Electrochemical Production of Hypochlorous Acid and Sodium Hydroxide Using Ion Exchange Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Electrodes
2.3. Ion Exchange Membranas
2.4. Analysis of Electrolyzed Water and Sodium Hydroxide
2.5. Calculation of Energy Consumption and Electrical Current Efficiency
2.6. Taguchi Experimental Design
2.7. Experimental Equipment
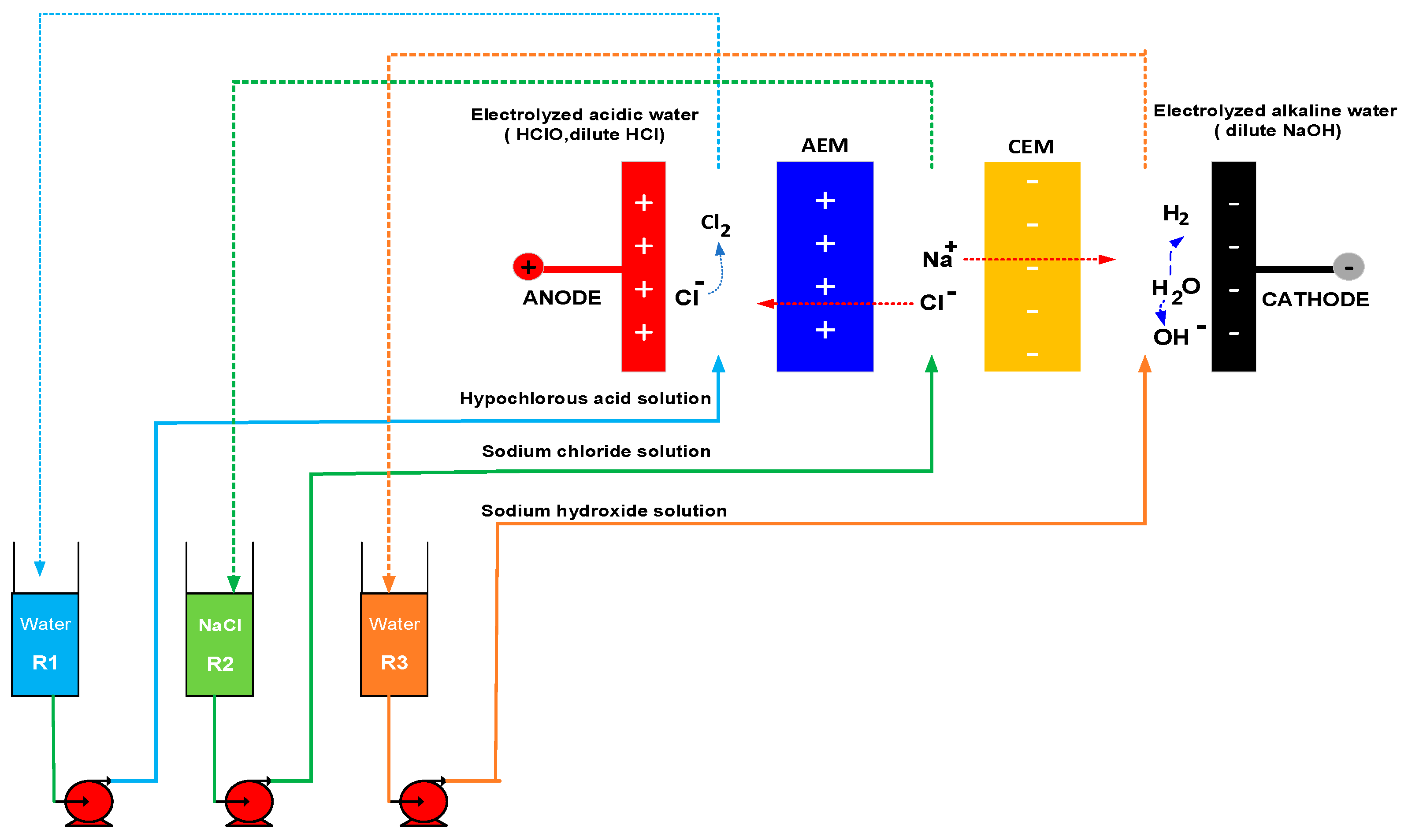
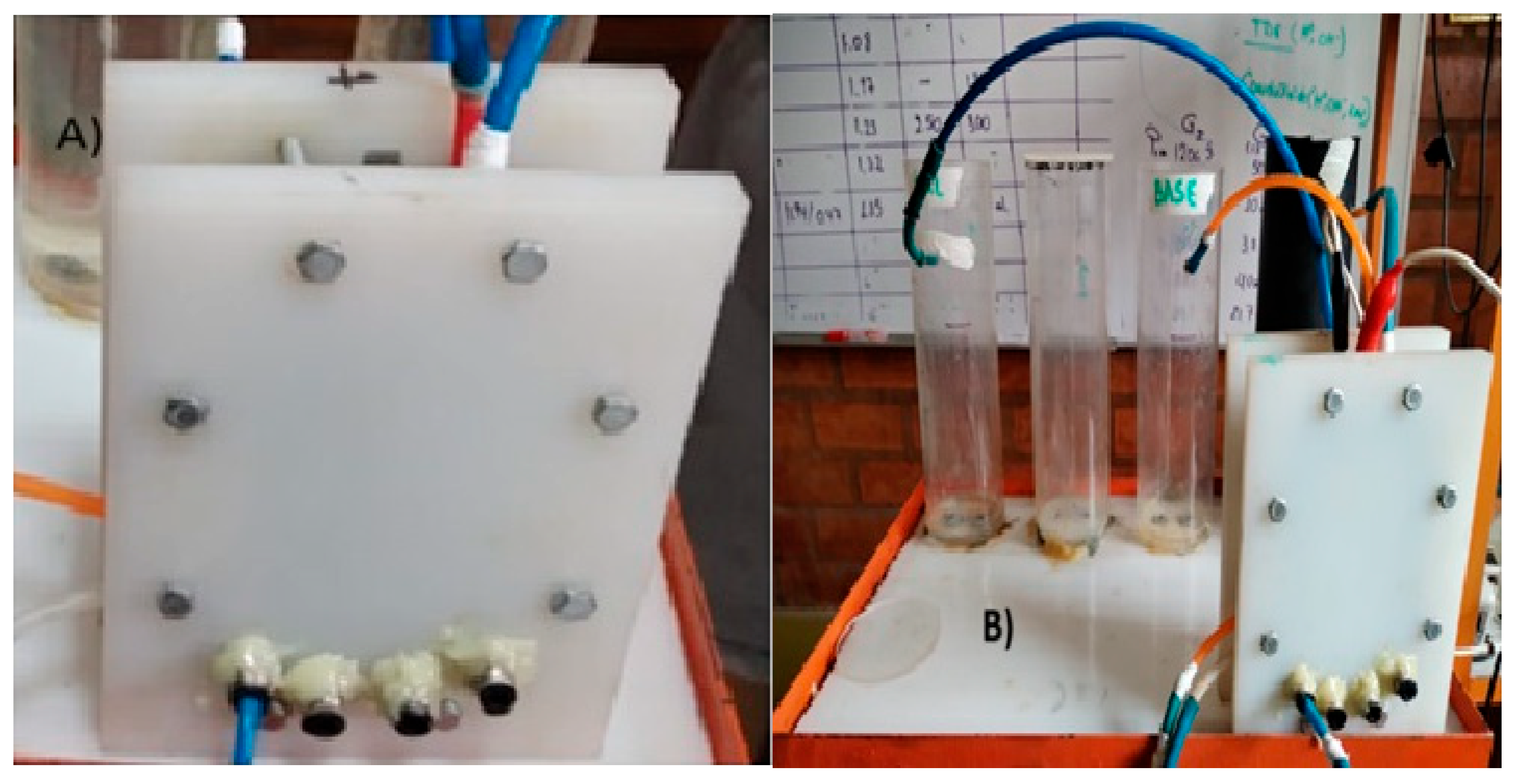
2.8. Cell Photograph and Schematic Diagram of Experimental Equipment
3. Results and Discussion
3.1. Results of the Studied Variables
3.2. Responses as a Function of Factors
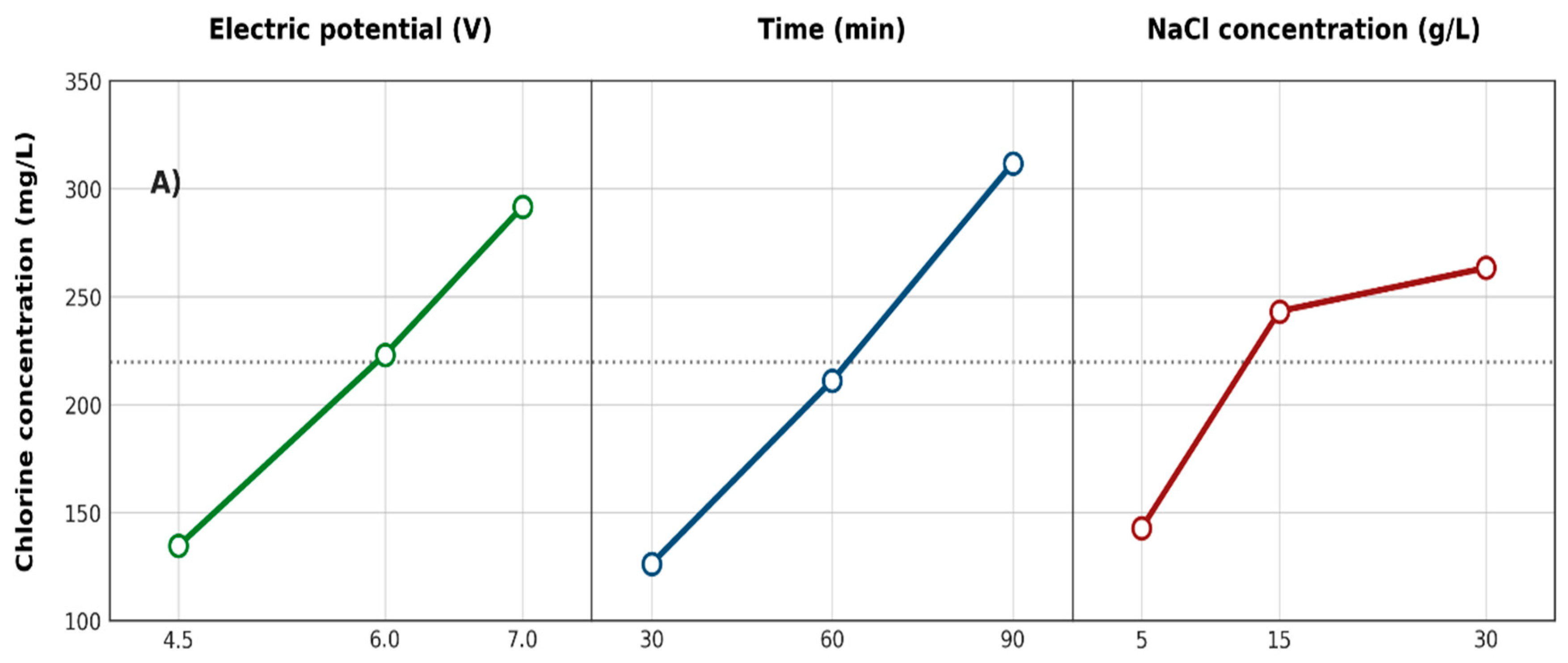
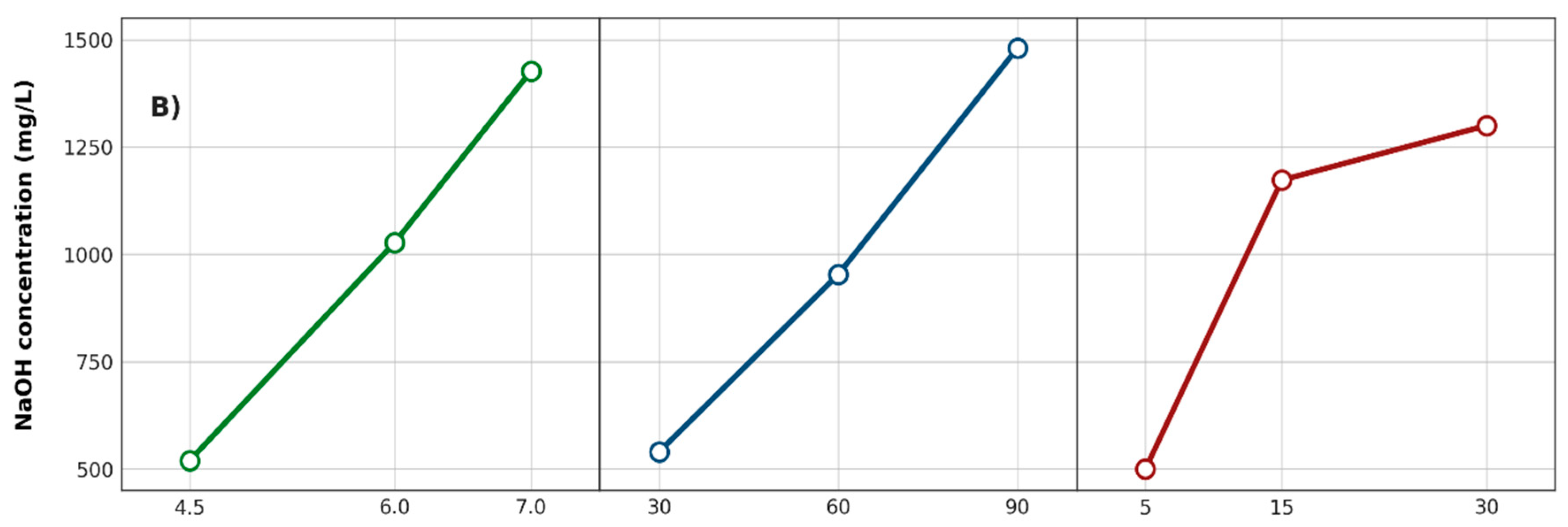
3.2.1. Active Chlorine and Sodium Hydroxide Concentrations
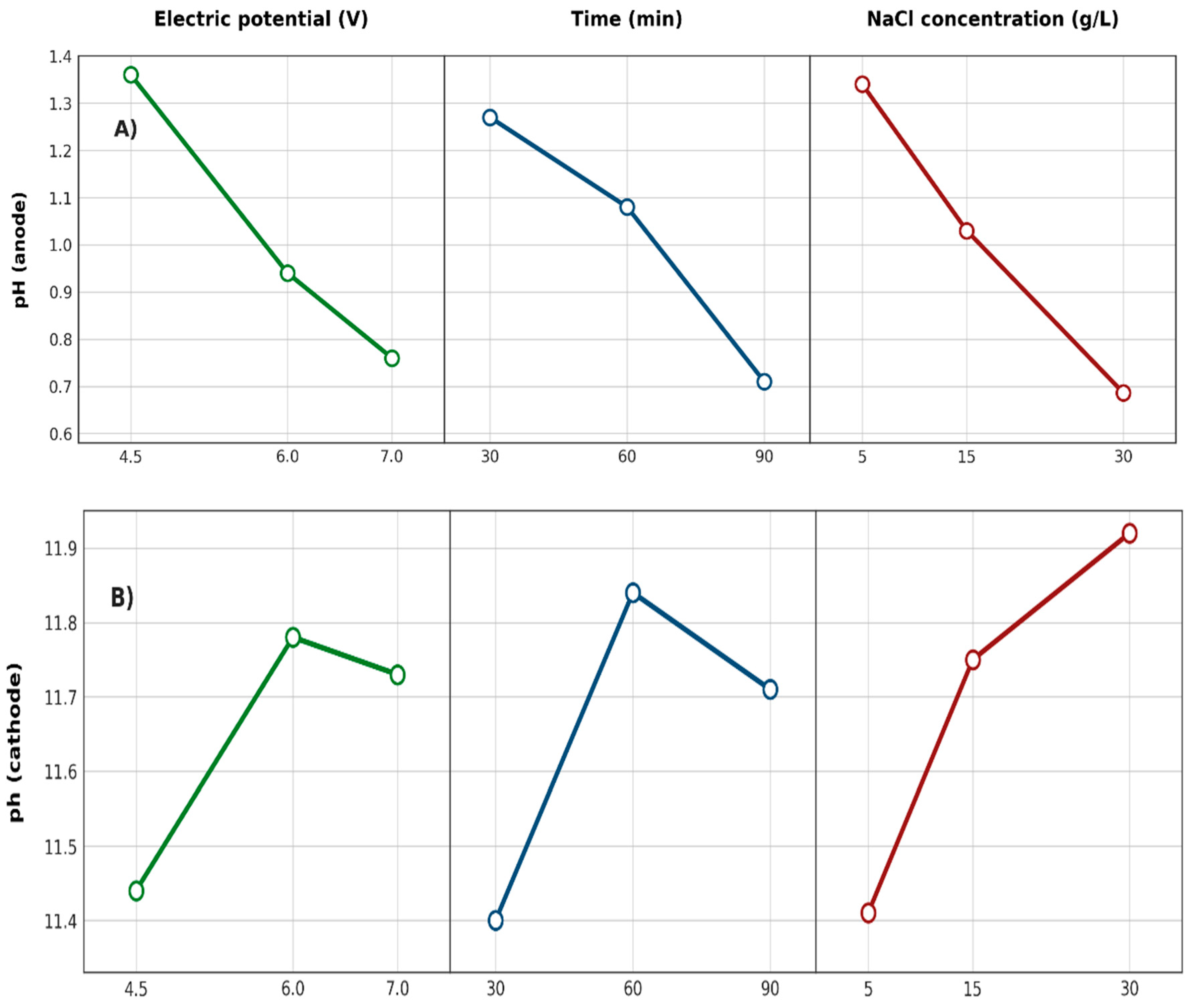
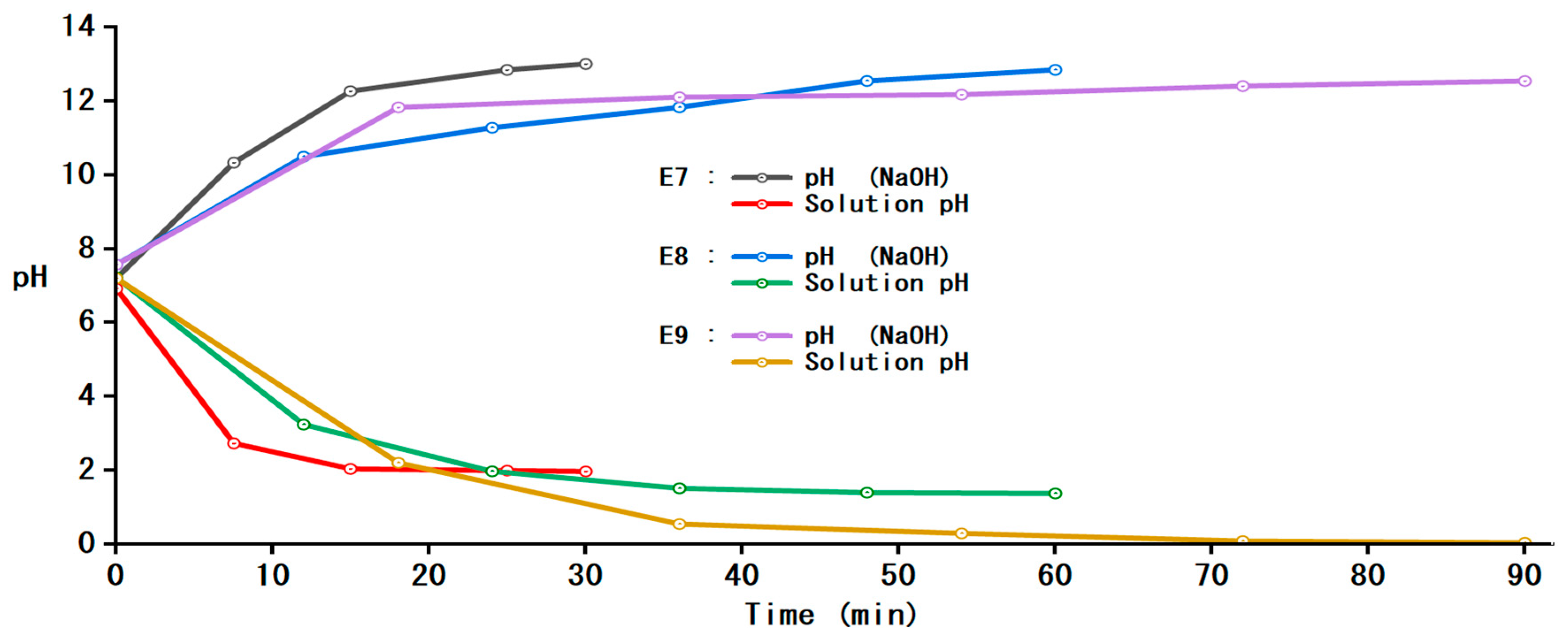
3.2.2. pH Indices of Solutions in Anodic and Cathodic Compartments
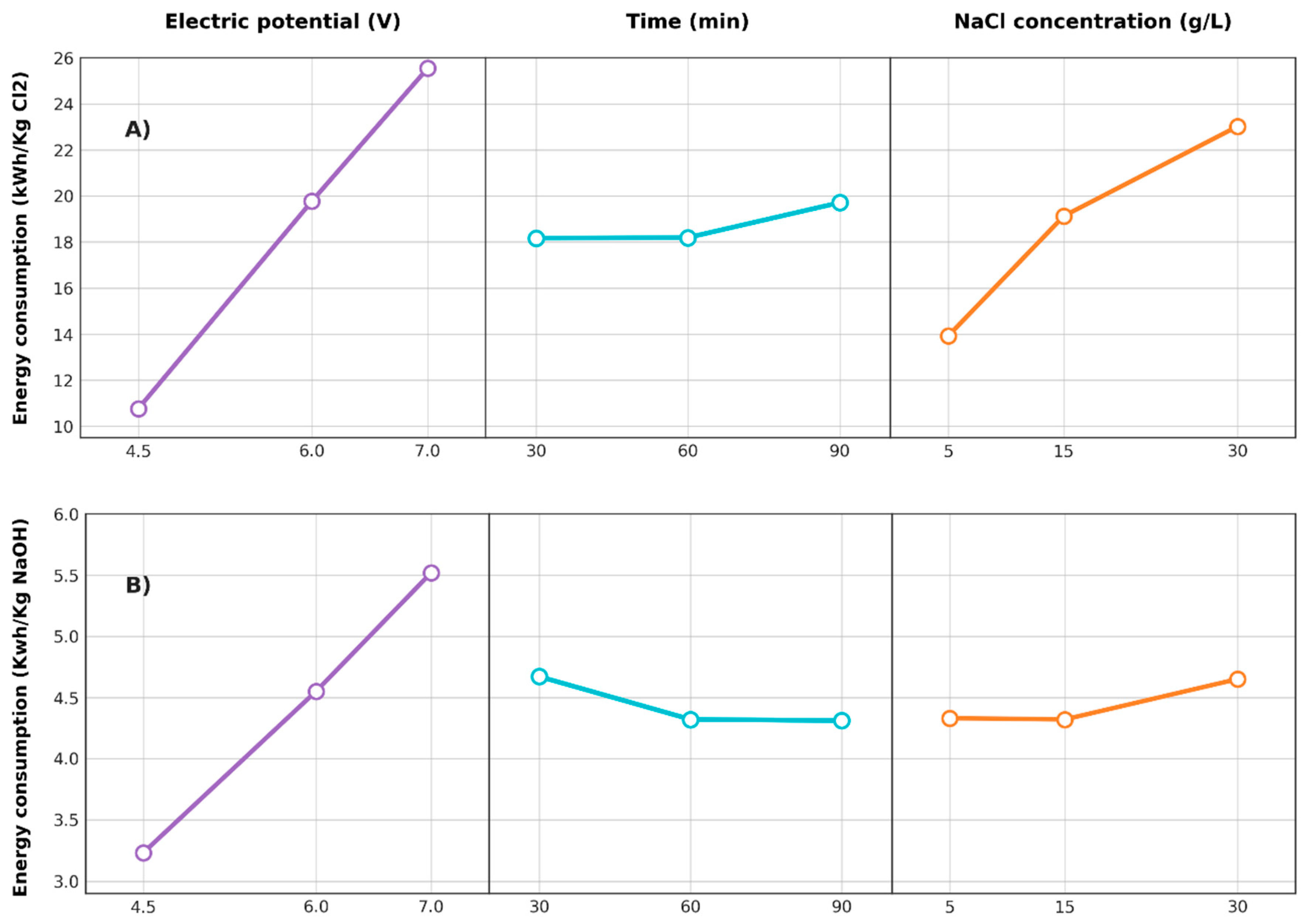
3.2.3. Energy Consumption
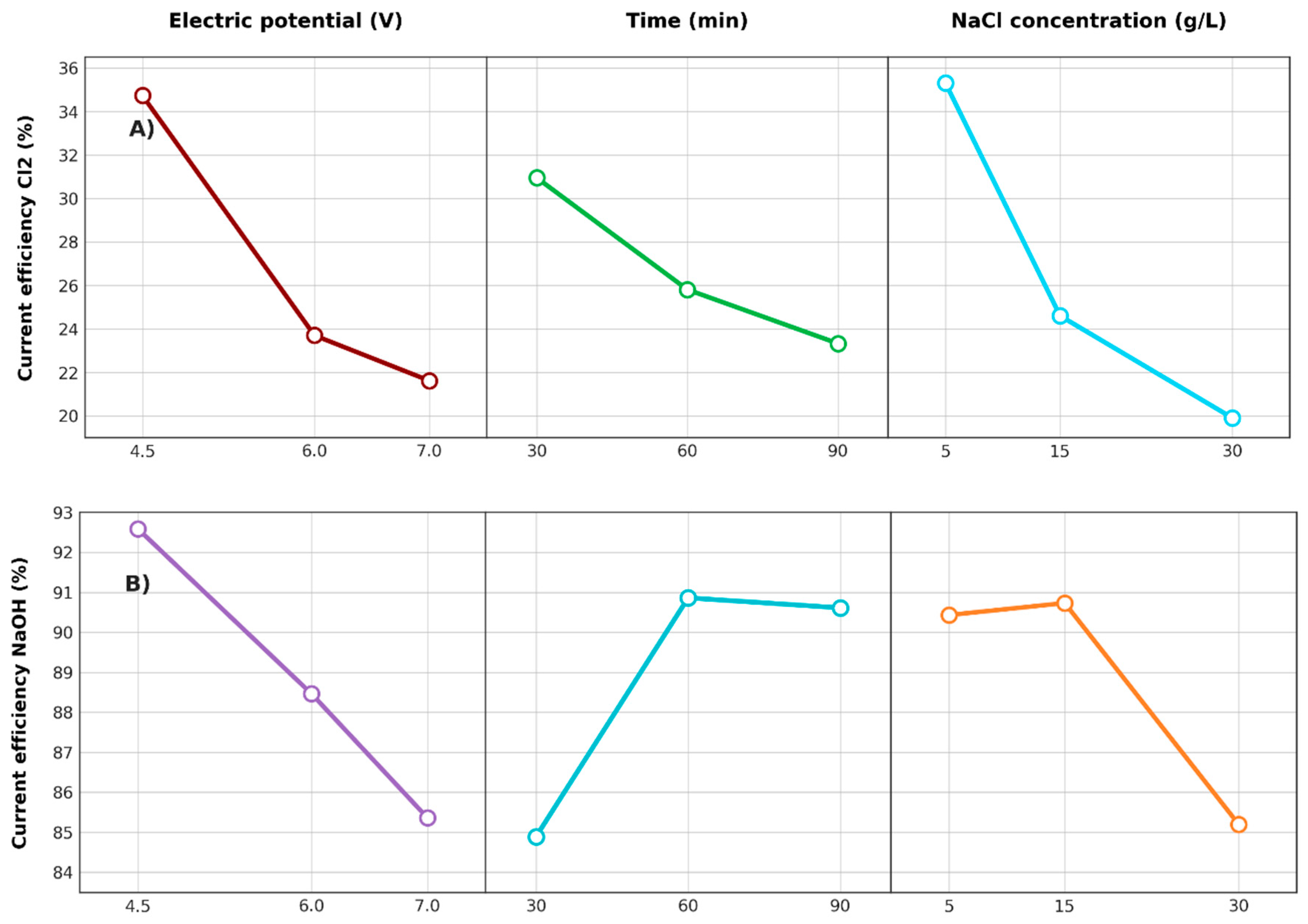
3.2.4. Efficiency of Electric Current
3.2.5. ANOVA Results
3.2.6. Effects of Factors on Responses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AEW | acidic electrolyzed water |
| SAEW | strongly acidic electrolyzed water |
| AcEW | strongly acidic electrolyzed water |
| BEW | basic electrolyzed water |
| ACC | available chlorine concentration |
| DOF | degrees of freedom |
References
- Yaqub, M.; Woo, C.; Lee, W. Optimization of hypochlorous acid generation by HCl electrolysis through response surface methodology and artificial neural networks. J. Environ. Chem. Eng. 2021, 9, 105826. [Google Scholar] [CrossRef]
- Li, X.; Yang, D.; Li, S.; Li, Z.; Yan, W.; Xu, H.; Yu, K. Revolutionizing Strongly Acidic Electrolyzed Water Production: A Breakthrough in Membrane-Free Pump-Suction Electrochemical System. Sep. Purif. Technol. 2025, 361, 131449. [Google Scholar] [CrossRef]
- Luo, C.-H.; Huang, H.-C.; Chuang, C.-Y.; Yang, S.; Hung, P.-C.; Chang, M.-Y.; Fang, W. Inactivation efficiency to Bacillus subtilis and Escherichia coli bacterial aerosols of spraying neutral electrolyzed water. J. Air Waste Manag. Assoc. 2013, 63, 1447–1456. [Google Scholar] [CrossRef]
- Lin, M.; Li, M.; Fang, L.; Tang, J.; Hung, Y.-C.; Jiang, X.; Sun, J.; Chen, Y.; Lin, Q.; Lin, H. Recent trends and applications of electrolyzed oxidizing water in fresh foodstuff preservation and safety control. Food Chem. 2022, 369, 130873. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, Q.; Yuan, M.; Cui, C.; Qiu, S.; Deng, F. Ehanced electrolytic production of hypochlorous acid using phosphorus-modified carbon felt electrodes: A study in disinfectant synthesis. Chemosphere 2024, 365, 143367. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Rao, B.; Smesrud, L.; Omole, D.O.; Mehare, M.; Hernandez, D.; N., C.; Gadgil, A.; Matange, V.; Álvarez-Mejía, C.; et al. Low-cost, local production of a safe and effective disinfectant for resource-constrained communities. PLoS Glob. Public Health 2024, 4, e0002213. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Ding, T.; Oh, D.H. Effectiveness of low concentration electrolyzed water to inactivate foodborne pathogens under different environmental conditions. Int. J. Food Microbiol. 2010, 139, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, S.; Yang, H. Recent advances on research of electrolyzed water and its applications. Curr. Opin. Food Sci. 2021, 41, 180–188. [Google Scholar] [CrossRef]
- Mąkinia, J.; Majtacz, J.; Al-Hazmi, H.E.; Hassan, G.K.; Drewnowski, J.; El-Gawad, H.A.; Kamal, R.M.; Mohamed, R.M.; Afify, A.A. Electrochemical Production of Sodium Hypochlorite from Salty Wastewater Using a Flow-by Porous Graphite Electrode. Energies 2023, 16, 4754. [Google Scholar] [CrossRef]
- Zaviska, F.; Drogui, P.; Pablo, G. Statistical optimization of active chlorine production from a synthetic saline effluent by electrolysis. Desalination 2012, 296, 16–23. [Google Scholar] [CrossRef]
- Nyamende, N.E.; Belay, Z.A.; Caleb, O.J. Recent advances in electrolyzed water treatments: Mechanisms of action and its effect on browning, bioactive compounds, and disinfection of fresh-cut fruit and vegetables–A review. Food Chem. Adv. 2023, 3, 100569. [Google Scholar] [CrossRef]
- Yan, P.; Daliri, E.B.M.; Oh, D.H. New clinical applications of electrolyzed water: A review. Microorganisms 2021, 9, 136. [Google Scholar] [CrossRef]
- Mohammadi, S.; Ebadi, T. Production of a water disinfectant by membrane electrolysis of brine solution and evaluation of its quality change during the storage time. Arab. J. Chem. 2021, 14, 102925. [Google Scholar] [CrossRef]
- Hao, J.; Wu, T.; Li, H.; Liu, H. Dfferences of Bactericidal Efficacy on Escherichia coli, Staphylococcus aureus, and Bacillus subtilis of Slightly and Strongly Acidic Electrolyzed Water. Food Bioprocess Technol. 2017, 10, 155–164. [Google Scholar] [CrossRef]
- Ampiaw, R.E.; Yaqub, M.; Lee, W. Eectrolyzed water as a disinfectant: A systematic review of factors affecting the production and efficiency of hypochlorous acid. J. Water Process. Eng. 2021, 43, 102228. [Google Scholar] [CrossRef]
- Athayde, D.R.; Flores, D.R.M.; Silva, J.S.; Silva, M.S.; Genro, A.L.G.; Wagner, R.; Campagnol, P.; Menezes, C.; Cichoski, A.J. Caracteristics and use of electrolyzed water in food industries. Int. Food Res. J. 2018, 25, 11–16. [Google Scholar]
- Dammak, L.; Ammar, R.B.; Ounissi, T.; Mofakhami, A.; Hmida, E.S.B.; Larchet, C.; Baklouti, L. A New Method Based on a Zero Gap Electrolysis Cell for Producing Bleach: Concept Validation. Membranes 2022, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Grabner, B.; Grimmer, I.; Höglinger, M.; Trattner, A.; Kartusch, S.; Ranz, M.; Rauh, J.; Macherhammer, M.-G.; Wallnöfer-Ogris, E. A review on understanding and identifying degradation mechanisms in PEM water electrolysis cells: Insights for stack application, development, and research. Int. J. Hydrogen Energy 2024, 65, 381–397. [Google Scholar] [CrossRef]
- Di Noto, V.; Jaouen, F.; Mustarelli, P.; Dekel, D.R.; Elbaz, L.; Santoro, C.; Lavacchi, A. What is Next in Anion-Exchange Membrane Water Electrolyzers? Bottlenecks, Benefits, and Future. ChemSusChem 2022, 15, e202200027. [Google Scholar] [CrossRef]
- Ren, Z.; Quan, S.; Gao, J.; Li, W.; Zhu, Y.; Liu, Y.; Chai, B.; Wang, Y. The electrocatalytic activity of IrO2-Ta2O5 anode materials and Electrolyzed Oxidizing Water preparation and sterilization effect. RSC Adv. 2015, 5, 8778–8786. [Google Scholar] [CrossRef]
- Waters, B.; Amazonas, M.; Hung, Y.-C. Eectrolyzed Oxidizing Water Generation Methods. J. Funct. Water 2012, 7, 1–13. [Google Scholar]
- García-López, I.; Maté, V.I.Á.; Aroca, S.C.; Garrido-Escudero, A. Wter disinfection via nature-inspired electrochemical flow cells in resource-limited settings. Next Sustain. 2025, 5, 100072. [Google Scholar] [CrossRef]
- Gomaa, F.A.; Nada, A.A.; Gomaa, H.E.M.; El-Maghrabi, H.H. Avancing green hydrogen: Innovations and challenges in seawater electrolysis for sustainable energy production. J. Environ. Chem. Eng. 2025, 13, 115644. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, X.; Li, B.; Hung, Y.C. Dsinfection efficacy of electrolyzed oxidizing water on brown rice soaking and germination. Food Control 2018, 89, 38–45. [Google Scholar] [CrossRef]
- Walsh, R.; Brennan, J.; Shergill, G.; Rosen, J.; Varley, R.; Hau, H.; Petran, R.; Gombas, D.; Zomorodi, B.; Khurana, K.; et al. Guidelines to validate control of cross-contamination during washing of fresh-cut leafy vegetables. J. Food Prot. 2017, 80, 312–330. [Google Scholar] [CrossRef]
- Chai, B.; Chen, J.; Deng, L.; He, S.; Zhao, G.; Zhu, Y.; Liu, Y.; Ren, Z. Preparation of electrolyzed oxidizing water by TiO2 doped IrO2-Ta2O5 electrode with high selectivity and stability for chlorine evolution. J. Electroanal. Chem. 2019, 832, 459–466. [Google Scholar] [CrossRef]
- Karlsson, R.K.B.; Cornell, A. Slectivity between Oxygen and Chlorine Evolution in the Chlor-Alkali and Chlorate Processes. Chem. Rev. 2016, 116, 2982–3028. [Google Scholar] [CrossRef]
- Ocasio, D.; Sedlak, D.L. Mmbrane-Assisted Electrochlorination for Zero-Chemical-Input Point-of-Use Drinking Water Disinfection. ACS ES&T Eng. 2022, 2, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Shin, D.M.; Rhim, J.W. Dsigning a high-efficiency hypochlorite ion generation system by combining cation exchange membrane aided electrolysis with chlorine gas recovery stream. J. Membr. Sci. 2021, 630, 119318. [Google Scholar] [CrossRef]
- Czarnetzki, L.R.; Janssen, L.J.J. Frmation of hypochlorite, chlorate and oxygen during NaCl electrolysis from alkaline solutions at an RuO2/TiO2 anode. J. Appl. Electrochem. 1992, 22, 315–324. [Google Scholar] [CrossRef]
- Jasper, J.T.; Yang, Y.; Hoffmann, M.R. Txic Byproduct Formation during Electrochemical Treatment of Latrine Wastewater. Environ. Sci. Technol. 2017, 51, 7111–7119. [Google Scholar] [CrossRef] [PubMed]
- Murrieta, M.F.; Nava, J.L. Electrosynthesis of hypochlorous acid in a filter-press electrolyzer and its modeling in dilute chloride solutions. J. Electroanal. Chem. 2021, 892, 115286. [Google Scholar] [CrossRef]
- Rodriguez, M.H.; Schmidt, U.; Büttner, C.; Bandte, M. Eectrolytic Disinfection of Irrigation Water for Intensive Crop Production in Greenhouses as Demonstrated on Tomatoes (Solanum lycopersicum Mill.). Horticulturae 2022, 8, 414. [Google Scholar] [CrossRef]
- Contreras, R.R.; Almarza, J.; Rincón, L.; Ruiz, C. Green sodium hydroxide for industrial purposes. A short review. J. Environ. Chem. Eng. 2025, 13, 114972. [Google Scholar] [CrossRef]
- Riaz, M.A.; Trogadas, P.; Aymé-Perrot, D.; Sachs, C.; Dubouis, N.; Girault, H.; Coppens, M.O. Water electrolysis technologies: The importance of new cell designs and fundamental modelling to guide industrial-scale development. R. Soc. Chem. 2025, 18, 5190–5214. [Google Scholar] [CrossRef]
- O’Brien, T.F. Slt, Chlor-Alkali, and Related Heavy Chemicals. In Handbook of Industrial Chemistry and Biotechnology; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 1267–1300. [Google Scholar] [CrossRef]
- Hu, M.; Yu, T.; Tan, K.; Zhou, A.; Luo, L.; Yin, S. Utralow Ru loading RuO2-TiO2 with strong oxide-support interaction for efficient chlorine evolution and ammonia-nitrogen-elimination. Chem. Eng. J. 2023, 465, 143001. [Google Scholar] [CrossRef]
- Saxena, J.; Williams, T. Eectrolysed water (hypochlorous acid) generation and efficacy against food-borne pathogens. Sustain. Food Technol. 2023, 1, 603–609. [Google Scholar] [CrossRef]
- León, T.; Abdullah Shah, S.; López, J.; Culcasi, A.; Jofre, L.; Cipollina, A.; Cortina, J.L.; Tamburini, A.; Micale, G. Electrodialysis with Bipolar Membranes for the Generation of NaOH and HCl Solutions from Brines: An Inter-Laboratory Evaluation of Thin and Ultrathin Non-Woven Cloth-Based Ion-Exchange Membranes. Membranes 2022, 12, 1204. [Google Scholar] [CrossRef]
- Khalid, N.I.; Sulaiman, S.; Ab Aziz, N.; Taip, F.S.; Sobri, S.; Nor-Khaizura, M.A.R. Electrolyzed water as a green cleaner: Chemical and physical characterization at different electrolysing parameters. Food Res. 2018, 2, 512–519. [Google Scholar] [CrossRef]
- Zayas, T.; Vega, M.; Soriano-Moro, G.; Handal, A.; Morales, M.; Salgado, L. Eectrogeneration of Hypochlorite Ions using a Dimensionally Stable Anode-Type (Ti/PtPd(10%)Ox) Electrode. J. Electrochem. Sci. Technol. 2024, 15, 268–275. [Google Scholar] [CrossRef]
- Khalid, N.I.; Sulaiman, N.S.; Aziz, N.A.; Taip, F.S.; Sobri, S.; Rashid, N.K.M.A. Otimization of electrolysis parameters for green sanitation chemicals production using response surface methodology. Processes 2020, 8, 792. [Google Scholar] [CrossRef]
- Huang, J.; Pei, L.; Ye, Z.; Zhao, C.; Zhu, L.; Yang, X.; Chen, Y.; Gao, L. Acidic Electrolyzed Water Inhibits the Viability of Gardnerella spp. via Oxidative Stress Response. Front. Med. 2022, 9, 817957. [Google Scholar] [CrossRef]
- Nyamende, N.E.; Hoff, J.W.; van Brede, V.; Belay, Z.A.; Oyenihi, A.B.; Caleb, O.J. Iolation and characterization culturable microbes on the surface of ‘Granny Smith’ apples treated with electrolyzed water during cold storage. Food Sci. Biotechnol. 2022, 31, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, G.; Zhao, X.; Xue, F.; Zhang, B.; Fu, B.; Shu, D.; Sun, L.; Zang, Y.; Hu, M.; et al. Antibacterial effect and mechanism of shorter ultraviolet light (222 nm) combined with slightly acidic electrolyzed water against Escherichia coli. LWT 2024, 193, 115761. [Google Scholar] [CrossRef]
- Ni, K.; Wang, X.; Zhang, P.; Bai, L.; Liu, Y.; Zhou, T.; Chang, G.; Luo, Z.; Zhang, C. Response surface methodology to optimize the sterilization process of slightly acidic electrolyzed water for Chinese shrimp (Fenneropenaeus chinensis) and to investigate its effect on shrimp quality. Food Chem. X 2024, 21, 101180. [Google Scholar] [CrossRef]
- Naka, A.; Nakamura, K.; Kurahashi, M. Sightly Acidic Electrolyzed Water to Remove Methylobacterium mesophilicum, Rhodotorula mucilaginosa and Cladosporium cladosporioides in Households. Appl. Microbiol. 2021, 1, 607–614. [Google Scholar] [CrossRef]
- Kang, M.; Park, B.; Ha, J.H. Knetic Modeling of Slightly Acidic Electrolyzed Water Decay Characteristics in Fresh Cabbage Disinfection Against Human Norovirus. Front. Microbiol. 2021, 12, 616297. [Google Scholar] [CrossRef]
- Satoh, K.; Nagayasu, T.; Nishida, N.; Sano, K.; Sakai, H.; Akamatsu, N.; Nakano, Y.; Fujita, Y.; Miyoshi, Y.; Nakayama, T.; et al. Sequential Washing with Electrolyzed Alkaline and Acidic Water Effectively Removes Pathogens from Metal Surfaces. PLoS ONE 2016, 11, e0156058. [Google Scholar] [CrossRef]
- Liu, Y.; Mei, J.; Xie, J. Efects of slightly acidic electrolyzed water and ginger (Zingiber officinale Roscoe) juice on the quality and myofibrillar protein structure of silver carp (Hypophthalmichthys molitrix) fillets during cold storage. LWT 2024, 204, 116451. [Google Scholar] [CrossRef]
- Oh, D.-H.; Liu, Y.; Zhong, H.; Liu, S.; Wang, Z.; Han, Z.; Sun, Q.; Liu, Y.; Khan, I.; Wei, S. Antibacterial mechanism of slightly acidic electrolyzed water against Pseudomonas arsenicoxydans A1 from greater amberjack (Seriola dumerili). LWT 2024, 194, 115814. [Google Scholar] [CrossRef]
- Lan, W.; Shao, Z.; Lang, A.; Xie, J. Efects of slightly acidic electrolyzed water combined with ԑ-polylysine-chitooligosaccharide Maillard reaction products treatment on the quality of vacuum packaged sea bass (Lateolabrax japonicas). Int. J. Biol. Macromol. 2024, 260, 129554. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Ye, X.-Q.; Xuan, X.-T.; Liu, D.-H.; Li, J.; Zhao, Y.; Ahn, J.-H.; Chen, S.-G. Estimation of growth parameters of Listeria monocytogenes after sublethal heat and slightly acidic electrolyzed water (SAEW) treatment. Food Control 2017, 71, 17–25. [Google Scholar] [CrossRef]
- Kumar, A.; Du, F.; Lienhard, J.H. Custic Soda Production, Energy Efficiency, and Electrolyzers. ACS Energy Lett. 2021, 6, 3563–3566. [Google Scholar] [CrossRef]
- Atrashkevich, A.; Alum, A.; Stirling, R.; Abbaszadegan, M.; Garcia-Segura, S. Aproaching easy water disinfection for all: Can in situ electrochlorination outperform conventional chlorination under realistic conditions? Water Res. 2024, 250, 121014. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Catalano, P.; Tanucci, G.; Giametta, F.; Bianchi, B. Dsign and Construction and Energy Consumption Study of a New Electrolyzed Water Cell Generator Prototype for Food Disinfection. Sci 2024, 6, 43. [Google Scholar] [CrossRef]
- Chai, B.; Zhang, A.; Shi, L.; Ming, R.; Wei, S.; Ren, Z.; Zhu, Y. Preparation of electrolyzed oxidizing water with a platinum electrode prepared by magnetron sputtering technique. RSC Adv. 2017, 7, 45377–45384. [Google Scholar] [CrossRef]
- Ampiaw, R.E.; Yaqub, M.; Woo, C.; Lee, W. Otimizing slightly acidic electrolyzed water (SAEW) production through experiments and gene expression programming. J. Water Process Eng. 2022, 47, 102736. [Google Scholar] [CrossRef]
- Thangappan, R.; Sampathkumaran, S.T. Eectrochlorination system: A unique method of prevention of biofouling in seawater desalination. Int. J. Nucl. Desalination 2008, 3, 135. [Google Scholar] [CrossRef]
- Maruyama, N.; Noda, M.; Goshima, H.; Okamoto, M.; Hirota, M. Eperimental Investigation on Hypochlorous Acid Water Production using Electrode Plates without a Barrier Membrane (Part I: Production conditions for high-concentration Hypochlorous acid water). IOSR J. Mech. Civ. Eng. (IOSR-JMCE) 2015, 12, 48–57. [Google Scholar] [CrossRef]
- Chai, B.; Wang, J.; Zhang, A.; Han, Y.; Deng, L.; Ming, R.; Ren, Z.; Zhu, Y. Effect of electrode material and electrolysis process on the preparation of electrolyzed oxidizing water. New J. Chem. 2018, 42, 12143–12151. [Google Scholar] [CrossRef]

| Type | Thickness of Wet Membrane, Microns | Exchange Capacity, meq/g Dry Membrane | Surface Resistance Escriba Aquí la Ecuación Ω·cm2 |
|---|---|---|---|
| MK-40 | 520 | 2 | 2 |
| MA-41 | 530 ± 20 | 1.6 | <11 |
| Factor | Notation | Unit | Level | ||
|---|---|---|---|---|---|
| Low | Medium | High | |||
| Electric potential | X1 | V | 4.5 | 6 | 7 |
| Electrolysis time | X2 | min | 30 | 60 | 90 |
| Sodium chloride concentration | X3 | g/L | 5 | 15 | 30 |
| Run | X1 | X2 | X3 | Concentration (ppm) | pH of Solution | Energy Consumption (kWh/kg) | Chloride Conversion (%) | Electrical Efficiency (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acid | Base | Acid | Base | Acid | Base | Acid | Base | |||||
| 1 | 4.5 | 30 | 5 | 38.98 | 80 | 1.98 | 10.78 | 6.84 | 3.33 | 2.37 | 49.75 | 90.44 |
| 2 | 4.5 | 60 | 15 | 116.9 | 420 | 1.39 | 11.75 | 11.09 | 3.01 | 4.31 | 30.68 | 97.61 |
| 3 | 4.5 | 90 | 30 | 248.0 | 1060 | 0.72 | 11.8 | 14.36 | 3.36 | 5.21 | 23.7 | 89.71 |
| 4 | 6 | 30 | 15 | 131.1 | 500 | 1.2 | 11.8 | 17.95 | 4.71 | 5.98 | 25.28 | 85.4 |
| 5 | 6 | 60 | 30 | 333.1 | 1800 | 0.71 | 11.94 | 24.96 | 4.62 | 9.31 | 18.18 | 87.04 |
| 6 | 6 | 90 | 5 | 205.5 | 780 | 0.91 | 11.61 | 16.41 | 4.33 | 24.11 | 27.64 | 92.94 |
| 7 | 7 | 30 | 30 | 209.0 | 1040 | 0.63 | 12.01 | 29.73 | 5.98 | 5.34 | 17.85 | 78.85 |
| 8 | 7 | 60 | 5 | 184.2 | 640 | 1.15 | 11.84 | 18.52 | 5.33 | 19.6 | 28.58 | 87.93 |
| 9 | 7 | 90 | 15 | 481.9 | 2600 | 0.51 | 11.72 | 28.37 | 5.26 | 25.37 | 18.65 | 89.17 |
| Response | N | Mean | Dev. Est. | Variance | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Chlorine concentration (mg/L) | 9 | 216.52 | 129.890 | 16,871.5 | 38.98 | 481.9 |
| NaOH concentration (mg/L) | 9 | 991.111 | 775.829 | 601,911 | 80 | 2600 |
| Chloride ion conversion (%) | 9 | 11.2889 | 9.11336 | 83.0534 | 2.37 | 25.37 |
| Current efficiency (NaOH) | 9 | 88.7878 | 5.15783 | 26.6032 | 78.85 | 97.61 |
| N° | Company | ACC (mg/L) | pH | R |
|---|---|---|---|---|
| 1 | V9 (Zhongshan Lady Li Electrical Co., Ltd., Zhongshan, Guangdong, China) | 98 | 6.01 | [45] |
| 2 | Harmony-II Electrolytic Water Generator (Rui Andre Environment Equipment Co., Ltd., Beijing, China) | 100 | 6.04 | [46] |
| 3 | Apia 270 (Hokuetsu Co., Ltd., Kanagawa, Japan) | 40 | 6.02 | [47] |
| 4 | SAEW Generator (Purester m-Clean; Morinaga Milk Industry Co., Ltd., Tokyo, Japan) | 26.65 | 5.46 | [48] |
| 5 | Y-9201T (Yuasa Membrane Systems Co., Ltd., Tokyo, Japan) | 18.67 ± 2 | 2.71 and 11.91 | [49] |
| 6 | SAEW Generator (FXSWS100, Fangxin Water Treatment Equipment Co., Ltd., Yantai, China) | 30.0 ± 1.54 | 6.35 ± 0.04 | [50] |
| 7 | Anywhere-320W (Ryande Environmental Protection Equipment Beijing Co., Ltd., Beijing, China) | 60 ± 1 | 6.00 ± 0.15 | [51] |
| 8 | SAEW Generator (Intercontinental Resources Environmental Science and Technology Co., Ltd., Beijing, China) | 30.0 ± 1.18 | 6.36 ± 0.03 | [52] |
| 9 | Beijing Intercontinental Resources and Environmental Protection Technology Co., Beijing, China | 200 ± 10 | 6.3 | [53] |
| Technology | Specific Energy Consumption | Reference |
|---|---|---|
| Membrane-based chlor-alkali process | 2.2–2.6 kWh/kg Cl2 | [54] |
| Electrochlorination with membrane | 0.054–0.11 kWh/m3 | [55] |
| Prototype membrane electrolyzed water cell generator | 0.11 kWh/L of electrolyzed water | [56] |
| Variable Response | Factor | DOF | Sum of Squares | Contribution (%) | SS Adjust. | MC Adjust. | F | p |
|---|---|---|---|---|---|---|---|---|
| Chlorine concentration (mg/L) | X1 | 2 | 37,211 | 27.5694218 | 37,211 | 18,605 | 1.77 | 0.361 |
| X2 | 2 | 51,700 | 38.3042409 | 51,700 | 25,850 | 2.45 | 0.289 | |
| X3 | 2 | 24,998 | 18.5208784 | 24,998 | 12,499 | 1.19 | 0.457 | |
| Error | 2 | 21,063 | 15.6054589 | 21,063 | 10,532 | |||
| Total | 8 | 134,972 | ||||||
| Concentration of NaOH (mg/L) | X1 | 2 | 1,238,756 | 25.7254757 | 1,238,756 | 619,378 | 1.09 | 0.478 |
| X2 | 2 | 1,331,822 | 27.6581946 | 1,331,822 | 665,911 | 1.17 | 0.460 | |
| X3 | 2 | 1,109,422 | 23.0395725 | 1,109,422 | 554,711 | 0.98 | 0.506 | |
| Error | 2 | 1,135,289 | 23.5767573 | 1,135,289 | 567,644 | |||
| Total | 8 | 4,815,289 | ||||||
| pH of acid | X1 | 2 | 0.57042 | 33.1994692 | 0.57042 | 0.285211 | 46.9 | 0.021 |
| X2 | 2 | 0.48162 | 28.0311496 | 0.48162 | 0.240811 | 39.6 | 0.025 | |
| X3 | 2 | 0.65396 | 38.0616473 | 0.65396 | 0.326978 | 53.8 | 0.018 | |
| Error | 2 | 0.01216 | 0.70773385 | 0.01216 | 0.00607 | |||
| Total | 8 | 1.71816 | ||||||
| pH of base | X1 | 2 | 0.2918 | 27.7798934 | 0.2918 | 0.14591 | 1.40 | 0.416 |
| X2 | 2 | 0.1484 | 14.1279513 | 0.1484 | 0.07418 | 0.71 | 0.583 | |
| X3 | 2 | 0.4025 | 38.3187357 | 0.4025 | 0.20124 | 1.94 | 0.340 | |
| Error | 2 | 0.2078 | 19.7829398 | 0.2078 | 0.10388 | |||
| Total | 8 | 1.0504 | ||||||
| Energy consumption (kWh/kgCl2) | X1 | 2 | 332.784 | 69.6779543 | 332.784 | 166.392 | 21.8 | 0.044 |
| X2 | 2 | 4.692 | 0.98240589 | 4.692 | 2.346 | 0.31 | 0.764 | |
| X3 | 2 | 124.922 | 26.1560334 | 124.922 | 62.461 | 8.22 | 0.109 | |
| Error | 2 | 15.204 | 3.18339709 | 15.204 | 7.602 | |||
| Total | 8 | 477.603 | ||||||
| Energy consumption (kWh/kg NaOH) | X1 | 2 | 7.92740 | 94.4097752 | 7.92740 | 3.96370 | 130 | 0.001 |
| X2 | 2 | 0.25207 | 3.00197694 | 0.25207 | 0.12603 | 41.5 | 0.024 | |
| X3 | 2 | 0.21127 | 2.51607755 | 0.21127 | 0.10563 | 34.8 | 0.028 | |
| Error | 2 | 0.00607 | 0.07228944 | 0.00607 | 0.00303 | |||
| Total | 8 | 8.39680 | ||||||
| Current efficiency in formation of NaOH | X1 | 2 | 79.763 | 37.4780337 | 79.763 | 39.881 | 11.7 | 0.078 |
| X2 | 2 | 68.230 | 32.0590529 | 68.230 | 34.115 | 10.0 | 0.090 | |
| X3 | 2 | 58.051 | 27.2762726 | 58.051 | 29.025 | 8.56 | 0.105 | |
| Total | 8 | 212.826 | ||||||
| Current efficiency in formation of Cl2 | X1 | 2 | 1.522 | 0.61172404 | 1.522 | 0.761 | 0.13 | 0.886 |
| X2 | 2 | 15.035 | 6.04288499 | 15.035 | 7.518 | 1.28 | 0.439 | |
| X3 | 2 | 220.480 | 88.6155825 | 220.480 | 110.240 | 18.7 | 0.051 | |
| Total | 8 | 248.805 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collana, J.T.M.; Guillen, K.A.; Martinez, E.W.V.; Dextre, C.A.; Venegas, L.C.; Taranco, O.R.; Herrera, J.L.; Bravo, P.D.; Loarte, J.P.; Pisfil, J.M. Electrochemical Production of Hypochlorous Acid and Sodium Hydroxide Using Ion Exchange Membranes. Sustainability 2025, 17, 5465. https://doi.org/10.3390/su17125465
Collana JTM, Guillen KA, Martinez EWV, Dextre CA, Venegas LC, Taranco OR, Herrera JL, Bravo PD, Loarte JP, Pisfil JM. Electrochemical Production of Hypochlorous Acid and Sodium Hydroxide Using Ion Exchange Membranes. Sustainability. 2025; 17(12):5465. https://doi.org/10.3390/su17125465
Chicago/Turabian StyleCollana, Juan Taumaturgo Medina, Kevin Azorza Guillen, Edgar Williams Villanueva Martinez, Carlos Ancieta Dextre, Luis Carrasco Venegas, Oscar Rodriguez Taranco, Jorge Lopez Herrera, Pablo Diaz Bravo, Jose Porlles Loarte, and Jorge Montaño Pisfil. 2025. "Electrochemical Production of Hypochlorous Acid and Sodium Hydroxide Using Ion Exchange Membranes" Sustainability 17, no. 12: 5465. https://doi.org/10.3390/su17125465
APA StyleCollana, J. T. M., Guillen, K. A., Martinez, E. W. V., Dextre, C. A., Venegas, L. C., Taranco, O. R., Herrera, J. L., Bravo, P. D., Loarte, J. P., & Pisfil, J. M. (2025). Electrochemical Production of Hypochlorous Acid and Sodium Hydroxide Using Ion Exchange Membranes. Sustainability, 17(12), 5465. https://doi.org/10.3390/su17125465






