Integration of Plant Electrophysiology and Time-Lapse Video Analysis via Artificial Intelligence for the Advancement of Precision Agriculture
Abstract
1. Introduction
1.1. Plant Electricity
1.2. Plant Organ Movements
1.3. Method
2. Artificial Intelligence Procedures in Plant Phenomics
3. Plant Electrograms and Electrome Concept
4. Effects of Environmental Factors and Stresses on Plant Electricity
4.1. Abiotic Factors and Stresses
| Plant | Environmental Factors | Electrogram Parameters | Statistics/ AI Analysis | Literature (Year) |
|---|---|---|---|---|
| Abiotic factors | ||||
| tomato | calcium (Ca), nitrogen (N), manganese (Mn), and iron (Fe) deficiencies | silver-coated copper wire electrodes continuous 3 weeks, 500 Hz | signal decomposition sample space reduction feature extraction | [28] (2022) |
| peppers | low and high urea fertilization | three stainless steel needle electrodes stem electrical resistance many days greenhouse and field experiment | average value of triplicates one-way ANOVA Duncan’s multi-range test principal component analysis (PCA) | [29] (2023) |
| tomato cabbage | exposure to chemicals such as sulfuric acid (H2SO4), sodium chloride (NaCl) and ozone (O3) | two stainless steel needle electrodes laboratory, Faraday cage 10 Hz | fifteen statistical features eight different classification algorithms, PCA | [30] (2023) |
| Hedera helix | ozone exposure | laboratory, Faraday cage 300 Hz | generic automatic toolchain machine learning models | [31] (2024) |
| Zamioculcas zamiifolia Solanum lycopersicum (tomato) | wind, heat, red light blue light | electrical potential and impedance many minutes lasting measures laboratory, 0.58 Hz | discriminant analysis deep-learning methods | [32] (2023) |
| grapevine | water status | two silver-plated needle electrodes many days climate chambers, 256 Hz. | two machine learning approaches based on classification and regression the prediction models | [33] (2024) |
| bean | water status | needle electrodes two hours measurements Faraday’s cage 62.5 Hz. | arithmetic average of voltage variation, skewness, kurtosis, probability density function (PDF), autocorrelation, power spectral density (PSD), approximate entropy (ApEn), fast Fourier transform (FFT), and multiscale approximate entropy (ApEn(s), machine learning (ML) | [34] (2024) |
| Clivia | water gradient | patch electrodes Faraday’s cage in the thermostatic and humidified incubator 60 min measurements 30 sec samples, 30 Hz | lightweight convolutional neural network (CNN) model (PlantNet) | [35] (2024) |
| Biotic factors | ||||
| barley (Hordeum vulgare) | fungal infection (Blumeria graminis, Bipolaris sorokiniana) | pair of needle electrodes Faraday cage, 48 h measurements | descriptive statistics, Bayesian change point (BCP) analysis, ApEn, autocorrelation, ML (cluster analysis) | [36] (2023) |
| tobacco | viral diseases (alfalfa mosaic virus) | two fine needle electrodes field conditions, 8 s at a sampling rate of 250 Hz | median, autoregressive coefficients, autocorrelation—ML models (support vector machine, k-nearest neighbours, random forest) | [37] (2024) |
| tomato | parasitic nematodes | many days measurements | ML model | [38] (2024) |
4.2. Biotic Stresses
5. Software for Time-Lapse Video Analysis for Investigation of Organ Movements in Plants
| Software Name | Plant | Types of Movement | Literature (Year) |
|---|---|---|---|
| OSCILLATOR | Arabidopsis thaliana Petunia hybrida Solanum lycopersicum | rhythmic leaf movements and growth | [52] (2012) |
| Circumnutation Tracker, https://circumnutation.umcs.lublin.pl/ct, accessed on 6 April 2025 | Helianthus annuus various plant species | analysis of the movements of various organs | [51] (2014) |
| 3D stereovision machine system | beans | nutation movements of climbing plants | [53] (2024) |
| PALMA (Plant Leaf Movement Analyzer), https://sourceforge.net/projects/palma-leafmov/ accessed on 6 April 2025 | Arabidopsis thaliana | periodic movements of leaves | [54] (2017) |
| TRiP (Tracking Rhythms in Plants), http://github.com/KTgreenham/TRiP, accessed on 6 April 2025 | Arabidopsis thaliana Brassica rapa Glycine max Cleome violacea Solanum lycopersicum Mimulus guttatus | whole-plant images and periodic movements of cotyledons and leaves | [55] (2015) |
| Oskam et al. system, https://github.com/Pierik-Lab, accessed on 6 April 2025 | Arabidopsis thaliana | nastic movements and growth of leaf as a part of the shade avoidance response | [56] (2024) |
| Rehman et al. system | Arabidopsis thaliana mutants | movements of plant leaves | [57] (2020) |
| Mao et al. tracker | Arabidopsis thaliana | circumnutation of flowering shoot apex | [58] (2023) |
| SLEAP (Social LEAP Estimates Animal Poses), https://zenodo.org/record/5764169#.YbCK0_FBxqt https://doi.org/10.5281/zenodo.5764169, accessed on 6 April 2025 | Arabidopsis thaliana sunflower bean | circumnutations, tropisms, twining | [59] (2022) |
| Gibbs et al. system | Triticum aestivum | wind-induced plant movement in field-grown wheat | [60] (2019) |
6. Discussion
6.1. Electrical Signals–Signatures
6.1.1. Abiotic Factors
6.1.2. Biotic Factors
6.1.3. Statistic and AI Analysis for Studying Plant Electrograms
6.2. Time-Lapse Video
6.3. Advantages and Difficulties
6.3.1. Advantages and Possibilities
- Application of measurement methods in laboratories, greenhouses, and field conditions;
- Successful attempts to record and analyse some electrograms and time-lapse videos in field conditions;
- Optimisation of universal measurement and analytical methods aimed at crop plants (not only the model plant Arabidopsis);
- Relatively cheap and either not at all or minimally invasive methods;
- Detection of stress and impact on the accuracy of biomass estimation;
- Real-time monitoring of environmental factors and stresses;
- Support for the decision-making process in agriculture in real time.
Difficulties and Problems
- Determination of electrogram and time-lapse video parameters;
- Elimination of artifacts and noise;
- Researcher supervision during method introduction;
- Impact of field and atmospheric conditions;
- Electrodes resistant to field conditions;
- Wireless connections enabling remote sensing;
- Selection of appropriate and standardised AI analyses;
- Integrated simultaneous bioelectrical studies and organ movement estimation;
- Standardisation of recording methods, electrode placement, sampling frequency, and sampling time during the day, taking into account the plant growth phase.
7. Conclusions
8. Future Directions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| AP | action potential |
| SLEAP | Social LEAP Estimates Animal Poses |
| TRiP | Tracking Rhythms in Plants |
| PALMA | Plant Leaf Movement Analyzer |
| PCA | principal component analysis |
| probability density function | |
| PSD | power spectral density |
| ApEn | approximate entropy |
| ApEn(s) | multiscale approximate entropy |
| FFT | fast Fourier transform |
| RF | random forest |
| ML | machine learning |
| DL | deep learning |
| CNN | convolutional neural network |
| SVM | support vector machine |
| KNN | k-nearest neighbours |
References
- Brzezicki, M. A Systematic Review of the Most Recent Concepts in Kinetic Shading Systems with a Focus on Biomimetics: A Motion/Deformation Analysis. Sustainability 2024, 16, 5697. [Google Scholar] [CrossRef]
- Balyan, S.; Jangir, H.; Tripathi, S.N.; Tripathi, A.; Jhang, T.; Pandey, P. Seeding a Sustainable Future: Navigating the Digital Horizon of Smart Agriculture. Sustainability 2024, 16, 475. [Google Scholar] [CrossRef]
- dos Reis, G.A.; Martínez-Burgos, W.J.; Pozzan, R.; Pastrana Puche, Y.; Ocán-Torres, D.; de Queiroz Fonseca Mota, P.; Rodrigues, C.; Lima Serra, J.; Scapini, T.; Karp, S.G.; et al. Comprehensive Review of Microbial Inoculants: Agricultural Applications, Technology Trends in Patents, and Regulatory Frameworks. Sustainability 2024, 16, 8720. [Google Scholar] [CrossRef]
- Li, P.; Han, Z.; Chepkorir, D.; Fang, W.; Ma, Y. Effect of Exogenous Jasmonates on Plant Adaptation to Cold Stress: A Comprehensive Study Based on a Systematic Review with a Focus on Sustainability. Sustainability 2024, 16, 10654. [Google Scholar] [CrossRef]
- Rogo, U.; Viviani, A.; Pugliesi, C.; Fambrini, M.; Usai, G.; Castellacci, M.; Simoni, S. Improving Crop Tolerance to Abiotic Stress for Sustainable Agriculture: Progress in Manipulating Ascorbic Acid Metabolism via Genome Editing. Sustainability 2025, 17, 719. [Google Scholar] [CrossRef]
- Sanberg, P.R. “Neural Capacity” in Mimosa pudica: A Review. Behav. Biol. 1976, 17, 435–452. [Google Scholar] [CrossRef]
- Applewhite, P.B. Plant and Animal Behavior: An Introductory Comparison. In Aneural Organisms in Neurobiology; Eisenstein, E.M., Ed.; Springer US: Boston, MA, USA, 1975; pp. 131–139. [Google Scholar]
- Simons, P.J. The Role of Electricity in Plant Movements. New Phytol. 1981, 87, 11–37. [Google Scholar] [CrossRef]
- Baluska, F. Recent Surprising Similarities between Plant Cells and Neurons. Plant Signal. Behav. 2010, 5, 87–89. [Google Scholar] [CrossRef]
- Borges, R.M. Do Plants and Animals Differ in Phenotypic Plasticity? J. Biosci. 2005, 30, 41–50. [Google Scholar] [CrossRef]
- Huey, R.B.; Carlson, M.; Crozier, L.; Frazier, M.; Hamilton, H.; Harley, C.; Hoang, A.; Kingsolver, J.G. Plants Versus Animals: Do They Deal with Stress in Different Ways? Integr. Comp. Biol. 2002, 42, 415–423. [Google Scholar] [CrossRef]
- Jones, A.M.; Chory, J.; Dangl, J.L.; Estelle, M.; Jacobsen, S.E.; Meyerowitz, E.M.; Nordborg, M.; Weigel, D. The Impact of Arabidopsis on Human Health: Diversifying Our Portfolio. Cell 2008, 133, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C.; Lal, M.A. Plant Movements. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2023; pp. 641–659. [Google Scholar]
- Darwin, C.; Darwin, F. The Power of Movement in Plants; John Murray: London, UK, 1880. [Google Scholar]
- Del Dottore, E.; Mondini, A.; Sadeghi, A.; Mattoli, V.; Mazzolai, B. An Efficient Soil Penetration Strategy for Explorative Robots Inspired by Plant Root Circumnutation Movements. Bioinspir. Biomim. 2018, 13, 015003. [Google Scholar] [CrossRef]
- Qin, K.; Tang, W.; Zong, H.; Guo, X.; Xu, H.; Zhong, Y.; Wang, Y.; Sheng, Q.; Yang, H.; Zou, J. Parthenocissus Inspired Soft Climbing Robots. Sci. Adv. 2025, 11, eadt9284. [Google Scholar] [CrossRef]
- Burdon-Sanderson, J.S. Note on the Electrical Phenomena Which Accompany Irritation of the Leaf of Dionæa muscipula. Proc. Roy. Soc. Lond. 1873, 21, 495–496. [Google Scholar]
- Tills, O.; Ibbini, Z.; Spicer, J.I. Bioimaging and-the Future of Whole-Organismal Developmental Physiology. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2024, 300, 111783. [Google Scholar] [CrossRef]
- Wang, Q. Understanding Plant Movement: From Kinematics to Machine Learning. PhD Thesis, Università degli Studi di Padova, Padova, Italy, 2024. [Google Scholar]
- de Toledo, G.R.; Parise, A.G.; Simmi, F.Z.; Costa, A.V.; Senko, L.G.; Debono, M.-W.; Souza, G.M. Plant Electrome: The Electrical Dimension of Plant Life. Theor. Exp. Plant Physiol. 2019, 31, 21–46. [Google Scholar] [CrossRef]
- De Loof, A. The Cell’s Self-Generated “Electrome”: The Biophysical Essence of the Immaterial Dimension of Life? Commun. Integr. Biol. 2016, 9, e1197446. [Google Scholar] [CrossRef]
- Reissig, G.N.; de Carvalho Oliveira, T.F.; Parise, A.G.; Posso, D.A.; Souza, G.M. An Electrifying View into the Life of Plants: The Plant Electrome. Front. Young Minds 2024, 12, 1400420. [Google Scholar] [CrossRef]
- Zhao, D.-J.; Wang, Z.-Y.; Li, J.; Wen, X.; Liu, A.; Huang, L.; Wang, X.-D.; Hou, R.-F.; Wang, C. Recording Extracellular Signals in Plants: A Modeling and Experimental Study. Math. Comput. Model. 2013, 58, 556–563. [Google Scholar] [CrossRef]
- Beilby, M.; Coster, H. The Action Potential in Chara Corallina III.* The Hodgkin-Huxley Parameters for the Plasmalemma. Funct. Plant Biol. 1979, 6, 337–353. [Google Scholar] [CrossRef]
- Beilby, M.J.; Al Khazaaly, S. Re-Modeling Chara Action Potential: II. The Action Potential Form under Salinity Stress. AIMS Biophys. 2017, 4, 298–315. [Google Scholar]
- Kisnieriene, V.; Lapeikaite, I.; Pupkis, V.; Beilby, M.J. Modeling the Action Potential in Characeae Nitellopsis obtusa: Effect of Saline Stress. Front. Plant Sci. 2019, 10, 82. [Google Scholar] [CrossRef]
- Shu, L.; Hancke, G.P.; Abu-Mahfouz, A.M. Guest Editorial: Sustainable and Intelligent Precision Agriculture. IEEE Trans. Ind. Inform. 2021, 17, 4318–4321. [Google Scholar] [CrossRef]
- Sai, K.; Sood, N.; Saini, I. Classification of Various Nutrient Deficiencies in Tomato Plants through Electrophysiological Signal Decomposition and Sample Space Reduction. Plant Physiol. Biochem. 2022, 186, 266–278. [Google Scholar] [CrossRef]
- Kim, H.N.; Seok, Y.J.; Park, G.M.; Vyavahare, G.; Park, J.H. Monitoring of Plant-Induced Electrical Signal of Pepper Plants (Capsicum annuum L.) According to Urea Fertilizer Application. Sci. Rep. 2023, 13, 291. [Google Scholar] [CrossRef]
- Bhadra, N.; Chatterjee, S.K.; Das, S. Multiclass Classification of Environmental Chemical Stimuli from Unbalanced Plant Electrophysiological Data. PLoS ONE 2023, 18, e0285321. [Google Scholar] [CrossRef]
- Aust, T.; Buss, E.; Mohr, F.; Hamann, H. Automated Phytosensing: Ozone Exposure Classification Based on Plant Electrical Signals. In Proceedings of the IEEE Symposia on Computational Intelligence for Energy, Transport and Environmental Sustainability (CIETES), Trondheim, Norway, 17–20 March 2025; pp. 1–7. [Google Scholar]
- Buss, E.; Aust, T.; Wahby, M.; Rabbel, T.-L.; Kernbach, S.; Hamann, H. Stimulus Classification with Electrical Potential and Impedance of Living Plants: Comparing Discriminant Analysis and Deep-Learning Methods. Bioinspir. Biomim. 2023, 18, 025003. [Google Scholar] [CrossRef]
- Cattani, A.; De Riedmatten, L.; Roulet, J.; Smit-Sadki, T.; Alfonso, E.; Kurenda, A.; Graeff, M.; Remolif, E.; Rienth, M. Water Status Assessment in Grapevines Using Plant Electrophysiology: This Article Is Published in Cooperation with the XVth International Terroir Congress, 18–22 November 2024, Mendoza, Argentina. Guest Editors: Federico Berli, Jorge Prieto and Martín Fanzone. OENO One 2024, 58, 4. [Google Scholar]
- de Toledo, G.R.; Reissig, G.N.; Senko, L.G.; Pereira, D.R.; da Silva, A.F.; Souza, G.M. Common Bean under Different Water Availability Reveals Classifiable Stimuli-Specific Signatures in Plant Electrome. Plant Signal. Behav. 2024, 19, 2333144. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Liu, C.; Wang, Q.; Shi, Y.; Xia, X.; Wang, H.; Sun, L.; Men, H. Clivia Biosensor: Soil Moisture Identification Based on Electrophysiology Signals with Deep Learning. Biosens. Bioelectron. 2024, 262, 116525. [Google Scholar] [CrossRef] [PubMed]
- Simmi, F.Z.; Dallagnol, L.J.; Almeida, R.O.; da Rosa Dorneles, K.; Souza, G.M. Barley Systemic Bioelectrical Changes Detect Pathogenic Infection Days before the First Disease Symptoms. Comput. Electron. Agric. 2023, 209, 107832. [Google Scholar] [CrossRef]
- Ghasemi, E.; Ebrahimie, E.; Niazi, A. Machine Learning for Early Detection of Plant Viruses: Analyzing Post-Infection Electrical Signal Patterns. Smart Agric. Technol. 2024, 9, 100668. [Google Scholar] [CrossRef]
- Kurenda, A.; Jenni, D.; Lecci, S.; Buchholz, A. Bringing Light into the Dark—Plant Electrophysiological Monitoring of Root Knot Nematode Infestation and Real-Time Nematicide Efficacy. J. Pest Sci. 2024, 98, 493–507. [Google Scholar] [CrossRef]
- Iosip, A.-L.; Scherzer, S.; Bauer, S.; Becker, D.; Krischke, M.; Al-Rasheid, K.A.; Schultz, J.; Kreuzer, I.; Hedrich, R. Dyscalculia, a Venus Flytrap Mutant without the Ability to Count Action Potentials. Curr. Biol. 2023, 33, 589–596.e5. [Google Scholar] [CrossRef]
- Scherzer, S.; Böhm, J.; Huang, S.; Iosip, A.L.; Kreuzer, I.; Becker, D.; Heckmann, M.; Al-Rasheid, K.A.; Dreyer, I.; Hedrich, R. A Unique Inventory of Ion Transporters Poises the Venus Flytrap to Fast-Propagating Action Potentials and Calcium Waves. Curr. Biol. 2022, 32, 4255–4263.e5. [Google Scholar] [CrossRef]
- Böhm, J.; Scherzer, S.; Krol, E.; Kreuzer, I.; von Meyer, K.; Lorey, C.; Mueller, T.D.; Shabala, L.; Monte, I.; Solano, R.; et al. The Venus Flytrap Dionaea muscipula Counts Prey-Induced Action Potentials to Induce Sodium Uptake. Curr. Biol. 2016, 26, 286–295. [Google Scholar] [CrossRef]
- Roblin, G.; Moyen, C.; Fleurat-Lessard, P.; Dédaldéchamp, F. Rapid Osmocontractile Response of Motor Cells of Mimosa pudica Pulvini Induced by Short Light Signals. Photochem. Photobiol. 2025, 101, 728–745. [Google Scholar] [CrossRef]
- Stolarz, M.; Trębacz, K. Spontaneous Rapid Leaf Movements and Action Potentials in Mimosa pudica L. Physiol. Plant. 2021, 173, 1882–1888. [Google Scholar] [CrossRef]
- Mahta, J.; Spangaro, A.; Lenartowicz, M.; Mattiazzi Usaj, M. From Pixels to Insights: Machine Learning and Deep Learning for Bioimage Analysis. BioEssays 2024, 46, 2300114. [Google Scholar]
- Boquet-Pujadas, A.; Olivo-Marin, J.-C.; Guillén, N. Bioimage Analysis and Cell Motility. Patterns 2021, 2, 100170. [Google Scholar] [CrossRef]
- Bragantini, J.; Theodoro, I.; Zhao, X.; Huijben, T.A.; Hirata-Miyasaki, E.; VijayKumar, S.; Balasubramanian, A.; Lao, T.; Agrawal, R.; Xiao, S.; et al. Ultrack: Pushing the Limits of Cell Tracking across Biological Scales. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ershov, D.; Phan, M.-S.; Pylvänäinen, J.W.; Rigaud, S.U.; Le Blanc, L.; Charles-Orszag, A.; Conway, J.R.; Laine, R.F.; Roy, N.H.; Bonazzi, D.; et al. Trackmate 7: Integrating State-of-the-Art Segmentation Algorithms into Tracking Pipelines. Nat. Methods 2022, 19, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Gallusser, B.; Weigert, M. Trackastra: Transformer-Based Cell Tracking for Live-Cell Microscopy. In Proceedings of the European Conference on Computer Vision, Paris, France, 26–27 March 2025; Springer Science & Business Media: Dordrecht, The Netherlands, 2025. [Google Scholar]
- Stolarz, M.; Król, E.; Dziubińska, H. Lithium Distinguishes between Growth and Circumnutation and Augments Glutamate-Induced Excitation of Helianthus annuus Seedlings. Acta Physiol. Plant. 2015, 37, 69. [Google Scholar] [CrossRef]
- Stolarz, M.; Krol, E.; Dziubinska, H.; Zawadzki, T. Complex Relationship between Growth and Circumnutations in Helianthus annuus Stem. Plant Signal. Behav. 2008, 3, 376–380. [Google Scholar] [CrossRef][Green Version]
- Stolarz, M.; Zuk, M.; Krol, E.; Dziubińska, H. Circumnutation Tracker: Novel Software for Investigation of Circumnutation. Plant Methods 2014, 10, 24. [Google Scholar] [CrossRef]
- Bours, R.; Muthuraman, M.; Bouwmeester, H.; van der Krol, A. Oscillator: A System for Analysis of Diurnal Leaf Growth Using Infrared Photography Combined with Wavelet Transformation. Plant Methods 2012, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Melero, D.R.; Ponkshe, A.; Calvo, P.; García-Mateos, G. The Development of a Stereo Vision System to Study the Nutation Movement of Climbing Plants. Sensors 2024, 24, 747. [Google Scholar] [CrossRef]
- Wagner, L.; Schmal, C.; Staiger, D.; Danisman, S. The Plant Leaf Movement Analyzer (PALMA): A Simple Tool for the Analysis of Periodic Cotyledon and Leaf Movement in Arabidopsis Thaliana. Plant Methods 2017, 13, 2. [Google Scholar] [CrossRef]
- Greenham, K.; Lou, P.; Remsen, S.E.; Farid, H.; McClung, C.R. Trip: Tracking Rhythms in Plants, an Automated Leaf Movement Analysis Program for Circadian Period Estimation. Plant Methods 2015, 11, 33. [Google Scholar] [CrossRef]
- Oskam, L.; Snoek, B.L.; Pantazopoulou, C.K.; van Veen, H.; Matton, S.E.; Dijkhuizen, R.; Pierik, R. A Low-Cost Open-Source Imaging Platform Reveals Spatiotemporal Insight into Leaf Elongation and Movement. Plant Physiol. 2024, 195, 1866–1879. [Google Scholar] [CrossRef]
- Rehman, T.U.; Zhang, L.; Wang, L.; Ma, D.; Maki, H.; Sánchez-Gallego, J.A.; Mickelbart, M.V.; Jin, J. Automated Leaf Movement Tracking in Time-Lapse Imaging for Plant Phenotyping. Comput. Electron. Agric. 2020, 175, 105623. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, H.; Wang, Y.; Brenner, E.D. A Deep Learning Approach to Track Arabidopsis Seedlings’ Circumnutation from Time-Lapse Videos. Plant Methods 2023, 19, 18. [Google Scholar] [CrossRef]
- Gall, G.E.C.; Pereira, T.D.; Jordan, A.; Meroz, Y. Fast Estimation of Plant Growth Dynamics Using Deep Neural Networks. Plant Methods 2022, 18, 21. [Google Scholar] [CrossRef]
- Gibbs, J.A.; Burgess, A.J.; Pound, M.P.; Pridmore, T.P.; Murchie, E.H. Recovering Wind-Induced Plant Motion in Dense Field Environments Via Deep Learning and Multiple Object Tracking. Plant Physiol. 2019, 181, 28–42. [Google Scholar] [CrossRef]
- Kunkowska, A.B. What They Do in the Shadows: A Low-Cost Imaging System for Recording Leaf Expansion and Movements. Plant Physiol. 2024, 195, 1745–1747. [Google Scholar] [CrossRef]
- Burgess, A.J.; Gibbs, J.A.; Murchie, E.H. A Canopy Conundrum: Can Wind-Induced Movement Help to Increase Crop Productivity by Relieving Photosynthetic Limitations? J. Exp. Bot. 2019, 70, 2371–2380. [Google Scholar] [CrossRef]
- Ufuktepe, D.K.; Palaniappan, K.; Elmali, M.; Baskin, T.I. Rtip: A Fully Automated Root Tip Tracker for Measuring Plant Growth with Intermittent Perturbations. In Proceedings of the 2020 IEEE International Conference on Image Processing (ICIP), Virtual Conference, 25–28 October 2020. [Google Scholar]
- Golka, W.; Arseniuk, E.; Golka, A.; Góral, T. Sztuczne Sieci Neuronowe I Teledetekcja W Ocenie Porażenia Pszenicy Jarej Fuzariozą Kłosów. Biuletyn Inst. Hod. Aklim. Rośl. 2020, 288, 67–75. [Google Scholar] [CrossRef]
- Fiaz, M.; Mahmood, A.; Javed, S.; Jung, S.K. Handcrafted and Deep Trackers: Recent Visual Object Tracking Approaches and Trends. ACM Comput. Surv. 2019, 52, 1–44. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gao, S.; Hassan, M.A.; Huang, Z.; Rasheed, A.; Hearne, S.; Prasanna, B.; Li, X.; Li, H. Artificial Intelligence in Plant Breeding. Trends Genet. 2024, 40, 891–908. [Google Scholar] [CrossRef]
- Gupta, D.K.; Pagani, A.; Zamboni, P.; Singh, A.K. AI-Powered Revolution in Plant Sciences: Advancements, Applications, and Challenges for Sustainable Agriculture and Food Security. Explor. Foods Foodomics 2024, 2, 443–459. [Google Scholar] [CrossRef]
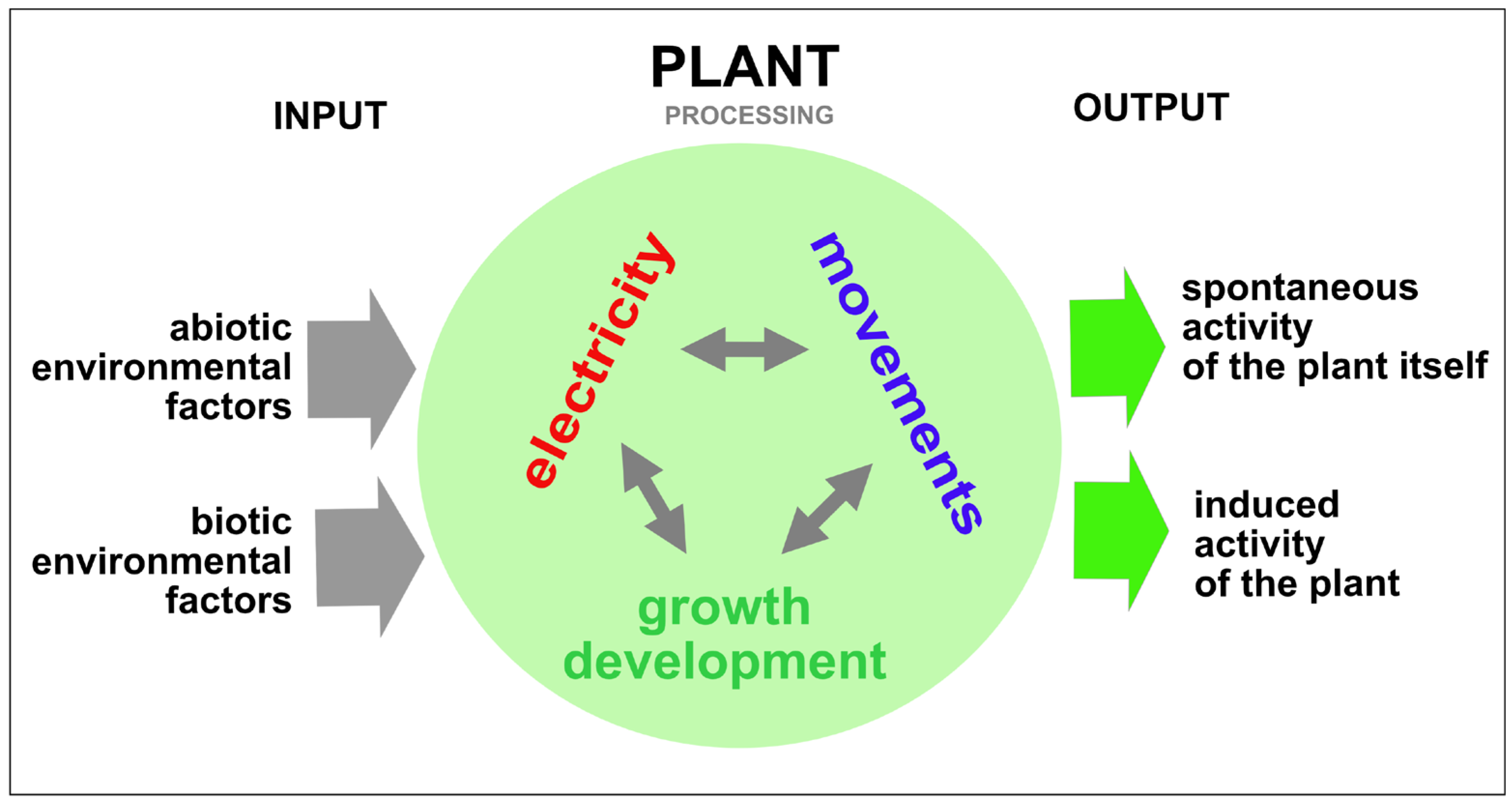
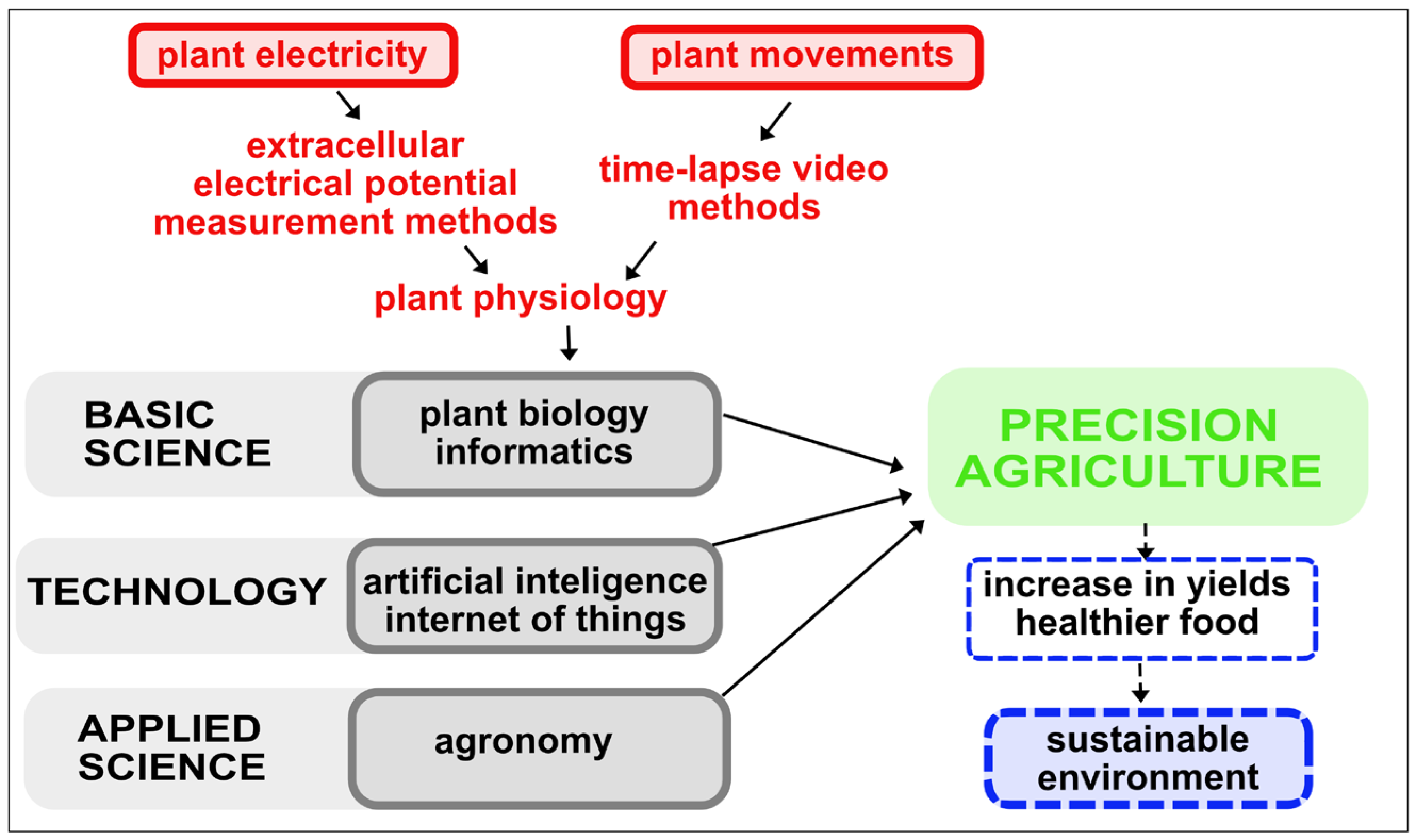

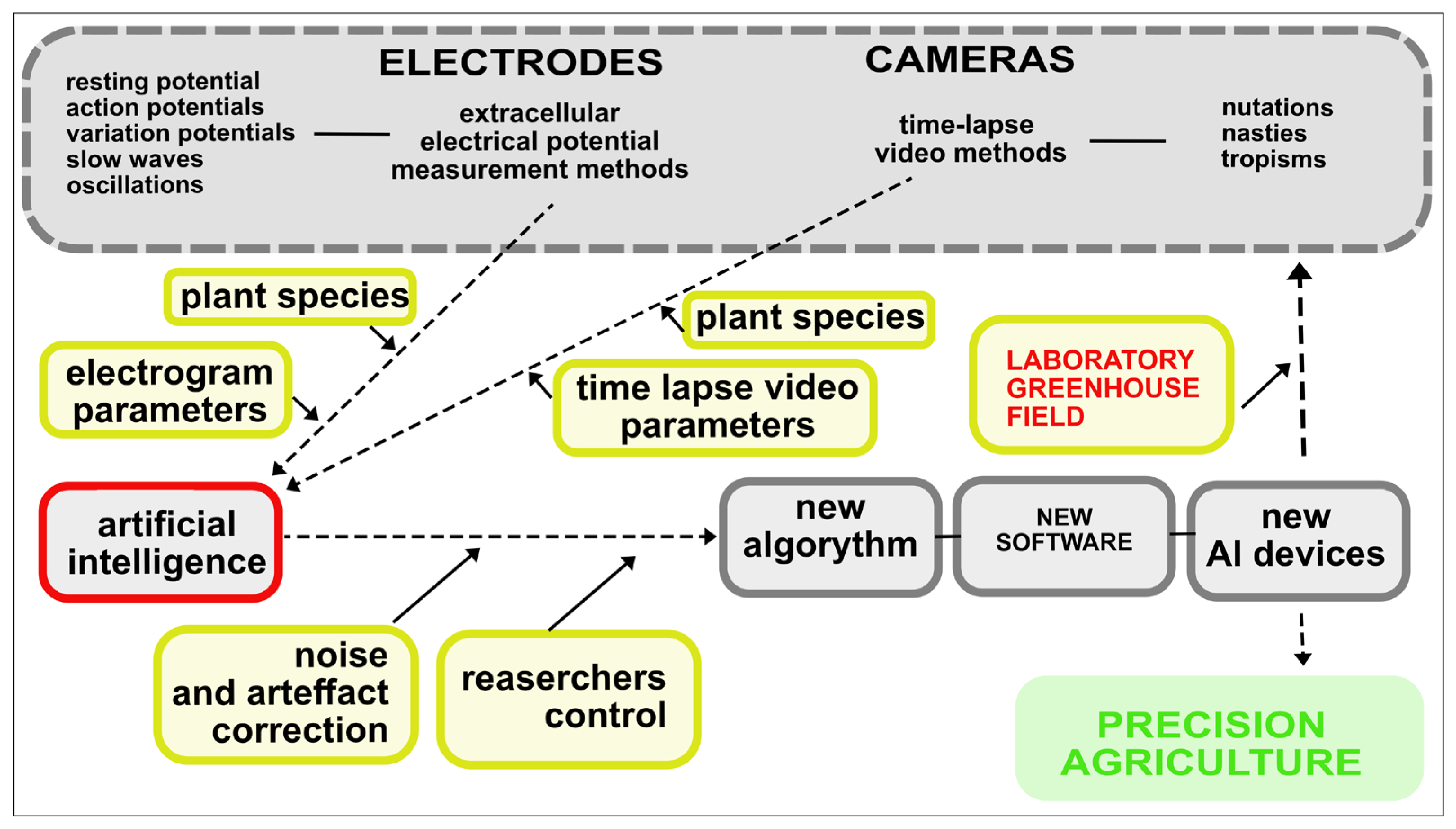
| Key Steps | Statistics/AI Analysis |
|---|---|
| data preparation and preprocessing | average value sample space reduction data |
| feature extraction and signal processing | signal decomposition |
| statistical features (arithmetic average, skewness, kurtosis, probability density function (PDF), autocorrelation, power spectral density (PSD)) | |
| transforms and entropies (fast Fourier transform (FFT), approximate entropy (ApEn), multiscale approximate entropy (ApEn(s)) | |
| statistical analysis of variation and differences | descriptive statistics, median, one-way ANOVA, Duncan’s multi-range test, Bayesian change point (BCP) analysis |
| multivariate analysis | principal component analysis (PCA) |
| machine learning methods (ML) | classification and regression (support vector machine (SVM), k-nearest neighbours (KNN), random forest (RF), discriminant analysis, different classification algorithms) |
| predictive models (construction of regression and classification models using selected features: median, autoregressive coefficients, autocorrelation) | |
| other ML techniques (cluster analysis) | |
| deep learning (DL) | lightweight convolutional neural network (lightweight CNN, e.g., PlantNet), deep-learning methods: neural networks with various architectures |
| automation and toolchains | automatic toolchain, integration of ML models within automated tools |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolarz, M. Integration of Plant Electrophysiology and Time-Lapse Video Analysis via Artificial Intelligence for the Advancement of Precision Agriculture. Sustainability 2025, 17, 5614. https://doi.org/10.3390/su17125614
Stolarz M. Integration of Plant Electrophysiology and Time-Lapse Video Analysis via Artificial Intelligence for the Advancement of Precision Agriculture. Sustainability. 2025; 17(12):5614. https://doi.org/10.3390/su17125614
Chicago/Turabian StyleStolarz, Maria. 2025. "Integration of Plant Electrophysiology and Time-Lapse Video Analysis via Artificial Intelligence for the Advancement of Precision Agriculture" Sustainability 17, no. 12: 5614. https://doi.org/10.3390/su17125614
APA StyleStolarz, M. (2025). Integration of Plant Electrophysiology and Time-Lapse Video Analysis via Artificial Intelligence for the Advancement of Precision Agriculture. Sustainability, 17(12), 5614. https://doi.org/10.3390/su17125614







