Assessment of Disinfection Efficiency of Chlorine and Bromine-Based Biocides for Marine Biofouling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Batch-Scale Biofilm Reactor Setup
2.3. Factors Affecting Biofilm Growth
2.4. Correlation Between Seawater Initial Cell Count and Biofilm Growth
2.5. Disinfection Study
2.5.1. Biofilm Annular Reactor for Disinfection Testing
2.5.2. Optimization Using Response Surface Methodology (RSM)
2.6. Biofilm Growth Quantification
2.6.1. Gravimetric Analysis of Biofilm Growth
2.6.2. Flow Cytometry for Cell Count
3. Results and Discussion
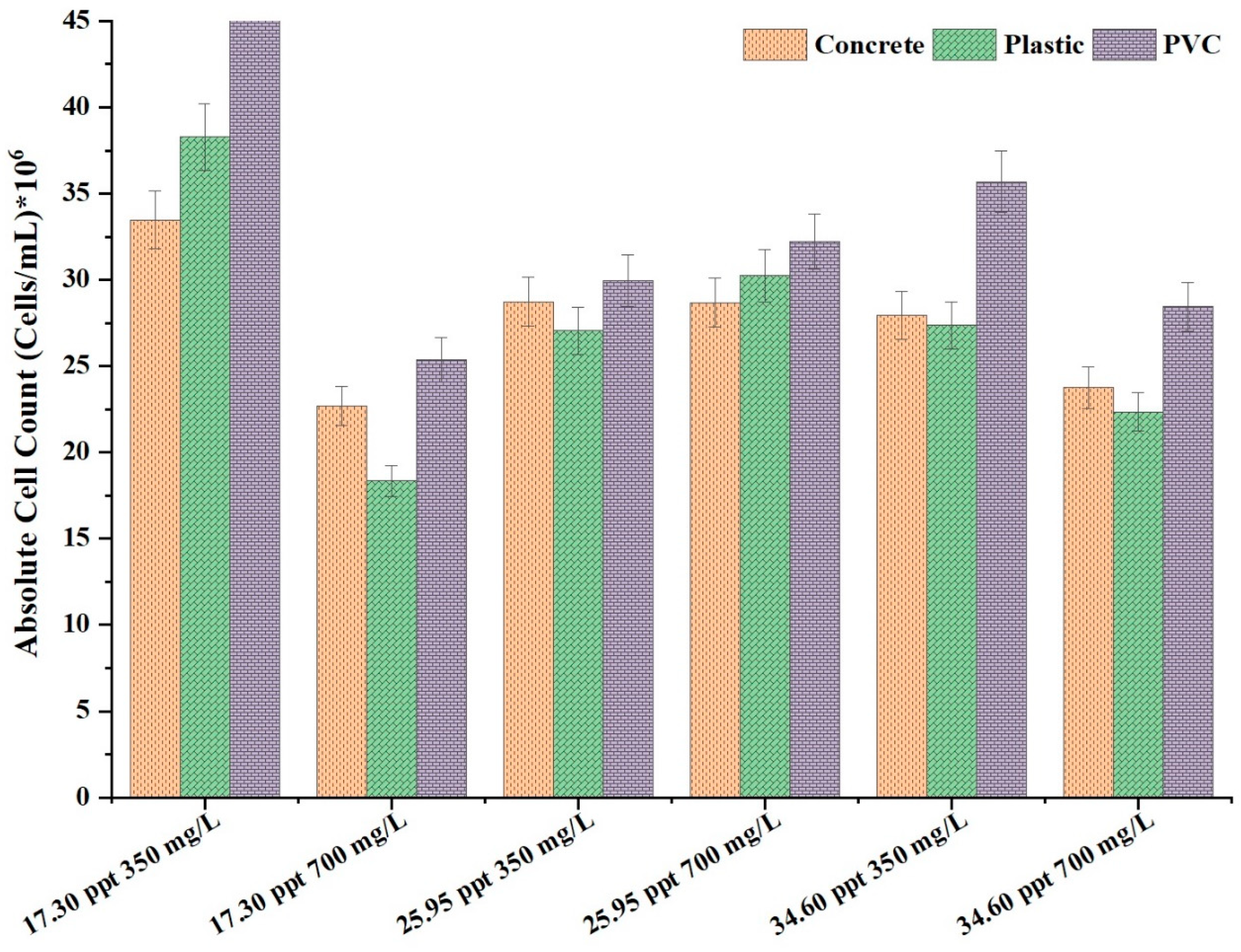
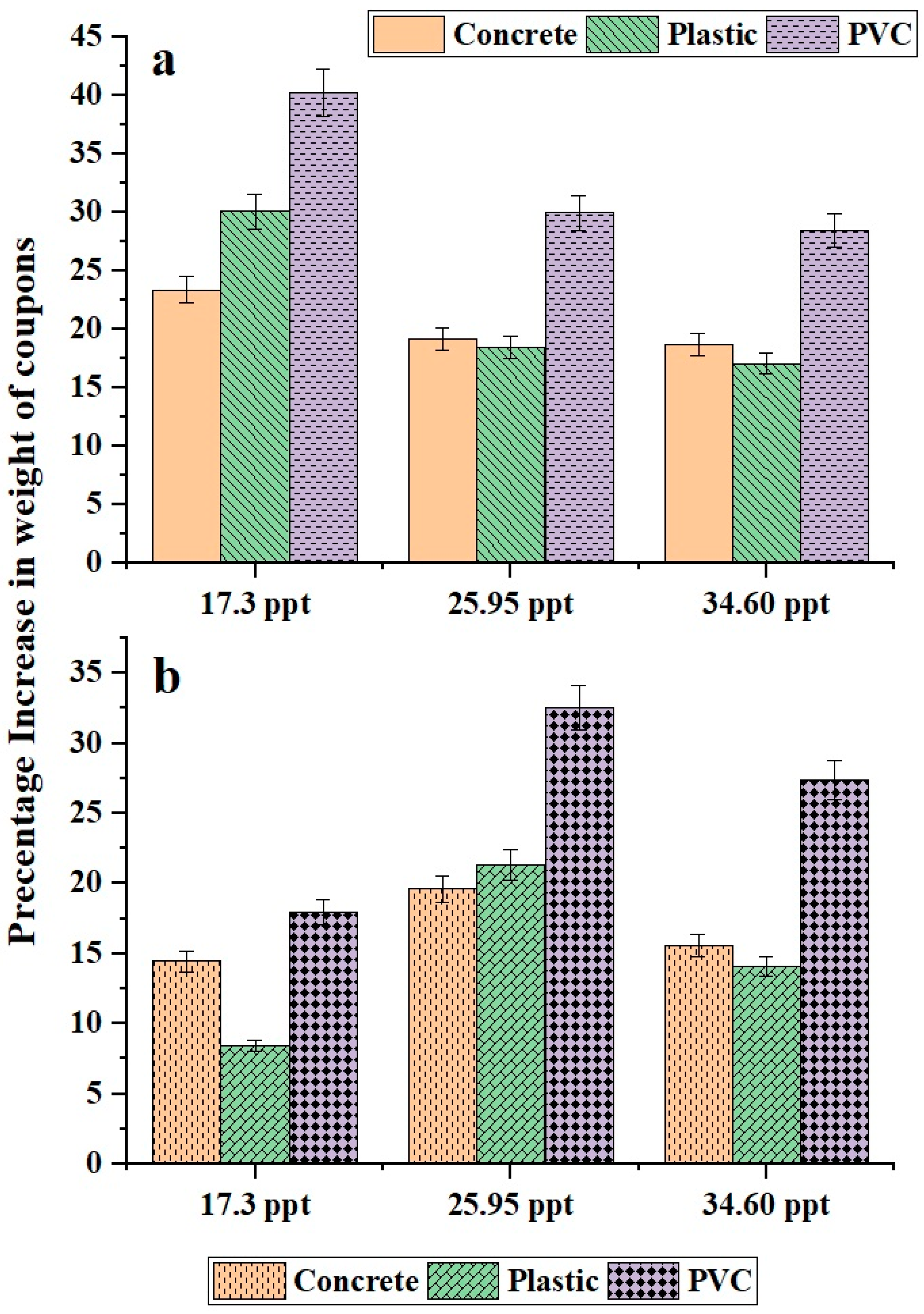
3.1. Biofilm Growth Trends Across Different Materials
3.2. Influence of Carbon Concentration and Salinity on Biofilm Growth
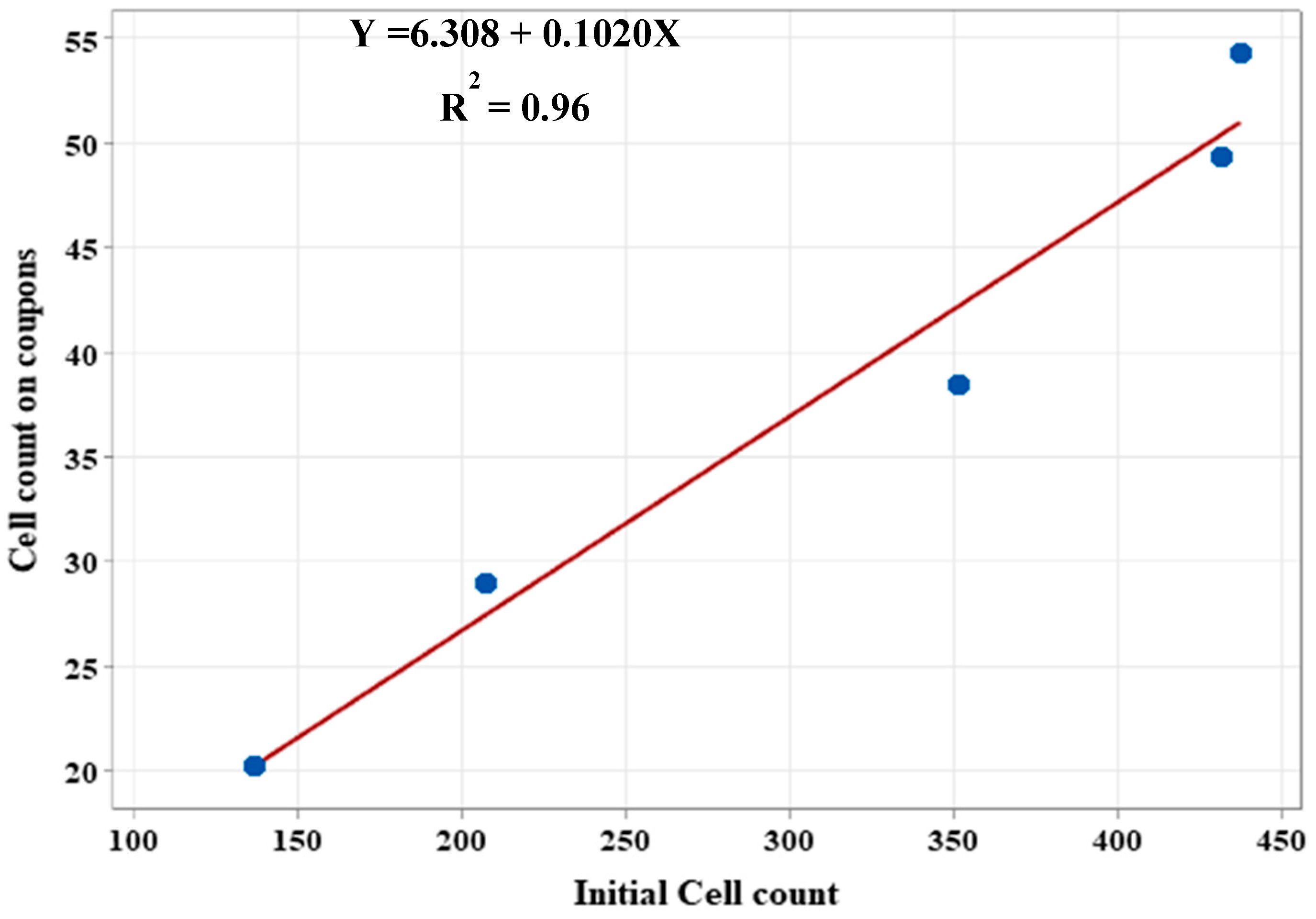
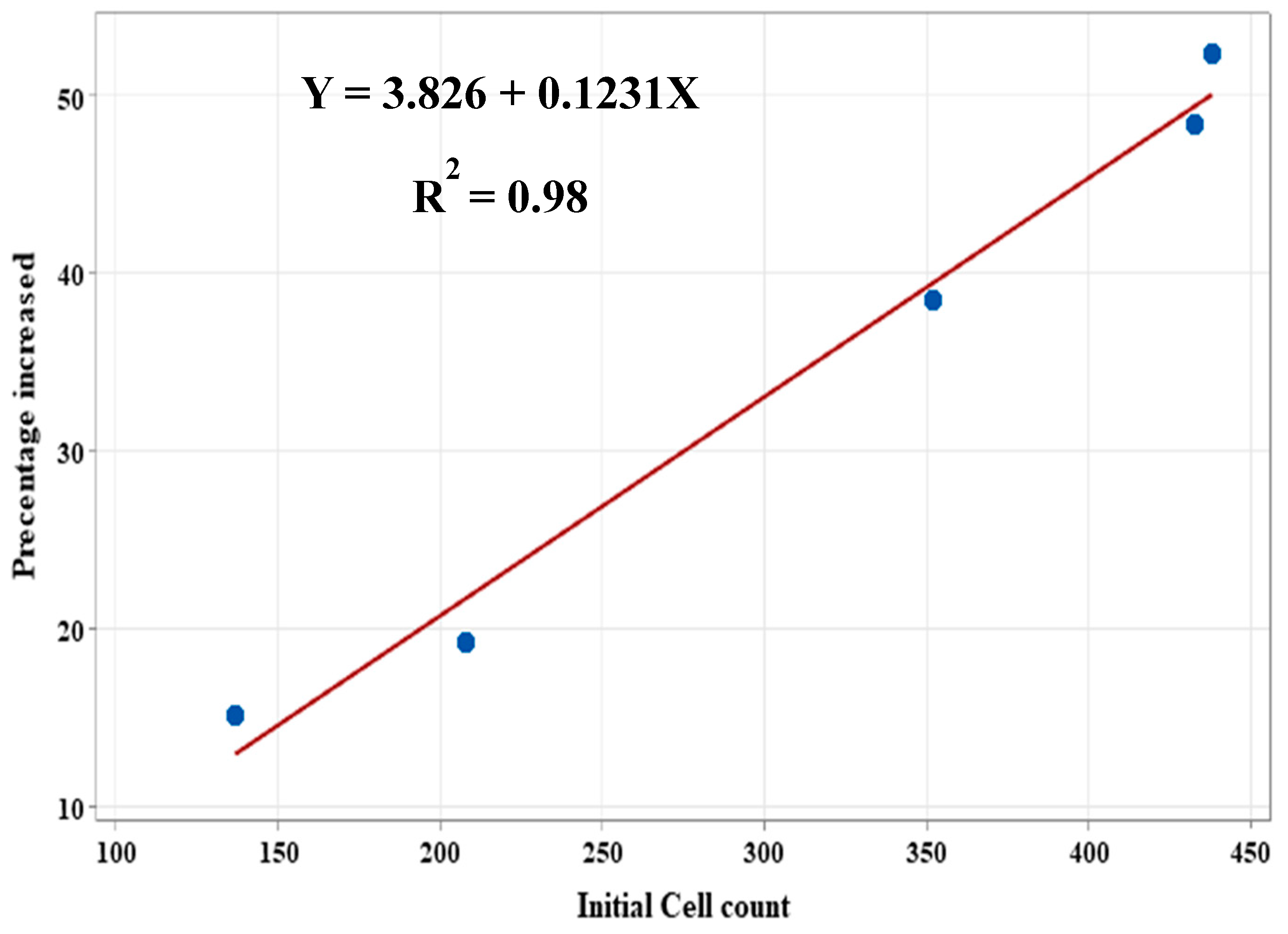
3.3. Correlation Between Initial Cell Count and Biofilm Growth
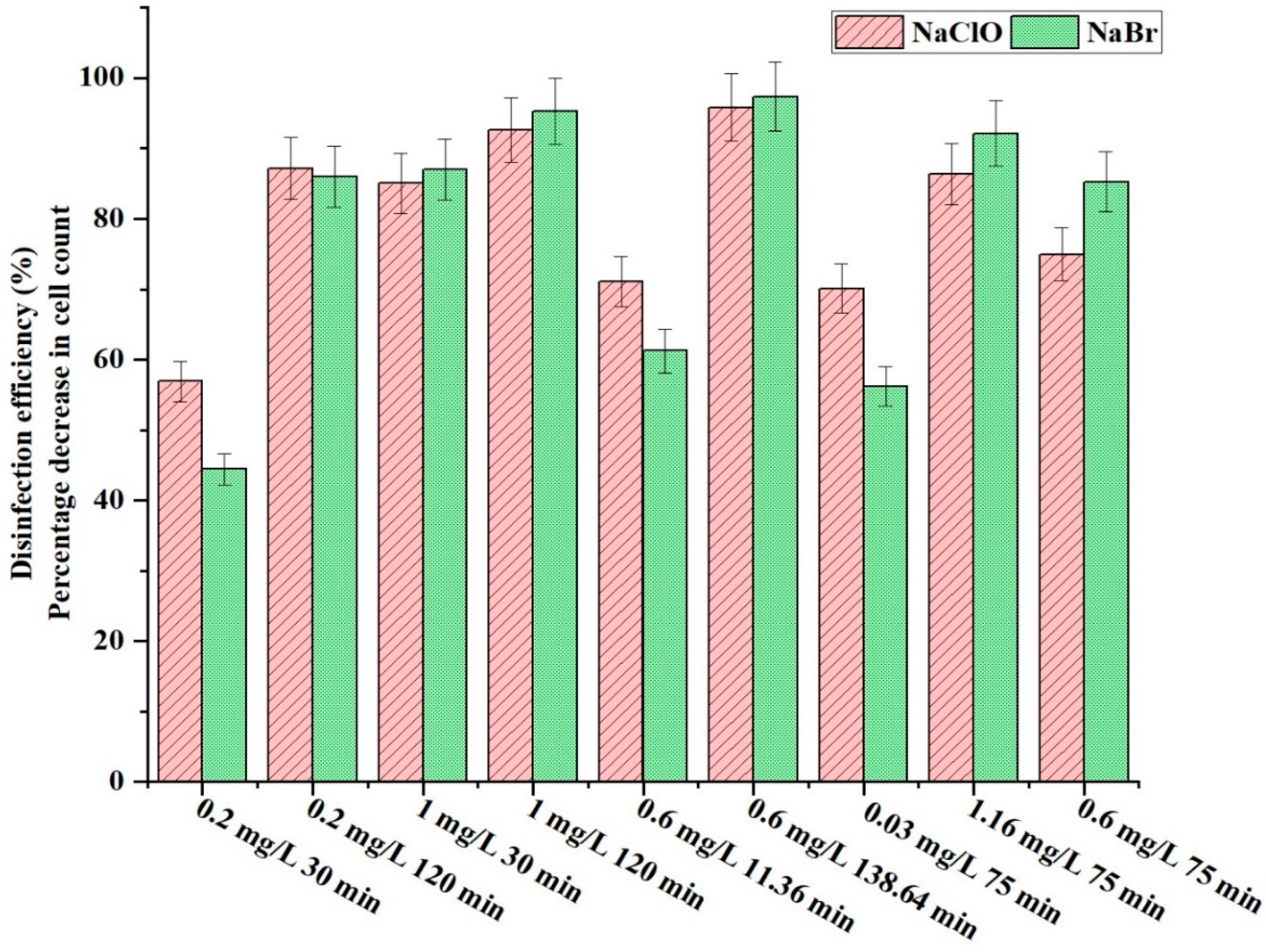
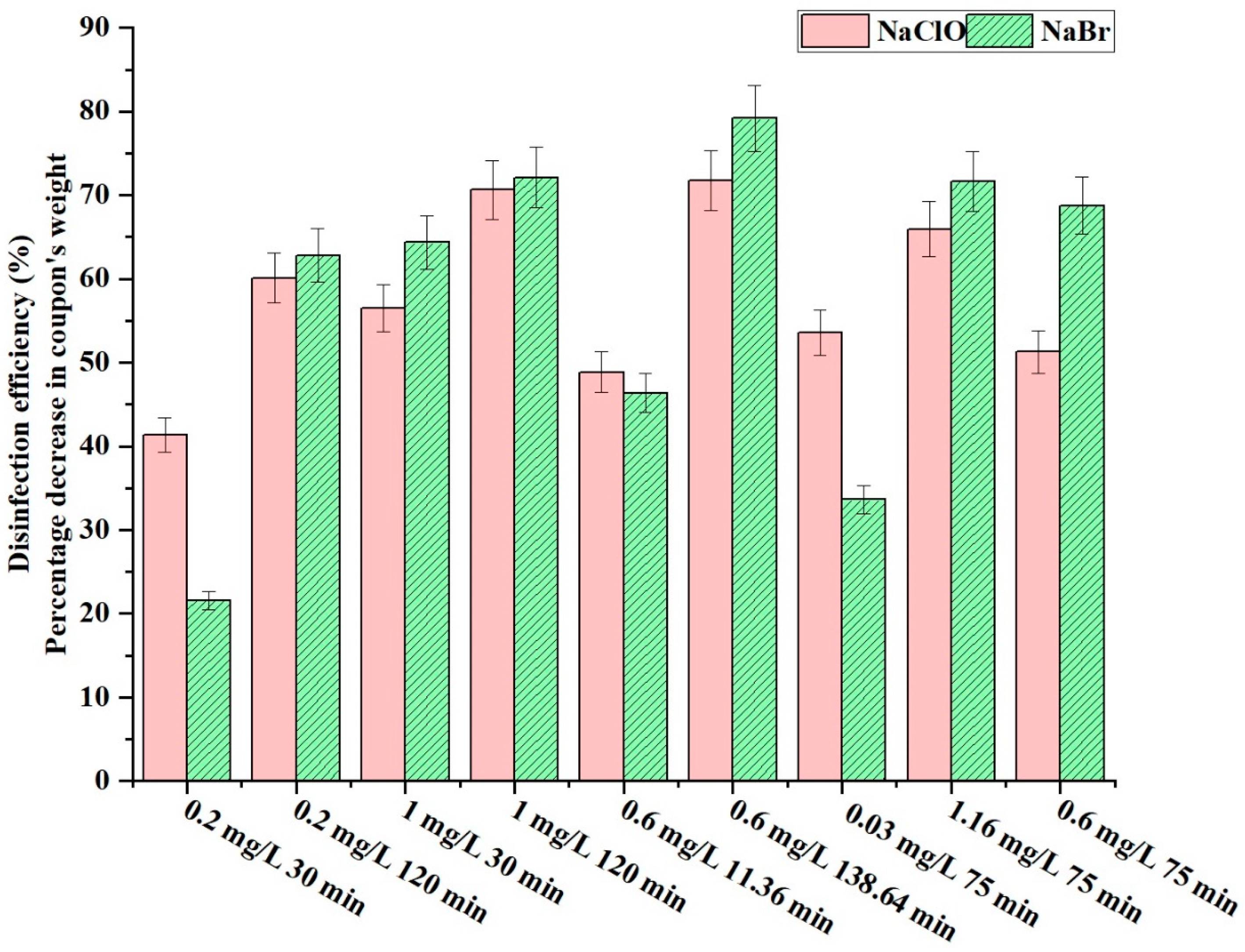
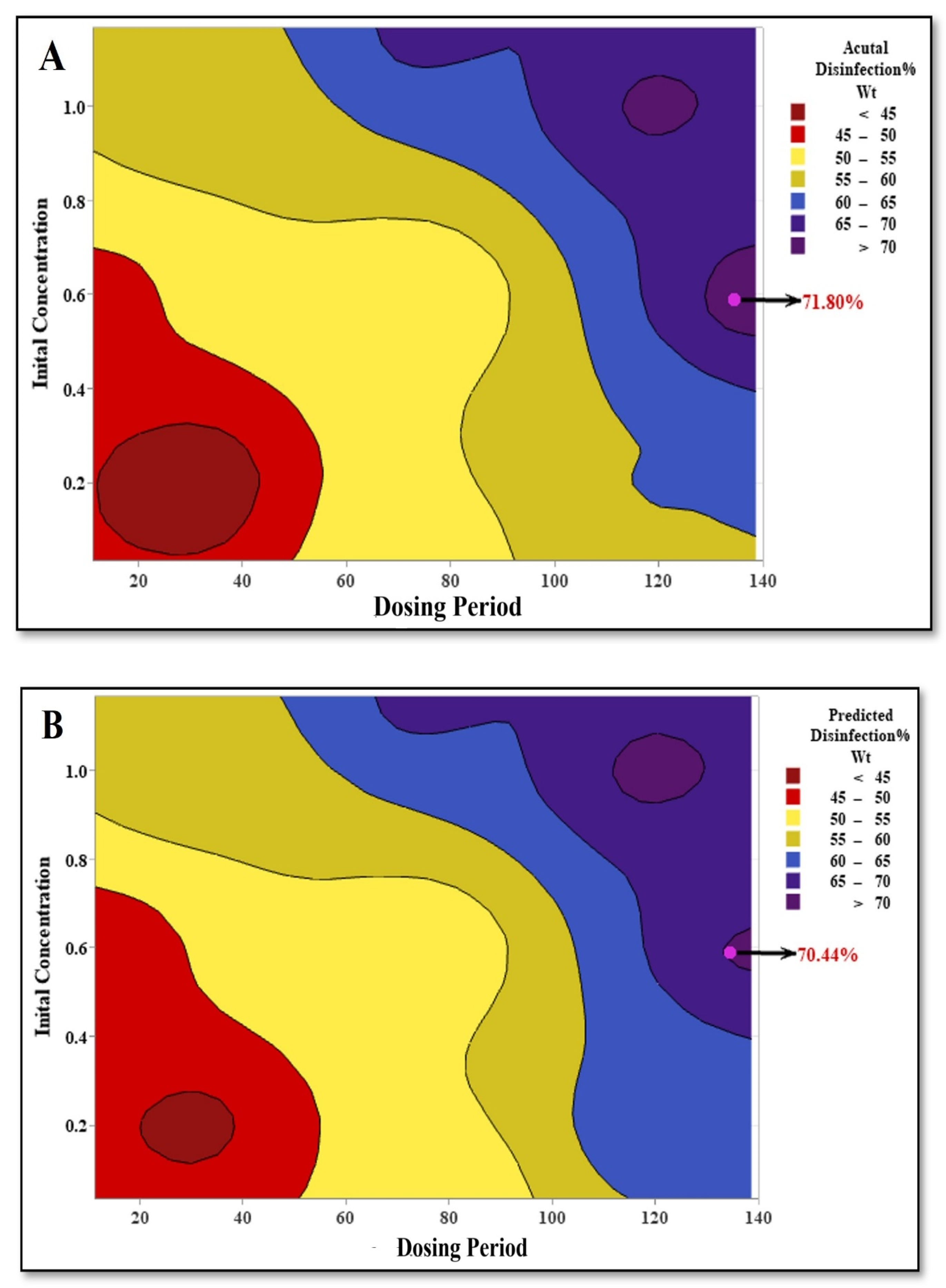
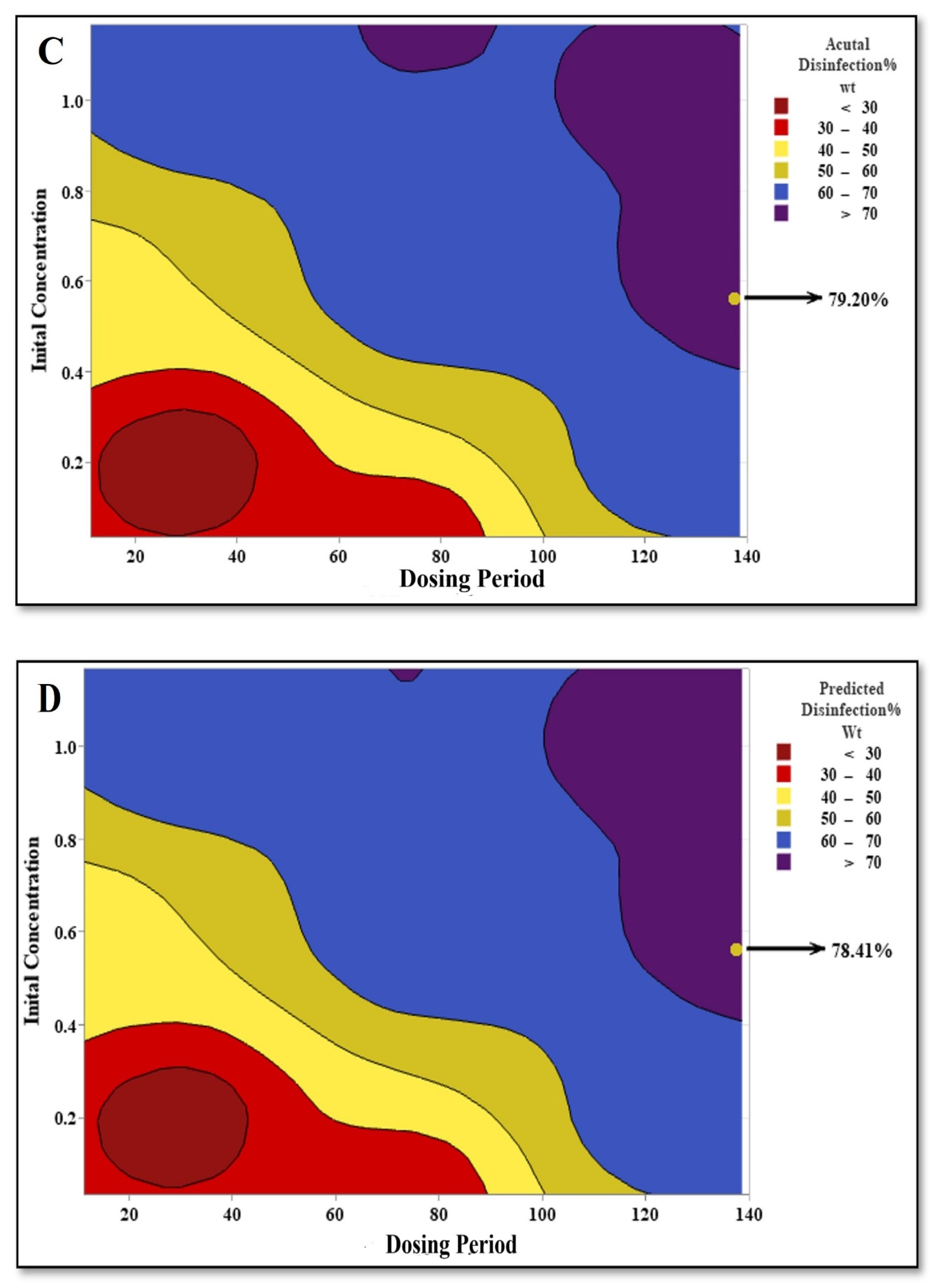
3.4. Comparison of Disinfection Efficiency: NaClO vs. NaBr
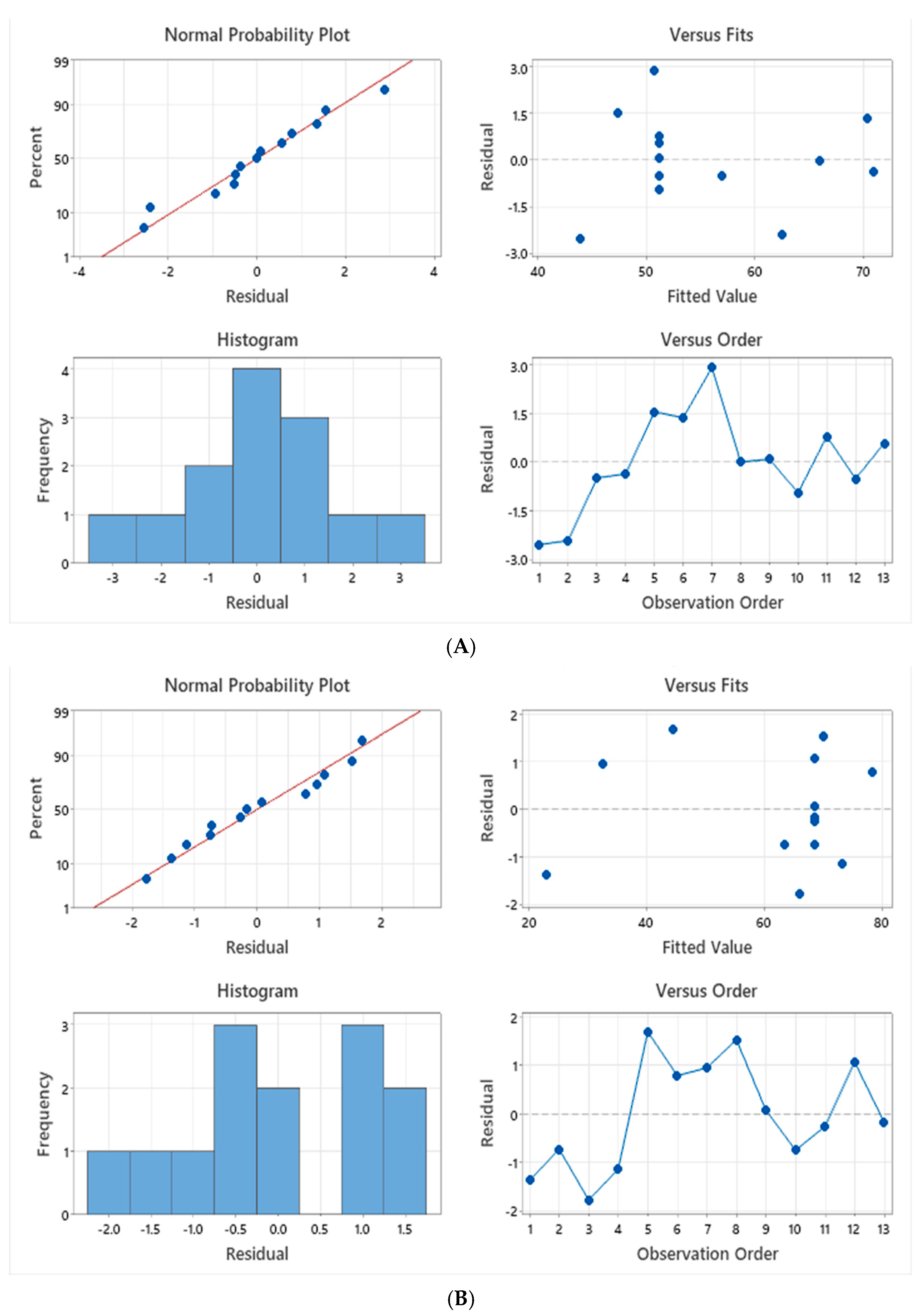
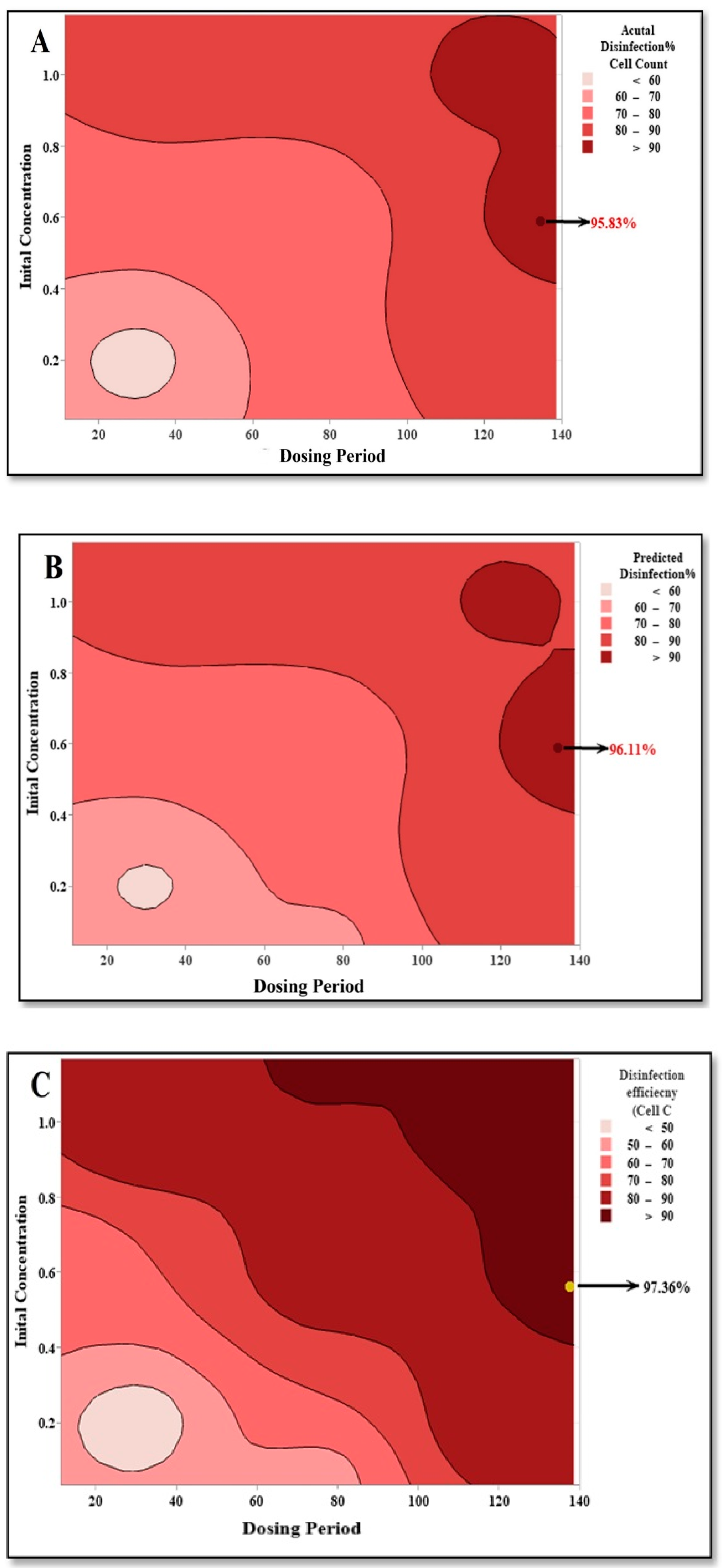
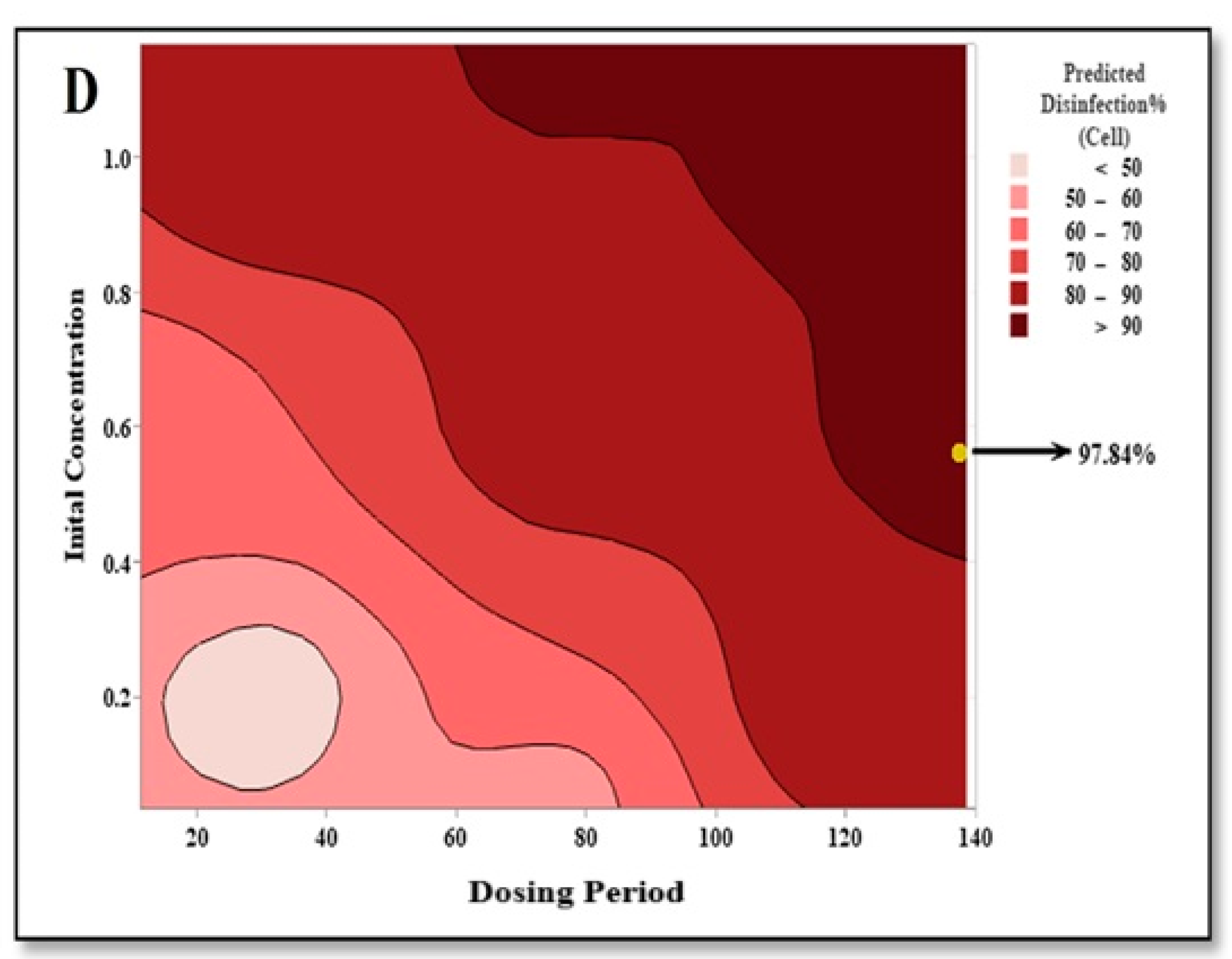
3.5. Optimization of Disinfection Parameters Using RSM
4. Conclusions
5. Limitations and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, L.; Wang, L.; Ou, D.; Jia, C.; Li, W.; Ding, Y.; You, C.; Liao, J.; Huang, H. The ecological mechanisms of Acetes blooms as a threat to the security of cooling systems in coastal nuclear power plants. J. Coast. Conserv. 2021, 25, 55. [Google Scholar] [CrossRef]
- Rubio, D.; López-Galindo, C.; Casanueva, J.F.; Nebot, E. Monitoring and assessment of an industrial antifouling treatment. Seasonal effects and influence of water velocity in an open once-through seawater cooling system. Appl. Therm. Eng. 2014, 67, 378–387. [Google Scholar] [CrossRef]
- Satpathy, K.K.; Mohanty, A.K.; Sahu, G.; Biswas, S.; Prasad, M.V.R.; Slvanayagam, M. Biofouling and its Control in Seawater Cooled Power Plant Cooling Water System—A Review. Nucl. Power 2010, 191–242. [Google Scholar] [CrossRef]
- Cristiani, P.; Perboni, G. Antifouling strategies and corrosion control in cooling circuits. Bioelectrochemistry 2014, 97, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Dobersek, D.; Goricanec, D. Influence of Water Scale on Thermal Flow Losses of Domestic Appliances. Int. J. Math. Models Methods Appl. Sci. 2007, 1, 55–61. [Google Scholar]
- Li, J.; Tang, M.; Ye, Z.; Chen, L.; Zhou, Y. Scale formation and control in oil and gas fields: A review. J. Dispers. Sci. Technol. 2016, 38, 661–670. [Google Scholar] [CrossRef]
- López-Galindo, C.; Casanueva, J.F.; Nebot, E. Efficacy of different antifouling treatments for seawater cooling systems. Biofouling 2010, 26, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Macadam, J.; Parsons, S.A. Calcium carbonate scale formation and control. Rev. Environ. Sci. Bio/Technol. 2004, 3, 159–169. [Google Scholar] [CrossRef]
- Barton, F.; Shaw, S.; Morris, K.; Graham, J.; Lloyd, J.R. Impact and control of fouling in radioactive environments. Prog. Nucl. Energy 2022, 148, 104215. [Google Scholar] [CrossRef]
- Faria, S.I.; Teixeira-Santos, R.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J. The association between initial adhesion and cyanobacterial biofilm development. FEMS Microbiol. Ecol. 2021, 97, fiab052. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, A.; Leyva-Diaz, J.C.; Poyatos, J.M.; Gonzalez-Lopez, J. Influent salinity conditions affect the bacterial communities of biofouling in hybrid MBBR-MBR systems. J. Water Process Eng. 2019, 30, 100650. [Google Scholar] [CrossRef]
- Xavier, E.A.; Almeida, A.C.S.; Nogueira, M.M.; Vieira, L.M. Effects of substratum type and orientation on the recruitment of bryozoans in an artificial area of the Western Atlantic. Biofouling 2023, 39, 748–762. [Google Scholar] [CrossRef]
- Yang, H.L.; Pan, J.R.; Huang, C.; Lin, J.C.T. The effect of feed salinity on the biofouling dynamics of seawater desalination. Biofouling 2011, 27, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Sahan, E.; Sabbe, K.; Creach, V.; Hernandez-Raquet, G.; Vyverman, W.; Stal, L.J.; Muyzer, G. Community structure and seasonal dynamics of diatom biofilms and associated grazers in intertidal mudflats. Aquat. Microb. Ecol. 2007, 47, 253–266. [Google Scholar] [CrossRef]
- Bartolomé, M.C.; Sánchez-Fortún, S. Effects of selected biocides used in the disinfection of cooling towers on toxicity and bioaccumulation in Artemia larvae. Env. Toxicol Chem 2005, 24, 3137–3142. [Google Scholar] [CrossRef]
- Cloete, T.E.; Jacobs, L.; Brözel, V.S. The chemical control of biofouling in industrial water systems. Biodegradation 1998, 9, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Abosaty, M.; Hefnawi, H.; Ayaat, N.; Abousaty, A. Assessment and Control of Microbial Induced Corrosion in Sea Water in Nuclear Power Plant Materials. Arab. J. Nucl. Sci. Appl. 2023, 56, 15–28. [Google Scholar] [CrossRef]
- Murthy, P.S.; Venkatesan, R.; Nair, K.V.K.; Ravindran, M. Biofilm control for plate heat exchangers using surface seawater from the open ocean for the OTEC power plant. Int. Biodeterior. Biodegrad. 2004, 53, 133–140. [Google Scholar] [CrossRef]
- Nebot, E.; Casanueva, J.F.; Casanueva, T.; Sales, D. Model for fouling deposition on power plant steam condensers cooled with seawater: Effect of water velocity and tube material. Int. J. Heat Mass Transf. 2007, 50, 3351–3358. [Google Scholar] [CrossRef]
- Chang, Y.S.; Munro, C.J.; Fortunato, L.; AlAli, A.; Marciulescu, C.; Harvey, S.L.; Vrouwenvelder, J.; Arafat, H.; Dumée, L.F. Macrofouling remediation strategies for water intakes of desalination and other industrial plants—A review. Desalination 2024, 590, 117987. [Google Scholar] [CrossRef]
- Allonier, A.S.; Khalanski, M.; Camel, V.; Bermond, A. Characterization of Chlorination By-products in Cooling Effluents of Coastal Nuclear Power Stations. Mar. Pollut. Bull. 1999, 38, 1232–1241. [Google Scholar] [CrossRef]
- Bartholomew, R.; Powell, S.T.; Bartholomew, R.D. Bromine-Based Biocides for Cooling Water Systems: A Literature Review. Combustion 1998, 2, 1–30. [Google Scholar]
- Shukla, S.K.; Rao, T.S.; M, N.; Mohan, T.V.K. Active-bromide and surfactant synergy for enhanced microfouling control. Arch. Microbiol. 2024, 206, 430. [Google Scholar] [CrossRef]
- Papale, M.; Fazi, S.; Severini, M.; Scarinci, R.; Dell’Acqua, O.; Azzaro, M.; Venuti, V.; Fazio, B.; Fazio, E.; Crupi, V.; et al. Structural properties and microbial diversity of the biofilm colonizing plastic substrates in Terra Nova Bay (Antarctica). Sci. Total. Environ. 2024, 943, 173773. [Google Scholar] [CrossRef]
- Cai, H.; Wang, Y.; Wu, K.; Guo, W. Enhanced hydrophilic and electrophilic properties of polyvinyl chloride (PVC) biofilm carrier. Polymers 2020, 12, 1240. [Google Scholar] [CrossRef] [PubMed]
- Almousa, R.; Wen, X.; Na, S.; Anderson, G.; Xie, D. Hydrophilic polymer-coated PVC surface for reduced cell and bacterial adhesions. Biosurface Biotribology 2022, 8, 34–43. [Google Scholar] [CrossRef]
- Roberts, D.J.; Nica, D.; Zuo, G.; Davis, J.L. Quantifying microbially induced deterioration of concrete: Initial studies. Int. Biodeterior. Biodegrad. 2002, 49, 227–234. [Google Scholar] [CrossRef]
- Huang, S.; Voutchkov, N.; Jiang, S. Balancing carbon, nitrogen and phosphorus concentration in seawater as a strategy to prevent accelerated membrane biofouling. Water Res. 2019, 165, 114978. [Google Scholar] [CrossRef]
- Bott, T.R. Biofouling control in cooling water. Int. J. Chem. Eng. 2009, 2009, 619873. [Google Scholar] [CrossRef]
- Fujioka, T.; Ngo, M.T.T.; Boivin, S.; Kawahara, K.; Takada, A.; Nakamura, Y.; Yoshikawa, H. Controlling biofouling and disinfection by-product formation during reverse osmosis treatment for seawater desalination. Desalination 2020, 488, 114507. [Google Scholar] [CrossRef]
- Burton, D.T.; Margrey, S.L. Control of Fouling Organisms in Estuarine Cooling Water Systems by Chlorine and Bromine Chloride. Environ. Sci. Technol. 1979, 13, 684–689. [Google Scholar] [CrossRef]
- Rajagopal, S. Chlorination and biofouling control in industrial cooling water systems. In Operational and Environmental Consequences of Large Industrial Cooling Water Systems; Springer: Greer, SC, USA, 2012; pp. 163–182. [Google Scholar] [CrossRef]
- Venkatesan, R.; Murthy, P.S. Macrofouling Control in Power Plants. In Marine and Industrial Biofouling; Flemming, H.C., Murthy, P.S., Venkatesan, R., Cooksey, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]



| SL. No | Salinity (ppt) | Ratio | COD (mg/L) |
|---|---|---|---|
| 1 | 17.30 | 1/2 | 2000 |
| 2 | 17.30 | 1/2 | 1000 |
| 3 | 25.95 | 3/4 | 1000 |
| 4 | 25.95 | 3/4 | 2000 |
| 5 | 34.60 | 1 | 2000 |
| 6 | 34.60 | 1 | 1000 |
| Salinity (ppt) | Ratio | Volume of Seawater (mL) | Volume of DI Water (mL) |
|---|---|---|---|
| 34.60 | 1 | 1000 | 0 |
| 25.95 | 3/4 | 750 | 250 |
| 17.30 | 1/2 | 500 | 500 |
| SL. No | Concentration (mg/L) | Dosing Period (min) |
|---|---|---|
| 1 | 0.2 | 30 |
| 2 | 3/4 | 750 |
| 3 | 1/2 | 500 |
| 4 | 1 | 120 |
| 5 | 0.03 | 75 |
| 6 | 1.16 | 75 |
| 7 | 0.6 | 11 |
| 8 | 0.6 | 138 |
| 9 | 0.6 | 75 |
| 10 | 0.6 | 75 |
| 11 | 0.6 | 75 |
| 12 | 0.6 | 75 |
| 13 | 0.6 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, S.N.; Alteneiji, A.A.; Alteneiji, A.B.; Alharmi, F.M.; Al Balushi, N.H.; Hassooni, S.K.; Aly Hassan, A.; Hamouda, M.A. Assessment of Disinfection Efficiency of Chlorine and Bromine-Based Biocides for Marine Biofouling. Sustainability 2025, 17, 4262. https://doi.org/10.3390/su17104262
James SN, Alteneiji AA, Alteneiji AB, Alharmi FM, Al Balushi NH, Hassooni SK, Aly Hassan A, Hamouda MA. Assessment of Disinfection Efficiency of Chlorine and Bromine-Based Biocides for Marine Biofouling. Sustainability. 2025; 17(10):4262. https://doi.org/10.3390/su17104262
Chicago/Turabian StyleJames, Susan N., Alya Ahmed Alteneiji, Ameera Badr Alteneiji, Fatema Mohammed Alharmi, Noura Hatem Al Balushi, Shahad K. Hassooni, Ashraf Aly Hassan, and Mohamed A. Hamouda. 2025. "Assessment of Disinfection Efficiency of Chlorine and Bromine-Based Biocides for Marine Biofouling" Sustainability 17, no. 10: 4262. https://doi.org/10.3390/su17104262
APA StyleJames, S. N., Alteneiji, A. A., Alteneiji, A. B., Alharmi, F. M., Al Balushi, N. H., Hassooni, S. K., Aly Hassan, A., & Hamouda, M. A. (2025). Assessment of Disinfection Efficiency of Chlorine and Bromine-Based Biocides for Marine Biofouling. Sustainability, 17(10), 4262. https://doi.org/10.3390/su17104262








