Optimization of Coagulation to Remove Turbidity from Surface Water Using Novel Nature-Based Plant Coagulant and Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
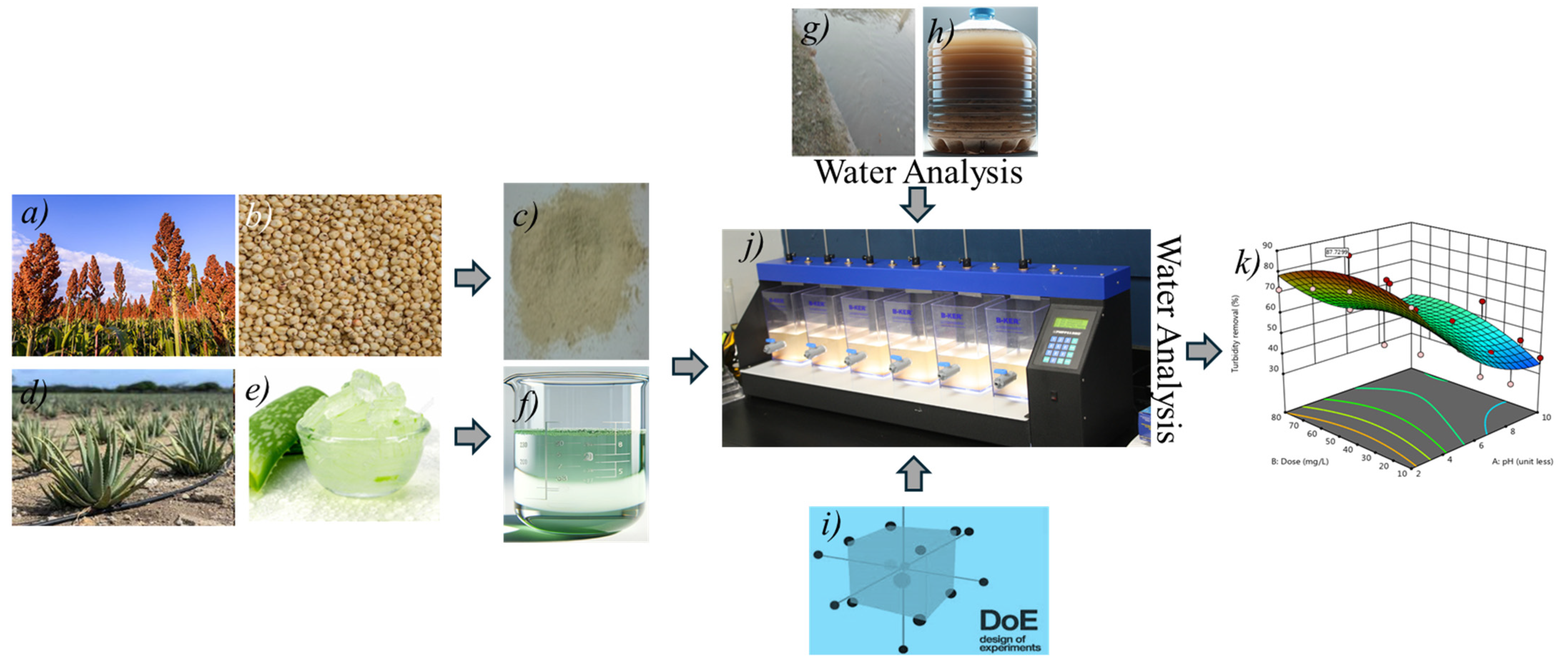
2.1. Sample Collection and Characterization
2.2. Preparation of Coagulants
2.2.1. Preparation of Stock Solution for Sorghum Coagulant
2.2.2. Preparation of Coagulant Aid
2.3. Design of Experiment Using Response Surface Methodology (RSM)
2.4. Jar Test
2.5. Fourier Transform Infrared Spectroscopy (FTIR) of Coagulant, Coagulant Aid, and Suspended Solids
2.6. Scanning Electron Microscopy (SEM) of Coagulant, Coagulant Aid, and Sludge
2.7. Statistical Analysis
3. Results and Discussion
3.1. Turbidity Removal by Sorghum (Great Millet)
3.1.1. Fitting of Statistical Models on Experimental Data for Sorghum
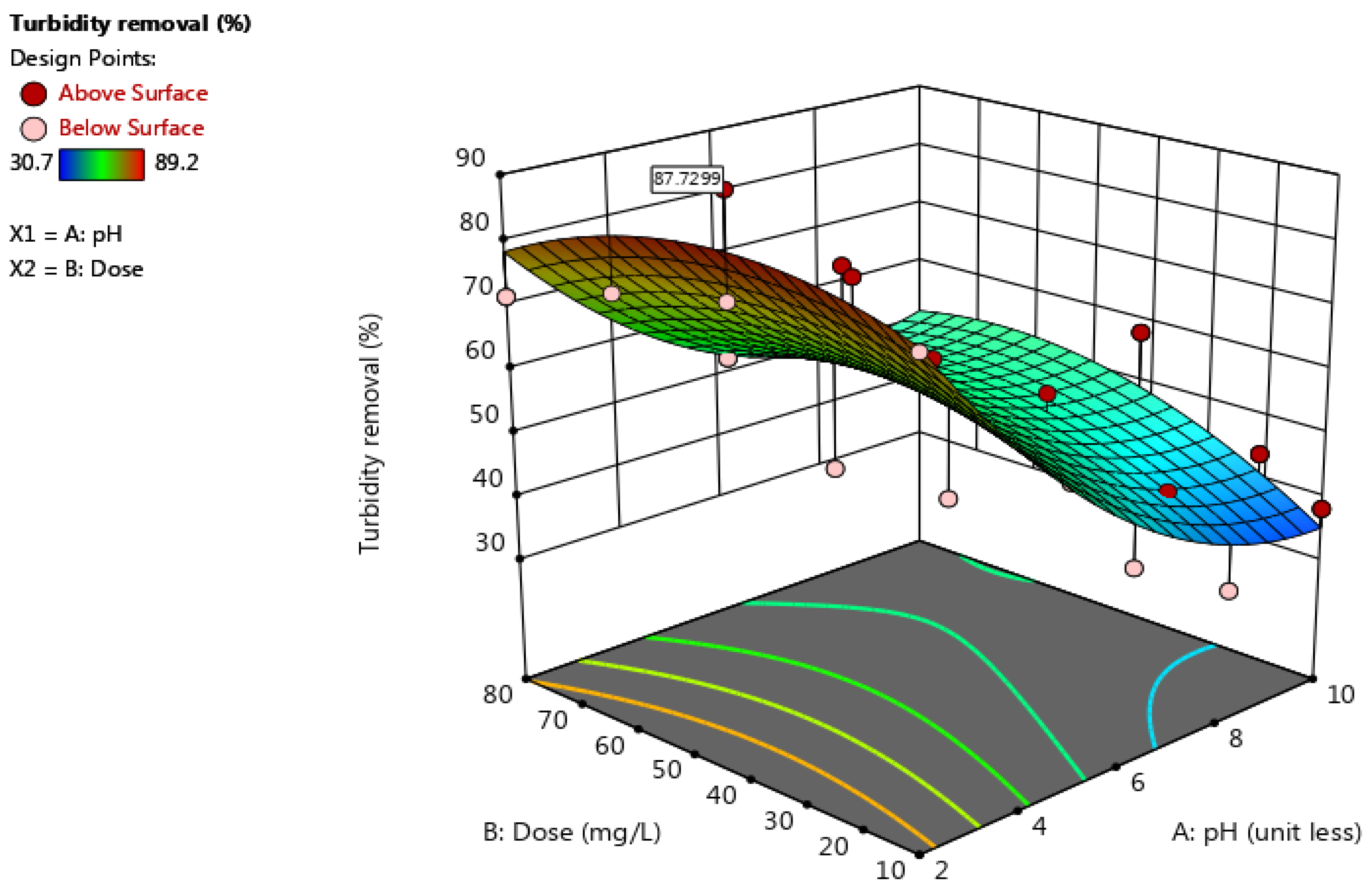
3.1.2. 3D Response Surface Plot for Sorghum Performance
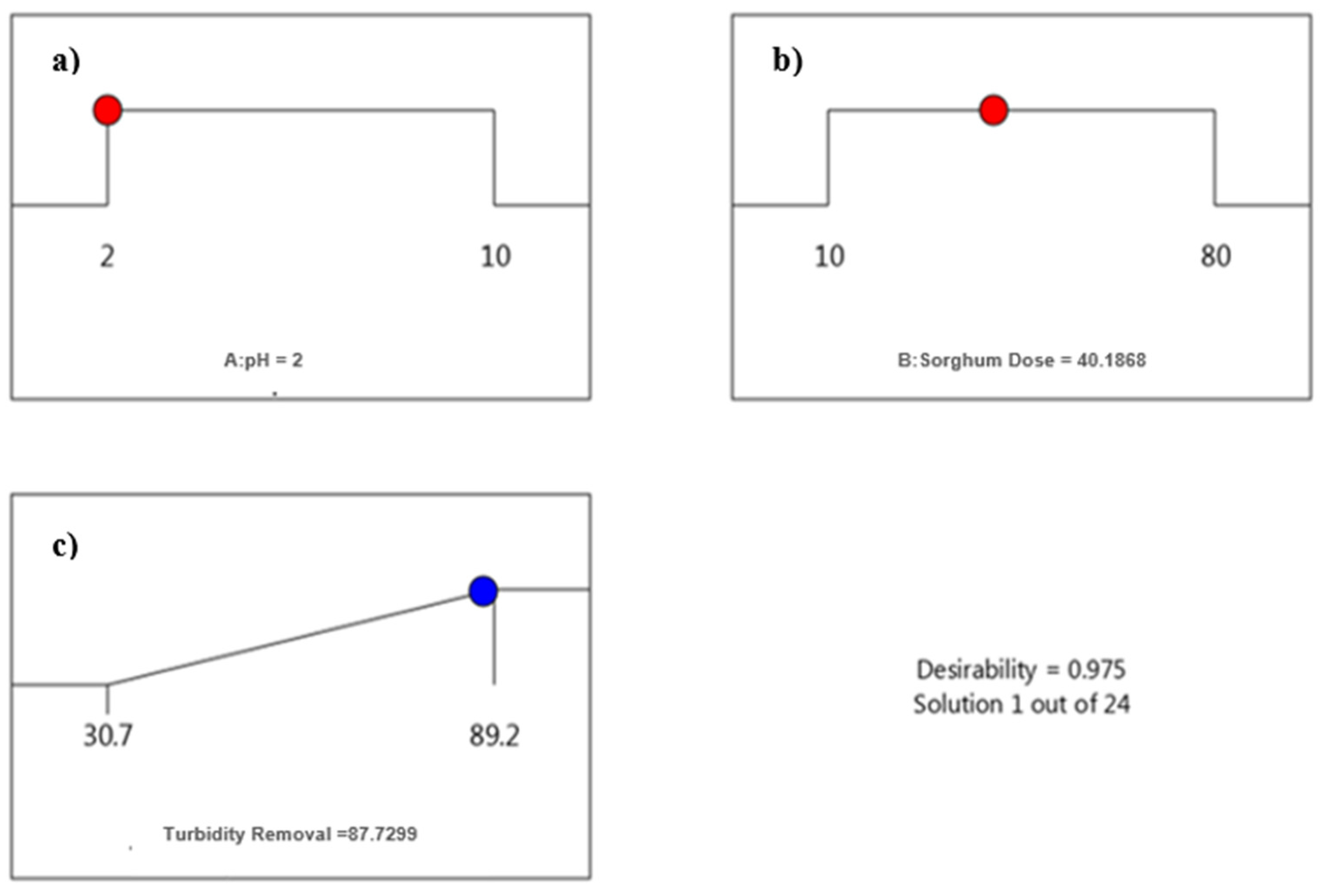
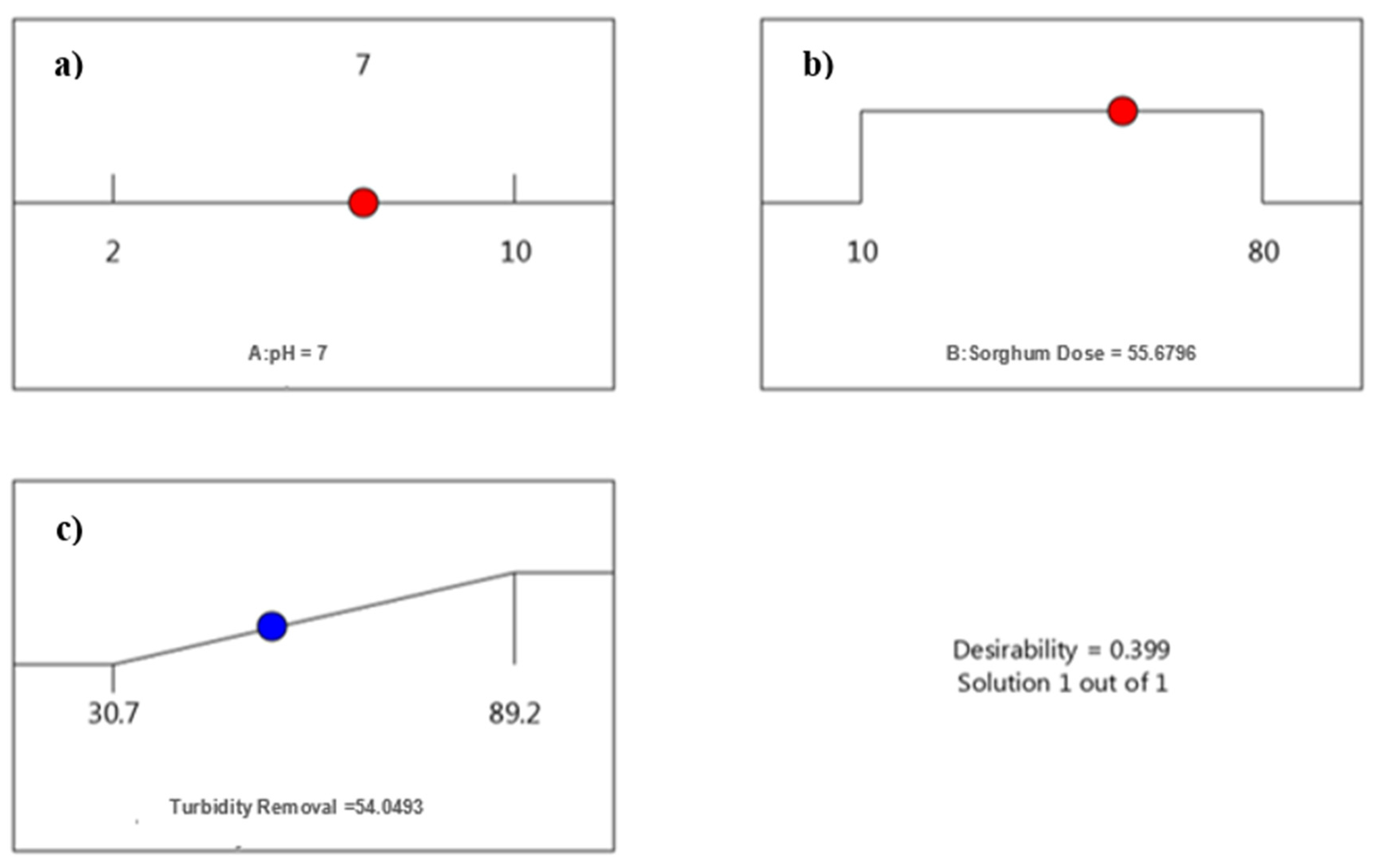
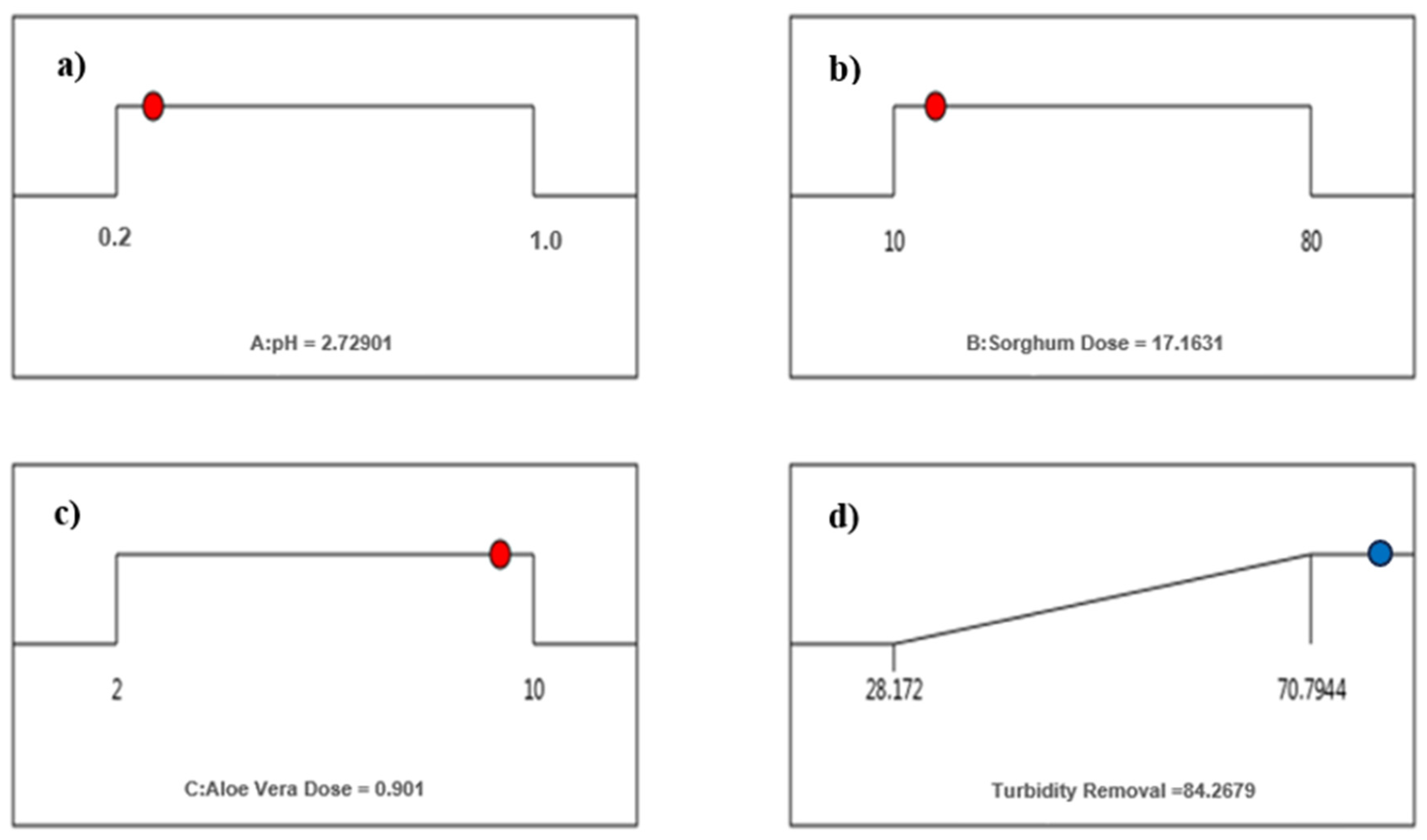
3.1.3. Optimization of pH and Dose for Sorghum
3.1.4. Analysis of Variance (ANOVA)
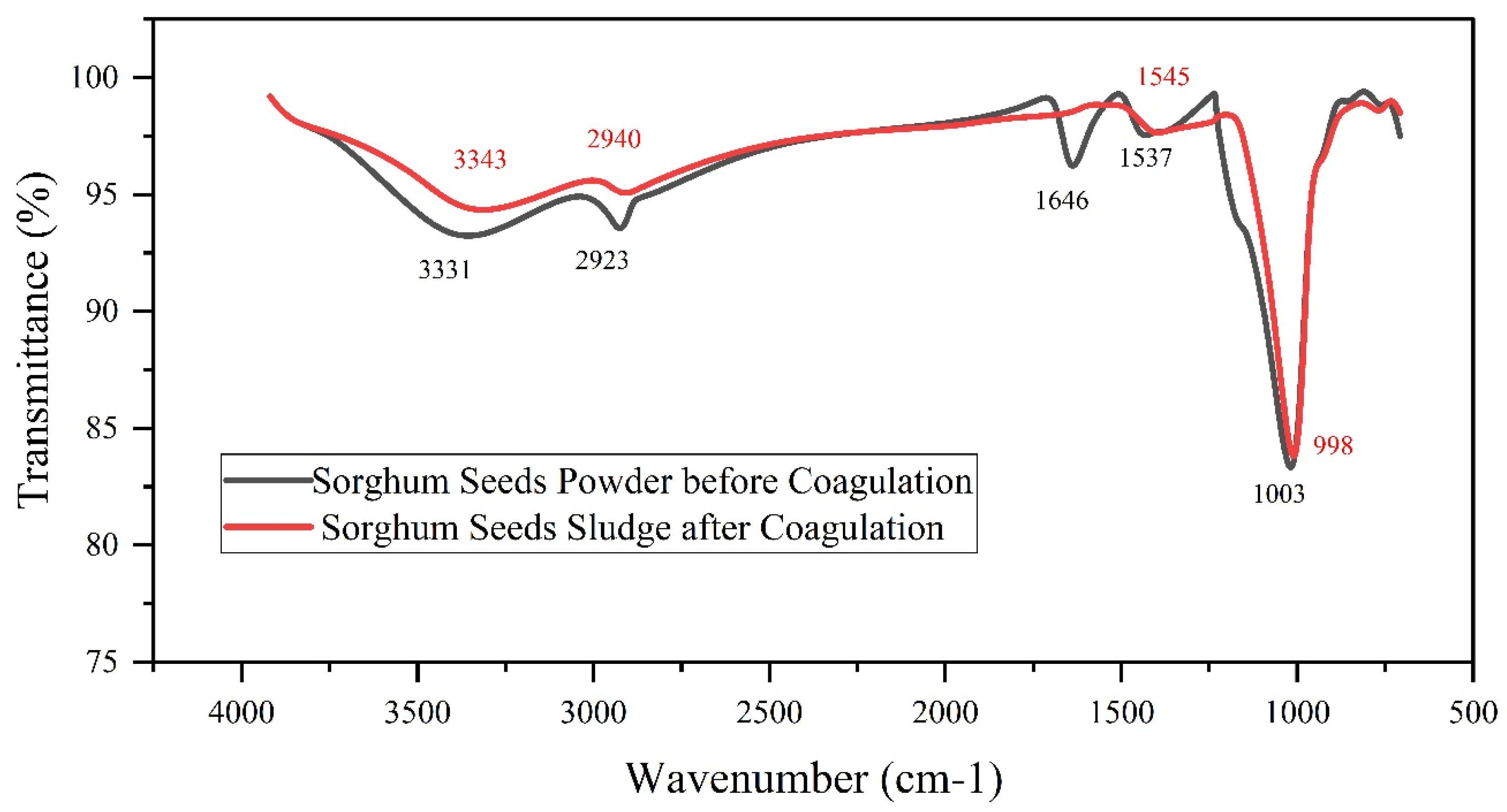
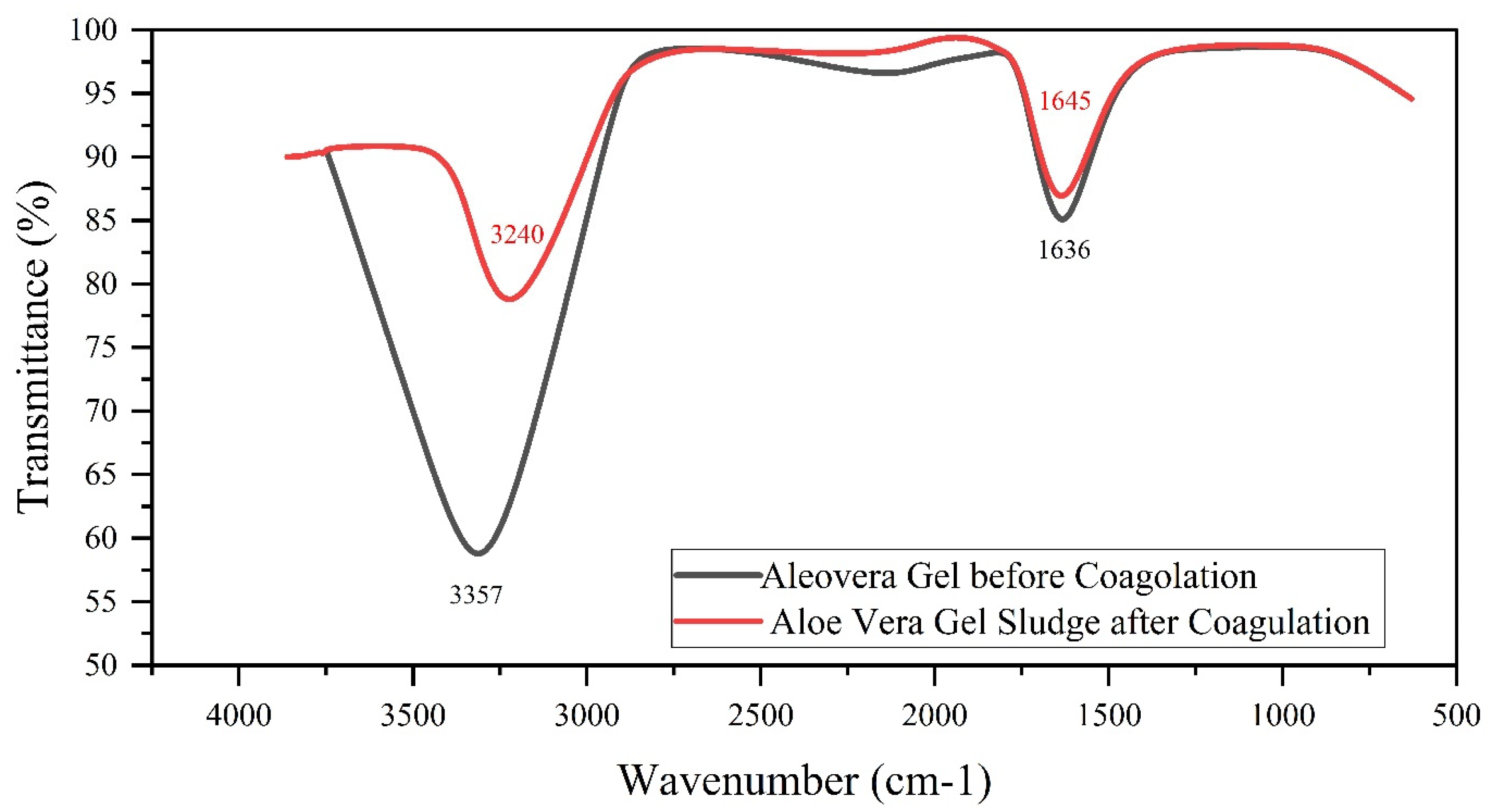
3.2. Identification of Functional Groups through FTIR Analysis
3.2.1. FTIR Analysis of Sorghum Powder
3.2.2. FTIR Analysis of Aloe Vera Gel
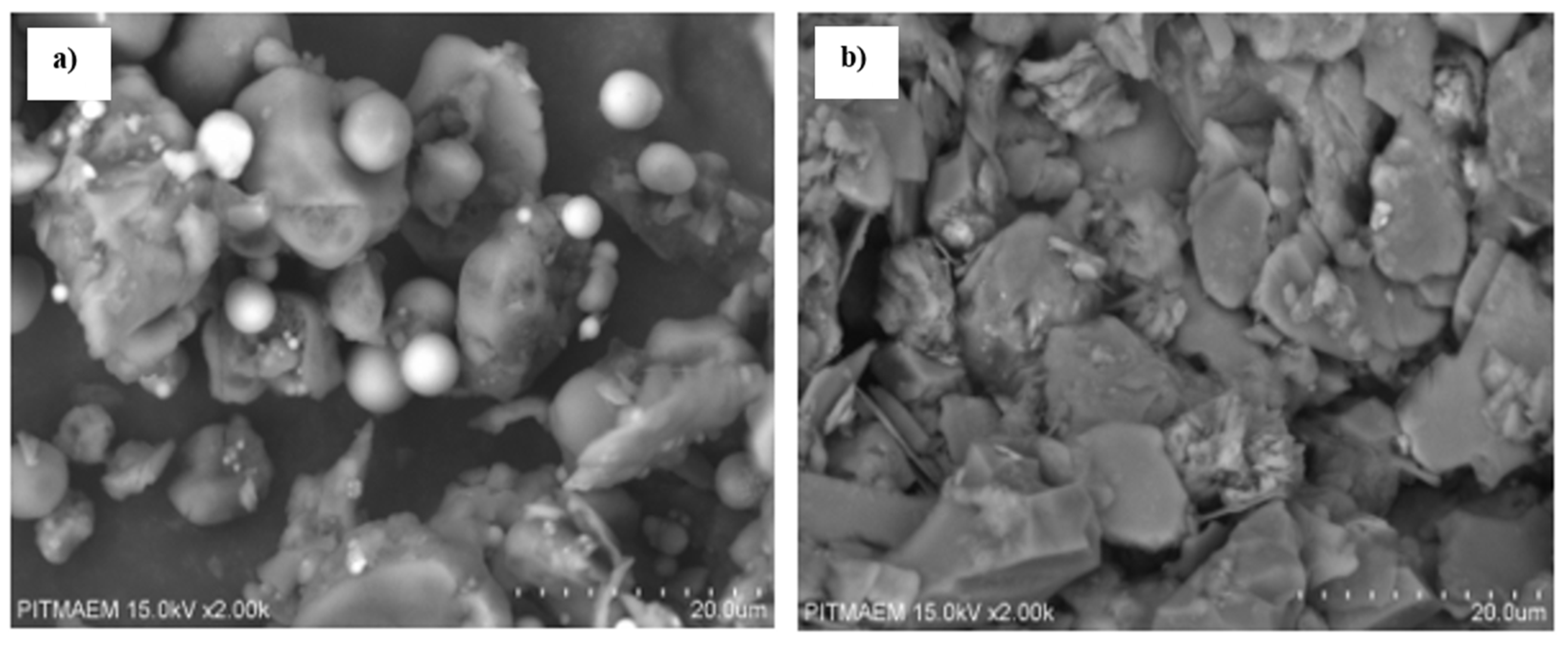
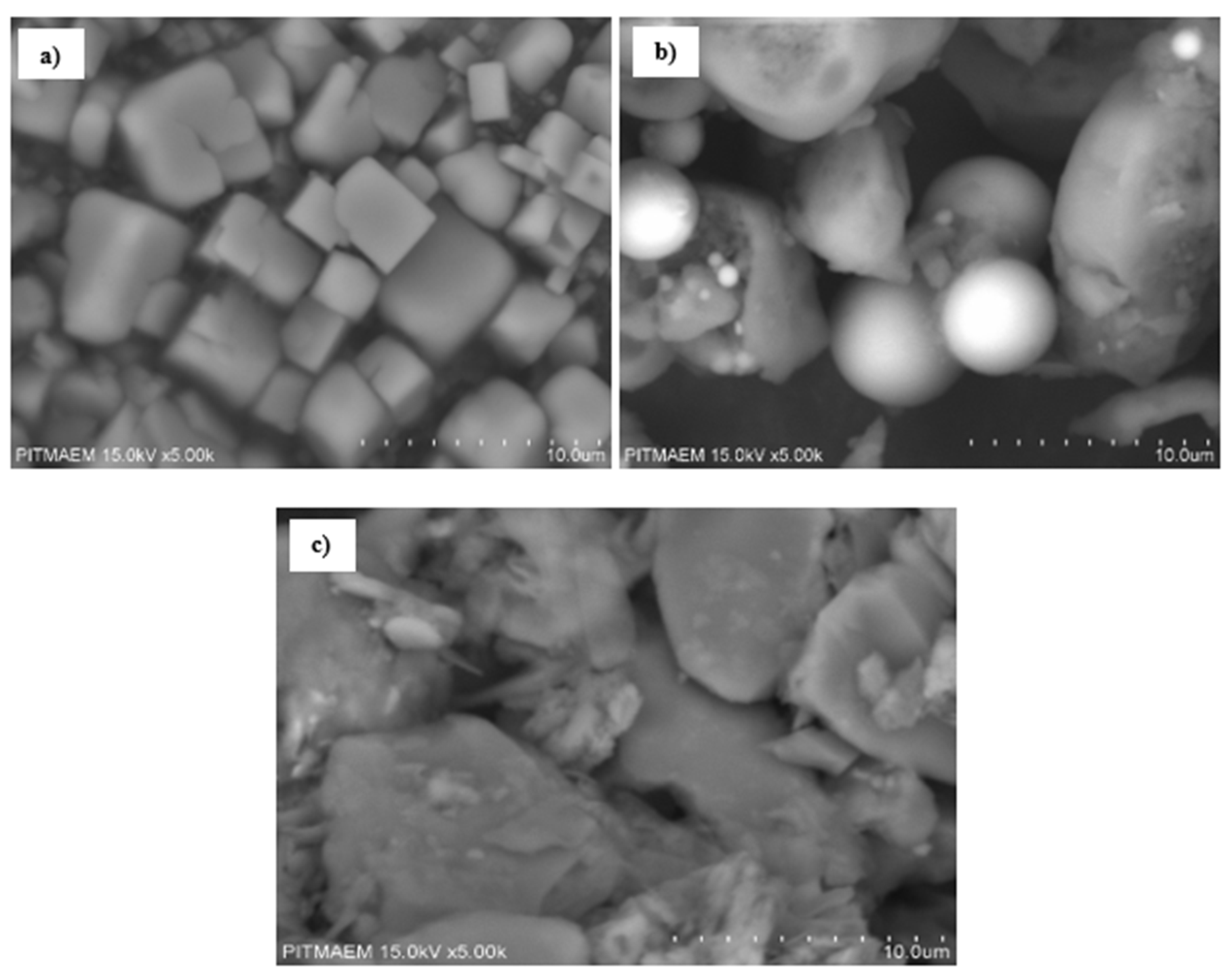
3.2.3. SEM Analysis
3.3. Turbidity Removal by Sorghum with Coagulant Aid (Aloe Vera)
3.3.1. Statistical Analysis of Experimental Data (Sorghum with Coagulant Aid)
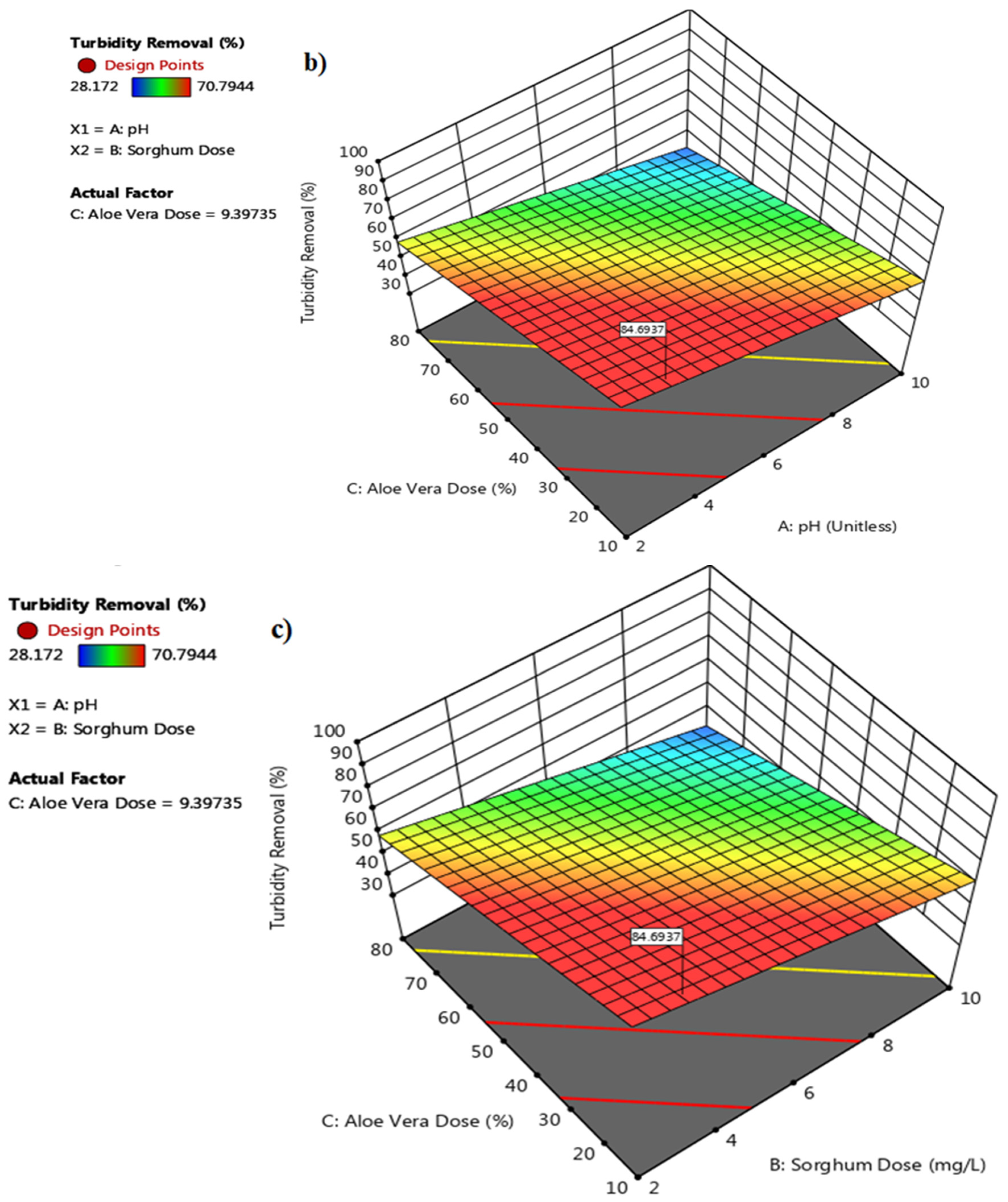
3.3.2. 3D Response Surface Plot for Sorghum with Aloe Vera
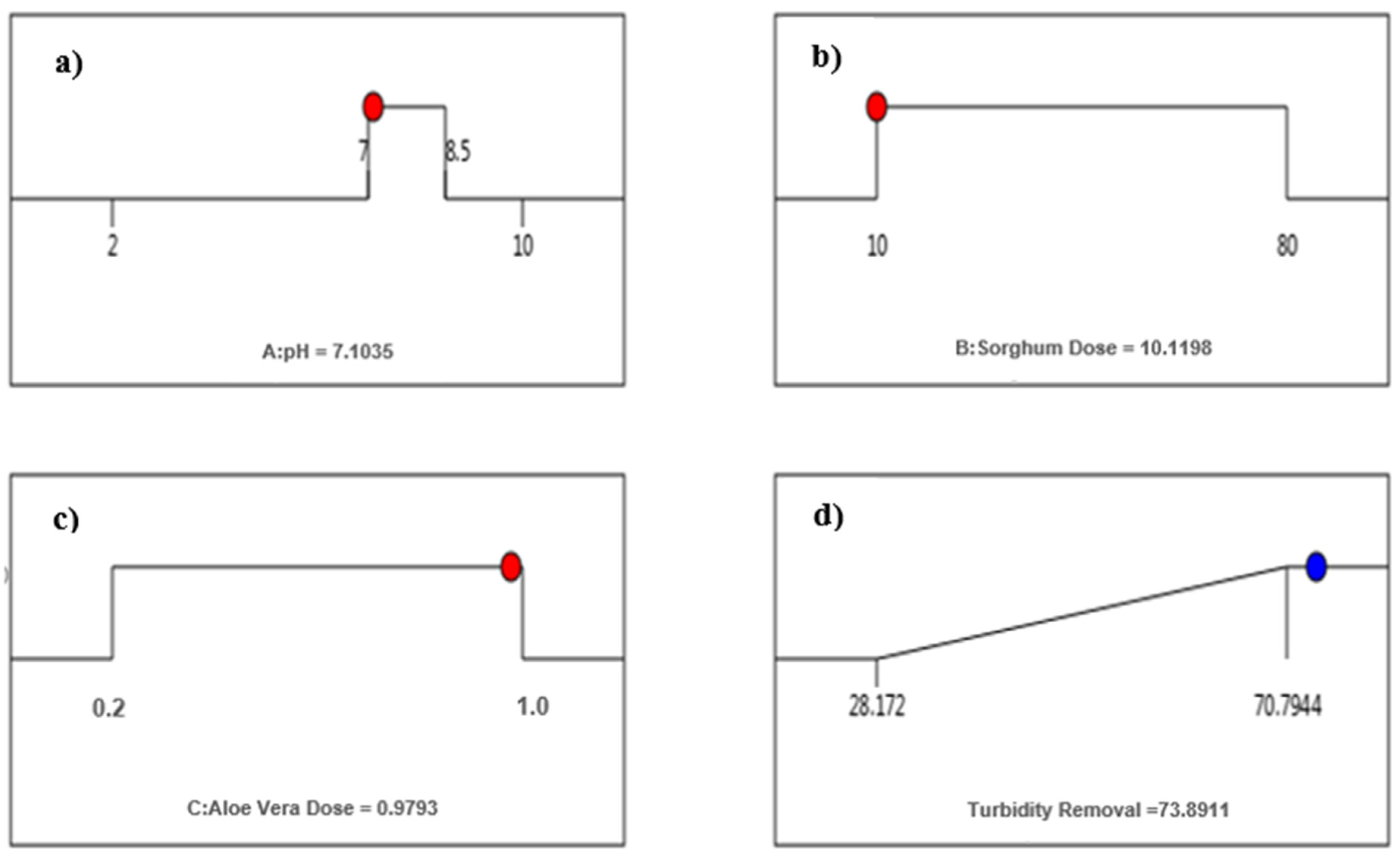
3.3.3. Optimization of pH and Dose for Sorghum with Aloe Vera
3.3.4. Analysis of Variance (ANOVA)
3.3.5. SEM Analysis
3.4. Cost of Treatment
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, D.; Baumgartner, D.; Gerba, C.; Fitzsimmons, K. Surface water pollution. In Environmental and Pollution Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 261–292. [Google Scholar]
- Al-Weshah, R.A.-M. Jordan’s water resources: Technical perspective. Water Int. 1992, 17, 124–132. [Google Scholar] [CrossRef]
- Tabraiz, S.; Nasreen, S.; Qureshi, L.A.; Zeeshan, M.; Ahmad, I.; Ahmed, S.; Hassan, Z. Sewage land disposal and unpaved drains: Threat to groundwater quality. Desalination Water Treat. 2016, 57, 20464–20469. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ali, O.; Tabraiz, S.; Ruhl, A.S. Seasonal variations in dissolved organic matter concentration and composition in an outdoor system for bank filtration simulation. J. Environ. Sci. 2024, 135, 252–261. [Google Scholar] [CrossRef]
- Asharuddin, S.M.; Othman, N.; Zin, N.S.M.; Tajarudin, H.A.; Din, M.F.M. Flocculation and antibacterial performance of dual coagulant system of modified cassava peel starch and alum. J. Water Process Eng. 2019, 31, 100888. [Google Scholar] [CrossRef]
- Kakoi, B.; Kaluli, J.W.; Ndiba, P.; Thiong’o, G. Banana pith as a natural coagulant for polluted river water. Ecol. Eng. 2016, 95, 699–705. [Google Scholar] [CrossRef]
- Arulmathi, P.; Jeyaprabha, C.; Sivasankar, P.; Rajkumar, V. Treatment of Textile Wastewater by Coagulation–Flocculation Process Using Gossypium herbaceum and Polyaniline Coagulants. CLEAN Soil Air Water 2019, 47, 1800464. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Li, A.; Yang, H. Enhanced coagulation of low-turbidity micro-polluted surface water: Properties and optimization. J. Environ. Manag. 2019, 233, 739–747. [Google Scholar] [CrossRef]
- Asif, M.B.; Majeed, N.; Iftekhar, S.; Habib, R.; Fida, S.; Tabraiz, S. Chemically enhanced primary treatment of textile effluent using alum sludge and chitosan. Desalination Water Treat. 2016, 57, 7280–7286. [Google Scholar] [CrossRef]
- Khan, M.D.; Singh, A.; Khan, M.Z.; Tabraiz, S.; Sheikh, J. Current perspectives, recent advancements, and efficiencies of various dye-containing wastewater treatment technologies. J. Water Process Eng. 2023, 53, 103579. [Google Scholar] [CrossRef]
- Yin, C.-Y. Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem. 2010, 45, 1437–1444. [Google Scholar] [CrossRef]
- Saleem, M.; Bachmann, R.T. A contemporary review on plant-based coagulants for applications in water treatment. J. Ind. Eng. Chem. 2019, 72, 281–297. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Letterman, R.D. Factors regulating residual aluminium concentrations in treated waters. Environmetrics 1995, 6, 287–305. [Google Scholar] [CrossRef]
- Gebbie, P. A dummy’s guide to coagulants. In Proceedings of the 68th Annual Water Industry Engineers and Operators’ Conference, Orlando, FL, USA, 9–13 October 2005; pp. 75–83. [Google Scholar]
- Marobhe, N.J. Effectiveness of crude extract and purified protein from Vigna unguiculata seed in purification of charco dam water for drinking in Tanzania. Int. J. Environ. Sci. 2013, 4, 259–273. [Google Scholar]
- Narasiah, K.; Vogel, A.; Kramadhati, N. Coagulation of turbid waters using Moringa oleifera seeds from two distinct sources. Water Sci. Technol. Water Supply 2002, 2, 83–88. [Google Scholar] [CrossRef]
- Amran, A.H.; Zaidi, N.S.; Muda, K.; Loan, L.W. Effectiveness of natural coagulant in coagulation process: A review. Int. J. Eng. Sci. Technol. 2018, 7, 34–37. [Google Scholar]
- Miller, S.M.; Fugate, E.J.; Craver, V.O.; Smith, J.A.; Zimmerman, J.B. Toward understanding the efficacy and mechanism of Opuntia spp. as a natural coagulant for potential application in water treatment. Environ. Sci. Technol. 2008, 42, 4274–4279. [Google Scholar] [CrossRef] [PubMed]
- Chaibakhsh, N.; Ahmadi, N.; Zanjanchi, M.A. Use of Plantago major L. as a natural coagulant for optimized decolorization of dye-containing wastewater. Ind. Crops Prod. 2014, 61, 169–175. [Google Scholar] [CrossRef]
- Okuda, T.; Baes, A.U.; Nishijima, W.; Okada, M. Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution. Water Res. 2001, 35, 405–410. [Google Scholar] [CrossRef]
- Siddique, M.; Soomro, S.A.; Aziz, S.; Jatoi, A.S.; Mengal, A.; Mahar, H. Removal of Turbidty from Turbid Water by Bio-cogulant Prepared from Walnut Shell. J. Appl. Emerg. Sci. 2016, 6, 66–68. [Google Scholar]
- Asrafuzzaman, M.; Fakhruddin, A.; Hossain, M.A. Reduction of turbidity of water using locally available natural coagulants. ISRN Microbiol. 2011, 2011, 632189. [Google Scholar] [CrossRef]
- Choy, S.; Prasad, K.; Wu, T.; Ramanan, R. A review on common vegetables and legumes as promising plant-based natural coagulants in water clarification. Int. J. Environ. Sci. Technol. 2015, 12, 367–390. [Google Scholar] [CrossRef]
- Hussain, G.; Haydar, S. Exploring potential of pearl millet (Pennisetum glaucum) and black-eyed pea (Vigna unguiculata subsp. unguiculata) as bio-coagulants for water treatment. Desalination Water Treat. 2019, 143, 184–191. [Google Scholar] [CrossRef]
- Imran, S.; Bukhari, L.N.; Ashraf, M. Spatial and Temporal Trends in River Water Quality of Pakistan; Pakistan Council of Research in Water Resources: Lahore, Pakistan, 2018. [Google Scholar]
- Momeni, M.M.; Kahforoushan, D.; Abbasi, F.; Ghanbarian, S. Using chitosan/CHPATC as coagulant to remove color and turbidity of industrial wastewater: Optimization through RSM design. J. Environ. Manag. 2018, 211, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Zanil, M.F.B.; Ramzan, L.A.B. Optimization of Coagulation Tank Processes through Interval Fuzzy Type 2 Logic System: A Study of Turbidity Reduction. ASEAN J. Process Control 2023, 2, 1–16. [Google Scholar]
- Raj, S.; Mahanty, B.; Hait, S. Coagulative removal of polystyrene microplastics from aqueous matrices using FeCl3-chitosan system: Experimental and artificial neural network modeling. J. Hazard. Mater. 2024, 468, 133818. [Google Scholar] [CrossRef] [PubMed]
- Singgalen, Y.A. Analysis and Design of Natural Spring Water Preservation and Monitoring System Using Rapid Application Development. J. Inf. Syst. Inform. 2024, 6, 118–135. [Google Scholar]
- Alenazi, M.; Hashim, K.S.; Hassan, A.A.; Muradov, M.; Kot, P.; Abdulhadi, B. Turbidity removal using natural coagulants derived from the seeds of strychnos potatorum: Statistical and experimental approach. IOP Conf. Ser. Mater. Sci. Eng. 2020, 888, 012064. [Google Scholar] [CrossRef]
- Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Raghunandan, M.E.; Ramanan, R.N. Utilization of plant-based natural coagulants as future alternatives towards sustainable water clarification. J. Environ. Sci. 2014, 26, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Doko, D. Biophysical Characterization and NMR Analysis of the PDI Fragment B’xa’c; University of Kent: Canterbury, UK, 2012. [Google Scholar]
- Ni, F.; Peng, X.; He, J.; Yu, L.; Zhao, J.; Luan, Z. Preparation and characterization of composite bioflocculants in comparison with dual-coagulants for the treatment of kaolin suspension. Chem. Eng. J. 2012, 213, 195–202. [Google Scholar] [CrossRef]
- Sellami, M.; Zarai, Z.; Khadhraoui, M.; Jdidi, N.; Leduc, R.; Ben Rebah, F. Cactus juice as bioflocculant in the coagulation–flocculation process for industrial wastewater treatment: A comparative study with polyacrylamide. Water Sci. Technol. 2014, 70, 1175–1181. [Google Scholar] [CrossRef]
- Szilagyi, I.; Polomska, A.; Citherlet, D.; Sadeghpour, A.; Borkovec, M. Charging and aggregation of negatively charged colloidal latex particles in the presence of multivalent oligoamine cations. J. Colloid Interface Sci. 2013, 392, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Novel magnetic amine functionalized carbon nanotube/metal-organic framework nanocomposites: From green ultrasound-assisted synthesis to detailed selective pollutant removal modelling from binary systems. J. Hazard. Mater. 2019, 368, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Fatombi, J.; Lartiges, B.; Aminou, T.; Barres, O.; Caillet, C. A natural coagulant protein from copra (Cocos nucifera): Isolation, characterization, and potential for water purification. Sep. Purif. Technol. 2013, 116, 35–40. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Akram, W.; Garud, N. Design expert as a statistical tool for optimization of 5-ASA-loaded biopolymer-based nanoparticles using Box Behnken factorial design. Future J. Pharm. Sci. 2021, 7, 146. [Google Scholar] [CrossRef]
- Halder, A. Bio-coagulants, a substitute of chemical coagulants. J. Adv. Sci. Res. 2021, 12, 58–67. [Google Scholar] [CrossRef]

| Parameters | Units | Values |
|---|---|---|
| pH | - | 7.7 ± 1 |
| Turbidity | NTU | 452 ± 45 |
| Conductivity | µS/cm | 230 ± 2 |
| Total alkalinity | mg/L as CaCO3 | 57.3 ± 7 |
| Total hardness | mg/L as CaCO3 | 221 ± 10 |
| Calcium (Ca2+) | mg/L | 49 ± 2 |
| Magnesium (Mg2+) | mg/L | 24.3 ± 3 |
| Chlorides (Cl1−) | mg/L | 41.7 ± 1 |
| Sulfates (SO42−) | mg/L | 33.2 ± 4 |
| Phosphates (PO43−) | mg/L | 7.89 ± 1 |
| Nitrates (NO31−) | mg/L | 11.3 ± 6 |
| Factor | Name | Unit | Type | Coded Low | Coded High | Factors Level | |
|---|---|---|---|---|---|---|---|
| 1st Trial | A | pH | unit less | Numeric | −1 ↔ 2.00 | +1 ↔ 10.00 | 2, 4, 6, 8, 10 |
| B | Sorghum Dose | mg/L | Numeric | −1 ↔ 10.00 | +1 ↔ 80.00 | 10, 20, 40, 60, 80 | |
| 2nd Trail | A | pH | unit less | Numeric | −1 ↔ 2.00 | +1 ↔ 10.00 | 2, 4, 6, 8, 10 |
| B | Sorghum Dose | mg/L | Numeric | −1 ↔ 10.00 | +1 ↔ 80.00 | 10, 20, 40, 60, 80 | |
| C | Aloe Vera Dose | % | Numeric | −1 ↔ 0.20 | +1 ↔ 1.00 | 0.2, 0.4, 0.6, 0.8, 1.0 |
| Experimental Run | Factors | Initial Turbidity T-1 | Response | ||
|---|---|---|---|---|---|
| A pH | B Dose | Residual Turbidity T-2 | Turbidity Removal | ||
| Units | - | mg/L | NTU | NTU | NTU (%) |
| 1 | 10 | 40 | 500 | 208.9 | 58.2 |
| 2 | 2 | 60 | 359 | 82.5 | 77.0 |
| 3 | 2 | 20 | 368 | 70.6 | 80.8 |
| 4 | 10 | 10 | 440 | 272.3 | 38.1 |
| 5 | 6 | 10 | 435 | 260 | 40.2 |
| 6 | 10 | 20 | 488 | 274 | 43.9 |
| 7 | 4 | 60 | 450 | 52.6 | 88.3 |
| 8 | 6 | 60 | 420 | 252 | 40.0 |
| 9 | 8 | 40 | 610 | 286.7 | 53.0 |
| 10 | 4 | 10 | 476 | 164.4 | 65.5 |
| 11 | 8 | 10 | 540 | 374 | 30.7 |
| 12 | 6 | 40 | 426 | 250 | 41.3 |
| 13 | 4 | 40 | 420 | 76.8 | 81.7 |
| 14 | 8 | 60 | 474 | 222 | 53.2 |
| 15 | 2 | 80 | 298 | 84.9 | 71.5 |
| 16 | 2 | 10 | 395 | 72.3 | 81.7 |
| 17 | 4 | 80 | 485 | 130 | 73.2 |
| 18 | 6 | 80 | 432 | 204.6 | 52.6 |
| 19 | 6 | 20 | 420 | 210 | 50.0 |
| 20 | 4 | 20 | 470 | 138.7 | 70.5 |
| 21 | 10 | 60 | 540 | 278 | 48.5 |
| 22 | 10 | 80 | 523 | 282 | 46.1 |
| 23 | 2 | 40 | 352 | 37.9 | 89.2 |
| 24 | 8 | 80 | 474 | 209 | 55.9 |
| 25 | 8 | 20 | 434 | 246.4 | 43.2 |
| Model | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | PRESS | |
|---|---|---|---|---|---|---|
| Linear | 11.11 | 0.6307 | 0.5971 | 0.5437 | 3355.37 | |
| 2FI | 11.00 | 0.6546 | 0.6052 | 0.5618 | 3222.62 | |
| Quadratic | 9.50 | 0.7667 | 0.7053 | 0.6244 | 2762.56 | Suggested |
| Cubic | 8.93 | 0.8372 | 0.7395 | 0.6399 | 2648.56 | |
| Quartic | 7.06 | 0.9323 | 0.8375 | 0.5972 | 2962.05 | |
| Fifth | 7.05 | 0.9595 | 0.8380 | −1.9686 | 21,831.96 | Aliased |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 5638.51 | 5 | 1127.70 | 12.49 | <0.0001 | significant |
| A: pH | 4229.80 | 1 | 4229.80 | 46.84 | <0.0001 | significant |
| B: dose | 131.66 | 1 | 131.66 | 1.46 | 0.2421 | |
| AB | 175.61 | 1 | 175.61 | 1.94 | 0.1793 | |
| A2 | 609.77 | 1 | 609.77 | 6.75 | 0.0176 | significant |
| B2 | 214.74 | 1 | 214.74 | 2.38 | 0.1396 | |
| Residual | 1715.81 | 19 | 90.31 |
| Experimental Run | Factors | Initial Turbidity T-1 | Response | |||
|---|---|---|---|---|---|---|
| A pH | B Dose Sorghum | C Dose Aloe Vera | Residual Turbidity T-2 | Turbidity Removal | ||
| Units | - | mg/L | ml/100 mL | NTU | NTU | NTU (%) |
| 1 | 10 | 40 | 0.6 | 400 | 257 | 35.8 |
| 2 | 2 | 60 | 0.8 | 440 | 247 | 43.9 |
| 3 | 2 | 40 | 0.6 | 440 | 200 | 54.5 |
| 4 | 10 | 10 | 0.2 | 400 | 248 | 38.0 |
| 5 | 6 | 10 | 0.2 | 544 | 289 | 46.9 |
| 6 | 10 | 20 | 0.4 | 400 | 254 | 36.5 |
| 7 | 4 | 60 | 0.8 | 424 | 138 | 67.5 |
| 8 | 6 | 60 | 0.8 | 540 | 234 | 56.7 |
| 9 | 8 | 40 | 0.6 | 468 | 307 | 34.4 |
| 10 | 4 | 10 | 0.2 | 424 | 162 | 61.8 |
| 11 | 8 | 10 | 0.2 | 470 | 304 | 35.3 |
| 12 | 6 | 40 | 0.6 | 544 | 229 | 57.9 |
| 13 | 4 | 40 | 0.6 | 428 | 125 | 70.8 |
| 14 | 8 | 60 | 0.8 | 470 | 323 | 31.3 |
| 15 | 2 | 80 | 1.0 | 450 | 183 | 59.3 |
| 16 | 2 | 10 | 0.2 | 444 | 211 | 52.5 |
| 17 | 4 | 80 | 1.0 | 424 | 150 | 64.6 |
| 18 | 6 | 80 | 1.0 | 540 | 246 | 54.4 |
| 19 | 6 | 20 | 0.4 | 540 | 270 | 50.0 |
| 20 | 4 | 20 | 0.4 | 424 | 146 | 65.6 |
| 21 | 10 | 60 | 0.8 | 400 | 257 | 35.8 |
| 22 | 10 | 80 | 1.0 | 400 | 262 | 34.5 |
| 23 | 2 | 20 | 0.4 | 440 | 168 | 61.8 |
| 24 | 8 | 80 | 1.0 | 465 | 334 | 28.2 |
| 25 | 8 | 20 | 0.4 | 465 | 295 | 36.6 |
| Model | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | PRESS | |
|---|---|---|---|---|---|---|
| Linear | 8.98 | 0.5919 | 0.5336 | 0.4513 | 2276.07 | Suggested |
| 2FI | 9.62 | 0.5980 | 0.4640 | 0.3072 | 2873.76 | |
| Quadratic | 9.24 | 0.6498 | 0.5057 | 0.3040 | 2887.11 | Aliased |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 2455.50 | 3 | 818.50 | 10.15 | 0.0002 | Significant |
| A: pH | 2416.18 | 1 | 2416.18 | 29.97 | <0.0001 | Significant |
| B: Sorghum dose | 39.22 | 1 | 39.22 | 0.4865 | 0.4931 | |
| C: Aloe Vera dose | 38.31 | 1 | 38.31 | 0.4753 | 0.4981 | |
| Residual | 1692.78 | 21 | 80.61 |
| Optimum Doses | Unit Cost * USD/kg | Total Cost ** USD/1000 m3 of Water | |
|---|---|---|---|
| Trial 1 (Sorghum) | |||
| pH-2 | 40 mg/L | 0.70 | 50.89 |
| pH-7 | 55 mg/L | 0.70 | 59.89 |
| Trial 1 (Sorghum along with Aloe Vera) | |||
| pH-2.7 | 17 mg/L, 0.9% | 0.27 | 37.19 |
| pH-7 | 10 mg/L, 1.0% | 0.27 | 32.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzadi, F.; Haydar, S.; Tabraiz, S. Optimization of Coagulation to Remove Turbidity from Surface Water Using Novel Nature-Based Plant Coagulant and Response Surface Methodology. Sustainability 2024, 16, 2941. https://doi.org/10.3390/su16072941
Shahzadi F, Haydar S, Tabraiz S. Optimization of Coagulation to Remove Turbidity from Surface Water Using Novel Nature-Based Plant Coagulant and Response Surface Methodology. Sustainability. 2024; 16(7):2941. https://doi.org/10.3390/su16072941
Chicago/Turabian StyleShahzadi, Fakhara, Sajjad Haydar, and Shamas Tabraiz. 2024. "Optimization of Coagulation to Remove Turbidity from Surface Water Using Novel Nature-Based Plant Coagulant and Response Surface Methodology" Sustainability 16, no. 7: 2941. https://doi.org/10.3390/su16072941
APA StyleShahzadi, F., Haydar, S., & Tabraiz, S. (2024). Optimization of Coagulation to Remove Turbidity from Surface Water Using Novel Nature-Based Plant Coagulant and Response Surface Methodology. Sustainability, 16(7), 2941. https://doi.org/10.3390/su16072941







