Abstract
The accelerated growth of the global population and rising food demand place increasing pressure on agricultural systems. While fertilizers have improved crop yields, they have also contributed to environmental degradation due to nutrient overuse, particularly nitrogen. Effective nitrogen management is therefore critical for achieving sustainable agricultural practices. This study investigates nitrogen dynamics in soil and reviews key analytical methods for monitoring total, mineral, and organic nitrogen. It evaluates protocols and technologies—including sensor-based systems—designed to optimize nitrogen application and reduce losses. The study demonstrates that the application of nitrogen fertilizers based on soil analysis and exploratory simulations, supported by Artificial Intelligence (AI) and the Internet of Things (IoT), can reduce inputs without compromising yield or quality parameters. Enhanced nitrogen monitoring techniques can significantly contribute to the goals of the Nitrates Directive (91/676/EEC) and promote sustainable farming, especially in regions facing environmental and technical limitations. Adoption, however, depends on overcoming challenges such as sensor affordability and farmer training. In conclusion, it emphasizes the crucial role of nitrogen management in maintaining soil health, optimizing crop yields, and minimizing environmental impact, ensuring that farming practices remain both productive and sustainable for future generations.
1. Introduction
Rising global food demands have made it increasingly challenging to balance agricultural inputs for optimal crop productivity with the imperative of environmental sustainability [1]. Among these inputs, nitrogen is indispensable due to its involvement in numerous physiological and biochemical processes essential for plant growth and development. It is a fundamental component of amino acids and proteins, plays a critical role in chlorophyll synthesis, and promotes overall plant growth [2].
The role of nitrogen in agriculture is indisputable; however, its use requires careful consideration of multiple factors. While the application of fertilizers aims to enhance crop yields, it can also result in unintended consequences such as soil, crop, and water pollution, as well as increased greenhouse gas emissions.
Of all essential nutrients, nitrogen is often the most limiting factor for crop production, and its application in the form of fertilizers has become vital for achieving higher yields—particularly in response to the growing global population over the past century. Monitoring soil nutrient levels is therefore crucial, as imbalances can reduce crop yield and quality while contributing to environmental pollution.
In the case of nitrogen, excessive application—often resulting from overfertilization—can lead to elevated nitrogen levels in the soil. This surplus nitrogen may run off into surface waters such as rivers and lakes or leach into groundwater, causing eutrophication and contamination of water sources [3,4,5].
Although significant progress has been made in nitrogen management strategies, existing practices often exhibit low nutrient use efficiency and raise environmental concerns. Conventional fertilizers typically lack precise delivery systems and the flexibility to adapt to real-time soil–crop interactions. To overcome these limitations, nano-fertilizers have been introduced. They represent a significant advancement in nutrient delivery due to their biodegradable nature and controlled nitrogen release, which improves plant nutrient uptake and consequently minimizes losses [6]. Recent studies have shown promising results in integrating Artificial Intelligence (AI) and the Internet of Things (IoT) for site-specific nitrogen management in various cropping systems. AI algorithms and automation technologies are used to interpret field images and enable the precise application of inputs, allowing for real-time feedback, reduced input waste, and improved crop yields [7,8].
Nitrogen pollution is expected to increase by 150% by the year 2050 relative to 2010 levels, with the agricultural sector contributing around 60% of this projected rise [9].
Furthermore, plants can accumulate excessive nitrate concentrations from the soil, and the consumption of nitrate-rich products may pose significant health risks to humans [10,11]. Nitrogen also contributes to greenhouse gas emissions, particularly in the form of nitrous oxide (N2O), which has a global warming potential significantly greater than that of carbon dioxide (CO2), thereby exacerbating climate change [12].
This paper explores strategies for controlling nitrogen in agricultural soils, including techniques for quantifying different nitrogen forms. Monitoring nitrogen levels in soil and crops is essential for sustainable agriculture—not just to improve plant health and maximize yields, but also to minimize input waste and reduce environmental impact [13].
It also highlights the importance of environmental monitoring, controlled fertilization, and effective nitrogen management as key elements for achieving long-term sustainability in agriculture. Sustainable nitrogen management relies on an integrated framework that combines precision agriculture, optimized fertilizer use, crop rotation, organic inputs, stabilizers, and nutrient management strategies—ensuring environmental protection, soil health, and sustained agricultural productivity.
The purpose of this paper is to analyze effective strategies for nitrogen management in agricultural soils, focusing on monitoring different nitrogen forms, applying controlled fertilization, integrating advanced technologies (such as AI and IoT), and promoting a sustainable framework to maintain agricultural productivity while protecting the environment.
2. Nitrogen Forms in the Soil
Nitrogen plays a crucial role in soil fertility, and it occurs in both organic and inorganic forms—primarily as ammonium (NH4+), nitrate (NO3−), and, to a lesser extent, nitrite (NO2−). In the following sections, these nitrogen forms, their significance, the relationships between them, the processes they are involved in, and their importance in relation to soil fertility will be discussed.
2.1. Total Nitrogen
Total nitrogen refers to the sum of all nitrogen forms (mineral, organic) present in the soil. It is crucial for soil fertility and provides a comprehensive measure of the soil’s nitrogen pool. It plays a key role in the formation of amino acids, proteins, and enzymes in plants. Regular monitoring of total nitrogen levels is used to assess nitrogen availability from natural decomposition of organic materials and helps farmers evaluate the overall nitrogen status of the soil, ensuring high-quality yields while minimizing environmental impacts.
The maximum total nitrogen content is found in the surface horizon, with its distribution varying across the profile. In some soils formed under forest vegetation, there is a much higher concentration of nitrogen near the surface compared to other soils formed under grassland vegetation. In most soils, the accumulation of organic matter and nitrogen is found in the 0–50 cm layer [14]. The plowed layer of most cultivated soils in temperate regions contains 0.1–4.0 g kg−1 total nitrogen. The exact amount of nitrogen present in each case depends on several factors: the influence of climate, vegetation type, and human activity, and the duration of these influencing factors. In deeper horizons, the average total nitrogen content is below 0.1 g kg−1, while in surface horizons, it rises to 0.8–4.0 g kg−1. Furthermore, total nitrogen content depends on soil texture and follows the order of sand < fine sand < sandy loam < loam < silt loam [14].
In the case of forest soils, total nitrogen content from soil is higher. For example, for forest soils from Romania, values of up to 4.4 g kg−1 were reported [15], whereas soil samples collected from Italian forests contained 3.7 g kg−1 [16].
The total nitrogen content plays a vital role in the sustainability of agricultural ecosystems and soil health.
2.2. Mineral Nitrogen
Mineral nitrogen, also referred to as assimilable nitrogen, includes soluble forms of ammonium (NH4+), nitrate (NO3−), and nitrite (NO2−). These are the forms that plants can directly absorb to synthesize proteins, enzymes, and chlorophyll. Generally, plants prefer to absorb nitrate ions over ammonium ions. Due to its size and negative charge, nitrate is highly mobile in the soil and can leach into groundwater if present in excess, which can be detrimental to the environment.
Ammonium ions (NH4+) are less mobile than nitrate ions (NO3−) because they bind to the soil’s adsorptive complex through electrostatic interactions. Mineral nitrogen is often added to soil through fertilizers, and monitoring its levels in soil helps farmers optimize fertilization. This can prevent overapplication of fertilizers, which can lead to environmental harm, such as water pollution and greenhouse gas emissions.
Generally, ammonia nitrogen in its soluble and exchangeable form, which is available to plants, is present in quantities below 10 mg kg−1, except during periods when ammonium-based fertilizers are applied. The nitrate nitrogen content in the arable layer is typically below 20 mg kg1 in unfertilized soils, between 20–40 mg kg−1 in fertilized soils, and can exceed 60 mg kg−1 in horticultural soils [17].
Nitrate ions periodically accumulate in the soil, typically after fertilization, following harvest when plant residues decompose through microbial activity, and during late winter before plant growth [18]. Additionally, nitrate levels may also increase after application of manures, crop residues, and ammonium fertilizers through mineralization and nitrification, as presented in Section 2.4.
Limits in interpreting nitrogen levels are related to crop type, and a compilation of data [17] to evaluate soil supply with nitrogen is presented in Table 1.

Table 1.
Interpretation of supply level of soils with nitrogen.
Many studies deal with pollution resulting from agriculture, mainly after fertilization practices, emphasizing the effects on soil, crops, and water [19,20,21,22,23]. Approximately 50% of the nitrogen fertilizer applied is absorbed by crops, while the remainder is mineralized and later taken up by plants, with the rest being lost through leaching [24].
Considering the danger posed by nitrates resulting from agricultural practices, the European Union introduced the Nitrates Directive (91/676/EEC) in 1991 [25] to safeguard water bodies from contamination. According to the aforementioned directive [25], EU member states are required to identify nitrate-vulnerable zones (NVZs), which are areas where surface or groundwater is contaminated with nitrates at levels exceeding 50 mg L−1. Furthermore, within these zones, there is a limit of 170 kg ha−1 year−1 of nitrogen from organic manure applied on farms.
Some studies evaluate the effect of nitrogen fertilization on nitrate accumulation in soil, plants, and water. For example, Mosaad and his team [26] investigated the effects of varying nitrogen application rates (0, 140, 280, 420 kg N ha−1) and irrigation intervals on clay soil properties and groundwater nitrate levels, with maize as the experimental crop. Their findings indicated that increasing nitrogen doses significantly increased plant-available soil mineral nitrogen after the first and second irrigations. Additionally, groundwater nitrate levels also increased with higher fertilization rates. However, longer intervals between irrigations significantly decreased groundwater nitrate levels. The study suggests that optimizing nitrogen fertilization combined with proper irrigation practices can mitigate nitrate contamination in groundwater, thereby supporting sustainable agricultural practices.
The adoption of appropriate fertilization practices strongly influences nitrate accumulation in soil without negatively impacting crop yield. For instance, Yan and colleagues [27] conducted an experiment to investigate the accumulation of nitrate in soil following different fertilization treatments, which included urea fertilization via split application (1), fertilization with a low nitrogen rate based on soil testing (2), and the application of controlled-release fertilizer (3). The results demonstrated that treatments (2) and (3) reduced nitrate nitrogen by 16.6% and 39.5%, respectively, in the 0–90 cm soil layer while maintaining relatively high maize yields compared to treatment (1). However, rainfall promoted nitrate leaching in all treatments. Therefore, strategies aimed at controlling nitrate leaching should consider fertilization methods, soil properties, and precipitation patterns.
One of the strategies that can promote sustainable practices by reducing nitrate accumulation in plant products is the use of organic nano-fertilizers. In this context, a study conducted by Abdelkader [28] demonstrated that the transition from mineral to organic nano-fertilizers resulted in a significant reduction in nitrate levels in fresh celery crops, decreasing concentrations from 342.4 mg kg−1 to 100 mg kg−1.
2.3. Organic Nitrogen
Organic nitrogen represents nitrogen bound in organic compounds (amino acids, peptides, amine and amino sugars) and can be categorizes into two types: (i) nitrogen from organic residues and (ii) nitrogen from soil organic matter or humus.
The primary sources of organic nitrogen in agricultural soils include manure, crop residues, roots, and root exudates, along with bacteria, fungi, and animal tissues [29]. Therefore, managing organic nitrogen through composting, cover cropping, and proper crop rotation can enhance soil fertility and long-term productivity [30].
This organic nitrogen is not immediately available to plants but becomes accessible through microbial decomposition and mineralization, a process in which soil microorganisms break down organic materials to release inorganic forms of nitrogen (like ammonium and nitrate) that plants can absorb (Figure 1).
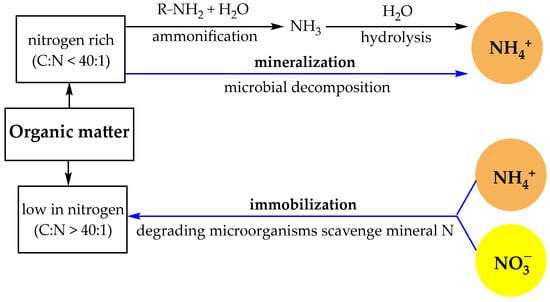
Figure 1.
Organic nitrogen transformations in the soil [31].
Various soil microorganisms break down organic matter, such as organic nitrogen, into inorganic forms that plants can use. Contrariwise, the immobilization process of nitrate (NO3−) and ammonium (NH4+) in soil refers to their conversion into organic forms that are incorporated into microbial biomass or organic matter [31]. The processes involved in the decomposition of organic matter (organic nitrogen) are depicted in Figure 1.
In the surface layer of most soils, more than 90% of nitrogen is found in organic forms [32].
The content of organic nitrogen in soil is constantly changing, and the amount present at any given time represents the difference between annual accumulations and losses.
The nitrogen in cultivated soils is mostly in organic form and accumulates through biological fixation processes [13]. Organic nitrogen is largely unavailable to growing plants. Notwithstanding, according to different studies [33,34], there are agricultural species that readily absorb organic nitrogen. Its uptake and assimilation by different crop species are investigated on the basis of stable isotope studies [29]. Hence, studies with 15δN have shown that in temperate regions, 20 to 40% of the applied nitrogen fertilizer is converted into organic forms within the first growing season [35].
The study of organic nitrogen in soil is achieved by analyzing fractions appearing after acid hydrolysis, which include hydrolysable total nitrogen, unidentified nitrogen, and acid-insoluble nitrogen fraction bound to soil minerals even after hydrolysis in acidic medium. Furthermore, hydrolysable nitrogen is composed of hydrolysable NH4+-N, amino sugars-N, and amino acids-N (Figure 2) [35,36].
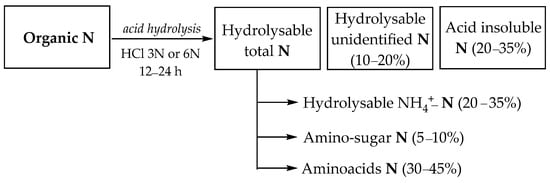
Figure 2.
Organic nitrogen fractions in soil after acid hydrolysis.
The evaluation of organic nitrogen fractions was used to investigate the effect of manure fertilization in relation to soil type and incubation time on soil nitrogen forms and availability [37]. In this regard, Adame et al. [37] conducted an experiment that included two soil types (clayey and sandy), the application of organic manure (20 t ha−1), and varying incubation times (14, 45, 90, and 180 days). The obtained results indicated that fertilization increased organic matter and mineral nitrogen in both soil types. Regarding the dynamics of organic nitrogen fractions, these varied depending on the soil type. For example, hydrolysable unidentified nitrogen was higher in clayey soils, whereas in sandy soils, the hydrolysable ammonium fraction showed a greater increase. In conclusion, manure fertilization had different effects on the dynamics of nitrogen forms, with the correlations between nitrogen forms behaving differently depending on the soil type.
Hydrolysable nitrogen levels, which varied depending on soil type and applied fertilizers, were also observed by Mazur and Mazur [38] in a long-term experiment where manure, slurry, and NPK fertilizer were applied to lessive and brown soils. Based on the results, manure was the most effective in increasing nitrogen levels, followed by slurry and mineral fertilization. The hydrolysable nitrogen content increased by 25.6% and 51.3% in lessive and brown soils, respectively, compared to the control variant.
In addition, soil organic nitrogen fractions were analyzed to assess the impact of land-use changes [39]. In this regard, total nitrogen, available nitrogen, and soil organic nitrogen fractions were examined in the topsoil (0–20 cm) and subsoil (20–40 cm) to investigate the effects of land-use changes on soil organic nitrogen fractions.
The experiment included natural grassland (uncultivated), farmland (converted from natural grassland), and artificial forestland (grain plots converted into forests) in the black soil region of Northeast China. The results indicated that the conversion of natural grassland to farmland reduced nitrogen accumulation, whereas the conversion of grain plots into forestland improved soil nitrogen storage capacity. However, the potential nitrogen supply did not reach the levels observed in uncultivated lands.
Drescher and co-workers [40] determined hydrolysable nitrogen under alkaline conditions using direct steam distillation for paddy soil samples collected from a depth of 0–20 cm. The obtained results ranged from 84 to 279 mg kg−1. Additionally, a positive correlation (r = 0.86) was found between hydrolysable nitrogen content and total nitrogen content. Another study [41] used a similar procedure for assessing hydrolysable nitrogen in soil samples collected from a depth of 0–20 cm (from Ghana). The obtained values varied slightly depending on whether NaOH or KOH was used for hydrolysis, as follows: 104.6–306.9 mg kg−1 and 143.5–371.3 mg kg−1, respectively. Furthermore, studying organic nitrogen fractions and their transformation over time could be useful for predicting the availability of nitrogen for crop plants. In this regard, it has been found that organic fertilization influences organic nitrogen fractions, but it is strongly related to soil type [37].
2.4. Concluding Remarks on Nitrogen in Soil
Annually, a fraction of the organic nitrogen in the soil undergoes mineralization, while another portion is immobilized in organic forms. The resulting forms, ammonium and nitrate, produced through mineralization and nitrification, respectively, are readily available for plant uptake and are essential for plant growth. A portion of nitrogen is lost to the atmosphere through denitrification as nitrogen gas (N2) and nitrous oxide (N2O), while another portion is leached, contributing to water pollution and its associated negative effects.
Denitrification has both positive and negative effects. On the positive side, it reduces nitrate leaching, thereby decreasing water pollution. On the negative side, it leads to the loss of nitrogen that could otherwise be used by plants and results in the production of nitrous oxide (N2O), a greenhouse gas with a lifetime of over 100 years and a global warming potential approximately 300 times greater than that of carbon dioxide (CO2) [18].
Additionally, some nitrogen may be lost through erosion or sequestered in clay minerals and organic compounds (such as humus and microorganisms).
Figure 3 illustrates nitrogen moves through soil in different chemical forms, impacted by biological activity and environmental processes—highlighting potential nutrient losses and pollution pathways [2].
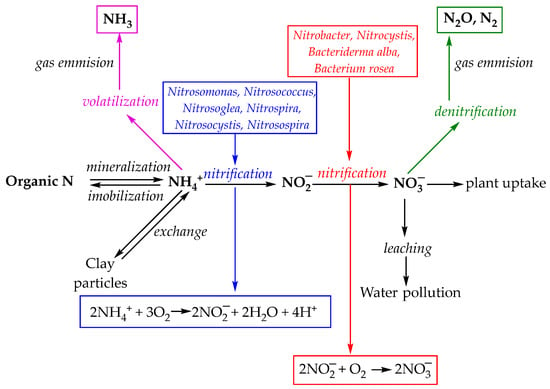
Figure 3.
Nitrogen transformation in soil [2].
The balance between mineral and organic nitrogen is crucial for maintaining soil fertility, as the organic nitrogen pool serves as a reservoir that can be converted into mineral forms over time.
The transformation rate of one nitrogen form to another is evaluated based on the “half-life” (the time required for the initial nitrogen quantity to decrease by 50% as a result of the transformations it undergoes) and is presented in Table 2 [42]. Mineralization is the slowest process, with a half-life of 100–200 days, while denitrification is the fastest, with just 7–15 days. In general, the processes that convert nitrogen from one form to another (from organic to inorganic, from ammonium to nitrate or gas) have different half-lives, reflecting the differences in their dynamics within the soil environment.

Table 2.
Transformations of nitrogen forms and corresponding half-life.
These transformations are crucial in the nitrogen cycle, impacting soil fertility, nitrogen availability, and greenhouse gas emissions such as N2O (nitrous oxide).
To synthesize the information presented in the previous subchapters, Table 3 provides a comparison of soil nitrogen forms, each with distinct characteristics and roles in fertility, plant availability, and long-term soil health.

Table 3.
Overview of nitrogen forms from soil.
3. Methods for Control of Nitrogen Levels in Soil
Sustainable agriculture relies on nutrient monitoring to balance crop performance with environmental responsibility. Efficient nitrogen management begins with accurate assessment, using methods that range from traditional soil testing to advanced precision technologies.
Assessment of nitrogen levels from soils depends strongly on several factors: objectives of the assessment (long-term monitoring, evaluation of nitrogen levels for immediate decision-making), available equipment, laboratory resources, and needed accuracy.
Laboratory testing is essential for soil nitrogen analysis, environmental monitoring purposes, research, and adopting correct nitrogen fertilization practices on farms.
Nowadays, soil testing can be performed in a laboratory after sampling and conditioning the sample by traditional methods, but advanced techniques (sensors, microdevices, remote sensing) can be employed to provide accurate results, which will help to adopt efficient and sustainable nitrogen management strategies.
Methodologies used for nitrogen assessment encompass a wide range of analytical techniques, each of which presents advantages and limitations (Table 4). Independent of the analytical method applied, key limitations include the need for technically trained personnel, time-consuming analytical procedures, and substantial costs associated with the acquisition and maintenance of instruments, as well as consumable materials.
Total nitrogen serves as an indicator of the soil’s potential for this element, but it does not reflect the amount available to plants. During growth and development, only about 0.5–2.5% (and rarely up to 5%) of total nitrogen is converted into forms accessible to plants, with this conversion being influenced by temperature, moisture, and microbiological activity.
Total nitrogen assessment is traditionally performed using the Kjeldahl method, but other methods are now also accepted and used due to their many advantages (Table 4). For example, Refs. [43,44] compare total nitrogen levels obtained by the Kjeldahl or dry combustion methods and report similar results. However, for soils rich in organic matter, the Kjeldahl method is recommended.
Hydrolysable organic nitrogen includes aminic organic compounds that easily hydrolyze in either acidic or basic conditions. On the basis of this form are assessed nitrogen species susceptible to mineralization [38]. Methods for determining hydrolysable nitrogen are based on the hydrolysis of organic nitrogen-containing species in either an alkaline or acidic medium and have advantages and limitations, as well (Table 4).
Mineral forms of nitrogen, such as NO3− and NH4+, are mobile in soil, and in some cases, unexpected nitrogen deficiencies may occur. Consequently, the results obtained from the analyses, which characterize the momentary status when the sample was collected, are valid for only half of the month, indicating short-term nitrogen availability. Therefore, the measures that need to be implemented following the analyses must be applied immediately.
The determination of mineral nitrogen is usually performed on fresh soil samples at harvest moisture. If this is not possible, it is recommended to use methods that slow down the microbiological activity of the soil [13]. In this regard, nitrification inhibitors are used to suppress the oxidation of ammonium to nitrate [45].
For example, one study [46] demonstrated that nitrate levels were 2 and 3.5 times higher in soil extracted with 1 M KCl the day after sampling. Additionally, soil samples stored for one month, either refrigerated or air-dried, had nitrate concentrations significantly higher than soil extracted in the field or the day after collection. Therefore, the storage of soil samples intended for nitrate determination should be avoided or minimized, if possible.
Additionally, there are studies that emphasize that freezing and air-drying soil samples are acceptable preservation methods for soil samples with nitrate and ammonium levels below 30 mg kg−1 and 3 mg kg−1, respectively. Also, samples with high organic matter content should be analyzed when they are fresh [47].
Nitrate, being soluble, is frequently determined in aqueous or saline extracts (Table 4). Most determination methods are based on the property of nitrate to form colored compounds with various organic molecules (frequently with phenol-2,4-disulphonic acid in alkaline medium), followed by spectrophotometric quantification.
However, there are some limitations due to the presence of certain ions, such as chloride ions, which can be removed by precipitation. In such cases, if spectrophotometric assessment is adopted, particular care must be taken, especially with saline soils. These limitations are overcome by using other quantification techniques. For example, Wada and his team [48] determined nitrate and chloride ions in aqueous extracts with average relative errors of 18% and 17%, respectively, by ion chromatography.
Unlike the extraction of nitrate nitrogen, for ammoniacal nitrogen this process is more problematic because ammonium ions exist in soil in both soluble and exchangeable forms, adsorbed by soil colloids or fixed in clay minerals. If the goal is to assess the nitrogen supply status for plants during a vegetation period, saline solutions can be used (Table 4). Quantitative determination of ammoniacal nitrogen is typically performed spectrophotometrically using Nessler’s reagent or, more recently, with selective ion electrodes.
Recently, new methods, or improvements to older ones, have been developed for the assessment of mineral nitrogen. For instance, a digital colorimetric method for determining mineral nitrogen (NO3−, NH4+) was reported [49]. This method involves the extraction of ions using a KCl solution, followed by spectrophotometric analysis. The original procedure was refined to enhance sensitivity, resulting in lower limits of detection and quantification: 0.42 and 1.4 mg kg−1 for NO3− and 1.1 and 3.7 mg kg−1 for NH4+, which are lower than those of standard methods.

Table 4.
Commonly used techniques for soil nitrogen assessment.
Table 4.
Commonly used techniques for soil nitrogen assessment.
| N | Soil Sample Preparation/Extraction | Quantification (Lab/Field Techniques) | Advantages (A) and Limitations (L) | Ref. |
|---|---|---|---|---|
| Total nitrogen | N is converted in NH4+ by digestion with H2SO4 conc. (Kjeldahl method) | Titration of NH3 resulted by distillation (lab technique) | A: Comprehensive for total nitrogen (organic + inorganic); accurate; widely used and accepted. L: Requires attention in handling hazardous chemicals (H2SO4); time consuming, laborious. | [14,50,51,52] |
| Combustion in the presence of CuO at high temperature (600 °C) (Dumas method) | Resulting N2 is quantified using a thermal conductivity detector (lab technique). | A: Rapid, can measure total nitrogen (organic + inorganic); no reagents used; high precision; safer than Kjeldahl. L: Specialized expensive instruments. | [50,52] | |
| Extraction with 0.025 M CaCl2 and digestion by Koroleff’s method | Spectrophotometric, on the basis of the protocol proposed by Merck (field technique) | A: Easy, precise measurement for total nitrogen. L: Total nitrogen test kits, reagents, and photometers need to be purchased. | [53,54] | |
| The soil sample is subjected to the removal of grass and plant debris and other impurities. | Near-infrared spectroscopy (NIRS) technique (lab technique) | A: No chemicals; little sample preparation; non-destructive; provides real-time data. L: Extensive research on calibration models. | [55,56] | |
| Soil samples are collected and air-dried, and plant residues and stones are removed from the soil. | Hyperspectral imaging technology (lab technique) | A: Rapid; accurate; allows the change in total nitrogen content in soil to be monitored in real time. L: Extensive calibration and validation; data interpretation requires specialized personnel. | [57,58,59] | |
| Hydrolysable nitrogen | Acidic hydrolysis (0.5 N H2SO4 or 3 N, 6 N HCl) Alkaline hydrolysis (2% H3BO3 and 0.25 N NaOH) Oxidative hydrolysis (1 N KMnO4, 1 N H2SO4 or chromic acid) With hot water | Distillation, titration (lab technique) | A: Simple, no expensive equipment. L: Laborious, time-consuming. | [14,35,36,38,60,61] |
| Ammonium nitrogen, NH4+-N | Extraction with saline solutions (2 N KCl, 0.1 N K2SO4, 1% K2SO4), distilled water using specific extraction ratios. Extraction with 2 M KCl, 0.1 M MgSO4 | Ammonium reacts with Nessler’s reagent to form a yellow color, which is measured spectrophotometrically (420 nm) (lab technique). | A: Simple, inexpensive. L: Limited sensitivity at low levels; interference from other species. | [14,61,62] |
| A specific ion-selective electrode (ISE) measures the concentration of ammonium ions in the soil extract (lab technique). | A: Fast, easy to operate. L: Requires specific electrode and equipment; sensitivity could be affected by the presence of other ions in soil extract. | [63] | ||
| Extraction with 2 M KCl | Spectrophotometric measurement at 636 nm of blue-colored compound resulted after the addition of phenol–nitroprusside and hypochlorite reagents to an aliquot of soil extract (lab technique). | A: Rapid, sensitive, accurate method; allows for the quantification of low levels of ammonium. L: Interference with other cations found in soil extract; long time for analysis. | [64] | |
| Extraction with 1 M KCl | Rapid detection kit coupled with a microplate reader (lab technique) | A: Simple reagent preparation, convenient operation, shorter detection time (96 samples in 30 min). L: Suitable for paddy soils only. | [65] | |
| Distilled water | Ammonium ions form with salicylic acid in the presence of sodium nitroprusside and sodium hypochlorite, a colored complex with absorption maxima at 697.5 nm (lab technique) | A: Suitable for trace amounts of ammonium, good selectivity. L: Interference with other ions from soil. | [66] | |
| Nitrate nitrogen, NO3−-N | Extraction with saline solutions (2 N KCl, 0.1 N K2SO4, 1% K2SO4), distilled water using specific extraction ratios | Nitrate ions react with specific reagents (phenol-2,4-disulphonic acid method) to develop colored nitro derivatives that absorb the light at certain wavelengths (420 nm) (lab technique). | A: Simple, commonly used for measuring nitrate nitrogen. L: Laborious, time-consuming, requires attention with used reagents. | [14,62] |
| Nitrate ions are reduced to nitrite on a cadmium column, and afterwards, nitrite ions are quantified spectrophotometrically after a diazotation reaction (lab technique). | A: Very sensitive, easy to operate. L: Careful handling due to the use of cadmium. | [14] | ||
| Extraction with distilled water, deionized water | A specific ion-selective electrode (ISE) measures the concentration of nitrate ions in the soil extract (lab technique). | A: Fast, easy to operate, efficient measurement. L: Requires specific electrode and equipment; interference from other ions encountered in soil extract. | [67] | |
| Extraction with 2 M KCl, 0.1 M MgSO4 | A specific ion-selective electrode (ISE) measures the concentration of nitrate ions in the soil extract (lab technique). | A: Fast, easy to operate. L: Requires specific electrode and equipment; sensitivity could be affected by the presence of other ions in soil extract. | [63] | |
| Extraction with 2 M KCl and cation exchange resin (Ag+) for Cl− precipitation and removal | Ion chromatography (lab technique) | A: Sensitive, precise, rapid technique. L: Laborious, interference with other ions found in soil extract. Requires specialized equipment; more expensive and time-consuming than other methods. | [68,69] | |
| Extraction with distilled water | Ion chromatography (lab technique) | A: Sensitive, precise, rapid technique. L: Laborious, interference with other ions found in soil extract. Requires specialized equipment; more expensive and time-consuming than other methods. | [48] | |
| N-NO3−, N-NO2− | Extraction with 5–25 mM K2SO4, distilled water (ultrasonic assisted extraction) | Ion chromatography (portable device) (field technique) | A: Real-time monitoring, on-site analysis; allows for the analysis of soil samples with a high content of organic matter; non-hazardous reagents. L: Specialized expensive instruments. | [70] |
Besides the above-mentioned laboratory methods, field-based kits can also be used for the quick and low-cost quantification of nitrogen species. However, the obtained results are generally less accurate than those from lab-based testing [71]. In this regard, one study [72] demonstrated that standard commercially available soil tests are relatively poor indicators of soil nutrient levels compared to laboratory-based analyses. Accordingly, it is recommended that fertilization decisions be informed by comprehensive laboratory testing rather than by on-site kits.
Lately, many microdevices derived from microelectronics have been developed for in situ soil analysis. For example, Joly and co-workers [73] constructed microdevices based on ChemFET technology, suitable for soil nitrogen analysis. The resulting sensors (pNH4-ISFET and pNO3-ISFET) were tested for acidic clay–silt soil where wheat crop was grown in southwest France. The initial tests using ChemFET sensors were conducted under laboratory conditions by simulating rainy conditions with deionized water and fertilization practices through the addition of ammonium nitrate at different levels. Soil monitoring was carried out over a 15-day period at an ambient temperature of 21 °C. Based on these sensors’ measurements, the application of ammonium nitrate was studied at different concentrations, fertilization kinetics, soil nitrification, and even ammonium inclusion into a clay–humus complex.
As nitrogen sensing in soil is important from both agronomic and environmental perspectives, many scientists have proposed alternatives to traditional nitrogen detection methods. One of the adopted variants is portable sensors based on voltametric methods. For example, Chen and his team [74] developed an on-site detection platform for nitrate capable of extracting and quantifying this nitrogen form. The sensing element, based on cadmium sulfide nanorod-modified screen-printed electrodes (CdS NRs-SPE), demonstrates excellent reproducibility, long-term stability, and high selectivity towards nitrate reduction. The analysis time is 10 min, and the cost of analysis is affordable. The excellent performance of the CdS NRs-SPE was demonstrated by testing nitrate levels in four different soil samples and comparing the results with those obtained using UV-VIS spectrometry. For example, using the new electrode, the measured nitrate concentrations were 464.05, 234.23, 198.79, and 128.22 mg kg−1, while the UV-VIS method yielded values of 504.45, 223.93, 219.05, and 131.04 mg kg−1, respectively. The new nitrate sensing platform is portable and has strong potential for successful application in precision agriculture.
Another team [75] proposed an ion-selective screen-printed electrode for real-time continuous in situ soil nitrate monitoring in the range of 5–512 mg kg−1. The calibration curves were built for clay, loamy clay, and sandy loam soils, and all of them had R2 > 0.957. A 7-day in situ soil study demonstrated the sensor’s ability to measure soil nitrate dynamically over time, with an error rate of less than 20%.
Additionally, Baumbauer and co-workers [76] developed printed potentiometric nitrate sensors based on an ion-selective electrode and a reference electrode functionalized with polymeric membranes. These electrodes were integrated into a printed sensor with a sensitivity of −48.0 ± 3.3 mV/dec between 0.62 and 6200 mg kg−1 nitrate in solution and −47 ± 4.1 mV/dec in peat soil. Investigation of the sensor’s selectivity revealed that it was influenced by high concentrations of calcium but was not impacted by sulfate, chloride, phosphate, nitrite, ammonium, potassium, or magnesium at concentrations typically found in soil.
Li and Smith [77] compared measurements with a commercial ion-selective electrode with continuous flow analysis (CFA) for nitrate determination at low concentrations, using as extractants saturated CaSO4 solution and 1 M KCl. The obtained results with the ion-selective electrode were highly correlated with those obtained by the CFA analyzer (R2 = 0.93). In addition, the CaSO4 solution was highly effective for the extraction of nitrate ions from air-dried soils.
Furthermore, another team [78] developed a self-designed prototype system for on-site soil nitrate assessment, employing an ISE for nitrate measurement and a soil moisture sensor. The results obtained with this system are in good agreement with those obtained in the laboratory using UV-VIS and ISE methods. The major advantage of this system is its short analysis time of 4–5 min per sample, which is 60% shorter than manual sample preparation.
Nitrate and nitrite in field evaluation of soil samples were analyzed by Mai and co-workers [70] using a portable ion chromatograph analyzer after ultrasonic-assisted extraction, either with K2SO4 (0–25 mM) or, for simplification of the methodology, with distilled water. The obtained results for soil analysis, which agreed with those obtained by laboratory ion chromatography, were 94.8 ± 4.3 mg kg−1 for nitrate and 5.4 mg kg−1 for nitrite. In addition, relative standard deviations were below 10%.
Monitoring nitrate levels in soil solution using an all-solid-state miniature potentiometric soil sensor has been reported by Ali and his team [79]. The electrode is composed of a nanocomposite of poly(3-octyl-thiophene) and molybdenum disulfide, coated on a patterned Au electrode and covered with a nitrate-selective membrane. The resulting sensor can measure nitrate levels ranging from 1 to 1500 mg kg−1, is selective for nitrate in the presence of other anions at significant concentrations, and can be deployed in the soil for up to 4 weeks of accurate monitoring.
Precision nitrogen management aimed at minimizing nitrogen losses can be assessed using various sensors. In one experiment, three non-destructive sensing devices—the Leaf Color Chart (LCC), Soil Plant Analyzer Development (SPAD) meter, and GreenSeeker—were evaluated under different nitrogen application rates (0, 90, 120, and 159 kg N ha−1) in wheat cultivars. The highest agronomic efficiency of nitrogen (21.2%) was achieved with the application of 120 kg N ha−1, split into two doses: the first applied after the first irrigation, and the second at the leaf development stage [7].
The use of precision agriculture technologies to monitor maize yield under different nitrogen fertilization rates and irrigation regimes was demonstrated by a study conducted in Hungary during the 2022 agricultural year by Szeles and co-workers [80]. By integrating Soil Plant Analysis Development (SPAD) measurements and the UAV-based Normalized Difference Vegetation Index (NDVI), the study concluded that the application of 120 kg N ha−1 before sowing led to the highest grain yield (8.649 t ha−1) under irrigated conditions.
Furthermore, a study conducted in Spain [81] evidenced the importance of precision agriculture tools—such as handheld optical sensors (GreenSeeker®) and the UAV-based Normalized Difference Vegetation Index (NDVI)—for accurate assessment of nitrogen status and the development of site-specific fertilization recommendations. Using hand-held crop sensors in two wheat fields in Spain, researchers observed average yields of approximately 3498 kg ha−1 and 3221 kg ha−1, alongside NDVI values of around 0.67 and 0.68, respectively. A strong positive correlation between yield and NDVI was found in both fields (r = 0.64 and 0.78, p < 0.0001). These preliminary results suggest that sensor-based nitrogen management can effectively improve wheat production and enhance long-term agronomic nitrogen use efficiency in Spain.
A new device, Stenon FarmLab, was reported for real-time assessment of mineral nitrogen by measuring nitrate and ammonium using spectral methods [82]. The data obtained were compared to those from traditional laboratory methods, revealing that Stenon FarmLab overestimated mineral nitrogen in 75% of cases. The average nitrate level measured with the Stenon FarmLab device across all fields was 107 ± 38 kg N ha−1, a value significantly higher than that obtained in the laboratory, which was 86 ± 66 kg N ha−1. This indicates that while Stenon FarmLab is time-saving, it should be used cautiously because its results, if used for further fertilization, are not accurate enough, and the product requires further improvements. The accuracy of the Stenon FarmLab was evaluated by comparing it with standard laboratory analyses across 15 sites in Bavaria, Germany. Of the 211 measurements taken, only 181 were valid within the device’s operational range (42–189 kg N ha−1). Results showed that the FarmLab overestimated mineral nitrogen (Nmin) in 75% of cases, with an average deviation of 38 kg N ha−1, corresponding to a 69% error. While the device offers a faster, field-based method for detecting relative differences in Nmin, it lacks the accuracy needed for precise, demand-driven fertilization decisions. Further technical improvements are necessary before it can replace laboratory reference methods.
Another study [83] evaluated the performance of the Stenon FarmLab handheld soil sensor by comparing its real-time measurements to high-resolution soil maps produced via a calibrated Vis-NIR online multi-sensor platform in a potato field in northeast Germany. While the Vis-NIR system demonstrated strong calibration and validation accuracy (mean R2 = 0.81 and 0.72, respectively), the Stenon FarmLab showed considerable variability and limited reliability. The Stenon FarmLab sensor showed poor to moderate correlations with reference measurements for soil parameters such as phosphorus, potassium, magnesium, and pH. Its performance for total organic carbon (TOC) was inconsistent, and while soil texture estimates were generally reliable, their broad classification limits their value for precision agriculture.
An alternative to colorimetric approaches for nitrate detection was proposed by Lavanya and co-workers [84], who introduced a novel chromotropic acid-based color development method. This method uses a 3D-printed device integrated with the rear-end camera of a smartphone and a standalone application called SMART NP. It is capable of instantly estimating nitrate levels in soil. The detection limit was 0.1 mg L−1, with a sensitivity of 0.26 mg L−1. The data obtained from this device were compared with those from laboratory measurements, which were encouraging for the device’s ability to accurately detect nitrate in soil and contribute to sustainable practices.
An innovative approach [85] for soil mineral nitrogen quantification is based on the dielectric response characteristics of soil when analyzed in an electromagnetic field. The complex dielectric spectra of red soil and yellow clay loam were measured using a vector network analyzer over a frequency range of 10 MHz to 4.5 GHz. The sensitivity bands for ammonium and nitrate were found to range from 136 to 159 MHz and from 97 to 129 MHz, respectively. The advantages of this method include the potential for real-time monitoring of soil mineral nitrogen and non-destructive analysis. Additionally, the issue of water interference, commonly encountered with this method, is overcome by a dual-frequency decoupling mechanism.
Isotope tracing of nitrogen in soil is a powerful technique for studying nitrogen cycling, transformations, and uptake by plants. Stable nitrogen isotopes (such as δ15N) are commonly used in research to trace nitrogen movement in soil and are a valuable tool for studying biological nitrogen (N2) fixation, nitrogen losses through denitrification, and nitrogen use efficiency. By applying a nitrogen source enriched with δ15N to soil, researchers can track how nitrogen moves through different pools (e.g., ammonium, nitrate, organic nitrogen) and its fate in the soil environment.
The values of δ15N for agricultural soils vary widely between +1‰ and +12‰, due to the application of isotopically distinct nitrogen sources and differences in the magnitude of nitrogen loss through various processes, such as ammonia volatilization, denitrification, and nitrate leaching. For instance, the application of 15N-depleted synthetic fertilizer can result in δ15N values below 0‰, while soils that have received 15N-enriched composted manure for more than five years show δ15N values of +8.8‰. Additionally, in uncultivated soils, δ15N of bulk soils ranges from −5‰ to +12‰, while in cultivated soils, δ15N ranges from +1‰ to +12‰ due to nitrogen inputs [86].
In addition, Mariotti and co-workers [87] have investigated the fractionation of the nitrogen isotopes δ14N and δ15N during the denitrification process for the step NO2− → N2O under anaerobic conditions on natural soils. Other researchers [88] studied soil N dynamics by δ15N composition under an erodible environment. The δ15N values of soil organic nitrogen (SON) were used to evidence soil nitrogen transformation processes that occur under agricultural soil erosion. The authors reported that δ15N values of SON for paddy soils range from −5‰ to 5‰ due to manure and synthetic fertilizers application.
Furthermore, stable isotopes δ15N and δ16O are used to track nitrate ions in groundwater [89]. Considering that natural and synthetic fertilizers are characterized by different isotopic signatures, it is possible to distinguish between them and identify the sources of nitrogen pollution [5]. Sources of nitrate pollution are characterized by a wide range of δ15N-NO3−: inorganic fertilizers from −8 to +7‰, soil organic matter from +3 to +8‰, and organic fertilizers from +6 to +30‰. In addition, the δ15N-NO3− of soil nitrate ranges between +3 and +8‰ [90].
Even though isotope tracing of nitrogen is very useful and can provide highly accurate, detailed information on nitrogen sources and transformations, it is expensive and typically used in research rather than routine field assessments.
Remote sensing technologies for nitrogen management allow continuous, real-time monitoring of nitrogen status and are non-destructive, enabling the evaluation of nitrogen levels without disturbing the soil. The technologies used are satellite imagery [91,92,93], unmanned aerial vehicle (UAV) platforms [94,95,96], and ground-based sensors [96], and although their application can be costly, they ultimately save money over time by improving nitrogen management efficiency.
Among the limitations, it is worth mentioning the impact of weather conditions and challenges in data interpretation, with the latter requiring expertise in both remote sensing and agriculture [97]. Furthermore, in addition to evaluating nitrogen levels in soil, remote sensing technologies can correlate nitrogen levels based on leaf chlorophyll content, which is influenced by nitrogen availability [98,99,100].
The advanced nitrogen detection techniques reviewed in this paper, including sensor-based systems and AI-driven monitoring, hold strong potential for integration into farm management platforms.
Although there are currently many methods available for determining soil nitrogen, significant limitations exist related to the type of soil being analyzed and the form of nitrogen of interest. Choosing the proper method suitable for the form of nitrogen of interest and applicable to a specific soil type is a determining factor for the accuracy of the result. Additionally, finding a method that provides accurate results without requiring lengthy analysis times and significant resources remains a challenge. Furthermore, regardless of the chosen analytical technique and available resources, the analyst plays a crucial role in interpreting the results. Subsequently, the implementation of the obtained results in the field depends on the farmer’s expertise.
4. Literature Data on Nitrogen Levels in Different Soils
The reported values for total and mineral nitrogen are collected from studies and reports and are dependent on many factors, such as soil texture, soil pH, organic matter decomposition, geographic location, land use, and cropping management (Table 5). For example, sandy soils have lower levels of nitrogen. In this regard, one study [101] reported total nitrogen and mineral nitrogen levels of 0.13 g kg−1 and 1.74 mg kg−1, respectively, for sandy soils. Higher values for total nitrogen and mineral nitrogen were found in loam, sandy loam, and sandy clay soils as follows: 1.48 g kg−1 and 23.99 mg kg−1, 1.51 g kg−1 and 37.32 mg kg−1, and 1.20 g kg−1 and 41.60 mg kg−1, respectively.
Soil pH influences nitrogen mineralization, as investigated in a long-term grassland experiment on mineral soils [102]. The results showed that higher pH increased the mineralization rate, which was favored by calcium carbonate, while lower pH led to ammonium accumulation.
In addition, fertilization, crop rotation, and agricultural technological practices (tillage, reduced tillage, no tillage) influence nitrogen in the case of agricultural soils.
Mineral nitrogen applied as fertilizer can remain unchanged for a long time if the soil is dry. The depth at which nitrates can be lost is arbitrary and depends on the crop, soil, and rainfall regime [14].
Du and co-workers [103] evidenced that the application of nitrogen fertilizers (urea: 375 kg ha−1 and urea-P2O5-K2O: 350–150–75 kg ha−1) significantly increased the total nitrogen content in the soil, with 25.41% in comparison with the control variant.
Long-term fertilization increases the levels of total, mineral, and organic nitrogen in the soil profile [61]. The developed experiment investigated the effect of organic and mineral fertilizers on nitrogen levels in the soil profile. It was found that the application of manure had the greatest impact on nitrogen content, while the least effect was observed with the application of mineral fertilizers. The total nitrogen content was 0.33 g kg−1 (control variant) and increased to 0.59 g kg−1 with the application of farmyard manure.
The importance of crop rotation on soil properties was studied by Mocanu and her team [104], and the results of the experiment highlighted that introducing a mixture of grasses with legumes in the rotation led to an increase in the total nitrogen content to up to 0.1487% from low values.
Assessment of total nitrogen up to a 30 cm soil depth during a long-term (34 years) experiment under organic and conventional management practices revealed a higher total nitrogen content in organic systems managed by tillage, compared to conventional systems that rely on chemical fertilizers. The use of organic manure under tillage resulted in total nitrogen contents of 3.8 g kg−1 (0–10 cm), 3.6 g kg−1 (10–20 cm), and 2.4 g kg−1 (20–30 cm), whereas in conventional agricultural practices, the results were 3.0 g kg−1 (0–10 cm), 2.7 g kg−1 (10–20 cm), and 1.9 g kg−1 (20–30 cm) [105].
In the case of agricultural soils, nitrogen content is influenced by fertilization practices and crop management. For instance, total soil nitrogen is higher in cropland and grassland than in urban soil. In this regard, one study [106] revealed nitrogen concentrations of 1.79 g kg−1 for cropland, 1.23 g kg−1 for grassland, and 0.51 g kg−1 for urban soil. The soil samples were collected from the surface topsoil (0–5 cm).
In the case of forest soils, the nitrogen content can vary depending on several factors, such as the type of forest (coniferous, deciduous), soil conditions (acidity, soil texture), climate, and forest management practices. For example, total nitrogen content was found to be 2.3 times higher in clayey and fine silty soils than in sandy soils [107].
Generally, forest soils tend to have a higher total nitrogen content compared to agricultural soils due to the natural decomposition processes of organic matter and nutrient accumulation. For example, total nitrogen content for soil samples (0–20 cm depth) collected from a forest located in Southern Italy covered a range between 0.8 and 7.7 g kg−1, with an average of 3.7 g kg−1 [16].

Table 5.
Nitrogen levels in soils from different countries.
Table 5.
Nitrogen levels in soils from different countries.
| Region | Soil Texture; Depth; Other Details | Total Nitrogen, g kg−1 | Mineral Nitrogen, mg kg−1 | Ref. | |
|---|---|---|---|---|---|
| NH4+-N | NO3−-N | ||||
| Brazil | 0–20 cm; p.s. | 0.6–2.2 | 15.4–170.7 | 0.6–23.9 | [40] |
| Clay; 0–20 cm; o.s. | 1.18 | 13.7 | 0.68 | [37] | |
| Loamy sand; 0–20 cm; o.s. | 0.82 | 18.6 | 0.89 | [37] | |
| China | Clay loam; 0–20 cm; a.s. | 1.32 | - | - | [108] |
| Clay loam; p.s. | 1.26 | - | - | [109] | |
| a.s. | 1.06 | - | - | [110] | |
| Ghana | 0–20 cm; a.s. | 1.4–5.1 | 15.9–42.7 | 23.1–46.2 | [41] |
| Greece | 0–30 cm | 0.67–1.82 | 60–128 | [111] | |
| Hungary | Sandy soil; 0–20 cm; a.s. | - | 6.8 | 12.5 | [112] |
| Loamy soil; 0–30 cm; a.s. | 1.4 | - | - | [113] | |
| Indonesia | p.s. | 1.1–1.7 | - | - | [114] |
| Italy | 0–25 cm; a.s. | 1.27 | 6.45 | 12.14 | [115] |
| Clay; 0–40 cm; a.s. | 0.8–1.9 | [116] | |||
| Sandy; 0–40 cm; a.s. | 1.1–1.8 | [116] | |||
| Sandy loam; 0–40 cm; a.s. | 0.05–0.15 | [116] | |||
| Sandy silty clay; 0–40 cm; a.s. | 0.4–1.3 | [116] | |||
| Silty loam; 0–40 cm; a.s. | 0.8–2.8 | [116] | |||
| Loam; 0–40 cm; a.s. | 0.7–1.9 | [116] | |||
| Sandy clay loam; 0–20 cm; o.s. | 1.34 | 12.51 | 67.68 | [117] | |
| Sandy clay loam; 20–40 cm; o.s. | 1.13 | 9.78 | 39.36 | [117] | |
| Loam; 0–10 cm; a.s. | 0.99 | 8.5 (including NO2−-N) | [118] | ||
| Loam; 10–20 cm; a.s. | 0.93 | 18.2 (including NO2−-N) | [118] | ||
| Loam; 20–30 cm; a.s. | 0.90 | 23 (including NO2−-N) | [118] | ||
| Loam; 30–40 cm; a.s. | 0.89 | 18.1 (including NO2−-N) | [118] | ||
| 0–5 cm; a.s. | 1.0–2.5 | [119] | |||
| 5–30 cm; a.s. | 0.8–1.7 | [119] | |||
| Lithuania | Loam; a.s. | 1.48 | 2.06 | 21.93 | [101] |
| Sandy loam; a.s. | 1.51 | 1.33 | 35.99 | [101] | |
| Sandy; a.s. | 0.13 | 0.98 | 0.76 | [101] | |
| Sandy clay; a.s. | 1.20 | 3.68 | 37.92 | [101] | |
| Poland | Sandy loams, loams, silt loams; a.s. | 0.4–0.8 | 0.9–1.9 | 4.3–15.1 | [120] |
| Heavy loamy silty sand underlain by light loam; 0–25 cm; a.s. | 0.58 | 9.4 (including NO2−-N) | [61] | ||
| 0–30 cm; a.s. | - | 18.24–18.31 (including NO2−-N) | [61] | ||
| 0–25 cm; a.s. | 0.846 | 8.25 | 4.65 | [38] | |
| 0–25 cm; a.s. | 0.428 | 7.50 | 2.65 | [38] | |
| Romania | 0–25 cm; a.s. | 2.9 | - | - | [121] |
| Spain | Silt, silt loam, sandy loam; 0–5 cm; a.s. | 1.79 | [106] | ||
| Thailand | 0–20 cm; a.s. | 1.7 | - | - | [55] |
| Turkey | Clay loam; 0–25 cm; a.s. | 1.74 | - | - | [122] |
| Clay loam; 0–25 cm; o.s. | 1.28 | - | - | [122] | |
| Clay; 0–25 cm; a.s. | 1.13 | - | - | [122] | |
| Sandy loam; 0–25 cm; a.s. | 0.57 | - | - | [122] | |
| 0–20 cm; a.s. | 1.38 | [123] | |||
a.s. = Agricultural soil; o.s. = orchard soil; p.s. = paddy soil.
To provide a better visualization of the data regarding total and mineral nitrogen contents, heat maps (Figure 4 and Figure 5) were constructed based on the average values reported in the literature (Table 5).
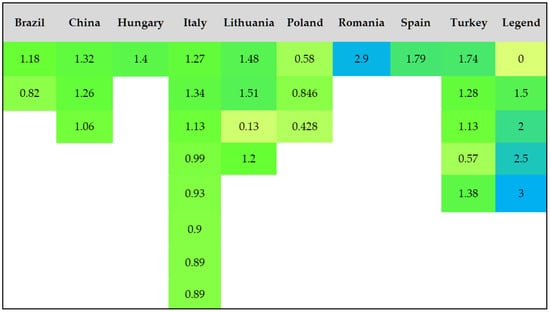
Figure 4.
Total nitrogen levels (g kg−1) for several locations.
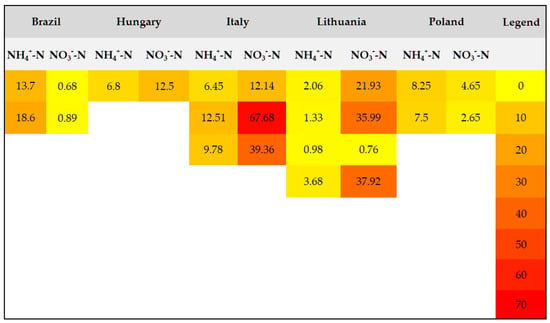
Figure 5.
Mineral nitrogen levels (mg kg−1) for several locations.
The total nitrogen and mineral nitrogen levels across various regions and soil types vary significantly (Table 5). Total nitrogen values range from as low as 0.05 g kg−1 in sandy loam soils to as high as 5.1 g kg−1 in agricultural soils from Ghana.
Mineral nitrogen, including ammonium (NH4+-N) and nitrate (NO3−-N), shows a wide range as well, with ammonium levels fluctuating from very small values in sandy soils in some regions (e.g., Poland) to over 80 mg kg−1 in sandy clay loam soils in Italy. Ammonium nitrogen (NH4+-N) concentrations also display significant variation, with some regions (Brazil) showing high nitrate levels (up to 170.7 mg kg−1), while others, like Lithuania and Poland, have lower values.
Fluctuations in nitrogen levels are influenced by factors such as soil texture, depth, agricultural practices, and local environmental conditions, and can vary significantly across different soil types and regions. For accurate nitrogen management, it is essential to conduct local soil testing, as nitrogen dynamics differ widely.
To ensure sustainable fertilizer application and enhance soil fertility while minimizing environmental impact, data specific to the soils in the area of interest must be gathered, laboratory analyses conducted, and the results used to adopt the most efficient practices that promote high yields.
5. Sustainable Approaches for Nitrogen Management in Agriculture
Sustainable management of nitrogen fertilizers is crucial, as they are essential for maximizing crop yields, but in excess, they can cause water pollution, greenhouse gas emissions, and biodiversity loss. Nitrogen leaching into waterways leads to eutrophication, depleting oxygen in aquatic ecosystems and harming marine life [124].
The main sustainable practices for nitrogen management are presented in Figure 6 and discussed in this chapter.

Figure 6.
Sustainable practices for nitrogen management.
Precision agriculture uses advanced technologies to apply the right type of nitrogen fertilizer, in the right doses, at the right time, and precisely in the right places (4R nutrient principle) [125]. Through the use of advanced technologies (remote sensing, optical sensors, variable rate application, GPS/GIS mapping, IoT) and data-driven approaches, precision agriculture improves nitrogen use efficiency, reducing waste and environmental impact while enhancing crop performance.
For example, fertilizer requirements and optimum nitrogen levels for horticultural species have been determined using non-destructive optical sensor technology (SPAD-502 and GreenSeekerTM). Based on this technology, the application of 20 g of Florikan Top-Dress fertilizer (12N-6P-8K) to Justicia brandegeana has been found to be effective, with this application rate helping to reduce agricultural nutrient runoff [126].
Nitrogen fertilization in winter wheat (Triticum aestivum L.) grown on a 16 ha field, located on an arable farm in the Netherlands, has been optimized through exploratory simulations, and the results indicated that fertilizer inputs could be reduced by 15–27% without affecting grain yield or quality parameters [127].
Optimization of nitrogen fertilizer application in olive orchards for each individual plant was achieved using a standardized methodology on a GIS platform, considering the spatial variability of soil nitrogen. Productivity, biometric, nutritional, and spectral data from olive trees provided valuable insights for determining the precise nitrogen requirements of each plant. Based on this methodology, a 31% reduction in applied nitrogen fertilizer was achieved compared to the control, supporting more sustainable agricultural practices [128].
The risk of nitrogen leaching and volatilization is reduced through the use of controlled-release or slow-release fertilizers. This strategy provides nitrogen to plants over a longer period, prevents the negative effects of over-fertilization, and ensures nitrogen availability when crops need it most. The advantages and disadvantages of using this type of fertilizer are widely discussed in terms of economic efficiency, implications for crop yield, and environmental impact [129,130,131,132].
The effect of slow-release nitrogen fertilizers (urea, neem-coated urea (NCU), sulfur-coated urea (SCU), and bioactive sulfur-coated urea (BSCU)) on the growth, productivity, and grain nutritional qualities of wheat crop was investigated by Ghafoor and co-workers [133]. The nitrogen levels of applied fertilizers were 130, 117, 104, and 94 kg ha−1. Among the fertilizers used, BSCU proved to be the most effective. Application of BSCU at a rate of 130 kg ha−1 increased dry matter accumulation and grain yield, with the highest grain NPK levels (3.54, 0.66, and 1.07%) being achieved under BSCU fertilization (130 kg ha−1).
To enhance nitrogen fertilizer efficiency, a lignin-based slow-release nitrogen fertilizer has been developed using lignin derived from agricultural and forestry waste. Soil column leaching tests have shown that it reduces nitrogen loss, thereby promoting sustainable agricultural practices. The reduced cumulative nitrogen loss indicates an effective slow-release behavior [134].
Crop rotation is an effective nitrogen management practice that helps maintain soil fertility, reduce nitrogen leaching, and minimize the need for synthetic fertilizers by alternating nitrogen-fixing crops with other plant species. This strategy can influence nitrogen mineralization dynamics or the transformation of organic nitrogen to mineral nitrogen.
The evaluation of diverse crop rotation schemes (rice–wheat (control), rice–rape, rice–hairy vetch, rice–barley, rice–faba bean, and rice–winter fallow) on rice growth, yield, and soil properties, including nitrogen level, was investigated [135]. The results indicated that legume-based rotations (rice–faba bean, rice–hairy vetch) significantly enhanced rice growth and yield compared to the rice–wheat control. In terms of soil nitrogen levels, legume-based rotations were found to increase nitrogen availability. The available nitrogen in the rice–wheat control was 24.38 mg kg−1 in spring and 21.73 mg kg−1 in autumn, both of which were lower than the nitrogen levels observed in the rice–faba bean rotation in spring (43.56 mg kg−1) and the rice–hairy vetch rotation in autumn (26.22 mg kg−1). The study evidenced that legume-based rotations increase nitrogen available levels and increase crop yield.
Furthermore, another study [136] investigated the effects of farming systems and applied crop rotation schemes on soil nutrient content (including nitrogen) and cabbage yield. In this study, sheep manure was used in the organic farming system, while an inorganic fertilizer (11-15-15 N-P2O5-K2O) was applied in the conventional system. The rotation schemes included pea–cabbage, faba bean–cabbage, and cabbage–cabbage. The results indicated that the highest levels of nitrate and total nitrogen in the soil were observed in the pea–cabbage and faba bean–cabbage rotations, particularly within the conventional farming system.
Integrated nutrient management (INM) combines organic and mineral sources of nitrogen according to crop needs, thereby enhancing nitrogen availability and reducing both nitrogen losses (leaching, runoff, volatilization) and environmental impacts (nitrate water contamination, greenhouse gas emissions) [137]. In addition, INM is a sustainable method for producing high-quality crops with higher yields [138], and it focuses on optimizing fertilizer application rates and timing. For example, the INM strategy has proven its efficacy, as it has increased crop yields by 8–150% compared to conventional agricultural practices [139]. One study [140] investigated the effect of the INM strategy on nitrogen loss and the impact on wheat yield in a field experiment with different doses of fertilizers combined with biofertilizers. It was found that NPK + Azotobacter + mycorrhiza (Tram) improved soil nitrogen dynamics. Additionally, the combination of inorganic fertilizers, organic manure, and biofertilizers increased the availability of nitrogen, phosphorus, and potassium in a field experiment with direct-seeded upland paddy (Oryza sativa L.). Among the treatments tested, the one that included inorganic fertilizers, biofertilizers, and 15 tons of FYM ha−1 resulted in enhanced crop productivity [141].
The effect of farmyard manure or composted olive-mill waste, combined with vegetative residues of cowpea, common bean, or faba bean, on soil mineral nitrogen and yield was investigated in an organic greenhouse tomato crop [142]. Even though mineral nitrogen levels increased in all treatments, the increase was more pronounced with green manuring using faba bean combined with farmyard manure. Additionally, a higher tomato fruit yield was obtained when faba bean was combined with farmyard manure compared to compost.
There are studies that demonstrate that integrated nutrient management strategies, which involve optimized fertilizer application, can significantly reduce nitrate leaching in agricultural soils. A 15-year field experiment [143] under a double-cropping system evaluated the effects of reduced nitrogen input and straw incorporation on mineral nitrogen leaching in plastic-shed greenhouse conditions. Results showed that reducing nitrogen input by 47% and incorporating straw decreased nitrogen leaching by 4–86%, without compromising tomato yield or nitrogen uptake.
A three-year field study [144] conducted in Henan Province, China, assessed the effects of different nitrogen fertilizer application rates, including a combination of chemical and organic fertilizers, on nitrate leaching, crop yield, and nitrogen use efficiency in a wheat–maize rotation system. Based on the results, an optimal nitrogen application range of 285–465 kg ha−1 was identified for this region. Within this range, the risk of nitrogen leaching was significantly reduced while maintaining high crop yields. Specifically, yields of 16.5–17.9 t ha−1 were achieved, with nitrate leaching limited to 12.6–30.5 kg ha−1, highlighting this approach as a sustainable nitrogen management strategy.
Nitrogen losses from fertilized soils can be mitigated by using nitrogen stabilizers, such as urease inhibitors, which inhibit urea hydrolysis, and nitrification inhibitors, which slow the transformation of ammonium to nitrate. These stabilizers are essential tools for sustainable nitrogen management, helping to reduce greenhouse gas emissions and minimize nitrogen losses through leaching and volatilization [145].
One study [146] investigated the efficacy of urease and nitrification inhibitors in reducing ammonia loss following urea application in maize and wheat crops over a two-year field trial. The results indicated that the urease inhibitor reduced ammonia loss by 85% and 96% for maize and by 41% and 65% for wheat. Additionally, the nitrification inhibitor suppressed nitrate formation, although its effectiveness was negatively affected by high temperatures. Further studies demonstrated that both inhibitors were effective in reducing emissions of NO, N2O, and CO2.
The influence of a nitrapyrin-based nitrification inhibitor was studied in relation to the growth of Brassica oleracea L. var. botrytis, which was fertilized with calcium nitrate. The experimental results demonstrated that the nitrification inhibitor reduced nitrogen losses, specifically decreasing nitrate leaching from 925 to 294 mg L−1 [147].
In conclusion, nitrification inhibitors and nitrogen stabilizers are essential tools for improving nitrogen use efficiency, reducing greenhouse gas emissions, and minimizing nitrogen losses through leaching and volatilization.
The use of organic fertilizers releases nitrogen slowly to crops, ensuring a proper supply with minimal risk of leaching, while also promoting long-term soil fertility, a reduction in environmental pollution, and support for ecosystem balance. Unlike chemical fertilizers, they align nutrient release with plant needs over time—making them more environmentally friendly and sustainable.
The nitrogen content of organic fertilizers varies widely depending on their source and form. In the case of animal manure, nitrogen levels depend on factors such as the animal species, diet, and whether the manure is fresh or composted (Table 6). Composted manures generally contain lower nitrogen levels compared to fresh variants. Chicken/poultry manure typically contains higher nitrogen levels than manure from other animal species. Poultry and blood-based fertilizers tend to have higher nitrogen concentrations, making them great for nitrogen-demanding crops.

Table 6.
Nitrogen content of various organic fertilizers.
Table 6.
Nitrogen content of various organic fertilizers.
| Fertilizer | Total N, % | Organic N, g kg−1 | NH4+-N, g kg−1 | Ref. |
|---|---|---|---|---|
| Plant based-organic fertilizers | ||||
| Algae fertilizer | 2.60 | - | - | [148] |
| Crop residue compost | 1.20 | 12 | - | [149] |
| 2.43 | - | - | [150] | |
| Green compost (fresh) | 2.12 | - | - | [151] |
| Green compost (matured) | 1.44 | - | - | [151] |
| Green seaweed fertilizer | 3.12 | - | - | [152] |
| Seaweed fertilizer (fermented) | 1.88 | - | - | [153] |
| Animal-based organic fertilizers | ||||
| Beef manure | 0.92 | - | 1.80 | [154] |
| 2.60 | - | - | [155] | |
| Blood meal fertilizer | 11.06 | - | 0.10 | [156] |
| 15.50 | - | - | [157] | |
| Cattle manure | 0.92 | - | - | [158] |
| 1.99 | - | - | [159] | |
| Cattle manure (composted) | 0.86 | - | 0.50 | [154] |
| Cattle manure (liquid) | 0.26–0.33 | 1.10–2.20 | 1.50–2.00 | [160] |
| Chicken manure | - | 21–40 | - | [161] |
| 4.60 | - | - | [155] | |
| 8.57 | - | - | [162] | |
| 5.54 | [163] | |||
| Chicken manure (compost) | 4.47 | - | - | [164] |
| Dairy manure | - | 9–24 | - | [165] |
| 0.72 | - | 1.50 | [154] | |
| 2.00–2.90 | - | - | [155] | |
| Dairy manure compost | 1.50 | 14 | - | [149] |
| 1.90–2.10 | - | - | [155] | |
| Farmyard manure | 1.86 | - | - | [151] |
| 0.55 | - | - | [164] | |
| 2.10 | - | - | [163] | |
| Feather meal fertilizer | 13.01 | - | - | [156] |
| 14.40 | - | - | [157] | |
| Fish emulsion | 0.045 | - | - | [157] |
| Goat manure | - | 14–23 | - | [161] |
| 1.04 | - | 2.80 | [154] | |
| Hog manure | 0.93 | - | 2.90 | [154] |
| Horse manure | 0.50 | - | 0.70 | [154] |
| - | 18–21 | - | [161] | |
| Ostrich manure | - | 13–19 | - | [161] |
| Pig manure | - | 17–26 | - | [161] |
| 3.90 | - | - | [155] | |
| Poultry manure | 2.09 | - | - | [157] |
| 3.89 | - | - | [158] | |
| 2.71 | - | 6.00 | [154] | |
| Poultry manure (pellet) | 4.00 | - | - | [166] |
| 4.70 | 39 | - | [149] | |
| 5.19 | - | 1.70 | [156] | |
| Poultry manure compost | 2.60–3.80 | 24–36 | - | [149] |
| Poultry manure liquid | 0.75–0.80 | 0.40–1.30 | 6.60–7.10 | [160] |
| Rabbit manure | - | 16–18 | - | [161] |
| Sheep manure | 0.87 | - | 2.80 | [154] |
| - | 14–24 | - | [155] | |
| 2.30 | - | - | [155] | |
| Sheep/goat manure | 1.45 | - | - | [159] |
| Shrimp meal fertilizer | 5.69 | - | 0.10 | [156] |
| Swine manure | 2.02 | - | - | [159] |
| Turkey manure | 2.53 | - | 8.30 | [154] |
| Veal manure (grain-fed) | 0.79 | - | 1.40 | [154] |
| Other organic sources | ||||
| Vermicompost | 2.20 | - | - | [167] |
| 1.50 | - | - | [148] | |
| 0.95 | - | - | [165] | |
| Guano | 20.70 | 5.64 | 0.39 | [168] |
| Insect’s frass (pellet) | 3.00 | - | - | [166] |
| Organic fertilizers from tannery and slaughterhouse by-products | 2.78–14.80 | <0.10 | 0.30–6.00 | [169] |
| Leonardite | 2.10 | - | - | [165] |
| Vinasse-based fertilizers (pellet) | 6.00 | - | - | [166] |
Plant-based organic fertilizers such as seaweed and algae fertilizers tend to have moderate nitrogen levels, while animal-based fertilizers show more variation. Poultry and blood meal fertilizers generally have the highest nitrogen concentrations, while manures from animals like cattle, sheep, and horses often contain lower nitrogen levels. Composted manures typically have reduced nitrogen content compared to fresh manures, with liquid manures containing more readily available nitrogen in the form of ammonium (NH4+-N).
Even though there are cons alongside the pros [170], the use of organic fertilizers represents an efficient sustainable practice for nitrogen management.
Understanding the nitrogen demand of crops is essential for optimizing fertilization strategies, achieving high yields, and minimizing environmental impacts. Nitrogen demand varies among crop types, with the highest requirements typically observed in cereals and sugarcane, followed by vegetables and fruit trees [171] (Table 7).

Table 7.
Nitrogen demand by various crops [171].
To ensure high-quality products and high crop yields without environmental pollution, some authors [171,172] have suggested that a combination of organic and mineral fertilization can achieve these goals. In addition, a nitrogen dose of 150 kg ha−1 may be favorable for most crops when using both organic and mineral fertilization, as it can increase soil nitrogen availability. Conversely, applying more than 300 kg ha−1 may be excessive, potentially leading to negative impacts on microbial activity and a decrease in soil nitrogen availability [173].
Sustainable nitrogen management through the optimization of fertilizer use efficiency and improved agronomic practices has been widely adopted in developed nations. Meanwhile, many developing countries continue to apply excessive amounts of fertilizer, contributing to soil degradation, nitrogen loss through leaching, and increased greenhouse gas emissions [174]. Given the importance of appropriate nitrogen fertilization recommendations, ten Western European countries conducted a comparative study [175] to calculate recommended nitrogen application rates for farmers.
The results revealed that fertilization recommendations vary across Europe, with differences of up to 100 kg N ha−1 in the recommended application rates. Additionally, there were significant variations in crop nitrogen uptake and the availability of nitrogen from manure among the countries studied.
The implementation of the above-discussed sustainable nitrogen management practices offers both economic and environmental benefits. These include cost savings, yield stability, long-term productivity, soil conservation, reduced greenhouse gas emissions, and overall environmental protection.
6. Conclusions and Future Prospects
Nitrogen is a nutrient of great importance in agriculture, playing a key role in plant growth, crop productivity, and overall soil fertility. Its efficient use is essential for sustainable agriculture, with the aim of optimizing crop yields while minimizing environmental impacts such as nitrogen loss through leaching and volatilization.
Therefore, the precise application of fertilizers—based on crop-specific requirements and soil nutrient status—supports long-term soil fertility, enhances ecosystem resilience, and contributes to the overall sustainability and efficiency of agroecosystems. Efficient nitrogen management is critical for reducing the environmental impact of agriculture and promoting climate-smart farming.
This paper has reviewed the multifaceted dynamics of nitrogen in agricultural soils, with an emphasis on its different forms encountered in soil. This is fundamental, as each form behaves differently in the soil environment and contributes uniquely to plant nutrition, loss pathways, and environmental risks. Additionally, the paper discusses quantification methodologies, highlighting both traditional and advanced techniques for nitrogen monitoring, along with their respective advantages and limitations. Implementing the insights gained from nitrogen monitoring helps boost crop yields, prevent over-application, lower input costs, and reduce emissions from fertilizer misuse.
The determination of different forms of nitrogen is a rather challenging task that requires not only technical knowledge of analytical methodologies, but also significant agronomic expertise to interpret the results comprehensively and to integrate them with field conditions. Despite advancements in soil nitrogen analysis, the choice of method must balance precision, resource availability, and ease of use. The need for skilled labor, specialized instruments, and potential chemical interferences remains a central limitation across most techniques.
Mitigating nitrogen losses through leaching, volatilization, and denitrification remains a significant challenge, as there are not only environmental concerns but also economic consequences associated with reduced crop yields. In this regard, sustainable agricultural strategies should be employed, including the adoption of precision agriculture tools, optimized fertilizer application, crop rotation, a combination of organic and mineral nitrogen sources based on crop needs, the use of nitrogen inhibitors, and the application of organic fertilizers.
By improving nitrogen monitoring at the field level, this research supports the objectives of the Nitrates Directive (91/676/EEC) [25], particularly in reducing nitrogen-related pollution. In developing countries, where nitrogen mismanagement poses both environmental and economic risks, low-cost sensing technologies could play a crucial role in controlling nitrogen levels on farms. However, the promotion of sensor-based technologies for nitrogen monitoring faces significant challenges, including high equipment costs, limited technical capacity, and farmer reluctance to adopt precision agriculture tools—often due to lack of training and concerns about affordability.
Future research should focus on:
- -
- Developing low-cost, user-friendly nitrogen sensors for wider adoption, particularly in smallholder and developing-country contexts;
- -
- Evaluating the long-term impacts of integrated nutrient strategies across diverse soil types and textures, multiple crops, a range of fertilizers, and varying climatic conditions;
- -
- The development of enhanced-efficiency fertilizers and organic alternatives that further contribute to reducing nitrogen losses;
- -
- Investigating behavioral and economic barriers to the adoption of precision technologies;
- -
- Establishing region-specific thresholds and indicators aligned with policies such as the EU Nitrates Directive.
A key insight from this review is that there is no one-size-fits-all solution for nitrogen management. Effective strategies must integrate real-time data, precision application, and tailored combinations of organic and inorganic sources. Advanced technologies like AI and IoT hold great promise for transforming nitrogen monitoring and field-level decision-making. Despite their potential, widespread adoption is hindered by challenges such as high costs, the need for user training, and infrastructural limitations. When effectively implemented, these technologies can greatly improve the efficiency of real-time nitrogen monitoring by facilitating accurate and timely data collection and analysis with minimal human input.
Author Contributions
Conceptualization, R.M.M. and G.V.S.; methodology, R.M.M.; validation, G.V.S.; formal analysis, R.M.M.; investigation, G.V.S.; resources, R.M.M.; data curation, G.V.S.; writing—original draft preparation, G.V.S.; writing—review and editing, R.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors sincerely thank the anonymous reviewers for their valuable comments and suggestions, which have significantly contributed to enhancing the quality of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Penuelas, J.; Coello, F.; Sardans, J. A better use of fertilizers is needed for global food security and environmental sustainability. Agric. Food Secur. 2023, 12, 5. [Google Scholar] [CrossRef]
- Madjar, R. Agrochemistry, Plant and Soil; INVEL-Multimedia: Ilfov, Romania, 2008. (In Romanian) [Google Scholar]
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and eutrophication: Where do we go from here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, Q.; Zhao, C.; Wang, L.; Li, Y.; Ma, X.; Guo, J.; Yang, H. Identification of nitrate sources in rivers in a complex catchment using a dual isotopic approach. Water 2021, 13, 83. [Google Scholar] [CrossRef]
- Madjar, R.M.; Vasile Scăețeanu, G.; Sandu, M.A. Nutrient water pollution from unsustainable patterns of agricultural systems, effects and measures of integrated farming. Water 2024, 16, 3146. [Google Scholar] [CrossRef]
- Mim, J.J.; Rahman, M.; Khan, F.; Paul, D.; Sikder, S.; Das, H.P.; Khan, S.; Orny, N.T.; Shuvo, R.H.; Hossain, N. Towards smart agriculture through nano-fertilizer—A review. Mater. Today Sustain. 2025, 30, 101100. [Google Scholar] [CrossRef]
- Kumar, B.; Shaloo; Bisht, H.; Meena, M.C.; Dey, A.; Dass, A.; Paramesh, V.; Babu, S.; Upadhyay, P.K.; Prajapati, V.K.; et al. Nitrogen management sensor optimization, yield, economics, and nitrogen use efficiency of different wheat cultivars under varying nitrogen levels. Front. Sustain. Food Syst. 2023, 7, 1228221. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, A.; Tselykh, A.; Bozhenyuk, A.; Choudhury, T.; Alomar, M.A.; Sanchez-Chero, M. Artificial intelligence and internet of things oriented sustainable precision farming: Towards modern agriculture. Open Life Sci. 2023, 18, 20220713. [Google Scholar] [CrossRef]
- Martínez-Dalmau, J.; Berbel, J.; Ordóñez-Fernández, R. Nitrogen Fertilization. A review of the risks associated with the inefficiency of its use and policy responses. Sustainability 2021, 13, 5625. [Google Scholar] [CrossRef]
- Luo, F.; Yan, X.-J.; Hu, X.-F.; Yan, L.-J.; Cao, M.-Y.; Zhang, W.-J. Nitrate quantification in fresh vegetables in Shanghai: Its dietary risks and preventive measures. Int. J. Environ. Res. Public Health 2022, 19, 14487. [Google Scholar] [CrossRef]
- Luetic, S.; Knezovic, Z.; Jurcic, K.; Majic, Z.; Tripkovic, K.; Sutlovic, D. Leafy vegetable nitrite and nitrate content: Potential health effects. Foods 2023, 12, 1655. [Google Scholar] [CrossRef]
- Basheer, S.; Wang, X.; Farooque, A.A.; Nawaz, R.A.; Pang, T.; Neokye, E.O. A review of greenhouse gas emissions from agricultural soil. Sustainability 2024, 16, 4789. [Google Scholar] [CrossRef]
- Ravikumar, S.; Villingiri, G.; Sellaperumal, P.; Pandian, K.; Sivasankar, A.; Sangchul, H. Real-time nitrogen monitoring and management to augment N use efficiency and ecosystem sustainability—A review. J. Hazard. Mater. Adv. 2024, 16, 100466. [Google Scholar] [CrossRef]
- Davidescu, D.; Davidescu, V.; Calancea, L.; Handra, M.; Petrescu, O. Nitrogen in Agriculture; Academy Publishing House: Bucharest, Romania, 1976. (In Romanian) [Google Scholar]
- Dincă, L.; Dincă, M. The characteristics of forest soils from Covasna County. Sci. Pap. Agron. Ser. 2021, 64, 123–126. [Google Scholar]
- Conforti, M.; Matteucci, G.; Buttafuoco, G. Organic carbon and total nitrogen topsoil stocks, biogenetic natural reserve ‘Marchesale’ (Calabria region, southern Italy). J. Maps 2017, 13, 91–99. [Google Scholar] [CrossRef]
- Lixandru, G.; Calancea, L.; Caramete, C.; Borlan, Z.; Goian, M.; Hera, C.; Rauta, C. Agrochemistry; Didactic and Pedagogic Publishing House: Bucharest, Romania, 1990. (In Romanian) [Google Scholar]
- Munch, J.C.; Velthof, G. Denitrification and agriculture. In Biology of the Nitrogen Cycle; Bothe, H., Ferguson, S.J., Newton, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Zhum, X.; Fu, W.; Kong, X.; Chen, C.; Liu, Z.; Chen, Z.; Zhou, J. Nitrate accumulation in the soil profile is the main fate of surplus nitrogen after land-use change from cereal cultivation to apple orchards on the Loess Plateau. Agric. Ecosyst. Environ. 2021, 319, 107574. [Google Scholar] [CrossRef]
- Qiu, W.; Wang, Z.; Huang, C.; Chen, B.; Yang, R. Nitrate accumulation in leafy vegetables and its relationship with water. J. Soil Sci. Plant Nutr. 2014, 14, 761–768. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Sheng, H.; Wang, X.; Zhao, H.; Feng, K. Excessive nitrogen fertilizer application causes rapid degradation of greenhouse soil in China. Pol. J. Environ. Stud. 2022, 31, 1527–1534. [Google Scholar] [CrossRef]
- Awadelkareem, W.; Haroun, M.; Wang, J.; Qian, X. Nitrogen interactions cause soil degradation in greenhouses: Their relationship to soil preservation in China. Horticulturae 2023, 9, 340. [Google Scholar] [CrossRef]
- Sandu, M.A.; Vîrsta, A.; Vasile Scăețeanu, G.; Nicolae, C.G.; Iliescu, A.I.; Ivan, I.; Stoian, M.; Madjar, R.M. Water quality monitoring of Moara Domnească pond, Ilfov County, using UAV-based RGB imaging. AgroLife Sci. J. 2023, 12, 191–201. [Google Scholar] [CrossRef]
- Singh, B.; Craswell, E. Fertilizers and nitrate pollution of surface and ground water and increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources. Available online: https://eur-lex.europa.eu/eli/dir/1991/676/oj (accessed on 23 March 2025).
- Mosaad, I.; El-Samit, R.; Seadh, A.; Abdelhamied, A.; Mustafa, A.; Elshikh, M. Quantitative nitrate leaching relationship models based on nitrogen fertilization and the intervals between maize irrigations in the salt-affected soil. J. King Saud Univ. Sci. 2024, 36, 103187. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, J.; Zhang, Z.; Abdelrahman, A.M.; Gao, Q. Effect of different fertilization managements on nitrate accumulation in a Mollisol of Northeast China. Biol. Technol. Agric. 2016, 3, 16. [Google Scholar] [CrossRef]
- Abdelkader, M.; Zargar, M.; Bayat, M.; Pakina, E.; Shehata, A.S.A.; Suliman, A.A. Biogenic nano-fertilizers as a sustainable approach to alleviate nitrate accumulation and enrich quality traits of vegetable crops. Horticulturae 2024, 10, 789. [Google Scholar] [CrossRef]
- Farzadfar, S.; Knight, D.; Congreves, K. Soil organic nitrogen: An overlooked but potentially significant contribution to crop nutrition. Plant Soil 2021, 462, 7–23. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Manuel, D.-B.; De Beeck, M.O.; Shahbaz, M.; Chen, Y.; Deng, X.; Xu, Z.; Liu, Z. Rotation cropping and organic fertilizer jointly promote soil health and crop production. J. Environ. Manag. 2022, 315, 115190. [Google Scholar] [CrossRef]
- Walworth, J. Nitrogen in Soil and the Environment; AZ1591 2013; The University of Arizona, College of Agriculture and Life Sciences: Tucson, AZ, USA, 2013. [Google Scholar]
- Da Silva, E.F.; Ferreira Melo, M.; Santos Sombra, K.E.; Severo Silva, T.; Ferreira de Freitas, D.; Da Costa, M.A.; Da Silva Santos, E.P.; Fernandes da Silva, L.; Pereira Serra, A.; De Morais Cavalcante Neitzke, P.R. Organic nitrogen in agricultural systems. In Nitrogen Fixation; Rigobelo, E.C., Pereira Serra, A., Eds.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Näsholm, T.; Huss-Danell, K.; Högberg, P. Uptake of organic nitrogen in the field by four agriculturally important plant species. Ecology 2000, 81, 1155–1161. [Google Scholar] [CrossRef]
- Näsholm, T.; Huss-Danell, K.; Högberg, P. Uptake of glycine by field grown wheat. New Phytol. 2002, 150, 59–63. [Google Scholar] [CrossRef]
- Kelley, K.R.; Stevenson, F.J. Forms and nature of organic N in soil. Fertil. Res. 1995, 42, 1–11. [Google Scholar] [CrossRef]
- Stevenson, F.J. Organic forms of soil nitrogen. In Nitrogen in Agricultural Soils; Stevenson, F.J., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA; Crop Science Society of America, Inc.: Madison, WI, USA; Soil Science Society of America Inc.: Madison, WI, USA, 1982; Volume 22, pp. 67–122. [Google Scholar]
- Adame, C.R.; Carlos, R.S.; Braos, L.B.; Ferreira, M.E.; da Cruz, M.C.P. Organic nitrogen forms in soils treated with cattle manure. Nitrogen 2024, 5, 91–105. [Google Scholar] [CrossRef]
- Mazur, Z.; Mazur, T. Effects of long-term organic and mineral fertilizer applications on soil nitrogen content. Pol. J. Environ. Stud. 2015, 24, 2073–2078. [Google Scholar] [CrossRef]
- He, Y.; Zhang, C.; Xue, T.; Chen, X.; Fu, Y. Effects of land-use changes on soil organic nitrogen fractions in the black soil region on Northeast China. Soil Use Manag. 2023, 39, 805–816. [Google Scholar] [CrossRef]
- Drescher, G.L.; Da Silva, L.S.; Sarfaraz, Q.; Dal Molin, G.; Marzari, L.B.; Lopes, A.F.; Cella, C.; Facco, D.B.; Hammerschitt, R.K. Alkaline hydrolysable nitrogen and properties that dictate its distribution in paddy soil profiles. Pedosphere 2020, 30, 326–335. [Google Scholar] [CrossRef]
- Dodor, D.E.; Kamara, M.S.; Asamoah-Bediako, A.; Adiku, S.G.K.; MacCarthy, D.S.; Kumahor, S.K.; Neina, D. Evaluation of alkaline hydrolyzable organic nitrogen as an index of nitrogen mineralization potential of some coastal savannah soils of Ghana. Nitrogen 2022, 3, 652–662. [Google Scholar] [CrossRef]
- Davidescu, D.; Davidescu, V. Evaluation of Fertility by Plant and Soil Analysis; Abacus Press: London, UK, 1982; pp. 437–438. [Google Scholar]
- Pereira, M.G.; Espindula, A.; Valladares, G.S.; Cunha don Anjos, L.H.; De Melo Benites, V.; Schultz, N. Comparison of total nitrogen methods applied for histosols and soil horizons with high organic matter content. Commun. Soil Sci. Plant Anal. 2006, 37, 939–943. [Google Scholar] [CrossRef]
- Gautam, V.P.; Mishra, S.; Ahmed, H. Comparison of total nitrogen estimations by Kjeldahl method and CHNS analyzer in dry tropical grassland. Int. J. Plant Environ. 2023, 9, 180–182. [Google Scholar] [CrossRef]
- Ren, B.; Ma, Z.; Zhao, B.; Liu, P.; Zhang, J. Nitrapyrin mitigates nitrous oxide emissions, and improves maize yield and nitrogen efficiency under waterlogged field. Plants 2022, 11, 1983. [Google Scholar] [CrossRef]
- Bailey, T.; Robinson, N.; Farrell, M.; Macdonald, B.; Weaver, T.; Antille, D.; Chin, A.; Brackin, R. Storage of soil samples leads to over-representation of the contribution of nitrate to plant-available nitrogen. Soil Res. 2022, 60, 22–32. [Google Scholar] [CrossRef]
- Allende-Montalban, R.; San-Juan-Heras, R.; Martin-Lammerding, D.; Del Mar Delgado, M.; Del Mar Albarran, M.; Gabriel, J. The soil sample conservation method and its potential impact on ammonium, nitrate and total mineral nitrogen measurements. Geoderma 2024, 448, 116963. [Google Scholar] [CrossRef]
- Wada, S.I.; Odahara, K.; Gunjikake, N.; Takada, S. Prediction of nitrate and chloride ion concentrations in soil solution using water extracts. Soil Sci. Plant Nutr. 2006, 52, 1–4. [Google Scholar] [CrossRef]
- Garmay, A.; Oskolok, K.; Monogarova, O.; Demidov, M. Determination of ammonium and nitrate in soils by digital colorimetry. Environ. Monit. Assess. 2024, 196, 948. [Google Scholar] [CrossRef]
- Nelson, D.; Sommers, L. Total nitrogen analysis of soil and plant tissues. J. Assoc. Off. Anal. Chem. 1980, 63, 770–778. [Google Scholar] [CrossRef]
- FAO. Standard Operating Procedure for Soil Nitrogen–Kjeldahl Method. Rome. 2021. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/17a0e476-f796-4608-a869-295a367c0b56/content (accessed on 17 April 2025).
- Mot, A.; Ion, V.A.; Madjar, R.M.; Badulescu, L. Dynamic Pregl-Dumas technique applied in nitrogen determination from inputs used in organic agriculture. Sci. Papers. Ser. A Agron. 2022, LXV, 105–110. [Google Scholar]
- Koroleff, F. Determination of Total Nitrogen in Natural Waters by Means of Persulfate Oxidation; ASC 1969–C–Hydrography Committee Report; ICES: Copenhagen, Denmark, 1969. [Google Scholar] [CrossRef]
- Merck. Available online: https://www.sigmaaldrich.com/RO/en/technical-documents/protocol/analytical-chemistry/photometry-and-reflectometry/determination-of-total-nitrogen-in-soil (accessed on 6 April 2025).
- Santasup, N.; Theanjumpol, P.; Santasup, C.; Kittiwachana, S.; Mawan, N.; Prantong, L.; Khongdee, N. Development of near-infrared spectroscopy for estimating organic matter, total carbon, and total nitrogen in agricultural soil. MethodsX 2024, 13, 102798. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; You, W.; Tian, S.; Xiao, T.; Wang, M.; Zheng, B.; Luo, L. Soil nitrogen content detection based on near-infrared spectroscopy. Sensors 2022, 22, 8013. [Google Scholar] [CrossRef]
- Li, H.; Jia, S.; Le, Z. Quantitative analysis of soil total nitrogen using hyperspectral imaging technology with extreme learning machine. Sensors 2019, 19, 4355. [Google Scholar] [CrossRef]
- Ma, J.; Cheng, J.; Wang, J.; Pan, R.; He, F.; Yan, L.; Xiao, J. Rapid detection of total nitrogen content in soil based on hyperspectral technology. Inf. Process. Agric. 2022, 9, 566–574. [Google Scholar] [CrossRef]
- Kim, M.-J.; Lee, J.-E.; Back, I.; Lim, K.J.; Mo, C. Estimation of total nitrogen content in topsoil based on machine and deep learning using hyperspectral imaging. Agriculture 2023, 13, 1975. [Google Scholar] [CrossRef]
- Gotoh, S.; Araragi, M.; Koga, H.; Ono, S.I. Hydrolysable organic forms of nitrogen in some rice soil profiles as affected by organic matter application. Soil Sci. Plant Nutr. 1986, 32, 535–550. [Google Scholar] [CrossRef]
- Sądej, W.; Przekwas, K. Fluctuations of nitrogen levels in soil profile under conditions of a long-term fertilization experiment. Plant Soil Environ. 2008, 54, 197–203. [Google Scholar] [CrossRef]
- Kuśmierz, S.; Skowrońska, M.; Tkaczyk, P.; Lipiński, W.; Mielniczuk, J. Soil organic carbon and mineral nitrogen contents in soils as affected by their ph, texture and fertilization. Agronomy 2023, 13, 267. [Google Scholar] [CrossRef]
- Fayose, T.; Thomas, E.; Radu, T.; Dillingham, P.; Ullah, S.; Radu, A. Concurrent measurement of nitrate and ammonium in water and soil samples using ion-selective electrodes: Tackling sensitivity and precision issues. Anal. Sci. Adv. 2021, 2, 2790288. [Google Scholar] [CrossRef] [PubMed]
- Dorich, R.A.; Nelson, D.W. Direct colorimetric measurement of ammonium in potassium chloride extracts of soils. Soil Sci. Soc. Am. J. 1983, 47, 8330836. [Google Scholar] [CrossRef]
- Liu, X.; Wu, D.; Abid, A.A.; Liu, Y.; Zhou, J.; Zhang, Q. Determination of paddy soil ammonia nitrogen using rapid detection kit coupled with microplate reader. Toxics 2022, 10, 725. [Google Scholar] [CrossRef]
- Qiu, X.-C.; Liu, G.-P.; Zhu, Y.-Q. Determination of water-soluble ammonium ion in soil by spectrophotometry. Analyst 1987, 112, 909–911. [Google Scholar] [CrossRef]
- Sibley, K.J.; Brewster, G.; Astatkie, T.; Adsett, J.; Struik, P. In-field measurement of soil nitrate using an ion-selective electrode. In Advances in Measurement Systems; From the Edited Volume; Sharma, M., Ed.; IntechOpen: London, UK, 2010. [Google Scholar]
- Stewart, B.M. Ion chromatographic determination of nitrate in 2M KCl soil extracts. J. Soil Sci. 1987, 38, 415–419. [Google Scholar] [CrossRef]
- Du Preez, C.C.; Laubscher, D.J.; Toit, P.J. Determination of nitrate in 2.0 M KCl soil extracts using ion chromatography. Commun. Soil Sci. Plant Anal. 1989, 20, 113–120. [Google Scholar] [CrossRef]
- Mai, Y.; Ghiasvand, A.; Gupta, V.; Edwards, S.; Cahoon, S.; Debruille, K.; Mikhail, I.; Murray, E.; Paull, B. Application of a portable ion chromatograph for real-time field analysis of nitrite and nitrate in soils and soil pore waters. Talanta 2024, 274, 126031. [Google Scholar] [CrossRef]
- Maggini, R.; Carmassi, G.; Incrocci, L.; Pardossi, A. Evaluation of quick test kits for the determination of nitrate, ammonium and phosphate in soil and hydroponic nutrient solutions. Agrochimica 2010, LIV, 331–341. [Google Scholar]
- Thomsen, E.O.; Reeve, J.R.; Culumber, C.M.; Alston, D.G.; Newhall, R.; Cardon, G. Simple soil tests for on-site evaluation of soil health in orchards. Sustainability 2019, 11, 6009. [Google Scholar] [CrossRef]
- Joly, M.; Marlet, M.; Barreau, D.; Jourdan, A.; Durieu, C.; Launay, J.; Temple-Boyer, P. Monitoring of ammonium and nitrate ions in soil using ion-sensitive potentiometric microsensors. Sensors 2024, 24, 7143. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Qian, M.; Liu, J.; Fang, Y. Low cost, portable voltammetric sensors for rapid detection of nitrate in soil. Electrochim. Acta 2023, 446, 142077. [Google Scholar] [CrossRef]
- Eldeeb, M.A.; Dhamu, V.N.; Paul, A.; Muthukumar, S.; Prasad, S. Electrochemical soil nitrate sensor for in situ real-time monitoring. Micromachines 2023, 14, 1314. [Google Scholar] [CrossRef] [PubMed]
- Baumbauer, C.L.; Goodrich, P.J.; Payne, M.E.; Anthony, T.; Beckstoffer, C.; Toor, A.; Silver, W.; Arias, A.C. Printed potentiometric nitrate sensors for use in soil. Sensors 2022, 22, 4095. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Smith, K.A. The rapid determination of nitrate at low concentrations in soil extracts: Comparison of ion-selective electrode with continuous-flow analysis. Commun. Soil Sci. Plant. Anal. 1984, 15, 1437–1451. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Chen, M.; Wang, M.; Zhang, M. An ISE-based on-site soil nitrate nitrogen detection system. Sensors 2019, 19, 4669. [Google Scholar] [CrossRef]
- Ali, M.A.; Wang, X.; Chen, Y.; Jiao, Y.; Mahal, N.; Moru, S.; Castellano, M.; Schnable, J.; Schnable, P.; Dong, L. Continuous monitoring of soil nitrate using a miniature sensor with poly(3-octyl-thiophene) and molybdenum disulfide nanocomposite. ACS Appl. Mater. Interfaces 2019, 11, 29195–29206. [Google Scholar] [CrossRef]
- Szeles, A.; Huzsvai, L.; Mohammed, S.; Nyeki, A.; Zagyi, P.; Horvath, E.; Simon, K.; Arshad, S.; Tamas, A. Precision agricultural technology for advanced monitoring of maize yield under different fertilization and irrigation regimes: A case study in Eastern Hungary (Debrecen). J. Agric. Food Res. 2024, 15, 100967. [Google Scholar] [CrossRef]
- Quebrajo, L.; Pérez-Ruiz, M.; Rodriguez-Lizana, A.; Agüera, J. An approach to precise nitrogen management using hand-held crop sensor measurements and winter wheat yield mapping in a Mediterranean environment. Sensors 2015, 15, 5504–5517. [Google Scholar] [CrossRef]
- Vikuk, V.; Spirkaneder, A.; Noack, P.; Duemig, A. Validation of a sensor-system for real-time measurement of mineralized nitrogen in soils. Smart Agric. Technol. 2024, 7, 100390. [Google Scholar] [CrossRef]
- Steiger, A.; Qaswar, M.; Bill, R.; Mouazen, A.; Grenzdörffer, G. Comparing the hanfheld Stenon FarmLab soil sensor with a Vis-NIR multi-sensor soil sensing platform. Smart Agric. Technol. 2025, 10, 100717. [Google Scholar] [CrossRef]
- Lavanya, V.; Nayak, A.; Deb Roy, P.; Dasgupta, S.; Dey, S.; Li, B.; Weindorf, D.C.; Chakraborty, S. A smartphone-enabled imaging device for chromotropic acid-based measurement of nitrate in soil samples. Sensors 2023, 23, 7345. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Han, X.; Hu, R.; Yu, J.; Yan, X.; Xu, J. Research on soil inorganic nitrogen detection technology based on dielectric response. Sustainability 2025, 17, 2491. [Google Scholar] [CrossRef]
- Choi, W.-J.; Kwak, J.-H.; Lim, S.-S.; Park, H.-J.; Chang, S.; Lee, S.-M.; Arshad, M.; Yunm, S.-I.; Kim, H.-Y. Synthetic fertilizer and livestock manure differently affect δ15N in the agricultural landscape: A review. Agric. Ecosyst. Environ. 2017, 237, 1–15. [Google Scholar] [CrossRef]
- Mariotti, A.; Germon, J.C.; Leclerc, A. Nitrogen isotope fractionation associated with the NO2−→N2O step of the denitrification in soils. Can. J. Soil Sci. 1982, 62, 227–241. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.; Li, X. Using stable nitrogen isotope to indicate soil nitrogen dynamics under agricultural soil erosion In the Mun River basin, Northeast Thailand. Ecol. Indic. 2021, 128, 107814. [Google Scholar] [CrossRef]
- Cui, M.; Zeng, L.; Qun, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263, 114553. [Google Scholar] [CrossRef]
- Nikolenko, O.; Jurado, A.; Borges, A.; Knöller, K.; Brouyére, S. Isotopic composition of nitrogen species in groundwater under agricultural areas: A review. Sci. Total Environ. 2018, 621, 1415–1432. [Google Scholar] [CrossRef]
- Mashaba-Munghemezulu, Z.; Chirima, G.J.; Munghemezulu, C. Modeling the spatial distribution of soil nitrogen content at smallholder maize farms using machine learning regression and Sentinel-2 data. Sustainability 2021, 13, 11591. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, M.; Zhang, Y.; Mao, D.; Li, F.; Wu, F.; Song, J.; Li, X.; Kou, C.; Li, C.; et al. Comparison of machine learning methods for predicting soil total nitrogen content using Landsat-8, Sentinel-1, and Sentinel-2 images. Remote Sens. 2023, 15, 2907. [Google Scholar] [CrossRef]
- Charishma, D.S.; Kuligod, V.B.; Gundlur, S.S.; Potdar, M.P.; Doddamani, M.B.; Nagaveni, H.C. Estimation of top soil properties by Sentinel-2 imaging. Geol. Ecol. Landsc. 2024, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Bao, N.; Li, W.; Liu, S.; Fu, Y.; Mao, Y. Soil nutrient estimation and mapping in farmland based on UAV imaging spectrometry. Sensors 2021, 21, 3919. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.A.; Diwakar, P.K.; Ragi, S. Total nitrogen estimation in agricultural soils via aerial multispectral imaging and LIBS. Sci. Rep. 2021, 11, 12693. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Sherrell, J.; Emami, A.; Khaleghian, M. Application of artificial intelligence and sensor fusion for soil organic matter prediction. Sensors 2024, 24, 2357. [Google Scholar] [CrossRef] [PubMed]
- Schard, P.C.; Schmidt, J.P.; Kitchen, N.R.; Sudduth, K.A.; Hong, S.Y.; Lory, J.A.; Davis, J.G. Remote sensing for nitrogen management. J. Soil Water Conserv. 2002, 57, 518–524. [Google Scholar] [CrossRef]
- Boegh, E.; Houborg, R.; Bienkowski, J.; Braban, C.; Dalgaard, T.; Van Dijk, C.; Dragosits, U.; Holmes, E.; Magliulo, V.; Schelde, K.; et al. Remote sensing of LAI, chlorophyll and leaf nitrogen pools of crop- and grasslands in five European landscapes. Biogesciences 2013, 10, 6279–6307. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Veeranampalayam Sivukumar, A.-N.; Schachtman, D. Elucidating sorghum biomass, nitrogen and chlorophyll contents with spectral and morphological traits derived from unmanned aircraft system. Front. Plant Sci. 2018, 9, 1406. [Google Scholar] [CrossRef]
- Croft, H.; Arabian, J.; Chen, J.; Shang, J.; Liu, J. Mapping within-field leaf chlorophyll content in agricultural crops for nitrogen management using Landsat-8 imagery. Precis. Agric. 2020, 21, 856–880. [Google Scholar] [CrossRef]
- Swify, S.; Mažeika, R.; Volungevičius, J. Mineral nitrogen release patterns in various soil and texture types and the impact of urea and coated urea potassium humate on barley biomass. Soil Syst. 2023, 7, 102. [Google Scholar] [CrossRef]
- Sapek, B. Impact of soil pH on nitrogen mineralization in grassland soils. In Progress in Nitrogen Cycling Studies. Developments in Plant and Soil Sciences; Van Cleemput, O., Hofman, G., Vermoesen, A., Eds.; Springer: Dordrecht, The Netherlands, 1996; Volume 68, pp. 271–276. [Google Scholar]
- Du, L.; Zhong, H.; Guo, X.; Li, H.; Xia, J.; Chen, Q. Nitrogen fertilization and soil nitrogen cycling: Unraveling the links among multiple environmental factors, functional genes, and transformation rates. Sci. Total Environ. 2024, 951, 175561. [Google Scholar] [CrossRef]
- Mocanu, V.; Voicu, V.; Dumitru, S.; Ignat, P.; Mocanu, V. The influence of mixed grass/legume pastures in crop rotation on soil quality—A study case on a cambisol from Southern Transylvania (Romania). AgroLife Sci. J. 2016, 5, 138–143. [Google Scholar]
- Lorenz, K.; Omondi, E.; Lal, R.; Das, S.; Smith, A. Soil organic carbon and total nitrogen after 34 years under conventional and organic management practices at the Rodale Institute Farming Systems Trial. Soil Sci. Soc. Am. J. 2025, 89, e14165. [Google Scholar] [CrossRef]
- Vidal, M.M.; Almendro-Candel, M.B.; Navarro-Pedreño, J. Carbon and nitrogen stocks in topsoil under different land use/land cover types in the Southeast of Spain. AgriEngineering 2024, 6, 396–408. [Google Scholar] [CrossRef]
- Spohn, M.; Stendahl, J. Soil carbon and nitrogen contents in forest soils are related to soil texture in interaction with pH and metal cations. Geoderma 2024, 441, 116746. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.; Zang, Q.; Li, Y.; Zhao, J.; Lu, X.; Jiang, M.; Zhuang, H.; Huang, L. The effects of cultivation patterns and nitrogen levels on fertility and bacterial community characteristics of surface and subsurface soil. Front. Microbiol. 2023, 14, 1072228. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Chen, W.-Q.; Jiao, Y.-Q.; Yang, R.; Niu, L.-L.; Chen, X.-R.; Ji, C.-Y.; Yin, D.-X. Effects of nitrogen fertilizer application on soil properties and arsenic mobilization in paddy soil. Sustainability 2024, 16, 5565. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Z.; Sun, X.; Yan, J.; Sun, Y.; Liu, P.; Chen, J. Mapping topsoil total nitrogen using random forest and modified regression kriging in agricultural areas of Central China. Plants 2023, 12, 1464. [Google Scholar] [CrossRef]
- Simonis, A.; Setatou, H. Investigations on nitrogen dynamics in red Mediterranean soils of Greece. In Proceedings of the World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 131–133. [Google Scholar]
- Kincses, I.; Melendez, J.R.; Ramírez-Cando, L.; Burbano-Salas, D.; Lowy, D.; Nevarez, G.C.; Talledo Solorzano, V.; Arteaga, J.M.; Mendoza, B.; Sandor, Z. Soluble nitrogen forms in sand soil of Pallag: A quantitative report. F1000Research 2020, 9, 781. [Google Scholar] [CrossRef]
- Balla Kovács, A.; Juhász, E.K.; Béni, Á.; Kincses, I.; Tállai, M.; Sándor, Z.; Kátai, J.; Rátonyi, T.; Kremper, R. Changes in microbial community and activity of chernozem soil under different management systems in a long-term field experiment in Hungary. Agronomy 2024, 14, 745. [Google Scholar] [CrossRef]
- Wibowo, H.; Kasno, A. Soil organic carbon and total nitrogen dynamics in paddy soils on the Java Island, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 648, 012192. [Google Scholar] [CrossRef]
- Zilio, M.; Pigoli, A.; Rizzi, B.; Goglio, A.; Tambone, F.; Giordano, A.; Maretto, L.; Squartini, A.; Stevanato, P.; Meers, E.; et al. Nitrogen dynamics in soils fertilized with digestate and mineral fertilizers: A full field approach. Sci. Total Environ. 2023, 868, 161500. [Google Scholar] [CrossRef]
- Benedetti, A.; Sebastiani, G. Determination of potentially mineralizable nitrogen in agricultural soil. Biol. Fertil. Soils 1996, 21, 114–120. [Google Scholar] [CrossRef]
- Mia, M.J.; Monaci, E.; Murri, G.; Massetani, F.; Facchi, J.; Neri, D. Soil nitrogen and weed biodiversity: An assessment under two orchard floor management practices in a nitrogen vulnerable zone in Italy. Horticulturae 2020, 6, 96. [Google Scholar] [CrossRef]
- Papini, R.; Valboa, G.; Piovanelli, C.; Brandi, G. Nitrogen and phosphorus in a loam soil of Central Italy as affected by 6 years of different tillage systems. Soil Tillage Res. 2007, 92, 175–180. [Google Scholar] [CrossRef]
- Badagliacca, G.; Romeo, M.; Lo Presti, E.; Gelsomino, A.; Monti, M. Factors governing total and permanganate oxidizable c pools in agricultural soils from Southern Italy. Agriculture 2020, 10, 99. [Google Scholar] [CrossRef]
- Podlasek, A.; Koda, E.; Vaverková, M.D. The variability of nitrogen forms in soils due to traditional and precision agriculture: Case studies in Poland. Int. J. Environ. Res. Public Health 2021, 18, 465. [Google Scholar] [CrossRef]
- Moigradean, D.; Lazureanu, A.; Poiana, M.-A.; Harmanescu, M.; Gogoasa, I.; Gergen, I. The influence of mineral fertilization about nitrogen content in soil, plant and tomato fruit. Bull. UASVM Hortic. 2008, 65, 172–177. [Google Scholar]
- Mayes, M.; Marin-Spiotta, E.; Szymanski, L.; Erdogan, A.; Ozdogan, M.; Clayton, M. Soil type mediates effects of land use on soil carbon and nitrogen in the Konya Basin, Turkey. Geoderma 2014, 232–234, 517–527. [Google Scholar] [CrossRef]
- Uygur, V.; Irvem, A.; Karanlik, S.; Akis, R. Mapping of total nitrogen, available phosphorus and potassium in Amik Plain, Turkey. Environ. Earth Sci. 2010, 59, 1129–1138. [Google Scholar] [CrossRef]
- Sandu, M.A.; Madjar, R.M.; Preda, M.; Vîrsta, A.; Stavrescu-Bedivan, M.-M.; Vasile Scăețeanu, G. Assessment of Water Quality and Parasitofauna, and a Biometric Analysis of the Prussian Carp of the Romanian Lentic Ecosystem in Moara Domnească, Ilfov County. Water 2023, 15, 3978. [Google Scholar] [CrossRef]
- Gu, B.; Zhang, X.; Lam, S.K.; Yu, Y.; van Grinsven, H.; Zhang, S.; Wang, X.; Bodirsky, B.L.; Wang, S.; Duan, J.; et al. Cost-effective mitigation of nitrogen pollution from global croplands. Nature 2023, 613, 77–84. [Google Scholar] [CrossRef]
- Freidenreich, A.; Barraza, G.; Jayachandran, K.; Khoddamzadeh, A.A. Precision agriculture application for sustainable nitrogen management of Justicia brandegeana using opti-cal sensor technology. Agriculture 2019, 9, 98. [Google Scholar] [CrossRef]
- Van Alphen, B.J. Precision nitrogen fertilization: A case study for Dutch arable farming. In Proceedings of the 5th International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Roma, E.; Laudicina, V.A.; Vallone, M.; Catania, P. Application of Precision Agriculture for the Sustainable Management of Fertilization in Olive Groves. Agronomy 2023, 13, 324. [Google Scholar] [CrossRef]
- AL Aasmi, A.; Li, J.; Hamoud, Y.A.; Lan, Y.; Alordzinu, K.E.; Appiah, S.A.; Shaghaleh, H.; Sheteiwy, M.; Wang, H.; Qiao, S.; et al. Impacts of slow-release nitrogen fertilizer rates on the morpho-physiological traits, yield, and nitrogen use efficiency of rice under different water regimes. Agriculture 2022, 12, 86. [Google Scholar] [CrossRef]
- Maduro Dias, C.; Machado, M.; Nunes, H.; Borba, A.; Madruga, J.; Monjardino, P. Nitrogen fertilization using conventional and slow-release fertilizers at multiple levels in Lolium multiflorum Lam. pastures. Agronomy 2024, 14, 2191. [Google Scholar] [CrossRef]
- Abhiram, G. Slow-Release Fertilisers Con-trol N Losses but negatively impact on agronomic performances of pasture: Evidence from a meta-analysis. Nitrogen 2024, 5, 1058–1073. [Google Scholar] [CrossRef]
- Li, X.; Li, Z. Global trends and current advances in slow/controlled-release fertilizers: A bibliometric analysis from 1990 to 2023. Agriculture 2024, 14, 1502. [Google Scholar] [CrossRef]
- Ghafoor, I.; Rahman, M.H.u.; Hasnain, M.U.; Ikram, R.M.; Khan, M.A.; Iqbal, R.; Hussain, M.I.; El Sabagh, A. Effect of slow-release nitrogenous fertilizers on dry matter accumulation, grain nutritional quality, water productivity and wheat yield under an arid environment. Sci. Rep. 2022, 12, 14783. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, G.; Wang, J.; She, D. Preparation of lignin-based slow-release nitrogen fertilizer. Sustainability 2024, 16, 10289. [Google Scholar] [CrossRef]
- Yang, R.; Shen, Y.; Kong, X.; Ge, B.; Sun, X.; Cao, M. Effects of diverse crop rotation sequences on rice growth, yield, and soil properties: A field study in Gewu station. Plants 2024, 13, 3273. [Google Scholar] [CrossRef]
- Yfantopoulos, D.; Ntatsi, G.; Karkanis, A.; Savvas, D. Evaluation of the role of legumes in crop rotation schemes of organic or conventionally cultivated cabbage. Agronomy 2024, 14, 297. [Google Scholar] [CrossRef]
- Graham, R.F.; Wortman, S.E.; Pittelkow, C.M. Comparison of organic and integrated nutrient management strategies for reducing soil N2O emissions. Sustainability 2017, 9, 510. [Google Scholar] [CrossRef]
- Ali, A.; Niu, G.; Masabni, J.; Ferrante, A.; Cocetta, G. Integrated nutrient management of fruits, vegetables, and crops through the use of biostimulants, soilless cultivation, and traditional and modern approaches—A mini review. Agriculture 2024, 14, 1330. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B. Integrated nutrient management (INM) for sustaining crop productivity and reducing environmental impact: A review. Sci. Total. Environ. 2015, 512, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Darjee, S.; Shrivastava, M.; Langyan, S.; Singh, G.; Pandey, R.; Sharma, A.; Khandelwal, A.; Singh, R. Integrated nutrient management reduced the nutrient losses and increased crop yield in irrigated wheat. Arch. Agron. Soil Sci. 2022, 69, 1298–1309. [Google Scholar] [CrossRef]
- Kharlukhi, D.G.; Upadhyaya, K.; Sahoo, U.K. Influence of integrated nutrient management on soil health, growth and yield of paddy in “jhum lands’ of north-eastern Himalayas. Discov. Agric. 2024, 2, 107. [Google Scholar] [CrossRef]
- Gatsios, A.; Ntatsi, G.; Celi, L.; Said-Pullicino, D.; Tampakaki, A.; Savvas, D. Impact of legumes as a pre-crop on nitrogen nutrition and yield in organic greenhouse tomato. Plants 2021, 10, 468. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, H.; Chen, F.; Wang, Q.; Li, J.; Chen, Q.; Liang, B. Optimizing nitrogen management reduces mineral nitrogen leaching loss mainly by decreasing water leakage in vegetable fields under plastic-shed greenhouse. Environ. Pollut. 2022, 308, 119616. [Google Scholar] [CrossRef]
- Luo, X.; Kou, C.; Wang, Q. Optimal fertilizer application reduced nitrogen leaching and maintained high yield in wheat-maize cropping system in North China. Plants 2022, 11, 1963. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, J.; Wei, Z.; Dodla, S.; Fultz, L.; Gaston, L.; Xiao, R.; Park, J.-H.; Scaglia, G. Nitrification inhibitors reduce nitrogen losses and improve soil health in a subtropical pastureland. Geoderma 2021, 388, 114947. [Google Scholar] [CrossRef]
- Sha, Z.; Ma, X.; Loick, N.; Lv, T.; Cardenas, L.; Ma, Y.; Liu, X.; Misselbrook, T. Nitrogen stabilizers mitigate reactive N and greenhouse gas emissions from an arable soil in North China Plain: Field and laboratory investigation. J. Clean. Prod. 2020, 258, 121025. [Google Scholar] [CrossRef]
- Triozzi, M.; Ilacqua, A.; Tumolo, M.; Ancona, V.; Losacco, D. Assessment of the nitrification inhibitor nitrapyrin on nitrogen losses and Brassica oleracea growth: A preliminary sustainable research. Nitrogen 2025, 6, 15. [Google Scholar] [CrossRef]
- Rey, C.S.; Oyege, I.; Shetty, K.G.; Jayachandran, K.; Balaji Bhaskar, M.S. Evaluation of vermicompost, seaweed, and algal fertilizers on soil fertility and plant production of sunn hemp. Soil Syst. 2024, 8, 132. [Google Scholar] [CrossRef]
- Hartz, T.K.; Mitchell, J.P.; Giannini, C. Nitrogen and carbon mineralization dynamics of manures and composts. HortScience 2000, 35, 209–212. [Google Scholar] [CrossRef]
- Cozzolino, E.; Salluzzo, A.; Piano, L.d.; Tallarita, A.V.; Cenvinzo, V.; Cuciniello, A.; Cerbone, A.; Lombardi, P.; Caruso, G. Effects of the application of a plant-based compost on yield and quality of industrial tomato (Solanum lycopersicum L.) grown in different soils. Appl. Sci. 2023, 13, 8401. [Google Scholar] [CrossRef]
- Strenner, M.; Chmelíková, L.; Hülsbergen, K.-J. Compost Fertilization in Organic Agriculture—A comparison of the impact on corn plants using field spectroscopy. Appl. Sci. 2023, 13, 3676. [Google Scholar] [CrossRef]
- Espinosa-Antón, A.A.; Zamora-Natera, J.F.; Zarazúa-Villaseñor, P.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Águila Alcántara, E.; Torres-Morán, M.I.; Velasco-Ramírez, A.P.; Hernández-Herrera, R.M. Application of seaweed generates changes in the substrate and stimulates the growth of tomato plants. Plants 2023, 12, 1520. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Kurniawan, N.S.H.; Kirana, I.A.P.; Ardiana, N.; Abidin, A.S.; Ilhami, B.T.K.; Jupri, A.; Widyastuti, S.; Sunarpi, H.; Nikmatullah, A. Seaweed fertilizer prepared by EM-fermentation increases abundance of beneficial soil microbiome in paddy (Oryzasativa L.) during vegetative stage. Fermentation 2022, 8, 46. [Google Scholar] [CrossRef]
- Brown, C. Available Nutrients and Value for Manure from Various Livestock Types. Factsheet. Order No.13-043, AGDEX 538, 2013. Available online: https://www.ontario.ca/page/available-nutrients-and-value-manure-various-livestock-types (accessed on 17 April 2025).
- Pratt, P.; Castellanos, J. Available nitrogen from animal manures. Calif. Agric. 1981, 35, 24. [Google Scholar]
- Dion, P.-P.; Jeanne, T.; Theriault, M.; Hogue, R.; Pepin, S.; Dorais, M. Nitrogen release from five organic fertilizers commonly used in greenhouse organic horticulture with contrasting effects on bacterial communities. Can. J. Soil Sci. 2020, 100, 120–135. [Google Scholar] [CrossRef]
- Sukor, A.; Qian, Y.; Davis, J.G. Organic nitrogen fertilizer selection influences water use efficiency in drip-irrigated sweet corn. Agriculture 2023, 13, 923. [Google Scholar] [CrossRef]
- Bakayoko, S.; Soro, D.; Nindjin, C.; Dao, D.; Tschannen, A.; Girardin, O.; Assa, A. Effects of cattle and poultry manures on organic matter content and adsorption complex of a sandy soil under cassava cultivation (Manihot esculenta, Crantz). Afr. J. Environ. Sci. Technol. 2009, 3, 190–197. [Google Scholar]
- Dalias, P.; Christou, A. Nitrogen supplying capacity of animal manures to the soil in relation to the length of their storage. Nitrogen 2020, 1, 52–66. [Google Scholar] [CrossRef]
- Beauchamp, E.G. Availability of nitrogen from three manures to corn in the field. Can. J. Soil Sci. 1986, 66, 713–720. [Google Scholar] [CrossRef]
- Moral, R.; Moreno-Caselles, J.; Perez-Murcia, M.D.; Perez-Espinosa, A.; Rufete, B.; Paredes, C. Characterization on the organic matter pool in manures. Bioresour. Technol. 2005, 96, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ksheem, A.; Bennett, J.; Antille, D.; Raine, S. Towards a method for optimized extraction of soluble nutrients from fresh and composted chicken manures. Waste Manag. 2015, 45, 76–90. [Google Scholar] [CrossRef]
- Li, X.; Kang, X.; Xi, L.; Dou, Q.; Shi, Z.; Liu, T.; Wang, L. Drying characteristics of chicken manure under a variable temperature process. Appl. Sci. 2025, 15, 4093. [Google Scholar] [CrossRef]
- Patil, S.B.; Balakrishna Reddy, B.C.; Chitgupekar, S.C.; Patil, B.B. Modern tillage and integrated nutrient management practices for improving soil fertility and productivity of groundnut (Arachis hypogaea L.) under rainfed farming system. Int. Lett. Nat. Sci. 2015, 29, 1–12. [Google Scholar] [CrossRef]
- Başay, S.; Dorak, S.; Aşik, B.B. The effects of organic fertilizer applications on the nutrient elements content of eggplant seeds. Agronomy 2025, 15, 439. [Google Scholar] [CrossRef]
- Cardarelli, M.; El Chami, A.; Iovieno, P.; Rouphael, Y.; Bonini, P.; Colla, G. Organic fertilizer sources distinctively modulate productivity, quality, mineral composition, and soil enzyme activity of greenhouse lettuce grown in degraded soil. Agronomy 2023, 13, 194. [Google Scholar] [CrossRef]
- Tascı, F.G.; Kuzucu, C.O. The effects of vermicompost and green manure use on yield and economic factors in broccoli. Horticulturae 2023, 9, 406. [Google Scholar] [CrossRef]
- Hadas, A.; Rosenberg, R. Guano as a nitrogen source for fertigation in organic farming. Fert. Res. 1992, 31, 209–214. [Google Scholar] [CrossRef]
- Rapisarda, S.; Di Biase, G.; Mazzon, M.; Ciavatta, C.; Cavani, L. Nitrogen availability in organic fertilizers from tannery and slaughterhouse by-products. Sustainability 2022, 14, 12921. [Google Scholar] [CrossRef]
- Panday, D.; Bhusal, N.; Das, S.; Ghalehgolabbehbahani, A. Rooted in Nature: The rise, challenges, and potential of organic farming and fertilizers in agroecosystems. Sustainability 2024, 16, 1530. [Google Scholar] [CrossRef]
- Rodrigues-Espinosa, T.; Papamichael, I.; Voukkali, I.; Gimeno, A.P.; Almendro Candel, M.B.; Navarro-Pedreno, J.; Zorpas, A.; Gomez Lucas, I. Nitrogen management in farming systems under the use of agricultural wastes and circular economy. Sci. Total Environ. 2023, 876, 162666. [Google Scholar] [CrossRef] [PubMed]
- Chatzistathis, T.; Kavvadias, V.; Sotiropoulos, T.; Papadakis, I.E. Organic fertilization and tree orchards. Agriculture 2021, 11, 692. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Hu, W.; Bai, H.; Ma, L.; Lv, X.; Zhou, Z.; Meng, Y. Crop residue return improved soil nitrogen availability by increasing amino acid and mineralization under appropriate N fertilization. Land Degrad. Dev. 2022, 33, 2197–2207. [Google Scholar] [CrossRef]
- Bhatt, R.; Kunal; Moulick, D.; Barek, V.; Brestic, M.; Gaber, A.; Skalicky, M.; Hossain, A. Sustainable strategies to limit nitrogen loss in agriculture through improving its use efficiency—Aiming to reduce environmental pollution. J. Agric. Food Res. 2025, 22, 101957. [Google Scholar] [CrossRef]
- Jordan-Meille, L.; Danoroy, P.; Dittert, K.; Cugnon, T.; Quemada, M.; Wall, D.; Bechini, L.; Marx, S.; Oenema, O.; Reijneveld, A.; et al. Comparison of nitrogen fertilization recommendations of West European Countries. Eur. J. Soil Sci. 2023, 74, e13436. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).