Abstract
Treating poultry manure with calcium compounds is the primary technique for inactivating toxic pathogens such as bacteria, fungi, or viruses and decreasing the risk of biological contaminant release into the environment. On the other hand, the preferable method for reducing its volume is incineration with the aim of obtaining highly concentrated fertilizer. This paper presents the optimization of the biological deactivation of fresh poultry manure using calcium hydroxide via central composite design and response surface methodology. The results revealed that the optimum parameters required to decrease the number of E. coli bacteria to below the acceptable level (1000 CFU/g) were 5.0 wt% Ca(OH)2 at 22 °C and an exposure time of 209 h. A regression analysis showed a good fit of the approximated parameters to the experimental data (R2 = 98%, Radj.2 = 97%). Additionally, laboratory tests involving ash samples obtained from the incineration of poultry manure with the addition of 5 wt% calcium hydroxide (T = 500 °C, t = 5 h) intended as a fertilizer for degraded soils were performed. The analysis revealed that the content of pure manure ash in the sample incinerated with Ca(OH)2 was approximately 47.5%. An X-ray diffraction analysis of the ash sample revealed that the main crystalline component was calcite (67.5 wt% CaCO3), the phases containing phosphorus were apatite (3 wt%) and hydroxyapatite (3 wt%), whereas the source of the bioavailable form of phosphorus was the amorphous phase (15.5 wt%). An analysis of the ash extracts in a 2% citric acid solution revealed that the phosphorus concentration (287 mg/L) was two times lower than that of potassium (661 mg/L). The best results of phytotoxicity tests with Sinapis alba were obtained for soils containing no more than 1.0 wt% ash with calcium hydroxide.
1. Introduction
Calcium compounds such as calcium hydroxide (Ca(OH)2), calcium oxide (CaO), and calcium carbonate (CaCO3) are traditionally used for enhanced plant growth in agriculture and land rehabilitation [1]. The addition of calcium-based chemical agents is a common practice in the remediation of acidic mine spoils to increase the soil pH [2,3] and reduce the level of acid mine drainage [4]. An increase in soil pH following the addition of calcium compounds has a positive effect on reducing the mobility of heavy metals, especially zinc (Zn), lead (Pb), chromium (Cr), and cadmium (Cd), in soils [5,6]. It also reduces the mobility of toxic aluminum (Al) to benefit aquatic and terrestrial ecosystems [7].
The application of poultry manure as an organic fertilizer in industrial degraded land is useful in terms of supplying essential plant nutrients, i.e., nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg) [8,9]. However, poultry manure is contaminated with numerous pathogenic microorganisms, including bacteria, fungi, viruses, and helminths, which exhibit a negative impact on both human health and the environment [10,11]. According to the Polish Ministry of Agriculture and Rural Development [12] and European Union regulations [13], the total level of Enterobacteriaceae or Escherichia coli in representative samples of organic fertilizers must not exceed 1000 CFU/g, whereas the threshold value for the number of Salmonella bacteria is zero. Nevertheless, the total number of zoonotic bacteria in raw poultry manure may exceed the permissible level, and the number of E. coli strains ranges from 105 to 108 CFU/g [14,15].
Diseases associated with animal waste contact remain a significant health problem [16,17]. According to the Polish National Institute of Public Health [18], a few thousand cases of diseases caused by zoonotic pathogens, which occur in poultry manure, are diagnosed in Poland each year. There are still many cases of infection with dangerous strains of Escherichia coli, including the verotoxigenic bacteria O157:H7 (A04.3), the primary toxic pathogen for public health safety [19]. The spread of biological contaminants into the environment can occur during storage, excessive land application, or even the transport of off-farm animal manure [20].
Treating poultry manure with calcium compounds may reduce the levels of enteric bacteria and parasite eggs to an acceptable level, thereby decreasing the risk of contracting human diseases caused by zoonotic pathogens. According to the literature [21], chemical stabilization should be performed for a few hours until the pH is at least 12 and the temperature is between 55 °C and 70 °C. Notably, during this process, significant amounts of harmful gases, such as ammonia (NH3) or hydrogen sulfide (H2S), are released into the environment [22]. In addition to chemical stabilization, several other methods are used to reduce pathogens in poultry manure, including composting, anaerobic digestion, and pasteurization [23,24]. However, the best method to completely deactivate pathogens contained in organic waste is through incineration. The chemical composition of the ash obtained from poultry manure, i.e., its high nutrient content, especially phosphorus and potassium, makes it possible to use it as a fertilizer. Research shows that the contents of P2O5 and K2O in poultry litter ash obtained in fluidized bed reactors range from 13.75 wt% to 25.20 wt% and 15.06 wt% to 20.49 wt%, respectively [25,26]. For comparison, the quantity of P2O5 in phosphorous raw materials located in Morocco, Tunisia, and the United States ranges between 30.69 wt% and 39.40 wt% [27]. Since the consumption of natural phosphate rocks is constantly decreasing, it is reasonable to obtain phosphorus from another source. Poultry manure incineration is used as a method of waste management; however, to ensure the efficiency of the process and decrease the cost of energy, excess moisture must be removed from the waste. Many experiments have demonstrated that the moisture content of raw poultry manure ranges between 70% and 75% [9,28,29].
When burning biomass, which often exhibits a higher moisture content and different physical properties than coal, adding Ca(OH)2 can enhance the combustion process by affecting the calorific value of the mixture. Therefore, treating organic waste with calcium compounds not only reduces the risk of pathogen transmission but also removes excess moisture from the waste before incineration. Studies on the co-incineration of animal manure with calcium compounds are not well known. Nevertheless, laboratory tests confirmed that the addition of CaCO3 to animal manure at 7.5 wt% improves the combustion process, reduces the degree of graphitization and increases the specific surface area of the resulting ash [30].
Other studies have shown that the combustion of coal and biomass fuels with the addition of Ca(OH)2 is a promising approach for reducing carbon dioxide (CO2) emissions into the atmosphere [31]. Carbon dioxide generated during organic material combustion is bound by Ca(OH)2 to form CaCO3.
The blending of ash from poultry litter incineration with inactive ingredients such as granular lime, calcic limestone, or gypsum from the desulfurization process has become the subject of other investigations [32,33]. Research carried out by Bauer et al. [32] revealed that adding calcic lime or gypsum to poultry litter ash did not negatively affect plant growth, soil-extractable P and K, or the uptake of these macronutrients by plants.
This study presents a novel approach to understanding the impact of calcium compounds (e.g., Ca(OH)2) on the incineration of poultry manure, addressing a gap in the literature. The authors hypothesized that poultry manure after biological deactivation and incineration can enhance the quality of degraded soils.
The main goals of our research were as follows: (i) to optimize the biological inactivation of poultry manure using calcium hydroxide (Ca(OH)2) via central composite design (CCD) and response surface methodology (RSM); (ii) to investigate the properties of poultry manure ash (PMA) after incineration at 500 °C, particularly its chemical composition and potential as a fertilizer for degraded soils; and (iii) to evaluate the phytotoxicity of PMA and PMACa (PMA with Ca(OH)2) using Sinapis alba as a test plant.
The results were intended to facilitate the better utilization of livestock waste as an environmentally friendly fertilizer that is safe for human health and suitable for reclaiming areas degraded by industrial activity. Our findings could benefit degraded land rehabilitation and contribute to the existing state of knowledge on the subject.
2. Materials and Methods
2.1. Organic Waste, Soil, and Chemical Reagents
The organic material used as a poultry manure (PM) sample was obtained from laying hens and delivered by a Polish chicken farm in the Silesia region during the summer season (50°11′ N, 18°73′ E). The samples were randomly collected from several locations within the fresh PM piles in a manner representative of the entire test sample and then mixed to obtain a pooled sample. Finally, the average weight of the 1 kg laboratory sample was transported to the laboratory and stored at 4–5 °C for 24 h until microbial analysis. The total moisture content (after drying at 105 °C) of the PM sample was 78.97%. The calculated C/N ratio was 6:1, which indicated that the PM was rich in nitrogen and may be available for plant uptake.
Soil samples were collected from two heavy-metal-contaminated sites in Poland, i.e., the zinc smelting plant “Miasteczko Śląskie” (S1) and the nonferrous metal plant “Szopienice” (S2). The physicochemical analysis results obtained for the poultry manure and soils used in this study were investigated in our previous work [34].
An analysis of the conducted tests indicated that the test soils S1 and S2 were characterized by low levels of K (7.2 and 4.8 g/kg), P (0.3 and 0.2 g/kg), Ca (1.6 and 0.6 g/kg), Mg (0.7 and 0.2 g/kg), and S (0.3 g/kg), which are essential plant nutrients. The contents of these elements in the PM sample were 56.7 g/kg for N, 20.1 g/kg for P, 23.6 g/kg for K, 24.0 g/kg for Ca, and 6.2 g/kg for S. In both the tested soil types, the concentrations of Zn (6920 and 746 mg/kg), Pb (942 and 387 mg/kg), Cd (35 and 12 mg/kg), and Cu (42 and 17 mg/kg) exceeded the permissible limits in soils classified under Group III (woodland and shrubland as well as green woodland areas) and Group IV (industrial land and mining grounds) as per the Polish Regulation on Soil Quality and Pollution Standards [35]. A chemical analysis of soils S1 and S2 revealed the presence of Cr (12.1 and 13.1 mg/kg, respectively), Co (3 and 5 mg/kg), and Ni (10. 1 and 7.0 mg/kg).
Additionally, technical grade 92% calcium hydroxide delivered by Brenntag Poland Ltd. was used for the chemical stabilization of the PM.
2.2. Microbial Analysis
The enumeration of E. coli was performed in accordance with standard ISO 6887-1: 2017-05 by adding 0.1 mL of the decimal solutions (from 10−1 to 10−7) to Petri dishes with Endo medium (BTL Ltd., Łódź, Poland) [36]. After incubation in Petri dishes at 37 ± 1 °C for 24 ± 2 h, the circular, black, and metallic sheen colonies of E. coli were counted. The analysis was performed in triplicate, and the mean value of the obtained results was expressed as log colony-forming units per gram (CFU/g) of poultry manure sample.
Microbiological analysis of fresh PM delivered to the laboratory revealed that the number of Escherichia coli bacteria was 5.8 × 108 CFU/g, which exceeded the permissible level (≤103 CFU/g) [12,13]. Neither Salmonella bacteria nor other harmful pathogens were observed in the tested samples.
2.3. Preparation of Poultry Manure Ash
Before incineration, both the raw PM sample and the PM with Ca(OH)2 were air dried (22 ± 1 °C) for 209 h. The moisture contents of the samples, which were determined according to the standard ISO 18134-2: 2017-03 [37], were 64.8% and 66.8%, respectively. Each sample was subsequently crushed and passed through a 2 mm sieve. The amount of Ca(OH)2 (5.0 wt%) and time (209 h) corresponded to the dose and contact time necessary to reduce the number of pathogens in fresh PM to below the level of 1000 CFU/g and were estimated via response surface methodology (Section 3).
The incineration process was carried out in an HT 16/16 high-temperature chamber furnace with a P310 controller (Nabertherm GmbH, Lilienthal, Germany). The raw poultry manure samples and poultry manure stabilized with calcium hydroxide were both burned at 500 ± 1 °C at a heating rate of 10 °C/min for 5 h. Before incineration, the samples were heated at 140 °C for 30 min to completely remove residual moisture.
2.4. Physicochemical, Mineralogical, and Microscopic Analyses of Ash
The pH and electrical conductivity (EC) values were measured in distilled water with a pH meter (CPC-411, Elmetron, Zabrze, Poland) with a combination electrode (IJ44AT, Elmetron, Zabrze, Poland) under standards ISO 10390: 2021 [38] and ISO 11265: 1994 [39], respectively.
The quantitative phase composition of the ash samples was analyzed via X-ray powder diffraction (XRD) in Bragg–Brentano geometry by means of a D8 DISCOVER diffractometer (Bruker, Billerica, MA, USA), using standard PN-EN 15309: 2010 [40].
The ash grain surface morphology and chemical composition in the microareas were analyzed by scanning electron microscopy (SEM) and X-ray energy dispersion spectroscopy (EDS) by means of an SU3500 SEM (Hitachi, Tokyo, Japan) operating in conjunction with an UltraDry EDS detector (Thermo Fisher Scientific, Waltham, MA, USA) under the following conditions: acceleration voltage—15 keV, detector—BSE, scanning time—60 s, and magnification in the range of ×500 to ×2500.
The ash grain size and shape were analyzed via the optical method with image analysis by means of a G3S-ID analyzer (Malvern Panalytical, GB, Malvern, UK) under the following conditions: dispersion medium—air, lenses—×20 and ×5, light—diascopic, and automatically calibrated.
The content of bioavailable ions in the fertilizers was estimated via the extraction of ash samples in 2% citric acid at a ratio of 1:100 (g/mL). The suspension was stirred for 30 min in a rotary mixer at a speed of 80 rpm (Vortex Classic, Velp Scientifica, Usmate Velate MB, Italy). After filtration with MCE membrane filters (pore size 0.45 μm; Whatman, Maidstone, UK), the concentrations of nutrients and trace elements (Al, Ba, B, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, MN, Na, Ni, Pb, Sr, S, and Zn) in the 2% citric acid solution were assayed via ICP—OES (Perkin Elmer Optima 5300, Perkin Elmer Inc., Waltham, MA, USA) in accordance with standard ISO 11885: 2007 [41].
The analysis was carried out at the Department of Environmental Monitoring (GIG-PIB).
2.5. Response Surface Methodology
Central composite design with response surface methodology (CCD/RSM) was applied to determine the optimal conditions for the microbial inactivation of livestock waste via Statistica 13.3 (Tibco Software Inc., Palo Alto, CA, USA). To optimize the poultry manure deactivation process, three independent factors were selected: (X1) Ca(OH)2 concentration, (X2) temperature, and (X3) contact time. All selected factors were included at three coded levels (−1, 0, and +1), representing the values for x1 (3.0; 5.0; and 7.0 wt %), x2 (12; 22; and 32°C) and x3 (48; 108; and 168 h), respectively. After a rotatability coefficient α = 1.68 was adopted, the ranges of the final variables (−α, 0, and +α) were calculated as follows: x1 (1.6; 5.0; and 8.4 wt%), x2 (5.2; 22.0; and 38.8 h), and x3 (7.1; 108.0; and 208.9 °C). The number of E. coli (denoted as Y and calculated as log CFU/g) was selected in response to the system.
A mathematical model was developed to predict the number of E. coli (Y) as a function of the Ca(OH)2 concentration, temperature, and time. The obtained data were analyzed via multiple linear regression with analysis of variance (ANOVA) (p < 0.05). To describe the mathematical model of the response function, the following quadratic Equation (1) was applied:
where β0, β1, …. β9 represent the estimated regression coefficients, i.e., constant (β0), linear (β1–β3), quadratic (β4–β6), and interaction effects (β7–β9); ε is a random experimental error of the normal distribution; and x1, x2, and x3 are the independent variables, i.e., the Ca(OH)2 concentration (wt%), temperature (°C), and contact time (h).
The CCD plan included 16 experimental trials with two trials in two replications at the central point, as presented in Table 1 (Section 3).

Table 1.
Experiment plan and results of CCD/RSM analysis.
2.6. Phytotoxicity Tests
The phytotoxicity test was carried out according to the standard method ISO 18763: 2016 [42]. The obtained samples of poultry manure ash (PMA) and poultry manure ash with calcium hydroxide (PMACa) were tested as fertilizers for contaminated soils (S1 and S2) at doses of 0.25%, 0.50%, 1.00%, 2.00%, and 3.00%, respectively. A 50.000 ± 0.001 g blend of soil and poultry manure ash was placed in a sterile Petri dish (90 mm diameter) and mixed with distilled water. Afterwards, 15 seeds of white mustard (Sinapis alba L.) were placed on filter paper (grade 292, Munktell, Ahlstrom Munksjö, Finland) close to the lid. All the samples were prepared in triplicate. Contaminated soil without ash was used as the control sample. The test plates with the seeds were incubated for 72 h at 25 ± 1 °C, and the length of the Sinapis alba roots was measured after incubation.
The statistical analysis of the experimental data was performed via Statistica 13.3 (StatsSoft, Kraków, Poland). The differences between multiple groups were compared via one-way analysis of variance (ANOVA) and post hoc Tukey’s honestly significant difference (HSD) tests.
3. Results and Discussion
3.1. Deactivation Model with CCD/RSM
The results of the 16 experiments performed with different Ca(OH)2 concentrations, temperatures, and contact times are presented in Table 1.
The experimental results revealed that adding Ca(OH)2 to a fresh sample of PM at doses ranging from 1.6 to 8.5% reduced the number of E. coli by 12.8 to 77.2%, respectively. However, the important factors are the time of exposure and the temperature. The data analysis revealed that the lowest number of E. coli bacteria (2 log CFU/g) was obtained for experiments no. 6, 8, and 10, with doses of 7.0 and 8.4 wt% Ca(OH)2. The tests where the number of bacteria was 2 log CFU/g were carried out at 12–32 °C for 108 and 168 h. Additionally, an acceptable level of E. coli was obtained in experiments no. 7, 12, and 14. A lower dose of Ca(OH)2 (3.0 wt%) and a short contact time (48 h) resulted in the lowest reduction in pathogens (7.6 log CFU/g). For the experiments performed in the center of the plan, with the same values of the input parameters (5.0 wt%, 22 °C, and 108 h), similar numbers of E. coli (4.70 log CFU/g for 15(C) and 4.63 log CFU/g for 16(C)) were observed.
The results revealed that a minimum concentration of 5 wt% Ca(OH)2 in poultry manure is crucial for effectively decreasing the number of E. coli bacteria to the maximum acceptable value of 3.0 log CFU/g. Extending the contact time from 108 to 209 h while maintaining a lower temperature (22 °C) can be more economical. Raising the temperature to 38.8 °C requires additional energy, which increases operating costs, including those related to the heating equipment and precise thermal control.
The ANOVA results after excluding the statistically insignificant linear (L) interaction effects of the Ca(OH)2 concentration relative to time (p = 0.5386) and temperature relative to time (p = 0.1010) are presented in Table 2. The calculated values of the coefficients R2 (98%) and Radj.2 (97%) suggest that the determined regression plane exhibited a good fit to the experimental data. The statistical analysis revealed four statistically significant parameters, i.e., the Ca(OH)2 concentration (L), temperature (L), temperature (Q), time (L), and one interaction of Ca(OH)2 concentration (L) relative to temperature (L). The Ca(OH)2 concentrations (Q) and time (Q) were statistically insignificant, with p values higher than 0.05. The results of the ANOVA revealed that the mean squared error (MS) was 0.0928.

Table 2.
Analysis of the E. coli deactivation model with CCD/RSM via ANOVA model coefficients.
Verification of the experimental data relative to the designed deactivation process model via a Pareto chart is presented graphically in Figure 1.
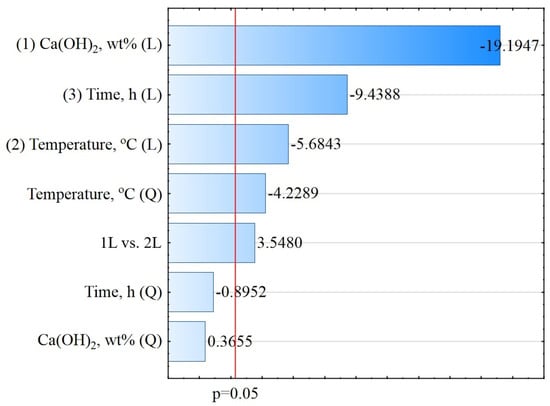
Figure 1.
Pareto chart for the absolute value of the standardized assessment concerning the effects on E. coli, log CFU/g.
The lengths of the horizontal bars represent the estimated standardized effect values from the highest to the lowest. The vertical line shows the absolute value of the standardized effect (p = 0.05).
The results of the model adequacy verification via an ANOVA test after excluding insignificant linear–linear interaction effects are presented in Table 3.

Table 3.
Analysis of variance (ANOVA) for the E. coli deactivation model with CCD/RSM.
A plot of the relationships between the estimated values and the observed values was constructed to verify the quality of the fit between the experimental data and the mathematical model.
Figure 2 shows that all the points denoting the experimental data are close to the red line, which represents the approximated values. This indicates a good fit between the developed model and the obtained experimental results. The response surface plots describing the influence of the Ca(OH)2 concentration, process temperature, and contact time on the number of E. coli bacteria are presented in Figure 3. The shapes of the prepared 3D plots revealed the influence of the discussed factors on the number of pathogens identified in the poultry manure samples.
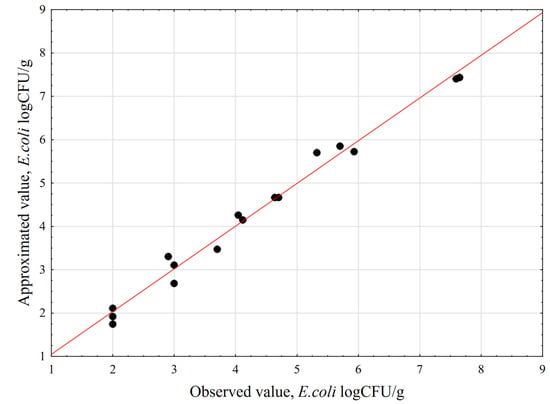
Figure 2.
Correlations between the estimated and observed values of E. coli, log CFU/g.
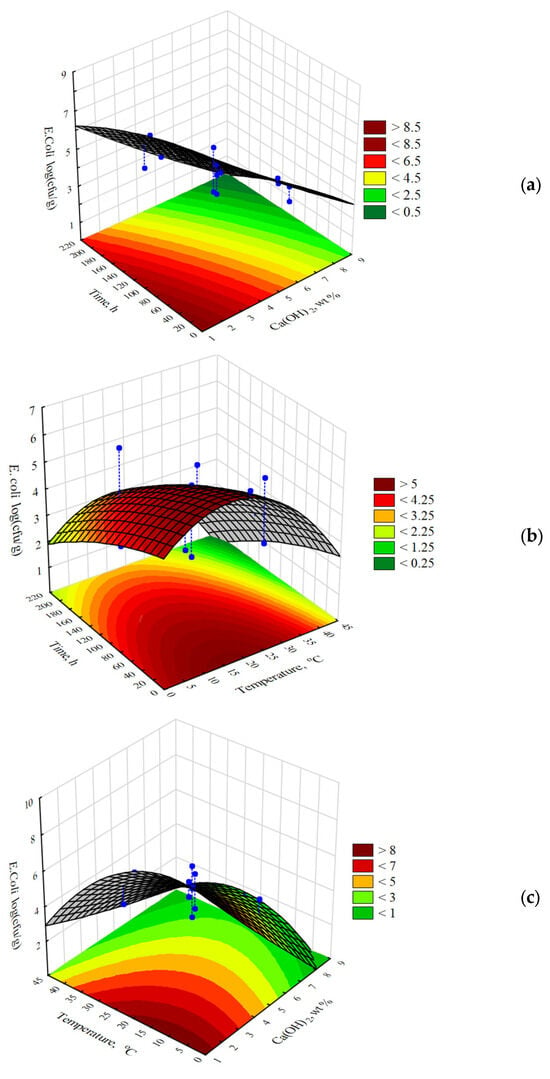
Figure 3.
Three-dimensional surface response plots showing the influence of (a) Ca(OH)2 concentration and exposure time, (b) temperature and exposure time, and (c) Ca(OH)2 concentration and temperature on the number of E. coli bacteria in poultry manure. The color scale from green to dark red indicates an increasing bacterial growth response.
The analysis revealed that the addition of 3.0 wt% Ca(OH)2, a stabilizing agent, to PM had a slight biocidal effect on the number of E. coli (4.0 log CFU/g). According to the literature, the concentration of CaO or Ca(OH)2 used for stabilizing solid manure is approximately 3 wt% [23]. However, this dose may not be sufficient for the complete deactivation of zoonotic bacteria and parasites. A reduction in the number of microorganisms below the acceptable threshold (3.0 log CFU/g) was observed when the Ca(OH)2 concentration was equal to or greater than 5.0 wt%, and the contact time exceeded 200 h. The addition of Ca(OH)2 to fresh PM at concentrations between 6.0 wt% and 7.0 wt% led to a reduction in E. coli to 4.5 log CFU/g after 40 h and to 3.0 log CFU/g after 100 h of exposure. The highest bacterial elimination, to a level below the acceptable threshold, was achieved in samples with Ca(OH)2 concentrations above 8.0 wt% after 180 h.
Equation (2) presents the complete equation of the model as a polynomial, which describes the change in the number of E. coli bacteria (Y) depending on the change in the input values, i.e., x1, x2, and x3.
This polynomial equation included linear, quadratic, and interaction terms. The negative coefficients for the Ca(OH)2 concentration (x1) and contact time (x3) suggest that increasing these factors by one unit decreases the inactivation efficiency of E. coli by 1.3594 and 0.0169 units, respectively. However, an increase in temperature (x2) positively reduces the number of bacteria, with a rise of 0.0106 units. The quadratic terms for x1 and x2 show that the effects of concentration and temperature are not linear, with diminishing returns at higher levels. On the basis of this model, the optimal conditions were 5 wt% Ca(OH)2, 22 °C, and 209 h, where the predicted inactivation efficiency was maximized. A literature review revealed that studies have investigated the antimicrobial effectiveness of chemical compounds, time, pH, or temperature via the RSM/CCD method. Iliadis et al. [43] presented the combined effects of temperature, pH, and sodium chloride on biofilm formation by Salmonella enterica, with an adjusted determination coefficient R2 ≥ 0.96. The impacts of temperature (15–35 °C), treatment time (1–5 min), and chlorine concentration of electrolyzed oxidizing water (30–70 mg/kg) on the reduction in Listeria monocytogenes in lettuce were investigated in China (Radj.2 ≥ 0.95) [44]. Similar results have been reported by Bidlas et al. [45]. They inhibited the growth of three species of pathogens via the use of sodium and potassium chloride. The use of E. coli in the biodegradation process for the removal of two dyes from contaminated water was the aim of a study by M-Ridha et al. [46], resulting in Radj.2 values ranging from 0.93 to 0.98. Our results (Radj.2 > 0.97) confirm that the use of RSM/CCD to analyze the effect of E. coli deactivation in poultry manure is highly effective and demonstrates the significance of the applied method.
3.2. Ash Sample Property Determination via XRD Analysis
The determination of the crystalline components in the ash samples with (PMA) and without the addition of 5.0 wt% calcium hydroxide (PMACa) is presented in Table 4.

Table 4.
Phase composition of poultry manure ash as determined via XRD.
The X-ray diffraction analysis revealed that K2SO4 and CaCO3 were the main crystalline components in the ash samples incinerated at 500 °C. However, the PMACa sample contained four times more CaCO3 than the PMA sample. Calcium carbonate and calcitic limestone (which contain between 80% and 90% CaCO3), inactive ingredients similar to sand, sawdust, or gypsum, are used as filler materials for organic fertilizers. The presence of CaCO3 enhances the shear strength of soil and decreases its porosity, which is advantageous for sandy soils [47]. The application of CaCO3 can optimize soil pH [48] by neutralizing soil acidity, especially in areas degraded by industry. Furthermore, CaCO3 strengthens soil aggregate stability, encourages organic matter accumulation, and regulates microbial communities. Studies show that limestone enhances the adsorption of organic compounds, thereby increasing the organic matter content in soil and improving soil fertility [49]. Calcium is an essential nutrient for plant growth and plays a crucial role in promoting plant development, building plant cell walls, facilitating photosynthesis, and enhancing resistance to environmental stress [50]. However, an excess amount of soluble Ca2+ ions in soil–water solutions promotes the fixation of available phosphate ions such as PO43−, HPO43− and H2PO4−, which are strongly likely to be consolidated as apatite forms Ca5(PO4)3(OH, F, Cl). For this reason, the increased availability of nutrients, such as nitrogen, phosphorus, and potassium, can be observed in soils enriched with insoluble calcium carbonate [32]. The analysis revealed three sources of phosphorus in the PMA sample: whitlockite (11.0 wt%), apatite (5.0 wt%), and hydroxyapatite (5.0 wt%). Our previous study [51] confirmed that the bioavailable form of P in poultry manure ash is the amorphous phase (24.5 wt%), which could also serve as a source of phosphorus. Apatite is one of the primary phosphate rock compounds used to produce phosphate fertilizer in the form of phosphoric acid (H3PO4) [52]. Furthermore, hydroxyapatite is considered a green fertilizer that is suitable for application in soils with low or acidic pH values, and it is effective at increasing plant biomass, even by 30% [53]. According to the phase composition, no whitlockite was observed in the PMACa sample. The sources of phosphorus in the PMACa sample were hydroxyapatite (3.0 wt%) and apatite (3.0 wt%). The amorphous phase content in this ash was 15.5 wt%. Ca(OH)2, which is added during the incineration process, may inhibit the crystallization of whitlockite and promote the formation of an amorphous phosphorus-containing phase, particularly under high pH conditions and an improper Ca/P ratio. After the ballast of calcite (53 wt%, Table 4) formed during the incineration of poultry manure with the addition of calcium hydroxide was subtracted, the amorphous phase content of this ash reached 33 wt%, which supports the above hypothesis. The XRD patterns of the final products are presented in Figure 4.
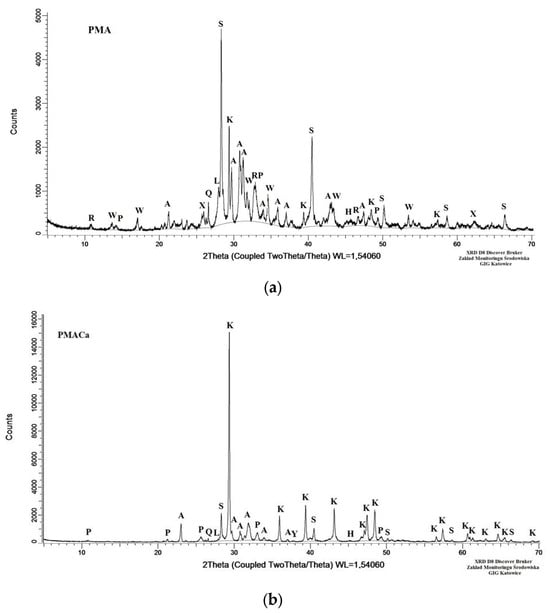
Figure 4.
X-ray diffractogram of poultry manure ash: (a) PMA sample and (b) PMACa sample. Explanations of the crystalline phase abbreviations are provided in Table 4.
3.3. Ash Samples Property Determination via Microscopic Analysis
The analyzed ash varied in terms of particle size distribution, shape, and surface morphology (Table 5). The largest grains observed exhibited CE diameters of 229 μm and 95 μm for samples PMA and PMACa, respectively, but, in both samples, grains above 45 μm accounted for approximately 0.1% of the total included grains. Fifty percent of the tested grains were characterized by a CE diameter under 2.1 μm and 2.4 μm for PMA and PMACa, respectively. Ninety percent of the tested grains were characterized by a CE diameter under 6.3 μm and 6.9 μm for PMA and PMACa, respectively.

Table 5.
Number and volume distribution and shape characterization of ash particles.
Large grains (>45 μm) were characterized by irregular shapes in both the samples. The mean circularity was 0.61 and 0.76 for PMA and PMACa, respectively. The grains ranging in size from 1 μm to 10 μm, which constitute the majority of the identified grains, were characterized by mean circularity values of 0.87 and 0.89 for PMA and PMACa, respectively.
The chemical composition of the ash grains in the selected grain microareas is presented in Table 6.

Table 6.
Chemical composition of the ash grains in the selected microareas.
The results indicate that phosphorus was identified in the microareas of all the analyzed grains, with a maximum amount of 13 wt% for PMACa. The maximum Ca/P ratios in the examined microareas were 3.3 and 3.8 for PMA and PMACa, respectively. In addition to calcium, phosphorus is accompanied by metals such as potassium, magnesium, sodium, and aluminum and, in the case of PMA, it is also accompanied by iron. XRD analysis also revealed the presence of an iron phase, iron(II) phosphate, in the PMA sample. Images of the selected ash grains in the ash samples are presented in Figure 5.
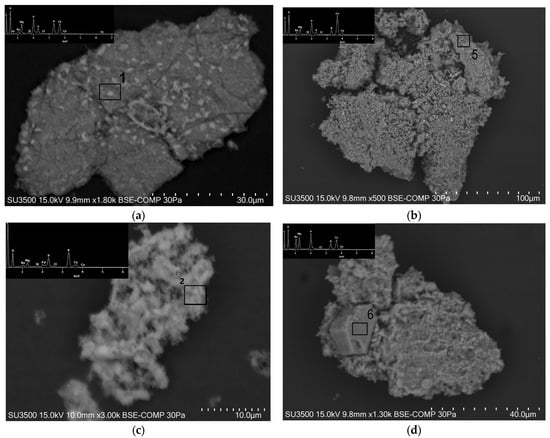
Figure 5.
Images of the selected ash grains: (a,c) sample PMA; (b,d) sample PMACa. Explanation: 1, 2, 5 and 6 are numbers of the microareas subjected to analysis, marked with a black square. The chemical compositions of these microareas are presented in Table 6.
3.4. Characteristics of Poultry Manure Ash and Its Extracts
The parameters of ash obtained after the incineration of poultry manure without (PMA) and with calcium hydroxide (PMACa) at 500 °C for 5 h are presented in Table 7.

Table 7.
Physicochemical parameters of poultry manure ash.
The incineration of poultry manure without and with the addition of 5 wt% Ca(OH)2 yielded ash with efficiencies of 13.58% and 26.15%, respectively. The pure manure ash content (without CaCO3 ballast) in the PMACa was approximately 47.5%.
The PMA sample contained 195 g/kg CaO, 167 g/kg K2O, and 250 g/kg P2O5 [51]. Our results are comparable to those reported by Lynch et al. [25] obtained from the combustion of poultry litter via small-scale fluidized bed combustion technology (224 g/kg CaO, 205 g/kg K2O, and 252 g/kg P2O5). The XRD results revealed that the amounts of Ca, K, and P in the crystalline phase (calculated as oxides) were 191 g/kg, 164 g/kg, and 98 g/kg, respectively. In the case of the PMACa sample, the content of CaO was significantly greater (387 g/kg), whereas the contents of K2O and P2O5 were 79 g/kg and 119 g/kg, respectively, which was associated with the presence of ballast in the form of CaCO3. In the crystalline phase of the PMACa sample, the contents of calcium, potassium and phosphorus (calculated as oxides) were 411 g/kg, 60 g/kg, and 22 g/kg, respectively. The presented calculations demonstrate that the majority of phosphorus—61% in the case of PMA and 82% in the case of PMACa—accumulates in the amorphous phase. Thus, bioavailable phosphorus was found mainly in the amorphous phase, as confirmed by other researchers [54,55].
The results revealed that the pH of the PMACa sample was greater than that of the PMA sample, i.e., 11.6 and 10.4, respectively. However, the determined EC value for the PMA sample (29.8 mS/cm) was two times greater than that of the PMACa sample (16.6 mS/cm). These findings suggest that the addition of Ca(OH)2 to fresh poultry manure results in the formation of insoluble calcium compounds.
Our previous research [51] revealed that poultry manure ash has great potential as a fertilizer because of its high content of valuable nutrients such as CaO, MgO, K2O, and P2O5. The use of ash from poultry litter incineration is applicable in the reclamation of areas degraded by mining activity. This material serves not only as a source of nutrients essential for metabolic processes in plants, such as P and K, but, also, owing to its high pH (10–13), can help mitigate soil acidity caused by the presence of acid mine drainage (AMD) [4].
According to the requirements of the European Union Regulation 2009/1009 [56] and PFC categorization, poultry manure ash can be classified as an inorganic macronutrient fertilizer designated PFC 1(C)(I), which means that the product contains one or more of the primary (N, P, and K) or secondary (Ca, Mg, Na, and S) macronutrients. The permissible levels of toxic elements must not exceed the following limit values: As (40 mg/kg), Cd (60 mg/kg), Cr (2 mg/kg), Ni (100 mg/kg), Pb (120 mg/kg), Cu (600 mg/kg), and Zn (1500 mg/kg). The chemical analysis of the PMA sample revealed no environmental risk associated with the application of ash to the soil. The determined concentrations of As, Co, and Cr were below the detection limits, and the concentrations of Cd, Cu, Ni, and Pb were within permissible thresholds. The exception was the high concentration of Zn (2809 mg/kg) for the PMA sample. However, considering that the concentration of pure ash in the PMACa sample was estimated at 47.5%, it can be assumed that the calculated content of Zn in PMACa (1334 mg/kg) should not exceed the permissible limit (<1500 mg/kg). Owing to the significantly high percentage of Ca in the ash sample with the addition of Ca(OH)2, it can be concluded that the concentrations of all toxic metals are at least two times lower than those in PMA.
The results of the chemical characterization of extracts from poultry manure ash treated with 2% citric acid are presented in Table 8. These findings indicate that poultry manure ash can serve as a valuable source of phosphorus when treated with citric acid, which increases its solubility and bioavailability.

Table 8.
Chemical composition of extracts from poultry manure ash in 2% citric acid.
Phosphorus and potassium are the main macronutrients that may be delivered to plants by poultry manure ash. The results obtained for PMA and PMACa confirmed the findings reported in the literature that, in contrast to P, almost 50% of the total K is soluble in citrate extracts [57].
Solid sample extraction in 2% citric acid is a widely used method for measuring the bioavailable phosphorus content in sewage sludge [58] or ash [26] samples. An analysis revealed that the content of potassium in the extract (1591.37 mg/L for PMA and 660.79 mg/L for PMACa) was approximately 2.2 times greater than that of phosphorus (731.87 mg/L for PMA and 296.33 mg/L for PMACa). The phosphorus concentration in the extract was 2.5 times lower for PMACa than for PMA.
However, the high concentration of K, similar to Na, is responsible for the high EC of the ash and may lead to soil salinity during long-term application. The sample incinerated with Ca(OH)2 was characterized by a lower sodium content (98.9 mg/L) than in PMA (271.4 mg/L).
The total phosphorus contents in the PMA and PMACa samples were 109.1 g/kg and 51.9 g/kg, respectively. The bioavailable phosphorus contents in the PMA and PMACa samples were 73.2 g/kg and 29.6 g/kg, respectively. The bioavailable phosphorus (citric acid soluble) thus accounted for 67% and 57% of the total phosphorus in the PMA and PMACa samples, respectively. On the other hand, the bioavailable calcium in the PMA and PMACa samples accounted for 82% and 96%, respectively. Notably, all the potassium contained in both of the analyzed ash samples was soluble in citric acid.
The concentrations of trace elements such as Cd, Co, Cr, Cu, Ni, Pb, and Zn in the extracts were 3.2, 2.2, 1.3, 2.4, and 2.4 times lower, respectively, for PMACa than for PMA.
The literature suggests that the addition of calcium compounds such as CaCO3 and Ca(OH)2 to soil results in the formation of insoluble calcium phosphate compounds, such as hydroxyapatite (Ca10(PO4)6OH2), which effectively immobilize phosphorus [59]. Moreover, Maguire et al. [60] reported that the treatment of layer manure and broiler litter with 5 wt% Ca(OH)2 reduced the P content in water extracts from 1854 mg/kg to 212 mg/kg and from 2750 mg/kg to 144 mg/kg, respectively. In the water extract, the amount of P extracted from poultry manure ash was lower than citric acid extract and lower than that extracted from a sample of fresh poultry manure. According to Faridullah et al. [61], the water-soluble P in poultry litter ash (600 °C for 2 h) was 44% lower than that in poultry litter samples. Our previous research [51] revealed that the concentration of P in water extracted from a poultry manure ash sample (500 °C for 5 h) was 0.9 mg/L, resulting in less than 90% water-soluble P being removed compared with that from a raw poultry manure sample (310 mg/L) [62]. This could suggest that the use of PMA as a stable mineral fertilizer may slowly release P in the soil as opposed to the application of organic materials.
The amorphous phosphorus phase could offer a controlled release of orthophosphate ions (PO43−) for long-term soil improvement. Research shows that amorphous calcium phosphate, an innovative macronutrient nanofertilizer, reduces nutrient losses and enhances agricultural sustainability [63,64]. Compared to traditional fertilizers, phosphorus is released from the amorphous phase into the soil for weeks or even months. Moreover, amorphous phosphorus is effective over a wide pH range (4–8), making it more suitable for the diverse soil conditions typically found in degraded areas. In contrast, traditional fertilizers are more sensitive to soil pH and may have reduced effectiveness.
The rapid loss of water-soluble P from fresh PM is responsible for the eutrophication of water bodies and requires special control [65]. The high concentration of water-extractable phosphorus in organic fertilizers can influence the risk of phosphorus (P) loss in runoff when they are land-applied. The application of fertilizers in the form of amorphous phosphorus, which is released in a controlled manner, decreases the risk of eutrophication. Consequently, amorphous phosphorus provides a more sustainable and effective solution for restoring degraded land by improving soil fertility.
3.5. Phytotoxicity Tests Analysis
The results of Sinapis alba toxicity tests for evaluating soils amended with both PMA and PMACa in the range of 0.25 wt% to 3.00 wt% are presented in Figure 6 and Figure 7.
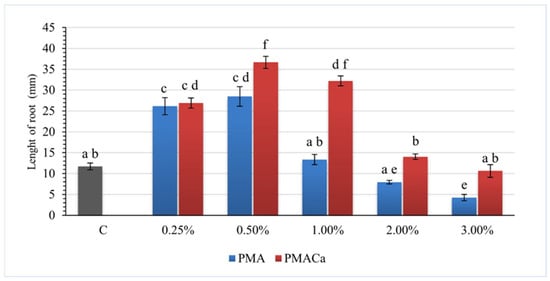
Figure 6.
Sinapis alba root lengths after the addition of poultry manure ash to S1 soil. a–f—Mean values marked with different letters indicate significant differences between the groups according to Tukey’s HSD test (p < 0.05, MS = 46.95, df = 319); C—contaminated S1 soil; bars on the graphs—standard deviation (SD).
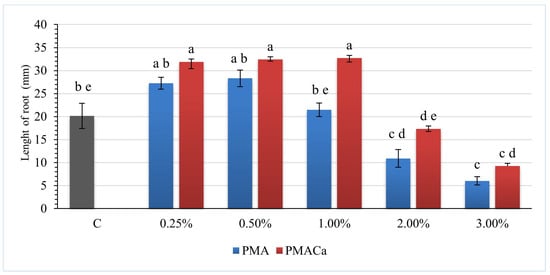
Figure 7.
Sinapis alba root lengths after the addition of poultry manure ash to S2 soil. a–e—Mean values marked with different letters indicate significant differences between the groups according to Tukey’s HSD test (p > 0.05, MS = 99.5, df = 319); C—contaminated S2 soil; bars on the graphs—standard deviation (SD).
The one-way ANOVA results indicated statistically significant (p < 0.01) differences in the growth of Sinapis alba among the different groups. Further post hoc examinations by Tukey’s honestly significant difference (HSD) tests revealed that the average growth rate differed significantly between the groups, highlighting the effect of the ash dose applied on plant root development.
The positive results for the soil samples with the addition of PMA were achieved at 0.25 wt% ash content (26.1 mm for S1 and 27.2 mm for S2) and 0.50 wt% ash content (28.5 mm for S1 and 28.3 mm for S2). The root lengths measured in soils S1 and S2 were 11.7 mm and 20.1 mm, respectively. The addition of an increased amount of PMA at 1.00 wt%, 2.00 wt%, and 3.00 wt% resulted in root growth to 13.3 mm, 7.9 mm, and 4.2 mm, respectively, for S1 and to 21.5 mm, 10.9 mm, and 6.0 mm, respectively, for S2. The root lengths of Sinapis alba grown in soil supplemented with 1.00 wt% PMA were comparable to the lengths in the reference soil. Under these conditions, the phosphorus content in the soil did not affect root growth, whereas increasing the ash dose had an inhibitory effect.
As presented in Figure 8, compared with the addition of PMA, the addition of PMACa, a product of poultry manure incineration with Ca(OH)2, increased the Sinapis alba root length in both the S1 and S2 soils. Positive results were obtained for soils containing 0.25 wt%, 0.50 wt%, and 1.00 wt% ash, i.e., 26.9 mm, 36.6 mm, and 32.2 mm for S1 and 20.1 mm, 31.9 mm, and 32.4 mm for S2, respectively. The soils with PMACa contents of 2.0 wt% and 3.0 wt% were characterized by lower root lengths, ranging between 14.0 mm and 10.6 mm for S1 and between 10.9 mm and 6.0 mm for S2. The root lengths of Sinapis alba grown in soil supplemented with 2.00 wt% PMACa were comparable to the root lengths in the contaminated soils used as the control samples.
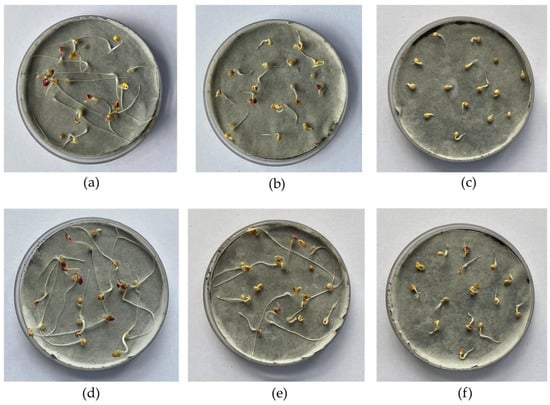
Figure 8.
Photos of Sinapis alba after 72 h of germination in S2 soil treated with poultry manure ash: (a) 0.5 wt% PMA; (b) 1.0 wt% PMA; (c) 3.0 wt% PMA; (d) 0.5 wt% PMACa; (e) 1.0 wt% PMACa; and (f) 3.0 wt% PMACa.
The root length of Sinapis alba grown in soil with the same ash dose was always greater when PMACa was used as an additive. For example, the lengths of the roots grown in soil S2 with the addition of 1.00 wt% ash were 21.5 mm and 32.7 mm for PMA and PMACa, respectively. PMACa contained less bioavailable phosphorus than PMA (see Table 8) but was characterized by a higher content of calcite (see Table 4), which may have a beneficial effect on Sinapis alba growth.
Moreover, the effect of ash addition on the germination of Sinapis alba seeds was inversely proportional to its concentration. In S1 soil, 93% and 100% germination were reached for samples containing 0.25% PMA and PMCa, respectively. However, in samples enriched with 3% PMA or PMACa, germination decreased to 67% and 93%, respectively. The results for S2 soil revealed that germination was 86% and 93% for 0.25% PMA and PMACa, respectively, whereas samples with 3% ash content presented reduced germination to 67% and 80% for PMA and PMACa, respectively.
Figure 8 presents photos of Petri dishes after the germination of Sinapis alba in one tested soil sample.
Extensive literature data show that Sinapis alba is the most widely used testing plant. The toxicity of soils and their components has been tested by measuring seed germination, root and shoot length, and biomass growth [66,67]. It is also used to detect heavy metals in bioremediation [68,69] or phytotoxicity of municipal waste leachate [70]. Cempa et al. [71] evaluated poultry manure products from the perspective of fertilizer use efficiency on anthropogenic land. The authors tested ash obtained from poultry manure incineration as well as a phosphate concentrate separated from the ash via dry sieving. Cassette biotests with Sinapis alba were also used to optimize the dose. In field cultivation, the yield of the obtained phosphorus concentrate at a dose of 95 g/m3 was comparable to that of commercial fertilizer at a dose of 75 g/m3. Unprocessed ash had to be used in larger amounts, i.e., 165 g/m3, to achieve a yield comparable to that of a commercial fertilizer [72]. However, the use of fertilizers in the form of ash exhibits another aspect. Poultry manure combustion is used as a means of energy production, providing a more environmentally friendly alternative to the burning of fossil fuels. The presence of Ca(OH)2 in the burned waste acts as a filter for CO2. The reaction that occurs during the burning of organic material with calcium-based additions such as Ca(OH)2 is presented in Equation (3):
The predominant product of the carbonation process reaction is calcium carbonate, as presented in Equation (4):
Zhang et al. [73] conducted studies on the CO2 absorption capacity of Ca(OH)2 particles. The Ca(OH)2 nanosuspension was more than eight times greater than that of the regular 2 µm particles. In addition to CO2 removal, nano-Ca(OH)2 also exhibits high potential for treating acidic gases such as hydrochloric acid (HCl), sulfur oxides (SOx), or nitrogen oxides (NOx) to improve air quality. This contributes to lowering the overall carbon footprint of the process, making it a more sustainable option for soil restoration.
Moreover, the addition of calcium compounds to organic material before the incineration process may increase the specific surface area of the ash [30], which can enhance the immobilization of heavy metals by promoting better absorption. However, additional research is still needed to confirm this. Notably, the impact on soil microbial communities can be both positive and negative. The addition of PMACa to soil provides essential nutrients that stimulate microbial activity, but high pH may adversely affect specific microbial populations, thereby influencing soil properties.
On the basis of a review of the relevant literature, poultry manure ash has been identified as a promising material for use in building materials or cement production because of its substantial content of Ca, Si, Mg, Fe, and Al [74]. The elevated concentration of Ca in PMACa may offer particular advantages for applications within the construction sector. Nevertheless, it is essential to note that the suitability of this type of material requires further comprehensive experimental studies to confirm these potential benefits.
4. Conclusions
The long-term storage of poultry manure poses a risk to human and animal health and leads to potential microbiological and chemical contamination of the environment. This study investigated the chemical deactivation of this waste via the addition of 5% Ca(OH)2, which makes it possible to decrease the number of pathogenic microorganisms to below the acceptable threshold. The optimization of the biological deactivation of fresh poultry manure was carried out via a central composite design for response surface methodology. The combustion of raw poultry manure and poultry manure with Ca(OH)2 resulted in ash with efficiencies of 13.6% (PMA) and 26.1% (PMACa), respectively.
The addition of Ca(OH)2 to poultry manure likely inhibited the crystallization of whitlockite and led to the formation of a phosphorus-rich amorphous phase after the incineration of this mixture at 500 °C. In the PMA, the main source of phosphorus was the crystalline phase (24.0% phosphorus-containing minerals). In the PMACa, the phosphorus-containing crystalline phase (apatite and hydroxyapatite) constituted 6% of the content, whereas whitlockite was absent. Therefore, the main source of phosphorus in this ash was the amorphous phase. The concentrations of trace elements such as Cd, Co, Cr, Cu, Ni, Pb, and Zn in the extracts (2% citric acid) from the obtained ash ranged from approximately 1.3 to 3.2 times lower for PMACa than for PMA. The phosphorus concentration in these extracts was approximately 2.5 times lower for PMACa than for PMA.
The ashes were applied as soil amendments in industrial areas. The root length of Sinapis alba grown in soil with the same ash dose was always greater when PMACa rather than PMA was used as an additive. On the basis of these studies, the optimal fertilizer additive was ash from the incineration of poultry manure in the presence of Ca(OH)2 at a dose of 0.50%.
Careful management is needed to ensure that the benefits of using the ash as a fertilizer are employed without adversely affecting soil biodiversity, microbial communities, and long-term fertility. One limitation of this study is the lack of long-term field experiments, which would provide more comprehensive results about the effects that PMACa could have on soil properties and plant growth over time. The use of ash-based fertilizers as a source of phosphorus is preferable in acidic soils such as coal mining waste dumps. Future studies could consider several pot and field tests that will be conducted using other plant species (including a grass mixture) in degraded soils, along with a technical and eco-nomic analysis. Further research is needed to assess the long-term effects of heavy metal immobilization in contaminated soils. Moreover, investigating the impact of PMACa on the bioaccumulation of heavy metals by plants would be beneficial.
Thus, the presented method of poultry manure disposal, which is safe for storage due to the absence of harmful microbiological contaminants, is a promising approach for producing fertilizers for enriching soils degraded by industrial activity.
Author Contributions
Conceptualization: A.W.-R. and M.C.; Methodology: A.W.-R., M.C. and M.T.; Software statistical analysis: A.W.-R. and M.T.; Validation: M.T. and B.B.; Formal analysis: A.W.-R. and M.C.; Investigation: A.W.-R., M.C. and M.T.; Writing—original draft: A.W.-R. and M.C.; Writing—review and editing: A.W.-R., M.C., M.T. and B.B.; Visualization: A.W.-R. and M.C.; Supervision: B.B.; Project administration: B.B.; Funding acquisition: B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish National Centre for Research and Development (NCBR) (ERA-MIN3/1/9/PHIGO/2022) under the ERA-NET co-fund ERA-MIN 3 under the project PHIGO “Thermal Processing of P-rich ashes aiming for HIGH-GRADE PHOSPHORUS Products”. Project value (cost of tasks carried out by Polish entities): 927,671.90 PLN, including national funding: 911,319.67 PLN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
The authors would like to thank the anonymous reviewers for their helpful comments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PM | poultry manure |
| PMA | poultry manure ash obtained from PM incineration |
| PMACa | poultry manure ash obtained from the incineration of a mixture (PM with 5.0 wt% calcium hydroxide) |
References
- Mays, D.A.; Sistani, K.R.; Soileau, J.M. Lime and Fertilizer Needs for Land Reclamation. Reclam. Drastically Disturb. Lands 2015, 41, 217–240. [Google Scholar] [CrossRef]
- Novak, J.M.; Ippolito, J.A.; Ducey, T.F.; Watts, D.W.; Spokas, K.A.; Trippe, K.M.; Sigua, G.C.; Johnson, M.G. Remediation of an Acidic Mine Spoil: Miscanthus Biochar and Lime Amendment Affects Metal Availability, Plant Growth, and Soil Enzyme Activity. Chemosphere 2018, 205, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and Challenges in the Use of Coal Fly Ash for Soil Improvements—A Review. J. Environ. Manag. 2014, 145, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, Treatment and Case Studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Karalić, K.; Lončarić, Z.; Popović, B.; Zebec, V.; Kerovec, D. Liming Effect on Soil Heavy Metals Availability. Poljoprivreda 2013, 19, 59–64. [Google Scholar]
- Nagiel, A.; Szulc, W. Effect of Liming on Cadmium Immobilization in the Soil and Content in Spring Wheat (Triticum aestivum L.). Soil Sci. Annu. 2020, 71, 93–96. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Burns, D.A.; Riva-Murray, K. A New Look at Liming as an Approach to Accelerate Recovery from Acidic Deposition Effects. Sci. Total Environ. 2016, 562, 35–46. [Google Scholar] [CrossRef]
- Chastain, J.P.; Camberato, J.J.; Skewes, P. Poultry Manure Production and Nutrient Content. In Chapter 3b in: Confined Animal Manure Managers Certification Program Manual B Poultry Version; Clemson University Cooperative Extension Service: Clemson, SC, USA, 2001; Volume 2, pp. 1–17. [Google Scholar]
- Ashworth, A.J.; Chastain, J.P.; Moore, P.A. Nutrient Characteristics of Poultry Manure and Litter. Anim. Manure Prod. Charact. Environ. Concerns Manag. 2020, 67, 63–87. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X. Microbiological Safety of Chicken Litter or Chicken Litter-Based Organic Fertilizers: A Review. Agriculture 2014, 4, 1–29. [Google Scholar] [CrossRef]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Basamba, T.A. How safe is chicken litter for land application as an organic fertilizer? A review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Development. Regulation of 18 June 2008 on the Implementation of Certain Provisions of Fertilizers and Fertilization. J. Law. Pol. 2008, 119, 6515–6520. Available online: https://dziennikustaw.gov.pl/DU/2008/s/119/765 (accessed on 25 May 2025). (In Polish).
- Council of the European Union; European Parliament. Regulation (EC) No 1774/2002 of the European Parliament and of the Council of the European Union of 3th October 2002. Laying down Health Rules Concerning Animal by-Products Not Intended for Human Consumption. In Official Journal of the European Communities; European Union: Brussels, Belgium, 2010. [Google Scholar]
- Park, G.W.; Diez-Gonzalez, F. Utilization of Carbonate and Ammonia-Based Treatments to Eliminate Escherichia coli O157:H7 and Salmonella typhimurium DT104 from Cattle Manure. J. Appl. Microbiol. 2003, 94, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Unc, A.; Goss, M.J. Transport of Bacteria from Manure and Protection of Water Resources. Appl. Soil Ecol. 2004, 25, 1–18. [Google Scholar] [CrossRef]
- Gerba, C.P.; Smith, J.E. Sources of Pathogenic Microorganisms and Their Fate during Land Application of Wastes. J. Environ. Qual. 2005, 34, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef]
- National Institute of Public Health Infectious Diseases and Poisonings in Poland in 2020. Warsaw. 2021. Available online: https://wwwold.pzh.gov.pl/oldpage/epimeld/index_a.html (accessed on 27 November 2024).
- Chekabab, S.M.; Paquin-Veillette, J.; Dozois, C.M.; Harel, J. The Ecological Habitat and Transmission of Escherichia coli O157:H7. FEMS Microbiol. Lett. 2013, 341, 1–12. [Google Scholar] [CrossRef]
- Mawdsley, J.L.; Bardgett, R.D.; Merry, R.J.; Pain, B.F.; Theodorou, M.K. Pathogens in Livestock Waste, Their Potential for Movement through Soil and Environmental Pollution. Appl. Soil Ecol. 1995, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Malej, J. Properties of Sewage Sludge and Selected Methods of Their Neutralization, Processing and Utilization. Annu. Set Environ. Prot. 2000, 2, 69–101. [Google Scholar]
- Baki Unal, H.; Hakan Bayraktar, Ö.; Alkan, I.; Cengiz Akdeniz, R. Evaluation Possibilities of Chicken Manure in Turkey. Agric. Eng. 2015, 2, 5–14. [Google Scholar] [CrossRef]
- Heinonen-Tanski, H.; Mohaibes, M.; Karinen, P.; Koivunen, J. Methods to Reduce Pathogen Microorganisms in Manure. Livest. Sci. 2006, 102, 248–255. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Mamphweli, S.N.; Meyer, E.L.; Makaka, G.; Simon, M.; Okoh, A.I. An Overview of the Control of Bacterial Pathogens in Cattle Manure. Int. J. Environ. Res. Public Health 2016, 13, 843. [Google Scholar] [CrossRef]
- Lynch, D.; Henihan, A.M.; Bowen, B.; Lynch, D.; McDonnell, K.; Kwapinski, W.; Leahy, J.J. Utilization of Poultry Litter as an Energy Feedstock. Biomass Bioenergy 2013, 49, 197–204. [Google Scholar] [CrossRef]
- Luyckx, L.; de Leeuw, G.H.J.; Van Caneghem, J. Characterization of Poultry Litter Ash in View of Its Valorization. Waste Biomass Valorization 2020, 11, 5333–5348. [Google Scholar] [CrossRef]
- Gorazda, K.; Kowalski, Z.; Nowak, A.K.; Wzorek, Z.; Krupa-Żuczek, K.; Kulczycka, J.; Henclik, A. Wastes. Alternative Raw Materials for Phoshorous Industry. Przem. Chem. 2013, 92, 761–766. [Google Scholar]
- Nicholson, F.A.; Chambers, B.J.; Smith, K.A. Nutrient Composition of Poultry Manures in England and Wales. Bioresour. Technol. 1996, 58, 279–284. [Google Scholar] [CrossRef]
- Quiroga, G.; Castrillón, L.; Fernández-Nava, Y.; Marañón, E. Physico-chemical analysis and calorific values of poultry manure. Waste Manag. 2010, 30, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Yuan, Q.; Cao, H.; Niu, W.; Wang, M.; Zhu, Y.; Yan, S. Effect of Alkali and Alkaline Earth Metal Species on the Combustion Characteristics of Cattle Manures. R. Soc. Chem. Adv. 2018, 8, 11705–11713. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, X.; Fisk, C.; Davies, M.; Wang, Y.; Yang, J.; du Plessis, C.; Cotton, L.; Zhang, Y.; Willmott, J. Combustion Inhibition of Biomass Charcoal Using Slaked Lime and Dolime Slurries. Fire Saf. J. 2023, 140, 103841. [Google Scholar] [CrossRef]
- Bauer, P.J.; Szogi, A.A.; Shumaker, P.D. Fertilizer Efficacy of Poultry Litter Ash Blended with Lime or Gypsum as Fillers. Environments 2019, 6, 50. [Google Scholar] [CrossRef]
- Codling, E.E.; Lewis, J.; Watts, D.B. Broiler Litter Ash and Flue Gas Desulfurization Gypsum Effects on Peanut Yield and Uptake of Nutrients. Commun. Soil Sci. Plant Anal. 2015, 46, 2553–2575. [Google Scholar] [CrossRef]
- Więckol-Ryk, A.; Thomas, M.; Białecka, B. Improving the Properties of Degraded Soils from Industrial Areas by Using Livestock Waste with Calcium Peroxide as a Green Oxidizer. Materials 2021, 14, 3132. [Google Scholar] [CrossRef]
- Regulation of the Minister of Environment on the Manner of Conducting Assessment of Pollution of the Ground Surface. J. Law Repub. Pol. Warsaw, Poland 2016. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001395 (accessed on 10 February 2025).
- ISO 6887-1:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. ISO: Geneva, Switzerland, 2017.
- ISO 18134-2:2017; Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 2: Total Moisture—Simplified Method. ISO: Geneva, Switzerland, 2017.
- ISO 10390:2021; Soil, Treated Biowaste and Sludge—Determination of pH. ISO: Geneva, Switzerland, 2021.
- ISO 11265:1994; Soil Quality—Determination of the Specific Electrical Conductivity. ISO: Geneva, Switzerland, 1994.
- PN-EN 15309: 2010; Characterization of Waste and Soil-Determination of Elemental Composition by X-ray Fluorescence. Polish Committee for Standardization: Warsaw, Poland, 2010.
- ISO 11885:2007; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO: Geneva, Switzerland, 2007.
- ISO 18763: 2016; Soil Quality—Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants. ISO: Geneva, Switzerland, 2016.
- Iliadis, I.; Daskalopoulou, A.; Simões, M.; Giaouris, E. Integrated Combined Effects of Temperature, PH and Sodium Chloride Concentration on Biofilm Formation by Salmonella enterica Ser. Enteritidis and Typhimurium under Low Nutrient Food-Related Conditions. Food Res. Int. 2018, 107, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Dong, Q.L.; Rahman, S.M.E.; Oh, D.H. Response Surface Modeling of Listeria monocytogenes Inactivation on Lettuce Treated with Electrolyzed Oxidizing Water. J. Food Process Eng. 2011, 34, 1729–1745. [Google Scholar] [CrossRef]
- Bidlas, E.; Lambert, R.J.W. Comparing the Antimicrobial Effectiveness of NaCl and KCl with a View to Salt/Sodium Replacement. Int. J. Food Microbiol. 2008, 124, 98–102. [Google Scholar] [CrossRef] [PubMed]
- M-Ridha, M.J.; Hussein, S.I.; Alismaeel, Z.T.; Atiya, M.A.; Aziz, G.M. Biodegradation of Reactive Dyes by Some Bacteria Using Response Surface Methodology as an Optimization Technique. Alex. Eng. J. 2020, 59, 3551–3563. [Google Scholar] [CrossRef]
- Kim, Y.; Roh, Y. Microbial Precipitation of Calcium Carbonate for Crack Healing and Stabilization of Sandy Soils. Appl. Sci. 2024, 14, 1568. [Google Scholar] [CrossRef]
- Pagani, A.; Mallarino, A.P. Change of Soil pH over Time as Affected by Lime Sources and Application Rates. Iowa State Research Farm Progress Reports. 248. 2011. Available online: https://www.iastatedigitalpress.com/farmreports/article/5197/galley/5059/view/ (accessed on 28 November 2024).
- Barreto, M.S.C.; Elzinga, E.J.; Ramlogan, M.; Rouff, A.A.; Alleoni, L.R.F. Calcium Enhances Adsorption and Thermal Stability of Organic Compounds on Soil Minerals. Chem. Geol. 2021, 559, 119804. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, S.; Yang, M.; Hao, Z.; Wang, X.; Shi, Y. Nano Calcium Carbonate Improves Wheat Nitrogen Accumulation and Grain Yield by Enhancing Soil Nitrogen Supply and Flag Leaf Photosynthetic Characteristics. Field Crop. Res. 2024, 310, 109341. [Google Scholar] [CrossRef]
- Więckol-Ryk, A.; Białecka, B.; Cempa, M.; Adamczyk, Z. Optimization of Chicken Manure Combustion Parameters in the Aspect of Phosphorus Recovery. Int. J. Recycl. Org. Waste Agric. 2020, 9, 273–285. [Google Scholar] [CrossRef]
- Rivera, R.M.; Chagnes, A.; Cathelineau, M.; Boiron, M.C. Conditioning of Poultry Manure Ash for Subsequent Phosphorous Separation and Assessment for a Process Design. Sustain. Mater. Technol. 2022, 31, e00377. [Google Scholar] [CrossRef]
- Asiandu, A.P.; Sari, W. The Utilization of Hydroxyapatite as Green Fertilizer Increasing Soil Fertility. Arch. Agric. Res. Technol. 2023, 4, 1–2. [Google Scholar] [CrossRef]
- Fahimi, A.; Bilo, F.; Assi, A.; Dalipi, R.; Federici, S.; Guedes, A.; Valentim, B.; Olgun, H.; Ye, G.; Bialecka, B.; et al. Poultry Litter Ash Characterisation and Recovery. Waste Manag. 2020, 111, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.L. LSU Digital Commons Using Poultry Litter Ash as a Fertilizer Source for Bermudagrass (Cynodon dactylon) Establishment and Loblolly Pine (Pinus taeda) Plantation. LSU Doctoral Dossertations. 5099. Louisiana State University and Agricultural and Mechanical College. 2019. Available online: https://digitalcommons.lsu.edu/gradschool_dissertations/5099 (accessed on 30 March 2025).
- EU Regulation of the European Parliament and of the council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending regulations. (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 170, 1–124. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R1009&qid=1736325125513 (accessed on 30 March 2025).
- Bernal, M.P.; Álvarez-Robles, M.J.; Bernal-Molina, P.; Clemente, R. Bottom Ash from Combustion of Chicken Manure as a Fertilizer Material. Front. Sustain. Food Syst. 2024, 8, 1392445. [Google Scholar] [CrossRef]
- Lee, C.G.; Alvarez, P.J.J.; Kim, H.G.; Jeong, S.; Lee, S.; Lee, K.B.; Lee, S.H.; Choi, J.W. Phosphorous Recovery from Sewage Sludge Using Calcium Silicate Hydrates. Chemosphere 2018, 193, 1087–1093. [Google Scholar] [CrossRef]
- Ann, Y.; Reddy, K.R.; Delfino, J.J. Influence of Chemical Amendments on Phosphorus Immobilization in Soils from a Constructed Wetland. Ecol. Eng. 1999, 14, 157–167. [Google Scholar] [CrossRef]
- Maguire, R.O.; Hesterberg, D.; Gernat, A.; Anderson, K.; Wineland, M.; Grimes, J. Liming Poultry Manures to Decrease Soluble Phosphorus and Suppress the Bacteria Population. J. Environ. Qual. 2006, 35, 849–857. [Google Scholar] [CrossRef]
- Faridullah; Irshad, M.; Yamamoto, S.; Ahmad, Z.; Endo, T.; Honna, T. Extractability and Bioavailability of Phosphorus from Soils Amended with Poultry Litter and Poultry Litter Ash. J. Food Agric. Environ. 2009, 7, 692–697. [Google Scholar]
- Więckol-Ryk, A.; Białecka, B.; Thomas, M. Effect of Green Oxidizing Agent on Inhibition of Escherichia coli Present in Livestock Wastes. Water. Air. Soil Pollut. 2020, 231, 1–16. [Google Scholar] [CrossRef]
- Carmona, F.J.; Guagliardi, A.; Masciocchi, N. Nanosized Calcium Phosphates as Novel Macronutrient Nano-Fertilizers. Nanomaterials 2022, 12, 2709. [Google Scholar] [CrossRef]
- Schaller, J.; Frei, S.; Rohn, L.; Gilfedder, B.S. Amorphous Silica Controls Water Storage Capacity and Phosphorus Mobility in Soils. Front. Environ. Sci. 2020, 8, 94. [Google Scholar] [CrossRef]
- Saleem, A.; Irshad, M.; Ping, A.; Haroon, B. Loss of Phosphorus by Runoff from Soils after Amendment with Poultry Litter Co-Composted with Crop Waste. Int. J. Recycl. Org. Waste Agric. 2018, 7, 211–215. [Google Scholar] [CrossRef]
- Fargašová, A. Phytotoxic Effect of Cd, Zn, Pb, Cu and Fe on Sinapis Alba L. Seedlings and Their Acucumulation in Roots and Shoots. Biol. Plantarium 2001, 44, 471–473. [Google Scholar] [CrossRef]
- Berta, K.M.; Kurdi, R.; Lukács, P.; Penk, M.; Somogyi, V. Red Mud with Other Waste Materials as Artificial Soil Substitute and Its Effect on Sinapis Alba. J. Environ. Manag. 2021, 287, 112311. [Google Scholar] [CrossRef] [PubMed]
- Krasnodębska-Ostręga, B.; Sadowska, M.; Biaduń, E.; Mazur, R.; Kowalska, J. Sinapis Alba as a Useful Plant in Bioremediation–Studies of Defense Mechanisms and Accumulation of As, Tl and PGEs. Int. J. Phytoremediation 2022, 24, 1475–1490. [Google Scholar] [CrossRef]
- Repkina, N.; Nilova, I.; Kaznina, N. Effect of Zinc Excess in Substrate on Physiological Responses of Sinapis alba L. Plants 2023, 12, 211. [Google Scholar] [CrossRef]
- Sindelar, O.; Vaverkova, M.D.; Adamcova, D. Analysis of the Phytotoxic Effect of Leachates from the Landfill of Municipal Waste in Zdounky on Higher Plants. In Proceedings of the MendelNet 2019: 26th International PhD Students Conference, Brno, Czech Republic, 6–7 November 2019; pp. 332–337. [Google Scholar]
- Cempa, M.; Olszewski, P.; Wierzchowski, K.; Kucharski, P.; Białecka, B. Ash from Poultry Manure Incineration as a Substitute for Phosphorus Fertiliser. Materials 2022, 15, 3023. [Google Scholar] [CrossRef]
- Billen, P.; Costa, J.; Van der Aa, L.; Van Caneghem, J.; Vandecasteele, C. Electricity from poultry manure: A cleaner alternative to direct land application. J. Clean. Prod. 2015, 96, 467–475. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Ning, T.; Lal, R. Higher CO2 absorption using a new class of calcium hydroxide (Ca(OH)2) nanoparticles. Environ. Chem. Lett. 2018, 16, 1095–1100. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Mahmud, S.; Tushar, M.; Leon, M. Ash Analysis of Poultry Litter, Willow and Oats for Combustion in Boilers. J. Biomass Biofuel. 2014, 1, 16–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).