Coastal Ecological Connectivity between Seagrass Bed and Marine Ranching 30 km Apart: A Case STUDY of Apostichopus japonicus Feeding on Seagrass Debris in the Bohai Sea, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sample Collection
2.3. Sample Analysis
2.4. Data Analysis
3. Results
3.1. Isotopic Distribution
3.2. Food Source Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- do Amaral Camara Lima, M.; Bergamo, T.F.; Ward, R.D.; Joyce, C.B. A review of seagrass ecosystem services: Providing nature-based solutions for a changing world. Hydrobiologia 2023, 850, 2655–2670. [Google Scholar] [CrossRef]
- Potouroglou, M.; Bull, J.C.; Krauss, K.W.; Kennedy, H.A.; Fusi, M.; Daffonchio, D.; Mangora, M.M.; Githaiga, M.N.; Diele, K.; Huxham, M. Measuring the role of seagrasses in regulating sediment surface elevation. Sci. Rep. 2017, 7, 11917. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.d.A.C.; Ward, R.D.; Joyce, C.B.; Kauer, K.; Sepp, K. Carbon stocks in southern England’s intertidal seagrass meadows. Estuar. Coast. Shelf Sci. 2022, 275, 107947. [Google Scholar] [CrossRef]
- de los Santos, C.B.; Scott, A.; Arias-Ortiz, A.; Jones, B.; Kennedy, H.; Mazarrasa, I.; McKenzie, L.; Nordlund, L.M.; de la Torre-Castro, M.d.l.T.; Unsworth, R.K. Seagrass ecosystem services: Assessment and scale of benefits. In Out of the Blue: The Value of Seagrasses to the Environment and to People; United Nations Environment: Nairobi, Kenya, 2020; pp. 19–21. [Google Scholar]
- Costanza, R.; d’Arge, R.; De Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’neill, R.V.; Paruelo, J. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L., Jr.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, R.K.F.; Nordlund, L.M.; Cullen-Unsworth, L.C. Seagrass meadows support global fisheries production. Conserv. Lett. 2019, 12, e12566. [Google Scholar] [CrossRef]
- Wang, F. Feeding Ecology of Two Megagrazers in Two Typical Bays. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2015. [Google Scholar]
- Reynolds, P.L.; Duffy, E.; Knowlton, N. Seagrass and seagrass beds. Ocean Portal 2018, 16, 10. [Google Scholar]
- Liu, X.; Zhou, Y.; Yang, H.; Ru, S. Eelgrass detritus as a food source for the sea cucumber Apostichopus japonicus Selenka (Echinidermata: Holothuroidea) in coastal waters of North China: An experimental study in flow-through systems. PLoS ONE 2013, 8, e58293. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.A.; Beltran, R.; Traveset, A.; Calleja, M.L.; Delgado-Huertas, A.; Marbà, N. Aeolian transport of seagrass (Posidonia oceanica) beach-cast to terrestrial systems. Estuar. Coast. Shelf Sci. 2017, 196, 31–44. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Zhang, Y.; Li, G. Strategic approach for mariculture to practice “Ocean Negative Carbon Emission”. Bull. Chin. Acad. Sci. 2021, 36, 252–258. [Google Scholar]
- Yu, Z.; Zhou, Y.; Yang, H.; Hu, C. Survival, growth, food availability and assimilation efficiency of the sea cucumber Apostichopus japonicus bottom-cultured under a fish farm in southern China. Aquaculture 2014, 426, 238–248. [Google Scholar] [CrossRef]
- Floren, A.S.; Hayashizaki, K.-I.; Putchakarn, S.; Tuntiprapas, P.; Prathep, A. A review of factors influencing the seagrass-sea cucumber association in tropical seagrass meadows. Front. Mar. Sci. 2021, 8, 696134. [Google Scholar] [CrossRef]
- Du, J.; Ye, G.; Zhou, Q.; Chen, B.; Hu, W.; Zheng, X. Progress and prospects of coastal ecological connectivity studies. Acta Ecol. Sin. 2015, 35, 6923–6933. [Google Scholar]
- Pittman, S.J.; Caldow, C.; Hile, S.D.; Monaco, M.E. Using seascape types to explain the spatial patterns of fish in the mangroves of SW Puerto Rico. Mar. Ecol. Prog. Ser. 2007, 348, 273–284. [Google Scholar] [CrossRef]
- Pittman, S.; McAlpine, C. Movements of marine fish and decapod crustaceans: Process, theory and application. Adv. Mar. Biol. 2003, 44, 205–294. [Google Scholar] [PubMed]
- Ahlbeck, I.; Hansson, S.; Hjerne, O. Evaluating fish diet analysis methods by individual-based modelling. Can. J. Fish Aquat. Sci. 2012, 69, 1184–1201. [Google Scholar] [CrossRef]
- França, S.; Vasconcelos, R.P.; Tanner, S.; Máguas, C.; Costa, M.J.; Cabral, H.N. Assessing food web dynamics and relative importance of organic matter sources for fish species in two Portuguese estuaries: A stable isotope approach. Mar. Environ. Res. 2011, 72, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.L.; Inger, R.; Bearhop, S.; Jackson, A.L.; Moore, J.W.; Parnell, A.C.; Semmens, B.X.; Ward, E.J. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool. 2014, 92, 823–835. [Google Scholar] [CrossRef]
- Blomberg, B.N.; Montagna, P.A. Meta-analysis of Ecopath models reveals secondary productivity patterns across the Gulf of Mexico. Ocean Coast. Manag. 2014, 100, 32–40. [Google Scholar] [CrossRef]
- Cresson, P.; Ruitton, S.; Harmelin-Vivien, M. Artificial reefs do increase secondary biomass production: Mechanisms evidenced by stable isotopes. Mar. Ecol. Prog. Ser. 2014, 509, 15–26. [Google Scholar] [CrossRef]
- Kang, H.Y.; Lee, B.-G.; Park, H.J.; Yun, S.-G.; Kang, C.-K. Trophic structures of artificial reef communities off the southern coast of the Korean peninsula as determined using stable isotope analyses. Mar. Pollut. Bull. 2021, 169, 112474. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.; Robert, M.; Cresson, P.; Le Bris, H.; Kopp, D. Food web structure in relation to environmental drivers across a continental shelf ecosystem. Limnol. Oceanogr. 2021, 66, 2563–2582. [Google Scholar] [CrossRef]
- Granek, E.F.; Compton, J.E.; Phillips, D.L. Mangrove-exported nutrient incorporation by sessile coral reef invertebrates. Ecosystems 2009, 12, 462–472. [Google Scholar] [CrossRef]
- Savage, C.; Thrush, S.F.; Lohrer, A.M.; Hewitt, J.E. Ecosystem services transcend boundaries: Estuaries provide resource subsidies and influence functional diversity in coastal benthic communities. PLoS ONE 2012, 7, e42708. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.L.; Vieira, J.P. Feeding strategy of Menticirrhus americanus and Menticirrhus littoralis (Perciformes: Sciaenidae) juveniles in a sandy beach surf zone of southern Brazil. Zoologia 2010, 27, 873–880. [Google Scholar] [CrossRef][Green Version]
- Niang, T.M.S.; Pessanha, A.L.M.; Araújo, F.G. Dieta de juvenis de Trachinotus carolinus (Actinopterygii, Carangidae) em praias arenosas na costa do Rio de Janeiro. Iheringia. Série Zool. 2010, 100, 35–42. [Google Scholar] [CrossRef][Green Version]
- Shanming, G.; Yuanfang, L.; Fengtueng, A.; Fengxin, L. The formation of sand bars on the Luanhe River Delta and the change of the coast line. Acta Oceanol. Sin. 1980, 2, 102–114. [Google Scholar]
- Ying, W.; Guanghe, F.; Yongzhan, Z. River-sea interactive sedimentation and plain morphological evolution. Quat. Sci. 2007, 27, 674–689. [Google Scholar]
- Lu, Y.; Zuo, L.; Ji, R.; Zhang, J. Effect of development of Caofeidian harbor area in Bohai Bay on hydrodynamic sediment environment. Adv. Water Sci. 2007, 18, 793–800. [Google Scholar]
- Yin, Y.H. Thoughts on large area reclamation of Caofeidian shoal in Tangshan, Hebei Province. Mar. Geol. Lett. 2007, 23, 1–10. [Google Scholar]
- Xu, S.; Xu, S.; Zhou, Y.; Yue, S.; Zhang, X.; Gu, R.; Zhang, Y.; Qiao, Y.; Liu, M.; Zhang, Y. Do adult eelgrass shoots rule seedling fate in a large seagrass meadow in a eutrophic bay in northern China? Mar. Pollut. Bull. 2022, 178, 113499. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, Y.; Song, X.; Zhang, Y.; Zhang, H. The distribution of large floating seagrass (Zostera marina) aggregations in northern temperate zones of Bohai Bay in the Bohai Sea, China. PLoS ONE 2019, 14, e0201574. [Google Scholar]
- Yang, X. The Community Characteristics and Ecological Functions of Artificial Oyster Reef at Xiangyun Bay Marine Ranching. Master’s Thesis, University of Chinese Academy of Sciences, Qingdao, China, 2019. [Google Scholar]
- Zhang, R. The Comparative Study of the Food Web Structure and Function between the Artificial and Natural Reefs in the Nearshore. Ph.D. Thesis, University of Chinese Academy of Sciences, Yantai, China, 2021. [Google Scholar]
- Stock, B.C.; Semmens, B.X. MixSIAR GUI User Manual. Version 3.1. 2016. Available online: https://github.com/brianstock/MixSIAR (accessed on 1 July 2023).
- Caut, S.; Angulo, E.; Courchamp, F. Variation in discrimination factors (Δ15N and Δ13C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- McClanahan, T.R. The near future of coral reefs. Environ. Conserv. 2002, 29, 460–483. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S. A global crisis for seagrass ecosystems. BioScience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; Cullen, L.C. Recognising the necessity for Indo-Pacific seagrass conservation. Conserv. Lett. 2010, 3, 63–73. [Google Scholar] [CrossRef]
- Green, E.P.; Short, F.T. World Atlas of Seagrasses; University of California Press: Berkeley, CA, USA, 2003. [Google Scholar]
- de la Torre-Castro, M.; Rönnbäck, P. Links between humans and seagrasses—An example from tropical East Africa. Ocean Coast. Manag. 2004, 47, 361–387. [Google Scholar] [CrossRef]
- Cullen-Unsworth, L.C.; Nordlund, L.M.; Paddock, J.; Baker, S.; McKenzie, L.J.; Unsworth, R.K. Seagrass meadows globally as a coupled social–ecological system: Implications for human wellbeing. Mar. Pollut. Bull. 2014, 83, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerken, I.; van der Velde, G.; Gorissen, M.W.; Meijer, G.J.; Van’t Hof, T.; den Hartog, C. Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuar. Coast. Shelf Sci. 2000, 51, 31–44. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Taylor, M.D.; Fry, B.; Becker, A.; Moltschaniwskyj, N. Recruitment and connectivity influence the role of seagrass as a penaeid nursery habitat in a wave dominated estuary. Sci. Total Environ. 2017, 584–585, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, R.K.; De León, P.S.; Garrard, S.L.; Jompa, J.; Smith, D.J.; Bell, J.J. High connectivity of Indo-Pacific seagrass fish assemblages with mangrove and coral reef habitats. Mar. Ecol. Prog. Ser. 2008, 353, 213–224. [Google Scholar] [CrossRef]
- Jenkins, G.; May, H.; Wheatley, M.; Holloway, M. Comparison of fish assemblages associated with seagrass and adjacent unvegetated habitats of Port Phillip Bay and Corner Inlet, Victoria, Australia, with emphasis on commercial species. Estuar. Coast. Shelf Sci. 1997, 44, 569–588. [Google Scholar] [CrossRef]
- Domínguez-Godino, J.A.; Santos, T.F.; Pereira, H.; Custódio, L.; González-Wangüemert, M. Seagrass debris as potential food source to enhance Holothuria arguinensis’ growth in aquaculture. Aquac. Res. 2020, 51, 1487–1499. [Google Scholar] [CrossRef]
- Zieman, J.; Fourqurean, J.W.; Iverson, R.L. Distribution, abundance and productivity of seagrasses and macroalgae in Florida Bay. Bull. Mar. Sci. 1989, 44, 292–311. [Google Scholar]
- Vandendriessche, S.; Messiaen, M.; O’Flynn, S.; Vincx, M.; Degraer, S. Hiding and feeding in floating seaweed: Floating seaweed clumps as possible refuges or feeding grounds for fishes. Estuar. Coast. Shelf Sci. 2007, 71, 691–703. [Google Scholar] [CrossRef]
- Mateo, M.A. Beach-cast Cymodocea nodosa along the shore of a semienclosed bay: Sampling and elements to assess its ecological implications. J. Coast. Res. 2010, 26, 283–291. [Google Scholar] [CrossRef]
- Purcell, S.W.; Mercier, A.; Conand, C.; Hamel, J.F.; Toral-Granda, M.V.; Lovatelli, A.; Uthicke, S. Sea cucumber fisheries: Global analysis of stocks, management measures and drivers of overfishing. Fish Fish. 2013, 14, 34–59. [Google Scholar] [CrossRef]
- Floren, A.; Hayashizaki, K.; Tuntiprapas, P.; Prathep, A. Contributions of seagrasses and other sources to sea cucumber diets in a tropical seagrass ecosystem. Chiang Mai J. Sci. 2021, 48, 1259–1270. [Google Scholar]
- Costa, V.; Mazzola, A.; Vizzini, S. Holothuria tubulosa Gmelin 1791 (Holothuroidea, Echinodermata) enhances organic matter recycling in Posidonia oceanica meadows. J. Exp. Mar. Biol. Ecol. 2014, 461, 226–232. [Google Scholar] [CrossRef]
- Ricart, A.M.; Dalmau, A.; Pérez, M.; Romero, J. Effects of landscape configuration on the exchange of materials in seagrass ecosystems. Mar. Ecol. Prog. Ser. 2015, 532, 89–100. [Google Scholar] [CrossRef]
- Boncagni, P.; Rakaj, A.; Fianchini, A.; Vizzini, S. Preferential assimilation of seagrass detritus by two coexisting Mediterranean sea cucumbers: Holothuria polii and Holothuria tubulosa. Estuar. Coast. Shelf Sci. 2019, 231, 106464. [Google Scholar] [CrossRef]
- Hart, M.W. Particle captures and the method of suspension feeding by echinoderm larvae. Biol. Bull. 1991, 180, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Q.; Zhao, Y.; Yang, H. Sea cucumber (Apostichopus japonicus) eukaryotic food source composition determined by 18s rDNA barcoding. Mar. Biol. 2016, 163, 153. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Shi, Y. Phytoplankton community and changes after reclamation in Caofeidian coastal waters. Mar. Environ. Sci. 2020, 39, 379–386. [Google Scholar]
- Liang, M.; Chen, Z.; Li, D.; Sun, L.; Jiang, Q.; Lu, B. Net-phytoplankton community structure characteristics and its correlation with environmental factors in coastal waters of Caofeidian. J. Appl. Oceanogr. 2019, 38, 252–265. [Google Scholar]
- Ward, M. Carbon Cycling and Climate Resilience in Coastal Habitats of California; University of California: Davis, CA, USA, 2020. [Google Scholar]

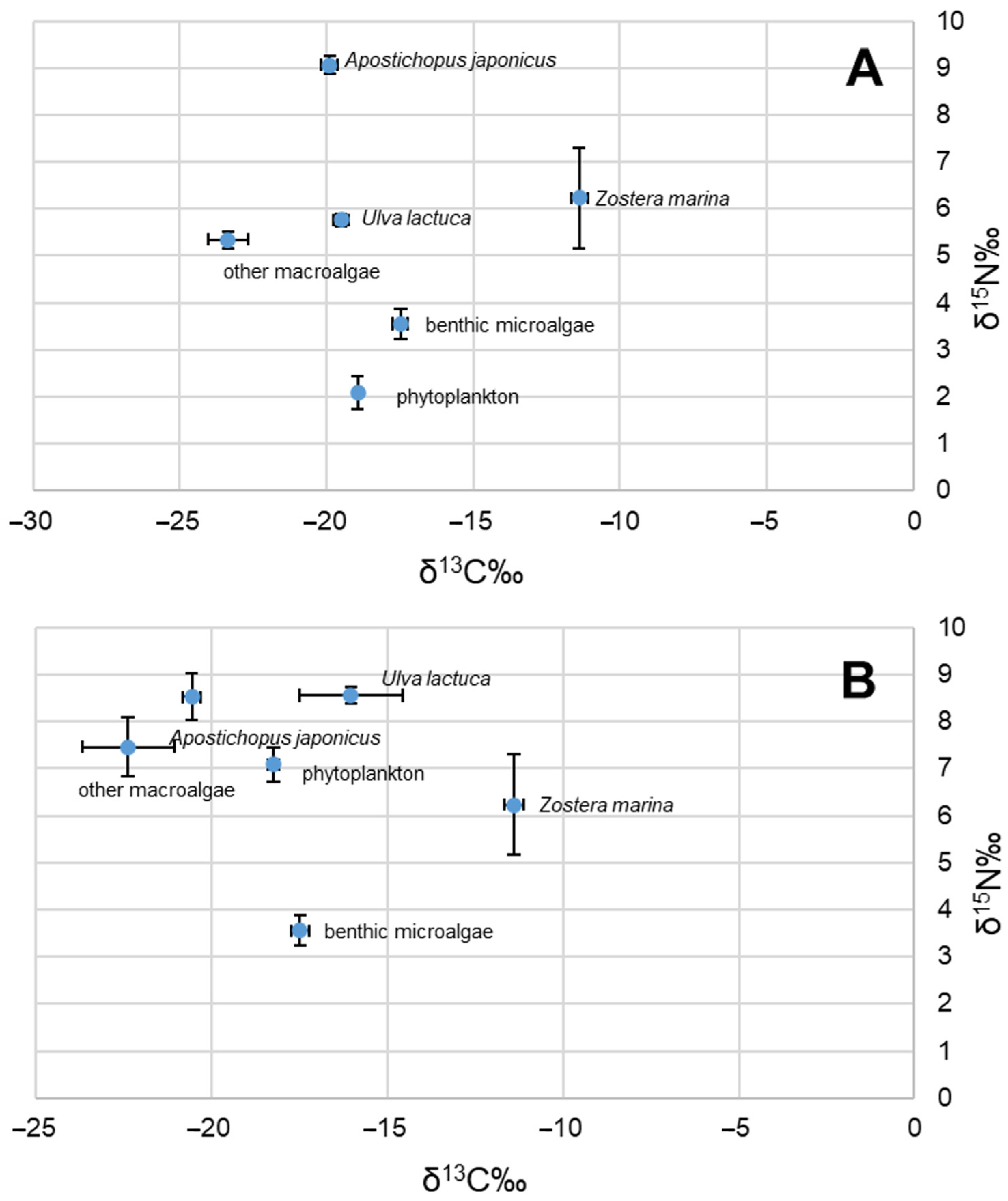


| Sampling Date | Apostichopus japonicus (n = 3) | Phytoplankton (n = 3) | Zostera marina (n = 3) | Ulva lactuca (n = 3) | Other Macroalgae (n = 3) | Benthic Microalgae (n = 3) | |
|---|---|---|---|---|---|---|---|
| δ13C values (‰) | September 2020 | −19.9 ± 0.28 | −18.94 ± 0.09 | −11.39 ± 0.28 * | −19.51 ± 0.25 | −23.37 ± 0.67 | −17.48 ± 0.26 + |
| March 2021 | −20.56 ± 0.25 | −18.24 ± 0.17 | −16.04 ± 1.46 | −22.38 ± 1.31 | |||
| δ15N values (‰) | September 2020 | 9.06 ± 0.19 | 2.09 ± 0.35 | 6.23 ± 1.07 * | 5.76 ± 0.12 | 5.34 ± 0.17 | 3.55 ± 0.32 + |
| March 2021 | 8.52 ± 0.49 | 7.09 ± 0.36 | 8.56 ± 0.17 | 7.46 ± 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Wang, X.; Yue, S.; Zhang, X.; Zhang, Y.; Lin, C.; Zhou, Y. Coastal Ecological Connectivity between Seagrass Bed and Marine Ranching 30 km Apart: A Case STUDY of Apostichopus japonicus Feeding on Seagrass Debris in the Bohai Sea, China. Sustainability 2024, 16, 2944. https://doi.org/10.3390/su16072944
Xu S, Wang X, Yue S, Zhang X, Zhang Y, Lin C, Zhou Y. Coastal Ecological Connectivity between Seagrass Bed and Marine Ranching 30 km Apart: A Case STUDY of Apostichopus japonicus Feeding on Seagrass Debris in the Bohai Sea, China. Sustainability. 2024; 16(7):2944. https://doi.org/10.3390/su16072944
Chicago/Turabian StyleXu, Shaochun, Xu Wang, Shidong Yue, Xiaomei Zhang, Yunling Zhang, Chenggang Lin, and Yi Zhou. 2024. "Coastal Ecological Connectivity between Seagrass Bed and Marine Ranching 30 km Apart: A Case STUDY of Apostichopus japonicus Feeding on Seagrass Debris in the Bohai Sea, China" Sustainability 16, no. 7: 2944. https://doi.org/10.3390/su16072944
APA StyleXu, S., Wang, X., Yue, S., Zhang, X., Zhang, Y., Lin, C., & Zhou, Y. (2024). Coastal Ecological Connectivity between Seagrass Bed and Marine Ranching 30 km Apart: A Case STUDY of Apostichopus japonicus Feeding on Seagrass Debris in the Bohai Sea, China. Sustainability, 16(7), 2944. https://doi.org/10.3390/su16072944







