Iron-Modified Biochar Derived from Poultry Manure for Efficient Removal of Methyl Orange Dye from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Sorbent Synthesis and Characterization: Biochar, Modified Biochar Preparation, and Characterization
2.2. Sorption Experiments
2.2.1. Influence of Solution pH on Dye Sorption
2.2.2. Kinetic Sorption Batches
- Pseudo-second-order
- 2.
- Elovich
- 3.
- Power function
2.2.3. Equilibrium Adsorption Batch Experiments
- 1.
- Langmuir
- 2.
- Freundlich
- 3.
- Temkin
- 4.
- Dubinin–Radushkevich
2.3. Partition Coefficient
3. Results and Discussion
3.1. Properties of the Absorbents
3.2. Methyl Orange Sorption Batches
3.2.1. Effects of pH on MB Sorption
3.2.2. Kinetic Sorption
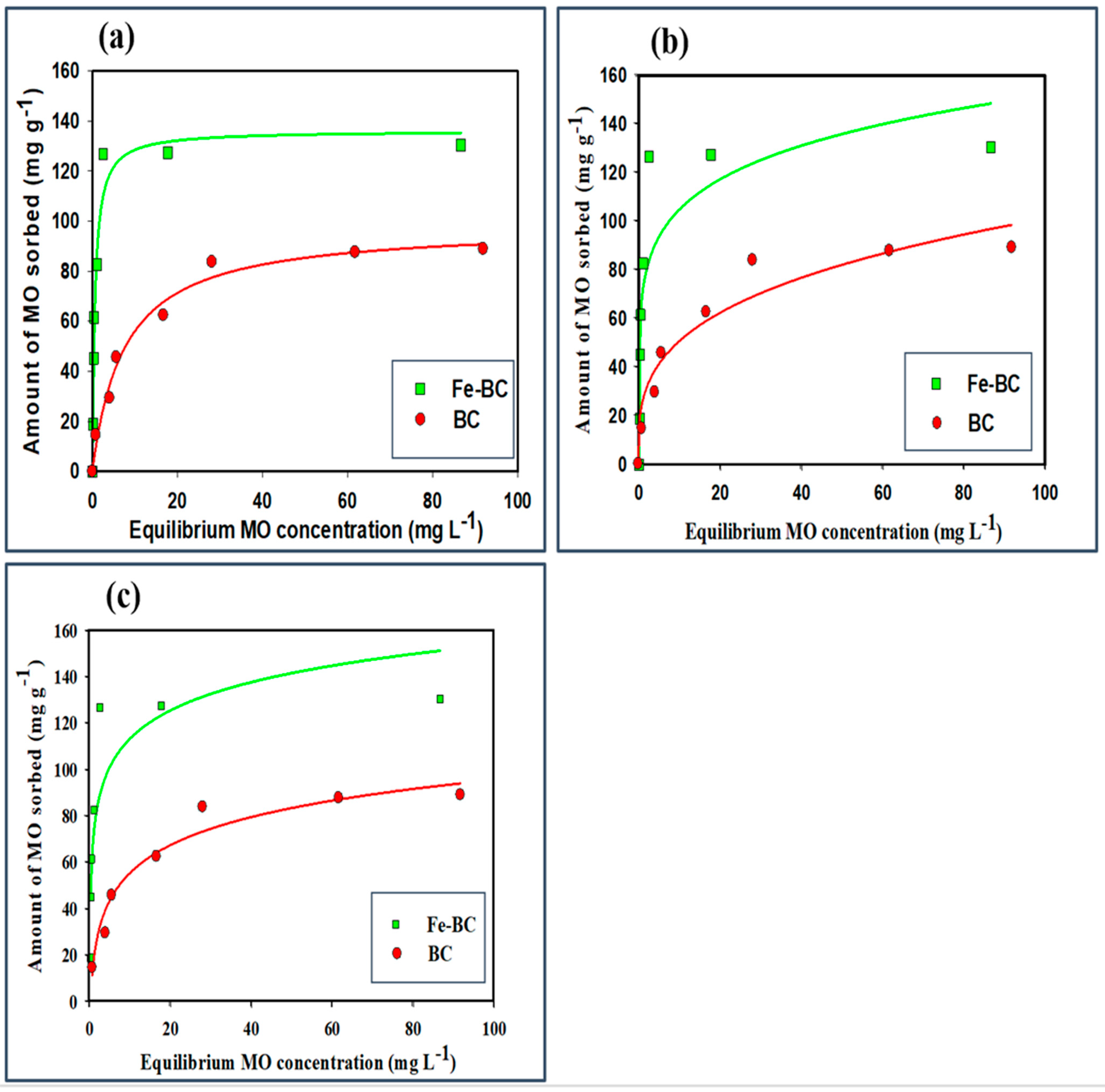
3.2.3. Equilibrium Sorption
3.3. Mechanism of Methyl Orange Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fobiri, G.K. Synthetic dye application in textiles: A review on the efficacies and toxicities involved. Text. Leather Rev. 2022, 5, 180–198. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02842. [Google Scholar] [CrossRef] [PubMed]
- Haldorai, Y.; Shim, J.J. An efficient removal of methyl orange dye from aqueous solution by adsorption onto chitosan/MgO composite: A novel reusable adsorbent. Appl. Surf. Sci. 2014, 292, 447–453. [Google Scholar] [CrossRef]
- Hazril, N.I.H.; Jalil, A.A.; Aziz, F.F.A.; Hassan, N.S.; Fauzi, A.A.; Khusnun, N.F.; Rajendran, S. Selective simultaneous photo-Fenton removal of Cr[VI] and methyl orange dye over critical raw material-free fibrous-silica irons catalyst. Sustain. Mater. Technol. 2024, 41, e00994. [Google Scholar] [CrossRef]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo dyes: Past, present and the future. Environ. Rev. 2011, 19, 350–371. [Google Scholar] [CrossRef]
- Feng, J.; Cerniglia, C.E.; Chen, H. Toxicological significance of azo dye metabolism by human intestinal microbiota. Front. Biosci. 2012, 4, 568. [Google Scholar]
- Nguyen, T.; Saleh, M.A. Detection of azo dyes and aromatic amines in women undergarment. J. Environ. Sci. Health A 2016, 51, 744–753. [Google Scholar] [CrossRef]
- Chung, K.T. Azo dyes and human health: A review. J. Environ. Sci. Health C 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Dwivedi, P.; Tomar, R.S. Bioremediation of textile effluent for degradation and decolourization of synthetic dyes: A review. Int. J. Curr. Res. Life Sci. 2018, 7, 1948–1951. [Google Scholar]
- Garg, S.; Roy, A. Phytoremediation: An alternative approach for removal of dyes. In Phytoremediation; Academic Press: Cambridge, MA, USA, 2022; pp. 369–386. [Google Scholar]
- Asadullah, M.; Kabir, M.S.; Ahmed, M.B.; Razak, N.A.; Rasid, N.S.A.; Aezzira, A. Role of microporosity and surface functionality of activated carbon in methylene blue dye removal from water. Korean J. Chem. Eng. 2013, 30, 2228–2234. [Google Scholar] [CrossRef]
- Shikuku, V.O.; Nyairo, W.N. Advanced oxidation processes for dye removal from wastewater. In Impact of Textile Dyes on Public Health and the Environment; IGI Global: Hershey, PA, USA, 2020; pp. 205–238. [Google Scholar]
- Gupta, R.; Pandit, C.; Pandit, S.; Gupta, P.K.; Lahiri, D.; Agarwal, D.; Pandey, S. Potential and future prospects of biochar-based materials and their applications in removal of organic contaminants from industrial wastewater. J. Mater. Cycles Waste Manag. 2022, 24, 852–876. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation [EC] science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Othman, N.; Asharuddin, S. Applications of natural coagulants to treat wastewater− a review. MATEC Web Conf. 2017, 103, 06016. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef] [PubMed]
- Kwapinski, W.; Byrne, C.M.; Kryachko, E.; Wolfram, P.; Adley, C.; Leahy, J.J.; Hayes, M.H. Biochar from biomass and waste. Waste Biomass Valorization 2010, 1, 177–189. [Google Scholar] [CrossRef]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an eco-friendly and economical adsorbent for the removal of colorants [dyes] from aqueous environment: A review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Mosa, A.; Natasha; Abdelrahman, H.; Niazi, N.K.; Antoniadis, V.; Rinklebe, J. Removal of toxic elements from aqueous environments using nano zero-valent iron- and iron oxide-modified biochar: A review. Biochar 2022, 4, 24. [Google Scholar] [CrossRef]
- Inal, A.; Gunes, A.Y.; Sahin, O.Z.; Taskin, M.B.; Kaya, E.C. Impacts of biochar and processed poultry manure, applied to a calcareous soil, on the growth of bean and maize. Soil Use Manag. 2015, 31, 106–113. [Google Scholar] [CrossRef]
- Dong, S.; Xu, W.; Wu, F.; Yan, C.; Li, D.; Jia, H. Fe-modified biochar improving transformation of arsenic form in soil and inhibiting its absorption of plant. Trans. Chin. Soc. Agric. Eng. 2016, 32, 204–212. [Google Scholar]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; Wang, H. Effect of biochar on the extractability of heavy metals [Cd, Cu, Pb, and Zn] and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Soil processes and the behaviour of metals. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 1995; pp. 13, 3488. [Google Scholar]
- Pérez, J.S.; Arzate, S.; Soriano-Molina, P.; Sánchez, J.G.; López, J.C.; Plaza-Bolaños, P. Neutral or acidic pH for the removal of contaminants of emerging concern in wastewater by solar photo-Fenton? A techno-economic assessment of continuous raceway pond reactors. Sci. Total Environ. 2020, 736, 139681. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L.; Zhao, L. An efficient biochar synthesized by iron-zinc modified corn straw for simultaneous immobilization of Cd in acidic and alkaline soils. Environ. Pollut. 2021, 291, 118129. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.M.; Dziubla, T.D.; Hilt, J.Z. Recent advances on iron oxide magnetic nanoparticles as sorbents of organic pollutants in water and wastewater treatment. Rev. Environ. Health 2017, 32, 111–117. [Google Scholar] [CrossRef]
- Li, X.C.; Yang, Z.Z.; Zhang, C.; Wei, J.J.; Zhang, H.Q.; Li, Z.H.; Hu, J.W. Effects of different crystalline iron oxides on immobilization and bioavailability of Cd in contaminated sediment. Chem. Eng. J. 2019, 373, 307–317. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A. Carbon-based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Huang, Q.; Song, S.; Chen, Z.; Hu, B.; Chen, J.; Wang, X. Biochar-based materials and their applications in removal of organic contaminants from wastewater: State-of-the-art review. Biochar 2019, 1, 45–73. [Google Scholar]
- Liu, Y.; Wang, L.; Liu, C.; Ma, J.; Ouyang, X.; Weng, L.; Chen, Y.; Li, Y. Enhanced Cadmium Removal by Biochar and Iron Oxides Composite: Material Interactions and Pore Structure. J. Environ. Manag. 2023, 330, 117136. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.; Ok, Y.S.; Hou, D. A Green Biochar/Iron Oxide Composite for Methylene Blue Removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Z.; Wang, C.C.; Zhang, M.Y.; Zong, X.B.; Huang, X.F.; Deng, Z.H.; Xiang, P. Advances and Prospectives of Iron/Biochar Composites: Application, Influencing Factors and Characterization Methods. Ind. Crops Prod. 2023, 205, 117496. [Google Scholar] [CrossRef]
- Chen, G.; Yin, Y.; Zhang, X.; Qian, A.; Pan, X.; Liu, F.; Li, R. Enhanced Adsorption of Methyl Orange from Aqueous Phase Using Chitosan–Palmer Amaranth Biochar Composite Microspheres. Molecules 2024, 29, 1836. [Google Scholar] [CrossRef]
- Salahshoori, I.; Jorabchi, M.N.; Ghasemi, S.; Golriz, M.; Wohlrab, S.; Khonakdar, H.A. Advancements in Wastewater Treatment: A Computational Analysis of Adsorption Characteristics of Cationic Dyes Pollutants on Amide Functionalized-MOF Nanostructure MIL-53 (Al) Surfaces. Sep. Purif. Technol. 2023, 319, 124081. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Zhang, M.; Zhou, R.; Li, J.; Zhao, W.; Wang, L. Optimize the Preparation of Fe3O4-Modified Magnetic Mesoporous Biochar and Its Removal of Methyl Orange in Wastewater. Environ. Monit. Assess. 2021, 193, 179. [Google Scholar] [CrossRef]
- Akawa, M.N.; Dimpe, K.M.; Nomngongo, P.N. An adsorbent composed of alginate, polyvinylpyrrolidone and activated carbon (AC@PVP@alginate) for ultrasound-assisted dispersive micro-solid phase extraction of nevirapine and zidovudine in environmental water samples. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100559. [Google Scholar]
- Baloo, L.; Isa, M.H.; Sapari, N.B.; Jagaba, A.H.; Wei, L.J.; Yavari, S.; Razali, R.; Vasu, R. Adsorptive removal of methylene blue and acid orange 10 dyes from aqueous solutions using oil palm wastes-derived activated carbons. Alex. Eng. J. 2021, 60, 5611–5629. [Google Scholar] [CrossRef]
- Li, H.; Sun, Z.; Zhang, L.; Tian, Y.; Cui, G.; Yan, S. A cost-effective porous carbon derived from pomelo peel for the removal of methyl orange from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 191–199. [Google Scholar] [CrossRef]
- Daffalla, S.; Da’na, E.; Taha, A.; El-Aassar, M.R. Synthesis of a Novel Magnetic Biochar from Lemon Peels via Impregnation-Pyrolysis for the Removal of Methyl Orange from Wastewater. Magnetochemistry 2024, 10, 95. [Google Scholar] [CrossRef]
- Raabe, T.; Rasser, H.; Nottelmann, S.; Groß, A.; Krause, H.; Kureti, S. Mechanistic study on H2S and subsequent O2 adsorption on iron oxides and hydroxides. Appl. Surf. Sci. 2021, 565, 150504. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H. Effect of Fe–N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, M.; Usman, A.R.; Al-Faraj, A.S.; Abduljabbar, A.S.; Al-Wabel, M.I. Biochar Composites with Nano Zerovalent Iron and Eggshell Powder for Nitrate Removal from Aqueous Solution with Coexisting Chloride Ions. Environ. Sci. Pollut. Res. 2018, 25, 25757–25771. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Patel, R.; Yang, D. FTIR spectroscopic analysis of biochar functionalized with transition metals. Chem. Eng. J. 2023, 429, 132947. [Google Scholar]
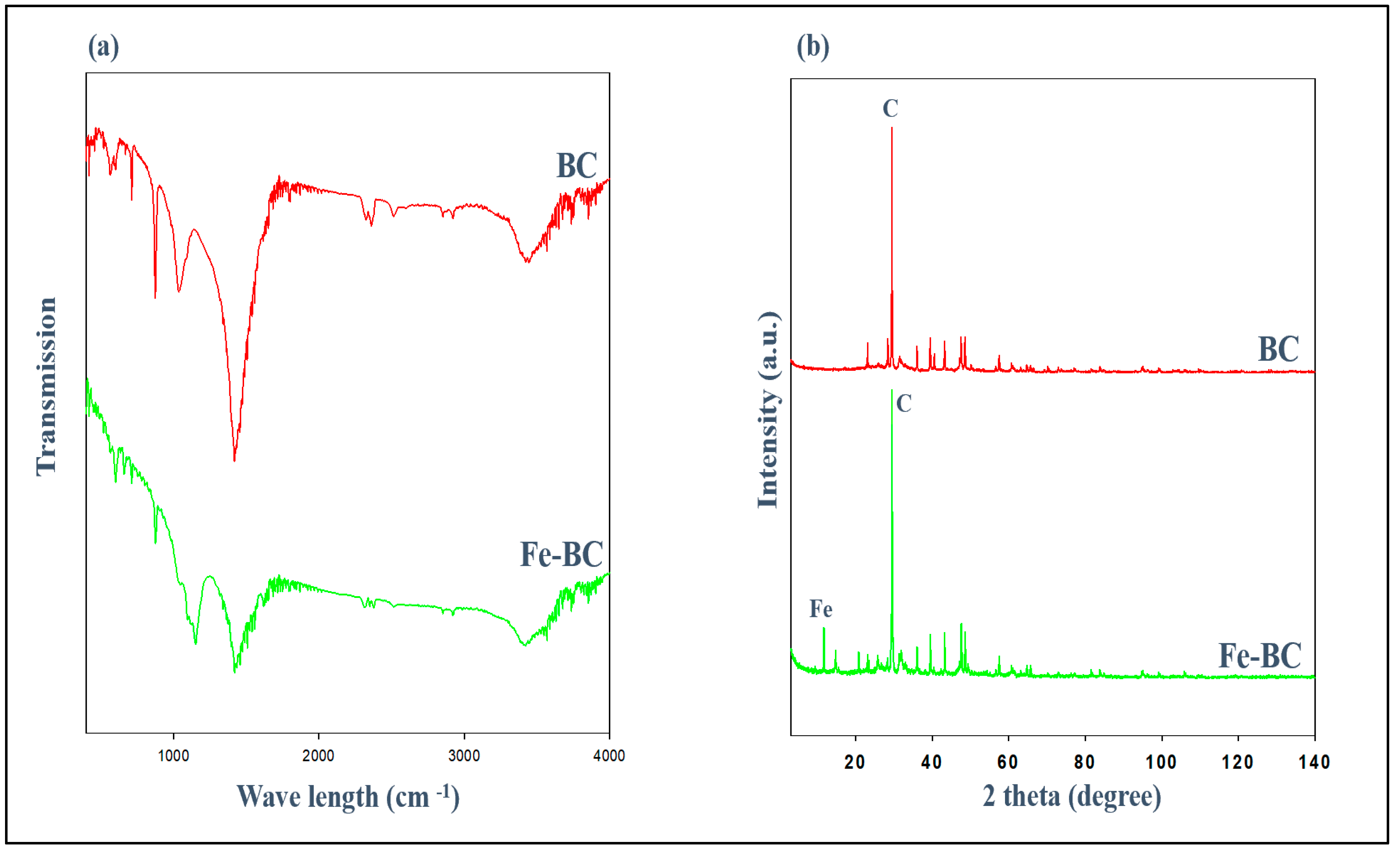
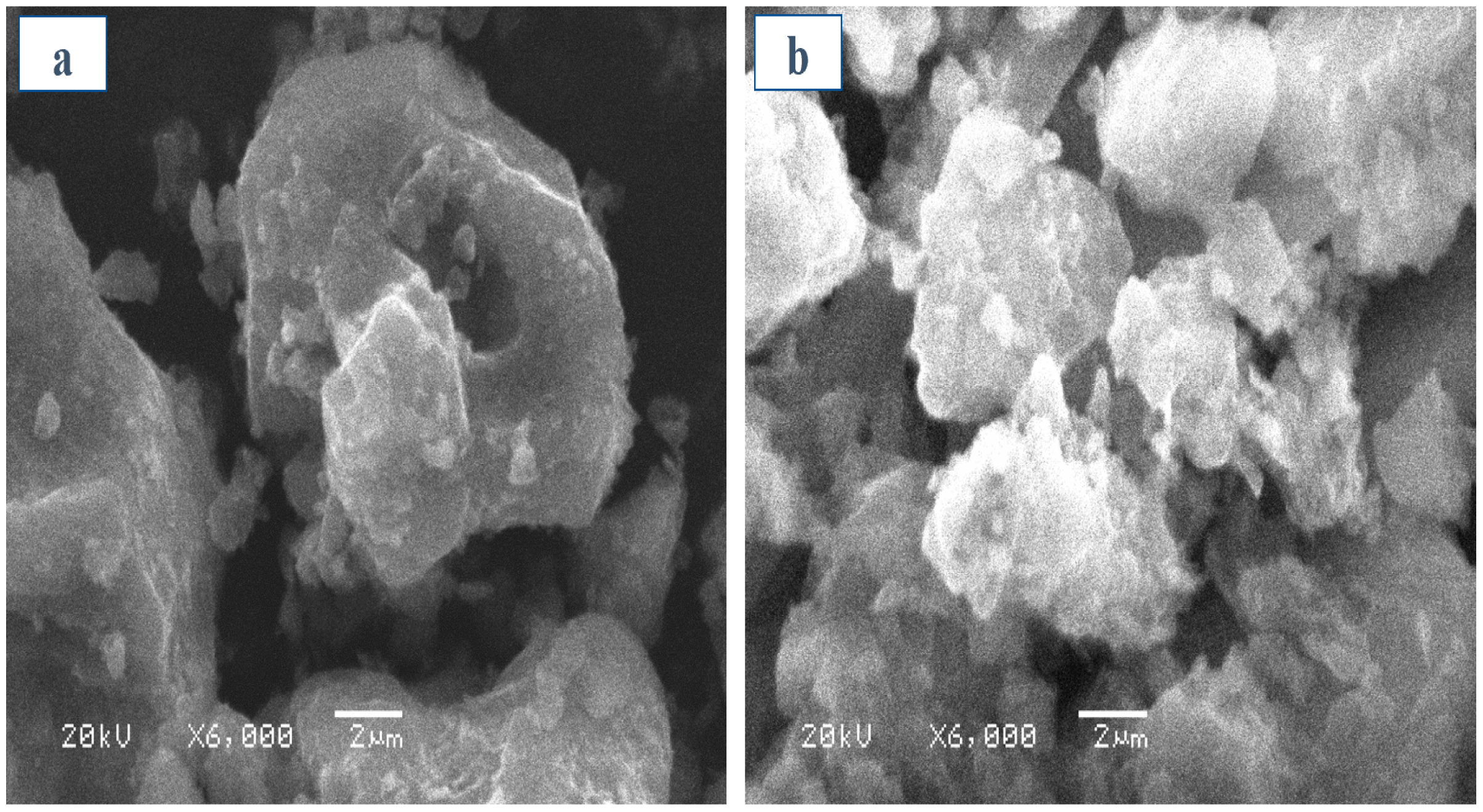
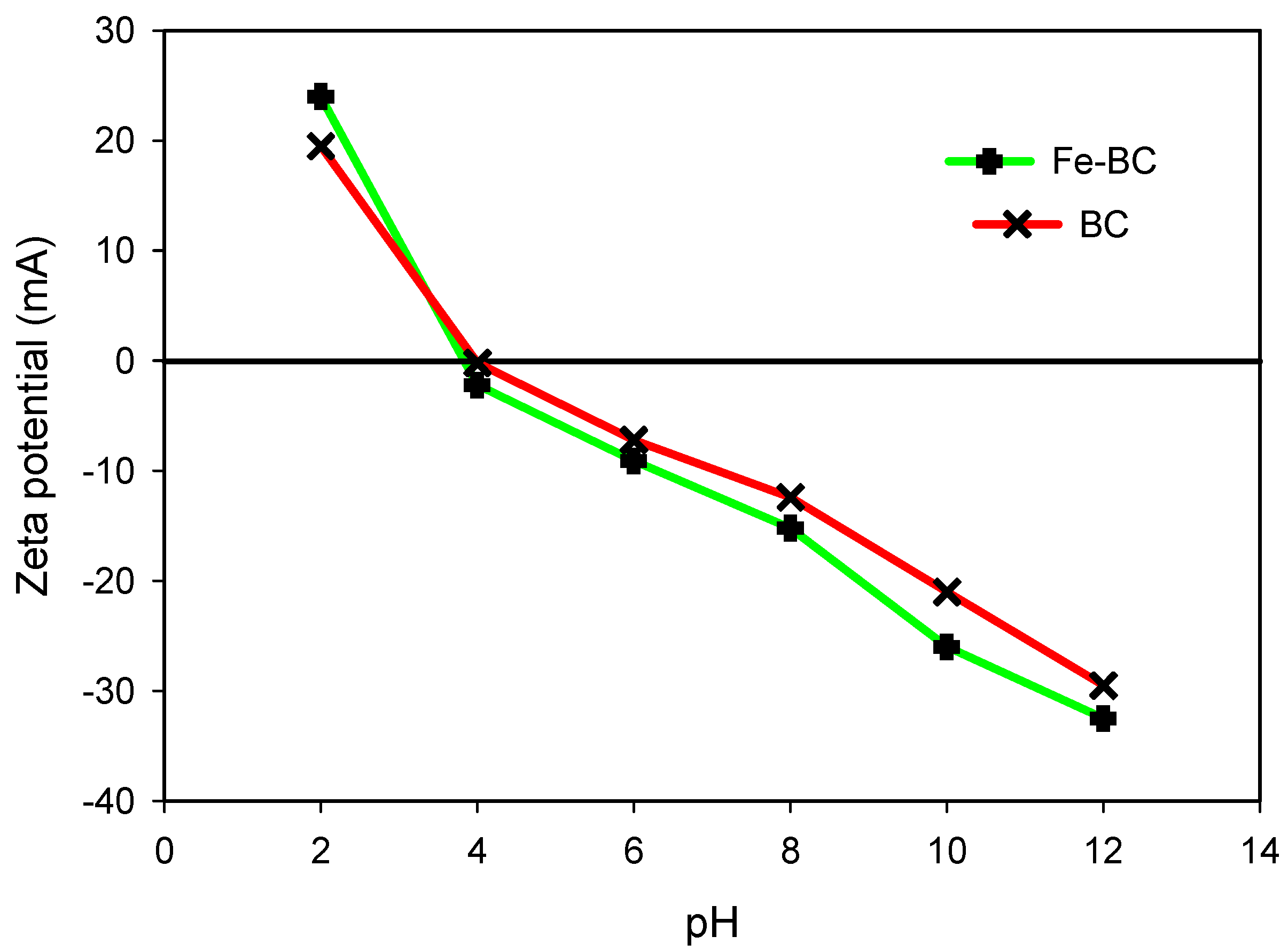
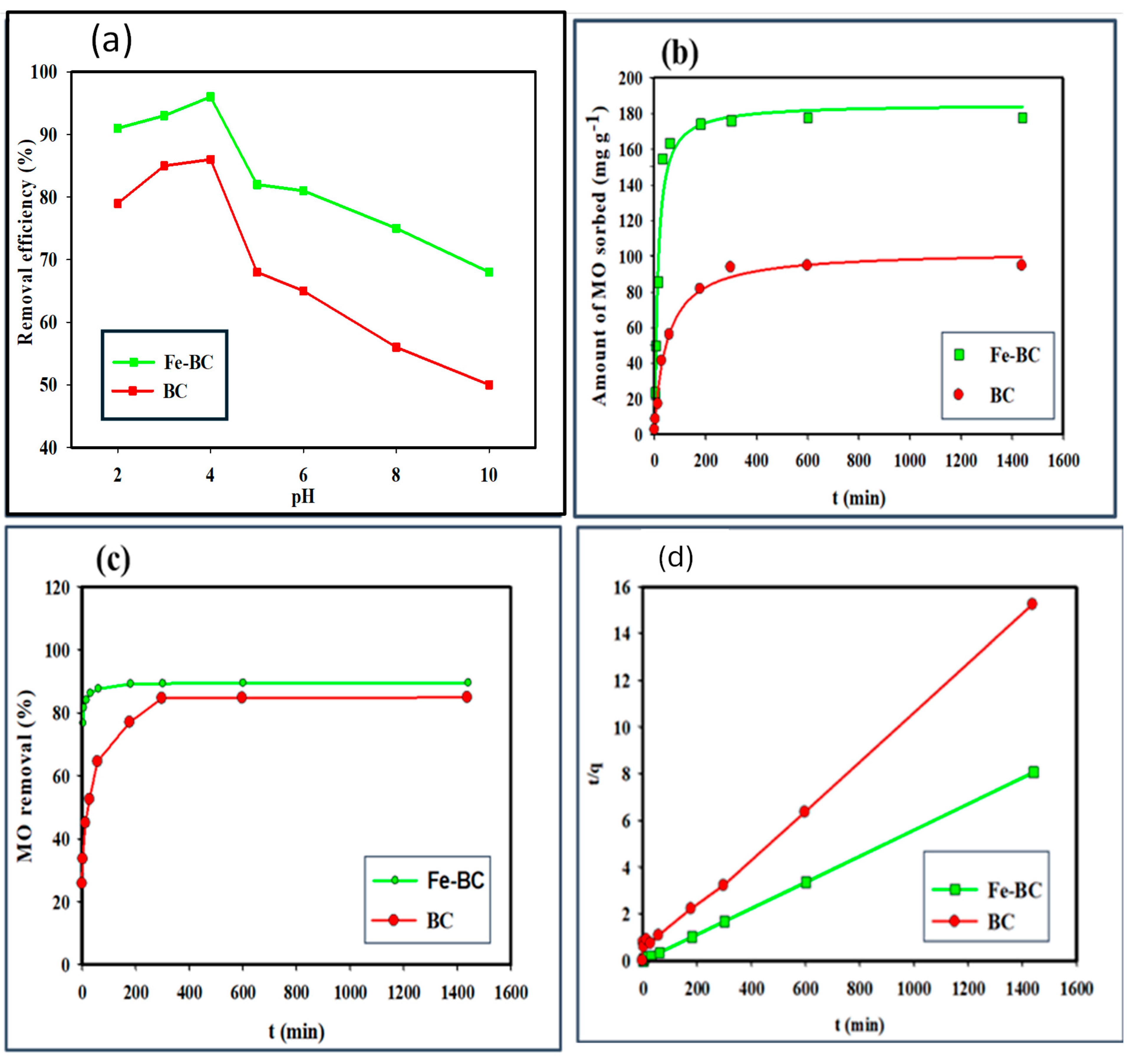
| Sorbents | pH (1:10) | CEC (meq/100 g) | Yield | Moisture | Volatile Matter | Ash | Fixed Carbon | Surface Area (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|---|---|---|---|---|
| % | |||||||||
| BC | 10.98 | 45.35 | 33.46 | 3.98 | 22.43 | 17.86 | 52.67 | 99.95 | 0.09 |
| BC-Fe | 8.25 | 75.59 | - | 4.93 | 29.29 | 13.59 | 28.65 | 90.68 | 0.15 |
| Order | Model | Parameters | Sorbents | |
|---|---|---|---|---|
| BC | Fe–BC | |||
| 1 | Elovich | A (mg·g−1·min−1) | 15.8547 | 27.0613 |
| Β (g·mg−1) | −9.8451 | 18.0115 | ||
| r2 | 0.9452 | 0.8762 | ||
| SEE (J/g) | 0.0037 | 0.0028 | ||
| 2 | Power function | kf (mg·g−1·min−1) | 0.6281 | 0.5246 |
| B | 0.8414 | 2.2908 | ||
| r2 | 0.8833 | 0.6222 | ||
| SEE (J/g) | 6.2744 × 10−6 | 5.8673 × 10−6 | ||
| 3 | Pseudo-second-order | H (min−1) | 2.3636 | 23.0312 |
| qe (mg·g−1) | 98.1917 | 179.1673 | ||
| k2 (g·mg−1·min−1) | 0.0002 | 0.0007 | ||
| r2 | 0.9973 | 0.9999 | ||
| SEE (J/g) | 0.0210 | 0.0244 | ||
| Isotherms | Parameters | BC | Fe–BC |
|---|---|---|---|
| Langmuir | QL (mg·g−1) | 98.23 | 136.25 |
| KL (L·g−1) | 0.13 | 1.67 | |
| R2 | 0.98 | 0.97 | |
| Freundlich | KF (L·g−1) | 24.98 | 72.54 |
| 1/n | 0.30 | 0.16 | |
| R2 | 0.94 | 0.75 | |
| Temkin | A (L·g−1) | 2.27 | 65.31 |
| B (J·mol−1) | 129.30 | 129.65 | |
| R2 | 0.95 | 0.78 | |
| Dubinin–Radushkevich | QD (mg·g−1) | 83.49 | 126.90 |
| BD (kJ·g−1) | 0.01 | 0.00 | |
| R2 | 0.91 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alasmary, Z.; Akanji, M.A. Iron-Modified Biochar Derived from Poultry Manure for Efficient Removal of Methyl Orange Dye from Aqueous Solution. Sustainability 2025, 17, 6008. https://doi.org/10.3390/su17136008
Alasmary Z, Akanji MA. Iron-Modified Biochar Derived from Poultry Manure for Efficient Removal of Methyl Orange Dye from Aqueous Solution. Sustainability. 2025; 17(13):6008. https://doi.org/10.3390/su17136008
Chicago/Turabian StyleAlasmary, Zafer, and Mutair A. Akanji. 2025. "Iron-Modified Biochar Derived from Poultry Manure for Efficient Removal of Methyl Orange Dye from Aqueous Solution" Sustainability 17, no. 13: 6008. https://doi.org/10.3390/su17136008
APA StyleAlasmary, Z., & Akanji, M. A. (2025). Iron-Modified Biochar Derived from Poultry Manure for Efficient Removal of Methyl Orange Dye from Aqueous Solution. Sustainability, 17(13), 6008. https://doi.org/10.3390/su17136008






