The Synergistic Effects of Rice Straw-Pyrolyzed Biochar and Compost on Acidity Mitigation and Carbon Sequestration in Acidic Soils: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Basic Properties of Soil and Straw Derivates
2.2. The Soil Incubation Experiment
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
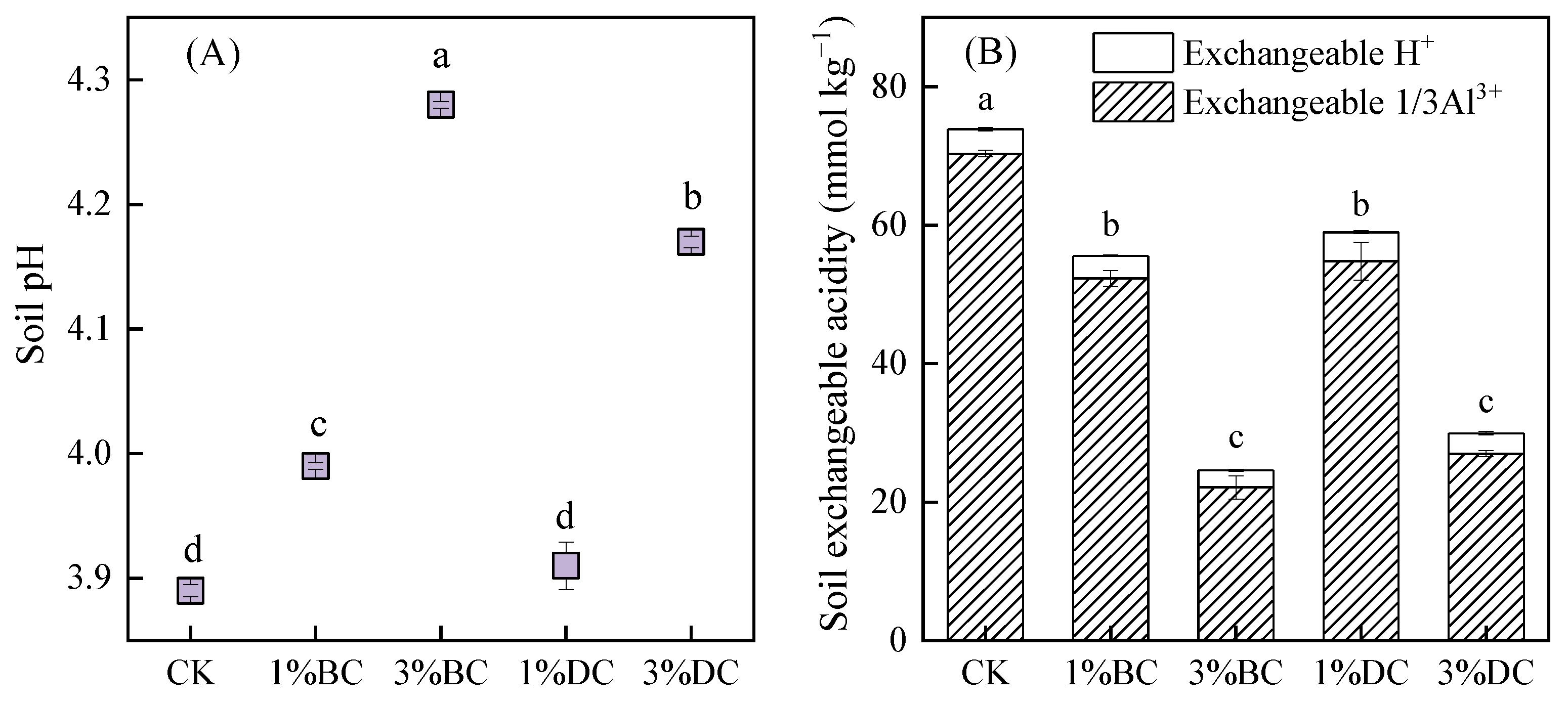
3.1. Effects of BC and DC on Soil Acidity
3.2. Changes in Soil Al Speciation After Applying BC and DC
3.3. Response of Soil Organic Carbon to BC and DC Application
3.4. Influences of BC and DC on Soil Exchangeable Base Cations
3.5. Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.Q.; Pan, X.Z.; Ma, H.Y.; Dong, X.Y.; Che, J.; Wang, C.; Shi, Y.; Liu, K.L.; Shen, R.F. Scientific Issues and Strategies of Acid Soil Use in China. Acta Pedol. Sin. 2023, 60, 1248–1263. (In Chinese) [Google Scholar] [CrossRef]
- Kocjan, A.; Kwasniewska, J.; Szurman-Zubrzycka, M. Understanding Plant Tolerance to Aluminum: Exploring Mechanisms and Perspectives. Plant Soil 2024, 507, 195–219. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Wang, M.Y.; Hu, S.J.; Zhang, X.D.; Ouyang, Z.; Zhang, G.L.; Huang, B.A.; Zhao, S.W.; Wu, J.S.; Xie, D.T.; et al. Economics- and Policy-Driven Organic Carbon Input Enhancement Dominates Soil Organic Carbon Accumulation in Chinese Croplands. Proc. Natl. Acad. Sci. USA 2018, 115, 4045. [Google Scholar] [CrossRef]
- Ma, Y.; Woolf, D.; Fan, M.; Qiao, L.; Li, R.; Lehmann, J. Global Crop Production Increase by Soil Organic Carbon. Nat. Geosci. 2023, 16, 1159–1165. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Q.; de Vries, W.; Ros, G.H.; Chen, X.; Muneer, M.A.; Zhang, F.; Wu, L. Effects of Soil Amendments on Soil Acidity and Crop Yields in Acidic Soils: A World-wide Meta-analysis. Environ. Manag. 2023, 345, 118531. [Google Scholar] [CrossRef]
- Shen, Z.; Han, T.F.; Huang, J.; Li, J.W.; Daba, N.A.; Gilbert, N.; Khan, M.N.; Shah, A.; Zhang, H.M. Soil Organic Carbon Regulation by pH in Acidic Red Soil Subjected to Long-Term Liming and Straw Incorporation. Environ. Manag. 2024, 367, 122063. [Google Scholar] [CrossRef]
- Moran-Rodas, V.E.; Joergensen, R.G.; Wachendorf, C. Does Liming Improve Microbial Carbon Use Efficiency after Maize Litter Addition in A Tropical Acidic Soil? Biol. Fertil. Soils 2023, 59, 619–627. [Google Scholar] [CrossRef]
- Liu, J.; Fang, L.C.; Qiu, T.Y.; Chen, J.; Wang, H.; Liu, M.X.; Zhang, H.L.; Wang, C.; Sardans, J.; Chen, L.; et al. Crop Residue Return Achieves Environmental Mitigation and Enhances Grain Yield: A Global Meta-Analysis. Agron. Sustain. Dev. 2023, 43, 78. [Google Scholar] [CrossRef]
- Paradelo, R.; Navarro-Pedreño, J.; Glaser, B.; Grobelak, A.; Kowalska, A.; Singh, B.R. Potential and Constraints of Use of Organic Amendments from Agricultural Residues for Improvement of Soil Properties. Sustainability 2023, 16, 158. [Google Scholar] [CrossRef]
- Liang, F.; Li, B.; Vogt, R.D.; Mulder, J.; Song, H.; Chen, J.; Guo, J. Straw Return Exacerbates Soil Acidification in Major Chinese Croplands. Resour. Conserv. Recycl. 2023, 198, 107176. [Google Scholar] [CrossRef]
- Liang, Y.; Al-Kaisi, M.; Yuan, J.C.; Liu, J.Z.; Zhang, H.X.; Wang, L.C.; Cai, H.G.; Ren, J. Effect of Chemical Fertilizer and Straw-Derived Organic Amendments on Continuous Maize Yield, Soil Carbon Sequestration and Soil Quality in A Chinese Mollisol. Agric. Ecosyst. Environ. 2021, 314, 107403. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Baquy, M.A.A.; Mia, S.; Shi, R.Y.; Kamran, M.A.; Mehmood, K.; Xu, R.K. A Critical-Systematic Review of the Interactions of Biochar with Soils and the Observable Outcomes. Sustainability 2021, 13, 13726. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Qian, W.; Wang, R.H. Comparison of the Ameliorating Effects on an Acidic Ultisol between Four Crop Straws and Their Biochars. J. Soils Sediments 2011, 11, 741–750. [Google Scholar] [CrossRef]
- Tusar, H.M.; Uddin, M.K.; Mia, S.; Suhi, A.A.; Wahid, S.B.A.; Kasim, S.; Sairi, N.A.; Alam, Z.; Anwar, F. Biochar-acid soil interactions—A review. Sustainability 2023, 15, 13366. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.N.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum Toxicity in Plants and its Possible Mitigation in Acid Soils by Biochar: A Review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B. Dual Role of Biochars as Adsorbents for Aluminum: The Effects of Oxygen-Containing Organic Components and The Scattering of Silicate Particles. Environ. Sci. Technol. 2013, 47, 8759–8768. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.; Nabel, M.; Roß-Nickoll, M.; Van Dongen, J.T. Comparing Straw, Compost, and Biochar Regarding Their Suitability as Agricultural Soil Amendments to Affect Soil Structure, Nutrient Leaching, Microbial Communities, and the Fate of Pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Shao, M.J.; Dou, S.; Xie, Z.B. Effects of Corn Straw and its Humified and Carbonized Materials Applying to the Black Soil with an Equal Mass of Carbon on Soil Humus. J. Agro-Environ. Sci. 2018, 37, 2202–2209. (In Chinese) [Google Scholar] [CrossRef]
- Biswas, B.; Balla, P.; Krishna, B.B.; Adhikari, S.; Bhaskar, T. Physiochemical Characteristics of Biochar Derived from Pyrolysis of Rice Straw under Different Temperatures. Biomass Convers. Bior. 2024, 14, 12775–12783. [Google Scholar] [CrossRef]
- Pan, X.Y.; Shi, R.Y.; Hong, Z.N.; Jiang, J.; He, X.; Xu, R.K.; Qian, W. Characteristics of Crop Straw-Decayed Products and Their Ameliorating Effects on an Acidic Ultisol. Arch. Agron. Soil Sci. 2021, 12, 1708–1721. [Google Scholar] [CrossRef]
- Zhang, N.; Xing, J.; Wei, L.; Liu, C.; Zhao, W.; Liu, Z.; Wang, Y.; Liu, E.; Ren, X.; Jia, Z.; et al. The Potential of Biochar to Mitigate Soil Acidification: A Global Meta-analysis. Biochar 2025, 7, 49. [Google Scholar] [CrossRef]
- Pansu, D.M.; Gautheyrou, J. Handbook of Soil Analysis; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Soon, Y.K. Fractionation of extractable aluminum in acid soils: A review and a proposed procedure. Commun. Soil Sci. Plant Anal. 1993, 24, 1683–1708. [Google Scholar] [CrossRef]
- Chen, X.M.; Zhang, D.Q.; Liang, G.H.; Qiu, Q.Y.; Liu, J.X.; Zhou, G.Y.; Liu, S.Z.; Chu, G.W.; Yan, J.H. Effects of Precipitation on Soil Organic Carbon Fractions in Three Subtropical Forests in Southern China. J. Plant Ecol. 2016, 1, 10–19. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L.; Lawrinenko, M. Characterization and Quantification of Biochar Alkalinity. Chemosphere 2017, 167, 367–373. [Google Scholar] [CrossRef]
- Cai, Z.; Xu, M.; Zhang, L.; Yang, Y.; Wang, B.; Wen, S.; Misselbrook, T.H.; Carswell, A.M.; Duan, Y.; Gao, S. Decarboxylation of Organic Anions to Alleviate Acidification of Red Soils from Urea Application. J. Soils Sediments 2020, 20, 3124–3135. [Google Scholar] [CrossRef]
- Yu, W.J.; Ren, T.S.; Duan, Y.H.; Huai, S.C.; Zhang, Q.Y.; Cai, Z.J.; Lu, C.A. Mechanism of Al Toxicity Alleviation in Acidic Red Soil by Rice-Straw Hydrochar Application and Comparison with Pyrochar. Sci. Total Environ. 2023, 877, 162849. [Google Scholar] [CrossRef]
- Shi, R.Y.; Ni, N.; Nkoh, J.N.; Dong, Y.; Zhao, W.R.; Pan, X.Y.; Li, J.Y.; Xu, R.K.; Qian, W. Biochar Retards Al Toxicity to Maize (Zea mays L. ) During Soil. Acidification: The Effects and Mechanisms. Sci. Total Environ. 2020, 719, 137448. [Google Scholar] [CrossRef]
- Du, X.; Wang, Y.; Su, X.; Li, J. Influences of pH Value on The Microstructure and Phase Transformation of Aluminum Hydroxide. Powder Technol. 2009, 192, 40–46. [Google Scholar] [CrossRef]
- Mandal, M.; Kamp, P.; Singh, M. Effect of Long Term Manuring on Carbon Sequestration Potential and Dynamics of Soil Organic Carbon Labile Pool Under Tropical Rice-Rice Agro-Ecosystem. Commun. Soil. Sci. Plant 2020, 51, 468–480. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, M.; Zhai, Z.; Dai, H.; Yang, M.; Zhang, Y.; Liang, T. Soil Organic Carbon, Carbon Fractions, and Microbial Community under Various Organic Amendments. Sci. Rep. 2024, 14, 25431. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, S.; Zhang, Q.; Zou, M.; Yin, Q.; Qiu, Y.; Qin, W. Effect of Organic Material Addition on Active Soil Organic Carbon and Microbial Diversity: A Meta-Analysis. Soil. Till. Res. 2024, 241, 106128. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Soil Organic Matter Priming: The pH Effects. Glob. Change Biol. 2024, 30, e17349. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yao, Z.Y.; Chen, Y.P.; Liu, N.; Teng, Z.R.; Huang, D.L.; Cao, W.D.; Kuzyakov, Y.; Shah, Y.H.; Zhao, N.; et al. Priming and Balance of Soil Organic Carbon Differ with Additive C: N Ratios and Long-Term Green Manuring. Appl. Soil. Ecol. 2024, 201, 105495. [Google Scholar] [CrossRef]
- Zhou, X.H.; Feng, Z.Q.; Yao, Y.X.; Liu, R.Q.; Shao, J.J.; Jia, S.X.; Gao, Y.N.; Xue, K.; Chen, H.Y.; Fu, Y.L.; et al. Nitrogen Input Alleviates the Priming Effects of Biochar Addition on Soil Organic Carbon Decomposition. Soil Biol. Biochem. 2025, 202, 109689. [Google Scholar] [CrossRef]
- Yuan, J.H.; E, S.Z.; Che, Z.X. Base Cation-Enhancing Role of Corn Straw Biochar in an Acidic Soil. Soil Use Manag. 2022, 38, 1322–1336. [Google Scholar] [CrossRef]
- Shi, R.Y.; Hong, Z.N.; Li, J.Y.; Jiang, J.; Baquy, M.A.A.; Xu, R.K.; Qian, W. Mechanisms for Increasing the pH Buffering Capacity of an Acidic Ultisol by Crop Residue-Derived Biochars. J. Agric. Food Chem. 2017, 65, 8111–8119. [Google Scholar] [CrossRef]
- Wang, H.F.; Yang, Y.; Wang, M.P.; Yuan, R.J.; Song, W.Y.; Wang, L.; Liang, N.; Shi, J.Y.; Li, J. Insights into the Roles of Surface Functional Groups and Micropores in the Sorption of Ofloxacin on Banana Pseudo-Stem Biochars. Sustainability 2024, 16, 2629. [Google Scholar] [CrossRef]
- Dietel, J.; Ufer, K.; Kaufhold, S.; Dohrmann, R. Crystal Structure Model Development for Soil Clay Minerals–II. Quantification and Characterization of Hydroxy-Interlayered Smectite (HIS) Using the Rietveld Refinement Technique. Geoderma 2019, 347, 1–12. [Google Scholar] [CrossRef]
- Li, J.Y.; Xu, R.K. Inhibition of Acidification of Kaolinite and an Alfisol by Aluminum Oxides through Electrical Double-Layer Interaction and Coating. Eur. J. Soil Sci. 2013, 64, 110–120. [Google Scholar] [CrossRef]
- Apostolović, T.; Gross, A.; Rodríguez, Á.F.G.; de la Rosa, J.M.; Glaser, B.; Knicker, H.; Maletić, S. Impact of Biochar Aging on Soil Physicochemical Properties. Agronomy 2024, 14, 3007. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in Climate Change Mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.G.; Jeong, S.T.; Gwon, H.S.; Kim, P.J.; Kim, G.W. Straw Recycling in Rice Paddy: Trade-off Between Greenhouse Gas Emission and Soil Carbon Stock Increase. Soil. Till. Res. 2020, 199, 104598. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Wang, C.; Wang, Y. Comparison of the Effect of NaOH Pretreatment and Microbial Agents on Rice Straw Decomposition. Agronomy 2023, 13, 816. [Google Scholar] [CrossRef]

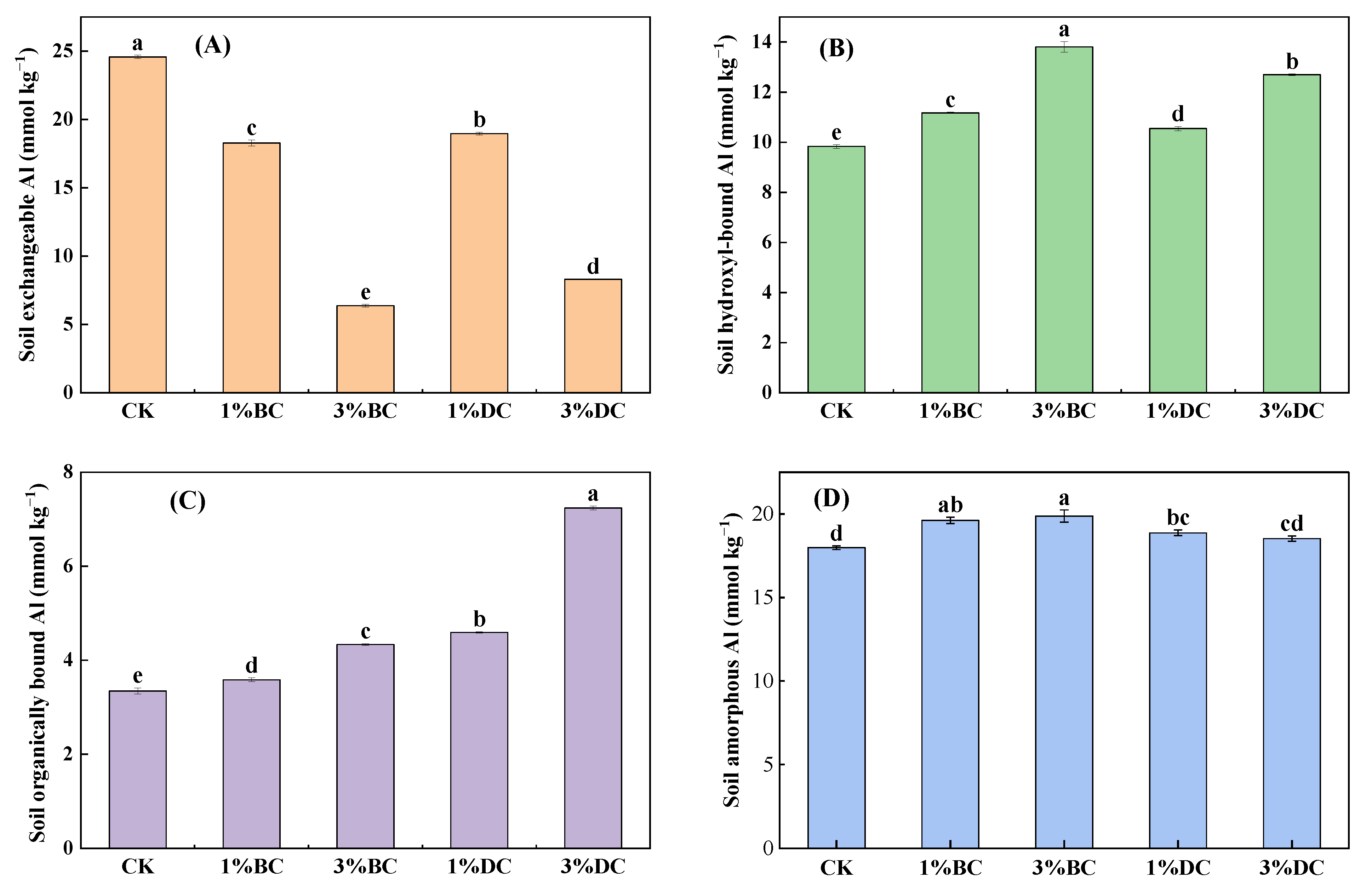


| Straw Derivates | pH | EC | Total C | Total N | Carbonate | ROC | DOC | Soluble Base Cations | Exchangeable Base Cations | CEC | Ash Alkalinity | BET Surface Area | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | K+ | Ca2+ | Mg2+ | |||||||||||
| ms cm−1 | --------%------- | ------------g kg−1----------- | ----------------------------------------------- (cmol(+) kg−1) ------------------------------------- | m2 g−1 | |||||||||||||
| BC | 9.41 | 11.17 | 51.35 | 1.08 | 73.99 | 31.21 | 13.38 | 108.65 | 46.46 | 1.44 | 1.37 | 39.81 | 49.04 | 19.11 | 138.73 | 148.51 | 3.57 |
| DC | 9.20 | 14.07 | 26.21 | 3.06 | 73.13 | 110.23 | 14.28 | 118.17 | 223.64 | 7.30 | 5.93 | 11.84 | 51.02 | 27.84 | 74.97 | 115.73 | 3.20 |
| Treatments | Soil Exchangeable Base Cations | ECEC | |||
|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | ||
| mmol(+) kg−1 | |||||
| CK | 2.48 ± 0.00 e | 3.79 ± 1.30 ab | 2.87 ± 0.37 d | 2.14 ± 0.04 e | 85.16 ± 1.06 b |
| 1%BC | 14.31 ± 0.14 c | 4.37 ± 0.00 ab | 8.39 ± 0.95 c | 4.85 ± 0.12 d | 87.48 ± 0.78 b |
| 3%BC | 37.2 ± 0.85 a | 4.94 ± 0.42 a | 18.49 ± 0.30 a | 9.95 ± 0.15 b | 95.23 ± 1.72 a |
| 1%DC | 11.24 ± 0.64 d | 2.29 ± 0.20 b | 8.54 ± 1.37 c | 5.87 ± 0.18 c | 86.91 ± 2.88 b |
| 3%DC | 32.94 ± 0.73 b | 5.06 ± 0.35 a | 16.82 ± 0.22 b | 13.33 ± 0.10 a | 98.10 ± 0.69 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Shu, T.; Shi, R.; Mao, X.; Li, J.; Nkoh, J.N.; Xu, R. The Synergistic Effects of Rice Straw-Pyrolyzed Biochar and Compost on Acidity Mitigation and Carbon Sequestration in Acidic Soils: A Comparative Study. Sustainability 2025, 17, 4408. https://doi.org/10.3390/su17104408
Pan X, Shu T, Shi R, Mao X, Li J, Nkoh JN, Xu R. The Synergistic Effects of Rice Straw-Pyrolyzed Biochar and Compost on Acidity Mitigation and Carbon Sequestration in Acidic Soils: A Comparative Study. Sustainability. 2025; 17(10):4408. https://doi.org/10.3390/su17104408
Chicago/Turabian StylePan, Xiaoying, Tianchu Shu, Renyong Shi, Xiaoxia Mao, Jiuyu Li, Jackson Nkoh Nkoh, and Renkou Xu. 2025. "The Synergistic Effects of Rice Straw-Pyrolyzed Biochar and Compost on Acidity Mitigation and Carbon Sequestration in Acidic Soils: A Comparative Study" Sustainability 17, no. 10: 4408. https://doi.org/10.3390/su17104408
APA StylePan, X., Shu, T., Shi, R., Mao, X., Li, J., Nkoh, J. N., & Xu, R. (2025). The Synergistic Effects of Rice Straw-Pyrolyzed Biochar and Compost on Acidity Mitigation and Carbon Sequestration in Acidic Soils: A Comparative Study. Sustainability, 17(10), 4408. https://doi.org/10.3390/su17104408









