Bio-Products Obtained from Broccoli and Cabbage Wastes Are Proposed as Functional Food Ingredients and Bioherbicides for Sustainable Weed Management
Abstract
1. Introduction
2. Materials and Methods
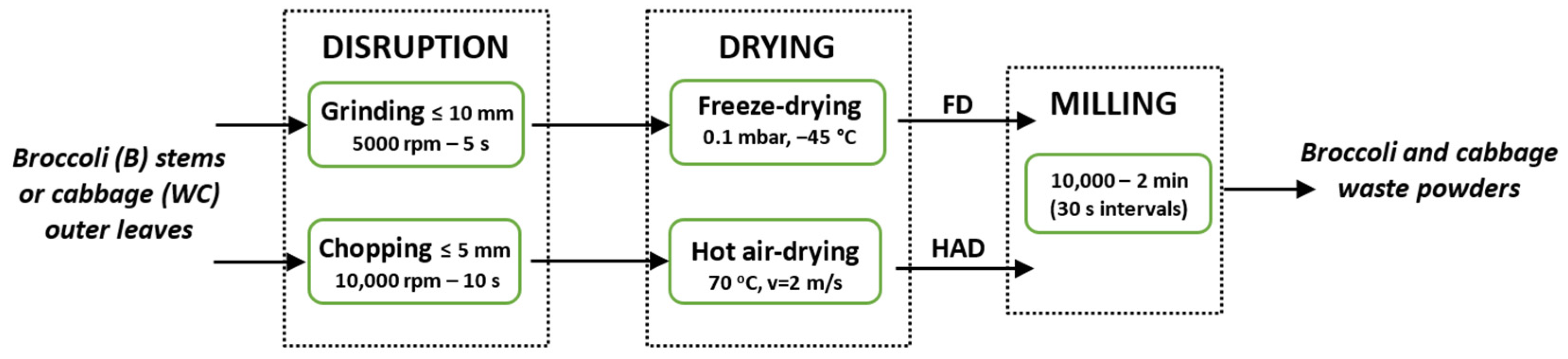
2.1. Plant Materials and Powders Obtention
2.2. Physicochemical Characterization of Powders
2.3. Simulated In Vitro Digestion Experiments for Functional Ingredients Assessment
2.4. Herbicidal Potential of Brassica Waste Powders Tests Under Greenhouse Conditions
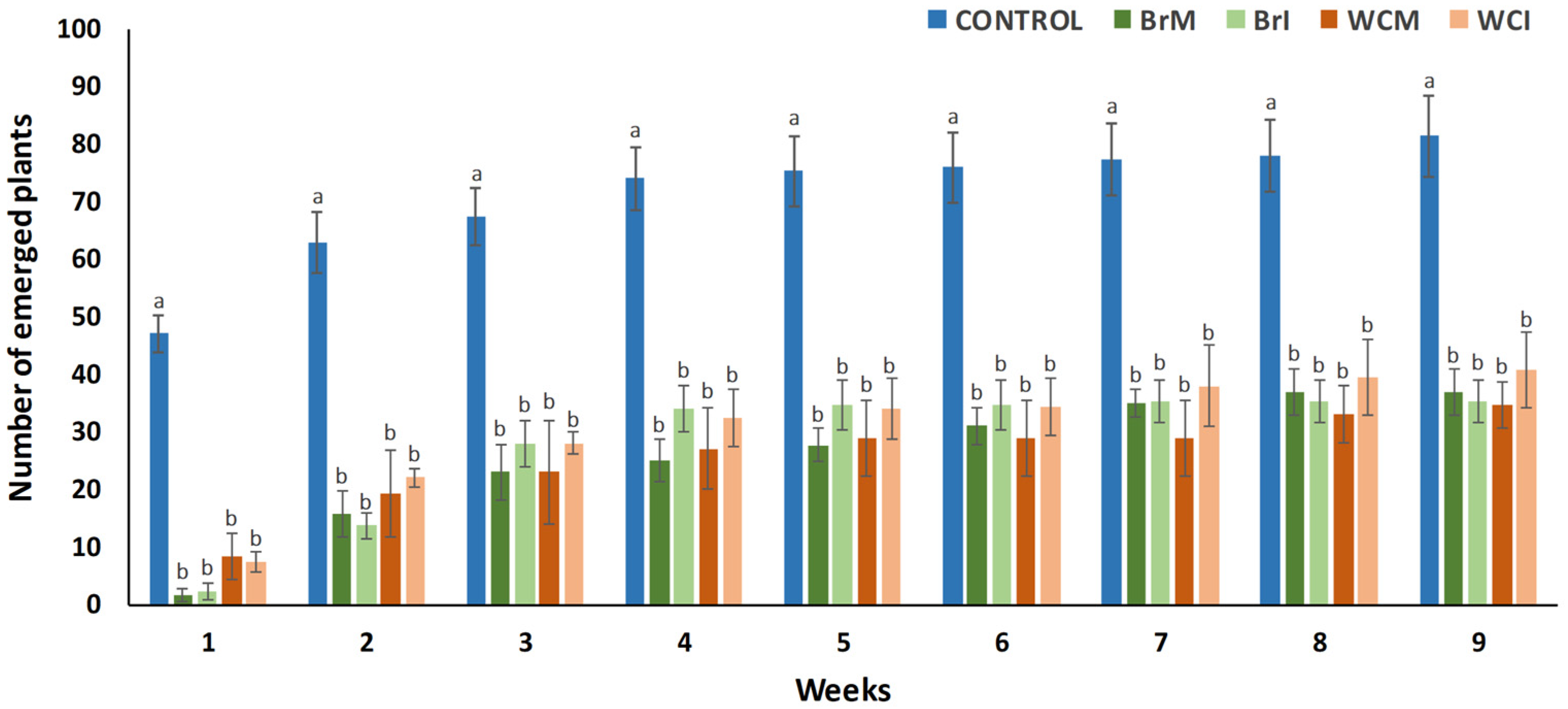
2.4.1. Pre-Emergence Herbicidal Trials Against the Weed Seedbank of a Soil Untreated with Herbicides
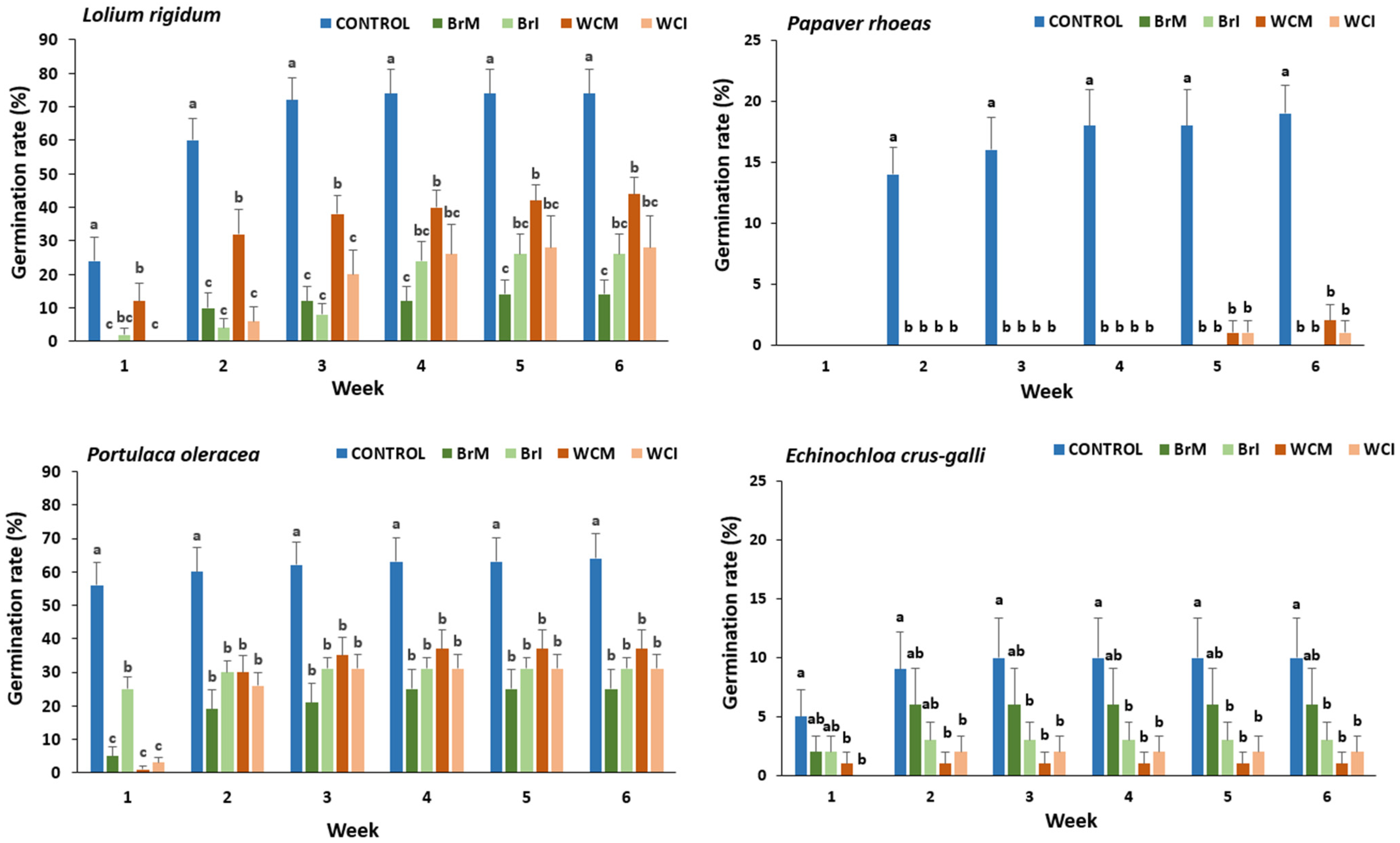
2.4.2. Pre-Emergence Herbicidal Trials of Brassica Waste Powders Against Selected Weed Species
2.4.3. Starting–End Dates and Climatic Conditions of the Greenhouse Experiments
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Attributes of Selected Broccoli and White Cabbage Waste Powders
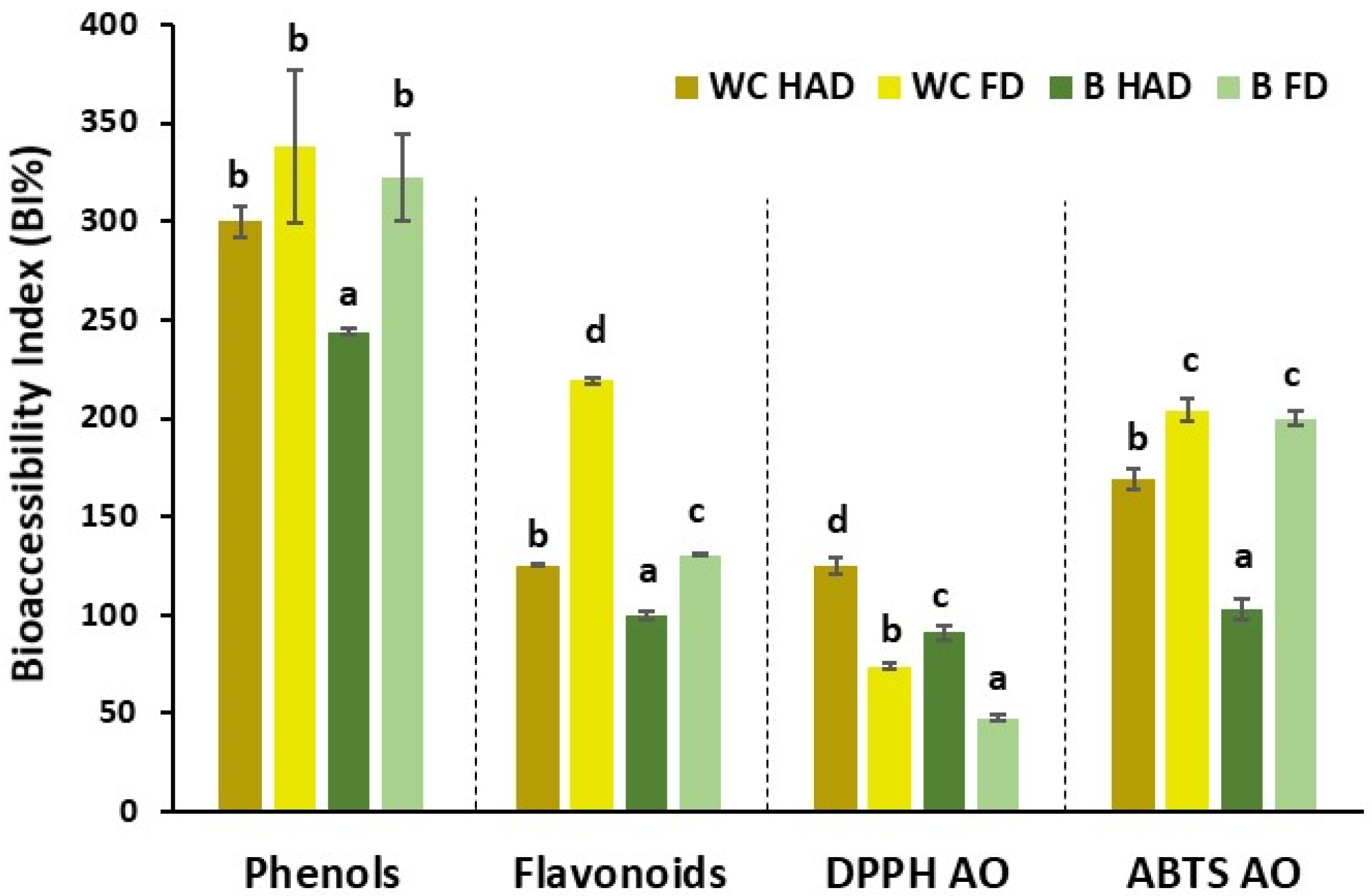
3.2. Response of Brassica Waste Powders to Simulated In Vitro Digestion
3.3. Herbicidal Potential of White Cabbage and Broccoli Waste Powders Against the Seed Weeds Present in the Soil Seedbank
3.3.1. Herbicidal Potential of White Cabbage and Broccoli Waste Powders Against Selected Weed Species
Lolium Rigidum
Papaver Rhoeas
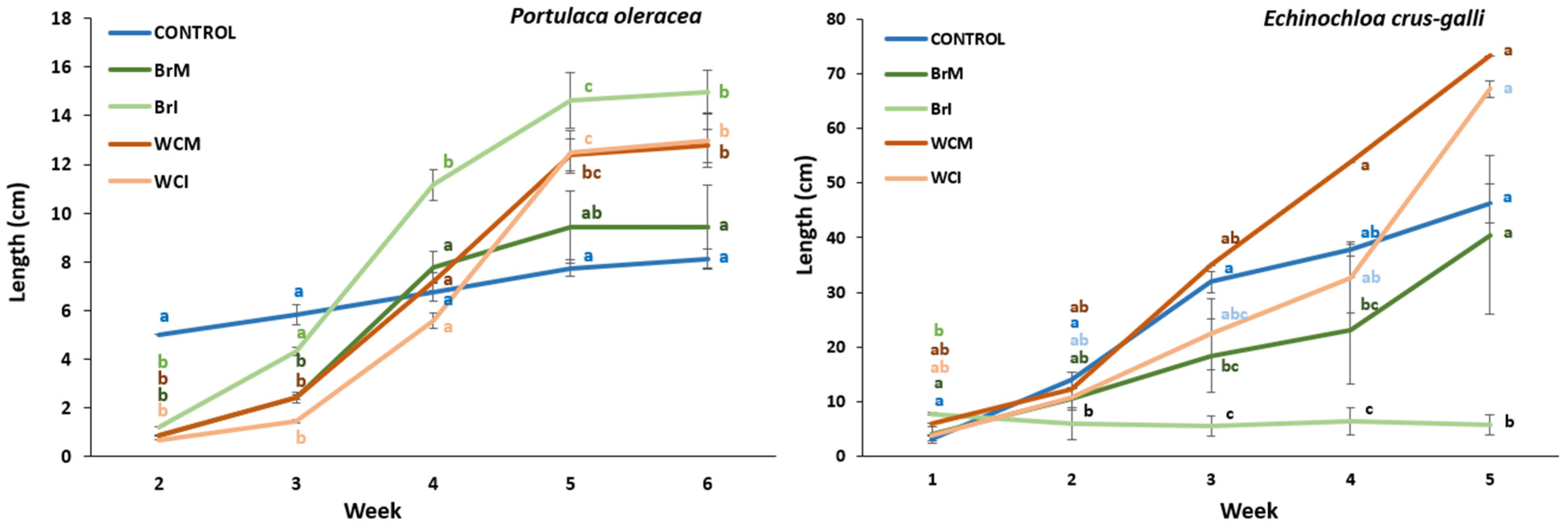
Portulaca Oleracea
Echinochloa Crus-Galli
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Froidmont-Görtz, I.d.; Faure, U.; Gajdzinska, M.; Haentjens, W.; Krommer, J.; Lizaso, M.; Lutzeyer, H.J.; Mangan, C.; Markakis, M.; Schoumacher, C.; et al. Food 2030 Pathways for Action: Research and Innovation Policy as a Driver for Sustainable, Healthy and Inclusive Food Systems; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Lopez, J.S.; Caldeira, C.; De Laurentiis, V.; Sala, S.; Avraamides, M. Brief on Food Waste in the European Union 12 Key Messages; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Caldeira, C.; De Laurentiis, V.; Corrado, S.; van Holsteijn, F.; Sala, S. Quantification of food waste per product group along the food supply chain in the European Union: A mass flow analysis. Resour. Conserv. Recycl. 2019, 149, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Cassani, L.; Gomez-Zavaglia, A. Sustainable Food Systems in Fruits and Vegetables Food Supply Chains. Front. Nutr. 2022, 9, 829061. [Google Scholar] [CrossRef]
- FAO Food and Agriculture Organization of the United Nations. The State of Food and Agriculture. 2019, Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Cagno, R.D. High-value compounds in fruit, vegetable and cereal byproducts: An overview of potential sustainable reuse and exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Martínez, E.J.; González, R.; Ellacuriaga, M.; Gómez, X. Valorization of Fourth-Range Wastes: Evaluating Pyrolytic Behavior of Fresh and Digested Wastes. Fermentation 2022, 8, 744. [Google Scholar] [CrossRef]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef] [PubMed]
- Ross, I.A. The Bioactive Components of Brassicaceae. In Plant-Based Therapeutics, Volume 2; Springer: Cham, Switzerland, 2024; pp. 17–95. [Google Scholar]
- Deng, Q.; Zinoviadou, K.G.; Galanakis, C.M.; Orlien, V.; Grimi, N.; Vorobiev, E.; Lebovka, N.; Barba, F.J. The Effects of Conventional and Non-conventional Processing on Glucosinolates and Its Derived Forms, Isothiocyanates: Extraction, Degradation, and Applications. Food Eng. Rev. 2015, 7, 357–381. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- FAO; WHO. Sustainable Healthy Diets—Guiding Principles; FAO: Rome, Italy, 2019. [Google Scholar]
- Nirmal, N.; Khanashyam, A.; Mundanat, A.; Shah, K.; Babu, K.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Walia, S.; Saha, S.; Tripathi, V.; Sharma, K.K. Phytochemical biopesticides: Some recent developments. Phytochem. Rev. 2017, 16, 989–1007. [Google Scholar] [CrossRef]
- Cluzet, S.; Mérillon, J.-M.; Ramawat, K.G. Specialized Metabolites and Plant Defence. In Plant Defence: Biological Control. Progress in Biological Control; Springer: Cham, Switzerland, 2020; pp. 45–80. [Google Scholar]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Doheny-Adams, T.; Redeker, K.; Kittipol, V.; Bancroft, I.; Hartley, S.E. Development of an efficient glucosinolate extraction method. Plant Methods 2017, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Eugui, D.; Velasco, P. Natural control of plant pathogens through glucosinolates: An effective strategy against fungi and oomycetes. Phytochem. Rev. 2020, 19, 1045–1059. [Google Scholar] [CrossRef]
- Sharma, S.; Rani, H.; Kaur, G.; Kumar, S.; Sheikh, S.; Samota, M.K. Comprehensive overview of glucosinolates in crucifers: Occurrence, roles, metabolism, and transport mechanisms—A review. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, J.-N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Nath, C.P.; Singh, R.G.; Choudhary, V.K.; Datta, D.; Nandan, R.; Singh, S.S. Challenges and Alternatives of Herbicide-Based Weed Management. Agronomy 2024, 14, 126. [Google Scholar] [CrossRef]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Abbas, T.; Zahir, Z.A.; Naveed, M.; Kremer, R.J. Limitations of Existing Weed Control Practices Necessitate Development of Alternative Techniques Based on Biological Approaches. Adv. Agron. 2018, 147, 239–280. [Google Scholar]
- Heap, I. Herbicide Resistant Weeds. In Integrated Pest Management; Springer: Dordrecht, The Netherlands, 2014; pp. 281–301. [Google Scholar]
- Mahmood, Q.; Bilal, M.; Jan, S. Herbicides, Pesticides, and Plant Tolerance. In Emerging Technologies and Management of Crop Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 423–448. [Google Scholar]
- United Nations Doeasapdivision. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; Working Paper No. ESA/P/WP/248; United Nations Doeasapdivision: Geneva, Switzerland, 2017. [Google Scholar]
- Tataridas, A.; Kanatas, P.; Chatzigeorgiou, A.; Zannopoulos, S.; Travlos, I. Sustainable Crop and Weed Management in the Era of the EU Green Deal: A Survival Guide. Agronomy 2022, 12, 589. [Google Scholar] [CrossRef]
- Mocniak, L.E.; Elkin, K.R.; Dillard, S.L.; Bryant, R.B.; Soder, K.J. Building comprehensive glucosinolate profiles for brassica varieties. Talanta 2023, 251, 123814. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef] [PubMed]
- Mawlong, I.; Sujith Kumar, M.S.; Gurung, B.; Singh, K.H.; Singh, D. A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Int. J. Food Prop. 2017, 20, 3274–3281. [Google Scholar] [CrossRef]
- Vargas, L.; Kapoor, R.; Nemzer, B.; Feng, H. Application of different drying methods for evaluation of phytochemical content and physical properties of broccoli, kale, and spinach. LWT 2022, 155, 112892. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Impact of Disruption and Drying Conditions on Physicochemical, Functional and Antioxidant Properties of Powdered Ingredients Obtained from Brassica Vegetable By-Products. Foods 2022, 11, 3663. [Google Scholar] [CrossRef]
- Hansson, D.; Morra, M.J.; Borek, V.; Snyder, A.J.; Johnson-Maynard, J.L.; Thill, D.C. Ionic thiocyanate (SCN-) production, fate, and phytotoxicity in soil amended with Brassicaceae seed meals. J. Agric. Food Chem. 2008, 56, 3912–3917. [Google Scholar] [CrossRef]
- AOAC BAM. Association of official analytical chemists. Official Methods of Analysis; AOAC International: Rockville, MD, USA, 1990. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Ravent6s, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, M.A.; Aruoma, O.I. Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of Cassia fistula. J. Agric. Food Chem. 2002, 50, 5042–5047. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Original Contribution Antioxidant Activity Applying an Improved Abts Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Ortega, N.; Macià, A.; Romero, M.P.; Reguant, J.; Motilva, M.J. Matrix composition effect on the digestibility of carob flour phenols by an in-vitro digestion model. Food Chem. 2011, 124, 65–71. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Turning agri-food cooperative vegetable residues into functional powdered ingredients for the food industry. Sustainability 2020, 12, 1284. [Google Scholar] [CrossRef]
- Que, F.; Mao, L.; Fang, X.; Wu, T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int. J. Food Sci. Technol. 2008, 43, 1195–1201. [Google Scholar] [CrossRef]
- Bernaert, N.; De Clercq, H.; Van Bockstaele, E.; De Loose, M.; Van Droogenbroeck, B. Antioxidant changes during postharvest processing and storage of leek (Allium ampeloprasum var. porrum). Postharvest Biol. Technol. 2013, 86, 8–16. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Zhao, Y.Y.; Luo, C.X.; Li, J.; Gao, Y.Q. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crops Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Campos-Vega, R.; Gaytán-Martínez, M.; Preciado-Ortiz, R.; Mendoza, S.; Loarca-Piña, G. Bioaccessibility and antioxidant activity of free phenolic compounds and oligosaccharides from corn (Zea mays L.) and common bean (Phaseolus vulgaris L.) chips during in vitro gastrointestinal digestion and simulated colonic fermentation. Food Res. Int. 2017, 100, 304–311. [Google Scholar] [CrossRef]
- Lee, H.N.; Jang, Y.; Koh, E. Effect of drying methods on in vitro digestion stability of anthocyanins and polyphenols from omija (Schisandra chinensis Baillon). J. Food Process Preserv. 2022, 46, e17055. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Effect of Processing and In Vitro Digestion on Bioactive Constituents of Powdered IV Range Carrot (Daucus carota, L.) Wastes. Foods 2023, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Change of phenolics, carotenoids, and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Capanoglu, E. Evaluating the invitro bioaccessibility of phenolics and antioxidant activity during consumption of dried fruits with nuts. LWT 2014, 56, 284–289. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Pinazo, A.; Heredia, A.; Andrés, A. Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. 2017, 214, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Bas-Bellver, C.; Andrés, C.; Seguí, L.; Barrera, C.; Jiménez-Hernández, N.; Artacho, A.; Betoret, N.; Gosalbes, M.J. Valorization of Persimmon and Blueberry Byproducts to Obtain Functional Powders: In Vitro Digestion and Fermentation by Gut Microbiota. J. Agric. Food Chem. 2020, 68, 8080–8090. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Dong, R.; Liu, S.; Xie, J.; Chen, Y.; Zheng, Y.; Zhang, X.; Zhao, E.; Wang, Z.; Xu, H.; Yu, Q. The recovery, catabolism and potential bioactivity of polyphenols from carrot subjected to in vitro simulated digestion and colonic fermentation. Food Res. Int. 2021, 143, 110263. [Google Scholar] [CrossRef] [PubMed]
- Bangarwa, S.K.; Norsworthy, J.K. Glucosinolate and Isothiocyanate Production for Weed Control in Plasticulture Production System. In Glucosinolates; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–35. [Google Scholar]
- Kunz, C.; Sturm, D.J.; Varnholt, D.; Walker, F.; Gerhards, R. Allelopathic effects and weed suppressive ability of cover crops. Plant Soil. Environ. 2016, 62, 60–66. [Google Scholar] [CrossRef]
- Bangarwa, S.K.; Norsworthy, J.K.; Mattice, J.D.; Gbur, E.E. Glucosinolate and Isothiocyanate Production from Brassicaceae Cover Crops in a Plasticulture Production System. Weed Sci. 2011, 59, 247–254. [Google Scholar] [CrossRef]
- Ullah, R.; Aslam, Z.; Maitah, M.; Zaman Q uz Bashir, S.; Hassan, W.; Chen, Z. Sustainable Weed Control and Enhancing Nutrient Use Efficiency in Crops through Brassica (Brassica compestris L.) Allelopathy. Sustainability 2020, 12, 5763. [Google Scholar] [CrossRef]
- Khamare, Y.; Chen, J.; Marble, S.C. Allelopathy and its application as a weed management tool: A review. Front. Plant Sci. 2022, 13, 1034649. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Gunasekaran, V.P.; Dominic, S.; Xuan, T.D. Phenolic Allelochemicals from Crops and Weed Management. In Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020; pp. 183–199. [Google Scholar]
- Asaduzzaman, M.; An, M.; Pratley, J.E.; Luckett, D.J.; Lemerle, D.; Coombes, N. The seedling root response of annual ryegrass (Lolium rigidum) to neighbouring seedlings of a highly-allelopathic canola (Brassica napus). Flora 2016, 219, 18–24. [Google Scholar] [CrossRef]
- Golmohammadzadeh, S.; Zaefarian, F.; Rezvani, M. Priming techniques, germination and seedling emergence in two Papaver species (P. rhoeas L. and P. dubium L., Papaveraceae). Braz. J. Bot. 2020, 43, 503–512. [Google Scholar] [CrossRef]
- Haramoto, E.R.; Gallandt, E.R. Brassica cover cropping for weed management: A review. Renew. Agric. Food Syst. 2004, 19, 187–198. [Google Scholar] [CrossRef]
| Sample | aw | Moisture Content (%, g water/100 g) | Particle Size * D[3,4] | Total Phenols (mgGAE/gdm) | Sulforaphane * (µg/gdm) |
|---|---|---|---|---|---|
| WC HAD | 0.23 ± 0.02 a | 2.5 ± 0.7 a | 294 ± 20 a | 4.86 ± 0.04 c | 73 ± 3 a |
| WC FD | 0.29 ± 0.02 b | 3.3 ± 0.2 b | 137 ± 3 b | 3.42 ± 0.10 a | 77 ± 4 a |
| B HAD | 0.29 ± 0.03 a | 3.0 ± 0.6 a | 445 ± 38 a | 5.91± 0.15 d | 461± 9 a |
| B FD | 0.28 ± 0.02 a | 2.6 ± 0.2 b | 145 ± 4 b | 4.52 ± 0.13 b | 506 ± 1 b |
| Sample | BD | Gastric PHASE (GP) | Intestinal PHASE (IP) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | P | Total | %RI | S | P | Total | %RI | |||
| Total phenol content (mg GAE/g) | WC_HAD | 4.71 ± 0.04 c | 9.65 ± 0.19 b | 14.9 ± 0.3 b | 24.58 ± 0.18 b | 521 ± 4 d | 4.25 ± 0.11 a | 21.7 ± 0.5 d | 25.9 ± 0.5 d | 550 ± 10 d |
| WC_FD | 3.32 ± 0.10 a | 5.4 ± 0.3 a | 9.1 ± 0.3 a | 14.5 ± 0.6 a | 437 ± 17 b | 6.9 ± 0.8 c | 8.93 ± 0.12 a | 15.8 ± 0.9 a | 476 ± 26 c | |
| B_HAD | 5.73 ± 0.15 d | 12.10 ± 0.08 c | 15.2 ± 0.9 b | 27.3 ± 0.9 c | 476 ± 16 c | 5.48 ± 0.04 b | 17.5 ± 0.2 c | 22.99 ± 0.16 c | 401 ± 2 a | |
| B_FD | 4.38 ± 0.13 b | 5.8 ± 0.2 a | 8.7 ± 0.3 a | 14.5 ± 0.4 a | 330 ± 8 a | 6.1 ± 0.4 bc | 13.3 ± 0.7 b | 19.4 ± 0.3 b | 444 ± 8 b | |
| Total flavonoid content (mg EQ/g) | WC_HAD | 5.43 ± 0.06 b | 4.036 ± 0.011 c | 8.62 ± 0.08 c | 12.65 ± 0.10 c | 232.9 ± 1.8 b | 2.042 ± 0.005 b | 13.02 ± 0.12 c | 15.06 ± 0.12 d | 277 ± 2 d |
| WC_FD | 2.29 ± 0.05 a | 2.87 ± 0.05 b | 3.68 ± 0.05 b | 6.55 ± 0.02 b | 285.7 ± 0.9 c | 3.06 ± 0.03 d | 3.12 ± 0.04 a | 6.18 ± 0.03 b | 269.6 ± 1.3 c | |
| B_HAD | 7.48 ± 0.07 c | 4.210 ± 0.004 d | 10.90 ± 0.09 d | 15.11 ± 0.09 d | 202.01 ± 1.17 a | 2.930 ± 0.007 c | 10.45 ± 0.16 b | 13.38 ± 0.15 c | 179 ± 2 a | |
| B_FD | 2.33 ± 0.09 a | 1.665 ± 0.006 a | 3.05 ± 0.07 a | 4.71 ± 0.07 a | 202 ± 3 a | 1.319 ± 0.006 a | 3.16 ± 0.04 a | 4.48 ± 0.04 a | 192.3 ± 1.8 b | |
| DPPH (mg TE/g) | WC_HAD | 6.7 ± 1.2 a | 3.87 ± 0.07 c | 32 ± 2 c | 36 ± 2 b | 541 ± 37 b | 2.50 ± 0.09 b | 62.7 ± 0.4 d | 64.2 ± 1.7 d | 961 ± 2.5 b |
| WC_FD | 5.9 ± 0.3 a | 1.61 ± 0.04 b | 27.4 ± 0.6 ab | 29.04 ± 0.65 a | 489 ± 11 b | 2.70 ± 0.08 c | 27.9 ± 0.3 a | 30.6 ± 0.4 a | 515 ± 6 a | |
| B_HAD | 9.3 ± 1.4 b | 4.77 ± 0.06 d | 31 ± 2 bc | 36 ± 2 b | 385 ± 24 a | 3.4 ± 0.2 d | 47 ± 2 c | 50 ± 2 c | 538 ± 20 a | |
| B_FD | 7.1 ± 1.4 a | 0.86 ± 0.16 a | 25 ± 3 s | 25 ± 3 a | 359 ± 47 a | 1.47 ± 0.04 a | 36.1 ± 0.5 b | 37.5 ± 0.4 b | 528 ± 6 a | |
| ABTS (mg TE/g) | WC_HAD | 56 ± 4 c | 49.1 ± 1.8 c | 329 ± 33 b | 378 ± 34 b | 673 ± 61 b | 28.4 ± 0.8 a | 555 ± 39 b | 584 ± 39 c | 1040 ± 69 c |
| WC_FD | 36 ± 2 a | 29.6 ± 1.6 b | 244 ± 6 a | 273 ± 8 a | 768 ± 22 c | 44.6 ± 1.3 c | 295 ± 5 a | 340 ± 6 a | 954 ± 16 b | |
| B_HAD | 94.7 ± 1.0 d | 51.2 ± 1.8 c | 414 ± 32 c | 465 ± 33 c | 491 ± 35 a | 38 ± 2 b | 550 ± 40 b | 588 ± 42 c | 621 ± 44 a | |
| B_FD | 45.9 ± 1.8 b | 20.8 ± 0.8 a | 270 ± 6 a | 291 ± 7 a | 635 ± 15 b | 39.7 ± 0.8 b | 474 ± 4 b | 512 ± 4 b | 1116 ± 9 | |
| Monocotyledonous | Aerial Parts | Root Parts | ||||
| Treatment | Fresh Weight (g) | Dry Weight (g) | Length (cm) | Fresh Weight (g) | Dry Weight (g) | Length (cm) |
| control | 6.30 ± 0.73 ab | 0.82 ± 0.26 ab | 18.72 ± 2.64 a | 1.15 ± 0.32 ab | 0.35 ± 0.08 ab | 8.98 ± 1.22 a |
| BrM | 3.75 ± 0.21 a | 0.56 ± 0.05 a | 20.11 ± 0.17 a | 0.60 ± 0.06 a | 0.33 ± 0.17 a | 8.17 ± 0.64 a |
| BrI | 4.19 ± 1.27 a | 0.64 ± 0.17 a | 21.62 ± 0.99 a | 0.95 ± 0.59 ab | 0.27 ± 0.11 a | 9.58 ± 1.57 a |
| WCM | 6.33 ± 3.07 ab | 0.94 ± 0.34 ab | 19.58 ± 1.03 a | 0.90 ± 0.53 ab | 0.39 ± 0.20 ab | 8.34 ± 0.34 a |
| WCI | 9.90 ± 1.50 b | 1.37 ± 0.22 b | 22.17 ± 1.53 a | 2.25 ± 0.47 b | 1.10 ± 0.35 b | 10.24 ± 0.96 a |
| Dicotyledonous | Aerial Parts | Root Parts | ||||
| Treatment | Fresh Weight (g) | Dry Weight (g) | Length (cm) | Fresh Weight (g) | Dry Weight (g) | Length (cm) |
| control | 5.45 ± 2.19 a | 0.56 ± 0.18 ab | 7.38 ± 0.86 a | 1.22 ± 0.42 a | 0.37 ± 0.21 a | 4.69 ± 1.09 a |
| BrM | 2.02 ± 0.73 a | 0.24 ± 0.07 a | 5.90 ± 1.08 a | 0.31 ± 0.12 b | 0.07 ± 0.02 b | 7.65 ± 2.27 a |
| BrI | 2.16 ± 0.28 a | 0.25 ± 0.01 a | 5.03 ± 0.75 a | 0.43 ± 0.11 b | 0.09 ± 0.01 ab | 6.50 ± 1.22 a |
| WCM | 3.73 ± 1.23 a | 0.76 ± 0.15 b | 6.07 ± 1.70 a | 0.56 ± 0.25 ab | 0.09 ± 0.02 ab | 4.17 ± 1.04 a |
| WCI | 1.82 ± 0.35 a | 0.24 ± 0.07 a | 4.70 ± 0.77 a | 0.26 ± 0.09 b | 0.06 ± 0.01 b | 4.75 ± 0.24 a |
| Lolium rigidum | Aerial Parts | Root Parts | ||||
| Treatment | Fresh Weight (g) | Dry Weight (g) | Length (cm) | Fresh Weight (g) | Dry Weight (g) | Length (cm) |
| control | 0.28 ± 0.04 a | 0.027 ± 0.001 a | 18.2 ± 0.7 a | 0.10 ± 0.02 a | 0.022 ± 0.003 a | 15.3 ± 0.8 a |
| BrM | 0.32 ± 0.12 a | 0.03 ± 0.01 a | 18 ± 3 abc | 0.03 ± 0.01 bc | 0.007 ± 0.002 b | 8.4 ± 1.5 bc |
| BrI | 0.13 ± 0.03 a | 0.017 ± 0.001 a | 17.8 ± 1.4 abc | 0.008 ± 0.001 c | 0.003 ± 0.001 b | 5.8 ± 0.6 bc |
| WCM | 0.70 ± 0.10 b | 0.06 ± 0.01 b | 21.5 ± 1.0 b | 0.07 ± 0.01 ab | 0.011 ± 0.002 b | 8.4 ± 0.7 b |
| WCI | 0.19 ± 0.06 a | 0.02 ± 0.01 a | 14 ± 2 c | 0.02 ± 0.01 bc | 0.004 ± 0.001 b | 5.7 ± 0.9 c |
| Papaver rhoeas | Aerial Parts | Root Parts | ||||
| Treatment | Fresh Weight (g) | Dry Weight (g) | Length (cm) | Fresh Weight (g) | Dry Weight (g) | Length (cm) |
| control | 0.07 ± 0.01 a | 0.006 ± 0.001 a | 3.39 ± 0.32 a | 0.005 ± 0.001 a | 0.0008 ± 0.0001 a | 3.7 ± 0.3 a |
| BrM | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| BrI | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| WCM | 0.0075 ± 0.0005 a | 0.0006 ± 0.0001 a | 1.05 ± 0.51 b | 0.0018 ± 0.0003 a | 0.0003 ± 0.0002 a | 2.5 ± 0.9 a |
| WCI | 0.01 *,a | 0.0009 *,a | 1.13 *,a,b | 0.004 *,a | 0.0002 *,a | 1.92 *,a |
| Portulaca oleracea | Aerial Parts | Root Parts | ||||
| Treatment | Fresh Weight (g) | Dry Weight (g) | Length (cm) | Fresh Weight (g) | Dry Weight (g) | Length (cm) |
| control | 0.62 ± 0.07 a | 0.11 ± 0.01 a | 5.9 ± 0.4 a | 0.06 ± 0.01 a | 0.018 ± 0.002 a | 7.8 ± 0.5 a |
| BrM | 4.2 ± 0.8 b | 0.51 ± 0.09 b | 10.4 ± 0.8 b | 0.28 ± 0.05 bc | 0.08 ± 0.01 c | 13.1 ± 1.8 c |
| BrI | 7.1 ± 1.4 c | 0.78 ± 0.16 c | 11.8 ± 1.6 b | 0.39 ± 0.08 c | 0.08 ± 0.02 c | 9.9 ± 1.5 bc |
| WCM | 3.4 ± 0.5 b | 0.34 ± 0.05 b | 10.8 ± 0.7 b | 0.22 ± 0.04 b | 0.04 ± 0.01 b | 11.5 ± 1.1 bc |
| WCI | 4.3 ± 0.8 b | 0.36 ± 0.07 b | 12.5 ± 1.0 b | 0.21 ± 0.04 b | 0.05 ± 0.01 b | 9.8 ± 1.1 b |
| Echicnochloa crus-galli | Aerial Parts | Root Parts | ||||
| Treatment | Fresh Weight (g) | Dry Weight (g) | Length (cm) | Fresh Weight (g) | Dry Weight (g) | Length (cm) |
| control | 2.0 ± 0.4 ab | 0.32 ± 0.05 ab | 43 ± 4 a | 2.1 ± 0.4 a | 0.21 ± 0.04 ab | 19.51± 1.1 a |
| BrM | 5 ± 3 bc | 0.6 ± 0.4 abc | 40± 15 a | 6 ± 3 a | 0.3 ± 0.2 ab | 145 ± 7 a |
| BrI | 0.0043 ± 0.002 a | 0.0022 ± 0.0009 a | 5.8 ± 1.9 b | 0.0005 ± 0.0004 a | 0.0002 ± 0.0002 a | 0.75 ± 0.03 b |
| WCM | 10.94 *,c | 1.26 *,c | 73.32 *,a | 15.89 *,b | 1.43 *,c | 26.46 *,a |
| WCI | 8 ± 2 c | 0.8 ± 0.3 bc | 67.2 ± 1.5 a | 7 ± 4 ab | 0.5 ± 0.2 b | 23 ± 2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bas-Bellver, C.; Melero-Carnero, N.; López-González, D.; Barrera, C.; Verdeguer, M.; Seguí, L. Bio-Products Obtained from Broccoli and Cabbage Wastes Are Proposed as Functional Food Ingredients and Bioherbicides for Sustainable Weed Management. Sustainability 2025, 17, 282. https://doi.org/10.3390/su17010282
Bas-Bellver C, Melero-Carnero N, López-González D, Barrera C, Verdeguer M, Seguí L. Bio-Products Obtained from Broccoli and Cabbage Wastes Are Proposed as Functional Food Ingredients and Bioherbicides for Sustainable Weed Management. Sustainability. 2025; 17(1):282. https://doi.org/10.3390/su17010282
Chicago/Turabian StyleBas-Bellver, Claudia, Nieves Melero-Carnero, David López-González, Cristina Barrera, Mercedes Verdeguer, and Lucía Seguí. 2025. "Bio-Products Obtained from Broccoli and Cabbage Wastes Are Proposed as Functional Food Ingredients and Bioherbicides for Sustainable Weed Management" Sustainability 17, no. 1: 282. https://doi.org/10.3390/su17010282
APA StyleBas-Bellver, C., Melero-Carnero, N., López-González, D., Barrera, C., Verdeguer, M., & Seguí, L. (2025). Bio-Products Obtained from Broccoli and Cabbage Wastes Are Proposed as Functional Food Ingredients and Bioherbicides for Sustainable Weed Management. Sustainability, 17(1), 282. https://doi.org/10.3390/su17010282









