Ethanol Content Increase in Gasoline Toward Sustainable Liquid Fuels Worldwide: Impacts on Manufacturing and Supply Chains via Discrete-Event Scenarios
Abstract
1. Introduction
- Increased gasoline production (by reducing reformate stream losses when in low-ON mode);
- Enhanced profitability (since the price of ETH is lower than mineral gasoline);
- Mitigating CO2 emissions via the incorporation of renewable fuel components [10];
- The potential to cease the utilization of ON boosters such as MTBE (which is already banned or restricted in most nations) [4].
- Comprehensive analysis of ETH integration: We evaluate the integration of ETH into gasoline blends, considering both manufacturing (refinery operations) and supply chain (external inputs such as MTBE and heavy naphtha) dimensions, and providing a holistic view of sustainable fuel production;
- Discrete-event simulation model: A discrete-event simulation model was developed to analyze over 512 scenarios, examining variations in refinery operations, supply chain inputs, and policy constraints. This approach allows for a detailed evaluation of gasoline production in terms of both quantity (yield) and quality (octane rating);
- Synergistic effects of ETH and other components: We explore the synergistic effects of combining ETH with other gasoline components, such as MTBE and reformate streams, across multiple refinery sites; this provides insights into how ETH can reduce or eliminate the reliance on MTBE while maintaining or improving octane ratings;
- Tailored solutions for different regions: The findings provide a framework for nations to adopt ETH in gasoline blends tailored to their unique regulatory and resource constraints. This is particularly relevant for regions with varying levels of ETH adoption, such as the Middle East, Europe, and North America;
- Carbon footprint reduction: The study highlights the potential for ETH to reduce the carbon footprint of gasoline production, aligning with global sustainability goals and reducing greenhouse gas emissions;
- Practical insights for refinery operations: This research offers practical insights for refinery operators, including strategies for adjusting reformate unit severity, optimizing gasoline blends, and reducing material losses, thereby enhancing profitability and sustainability.
2. ETH and Gasoline Interplay with Geopolitics
- Arable land for ETH production;
- Public policies toward renewable energy and de-risking dependence on fossil fuels;
- Overproduction of light petroleum (crude oil and condensate streams);
- Banning or restricting MTBE from the gasoline pool.
3. ETH and Octane Rating Boosters in Gasoline
3.1. ETH and MTBE
3.2. ETH and Carbon-Chain-Rearranged Naphtha
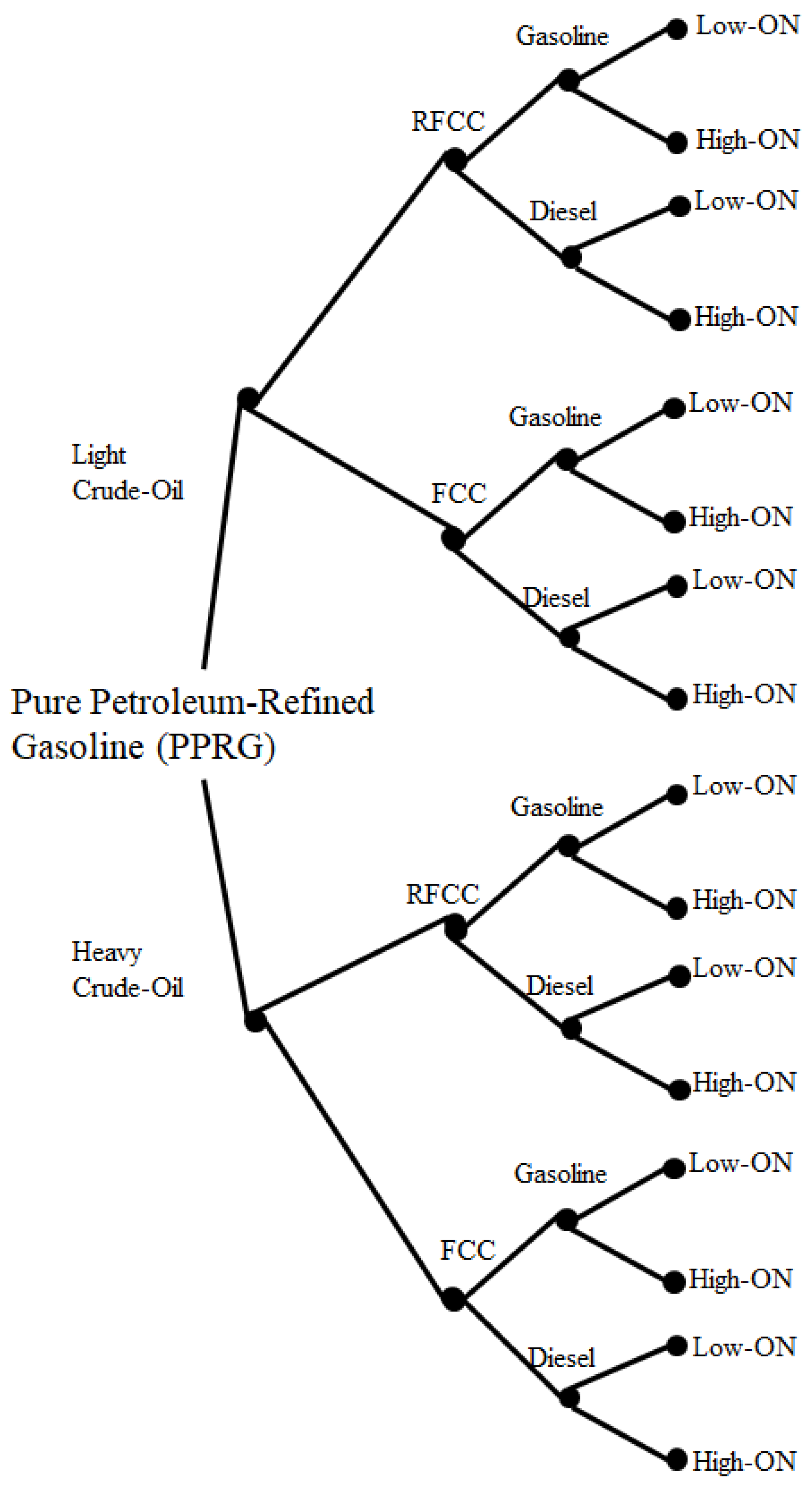
4. Problem Statement: Gasoline Recipes
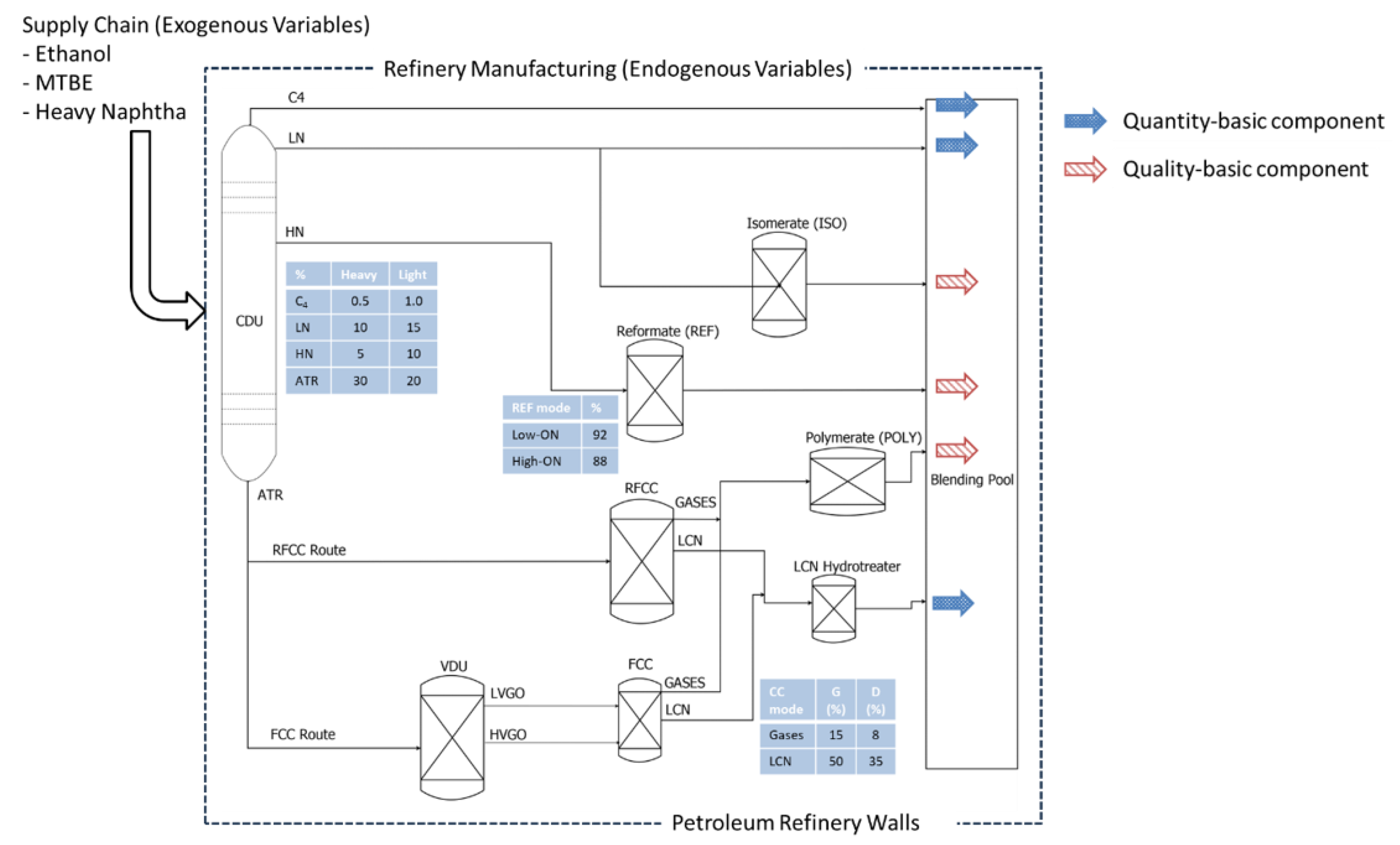
4.1. Gasoline Recipes and Component Dimensions
- Crude oils: light or heavy;
- ATR route: RFCC or VDU/FCC;
- Catalytic cracking (CC) mode: gasoline or diesel;
- Reformate unit mode: low-ON or high-ON.
- Isomerate: yes or no;
- Polymerate: yes or no.
- ON boosters (MTBE);
- ETH;
- Additional carbon-chain-rearranged naphtha (CCRN).
4.2. Pseudo-Code Algorithm to Simulate Scenarios
| Algorithm 1 Result Plots |
|
| Algorithm 2 Calculation |
|
- Main components (always included): Butane (C4), light naphtha (LN), reformate (REF) streams, and HTLCN;
- Marginal or secondary components (included via IF–THEN clauses): Isomerate (ISO) and polymerate (POLY).
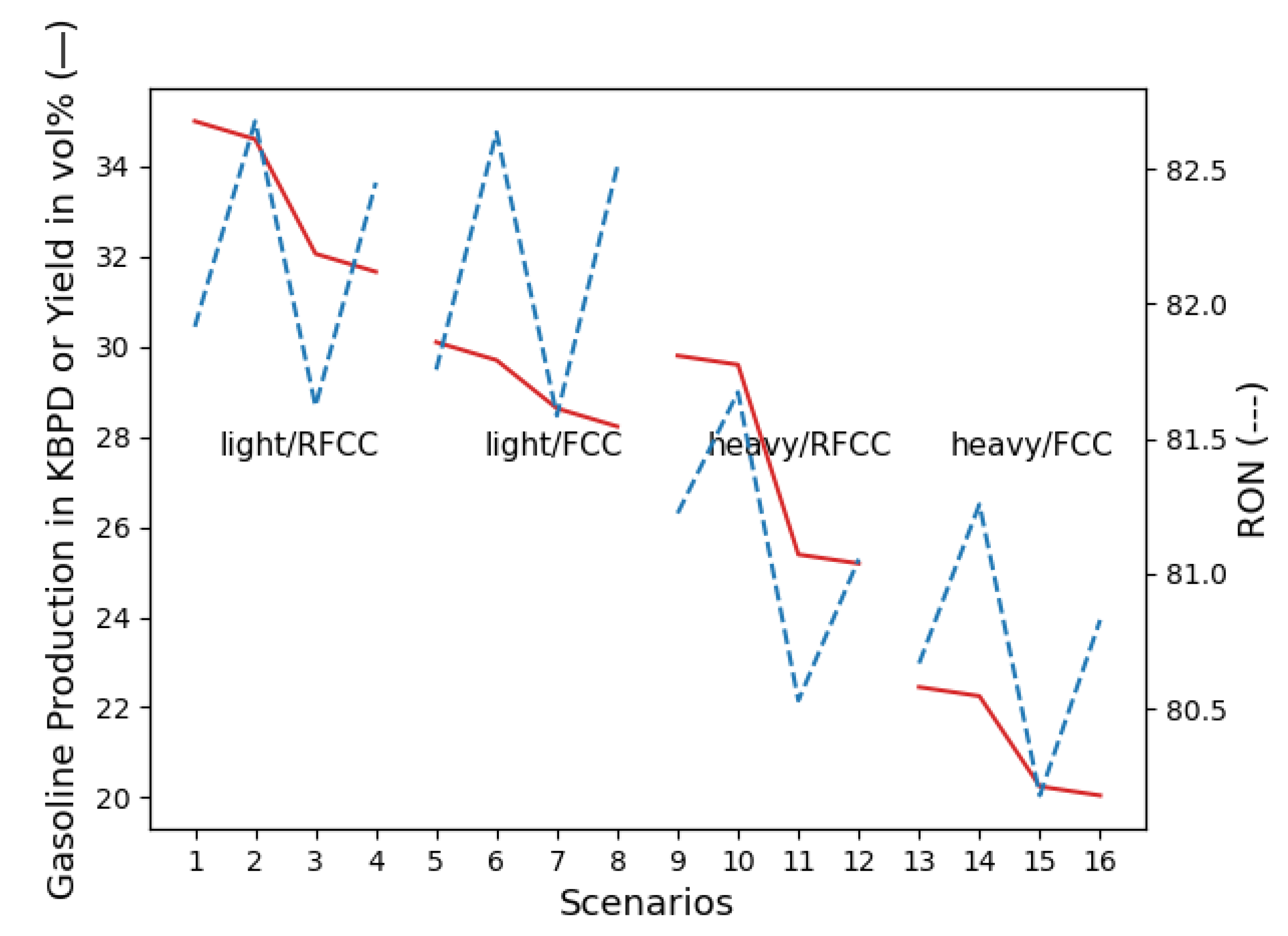
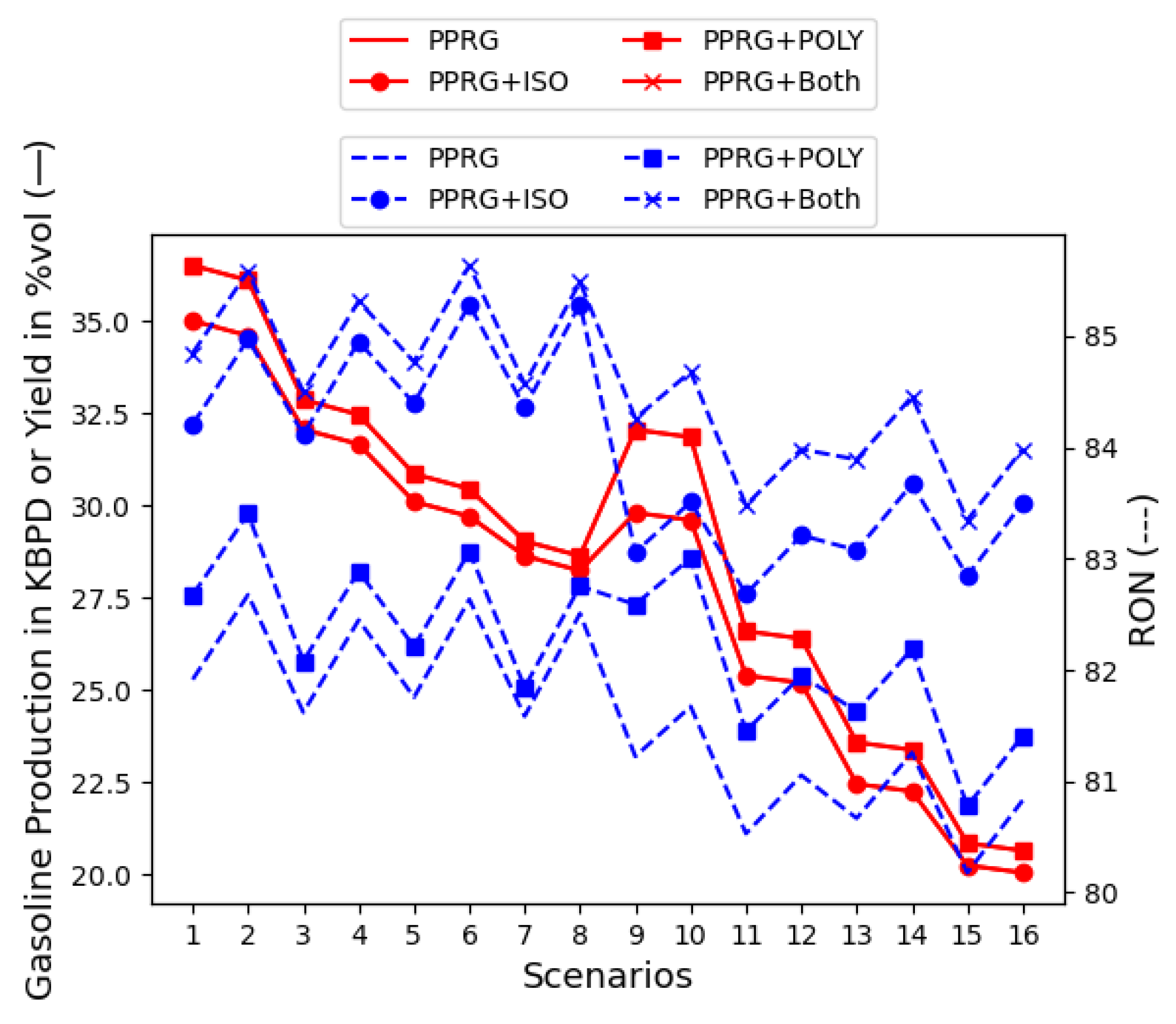


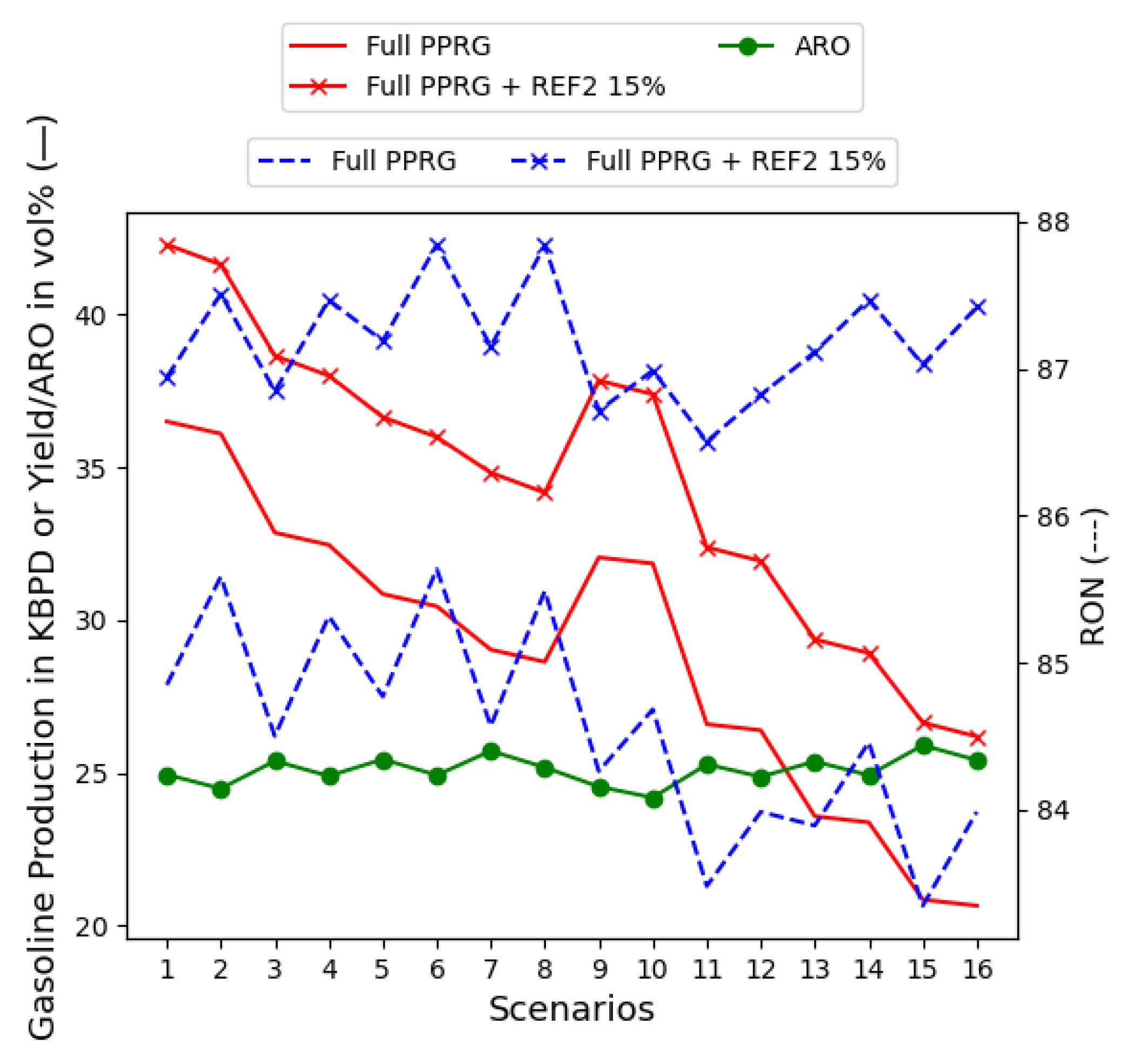
4.3. Gasoline Component Quantities: Process Scenario Flows
- Butane (C4);
- Light naphtha (LN);
- Isomerate (ISO);
- Reformate (REF);
- Polymerate (POLY);
- HTLCN.
- Crude selection: light () or heavy () petroleum;
- Reformate mode of operation: low () or high () severity;
- ATR-to-CC route: RFCC () or FCC ();
- CC mode of operation: gasoline () or diesel ().
4.4. Gasoline Components and Blending Formulas
4.5. Gasoline Component Properties: Process Scenario Qualities
4.6. Uncertainty and Error Analysis
Error Sources in Model Assumptions
- Blending Interactions: This study applies volume-based property blending rules, but real-world gasoline blending exhibits nonlinear interactions, particularly with oxygenates like ethanol and MTBE [40].
- Process Yield Assumptions: The reformate unit’s severity levels (low vs. high ON) assume fixed conversion efficiencies, whereas actual refinery operations may adjust hydrogen production and reformate composition based on feed variations.
- Component Stability: Ethanol volatility and water absorption were not explicitly modeled, potentially impacting RVP calculations in high-ethanol blends.
- Supply Chain Variability: The model assumes consistent ethanol availability, but geopolitical factors, production constraints, and biofuel mandates can influence supply reliability.
5. Gasoline Production: Recipe Results
5.1. Refined Gasoline Production
5.2. PPRG with Isomerate and Polymerate Units (Full PPRG)
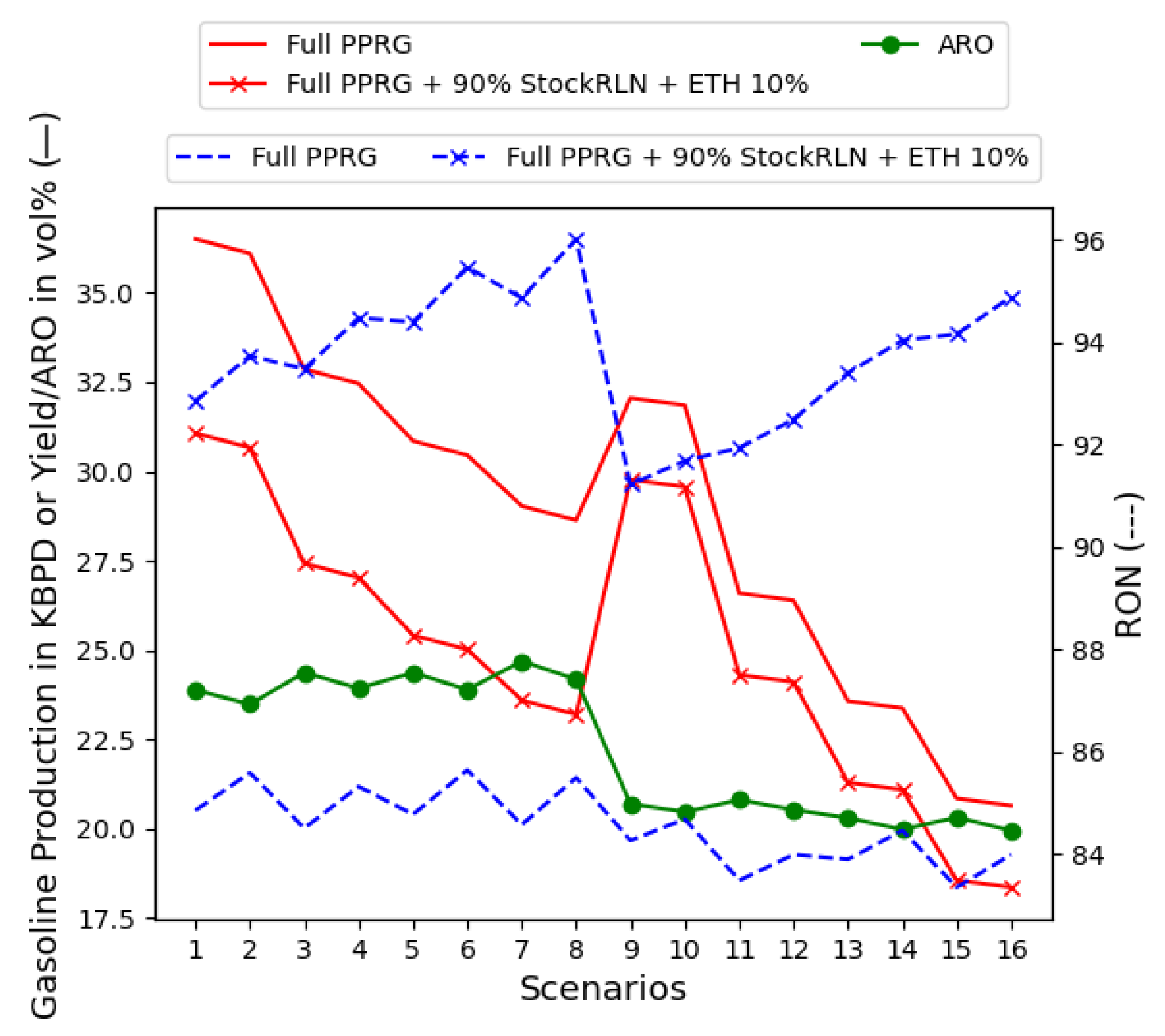
5.3. The Full PPRG with Supply-Chain-Independent Variables
5.3.1. MTBE in the Gasoline Blend
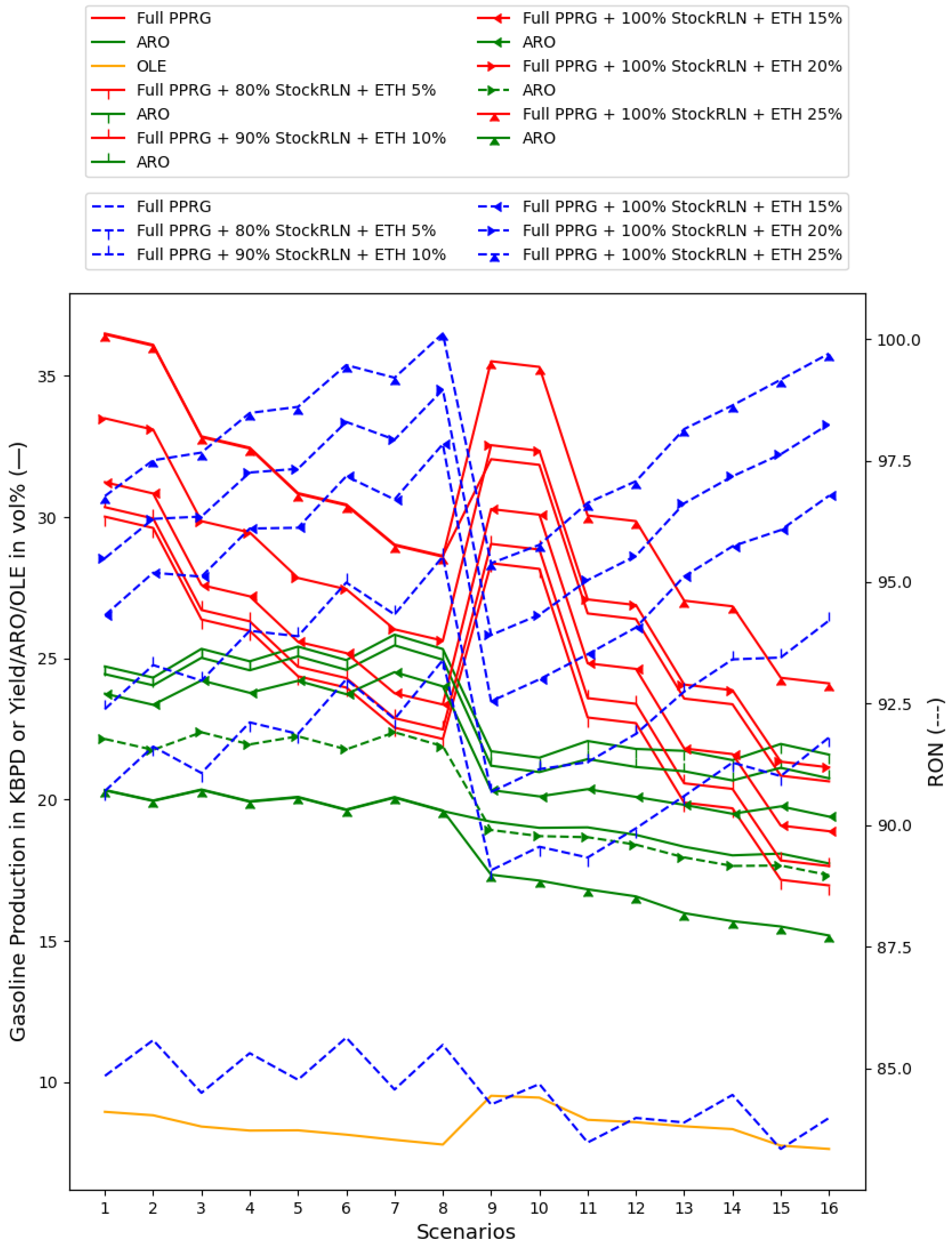
5.3.2. ETH in the Gasoline Blend
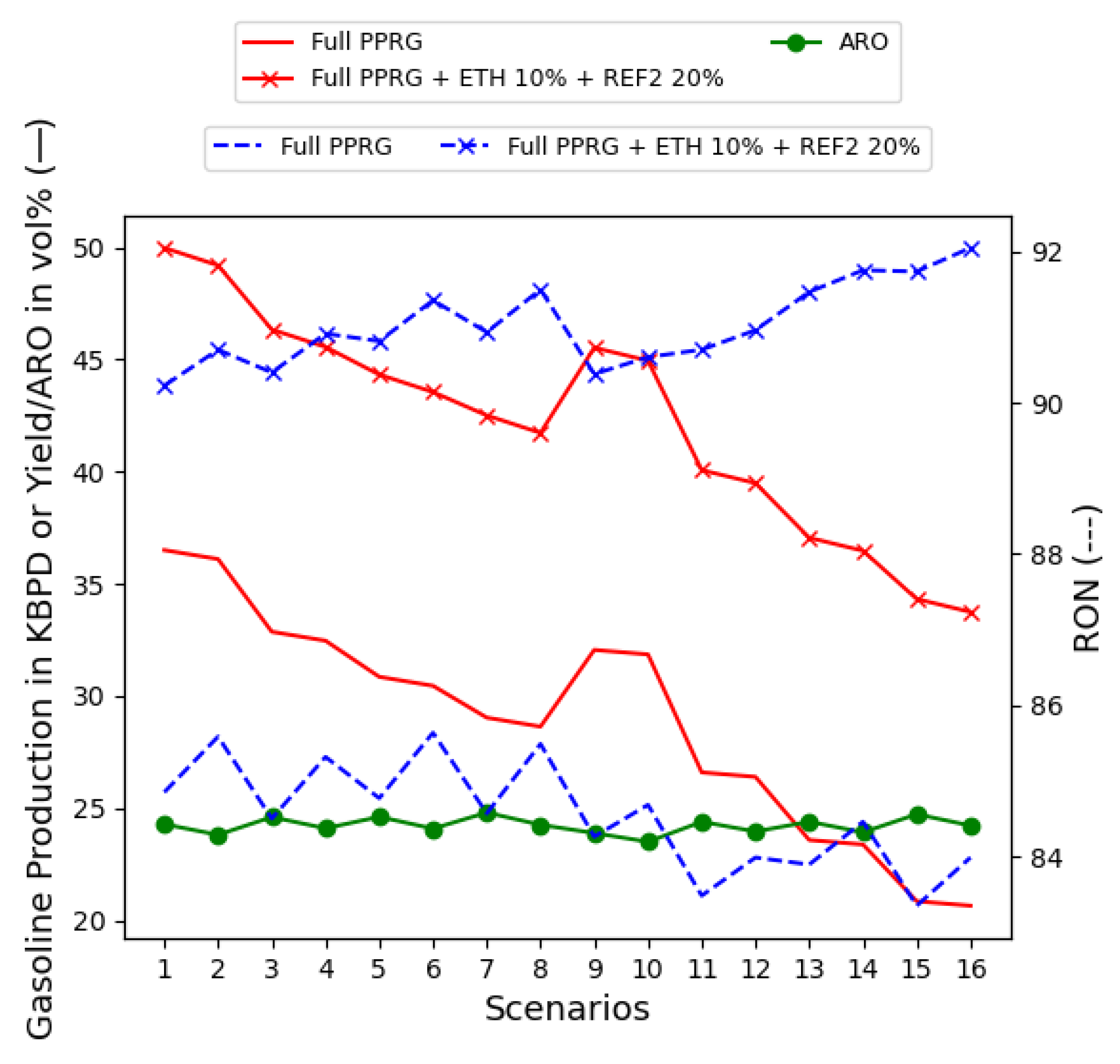
5.3.3. Extra Reformate (REF2) Unit in the Gasoline Blend
6. Interplay of Manufacturer-Made and Supply Chain-Related Dimensions
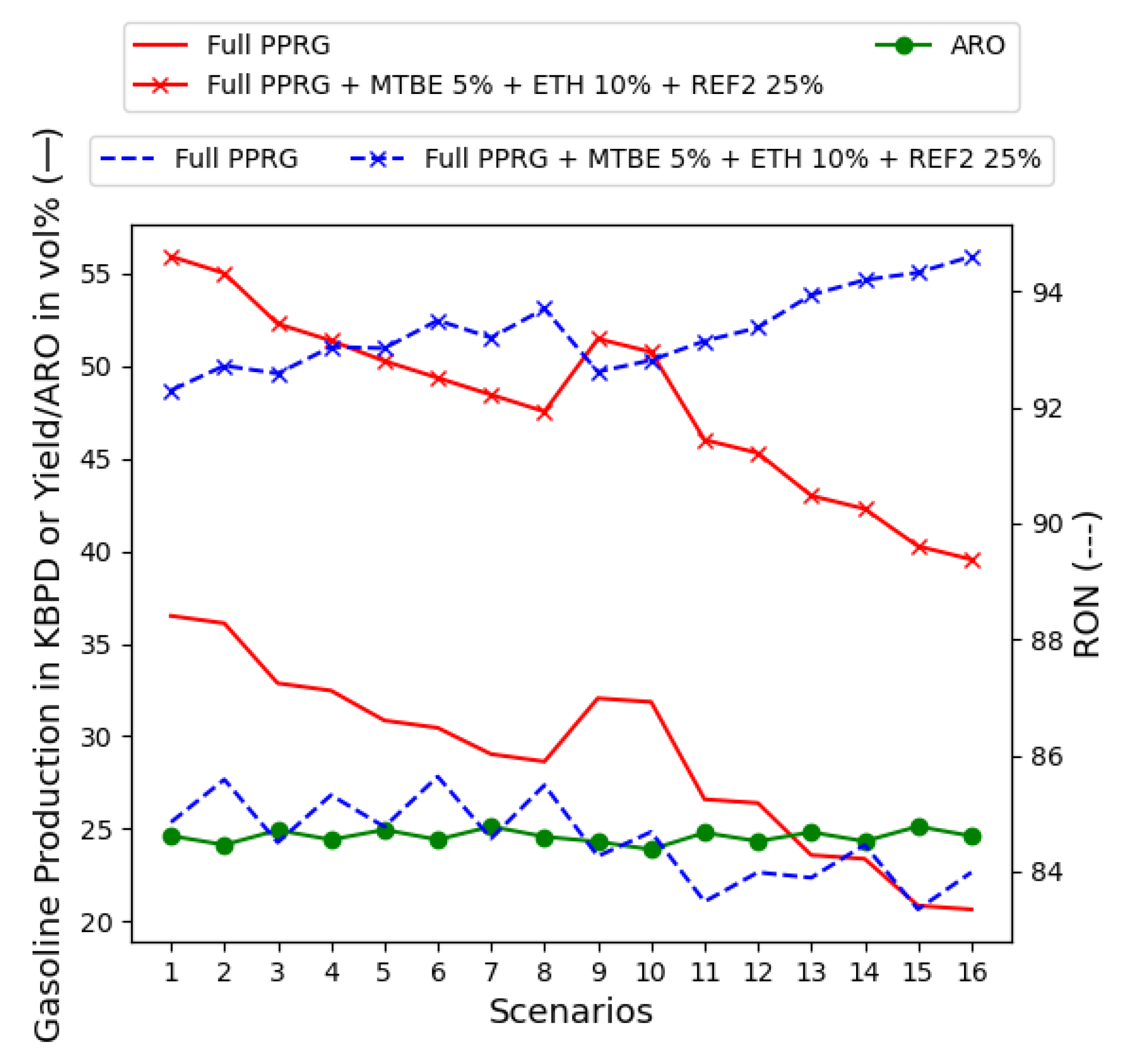
- MTBE at 5% instead of 15–20%, as per today’s recipes. A few points are plotted in Figure 8;
- ETH at 10%, with 5% and 10% plotted in Figure 9;
- An extra reformate (REF2) unit can be added until it reaches the aromatic content limit of 25% by volume, which is the typical maximum allowed content, as in Figure 10.
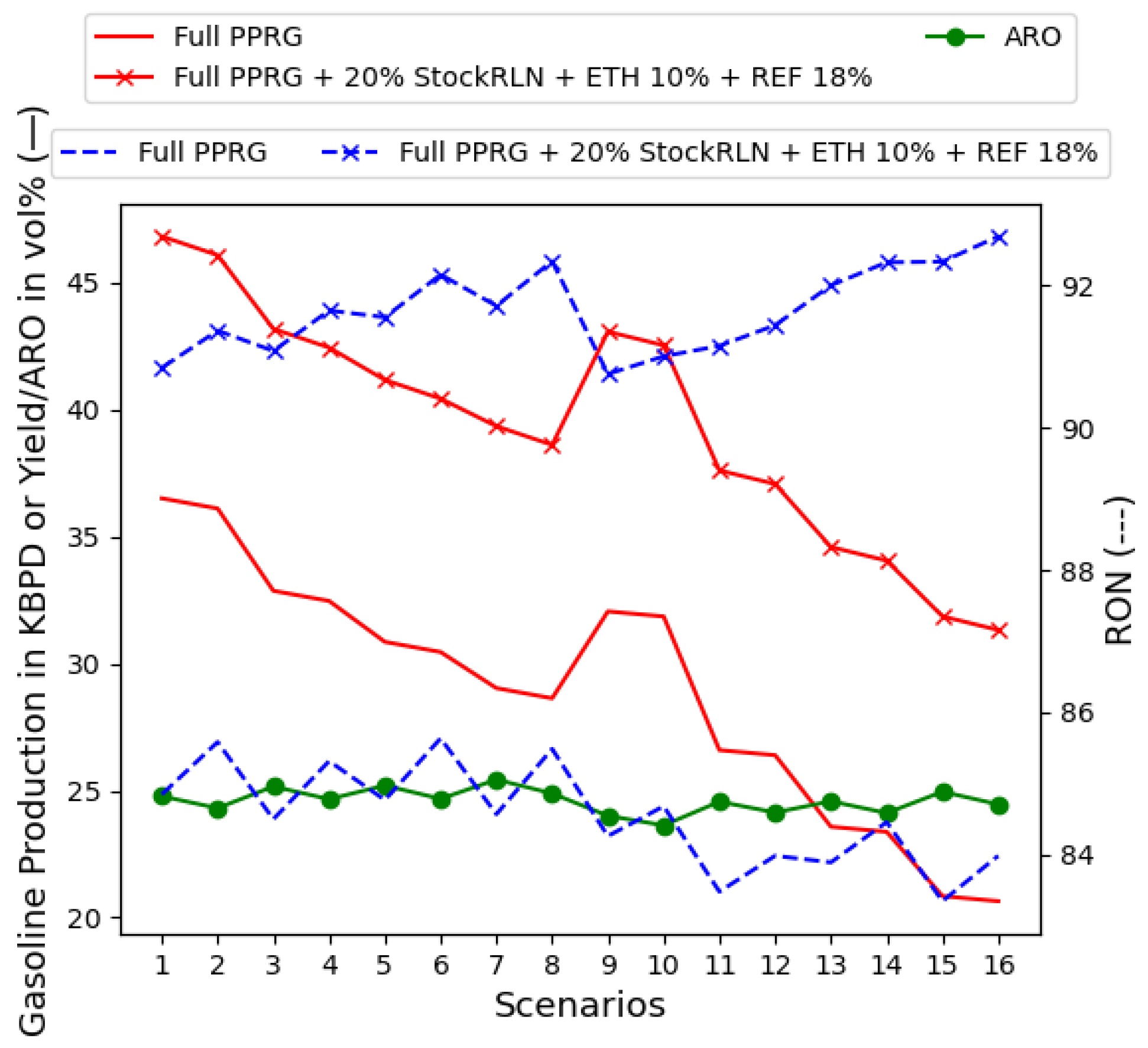
- MTBE at 5% and ETH at 10%, as in Figure 11;
- ETH at 10% and REF2 unit until ARO bounds around 25% by volume, which is found when REF2 is at 20% by volume, as in Figure 12;
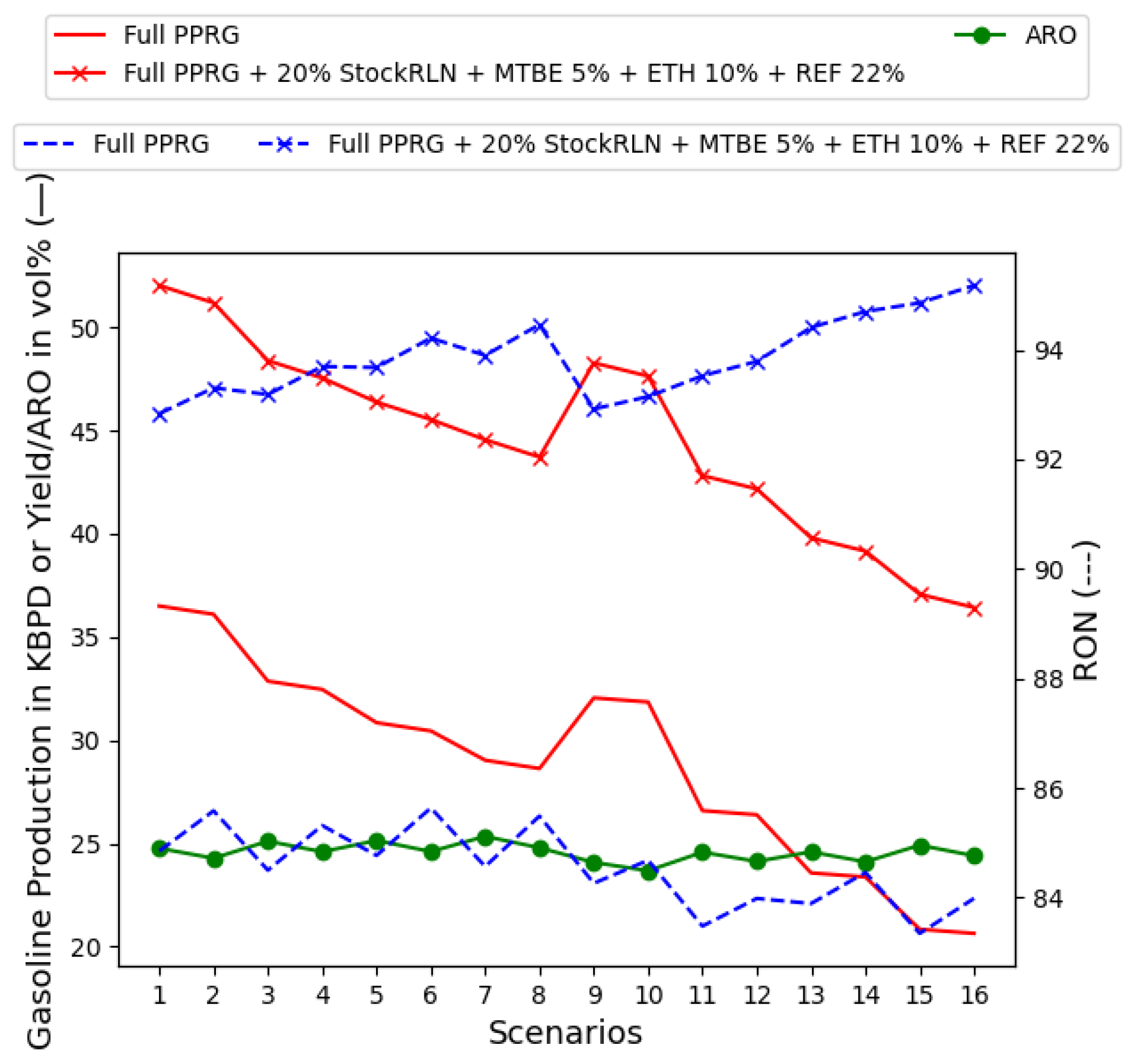
- Mixing all supply chain-related dimensions, MTBE at 5%, ETH at 10%, and REF2 content, until the gasoline mix is bounded by 25% of ARO. This occurs at 25% of REF2 in the gasoline recipe, as in Figure 13.
Reduction in Light Naphtha for Higher-ON Grades of Gasoline
7. Final Remarks
7.1. Banning MTBE from Gasoline
7.2. Reduction in CO2 Footprint Using ETH in Gasoline
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Atmospheric residue |
| ARO | Aromatic |
| BPD | Barrels per day |
| BTX | Benzene-Toluene-Xylene |
| °C | Degrees Celsius |
| C4 | Butane |
| CC | Catalytic cracking |
| CCR | Continuous catalytic reforming |
| CDU | Crude distillation column |
| CCRN | Carbon-chain-rearranged naphtha |
| CH2-OH | Alcohols |
| CH3-CH2-OH | ETH |
| CN | Cracked naphtha |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| E5 | Gasoline with 5% ETH by volume |
| E10 | Gasoline with 10% ETH by volume |
| E15 | Gasoline with 15% ETH by volume |
| E20 | Gasoline with 20% ETH by volume |
| E25 | Gasoline with 25% ETH by volume |
| E73 | Gasoline with 73% ETH by volume |
| ENDO-IVs | Endogenous independent |
| ETBE | Ethyl Tertiary-Butyl Ether |
| ETH | Ethanol |
| EPA | Environmental Protection Agency |
| °F | Fahrenheit |
| FCC | Fuel catalytic cracker |
| GCC | Gulf Cooperation Countries |
| GHG | Greenhouse gases |
| H2 | Hydrogen |
| HN | Heavy naphtha |
| HT | Hydrotreating unit |
| HTLCN | Hydrotreated light cracked naphtha |
| HVGO | Heavy vacuum gasoil |
| ISO | Isomerate stream |
| ISOU | Isomerate unit |
| LCN | Light cycle naphtha |
| LH2 | Liquid hydrogen |
| LN | Light naphtha |
| LNG | Liquid natural gas |
| LP | Linear programming |
| LPG | Liquified petroleum gas |
| LVGO | Light vacuum gasoil |
| MAN | Manufacturing |
| MILP | Mixed-integer linear programming |
| MILP | Mixed-integer nonlinear programming |
| MON | Motor octane number |
| MTBE | Methyl Tertiary Butyl Ether |
| NLP | Nonlinear programming |
| OLE | Olefin |
| ON | Octane number |
| POLY | Polymerate stream |
| POLYU | Polymerate unit |
| PPRG | Pure petroleum-refined gasoline |
| QTB-GC | Quantity-basic gasoline component |
| QLB-GC | Quality-basic gasoline component |
| REF | Reformer unit (reforming units) |
| REFOR | Reformate stream |
| RFCC | Residue fuel catalytic cracker |
| RFN | Reformed naphtha |
| RON | Research octane number |
| RVP | Reid vapor pressure |
| RWN | Raw naphtha |
| SC | Supply chain |
| SC-IVs | Supply chain independent variables |
| SG | Specific gravity |
| SUL | Sulfur |
| USA | United States of America |
| TAME | Tertiary-Amyl Methyl Ether |
| VDU | Vacuum distillation unit |
| 1G | 1 Generation (ethanol made from seeds (wheat corn) |
| 1.5G | 1.5 Generation (ethanol made from cassava |
| 2G | 2 Generation (ethanol made from biomass and lignin) |
| Coefficients representing molecular interaction effects | |
| Aromatic and olefin content of component i | |
| Volume-based aromatic and olefin content of the blend | |
| Secondary aromatic content property of the blend | |
| Ratio of LN diverted to isomerate | |
| Ratio of the stream diverted to the polymerate unit | |
| Refinery throughputs | |
| Volume flow of component i | |
| Butane flowrate to the gasoline pool | |
| Light naphtha flowrate to the gasoline pool | |
| Isomerate flowrate to the gasoline pool | |
| Reformate flowrate to the gasoline pool | |
| Butane output from distillation column | |
| Light naphtha output from distillation column | |
| Heavy naphtha output from distillation column | |
| Atmospheric residue output from distillation column | |
| Total feed flow rate to the conversion complex (CC) | |
| Flow rate of GASES from the CC | |
| Flow rate of LCN from the CC | |
| Flow rate of hydrotreated light cycle naphtha (HT LCN) | |
| Total HT LCN contribution to the gasoline pool | |
| Light cycle naphtha feed flow rate from the conversion complex | |
| Flow rate of the polymerate stream | |
| GASES feed flow rate from the conversion complex | |
| Discrete-event selection when ATR route is via RFCC | |
| Discrete-event selection when ATR route is via FCC | |
| Discrete-event selection for gasoline mode in the CC (RFCC or FCC) | |
| Discrete-event selection for diesel mode in the CC (RFCC or FCC) | |
| Discrete-event selection for low-severity operational modes of the REFU | |
| Discrete-event selection for high-severity operational modes of the REFU | |
| Discrete-event selection for the isomerate unit inclusion | |
| Discrete-event selection for the polymerate unit inclusion | |
| Butane yield for light petroleum (when light crude is selected) | |
| Light naphtha yield for light petroleum | |
| Heavy naphtha yield for light petroleum | |
| Atmospheric residue yield for light petroleum | |
| Butane yield for heavy petroleum | |
| Light naphtha yield for heavy petroleum | |
| Heavy naphtha yield for heavy petroleum | |
| Atmospheric residue yield for heavy petroleum | |
| Reformate yield for low severity | |
| Reformate yield for high severity | |
| Yield of ATR converted to vacuum gas oil (VGO) in the vacuum distillation unit. | |
| Yield of GASES in gasoline mode | |
| Yield of GASES in diesel mode | |
| Yield of LCN in gasoline mode | |
| Yield of LCN in diesel mode | |
| Yield of HT LCN from the stream | |
| Blended specific gravity | |
| Blended Reid vapor pressure (transformed as ) | |
| Blended sulfur concentration | |
| Specific gravity of input i | |
| Transformed RVP property index of input i | |
| Sulfur concentration of input i | |
| IN | Set of all inputs considered in the blend |
| Fixed RON and MON values for component i | |
| Octane number sensitivity () | |
| Sensitivity for the blend | |
| Blended RON and MON values of gasoline | |
| Shifts in RON and MON due to REFU operational severity | |
| Shifts in RON and MON due to CC operational mode | |
| RON and MON reductions due to hydrotreating |
References
- Foong, T.M.; Morganti, K.J.; Brear, M.J.; da Silva, G.; Yang, Y.; Dryer, F.L. The octane numbers of ethanol blended with gasoline and its surrogates. Fuel 2014, 115, 727–739. [Google Scholar] [CrossRef]
- Government of Canada. Deployment of Mid-Level Ethanol Blends; Government of Canada: Ottawa, ON, Canada, 2024.
- Ethanol Producer Magazine. Brazil to Increase Ethanol Blend to 27 Percent. 2015. Available online: https://ethanolproducer.com/articles/brazil-to-increase-ethanol-blend-to-27-percent-12020 (accessed on 27 October 2023).
- Concave. Gasoline Ether Oxygenate Occurrence in Europe, and a Review of Their Rate and Transport Characteristics in the Environment; Technical Report 4/12; Concave: Brussels, Belgium, 2012. [Google Scholar]
- Menezes, B.C.; Moro, L.F.L.; Lin, W.O.; Medronho, R.A.; Pessoa, F.L.P. Nonlinear Production Planning of Oil-Refinery Units for the Future Fuel Market in Brazil: Process Design Scenario-Based Model. Ind. Eng. Chem. Res. 2014, 53, 4352–4365. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Oil Market Report—April 2021; International Energy Agency (IEA): Paris, France, 2021. [Google Scholar]
- U.S. Energy Information Administration (EIA). Refinery Capacity Report; U.S. Energy Information Administration (EIA): Washington, DC, USA, 2021.
- BP. BP Statistical Review of World Energy 2021; BP: London, UK, 2021. [Google Scholar]
- QatarEnergy. Super Gasoline 95 RON. 2024. Available online: https://www.qatarenergy.qa/en/MarketingAndTrading/Marketing/Pages/OurProducts.aspx (accessed on 19 August 2024).
- Jiao, J.; Li, J.; Bai, Y. Ethanol as a vehicle fuel in China: A review from the perspectives of raw material resource, vehicle, and infrastructure. J. Clean. Prod. 2018, 180, 832–845. [Google Scholar] [CrossRef]
- IEA Advanced Motor Fuels. Ethanol Properties. Available online: https://www.iea-amf.org/content/fuel_information/fuel_info_home/ethanol/e10/ethanol_properties (accessed on 30 October 2023).
- SP Global. Brazil to Study Increase in Ethanol-Gasoline Pump Blend to 30Minister. 2023. Available online: https://www.spglobal.com/commodityinsights/en/market-insights/latest-news/agriculture/050123-brazil-to-study-increase-in-ethanol-gasoline-pump-blend-to-30-minister (accessed on 30 October 2023).
- International Energy Agency (IEA). Global Ethanol Production Statistics. IEA Rep. 2022, 1, 1–50. [Google Scholar]
- U.S. Department of Energy. Alternative Fuels Data Center. 2021. Available online: https://afdc.energy.gov/data/10331 (accessed on 27 October 2023).
- Oil & Gas Journal. Persian Gulf Crisis Revives Alcohol Fuels Program in Brazil. Oil Gas J. 1990. Available online: https://www.ogj.com/refining-processing/article/17213479/persian-gulf-crisis-revives-alcohol-fuels-program-in-brazil (accessed on 27 October 2023).
- Aba, M.M.; Le Roux, G.A.; Menezes, B.C. Integrated ethanol and gasoline supply chain planning under environmental constraints: A case study of Brazil. Comput. Chem. Eng. 2022, 164, 107875. [Google Scholar] [CrossRef]
- U.S. Grains Council. United Arab Emirates Ethanol Market Profile. 2019. Available online: https://grains.org/bioethanol/ethanol-market-profiles/united-arab-emirates/ (accessed on 31 October 2023).
- IEA. Share of Global Ethanol Output by Country Between 2017 and 2023. 2024. Available online: https://www.iea.org/data-and-statistics/charts/share-of-global-ethanol-output-by-country-between-2017-and-2023 (accessed on 9 September 2024).
- Zhang, Y. Biofuel Integration in China: Challenges and Opportunities. Energy Policy Inst. 2021, 45, 123–135. [Google Scholar]
- Liu, J. Sustainable Biofuel Crops for Marginal Lands. Renew. Energy J. 2019, 45, 123–135. [Google Scholar]
- Ministry of Industry and Information Technology of China. Pilot Program for E10 Gasoline Implementation. MIIT Rep. 2020, 1, 1–20. [Google Scholar]
- Wang, L. Subsidy Policies for Biofuel Production in China. Energy Policy 2021, 156, 112–120. [Google Scholar]
- Badra, J.; Alowaid, F.; Alhussaini, A.; Alnakhil, A.; AlRamadan, A.S. Understanding of the octane response of gasoline/MTBE blends. Fuel 2022, 318, 123647. [Google Scholar] [CrossRef]
- Hamid, S.H.; Ali, M.A. Effect of MTBE blending on the properties of gasoline. Fuel Sci. Technol. Int. 1995, 13, 509–544. [Google Scholar] [CrossRef]
- Kostin, A.; Macowski, D.H.; Pietrobelli, J.M.; Guillén-Gosálbez, G.; Jiménez, L.; Ravagnani, M.A. Optimization-based approach for maximizing profitability of bioethanol supply chain in Brazil. Comput. Chem. Eng. 2018, 115, 121–132. [Google Scholar] [CrossRef]
- Akbarian-Saravi, N.; Mobini, M.; Rabbani, M. Development of a comprehensive decision support tool for strategic and tactical planning of a sustainable bioethanol supply chain: Real case study, discussions and policy implications. J. Clean. Prod. 2020, 244, 118871. [Google Scholar] [CrossRef]
- Gary, J.H.; Handwerk, J.H.; Kaiser, M.J. Petroleum Refining: Technology and Economics, 4th ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Speight, J.G. The Chemistry and Technology of Petroleum, 5th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Jones, D.S.; Pujadó, P.R. Handbook of Petroleum Processing; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Maples, R.E. Petroleum Refinery Process Economics; PennWell Books: Tulsa, OK, USA, 2000. [Google Scholar]
- Anderson, J.E.; Leone, T.G.; Shelby, M.H.; Wallington, T.J.; Bizub, J.J.; Foster, M.; Lynskey, M.G.; Polovina, D. Octane Numbers of Ethanol-Gasoline Blends: Measurements and Modeling. SAE Int. J. Fuels Lubr. 2012, 5, 647–659. [Google Scholar]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Tomas, M.; Vakili, M. A Review on Production of Light Olefins via Fluid Catalytic Cracking. Energies 2021, 14, 1089. [Google Scholar] [CrossRef]
- Sadeghbeigi, R. Fluid Catalytic Cracking Handbook: An Expert Guide to the Practical Operation, Design, and Optimization of FCC Units; Butterworth-Heinemann: Oxford, UK, 2012. [Google Scholar]
- Antos, G.J.; Aitani, A.M. Catalytic Naphtha Reforming; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Stratiev, D.; Nikolaychuk, E.; Shishkova, I.; Bonchev, I.; Marinov, I.; Dinkov, R.; Yordanov, D.; Tankov, I.; Mitkova, M. Evaluation of accuracy of literature gasoline blending models to predict octane numbers of gasoline blends. Pet. Sci. Technol. 2017, 35, 1146–1153. [Google Scholar] [CrossRef]
- Owen, K.; Coley, T. Gasoline and Diesel Fuel Additives; Wiley: Hoboken, NJ, USA, 1995. [Google Scholar]
- Kelly, J.D.; Mann, J.L. Crude Oil Blend Scheduling Optimization: An Application with Multi-Million Dollar Benefits. Hydrocarb. Process. 2003, 82, 47–53. [Google Scholar]
- Ali, T.; Franzoi, R.E.; Menezes, B.C. Surrogate modeling for nonlinear gasoline blending operations. In Proceedings of the 14th International Symposium on Process Systems Engineering, Kyoto, Japan, 19–23 June 2022; Computer Aided Chemical Engineering. Yamashita, Y., Kano, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 49, pp. 1783–1788. [Google Scholar] [CrossRef]
- Menezes, B.C.; Kelly, J.D.; Grossmann, I.E. Improved Swing-Cut Modeling for Planning and Scheduling of Oil-Refinery Distillation Units. Ind. Eng. Chem. Res. 2013, 52, 18324–18333. [Google Scholar] [CrossRef]
- Franzoi, R.E.; Ali, T.; Al-Hammadi, A.; Menezes, B.C. Surrogate Modeling Approach for Nonlinear Blending Processes. In Proceedings of the 2021 1st International Conference on Emerging Smart Technologies and Applications (eSmarTA), Sana’a, Yemen, 10–12 August 2021; pp. 1–8. [Google Scholar] [CrossRef]






| Components (i) | RON | RVP (Psi) | MON | ARO (v%) | OLE (v%) |
|---|---|---|---|---|---|
| C4 | 93.8 | 7.4704 | 90 | 0 | 0 |
| Light Naphtha | 69.1 | 13.0848 | 67.1 | 0 | 0 |
| Isomerate | 87 | 12.325 | 82 | 0 | 0 |
| Reformate | 98 | 9.425 | 90 | 54 | 20 |
| Polymerate | 96 | 7.54 | 81.78 | 0 | 29 |
| Light Cracked Naphtha | 93 | 7.975 | 82 | 25 | 10 |
| ETH | 108 | 10.005 | 90.7 | 0 | 0 |
| MTBE | 116 | 2.465 | 101 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmednooh, M.; Menezes, B. Ethanol Content Increase in Gasoline Toward Sustainable Liquid Fuels Worldwide: Impacts on Manufacturing and Supply Chains via Discrete-Event Scenarios. Sustainability 2025, 17, 4884. https://doi.org/10.3390/su17114884
Ahmednooh M, Menezes B. Ethanol Content Increase in Gasoline Toward Sustainable Liquid Fuels Worldwide: Impacts on Manufacturing and Supply Chains via Discrete-Event Scenarios. Sustainability. 2025; 17(11):4884. https://doi.org/10.3390/su17114884
Chicago/Turabian StyleAhmednooh, Mahmoud, and Brenno Menezes. 2025. "Ethanol Content Increase in Gasoline Toward Sustainable Liquid Fuels Worldwide: Impacts on Manufacturing and Supply Chains via Discrete-Event Scenarios" Sustainability 17, no. 11: 4884. https://doi.org/10.3390/su17114884
APA StyleAhmednooh, M., & Menezes, B. (2025). Ethanol Content Increase in Gasoline Toward Sustainable Liquid Fuels Worldwide: Impacts on Manufacturing and Supply Chains via Discrete-Event Scenarios. Sustainability, 17(11), 4884. https://doi.org/10.3390/su17114884







