Application of Environmental DNA in the Air for Monitoring Biodiversity
Abstract
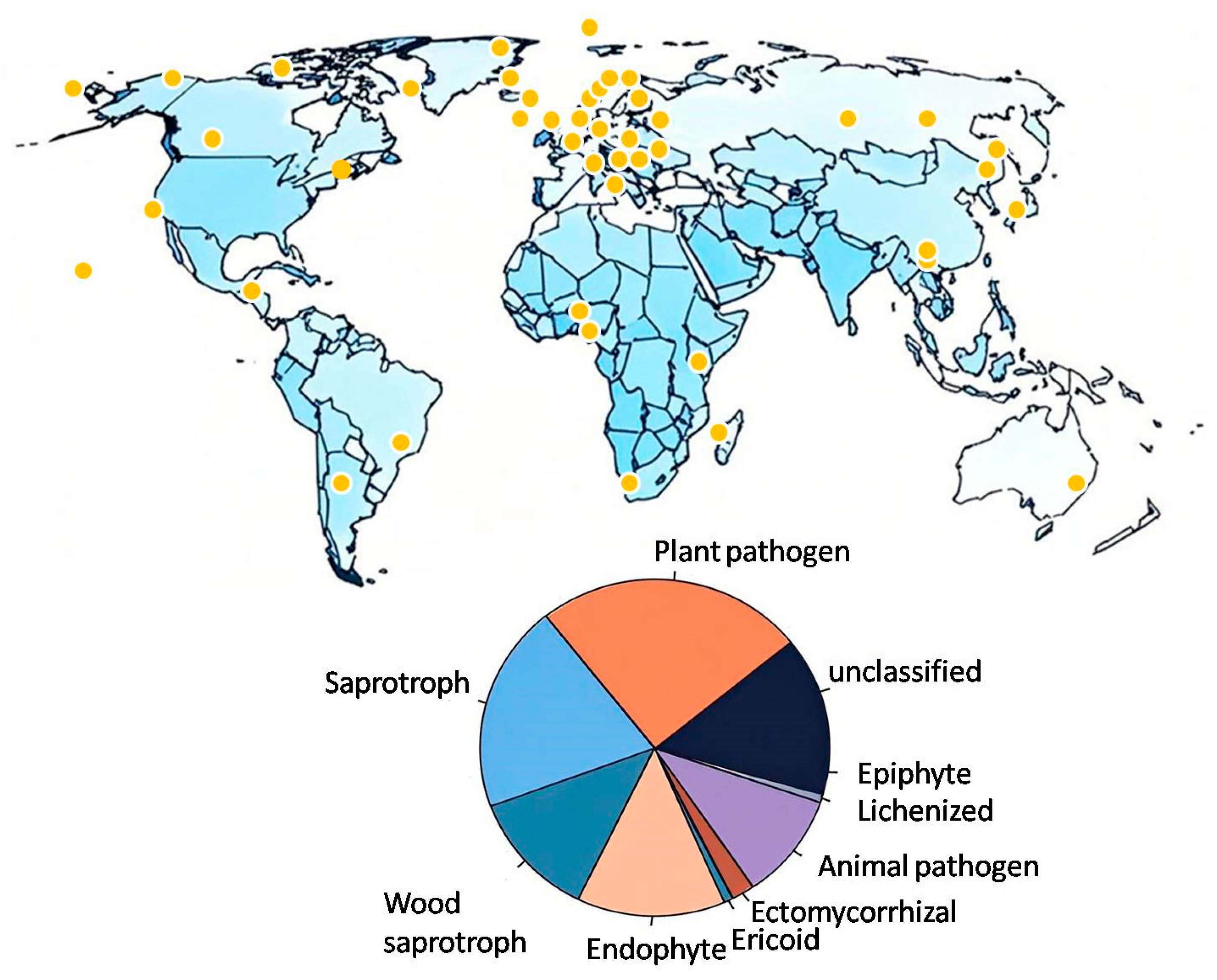
1. Introduction
2. Methods for Detecting eDNA in the Air
2.1. Sources and Release Mechanisms of eDNA
2.2. Sampling Methods
2.2.1. Active Sampling
| Sampling Method | Capture Efficiency | Cost | Best Use Case | Limitations |
|---|---|---|---|---|
| Filter samplers | High (0.22–1.2 µm) | Low | General biodiversity | Clogging in high-particle areas |
| Water-based samplers | Moderate | Medium | Pollen/microorganisms | DNA dilution in liquid |
| Electrostatic adsorption | Very high (small particles) | High | Microorganisms | High maintenance |
2.2.2. Passive Sampling
2.3. Molecular Analysis Techniques
2.3.1. DNA Extraction and Contamination Control
2.3.2. Primer Design and Amplification
2.3.3. High-Throughput Sequencing and Bioinformatics
3. Case Studies
3.1. Synchronous Monitoring of Multiple Groups
3.2. Comparison with Traditional Methods
3.3. Forest Ecosystem Monitoring
3.4. Urban and Farmland Bird Diversity
3.5. Dynamic Changes in Biodiversity Across Seasons
4. Challenges and Limitations
4.1. Production, Transport, and Degradation of eDNA in the Ambient Environment
4.1.1. Factors Affecting eDNA Production
4.1.2. Influencing Factors in eDNA Transport
4.1.3. Factors Affecting the Degradation of eDNA
4.2. Factors Affecting the Accuracy of eDNA Detection
4.2.1. Contamination Sources
4.2.2. Measures for Control Contamination Sources
4.3. Detection Sensitivity and False Negatives
4.3.1. Risk of Missing Low-Abundance Species
4.3.2. Impact of Primer Bias
4.4. Limitations of Quantitative Analysis
5. Future Directions
5.1. Technical Optimization
5.2. Standardization and Database Construction
- Sampling protocols
- (a)
- Clear specifications regarding sampler selection, including operational parameters and performance criteria
- (b)
- Defined standard operating parameters (e.g., sampling duration, flow rate, and frequency)
- Molecular processing
- (a)
- Optimized DNA extraction methodologies with prescribed reagents and protocols
- (b)
- Standardized primer design principles with validation requirements
- Bioinformatics pipeline
- (a)
- Implementation of uniform data processing and analytical frameworks
- (b)
- Quality control metrics to enable cross-study data integration
- (a)
- Coordinated large-scale specimen collection and sequencing initiatives
- (b)
- Enhanced quality assurance systems for reference sequences
- (c)
- Rigorous vetting procedures for newly submitted sequences to ensure database integrity
5.3. Integration of Multiple Technologies
5.4. Emerging Application Scenarios
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes-Lima, M.; Lyet, A.; Prié, V.; Walters, M.; Lindeque, P.; Kamanja, S.M.; Brosse, S.; Martins, F.M.S.; Beytell, P.; Becker, F.; et al. A stakeholder empowerment framework to advance eDNA biodiversity monitoring in Africa: Perspectives from Namibia. One Earth 2025, 8, 101244. [Google Scholar] [CrossRef]
- Johnson, C.N.; Balmford, A.; Brook, B.W.; Buettel, J.C.; Galetti, M.; Guangchun, L.; Wilmshurst, J.M. Biodiversity losses and conservation responses in the Anthropocene. Science 2017, 356, 270. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Filho, J.A.F.; Bini, L.M.; Targueta, C.P.; Telles, M.P.d.C.; Jardim, L.; Machado, K.B.; Nabout, J.C.; Nunes, R.; Vieira, L.C.G.; Soares, T.N. Environmental DNA and biodiversity patterns: A call for a community phylogenetics approach. Perspect. Ecol. Conserv. 2024, 22, 15–23. [Google Scholar] [CrossRef]
- Callaghan, C.T.; Santini, L.; Spake, R.; Bowler, D.E. Population abundance estimates in conservation and biodiversity research. Trends Ecol. Evol. 2024, 39, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Silveira, P.; Santos, J.S.d.; Pena, J.C.; de Lima, N.E.; Vitorino, L.C.; Martello, F.; Guimarães, R.A.; Moreira, J.W.; Gomes, J.C.; de Araújo, L.M.; et al. The loss of an unknown biodiversity: Spatial gaps in plant survey and conservation in a Brazilian hotspot of biodiversity. Biol. Conserv. 2025, 305, 111098. [Google Scholar] [CrossRef]
- Burrascano, S.; Trentanovi, G.; Paillet, Y.; Heilmann-Clausen, J.; Giordani, P.; Bagella, S.; Bravo-Oviedo, A.; Campagnaro, T.; Campanaro, A.; Chianucci, F.; et al. Handbook of field sampling for multi-taxon biodiversity studies in European forests. Ecol. Indic. 2021, 132, 108266. [Google Scholar] [CrossRef]
- Suuri, B.; Baatargal, O.; Badamdorj, B.; Reading, R.P. Assessing wildlife biodiversity using camera trap data on the Mongolian marmot (Marmota sibirica) colonies. J. Arid. Environ. 2021, 188, 104409. [Google Scholar] [CrossRef]
- Ramesh, V.; Hariharan, P.; Akshay, V.A.; Choksi, P.; Khanwilkar, S.; DeFries, R.; Robin, V.V. Using passive acoustic monitoring to examine the impacts of ecological restoration on faunal biodiversity in the Western Ghats. Biol. Conserv. 2023, 282, 110071. [Google Scholar] [CrossRef]
- Abrego, N.; Furneaux, B.; Hardwick, B.; Somervuo, P.; Palorinne, I.; Aguilar-Trigueros, C.A.; Andrew, N.R.; Babiy, U.V.; Bao, T.; Bazzano, G.; et al. Airborne DNA reveals predictable spatial and seasonal dynamics of fungi. Nature 2024, 631, 835–842. [Google Scholar] [CrossRef]
- Doi, H.; Inui, R.; Akamatsu, Y.; Kanno, K.; Yamanaka, H.; Takahara, T.; Minamoto, T. Environmental DNA analysis for estimating the abundance and biomass of stream fish. Freshw. Biol. 2017, 62, 30–39. [Google Scholar] [CrossRef]
- Vanni, M.J.; McIntyre, P.B. Predicting nutrient excretion of aquatic animals with metabolic ecology and ecological stoichiometry: A global synthesis. Ecology 2016, 97, 3460–3471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pavlovska, M.; Stoica, E.; Prekrasna, I.; Yang, J.; Slobodnik, J.; Zhang, X.; Dykyi, E. Holistic pelagic biodiversity monitoring of the Black Sea via eDNA metabarcoding approach: From bacteria to marine mammals. Environ. Int. 2020, 135, 105307. [Google Scholar] [CrossRef]
- Bohmann, K.; Lynggaard, C. Transforming terrestrial biodiversity surveys using airborne eDNA. Trends Ecol. Evol. 2023, 38, 119–121. [Google Scholar] [CrossRef]
- Ke, Y.; Liu, T.; Han, C.; Yu, X.; Wang, J.; Ding, L.; Pan, H.; Mo, X.; Lu, X. A review of eDNA technology in avian monitoring: Current status, challenges and future perspectives. Avian Res. 2025, 16, 100235. [Google Scholar] [CrossRef]
- Shuai, H.; Liu, X.; Zhang, Y.; Sun, Y.; Li, H.; Li, Z. eDNA enhances detection efficiency but reveals lower waterbird diversity: A comparison with point counting method. Avian Res. 2025, 16, 100236. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Zhao, J.; Yao, M. Passive eDNA sampling facilitates biodiversity monitoring and rare species detection. Environ. Int. 2024, 187, 108706. [Google Scholar] [CrossRef]
- Naputo, C.F.P.; Isowa, Y.; Gerona-Daga, M.E.; Artigas, M.D.; Kajita, T.; Salmo Iii, S.G. Application of eDNA metabarcoding in the assessment of fish biodiversity in Philippine mangroves: Challenges and opportunities. Reg. Stud. Mar. Sci. 2024, 77, 103642. [Google Scholar] [CrossRef]
- Lynggaard, C.; Bertelsen, M.F.; Jensen, C.V.; Johnson, M.S.; Frøslev, T.G.; Olsen, M.T.; Bohmann, K. Airborne environmental DNA for terrestrial vertebrate community monitoring. Curr. Biol. 2022, 32, 701–707. [Google Scholar] [CrossRef]
- Bell, K.L.; Campos, M.; Hoffmann, B.D.; Encinas-Viso, F.; Hunter, G.C.; Webber, B.L. Environmental DNA methods for biosecurity and invasion biology in terrestrial ecosystems: Progress, pitfalls, and prospects. Sci. Total Environ. 2024, 926, 171810. [Google Scholar] [CrossRef]
- Johnson, M.D.; Cox, R.D.; Barnes, M.A. The detection of a non-anemophilous plant species using airborne eDNA. PLoS ONE 2019, 14, e0225262. [Google Scholar] [CrossRef]
- de Groot, G.A.; Geisen, S.; Wubs, E.R.J.; Meulenbroek, L.; Laros, I.; Snoek, L.B.; Lammertsma, D.R.; Hansen, L.H.; Slim, P.A. The aerobiome uncovered: Multi-marker metabarcoding reveals potential drivers of turn-over in the full microbial community in the air. Environ. Int. 2021, 154, 106551. [Google Scholar] [CrossRef] [PubMed]
- Cananzi, G.; Tatini, I.; Li, T.; Montagna, M.; Serra, V.; Petroni, G. Active or passive? A multi-marker approach to compare active and passive eDNA sampling in riverine environments. Sci. Total Environ. 2025, 974, 179247. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, B.; Hussain, S.; Rasool, K.; Hassan, M.; Almomani, F. Comprehensive insights into advances in ambient bioaerosols sampling, analysis and factors influencing bioaerosols composition. Environ. Pollut. 2023, 336, 122473. [Google Scholar] [CrossRef] [PubMed]
- Clare, E.L.; Economou, C.K.; Bennett, F.J.; Dyer, C.E.; Adams, K.; McRobie, B.; Drinkwater, R.; Littlefair, J.E. Measuring biodiversity from DNA in the air. Curr. Biol. 2022, 32, 693–700. [Google Scholar] [CrossRef]
- Gusareva, E.S.; Acerbi, E.; Lau, K.J.X.; Luhung, I.; Premkrishnan, B.N.V.; Kolundžija, S.; Purbojati, R.W.; Wong, A.; Houghton, J.N.I.; Miller, D.; et al. Microbial communities in the tropical air ecosystem follow a precise diel cycle. Proc. Natl. Acad. Sci. USA 2019, 116, 23299–23308. [Google Scholar] [CrossRef]
- Newton, J.P.; Bateman, P.W.; Heydenrych, M.J.; Kestel, J.H.; Dixon, K.W.; Prendergast, K.S.; White, N.E.; Nevill, P. Monitoring the birds and the bees: Environmental DNA metabarcoding of flowers detects plant–animal interactions. Environ. DNA 2023, 5, 488–502. [Google Scholar] [CrossRef]
- Johnson, M.D.; Barnes, M.A.; Garrett, N.R.; Clare, E.L. Answers blowing in the wind: Detection of birds, mammals, and amphibians with airborne environmental DNA in a natural environment over a yearlong survey. Environ. DNA 2023, 5, 375–387. [Google Scholar] [CrossRef]
- Sahu, A.; Singh, M.; Amin, A.; Malik, M.M.; Qadri, S.N.; Abubakr, A.; Teja, S.S.; Dar, S.A.; Ahmad, I. A systematic review on environmental DNA (eDNA) science: An eco-friendly survey method for conservation and restoration of fragile ecosystems. Ecol. Indic. 2025, 173, 113441. [Google Scholar] [CrossRef]
- Duarte, S.; Simões, L.; Costa, F.O. Current status and topical issues on the use of eDNA-based targeted detection of rare animal species. Sci. Total Environ. 2023, 904, 166675. [Google Scholar] [CrossRef]
- Varrella, S.; Livi, S.; Corinaldesi, C.; Castriota, L.; Maggio, T.; Vivona, P.; Pindo, M.; Fava, S.; Danovaro, R.; Dell’Anno, A. A comprehensive assessment of non-indigenous species requires the combination of multi-marker eDNA metabarcoding with classical taxonomic identification. Environ. Int. 2025, 199, 109489. [Google Scholar] [CrossRef]
- Rishan, S.T.; Kline, R.J.; Rahman, M.S. Applications of environmental DNA (eDNA) to detect subterranean and aquatic invasive species: A critical review on the challenges and limitations of eDNA metabarcoding. Environ. Adv. 2023, 12, 100370. [Google Scholar] [CrossRef]
- Berezin, C.-T.; Peccoud, S.; Kar, D.M.; Peccoud, J. Cryptographic approaches to authenticating synthetic DNA sequences. Trends Biotechnol. 2024, 42, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Waymire, E.; Samake, J.N.; Gunarathna, I.; Carter, T.E. A decade of invasive Anopheles stephensi sequence-based identification: Toward a global standard. Trends Parasitol. 2024, 40, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Ghosh, P.K.; Basu, S.; Mukherjee, T.; Mandal, B.; Sinha, P.; Mukherjee, A. Microplastic contamination in threatened wild felids of India: Understanding environmental uptake, feeding implications, and associated risks. Environ. Res. 2025, 273, 121218. [Google Scholar] [CrossRef]
- Feist, S.M.; Lance, R.F. Genetic detection of freshwater harmful algal blooms: A review focused on the use of environmental DNA (eDNA) in Microcystis aeruginosa and Prymnesium parvum. Harmful Algae 2021, 110, 102124. [Google Scholar] [CrossRef]
- Sahu, A.; Kumar, N.; Pal Singh, C.; Singh, M. Environmental DNA (eDNA): Powerful technique for biodiversity conservation. J. Nat. Conserv. 2023, 71, 126325. [Google Scholar] [CrossRef]
- Pumkaeo, P.; Takahashi, J.; Iwahashi, H. Detection and monitoring of insect traces in bioaerosols. PeerJ 2021, 9, e10862. [Google Scholar] [CrossRef]
- Littlefair, J.E.; Allerton, J.J.; Brown, A.S.; Butterfield, D.M.; Robins, C.; Economou, C.K.; Garrett, N.R.; Clare, E.L. Air-quality networks collect environmental DNA with the potential to measure biodiversity at continental scales. Curr. Biol. 2023, 33, R426–R428. [Google Scholar] [CrossRef]
- Polling, M.; Buij, R.; Laros, I.; de Groot, G.A. Continuous daily sampling of airborne eDNA detects all vertebrate species identified by camera traps. Environ. DNA 2024, 6, e591. [Google Scholar] [CrossRef]
- Weidlich-Rau, M.; Navine, A.K.; Chaopricha, P.T.; Günther, F.; Kahl, S.; Wilhelm-Stein, T.; Mack, R.C.; Reers, H.; Rice, A.N.; Eibl, M.; et al. Continuous Real-Time Acoustic Monitoring of endangered bird species in Hawai‘i. Ecol. Inform. 2025, 87, 103102. [Google Scholar] [CrossRef]
- Lynggaard, C.; Frøslev, T.G.; Johnson, M.S.; Olsen, M.T.; Bohmann, K. Airborne environmental DNA captures terrestrial vertebrate diversity in nature. Mol. Ecol. Resour. 2024, 24, e13840. [Google Scholar] [CrossRef]
- Granqvist, E.; Goodsell, R.M.; Töpel, M.; Ronquist, F. The transformative potential of eDNA-based biodiversity impact assessment. Curr. Opin. Environ. Sustain. 2025, 73, 101517. [Google Scholar] [CrossRef]
- Huang, S.; Yoshitake, K.; Watabe, S.; Asakawa, S. Environmental DNA study on aquatic ecosystem monitoring and management: Recent advances and prospects. J. Environ. Manag. 2022, 323, 116310. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, M.; Furukawa, T.; Nakajima, F.; Sei, K. Pathogens and disease vectors/hosts monitoring in aquatic environments: Potential of using eDNA/eRNA based approach. Sci. Total Environ. 2021, 796, 148810. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019, 17, e00547. [Google Scholar] [CrossRef]
- Lunghi, E.; Valle, B.; Guerrieri, A.; Bonin, A.; Cianferoni, F.; Manenti, R.; Ficetola, G.F. Environmental DNA of insects and springtails from caves reveals complex processes of eDNA transfer in soils. Sci. Total Environ. 2022, 826, 154022. [Google Scholar] [CrossRef]
- Saccò, M.; Guzik, M.T.; van der Heyde, M.; Nevill, P.; Cooper, S.J.B.; Austin, A.D.; Coates, P.J.; Allentoft, M.E.; White, N.E. eDNA in subterranean ecosystems: Applications, technical aspects, and future prospects. Sci. Total Environ. 2022, 820, 153223. [Google Scholar] [CrossRef]
- Jo, T.S.; Murakami, H.; Nakadai, R. Spatial dispersal of environmental DNA particles in lentic and marine ecosystems: An overview and synthesis. Ecol. Indic. 2025, 174, 113469. [Google Scholar] [CrossRef]


| Method | Advantages | Limitations |
|---|---|---|
| Camera traps | High-resolution images, individual identification | Only suitable for medium–large-sized animals, high installation cost |
| Auditory survey | Applicable to songbird monitoring | Subject to interference from environmental noise |
| Air eDNA | Simultaneous detection of multiple taxa, non-invasive | Difficult to conduct quantitative analysis, susceptible to contamination influence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q. Application of Environmental DNA in the Air for Monitoring Biodiversity. Sustainability 2025, 17, 5530. https://doi.org/10.3390/su17125530
Liu Q. Application of Environmental DNA in the Air for Monitoring Biodiversity. Sustainability. 2025; 17(12):5530. https://doi.org/10.3390/su17125530
Chicago/Turabian StyleLiu, Qingyang. 2025. "Application of Environmental DNA in the Air for Monitoring Biodiversity" Sustainability 17, no. 12: 5530. https://doi.org/10.3390/su17125530
APA StyleLiu, Q. (2025). Application of Environmental DNA in the Air for Monitoring Biodiversity. Sustainability, 17(12), 5530. https://doi.org/10.3390/su17125530






