Identification of Heavy Metal Sources and Health Risk Assessment in Coal Mining Area Soils Using Mercury Isotopes and Positive Matrix Factorization (PMF) Model
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Area
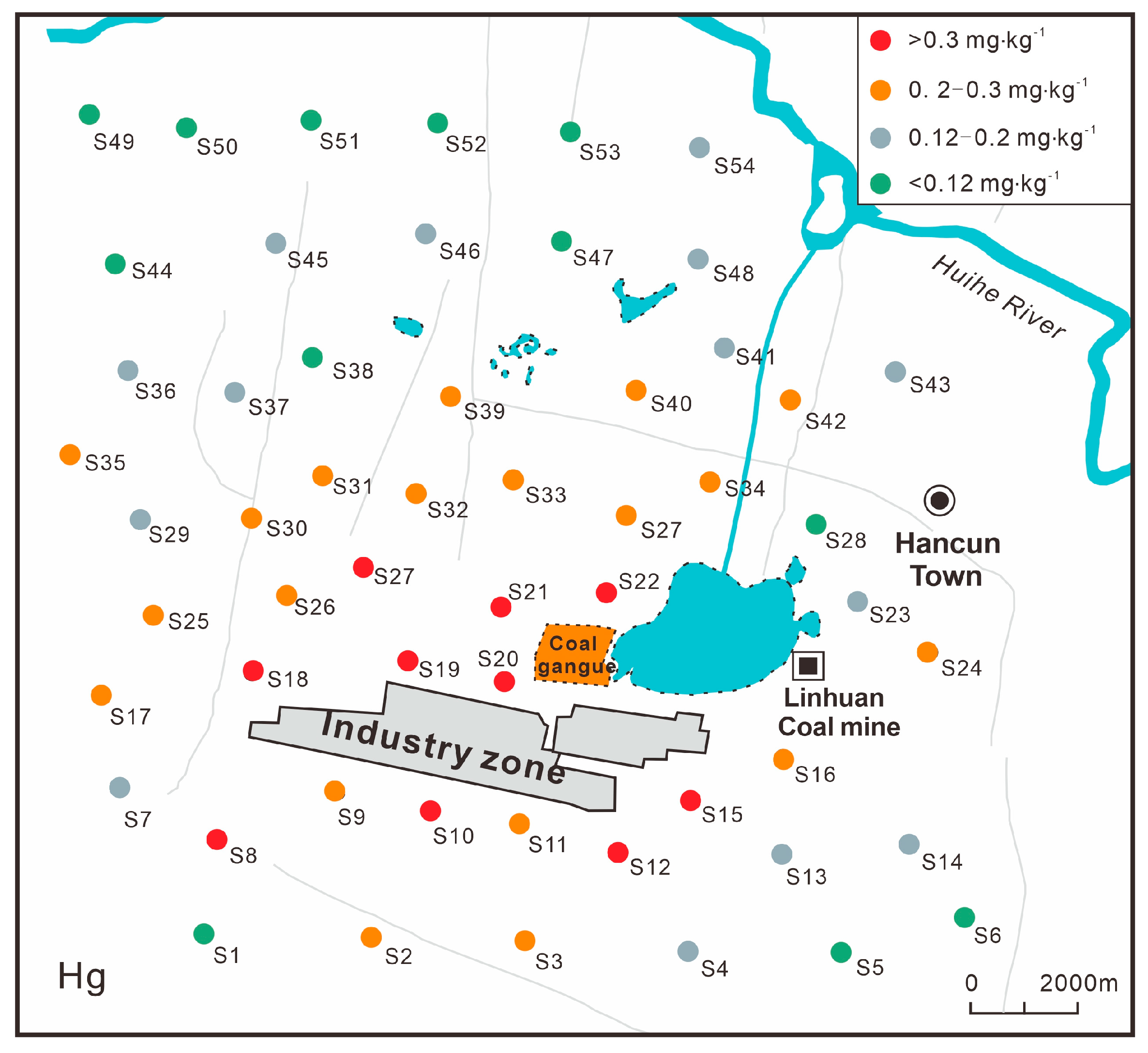
2.2. Sample Collection
2.3. Sample Analysis
2.4. Determination of Mercury Isotopes
2.5. PMF Model
2.6. Health Risk Assessment Model
3. Results
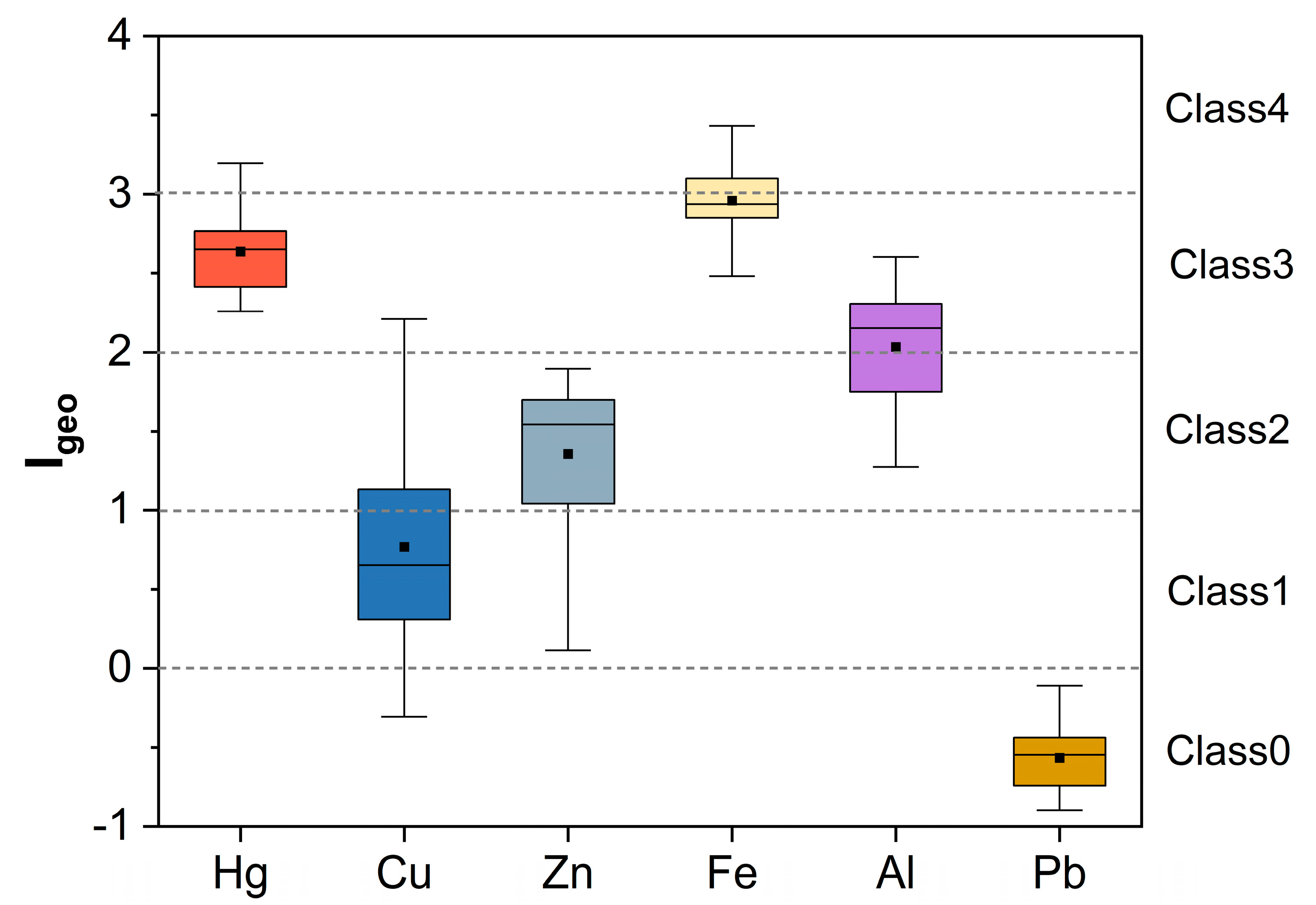
3.1. Descriptive Statistics of Heavy Metal Contents in Soil
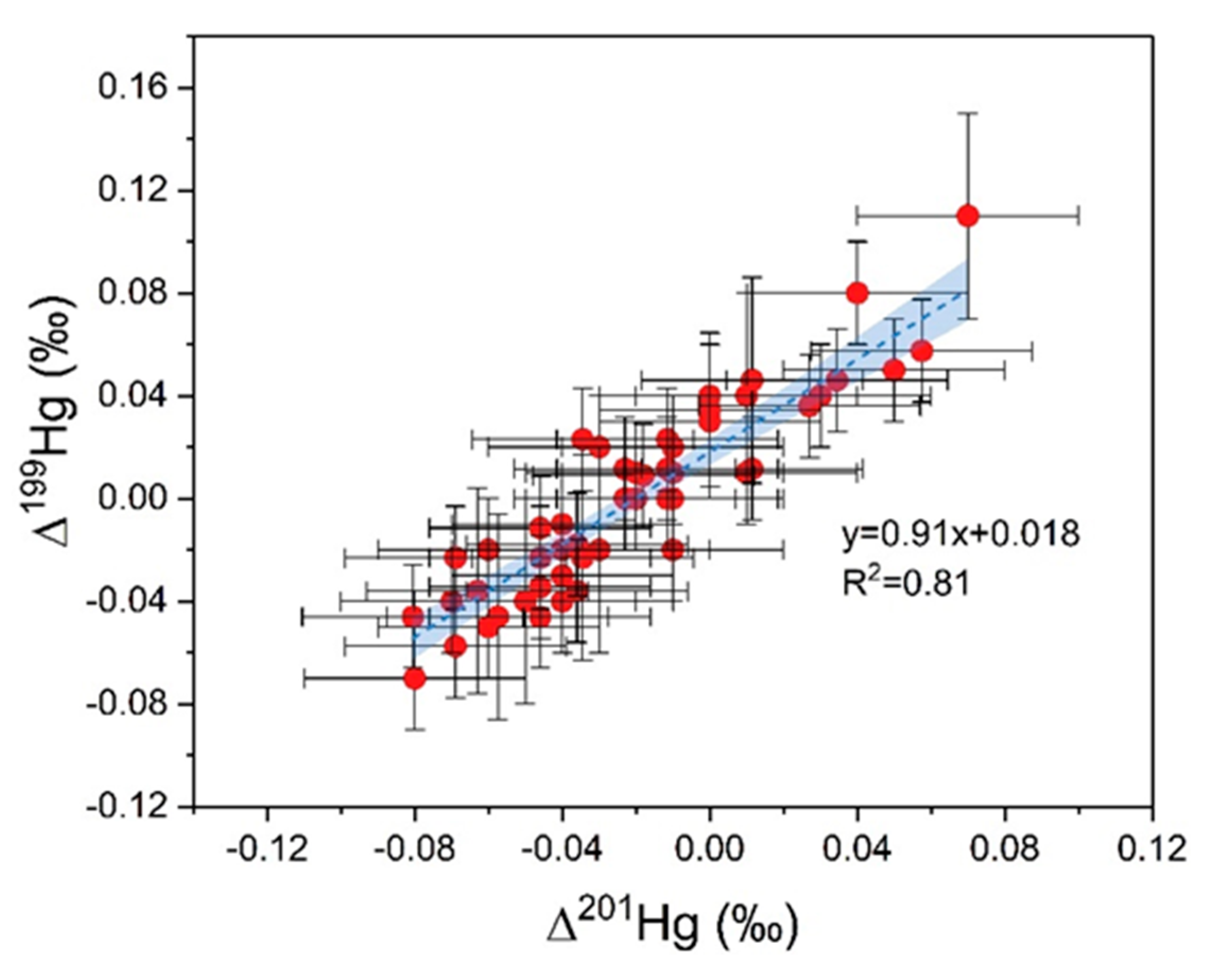
3.2. Characteristics of Hg Isotope in Soil
4. Discussion
4.1. Sources and Transformation of Hg
4.2. Identification of Heavy Metal Sources
4.3. Human Health Risks from Specific Sources
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Qian, Y.; Hong, X.; Luo, Z.; Gao, X.; Liang, H. Contamination characteristics of polycyclic aromatic compounds from coal sources in typical coal mining areas in Huaibei area. China Sci. Total Environ. 2023, 873, 162311. [Google Scholar] [CrossRef]
- You, M.; Hu, Y.; Wang, Z.; Zhang, W. Characterization and Ecological Risk Assessment of Toxic Metal Contaminants in the Soil Around the Coal Gangue Hill in Huainan, Central China. Water Air Soil Pollut. 2023, 234, 667. [Google Scholar] [CrossRef]
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Fang, J.; He, Y.; Huang, N.T.; Zhi, Y.Y.; Fu, W.J. Integrated Analysis on Source-exposure Risk of Heavy Metals in Farmland Soil Based on PMF Model: A Case Study in the E-waste Dismantling Area in Zhejiang Province. Environ. Sci. 2023, 44, 4027–4038. [Google Scholar]
- Mielcarek, K.; Nowakowski, P.; Puścion-Jakubik, A.; Gromkowska-Kępka, K.J.; Soroczyńska, J.; Markiewicz-Żukowska, R.; Naliwajko, S.K.; Grabia, M.; Bielecka, J.; Żmudzińska, A.; et al. Arsenic, cadmium, lead and mercury content and health risk assessment of consuming freshwater fish with elements of chemometric analysis. Food Chem. 2022, 379, 132167. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Huang, R.; Mao, P.; Lei, L.; Li, Y.; Li, Y.; Xia, H.; Li, Z.; Zhuang, P. Immobilization of Cadmium by Molecular Sieve and Wollastonite Is Soil pH and Organic Matter Dependent. Int. J. Environ. Res. Public Health 2021, 18, 5128. [Google Scholar] [CrossRef]
- Schroeder, W.H.; Munthe, J. Atmospheric mercury—An overview. Atmos. Environ. 1998, 32, 809–822. [Google Scholar] [CrossRef]
- Selin, N.E. Global Biogeochemical Cycling of Mercury: A Review. Annu. Rev. Env. Resour. 2009, 34, 43–63. [Google Scholar] [CrossRef]
- Teng, D.Y.; Mao, K.; Ali, W.; Xu, G.M.; Huang, G.P.; Niazi, N.K. Describing the toxicity and sources and the remediation technologies for mercury-contaminated soil. RSC Adv. 2020, 10, 23221–23232. [Google Scholar] [CrossRef]
- Habib, M.A.; Islam, A.R.M.T.; Varol, M.; Phoungthong, K.; Khan, R.; Islam, M.S.; Hasanuzzaman, M.; Mia, M.Y.; Costache, R.; Pal, S.C. Receptor model-based source-specific health risks of toxic metal(loid)s in coal basin-induced agricultural soil in northwest Bangladesh. Environ. Geochem. Health 2023, 45, 8539–8564. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, B.; Wang, Z.; Wang, S.; Liu, L.; Gao, Y.; Zhang, H. Characteristics and pollution risks of potentially toxic elements and nematode community structure on farm soil near coal mines. Environ. Geochem. Health 2023, 45, 6835–6852. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Yang, J.; Wang, H.; Li, Y.; Shi, Y.; Li, D.; Holm, P.E.; Ou, Q.; Hu, W. Quantitative source apportionment, risk assessment and distribution of heavy metals in agricultural soils from southern Shandong Peninsula of China. Sci. Total Environ. 2021, 767, 144879. [Google Scholar] [CrossRef] [PubMed]
- Lv, J. Multivariate receptor models and robust geostatistics to estimate source apportionment of heavy metals in soils. Environ. Pollut. 2019, 244, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Yuanan, H.; He, K.; Sun, Z.; Chen, G.; Cheng, H. Quantitative source apportionment of heavy metal(loid)s in the agricultural soils of an industrializing region and associated model uncertainty. J. Hazard. Mater. 2020, 391, 122244. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, J.; Lv, J. Identifying quantitative sources and spatial distributions of potentially toxic elements in soils by using three receptor models and sequential indicator simulation. Chemosphere 2020, 242, 125266. [Google Scholar] [CrossRef]
- Liao, R.; Ratié, G.; Shi, Z.; Šípková, A.; Vaňková, Z.; Chrastný, V.; Zhang, J.; Komárek, M. Cadmium isotope systematics for source apportionment in an urban–rural region. Appl. Geochem. 2022, 137, 105196. [Google Scholar] [CrossRef]
- Salmanzadeh, M.; Hartland, A.; Stirling, C.H.; Balks, M.R.; Schipper, L.A.; Joshi, C.; George, E. Isotope Tracing of Long-Term Cadmium Fluxes in an Agricultural Soil. Environ. Sci. Technol. 2017, 51, 7369–7377. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Y.; Wadood, S.A.; Sun, Q.; Guo, B. Source apportionment of cadmium pollution in agricultural soil based on cadmium isotope ratio analysis. Appl. Geochem. 2020, 123, 104776. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, F.; Liu, S.; Tan, Q.; Wang, L.; Yue, Y.; Li, C. Tracing concealed gold deposits using mercury isotopes in surface soils: A study from the Shuiyindong gold deposit, Guizhou province, China. J. Geochem. Explor. 2022, 238, 107015. [Google Scholar] [CrossRef]
- Zhang, K.; Liao, L.; Li, Z.; Lin, Y.; Meng, B.; Feng, X. Mercury isotopes trace historical mercury pollution from Pb–Zn smelter in China. Chem. Geol. 2023, 635, 121622. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, G.; Zhang, D.; Lei, M. An integrated method for source apportionment of heavy metal(loid)s in agricultural soils and model uncertainty analysis. Environ. Pollut. 2021, 276, 116666. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Regional geochemical baseline based pollution assessment and dominant factors influencing metals in riparian sediment of Yangtze River, China. Catena 2023, 230, 107259. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, L.; Chen, X.; Hu, J.; Li, C.; Zhang, L.; Cheng, H. Distribution of mercury and methylmercury in aquacultured fish in special waters formed by coal mining subsidence. Ecotoxicol. Environ. Saf. 2024, 280, 116546. [Google Scholar] [CrossRef]
- Hu, J.; Zheng, L.; Liu, S.; Chen, Y.; Li, C.; Ni, J.; Chen, Y.; An, S. Quantifying the impacts of coal mining activities on topsoil using Hg stable isotope: A case study of Guqiao mining area, Huainan City. Environ. Pollut. 2023, 332, 122378. [Google Scholar] [CrossRef]
- Li, H.J. Comprehensive Utilization of Coal Gangue; Chemical Industry Press: Beijing, China, 2010. [Google Scholar]
- Yin, R.S.; Feng, X.B.; Shi, W.F. Application of the stable-isotope system to the study of sources and fate of Hg in the environment: A review. Appl. Geochem. 2010, 25, 1467–1477. [Google Scholar] [CrossRef]
- Sun, R.Y.; Sonke, J.E.; Liu, G.J.; Zheng, L.G.; Wu, D. Variations in the stable isotope composition of mercury in coal-bearing sequences: Indications for its provenance and geochemical processes. Int. J. Coal. Geol. 2014, 133, 13–23. [Google Scholar] [CrossRef]
- Bergquist, B.A.; Blum, J.D. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 2007, 318, 417–420. [Google Scholar] [CrossRef]
- Liang, J.H.; Tian, Y.Q.; Fei, Y.; Liu, Z.Y.; Shi, H.D.; Qi, J.X.; Mo, L. Source apportionment and potential ecological risk assessment of soil heavy metals in typical industrial and mining towns in north China. Environ. Sci. 2023, 44, 5657–5665. [Google Scholar]
- Zhou, W.; Li, Z.; Liu, Y.; Shen, C.; Tang, H.; Huang, Y. Soil type data provide new methods and insights for heavy metal pollution assessment and driving factors analysis. J. Hazard. Mater. 2024, 480, 135868. [Google Scholar] [CrossRef]
- CEMS. Soil Background Values of China. Chinese Environmental Monitoring Station; Chinese Environmental Science Press: Beijing, China, 1990; (In Chinese with English Abstract). [Google Scholar]
- Yu, L.J.; Ma, H.J.; Wang, C.P. Characteristics, Ecological Risk Assessment, and Source Apportionment of Soil Heavy Metals in the Yellow River Floodplain of Yinchuan City. Environ. Sci. 2023, 45, 2971–2982. [Google Scholar]
- Zhang, C.H.; Wang, M.S.; Sun, A.; Hang, Q.; Chen, C.; Liu, D.; Mao, P.; Feng, Q.Q.; Wang, M.Y. Quantitative effects of anthropogenic and natural interactions on heavy metal pollution and spatial distribution in sediment of Qinhe River. Acta Sci. Circumstantiae 2023, 43, 176–186. [Google Scholar]
- Tao, L.; Xiao, G.; Song, L. Risk assessment of filling pipeline failure based on variation coefficient method and TOPSIS. Ind. Miner. Process. 2019, 48, 23–26. [Google Scholar]
- Hao, W.D. Environmental Impacts of Coal Mine Wastes Stacking on the Soil, Waterbody and Vegetation—Cases study. Master’s Thesis, China University of Mining and Technology, Xuzhou, China, 2016. [Google Scholar]
- Stamenkovic, J.; Gustin, M.S. Nonstomatal versus Stomatal Uptake of Atmospheric Mercury. Environ. Sci. Technol. 2009, 43, 1367–1372. [Google Scholar] [CrossRef]
- Laacouri, A.; Nater, E.A.; Kolka, R.K. Distribution and uptake dynamics of mercury in leaves of common deciduous tree species in Minnesota, U.S.A. Environ. Sci. Technol. 2013, 47, 10462–10470. [Google Scholar] [CrossRef]
- Demers, J.D.; Blum, J.D.; Zak, D.R. Mercury isotopes in a forested ecosystem: Implications for air-surface exchange dynamics and the global mercury cycle. Glob. Biogeochem. Cycles 2013, 27, 222–238. [Google Scholar] [CrossRef]
- Wang, S.B.; Luo, K.L.; Wang, X.; Sun, Y.Z. Estimate of sulfur, arsenic, mercury, fluorine emissions due to spontaneous combustion of coal gangue: An important part of Chinese emission inventories. Environ. Pollut. 2016, 209, 107–113. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, R.; Hintelmann, H.; Zhu, J.; Wang, R.; Sonke, J.E. Mercury stable isotope compositions in magmatic-affected coal deposits: New insights to mercury sources, migration and enrichment. Chem. Geol. 2018, 479, 86–101. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Liu, J.; Bi, X.; Ning, Y.; Yang, S.; Yang, X. Apportionment of sources of heavy metals to agricultural soils using isotope fingerprints and multivariate statistical analyses. Environ. Pollut. 2019, 249, 208–216. [Google Scholar] [CrossRef]
- Ren, M.; Zheng, L.; Wang, D.; Chen, X.; Dong, X.; Wei, X.; Cheng, H. Copper isotope ratios allowed for quantifying the contribution of coal mining and combustion to total soil copper concentrations in China. Environ. Pollut. 2022, 308, 119613. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, K.; Wang, Y.; Hu, W.; Liu, B.; Yuan, X.; Huang, B.; Wu, L. Identification of sources and their potential health risk of potential toxic elements in soils from a mercury-thallium polymetallic mining area in Southwest China: Insight from mercury isotopes and PMF model. Sci. Total Environ. 2023, 869, 161774. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, L.; Sun, R.; Liu, S.; Li, C.; Chen, Y.; Xu, Y. Mercury in sediment reflecting the intensive coal mining activities: Evidence from stable mercury isotopes and Bayesian mixing model analysis. Ecotoxicol. Environ. Saf. 2022, 234, 113392. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, C.; Ding, L.; Chen, M.; Hu, Y.; Li, P.; Zhang, L.; Feng, X. Soil mercury pollution caused by typical anthropogenic sources in China: Evidence from stable mercury isotope measurement and receptor model analysis. J. Clean. Prod. 2021, 288, 125687. [Google Scholar] [CrossRef]
- Cao, F.; Meng, M.; Shan, B.; Sun, R. Source apportionment of mercury in surface soils near the Wuda coal fire area in Inner Mongolia, China. Chemosphere 2021, 263, 128348. [Google Scholar] [CrossRef]
- Liu, H.; Shao, J.; Yu, B.; Liang, Y.; Duo, B.; Fu, J.; Yang, R.; Shi, J.; Jiang, G. Mercury isotopic compositions of mosses, conifer needles, and surface soils: Implications for mercury distribution and sources in Shergyla Mountain, Tibetan Plateau. Ecotoxicol. Environ. Saf. 2019, 172, 225–231. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Q.; Zheng, G.; Zeng, Y.; Wang, H.; Mei, A.; Gao, S.; Zhang, X.; Zhang, Y. Quantitative Source Apportionment and Uncertainty Analysis of Heavy Metal(loid)s in the Topsoil of the Nansi Lake Nature Reserve. Sustainability 2022, 14, 6679. [Google Scholar] [CrossRef]
- Gao, H.; Huang, Y.; Li, W.; Li, J.; Ouyang, S.; Song, T.; Lv, F.; Zhai, W.; Ma, K. Explanation of heavy metal pollution in coal mines of china from the perspective of coal gangue geochemical characteristics. Environ. Sci. Pollut. Res. 2021, 28, 65363–65373. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, L.; Jiang, C.; Chen, X.; Ding, S. Characteristics of leaching of heavy metals from low-sulfur coal gangue under different conditions. Int. J. Coal Sci. Technol. 2021, 8, 780–789. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, L.; Ren, M.; Li, C.; Dong, X.; Wei, X.; Zhou, W.; Cui, J. Zinc in soil reflecting the intensive coal mining activities: Evidence from stable zinc isotopes analysis. Ecotoxicol. Environ. Saf. 2022, 239, 113669. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, H.; Chen, X.; Liu, G.; Jiang, C.; Zheng, L. Study of the micromorphology and health risks of arsenic in copper smelting slag tailings for safe resource utilization. Ecotoxicol. Environ. Saf. 2021, 219, 112321. [Google Scholar] [CrossRef]
- Shentu, J.; Fang, Y.; Wang, Y.; Cui, Y.; Zhu, M. Bioaccessibility and reliable human health risk assessment of heavy metals in typical abandoned industrial sites of southeastern China. Ecotoxicol. Environ. Saf. 2023, 256, 114870. [Google Scholar] [CrossRef]




| pH | SOM | Hg | Cu | Zn | Fe | Al | Pb | |
|---|---|---|---|---|---|---|---|---|
| Max | 8.9 | 34.5 | 0.479 | 134 | 327 | 48,700 | 59,990 | 36.1 |
| Min | 6.5 | 8.9 | 0.098 | 23.4 | 95 | 25,200 | 23,890 | 20.9 |
| Median | 7.3 | 16.45 | 0.260 | 45.5 | 256.5 | 34,541.5 | 43,874.5 | 26.7 |
| Mean | 7.52 | 16.60 | 0.262 | 54.1 | 235.3 | 35,477.4 | 41614 | 26.7 |
| SD | 0.7 | 6.16 | 4.87 | 26.70 | 65.22 | 5262.56 | 9784.44 | 4.25 |
| CV | 0.09 | 0.37 | 0.39 | 0.49 | 0.28 | 0.15 | 0.24 | 0.16 |
| Background value [31] | - | - | 0.0276 | 19.3 | 58.6 | 3010 | 6580 | 26.0 |
| Hg | Cu | Zn | Fe | Al | Pb | |
|---|---|---|---|---|---|---|
| Hg | 1 | |||||
| Cu | −0.05 | 1 | ||||
| Zn | 0.56 * | 0.49 * | 1 | |||
| Fe | −0.20 | 0.13 | −0.22 | 1 | ||
| Al | −0.12 | 0.04 | −0.10 | 0.68 ** | 1 | |
| Pb | 0.46 * | −0.22 | 0.39 * | −0.47 * | −0.21 | 1 |
| Study Area | δ202Hg (‰) | Δ199Hg (‰) | Δ201Hg (‰) | Reference | |||
|---|---|---|---|---|---|---|---|
| Range | Mean | Range | Mean | Range | Mean | ||
| Guizhou | −0.30~0.41 | 0.03 ± 0.36 | 0.00~0.02 | 0.01 ± 0.01 | −0.05~−0.01 | −0.03 ± 0.02 | [43] |
| Anhui | −0.79~0.02 | −0.45 ± 0.27 | −0.05~0.05 | 0.01 ± 0.03 | −0.07~0.01 | −0.02 ± 0.03 | [44] |
| Southwest China | −1.98~0.08 | −0.90 ± 0.57 | - | −0.31 ± 0.05 | - | - | [45] |
| Nei Monggol | −1.71~−0.26 | −1.19 ± 0.28 | −0.26~−0.07 | −0.02 ± 0.03 | - | - | [46] |
| Qinghai Tibet | −1.65~−0.16 | −1.15 ± 0.44 | −0.31~−0.06 | −0.20 ± 0.07 | - | - | [47] |
| This study | −1.27~0.04 | −0.41 ± 0.31 | −0.07~0.11 | 0.01 ± 0.03 | −0.08~0.07 | −0.03 ± 0.03 | - |
| Total | Adult | Children | ||||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | Total | F1 | F2 | F3 | Total | |
| Non-carcinogenic | ||||||||
| Hg | 0.21 | 0.12 | 0.37 | 0.70 | 0.19 | 0.11 | 0.33 | 0.62 |
| Cu | 4.66 × 10−7 | 8.47 × 10−4 | 4.34 × 10−4 | 1.28 × 10−3 | 4.00 × 10−7 | 7.28 × 10−4 | 3.73 × 10−4 | 1.10 × 10−3 |
| Zn | 2.75 × 10−5 | 1.61 × 10−4 | 4.97 × 10−4 | 6.85 × 10−4 | 2.36 × 10−5 | 1.38 × 10−4 | 4.27 × 10−4 | 5.89 × 10−4 |
| Pb | 2.40 × 10−3 | 1.27 × 10−3 | 3.79 × 10−3 | 7.46 × 10−3 | 2.20 × 10−3 | 1.16 × 10−3 | 3.46 × 10−3 | 6.82 × 10−3 |
| THI | 0.22 | 0.12 | 0.37 | 0.71 | 0.19 | 0.11 | 0.33 | 0.63 |
| carcinogenic | ||||||||
| Pb | 4.39 × 10−10 | 2.32 × 10−10 | 6.93 × 10−10 | 1.36 × 10−9 | 3.92 × 10−10 | 2.08 × 10−10 | 6.19 × 10−10 | 1.22 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Chen, X.; Cheng, H.; Zheng, L. Identification of Heavy Metal Sources and Health Risk Assessment in Coal Mining Area Soils Using Mercury Isotopes and Positive Matrix Factorization (PMF) Model. Sustainability 2025, 17, 4334. https://doi.org/10.3390/su17104334
Li C, Chen X, Cheng H, Zheng L. Identification of Heavy Metal Sources and Health Risk Assessment in Coal Mining Area Soils Using Mercury Isotopes and Positive Matrix Factorization (PMF) Model. Sustainability. 2025; 17(10):4334. https://doi.org/10.3390/su17104334
Chicago/Turabian StyleLi, Chang, Xing Chen, Hua Cheng, and Liugen Zheng. 2025. "Identification of Heavy Metal Sources and Health Risk Assessment in Coal Mining Area Soils Using Mercury Isotopes and Positive Matrix Factorization (PMF) Model" Sustainability 17, no. 10: 4334. https://doi.org/10.3390/su17104334
APA StyleLi, C., Chen, X., Cheng, H., & Zheng, L. (2025). Identification of Heavy Metal Sources and Health Risk Assessment in Coal Mining Area Soils Using Mercury Isotopes and Positive Matrix Factorization (PMF) Model. Sustainability, 17(10), 4334. https://doi.org/10.3390/su17104334






