The Suitability of Selected Naturally Growing Plant Species for the Phytostabilization of Heavy Metals at Different Locations on the Slopes of a Zinc Smelting Waste Landfill: The Second Case Study
Abstract
1. Introduction
2. Research Methods
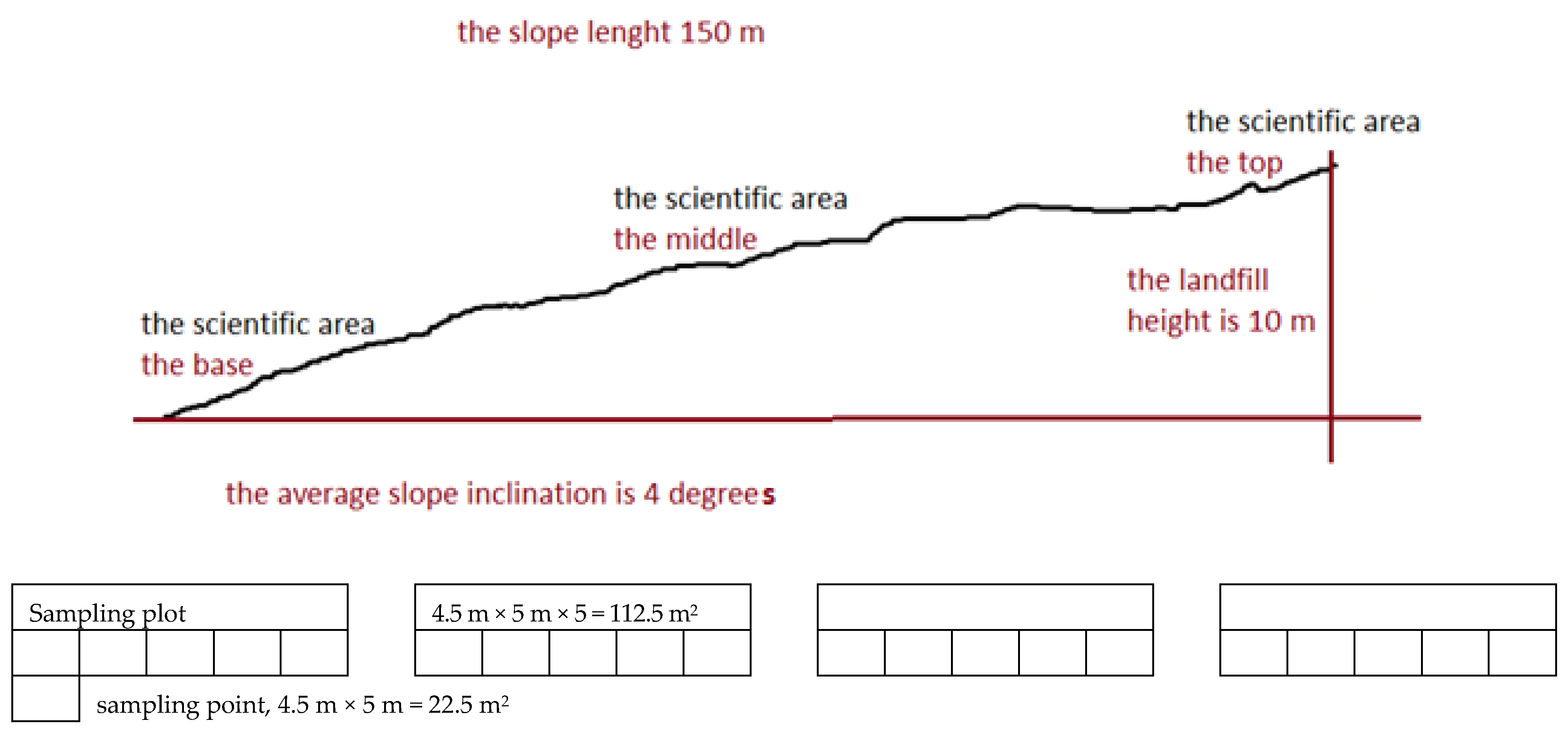
2.1. Research Site
2.2. General Description of Research Methods
3. Results and Discussion
3.1. Results and Overall Assessment
3.2. Detailed Assessment
- Location, i.e., the site of growth on the slope of the landfill, affects the bioaccumulation of the metal.
- The species of plant affects the bioaccumulation of the metal.
- The species and location affect the bioaccumulation of the metal jointly and simultaneously.
3.2.1. Effect of Location
3.2.2. Plant Species or Organ Without Location
- -
- For the BCF of copper—oak shoots, followed by oak leaves; these values differed statistically significantly.
- -
- For the BCF of zinc—oak shoots, followed by herbaceous plants; here too the differences were statistically significant.
- -
- For the BCF of cadmium—rowan shoots, followed by oak leaves; in this case, however, the differences were not statistically significant.
- -
- For the BCF of lead—oak shoots and oak leaves; these values did not differ statistically significantly.
3.2.3. Interaction of Location and Plant Species/Organ
- Differences in the species composition of the plants growing on the landfills;
- Differences in area cover—a parameter in phytosociological research which presents the total area of rectangular projections of vegetation onto the landfill surface;
- Different types of industrial waste;
- Different ages of the landfills—200 vs. 50 years;
- Different technology used to build the landfills;
- Different surroundings;
- Differently calculated interaction—location–species in Krze vs. species–location in Skawina.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qdais, H.A.; Begday, I.V.; Katorgin, I.Y.; Shkarlet, K.Y.; Kharin, K.V.; Bluzhina, A.S.; Likhovid, A.A. Assessment of metals pollution from tailing sites in the North Caucuses Region, Russia. Mine Water Environ. 2018, 37, 815–824. [Google Scholar] [CrossRef]
- Yang, B.; Ren, J.; Wang, M.; Luo, H.; Cao, Y. Concentrations and chemical fractions of Cu, Zn, Cd, and Pb at ten metallurgical sites in China. Environ. Sci. Pollut. Res. 2019, 26, 3603–3611. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Muhammad, F.; Zeng, L.; Li, S.; Huang, X.; Jiao, B.; Shiau, Y.C.; Li, D. Solidification/stabilization of lead-zinc smelting slag in composite based geopolymer. J. Clean. Prod. 2019, 209, 1206–1215. [Google Scholar] [CrossRef]
- Kędzior, R.; Szwalec, A.; Mundała, P.; Skalski, T. Ground beetle (Coleoptera, Carabidae) life history traits as indicators of habitat recovering processes in postindustrial areas. Ecol. Eng. 2020, 142, 1–9. [Google Scholar] [CrossRef]
- Szwalec, A.; Mundała, P.; Kędzior, R. Suitability of selected plant species for phytoremediation: A case study of a coal combustion ash landfill. Sustainability 2022, 14, 1–15. [Google Scholar] [CrossRef]
- Sun, C.; Wu, P.; Wang, G.; Kong, X. Improvement of plant diversity along the slope of an historical Pb–Zn slag heap ameliorates the negative effect of heavy metal on microbial communities. Plant Soil 2022, 473, 473–487. [Google Scholar] [CrossRef]
- Aloway, B.J. Environmental Pollution 22. In Heavy Metals in Soils: Trace Metals and Metaloids in Soils and Their Bioavailability, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Burges, A.; Epelde, L.; Garbisu, C. Impact of repeated single-metal and multi-metal pollution events on soil quality. Chemosphere 2015, 120, 8–15. [Google Scholar] [CrossRef]
- Balmuri, S.R.; Selvaraj, U.; Kumar, V.V.; Anthony, S.P.; Tsatsakis, A.M.; Golokhvast, K.S.; Raman, T. Effect of surfactant in mitigating cadmium oxide nanoparticle toxicity: Implications for mitigating cadmium toxicity in environment. Environ. Res. 2017, 152, 141–149. [Google Scholar] [CrossRef]
- Johnson, A.W.; Gutiérrez, M.; Gouzie, D.; McAliley, L.R. State of remediation and metal toxicity in the Tri-State Mining District, USA. Chemosphere 2016, 144, 1132–1141. [Google Scholar] [CrossRef]
- Bi, C.; Zhou, Y.; Chen, Z.; Jia, J.; Bao, X. Heavy metals and lead isotopes in soils, road dust and leafy vegetables and health risks via vegetable consumption in the industrial areas of Shanghai, China. Sci. Total Environ. 2018, 619–620, 1349–1357. [Google Scholar] [CrossRef]
- Dong, R.; Jia, Z.; Li, S. Risk assessment and sources identification of soil heavy metals in a typical county of Chongqing Municipality, Southwest China. Process Saf. Environ. Prot. 2018, 113, 275–281. [Google Scholar] [CrossRef]
- Szwalec, A.; Mundała, P.; Kędzior, R.; Telk, M.; Lasota, A. The impact of the combustion waste landfill of the Skawina Power Plant on selected elements of the agricultural production area. Geol. Geophys. Environ. 2015, 41, 199–206. [Google Scholar] [CrossRef]
- Civeira, M.S.; Pinheiro, R.N.; Gredilla, A.; de Vallejuelo, S.F.O.; Oliveira, M.L.S.; Ramos, C.G.; Taffarel, S.R.; Kautzmann, R.M.; Madariaga, J.M.; Silva, L.F.O. The properties of the nano-minerals and hazardous elements: Potential environmental impacts of Brazilian coal waste fire. Sci. Total Environ. 2016, 544, 892–900. [Google Scholar] [CrossRef]
- Chaabani, S.; Abdelmalek-Babbou, C.; Ahmed, B.H.; Chaabani, A.; Sebei, A. Phytoremediation assessment of native plants growing on Pb-Zn mine site Northern Tunisia. Environ. Earth Sci. 2017, 76, 2–15. [Google Scholar] [CrossRef]
- Rumayor, M.; Gallego, J.R.; Rodríguez-Valdés, E.; Díaz-Somoano, M. An assessment of the environmental fate of mercury species in highly polluted brownfields by means of thermal. J. Hazard. Mater. 2017, 325, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cortada, U.; Hidalgo, M.C.; Martínez, J.; Rey, J. Impact in soils caused by metal(loid)s in lead metallurgy. The case of La Cruz Smelter (Southern Spain). J. Geochem. Explor. 2018, 190, 302–313. [Google Scholar] [CrossRef]
- Jelenová, H.; Majzlan, J.; Amoako, F.Y.; Drahota, P. Geochemical and mineralogical characterization of the arsenic-, iron-, and sulphur-rich mining waste dumps near Kaňk, Czech Republic. Appl. Geochem. 2018, 97, 247–255. [Google Scholar] [CrossRef]
- Szwalec, A.; Lasota, A.; Kędzior, R.; Mundała, P. Variation in heavy metal content in plants growing on a zinc and lead tailings dump. Appl. Ecol. Environ. Res. 2018, 16, 5081–5094. [Google Scholar] [CrossRef]
- Pandey, V.C. Invasive species based efficient green technology for phytoremediation of fly ash deposits. J. Geoch. Explor. 2012, 123, 13–18. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Antonkiewicz, J.; Gruba, P.; Pająk, M. Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill. Open Chem. 2018, 16, 1143–1152. [Google Scholar] [CrossRef]
- Wójcik, M.; Sugier, P.; Siebielec, P. Metal accumulation strategies in plants spontaneously inhabiting Zn-Pb waste deposits. Sci. Total Environ. 2014, 487, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Woch, M.; Kapusta, P.; Stefanowicz, A. Variation in dry grassland communities along a heavy metals gradient. Ecotoxicology 2016, 25, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization: A green approach to contaminant containment. Adv. Agron. 2011, 112, 145–204. [Google Scholar]
- Phusantisampan, T.; Meeinkuirt, W.; Saengwilai, P.; Pichtel, J.; Chaiyarat, R. Phytostabilization potential of two ecotypes of Vetiveria zizanioides in cadmium-contaminated soils: Greenhouse and field experiments. Environ. Sci. Pollut. Res. 2016, 23, 20027–20038. [Google Scholar] [CrossRef]
- Chunwichit, S.; Phusantisampan, T.; Thongchai, A.; Taeprayoon, P.; Pechampai, N.; Kubola, J.; Pichtel, J.; Meeinkuirt, W. Influence of soil amendments on phytostabilization, localization and distribution of zinc and cadmium by marigold varieties. Sci. Total Environ. 2024, 919, 170791. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Hanus-Fajerska, E.; Gambuś, F.; Muszyńska, E.; Czech, T. Phytostabilization of Zn-Pb ore flotation tailings with Dianthus carthusianorum and Biscutella laevigata after amending with mineral fertilizers or sewage sludge. J. Environ. Manag. 2017, 189, 75–83. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, X.; Tian, C.; Zhang, X.; Liu, J. Effects of amendments and aided phytostabilization of an energy crop on the metal availability and leaching in mine tailings using a pot test. Environ. Sci. Pollut. Res. 2020, 27, 2745–2759. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Stanek, M.; Woch, M.W. High concentrations of heavy metals in beech forest understory plants growing on waste heaps left by Zn-Pb ore mining. J. Geochem. Explor. 2016, 169, 157–162. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Sugier, P.; Woźniak, M.; Grządziel, J.; Gałązka, A.; Stuczyński, T. Activity and diversity of microorganisms in root zone of plant species spontaneously inhabiting smelter waste piles. Molecules 2020, 25, 5638. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Zeng, G.; Sun, C. The role of plant-specific rhizosphere bacterial biomarkers in enhancing soil nutrient cycling in Pb–Zn waste slag. Plant Soil 2025, 1–19. [Google Scholar] [CrossRef]
- Geoportal. Town of Trzebina and Community GeoPortal. Available online: https://trzebinia.geoportal2.pl/map/www/mapa.php?CFGF=wms&mylayers=+granice+OSM+&INFO=startx (accessed on 5 February 2025).
- Kiryk, F. (Ed.) Trzebinia Town, An Outline of the History of the Town and Region; Secesja: Kraków, Poland, 1994. (In Polish) [Google Scholar]
- Suwarzyński, M.; Kryza, A. The problem of flotation waste in zinc and lead ore mining in the Silesian-Cracow deposit province. Przegląd Geolog. 1993, 41, 629–633. (In Polish) [Google Scholar]
- Suwarzyński, M. An outline of the history of the Chrzanów Town and region; Muzeum Maziarskich: Chrzanów, Poland, 2009; Volume III. (In Polish) [Google Scholar]
- Dobis, N. Zinc and lead industry in Poland. Min. Metall. Calendar. Katow. 1938, 1–36. [Google Scholar]
- Szöcs, E.; Schäfer, R.B. Ecotoxicology is not normal. A comparison of statistical approaches for analysis of count and proportion data in ecotoxicology. Environ. Sci. Pollut. Res. 2015, 22, 13990–13999. [Google Scholar] [CrossRef]
- PTG. Particle size distribution and textural classes of soils and mineral materials-classification of Polish Society of Soil Science. Soil Sci. Annual. 2012, 60, 5–16. (In Polish) [Google Scholar]
- Kabata-Pendias, A.; Piotrowska, M.; Motowicka-Terelak, T.; Maliszewska-Kordybach, B.; Filipiak, K.; Krakowiak, A.; Pietruch, C.Z. Guidelines for Assessment of Soil Contamination-Heavy Metals, Sulphur and PAHs; Bibliotheca of Environmental Monitoring: Warszawa, Poland, 1995. [Google Scholar]
- Kłos, A.; Rajfur, M.; Šrámek, I.; Wacławek, M. Use of lichen and moss in assessment of forest contamination with heavy metals in Praded and Glacensis Euroregions (Poland and Czech Republic). Water Air Soil Pollut. 2011, 222, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y. Comparison of moss and pine needles as bioindicators of transboundary polycyclic aromatic hydrocarbon pollution in central Japan. Environ. Pollut. 2018, 234, 330–338. [Google Scholar] [CrossRef]
- Kosior, G.; Přibylová, P.; Vaňková, L.; Kukučka, P.; Audy, O.; Klánová, J.; Samecka-Cymerman, A.; Mróz, L.; Kempers, A.J. Bioindication of PBDEs and PCBs by native and transplanted moss Pleurozium schreberi. Ecotoxicol. Environ. Saf. 2017, 143, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Radziemska, M.; Mazur, Z.; Bes, A.; Majewski, G.; Gusiatin, Z.M.; Brtnicky, M. Using mosses as bioindicators of potentially toxic element contamination in ecologically valuable areas located in the vicinity of a road: A case study. Int. J. Environ. Res. Public Health 2019, 16, 3963. [Google Scholar] [CrossRef]
- Pajak, M.; Jasik, M. Heavy metal (Zn, Pb, Cd) concentration in soil and moss (Pleurozium schreberii) in the Brynica district, southern Poland. iForest-Biogeosci. For. 2011, 4, 176–180. [Google Scholar] [CrossRef]
- Ite, A.E.; Ubong, U.U.; Etesin, U.M.; Nsi, E.W.; Ukpong, E.J.; Ekanem, A.N.; Ufot, U.F.; Udo, A.I. Heavy metals in epiphytic lichens and mosses of oil–producing communities of Eket and Ibeno, Akwa Ibom State-Nigeria. Am. J. Environ. Prot. 2016, 4, 38–47. [Google Scholar]
- Macedo-Miranda, G.; Avila-Pérez, P.; Gil-Vargas, P.; Zarazúa, G.; Sánchez-Meza, J.C.; Zepeda-Gómez, C.; Tejeda, S. Accumulation of heavy metals in mosses: A biomonitoring study. Springerplus 2016, 5, 715. [Google Scholar] [CrossRef]
- Dmuchowski, W.; Gozdowski, D.; Baczewska, A.H.; Bragoszewska, P. Evaluation of various bioindication methods used for measuring zinc environmental pollution. Int. J. Environ. Pollut. 2013, 51, 238–254. [Google Scholar] [CrossRef]
- Dmuchowski, W.; Gozdowski, D.; Brągoszewska, P.; Baczewska, A.H.; Suwara, I. Phytoremediation of zinc contaminated soils using silver birch (Betula pendula Roth). Ecol. Eng. 2014, 71, 32–35. [Google Scholar] [CrossRef]
- Marguí, E.; Queralt, I.; Carvalho, M.L.; Hidalgo, M. Assessment of metal availability to vegetation (Betula pendula) in Pb-Zn ore concentrate residues with different features. Environ. Pollut. 2007, 145, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Reimann, C.; Arnoldussen, A.; Boyd, R.; Finne, T.E.; Koller, F.; Nordgulen, Ø.; Englmaier, P. Element contents in leaves of four plant species (birch, mountain ash, fern and spruce) along anthropogenic and geogenic concentration gradients. Sci. Total Environ. 2007, 377, 416–433. [Google Scholar] [CrossRef]
- Aboal, R.; Fernández, J.A.; Carballeira, A. Oak leaves and pine needles as biomonitors of airborne trace elements pollution. Environ. Exp. Bot. 2004, 51, 215–225. [Google Scholar] [CrossRef]
- Stojnić, S.; Čehova, A.; Kebert, M.; Drekić, M.; Galić, Z.; Kesić, L.; Tepavac, A.; Orlović, S. Heavy metals content in foliar litter and branches of Quercus petraea (Matt.) Liebl. and Quercus robur L. observed at two ICP forests monitoring plots. South-East Eur. For. 2019, 10, 151–157. [Google Scholar] [CrossRef]
- Jankowski, K.; Malinowska, E.; Ciepiela, G.A.; Jankowska, J.; Wiśniewska-Kadżajan, B.; Sosnowski, J. Lead and cadmium content in grass growing near an expressway. Arch. Environ. Contam. Toxicol. 2019, 76, 66–75. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Heavy metals in crop plants: Transport and redistribution processes on the whole plant level. Agronomy 2015, 5, 447463. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Chopra, P.; Chhillar, H.; Ahanger, M.A.; Hussain, S.J.; Maheshwari, C. Regulatory hubs and strategies for improving heavy metal tolerance in plants: Chemical messengers, omics and genetic engineering. Plant Physiol. Biochem. 2021, 164, 260–278. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, F.; Liu, J.L.; Wu, H.T.; Yang, H.; Shi, Y.; Liu, J.; Zhang, Y.F.; Luo, Y.R.; Chen, K.M. Heavy metal transporters: Functional mechanisms, regulation, and application in phytoremediation. Sci. Total Environ. 2022, 809, 151099. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Li, X.; Xiao, J.; Salam, M.M.A.; Chen, G. Evaluation of dendroremediation potential of ten Quercus spp. for heavy metals contaminated soil: A three-year field trial. Sci. Total Environ. 2022, 851, 158232. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.T.; Madrid, F.; Marañón, T.; Murillo, J.M. Cadmium availability in soil and retention in oak roots: Potential for phytostabilization. Chemosphere 2009, 76, 480–486. [Google Scholar] [CrossRef]
- Dong, S.; Li, L.; Chen, W.; Chen, Z.; Wang, Y.; Wang, S. Evaluation of heavy metal speciation distribution in soil and the accumulation characteristics in wild plants: A study on naturally aged abandoned farmland adjacent to tailings. Sci. Total Environ. 2024, 917, 170594. [Google Scholar] [CrossRef]




| Cd BCF | Cu BCF | Pb BCF | Zn BCF | |
|---|---|---|---|---|
| Probability Value | ||||
| Location | 4.28 × 10−6 | 7.2 × 10−166 | 7.9 × 10−107 | 6.9 × 10−241 |
| Plant species/part | 1.7 × 10−264 | 8 × 10−289 | 2.7 × 10−307 | 9.5 × 10−300 |
| Interaction | 5.98 × 10−6 | 4.2 × 10−176 | 1.1 × 10−201 | 4.3 × 10−240 |
| HSD BCF Cd | 0.002 | s.s.d | HSD BCFPb | 0.001141 | s.s.d |
|---|---|---|---|---|---|
| Base | 0.066 | a | Top | 0.074119 | a |
| Middle | 0.055 | b | Base | 0.031613 | b |
| Top | 0.053 | c | Middle | 0.023417 | c |
| HSD BCFCu | 0.0019 | s.s.d | HSD BCFZn | 0.0016 | s.s.d |
| Top | 0.2509 | a | Top | 0.2570 | a |
| Base | 0.1374 | b | Base | 0.1138 | b |
| Middle | 0.1224 | c | Middle | 0.0641 | c |
| BCFCd HSD | 0.004 | s.s.d | BCFPb HSD | 0.00242 | s.s.d |
|---|---|---|---|---|---|
| moss | 0.364 | a | moss | 0.29467 | a |
| herbaceous.plants | 0.023 | b | birch.shoot | 0.01281 | b |
| birch.shoot | 0.020 | b,c | birch.leaves | 0.00875 | c |
| birch.leaves | 0.018 | c | herbaceous.plants | 0.00822 | c |
| rowan.leaves | 0.010 | d | rowan.shoot | 0.00744 | c,d |
| oak.shoot | 0.010 | d | rowan.leaves | 0.00556 | d,e |
| oak.leaves | 0.009 | d | oak.leaves | 0.00428 | e,f |
| rowan.shoot | 0.008 | d | oak.shoot | 0.00267 | f |
| BCFCu HSD | 0.0039 | s.s.d | BCFZn HSD | 0.0033 | s.s.d |
| moss | 0.5311 | a | moss | 0.3593 | a |
| rowan.leaves | 0.1366 | b | birch.leaves | 0.3428 | b |
| herbaceous.plants | 0.1312 | c | birch.shoot | 0.1645 | c |
| rowan.shots | 0.1280 | c | oak.leaves | 0.0839 | d |
| birch.shoot | 0.1220 | d | rowan.shoot | 0.0677 | e |
| birch.leaves | 0.1122 | e | rowan.leaves | 0.0632 | f |
| oak.leaves | 0.1119 | e | herbaceous.plants | 0.0497 | g |
| oak.shoot | 0.0885 | f | oak.shoot | 0.0284 | h |
| BCFCd HSD | 0.005 | s.s.d | BCFPb HSD | 0.0032 | s.s.d |
|---|---|---|---|---|---|
| base-moss | 0.389 | A | top-moss | 0.5071 | a |
| middle-moss | 0.369 | B | base-moss | 0.2117 | b |
| top-moss | 0.335 | C | middle-moss | 0.1652 | c |
| base-birch.shoot | 0.034 | D | top-birch.shoot | 0.0199 | d |
| top-herbaceous.plants | 0.034 | D | top-birch.leaves | 0.0178 | d |
| base-birch.leaves | 0.028 | E | top-herbaceous.plants | 0.0168 | d |
| middle-herbceous.plants | 0.019 | F | top-rowan.shoot | 0.0134 | e |
| top-birch.leaves | 0.018 | f,g | base-birch.shoot | 0.0133 | e |
| base-rowan.leaves | 0.017 | f,g | top-rowan.leaves | 0.0083 | f |
| base-herbaceous.plants | 0.017 | f,g | top-oak.leaves | 0.0065 | f |
| base-rowan.shoot | 0.016 | f,g,h | base-rowan.shoot | 0.0061 | f,g |
| base-oak.shoot | 0.015 | f,g,h | base-birch.leaves | 0.0061 | f,g |
| middle-birch.shoot | 0.014 | h,i | middle-birch.shoot | 0.0053 | f,g |
| top-birch.shoot | 0.013 | h,i,j | middle-herbaceous.plants | 0.0050 | g,h |
| base-oak.leaves | 0.011 | i,j,k | base-rowan.leaves | 0.0048 | g,h,i |
| middle-birch.leaves | 0.009 | j,k | base-oak.leaves | 0.0046 | g,h,i |
| middle-oak.shoot | 0.009 | j,k,l | middle-rowan.leaves | 0.0036 | g,h,i |
| top-oak.leaves | 0.008 | j,k,l | base-oak.shoot | 0.0033 | g,h,i |
| middle-oak.leaves | 0.008 | j,k,l | top-oak.shoot | 0.0032 | g,h,i |
| top-oak.shoot | 0.007 | k,l,m | base-herbaceous.plants | 0.0029 | h,i |
| top-rowan.leaves | 0.007 | k,l,m | middle-rowan.shoot | 0.0028 | h,i |
| middle-rowan.leaves | 0.007 | k,l,m | middle-birch.leaves | 0.0023 | h,i |
| top-rowan.shoot | 0.006 | l,m | middle-oak.leaves | 0.0017 | i |
| middle-rowan.shoot | 0.003 | m | middle-oak.shoot | 0.0016 | i |
| BCFCu HSD | 0.005 | s.s.d | BCFZn HSD | 0.004 | s.s.d |
| top-moss | 0.829 | a | top-birch.leaves | 0.707 | a |
| base-moss | 0.457 | b | top-moss | 0.617 | b |
| middle-moss | 0.307 | c | base-moss | 0.319 | c |
| top-herbaceous.plants | 0.249 | d | top-birch.shoot | 0.214 | d |
| top-oak.leaves | 0.175 | e | base-birch.leaves | 0.209 | e |
| top-birch.leaves | 0.160 | f | base-birch.shoot | 0.172 | f |
| top-birch.shoot | 0.155 | f | top-oak.leaves | 0.144 | g |
| top-rowan.shoot | 0.154 | g | middle-moss | 0.142 | g |
| top-rowan.leaves | 0.154 | g | top-rowan.shoot | 0.131 | h |
| base-rowan.shoot | 0.148 | h | top-rowan.leaves | 0.119 | i |
| middle-rowan.leaves | 0.144 | h | middle-birch.leaves | 0.113 | j |
| top-oak.shoot | 0.130 | i | middle-birch.shoot | 0.107 | k |
| base-birch.shoot | 0.113 | j | top-herbaceous.plants | 0.081 | l |
| base-rowan.leaves | 0.112 | j | base-oak.leaves | 0.080 | l |
| middle-herbaceous.plants | 0.098 | k | top-oak.shoot | 0.044 | m |
| middle-birch.shoot | 0.097 | k | middle-rowan.leaves | 0.041 | m |
| middle-oak.leaves | 0.096 | k | base-rowan.shoot | 0.040 | m |
| middle-birch.leaves | 0.094 | k | base-herbaceous.plants | 0.035 | o |
| base-birch.leaves | 0.082 | l | middle-herbaceous.plants | 0.033 | o,p |
| middle-rowan.shoot | 0.081 | l | middle-rowan.shoot | 0.033 | o,p |
| base-oak.shoot | 0.075 | m | base-rowan.lives | 0.030 | p,r |
| base-oak.leaves | 0.065 | n | middle-oak.lives | 0.028 | r,s |
| middle-oak.shoot | 0.061 | n | base-oak.shoot | 0.025 | s |
| base-herbaceous.plants | 0.047 | o | middle-oak.shoot | 0.016 | t |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwalec, A.; Mundała, P. The Suitability of Selected Naturally Growing Plant Species for the Phytostabilization of Heavy Metals at Different Locations on the Slopes of a Zinc Smelting Waste Landfill: The Second Case Study. Sustainability 2025, 17, 4692. https://doi.org/10.3390/su17104692
Szwalec A, Mundała P. The Suitability of Selected Naturally Growing Plant Species for the Phytostabilization of Heavy Metals at Different Locations on the Slopes of a Zinc Smelting Waste Landfill: The Second Case Study. Sustainability. 2025; 17(10):4692. https://doi.org/10.3390/su17104692
Chicago/Turabian StyleSzwalec, Artur, and Paweł Mundała. 2025. "The Suitability of Selected Naturally Growing Plant Species for the Phytostabilization of Heavy Metals at Different Locations on the Slopes of a Zinc Smelting Waste Landfill: The Second Case Study" Sustainability 17, no. 10: 4692. https://doi.org/10.3390/su17104692
APA StyleSzwalec, A., & Mundała, P. (2025). The Suitability of Selected Naturally Growing Plant Species for the Phytostabilization of Heavy Metals at Different Locations on the Slopes of a Zinc Smelting Waste Landfill: The Second Case Study. Sustainability, 17(10), 4692. https://doi.org/10.3390/su17104692







