Use of Sands from Wastewater Treatment Plants as a Substitute for Natural Aggregate in the Context of a Circular Economy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
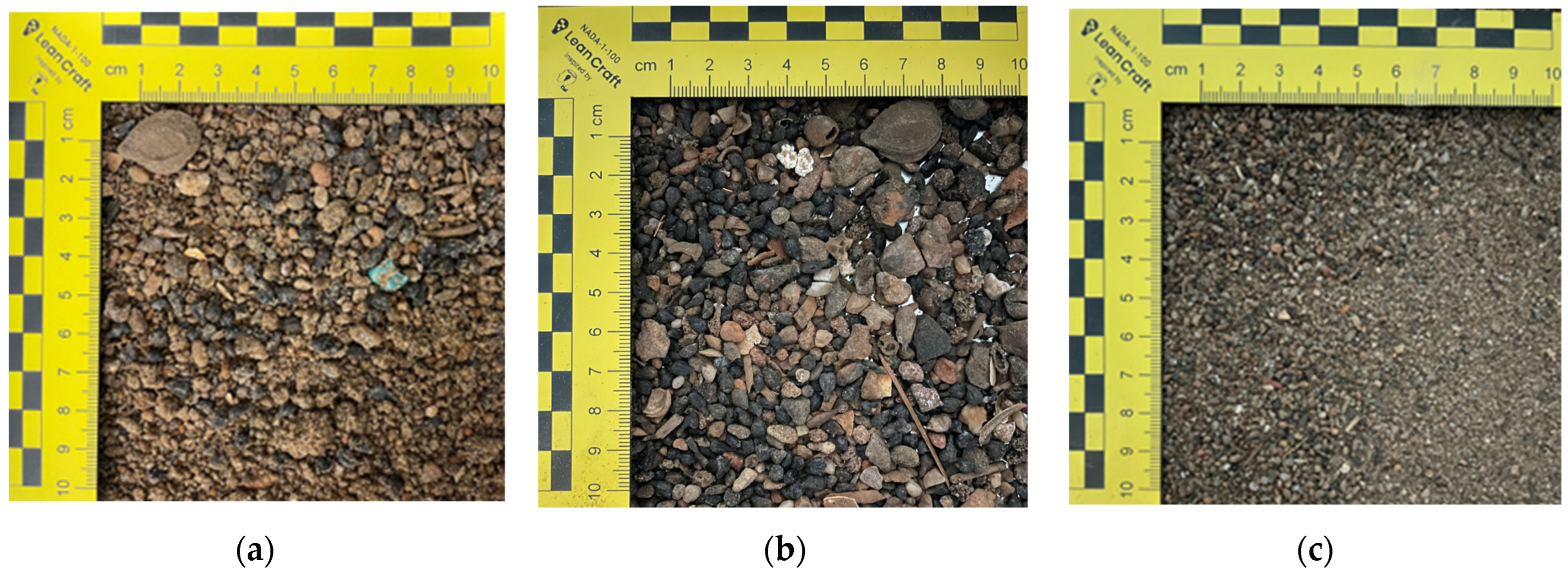
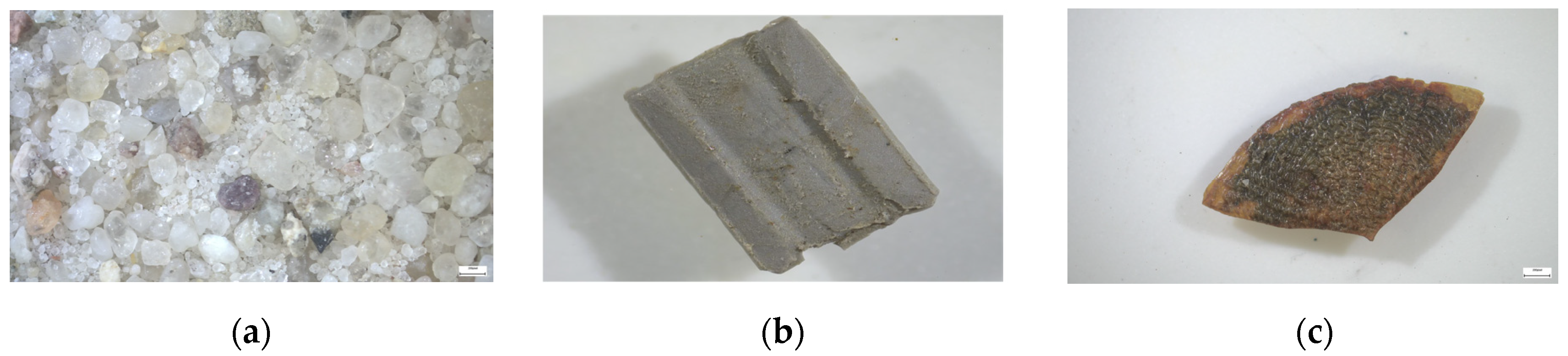
2.2.1. Sample Preparation and Physicochemical Characterization of Raw Sand
2.2.2. Leaching Procedure and Analysis of Aqueous Extracts
2.2.3. Granulometric Analysis of Waste Sand
2.2.4. Preparation and Environmental Assessment of Concrete Mortars
3. Results and Discussion
4. Conclusions
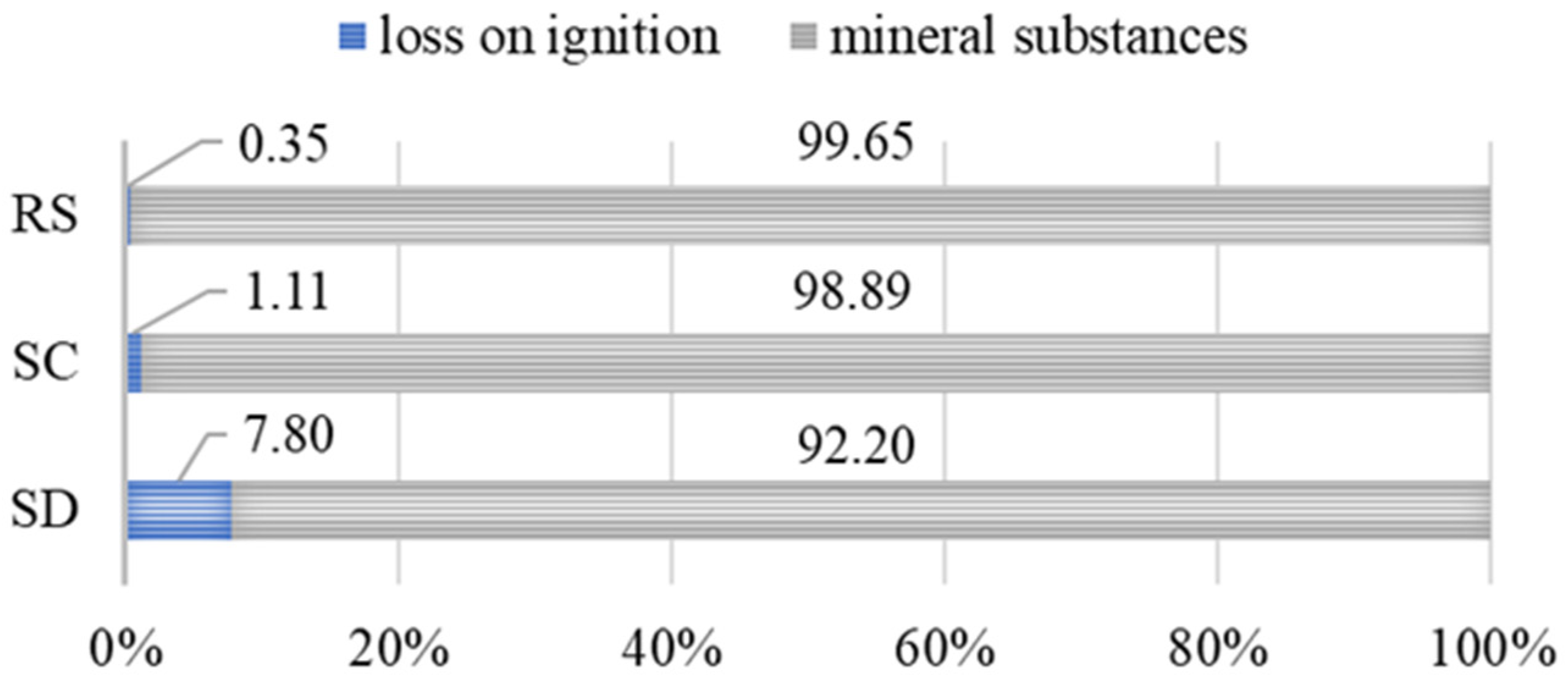
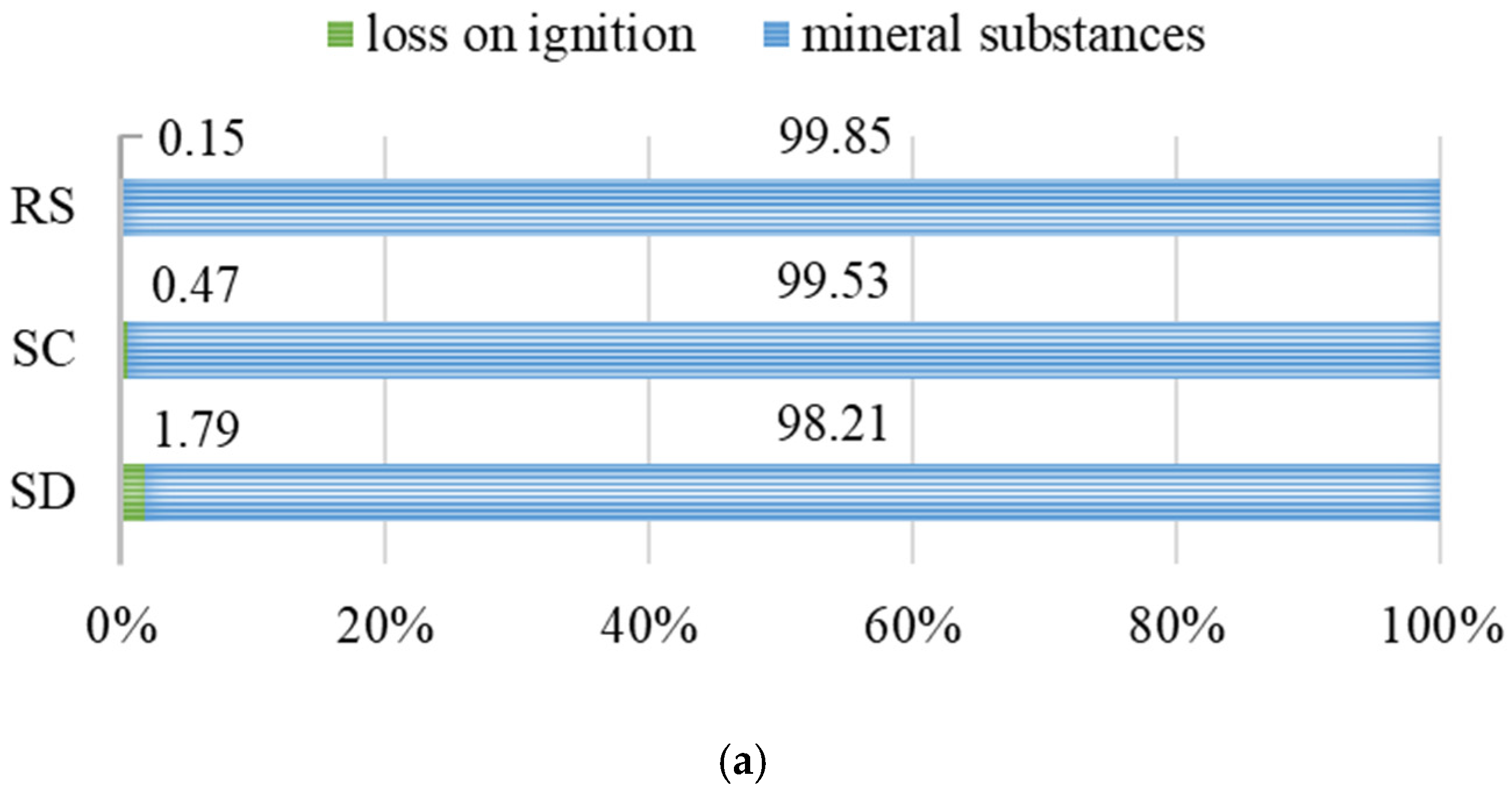
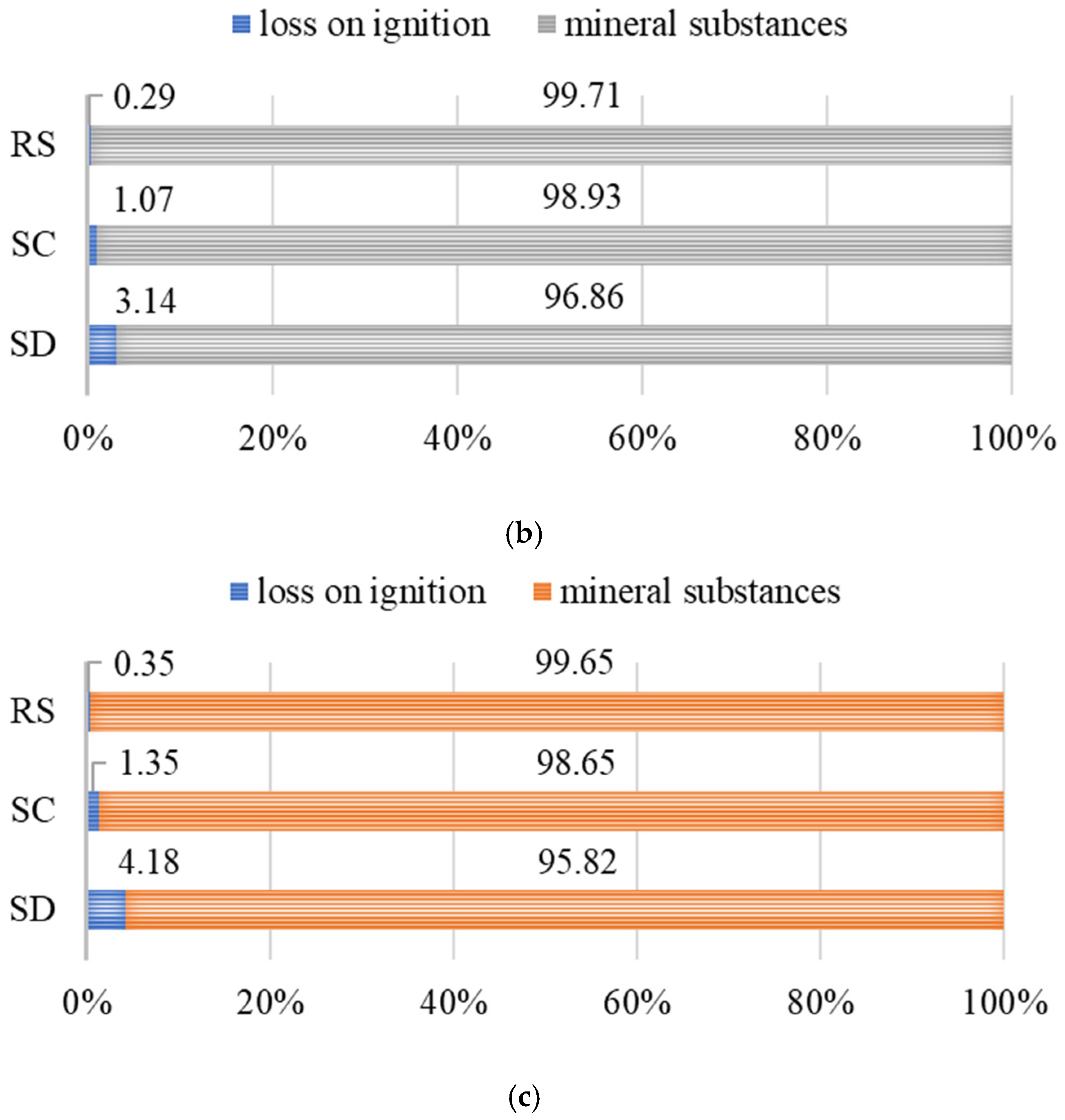
- The loss on ignition (LOI) analysis at 950 °C revealed that sample SD exceeds the recommended threshold for organic matter content in construction applications (4.18%), which may significantly limit its suitability for structural concrete production. In contrast, sample SC meets the required quality criteria (1.35%), indicating its potential applicability in building materials.
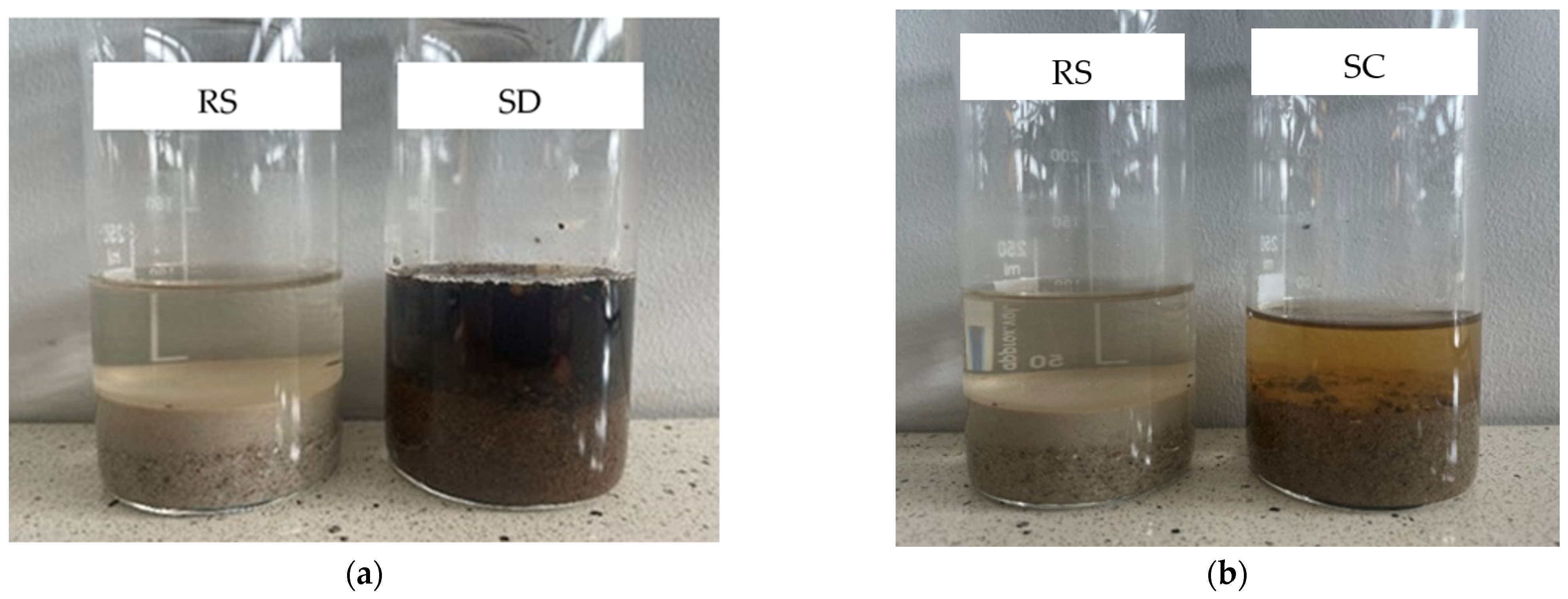
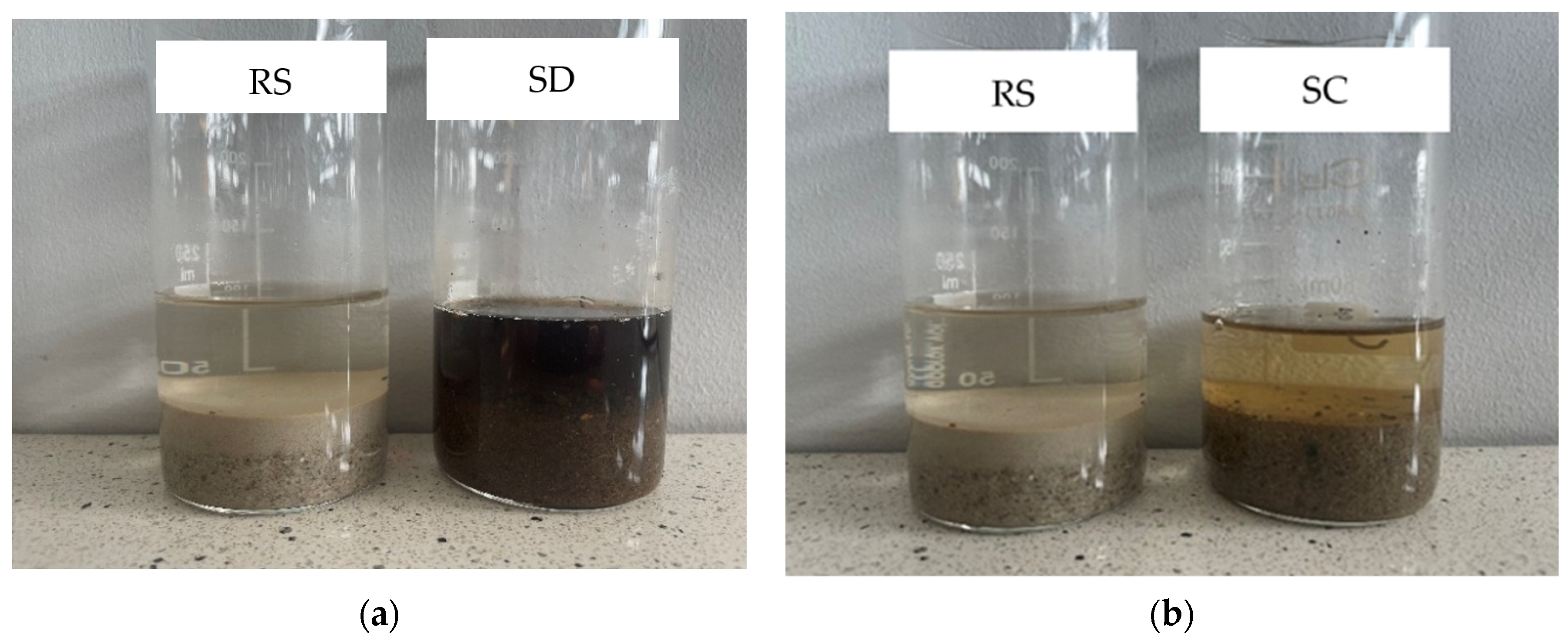
- Visual evaluation of the humic substance content showed a significantly higher concentration of humic compounds in the SD sample compared to the SC sample. A high content of humic substances may affect the physicochemical properties and long--term stability of the building material under its operating conditions. Additionally, humic substances can form water-repellent films on sand-grain surfaces, which may disrupt the interaction between the cementitious matrix and the aggregate, thereby reducing the quality of the interfacial transition zone.
- The waste sands examined that originated from wastewater treatment plants (SD and SC) comply with the current legal regulations establishing permissible levels of harmful substances, including heavy metals. This confirms their potential suitability as secondary raw materials from an environmental protection perspective.
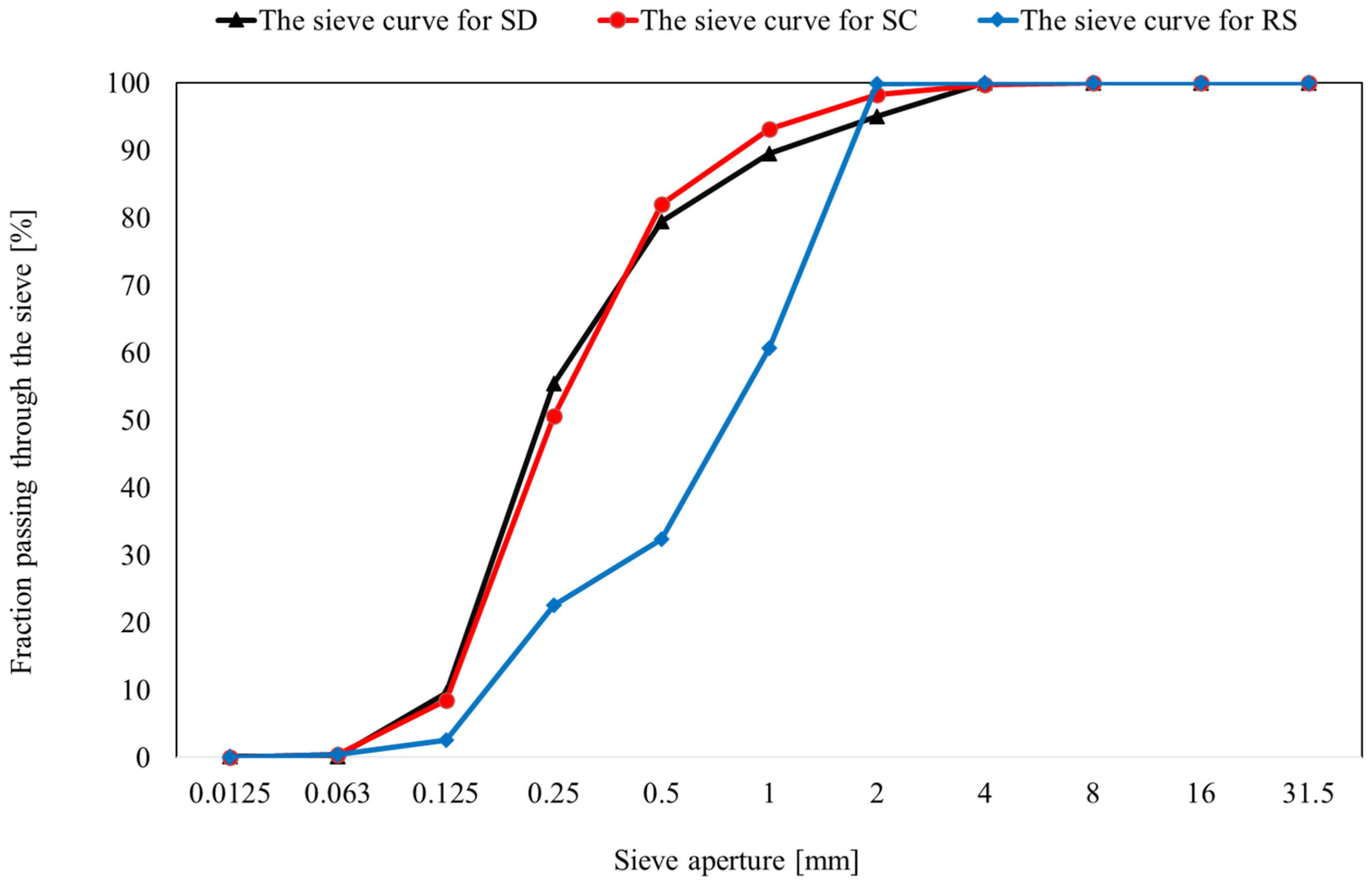
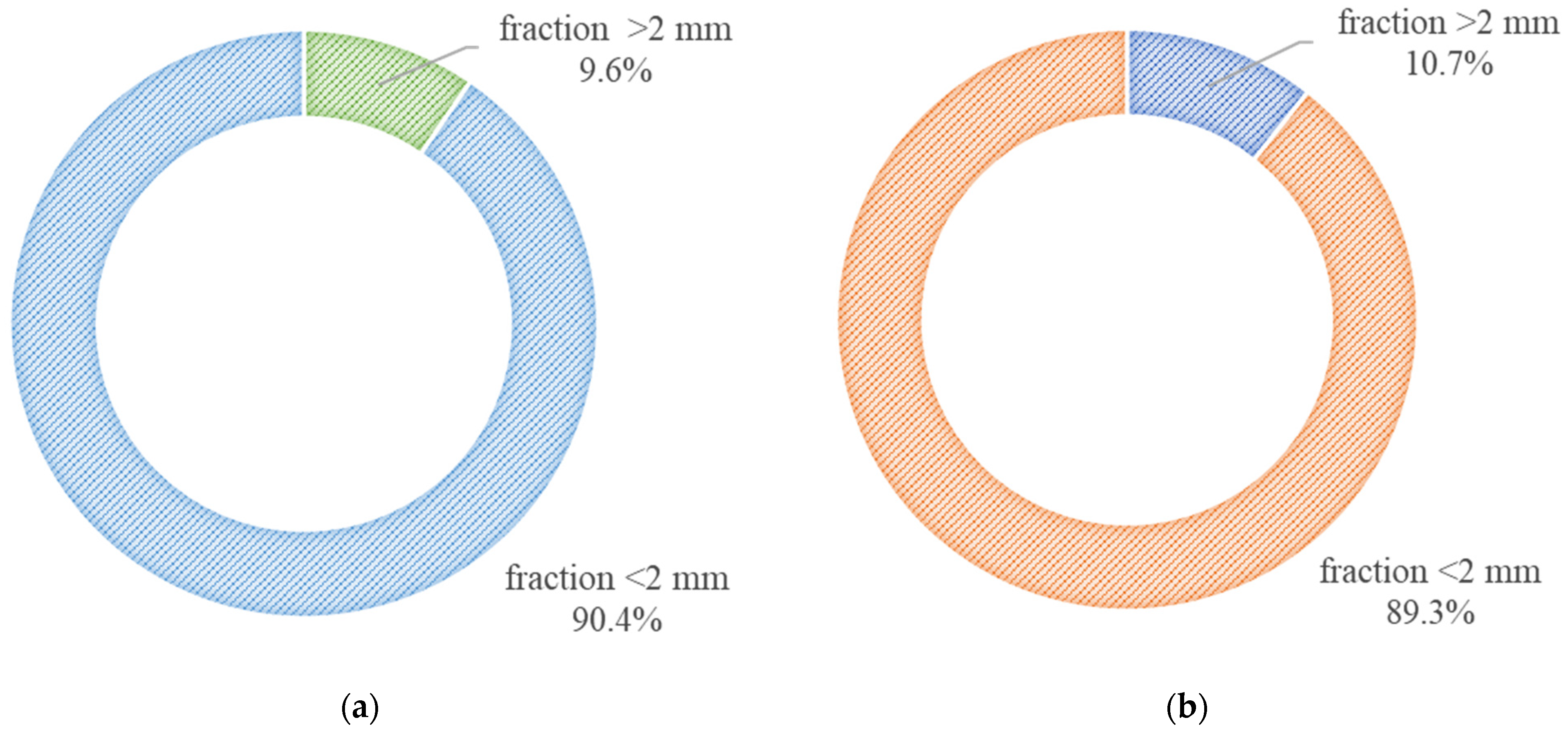
- Both analyzed samples exhibited a high content of fine fractions (<2 mm). However, the SC sample is characterized by a noticeably greater proportion of very fine (<0.063 mm) and fine (<2 mm) particles, which may indicate a higher degree of washing and a generally finer material composition. In contrast, the SD sample contains a higher percentage of particles within the 0.125–0.25 mm range, which may significantly influence its filtration properties. The observed differences in grain size distribution between the samples suggest distinct functional characteristics that are relevant in their potential engineering applications.
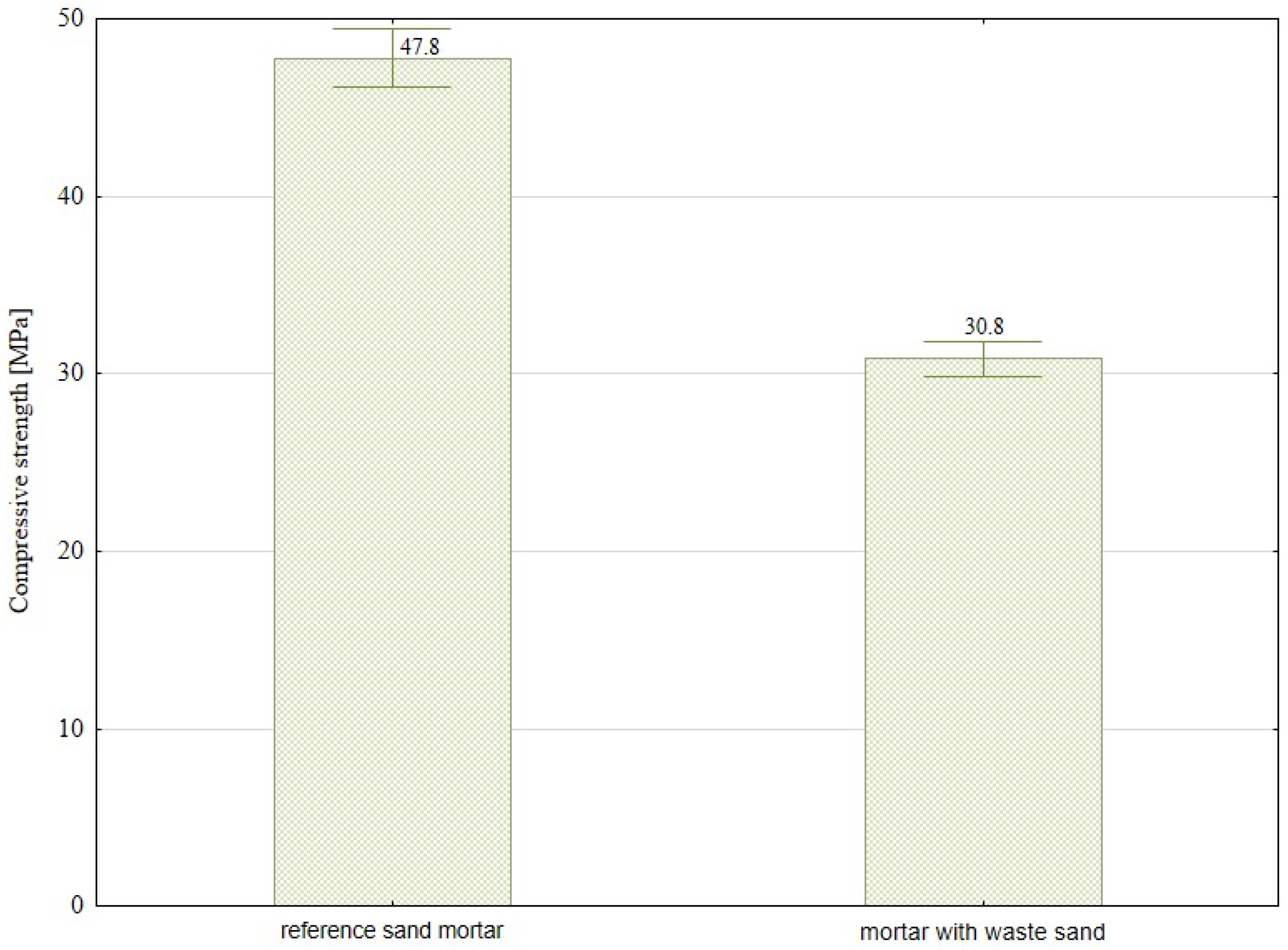
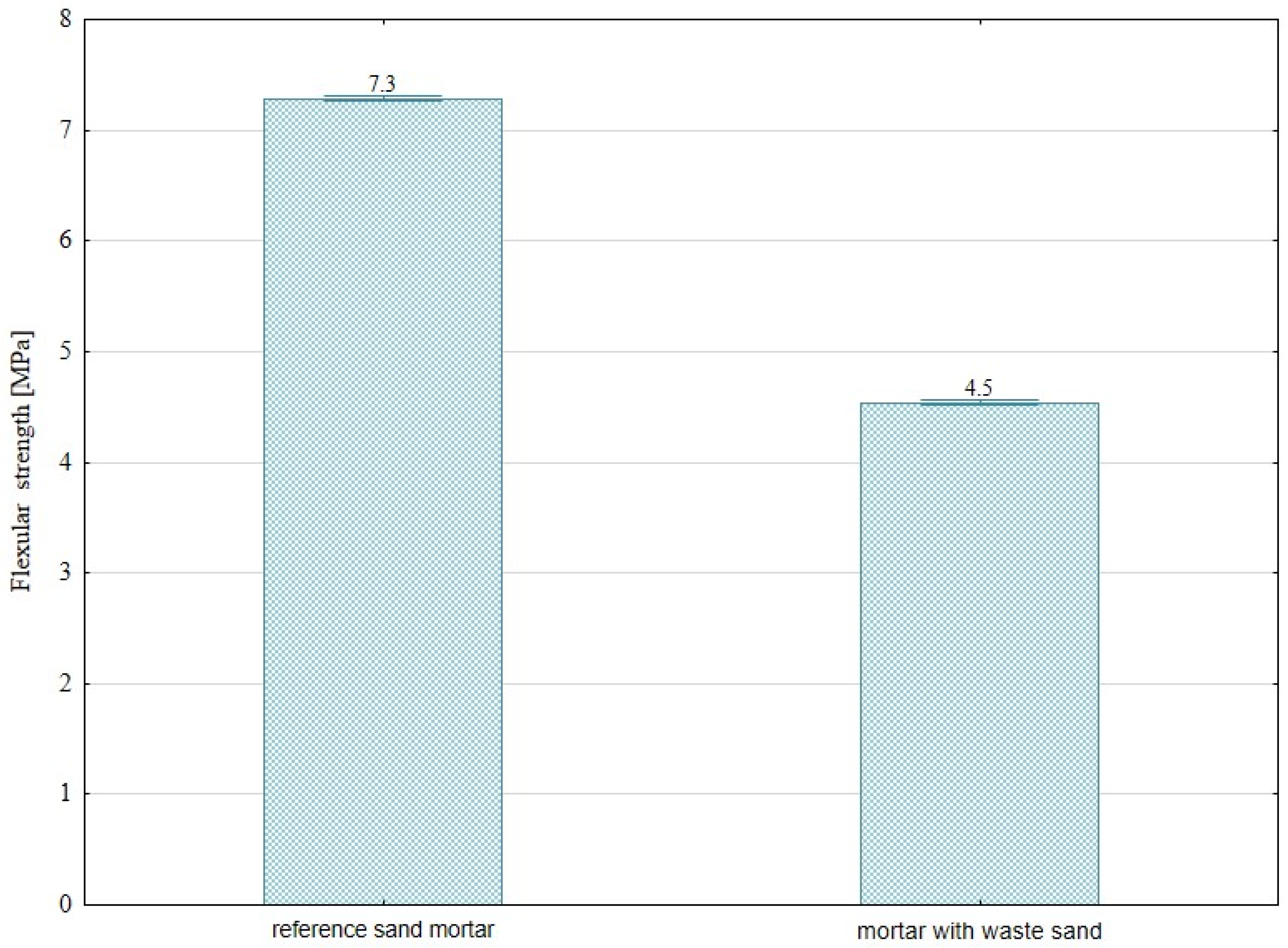
- The substitution for natural sand with waste sand derived from sewage cleaning processes in cement mortar formulations results in a significant reduction in mechanical performance. Specifically, declines of approximately 35% in compressive strength and 38% in flexural strength have been observed. This deterioration can be attributed to several factors, including the presence of organic and inorganic contaminants commonly found in sewage-derived sand. Moreover, the irregular morphology and suboptimal granulometric distribution of the particles may adversely affect the compaction behavior of the mix and hinder the bond development between the cement paste and the aggregate. To mitigate these negative effects and enhance the suitability of this secondary raw material, appropriate pretreatment measures should be considered prior to its incorporation into mortar mixtures.
- Despite its lower levels of mechanical performance, waste sand may still be considered for use in sub-base layers and other non-structural applications. Such an approach aligns with the principles of the circular economy and contributes to the reduction of natural aggregate exploitation.
- Modification of recycled concrete composition: Further research is required on the application of specialized chemical additives to enhance the mechanical properties of concrete in which natural aggregate has been entirely replaced with waste materials. Particular attention should be given to improving compressive strength and durability, while considering the long-term operational effects.
- Optimization of wastewater treatment plant sand content in recycled aggregate: Another potential research direction involves determining the optimal proportion of sand derived from wastewater treatment plants in concrete mixtures, with particular consideration of the compositional variability resulting from the diverse sources. Analyses should focus on the impacts of chemical and granulometric composition on the mechanical properties and durability of concrete.
- Innovative uses of waste sands in next-generation concrete: It would be worthwhile to simultaneously conduct research on the possibility of using sands from sewage treatment plants in innovative building materials, such as geopolymers or self-healing concrete. In the context of sustainable development, it is particularly important to determine the impacts of these materials on the reduction of CO2 emissions and with reference to improvements in durability and resistance to environmental factors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nilimaa, J. Smart materials and technologies for sustainable concrete construction. Dev. Built Environ 2023, 15, 100177. [Google Scholar] [CrossRef]
- Eštoková, A.; Wolfová Fabiánová, M.; Ondová, M. Concrete Structures and Their Impacts on Climate Change and Water and Raw Material Resource Depletion. Int. J. Civ. Eng 2022, 20, 735–747. [Google Scholar] [CrossRef]
- Jiménez, L.F.; Domínguez, J.A.; Vega-Azamar, R.E. Carbon Footprint of Recycled Aggregate Concrete. Adv. Civ. Eng 2018, 2018, 7949741. [Google Scholar] [CrossRef]
- Huang, L.; Krigsvoll, G.; Johansen, F.; Liu, Y.; Zhang, X. Carbon emission of global construction sector. Renew. Sustain. Energy Rev 2018, 81, 1906–1916. [Google Scholar] [CrossRef]
- Bujnarowski, K.; Grygo, R. Properties of lightweight aggregates for use in construction. Instal 2022, 7, 71–75. (In Polish) [Google Scholar]
- RILEM TC 121-DRG. Specifications for concrete with recycled aggregates. Mater. Struct 1994, 27, 557–559. [Google Scholar] [CrossRef]
- Giergiczny, Z.; Machniak, Ł.; Golda, A.; Witczak, S.; Adamski, G.; Bukowski, L.; Szewczyk, E.; Nowek, M.; Brykalski, W. Technical Guidelines for the Classification of Domestic Aggregates and the Prevention of Alkali--Silica Reaction in Concrete Used in Road Pavements and Highway Engineering Structures, Prepared by the General Directorate for National Roads and Motorways (GDDKiA) in Poland, 2022. Available online: https://www.gov.pl/attachment/15a40897941a41278bbe959c04e97a27 (accessed on 3 March 2025). (In Polish)
- Mathews, G.; Dalesandro, K.; Young, M.; Soliman, M. Waste-to-Energy reclaimed sands as lightweight aggregates for internally cured self-consolidated precast concrete. Constr. Build. Mater 2021, 280, 122545. [Google Scholar] [CrossRef]
- de Andrade Salgado, F.; de Andrade Silva, F. Recycled aggregates from construction and demolition waste towards an application on structural concrete: A review. J. Build. Eng 2022, 52, 104452. [Google Scholar] [CrossRef]
- Segui, P.; Safhi, A.e.M.; Amrani, M.; Benzaazoua, M. Mining Wastes as Road Construction Material: A Review. Minerals 2023, 13, 90. [Google Scholar] [CrossRef]
- Vo, T.L.; Nash, W.; Galdo, M.D.; Rezania, M.; Crane, R.; Mousavi Nezhad, M.; Ferrara, L. Coal mining wastes valorization as raw geomaterials in construction: A review with new perspectives. J. Clean. Prod 2022, 336, 130213. [Google Scholar] [CrossRef]
- Chadee, A.; Maharaj, D.; Rajapakse, Y.; Rajack, E.; Mendis, S.; Azamathulla, H.M.; Mehta, D.J.; Rathnayake, U. Sustainable Use of Electric Arc Furnace Slag as a Fine Aggregate Replacement for Concrete. J. Sustain. Res 2025, 7, e250012. [Google Scholar] [CrossRef]
- Kumar, S.; Silori, R.; Sethy, S.K. Insight into the perspectives of waste foundry sand as a partial or full replacement of fine aggregate in concrete. Total Environ. Res. Themes 2023, 6, 100048. [Google Scholar] [CrossRef]
- Cioli, F.; Abbà, A.; Alias, C.; Sorlini, S. Reuse or Disposal of Waste Foundry Sand: An Insight into Environmental Aspects. Appl. Sci 2022, 12, 6420. [Google Scholar] [CrossRef]
- Aligholi, H.Z.; Yıldırım, A.K. Properties of Recycled Aggregates and Their Effects on Concrete. Int. J. Eng. Sci. Appl 2023, 7, 48–55. [Google Scholar]
- Karło, A.; Gembołyś, B.; Pieczykolan, M.; Bacza, T. Sand from grit chambers from waste to raw material. Forum Eksploatatora 2018, 98, 38–40. (In Polish) [Google Scholar]
- Czop, M.; Petryk, A.; Balcerzak, L.; Jamry, A.; Kopiec, W.; Nowak, K.; Zackiewicz, W. Testing and evaluation of physical and chemical properties of waste from desanding. Inżynieria Miner 2023, 2, 129–134. [Google Scholar]
- Announcement of the Marshal of the Sejm of the Republic of Poland of 7 July 2023 on the Announcement of the Uniform Text of the Waste Act, Journal of Laws 2023, Item 1587. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20230001587 (accessed on 12 February 2025). (In Polish)
- Regulation of the Minister of Climate of 2 January 2020 on the Waste Catalogue. Journal of Laws 2020. Item 10. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20200000010 (accessed on 12 February 2025). (In Polish)
- PN-EN 15934; Sewage Sludge, Treated Bio-Waste, Soil and Waste. Determination of Dry Matter by Determining the Dry Residue Content or Water Content. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-EN 1097-3:2000; Tests of the Mechanical and Physical Properties of Aggregates. Determination of Bulk Density and Cavernity. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-Z-15011-3:2001; Determination of: pH, Organic Matter Content, Organic Carbon, Nitrogen, Phosphorus and Potassium. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-ISO 9964-3:1994; Determination of Sodium and Potassium by Flame Emission Spectrometry. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-EN 1744-1+A1:2013-05; Tests for Chemical Properties of Aggregates. Part 1: Chemical Analysis. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-EN 15935:2022-01; Soil. Waste. Treated Biowaste and Sewage Sludge. Determination of Losses on Ignition. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-EN 12457-2:2006; Characterization of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludges. Part 2: One-Step Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with a Particle Size of Less than 4 mm (Without or with Size Reduction). Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-ISO 9297:1994; Water Quality—Determination of Chloride-Silver Nitrate Titration with Chromate Indicator (Mohr’s Method). Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-ISO 9280; Water Quality. Determination of Sulfates (VI). Gravimetric Method with Barium Chloride. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-EN ISO 6878:2006; Water Quality. Determination of Phosphorus. Spectrometric Method with Ammonium Molybdate. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- REF 918142; Test 1-42; Standard Methods for the Examination of Water and Wastewater (4500-F-D). Fluoride. Polish Committee for Standardization: Warsaw, Poland.
- PN-EN 933-2:2021-01; Tests for Geometrical Properties of Aggregates. Part 2: Determination of Particle Size Distribution. Test Sieves, Nominal Size of Apertures. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-EN 197-1:2012; Cement. Part 1: Composition, Requirements and Conformity Criteria for Common Cements. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-EN 12457-4:2006; Characterization of Waste. Leaching. Compatibility Test for the Leaching of Granular Waste Materials and Sludge. Part 4: One-Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with a Particle Size of Less than 10 mm (with or Without Size Reduction). Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- Regulation of the Minister of Maritime Affairs and Inland Navigation of 12 July 2019 on Substances Particularly Harmful to the Aquatic Environment and Conditions to be Met when Discharging Waste Water into Waters or into the Ground and When Discharging Rainwater or Snowmelt into Waters or into Water Facilities. Journal of Laws 2019. Item 1311. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20190001311 (accessed on 12 February 2025). (In Polish)
- PN-EN 12620+A1:2010; Aggregates for Concrete. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- PN-EN 196-1: 2016; Methods of Testing Cement. Part 1. Determination of Strength. Polish Committee for Standardization: Warsaw, Poland. (In Polish)
- Kępniak, M.; Łukowski, P. Multicriteria Analysis of Cement Mortar with Recycled Sand. Sustainability 2024, 16, 1773. [Google Scholar] [CrossRef]
- Chen, H.J.; Yen, T.; Chen, K.H. Use of building rubbles as recycled aggregates. Cem. Concr. Res 2003, 33, 125–132. [Google Scholar] [CrossRef]
- Verian, K.P.; Ashraf, W.; Cao, Y. Properties of recycled concrete aggregate and their influence in new concrete production. Resour. Conserv. Recycl 2018, 133, 30–49. [Google Scholar] [CrossRef]
- Li, L.; Zhan, B.J.; Lu, J.; Poon, C.S. Systematic evaluation of the effect of replacing river sand by different particle size ranges of fine recycled concrete aggregates (FRCA) in cement mortars. Constr. Build. Mater 2019, 209, 147–155. [Google Scholar] [CrossRef]
- Garbacz, A.; Sokołowska, J.J. Concrete-like polymer composites with fly ashes—Comparative study. Constr. Build. Mater 2013, 38, 689–699. [Google Scholar] [CrossRef]



| Parameter | Unit | SD | SC |
|---|---|---|---|
| Total humidity (Htot.) | % | 13.50 | 2.98 |
| Analytical humidity (Ha) | % | 0.74 | 0.45 |
| Bulk density in a dry state (pn) | kg/m3 | 1330 | 1350 |
| Degradable organic substances (DOS) | % | 8.85 | 3.79 |
| Total organic carbon (TOC) | % | 2.75 | 1.78 |
| Total nitrogen (Ntot.) | % | 0.17 | 0.09 |
| Sodium (Na) | % | 0.03 | 0.02 |
| Calcium (Ca) | % | 0.34 | 0.10 |
| Potassium (K) | % | 0.05 | 0.03 |
| Lithium (Li) | % | blq 1 | blq 1 |
| Bar (Ba) | % | 0.10 | 0.01 |
| Substance Name | SD | SC | Permissible Value According to [34] |
|---|---|---|---|
| pH, - | 7.0 | 8.6 | 6.5–9.0 |
| Total suspended solids (TSS), mg/L | 24 | 18 | 35 |
| Chlorides (Cl−), mg/L | 50.69 | 9.22 | 1000 |
| Sulphates (SO42−), mg/L | 98.05 | 41.41 | 500 |
| Total nitrogen (Ntot.), mg/L | 0.56 | 3.68 | 10 |
| Phosphorus (P), mg/L | 0.09 | 0.05 | 1 |
| Potassium (K), mg/L | 4.50 | 2.69 | 80 |
| Calcium (Ca), mg/L | 27.12 | 9.95 | ng 1 |
| Sodium (Na), mg/L | 4.19 | 1.82 | 800 |
| Fluoride (F−), mg/L | 1.26 | <0.05 | 25 |
| Barium (Ba), mg/L | 1.91 | 1.36 | 2 |
| Total organic carbon (TOC), mg/L | 27.76 | 4.21 | 30 |
| Total carbon (TC), mg/L | 31.16 | 9.14 | ng 1 |
| Inorganic carbon (IC), mg/L | 3.40 | 4.93 | ng 1 |
| Zinc (Zn), mg/L | 0.08 | 0.23 | 2 |
| Copper (Cu), mg/L | 0.02 | 0.03 | 0.5 |
| Lead (Pb), mg/L | <0.05 | <0.05 | 0.5 |
| Cadmium (Cd), mg/L | 0.03 | <0.005 | ng 1 |
| Chromium (Cr), mg/L | <0.05 | <0.05 | 0.1 |
| Cobalt (Co), mg/L | <0.05 | 0.06 | 1 |
| Manganese (Mn), mg/L | 0.06 | 0.03 | ng 1 |
| Iron (Fe), mg/L | 0.18 | 0.15 | 10 |
| Nickel (Ni), mg/L | <0.04 | <0.04 | 0.5 |
| Parameter | Unit | SD | SC | RS |
|---|---|---|---|---|
| Analytical humidity (Ha) | % | 0.66 | 0.46 | 0.33 |
| Bulk density in a dry state (pb) | kg/m3 | 1333 | 1350 | 1329 |
| Degradable organic substances (DOS) | % | 3.79 | blq 1 | blq 1 |
| Total organic carbon (TOC) | % | 1.78 | blq 1 | blq 1 |
| Total nitrogen (Ntot.) | % | 0.14 | 0.04 | blq 1 |
| Phosphorus (P) | % | 0.06 | 0.01 | blq 1 |
| Sodium (Na) | % | 0.02 | 0.02 | 0.01 |
| Calcium (Ca) | % | 0.03 | 0.05 | blq 1 |
| Potassium (K) | % | 0.03 | 0.02 | 0.01 |
| Lithium (Li) | % | blq 1 | blq 1 | blq 1 |
| Bar (Ba) | % | blq 1 | 0.01 | blq 1 |
| Substance Name | SD | SC | RS | Permissible Value According to [34] |
|---|---|---|---|---|
| pH, - | 7.3 ± 0.1 | 6.9 ± 0.1 | 8.9 ± 0.1 | 6.5–9.0 |
| Chlorides (Cl−), mg/L | 69.12 ± 0.01 | blq 1 | blq 1 | 1000 |
| Sulphates (SO42−), mg/L | 44.29 ± 5.21 | 47.86 ± 5.21 | 2.88 ± 0.41 | 500 |
| Total phosphorus (Ptot.), mg/L | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.44 ± 0.01 | 1 |
| Potassium (K), mg/L | 2.59 ± 0.01 | 1.03 ± 0.01 | 0.72 ± 0.01 | 80 |
| Calcium (Ca), mg/L | 15.85 ± 0.02 | 12.19 ± 0.02 | 5.15 ± 0.02 | ng 2 |
| Sodium (Na), mg/L | 3.08 ± 0.01 | 1.27 ± 0.01 | 0.51 ± 0.01 | 800 |
| Fluorides (F−), mg/L | 0.40 ± 0.04 | 0.37 ± 0.04 | 0.13 ± 0.04 | 25 |
| Barium (Ba), mg/L | blq 1 | 0.12 ± 0.01 | 0.15 ± 0.01 | 2 |
| Total organic carbon (TOC), mg/L | 18.63 | 2.17 | 1.92 | 30 |
| Total carbon (TC), mg/L | 25.30 | 7.78 | 9.29 | ng 2 |
| Inorganic carbon (IC), mg/L | 6.67 | 5.61 | 7.37 | ng 2 |
| Zinc (Zn), mg/L | 0.11 | 0.23 | <0.01 | 2 |
| Copper (Cu), mg/L | 0.08 | <0.02 | <0.02 | 0.5 |
| Lead (Pb), mg/L | <0.05 | <0.05 | <0.05 | 0.5 |
| Cadmium (Cd), mg/L | 0.02 | <0.005 | <0.005 | ng 2 |
| Chromium (Cr), mg/L | <0.05 | <0.05 | <0.05 | 0.1 |
| Cobalt (Co), mg/L | <0.05 | 0.06 | <0.05 | 1 |
| Manganese (Mn), mg/L | <0.02 | 0.03 | <0.02 | ng 2 |
| Iron (Fe), mg/L | <0.04 | 0.15 | <0.04 | 10 |
| Nickel (Ni), mg/L | 0.11 | <0.04 | <0.04 | 0.5 |
| Type of Waste | CEM I | Water | Sand acc. PN-EN 196-1 [36] | Sand from Cleaning the Sewage System |
|---|---|---|---|---|
| Reference sand mortar | 450 | 225 | 1350 | 0 |
| Mortar with waste sand | 450 | 225 | 0 | 1350 |
| Substance Name | Mortar with Waste Sand | Reference Sand Mortar | Permissible Value According to [34] |
|---|---|---|---|
| pH, - | 10.4 ± 0.1 | 10.3 ± 0.1 | 6.5–9.0 |
| Chlorides (Cl−), mg/L | 23.03 ± 0.01 | 34.56 ± 0.01 | 1000 |
| Sulphates (SO42−), mg/L | 1.44 ± 0.29 | 13.78 ± 0.87 | 500 |
| Total phosphorus (Ptot.). mg/L | 0.44 ± 0.01 | 0.93 ± 0.01 | 1 |
| Potassium (K), mg/L | 14.01 ± 0.12 | 1.89 ± 0.01 | 80 |
| Calcium (Ca), mg/L | 7.31 ± 0.10 | 8.72 ± 0.02 | ng 1 |
| Sodium (Na), mg/L | 3.36 ± 0.01 | 1.89 ± 0.01 | 800 |
| Fluorides (F−), mg/L | 0.51 ± 0.02 | 0.61 ± 0.03 | 25 |
| Barium (Ba), mg/L | 4.43 ± 0.06 | 0.36 ± 0.06 | 2 |
| Total organic carbon (TOC), mg/L | 2.29 | 2.85 | 30 |
| Total carbon (TC), mg/L | 7.42 | 6.29 | ng 1 |
| Inorganic carbon (IC), mg/L | 5.12 | 3.44 | ng 1 |
| Zinc (Zn), mg/L | <0.01 | <0.01 | 2 |
| Copper (Cu), mg/L | 0.03 | 0.03 | 0.5 |
| Lead (Pb), mg/L | 0.05 | <0.05 | 0.5 |
| Cadmium (Cd), mg/L | <0.005 | <0.005 | ng 1 |
| Chromium (Cr), mg/L | <0.05 | <0.05 | 0.1 |
| Cobalt (Co), mg/L | <0.05 | <0.05 | 1 |
| Manganese (Mn), mg/L | <0.02 | <0.02 | ng 1 |
| Iron (Fe), mg/L | <0.04 | <0.04 | 10 |
| Nickel (Ni), mg/L | <0.04 | <0.04 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czop, M.; Zajusz-Zubek, E.; Łaźniewska-Piekarczyk, B. Use of Sands from Wastewater Treatment Plants as a Substitute for Natural Aggregate in the Context of a Circular Economy. Sustainability 2025, 17, 5471. https://doi.org/10.3390/su17125471
Czop M, Zajusz-Zubek E, Łaźniewska-Piekarczyk B. Use of Sands from Wastewater Treatment Plants as a Substitute for Natural Aggregate in the Context of a Circular Economy. Sustainability. 2025; 17(12):5471. https://doi.org/10.3390/su17125471
Chicago/Turabian StyleCzop, Monika, Elwira Zajusz-Zubek, and Beata Łaźniewska-Piekarczyk. 2025. "Use of Sands from Wastewater Treatment Plants as a Substitute for Natural Aggregate in the Context of a Circular Economy" Sustainability 17, no. 12: 5471. https://doi.org/10.3390/su17125471
APA StyleCzop, M., Zajusz-Zubek, E., & Łaźniewska-Piekarczyk, B. (2025). Use of Sands from Wastewater Treatment Plants as a Substitute for Natural Aggregate in the Context of a Circular Economy. Sustainability, 17(12), 5471. https://doi.org/10.3390/su17125471








