Changes in Microbial Activity Associated with the Nitrogen Biogeochemical Cycle in Differently Managed Soils, Including Protected Areas and Those Reclaimed with Gangue
Abstract
1. Introduction
2. Materials and Methods
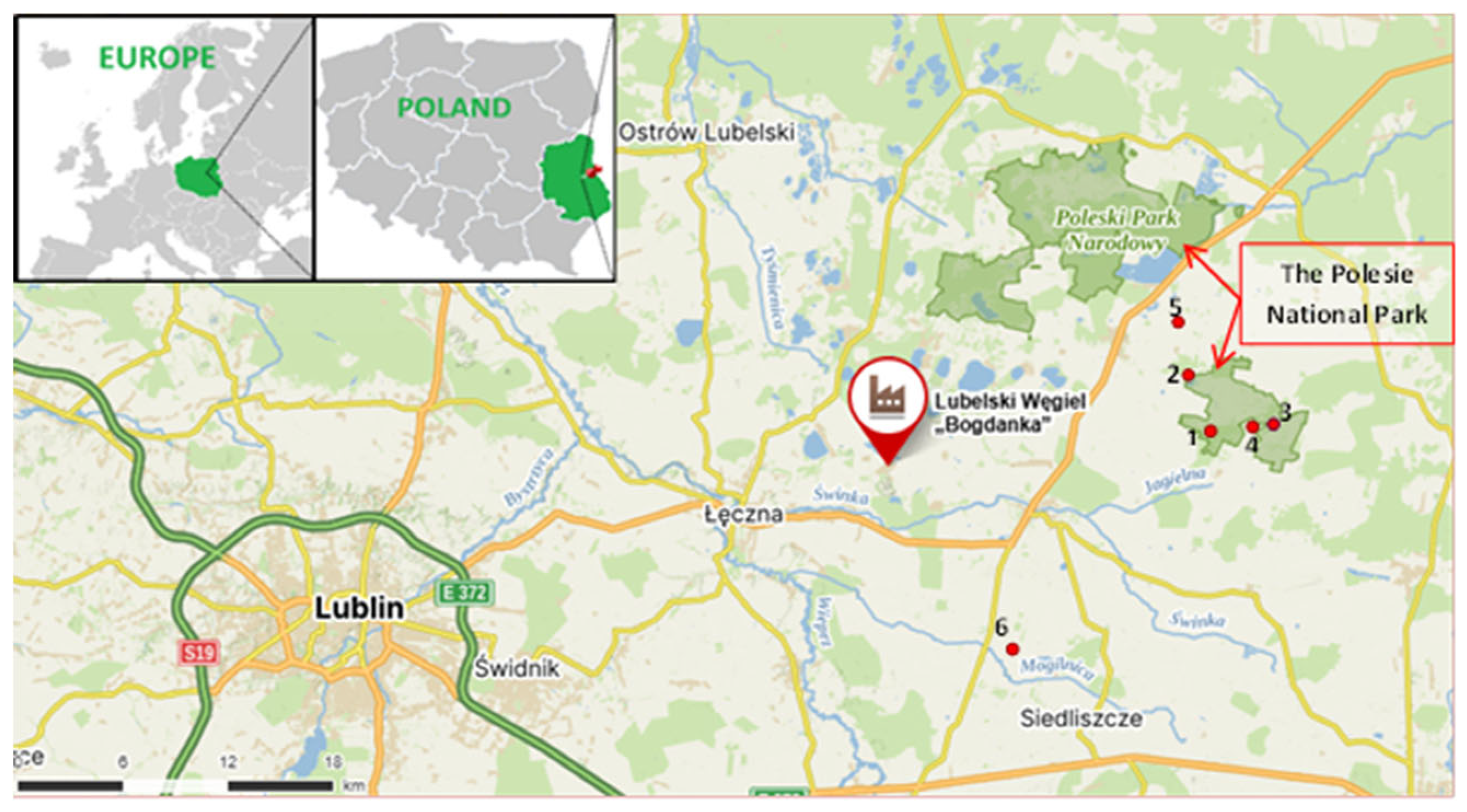
2.1. Sampling Location
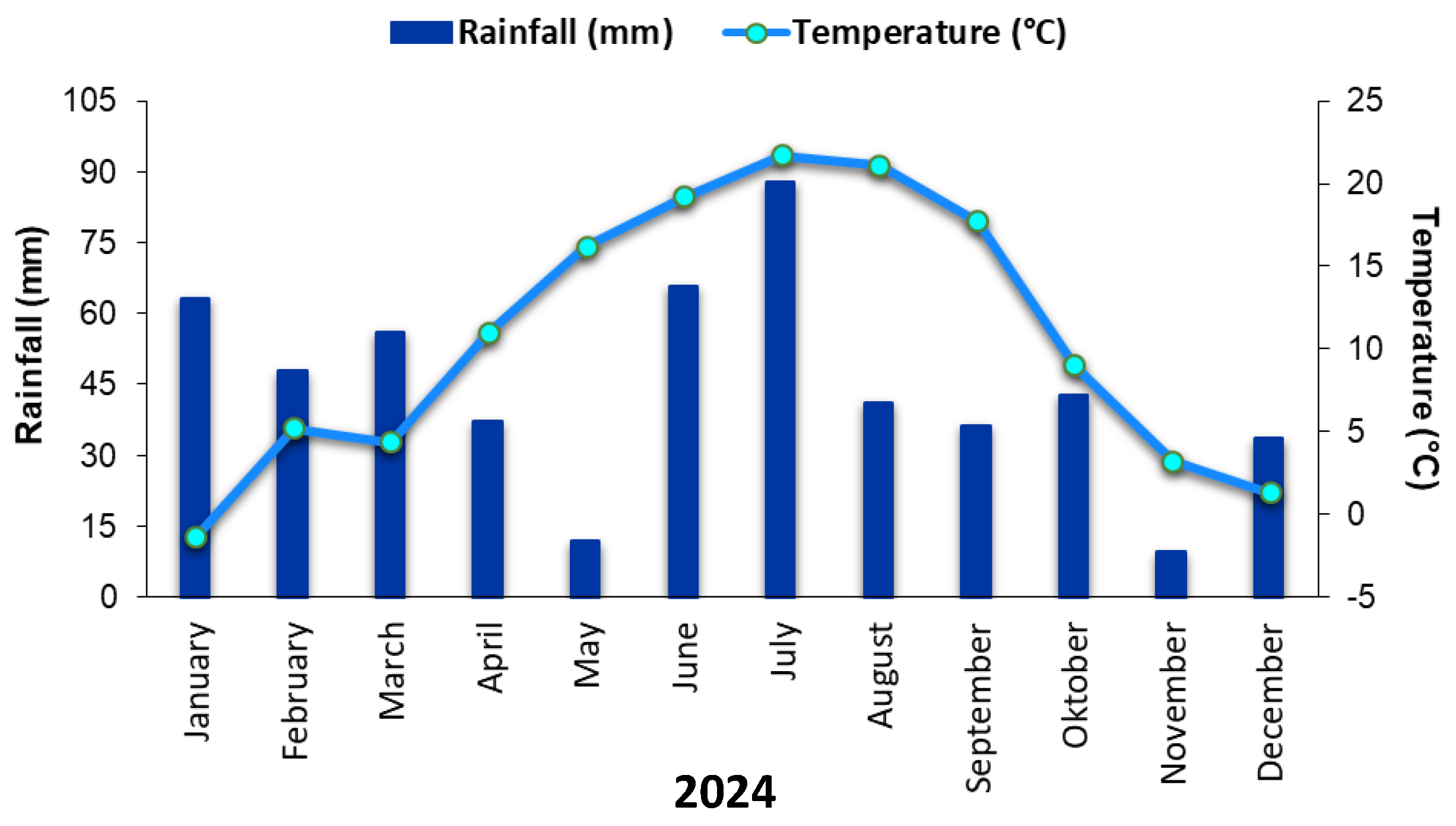
2.2. Study Area Characteristics
2.3. Soil Sample Collection
2.4. Biochemical Analysis of N-Related Soil Processes
2.5. Enzymatic Analyses
2.6. Statistical Analysis
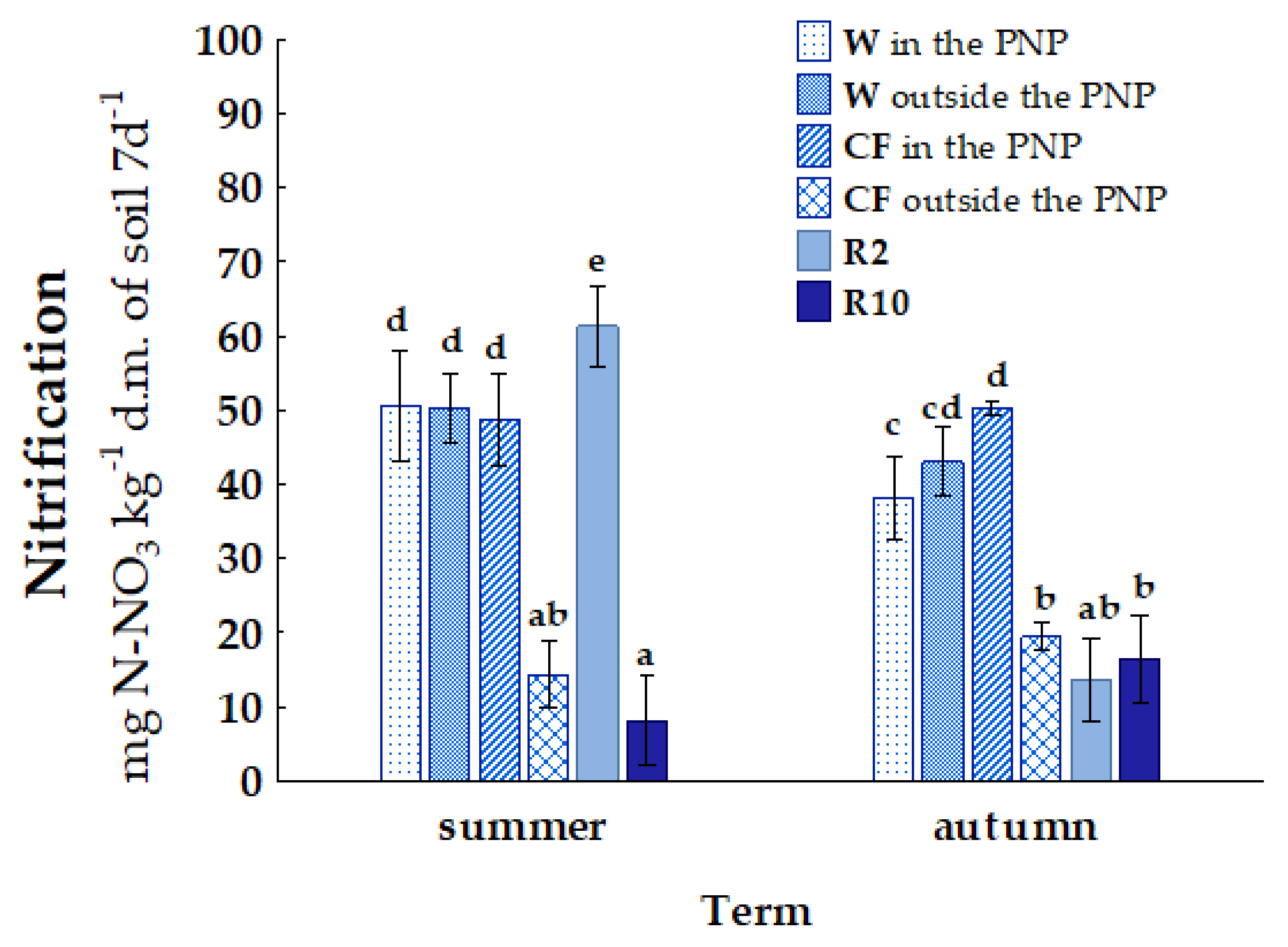
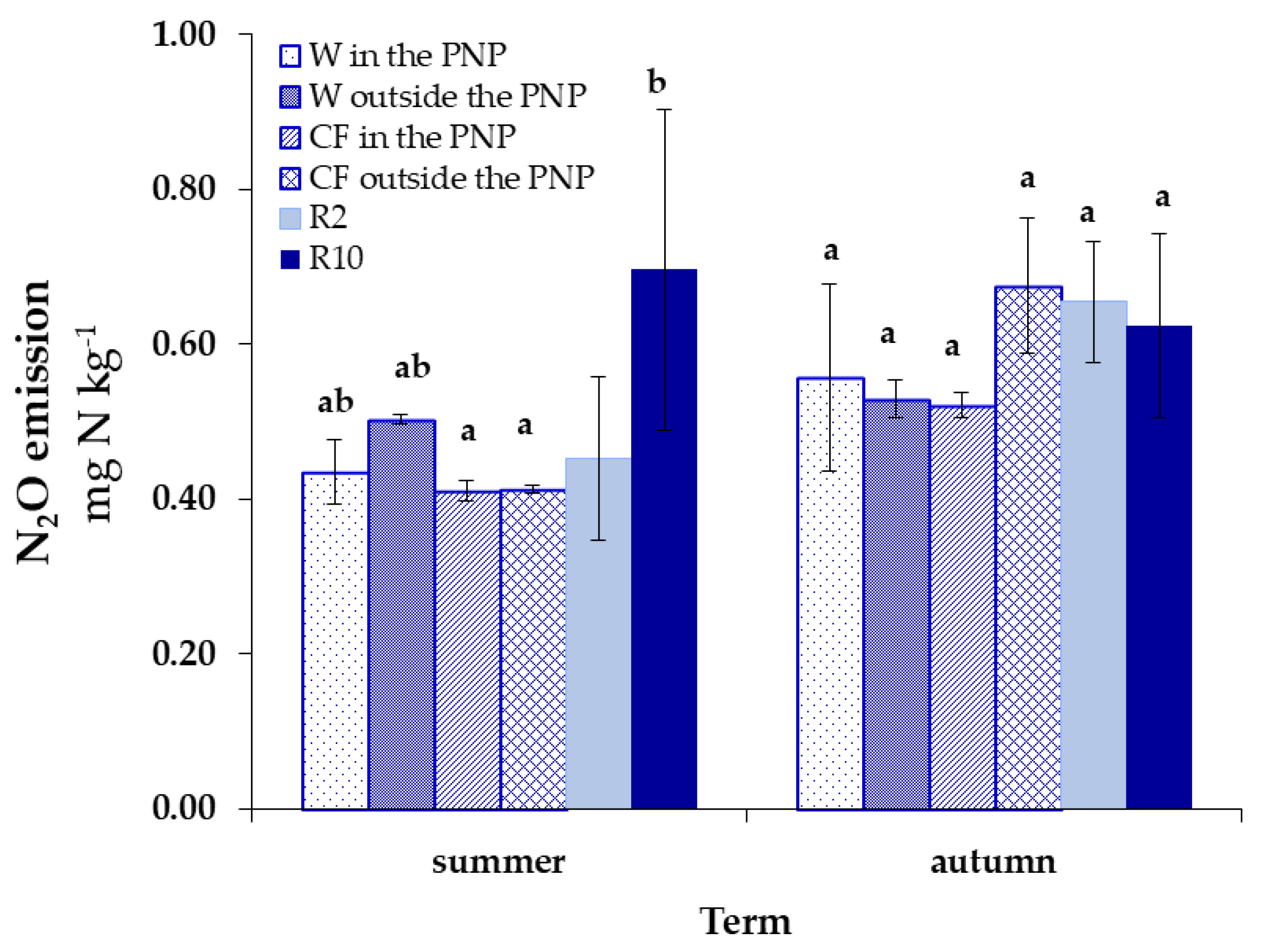
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Zhen, J.; Hu, W.; Chen, S.; Lizaga, I.; Zeraatpisheh, M.; Yang, X. Remote sensing of soil degradation: Progress and perspective. Int. Soil Water Conserv. Res. 2023, 11, 429–454. [Google Scholar] [CrossRef]
- FAO Soils Portal. Available online: https://www.fao.org/soils-portal/soil-degradation-restoration/en/ (accessed on 27 December 2024).
- Keesstra, S.; Mol, G.; De Leeuw, J.; Okx, J.; Molenaar, C.; De Cleen, M.; Visser, S. Soil-related sustainable development goals: Four concepts to make land degradation neutrality and restoration work. Land 2018, 7, 133. [Google Scholar] [CrossRef]
- Song, W.; Xu, R.; Li, X.; Min, X.; Zhang, J.; Zhang, H.; Hu, X.; Li, J. Soil reconstruction and heavy metal pollution risk in reclaimed cultivated land with coal gangue filling in mining areas. Catena 2023, 228, 107147. [Google Scholar] [CrossRef]
- Worlanyo, A.S.; Jiangfeng, L. Evaluating the environmental and economic impact of mining for post-mined land restoration and land-use: A review. J Environ. Manag. 2021, 279, 111623. [Google Scholar] [CrossRef]
- Agboola, O.; Babatunde, D.E.; Fayomi, O.S.I.; Sadiku, E.R.; Popoola, P.; Moropeng, L.; Moropeng, L.; Mamudu, O.A. A review on the impact of mining operation: Monitoring, assessment and management. Results Eng. 2020, 8, 100181. [Google Scholar] [CrossRef]
- Vriens, B.; Plante, B.; Seigneur, N.; Jamieson, H. Mine Waste rock: Insights for sustainable hydrogeochemical management. Minerals 2020, 10, 728. [Google Scholar] [CrossRef]
- Jin, L.; Li, X.; Sun, H.; Zhang, J.; Zhang, Y.; Wang, R. Responses of soil microbial activities to soil overburden thickness in restoring a coal gangue mound in an alpine mining area. Ecol. Indic. 2023, 151, 110294. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.W.; Lai, J.L.; Zhang, Y.; Luo, X.G. Effects of long-term herbaceous plant restoration on microbial communities and metabolic profiles in coal gangue-contaminated soil. Environ. Res. 2023, 234, 116491. [Google Scholar] [CrossRef]
- Capasso, I.; Lirer, S.; Flora, A.; Ferone, C.; Cioffi, R.; Caputo, D.; Liguori, B. Reuse of mining waste as aggregates in fly ash-based geopolymers. J. Clean. Prod. 2019, 220, 65–73. [Google Scholar] [CrossRef]
- Pactwa, K.; Woźniak, J.; Dudek, M. Coal mining waste in Poland in reference to circular economy principles. Fuel 2020, 270, 117493. [Google Scholar] [CrossRef]
- Klojzy-Karczmarczyk, B.; Mazurek, J. Legal and environmental conditions for the reclamation of opencast mines using coal mining waste or gangue-based raw materials. Bulletin of The Mineral and Energy Economy Research Institute of the Polish Academy of Sciences. 2015, Volume 90, pp. 67–77. (In Polish). Available online: https://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-7b8b9097-4a20-48f4-832a-88a564b3f857.
- Bogdanka, S.A. Available online: https://lw.com.pl/zrownowazony-rozwoj-bogdanki (accessed on 29 December 2024).
- Jeznach, J.; Urban, D.; Michalczyk, Z.; Kulik, M.; Sugier, P.; Grzywaczewski, G. Characteristic features of the Polish Part of Polesie. In Handbook of Research on Improving the Natural and Ecological Conditions of the Polesie Zone; IGI Global: Hershey, PA, USA, 2023; pp. 21–29. [Google Scholar] [CrossRef]
- Joniec, J.; Kwiatkowska, E.; Walkiewicz, A.; Grzywaczewski, G.; Garbacz, A. Assessment of N-related microbial processes in the soil of the Polesie National Park and adjacent areas, including reclaimed land. J. Environ. Manag. 2025, 373, 124002. [Google Scholar] [CrossRef] [PubMed]
- Inatomi, M.; Hajima, T.; Ito, A. Fraction of nitrous oxide production in nitrification and its effect on total soil emission: A meta-analysis and global-scale sensitivity analysis using a process-based model. PLoS ONE 2019, 14, e0219159. [Google Scholar] [CrossRef] [PubMed]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Chataut, G.; Bhatta, B.; Joshi, D.; Subedi, K.; Kafle, K. Greenhouse gases emission from agricultural soil: A review. J. Agric. Food Res. 2023, 11, 100533. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, R.; Zhao, D.; Liu, S.; Fu, J.; Zhang, Y.; Dai, N.; Song, D.; Ding, H. Microbial-mediated emissions of greenhouse gas from farmland soils: A Review. Processes 2022, 10, 2361. [Google Scholar] [CrossRef]
- Ayiti, O.E.; Babalola, O.O. Factors influencing soil nitrification process and the effect on environment and health. Front. Sustain. Food Syst. 2022, 6, 821994. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Chang, S.X.; Xu, Q.; Li, Y.; Ma, Z.; Qin, H.; Cai, Y. Linking enhanced soil nitrogen mineralization to increased fungal decomposition capacity with Moso bamboo invasion of broadleaf forests. Sci. Total Environ. 2021, 771, 144779. [Google Scholar] [CrossRef]
- Furtak, K.; Gałązka, A. Enzymatic activity as a popular parameter used to determine the quality of the soil environment. Pol. J. Agron. 2019, 37, 22–30. [Google Scholar] [CrossRef]
- Kujur, M.; Patel, A.K. Kinetics of soil enzyme activities under different ecosystems: An index of soil quality. Chil. J. Agric. Res. 2014, 74, 96–104. [Google Scholar] [CrossRef]
- Vranova, V.; Rejsek, K.; Formanek, P. Proteolytic activity in soil: A review. Appl. Soil Ecol. 2013, 70, 23–32. [Google Scholar] [CrossRef]
- Zhang, T.; Wan, S.; Kang, Y.; Feng, H. Urease activity and its relationships to soil physiochemical properties in a highly saline-sodic soil. J. Soil Sci. Plant Nutr. 2014, 14, 304–315. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, X.; Ni, K.; Ma, L.; Shi, Y.; Wang, Y.; Cai, Y.; Ma, Q.; Ruan, J. Nitrogen addition reduces phosphorus availability and induces a shift in soil phosphorus cycling microbial community in a tea (Camellia sinensis L.) plantation. J. Environ. Manag. 2023, 342, 118207. [Google Scholar] [CrossRef] [PubMed]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny-Horas, R.; Fernández-Martínez, M.; Janssens, I.A.; Richter, A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. The effect of global change on soil phosphatase activity. Glob. Change Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef] [PubMed]
- Patle, P.N.; Navnage, N.P.; Barange, P.K. Fluorescein diacetate (FDA): Measure of total microbial activity and as indicator of soil quality. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2103–2107. [Google Scholar] [CrossRef]
- Jezierska-Tys, S.; Joniec, J.; Mocek-Płóciniak, A.; Gałązka, A.; Bednarz, J.; Furtak, K. Microbial activity and community level physiological profiles (CLPP) of soil under the cultivation of spring rape with the Roundup 360 SL herbicide. J. Environ. Health Sci. Eng. 2021, 19, 2013–2026. [Google Scholar] [CrossRef]
- Joniec, J.; Oleszczuk, P.; Jezierska-Tys, S.; Kwiatkowska, E. Effect of reclamation treatments on microbial activity and phytotoxicity of soil degraded by the sulphur mining industry. Environ. Pollut. 2019, 252, 1429–1438. [Google Scholar] [CrossRef]
- Joniec, J.; Żukowska, G.; Bik-Małodzińska, M.; Kwiatkowska, E.; Rojek, K. Reaction of microorganisms to long-term waste reclamation of soil degraded by the sulfur mining industry. Minerals 2021, 11, 1226. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Joniec, J.; Kwiatkowski, C.A. Involvement of soil microorganisms in C, N and P transformations and phytotoxicity in soil from post-industrial areas treated with chemical industry waste. Minerals 2023, 13, 12. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.S.; Kim, J.G.; Kim, S.O. Use of Soil Enzymes as Indicators for Contaminated Soil Monitoring and Sustainable Management. Sustainability 2020, 12, 8209. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaite, R. Role of Soil Microbiota Enzymes in Soil Health and Activity Changes Depending on Climate Change and the Type of Soil Ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef]
- Kar, S.; Sundberg, T.; Satpati, L.; Mukherjee, S. Reappraising Natures and Perspectives of Wasteland in the Developing World with a Focus on India. Environments 2024, 11, 111. [Google Scholar] [CrossRef]
- Chmielewski, T.J.; Szymański, J.; Weigle, A. Poleski National Park: Heritage and Future; UMCS: Lublin, Poland, 2020. (In Polish) [Google Scholar]
- Priha, O.; Smolander, A. Nitrogen transformations in soil under Pinus sylvestris, Picea abies and Betula pendula at two forest sites. Soil Biol. Biochem. 1999, 31, 965–977. [Google Scholar] [CrossRef]
- Tutiempo. World Weather and Local Weather Forecast. 2024. Available online: https://en.tutiempo.net/climate/ws-124970.html (accessed on 10 January 2025).
- Nowosielski, O. Methods for Determination of Fertilization Requirements, 2nd ed.; PWRiL: Warsaw, Poland, 1974. (In Polish) [Google Scholar]
- Yu, Z.; Chen, L.; Pan, S.; Li, Y.; Kuzyakov, Y.; Xu, J.; Brookes, P.; Luo, Y. Feedstock determines biochar-induced soil priming effects by stimulating the activity of specific microorganisms. Eur. J. Soil Sci. 2018, 69, 521–534. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.A.H. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Zantua, M.J.; Bremner, J.M. Comparison of methods of assaying urease activity in soils. Soil Biol. Biochem. 1975, 7, 291–295. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Schnurer, J.; Rosswall, T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Liu, C.; Wang, B.; Zhu, Y.; Qu, T.; Xue, Z.; Li, X.; Zhou, Z.; An, S. Eco-enzymatic stoichiometry and microbial non-homeostatic regulation depend on relative resource availability during litter decomposition. Ecol. Indic. 2022, 145, 109729. [Google Scholar] [CrossRef]
- Act of April 16, 2004 on Nature Conservation, Art. 15(1) (Journal of Laws of 2023, Item 1336, as Amended). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20230001336/U/D20231336Lj.pdf (accessed on 1 April 2025).
- Joniec, J. Enzymatic activity as an indicator of regeneration processes in degraded soil reclaimed with various types of waste. Int. J. Environ. Sci. Technol. 2018, 15, 2241–2252. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Joniec, J.; Kwiatkowski, C.A.; Kowalczyk, K.; Nowak, M.; Leśniowska-Nowak, J. Assessment of the impact of spent mushroom substrate on biodiversity and activity of soil bacterial and fungal populations based on classical and modern soil condition indicators. Int. Agrophys. 2024, 38, 139–154. [Google Scholar] [CrossRef]
- Mouradi, B.; Farissic, M.; Makoudia, B.; Bouizgarenb, A.; Ghoulam, C. Effect of faba bean (Vicia faba L.)—Rhizobia symbiosis on barley’s growth, phosphorus uptake and acid phosphatase activity in the intercropping system. Ann. Agrar. Sci. 2018, 16, 297–303. [Google Scholar] [CrossRef]
- Campdelacreu Rocabruna, P.; Domene, X.; Preece, C.; Peñuelas, J. Relationship among soil biophysicochemical properties, agricultural practices and climate factors influencing soil phosphatase activity in agricultural land. Agriculture 2024, 14, 288. [Google Scholar] [CrossRef]
- Arora, S.; Sahni, D. Pesticides effect on soil microbial ecology and enzyme activity—An overview. J. Appl. Nat. Sci. 2016, 8, 1126–1132. [Google Scholar] [CrossRef]
- Opdyke, M.R.; Ostrom, N.E.; Ostrom, P.H. Evidence for the predominance of denitrification as a source of N2O in temperate agricultural soils based on isotopologue measurements. Glob. Biogeochem. Cycles 2009, 23, 4. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. The significance of microbial transformation of nitrogen compounds in the light of integrated crop management. Agronomy 2021, 11, 1415. [Google Scholar] [CrossRef]
- Breugem, A.J.; Kros, J.; de Vries, W. Impacts of pH on Mechanisms and Rates of Carbon and Nitrogen Mineralisation: A Review; Report 3342; Wageningen Environmental Research: Wageningen, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef]
- Nsabimana, D.; Haynes, R.J.; Wallis, F.M. Size, activity and catabolic diversity of the soil microbial biomass as affected by land use. Appl. Soil Ecol. 2004, 26, 81–92. [Google Scholar] [CrossRef]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim Change 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Cuhel, J.; Simek, M.; Laughlin, R.J.; Bru, D.; Cheneby, D.; Watson, C.J.; Philippot, L. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microbiol. 2010, 76, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, L.; Hu, R.; Ma, H.; Liu, B.; Zhang, W.; Wu, L. Patterns and determinants of nitrification and denitrification potentials across 24 rice paddy soils in subtropical China. Agric. Ecosyst. Environ. 2024, 361, 108799. [Google Scholar] [CrossRef]
- Nan, Y.-C.; Yang, Y.-G.; Wang, Z.-Q.; Zhou, Y.; Su, Q.-M. Effects of Coal Gangue on Soil Property and Plant Growth in Mining Area. Ying Yong Sheng Tai Xue Bao 2023, 34, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Ahirwal, J.; Maiti, S.K.; Reddy, M.S. Development of carbon, nitrogen and phosphate stocks of reclaimed coal mine soil within 8 years after forestation with Prosopis juliflora (Sw.) Dc. Catena 2017, 156, 42–50. [Google Scholar] [CrossRef]
- Alvarenga, P.; Rodrigues, D.; Mourinha, C.; Palma, P.; de Varennes, A.; Cruz, N.; Tarelho, L.A.C.; Rodrigues, S. Use of wastes from the pulp and paper industry for the remediation of soils degraded by mining activities: Chemical, biochemical and ecotoxicological effects. Sci. Total Environ. 2019, 686, 1152–1163. [Google Scholar] [CrossRef]
- Sekaran, U.; Sandhu, S.S.; Qiu, Y.; Kumar, S.; Gonzalez Hernandez, J.L. Biochar and manure addition influenced soil microbialcommunity structure and enzymatic activities at eroded and depositional landscape positions. Land Degrad. Dev. 2020, 31, 894–908. [Google Scholar] [CrossRef]
- Zornoza, R.; Acosta, J.A.; Faz, A.; Bååth, E. Microbial growth and community structure in acid mine soils after addition of different amendments for soil reclamation. Geoderma 2016, 272, 64–72. [Google Scholar] [CrossRef]
- Iticescu, C.; Georgescu, L.P.; Murariu, G.; Circiumaru, A.; Timofti, M. The characteristics of sewage sludge used on agricultural lands. In Proceedings of the AIP Conference Proceedings, Seoul, Republic of Korea, 4–8 July 2022; Volume 1, p. 020001. [Google Scholar] [CrossRef]
- Castagnoli, A.; Pasciucco, F.; Iannelli, R.; Meoni, C.; Pecorini, I. Keu Contamination in Tuscany: The Life Cycle Assessment of Remediation Project as a Decision Support Tool for Local Administration. Sustainability 2022, 14, 14828. [Google Scholar] [CrossRef]
- Pasciucco, E.; Pasciucco, F.; Castagnoli, A.; Iannelli, R.; Pecorini, I. Removal of heavy metals from dredging marine sediments via electrokinetic hexagonal system: A pilot study in Italy. Heliyon 2024, 10, e27616. [Google Scholar] [CrossRef]
- Rafique, N.; Khalil, S.; Cardinale, M.; Rasheed, A.; Zhao, F.; Abideen, Z. A comprehensive evaluation of the potential of plant growth-promoting rhizobacteria for applications in agriculture in stressed environments. Pedosphere 2025, 35, 229–248. [Google Scholar] [CrossRef]
- Han, R.; Guo, X.; Guan, J.; Yao, X.; Hao, Y. Activation Mechanism of Coal Gangue and Its Impact on the Properties of Geopolymers: A Review. Polymers 2022, 14, 3861. [Google Scholar] [CrossRef] [PubMed]
- Narendrula-Kotha, R.; Nkongolo, K.K. Changes in enzymatic activities in metal contaminated and reclaimed lands in Northern Ontario (Canada). Ecotoxicol. Environ. Saf. 2017, 140, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, K.; Heidari, G.; Khalesro, S.; Sohrabi, Y. Soil management, microorganisms and organic matter interactions: A review. Afr. J. Biotechnol. 2011, 10, 19840–19849. [Google Scholar] [CrossRef]

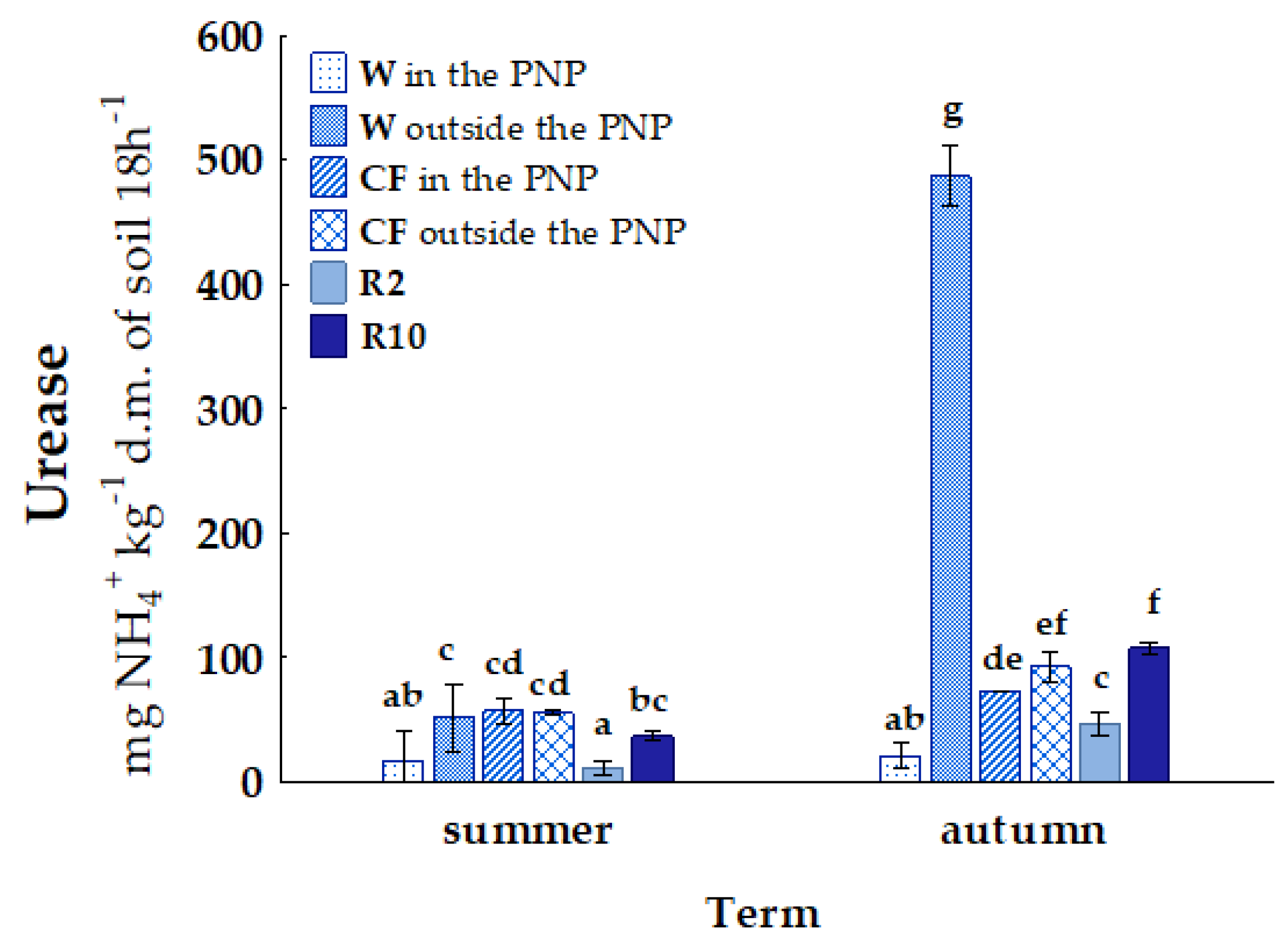
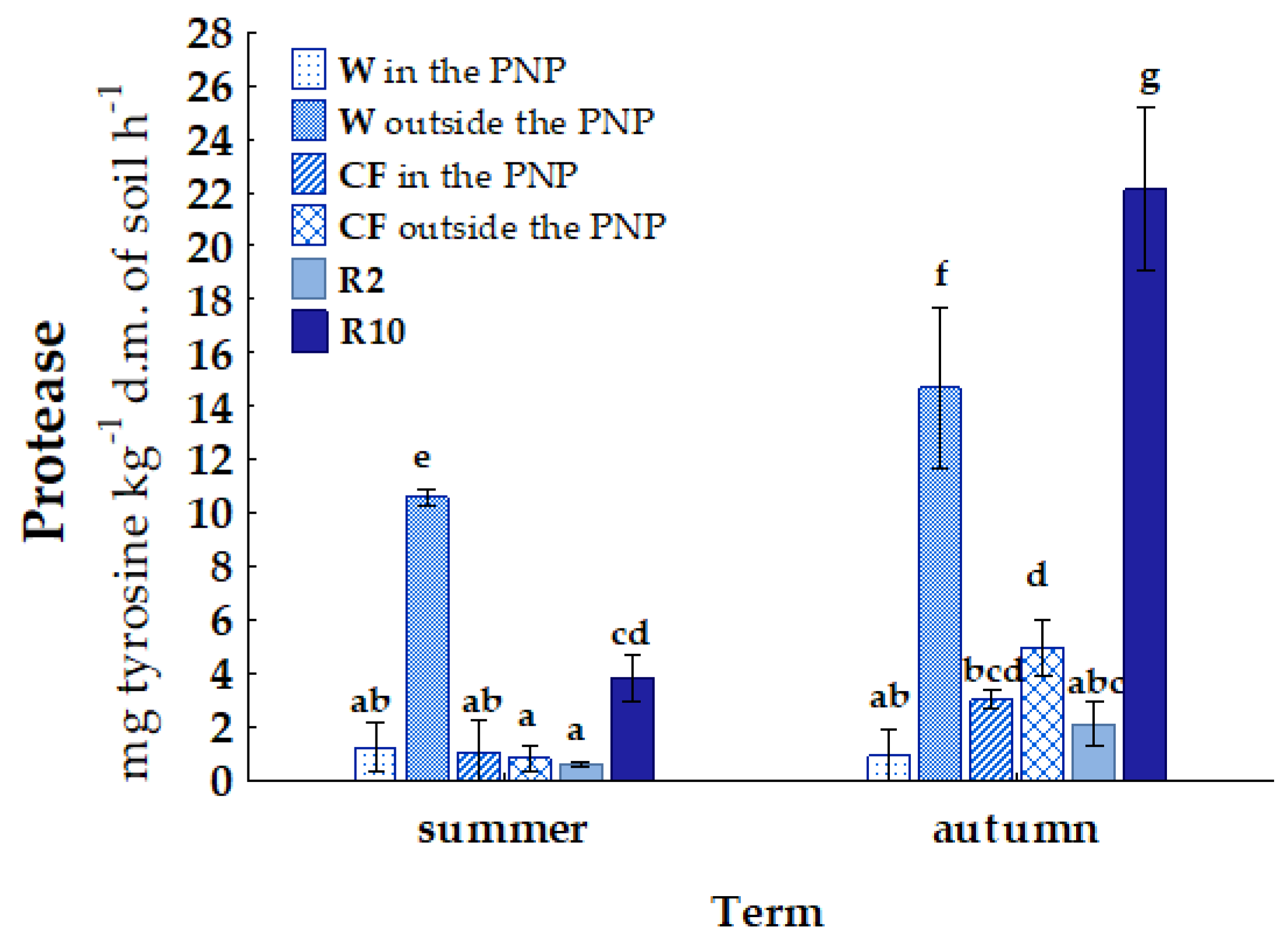
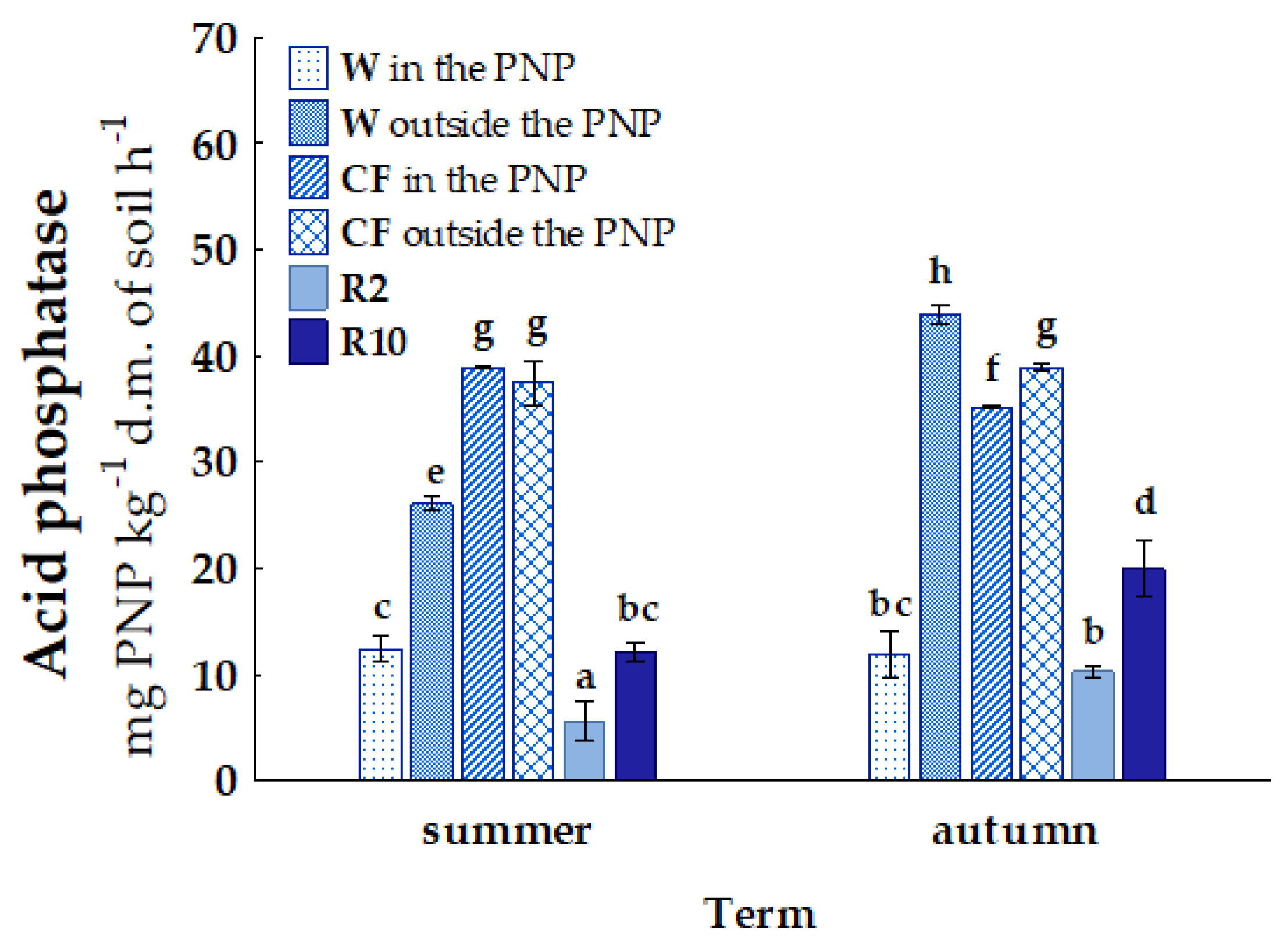
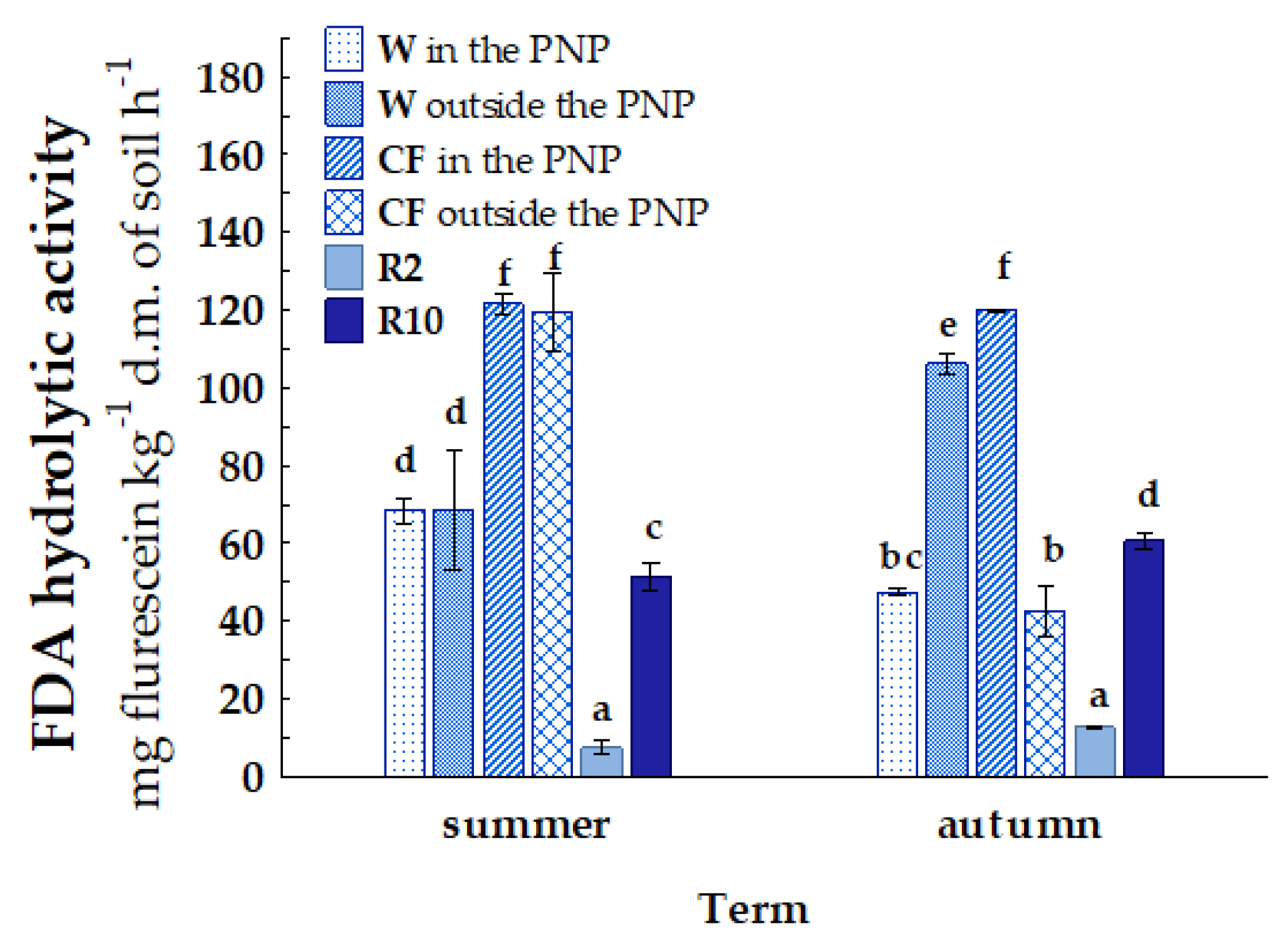
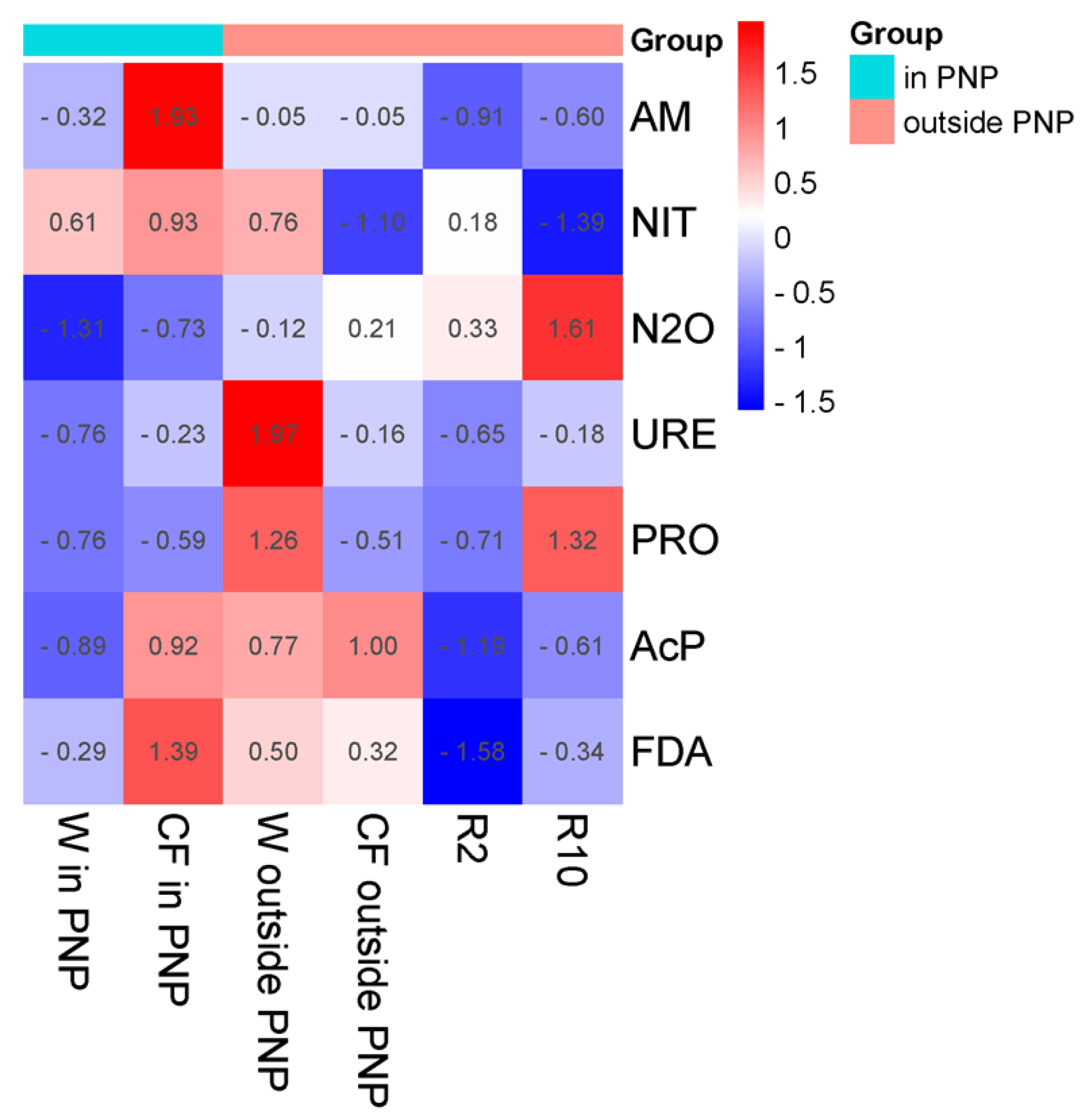

| Term | Wasteland | Cultivated Field | Reclaimed Land 2 Years | Reclaimed Land 10 Years | |||
|---|---|---|---|---|---|---|---|
| Area | Area | Area Outside the PNP | Area Outside the PNP | ||||
| in | Outside | in | Outside | ||||
| the PNP | the PNP | ||||||
| pH 1 mol KCl | summer | 5.04 | 6.18 | 4.27 | 4.14 | 7.15 | 8.25 |
| autumn | 3.95 | 7.33 | 4.15 | 5.94 | 7.16 | 8.20 | |
| TOC g kg−1 | summer | 0.20 | 0.60 | 7.11 | 9.63 | 4.39 | 13.74 |
| autumn | 0.20 | 1.60 | 6.82 | 12.2 | 2.47 | 25.87 | |
| TN g kg−1 | summer | 4.7 | 7.4 | 0.8 | 0.7 | 0.1 | 0.6 |
| autumn | 3.9 | 20.4 | 0.7 | 0.4 | 0.3 | 0.8 | |
| WHC g H2O g dry soil−1 | 0.34 | 0.50 | 0.33 | 0.43 | 0.24 | 0.33 | |
| Habitat | Location | AM | NIT | N2O | URE | PRO | AcP | FDA |
|---|---|---|---|---|---|---|---|---|
| W | area in the PNP | a 42.11 D | a 44.35 A | a 0.50 A | a 19.46 B | a 1.09 A | a 12.14 C | a 57.99 A |
| area outside the PNP | b 51.72 A | a 46.68 AB | a 0.52 A | b 269.45 C | b 12.65 C | b 34.95 E | b 87.44 B | |
| CF | area in the PNP | b 123.50 E | b 49.44 B | a 0.47 A | a 65.24 A | a 2.06 AB | a 37.04 A | b 120.78 D |
| area outside the PNP | a 51.86 A | a 16.86 D | b 0.54 A | b 74.08 A | b 2.90 B | b 38.17 A | a 80.90 B | |
| R2 | area outside the PNP | a 20.62 B | b 37.46 E | a 0.55 A | a 29.18 B | a 1.37 A | a 7.92 B | a 9.98 C |
| R10 | b 31.97 C | a 12.28 C | a 0.66 A | b 72.41 A | b 12.98 C | b 15.99 D | b 56.05 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joniec, J.; Kwiatkowska, E.; Walkiewicz, A.; Grzywaczewski, G. Changes in Microbial Activity Associated with the Nitrogen Biogeochemical Cycle in Differently Managed Soils, Including Protected Areas and Those Reclaimed with Gangue. Sustainability 2025, 17, 4343. https://doi.org/10.3390/su17104343
Joniec J, Kwiatkowska E, Walkiewicz A, Grzywaczewski G. Changes in Microbial Activity Associated with the Nitrogen Biogeochemical Cycle in Differently Managed Soils, Including Protected Areas and Those Reclaimed with Gangue. Sustainability. 2025; 17(10):4343. https://doi.org/10.3390/su17104343
Chicago/Turabian StyleJoniec, Jolanta, Edyta Kwiatkowska, Anna Walkiewicz, and Grzegorz Grzywaczewski. 2025. "Changes in Microbial Activity Associated with the Nitrogen Biogeochemical Cycle in Differently Managed Soils, Including Protected Areas and Those Reclaimed with Gangue" Sustainability 17, no. 10: 4343. https://doi.org/10.3390/su17104343
APA StyleJoniec, J., Kwiatkowska, E., Walkiewicz, A., & Grzywaczewski, G. (2025). Changes in Microbial Activity Associated with the Nitrogen Biogeochemical Cycle in Differently Managed Soils, Including Protected Areas and Those Reclaimed with Gangue. Sustainability, 17(10), 4343. https://doi.org/10.3390/su17104343







