Abstract
Sustainable viticulture practices could be useful tools for ensuring grape and wine quality, especially in the context of climate change. A promising and innovating approach is the use of bioelicitors in order to stimulate productivity and metabolite biosynthesis in an environmentally friendly way. However, the result depends on the variety, the phenological stage, concentration of the biomolecule applied, and climate conditions. The present study examined the impact of the plant hormone abscisic acid on the phenolic compound accumulation in the autochthonous, red-colored Greek grapevine variety Mouhtaro. During 2018 and 2019 vintages berry quality characteristics, and metabolome were evaluated at three stages: véraison, beginning and mid, and harvest. Abscisic acid (ABA) was given at doses of 0.04% w/v and 0.08% w/v during the véraison stage. According to the results, the ABA-treated grape berries were smaller and exhibited lower total soluble solid levels and increased titratable acidity compared to the control. Although no significant differences were observed in amino acids or anthocyanin and stilbene accumulation upon ABA treatment, application of ABA at the higher dose resulted in increased concentrations of phenolic acids, flavan-3-ols, and flavonols. Therefore, the application of ABA could be considered as a promising method for improving the grape quality characteristics of Mouhtaro.
1. Introduction
Climate change has already induced noticeable changes in viticulture and enology. These impacts include fluctuations in phenological timing, alterations in grape and wine composition, heterogeneous effects on grapevine yield, and decoupling grape maturity from soluble solids and secondary metabolites [1,2,3]. The predicted increase in average temperature in the coming decades will shift the suitability of wine-growing regions, favoring grapevine cultivation in northern countries but also threatening the established quality characteristic of traditional cultivars in Mediterranean region [4,5,6]. Given the anticipated negative effects of prolonged summer drought and rising temperatures on grape production and quality, developing and implementing sustainable practices is crucial for the resilience of Mediterranean viticulture.
A promising strategy to address the effects of climate change in viticulture is the application of bioelicitors. In order to promote plant growth and productivity, especially in response to abiotic challenges like heat, salinity, and drought, this approach uses biomolecules from several sources, including proteins, oligo-polysaccharides, plant hormones, peptides, and lipids [7,8,9]. Bioelicitors trigger diverse plant responses, including the regulation of specialized metabolism and antioxidant defenses, by regulating the expression of related genes [10,11,12,13,14,15]. The application of these compounds at the appropriate plant growth stage and the optimal concentration can enhance crop yield and quality characteristics under stress conditions [16].
Abscisic acid is a key plant hormone involved in various developmental processes, including fruit ripening and drought response [17,18,19]. ABA treatments on grapevine improve berry quality features and enhance the biosynthesis of tannins, flavonoids, anthocyanins, and stilbenes [17,20,21,22]. Moreover, ABA treatment was found to improve soluble sugar and phenolic compound accumulation under stress conditions [23,24].
Although research on bioelicitors is extensive and continually expanding [25,26], our understanding of their optimal application, crop-specific effects, and interactions with environmental conditions remains incomplete. This study seeks to assess the impact of ABA application on grape berry secondary metabolism of the autochthonous, red-colored variety Mouhtaro in the challenging conditions of a Greek vineyard. Only the Muses Valley in Askri, Central Greece, is home to the grapevine variety “Mouhtaro”, which yields few concentrated grapes. The berry is small to medium in size and is found in a medium-sized bunch. Additionally, grapevines are rigorous and distinguished by their late ripening and late bud break. There are currently no available scientific data about DNA genotyping. A two-vintage comprehensive trial was conducted and berry quality traits combined with metabolomic data were assessed throughout the ripening period. The observed alterations in the phenolic composition of grape berries subsequent to ABA treatment can potentially enhance our understanding and contribute to the development of more sustainable viticulture practices, ultimately aimed at ensuring improvement in grape production quality across diverse agricultural settings. This research holds significant implications for viticulture, as it not only addresses existing gaps in knowledge but also proposes innovative approaches that could lead to improved sustainability and quality in grape cultivation. Furthermore, the insights gained from this study may serve as a valuable reference for future research endeavors aimed at optimizing the utilization of bioelicitors in various horticultural applications. Thus, through this investigation, we aspire to contribute to the broader discourse regarding sustainable agricultural practices and the complex interactions among plant physiology and environmental factors.
2. Materials and Methods
2.1. Experimental Design
Experiment was carried out in productive vineyard in Muses Valley, near the town of Thiva, in Central Greece. Vineyard is located at GPS 38°19′30.9″ N and 23°05′37.7″ E, at an altitude of 450 m. The vineyard was planted in 2007; the training system is double cordon and planted on R110 rootstocks with a planting density of 2.5 m between and 1.2 in rows. All experimental vines were pruned uniquely; each cordon has three spurs. Without irrigation, the experimental plot’s vineyard management was consistent and followed the regionally advised agricultural techniques. Three treatments were compared in the trial, resulting from a combination of two levels of ABA and the control. More specifically: the untreated vines (control), and the vines sprayed with 0.04% w/v (low dose) and 0.08% w/v (high dosage) abscisic acid (s-abscisic acid 10.4% w/v, Protone SL, Hellafarm, Peania, Greece). These doses and the timing were chosen on the basis of previous studies conducted on other red grapevine varieties [20,21,22,23] as well as according to the suppliers’ instruction. The grape zone (all bunches of the experimental vines) was sprayed with ABA during véraison (81–85, based on the BBCH scale) [27], then again three and six days following the initial application. The different treatments did not sample buffer portions, which had more than three grapevine plants per row. In three completely randomized blocks, each treatment was given in triplicate, with 10 vines in a row for each treatment [28].
2.2. Meteorological Data
The meteorological data concerning the experimental period of both vintages (i.e., from July to September) were acquired from the Automatic Network of the National Observatory of Athens [29].
2.3. Grape Sampling and Physicochemical Must Analysis
The sampling dates were performed as shown in Table S1 (three distinct maturity levels: at véraison, immediately prior to the 1st treatment application, at middle véraison, and at the optimum grape maturity stage), and grapes were harvested on 30 August 2018 and on 3 September 2019, respectively. A weekly sample approach was used to track the ripening process and determine the optimum grape level for harvest. Fifty berries were randomly selected from each experimental treatment at each sample point, and the fresh weight of the berries was measured on an analytical balance. The sampled berries were collected on both sides of each of the ten plants, in both direct sunshine and shade. Prior to their chemical analysis, grape samples were transported on dry ice and were carefully stored at −80 °C. Weekly measurements were made to track the level of grape maturity. The Compendium of International Methods of Wine and Must Analysis’s official procedures were followed to evaluate the pH, titratable acidity (g L−1 tartaric acid), and total soluble solids content (°Brix) [30].
2.4. UPLC–MS-Based Metabolic Profiling
Using liquid nitrogen, the frozen (−80 °C) grape berries were ground into powder and utilized for metabolic profiling using modified techniques. [15,31]. Briefly, 50 mg of grounded dry berries was extracted using in a methanol/water mixture (80/20; v/v). Following sonication, the samples were extracted for 15 h at 4 °C. UPLC–MS analyses were performed using an ACQUITY™ electrospray ionization (ESI) source that is managed by Masslynx 4.1 software (Waters, Milford, MA, USA), which is connected to an Ultra Performance Liquid Chromatography system via a Xevo TQD mass spectrometer. As stated in Miliordos et al. [28], the peak annotation was carried out using retention times, m/z values, and UV spectra, as well as by comparison with pure standards, own purified substances, or data from the literature. Data collection was carried out with selected ion monitoring (SIM). Absolute quantifications were conducted using pure standards and using a five-point calibration curve (0–20 ppm).
2.5. Statistical Analysis
All of the values are presented as the mean standard deviation. The statistical analysis was carried out with GraphPad Prism (GraphPad Software, version 8.0.1). One-way ANOVA with Tukey’s test or an unpaired t-test were used to assess the results’ significance. Additionally, SIMCA P+ version 15 (Umetrics AB, Umeå, Sweden) was used to conduct a multivariate statistical data analysis (MVA) of the samples.
3. Results
3.1. Meteorological Conditions
The research took place in a Mouhtaro single vineyard located in a narrow valley at 450 m altitude in Central Greece, roughly 100 km northwest of the city of Athens. Muses Valley has a Mediterranean climate with mild, wet winters and dry summers. Considering that Mouhtaro is an early-harvested cultivar, the period of ripening (from véraison to harvest) is limited to July and August. In the study presented, the grapes were harvested on 30 August and 3 September of 2018 and 2019, respectively. Using meteorological data from the neighboring town of Askri, (38°18′51.1″ N 23°6′42.8″ E), monthly maximum temperatures were higher during August in 2019 vintage (39.5 °C) compared to 2018 (35 °C) (Figure S1). In comparison, the total precipitation amount in July and August was higher in 2018 (66 mm) compared to 2019 (35 mm) (Figure S1). Specifically, the precipitation during the ripening period of 2019 was observed two weeks prior to véraison, while most of the rainfall of 2018 was recorded the week after véraison and the days prior to harvest (Figure 1).
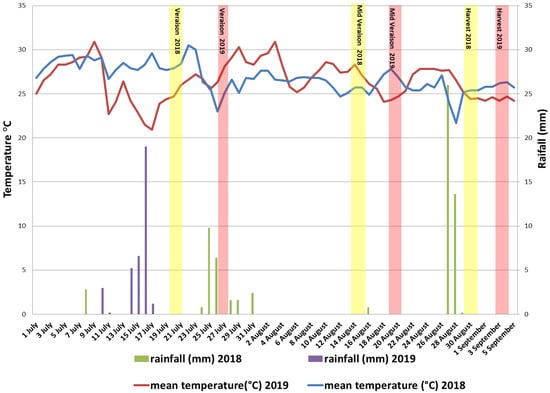
Figure 1.
Meteorological data from July 1st to September 5th in Muses Valley during 2018 and 2019 vintages. Purple and green bars indicate the daily amount of rainfall, while the red and blue lines on the plot show the evolution of the daily mean temperature. The yellow and pink bars show the three sampling points (i.e., véraison, middle véraison, and harvest) of 2018 and 2019, respectively.
3.2. Grape Weight and Must Conventional Analysis
Berry size was constantly increased during maturation in both control and treated plants. The ABA treatment resulted in significantly decreased berry size only at harvest stage of both vintages, while no difference was observed at the two previous stages (Table 1). The differences between the high and low dose of applied ABA were negligible. Surprisingly, compared to 2018, berry size in both control and ABA-treated vines grew considerably at all sample dates during ripening and at the harvest stage in 2019 (Table 1). The increased berry size observed in 2019 samples could be the result of the amount of rainfall recorded prior to véraison (Figure 1). During this period, grape berries accumulate a substantial amount of water, leading to an increase in berry weight, which may account for the differences observed between the two vintages

Table 1.
Physicochemical characteristics of Mouhtaro grapes at three phenological stages (i.e., véraison, middle véraison, and harvest). Data represent the mean ± std. deviation (n = 3). According to the t-test at the 5% probability level, different letters in the same column and phenological stage indicate significant differences.
The composition of the grape must, which is primarily determined by the pH, titratable acidity (TA, stated in tartaric acid g/L), and total soluble solids (TSS, expressed as °Brix), is a significant determinant in berry quality. Throughout their development, grape berries from both vintages showed a steady rise in total soluble solids. At the harvest stage of both vintages, the TSS content in ABA-treated plants was significantly lower than that of the controls. At the earlier sample points, no changes were seen (Table 1). Additionally, the 2019 vintage had a greater TSS content throughout maturity than the 2018 vintage (Table 1), suggesting that the weather circumstances were different.
Titratable acidity values were gradually decreased during the ripening period, while the pH content was increased in all treatments. Only at the harvest stages of both vintages were there statistically significant variations in titratable acidity and pH between ABA-treated berries and control samples; no differences were observed at the two earlier stages. (Table 1). In particular, as compared to controls, TA values were noticeably greater for both administered ABA dosages. However, only grapes treated with the higher dose of ABA showed noticeably different pH values, and the impact varied by vintage (i.e., higher and lower pH values in 2018 and 2019, respectively, compared to control and ABA medium dose) (Table 1). In contrast to the TSS profile of the two vintages, the 2018 samples had greater TA and pH values than the 2019 samples (Table 1).
Taken together, the results of berry size measurements and the conventional must analysis indicate that ABA application resulted in smaller grape berries, with lower TSS content and pH and higher TA. It is worth mentioning that the remarkable impact of the weather conditions on berry size and composition was also evident in the two vintages.
3.3. UPLC_MS Analysis of Grape Metabolites Univariate Statistics
At véraison, middle véraison, and harvest, grape samples were harvested from ABA-treated and untreated grapevines, and UPLC-MS analysis were carried out. UPLC-MS revealed 42 compounds in berry samples (Table S2), including one organic compound (citric acid), one stilbene (E-piceid), nine flavonols, eight flavan-3-ols, five anthocyanins diOH, eight anthocyanins triOH, six amino acids, and four phenolic acids.
The application of ABA on grape berries had no effect on the amino acid concentration at harvest in 2018 and throughout ripening in 2019. Further, amino acid levels found to be similar at harvest stage of both vintages (Figure 2A–C). On the contrary, the effect of ABA treatment was evident on phenolic acids content. Both doses of ABA in 2018 and the higher dose in 2019 resulted in increased phenolic acids level at véraison and harvest stages, compared to the controls. Worth mentioning, phenolic acid content was higher at the harvest stage of 2018 compared to the next vintage (Figure 2D–F).
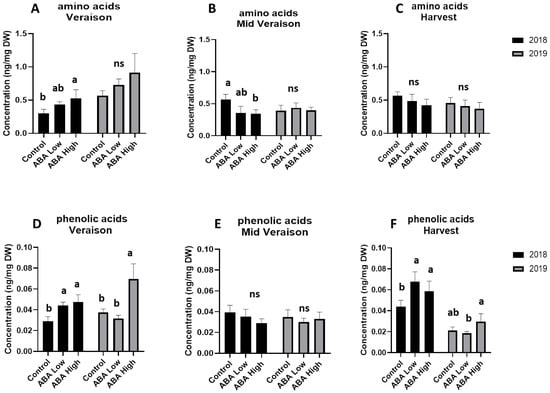
Figure 2.
Total quantity of amino acids (upper panel) and phenolic acids (lower panel) in grape berries at véraison (A,D), middle véraison (B,E), and at harvest (C,F) stage in 2018 (black bars) and 2019 (gray bars) in control and ABA-treated vines. Statistically significant differences between mean values (n = 6) are indicated with different letters (one way ANOVA, p-value < 0.05). Error bars represent the standard deviation.
Anthocyanins are responsible for giving grape berries their red color. According to our metabolomic analysis, there were no significant differences in anthocyanin di-OH and tri-OH levels between control and ABA-treated berries at harvest stages of both vintages (Figure 3). Similarly, no significant difference was observed in anthocyanin content at harvest stages of 2018 and 2019 vintage (Figure 3).
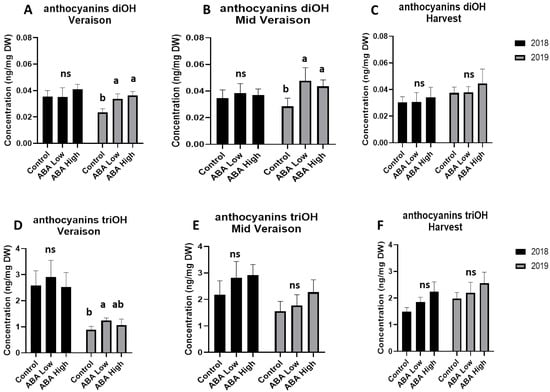
Figure 3.
Total concentrations of anthocyanins diOH (upper panel) and anthocyanin triOH (lower panel) in grape berries at véraison (A,D), middle véraison (B,E), and at harvest (C,F) stage in 2018 (black bars) and 2019 (gray bars) in control and ABA-treated vines. Statistically significant differences between mean values (n = 6) are indicated with different letters (one-way ANOVA, p-value < 0.05). Error bars represent the standard deviation.
The UPLC-MS analysis also revealed that ABA treatment altered the content of flavan-3-ols and flavonols during ripening. The application of ABA did not affect flavan-3-ol levels at the véraison and middle véraison stages in 2018 but resulted in a significant increase at harvest (Figure 4A–C). The same trend was also observed during the 2019 vintage with the higher dose ABA-treated berries recording higher flavan-3-ol concentrations than the control and the lower dose-treated berries at the véraison and harvest stages (Figure 4A–C). On the other hand, the application of ABA had diverse effects on total flavonol levels in the two vintages of the trial. During 2018 vintage, the ABA-treated grapes exhibited higher level of flavonols at the two first stages while no difference to the controls was observed at harvest (Figure 4D–F). On the contrary, ABA treatment increased the flavonols level at harvest stage of 2019 (Figure 4D–F). Noteworthy, flavonol content was higher in both control and ABA-treated grapes at harvest stage of 2019 compared to 2018 (Figure 4D–F).
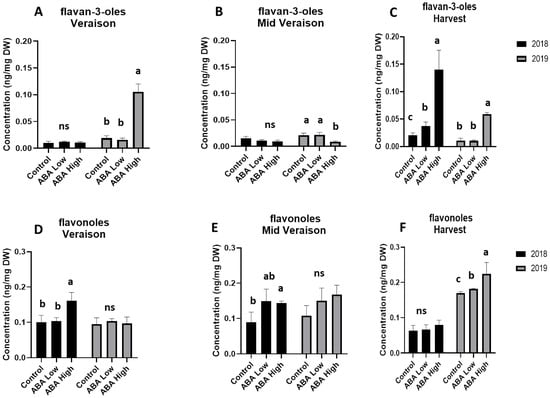
Figure 4.
Total concentrations of flavan-3-ols (upper panel) and flavonols (lower panel) in grape berries at véraison (A,D), middle véraison (B,E), and at harvest (C,F) stage in 2018 (black bars) and 2019 (gray bars) in control and ABA-treated vines. Statistically significant differences between mean values (n = 6) are indicated with different letters (one-way ANOVA, p-value < 0.05). Error bars represent the standard deviation.
A compound belonging to the stilbenes class, E-piceid, was also detected by UPLC-MS. The application of both a low and high dose of ABA resulted in higher levels of E-piceid compared to the control at middle véraison stage of both vintages (Figure 5). However, no statistically significant differences were observed between control and ABA-treated vines at harvest stage (Figure 5). It is worth mentioning that E-piceid content was higher at the harvest stage of 2019 compared to the 2018 (Figure 5).
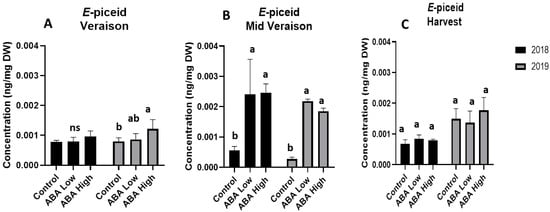
Figure 5.
Total concentrations of E-piceid in grape berries at véraison (A), middle véraison (B), and at harvest (C) stage in 2018 (black bars) and 2019 (gray bars) in control and ABA-treated vines. Statistically significant differences between mean values (n = 6) are indicated with different letters (one-way ANOVA, p-value < 0.05). Error bars represent the standard deviation.
Taken together, the metabolomic analysis showed that ABA treatment resulted in increased concentrations of phenolic acids and flavan-3-ols at harvest stage, regardless of the vintage. The flavonol levels were increased upon ABA treatment only the second vintage of the trial, while no significant effect was observed on amino acid, anthocyanin, and stilbene concentrations. Additionally, the flavonols may benefit from the lower daily temperatures that were recorded throughout the 2019 ripening season. High temperatures are known to reduce the formation of phenolic compounds, and it has been proposed that some secondary metabolites, such as kaempferol and flavonols in general, may serve as markers of warming-related declines in fruit quality. Under mild climatic circumstances, exogenously applied biostimulants can have a positive impact on grape composition.
3.4. Multivariate Statistics
In order to gain an overall view of the results and to examine the variability among the control and ABA-treated samples, different subgroups of metabolic data were independently analyzed and PCA plots on grape berries from the véraison, middle véraison (Figure S1), and harvest (Figure 6) stages from 2018 and 2019 vintages were produced. The vintage effect was evident at both phenological phases with a distinct separation along the PC1 axis, but the PCA score plots at véraison and middle véraison did not provide any distinction between the control and ABA-treated grape samples (Figure S1). The samples were discriminated along PC2, indicating the impact of the greater dose of ABA treatment on berry metabolism, in addition to the vintage effect (i.e., the separation along the PC1 axis) (Figure 6A).
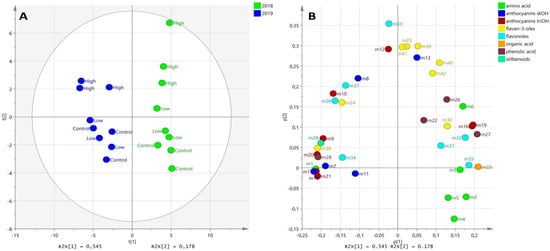
Figure 6.
Unsupervised classification using PCA on metabolomic data from control and ABA-treated grape berries at harvest stage in 2018 and 2019. Samples in the score plot (A) were colored according to the vintage, and variables in the loading plot (B) were colored according to the metabolic class. Numbers indicate the ID of metabolites, as follows: L-proline (m1); L-leucine (m2); L-isoleucine (m3); L-phenylalanine (m4;) L-tyrosine (m5); L-tryptophan (m6); cyanidin-3-O-glucoside (m7); peonidin-3-O-glucoside (m8); delphinidin-3-O-glucoside (m9); petunidin-3-O-glucoside (m10); cyanidin-3-O-(6-O-acetyl)-glucoside (m11); malvidin-3-O-glucoside (m12); peonidin-3-O-(6-acetyl-glucoside) (m13); dimethyl quercetin hexoside (m15); petunidin-3-O-(6-O-acetyl)-glucoside (m16); malvidin-3-O-(6-O-acetyl)-glucoside (m17); peonidin-3-O-(6-p-coumaroyl-glucoside) (m18); petunidin-3-O-(6-p-coumaroyl)-glucoside (m19); malvidin-3-O-(6-p-coumaroyl)-glucoside (m20); malvidin-3,5-O-diglucoside (m21); gallic acid (m22); citric acid (m23); catechin (m24); epicatechin (m25); coutaric acid ( m26); caftaric acid (m27); fertaric acid (m28); E-piceid (m29); catechin gallate (m30); kaempferol-3-O-glucoside (m31); quercetin-3-O-glucoside (m32); quercetin-O-gluronide m33); quercetin-3-O-glucuronide (m34); myricetin hexoside 1 (m35); myricetin glucoside (m36); quercetin (m37); procyanidinB1 (m38); procyanidinB2 (m39); procyanidinB3 ( m40); procyanidin B4 (m41); procyanidin gallate (m42).
The PCA score plot of the grape berries at harvest stage of the 2018 and 2019 vintages explained 72.8% of the variance. The metabolites that caused this separation were also shown in the loading plot (Figure 6B). The metabolites were mostly grouped according to their structural class, especially phenolic acids, flavonols, and flavan-3-ols (Figure 6B). The PC2 positive axis projection of flavan-3-ols, flavonols, and phenolic acids demonstrated that these substances were in charge of differentiating between samples treated with greater doses of ABA. This analysis confirmed that the higher dose treatment with ABA increased the concentrations of some phenolic compounds in the treated grapes at harvest stage, independently of the meteorological conditions during both vintages.
The amino acid and anthocyanin di-OH accumulation was correlated with the control and in lower dose ABA-treated berries, while flavan-3-ols were correlated with the berries treated with the higher dose of ABA (Figure 6B). The levels of specific flavonols (i.e., quercetin-O-glucuronide, myricetin glucoside, and quercetin), flavan-3-ols (i.e., procyanidins B2, B3, and B4, and procyanidin gallate) and anthocyanins (i.e., petunidin-3-O-glucoside and malvidin-3-O-glucoside) were significantly affected by the higher dose ABA treatment (Figure 6B).
4. Discussion
Central Greece’s Muses Valley is defined by extinguishing climatic and topographical characteristics that have fostered grapevine cultivation since antiquity. Nearly 90% of the area vineyards are Mouhtaro, an autochthonous red winemaking variety. It is an early/mid-harvested red variety, and the wine produced is characterized by middle to high color intensity and low astringency, while regarding the aroma is characterized by caramel, strawberry, and vegetal notes and forest fruit aromas [32].
There was a discernible vintage effect on the physicochemical properties of the grape berries at harvest as a result of the two years varying climatic circumstances, which affected the maturation process. For example, compared to the previous vintage, the 2019 vintage’s berries were larger because to the higher rainfall before véraison. Additionally, both control and ABA-treated grapes were characterized by higher TSS levels and decreased TA and pH values compared to 2018 vintage.
The results of berry size measurements and the conventional must analysis showed that ABA application affected the physiochemical characteristics of grape berries. The ABA-treated berries’ weight at harvest stage was lower, with lower TSS content and higher TA compared to the controls. These observations imply a delay in maturation upon the treatment. Although plant hormone application was found to have limited effect on berry sugar content [33,34], similar results to our study were obtained upon application of another bioelicitor, benzothiadiazole (BTH), on Mouhtaro and ABA on the white-colored Savvatiano grapes in trials conducted in the same vineyard [18,35].
Phenolic compounds represent a class of intensively studied bioactive molecules. They are related with the organoleptic characteristics of wines, like color, astringency, and bitterness. Moreover, they possess numerous health benefits and for this reason are used in the pharmaceutical and food industry [36,37,38]. The metabolomics analysis showed that ABA treatment affected the accumulation of phenolic compounds. More specifically, the ABA-treated grapes exhibited increased concentrations of phenolic acids and flavan-3-ols at harvest stage, regardless of the vintage, while the flavonols level was increased upon ABA treatment only for the second vintage of the trial. On the other hand, no significant differences to the controls were observed in amino acid, anthocyanin, and stilbene concentrations. Remarkably, BTH application on Mouhtaro grapes also had no effect on anthocyanins but, on the contrary, stilbenes level was increased [35], as well as upon ABA application on Savvatiano [28].
Earlier research has demonstrated that ABA application increased the concentration of phenolic compounds in grape berries, leading to improved attributes [21,22,23,39,40,41,42,43]. However, the diverse effects observed in various studies suggest that the timing, the concentration of ABA application, and the environmental conditions are critical factors in optimizing phenolic compounds accumulation in grape berries [12,44,45]. The application of ABA on Savvatiano grapes in a previous trial showed that another crucial parameter is the cultivar. Studies have shown that ABA treatment can enhance the content of anthocyanins, flavonols, and stilbenes in various grape varieties. For instance, in Malbec grapes, ABA increased low molecular weight polyphenols and stilbenes like trans-resveratrol and piceid [22]. Similarly, in Yan 73 and Cabernet Sauvignon, ABA treatment enhanced anthocyanins and non-anthocyanin phenolics, with varying effects depending on the variety (Luan et al., 2014) (Xi et al., 2013) [46,47]. For instance, contrary to ABA-treated grapes of Mouhtaro, which exhibited increased levels of flavan-3-ols and flavonols in the 2019 vintage compared to the controls, their concentrations were decreased or unaffected in Savvatiano. On the other hand, E-piceid was increased in the white variety upon ABA application whereas no effect was observed in Mouhtaro [28]. ABA treatment has been consistently shown to increase anthocyanin content in grape berries. For instance, studies on the Savvatiano and Kyoho white grape varieties demonstrated that ABA upregulates genes involved in anthocyanin biosynthesis, such as UDP-glucose:flavonoid 3-O-glucosyltransferase (UFGT) and glutathione S-transferase 4 (GST4), leading to higher anthocyanin accumulation [28,48,49]. Specifically, ABA treatment was found to enhance the production of petunidin- and malvidin-type anthocyanins, which are associated with deeper berry color [49].
The principal component analyses of the metabolomics data confirmed that both the vintage and the ABA treatment had a strong impact on grape berry phenolic composition. The PCA score plots at véraison and middle véraison showed that the first discrimination among the samples was due to the vintage. Although the vintage effect was also evident at harvest stage, an additional discrimination between controls/ABA lower dose and higher dose ABA samples indicated the impact of the treatment. Further, a more detailed analysis showed that the treatment with the higher dose of ABA increased the concentrations of specific phenolic compounds in the treated grapes at harvest stage, independently of the weather conditions during the two vintages.
The combination of a variety’s genetics and environmental conditions, including soil, terrain, and climate, determines its distinctive grape and wine quality traits. Given that grapevines have historically been grown in the Mediterranean region, the anticipated global warming scenario puts existing cultivation techniques to the test. As a result, sustainable agronomic practices may be helpful in reducing the adverse consequences of climate change while maintaining the high standards of the historically grown cultivars. Utilizing plant hormones and bioelicitors in viticulture provides a sustainable way to reduce the need for pesticides and fertilizers while improving the qualitative characteristics of grape berries in an eco-friendly way.
5. Conclusions
Applications of biostimulants are advantageous and successful viticultural techniques for improving grape and wine quality, particularly in view of the difficult climate change scenario. However, it is crucial to ascertain the best time and focus for their use. While ABA treatment reduced size and TSS in our two-vintage study, it also enhanced levels of phenolic chemicals, including flavonols, phenolic acids, and flavan-3-ols. As a result, using ABA may be regarded as a viable agronomic approach to improving the quality of grape berries. Further comprehensive studies, incorporating a wider range of indigenous grapevine varieties and local climate conditions, are needed to clarify grapevine reactions to various biostimulants and their unique impacts on each cultivar’s metabolic profiles requires taking into account a greater variety of indigenous grapevine varieties and local climate circumstances.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17104385/s1, Table S1: ABA applications and sampling dates during 2018 and 2019 vintages; Table S2: Metabolites identified by UPLC-MS in grape berry samples; Figure S1: The monthly climate conditions during the 2018 and 2019 vintages; Figure S2: Unsupervised classification using PCA on metabolomic data from control and ABA-treated grape berries at véraison and middle véraison stages in 2018 and 2019.
Author Contributions
Conceptualization, D.-E.M.; methodology, D.-E.M. and N.K.; software, D.-E.M.; validation, D.-E.M., A.A., N.K., K.N., P.H., A.L. and Y.K.; formal analysis, D.-E.M. and A.A.; investigation, D.-E.M. and A.A.; resources, D.-E.M. and A.A.; data curation, M.U. and D.-E.M.; writing—original draft preparation, D.-E.M. and A.A.; writing—review and editing, D.-E.M., A.A., N.K, K.N., P.H., A.L. and Y.K.; visualization, D.-E.M. and A.A.; supervision, D.-E.M.; project administration, D.-E.M.; funding acquisition, D.-E.M., A.L. and Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
The Project is Co Funded by Measure 16 Cooperation: Rural Development Program 2014–2020, Sub-measure 16.1–16.2 “Establishment and Functioning of Business Groups of the European Innovation Partnership for Agricultural Productivity and Sustainability” (project code: Μ16SΥΝ2-00292). This work was supported by the French Embassy in AthensGreece with the program “Programme de bourses pour séjour scientifique de haut niveau (SSHN) en France 2020–2021”. The authors wish to thank C-Valo for the grant to LOCASARM project funded by FEDER and Région Centre-Val de Loire.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors (pending privacy and ethical considerations).
Acknowledgments
The authors would like to thank the winemaker Nikos Zacharias of the winery Muses Estate for providing the Mouhtaro single vineyard in Muses Valley.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cabre, F.; Mario, N.; Nuñez, M. Impacts of climate change on viticulture in Argentina. Reg. Environ. Change 2020, 20, 12. [Google Scholar] [CrossRef]
- Droulia, F.; Charalampopoulos, I. Future climate change impacts on European viticulture: A review on recent scientific advances. Atmosphere 2021, 12, 495. [Google Scholar] [CrossRef]
- Jones, G.V.; Edwards, E.J.; Bonada, M.; Sadras, V.O.; Krstic, M.; Herderich, M. Climate change and its consequences for viticulture. In Managing Wine Quality, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2022; pp. 727–778. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef] [PubMed]
- Drappier, J.; Thibon, C.; Rabot, A.; Geny-Denis, L. Relationship between wine composition and temperature: Impact on Bordeaux wine typicity in the context of global warming. Crit. Rev. Food Sci. Nutr. 2017, 59, 14–30. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Ratnakumar, P.; Khan, M.L.R.; Minhas, P.S.; Farooq, M.; Sultana, R.; Per, T.S.; Deokate, P.P.; Khan, N.A.; Rane, J. Can plant bio-regulators minimize crop productivity losses caused by drought, heat and salinity stress? An integrated review. J. Appl. Bot. Food Qual. 2016, 89, 113–125. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.-T.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef]
- Small, C.C.; Degenhardt, D. Plant growth regulators for enhancing revegetation success in reclamation: A review. Ecol. Eng. 2018, 118, 43–51. [Google Scholar] [CrossRef]
- Dufour, M.C.; Lambert, C.; Bouscauta, J.; Merillon, J.M.; Corio-Costet, M.F. Benzothiadiazole-primed defence responses and enhanced differential expression of defence genes in Vitis vinifera infected with biotrophic pathogens Erysiphe necator and Plasmopara viticola. Plant Pathol. 2013, 62, 370–382. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Improving phenolic and chromatic characteristics of Monastrell, Merlot and Syrah wines by using methyl jasmonate and benzothiadiazole. J. Int. Sci. Vigne Vin 2017, 51, 17–27. [Google Scholar] [CrossRef]
- Koyama, R.; Roberto, S.R.; de Souza, R.T.; Borges, W.F.S.; Anderson, M.; Waterhouse, A.L.; Cantu, D.; Fidelibus, M.W.; Blanco-Ulate, B. Exogenous Abscisic Acid Promotes Anthocyanin Biosynthesis and Increased Expression of Flavonoid Synthesis Genes in Vitis vinifera X Vitis labrusca Table Grapes in a Subtropical Region. Front. Plant Sci. 2018, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Soares, B.; Goufo, P.; Castro, I.; Cosme, F.; Pinto-Sintra, A.L.; Inês, A.; Oliveira, A.A.; Falco, V. Chitosan Upregulates the Genes of the ROS Pathway and Enhances the Antioxidant Potential of Grape (Vitis vinifera L. ‘Touriga Franca’ and ‘Tinto Cão’) Tissues. Antioxidants 2019, 8, 525. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Martins, V.; Soares, B.; Castro, I.; Falco, V. Chitosan Application in Vineyards (Vitis vinifera L. cv. Tinto Cão) Induces Accumulation of Anthocyanins and Other Phenolics in Berries, Mediated by Modifications in the Transcription of Secondary Metabolism Genes. Int. J. Mol. Sci. 2020, 21, 306. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.; Unlubayir, M.; Teixeira, A.; Gerós, H.; Lanoue, A. Calcium and methyl jasmonate cross-talk in the secondary metabolism of grape cells. Plant Physiol. Biochem. 2021, 165, 228–238. [Google Scholar] [CrossRef]
- Sabir, A.; Kucukbasmaci, A.; Taytak, M.; Bilgin, O.F.; Jawshle, A.I.M.; Mohammed, O.J.M.; Gayretli, Y. Sustainable viticulture practices on the face of climate change. Agric. Res. Technol. 2018, 17, 133–137. [Google Scholar] [CrossRef]
- Koyama, L.; Sadamatsu, K.; Goto-Yamamoto, N. Abscisic acid stimulated ripening and gene expression in berry skins of the Cabernet Sauvignon grape. Funct. Integr. Genom. 2010, 10, 367–381. [Google Scholar] [CrossRef]
- Ferrandino, A.; Lovisolo, C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ. Exp. Bot. 2014, 103, 138–147. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Peppi, M.C.; Walker, M.A.; Fidelibus, M.W. Application of abscisic acid rapidly upregulated UFGT gene expression and improved color of grape berries. Vitis J. Grapevine Res. 2008, 47, 11–14. [Google Scholar]
- Villalobos-González, L.; Peña-Neira, A.; Ibáñez, F.; Pastenes, C. Long-term effects of abscisic acid (ABA) on the grape berry phenylpropanoid pathway: Gene expression and metabolite content. Plant Physiol. Biochem. 2016, 105, 213–223. [Google Scholar] [CrossRef]
- Alonso, R.; Berli, F.J.; Fontana, A.; Piccoli, P.; Bottini, R. Abscisic acid’s role in the modulation of compounds that contribute to wine quality. Plants 2021, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.J.D.; Junior, A.P.; Borges, C.V.; Cunha, S.R.; Callili, D.; Lima, G.P.P.; Roberto, S.R.; Leonel, S.; Tecchio, M.A. The exogenous application of abscisic acid induce accumulation of anthocyanins and phenolic compounds of the ‘Rubi’ grape. Am. J. Plant Sci. 2017, 8, 2422–2432. [Google Scholar] [CrossRef]
- Karimi, R.; Ebrahimi, M.; Amerian, M. Abscisic acid mitigates NaCl toxicity in grapevine by influencing phytochemical compounds and mineral nutrients in leaves. Sci. Hortic. 2021, 288, 110366. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Romanazzi, G.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. A review of the use of biostimulants in the vineyard for improved grape and wine quality: Effects on prevention of grapevine diseases. J. Sci. Food Agric. 2019, 99, 1001–1009. [Google Scholar] [CrossRef]
- Monteiro, E.; Concalves, B.; Cortez, I.; Castro, I. The Role of biostimulants as alleviators of biotic and abiotic stresses in grapevine: A review. Plants 2022, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Miliordos, D.E.; Alatzas, A.; Kontoudakis, N.; Kouki, A.; Unlubayir, M.; Gémin, M.-P.; Tako, A.; Hatzopoulos, P.; Lanoue, A.; Kotseridis, Y. Abscisic Acid and Chitosan Modulate Polyphenol Metabolism and Berry Qualities in the Domestic White-Colored Cultivar Savvatiano. Plants 2022, 11, 1648. [Google Scholar] [CrossRef]
- Lagouvardos, K.; Kotroni, V.; Bezes, A.; Koletsis, I.; Kopania, T.; Lykoudis, S.; Mazarakis, N.; Papagiannaki, K.; Vougioukas, S. The automatic weather stations NOANN network of the National Observatory of Athens: Operation and database. Geosci. Data J. 2017, 4, 4–16. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis of Wines and Musts, Methods, OIVMA–AS313–01; OIV–MA–AS2–10; International Organisation of Vine and Wine: Paris, France, 2018; Volume 2, Available online: https://www.oiv.int/public/medias/3731/oiv-ma-as313-01.pdf (accessed on 31 March 2025).
- Billet, K.; Delanoue, G.; Arnault, I.; Besseau, S.; Oudin, A.; Courdavault, V.; Marchand, P.A.; Giglioli-Guivarc’h, N.; Guérin, L.; LaNoue, A. Vineyard evaluation of stilbenoid-rich grape cane extracts against downy mildew: A large-scale study. Pest Manag. Sci. 2018, 75, 1252–1257. [Google Scholar] [CrossRef]
- Miliordos, D.-E.; Tsiknia, M.; Kontoudakis, N.; Zaharias, N.; Kotseridis, Y. A holistic approach to using biostimulants on the red grapevine variety, Mouhtaro—from grape to wine: Sourced from the research article: “Impact of Application of Abscisic Acid, Benzothiadiazole and Chitosan on Berry Quality Characteristics and Plant Associated Microbial Communities of Vitis vinifera L var. Mouhtaro Plants.” (Sustainability, 2021). Original language of the article: English. IVES Tech. Rev. Vine Wine 2022. [Google Scholar] [CrossRef]
- Jeong, S.; Goto-Yamamoto, N.; Kobayashi, S.; Esaka, M. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci. 2004, 167, 247–252. [Google Scholar] [CrossRef]
- Cocco, M.; Mercenaro, L.; Lo Cascio, M.; Nieddu, G. Effects of Vine Water Status and Exogenous Abscisic Acid on Berry Composition of Three Red Wine Grapes Grown under Mediterranean Climate. Horticulturae 2020, 6, 12. [Google Scholar] [CrossRef]
- Miliordos, D.E.; Alatzas, A.; Kontoudakis, N.; Unlubayir, M.; Hatzopoulos, P.; Lanoue, A.; Kotseridis, Y. Benzothiadiazole Affects Grape Polyphenol Metabolism and Wine Quality in Two Greek Cultivars: Effects during Ripening Period over Two Years. Plants 2023, 12, 1179. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Malinowska, M.; Billet, K.; Drouet, S.; Munsch, T.; Unlubayir, M.; Tungmunnithum, D.; Giglioli-Guivarch, N.; Hano, C.; Lanoue, A. Grape cane extracts as multifunctional rejuvenating cosmetic ingredient: Evaluation of sirtuin activity, tyrosinase inhibition and bioavailability potential. Molecules 2020, 25, 2203. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Romero-Cascales, I.; Gómez-Plaza, E. Increasing the Phenolic Compound Content of Grapes by Preharvest Application of Abcisic Acid and a Combination of Methyl Jasmonate and Benzothiadiazole. J. Agric. Food Chem. 2013, 61, 3978–3983. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Q.; Xi, B.; Dai, H. Study on the regulation of anthocyanin biosynthesis by exogenous abscisic acid in grapevine. Sci. Hortic. 2019, 250, 294–301. [Google Scholar] [CrossRef]
- Alenazi, M.M.; Shafiq, M.; Alobeed, R.S.; Alsdon, A.A.; Abbasi, N.A.; Ali, I.; Mubushar, M.; Javed, I. Application of abscisic acid at véraison improves red pigmentation and accumulation of dietary antioxidants in red table grapes cv. Red Globe at harvest. Sci. Hortic. 2019, 257, 108672. [Google Scholar] [CrossRef]
- He, L.; Ren, Z.-Y.; Wang, Y.; Fu, Y.-Q.; Li, Y.; Meng, N.; Pan, Q.-H. Variation of Growth-to-Ripening Time Interval Induced by Abscisic Acid and Synthetic Auxin Affecting Transcriptome and Flavor Compounds in Cabernet Sauvignon Grape Berry. Plants 2020, 9, 630. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Wang, S.; Wang, W.; Xu, X.; Wu, J.; Fang, Y.; Ju, Y. Effects of strigolactone and abscisic acid on the quality and antioxidant activity of grapes (Vitis vinifera L.) and wines. Food Chem. X 2022, 16, 100496. [Google Scholar] [CrossRef] [PubMed]
- Malovini, E.; Arancibia, C.; Durán, M.; Fontana, A.; de Rosas, M.I.; Deis, L.; Gargantini, R.; Bottini, R.; Cavagnaro, B.; Martínez, L. Abscisic acid and methyl jasmonic acid module anthocyanins and trans-resveratrol accumulation in berry skin of five red Vitis vinifera cultivars in two contrasting viticultural regions of mendoza-Argentina. Rev. Fac. Cienc. Agrar. 2019, 51, 451–460. [Google Scholar]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Martínez-Moreno, A.; Gil-Muñoz, R. Elicitors and Pre-Fermentative Cold Maceration: Effects on Polyphenol Concentration in Monastrell Grapes and Wines. Biomolecules 2019, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.Y.; Zhang, Z.W.; Xi, Z.M.; Huo, S.S.; Ma, L.N. Comparing the effects of exogenous abscisic acid on the phenolic composition of Yan 73 and Cabernet Sauvignon (Vitis vinifera L.) wines. Eur. Food Res. Technol. 2014, 239, 203–213. [Google Scholar] [CrossRef]
- Xi, Z.M.; Meng, J.F.; Huo, S.S.; Luan, L.Y.; Ma, L.N.; Zhang, Z.W. Exogenously applied abscisic acid to Yan73 (V. vinifera) grapes enhances phenolic content and antioxidant capacity of its wine. Int. J. Food Sci. Nutr. 2013, 64, 444–451. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, M.; Feng, M.; Liu, G.; Torregrosa, L.; Tao, X.; Xu, T. miR156b-targeted VvSBP8/13 functions downstream of the abscisic acid signal to regulate anthocyanins biosynthesis in grapevine fruit under drought. Hortic. Res. 2024, 11, uhad293. [Google Scholar] [CrossRef]
- Katayama-Ikegami, A.; Sakamoto, T.; Shibuya, K.; Katayama, T.; Gao-Takai, M. Effects of abscisic acid treatment on berry coloration and expression of flavonoid biosynthesis genes in grape. Am. J. Plant Sci. 2016, 7, 1325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).