Biochar, Compost, and Effective Microorganisms: Evaluating the Recovery of Post-Clay Mining Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experiment
- BEM—Biochar (B) derived from pine wood (Cupressus sp.) at a rate of 12 tons per hectare, combined with effective microorganisms (EMs);
- CEM—Commercial compost (C) at a rate of 12 tons per hectare, also combined with Ems;
- BCEM—A mixture of both amendments, consisting of 50% biochar and 50% compost, along with Ems;
- Control (CT)—A group without any amendments or EMs added.
2.3. Statistical Analysis
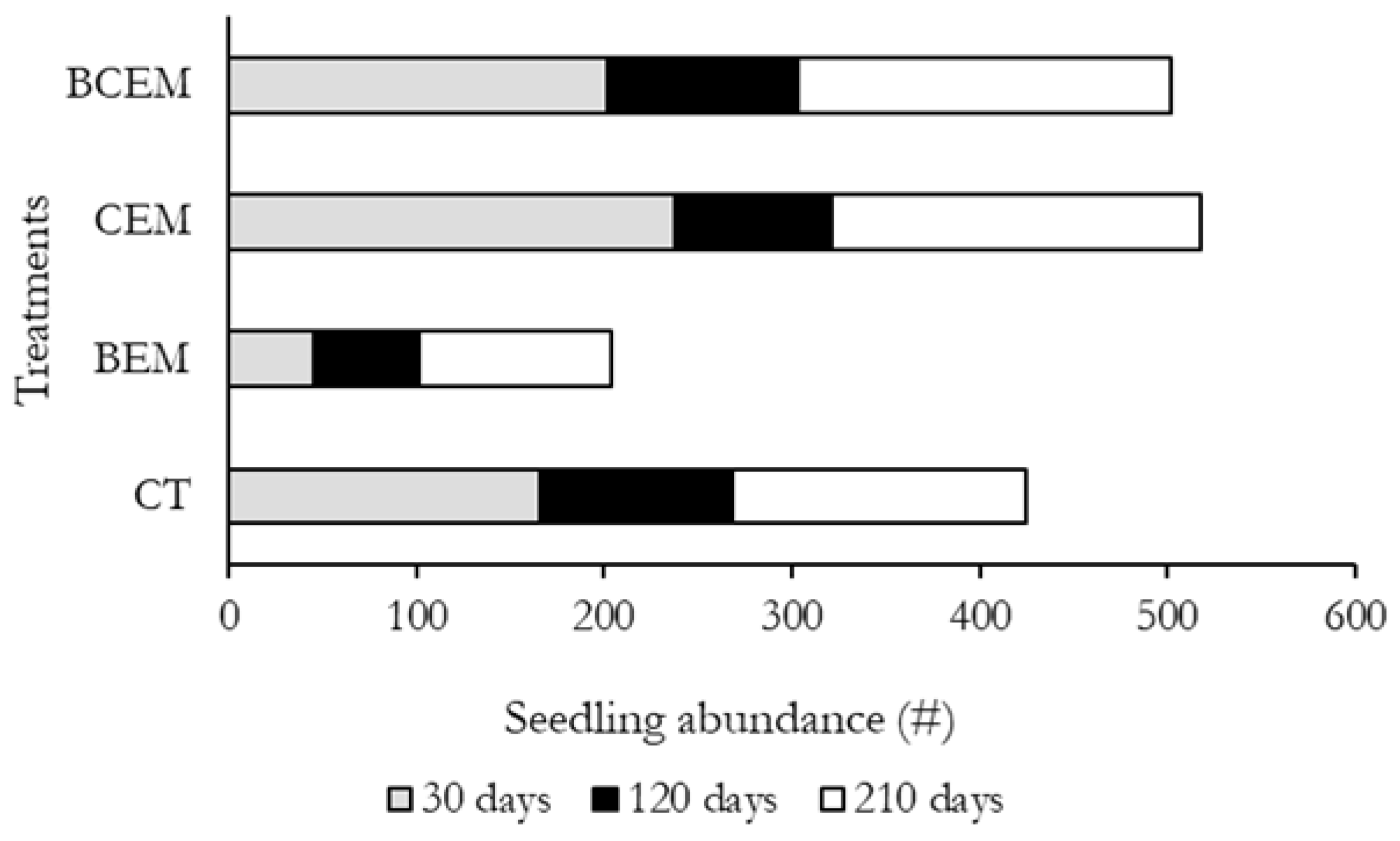
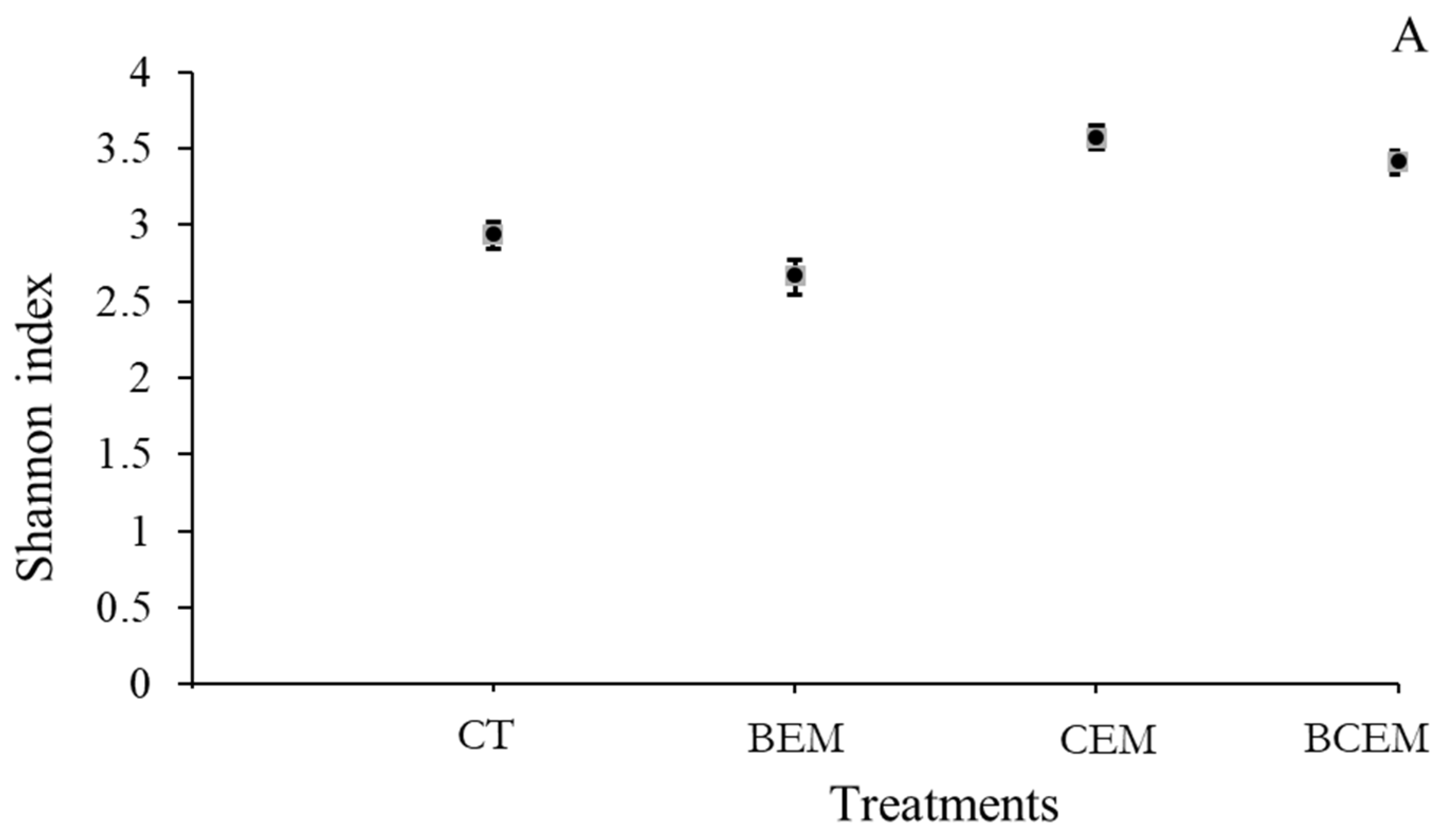
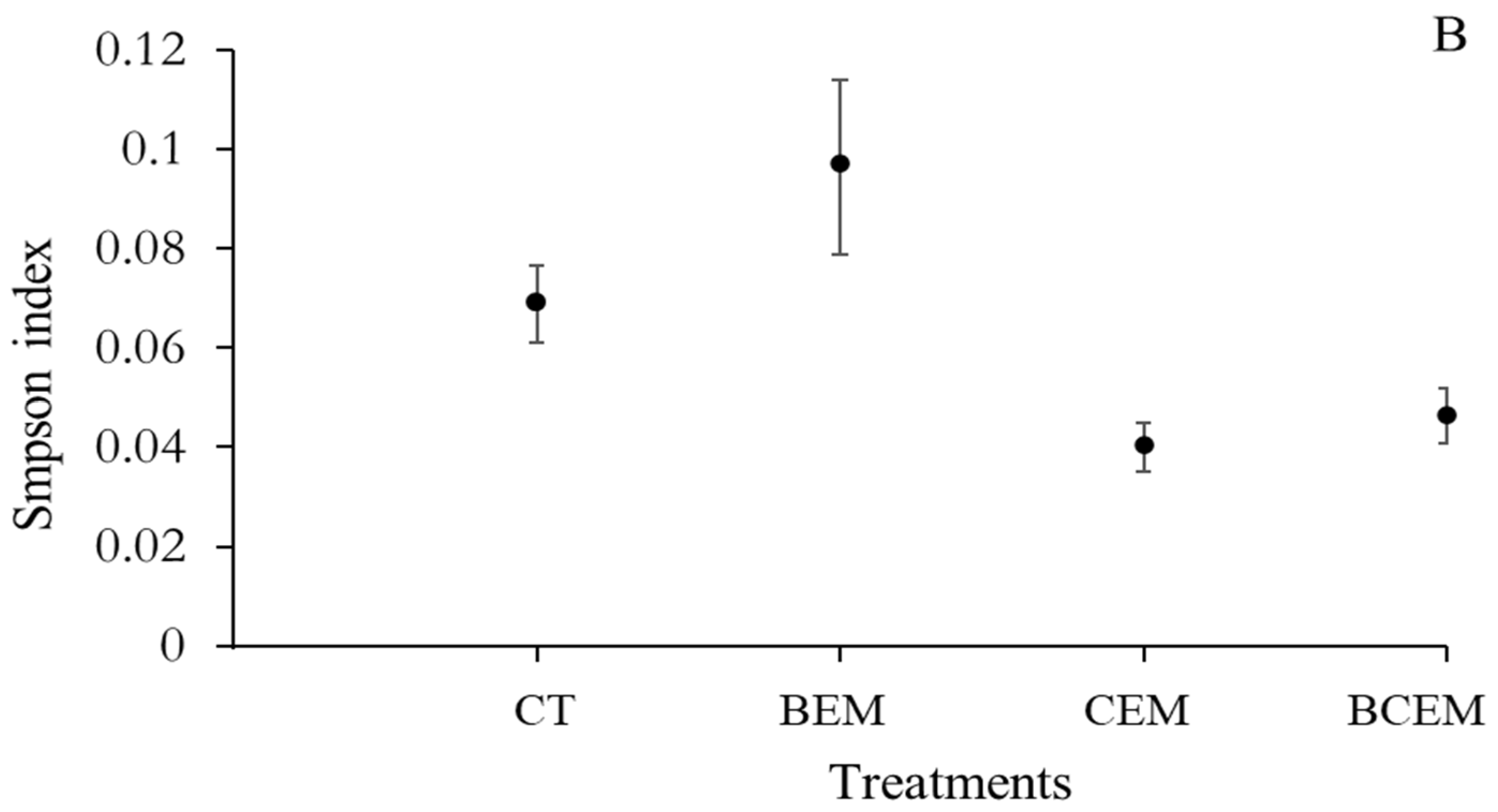
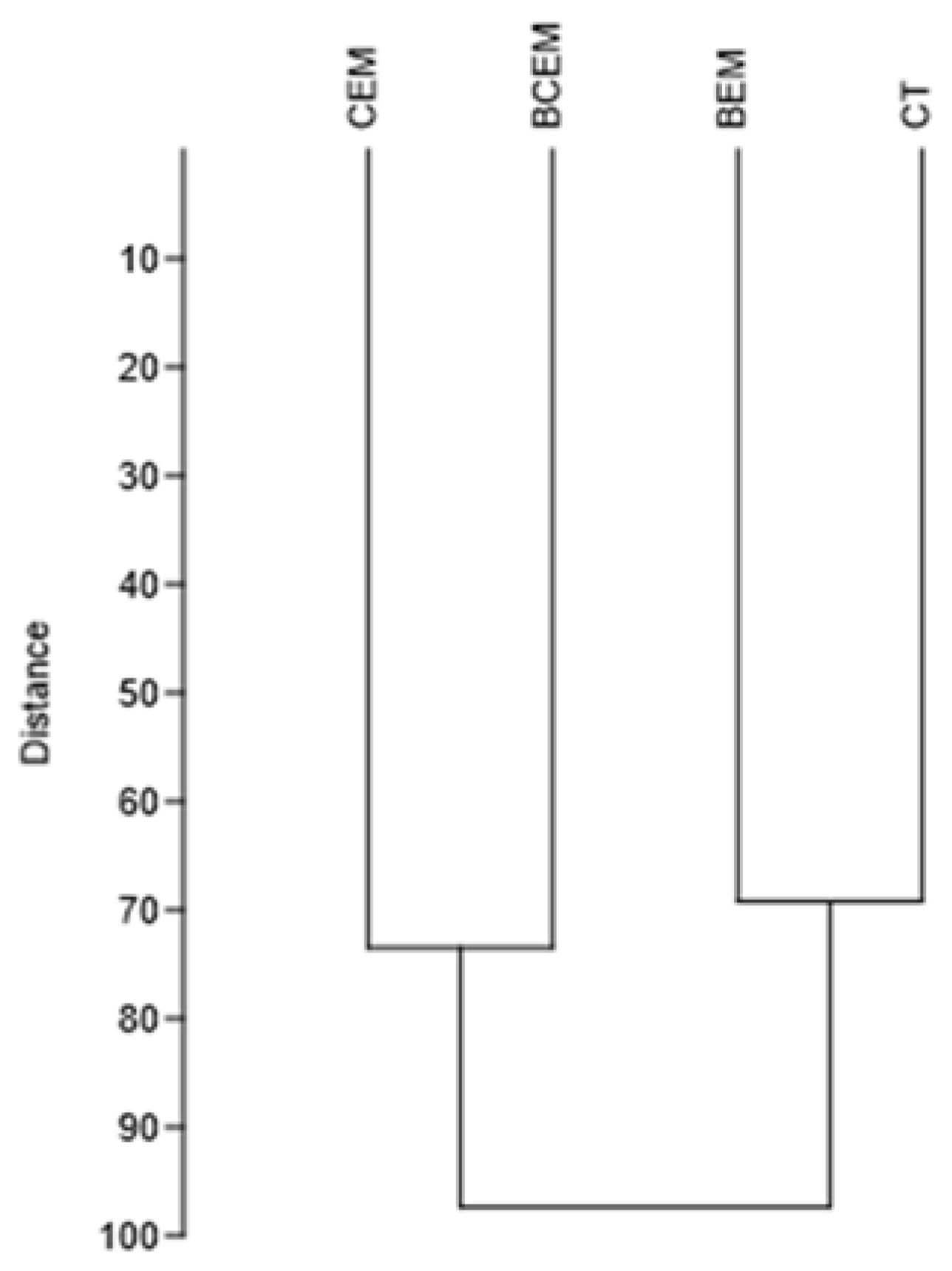
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, S.E.; Shahriar, M.M.; Nahar, N.; Haque, M.S. Impact of brick kiln emissions on soil quality: A case study of Ashulia brick kiln cluster, Bangladesh. Environ. Chall. 2022, 9, 100640. [Google Scholar] [CrossRef]
- Madavi, A.B. Environmental Impact of Bricks Making Process in Umbraj and Masur Area. In Environment Conservation, Challenges Threats in Conservation of Biodiversity; Barwant, M.M., Nanam, V.K., Eds.; Scieng Publications: Marakkanam, India, 2022; Volume III, pp. 196–202. [Google Scholar]
- Worlanyo, A.S.; Jiangfeng, L. Evaluating the environmental and economic impact of mining for post-mined land restoration and land-use: A review. J. Environ. Manag. 2021, 279, 111623. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Harris, J.; Zhang, Z. Responses of natural microorganisms to land reclamation and applications of functional microorganisms in biorestoration of coal mining area. Diversity 2024, 16, 86. [Google Scholar] [CrossRef]
- Hu, X.F.; Jiang, Y.; Shu, Y.; Hu, X.; Liu, L.; Luo, F. Effects of mining wastewater discharges on heavy metal pollution and soil enzyme activity of the paddy fields. J. Geochem. Explor. 2014, 147, 139–150. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Gao, Y.; Tan, X.; Sun, Y.; Chen, W. Effects of coal mining activities on the changes in microbial community and geochemical characteristics in different functional zones of a deep underground coal mine. Water 2024, 16, 1836. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Yadav, S.K.; Thawale, P.R.; Kumar, P.; Singh, S.K.; Chakrabarti, T. Developmental strategies for sustainable ecosystem on mine spoil dumps: A case of study. Environ. Monit. Assess. 2009, 157, 471–481. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Nandillon, R.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Biochar effect associated with compost and iron to promote Pb and As soil stabilization and Salix viminalis L. growth. Chemosphere 2019, 222, 810–822. [Google Scholar] [CrossRef]
- Quian, S.; Zhou, X.; Fu, Y.; Song, B.; Yan, H.; Chen, Z.; Sun, Q.; Ye, H.; Qin, L.; Lai, C. Biochar-compost as a new option for soil improvement: Application in various problem soils. Sci. Total Environ. 2023, 870, 15013–15032. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Hattab-Hambli, N.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Effect of different tissue biochar amendments on As and Pb stabilization and phytoavailability in a contaminated mine technosol. Sci. Total Environ. 2020, 707, 135657. [Google Scholar] [CrossRef]
- Zeng, Y.; Farooq, T.H.; Yuan, C.; Li, W.; Farooq, A.; Wang, G.; Fang, Y.; Wang, J.; Yan, W. Organic-based remediation of heavy metal-contaminated soils in the Taojia river basin affected by long-term non-ferrous mining and logging activities. Front. Plant Sci. 2025, 16, 1486575. [Google Scholar] [CrossRef]
- Asensio, V.; Vega, F.A.; Covelo, F. Changes in the phytoavailability of nutrients in mine soils after planting trees and amending with wastes. Water Air Soil Pollut. 2014, 225, 995. [Google Scholar] [CrossRef]
- Varkolu, M.; Gundekari, S.; Palla, V.C.; Kumar, P.; Bhattacharjee, S.; Vinodkumar, T. Recent advances in biochar production, characterization, and environmental applications. Catalysts 2025, 15, 243. [Google Scholar] [CrossRef]
- Sayara, T.; Hawamde, F.; Sánchez, A. Recycling of organic wastes through composting: Process performance and compost application in agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, C.; Thami Alami, I. Composting parameters and compost quality: A literature review. Org. Agr. 2018, 8, 141–158. [Google Scholar] [CrossRef]
- Thiery, E.; Brunschwig, G.; Veysset, P.; Mosnier, C. Estimation of short-and long-term floor and ceiling prices for manure in a crop and livestock farms exchange. Renew. Agric. Food Syst. 2023, 38, e21. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Cayuela, M.L.; Sánchez-García, M.; Vandecasteele, B.; D’Hose, T.; López, G.; Martínez-Gaitán, C.; Kuikman, P.J.; Sinicco, T.; Mondini, C. Agronomic evaluation of biochar, compost and biochar-blended compost across different cropping systems: Perspective from the European project FERTIPLUS. Agronomy 2019, 9, 225. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Ahmad Bhat, S.; Kuriqi, A.; Dar, M.U.D.; Bhat, O.; Sammen, S.S.; Towfiqul Islam, A.R.M.; Elbeltagi, A.; Shah, O.; AI-Ansari, N.; Ali, R.; et al. Application of biochar for improving physical, chemical, and hydrological soil properties: A systematic review. Sustainability 2022, 14, 11104. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Isotherm, kinetics, thermo-dynamics studies and effects of carbonization temperature on adsorption of Indigo Carmine (IC) dye using C. odorata biochar. Chem. Data Collect. 2021, 33, 100673. [Google Scholar] [CrossRef]
- Garau, M.; Pinna, M.V.; Nieddu, M.; Castaldi, P.; Garau, G. Mixing compost and biochar can enhance the chemical and biological recovery of soils contaminated by potentially toxic elements. Plants 2024, 13, 284. [Google Scholar] [CrossRef]
- Ajibade, S.; Nnadozie, E.C.; Iwai, C.B.; Ghotekar, S.; Chang, S.W.; Ravindran, B.; Awasthi, M.K. Biochar-based compost: A bibliometric and visualization analysis. Bioengineered 2022, 3, 15013–15032. [Google Scholar] [CrossRef] [PubMed]
- Maftukhah, R.; Keiblinger, K.M.; Ngadisih, N.; Murtiningrum, M.; Kral, R.M.; Mentler, A.; Hood-Nowotny, R. Post-Tin-mining agricultural soil regeneration using local organic amendments improve nitrogen fixation and uptake in a legume–cassava intercropping system. Land 2023, 12, 1107. [Google Scholar] [CrossRef]
- Kaudal, B.B.; Weatherley, A.J. Agronomic effectiveness of urban biochar aged through co-composting with food waste. Waste Manag. 2018, 77, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Vamvuka, D.; Esser, K.; Komnitsas, K. Investigating the suitability of grape husks biochar, municipal solid wastes compost and mixtures of them for agricultural applications to mediterranean soils. Resources 2020, 9, 33. [Google Scholar] [CrossRef]
- Sommerville, P.D.; Farrell, C.; May, P.B.; Livesley, S.J. Tree water use strategies and soil type determine growth responses to biochar and compost organic amendments. Soil Till. Res. 2019, 192, 12–21. [Google Scholar] [CrossRef]
- Joshi, H.; Somduttand, C.P.; Mundra, S.L. Role of Effective Microorganisms (EM) in sustainable agriculture. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 172–181. [Google Scholar] [CrossRef]
- de Araujo Avila, G.M.; Gabardo, G.; Clock, D.C.; de Lima Junior, O.S. Use of efficient microorganisms in agriculture. Res. Soc. Dev. 2021, 10, e40610817515. [Google Scholar] [CrossRef]
- Higa, T.; Parr, J.F. Beneficial and Effective Microorganisms for Sustainable Agriculture and Environment; International Nature Farming Research Center: Atami, Japan, 1994; p. 4. [Google Scholar]
- Morocho, M.T.; Leiva-Mora, M. Efficient microorganisms, functional properties and agricultural applications. Cent. Agrícola 2019, 46, 93–103. [Google Scholar]
- Schenck zu Schweinsberg-Mickan, M.; Müller, T. Impact of effective microorganisms and other biofertilizers on soil microbial characteristics, organic-matter decomposition and plant growth. J. Plant Nutr. Soil Sci. 2009, 172, 704–712. [Google Scholar] [CrossRef]
- Weatherspark 2024. Available online: https://es.weatherspark.com/y/24295/Clima-promedio-en-Cogua-Colombia-durante-todo-el-a%C3%B1o (accessed on 23 October 2024).
- IGAC—Institute Geográfico Agustín Codazzi. Detailed Soil Survey in Flat Areas of Municipalities of Cogua, El Rosal, Nemocón, Subachoque, Suesca, Zipacón and Zipaquirá in the Department of Cundinamarca; Instituto Geográfico Agustín Codazzi: Bogotá, Colombia, 2013; pp. 70, 85, 96, 154. [Google Scholar]
- Andrades Rodríguez, M. Edaphology and Climatology Practices; University of la Rioja: Logroño, España, 2012; pp. 13–16, 19–22, 33–36. [Google Scholar]
- USDA Soil Science Division Staff. Soil survey manual. In Agriculture Handbook No. 18, 4th ed.; USDA: Washington, DC, USA, 2017; pp. 120–125. [Google Scholar]
- Cremona, M.V.; Enriquez, A.S. Some soil properties that condition its behaviour: pH and electric conductivity. Presencia 2020, 73, 5–8. [Google Scholar]
- Hao, X.; Ball, B.C.; Culley, J.B.L.; Cater, M.R.; Parkin, G.W. Soil density and porosity. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 749–751. [Google Scholar]
- Williams, J.M.; Thomas, S.C. Effects of high-carbon wood ash biochar on volunteer vegetation establishment and community composition on metal mine tailings. Restor. Ecol. 2022, 31, e13861. [Google Scholar] [CrossRef]
- Nikita, E. Statistical Methods in Human Osteology. In Osteoarchaeology. A Guide to the Macroscopic Study of Human Skeletal Remains, 1st ed.; Nikita, E., Ed.; Academic Press: London, UK, 2017; pp. 375–376. [Google Scholar]
- Moreno, C.E.; Barragán, F.; Pineda, E.; Pavón, N.P. Reanalyzing alpha diversity: Alternatives to understand and compare information about ecological communities. Rev. Mex. Biodivers. 2011, 82, 1249–1261. [Google Scholar]
- Moreno, C.E. Methods for biodiversity measurement. In M&T SEA, 1st ed.; Sociedad Entomológica Aragonesa: Zaragoza, España, 2001; Volume 1, pp. 41–46, 56–57. [Google Scholar]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson Education, Inc.: Columbus, OH, USA, 2017; pp. 386–389. [Google Scholar]
- Pritchett, W. Forest Soils: Properties, Conservation and Improvement; Editorial Limusa S.A.: Ciudad de México, México, 1990; pp. 132–213. [Google Scholar]
- Porta, J.; López-Acevedo, M.; Roquero, C. Edaphology for Agriculture and Environment, 3rd ed.; Mundi-Prensa: Madrid, España, 2003; pp. 93–99. [Google Scholar]
- Panagos, P.; De Rosa, D.; Liakos, L.; Labouyrie, M.; Borrelli, P.; Ballabio, C. Soil bulk density assessment in Europe. Agric. Ecosyst. Environ. 2024, 364, 108907. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Shahzad, B.; Yang, G.; Fahad, S.; Ali, S.; Bukhari, M.A.; Tung, S.A.; Hafeez, A.; Souliyanonh, B. Soil compaction effects on soil health and crop productivity: An overview. Environ. Sci. Pollut. Res. 2017, 24, 10056–10067. [Google Scholar] [CrossRef]
- Fitzpatrick, E.A. Soils, Its Origin, Classification and Distribution; Compañía Editorial Compañia Editorial Continental, S.A.: Ciudad de México, México, 1987; p. 230. [Google Scholar]
- Adusu, D.; Abugre, S.; Dei-Kusi, D. Potential of biochar for minesoil amendment and floristic diversity enhancement at the Yongwa quarry site in the eastern region of Ghana. Agric. Sci. Dig. 2020, 41, 61–65. [Google Scholar] [CrossRef]
- Banu, M.R.; Rani, B.; Kavya, S.R.; Nihala Jabin, P.P. Biochar: A black carbon for sustainable agriculture. Int. J. Environ. Clim. Change 2023, 13, 418–432. [Google Scholar] [CrossRef]
- Haider, F.U.; Coulter, J.A.; Liqun, C.A.I.; Hussain, S.; Cheema, S.A.; Jun, W.U.; Zhang, R. An overview on biochar production, its implications, and mechanisms of biochar-induced amelioration of soil and plant characteristics. Pedosphere 2022, 32, 107–130. [Google Scholar] [CrossRef]
- Castellini, M.; Giglio, L.; Niedda, M.; Palumbo, A.D.; Ventrella, D. Impact of biochar addition on the physical and hydraulic properties of a clay soil. Soil Till. Res. 2015, 154, 1–13. [Google Scholar] [CrossRef]
- Anyebe, O.; Sadiq, F.K.; Manono, B.O.; Matsika, T.A. Biochar characteristics and application: Effects on soil ecosystem services and nutrient dynamics for enhanced crop yields. Nitrogen 2025, 6, 31. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, G.; Feng, H.; Sun, B.; Zhao, Y.; Chen, H.; Chen, J.; Dyck, M.; Wang, X.; Zhang, J.; et al. Effects of straw and biochar amendments on aggregate stability, soil organic carbon, and enzyme activities in the Loess Plateau, China. Environ. Sci. Pollut. Res. 2017, 24, 10108–10120. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Gundale, M.J.; Nilsson, M.C.; Pluchon, N.; Wardle, D.A. The effect of biochar management on soil and plant community properties in a boreal forest. Glob. Change Biol. Bioenergy 2016, 8, 777–789. [Google Scholar] [CrossRef]
- Ochoa-Carreño, A.C.; Barrera-Cataño, J.I. Effect of the application of biosolids on the development of vegetation in the first successional stages, in the Soratama quarry, Usaquén, Bogotá. Univ. Sci. 2007, 12, 57–72. [Google Scholar]
- Gutiérrez Acevedo, E.; Cortés Pérez, F.; Gómez Albarran, N.L. Compost as inductor of vegetal succession in an area affected by open mining in rio La Vega basin, Tunja, Boyacá. Colom. For. 2015, 18, 241–254. [Google Scholar] [CrossRef]
- Spargo, A.; Doley, D. Selective coal mine overburden treatment with topsoil and compost to optimize pasture or native vegetation establishment. J. Environ. Manag. 2016, 182, 342–350. [Google Scholar] [CrossRef]
- Sizmur, T.; Wingate, J.; Hutchings, T.; Hodson, M. Lumbricus terrestris L. does not impact on the remediation efficiency of compost and biochar amendments. Pedobiologia 2011, 54, 211–216. [Google Scholar] [CrossRef]
- Zhu, S.-C.; Zheng, H.-X.; Liu, W.-S.; Liu, C.; Guo, M.-N.; Huo, H.; Morel, J.L.; Qiu, R.-L.; Chao, Y.; Tang, Y.-T. Plant-soil feedbacks for the restoration of degraded mine lands: A review. Front. Microbiol. 2022, 12, 751794. [Google Scholar] [CrossRef]
- Li, S.; Liber, K. Influence of different revegetation choices on plant community and soil development nine years after initial planting on a reclaimed coal gob pile in the Shanxi mining area, China. Sci. Total Environ. 2018, 618, 1314–1323. [Google Scholar] [CrossRef]
- Sun, X.; Kong, T.; Ha, M.M.; Kolton, M.; Li, F.; Dong, Y.; Huang, Y.; Li, B.; Sun, W. Chemolithoautotropic diazotrophy dominates the nitrogen fixation process in mine tailings. Environ. Sci. Technol. 2020, 54, 6082–6093. [Google Scholar] [CrossRef]
- Wikskandar, W.; Ajidirman, A. Effect of biochar and Tithonia compost on physical properties of post-coal mining soil. J. Degrad. Min. Lands Manag. 2024, 11, 5829–5838. [Google Scholar] [CrossRef]
- Ghanbari, J.; Khajoei-Nejad, G. The effect of compost and combination of compost and biochar application in soil bulk density of planting bed, seedling emergence rate and early growth of saffron ecotypes. Saffron Agron. Technol. 2018, 6, 17–33. [Google Scholar] [CrossRef]
- Malińska, K.; Zabochnicka, M.; Dach, J. Effects of biochar amendment on ammonia emission during composting of sewage sludge. Ecol. Eng. 2014, 71, 474–478. [Google Scholar] [CrossRef]
- Mikajlo, I.; Lerch, T.Z.; Louvel, B.; Hynšt, J.; Záhora, J.; Pourrut, B. Composted biochar versus compost with biochar: Effects on soil properties and plant growth. Biochar 2024, 6, 85. [Google Scholar] [CrossRef]
- Gale, N.V.; Thomas, S.C. Dose-dependence of growth and ecophysiological responses of plants to biochar. Sci. Total Environ. 2019, 658, 1344–1354. [Google Scholar] [CrossRef] [PubMed]

| Physical/Chemical Characteristics | Experimental Soil | Compost | Biochar |
|---|---|---|---|
| Bulk density (g/cm3) | 2.37 | 0.58 | 0.30 |
| Organic matter (%) | 12.9 | 24.48 | 15.79 |
| Humidity (%) | 37.6 | 40.21 | 5.20 |
| pH | 5.7 | 6.8 | 8.6 |
| Corg (%) | 17.4 | 22.60 | 34.42 |
| Ntot (%) | 1.1 | 1.40 | 0.70 |
| P (mg/kg) | 4.0 | 3.00 | 10.33 |
| CEC (cmol + /kg) | 26.9 | 29.50 | 28.60 |
| Ca (cmol/kg) | 18.5 | 39.67 | 4.30 |
| Mg (cmol/kg) | 3.6 | 1.60 | 0.64 |
| K (cmol/kg) | 0.38 | 4.40 | 0.75 |
| Na (cmol/kg) | 1.4 | 0.80 | 0.90 |
| Mn (mg/kg) | 30.0 | 4.80 | 0.10 |
| Fe (mg/kg) | 142.0 | 36.60 | 17.20 |
| Zn (mg/kg) | 3.0 | 3.70 | 0.23 |
| Cu (mg/kg) | 3.2 | 1.23 | 0.10 |
| B (mg/kg) | 1.5 | 0.78 | 0.14 |
| Physicochemical Soil Property | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aggregate Size Ratio (%) | ||||||||||
| pH | Humidity (%) | OM (%) | 1.16 mm | 600 µ | 300 µ | 54 µ | BD (g/cm3) | AD (g/cm3) | Porosity (%) | EC (µS/cm) |
| 5.16 ± 0.5 | 36.30 ± 2.7 | 5.16 ± 0.5 | 49.57 ± 12.2 | 20.0 ± 5.5 | 6.41 ± 2.3 | 11.31 ± 5.1 | 1.61 ± 0.3 | 2.32 ± 0.2 | 30 ± 0.1 | 28.7 ± 15.1 |
| Variable/Treatment | BEM | CEM | BCEM | CT |
|---|---|---|---|---|
| pH | 5.30 ± 0.6 | 5.53 ± 0.8 | 5.49 ± 0.6 | 5.37 ± 0.6 |
| Humidity (%) | 24.4 ± 11.3 | 28.31 ± 8.9 | 24.83 ± 9.1 | 24.24 ± 9.6 |
| OM (%) | 4.5 ± 1.5 | 4.30 ± 1.35 | 4.37 ± 1.2 | 4.6 ± 1.6 |
| Aggregates 1.16 mm (%) | 45.70 ± 13.2 | 44.94 ± 12.8 | 45.33 ± 17.2 | 48.82 ± 14.0 |
| Aggregates 600 µ (%) | 20.67 ± 5.4 | 19.46 ± 5.7 | 18.58 ± 7.5 | 21.43 ± 7.0 |
| Aggregates 300 µ (%) | 6.78 ± 2.2 | 7.71 ± 2.7 | 6.4 ± 2.8 | 7.54 ± 2.8 |
| Aggregates 54 µ (%) | 11.34 ± 4.2 | 15.94 ± 7.9 | 13.9 ± 9.9 | 13.5 ± 4.9 |
| BD (g/cm3) | 1.46 ± 0.18 | 1.65 ± 0.37 | 1.45 ± 0.31 | 1.70 ± 0.25 |
| AD (g/cm3) | 2.24 ± 0.3 | 1.65 ± 0.4 | 2.25 ± 0.4 | 2.15 ± 0.2 |
| Porosity (%) | 33.98 ± 11.7 | 31.31 ± 14.4 | 33.71 ± 18.7 | 22.8 ± 12.7 |
| EC (µS/cm) | 24.44 ± 10.4 | 29.95 ± 18.1 | 33.45 ± 18.7 | 28.28 ± 14.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela, A.; Pineda Herrera, J.C.; Vanegas, J.; Soler, J.; Peña, J.; Pérez, P.; Pinilla, J. Biochar, Compost, and Effective Microorganisms: Evaluating the Recovery of Post-Clay Mining Soil. Sustainability 2025, 17, 6088. https://doi.org/10.3390/su17136088
Varela A, Pineda Herrera JC, Vanegas J, Soler J, Peña J, Pérez P, Pinilla J. Biochar, Compost, and Effective Microorganisms: Evaluating the Recovery of Post-Clay Mining Soil. Sustainability. 2025; 17(13):6088. https://doi.org/10.3390/su17136088
Chicago/Turabian StyleVarela, Amanda, Juan Camilo Pineda Herrera, Jennifer Vanegas, Jonathan Soler, Javier Peña, Paula Pérez, and Janis Pinilla. 2025. "Biochar, Compost, and Effective Microorganisms: Evaluating the Recovery of Post-Clay Mining Soil" Sustainability 17, no. 13: 6088. https://doi.org/10.3390/su17136088
APA StyleVarela, A., Pineda Herrera, J. C., Vanegas, J., Soler, J., Peña, J., Pérez, P., & Pinilla, J. (2025). Biochar, Compost, and Effective Microorganisms: Evaluating the Recovery of Post-Clay Mining Soil. Sustainability, 17(13), 6088. https://doi.org/10.3390/su17136088






