103. https://doi.org/10.3390/su17136103








