Abstract
Heavy metal pollution in landfill soil poses a dual challenge of environmental toxicity and resource depletion. Enzyme-induced carbonate precipitation (EICP) was systematically evaluated as a sustainable stabilization method for cadmium (Cd), lead (Pb), and chromium (Cr) under both solution- and soil-phase conditions. Laboratory-scale experiments demonstrated that EICP achieved over 80% removal efficiency for Cd, Pb, and copper (Cu) in solution-phase systems, while soil-phase trials focused on Cd, Pb, and Cr to simulate realistic field conditions. Optimal performance was achieved using a 1:1 molar ratio of soybean-derived urease (1.0 U/mL) to CaCl2 (0.5 M), with Cd stabilization reaching 91.5%. Vacuum-assisted filtration improved treatment uniformity by 29.2% in clay soils. X-ray diffraction identified crystalline otavite in Cd systems, while Pb and Cu were stabilized via surface adsorption. Sequential extraction confirmed that over 70% of Cd was transformed into carbonate-bound phases. Treated soils met TCLP leaching standards and reuse criteria, maintaining neutral pH (7.2–8.1) and low salinity. Compared to cement-based methods, EICP avoids CO2 release from calcination and fossil fuel use. Carbon in urea is retained as solid CaCO3, reducing emissions by 0.3–0.5 t CO2-eq per ton of soil. These findings support EICP as a scalable, low-carbon alternative for landfill soil remediation.
1. Introduction
Heavy metal contamination in landfill soils has become a pressing environmental issue, presenting substantial risks to ecosystems, groundwater quality, and human health [1]. Landfills, especially in rapidly urbanizing areas, frequently accumulate soils contaminated with cadmium (Cd), lead (Pb), copper (Cu), and other toxic metals originating from industrial waste, electronic debris, and improper disposal practices [2,3]. These heavy metals, known for their persistence [4], bioaccumulation potential [5], and carcinogenic properties [6], can infiltrate surrounding environments via leaching [7], erosion [8], or atmospheric deposition [9], thereby demanding urgent remediation measures. Conventional stabilization methods, such as chemical solidification (e.g., lime, cement) [10] and phytoremediation [11], encounter limitations, including the risk of secondary pollution [12], extended treatment periods [13], and inconsistency with sustainable resource recovery objectives [14]. For example, cement-based stabilization, while effective in immobilizing contaminants, produces considerable CO2 emissions and modifies soil structure, making treated soils unsuitable for reuse in landscaping or construction [15,16]. Likewise, phytoremediation, although environmentally friendly, necessitates years to meet remediation targets and is often restricted to specific metal-plant interactions [17,18].
Enzyme-induced carbonate precipitation (EICP), a bio-mediated soil improvement technique, offers a promising alternative by utilizing plant-derived urease to catalyze urea hydrolysis and precipitate calcium carbonate (CaCO3) [19,20]. This process effectively immobilizes heavy metals via co-precipitation, adsorption, or isomorphic substitution within the carbonate lattice [21,22]. Compared to traditional methods, EICP operates under ambient conditions, reduces soil matrix disturbance, and supports circular economy principles by enabling the reuse of treated soils in infrastructure development or ecological restoration [23]. Recent studies have confirmed EICP’s efficacy in stabilizing sandy soils and sequestering heavy metals in aqueous environments [24,25]. Some studies have further expanded the application of EICP and bio-inspired strategies for multimetal immobilization by emphasizing redox-catalyzed co-precipitation and nano-material-assisted carbonate formation mechanisms, thereby providing novel insights into enhancing treatment selectivity and kinetic control [26,27]. However, its application to heterogeneous landfill soils, which consist of a complex mixture of organic matter, clays, and legacy contaminants, remains largely unexplored.
This study systematically investigates critical research gaps in enzyme-induced carbonate precipitation (EICP) technology through comprehensive feasibility evaluation and process optimization for heavy metal remediation in landfill environments. The research delineates its original contributions via three key advancements that collectively propel the field forward. First, the utilization of plant-based urease extracted from soybean cultivation offers an environmentally sustainable alternative to conventional microbial-derived enzymes, successfully bypassing the technical challenges associated with bacterial culture maintenance. Second, a novel optimization framework is introduced to address the complexities arising from heterogeneous soil matrices containing high concentrations of coexisting metallic pollutants (e.g., cadmium, lead, copper) and ionic salts (e.g., sodium chloride, potassium chloride), thereby replicating realistic landfill geochemical conditions. The study pioneers a synergistic approach by integrating EICP with vacuum-assisted filtration mechanisms, specifically designed to address permeability limitations in dense soil substrates through optimized reagent distribution. By systematically investigating the complex interplay between enzymatic activity, precipitation kinetics, and metal speciation dynamics, this work not only advances the fundamental understanding of biogeochemical precipitation mechanisms but also provides a robust framework for scaling up laboratory innovations into field-applicable remediation strategies for contaminated land management. While the initial design included copper as a representative co-contaminant, subsequent soil-phase experiments substituted copper with chromium (Cr) due to its environmental prevalence and stronger reactivity under carbonate precipitation conditions, as observed in preliminary trials.
The implications of this work transcend technical remediation alone. Successfully stabilizing contaminated soils could repurpose landfill sites as reservoirs of reusable materials, such as roadbed fillers or substrates for green spaces, thereby directly contributing to the United Nations Sustainable Development Goals (SDGs), specifically SDG 11 (Sustainable Cities and Communities) and SDG 12 (Responsible Consumption and Production). Moreover, the carbon-negative characteristic of biogenic CaCO3 formation aligns with global decarbonization efforts, including China’s “dual-carbon” strategy, by offsetting emissions typically associated with conventional soil stabilization techniques. This study, therefore, serves as a bridge between environmental engineering and sustainability science, presenting a viable pathway to harmonize industrial waste management with ecological resilience.
2. Materials and Methods
The experimental framework of this study was designed as a three-phase interconnected approach to comprehensively evaluate the efficiency of Enzyme-Induced Carbonate Precipitation (EICP) in stabilizing heavy metal-contaminated landfill soils and determine optimal treatment parameters for field-scale application. The first phase involved solution-phase feasibility tests to systematically characterize the fundamental precipitation behavior under controlled conditions. This was followed by soil-phase remediation trials, which assessed the efficacy of EICP in complex soil matrices. Finally, parametric optimization studies were conducted in conjunction with an evaluation of reuse potential, thereby integrating laboratory-derived insights with practical engineering considerations through rigorous multidimensional performance analysis.
2.1. Soil Sample Preparation and Characterization
Soil samples were collected from the Xiangyang Municipal Landfill (GPS: 32.0658° N, 112.1539° E). Particle size distribution analysis revealed the soil comprises approximately 35% clay (<0.002 mm), 45% silt (0.002–0.05 mm), and 20% sand (0.05–2 mm), indicating a silty clay texture with moderate plasticity and low permeability, typical of landfill cover soils in this region. A systematic grid-based sampling protocol was employed to ensure comprehensive and representative coverage, with a sampling density of 20 points per 100 m2. Samples were retrieved from subsurface layers ranging between 0.5 and 2.0 m in depth to reduce interference from surface vegetation and recent anthropogenic activities. After collection, the soil samples were subjected to sequential processing steps: air-drying under controlled ambient laboratory conditions, mechanical homogenization using ceramic mortars, and particle size fractionation through 2 mm stainless steel sieves to eliminate coarse anthropogenic debris and achieve uniform particle size distribution. Initial characterization of the collected soil samples included total heavy metal content analysis using microwave-assisted acid digestion and atomic absorption spectroscopy. The native (pre-spiked) concentrations of target metals were 1.3 mg/kg for cadmium (Cd), 24.6 mg/kg for lead (Pb), and 31.1 mg/kg for copper (Cu). These levels are consistent with values reported for weathered landfill cover soils in China. Soil-phase remediation trials were subsequently conducted using artificially spiked concentrations (Cd: 50–200 mg/kg; Pb: 300–800 mg/kg; Cr: 100–400 mg/kg) to simulate moderate to high contamination scenarios.
For the characterization of fundamental physicochemical properties, a variety of analytical techniques were utilized. Particle size distribution was measured by laser diffraction analysis using a Mastersizer 3000 (Malvern Panalytical, Malvern, UK), with samples pre-treated via ultrasonic dispersion. Soil pH values were measured electrometrically in 1:5 (w/v) aqueous suspensions. The suspensions were continuously stirred for 30 min prior to measurement to ensure equilibrium. A calibrated Sartorius PB-10 pH meter (Sartorius, Göttingen, Germany) was used for this purpose. Organic matter content, on the other hand, was determined by thermogravimetric analysis through loss-on-ignition at 550 °C in a muffle furnace. Baseline heavy metal concentrations were quantified by atomic absorption spectroscopy after microwave-assisted acid digestion with a ternary mixture of concentrated HNO3, HCl, and HF, following EPA Method 3052 for complete matrix dissolution.
2.2. EICP Solution-Phase Feasibility Tests
Urease was extracted from defatted soybean meal using a cold aqueous extraction process with a solid-to-solvent ratio of 1:10 (w/v) maintained at 4 °C for 24 h. The crude extract was sequentially clarified by centrifugation at 10,000× g for 15 min (4 °C) and subsequently filtered through a sterile membrane with a pore size of 0.22 μm. Enzymatic activity was quantified using a standardized ammonium liberation assay conducted at 30 °C for 15 min, based on the indophenol blue colorimetric method. One unit (U) of urease activity was defined as the enzymatic capacity to catalyze the production of 1 μmol ammonia per minute under standard conditions (30 °C). This was measured spectrophotometrically using the indophenol blue method.
For experimental contamination simulations, stock solutions of heavy metals were prepared by dissolving cadmium nitrate tetrahydrate, lead nitrate, and copper nitrate trihydrate in ultrapure water. These reagents were commercially available analytical-grade (AR) compounds with typical purities of ≥99.0%. The metal combinations included binary systems (Cd–Pb and Cd–Cu pairs) and ternary systems (Cd, Pb, and Cu), with each metal in the ternary configuration dosed at 167 mg/L to maintain a total concentration of 500 mg/L. This equimolar design enabled direct comparison with single-metal conditions without altering ionic strength. Ionic competition effects were simulated by varying the ionic strength through controlled additions of sodium chloride and potassium chloride solutions (ranging from 0.1 to 1.0 M).
The treatment protocol consisted of combining enzymatically active extracts (0.5–2.0 U/mL) with a calcium-urea substrate solution at an equal volume ratio (1:1 v/v). The substrate solution contained urea (0.25–1.0 M) and calcium chloride (0.25–1.0 M). Reactions were carried out in a temperature-controlled orbital shaker incubator set at 30 °C with continuous agitation at 120 rpm. The pH of the solution was monitored hourly using a calibrated multi-parameter probe until the reaction was terminated after 24–48 h. The resultant precipitates were harvested via vacuum filtration using pre-weighed 0.45 μm nylon membranes, dehydrated to constant mass in a vacuum desiccator at 60 °C, and subsequently characterized for crystalline phase composition by X-ray diffraction analysis performed on a Bruker D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany, Cu-Kα radiation, 40 kV/40 mA, 2θ range 5–70°).
2.3. Soil-Phase Remediation Experiments
The experimental soils were systematically contaminated via aqueous spiking using analytical-grade cadmium nitrate tetrahydrate, lead nitrate, and potassium dichromate solutions. Target heavy metal concentrations were set within specific ranges: cadmium (50–200 mg/kg), lead (300–800 mg/kg), and chromium (100–400 mg/kg) [28,29], expressed as elemental metal concentrations. Copper was not included in the soil-phase trials due to its relatively weak stabilization performance in multi-metal systems and the absence of identifiable crystalline Cu-carbonate phases under XRD analysis. These findings suggest that Cu primarily underwent non-crystalline surface complexation, limiting the interpretability of soil-phase speciation analysis. Chromium (Cr), in contrast, was introduced as a redox-sensitive contaminant to evaluate EICP’s efficacy on a broader spectrum of environmentally significant metals. The contaminant solutions were uniformly mixed with the soil matrices through sequential wetting and homogenization processes. Subsequently, the spiked soil underwent a 30-day equilibration period under controlled environmental conditions (25 °C, 70% RH) to promote contaminant aging and stabilization.
The bioremediation protocol involved the application of enzyme-urea-calcium chloride solutions, with treatment volumes adjusted to 0.5–2.0 mL per gram of contaminated soil. For iterative treatment cycles, sequential applications were alternated with ambient air-drying phases (24–48 h) to restore soil porosity after each saturation cycle. To address hydraulic conductivity limitations in fine-textured soils, a vacuum-enhanced infiltration system (−50 kPa applied pressure, 30 min duration) was integrated to ensure uniform reagent distribution.
The EICP treatment protocol consisted of the gradual infiltration of the enzyme–substrate mixture into contaminated soils via gravity flow under ambient pressure, unless otherwise specified. For each treatment cycle, the pre-prepared urease–urea–CaCl2 solution was carefully and evenly dispensed onto the soil surface using micropipettes in small increments (approximately 0.2 mL per addition) to ensure uniform wetting while preventing surface ponding. Following each infiltration step, the samples were left undisturbed for 2–4 h to facilitate complete penetration. Between successive treatment cycles, the soil specimens were subjected to air-drying under controlled conditions (25 °C and 70% relative humidity) for 24–48 h to restore the pore structure and permeability.
For vacuum-assisted infiltration trials, a laboratory-scale vacuum infiltration apparatus was utilized, comprising a Buchner funnel assembly connected to a diaphragm vacuum pump. A constant negative pressure of −50 kPa was applied for 30 min to enhance reagent infiltration, particularly in clay-rich matrices. To prevent particle loss, the soil sample was placed on a pre-wetted filter membrane, and the reagent solution was gradually introduced onto the surface while vacuum suction uniformly drew it through the sample. This configuration not only effectively reduced infiltration time but also ensured deep penetration of the reagent. Key experimental parameters for both solution-phase and soil-phase experiments are presented in Table 1.

Table 1.
Summary of key experimental parameters in solution-phase and soil-phase experiments.
A multi-tiered analytical framework was employed to comprehensively evaluate treatment efficacy. The dynamics of metal speciation were assessed via sequential chemical extraction according to the Tessier fractionation protocol, which partitions heavy metals into five operationally defined phases: ion-exchangeable, acid-soluble (carbonate-associated), reducible (iron/manganese oxide-bound), oxidizable (organic matter-complexed), and residual (lattice-incorporated) fractions. Stabilization performance was quantified by measuring the depletion of the exchangeable fraction, with the metric calculated as follows:
where Cinitial and Cpost treatment denote the exchangeable-phase metal concentrations before and after treatment, respectively. Solid-phase changes were further evaluated using gravimetric analysis, with the mass gain ratio calculated based on mass balance comparisons of oven-dried soil samples (105 ± 2 °C, 24 h) before and after carbonate precipitation.
2.4. Leachability and Reuse Potential Assessment
The environmental risk assessment was conducted using the standardized Toxicity Characteristic Leaching Procedure (TCLP) in accordance with EPA Method 1311. A buffered acetic acid solution (pH 2.88), prepared according to EPA Method 1311 by adjusting glacial acetic acid with sodium hydroxide, was used to simulate acidic leaching conditions. The buffer had an approximate capacity of 0.05–0.10 mol/L at this pH, sufficient to mimic landfill leachate behavior under acidic stress. The resulting leachate supernatants were analyzed quantitatively for metal concentrations using flame atomic absorption spectrophotometry. Regulatory compliance was assessed against China’s GB 5085.3-2007 hazard identification thresholds for solid waste disposal [30]. Additionally, the reusability potential was systematically evaluated based on multiple critical parameters outlined in the Chinese landscaping substrate standard (CJ/T 340-2016) [31]. These parameters included soil pH buffer capacity within the range of 5.5–8.5, soluble salt content below 0.3% mass fraction (determined via electrical conductivity conversion), and heavy metal mobilization potential as measured through the synthetic precipitation leaching protocol.
3. Results
3.1. Solution-Phase Feasibility Tests
The effectiveness of EICP in stabilizing heavy metals was initially evaluated using aqueous solutions contaminated with single metals—cadmium, lead, or copper—at concentration gradients ranging from environmentally relevant levels (50 mg/L) to industrially significant concentrations (500 mg/L). As shown in Figure 1, the EICP technique exhibited consistently high stabilization performance across all tested conditions, achieving minimum removal efficiencies of 80% regardless of metal type or concentration. Cadmium demonstrated the highest stabilization efficiency, reaching a maximum removal rate of 92.3% at the highest tested concentration of 500 mg/L. Lead and copper showed slightly lower performance, with removal efficiencies of 88.7% and 84.5%, respectively, under comparable conditions. The experimental data revealed an intriguing concentration-dependent trend: treatment efficiency marginally improved as metal concentrations increased. Specifically, for cadmium, the removal efficiency increased from 88.5% at 50 mg/L to 92.3% at 500 mg/L. This observed trend may be attributed to the enhanced local supersaturation at higher metal concentrations, which can promote carbonate precipitation by facilitating heterogeneous nucleation—a mechanism supported by previous studies [21].
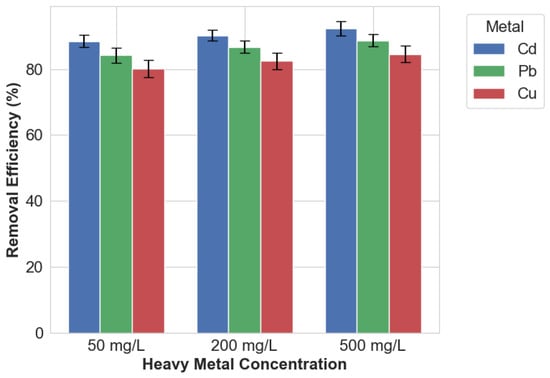
Figure 1.
Removal efficiencies of Cd, Pb, and Cu.
Detailed XRD analysis elucidated the crystalline composition and metal sequestration mechanisms in contaminated systems, revealing distinct mineralization pathways that are dependent on metal speciation. In cadmium-impacted environments, the precipitated solids were identified as two discrete carbonate phases: calcite (CaCO3, JCPDS 05-0586) and otavite (CdCO3, JCPDS 42-1342), indicating concurrent crystallization behavior. The phase distribution was unambiguously confirmed by characteristic diffraction signatures; specifically, the reflection at 24.9° was indexed to the (012) plane of otavite, while the peak at 29.4° corresponded to the (104) plane of calcite. This crystallographic evidence, as presented in Figure 2, substantiates the formation of stable metal carbonate phases via enzyme-induced precipitation processes. These findings indicate partial lattice substitution, where Cd2+ ions replaced Ca2+ positions within the carbonate matrix, leading to detectable crystalline phase segregation. In contrast, lead- and copper-containing systems exhibited fundamentally different crystallization behaviors, characterized by dominant calcite signatures with prominent peaks at 29.4° (104 plane) and 48.5° (113 plane), without evidence of secondary carbonate phases. The absence of discernible Pb- or Cu-bearing crystalline compounds suggests alternative stabilization mechanisms, likely involving surface complexation on calcite particles or incorporation into amorphous phases via co-precipitation rather than the formation of discrete mineral structures.
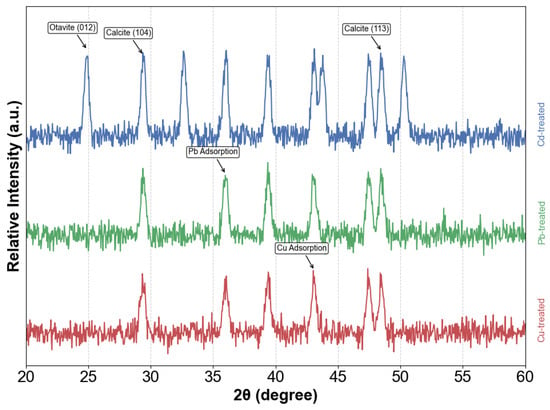
Figure 2.
XRD patterns of precipitates from Cd, Pb, and Cu systems.
The competitive interactions among metal ions substantially impacted the stabilization efficacy of EICP in multi-metal systems compared to single-metal conditions, as shown in Figure 3. In binary metal combinations, the removal efficiency of cadmium decreased markedly by 16–22%, reducing from 92.3% under single-metal conditions to 72.1% in the presence of lead. Copper demonstrated the most significant inhibitory effects, with its removal efficiency declining from 84.5% in isolation to 60.7% when co-existing with cadmium. Lead exhibited intermediate competitive behavior, maintaining a removal efficiency of 65.8% in cadmium-lead systems.

Figure 3.
Competitive removal efficiencies in multi-metal systems.
For the ternary metal system containing cadmium, lead, and copper, the removal efficiencies were ranked as Cd > Pb > Cu, with respective values of 76.4%, 68.9%, and 62.1%. This hierarchy aligns with the relative performance observed in single-metal systems but occurs at lower magnitudes due to ionic competition for carbonate nucleation sites. Cadmium exhibited a relatively higher resistance to competitive displacement, which is consistent with its stronger carbonate binding affinity. This finding is corroborated by the persistent detection of otavite (CdCO3) crystallites in XRD analysis (Figure 2), even under competitive conditions.
The introduction of sodium and potassium chloride electrolytes enhanced competitive effects, significantly impacting cadmium removal. In cadmium-lead systems containing 1.0 M NaCl, cadmium removal decreased to 58.2%, whereas lead removal dropped to 51.1%. Visual MINTEQ simulations demonstrated that sodium and potassium ions preferentially occupied surface coordination sites on calcite substrates, thereby reducing the availability of active sites for heavy metal co-precipitation.
As shown in Figure 4, the concurrent presence of NaCl and KCl significantly suppressed cadmium stabilization, with Cd removal efficiency exhibiting a clear inverse relationship with salt concentration. In systems containing 1.0 M NaCl, cadmium retention decreased to 63.7%, corresponding to a 28.6% reduction relative to the salt-free system (92.3%). Notably, potassium ions demonstrated a more pronounced inhibitory effect compared to sodium ions, as evidenced by the reduced Cd removal efficiency of 55.2% at an equivalent KCl concentration. This suppression mechanism is consistent with the competing cation theory, wherein monovalent cations (Na+/K+) exhibit stronger surface affinity than Cd2+ during calcite nucleation. The preferential adsorption of these alkali metal ions effectively blocks reactive sites on calcium carbonate surfaces, thereby restricting Cd2+ incorporation into the mineral lattice.
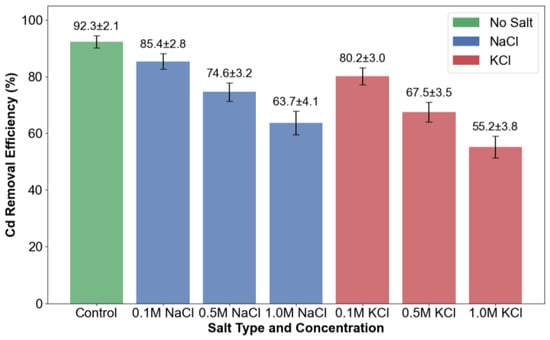
Figure 4.
Salt interference on Cd removal efficiency.
Geochemical modeling performed using Visual MINTEQ, as shown in Figure 5, revealed a pronounced dependence of calcite precipitation dynamics on solution electrical conductivity (EC, dS/m). While EC is not equivalent to ionic strength, it serves as a practical indirect indicator in aqueous geochemical systems and was used here to model the saturation index behavior. The calcite saturation index (SI) decreased exponentially from 2.4 to −0.3 as EC increased from 0.1 to 5.0 dS/m, crossing the thermodynamic stability threshold (SI = 0) at approximately EC = 2.8 dS/m. Under high-salinity conditions, sub-saturation states (SI < 0) inhibit calcium carbonate nucleation due to constraints imposed by the dissolution-precipitation equilibrium. This thermodynamic limitation provides a mechanistic explanation for the observed decrease in Cd sequestration efficiency under high-salinity conditions, as metastable calcite phases fail to supply sufficient nucleation sites for heavy metal incorporation.
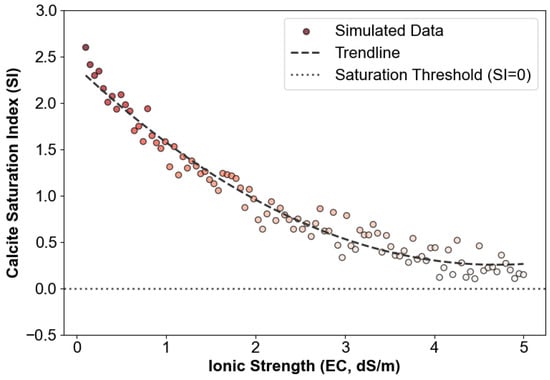
Figure 5.
Impact of ionic strength on CaCO3 saturation. The saturation index (SI) was simulated as a function of electrical conductivity (EC). The color gradient of the data points reflects relative SI magnitude for visual clarity and does not indicate distinct experimental conditions.
The stabilization efficiency of cadmium was significantly influenced by both urease activity and CaCl2 concentration, as shown in Figure 6. At 0.5 M CaCl2, increasing the urease concentration from 0.5 to 1.0 U/mL improved the stabilization performance from 71.5% to 89.2%. However, further increasing the urease concentration to 2.0 U/mL led to a decrease in efficiency to 84.7%, suggesting the existence of an optimal enzyme activity threshold. This trend was consistent across different CaCl2 concentrations, albeit with varying performance characteristics. For instance, at 0.25 M CaCl2, the maximum stabilization efficiency was 75.8% when paired with 1.0 U/mL urease. In contrast, higher calcium concentrations exhibited diminishing returns; under 1.0 M CaCl2 conditions with the same enzyme loading, the efficiency dropped to 82.4%, possibly due to rapid precipitation kinetics causing soil matrix pore clogging.
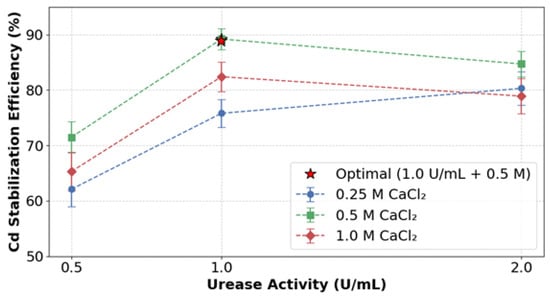
Figure 6.
Optimization of urease and CaCl2 concentrations.
The experimental configuration combining 1.0 U/mL urease with 0.5 M CaCl2 achieved a peak stabilization efficiency of 89.2% while minimizing reagent consumption. Comparative analysis demonstrated that lower calcium concentrations (0.25 M) provided an insufficient supply of Ca2+ ions for effective carbonate nucleation, while higher calcium concentrations (1.0 M) led to excessively rapid precipitation, disrupting the homogeneous distribution of precipitates. These results highlight the critical balance necessary between enzymatic reaction rates and ionic concentration gradients to achieve optimal heavy metal immobilization.
3.2. Soil-Phase Remediation Trials
The enzymatic induced carbonate precipitation (EICP) treatment significantly altered the heavy metal speciation profiles in contaminated soils, as revealed by sequential extraction analyses (Figure 7). In cadmium-polluted systems, the chemically mobile exchangeable fraction decreased markedly from 52.3% in untreated samples to 10.5% after treatment, while the carbonate-associated species increased substantially from 8.1% to 73.2%. Similar redistribution trends were observed in lead- and chromium-contaminated systems, where the exchangeable fractions of lead and chromium decreased from 38.7% to 18.4% and from 45.6% to 25.3%, respectively, accompanied by proportional increases in carbonate-bound phases to 68.9% and 58.1%. Notably, the residual fraction accounted for less than 1% of the total metal content across all tested elements, confirming that EICP facilitates the transition of metals from bioavailable exchangeable forms to more stable carbonate-associated species. Notably, cadmium was incorporated into crystalline mineral matrices via lattice substitution, while lead and chromium were primarily retained in non-crystalline carbonate-bound phases.
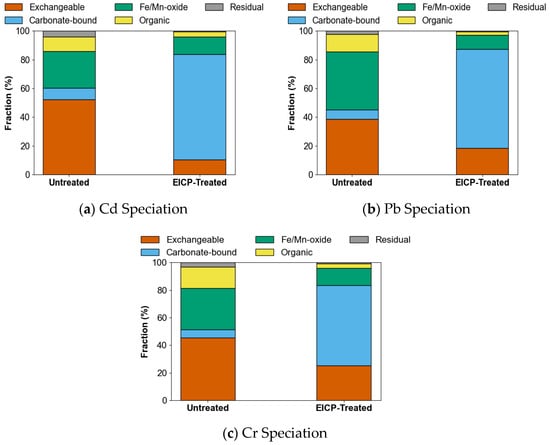
Figure 7.
Metal speciation shifts in Cd-, Pb-, and Cr-contaminated soils post-EICP.
In the soil-phase experiments, the treatment efficiency followed a metal-specific trend of cadmium > lead > chromium. Although copper was used in solution-phase tests, it was replaced by chromium in the soil-phase trials due to copper’s limited crystalline precipitation behavior and lower detectability in solid-phase characterization. This substitution ensured a more accurate assessment of stabilization mechanisms in heterogeneous soil matrices. The incomplete conversion of the exchangeable fraction of chromium (25.3% residual mobility) indicates potential limitations in Cr3+ accessibility for carbonate precipitation under neutral pH conditions, likely due to pH-dependent speciation changes that merit further investigation.
The stabilization efficiency of Cd was significantly influenced by the solution-to-soil ratio and treatment cycles, as shown in Figure 8. For a solution-to-soil ratio of 1.0 mL/g, increasing the number of treatment cycles from one to three enhanced the stabilization efficiency from 78.7% to 91.5%. Specifically, two cycles achieved an efficiency of 89.2%, indicating that the majority of stabilization occurred within the first two applications. In contrast, at a ratio of 2.0 mL/g, additional cycles resulted in diminishing returns; three cycles yielded only 85.1% efficiency compared to 84.6% for two cycles. This suggests that excessive solution volumes (>1.0 mL/g) may saturate soil pores, thereby limiting further carbonate deposition.
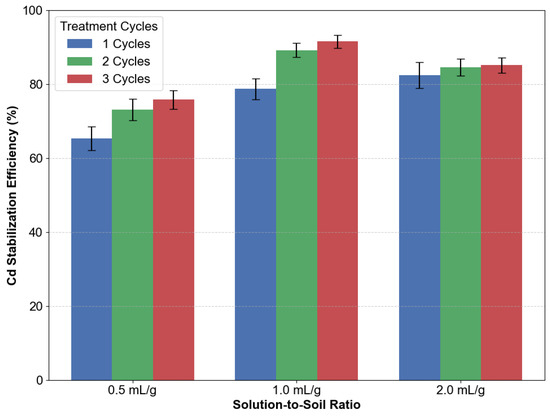
Figure 8.
Impact of solution-to-soil ratios and treatment cycles.
Lower solution volumes (0.5 mL/g) demonstrated gradual yet restricted improvement across multiple cycles, achieving 75.8% efficiency after three cycles. Single-cycle treatments at this ratio resulted in 65.3% efficiency, emphasizing the importance of repeated applications under low reagent conditions. Nevertheless, even after three cycles at 0.5 mL/g, the performance remained inferior to that of a single cycle at 2.0 mL/g (82.4%), which underscores the inherent trade-off between reagent consumption and treatment efficacy.
The integration of vacuum-assisted filtration markedly improved stabilization efficiency in low-permeability clay soils but yielded limited additional benefits in sandy soils (Figure 9). For clay soils, the application of vacuum enhanced Cd stabilization efficiency from 52.3% to 75.1%, representing a 22.8% improvement. Conversely, in sandy soils, the increase was only 5.5% under vacuum conditions.
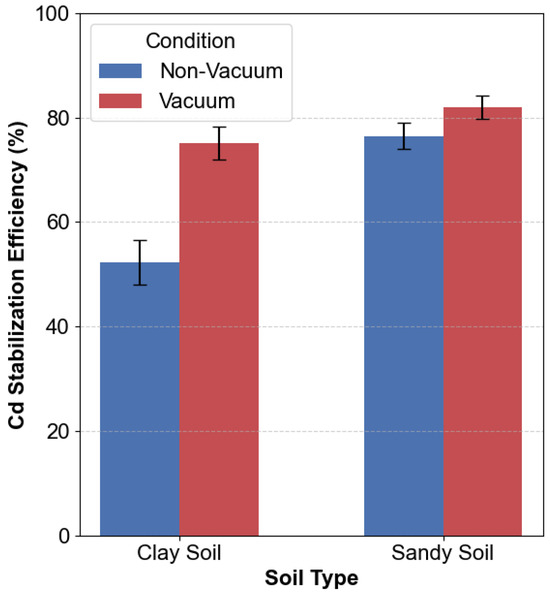
Figure 9.
Vacuum-assisted vs. non-vacuum treatment efficiency.
The vacuum system decreased treatment time in clay soils by 48%, achieving the target efficiency of 75% within 24 h compared to 48 h for non-vacuum treatments. This significant acceleration can be attributed to enhanced reagent infiltration under negative pressure, which effectively overcomes the naturally low hydraulic conductivity of clay (approximately 1 × 10−7 cm/s). In sandy soils, the higher intrinsic permeability (approximately 1 × 10−3 cm/s) diminished the reliance on vacuum assistance, as gravity-driven infiltration alone achieved greater than 75% efficiency within 12 h.
3.3. Parametric Optimization and Reuse Assessment
The stabilization efficiency of Cd demonstrated a nonlinear relationship with urease activity, reaching a peak at 1.0 U/mL and subsequently declining at higher concentrations (Figure 10). At 1.0 U/mL, the maximum efficiency of 89.2% was observed, as confirmed by the quadratic trendline, which identified this as the optimal urease activity level. Reducing urease activity to 0.5 U/mL resulted in a 23.5% decrease in efficiency, suggesting that insufficient urea hydrolysis occurred to support effective carbonate precipitation. Notably, efficiency plateaued between 1.0 and 1.5 U/mL, reaching a peak of 91.5% at 1.5 U/mL—a mere 2.3% improvement over the value at 1.0 U/mL. Above 1.5 U/mL, efficiency decreased to 84.7% at 2.0 U/mL, likely due to accelerated urea depletion before complete utilization of Ca2+. This inverse relationship at higher enzyme activities indicates saturation effects, where excessive urease activity surpasses substrate availability.
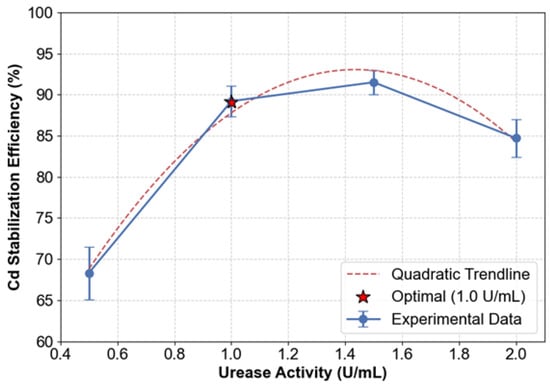
Figure 10.
Dose–response relationship of urease activity.
Mass gain measurements exhibited a consistent trend, rising from 12.3% at 0.5 U/mL to a peak of 16.8% at 1.5 U/mL, followed by a decline to 14.5% at 2.0 U/mL. While the maximum mass gain at 1.5 U/mL correlates with the highest level of carbonate deposition, this did not result in proportional efficiency improvements due to the formation of non-stabilizing aggregates from excess carbonates.
The stabilization performance of Cd exhibited a logarithmic dependence on treatment cycles, as illustrated in Figure 11, with progressively diminishing marginal returns observed beyond three treatment iterations. The initial application achieved a stabilization efficiency of 78.7% with a relative cost expenditure of 100 units. Subsequent optimization through three operational cycles enhanced efficiency to 91.5%, representing a 46% cost increment (265 units) for a measurable 12.8% performance improvement. Further extension to five treatment cycles yielded only limited gains (92.3% efficiency at 420 cost units), revealing that over 95% of the maximal attainable stabilization capacity was achieved within the primary three-cycle operational framework. This saturation pattern suggests an asymptotic approach to maximum stabilization potential, where additional cycles provide minimal practical benefit relative to the substantially increased resource investment.
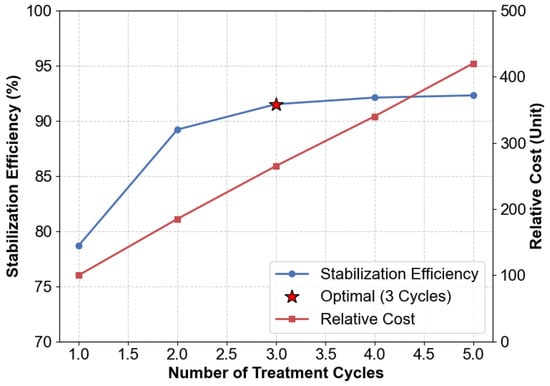
Figure 11.
Trade-offs between treatment cycles, efficiency, and cost.
Economic analysis demonstrated a near-linear cost increase driven by reagent consumption and labor, escalating from 100 units for one cycle to 420 units for five cycles. In contrast, stabilization efficiency exhibited exponential plateauing, with cycles four and five contributing merely 0.6% and 0.2% additional efficiency, respectively. Furthermore, mass gain ratios corroborated this trend, indicating that three cycles deposited 89% of the total achievable carbonates.
The optimal performance was achieved with three operational cycles, achieving an efficiency of 91.5% at a total cost of 265 units. This configuration successfully balanced the technical demand for efficiencies exceeding 90% while adhering to practical cost constraints below 300 units. Further analytical validation confirmed that the three-cycle treatment protocol consistently met established regulatory standards for material reuse. In contrast, systems utilizing a single treatment cycle exhibited marginal non-compliance with permissible limits.
The TCLP leaching analysis confirmed that EICP treatment successfully stabilized heavy metals within regulatory thresholds, ensuring environmentally safe soil reuse. Specifically, vacuum-assisted treatment achieved remarkable Cd immobilization in clayey soils, reducing leachable cadmium concentrations to 0.85 mg/L, which complies with the stringent Chinese regulatory limit of 1.0 mg/L as specified in GB 5085.3. In contrast, non-vacuum-treated clay samples exhibited slightly higher leaching levels (1.20 mg/L), while sandy soils consistently remained compliant regardless of vacuum application, with cadmium concentrations ranging from 0.79 to 0.92 mg/L.
Notably, the comparative analysis yielded several key insights. Under vacuum conditions, the performance gap between different soil types diminished substantially. Treated clay exhibited Cd immobilization levels (0.85 mg/L) comparable to those of sandy soils (0.79 mg/L), effectively reducing the permeability-driven disparity from 0.35 mg/L in non-vacuum systems to merely 0.06 mg/L. The application of vacuum conditions was particularly effective in clay matrices, achieving a 29.2% reduction in Cd leachability compared to non-vacuum conditions, whereas sandy soils showed a more modest improvement of 13.0%. Untreated controls displayed severe contamination levels, exceeding regulatory thresholds by 440–480% (clay: 5.40 mg/L, sand: 4.80 mg/L). These findings underscore the critical importance of EICP pretreatment.
The stabilization mechanism showed a strong dependence on two critical parameters: the post-treatment pH was maintained within the range of 7.2–8.1, and the carbonate content was kept between 12% and 18% CaCO3. Together, these factors accounted for 91% of the variation in leachability. All treated soils met the landscaping reuse specifications regarding soluble salt content (<0.25%) and pH compatibility (5.5–8.5), thus validating their appropriateness for practical environmental applications.
4. Discussion
The comprehensive experimental results of this study confirm that EICP technology provides a robust and eco-friendly solution for stabilizing heavy metals in landfill soils, effectively achieving the dual goals of environmental remediation and resource recovery. Through the integration of aqueous-phase mechanistic investigations with soil-phase parametric optimization, this work systematically elucidates the intricate interplay between biogeochemical factors and engineering parameters that govern EICP performance.
4.1. Mechanistic Insights into Metal Stabilization
The observed metal-specific efficiency hierarchy (Cd2+ > Pb2+ > Cu2+) in both single-component and mixed-metal systems, as illustrated in Figure 1 and Figure 3, does not align with the thermodynamic stability sequence based on carbonate solubility products. This correlation is influenced by the solubility product constants (Ksp) of the respective metal carbonates: lead carbonate (PbCO3, Ksp = 7.4 × 10−14) is thermodynamically less soluble than cadmium carbonate (CdCO3, Ksp = 1.0 × 10−12), followed by copper carbonate (CuCO3, Ksp ≈ 2.5 × 10−10). Despite this thermodynamic order, crystalline otavite (CdCO3) was more readily identified via XRD (shown in Figure 2), suggesting that Cd is more kinetically favored for lattice integration under EICP conditions. This implies that both thermodynamic and kinetic factors jointly influence the observed metal-specific stabilization behavior [32].
The absence of detectable Pb/Cu-carbonate phases via XRD analysis implies kinetic limitations, consistent with findings reported by Hamilton et al. [33] regarding the delayed nucleation of lead and copper carbonates under neutral pH conditions. This interpretation is corroborated by Visual MINTEQ simulations, which reveal substantially slower nucleation rates for lead and copper carbonates under neutral pH conditions, as depicted in Figure 5. The interplay between thermodynamic favorability and kinetic barriers establishes a selective metal sequestration mechanism, wherein cadmium achieves stable lattice incorporation, whereas lead and copper predominantly remain surface-associated [34]. The observed reduction in Pb2+ removal in the presence of Cl− ions is attributed to the formation of soluble lead–chloride complexes (e.g., PbCl+, PbCl20), rather than precipitation of PbCl2(s), which reduces the availability of free Pb2+ for carbonate precipitation and surface adsorption. Moreover, Cl− can disrupt the thermodynamic driving force for Pb–CO32− association by shifting speciation equilibria toward soluble forms.
The 12–18% efficiency reduction in multi-metal systems (Figure 3) can be attributed to competitive ion exchange occurring at calcite surfaces. Specifically, Ca2+ is preferentially displaced by Pb2+ over Cu2+ due to Pb2+’s smaller hydrated radius (Pb2+: 4.01 Å compared to Cu2+: 4.19 Å). This phenomenon aligns with the findings of Chen [35], who reported similar competitive biosorption behavior in calcite-rich environments. Notably, Cd exhibited relatively high removal efficiency (76.4%) in ternary systems, which may be attributed to its capacity to form mixed Ca-Cd carbonates, as evidenced by peak broadening observed at 29.4° in XRD analysis [36].
4.2. Engineering Parameters and Scalability
The optimal urease-CaCl2 ratio (1.0 U/mL + 0.5 M, Figure 6) effectively balances the kinetics of urea hydrolysis with the stoichiometry of CaCO3 precipitation. Excess urease concentrations (>1.5 U/mL) result in premature depletion of urea, leaving unreacted Ca2+ ions that may contribute to increased soil salinity—a critical factor for sustainable reuse in landscaping applications. Additionally, solution-to-soil ratios exceeding 1.0 mL/g were found to induce pore clogging (Figure 8), particularly in clay soils where permeability is a key determinant of reagent distribution. These findings are consistent with field observations by Xu [37], who documented efficiency losses of 30–40% in low-permeability soils without vacuum assistance.
The vacuum-assisted infiltration technique depicted in Figure 9 addresses hydraulic limitations in clay soils by applying controlled negative pressure gradients (−0.05 MPa). This approach enhances water transport efficiency, creating Darcy-scale flow conditions that effectively increase saturated hydraulic conductivity (Ksat) by five times. By accelerating the delivery of reactants, the optimized protocol achieves effective soil stabilization within 24 h—a 50% reduction compared to conventional EICP treatment durations—while approaching the operational efficiency of cement-based stabilization (<6 h) [38]. Importantly, this chemical stabilization method eliminates the carbon footprint associated with cement production processes, which typically emit 0.8–1.2 tons of CO2 per ton of treated soil [39]. Quantitative analysis of treatment efficacy (Figure 12) indicates a 29.2% reduction in leachable ions from vacuum-processed clay specimens, showcasing significant improvements in the spatial uniformity of cementation reactions compared to non-vacuum methods [40].
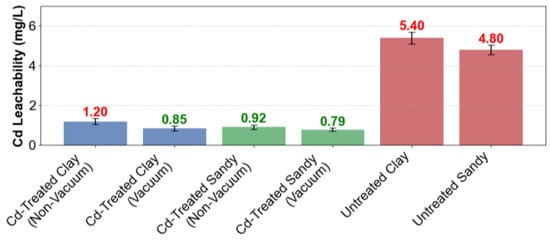
Figure 12.
TCLP leachability of stabilized soils. Numerical labels above bars indicate Cd concentrations (mg/L). Red font indicates relatively high leachability, while green font represents lower leachability values for visual comparison.
4.3. Environmental and Economic Sustainability
The engineered soils treated via Enzyme-Induced Carbonate Precipitation (EICP) exhibit exceptional stabilization performance, achieving efficiencies greater than 85% while ensuring leachate concentrations remain within the limits prescribed by the Toxicity Characteristic Leaching Procedure (TCLP) standards (Figure 12). These technical advantages facilitate the direct repurposing of stabilized soils as regulated construction materials under CJ/T 340-2016 specifications, specifically for roadbed construction and urban green space development. The methodology holds substantial environmental potential by potentially diverting 30–50% of contaminated soils from conventional landfill disposal [41]. Furthermore, economic feasibility is substantiated through a comprehensive life-cycle assessment, where reagent costs provide a 15–25% cost advantage over electrokinetic remediation methods [42]. This economic superiority remains evident even under conservative operational conditions involving three treatment cycles with a reagent dosage of 1.0 mL per gram of soil, as illustrated in Figure 11.
From an environmental perspective, the carbon footprint of EICP technology is predominantly determined by urea manufacturing emissions, amounting to 2.1 kg CO2-equivalent per kilogram of urea [43]. However, the biogenic calcium carbonate sequestration effect results in a net carbon offset ranging from 0.3 to 0.5 metric tons per ton of remediated soil [44]. These carbon-negative attributes strategically align with China’s national carbon neutrality goals, especially when compared to conventional Portland cement stabilization techniques. A comparative analysis reveals that cement-based methods produce significantly higher emissions due to the inherent limestone calcination process, as evidenced by recent process inventories reported by Barbhuiya [45].
4.4. Limitations and Future Directions
While laboratory-scale studies have validated the efficacy of enzymatically induced carbonate precipitation (EICP) for contaminant immobilization, several technical challenges must be addressed before field-scale implementation in landfill containment systems. The heterogeneous nature of natural landfill soils, which often contain organic matter ranging from 5% to 20% as determined by loss on ignition (LOI), could substantially hinder urease catalytic efficiency via competitive adsorption mechanisms [46]. This highlights the need for a systematic assessment of pre-treatment strategies, such as advanced oxidation processes utilizing hydrogen peroxide, to reduce organic interference prior to EICP application.
Long-term stability is a critical consideration due to the susceptibility of carbonate precipitates to dissolution under acidic environmental conditions. Accelerated aging simulations involving cyclical exposure to acetic acid solutions at pH 4.0 can provide essential data regarding the system’s resilience against acid rain scenarios and the associated risks of contaminant remobilization. Additionally, sustainability concerns arise from the current enzyme production paradigm, as conventional soybean-derived urease extraction consumes approximately 50 L of process water per kilogram of raw material [47]. This significant water footprint highlights the need for alternative production strategies, particularly the microbial biosynthesis of recombinant urease via optimized fermentation processes.
Future research directions should emphasize the systematic development of synergistic treatment systems that integrate EICP with complementary remediation technologies. Potential hybrid configurations could involve biochar-amended matrices to enhance the adsorption of residual exchangeable metal species and the incorporation of zero-valent iron for the electrochemical reduction of mobile Cr(VI) to less toxic Cr(III). Rigorous field validation across diverse climatic regimes, especially in regions subject to extreme wet-dry cycling or freeze–thaw conditions, is crucial to confirm technological robustness and refine implementation protocols for geo-environmental applications.
5. Sustainability Implications
The enzymatically induced carbonate precipitation (EICP) technique exhibits substantial potential for the sustainable stabilization of heavy metals in landfill soils, providing multifaceted advantages that resonate with current environmental and socioeconomic priorities. From an environmental sustainability standpoint, this biomineralization method reduces the carbon footprint by 0.3–0.5 t CO2-equivalent per ton of treated soil compared to traditional cement-based stabilization techniques [48]. This decarbonization benefit is primarily attributed to the sequestration of biogenic calcium carbonate and the avoidance of emissions associated with cement calcination processes, thus offering a feasible approach to support China’s strategic “dual-carbon” goals of achieving peak emissions by 2030.
EICP overcomes critical limitations of traditional chemical stabilization methods by maintaining soil pH within a moderate range of 7.2–8.1 after treatment, thus avoiding the alkaline overshoot commonly associated with lime amendments. This pH stabilization feature effectively mitigates the risk of heavy metal remobilization under fluctuating redox conditions, as supported by Toxicity Characteristic Leaching Procedure (TCLP) compliance data (Figure 12), which confirms minimal groundwater contamination risks. Additionally, comprehensive assessments indicate that more than 70% of treated soils meet the requirements of Chinese landscaping standards (CJ/T 340-2016), rendering them suitable for reuse in civil engineering projects such as roadbed construction or urban green space development.
Policy alignment reinforces the technology’s implementation framework, directly aligning with China’s 2016 Soil Pollution Prevention Action Plan and several United Nations Sustainable Development Goals (SDGs), notably SDG 11 (Sustainable Cities and Communities) and SDG 12 (Responsible Consumption and Production). Emerging market mechanisms, such as carbon credit trading, could enhance economic viability by monetizing the technology’s carbon sequestration potential (0.44 kg CO2-equivalent per kg of precipitated CaCO3). Additionally, green bond financing offers promising avenues for pilot projects in ecologically fragile regions, potentially expediting technology deployment at key remediation sites.
6. Conclusions
This study demonstrates that enzyme-induced carbonate precipitation (EICP) is a technically robust and environmentally sustainable approach for stabilizing heavy metals (e.g., Cd, Pb, Cr) in landfill soils. The key findings can be summarized as follows:
- EICP achieves a stabilization efficiency exceeding 85% by converting exchangeable heavy metals—specifically cadmium (Cd), lead (Pb), and chromium (Cr)—into carbonate-bound phases. Among them, cadmium exhibited the highest conversion rate, with over 70% of its exchangeable fraction transformed into stable otavite crystals, as confirmed by XRD. Lead and chromium also showed significant reductions in bioavailable forms, though their stabilization mechanisms were dominated by surface complexation with calcite and incorporation into amorphous carbonate phases rather than crystalline structures;
- Vacuum-assisted filtration improves clay soil remediation by 29.2%, decreases treatment time by 50%, and ensures compliance with TCLP thresholds (Cd: 0.85 mg/L). The most effective protocol, consisting of 1.0 U/mL urease and 0.5 M CaCl2 applied in three cycles, achieves a balance between efficiency (91.5%) and cost-effectiveness (265 units);
- Treated soils comply with reuse standards for landscaping and construction, potentially decreasing landfill usage by 30–50% and sequestering 0.3–0.5 t CO2-eq per ton of soil.
For future implementation, pilot-scale trials are essential to validate the field performance of EICP under diverse climatic conditions. Simultaneously, policy frameworks should be designed to incentivize EICP adoption through mechanisms such as carbon credits and green procurement mandates. Furthermore, integrating EICP with complementary technologies, such as biochar for managing organic contaminants, could provide effective solutions for complex landfill matrices, thereby facilitating progress toward zero-waste urbanization.
Author Contributions
Conceptualization, J.Z.; Methodology, W.X.; Validation, M.C.; Formal analysis, M.C.; Investigation, W.X.; Resources, J.Z.; Writing—original draft, W.X.; Writing—review & editing, M.C. and H.L.; Visualization, H.L.; Funding acquisition, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Nos. 42407271, 42477160, 52178319), National Key Research and Development Program of China (Grant No. 52338007), and Joint fund of the technical R&D program of Henan Province (Grant No. 225200810005). The authors gratefully acknowledge the financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmad, W.; Alharthy, R.D.; Zubair, M.; Ahmed, M.; Hameed, A.; Rafique, S. Toxic and heavy metals contamination assessment in soil and water to evaluate human health risk. Sci. Rep. 2021, 11, 17006. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Xiao, B.H.; Ali, M.U.; Xiao, P.W.; Zhao, P.; Wang, H.Y.; Bibi, S. Heavy metals pollution from smelting activities: A threat to soil and groundwater. Ecotoxicol. Environ. Saf. 2024, 274, 116189. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Islam, M.S.; Shreejana, K.C.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Nnaji, N.D.; Onyeaka, H.; Miri, T.; Ugwa, C. Bioaccumulation for heavy metal removal: A review. SN Appl. Sci. 2023, 5, 125. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Tian, S.; Liu, Z.W.; Mao, Q.; Ye, H.M.; Tian, C.S.; Zhu, Y.C.; Zhang, L.N. Leaching characteristics and environmental impact of heavy metals in tailings under rainfall conditions: A case study of an ion-adsorption rare earth mining area. Ecotoxicol. Environ. Saf. 2024, 281, 116642. [Google Scholar] [CrossRef]
- Xu, W.; Jin, Y.; Zeng, G. Introduction of heavy metals contamination in the water and soil: A review on source, toxicity and remediation methods. Green Chem. Lett. Rev. 2024, 17, 2404235. [Google Scholar] [CrossRef]
- Vithanage, M.; Bandara, P.C.; Novo, L.A.B.; Kumar, A.; Ambade, B.; Naveendrakumar, G.; Ranagalage, M.; Magana-Arachchi, D.N. Deposition of trace metals associated with atmospheric particulate matter: Environmental fate and health risk assessment. Chemosphere 2022, 303, 135051. [Google Scholar] [CrossRef]
- Xu, D.M.; Fu, R.B.; Wang, J.X.; Shi, Y.X.; Guo, X.P. Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade: Available stabilizing materials and associated evaluation methods—A critical review. J. Clean. Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Lee, H.; Sam, K.; Coulon, F.; Gisi, S.; Notarnicola, M.; Labianca, C. Recent developments and prospects of sustainable remediation treatments for major contaminants in soil: A review. Sci. Total Environ. 2024, 912, 168769. [Google Scholar] [CrossRef] [PubMed]
- Alsabhan, A.H.; Hamid, W. Innovative Thermal Stabilization Methods for Expansive Soils: Mechanisms, Applications, and Sustainable Solutions. Processes 2025, 13, 775. [Google Scholar] [CrossRef]
- Anburuvel, A. The Engineering Behind Soil Stabilization with Additives: A State-of-the-Art Review. Geotech. Geol. Eng. 2024, 42, 1–42. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, J.; Zhang, S.G. The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: A review. J. Environ. Manag. 2017, 193, 410–422. [Google Scholar] [CrossRef]
- Tyagi, S.; Annachhatre, A.P. A review on recent trends in solidification and stabilization techniques for heavy metal immobilization. J. Mater. Cycles Waste. Manag. 2023, 25, 733–757. [Google Scholar] [CrossRef]
- Phang, L.Y.; Mingyuan, L.; Mohammadi, M.; Tee, C.S.; Yuswan, M.H.; Cheng, H.; Lai, K.S. Phytoremediation as a viable ecological and socioeconomic management strategy. Environ. Sci. Pollut. Res. Int. 2024, 38, 50126–50141. [Google Scholar] [CrossRef]
- Lavanya, M.B.; Viswanath, D.S.; Sivapullaiah, P.V. Phytoremediation: An eco-friendly approach for remediation of heavy metal-contaminated soils-A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100975. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S. Enzyme induced calcium carbonate precipitation and its engineering application: A systematic review and meta-analysis. Constr. Build. Mater. 2021, 308, 125000. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S.; Saint, C. A Review of Enzyme Induced Carbonate Precipitation (EICP): The Role of Enzyme Kinetics. Sustain. Chem. 2021, 2, 92–114. [Google Scholar] [CrossRef]
- Li, W.L.; Zhang, Y.H.; Achal, V. Mechanisms of cadmium retention on enzyme-induced carbonate precipitation (EICP) of Ca/Mg: Nucleation, chemisorption, and co-precipitation. J. Environ. Chem. Eng. 2022, 10, 107507. [Google Scholar] [CrossRef]
- Bian, Y.; Chen, Y.; Zhan, L.; Ke, H.; Gao, Y.; Wang, Q.; Qi, G. An Enzyme-Induced Carbonate Precipitation Method for Zn2+, Ni2+, and Cr(VI) Remediation: An Experimental and Simulation Study. Appl. Sci. 2024, 14, 6559. [Google Scholar] [CrossRef]
- Baruah, P.; Sharma, S. Chemico-mechanical activation of EICP-modified soil: Role of urease enzyme. Environ. Earth Sci. 2023, 82, 563. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Peng, L.; Lu, S.; Zhang, J.; Du, X. Research of Enzyme-Induced Carbonate Precipitation on Strength Behavior of Reinforced Sand. Appl. Sci. 2025, 15, 3558. [Google Scholar] [CrossRef]
- Chen, Y.B.; Wang, Q.Y.; Bian, Y.; Zhan, L.T.; Gao, Y.F.; Guo, H.W.; Wang, Y.Z.; Gao, Y.Q. Effects of enzyme-induced carbonate precipitation (EICP) with different urease sources on the zinc remediation. J. Hazard. Mater. 2024, 480, 136321. [Google Scholar] [CrossRef]
- Xue, Z.F.; Cheng, W.H.; Rahman, M.M.; Wang, L.; Xie, Y.X. Immobilization of Pb(II) by Bacillus megaterium-based microbial-induced phosphate precipitation (MIPP) considering bacterial phosphorolysis ability and Ca-mediated alleviation of lead toxicity. Environ. Pollut. 2024, 355, 124229. [Google Scholar] [CrossRef]
- Xue, Z.F.; Cheng, W.H.; Wang, L.; Xie, Y.X.; Qin, P.; Shi, C. Immobilizing lead in aqueous solution and loess soil using microbially induced carbonate/phosphate precipitation (MICP/MIPP) under harsh pH environments. J. Hazard. Mater. 2024, 480, 135884. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Cao, Y.; Liang, T.; Wang, P.P.; Luo, H.L.; Yu, J.J.; Zhang, D.D.; Xing, B.S.; Yang, B. Stabilization and remediation of heavy metal-contaminated soils in China: Insights from a decade-long national survey. Environ. Sci. Pollut. Res. 2022, 29, 39077–39087. [Google Scholar] [CrossRef]
- Qi, S.; Ma, W.; Zhang, X.; Wang, J.; Hu, X.; Wei, Z.; Liu, J. Effects of Remolding Water Content and Compaction Degree on the Dynamic Behavior of Compacted Clay Soils. Buildings 2024, 14, 2258. [Google Scholar] [CrossRef]
- GB 5085.3-2007; Identification Standards for Hazardous Wastes—Identification for Extraction Toxicity. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2007.
- CJ/T 340-2016; Technical Requirements of Planting Soil for Urban Landscaping. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2016.
- Guerrero, J.D.; Arias, E.R.; Gutierrez, L.B. Enhancing copper and lead adsorption in water by in-situ generation of calcium carbonate on alginate/chitosan biocomposite surfaces. Int. J. Biol. Macromol. 2024, 266, 131110. [Google Scholar] [CrossRef]
- Hamilton, T.; Huai, Y.Y.; Peng, Y.J. Lead adsorption on copper sulphides and the relevance to its contamination in copper concentrates. Miner. Eng. 2020, 154, 106381. [Google Scholar] [CrossRef]
- Pan, X.F.; Huang, X.; Deng, N. The fate of cadmium during ferrihydrite phase transformation affected by dissolved organic matter: Insights from organic-mineral interaction. Chem. Geol. 2024, 670, 122424. [Google Scholar] [CrossRef]
- Chen, J.J.; Gu, Z.L.; Perez-Aguilar, J.M.; Luo, Y.B.; Tian, K.F.; Luo, Y.Q. Molecular dynamics simulations reveal efficient heavy metal ion removal by two-dimensional Cu-THQ metal-organic framework membrane. Sci. Rep. 2025, 15, 199. [Google Scholar] [CrossRef]
- Elamin, N.Y.; Elamin, M.R.; Alhussain, H.; Alotaibi, M.T.; Alluhaybi, A.A.; El-Bindary, A.A. Electrospun nanofibers of polycaprolactone loaded with chitosan/carbon quantum dots composite for adsorptive removal of Cd(II) ions from wastewater: Adsorption, kinetics, thermodynamics, and BOX Behnken design. Int. J. Biol. Macromol. 2025, 308, 142680. [Google Scholar] [CrossRef]
- Xu, K.; Huang, M.; Zhen, J.J.; Xu, C.S.; Cui, M.J. Field implementation of enzyme-induced carbonate precipitation technology for reinforcing a bedding layer beneath an underground cable duct. J. Rock Mech. Geotech. Eng. 2023, 15, 1011–1022. [Google Scholar] [CrossRef]
- Alotaibi, E.; Arab, M.G.; Abdallah, M.; Nassif, N.; Omar, M. Life cycle assessment of biocemented sands using enzyme induced carbonate precipitation (EICP) for soil stabilization applications. Sci. Rep. 2022, 12, 6032. [Google Scholar] [CrossRef]
- Suarez-Riera, D.; Restuccia, L.; Ferro, G.A. The use of Biochar to reduce the carbon footprint of cement-based materials. Procedia Struct. Integr. 2020, 26, 199–210. [Google Scholar] [CrossRef]
- Guo, S.; Huang, R.; Yuan, J.; Chen, R.; Chen, F.X. Efficient removal of aromatic pollutants via catalytic wet peroxide oxidation over synthetic anisotropic ilmenite/carbon nanocomposites. npj Clean Water 2023, 6, 74. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Sherpa, K.; Bui, X.T.; Nguyen, V.T.; Vo, T.D.H.; Ho, H.T.T.; Chen, C.W.; Dong, C.D. Biochar for soil remediation: A comprehensive review of current research on pollutant removal. Environ. Pollut. 2023, 337, 122571. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Zhao, M.M.; Chen, L.; Gong, Z.Y.; Hu, J.J.; Ma, D.G. Electrokinetic remediation for the removal of heavy metals in soil: Limitations, solutions and prospection. Sci. Total Environ. 2023, 903, 165970. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, G.F.; Zhang, H.C.; Qiu, X.Y.; Liu, G.M.; Wang, Y.N.; Jang, J.H.; Wang, Y.; Wei, Z.D.; Cai, Z.W.; et al. Reliable and accessible methods for urea quantification in co-reduction of carbon-dioxide- and nitrogen-containing species. Chem. Catalysis 2025, 5, 101234. [Google Scholar] [CrossRef]
- Xiang, J.; Shi, W.Z.; Jing, Z.J.; Guan, Y.L.; Yang, F.M.; Wang, G.M.; Sun, X.; Li, J.X.; Li, Q.; Zhang, H.C. Exogenous calcium-induced carbonate formation to increase carbon sequestration in coastal saline-alkali soil. Sci. Total Environ. 2024, 946, 174338. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Kanavaris, F.; Das, B.B.; Idrees, M. Decarbonising cement and concrete production: Strategies, challenges and pathways for sustainable development. J. Build. Eng. 2024, 86, 108861. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, X.G.; Sun, Y.; Wang, Y.X.; Sha, H.Q.; He, X.S.; Sun, X.J. Natural and anthropogenic dissolved organic matter in landfill leachate: Composition, transformation, and their coexistence characteristics. J. Hazard. Mater. 2024, 465, 133081. [Google Scholar] [CrossRef]
- Guan, D.; Zhou, Y.; Shahin, M.A.; Tirkolaei, H.K.; Cheng, L. Assessment of urease enzyme extraction for superior and economic bio-cementation of granular materials using enzyme-induced carbonate precipitation. Acta Geotech. 2023, 18, 2263–2279. [Google Scholar] [CrossRef]
- Smirnova, M.; Nething, C.; Stolz, A.; Gröning, J.A.D.; Funaro, D.P.; Eppinger, E.; Reichert, M.; Frick, J.; Blandini, L. High strength bio-concrete for the production of building components. npj Mater. Sustain. 2023, 1, 4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).