Utilization of Poultry Manure After Biological Deactivation and Incineration to Enhance the Quality of Degraded Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Organic Waste, Soil, and Chemical Reagents
2.2. Microbial Analysis
2.3. Preparation of Poultry Manure Ash
2.4. Physicochemical, Mineralogical, and Microscopic Analyses of Ash
2.5. Response Surface Methodology
2.6. Phytotoxicity Tests
3. Results and Discussion
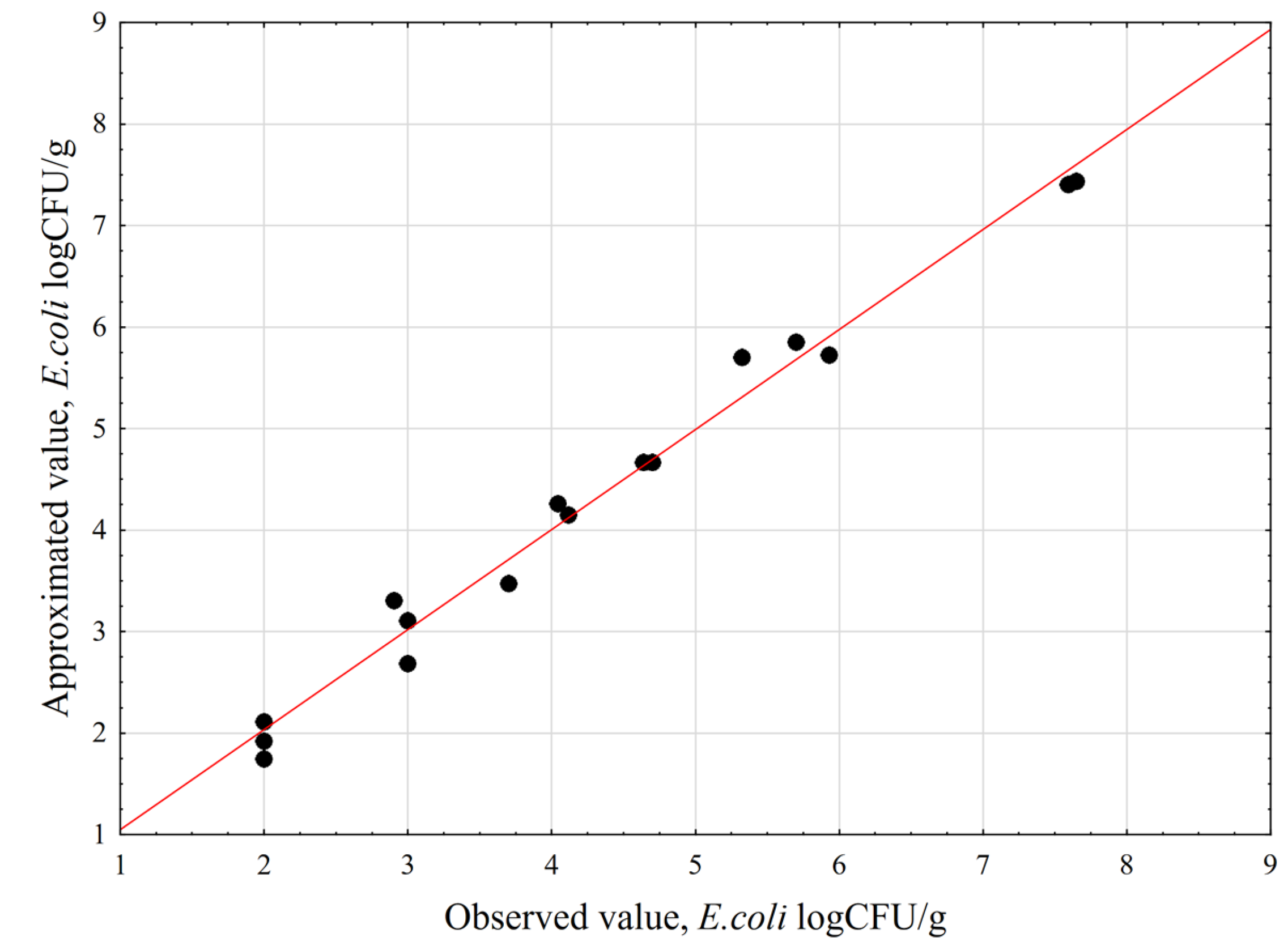
3.1. Deactivation Model with CCD/RSM
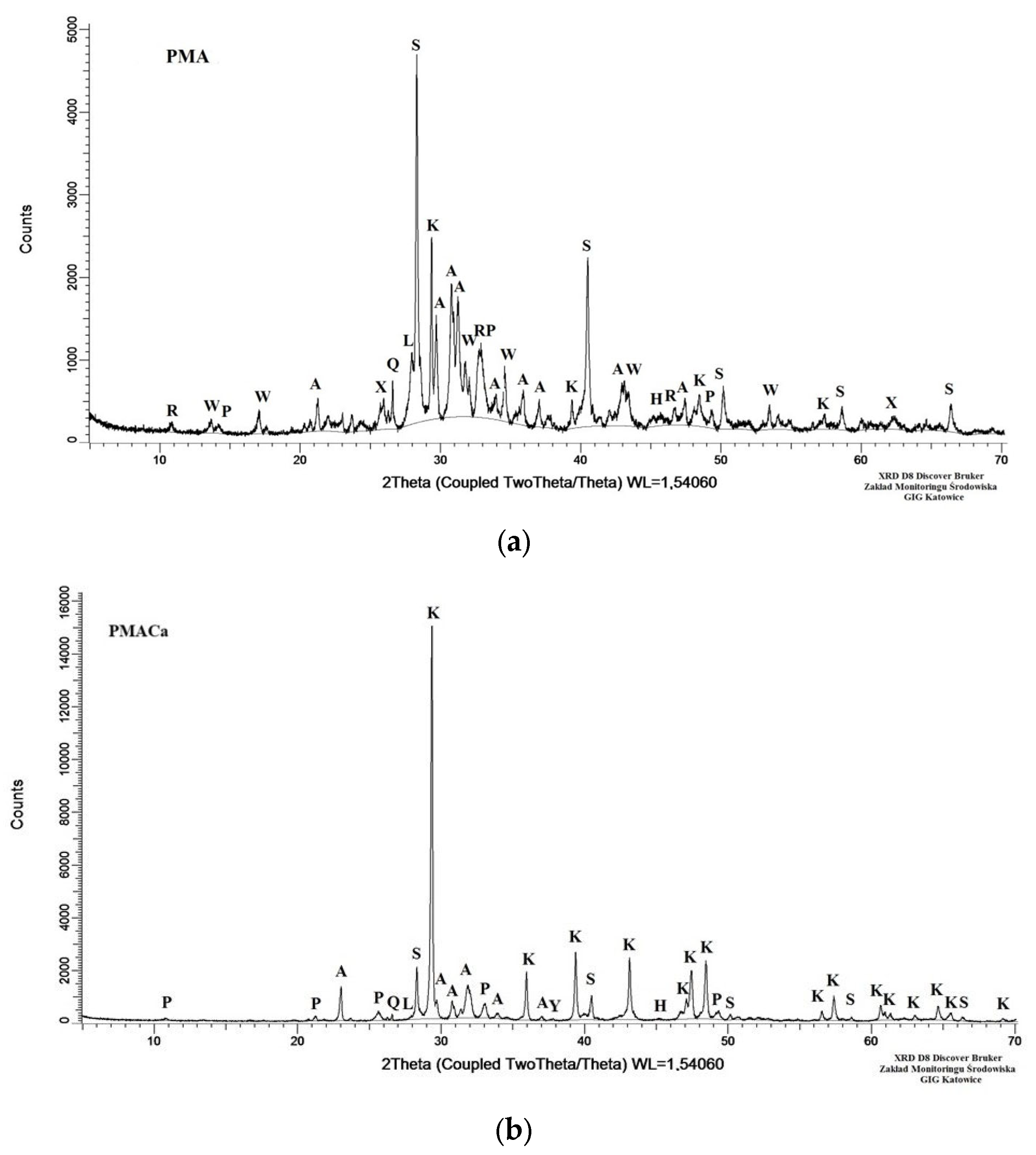
3.2. Ash Sample Property Determination via XRD Analysis
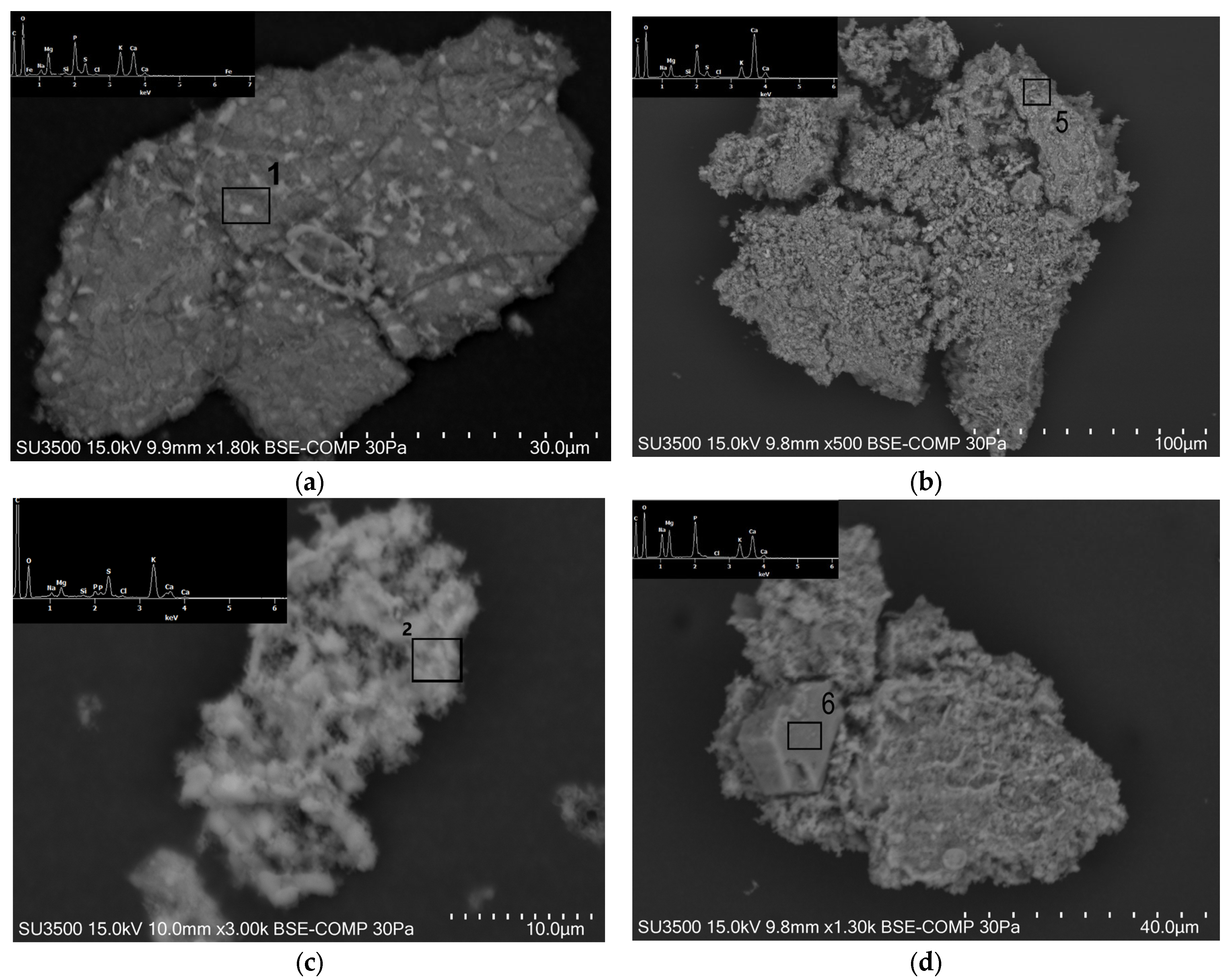
3.3. Ash Samples Property Determination via Microscopic Analysis
3.4. Characteristics of Poultry Manure Ash and Its Extracts
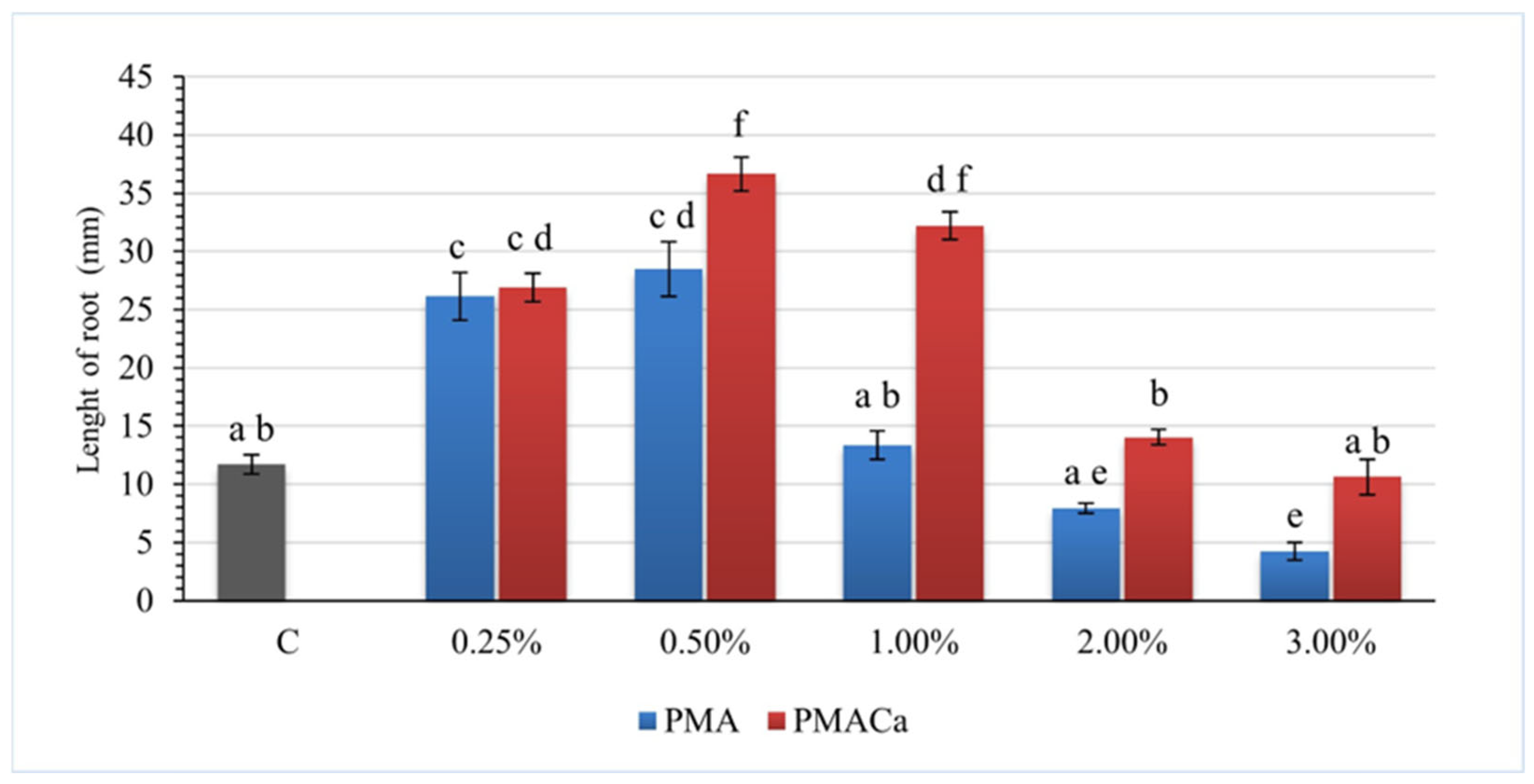
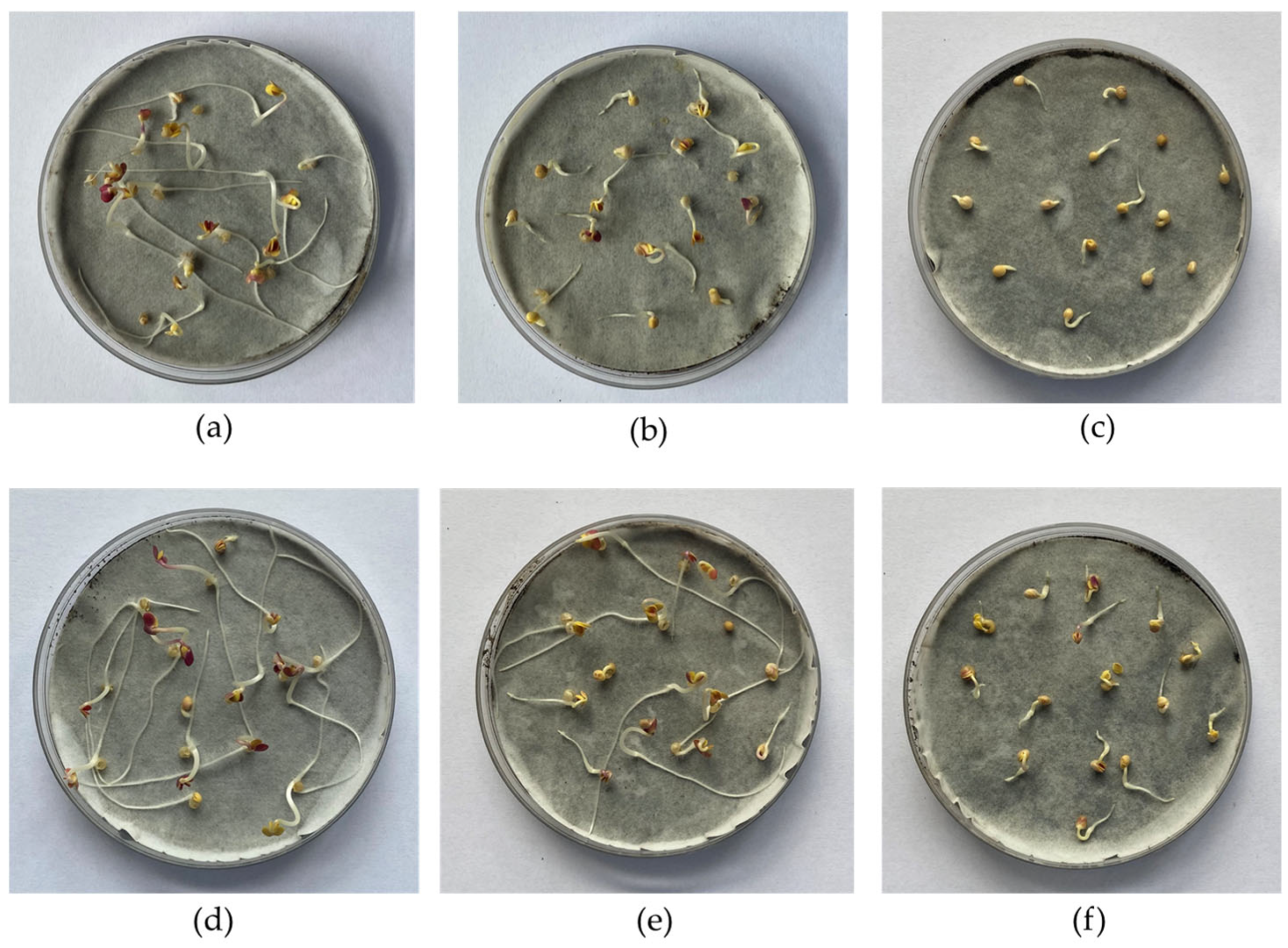
3.5. Phytotoxicity Tests Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PM | poultry manure |
| PMA | poultry manure ash obtained from PM incineration |
| PMACa | poultry manure ash obtained from the incineration of a mixture (PM with 5.0 wt% calcium hydroxide) |
References
- Mays, D.A.; Sistani, K.R.; Soileau, J.M. Lime and Fertilizer Needs for Land Reclamation. Reclam. Drastically Disturb. Lands 2015, 41, 217–240. [Google Scholar] [CrossRef]
- Novak, J.M.; Ippolito, J.A.; Ducey, T.F.; Watts, D.W.; Spokas, K.A.; Trippe, K.M.; Sigua, G.C.; Johnson, M.G. Remediation of an Acidic Mine Spoil: Miscanthus Biochar and Lime Amendment Affects Metal Availability, Plant Growth, and Soil Enzyme Activity. Chemosphere 2018, 205, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and Challenges in the Use of Coal Fly Ash for Soil Improvements—A Review. J. Environ. Manag. 2014, 145, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, Treatment and Case Studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Karalić, K.; Lončarić, Z.; Popović, B.; Zebec, V.; Kerovec, D. Liming Effect on Soil Heavy Metals Availability. Poljoprivreda 2013, 19, 59–64. [Google Scholar]
- Nagiel, A.; Szulc, W. Effect of Liming on Cadmium Immobilization in the Soil and Content in Spring Wheat (Triticum aestivum L.). Soil Sci. Annu. 2020, 71, 93–96. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Burns, D.A.; Riva-Murray, K. A New Look at Liming as an Approach to Accelerate Recovery from Acidic Deposition Effects. Sci. Total Environ. 2016, 562, 35–46. [Google Scholar] [CrossRef]
- Chastain, J.P.; Camberato, J.J.; Skewes, P. Poultry Manure Production and Nutrient Content. In Chapter 3b in: Confined Animal Manure Managers Certification Program Manual B Poultry Version; Clemson University Cooperative Extension Service: Clemson, SC, USA, 2001; Volume 2, pp. 1–17. [Google Scholar]
- Ashworth, A.J.; Chastain, J.P.; Moore, P.A. Nutrient Characteristics of Poultry Manure and Litter. Anim. Manure Prod. Charact. Environ. Concerns Manag. 2020, 67, 63–87. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X. Microbiological Safety of Chicken Litter or Chicken Litter-Based Organic Fertilizers: A Review. Agriculture 2014, 4, 1–29. [Google Scholar] [CrossRef]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Basamba, T.A. How safe is chicken litter for land application as an organic fertilizer? A review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Development. Regulation of 18 June 2008 on the Implementation of Certain Provisions of Fertilizers and Fertilization. J. Law. Pol. 2008, 119, 6515–6520. Available online: https://dziennikustaw.gov.pl/DU/2008/s/119/765 (accessed on 25 May 2025). (In Polish).
- Council of the European Union; European Parliament. Regulation (EC) No 1774/2002 of the European Parliament and of the Council of the European Union of 3th October 2002. Laying down Health Rules Concerning Animal by-Products Not Intended for Human Consumption. In Official Journal of the European Communities; European Union: Brussels, Belgium, 2010. [Google Scholar]
- Park, G.W.; Diez-Gonzalez, F. Utilization of Carbonate and Ammonia-Based Treatments to Eliminate Escherichia coli O157:H7 and Salmonella typhimurium DT104 from Cattle Manure. J. Appl. Microbiol. 2003, 94, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Unc, A.; Goss, M.J. Transport of Bacteria from Manure and Protection of Water Resources. Appl. Soil Ecol. 2004, 25, 1–18. [Google Scholar] [CrossRef]
- Gerba, C.P.; Smith, J.E. Sources of Pathogenic Microorganisms and Their Fate during Land Application of Wastes. J. Environ. Qual. 2005, 34, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef]
- National Institute of Public Health Infectious Diseases and Poisonings in Poland in 2020. Warsaw. 2021. Available online: https://wwwold.pzh.gov.pl/oldpage/epimeld/index_a.html (accessed on 27 November 2024).
- Chekabab, S.M.; Paquin-Veillette, J.; Dozois, C.M.; Harel, J. The Ecological Habitat and Transmission of Escherichia coli O157:H7. FEMS Microbiol. Lett. 2013, 341, 1–12. [Google Scholar] [CrossRef]
- Mawdsley, J.L.; Bardgett, R.D.; Merry, R.J.; Pain, B.F.; Theodorou, M.K. Pathogens in Livestock Waste, Their Potential for Movement through Soil and Environmental Pollution. Appl. Soil Ecol. 1995, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Malej, J. Properties of Sewage Sludge and Selected Methods of Their Neutralization, Processing and Utilization. Annu. Set Environ. Prot. 2000, 2, 69–101. [Google Scholar]
- Baki Unal, H.; Hakan Bayraktar, Ö.; Alkan, I.; Cengiz Akdeniz, R. Evaluation Possibilities of Chicken Manure in Turkey. Agric. Eng. 2015, 2, 5–14. [Google Scholar] [CrossRef]
- Heinonen-Tanski, H.; Mohaibes, M.; Karinen, P.; Koivunen, J. Methods to Reduce Pathogen Microorganisms in Manure. Livest. Sci. 2006, 102, 248–255. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Mamphweli, S.N.; Meyer, E.L.; Makaka, G.; Simon, M.; Okoh, A.I. An Overview of the Control of Bacterial Pathogens in Cattle Manure. Int. J. Environ. Res. Public Health 2016, 13, 843. [Google Scholar] [CrossRef]
- Lynch, D.; Henihan, A.M.; Bowen, B.; Lynch, D.; McDonnell, K.; Kwapinski, W.; Leahy, J.J. Utilization of Poultry Litter as an Energy Feedstock. Biomass Bioenergy 2013, 49, 197–204. [Google Scholar] [CrossRef]
- Luyckx, L.; de Leeuw, G.H.J.; Van Caneghem, J. Characterization of Poultry Litter Ash in View of Its Valorization. Waste Biomass Valorization 2020, 11, 5333–5348. [Google Scholar] [CrossRef]
- Gorazda, K.; Kowalski, Z.; Nowak, A.K.; Wzorek, Z.; Krupa-Żuczek, K.; Kulczycka, J.; Henclik, A. Wastes. Alternative Raw Materials for Phoshorous Industry. Przem. Chem. 2013, 92, 761–766. [Google Scholar]
- Nicholson, F.A.; Chambers, B.J.; Smith, K.A. Nutrient Composition of Poultry Manures in England and Wales. Bioresour. Technol. 1996, 58, 279–284. [Google Scholar] [CrossRef]
- Quiroga, G.; Castrillón, L.; Fernández-Nava, Y.; Marañón, E. Physico-chemical analysis and calorific values of poultry manure. Waste Manag. 2010, 30, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Yuan, Q.; Cao, H.; Niu, W.; Wang, M.; Zhu, Y.; Yan, S. Effect of Alkali and Alkaline Earth Metal Species on the Combustion Characteristics of Cattle Manures. R. Soc. Chem. Adv. 2018, 8, 11705–11713. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, X.; Fisk, C.; Davies, M.; Wang, Y.; Yang, J.; du Plessis, C.; Cotton, L.; Zhang, Y.; Willmott, J. Combustion Inhibition of Biomass Charcoal Using Slaked Lime and Dolime Slurries. Fire Saf. J. 2023, 140, 103841. [Google Scholar] [CrossRef]
- Bauer, P.J.; Szogi, A.A.; Shumaker, P.D. Fertilizer Efficacy of Poultry Litter Ash Blended with Lime or Gypsum as Fillers. Environments 2019, 6, 50. [Google Scholar] [CrossRef]
- Codling, E.E.; Lewis, J.; Watts, D.B. Broiler Litter Ash and Flue Gas Desulfurization Gypsum Effects on Peanut Yield and Uptake of Nutrients. Commun. Soil Sci. Plant Anal. 2015, 46, 2553–2575. [Google Scholar] [CrossRef]
- Więckol-Ryk, A.; Thomas, M.; Białecka, B. Improving the Properties of Degraded Soils from Industrial Areas by Using Livestock Waste with Calcium Peroxide as a Green Oxidizer. Materials 2021, 14, 3132. [Google Scholar] [CrossRef]
- Regulation of the Minister of Environment on the Manner of Conducting Assessment of Pollution of the Ground Surface. J. Law Repub. Pol. Warsaw, Poland 2016. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001395 (accessed on 10 February 2025).
- ISO 6887-1:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. ISO: Geneva, Switzerland, 2017.
- ISO 18134-2:2017; Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 2: Total Moisture—Simplified Method. ISO: Geneva, Switzerland, 2017.
- ISO 10390:2021; Soil, Treated Biowaste and Sludge—Determination of pH. ISO: Geneva, Switzerland, 2021.
- ISO 11265:1994; Soil Quality—Determination of the Specific Electrical Conductivity. ISO: Geneva, Switzerland, 1994.
- PN-EN 15309: 2010; Characterization of Waste and Soil-Determination of Elemental Composition by X-ray Fluorescence. Polish Committee for Standardization: Warsaw, Poland, 2010.
- ISO 11885:2007; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO: Geneva, Switzerland, 2007.
- ISO 18763: 2016; Soil Quality—Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants. ISO: Geneva, Switzerland, 2016.
- Iliadis, I.; Daskalopoulou, A.; Simões, M.; Giaouris, E. Integrated Combined Effects of Temperature, PH and Sodium Chloride Concentration on Biofilm Formation by Salmonella enterica Ser. Enteritidis and Typhimurium under Low Nutrient Food-Related Conditions. Food Res. Int. 2018, 107, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Dong, Q.L.; Rahman, S.M.E.; Oh, D.H. Response Surface Modeling of Listeria monocytogenes Inactivation on Lettuce Treated with Electrolyzed Oxidizing Water. J. Food Process Eng. 2011, 34, 1729–1745. [Google Scholar] [CrossRef]
- Bidlas, E.; Lambert, R.J.W. Comparing the Antimicrobial Effectiveness of NaCl and KCl with a View to Salt/Sodium Replacement. Int. J. Food Microbiol. 2008, 124, 98–102. [Google Scholar] [CrossRef] [PubMed]
- M-Ridha, M.J.; Hussein, S.I.; Alismaeel, Z.T.; Atiya, M.A.; Aziz, G.M. Biodegradation of Reactive Dyes by Some Bacteria Using Response Surface Methodology as an Optimization Technique. Alex. Eng. J. 2020, 59, 3551–3563. [Google Scholar] [CrossRef]
- Kim, Y.; Roh, Y. Microbial Precipitation of Calcium Carbonate for Crack Healing and Stabilization of Sandy Soils. Appl. Sci. 2024, 14, 1568. [Google Scholar] [CrossRef]
- Pagani, A.; Mallarino, A.P. Change of Soil pH over Time as Affected by Lime Sources and Application Rates. Iowa State Research Farm Progress Reports. 248. 2011. Available online: https://www.iastatedigitalpress.com/farmreports/article/5197/galley/5059/view/ (accessed on 28 November 2024).
- Barreto, M.S.C.; Elzinga, E.J.; Ramlogan, M.; Rouff, A.A.; Alleoni, L.R.F. Calcium Enhances Adsorption and Thermal Stability of Organic Compounds on Soil Minerals. Chem. Geol. 2021, 559, 119804. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, S.; Yang, M.; Hao, Z.; Wang, X.; Shi, Y. Nano Calcium Carbonate Improves Wheat Nitrogen Accumulation and Grain Yield by Enhancing Soil Nitrogen Supply and Flag Leaf Photosynthetic Characteristics. Field Crop. Res. 2024, 310, 109341. [Google Scholar] [CrossRef]
- Więckol-Ryk, A.; Białecka, B.; Cempa, M.; Adamczyk, Z. Optimization of Chicken Manure Combustion Parameters in the Aspect of Phosphorus Recovery. Int. J. Recycl. Org. Waste Agric. 2020, 9, 273–285. [Google Scholar] [CrossRef]
- Rivera, R.M.; Chagnes, A.; Cathelineau, M.; Boiron, M.C. Conditioning of Poultry Manure Ash for Subsequent Phosphorous Separation and Assessment for a Process Design. Sustain. Mater. Technol. 2022, 31, e00377. [Google Scholar] [CrossRef]
- Asiandu, A.P.; Sari, W. The Utilization of Hydroxyapatite as Green Fertilizer Increasing Soil Fertility. Arch. Agric. Res. Technol. 2023, 4, 1–2. [Google Scholar] [CrossRef]
- Fahimi, A.; Bilo, F.; Assi, A.; Dalipi, R.; Federici, S.; Guedes, A.; Valentim, B.; Olgun, H.; Ye, G.; Bialecka, B.; et al. Poultry Litter Ash Characterisation and Recovery. Waste Manag. 2020, 111, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.L. LSU Digital Commons Using Poultry Litter Ash as a Fertilizer Source for Bermudagrass (Cynodon dactylon) Establishment and Loblolly Pine (Pinus taeda) Plantation. LSU Doctoral Dossertations. 5099. Louisiana State University and Agricultural and Mechanical College. 2019. Available online: https://digitalcommons.lsu.edu/gradschool_dissertations/5099 (accessed on 30 March 2025).
- EU Regulation of the European Parliament and of the council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending regulations. (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 170, 1–124. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R1009&qid=1736325125513 (accessed on 30 March 2025).
- Bernal, M.P.; Álvarez-Robles, M.J.; Bernal-Molina, P.; Clemente, R. Bottom Ash from Combustion of Chicken Manure as a Fertilizer Material. Front. Sustain. Food Syst. 2024, 8, 1392445. [Google Scholar] [CrossRef]
- Lee, C.G.; Alvarez, P.J.J.; Kim, H.G.; Jeong, S.; Lee, S.; Lee, K.B.; Lee, S.H.; Choi, J.W. Phosphorous Recovery from Sewage Sludge Using Calcium Silicate Hydrates. Chemosphere 2018, 193, 1087–1093. [Google Scholar] [CrossRef]
- Ann, Y.; Reddy, K.R.; Delfino, J.J. Influence of Chemical Amendments on Phosphorus Immobilization in Soils from a Constructed Wetland. Ecol. Eng. 1999, 14, 157–167. [Google Scholar] [CrossRef]
- Maguire, R.O.; Hesterberg, D.; Gernat, A.; Anderson, K.; Wineland, M.; Grimes, J. Liming Poultry Manures to Decrease Soluble Phosphorus and Suppress the Bacteria Population. J. Environ. Qual. 2006, 35, 849–857. [Google Scholar] [CrossRef]
- Faridullah; Irshad, M.; Yamamoto, S.; Ahmad, Z.; Endo, T.; Honna, T. Extractability and Bioavailability of Phosphorus from Soils Amended with Poultry Litter and Poultry Litter Ash. J. Food Agric. Environ. 2009, 7, 692–697. [Google Scholar]
- Więckol-Ryk, A.; Białecka, B.; Thomas, M. Effect of Green Oxidizing Agent on Inhibition of Escherichia coli Present in Livestock Wastes. Water. Air. Soil Pollut. 2020, 231, 1–16. [Google Scholar] [CrossRef]
- Carmona, F.J.; Guagliardi, A.; Masciocchi, N. Nanosized Calcium Phosphates as Novel Macronutrient Nano-Fertilizers. Nanomaterials 2022, 12, 2709. [Google Scholar] [CrossRef]
- Schaller, J.; Frei, S.; Rohn, L.; Gilfedder, B.S. Amorphous Silica Controls Water Storage Capacity and Phosphorus Mobility in Soils. Front. Environ. Sci. 2020, 8, 94. [Google Scholar] [CrossRef]
- Saleem, A.; Irshad, M.; Ping, A.; Haroon, B. Loss of Phosphorus by Runoff from Soils after Amendment with Poultry Litter Co-Composted with Crop Waste. Int. J. Recycl. Org. Waste Agric. 2018, 7, 211–215. [Google Scholar] [CrossRef]
- Fargašová, A. Phytotoxic Effect of Cd, Zn, Pb, Cu and Fe on Sinapis Alba L. Seedlings and Their Acucumulation in Roots and Shoots. Biol. Plantarium 2001, 44, 471–473. [Google Scholar] [CrossRef]
- Berta, K.M.; Kurdi, R.; Lukács, P.; Penk, M.; Somogyi, V. Red Mud with Other Waste Materials as Artificial Soil Substitute and Its Effect on Sinapis Alba. J. Environ. Manag. 2021, 287, 112311. [Google Scholar] [CrossRef] [PubMed]
- Krasnodębska-Ostręga, B.; Sadowska, M.; Biaduń, E.; Mazur, R.; Kowalska, J. Sinapis Alba as a Useful Plant in Bioremediation–Studies of Defense Mechanisms and Accumulation of As, Tl and PGEs. Int. J. Phytoremediation 2022, 24, 1475–1490. [Google Scholar] [CrossRef]
- Repkina, N.; Nilova, I.; Kaznina, N. Effect of Zinc Excess in Substrate on Physiological Responses of Sinapis alba L. Plants 2023, 12, 211. [Google Scholar] [CrossRef]
- Sindelar, O.; Vaverkova, M.D.; Adamcova, D. Analysis of the Phytotoxic Effect of Leachates from the Landfill of Municipal Waste in Zdounky on Higher Plants. In Proceedings of the MendelNet 2019: 26th International PhD Students Conference, Brno, Czech Republic, 6–7 November 2019; pp. 332–337. [Google Scholar]
- Cempa, M.; Olszewski, P.; Wierzchowski, K.; Kucharski, P.; Białecka, B. Ash from Poultry Manure Incineration as a Substitute for Phosphorus Fertiliser. Materials 2022, 15, 3023. [Google Scholar] [CrossRef]
- Billen, P.; Costa, J.; Van der Aa, L.; Van Caneghem, J.; Vandecasteele, C. Electricity from poultry manure: A cleaner alternative to direct land application. J. Clean. Prod. 2015, 96, 467–475. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Ning, T.; Lal, R. Higher CO2 absorption using a new class of calcium hydroxide (Ca(OH)2) nanoparticles. Environ. Chem. Lett. 2018, 16, 1095–1100. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Mahmud, S.; Tushar, M.; Leon, M. Ash Analysis of Poultry Litter, Willow and Oats for Combustion in Boilers. J. Biomass Biofuel. 2014, 1, 16–26. [Google Scholar] [CrossRef]



| Run | Experimental Conditions | Experiment Results | |||
|---|---|---|---|---|---|
| Ca(OH)2 Concentration, wt % x1 | Temperature, °C x2 | Contact Time, h x3 | E. coli, log CFU/g Y | E. coli Reduction % | |
| 1 | 3.0 | 12.0 | 48 | 7.5911 | 13.38 |
| 2 | 3.0 | 12.0 | 168 | 5.6990 | 34.97 |
| 3 | 3.0 | 32.0 | 48 | 5.3222 | 39.27 |
| 4 | 3.0 | 32.0 | 168 | 4.1139 | 53.06 |
| 5 | 7.0 | 12.0 | 48 | 3.6990 | 57.79 |
| 6 | 7.0 | 12.0 | 168 | 2.0000 | 77.18 |
| 7 | 7.0 | 32.0 | 48 | 2.9031 | 66.87 |
| 8 | 7.0 | 32.0 | 168 | 2.0000 | 77.18 |
| 9 | 1.6 | 22.0 | 108 | 7.6435 | 12.78 |
| 10 | 8.4 | 22.0 | 108 | 2.0000 | 77.18 |
| 11 | 5.0 | 5.2 | 108 | 4.0414 | 53.88 |
| 12 | 5.0 | 38.8 | 108 | 3.0000 | 65.77 |
| 13 | 5.0 | 22.0 | 7 | 5.9294 | 32.34 |
| 14 | 5.0 | 22.0 | 209 | 3.0000 | 65.77 |
| 15(C) | 5.0 | 22.0 | 108 | 4.6990 | 46.38 |
| 16(C) | 5.0 | 22.0 | 108 | 4.6335 | 47.13 |
| Parameter | Effect Evaluation, E. coli Log CFU/g, R2 = 0.9850, Radj.2 = 0.9719, 3 Parameters, 1 Block, 16 Experiments, MS = 0.0928 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | Standard Error | p Value * | −95% Confidence Interval | +95% Confidence Interval | Factor | Standard Error of Factor | Lower Confidence Interval | Upper Confidence Interval | |
| Constant value | 4.6738 | 0.2148 | 0.0001 | 4.1784 | 5.1693 | 4.6738 | 0.2148 | 4.1784 | 5.1693 |
| Ca(OH)2, wt% (L) | −3.1655 | 0.1649 | 0.0001 | −3.5458 | −2.7852 | −1.5827 | 0.0825 | −1.7729 | −1.3926 |
| Ca(OH)2, wt% (Q) | 0.0732 | 0.2002 | 0.7242 | −0.3885 | 0.5349 | 0.0366 | 0.1001 | −0.1943 | 0.2675 |
| Temperature, °C (L) | −0.9374 | 0.1649 | 0.0005 | −1.3177 | −0.5571 | −0.4687 | 0.0825 | −0.6589 | −0.2786 |
| Temperature, °C (Q) | −0.8468 | 0.2002 | 0.0029 | −1.3085 | −0.3850 | −0.4234 | 0.1001 | −0.6543 | −0.1925 |
| Time, h (L) | −1.5566 | 0.1649 | 0.0001 | −1.9369 | −1.1763 | −0.7783 | 0.0825 | −0.9684 | −0.5882 |
| Time, h (Q) | −0.1793 | 0.2002 | 0.3968 | −0.6410 | 0.2825 | −0.0896 | 0.1001 | −0.3205 | 0.1412 |
| Ca(OH)2 (L) relative to Temperature (L) | 0.7645 | 0.2155 | 0.0075 | 0.2676 | 1.2614 | 0.3822 | 0.1077 | 0.1338 | 0.6307 |
| Parameter | Effect Evaluation, E. coli Log CFU/g, R2 = 0.9850, Radj2 = 0.9719, 3 Parameters, 1 Block, 16 Experiments, MS = 0.0928 | ||||
|---|---|---|---|---|---|
| SS | DF | MS | F | p Value * | |
| Ca(OH)2, wt% (L) | 34.2113 | 1 | 34.2113 | 368.4374 | <0.0001 |
| Ca(OH)2, wt% (Q) | 0.0124 | 1 | 0.0124 | 0.1336 | 0.7242 |
| Temperature, °C (L) | 3.0003 | 1 | 3.0003 | 32.3117 | 0.0005 |
| Temperature, °C (Q) | 1.6607 | 1 | 1.6607 | 17.8844 | 0.0029 |
| Contact time, h (L) | 8.2726 | 1 | 8.2726 | 89.0915 | <0.0001 |
| Contact time, h (Q) | 0.0744 | 1 | 0.0744 | 0.8015 | 0.3968 |
| Ca(OH)2 (L) relative to Temperature (L) | 1.1689 | 1 | 1.1689 | 12.5886 | 0.0075 |
| Error | 0.7428 | 8 | 0.0929 | ||
| SS | 49.5841 | 15 | |||
| Symbol | Chemical Formula of Crystalline Phase | Content, wt% | Uncertainty, wt% | ||
|---|---|---|---|---|---|
| PMA | PMACa | ||||
| A | arcanite | K2SO4 | 16.0 | 4.0 | ±1.0 |
| K | calcite | CaCO3 | 14.5 | 67.5 b | ±0.5 |
| S | sylvite | KCl | 12.0 | 6.0 | ±1.0 |
| W | whitlockite | Ca3(PO4)2 | 11.0 | bdl | ±1.0 |
| P | hydroxyapatite | Ca5(OH)(PO4)3 | 5.0 | 3.0 | ±1.0 |
| R | apatite | Ca5(PO4,CO3,OH)3 | 5.0 | 3.0 | ±1.0 |
| L | Feldspars a | - | 4.0 | 1.0 | ±1.0 |
| Q | quartz | SiO2 | 3.5 | 1.5 | ±0.5 |
| X | iron(II) phosphate | Fe3(PO4)2 | 3.0 | bdl | ±1.0 |
| H | halite | NaCl | <1.0 | <1.0 | |
| Y | calcium hydroxide | Ca(OH)2 | bdl | <1.0 | |
| Total amorphous phase | 24.5 | 15.5 | ±0.5 | ||
| Crystalline phase containing phosphorus | 24.0 | 6.0 | |||
| Total crystalline phase | 75.5 | 84.5 | |||
| Parameter (a) | PMA | PMACa |
|---|---|---|
| CE Diameter D[n, 0.1], μm | 0.9 | 0.9 |
| CE Diameter D[n, 0.5], μm | 2.1 | 2.4 |
| CE Diameter D[n, 0.9], μm | 6.1 | 6.9 |
| CE Diameter D [3, 2], μm | 31.2 | 20.9 |
| CE Diameter D[4, 3], μm | 77.5 | 41.3 |
| Mean Circularity | 0.86 | 0.88 |
| Mean Circularity for grains < 1 μm, | 0.84 | 0.78 |
| Mean Circularity for grains 1–10 μm | 0.87 | 0.89 |
| Mean Circularity for grains 10–45 μm | 0.76 | 0.85 |
| Mean Circularity for grains > 45 μm | 0.61 | 0.76 |
| Aspect Ratio | 0.77 | 0.77 |
| Aspect Ratio for grains < 1 μm, | 0.79 | 0.74 |
| Aspect Ratio for grains 1–10 μm | 0.77 | 0.78 |
| Aspect Ratio for grains 10–45 μm | 0.68 | 0.71 |
| Aspect Ratio for grains > 45 μm | 0.66 | 0.75 |
| Sample | Microarea No. | Composition, wt% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Na | Mg | Si | P | S | Cl | K | Ca | Fe | ||
| PMA | 1 | 15.3 | 40.6 | 1.6 | 5.1 | 0.3 | 8.2 | 3.0 | 0.5 | 10.6 | 13.3 | 1.5 |
| 2 | 43.1 | 29.0 | 0.9 | 1.9 | 0.3 | 0.9 | 5.0 | 0.6 | 15.3 | 3.0 | nd | |
| 3 | 16.0 | 36.1 | 3.3 | 0.5 | nd | 11.8 | 0.7 | 0.3 | 15.3 | 16.0 | nd | |
| PMACa | 4 | 15.4 | 41.6 | 2.8 | 11.2 | nd | 12.7 | nd | 0.6 | 15.8 | nd | nd |
| 5 | 13.0 | 41.8 | 1.8 | 2.9 | 0.3 | 7.0 | 1.4 | 0.5 | 4.9 | 26.5 | nd | |
| 6 | 15.9 | 37.9 | 7.2 | 7.1 | nd | 10.0 | nd | 0.2 | 7.3 | 14.4 | nd | |
| Parameter | Uncertainty c, % | Unit | PMA | PMACa |
|---|---|---|---|---|
| Ash efficiency | ±2 | % | 13.58 | 26.15 |
| EC | ±5 | mS/cm | 29.80 | 16.60 |
| pH | ±2 | - | 10.4 | 11.6 |
| CaO | ±10 | g/kg | 195 a | 387 b |
| MgO | ±10 | 65 a | 31 b | |
| K2O | ±10 | 167 a | 79 b | |
| P2O5 | ±14 | 250 a | 119 b | |
| Cd | ±20 | mg/kg | 11 a | 5 b |
| Co | ±35 | bdl (<2) | bdl (<2) | |
| Cr | ±35 | bdl (<3) | bdl (<3) | |
| Cu | ±20 | 352 a | 167 b | |
| Ni | ±20 | 25 a | 12 b | |
| Pb | ±20 | 15 a | 7 b | |
| Zn | ±20 | 2809 a | 1334 b |
| Parameter | Uncertainty a | Composition b, | Composition c, | ||
|---|---|---|---|---|---|
| PMA | PMACa | PMA | PMACa | ||
| % | mg/L | g/kg (d.m) | |||
| P | ±10 | 732 | 296 | 73.2 | 29.6 |
| K | ±10 | 1591 | 660 | 159 | 66.1 |
| Ca | ±10 | 1142 | 2674 | 114 | 267 |
| Mg | ±10 | 471 | 209 | 47.1 | 20.9 |
| S | ±20 | 336 | 175 | 33.6 | 17.5 |
| Na | ±10 | 271 | 98.9 | 27.1 | 9.89 |
| Fe | ±10 | 30.0 | 15.2 | 3.00 | 1.52 |
| Mn | ±10 | 29.5 | 14.1 | 2.95 | 1.41 |
| % | mg/L | mg/kg | |||
| Ba | ±10 | 0.88 | 0.42 | 88 | 42 |
| Sr | ±10 | 1.94 | 1.89 | 194 | 189 |
| B | ±10 | 1.74 | 0.66 | 174 | 66 |
| Al | ±10 | 12.1 | 6.68 | 1210 | 668 |
| Cd | ±25 | 0.015 | 0.0047 | 1.5 | 0.5 |
| Co | ±25 | 0.074 | 0.034 | 7.4 | 3.4 |
| Cr | ±25 | 0.057 | 0.044 | 5.7 | 4.4 |
| Cu | ±10 | 4.29 | 1.76 | 429 | 176 |
| Ni | ±20 | 0.26 | 0.11 | 26 | 11 |
| Pb | ±25 | <0.05 | <0.05 | <5 | <5 |
| Zn | ±10 | 31.4 | 13.3 | 3140 | 1330 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cempa, M.; Więckol-Ryk, A.; Thomas, M.; Białecka, B. Utilization of Poultry Manure After Biological Deactivation and Incineration to Enhance the Quality of Degraded Soils. Sustainability 2025, 17, 4976. https://doi.org/10.3390/su17114976
Cempa M, Więckol-Ryk A, Thomas M, Białecka B. Utilization of Poultry Manure After Biological Deactivation and Incineration to Enhance the Quality of Degraded Soils. Sustainability. 2025; 17(11):4976. https://doi.org/10.3390/su17114976
Chicago/Turabian StyleCempa, Magdalena, Angelika Więckol-Ryk, Maciej Thomas, and Barbara Białecka. 2025. "Utilization of Poultry Manure After Biological Deactivation and Incineration to Enhance the Quality of Degraded Soils" Sustainability 17, no. 11: 4976. https://doi.org/10.3390/su17114976
APA StyleCempa, M., Więckol-Ryk, A., Thomas, M., & Białecka, B. (2025). Utilization of Poultry Manure After Biological Deactivation and Incineration to Enhance the Quality of Degraded Soils. Sustainability, 17(11), 4976. https://doi.org/10.3390/su17114976







