Co-Hydrothermal Carbonization of Swine Manure and Soybean Hulls: Synergistic Effects on the Potential Use of Hydrochar as a Biofuel and Soil Improver
Abstract
1. Introduction
2. Materials and Methods
2.1. HTC and Co-HTC Experiments
2.2. Characterization of Feedstock, HTC and Co-HTC Products
2.2.1. Feedstock and Hydrochar Characterization
2.2.2. Process Water Characterization
2.2.3. Combustion Analysis
2.2.4. Statistical Analysis
3. Results and Discussion
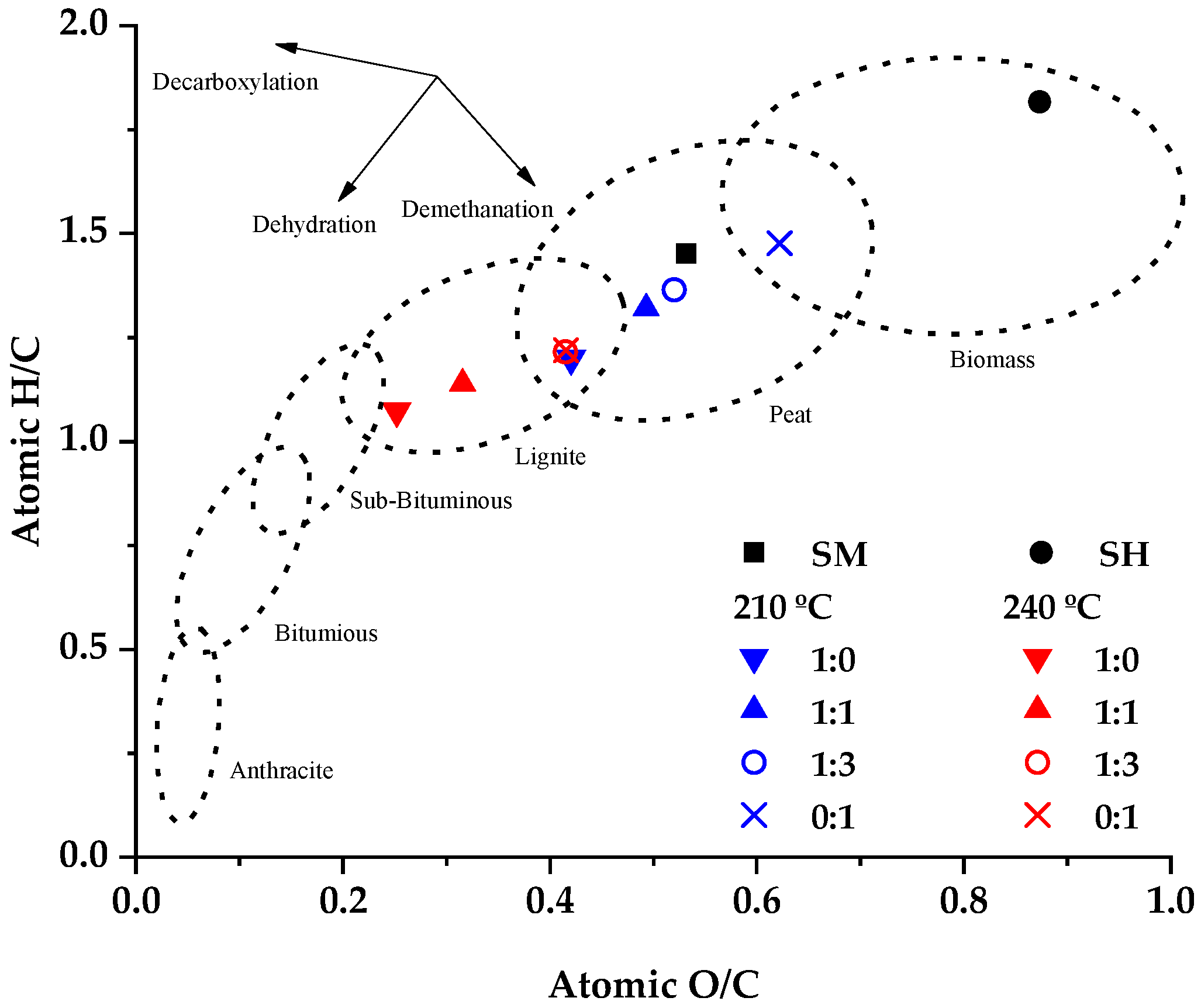
3.1. Characterization of Feedstock and HTC/Co-HTC Products
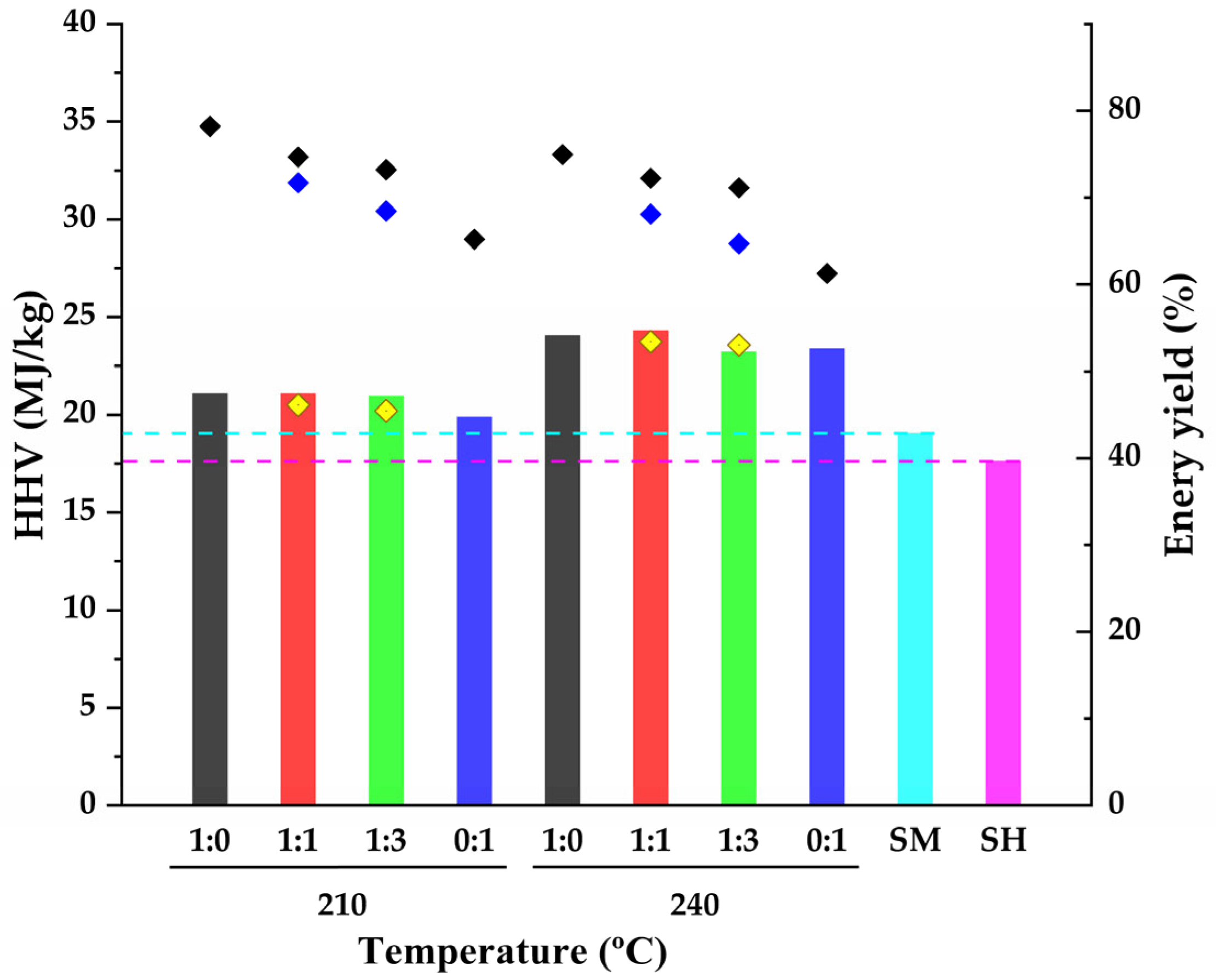
3.2. Analysis of Hydrochars for Biofuel Applications
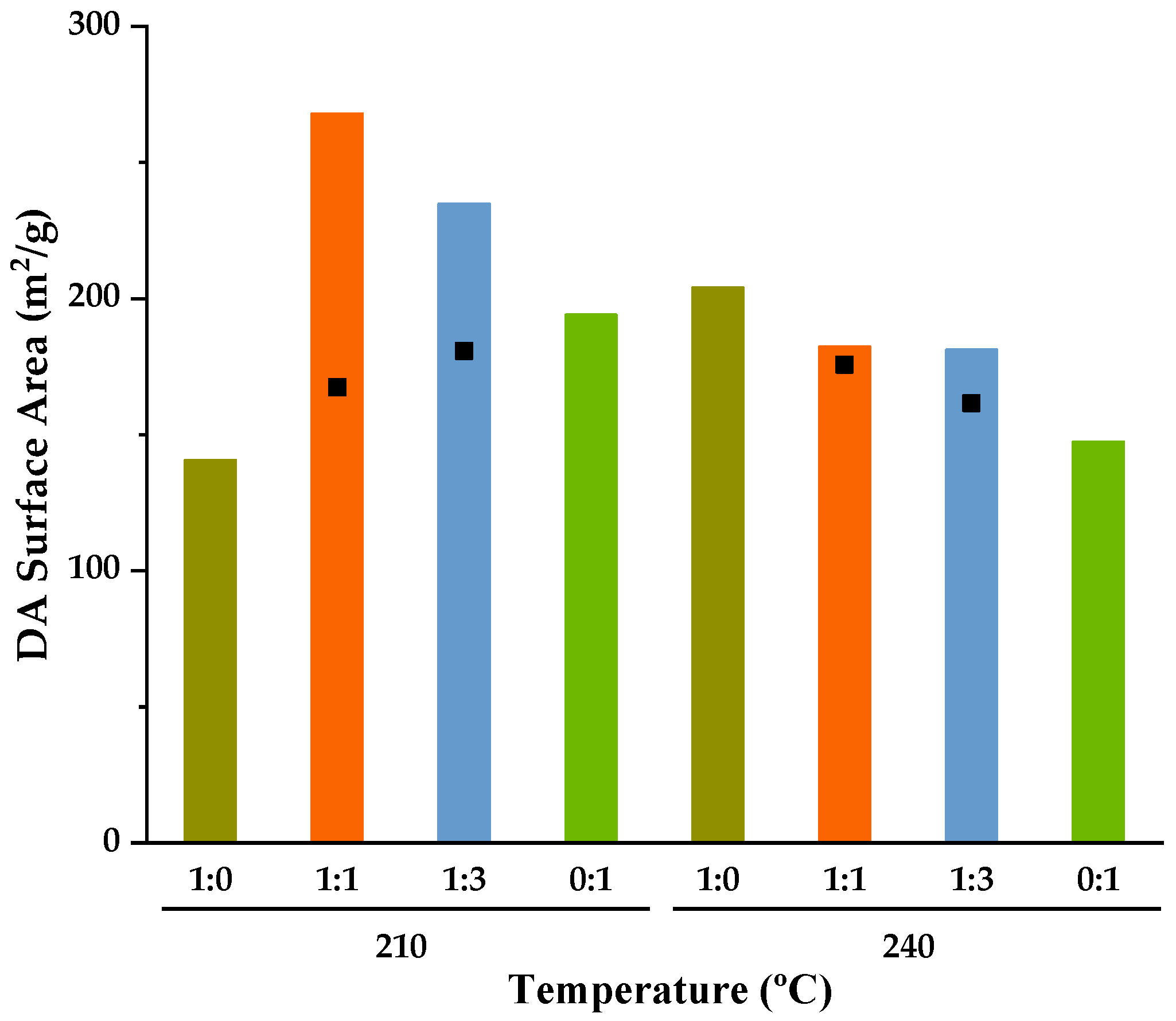
3.3. Evaluation of Hydrochars for Soil Improvement Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HTC | Hydrothermal carbonization |
| Co-HTC | Co-hydrothermal carbonization |
| SM | Swine manure |
| SH | Soybean hull |
| HC | Hydrochar |
| PW | Process water |
| TS | Total solids |
| FC | Fixed carbon |
| VM | Volatile matter |
| TOC | Total organic carbon |
| TN | Total nitrogen |
| TKN | Total Kjeldal nitrogen |
| CV | Calculated values |
References
- Mei, C.; Cheng, M.; Xie, M.; Yang, R.; Liu, T.; Huang, Z.; Zhou, T.; Zhao, Y.; Liu, Z.; Li, B. Recent Advances in Thermochemical Conversion Technology for Anaerobic Digestate from Food Waste. Bioresour. Technol. 2024, 413, 131527. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Norouzi, O.; MacDermid-Watts, K.; Acharya, B.; Zhang, Y.; Dutta, A. Product Evaluation of Hydrothermal Carbonization of Biomass: Semi-Continuous vs. Batch Feeding. Biomass-Convers. Biorefinery 2022, 12, 15–25. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An Overview of Effect of Process Parameters on Hydrothermal Carbonization of Biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Premchand; Javed, M.; Saeed, S.; Abro, R.; Mazari, S.A.; Mubarak, N.M.; Siddiqui, M.T.H.; Baloch, H.A.; Nizamuddin, S. Hydrothermal Carbonization of Oil Palm Trunk via Taguchi Method. Korean J. Chem. Eng. 2021, 38, 797–806. [Google Scholar] [CrossRef]
- Ipiales, R.P.; Pimentel-Betancurt, D.; Diaz, E.; de la Rubia, A.; Rodríguez, J.J.; Mohedano, A.F. Energy Recovery from Garden and Park Waste by Hydrothermal Carbonization with Process Water Recycling. ACS Sustain. Chem. Eng. 2024, 12, 5229–5240. [Google Scholar] [CrossRef]
- Gebretsadkan, A.A.; Belete, Y.Z.; Krounbi, L.; Gelfand, I.; Bernstein, R.; Gross, A. Soil Application of Activated Hydrochar Derived from Sewage Sludge Enhances Plant Growth and Reduces Nitrogen Loss. Sci. Total Environ. 2024, 949, 174965. [Google Scholar] [CrossRef]
- Qaramaleki, S.V.; Mohedano, Á.F.; Coronella, C.J. Phosphorus Recovery from Aqueous Product of Hydrothermal Carbonization of Cow Manure. Waste Manag. 2023, 168, 301–310. [Google Scholar] [CrossRef]
- Sarrion, A.; Ipiales, R.P.; de la Rubia, M.A.; Mohedano, A.F.; Diaz, E. Chicken Meat and Bone Meal Valorization by Hydrothermal Treatment and Anaerobic Digestion: Biofuel Production and Nutrient Recovery. Renew. Energy 2023, 204, 652–660. [Google Scholar] [CrossRef]
- Diez, M.P.; Barahona, E.; de la Rubia, M.A.; Mohedano, A.F.; Diaz, E. Valorization of Process Water from Hydrothermal Carbonization of Food Waste by Dark Fermentation. Int. J. Hydrogen Energy 2024, 89, 1383–1393. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, Y.; He, M.; Lei, H.; Song, H.; Alessi, D.S.; Tsang, D.C.W. Improved Energy Recovery from Yard Waste by Water-Starved Hydrothermal Treatment: Effects of Process Water and Pressure. Bioresour. Technol. 2024, 394, 130211. [Google Scholar] [CrossRef]
- Kambo, H.S.; Minaret, J.; Dutta, A. Process Water from the Hydrothermal Carbonization of Biomass: A Waste or a Valuable Product? Waste Biomass Valorization 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Ebrahim Malool, M.; Keshavarz Moraveji, M.; Shayegan, J. Co-Hydrothermal Carbonization of Digested Sewage Sludge and Sugarcane Bagasse: Integrated Approach for Waste Management, Optimized Production, Characterization and Pb(II) Adsorption. Alex. Eng. J. 2023, 74, 79–105. [Google Scholar] [CrossRef]
- García-Morato, R.; Román, S.; Ledesma, B.; Coronella, C. Co-Hydrothermal Carbonization of Grass and Olive Stone as a Means to Lower Water Input to HTC. Resources 2023, 12, 85. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Codignole Lùz, F.; Malik, W.; Volpe, R.; Messineo, A. Co-Hydrothermal Carbonization with Process Water Recirculation as a Valuable Strategy to Enhance Hydrochar Recovery with High Energy Efficiency. Waste Manag. 2024, 175, 101–109. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Wang, J.; Hu, Z.; Xu, M.; Qiao, Y. Co-Hydrothermal Carbonization of Sludge and Food Waste for Hydrochar Valorization: Effect of Mutual Interaction on Sulfur Transformation. Sci. Total Environ. 2023, 905, 167318. [Google Scholar] [CrossRef]
- Pampuro, N.; Dinuccio, E.; Balsari, P.; Cavallo, E. Gaseous Emissions and Nutrient Dynamics during Composting of Swine Solid Fraction for Pellet Production. Appl. Math. Sci. 2014, 8, 6459–6468. [Google Scholar] [CrossRef]
- Köninger, J.; Lugato, E.; Panagos, P.; Kochupillai, M.; Orgiazzi, A.; Briones, M.J.I. Manure Management and Soil Biodiversity: Towards More Sustainable Food Systems in the EU. Agric. Syst. 2021, 194, 103251. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Wang, S.; Wang, Z.; Liu, Y.; Hu, Z.; Zhan, X. Environmental Sustainability Assessment of Pig Manure Mono- and Co-Digestion and Dynamic Land Application of the Digestate. Renew. Sustain. Energy Rev. 2021, 137, 110476. [Google Scholar] [CrossRef]
- da Rosa, G.M.; Gabriel, M.; da Silva, J.C.; Mendonça, A.M.; Junior, J.A.C.; Wastowski, A.D. Leaching of the Different Forms of Nitrogen by the Application of Poultry Litter, Swine Waste, and Mineral Nitrogen on Corn Cultures (Zea mays L.). Environ. Qual. Manag. 2018, 28, 131–138. [Google Scholar] [CrossRef]
- Raj, C.; Chakraborty, D.; Watts, D.B.; Horvath, T.; Ketterings, Q.M.; Blersch, D.; Tomasek, A.A.; Cordoba, B.C.; Prasad, R. Impact of Broiler Litter and Swine Liquid Manure on Nutrient Loss in Runoff from Three Consecutive One Acre-Inch Rainfall Events. Heliyon 2024, 10, e40062. [Google Scholar] [CrossRef]
- Zhuang, M.; Shan, N.; Wang, Y.; Caro, D.; Fleming, R.M.; Wang, L. Different Characteristics of Greenhouse Gases and Ammonia Emissions from Conventional Stored Dairy Cattle and Swine Manure in China. Sci. Total Environ. 2020, 722, 137693. [Google Scholar] [CrossRef] [PubMed]
- EUROSTAT. Agri-Environmental Indicator—Livestock Patterns; EUROSTAT: Luxembourg, 2023.
- Ipiales, R.P.; Díaz, E.; Mohedano, A.F.; de la Rubia, M.A. Material and Energy Recovery from Animal Manure by Hydrothermal Carbonization. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Ipiales, R.P.; Mohedano, A.F.; Diaz-Portuondo, E.; Diaz, E.; de la Rubia, M.A. Co-Hydrothermal Carbonization of Swine Manure and Lignocellulosic Waste: A New Strategy for the Integral Valorization of Biomass Wastes. Waste Manag. 2023, 169, 267–275. [Google Scholar] [CrossRef]
- Lang, Q.; Guo, Y.; Zheng, Q.; Liu, Z.; Gai, C. Co-Hydrothermal Carbonization of Lignocellulosic Biomass and Swine Manure: Hydrochar Properties and Heavy Metal Transformation Behavior. Bioresour. Technol. 2018, 266, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Luo, Y.; Shangguan, W.; Deng, Y.; Li, R.; Song, D.; Zhang, M.; Li, Z.; Xiao, R. Co-Hydrothermal Carbonization of Lignocellulosic Biomass and Swine Manure: Optimal Parameters for Enhanced Nutrient Reclamation, Carbon Sequestration, and Heavy Metals Passivation. Waste Manag. 2024, 190, 174–185. [Google Scholar] [CrossRef]
- Świątkiewicz, M.; Witaszek, K.; Sosin, E.; Pilarski, K.; Szymczyk, B.; Durczak, K. The Nutritional Value and Safety of Genetically Unmodified Soybeans and Soybean Feed Products in the Nutrition of Farm Animals. Agronomy 2021, 11, 1105. [Google Scholar] [CrossRef]
- Gaffield, K.N.; Goodband, R.D.; DeRouchey, J.M.; Tokach, M.D.; Woodworth, J.C.; Denny, G.; Gebhardt, J.T. A Review of Soybean Processing Byproducts and Their Use in Swine and Poultry Diets. Transl. Anim. Sci. 2024, 8, txae063. [Google Scholar] [CrossRef]
- MAPA Estadística Digital. Available online: https://servicio.mapama.gob.es/es/estadistica/temas/estadistica-digital/powerbi-cultivos.aspx (accessed on 25 April 2025).
- Cassales, A.; de Souza-Cruz, P.B.; Rech, R.; Záchia Ayub, M.A. Optimization of Soybean Hull Acid Hydrolysis and Its Characterization as a Potential Substrate for Bioprocessing. Biomass Bioenergy 2011, 35, 4675–4683. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Porto de Souza Vandenberghe, L.; Karp, S.G.; Murawski de Mello, A.F.; Thomaz Soccol, V.; Soccol, C.R. Microbial Lipid Production from Soybean Hulls Using Lipomyces Starkeyi LPB53 in a Circular Economy. Bioresour. Technol. 2023, 372, 128650. [Google Scholar] [CrossRef]
- Riaz, M.N. Soy Beans: Processing. In Encyclopedia of Food and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 48–53. ISBN 9780123849533. [Google Scholar]
- ASTM E1756; Standard Test Method for Determination of Total Solids in Biomass. ASTM International: West Conshohocken, PA, USA, 2020.
- Sarrion, A.; Diaz, E.; de la Rubia, M.A.; Mohedano, A.F. Fate of Nutrients during Hydrothermal Treatment of Food Waste. Bioresour. Technol. 2021, 342, 125954. [Google Scholar] [CrossRef]
- ASTM D3173-11; Standard Test Method for Moisture in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM D3174-11; Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM D3175-11; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- Channiwala, S.A.; Parikh, P.P. A Unified Correlation for Estimating HHV of Solid, Liquid and Gaseous Fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Park, K.Y.; Lee, K.; Kim, D. Characterized Hydrochar of Algal Biomass for Producing Solid Fuel through Hydrothermal Carbonization. Bioresour. Technol. 2018, 258, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Cequier-Sánchez, E.; Rodríguez, C.; Ravelo, Á.G.; Zárate, R. Dichloromethane as a Solvent for Lipid Extraction and Assessment of Lipid Classes and Fatty Acids from Samples of Different Natures. J. Agric. Food Chem. 2008, 56, 4297–4303. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Hindrichsen, I.K.; Kreuzer, M.; Madsen, J.; Bach Knudsen, K.E. Fiber and Lignin Analysis in Concentrate, Forage, and Feces: Detergent Versus Enzymatic-Chemical Method. J. Dairy Sci. 2006, 89, 2168–2176. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z.; Fang, S.; Lin, Y.; Fan, Y. Combustion, Pyrolysis and Char CO2-Gasification Characteristics of Hydrothermal Carbonization Solid Fuel from Municipal Solid Wastes. Fuel 2016, 181, 905–915. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Peng, X.; Lin, Y.; Yao, Z. Conversion of Sweet Potato Waste to Solid Fuel via Hydrothermal Carbonization. Bioresour. Technol. 2018, 249, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Zhou, S.; Meng, B.; Wu, T. A Review on Thermogravimetric Analysis-Based Analyses of the Pyrolysis Kinetics of Oil Shale and Coal. Energy Sci. Eng. 2024, 12, 329–355. [Google Scholar] [CrossRef]
- Ma, B.G.; Li, X.G.; Xu, L.; Wang, K.; Wang, X.G. Investigation on Catalyzed Combustion of High Ash Coal by Thermogravimetric Analysis. Thermochim. Acta 2006, 445, 19–22. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Hameed, Z.; Ali, I.; Naqvi, M.; Chen, W.H.; Ceylan, S.; Rashid, H.; Ahmad, J.; Taqvi, S.A.; et al. Pyrolysis of High Ash Sewage Sludge: Kinetics and Thermodynamic Analysis Using Coats-Redfern Method. Renew. Energy 2019, 131, 854–860. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, J.; Mao, Q.; Zhang, Z.; Yao, Z.; Zhu, X.; Ye, S.; Zhong, Q. Thermogravimetric Analysis of the Combustion Characteristics and Combustion Kinetics of Coals Subjected to Different Chemical Demineralization Processes. ACS Omega 2022, 7, 13998–14008. [Google Scholar] [CrossRef]
- Tortosa Masiá, A.A.; Buhre, B.J.P.; Gupta, R.P.; Wall, T.F. Characterising Ash of Biomass and Waste. Fuel Process. Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- Cao, Z.; Hülsemann, B.; Wüst, D.; Oechsner, H.; Lautenbach, A.; Kruse, A. Effect of Residence Time during Hydrothermal Carbonization of Biogas Digestate on the Combustion Characteristics of Hydrochar and the Biogas Production of Process Water. Bioresour. Technol. 2021, 333, 125110. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Alavi, S.; Vadlani, P.; Amanor-Boadu, V. Thermo-Mechanical Extrusion Pretreatment for Conversion of Soybean Hulls to Fermentable Sugars. Bioresour. Technol. 2011, 102, 7583–7590. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.J.; Trabue, S.L.; van Weelden, M.B.; Andersen, D.S.; Pepple, L.M. Impact of Narasin on Manure Composition, Microbial Ecology, and Gas Emissions from Finishing Pigs Fed Either a Corn-Soybean Meal or a Corn-Soybean Meal-Dried Distillers Grains with Solubles Diets. J. Anim. Sci. 2018, 96, 1317–1329. [Google Scholar] [CrossRef]
- Wang, R.; Lin, Z.; Meng, S.; Liu, S.; Zhao, Z.; Wang, C.; Yin, Q. Effect of Lignocellulosic Components on the Hydrothermal Carbonization Reaction Pathway and Product Properties of Protein. Energy 2022, 259, 125063. [Google Scholar] [CrossRef]
- Hu, J.; Shen, D.; Wu, S.; Zhang, H.; Xiao, R. Effect of Temperature on Structure Evolution in Char from Hydrothermal Degradation of Lignin. J. Anal. Appl. Pyrolysis 2014, 106, 118–124. [Google Scholar] [CrossRef]
- Pauline, A.L.; Joseph, K. Hydrothermal Carbonization of Organic Wastes to Carbonaceous Solid Fuel—A Review of Mechanisms and Process Parameters. Fuel 2020, 279, 118472. [Google Scholar] [CrossRef]
- Ipiales, R.P.; Lelli, G.; Diaz, E.; Diaz-Portuondo, E.; Mohedano, A.F.; de la Rubia, M.A. Study of Two Approaches for the Process Water Management from Hydrothermal Carbonization of Swine Manure: Anaerobic Treatment and Nutrient Recovery. Environ. Res. 2024, 246, 118098. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Ledesma, B.; Román, S.; Olivares-Marín, M. Insights about the Formation of Secondary Char during HTC Processes. Sustain. Chem. Pharm. 2024, 37, 101420. [Google Scholar] [CrossRef]
- Yu, S.; Xie, M.; Li, Q.; Zhang, Y.; Zhou, H. Evolution of Kraft Lignin during Hydrothermal Treatment under Different Reaction Conditions. J. Energy Inst. 2022, 103, 147–153. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.M. Morphological and Structural Differences between Glucose, Cellulose and Lignocellulosic Biomass Derived Hydrothermal Carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- ISO 17225-8:2023; Solid Biofuels—Fuel Specifications and Classes—Part 8: Graded Firewood. International Organization for Standardization: Geneva, Switzerland, 2023.
- Liu, X.; Peng, L.; Deng, P.; Xu, Y.; Wang, P.; Tan, Q.; Zhang, C.; Dai, X. Co-Hydrothermal Carbonization of Sewage Sludge and Rice Straw to Improve Hydrochar Quality: Effects of Mixing Ratio and Hydrothermal Temperature. Bioresour. Technol. 2025, 415, 131665. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ma, X.; Qin, Z.; Chen, X.; Yue, W. Co-Hydrothermal Carbonization of Sewage Sludge and Swine Manure: Hydrochar Properties and Heavy Metal Chemical Speciation. Fuel 2022, 330, 125573. [Google Scholar] [CrossRef]
- Ghazidin, H.; Suyatno, S.; Prismantoko, A.; Ruhiyat, A.S.; Prabowo; Darmawan, A.; Soleh, M.; Aziz, A.; Asmanto, P.; Vuthaluru, H.; et al. Control of Slagging and Fouling during Co-Firing of Solid Recovered Fuel and High-Sodium Coal Using Aluminum and Magnesium-Based Additives. Energy 2025, 318, 134873. [Google Scholar] [CrossRef]
- Riaza, J.; Mason, P.; Jones, J.M.; Gibbins, J.; Chalmers, H. High Temperature Volatile Yield and Nitrogen Partitioning during Pyrolysis of Coal and Biomass Fuels. Fuel 2019, 248, 215–220. [Google Scholar] [CrossRef]
- Wang, Z.W.; Zhu, M.Q.; Li, M.F.; Wang, J.Q.; Wei, Q.; Sun, R.C. Comprehensive Evaluation of the Liquid Fraction During the Hydrothermal Treatment of Rapeseed Straw. Biotechnol. Biofuels 2016, 9, 142. [Google Scholar] [CrossRef]
- Ferrentino, R.; Sacchi, G.; Scrinzi, D.; Andreottola, G.; Fiori, L. Valorization of Swine Manure for a Circular Approach through Hydrothermal Carbonization. Biomass Bioenergy 2023, 168, 106689. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.Y. Conversion of Sewage Sludge to Clean Solid Fuel Using Hydrothermal Carbonization: Hydrochar Fuel Characteristics and Combustion Behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Khosravi, A.; Zheng, H.; Liu, Q.; Hashemi, M.; Tang, Y.; Xing, B. Production and Characterization of Hydrochars and Their Application in Soil Improvement and Environmental Remediation. Chem. Eng. J. 2022, 430, 133142. [Google Scholar] [CrossRef]
- Islam, M.A.; Sharif, M.; Limon, H.; Romić, M.; Islam, A. Hydrochar-Based Soil Amendments for Agriculture: A Review of Recent Progress. Arab. J. Geosci. 2021, 14, 102. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, Z.; Zhang, Z.; Wang, W.; Li, D.; Zhang, T.; Zhu, Z. Applying Hydrochar Affects Soil Carbon Dynamics by Altering the Characteristics of Soil Aggregates and Microbes. Agronomy 2024, 14, 1015. [Google Scholar] [CrossRef]


| SM | SH | SM | SH | ||
|---|---|---|---|---|---|
| FC (%) | 6.9 | 5.7 | Al (g/kg) | 0.4 | 0.3 |
| VM (%) | 80.2 | 91.0 | Ca (g/kg) | 19.7 | 5.5 |
| Ash (%) | 12.9 | 3.4 | Fe (g/kg) | 1.3 | 0.6 |
| C (%) | 46.0 | 42.2 | K (g/kg) | 4.2 | 13.7 |
| H (%) | 5.6 | 6.5 | Mg (g/kg) | 2.9 | 1.9 |
| N (%) | 2.2 | 2.9 | Na (g/kg) | 1.3 | <0.01 |
| S (%) | 0.8 | 0.1 | P (g/kg) | 12.6 | 1.3 |
| O * (%) | 32.5 | 44.9 | Cd (mg/kg) | 0.2 | 0.1 |
| HHV (MJ/kg) | 19.1 | 17.7 | Co (mg/kg) | 0.9 | 0.7 |
| Hemicellulose (%) | 12.4 | 11.6 | Cu (mg/kg) | 126.3 | 16.1 |
| Cellulose (%) | 16.9 | 44.5 | Ni (mg/kg) | 6.1 | 2.5 |
| Lignin (%) | 33.2 | 6.1 | Pb (mg/kg) | 1.2 | 0.2 |
| Lipids (%) | 2.3 | 1.5 | Cr (mg/kg) | 12.7 | 3.4 |
| Proteins (%) | 13.2 | 11.1 | Zn (mg/kg) | 418.1 | 48.1 |
| SM:SH | 210 °C | 240 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| 1:0 | 1:1 | 1:3 | 0:1 | 1:0 | 1:1 | 1:3 | 0:1 | |
| pH | 4.27 | 4.30 | 4.33 | 4.25 | 4.17 | 4.30 | 4.33 | 4.29 |
| Conductivity (mS/cm) | 6.1 | 6.4 | 6.6 | 7.9 | 5.2 | 6.1 | 6.6 | 7.3 |
| TS (g/L) | 17.5 | 19.1 | 21.2 | 24.7 | 15.6 | 20.4 | 22.2 | 25.5 |
| VS (g/L) | 4.0 | 5.3 | 4.9 | 5.9 | 2.8 | 3.5 | 4.8 | 6.1 |
| TOC (g/L) | 12.3 | 14.3 | 15.0 | 16.0 | 13.0 | 14.7 | 12.0 | 17.3 |
| TKN (mg/L) | 1171.4 | 937.4 | 1084.5 | 1121.2 | 1003.4 | 867.0 | 943.0 | 931.6 |
| NH4-N (mg/L) | 198.9 | 160.7 | 161.0 | 162.0 | 159.0 | 139.8 | 128.3 | 116.0 |
| SM:SH Ratio | Ti (°C) | Tb (°C) | CCI·107 | AI | Rb/a | SI | FI | |
|---|---|---|---|---|---|---|---|---|
| 210 °C | 1:0 | 290 | 555 | 1.3 | 0.10 | 72.6 | 50.8 | 15.8 |
| 1:1 | 280 | 512 | 7.3 | 0.22 | 24.0 | 7.2 | 11.3 | |
| 1:3 | 288 | 516 | 1.8 | 0.14 | 19.1 | 7.6 | 5.8 | |
| 0:1 | 277 | 473 | 6.2 | 0.19 | 8.3 | 0.8 | 3.2 | |
| 240 °C | 1:0 | 292 | 547 | 0.7 | 0.08 | 29.6 | 31.7 | 7.6 |
| 1:1 | 261 | 512 | 4.3 | 0.11 | 19.9 | 9.9 | 5.4 | |
| 1:3 | 283 | 526 | 1.0 | 0.19 | 19.8 | 7.9 | 8.8 | |
| 0:1 | 268 | 500 | 3.9 | 0.18 | 8.8 | 1.8 | 3.7 | |
| SM:SH Ratio | Mineral Species (g/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Al | Ca | Fe | K | Mg | Na | P | ||
| 210 °C | 1:0 | 0.7 (0.1) | 29.2 (0.2) | 2.0 (0.0) | 1.2 (0.1) | 2.0 (0.0) | 0.5 (0.0) | 18.7 (0.2) |
| 1:1 | 0.7 (0.1) | 16.8 (0.1) | 1.1 (0.0) | 2.3 (0.1) | 1.5 (0.0) | 0.2 (0.0) | 7.7 (0.1) | |
| 1:3 | 0.7 (0.2) | 9.1 (0.2) | 1.1 (0.0) | 3.8 (0.2) | 1.8 (0.1) | 0.1 (0.0) | 6.5 (0.1) | |
| 0:1 | 0.5 (0.1) | 4.4 (0.1) | 0.8 (0.1) | 3.2 (0.0) | 0.7 (0.0) | 0.1 (0.0) | 1.6 (0.1) | |
| 240 °C | 1:0 | 0.9 (0.0) | 33.4 (0.1) | 2.3 (0.1) | 1.0 (0.0) | 2.5 (0.2) | 0.6 (0.0) | 16.5 (0.1) |
| 1:1 | 0.9 (0.1) | 20.2 (0.1) | 1.6 (0.1) | 2.1 (0.1) | 1.7 (0.0) | 0.2 (0.0) | 9.7 (0.1) | |
| 1:3 | 0.9 (0.1) | 10.7 (0.2) | 1.4 (0.0) | 3.5 (0.1) | 2.0 (0.1) | 0.2 (0.0) | 6.5 (0.1) | |
| 0:1 | 0.8 (0.1) | 5.9 (0.2) | 1.1 (0.1) | 3.5 (0.1) | 1.0 (0.1) | 0.1 (0.0) | 2.6 (0.0) | |
| SM:SH Ratio | Heavy Metals (mg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Co | Cr | Cu | Ni | Pb | Si | Ti | Zn | ||
| 210 °C | 1:0 | 0.2 (0.0) | 1.1 (0.1) | 52.9 (1.3) | 175.5 (3.2) | 12.9 (1.2) | 2.6 (0.1) | 240.1 (5.3) | 50.1 (5.3) | 583.3 (3.1) |
| 1:1 | 0.2 (0.0) | 0.6 (0.0) | 9.9 (0.6) | 93.4 (2.5) | 4.3 (0.6) | 1.3 (0.0) | 365.5 (6.1) | 33.3 (2.6) | 261.9 (1.1) | |
| 1:3 | 0.1 (0.0) | 0.6 (0.0) | 8.9 (0.3) | 46.6 (1.7) | 3.9 (0.7) | 1.0 (0.0) | 392.7 (4.2) | 28.6 (2.5) | 213.3 (1.3) | |
| 0:1 | 0.1 (0.0) | 0.6 (0.0) | 7.2 (0.4) | 16.5 (1.9) | 4.5 (0.4) | 0.7 (0.0) | 627.1 (7.5) | 48.9 (4.3) | 60.6 (0.5) | |
| 240 °C | 1:0 | 0.3 (0.0) | 1.6 (0.1) | 94.9 (2.4) | 205.8 (4.2) | 19.7 (1.4) | 1.6 (0.0) | 760.4 (8.8) | 33.7 (2.1) | 665.1 (2.3) |
| 1:1 | 0.3 (0.0) | 1.0 (0.1) | 18.2 (0.3) | 134.9 (2.8) | 8.2 (0.7) | 1.0 (0.0) | 611.0 (6.9) | 45.8 (2.1) | 425.4 (1.6) | |
| 1:3 | 0.1 (0.0) | 0.7 (0.0) | 13.3 (0.3) | 58.2 (2.4) | 8.1 (0.7) | 1.2 (0.1) | 489.1 (4.7) | 35.3 (1.7) | 256.2 (2.2) | |
| 0:1 | 0.2 (0.0) | 0.7 (0.0) | 12.5 (0.2) | 19.1 (1.3) | 8.1 (0.8) | 0.9 (0.0) | 713.5 (7.9) | 62.7 (3.1) | 79.4 (1.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiguano-Tapia, B.; Diaz, E.; de la Rubia, M.A.; Mohedano, A.F. Co-Hydrothermal Carbonization of Swine Manure and Soybean Hulls: Synergistic Effects on the Potential Use of Hydrochar as a Biofuel and Soil Improver. Sustainability 2025, 17, 5022. https://doi.org/10.3390/su17115022
Chiguano-Tapia B, Diaz E, de la Rubia MA, Mohedano AF. Co-Hydrothermal Carbonization of Swine Manure and Soybean Hulls: Synergistic Effects on the Potential Use of Hydrochar as a Biofuel and Soil Improver. Sustainability. 2025; 17(11):5022. https://doi.org/10.3390/su17115022
Chicago/Turabian StyleChiguano-Tapia, Bryan, Elena Diaz, M. Angeles de la Rubia, and Angel F. Mohedano. 2025. "Co-Hydrothermal Carbonization of Swine Manure and Soybean Hulls: Synergistic Effects on the Potential Use of Hydrochar as a Biofuel and Soil Improver" Sustainability 17, no. 11: 5022. https://doi.org/10.3390/su17115022
APA StyleChiguano-Tapia, B., Diaz, E., de la Rubia, M. A., & Mohedano, A. F. (2025). Co-Hydrothermal Carbonization of Swine Manure and Soybean Hulls: Synergistic Effects on the Potential Use of Hydrochar as a Biofuel and Soil Improver. Sustainability, 17(11), 5022. https://doi.org/10.3390/su17115022









