Abstract
The tasar silk production of India’s sericulture industry supports tribal livelihoods and economic sustainability. However, Antheraea mylitta Drury, 1773, the primary species for tasar silk, faces habitat threats due to deforestation, climate change, and anthropogenic pressures. This study evaluates the distribution and habitat suitability of wild tasar silkworm using multi-criteria approach, Geographic Information System (GIS), Remote Sensing (RS), and ecological niche modeling using the MaxEnt algorithm. Field surveys were conducted to collect cocoon samples, and the analysis of environmental parameters and assessment of soil micronutrient influences were also carried out. The MaxEnt model predictions indicate that the Central, Western, and Southern zones of Mayurbhanj, encompassing the Similipal Biosphere Reserve, provide the most suitable habitats. The jackknife test confirmed that these climatic variables collectively contributed 68.7% to the habitat suitability model. This study highlights the impact of habitat fragmentation and deforestation on tasar silkworm populations, emphasizing the need for conservation strategies, sustainable forest management, and afforestation programs. The findings highlight the following key conservation strategies: restoring habitats in Similipal, enforcing anti-deforestation laws, promoting community-led planting of host trees, and adopting climate-resilient silk farming to protect biodiversity and support tribal livelihoods.
1. Introduction
Global biodiversity is increasingly threatened by habitat destruction, climate change, and unsustainable resource exploitation, which leads to the decline of many economically and ecologically significant species [1,2]. Among them, the wild tasar silkworm (Bombyx mori), Antheraea mylitta Drury, 1773, plays a crucial role in sustaining rural livelihoods while maintaining forest ecosystems [3,4]. Tasar silk, derived from this species, is a vital natural fiber and a cornerstone of India’s non-mulberry sericulture economy, supporting the socio-economic wellbeing of tribal communities [5,6,7].
India is unique as the only country that produces all four types of commercial silk, mulberry (Morus spp.), tasar (Antheraea mylitta), eri (Samia ricini), and muga (Antheraea assamensis), and it ranks as the world’s second-largest silk producer [8,9,10]. Sericulture is a vital industry in India, employing approximately 7.85 million people, primarily in rural and semi-urban areas, with significant benefits for economically weaker sections and women [11]. Tasar silk constitutes 95% of global non-mulberry silk production [12].
Antheraea mylitta is polyphagous, feeding on host plants such as Terminalia arjuna, T. tomentosa, and Shorea robusta [13]. It is reared by tribal communities across multiple Indian states, sustaining approximately 2.5 lakh families [14,15]. Several ecoraces, including Daba, Sarihan, Bhandara, and Sukinda, have adapted to different ecological conditions [16,17]. A. mylitta undergoes complete metamorphosis, with a life cycle varying in the range of 30–45 days depending on the season.
The species has adapted to diverse ecological niches, forming 44 identified ecoraces, of which 10 are commercially significant [18,19]. Environmental factors, such as temperature, humidity, and food plant availability, influence its distribution and behavior, shaping its growth and productivity [20]. Despite its adaptability and commercial value, A. mylitta faces growing threats due to habitat destruction, land use changes, and environmental stressors [21,22]. Key challenges include uncontrolled harvesting of natural populations, industrial encroachments, and introduction of non-native ecoraces, which have led to genetic dilution and population fragmentation [23]. Habitat destruction results in ecological disruption, loss of host plants, and reduced species connectivity, ultimately threatening genetic diversity, essential for survival and long-term adaptability [24,25,26,27,28].
The Similipal Biosphere Reserve, located in Mayurbhanj district, Odisha, is a critical habitat and biodiversity hotspot supporting several tasar silkworm ecoraces, including Modal, Jata, and Nalia [29]. Modal, a Shorea-based ecorace, once dominant in the region (Table S2), declined after 1965 due to the introduction of Daba and Sukinda races, overharvesting, and anthropogenic pressures [30,31]. The fragmentation and degradation of forest ecosystems in Similipal have drastically reduced the availability of host plants and disrupted the life cycle of wild A. mylitta [32].
Although Similipal has historically supported a rich diversity of A. mylitta, increasing anthropogenic pressure necessitates proactive conservation measures and scientific habitat assessments to preserve these populations. Cocoon traits are widely used to assess the commercial quality of silk and the ecological fitness of silkworms. Parameters such as volume, weight, shell ratio, and pupal weight reflect how environmental and genetic factors shape growth, survival, and silk yield potential of A. mylitta.
Soil micronutrients also influence the quality of host plants, which in turn affect the larval development and cocoon traits of A. mylitta. Elements such as magnesium (Mg) and zinc (Zn) regulate chlorophyll content and enzyme activity, while boron (B) and calcium (Ca) influence cell structure and nutrient transport. Sulphur (S), Copper (Cu), and Iron (Fe) are involved in protein synthesis and directly impact cocoon shell weight and silk strength [33].
Most previous studies have emphasized cocoon biometry, grainage behavior, and genetic diversity of A. mylitta, while the broader ecological threats, such as habitat degradation and climate variability, remain underexplored [34,35,36,37,38,39]. To address these gaps, spatial modeling through GIS and Remote Sensing has emerged as a crucial approach to assess species distribution and habitat suitability [40,41,42,43,44]. Predictive tools, such as ecological niche models, particularly MaxEnt, enable habitat forecasting based on climatic and environmental parameters [45,46,47,48].
This study focuses on the habitat suitability of A. mylitta in the Similipal Biosphere Reserve, integrating field surveys, cocoon trait analysis, soil micronutrient profiling, and ecological niche modeling using the MaxEnt algorithm. Similipal, a critical habitat for the Modal, Nalia, and Jata races, has witnessed significant distribution shrinkage. The objectives of this study include assessing the spatial distribution of A. mylitta across Mayurbhanj using GIS and MaxEnt modeling; identifying key environmental variables influencing habitat suitability; and preparing habitat suitability maps to guide conservation planning and sustainable sericulture initiatives.
By offering a scientific, geospatially informed framework, this study aims to support biodiversity conservation and improve economic outcomes for dependent communities. The findings can guide policymakers, forest departments, and sericulture stakeholders to develop sustainable, climate-resilient management strategies for A. mylitta conservation [49,50,51].
2. Materials and Methods
2.1. Study Area
This study was conducted in Mayurbhanj district of Odisha, which lies between 21°17′ and 22°34′ N latitude and 85°40′ and 87°10′ E longitude as shown in Figure 1. The figure illustrates the spatial context of the study area, the Similipal Biosphere Reserve (SBR), located in Eastern India. Figure 1a shows the national map of India with the state of Odisha highlighted in red. Figure 1b zooms into Odisha, marking the Mayurbhanj district in red. Figure 1c further narrows the focus to show the Similipal Biosphere Reserve highlighted within Mayurbhanj district. Finally, Figure 1d presents a high-resolution satellite image of the Similipal Biosphere Reserve, with its official boundary delineated in red. It falls within Survey of India toposheets 73F, 73G, 73J, and 73K. Covering an area of 10,418 km2, the district is bordered by Jharkhand, West Bengal, Balasore, and Keonjhar. It comprises the four administrative subdivisions of Sadar (Baripada), Bamanghati (Rairangpur), Panchpir (Karanjia), and Kaptipada (Udala) (Figure 1). It consists of 26 blocks and 3945 villages, out of which 227 villages remain uninhabited. The terrain varies from plains at 40 m to the highest point at Meghasani Peak (1165 m) in Similipal. Major rivers, including Budhabalanga, Kharkai, and Jamira, drain the region in a dendritic pattern, influenced by underlying geological structures. The inhabitants of the study area rely heavily on forest products for their livelihood, with minimal dependence on agriculture [52].
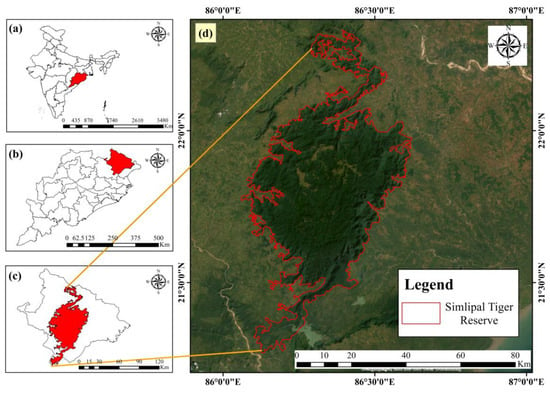
Figure 1.
Location map: (a) India (country map), (b) Odisha (state map), (c) Mayurbhanj (district map), and (d) the study area: Simlipal Biosphere Reserve (taken with Google Earth imagery).
Mayurbhanj experiences a tropical to subtropical climate, with temperatures ranging from 6 °C in winter to 47 °C in peak summer. The district receives an average annual rainfall level of 1197 mm, with monsoons from June to September. Nearly 38.6% of the land (88,589 hectares) is under forest cover, largely within the Similipal Biosphere Reserve, a UNESCO-recognized biodiversity hotspot supporting rich flora and fauna. The region is geologically diverse, hosting rock formations from the early crust to recent geological periods. The district is also known for its traditional tasar silk production, with tribal communities engaging in silkworm rearing on Sal-rich forest plantations, contributing to sustainable livelihoods.
2.2. Data Source
Field surveys were conducted across 36 selected locations based on the feasibility and accessibility within the Mayurbhanj district to ensure comprehensive habitat representation. A stratified random sampling approach was used, ensuring coverage across diverse ecological conditions. The site selection was based on historical records of A. mylitta occurrence, covering five ecological zones, i.e., Central, Western, Southern, Northern, and Eastern. The selection ensured representation across different ecoregions with varied host plant density and topography. At each site, GPS coordinates, altitude, and microclimatic parameters were recorded. Cocoon samples were collected, and key morphological traits, such as cocoon volume, weight, shell ratio, and pupal weight, were measured for statistical analysis.
Environmental data were obtained from multiple sources to support habitat modeling. Satellite imagery, including the Indian Remote Sensing Satellite P6 Linear Imaging Self-Scanning Sensor 3 or IRS P6 LISS III from National Remote Sensing Centre (NRSC), was chosen for its high-resolution vegetation mapping capabilities, while the Cartosat Digital Elevation Model (DEM) from the Bhuvan portal provided accurate topographic data essential for habitat suitability assessments. Land Use Land Cover maps from the years 2011-12 were incorporated to assess habitat conditions. Additional meteorological and soil data were collected from government sources to analyze the influence of climatic and edaphic factors on tasar silkworm distribution. Nineteen bioclimatic variables were obtained from the WorldClim database (version 2.1) at a spatial resolution of 1 km. This study followed a multi-step framework integrating field surveys, Remote Sensing, soil analysis, and ecological niche modeling (see Figure S1 for the complete methodological workflow).
2.3. Data Collection and Processing
2.3.1. GIS and Remote Sensing
Satellite imagery and spatial datasets were processed using ERDAS Imagine 2015 and ArcGIS 10.2 to prepare the environmental layers required for habitat suitability modeling. The images underwent radiometric and geometric corrections in ERDAS Imagine to ensure consistent reflectance values and accurate georeferencing. From these corrected images and ancillary datasets, several analytical layers were derived. Land Use Land Cover (LULC) classification was carried out using supervised classification with the maximum likelihood algorithm. The resulting classes were validated using field-collected ground truth points and cross-checked with forest inventory data. Forest-type information was extracted from the classified LULC maps and further refined using NDVI thresholding to distinguish between dense and open forest cover.
Topographic variables, such as elevation, slope, and aspect, were generated from Cartosat DEM data (30 m resolution) using the Spatial Analyst extension in ArcGIS. These topographic layers were resampled to a standard cell size of 1 km to maintain consistency with the bioclimatic variables. Soil types were digitized and rasterized from the Soil Atlas of Odisha and reprojected to the same coordinate system as other layers. All environmental and topographic raster layers were clipped to the Mayurbhanj district boundary using the ‘Extract by Mask’ tool in ArcGIS and reprojected to WGS 84 UTM Zone 45N. Each raster layer was carefully examined for null values or missing cells and then exported in the ASCII format compatible with MaxEnt input requirements.
2.3.2. Species Distribution Modeling
In this study, MaxEnt version 3.3.3k was utilized to model habitat suitability by incorporating 19 bioclimatic (BIOCLIM from WorldClim Organisation) variables along with additional continuous and categorical predictors, including forest type, soil type, slope, aspect, and elevation [53]. All environmental variables were standardized in the raster format and converted to ASCII to ensure compatibility with MaxEnt. To reduce redundancy and avoid multicollinearity among predictor variables, a pairwise Pearson correlation analysis was conducted on the 19 bioclimatic variables. Predictors showing a high correlation (|r| > 0.8) were excluded from the model to ensure statistical independence and improve prediction robustness. In addition to jackknife tests, a permutation importance test was used to assess the relative contribution of each variable to model performance. In this method, the values of each environmental variable are randomly permuted while keeping the others unchanged. The model is then rerun, and the drop-in training AUC is recorded and normalized to percentages. This approach highlights the sensitivity of the model to each predictor, but as with jackknife tests, its interpretation should be cautious when predictor variables are correlated.
To enhance prediction robustness, 25% of the presence data was reserved for model validation, and the model was run using five replicates with a subsampling approach. The maximum number of iterations was set at 5000 to ensure model convergence. The logistic output format was selected to generate habitat suitability probabilities ranging from 0 to 1, with a cutoff value of 0.5 used to differentiate between suitable and marginal habitats based on species presence patterns. Key model outputs included habitat suitability maps, response curves explaining environmental influence, jackknife variable importance tests, and ROC curve analysis for model performance assessment using Area Under the Curve (AUC) metrics [54,55]. Greater AUC values indicate better model robustness [56]. The final logistic output maps were exported as ASCII files, multiplied by 100, and converted to integer raster format to reduce the file size while preserving spatial accuracy. Suitability scores above 50 indicated presence zones, while values below 50 denoted unsuitable habitats.
2.4. Soil Micronutrient Analysis
Soil micronutrients influence host plant growth, directly impacting the health, growth, and silk-producing capacity of A. mylitta larvae. This study analyzed eight key micronutrients (B, Ca, Cu, Fe, Mg, Manganese (Mn), S, and Zn), which were selected based on their influence on plant physiology and documented effects on sericulture productivity. Soil samples were air-dried, sieved, and analyzed using Atomic Absorption Spectroscopy (AAS) [57]. To examine the relationship between soil micronutrients and tasar silkworm habitat suitability, secondary data from the Directorate of Agriculture and Food Product, Government of Odisha, were used to generate soil maps of Mayurbhanj. To evaluate their ecological significance, spatial layers of these micronutrients were incorporated into the MaxEnt model as environmental predictors. This integration enabled the generation of response curves that highlighted the individual contribution of each micronutrient to the habitat suitability of A. mylitta. Such an analysis facilitated a better understanding of how soil fertility conditions may indirectly influence species occurrence and habitat quality, thereby enriching the ecological interpretation of the distribution model.
2.5. Statistical Analysis
Descriptive statistics, including mean, standard deviation, and coefficient of variation, were computed to summarize the cocoon trait data. To assess significant differences in cocoon traits among the study locations, an Analysis of Variance (ANOVA) was performed. Additionally, relationships between environmental factors and cocoon characteristics were evaluated using Pearson correlation analysis [58].
The measurements and analysis of cocoons and pupae were carried out using a digital Vernier caliper with a precision of ±0.01 mm to ensure better accuracy. The analysis identified temperature and precipitation as the most influential factors shaping tasar silkworm distribution. Key environmental variables included temperature seasonality (BIO4), precipitation of the driest month (BIO14), mean temperature of the wettest quarter (BIO8), and mean temperature of the warmest quarter (BIO10). Soil micronutrients, such as Mg, Zn, and B, exhibited minor contributions to habitat suitability.
3. Results
3.1. Wild Tasar Populations
This study surveyed 36 locations for tasar cocoon collection, primarily within and around the Similipal Biosphere Reserve. These sites were categorized into five zones, Central, Western, Southern, Northern, and Eastern, for analysis. The altitude of collection sites varied significantly, with the lowest recorded at Suliapada (77 m a.s.l.) and the highest at Patbil (971 m a.s.l.). Suliapada, an isolated forest patch in the Eastern part of Mayurbhanj, is detached from the main Similipal forest. Other high-altitude locations included Gudgudia (843 m a.s.l.), Bahaghar (848 m a.s.l.), Chandikhaman (877 m a.s.l.), and Nuagaon (887 m a.s.l.), primarily located in the Central and Western zones of Similipal.
This study analyzed five key traits, cocoon volume, cocoon weight, shell weight, pupal weight, and shell ratio for both male and female cocoons, as presented in Tables S2 and S3. Figure 2, Figure 3 and Figure 4 illustrate the variations in these traits graphically. Statistical analysis revealed significant differences in almost all cocoon traits among male populations, with F-values ranging from 2.912 (p < 0.01) to 21.871 (p < 0.001). The Central and Western populations formed a distinct group, while the Southern populations showed similarities to them. Central-zone males exhibit the highest values in cocoon volume (23.95 cm3), cocoon weight (12.06 g), shell weight (2.34 g), pupal weight (9.72 g), and shell ratio (19.43%). A decreasing trend in all traits is observed from the Central to Eastern zone, indicating that altitude and environmental conditions in the Central zone are more favorable for larval development and silk production. Similarly, female silkworms in the Central zone outperform others, showing the highest values in cocoon volume (37.20 cm3), cocoon weight (16.39 g), shell weight (3.09 g), and pupal weight (13.30 g). However, the highest shell ratio (18.48%) is observed in the Eastern zone, suggesting possible morphological trade-offs between different traits. Northern and Eastern zones show the lowest trait values, consistent with the modeled marginal habitat zones. However, Northern and Eastern populations exhibited significant differences from the rest across all five traits.
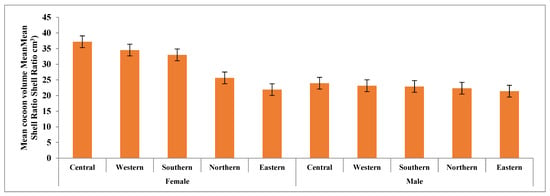
Figure 2.
Mean cocoon volume (cm3) in the cocoons of wild tasar silkworm populations collected from five regions in Similipal and the adjoining forests in Mayurbhanj.
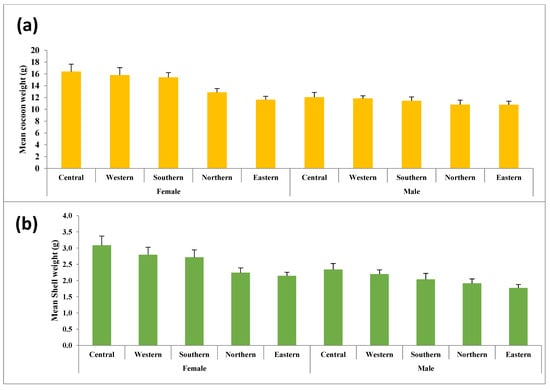
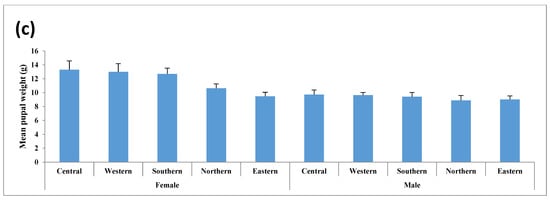
Figure 3.
Weight-related traits of wild Antheraea mylitta cocoons collected from five regions in Similipal and the adjoining forests: (a) mean cocoon weight (g), (b) mean shell weight (g), and (c) mean pupal weight (g).
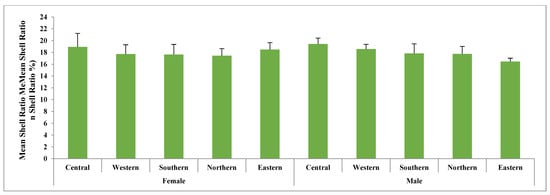
Figure 4.
Mean shell ratio (%) in the cocoons of wild tasar silkworm populations collected from five regions in Similipal and the adjoining forests.
In female cocoons, the observed trends were similar to those in males, except for the shell ratio, where differences were not statistically significant. Notable variations were observed in cocoon size across different zones. The largest cocoons were recorded in the Central and Western zones, with mean volumes of 37.2 cm3 and 34.55 cm3, respectively, whereas the smallest cocoons were found in the Eastern zone (21.9 cm3). The differences in cocoon volume were highly significant (F = 225.7, p < 0.001). Additionally, significant differences were observed between male and female cocoons, with females consistently exhibiting higher values for all traits except the shell ratio.
3.2. Soil Micronutrients
These maps illustrate the spatial distribution of key micronutrients, including B, Ca, Cu, Fe, Mg, Mn, S, and Zn, as presented in Figure 5. Understanding the availability of these micronutrients is crucial, as they influence soil fertility, plant health, and consequently, the suitability of the environment for A. mylitta. The analysis of the soil maps revealed that Boron levels were consistently low across Mayurbhanj, with slightly higher concentrations in the Central and Western zones. Calcium was found to be critically deficient in the Eastern zone, whereas in other parts of the district, it remained at an adequate level. Essential micronutrients, such as Copper, Iron, Manganese, and Sulphur were present in sufficient quantities across all zones, indicating favorable conditions for plant growth in these areas. However, Magnesium levels were critically low in the Northern and Eastern zones, which could negatively impact the health of host plants and, in turn, tasar silkworm development. Similarly, Zinc concentrations were also low in the Northern and Eastern zones, potentially affecting nutrient uptake in plants.
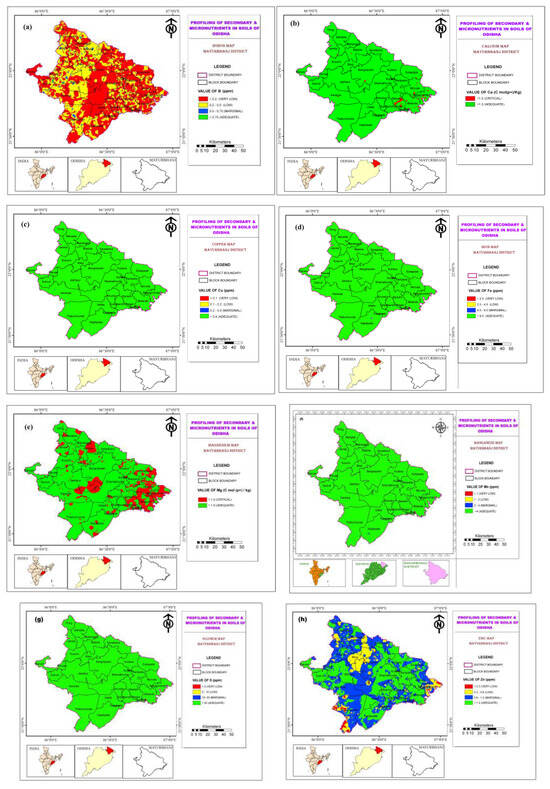
Figure 5.
Soil map of Mayurbhanj district showing (a) Boron content, (b) Calcium content, (c) Copper content, (d) Iron content, (e) Magnesium content, (f) Manganese content, (g) Sulphur content, and (h) Zinc content.
The lower availability of Magnesium and Zinc in the Northern and Eastern zones may contribute to reduced cocoon quality and yield. Conversely, the Central and Western zones, with relatively better micronutrient availability, may offer more suitable conditions for tasar silkworm rearing.
3.3. MaxEnt Model Outputs
3.3.1. Prediction Maps
Figure 6 illustrates the spatial patterns of habitat suitability across the Mayurbhanj district and also highlights areas with a high predicted presence of A. mylitta. These maps illustrate the spatial probability distribution of suitable habitats for A. mylitta, based on ecological and environmental variables. The point-wise mean and standard deviation of 15 output grids is represented, providing insights into habitat suitability variations across the district.
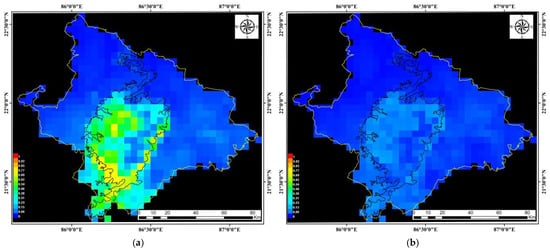
Figure 6.
Prediction maps of Mayurbhanj for wild tasar silkworms: (a) Spatial probability distribution of habitat suitability, (b) Standard deviation map of habitat suitability predictions.
The findings indicate that the most favorable tasar silkworm habitats lie in the Central, Western, and Southern zones of Mayurbhanj, encompassing the Similipal Biosphere Reserve. Thee Eastern part of Similipal exhibits climatic variations, as evidenced in the predictive map. High-altitude regions correlate with better-performing silkworm cocoon traits, reinforcing their ecological suitability. Additional summary grids, including minimum, maximum, and median values, further enhance the understanding of spatial trends and model uncertainty. The results indicate that the Central and Western zones exhibit the highest suitability, aligning with the observed tasar populations in these regions.
The model’s omission rate and AUC score (0.858 ± 0.050), as shown in Figures S7 and S8, demonstrate strong calibration and discrimination capability, confirming the MaxEnt model’s reliability across multiple replicate runs. These validation metrics further support the model’s robustness in predicting A. mylitta habitat suitability within diverse environmental settings of Mayurbhanj.
3.3.2. Response Curves
The influence of individual environmental variables on the MaxEnt prediction is illustrated through response curves in Figure S2. These response curves represent how the logistic prediction changes when a single variable is varied while keeping all other variables at their average sample value. The red curve indicates the mean response across 15 replicate MaxEnt runs, while the blue shading represents ± one standard deviation.
MaxEnt response curves show that A. mylitta distribution is most sensitive to variables such as BIO4 (temperature seasonality), BIO8 (mean temperature of the wettest quarter), BIO15 (precipitation seasonality), and BIO19 (precipitation of the coldest quarter). These variables exhibited strong nonlinear trends, confirming the species’ preference for thermally and hydrologically stable environments. In contrast, several other bioclimatic variables showed minimal influence, as indicated by their flat response curves, suggesting they contribute little explanatory power when isolated.
It is important to note that if environmental variables are strongly correlated, the response curves may be difficult to interpret. This is because the MaxEnt model can leverage relationships between variables that may not be apparent in a marginal response analysis. In contrast, Figure S3 presents response curves from individual MaxEnt models using only a single environmental variable. Here, BIO4 and BIO15 show strong negative trends, suggesting that temperature and precipitation variability are limiting factors for A. mylitta. BIO14 exhibited a unimodal curve, indicating an optimal range of dry-season precipitation beyond which habitat suitability declines. In contrast, BIO3 and BIO5 contributed limited discriminatory values when modeled in isolation.
Table S1 presents the estimated relative contributions of environmental variables to the MaxEnt model. BIO4 had the highest influence (20.6% contribution, 25.8% permutation importance), followed by BIO14 and BIO8, though BIO14′s lower permutation score suggests a possible indirect role. Variables such as BIO11 and BIO7 showed high permutation importance despite low training contribution, implying ecological relevance through the correlation with other predictors.
The results of the jackknife test are shown in Figures S4–S6. BIO9 (mean temperature of the driest quarter) exhibits the highest training gain when used alone and the greatest reduction when omitted, suggesting strong standalone and unique predictive power. Test gain and AUC-based jackknife evaluations confirmed the relevance of BIO9, BIO19, BIO2, and BIO14, underlining their predictive consistency across both training and testing contexts.
4. Discussion and Interpretation
4.1. Discussion
The jackknife test of variable importance in MaxEnt identified four key environmental variables: temperature seasonality (BIO4), precipitation of the driest month (BIO14), mean temperature of the wettest quarter (BIO8), and mean temperature of the warmest quarter (BIO10). These collectively contributed 68.7% to the MaxEnt prediction for tasar silkworm distribution. The habitat suitability modeling results identify BIO4 (temperature seasonality) and BIO14 (precipitation of the driest month) as the most influential climatic variables governing the distribution of A. mylitta. This finding aligns with earlier studies from regions such as Jharkhand, Chhattisgarh, and parts of Maharashtra, where seasonal variations in temperature and precipitation have been shown to critically affect the larval development and host plant phenology of A. mylitta and other tasar silkworm species [59]. Similar patterns were observed by other studies, which reported that tasar silkworm populations were highly sensitive to shifts in seasonal rainfall and temperature extremes [60,61]. Ecological niche models developed for related species, including Antheraea assamensis and Samia ricini, also emphasized the strong correlation between species distribution and seasonal climatic extremes [62]. Another study on Samia cynthia highlighted BIO14 and BIO8 as primary drivers, which further validates the relevance of these predictors across non-mulberry silkworm taxa [63]. The consistency of these predictors across taxa supports their ecological significance and strengthens their use in conservation planning.
The findings also reveal that at higher altitudes with dense canopy cover, tasar silkworm fitness traits, such as cocoon size, shell weight, and pupal weight, reach maximum values. Earlier studies reported similar trends, with enhanced quantitative traits at higher elevations in Similipal [64,65]. The MaxEnt model corroborates these findings, reinforcing the role of environmental variables in shaping tasar silkworm distribution.
While this study does not project future climate scenarios, the high sensitivity of A. mylitta to seasonal temperature and precipitation variations suggests that climate-induced habitat shifts are possible. Thus, periodic ecological monitoring is essential. This is supported by observations from similar studies across India, which emphasize regional variability in climate responses and underscore the need for region-specific conservation models [66,67].
While the MaxEnt model has effectively predicted habitat suitability for A. mylitta, certain limitations must be acknowledged. Its reliance on presence-only data introduces potential sampling bias, and it does not account for ecological complexities, such as interspecific interactions, disease prevalence, or genetic variability within populations. Moreover, continuous field validation and inclusion of factors, like land use change and anthropogenic pressures, could enhance model accuracy. Future studies should incorporate multi-seasonal monitoring and broader environmental variables to support more resilient and actionable conservation planning.
4.2. Conservation Implications
The Similipal Biosphere Reserve in Odisha, India, encompasses unique local environmental and social factors that impact the habitat and conservation of A. mylitta, or the tasar silk moth. Among environmental factors, the reserve’s specific climate, vegetation, and topography sustain rich biological diversity. Social factors include the use of the forest products and resources by local communities and their customs, as well as their level of awareness toward conservation. The reserve’s inscription as a UNESCO Biosphere Reserve, along with its rich cultural landscape, necessitates conservation action as well as diversified economic activities for the local people. Grasping these elements is important to anticipate possible habitats and devise tailored conservation plans for A. mylitta.
Building upon the habitat suitability findings, this study proposes a set of spatially informed and community-driven conservation strategies for A. mylitta. Firstly, habitat fragmentation analysis and model overlay have revealed multiple fragmented yet climatically suitable zones, particularly in the Eastern and Northern sectors of the Similipal Biosphere Reserve, where the historical presence of A. mylitta has dwindled. These areas should be prioritized for habitat restoration through assisted natural regeneration and protection of native host species, such as Terminalia arjuna, T. tomentosa, and Shorea robusta. Secondly, afforestation programs should be strategically conducted along the degraded fringes of Similipal, especially in the Southern zones with higher anthropogenic pressures. Thirdly, participatory rearing initiatives should be promoted to revive ecoraces like Modal and Nalia through decentralized silkworm rearing clusters embedded in community forest areas. These initiatives not only support conservation but also strengthen tribal livelihoods and cultural continuity. Fourthly, we propose integrating this study’s spatial outputs into district-level biodiversity action plans and the Odisha Forest Department’s micro-planning efforts to ensure policy-level responsiveness. Finally, considering the limitations of occurrence data, long-term ecological monitoring should be institutionalized. This can be complemented by citizen science initiatives, such as mobile-based applications and local forest surveillance schemes, to enhance species tracking and occurrence reporting at the grassroots level.
Furthermore, given Similipal’s genetic richness and the presence of semi-wild A. mylitta populations, it also holds potential as an in situ genetic reservoir for domestic tasar strains. This natural gene pool can contribute to future breeding programs by enhancing disease resistance and silk quality, especially under climate-induced stress scenarios [29,68]. In the context of conservation, the primary concerns include ongoing deforestation, forest fragmentation, and reduction in host plant density, especially Terminalia tomentosa and Terminalia arjuna. Feasible conservation actions must include (i) zoning of priority habitat restoration areas based on the suitability map, (ii) establishment of controlled rearing zones within buffer areas, (iii) seedling propagation and reforestation of host trees, and (iv) active collaboration with tribal communities through incentive-based afforestation schemes and participatory forest monitoring [23]. Additionally, linking forest-based livelihood strategies with sustainable tasar farming can promote community stewardship and minimize anthropogenic pressures on core silkworm habitats.
Altogether, these targeted, locally grounded strategies combine scientific insight with practical action, offering a robust pathway for sustainable A. mylitta conservation not only in Similipal but also in comparable habitats across India.
5. Conclusions and Recommendations
This study presents an integrated approach to assess the habitat suitability of Antheraea mylitta in the Similipal Biosphere Reserve by combining field-based cocoon trait analysis, soil micronutrient profiling, and ecological niche modeling through MaxEnt. The findings indicate that higher and mid-altitude regions with favorable climatic stability, dense canopy, and host plant presence offer the most suitable habitats for A. mylitta. Conversely, large areas in Eastern and Southern Mayurbhanj remain ecologically degraded, highlighting the urgent need for targeted conservation. The habitat suitability maps generated in this study can be directly utilized by policymakers and forest departments for biodiversity micro-planning, zoning of rearing zones, and habitat restoration under Odisha’s biodiversity action plans. Tribal communities, who are key custodians of tasar culture, may leverage these spatial tools to decentralize silkworm rearing and revive locally adapted ecoraces, like Modal and Nalia. Sericulture extension agencies and NGOs can prioritize afforestation and host plant regeneration in high-suitability zones, aligning efforts with local livelihoods and conservation. Furthermore, this study underscores the importance of participatory conservation grounded in spatial evidence. The integration of such predictive modeling with grassroots-level initiatives, such as incentive-based afforestation and citizen science monitoring, can enhance the long-term tracking of silkworm populations. While model limitations exist, such as the exclusion of future climate projections or genetic variance, its application still offers a valuable scientific foundation for ecosystem-based planning. By aligning ecological data with stakeholder actions, this study contributes to sustainable tasar sericulture, the conservation of native ecoraces, and biodiversity resilience, furthering the objectives of SDG 15 and India’s forest-based rural development goals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17135824/s1. Supplementary Table S1. Relative contributions of the environmental variables to the MaxEnt model. Supplementary Table S2. Cocoon quantitative traits of male wild tasar silkworm populations collected from five regions of Similipal and adjoining forests in Mayurbhanj. Supplementary Table S3. Cocoon quantitative traits of female wild tasar silkworm populations collected from five regions of Similipal and adjoining forests in Mayurbhanj. Supplementary Figure S1. Methodological Flowchart. Supplementary Figure S2: Response curves of each environmental variable for Maxent prediction. Supplementary Figure S3: Response curves of correlated environmental variables for MaxEnt prediction. Supplementary Figure S4. Jackknifing of regularized training gain for tasar silkworm. Supplementary Figure S5. Jackknifing of regularized test gain for tasar silkworm. Supplementary Figure S6. Jackknifing of area under curve (AUC) for tasar silkworm. Supplementary Figure S7. Average omission and predicted area. Supplementary Figure S8. Average sensitivity vs specificity.
Author Contributions
Conceptualization, R.R.T. and D.N.; methodology, D.K.B.; software, R.B.; validation, D.N. and D.K.B.; formal analysis, R.R.T.; investigation, P.M. and M.Z.; resources, D.N.; data curation, D.N.; writing-original draft preparation, R.R.T.; writing—review and editing, S.S., F.F.B.H., M.K. and M.Z.; visualization, R.R.T.; supervision, D.N.; project administration, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R675), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be made available on reasonable request.
Acknowledgments
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R675), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The first author and co-authors would like to extend their heartfelt gratitude to their seniors and colleagues for their invaluable guidance, scientific and technical discussions, and unwavering support throughout the preparation of this manuscript. We would like to extend our gratefulness to the Directorate of Agriculture and Food Production, Government of Odisha for sharing their datasets for the research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, Z.; Wang, T.; Zhang, X.; Wang, J.; Yang, Y.; Sun, Y.; Guo, X.; Wu, Q.; Nepovimova, E.; Watson, A.E.; et al. Biodiversity Conservation in the Context of Climate Change: Facing Challenges and Management Strategies. Sci. Total Environ. 2024, 937, 173377. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, K.V.; Kurup, M.B. Fish Biodiversity of Western Ghats in the Context of Climate Change and Strategies for Conservation. In Impact of Climate Change on Hydrological Cycle, Ecosystem, Fisheries and Food Security; CRC Press: Boca Raton, FL, USA, 2022; pp. 301–319. [Google Scholar] [CrossRef]
- Mahanta, D.K.; Komal, J.; Samal, I.; Bhoi, T.K.; Dubey, V.K.; Pradhan, K.; Nekkanti, A.; Gouda, M.N.R.; Saini, V.; Negi, N.; et al. Nutritional Aspects and Dietary Benefits of “Silkworms”: Current Scenario and Future Outlook. Front. Nutr. 2023, 10, 1121508. [Google Scholar] [CrossRef] [PubMed]
- Sobti, R.C. Biodiversity: Threats and Conservation; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Koldasbayeva, D.; Tregubova, P.; Gasanov, M.; Zaytsev, A.; Petrovskaia, A.; Burnaev, E. Challenges in Data-Driven Geospatial Modeling for Environmental Research and Practice. Nat. Commun. 2024, 15, 10700. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bhargava, J. Tasar sericulture: A sustainable economical booster. Ann. Sci. Allied Res. 2023, 1, 97–107. [Google Scholar]
- Rani, A.; Pandey, J.; Rajawat, D.; Pandey, D.; Chowdary, N. The Tasar Silk Industry: A gateway to a plethora of opportunities. Plant Arch. 2024, 24, 247–266. [Google Scholar] [CrossRef]
- Raju, P.J.; Mamatha, D.M.; Seshagiri, S.V.; Women’s University. Sericulture Industry’. In Environmental and Agricultural Informatics; IGI Global: Hershey, PA, USA, 2020; pp. 366–387. [Google Scholar] [CrossRef]
- Mohanty, P.K.; Mahānti, P.K. Tropical Wild Silk Cocoons of India; Daya Books: New Delhi, India, 2003. [Google Scholar]
- Chauhan, T.P.S.; Tayal, M.K. Mulberry Sericulture. Ind. Entomol. 2017, 197–263. [Google Scholar] [CrossRef]
- Subbulakshmi, V.; Yadava, N.; Soni, B.M.; Renjith, K.S.P. Colophospermum Mopane—A Potential Host for Rearing Wild Silk Worm (Gonometa Rufobrunnea) in Arid Rajasthan. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 549–560. [Google Scholar] [CrossRef]
- Bukhari, R.; Kour, H. Background, Current Scenario and Future Challenges of the Indian Silk Industry. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2448–2463. [Google Scholar] [CrossRef]
- Jena, K.; Kumari, R.; Pandey, J.; Kar, P.; Akhtar, J.; Gupta, V.; Sinha, A. Biochemical Characterization of Sericin Isolated from Cocoons of Tropical Tasar Silkworm Antheraea Mylitta Raised on Three Different Host Plants for Its Prospective Utilization. J. Asia-Pacific Èntomol. 2021, 24, 903–911. [Google Scholar] [CrossRef]
- Ravi Kumara, R.; Sneha, M.V. Breeding in Host Trees of Tasar Silkworm for Higher Foliage Productivity. J. Plant Dev. Sci. 2022, 14, 885–896. [Google Scholar]
- Bara, M.S.; Gilwax, P.I.; Muzeruddin, B.M.; Alok, S. Influence of Shorea Robusta Leaf Extract Treatment on Terminalia Arjuna Plants over Tasar Silkworm Growth and Economic Traits. J. Entomol. Zool. Stud. 2020, 8, 1095–1101. [Google Scholar]
- Bage, D.R.; Vishaka, G.V.; Mittal, V.; Chowdary, N.B.; Selvakumar, T. A Comparative Performance of Tasar Silkworm (Antheraea mylitta D.) Daba Ecorace and Bdr-10- An Only Authorized Race. Plant Arch. 2024, 24, 184–191. [Google Scholar] [CrossRef]
- Sinha, A.K. Variability in the Ecoraces of Tropical Tasar Sillkworm Antheraea mylitta Drury. Nat. Preced. 2011. [Google Scholar] [CrossRef]
- Saha, M.; Kundu, S.C. Molecular identification of tropical tasar silkworm (Antheraea mylitta) ecoraces with RAPD and SCAR markers. Biochem. Genet. 2006, 44, 72–85. [Google Scholar] [CrossRef]
- Chakraborty, S.; Muthulakshmi, M.; Vardhini, D.; Jayaprakash, P.; Nagaraju, J.; Arunkumar, K.P. Genetic Analysis of Indian Tasar Silkmoth (Antheraea mylitta) Populations. Sci. Rep. 2015, 5, 15728. [Google Scholar] [CrossRef]
- Barsagade, D.D.; Tbakre, M.P.; Meshram, H.M.; Gathalkar, G.B.; Gharade, S.A.; Thakre, R.P. Vanya Tasar Silk-Worm, Antheraea mylitta Eco-Race Bhandara, the Local Race and Its Conservation Strategy (Lepidoptera: Saturniidae). J. Sci. Inf. 2012, 3, 17–23. [Google Scholar]
- Prasad, R.; Ehrar, O.; Kumar, K.; Sukhija, N.; Manjappa, M.; Baig, M.M.; Gadad, H.; Singh, J.; Pandey, J.P.; Chowdary, N.B.; et al. Molecular interventions in sericulture: A focus on the tropical tasar silkworm. Plant Arch. 2024, 24, 111–119. [Google Scholar] [CrossRef]
- Omkar. An Introduction to Industrial Entomology. In Industrial Entomology; Springer: Singapore, 2017; pp. 1–3. [Google Scholar] [CrossRef]
- Chatterjee, S.N.; Vijayan, K.; Roy, G.C.; Nair, C.V. ISSR Profiling of Genetic Variability in the Ecotypes of Antheraea mylitta Drury, the Tropical Tasar Silkworm. Russ. J. Genet. 2004, 40, 152–159. [Google Scholar] [CrossRef]
- Hogue, A.S.; Breon, K. The Greatest Threats to Species. In Conservation Science and Practice; Wiley: Hoboken, NJ, USA, 2022; Volume 4. [Google Scholar] [CrossRef]
- Banks-Leite, C.; Ewers, R.M.; Folkard-Tapp, H.; Fraser, A. Countering the Effects of Habitat Loss, Fragmentation, and Degradation through Habitat Restoration. One Earth 2020, 3, 672–676. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat Fragmentation and Its Lasting Impact on Earth’s Ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Chung, M.Y.; Merilä, J.; Li, J.; Mao, K.; López-Pujol, J.; Tsumura, Y.; Chung, M.G. Neutral and Adaptive Genetic Diversity in Plants: An Overview. Front. Ecol. Evol. 2023, 13, e9926. [Google Scholar] [CrossRef]
- Hoban, S.; Campbell, C.D.; da Silva, J.M.; Ekblom, R.; Funk, W.C.; Garner, B.A.; Godoy, J.A.; Kershaw, F.; MacDonald, A.J.; Mergeay, J.; et al. Genetic Diversity Is Considered Important but Interpreted Narrowly in Country Reports to the Convention on Biological Diversity: Current Actions and Indicators Are Insufficient. Biol. Conserv. 2021, 261, 109233. [Google Scholar] [CrossRef]
- Alam, K.; Raviraj, V.S.; Kar, P.K.; Chacroborty, S. Diversity in Wild Tasar (Antheraea mylitta d.) Ecoraces of Simlipal Biosphere Reserve with Respect to Cocoon and Associated Parameters. Plant Arch. 2022, 22, 36–39. [Google Scholar] [CrossRef]
- Sinha, A.K. Conservation Package for Modal Ecorace. Nat. Preced. 2011. [Google Scholar] [CrossRef]
- Foucher, A.; Evrard, O.; Rabiet, L.; Cerdan, O.; Landemaine, V.; Bizeul, R.; Chalaux-Clergue, T.; Marescaux, J.; Debortoli, N.; Ambroise, V.; et al. Uncontrolled Deforestation and Population Growth Threaten a Tropical Island’s Water and Land Resources in Only 10 Years. Sci. Adv. 2024, 10, eadn5941. [Google Scholar] [CrossRef]
- Lele, A.A. Importance of Forests Outside Protected Area Networks for Large-Seeded Tree Species and Their Large-Bodied Avian Frugivores–A Study in Vazhachal Reserve Forest, India; University of Arkansas: Fayetteville, AR, USA, 2018. [Google Scholar]
- Papadakis, I.E.; Antonopoulou, C.; Sotiropoulos, T.; Chatzissavvidis, C.; Therios, I. Effect of Magnesium on Mineral Nutrition, Chlorophyll, Proline and Carbohydrate Concentrations of Sweet Orange (Citrus sinensis Cv. Newhall) Plants. Appl. Sci. 2023, 13, 7995. [Google Scholar] [CrossRef]
- Dubey, H.; Pradeep, A.; Neog, K.; Debnath, R.; Aneesha, P.; Shah, S.K.; Kamatchi, I.; Ponnuvel, K.; Ramesha, A.; Vijayan, K.; et al. Genome sequencing and assembly of Indian golden silkmoth, Antheraea assamensis Helfer (Saturniidae, Lepidoptera). Genomics 2024, 116, 110841. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Chowdhury, S.K.; Dey, S.; Moses, J.C.; Mandal, B.B. Silk: A Promising Biomaterial Opening New Vistas towards Affordable Healthcare Solutions. J. Indian Inst. Sci. 2019, 99, 445–487. [Google Scholar] [CrossRef]
- Martínez-Domínguez, L.; Nicolalde-Morejón, F.; Vergara-Silva, F.; Stevenson, D.W. A Review of Taxonomic Concepts and Species Delimitation in Cycadales. Bot. Rev. 2023, 90, 33–66. [Google Scholar] [CrossRef]
- Ullah, F.; Gao, Y.; Sari, I.; Jiao, R.-F.; Saqib, S.; Gao, X.-F. Macro-Morphological and Ecological Variation in Rosa Sericea Complex. Agronomy 2022, 12, 1078. [Google Scholar] [CrossRef]
- Hansen, L.S.; Laursen, S.F.; Bahrndorff, S.; Sørensen, J.G.; Sahana, G.; Kristensen, T.N.; Nielsen, H.M. The Unpaved Road towards Efficient Selective Breeding in Insects for Food and Feed—A Review. Èntomol. Exp. Appl. 2024, 17, 498–521. [Google Scholar] [CrossRef]
- Zilch, K.C.F.; Jahnke, S.M.; Köhler, A.; Bender, E. Effect of Diet, Photoperiod and Host Density on Parasitism of Anisopteromalus calandrae on the Tobacco Beetle and Biological Parameters of the Parasitoid. Am. J. Plant Sci. 2017, 8, 3218–3232. [Google Scholar] [CrossRef]
- Mishra, P.; Jena, D.; Thakur, R.R.; Chand, S.; Javed, B.; Shukla, A.K. Peri-Urban Floodscapes: Identifying and Analyzing Flood Risk Areas in North Bhubaneswar in Eastern India. Water 2024, 16, 3019. [Google Scholar] [CrossRef]
- Pandey, M.; Thakur, R.R.; Nandi, D.; Bera, D.K.; Beuria, R.; Kumari, M.; Kasawnea, A.M.; Zhran, M. Geospatial Monitoring of Environmental Sustainability: A Remote Sensing-Based Approach for Assessing Mining-Induced Impacts in Eastern India. Results Eng. 2025, 26, 104692. [Google Scholar] [CrossRef]
- Das, S.; Nandi, D.; Thakur, R.R.; Bera, D.K.; Behera, D.; Đurin, B.; Cetl, V. A Novel Approach for Ex Situ Water Quality Monitoring Using the Google Earth Engine and Spectral Indices in Chilika Lake, Odisha, India. ISPRS Int. J. Geo-Inf. 2024, 13, 381. [Google Scholar] [CrossRef]
- Dangayach, R.; Pandey, A.K. Technologies and methods for land use and land cover: A comprehensive review. In Remote Sensing and GIS Application in Forest Conservation Planning; Advances in Geographical and Environmental Sciences; Springer: Singapore, 2025; pp. 369–390. [Google Scholar] [CrossRef]
- Pettorelli, N.; Williams, J.; Bühne, H.S.T.; Crowson, M. Deep Learning and Satellite Remote Sensing for Biodiversity Monitoring and Conservation. Remote. Sens. Ecol. Conserv. 2024, 11, 123–132. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Štípková, Z.; Rejmánek, M.; Kindlmann, P. Predictions of Species Distributions Based Only on Models Estimating Future Climate Change Are Not Reliable. Sci. Rep. 2024, 14, 25778. [Google Scholar] [CrossRef]
- Sanguet, A.; Wyler, N.; Petitpierre, B.; Honeck, E.; Poussin, C.; Martin, P.; Lehmann, A. Beyond Topo-Climatic Predictors: Does Habitats Distribution and Remote Sensing Information Improve Predictions of Species Distribution Models? Glob. Ecol. Conserv. 2022, 39, e02286. [Google Scholar] [CrossRef]
- Giora, D.; Assirelli, A.; Cappellozza, S.; Sartori, L.; Saviane, A.; Marinello, F.; Martínez-Casasnovas, J.A. Remote Sensing Imaging as a Tool to Support Mulberry Cultivation for Silk Production. Remote. Sens. 2022, 14, 5450. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Komal, J.; Gowrisankar, R.; GV, V.; Nadaf, H.; Samal, I.; Kumar, P.V.D.; Selvaraj, C.; Reddy, B.T.; Selvakumar, T.; Mahanta, D.K.; et al. Bibliometric Trends and Patterns in Tasar Silkworm (Antheraea mylitta) Research: A Data Report (1980–2024). Front. Insect Sci. 2025, 5, 1533267. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, P.X.; Barros, S.; Mejía, D.; Villegas, F.R.; Siddons, D.C.; Latta, S.C. Using surrogate species and MaxEnt modeling to prioritize areas for conservation of a páramo bird community in a tropical high Andean biosphere reserve. Arctic Antarct. Alp. Res. 2024, 56, 2299362. [Google Scholar] [CrossRef]
- Konowalik, K.; Nosol, A. Evaluation Metrics and Validation of Presence-Only Species Distribution Models Based on Distributional Maps with Varying Coverage. Sci. Rep. 2021, 11, 1482. [Google Scholar] [CrossRef] [PubMed]
- Majhi, B.K.; Jena, P.; Prusty, B.A.K.; Mishra, A.K. Directive Strategies for Conservation and Threat Mitigation in Similipal Biosphere Reserve. Int. J. Ecol. Environ. Sci. 2022, 48, 801–815. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Wan, G.-Z.; Li, Q.-Q.; Jin, L.; Chen, J. Integrated Approach to Predicting Habitat Suitability and Evaluating Quality Variations of Notopterygium franchetii under Climate Change. Sci. Rep. 2024, 14, 26927. [Google Scholar] [CrossRef]
- Ahmadipari, M.; Yavari, A.; Ghobadi, M. Ecological Monitoring and Assessment of Habitat Suitability for Brown Bear Species in the Oshtorankooh Protected Area, Iran. Ecol. Indic. 2021, 126, 107606. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Singh, V.; Agrawal, H. Qualitative Soil Mineral Analysis by EDXRF, XRD and AAS Probes. Radiat. Phys. Chem. 2012, 81, 1796–1803. [Google Scholar] [CrossRef]
- Song, W.; Zhang, C.; Wang, Z. Investigation of the Microstructural Characteristics and the Tensile Strength of Silkworm Cocoons Using X-Ray Micro Computed Tomography. Mater. Des. 2021, 199, 109436. [Google Scholar] [CrossRef]
- Suryanarayana, N.; Srivastava, A.K. Monograph on Tropical Tasar Silkworm; Central Tasar Research and Training Institute, Central Silk Board: Bengaluru, India, 2005. [Google Scholar]
- Rai, M.M.; Rathod, M.; Wazalwar, S.; Raina, S.K. Tropical Tasar Insect. In Commercial Insects; CRC Press: Boca Raton, FL, USA, 2023; pp. 49–81. [Google Scholar]
- Singhvi, N.R.; Kushwaha, R.V.; Mathur, S.K.; Suranarayana, N. Role of Weather Factors on Disease and Pest Incidence on Tasar Food Plants and Silkworms and Developing Operational Disease and Pest Forewarning System for Tasar Sericulture; Department of Zoology: Muzaffarnagar, India, 2007. [Google Scholar]
- Ahmad, R.; Khuroo, A.A.; Hamid, M.; Charles, B.; Rashid, I. Predicting Invasion Potential and Niche Dynamics of Parthenium hysterophorus (Congress Grass) in India under Projected Climate Change. Biodivers. Conserv. 2019, 28, 2319–2344. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.D.; Pagán, A.; Candel, M.J.; Pérez-Rigueiro, J.; Cenis, J.L. Silkworm Gut Fibres from Silk Glands of Samia cynthia ricini—Potential Use as a Scaffold in Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 3888. [Google Scholar] [CrossRef] [PubMed]
- GV, V.; MS, R.; Nadaf, H.; SS, M.; DM, B.; SM, M.; Gedam, P.C.; NB, C. Spatial Variation in Cocoon Yield in Tropical Tasar Silkworm: An Influence of Insect-Predators and Pathogens. Plant Arch. 2022, 22, 40–44. [Google Scholar] [CrossRef]
- Jena, L.K.; Dash, A.K.; Behera, B. Host Plant Suitability and Altitudinal Variation in Cocoon Size of the Indian Tasar Silk Moth Antheraea mylitta Drury (Lepidoptera: Saturniidae). J. Lepid. Soc. 2017, 71, 182–188. [Google Scholar]
- Mazumdar, S.M.; Reddy, B.T.; Chandrashekharaiah, M.; Chowdary, N.B.; Chattopadhyay, S.; Rathore, M.S.; Sathyanarayana, K. Influence of abiotic factors on seasonal and nonseasonal emergence of Tasar silkworm, Antheraea mylitta Drury. J. Environ. Biol. 2023, 44, 445–451. [Google Scholar] [CrossRef]
- Sorte, C.J.; Jones, S.J.; Miller, L.P. Geographic variation in temperature tolerance as an indicator of potential population responses to climate change. J. Exp. Mar. Biol. Ecol. 2011, 400, 209–217. [Google Scholar] [CrossRef]
- Kar, P.; Vijayan, K.; Mohandas, T.; Nair, C.; Saratchandra, B.; Thangavelu, K. Genetic Variability and Genetic Structure of Wild and Semi-Domestic Populations of Tasar Silkworm (Antheraea mylitta) Ecorace Daba as Revealed Through ISSR Markers. Genetica 2005, 125, 173–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).