Superabsorbent Hydrogels in the Agriculture and Reclamation of Degraded Areas
Abstract
1. Introduction
2. Polymers: Structure and Preparation
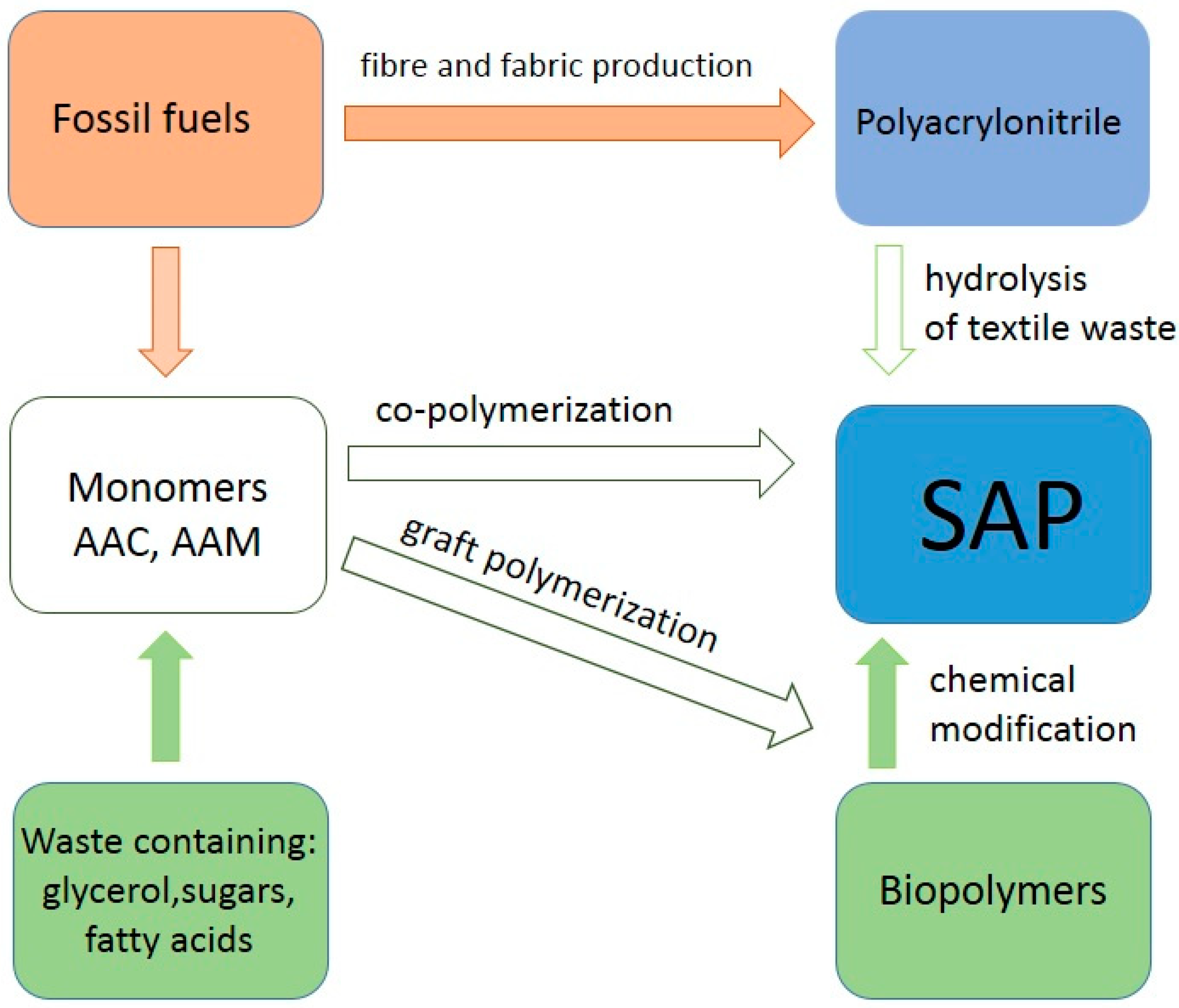
2.1. Chemical Composition of SAP
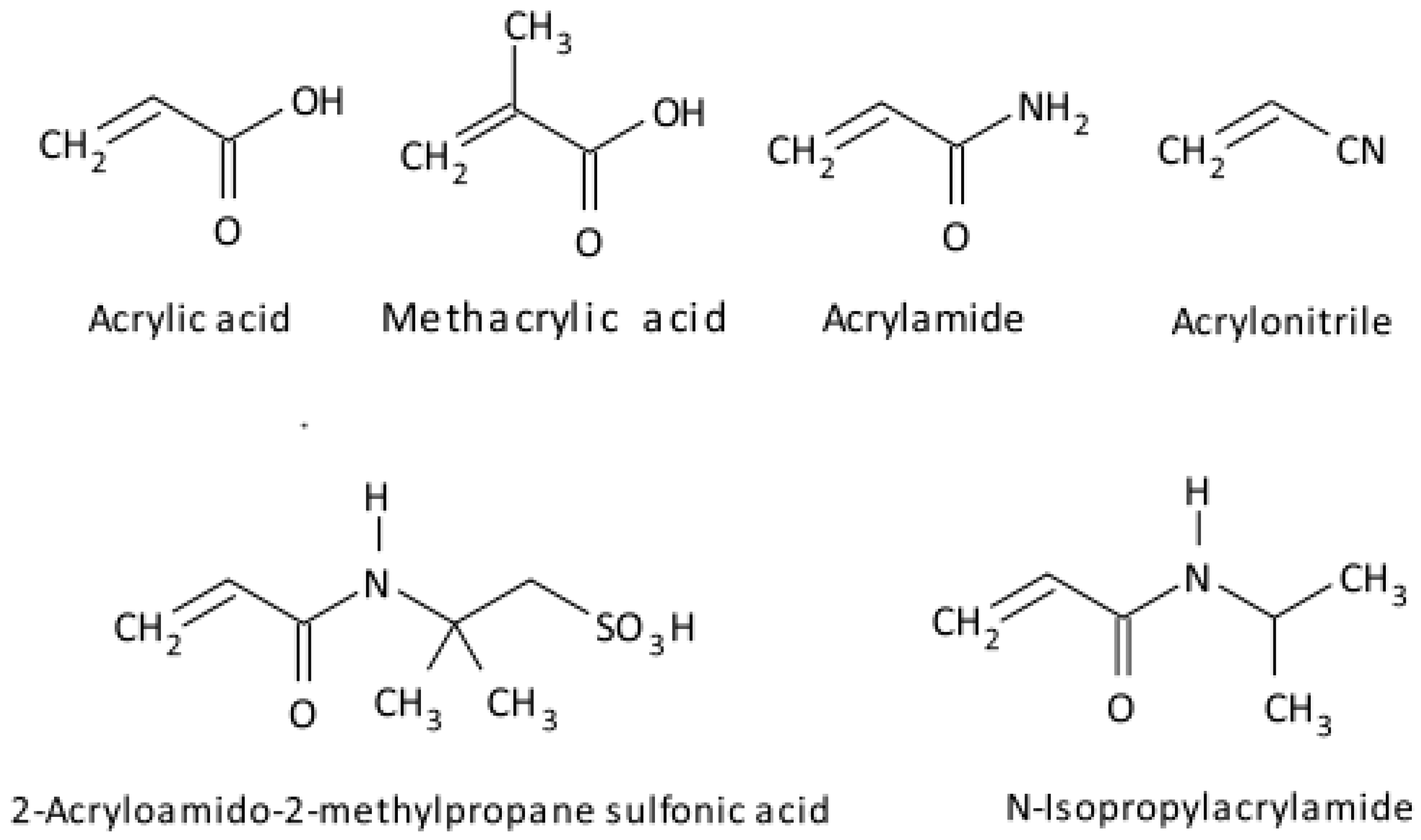
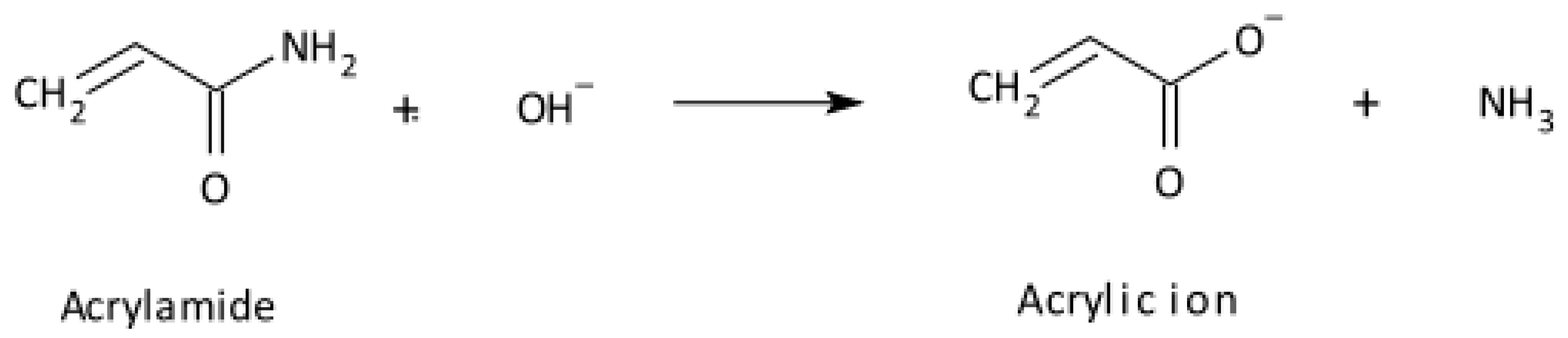
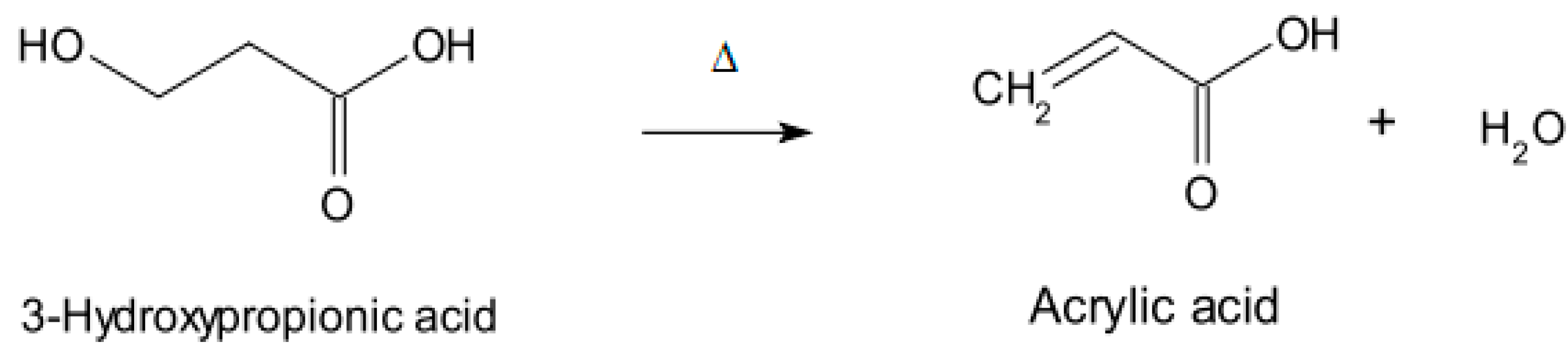
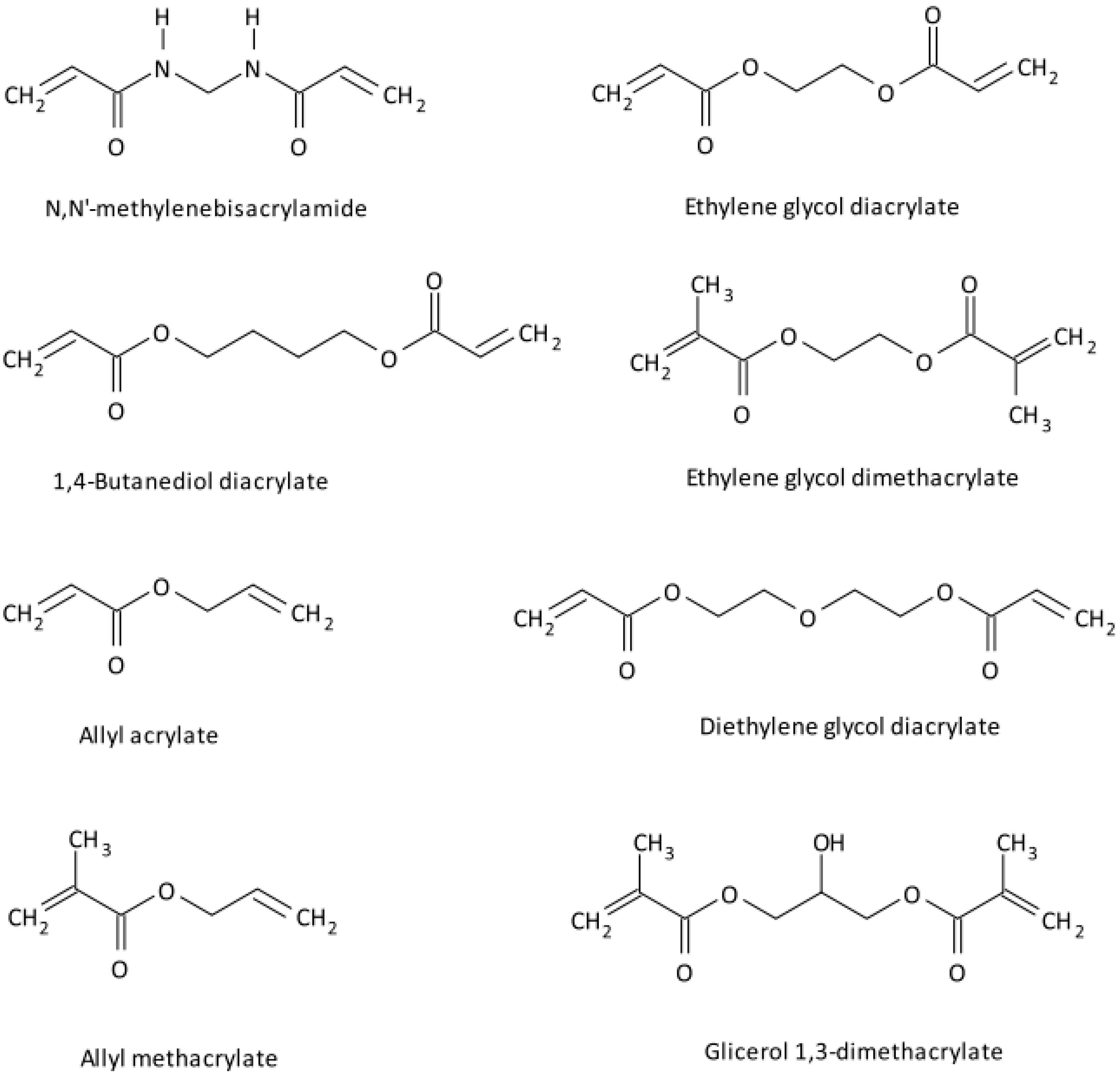
2.2. Vinyl Monomers
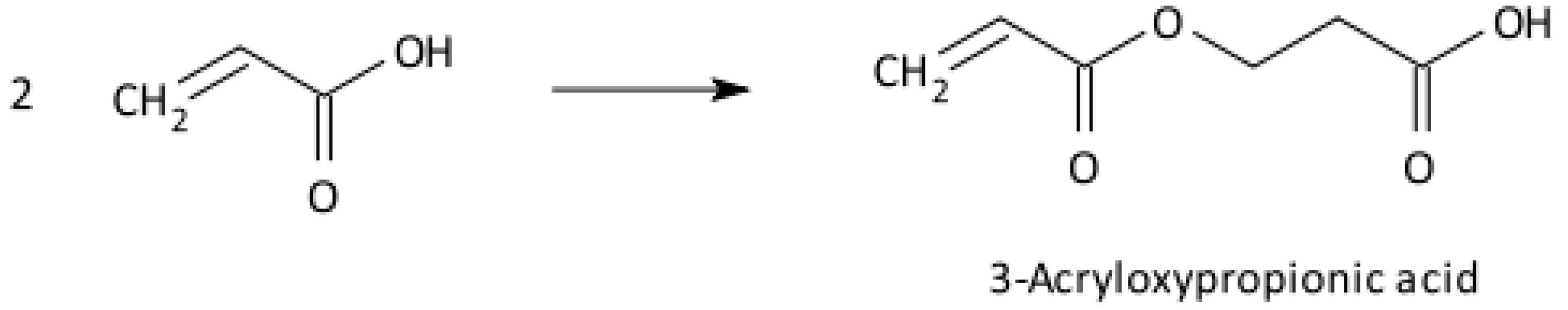
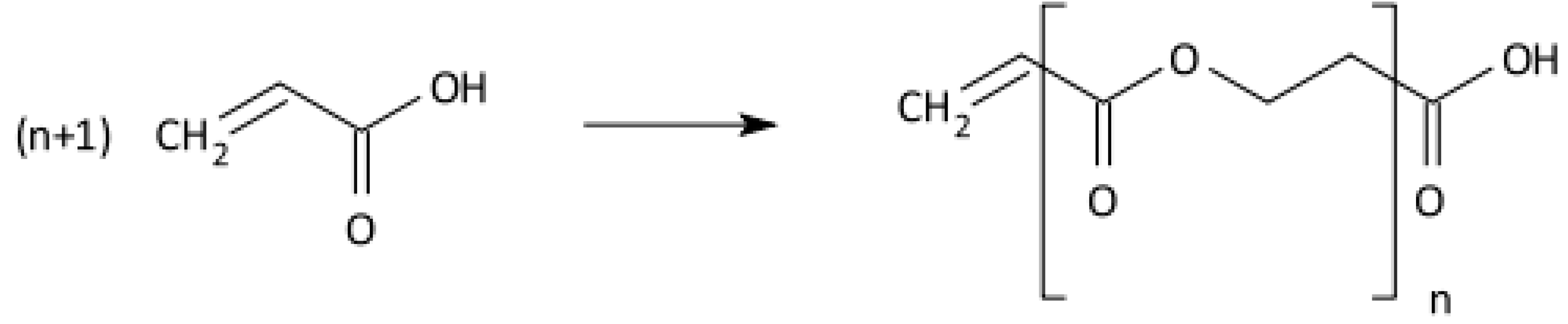
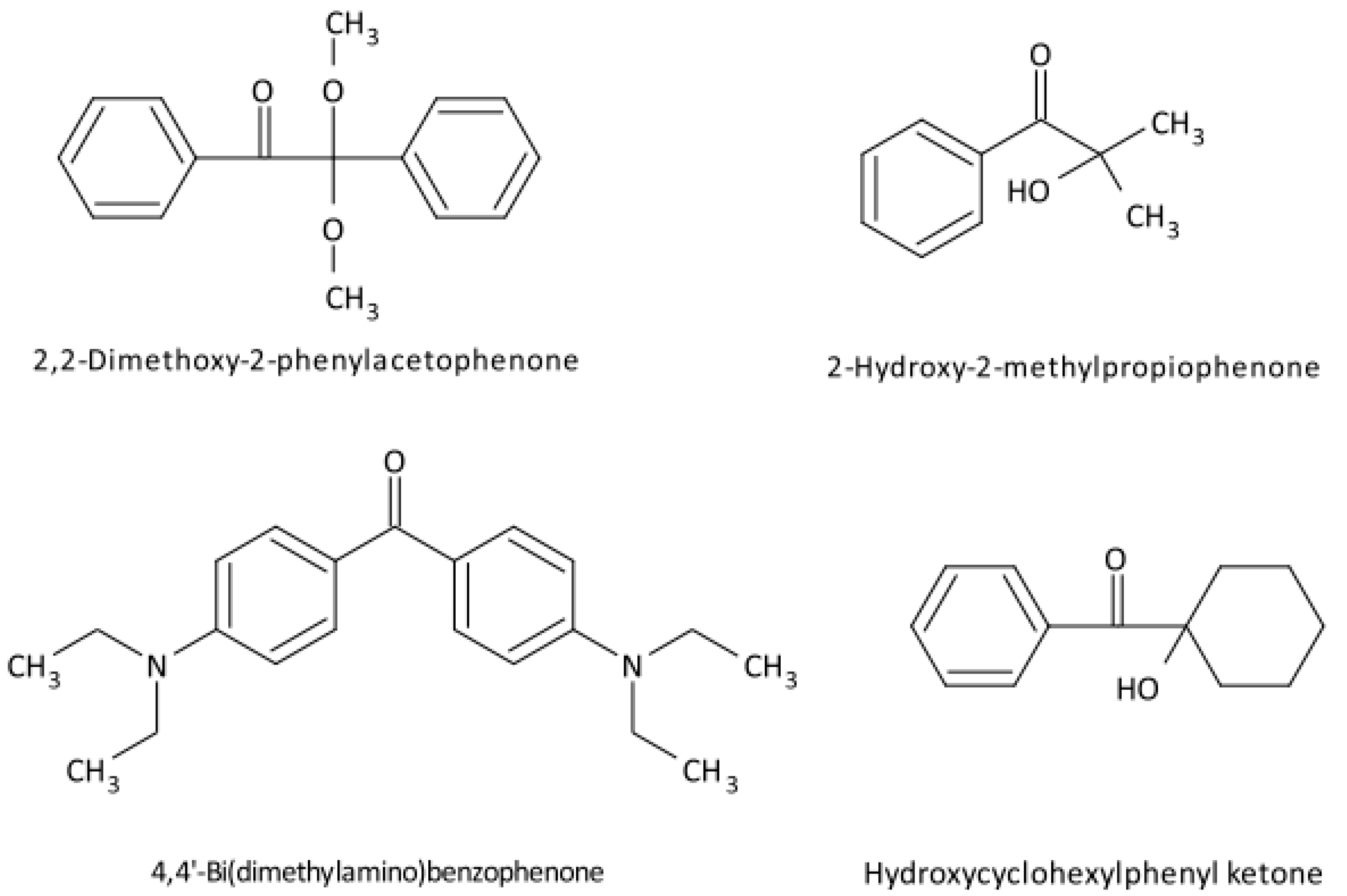
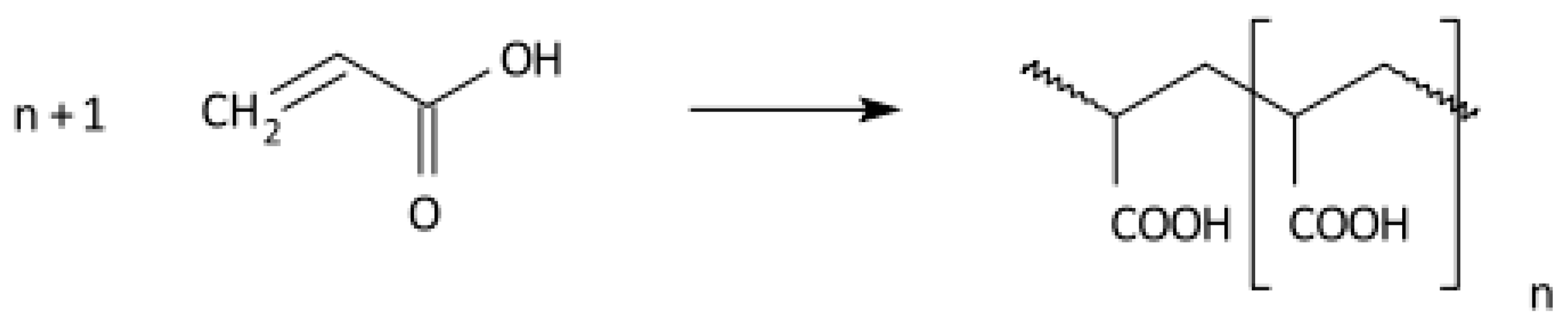
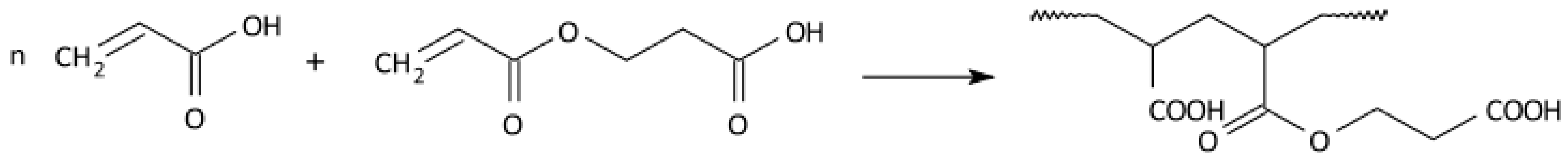
2.3. Radical Polymerisation of Vinyl Monomers
2.4. Chemical Properties of SAPs
3. Agricultural and Degraded Land Reclamation Applications
3.1. Agricultural Application
3.1.1. Influence of SAPs on Soil Structure and Soil Water Retention
3.1.2. Fertiliser Release into the Soil by SAPs
3.2. Reclamation
3.3. The Influence of Superabsorbents on the Microbiological Activity of Soils
3.4. New SAPs Environmentally Friendly and Plant-Friendly
3.5. Restrictions on the Use of SAPs
3.6. Degradation of SAPs in Soils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinke, K.; Martin, M.; Adams, S.; Baarsch, F.; Bondeau, A.; Coumou, D.; Donner, R.; Menon, A.; Perrette, M.; Rehfeld, K.; et al. Climatic Risks and Impacts in South Asia: Extremes of Water Scarcity and Excess. Reg. Environ. Chang. 2017, 17, 1569–1583. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, V.P. A Review of Drought Concepts. J. Hydrol. 2010, 391, 202–216. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Abrisham, E.S.; Jafari, M.; Tavili, A.; Rabii, A.; Zare Chahoki, M.A.; Zare, S.; Egan, T.; Yazdanshenas, H.; Ghasemian, D.; Tahmoures, M. Effects of a Super Absorbent Polymer on Soil Properties and Plant Growth for Use in Land Reclamation. Arid Land Res. Manag. 2018, 32, 407–420. [Google Scholar] [CrossRef]
- Emadodin, I.; Reinsch, T.; Taube, F. Drought and Desertification in Iran. Hydrology 2019, 6, 66. [Google Scholar] [CrossRef]
- Lei, T.; Feng, J.; Zheng, C.; Li, S.; Wang, Y.; Wu, Z.; Lu, J.; Kan, G.; Shao, C.; Jia, J.; et al. Review of Drought Impacts on Carbon Cycling in Grassland Ecosystems. Front. Earth Sci. 2020, 14, 462–478. [Google Scholar] [CrossRef]
- Bana, R.S.; Grover, M.; Singh, D.; Bamboriya, S.; Godara, S.; Kumar, M.; Kumar, A.; Sharma, S.; Shekhawat, P.S.; Lomte, D.; et al. Enhanced Pearl Millet Yield Stability, Water Use Efficiency and Soil Microbial Activity Using Superabsorbent Polymers and Crop Residue Recycling across Diverse Ecologies. Eur. J. Agron. 2023, 148, 126876. [Google Scholar] [CrossRef]
- Malik, S.; Chaudhary, K.; Malik, A.; Punia, H.; Sewhag, M.; Berkesia, N.; Nagora, M.; Kalia, S.; Malik, K.; Kumar, D.; et al. Superabsorbent Polymers as a Soil Amendment for Increasing Agriculture Production with Reducing Water Losses under Water Stress Condition. Polymers 2023, 15, 161. [Google Scholar] [CrossRef]
- Buchholz, F.L. Superabsorbent Polymers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; ISBN 978-0-471-44026-0. [Google Scholar]
- Djafari Petroudy, S.R.; Arjmand Kahagh, S.; Vatankhah, E. Environmentally Friendly Superabsorbent Fibers Based on Electrospun Cellulose Nanofibers Extracted from Wheat Straw. Carbohydr. Polym. 2021, 251, 117087. [Google Scholar] [CrossRef]
- Mignon, A.; De Belie, N.; Dubruel, P.; Van Vlierberghe, S. Superabsorbent Polymers: A Review on the Characteristics and Applications of Synthetic, Polysaccharide-Based, Semi-Synthetic and ‘Smart’ Derivatives. Eur. Polym. J. 2019, 117, 165–178. [Google Scholar] [CrossRef]
- Ma, X.; Wen, G. Development History and Synthesis of Super-Absorbent Polymers: A Review. J. Polym. Res. 2020, 27, 136. [Google Scholar] [CrossRef]
- Mistry, P.A.; Konar, M.N.; Latha, S.; Chadha, U.; Bhardwaj, P.; Eticha, T.K. Chitosan Superabsorbent Biopolymers in Sanitary and Hygiene Applications. Int. J. Polym. Sci. 2023, 2023, e4717905. [Google Scholar] [CrossRef]
- Wiegand, C.; Hipler, U.-C. A Superabsorbent Polymer-Containing Wound Dressing Efficiently Sequesters MMPs and Inhibits Collagenase Activity in Vitro. J. Mater. Sci. Mater. Med. 2013, 24, 2473–2478. [Google Scholar] [CrossRef]
- Lodhi, B.A.; Hussain, M.A.; Sher, M.; Haseeb, M.T.; Ashraf, M.U.; Hussain, S.Z.; Hussain, I.; Bukhari, S.N.A. Polysaccharide-Based Superporous, Superabsorbent, and Stimuli Responsive Hydrogel from Sweet Basil: A Novel Material for Sustained Drug Release. Adv. Polym. Technol. 2019, 2019, e9583516. [Google Scholar] [CrossRef]
- Sroka, P.; Satora, P.; Tarko, T.; Duda-Chodak, A. The Influence of Yeast Immobilization on Selected Parameters of Young Meads. J. Inst. Brew. 2017, 123, 289–295. [Google Scholar] [CrossRef]
- Poreda, A.; Tuszyński, T.; Zdaniewicz, M.; Sroka, P.; Jakubowski, M. Support Materials for Yeast Immobilization Affect the Concentration of Metal Ions in the Fermentation Medium. J. Inst. Brew. 2013, 119, 164–171. [Google Scholar] [CrossRef]
- Joshi, P.P.; Van Cleave, A.; Held, D.W.; Howe, J.A.; Auad, M.L. Preparation of Slow Release Encapsulated Insecticide and Fertilizer Based on Superabsorbent Polysaccharide Microbeads. J. Appl. Polym. Sci. 2020, 137, 49177. [Google Scholar] [CrossRef]
- Saha, A.; Sekharan, S.; Manna, U. Superabsorbent Hydrogel (SAH) as a Soil Amendment for Drought Management: A Review. Soil Tillage Res. 2020, 204, 104736. [Google Scholar] [CrossRef]
- Parsakhoo, A.; Jajouzadeh, M.; Rezaee, M. Effect of Hydroseeding on Grass Yield and Water Use Efficiency on Forest Road Artificial Soil Slopes. J. For. Sci. 2018, 64, 157–163. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent Hydrogels Based on Polysaccharides for Application in Agriculture as Soil Conditioner and Nutrient Carrier: A Review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- El Idrissi, A.; Channab, B.; Essamlali, Y.; Zahouily, M. Superabsorbent Hydrogels Based on Natural Polysaccharides: Classification, Synthesis, Physicochemical Properties, and Agronomic Efficacy under Abiotic Stress Conditions: A Review. Int. J. Biol. Macromol. 2024, 258, 128909. [Google Scholar] [CrossRef]
- Cheng, B.; Pei, B.; Wang, Z.; Hu, Q. Advances in Chitosan-Based Superabsorbent Hydrogels. RSC Adv. 2017, 7, 42036–42046. [Google Scholar] [CrossRef]
- Channab, B.-E.; El Idrissi, A.; Essamlali, Y.; Zahouily, M. Nanocellulose: Structure, Modification, Biodegradation and Applications in Agriculture as Slow/Controlled Release Fertilizer, Superabsorbent, and Crop Protection: A Review. J. Environ. Manag. 2024, 352, 119928. [Google Scholar] [CrossRef]
- Venkatachalam, D.; Kaliappa, S. Superabsorbent Polymers: A State-of-Art Review on Their Classification, Synthesis, Physicochemical Properties, and Applications. Rev. Chem. Eng. 2021, 39, 127–171. [Google Scholar] [CrossRef]
- Guan, H.; Li, J.; Zhang, B.; Yu, X. Synthesis, Properties, and Humidity Resistance Enhancement of Biodegradable Cellulose-Containing Superabsorbent Polymer. J. Polym. 2017, 2017, e3134681. [Google Scholar] [CrossRef]
- Czarnecka, E.; Nowaczyk, J. Semi-Natural Superabsorbents Based on Starch-g-Poly(Acrylic Acid): Modification, Synthesis and Application. Polymers 2020, 12, 1794. [Google Scholar] [CrossRef]
- Matmin, J.; Ibrahim, S.I.; Mohd Hatta, M.H.; Ricky Marzuki, R.; Jumbri, K.; Nik Malek, N.A.N. Starch-Derived Superabsorbent Polymer in Remediation of Solid Waste Sludge Based on Water-Polymer Interaction. Polymers 2023, 15, 1471. [Google Scholar] [CrossRef]
- Supare, K.; Mahanwar, P. Starch-Derived Superabsorbent Polymers in Agriculture Applications: An Overview. Polym. Bull. 2022, 79, 5795–5824. [Google Scholar] [CrossRef]
- Arredondo, R.; Yuan, Z.; Sosa, D.; Johnson, A.; Beims, R.F.; Li, H.; Wei, Q.; Xu, C.C. Performance of a Novel, Eco-Friendly, Cellulose-Based Superabsorbent Polymer (Cellulo-SAP): Absorbency, Stability, Reusability, and Biodegradability. Can. J. Chem. Eng. 2023, 101, 1762–1771. [Google Scholar] [CrossRef]
- Jafari, M.; Najafi, G.R.; Sharif, M.A.; Elyasi, Z. Superabsorbent Polymer Composites Derived from Polyacrylic Acid: Design and Synthesis, Characterization, and Swelling Capacities. Polym. Polym. Compos. 2021, 29, 733–739. [Google Scholar] [CrossRef]
- Alam, M.N.; Islam, M.S.; Christopher, L.P. Sustainable Production of Cellulose-Based Hydrogels with Superb Absorbing Potential in Physiological Saline. ACS Omega 2019, 4, 9419–9426. [Google Scholar] [CrossRef]
- Hemvichian, K.; Chanthawong, A.; Suwanmala, P. Synthesis and Characterization of Superabsorbent Polymer Prepared by Radiation-Induced Graft Copolymerization of Acrylamide onto Carboxymethyl Cellulose for Controlled Release of Agrochemicals. Radiat. Phys. Chem. 2014, 103, 167–171. [Google Scholar] [CrossRef]
- Sawut, A.; Yimit, M.; Sun, W.; Nurulla, I. Photopolymerisation and Characterization of Maleylatedcellulose-g-Poly(Acrylic Acid) Superabsorbent Polymer. Carbohydr. Polym. 2014, 101, 231–239. [Google Scholar] [CrossRef]
- Barleany, D.R.; Lestari, R.; Yulvianti, M.; Susanto, T.R.; Shalina, S.; Erizal, E. Acrylic Acid Neutralization for Enhancing the Production of Grafted Chitosan Superabsorbent Hydrogel. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 702–708. [Google Scholar] [CrossRef][Green Version]
- Jayanudin; Lestari, R.S.D.; Barleany, D.R.; Pitaloka, A.B.; Yulvianti, M.; Prasetyo, D.; Anggoro, D.V.; Ruhiatna, A. Chitosan-Graft-Poly(Acrylic Acid) Superabsorbent’s Water Holding in Sandy Soils and Its Application in Agriculture. Polymers 2022, 14, 5175. [Google Scholar] [CrossRef]
- Ge, H.; Wang, S. Thermal Preparation of Chitosan–Acrylic Acid Superabsorbent: Optimization, Characteristic and Water Absorbency. Carbohydr. Polym. 2014, 113, 296–303. [Google Scholar] [CrossRef]
- Gao, L.; Wang, S.; Zhao, X. Synthesis and Characterization of Agricultural Controllable Humic Acid Superabsorbent. J. Environ. Sci. 2013, 25, S69–S76. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kurdtabar, M.; Mahdavinia, G.R.; Hosseinzadeh, H. Synthesis and Super-Swelling Behavior of a Novel Protein-Based Superabsorbent Hydrogel. Polym. Bull. 2006, 57, 813–824. [Google Scholar] [CrossRef]
- Capezza, A.J.; Muneer, F.; Prade, T.; Newson, W.R.; Das, O.; Lundman, M.; Olsson, R.T.; Hedenqvist, M.S.; Johansson, E. Acylation of Agricultural Protein Biomass Yields Biodegradable Superabsorbent Plastics. Commun. Chem. 2021, 4, 52. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Lin, J.; Fan, S.; Hu, D. A Multifunctional Poly(Acrylic Acid)/Gelatin Hydrogel. J. Mater. Res. 2009, 24, 1653–1661. [Google Scholar] [CrossRef]
- El Idrissi, A.; Dardari, O.; Metomo, F.N.N.N.; Essamlali, Y.; Akil, A.; Amadine, O.; Aboulhrouz, S.; Zahouily, M. Effect of Sodium Alginate-Based Superabsorbent Hydrogel on Tomato Growth under Different Water Deficit Conditions. Int. J. Biol. Macromol. 2023, 253, 127229. [Google Scholar] [CrossRef]
- Agnihotri; Singhal, R. Effect of Sodium Alginate Content in Acrylic Acid/Sodium Humate/Sodium Alginate Superabsorbent Hydrogel on Removal Capacity of MB and CV Dye by Adsorption. J. Polym. Environ. 2019, 27, 372–385. [Google Scholar] [CrossRef]
- Thakur, S.; Arotiba, O. Synthesis, Swelling and Adsorption Studies of a pH-Responsive Sodium Alginate–Poly(Acrylic Acid) Superabsorbent Hydrogel. Polym. Bull. 2018, 75, 4587–4606. [Google Scholar] [CrossRef]
- Yoshimura, T. Alginate-Based Superabsorbent Hydrogels Composed of Carboxylic Acid-Amine Interaction. E-Polymers 2009, 9, 80. [Google Scholar] [CrossRef][Green Version]
- Hasija, V.; Sharma, K.; Kumar, V.; Sharma, S.; Sharma, V. Green Synthesis of Agar/Gum Arabic Based Superabsorbent as an Alternative for Irrigation in Agriculture. Vacuum 2018, 157, 458–464. [Google Scholar] [CrossRef]
- Liu, S.; Gao, C.; Mosquera-Giraldo, L.I.; Taylor, L.S.; Edgar, K.J. Selective Synthesis of Curdlan ω-Carboxyamides by Staudinger Ylide Nucleophilic Ring-Opening. Carbohydr. Polym. 2018, 190, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Kazimierczak, P.; Benko, A.; Palka, K.; Vivcharenko, V.; Przekora, A. Superabsorbent Curdlan-Based Foam Dressings with Typical Hydrocolloids Properties for Highly Exuding Wound Management. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112068. [Google Scholar] [CrossRef] [PubMed]
- Nurzynska, A.; Klimek, K.; Michalak, A.; Dos Santos Szewczyk, K.; Arczewska, M.; Szalaj, U.; Gagos, M.; Ginalska, G. Do Curdlan Hydrogels Improved with Bioactive Compounds from Hop Exhibit Beneficial Properties for Skin Wound Healing? Int. J. Mol. Sci. 2023, 24, 10295. [Google Scholar] [CrossRef]
- Jabeen, S.; Alam, S.; Shah, L.A.; Zahoor, M.; Naveed Umar, M.; Ullah, R. Novel Hydrogel Poly (GG-Co-Acrylic Acid) for the Sorptive Removal of the Color Rhodamine-B from Contaminated Water. Heliyon 2023, 9, e19780. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Ding, S.; Xu, B.; Chen, X.; Shen, X. Synthesis and Characterization of Polyacrylic Acid/Xanthan Gum/Bentonite Superabsorbent Polymer. Appl. Mech. Mater. 2014, 670–671, 148–152. [Google Scholar] [CrossRef]
- Sorze, A.; Valentini, F.; Dorigato, A.; Pegoretti, A. Development of a Xanthan Gum Based Superabsorbent and Water Retaining Composites for Agricultural and Forestry Applications. Molecules 2023, 28, 1952. [Google Scholar] [CrossRef] [PubMed]
- Elbedwehy, A.M.; Atta, A.M. Novel Superadsorbent Highly Porous Hydrogel Based on Arabic Gum and Acrylamide Grafts for Fast and Efficient Methylene Blue Removal. Polymers 2020, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Fávaro, S.; De Oliveira, F.; Reis, A.; Guilherme, M.; Muniz, E.; Tambourgi, E. Superabsorbent Hydrogel Composed of Covalently Crosslinked Gum Arabic with Fast Swelling Dynamics. J. Appl. Polym. Sci. 2008, 107, 1500–1506. [Google Scholar] [CrossRef]
- Wang, K.; Cen, R.; Shu, W. Preparation and Performance of Super-Absorbent Resin Using Polyacrylonitrile Fiber Wastes. Adv. Mater. Res. 2015, 1120–1121, 498–501. [Google Scholar] [CrossRef]
- Sadeghi, M. Synthesis of Alginate-Polyacrylonitrile Superabsorbent Hydrogel. Orient. J. Chem. 2011, 27, 469–476. [Google Scholar]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Reyhanitabar, A. Synthesis, Characterization, and Swelling Kinetic Study of Porous Superabsorbent Hydrogel Nanocomposite Based on Sulfonated Carboxymethylcellulose and Silica Nanoparticles. J. Porous Mater. 2018, 25, 1325–1335. [Google Scholar] [CrossRef]
- Kwon, Y.R.; Hong, S.J.; Lim, S.H.; Kim, J.S.; Chang, Y.W.; Choi, J.; Kim, D.H. Ionic-Bonded Superabsorbent Polymers and Their Surface-Crosslinking Using Modified Silica. Polym.-Plast. Technol. Mater. 2021, 60, 724–733. [Google Scholar] [CrossRef]
- Li, A.; Wang, A.; Chen, J. Studies on Poly(Acrylic Acid)/Attapulgite Superabsorbent Composite. I. Synthesis and Characterization. J. Appl. Polym. Sci. 2004, 92, 1596–1603. [Google Scholar] [CrossRef]
- Tao, W.; Xiaoqing, W.; Yi, Y.; Wenqiong, H. Preparation of Bentonite–Poly[(Acrylic Acid)-Acrylamide] Water Superabsorbent by Photopolymerization. Polym. Int. 2006, 55, 1413–1419. [Google Scholar] [CrossRef]
- Li, D.; Guo, J.; Wang, X.; Pei, L.; Li, W.; Liu, Y.; Deng, Y.; Chen, Z. Synthesis and Characterization of a Novel Bentonite Composite Superabsorbent Resin Based on Starch. Adv. Mater. Sci. Eng. 2022, 2022, 9038912. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Zhang, C.; Cui, B.; Li, Z.; Zhao, D.; Wang, Z. Kaolin-Enhanced Superabsorbent Composites: Synthesis, Characterization and Swelling Behaviors. Polymers 2021, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Joo, S.-W.; Hu, Y.; Shinde, V.V.; Cho, E.; Jung, S. Carboxymethyl Cellulose-Based Superabsorbent Hydrogels Containing Carboxymehtyl β-Cyclodextrin for Enhanced Mechanical Strength and Effective Drug Delivery. Eur. Polym. J. 2018, 105, 17–25. [Google Scholar] [CrossRef]
- Choi, J.; Jeon, H. Manufacture and Characterization of Alginate-CMC-Dextran Hybrid Double Layer Superabsorbent Scaffolds. Appl. Sci. 2021, 11, 11573. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Yoon, K.-J.; Ko, S.-W. Preparation and Properties of Alginate Superabsorbent Filament Fibers Crosslinked with Glutaraldehyde. J. Appl. Polym. Sci. 2000, 78, 1797–1804. [Google Scholar] [CrossRef]
- Dispat, N.; Poompradub, S.; Kiatkamjornwong, S. Synthesis of ZnO/SiO2-Modified Starch-Graft-Polyacrylate Superabsorbent Polymer for Agricultural Application. Carbohydr. Polym. 2020, 249, 116862. [Google Scholar] [CrossRef]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Water Absorption, Retention and the Swelling Characteristics of Cassava Starch Grafted with Polyacrylic Acid. Carbohydr. Polym. 2014, 103, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Sand, A.; Vyas, A. Superabsorbent Polymer Based on Guar Gum-Graft-Acrylamide: Synthesis and Characterization. J. Polym. Res. 2020, 27, 43. [Google Scholar] [CrossRef]
- Omidian, H.; Hashemi, S.A.; Sammes, P.G.; Meldrum, I.G. Modified Acrylic-Based Superabsorbent Polymers. Effect of Temperature and Initiator Concentration. Polymer 1998, 39, 3459–3466. [Google Scholar] [CrossRef]
- Sunitha, K.; Sadhana, R.; Mathew, D.; Reghunadhan Nair, C.P. Novel Superabsorbent Copolymers of Partially Neutralized Methacrylic Acid and Acrylonitrile: Synthesis, Characterization and Swelling Characteristics. Des. Monomers Polym. 2015, 18, 512–523. [Google Scholar] [CrossRef]
- Limparyoon, N.; Seetapan, N.; Kiatkamjornwong, S. Acrylamide/2-Acrylamido-2-Methylpropane Sulfonic Acid and Associated Sodium Salt Superabsorbent Copolymer Nanocomposites with Mica as Fire Retardants. Polym. Degrad. Stab. 2011, 96, 1054–1063. [Google Scholar] [CrossRef]
- Aday, A.N.; Matar, M.G.; Osio-Norgaard, J.; Srubar, W.V. Thermo-Responsive Poly(N-Isopropylacrylamide) (PNIPAM) Hydrogel Particles Improve Workability Loss and Autogenous Shrinkage in Cement Paste. Cement 2022, 10, 100049. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Hosseinzadeh, H. Synthesis and Properties of Partially Hydrolyzed Acrylonitrile-Co-Acrylamide Superabsorbent Hydrogel. Bull. Korean Chem. Soc. 2010, 31, 3163–3172. [Google Scholar] [CrossRef]
- Škugor Rončević, I.; Krivić, D.; Buljac, M.; Vladislavić, N.; Buzuk, M. Polyelectrolytes Assembly: A Powerful Tool for Electrochemical Sensing Application. Sensors 2020, 20, 3211. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Sousa, H.; Lima, I.S.; Neris, L.M.L.; Silva, A.S.; Santos Nascimento, A.M.S.; Araújo, F.P.; Ratke, R.F.; Silva, D.A.; Osajima, J.A.; Bezerra, L.R.; et al. Superabsorbent Hydrogels Based to Polyacrylamide/Cashew Tree Gum for the Controlled Release of Water and Plant Nutrients. Molecules 2021, 26, 2680. [Google Scholar] [CrossRef] [PubMed]
- Kalaleh, H.-A.; Atassi, Y. Up-Scalable Synthesis of High Porous Superabsorbent Polymer via Alkaline Hydrolysis of Acrylamide Using Microwave Irradiation: Application in Agriculture. J. Mater. Environ. Sci. 2018, 9, 955–963. [Google Scholar]
- Choudhary, M.S. Inverse Suspension Polymerization of Partially Neutralized and Lightly Cross-Linked Acrylic Acid: Effect of Reaction Parameters. Macromol. Symp. 2009, 277, 171–176. [Google Scholar] [CrossRef]
- Ceylan, Ö.; Kaya, M.A.; Sarac, A. Preparation of Partially Neutralized Poly(Acrylic Acid) Microspheres via Inverse Pickering Suspension Polymerization. Polym. Eng. Sci. 2019, 59, 162–169. [Google Scholar] [CrossRef]
- Mazloom, G.; Alavi, S.M. Different Catalytic Reactor Technologies in Selective Oxidation of Propane to Acrylic Acid and Acrolein. Part. Sci. Technol. 2018, 36, 61–71. [Google Scholar] [CrossRef]
- Dishisha, T.; Pyo, S.-H.; Hatti-Kaul, R. Bio-based 3-hydroxypropionic- and Acrylic Acid Production from Biodiesel Glycerol via Integrated Microbial and Chemical Catalysis. Microb. Cell Factories 2015, 14, 200. [Google Scholar] [CrossRef]
- Chang, Z.; Dai, W.; Mao, Y.; Cui, Z.; Wang, Z.; Chen, T. Engineering Corynebacterium glutamicum for the Efficient Production of 3-Hydroxypropionic Acid from a Mixture of Glucose and Acetate via the Malonyl-CoA Pathway. Catalysts 2020, 10, 203. [Google Scholar] [CrossRef]
- Liu, B.; Xiang, S.; Zhao, G.; Wang, B.; Ma, Y.; Liu, W.; Tao, Y. Efficient Production of 3-Hydroxypropionate from Fatty Acids Feedstock in Escherichia Coli. Metab. Eng. 2018, 51, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.S.; Li, Y.; Cortés-Peña, Y.R.; Brace, E.C.; Martin, T.A.; Zhao, H.; Guest, J.S. Sustainable Production of Acrylic Acid via 3-Hydroxypropionic Acid from Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 16659–16669. [Google Scholar] [CrossRef]
- Yin, G.; Zhong, H.; Yao, G.; Jin, F.; Zhao, J. Production of Acrylic Acid from Biomass-Derived Fumaric Acid under Hydrothermal Conditions. Energies 2021, 14, 5456. [Google Scholar] [CrossRef]
- Hussain, Y.A.; Liu, T.; Roberts, G.W. Synthesis of Cross-Linked, Partially Neutralized Poly(Acrylic Acid) by Suspension Polymerization in Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2012, 51, 11401–11408. [Google Scholar] [CrossRef]
- Cutie, S.S.; Henton, D.E.; Powell, C.; Reim, R.E.; Smith, P.B.; Staples, T.L. The Effects of MEHQ on the Polymerization of Acrylic Acid in the Preparation of Superabsorbent Gels. J. Appl. Polym. Sci. 1997, 64, 577–589. [Google Scholar] [CrossRef]
- Levy, L.B. Inhibition of Acrylic Acid Polymerization by Phenothiazine and P-Methoxyphenol. J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 1505–1515. [Google Scholar] [CrossRef]
- Becker, H.; Vogel, H. Phenothiazine as Stabilizer for Acrylic Acid. Chem. Eng. Technol. 2006, 29, 931–936. [Google Scholar] [CrossRef]
- Maafa, I.M. Inhibition of Free Radical Polymerization: A Review. Polymers 2023, 15, 488. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Schork, F. Modeling of the Inhibition Mechanism of Acrylic Acid Polymerization. Ind. Eng. Chem. Res. 2006, 45, 3001–3008. [Google Scholar] [CrossRef]
- Levy, L.B.; Penrod, J.D. The Anatomy of an Acrylic Acid Runaway Polymerization. Plant/Oper. Prog. 1989, 8, 105–108. [Google Scholar] [CrossRef]
- Fujita, M.; Iizuka, Y.; Miyake, A. Thermal and Kinetic Analyses on Michael Addition Reaction of Acrylic Acid. J. Therm. Anal. Calorim. 2017, 128, 1227–1233. [Google Scholar] [CrossRef]
- Li, Y.; Sawut, A.; Hou, G.; He, M.; Yimit, M. UV Polymerization and Property Analysis of Maleacylated Methyl Cellulose Acrylic Acid Absorbent Resin. Pol. J. Chem. Technol. 2020, 22, 34–41. [Google Scholar] [CrossRef]
- Jockusch, S.; Turro, N.; Mitsukami, Y.; Matsumoto, M.; Iwamura, T.; Lindner, T.; Flohr, A.; Massimo, G. Photoinduced Surface Crosslinking of Superabsorbent Polymer Particles. J. Appl. Polym. Sci. 2009, 111, 2163–2170. [Google Scholar] [CrossRef]
- Frenzel, R.; Morales, D.; Romanelli, G.; Sathicq, G.; Blanco, M.; Pizzio, L. Synthesis, Characterization and Catalytic Evaluation of H3PW12O40 Included in Acrylic Acid/Acrylamide Polymer for the Selective Oxidation of Sulfides. J. Mol. Catal. A Chem. 2016, 420, 124–133. [Google Scholar] [CrossRef]
- Wong, D.; Hellwig, T.; Halfar, A.H.; Moritz, H.-U.; Pauer, W. Design of Particle Morphology for Redox-Initiated Spray Polymerization of Acrylic Acid and Sodium Acrylate by Investigating Levitated Single Droplet. Macromol. Symp. 2016, 370, 128–134. [Google Scholar] [CrossRef]
- Kabiri, K.; Zohuriaan-Mehr, M.J.; Bouhendi, H.; Jamshidi, A.; Ahmad-Khanbeigi, F. Residual Monomer in Superabsorbent Polymers: Effects of the Initiating System. J. Appl. Polym. Sci. 2009, 114, 2533–2540. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Mu, B.; Wang, X.; Wang, A. Fenton-like Redox-Initiated Synthesis of Superabsorbent Composites with Excellent Water Retention and Swelling Properties Based on Green Tea and Oil Shale Semi-Coke. Eur. Polym. J. 2023, 182, 111716. [Google Scholar] [CrossRef]
- Mohammed, A.D.; Young, D.A.; Vosloo, H.C.M. Synthesis of High-Performance Superabsorbent Glycerol Acrylate-Cross-Linked Poly (Acrylic Acid). Res. Chem. Intermed. 2017, 43, 2187–2200. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Flores, Y.; Flores, R.; Alvarez-Gallegos, A. Heterogeneous Catalysis in the Fenton-Type System Reactive Black 5/H2O2. J. Mol. Catal. A Chem. 2008, 281, 184–191. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, Z.; Zhao, T.; Liu, M.; Hu, M.; Li, J. Synthesis, Characterization, and Swelling Behaviors of Salt-Sensitive Maize Bran–Poly(Acrylic Acid) Superabsorbent Hydrogel. J. Agric. Food Chem. 2014, 62, 8867–8874. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, M.; Shangguan, L. Synthesis of a Novel and Salt Sensitive Superabsorbent Hydrogel Using Soybean Dregs by UV-Irradiation. Materials 2018, 11, 2198. [Google Scholar] [CrossRef]
- Liu, L.; Sawut, A.; Abliz, S.; Nurulla, I.; Dolat, B.; Yimit, M. Ultraviolet-Induced Polymerization of Superabsorbent Composite Based on Sodium Humate and Its Urea Release Behavior. RSC Adv. 2016, 6, 101123–101132. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, X.; Gauthier, D.E.; An, X.; Cheng, D.; Ni, Y.; Yin, L. Development of Poly(Acrylic Acid)/Nanofibrillated Cellulose Superabsorbent Composites by Ultraviolet Light Induced Polymerization. Cellulose 2015, 22, 2499–2506. [Google Scholar] [CrossRef]
- Wan, T.; Liao, L.; Huang, R.; Wu, D.; Tang, L.; Xiong, J.; Chen, Q. Synthesis and Swelling Behaviors of Microcrystal Muscovite Composite Superabsorbent by Photopolymerization. J. Wuhan Univ. Technol.-Mat. Sci. Ed. 2016, 31, 151–156. [Google Scholar] [CrossRef]
- Tomida, T.; Hamaguchi, K.; Tunashima, S.; Katoh, M.; Masuda, S. Binding Properties of a Water-Soluble Chelating Polymer with Divalent Metal Ions Measured by Ultrafiltration. Poly(Acrylic Acid). Ind. Eng. Chem. Res. 2001, 40, 3557–3562. [Google Scholar] [CrossRef]
- Chakrapani, G.; Zare, M.; Ramakrishna, S. Intelligent Hydrogels and Their Biomedical Applications. Mater. Adv. 2022, 3, 7757–7772. [Google Scholar] [CrossRef]
- Rizwan, M.; Rubina Gilani, S.; Iqbal Durani, A.; Naseem, S. Materials Diversity of Hydrogel: Synthesis, Polymerization Process and Soil Conditioning Properties in Agricultural Field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef]
- Shukla, N.B.; Madras, G. Photo, Thermal, and Ultrasonic Degradation of EGDMA-Crosslinked Poly(Acrylic Acid-Co-Sodium Acrylate-Co-Acrylamide) Superabsorbents. J. Appl. Polym. Sci. 2012, 125, 630–639. [Google Scholar] [CrossRef]
- Mazlan, S.N.A.; Abd Rahim, S.; Ghazali, S.; Jamari, S.S. Optimization of N,N′-Methylenebis(Acrylamide), and Ammonium Persulfate Content in Carbonaceous/Acrylic Acid-Co-Acrylamide Superabsorbent Polymer. Mater. Today Proc. 2022, 57, 1088–1094. [Google Scholar] [CrossRef]
- Mullen, B.; Rodwogin, M.; Stollmaier, F.; Yontz, D.; Leibig, C. New Bio-Derived Superabsorbents from Nature. Green Mater. 2013, 1, 186–190. [Google Scholar] [CrossRef]
- Savaskan Yilmaz, S.; Yildirim, N.; Misir, M.; Misirlioglu, Y.; Celik, E. Synthesis, Characterization of a New Polyacrylic Acid Superabsorbent, Some Heavy Metal Ion Sorption, the Adsorption Isotherms, and Quantum Chemical Investigation. Materials 2020, 13, 4390. [Google Scholar] [CrossRef] [PubMed]
- Kiatkamjornwong, S. Superabsorbent Polymers and Superabsorbent Polymer Composites. Sci. Asia 2007, 33, 39–43. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, Y.; Wang, H.; Wang, Y.; Zhu, H.; Chen, Y.; Chen, S.; Jin, S.; Yang, Z.; Li, P.; et al. Preparation of the Sodium Alginate-g-(Polyacrylic Acid-Co-Allyltrimethylammonium Chloride) Polyampholytic Superabsorbent Polymer and Its Dye Adsorption Property. Mar. Drugs 2018, 16, 476. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Wang, A. Synthesis, Characterization and Swelling Behaviors of Sodium Alginate-g-Poly(Acrylic Acid)/Sodium Humate Superabsorbent. Carbohydr. Polym. 2009, 75, 79–84. [Google Scholar] [CrossRef]
- Islam, N.; Islam, M. Preparation and Characterization of Superabsorbent Polymer (SAP) by Graft Polymerization of Carboxymethyl Cellulose. Int. Lett. Chem. Phys. Astron. 2016, 70, 27–32. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Roh, H.; Oh, S.; Kim, S.; Kim, M.; Kim, D.; Park, J. Preparation and Characterization of Superabsorbent Polymers Based on Starch Aldehydes and Carboxymethyl Cellulose. Polymers 2018, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Wampler III, F.M. Formation of Diacrylic Acid during Acrylic Acid Storage. Plant/Oper. Prog. 1988, 7, 183–189. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on Hydrogel-Based pH Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef]
- Dąbrowska, J.; Lejcuś, K. Charakterystyka Wybranych Właściwości Superabsorbentów (Characteristics of Selected Properties of Superabsorbents). Infrastruct. Ecol. Rural Areas 2012, 3/IV, 59–68. [Google Scholar]
- Bajpai, S.; Johnson, S. Removal of Ni2+ Ions from Aqueous Solution by Sorption into Poly(Acrylamide–Co–Sodium Acrylate) Hydrogels. J. Macromol. Sci. 2007, 44, 285–290. [Google Scholar] [CrossRef]
- Bajpai, S.; Johnson, S. Superabsorbent Hydrogels for Removal of Divalent Toxic Ions. Part I: Synthesis and Swelling Characterization. React. Funct. Polym. 2005, 62, 271–283. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Theodoropoulos, A.G.; Drossopoulos, J.B. Designing Synthetic Polymers as Soil Conditioners. Commun. Soil Sci. Plant Anal. 1995, 26, 1455–1480. [Google Scholar] [CrossRef]
- Yakupoglu, T.; Rodrigo-Comino, J.; Cerdà, A. Potential Benefits of Polymers in Soil Erosion Control for Agronomical Plans: A Laboratory Experiment. Agronomy 2019, 9, 276. [Google Scholar] [CrossRef]
- Panova, I.G.; Ilyasov, L.O.; Khaidapova, D.D.; Bashina, A.S.; Smagin, A.V.; Ogawa, K.; Adachi, Y.; Yaroslavov, A.A. Soil Conditioners Based on Anionic Polymer and Anionic Micro-Sized Hydrogel: A Comparative Study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125635. [Google Scholar] [CrossRef]
- Mamedov, A.I.; Huang, C.; Aliev, F.A.; Levy, G.J. Aggregate Stability and Water Retention Near Saturation Characteristics as Affected by Soil Texture, Aggregate Size and Polyacrylamide Application. Land Degrad. Dev. 2017, 28, 543–552. [Google Scholar] [CrossRef]
- Sojka, R.E.; Entry, J.A.; Fuhrmann, J.J. The Influence of High Application Rates of Polyacrylamide on Microbial Metabolic Potential in an Agricultural Soil. Appl. Soil Ecol. 2006, 32, 243–252. [Google Scholar] [CrossRef]
- Tian, X.; Fan, H.; Wang, J.; Ippolito, J.; Li, Y.; Feng, S.; An, M.; Zhang, F.; Wang, K. Effect of Polymer Materials on Soil Structure and Organic Carbon under Drip Irrigation. Geoderma 2019, 340, 94–103. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y. New Material for Transforming Degraded Sandy Land into Productive Farmland. Land Use Policy 2020, 92, 104477. [Google Scholar] [CrossRef]
- Yirdaw, E.; Tigabu, M.; Monge, A. Rehabilitation of Degraded Dryland Ecosystems—Review. Silva Fenn. 2017, 51, 1673. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z.; Song, W.; Bai, S. Effects of Superabsorbent Polymers on Moisture Migration and Accumulation Behaviors in Soil. J. Clean. Prod. 2021, 279, 123841. [Google Scholar] [CrossRef]
- Derbali, I.; Derbali, W.; Gharred, J.; Manaa, A.; Slama, I.; Koyro, H.-W. Mitigating Salinity Stress in Quinoa (Chenopodium Quinoa Willd.) with Biochar and Superabsorber Polymer Amendments. Plants 2024, 13, 92. [Google Scholar] [CrossRef]
- Paluszek, J. Ksztaltowanie syntetycznymi polimerami wlasciwosci gleb erodowanych terenow lessowych. Rozpr. Naukowe. Akad. Rol. W Lublinie 2003, 277, 1–153. [Google Scholar]
- Paluszek, J. Effect of terracottem polymer on the structure of eroded luvisol. Acta Agroph. 2009, 14, 713–724. [Google Scholar]
- Paluszek, J. Wpływ dodatku AgroAquaGelu 420 na fizyczne właściwości erodowanej gleby płowej. Ochr. Sr. I Zasobów Nat. 2010, 44, 107–116. [Google Scholar]
- Awad, Y.M.; Lee, S.S.; Kim, K.-H.; Ok, Y.S.; Kuzyakov, Y. Carbon and Nitrogen Mineralization and Enzyme Activities in Soil Aggregate-Size Classes: Effects of Biochar, Oyster Shells, and Polymers. Chemosphere 2018, 198, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cao, T.; Dou, P.; Sheng, J.; Luo, M. Effect of Various Concentrations of Superabsorbent Polymers on Soil Particle-Size Distribution and Evaporation with Sand Mulching. Sci. Rep. 2019, 9, 3511. [Google Scholar] [CrossRef] [PubMed]
- Seybold, C.A. Polyacrylamide Review: Soil Conditioning and Environmental Fate. Commun. Soil Sci. Plant Anal. 1994, 25, 2171–2185. [Google Scholar] [CrossRef]
- Agaba, H.; Orikiriza, L.J.B.; Obua, J.; Kabasa, J.D.; Worbes, M.; Hüttermann, A. Hydrogel Amendment to Sandy Soil Reduces Irrigation Frequency and Improves the Biomass of Agrostis Stolonifera. Agric. Sci. 2011, 2, 544. [Google Scholar] [CrossRef]
- Narjary, B.; Aggarwal, P. Evaluation of Soil Physical Quality under Amendments and Hydrogel Applications in a Soybean–Wheat Cropping System. Commun. Soil Sci. Plant Anal. 2014, 45, 1167–1180. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, S.; Singh, V.; Kumar, M.; Kumar, M. Hydrogel and Its Effect on Soil Moisture Status and Plant Growth: A Review. J. Pharmacogn. Phytochem. 2020, 9, 1746–1753. [Google Scholar]
- Jamnongkan, T.; Kaewpirom, S. Potassium Release Kinetics and Water Retention of Controlled-Release Fertilizers Based on Chitosan Hydrogels. J. Polym. Environ. 2010, 18, 413–421. [Google Scholar] [CrossRef]
- Arbona, V.; Iglesias, D.J.; Jacas, J.; Primo-Millo, E.; Talon, M.; Gómez-Cadenas, A. Hydrogel Substrate Amendment Alleviates Drought Effects on Young Citrus Plants. Plant Soil 2005, 270, 73–82. [Google Scholar] [CrossRef]
- Mazen, A.M.; Radwan, D.E.M.; Ahmed, A.F. Growth Responses of Maize Plants Cultivated in Sandy Soil Amended by Different Superabsorbant Hydrogels. J. Plant Nutr. 2015, 38, 325–337. [Google Scholar] [CrossRef]
- Bakass, M.; Mokhlisse, A.; Lallemant, M. Absorption and Desorption of Liquid Water by a Superabsorbent Polymer: Effect of Polymer in the Drying of the Soil and the Quality of Certain Plants. J. Appl. Polym. Sci. 2002, 83, 234–243. [Google Scholar] [CrossRef]
- El-Rehim, H.; Hegazy, E.-S.; Abd El-Mohdy, H. Effect of Various Environmental Conditions on the Swelling Property of PAAm/PAAcK Superabsorbent Hydrogel Prepared by Ionizing Radiation. J. Appl. Polym. Sci. 2006, 101, 3955–3962. [Google Scholar] [CrossRef]
- Čechmánková, J.; Sedlařík, V.; Duřpeková, S.; Drbohlav, J.; Šalaková, A.; Vácha, R. Assessing the Effects of Whey Hydrogel on Nutrient Stability in Soil and Yield of Leucosinapis Alba and Hordeum Vulgare. Sustainability 2024, 16, 45. [Google Scholar] [CrossRef]
- Ali, M.A.; Mohamed, H.M.A.; Elsayed, S.A.; Sillanpää, M.; Al-Farraj, S.; El-sayed, M.E.A. Effect of Integrate Water Shortage and Soil Conditioners on Water Productivity, Growth, and Yield of Red Globe Grapevines Grown in Sandy Soil. Open Agric. 2023, 8, 20220240. [Google Scholar] [CrossRef]
- Hüttermann, A.; Zommorodi, M.; Reise, K. Addition of Hydrogels to Soil for Prolonging the Survival of Pinus Halepensis Seedlings Subjected to Drought. Soil Tillage Res. 1999, 50, 295–304. [Google Scholar] [CrossRef]
- Pery, R.S.M.; Marfà, O.; Serrano, L. The Effect of a Hydrophilic Polymer on Plant Water Status and Survival of Transplanted Pine Seedlings. HortTechnology 1995, 5, 141–143. [Google Scholar] [CrossRef]
- Hüttermann, A.; Orikiriza, L.J.B.; Agaba, H. Application of Superabsorbent Polymers for Improving the Ecological Chemistry of Degraded or Polluted Lands. CLEAN—Soil Air Water 2009, 37, 517–526. [Google Scholar] [CrossRef]
- Agaba, H.; Baguma Orikiriza, L.J.; Osoto Esegu, J.F.; Obua, J.; Kabasa, J.D.; Hüttermann, A. Effects of Hydrogel Amendment to Different Soils on Plant Available Water and Survival of Trees under Drought Conditions. CLEAN—Soil Air Water 2010, 38, 328–335. [Google Scholar] [CrossRef]
- Lejcus, K.; Orzeszyna, H.; Pawlowski, A.; Garlikowski, D. Wykorzystanie superabsorbentów w zabezpieczeniach przeciwerozyjnych. Infrastrukt. Ekol. Teren. Wiej. 2008, 9, 189–194. [Google Scholar]
- Nnadi, F.; Brave, C. Environmentally Friendly Superabsorbent Polymers for Water Conservation in Agricultural Lands. J. Soil Sci. Environ. Manag. 2011, 2, 206–211. [Google Scholar]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, e435073. [Google Scholar] [CrossRef]
- Dar, S.; Mishra, D.; Rashid, Z.; Afshana, B.B. Hydrogel: To Enhance Crop Productivity Per Unit Available Water Under Moisture Stress Agriculture. Bull. Environ. Pharmacol. Life Sci. 2017, 6, 123–129. [Google Scholar]
- Singh, S.P.; Singh, R.K.; Kumar, S. Response of Irrigation Schedule, Mulching and Hydrogel on Various Growth Analysis Attributes and Nutrient Uptake of Wheat (Triticum aestivum L.). J. Pharmacogn. Phytochem. 2017, 6, 2569–2573. [Google Scholar]
- Bao, Y.; Ma, J.; Li, N. Synthesis and Swelling Behaviors of Sodium Carboxymethyl Cellulose-g-Poly(AA-Co-AM-Co-AMPS)/MMT Superabsorbent Hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in Non-Hygienic Applications of Superabsorbent Hydrogel Materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Salimi, M.; Channab, B.; El Idrissi, A.; Zahouily, M.; Motamedi, E. A Comprehensive Review on Starch: Structure, Modification, and Applications in Slow/Controlled-Release Fertilizers in Agriculture. Carbohydr. Polym. 2023, 322, 121326. [Google Scholar] [CrossRef]
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Hilonga, A. Meticulous Overview on the Controlled Release Fertilizers. Adv. Chem. 2014, 2014, e363071. [Google Scholar] [CrossRef]
- Noordin, N.; Ghazali, S.; Adnan, N. Impact of Sap-Biochar Incorporation on Controlled Release Water Retention Fertilizer (CRWR) towards Growth of Okras (Abelmoschus esculentus). Mater. Today Proc. 2018, 5, 21911–21918. [Google Scholar] [CrossRef]
- Jing, Y.; Krauss, M.; Zschieschang, S.; Miltner, A.; Butkovskyi, A.; Eggen, T.; Kästner, M.; Nowak, K.M. Superabsorbent Polymer as a Supplement Substrate of Constructed Wetland to Retain Pesticides from Agricultural Runoff. Water Res. 2021, 207, 117776. [Google Scholar] [CrossRef] [PubMed]
- Rather, R.A.; Bhat, M.A.; Shalla, A.H. An Insight into Synthetic and Physiological Aspects of Superabsorbent Hydrogels Based on Carbohydrate Type Polymers for Various Applications: A Review. Carbohydr. Polym. Technol. Appl. 2022, 3, 100202. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of Acrylamide/Acrylic Acid Cellulose Hydrogels for the Adsorption of Heavy Metal Ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.O.; De Varennes, A. Remediation of a Sandy Soil Artificially Contaminated with Copper Using a Polyacrylate Polymer. Soil Use Manag. 1998, 14, 106–110. [Google Scholar] [CrossRef]
- Qu, G.; de Varennes, A.; Cunha-Queda, C. Use of Insoluble Polyacrylate Polymers to Aid Phytostabilization of Mine Soils: Effects on Plant Growth and Soil Characteristics. J. Environ. Qual. 2010, 39, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Guiwei, Q.; De Varennes, A.; Cunha-Queda, C. Remediation of a Mine Soil with Insoluble Polyacrylate Polymers Enhances Soil Quality and Plant Growth. Soil Use Manag. 2008, 24, 350–356. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher-Jenull, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial Diversity and Soil Functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Lal, B.; Gautam, P.; Nayak, A.K.; Panda, B.B.; Bihari, P.; Tripathi, R.; Shahid, M.; Guru, P.K.; Chatterjee, D.; Kumar, U.; et al. Energy and Carbon Budgeting of Tillage for Environmentally Clean and Resilient Soil Health of Rice-Maize Cropping System. J. Clean. Prod. 2019, 226, 815–830. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Dick, W.A. Enzymes in Soil: Research and Developments in Measuring Activities. Enzym. Environ. Act. Ecol. Appl. 2002, 567–596. [Google Scholar]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil Biodiversity and Soil Community Composition Determine Ecosystem Multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Scherer-Lorenzen, M.; Palmborg, C.; Prinz, A.; Schulze, E.-D. The Role of Plant Diversity and Composition for Nitrate Leaching in Grasslands. Ecology 2003, 84, 1539–1552. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme Activities as a Component of Soil Biodiversity: A Review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of Phosphatase Enzymes in Soil. In Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling; Bünemann, E., Oberson, A., Frossard, E., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–243. ISBN 978-3-642-15271-9. [Google Scholar]
- Furtak, K.; Gajda, A.M. Biochemiczne metody oceny różnorodności funkcjonalnej i strukturalnej mikroorganizmów glebowych. Postępy Mikrobiol. 2018, 57, 194–202. [Google Scholar] [CrossRef]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S. Different Approaches to Evaluating Soil Quality Using Biochemical Properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Pascual, J.A.; Garcia, C.; Hernandez, T.; Moreno, J.L.; Ros, M. Soil Microbial Activity as a Biomarker of Degradation and Remediation Processes. Soil Biol. Biochem. 2000, 32, 1877–1883. [Google Scholar] [CrossRef]
- Izquierdo, I.; Caravaca, F.; Alguacil, M.M.; Hernández, G.; Roldán, A. Use of Microbiological Indicators for Evaluating Success in Soil Restoration after Revegetation of a Mining Area under Subtropical Conditions. Appl. Soil Ecol. 2005, 30, 3–10. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil Enzymes in a Changing Environment: Current Knowledge and Future Directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Mocek-Płóciniak, A. Wykorzystanie aktywności enzymatycznej do oceny wpływu antropogenicznych zmian wywołanych przez metale ciężkie w środowisku glebowym. Nauka Przyr. Technol. 2010, 4, 86. [Google Scholar]
- Orczewska, A.; Piotrowska, A.; Lemanowicz, J. Soil Acid Phosphomonoesterase Activity and Phosphorus Forms in Ancient and Post-Agricultural Black Alder [Alnus Glutinosa (L.) Gaertn.] Woodlands. Acta Soc. Bot. Pol. 2012, 81, 81–86. [Google Scholar] [CrossRef]
- Kay-Shoemake, J.L.; Watwood, M.E.; Sojka, R.E.; Lentz, R.D. Polyacrylamide as a Substrate for Microbial Amidase in Culture and Soil. Soil Biol. Biochem. 1998, 30, 1647–1654. [Google Scholar] [CrossRef]
- Oksińska, M.P.; Magnucka, E.G.; Lejcuś, K.; Pietr, S.J. Biodegradation of the Cross-Linked Copolymer of Acrylamide and Potassium Acrylate by Soil Bacteria. Environ. Sci. Pollut. Res. 2016, 23, 5969–5977. [Google Scholar] [CrossRef] [PubMed]
- Awad, Y.M.; Blagodatskaya, E.; Ok, Y.S.; Kuzyakov, Y. Effects of Polyacrylamide, Biopolymer, and Biochar on Decomposition of Soil Organic Matter and Plant Residues as Determined by 14C and Enzyme Activities. Eur. J. Soil Biol. 2012, 48, 1–10. [Google Scholar] [CrossRef]
- Basanta, R.; de Varennes, A.; Díaz-Raviña, M. Microbial Community Structure and Biomass of a Mine Soil with Different Organic and Inorganic Treatments and Native Plants. J. Soil Sci. Plant Nutr. 2017, 17, 839–852. [Google Scholar] [CrossRef]
- Qu, G.; de Varennes, A. Use of Hydrophilic Polymers from Diapers to Aid the Establishment of Spergularia Purpurea in a Mine Soil. J. Hazard. Mater. 2010, 178, 956–962. [Google Scholar] [CrossRef]
- de Varennes, A.; Qu, G.; Cordovil, C.; Gonçalves, P. Soil Quality Indicators Response to Application of Hydrophilic Polymers to a Soil from a Sulfide Mine. J. Hazard. Mater. 2011, 192, 1836–1841. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A Mini-Review on Chitosan-Based Hydrogels with Potential for Sustainable Agricultural Applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Clark, A.H.; Adams, S. Swelling and Mechanical Properties of Biopolymer Hydrogels Containing Chitosan and Bovine Serum Albumin. Biomacromolecules 2006, 7, 2961–2970. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Li, P.; Xing, R.; Liu, S.; Qin, Y.; Yu, H.; Chen, X.; Li, K. Synthesis of Chitosan Derivative Graft Acrylic Acid Superabsorbent Polymers and Its Application as Water Retaining Agent. Int. J. Biol. Macromol. 2018, 115, 754–761. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Raffa, P.; Picchioni, F.; Koning, C.E. Superabsorbent Polymers: From Long-Established, Microplastics Generating Systems, to Sustainable, Biodegradable and Future Proof Alternatives. Prog. Polym. Sci. 2022, 125, 101475. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S.; Chen, Z.; Wang, M.; Cao, J.; Wang, R. Preparation and Characterization of Superabsorbent Polymers Based on Sawdust. Polymers 2019, 11, 1891. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Seggiani, M.; Hosny, A.; Rashad, M.; Cinelli, P.; Saad-Allah, K.M.; El-Sharnouby, M.; Shendy, S.; Azaam, M.M. Superabsorbent Composites Based on Rice Husk for Agricultural Applications: Swelling Behavior, Biodegradability in Soil and Drought Alleviation. J. Saudi Chem. Soc. 2021, 25, 101254. [Google Scholar] [CrossRef]
- Wang, W.B.; Wang, A.Q. Preparation, Swelling and Water-Retention Properties of Crosslinked Superabsorbent Hydrogels Based on Guar Gum. Adv. Mater. Res. 2010, 96, 177–182. [Google Scholar] [CrossRef]
- Wang, Z.; Ning, A.; Xie, P.; Gao, G.; Xie, L.; Li, X.; Song, A. Synthesis and Swelling Behaviors of Carboxymethyl Cellulose-Based Superabsorbent Resin Hybridized with Graphene Oxide. Carbohydr. Polym. 2016, 157, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, E.; Motesharezedeh, B.; Shirinfekr, A.; Samar, S.M. Synthesis and Swelling Behavior of Environmentally Friendly Starch-Based Superabsorbent Hydrogels Reinforced with Natural Char Nano/Micro Particles. J. Environ. Chem. Eng. 2020, 8, 103583. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable Agriculture Systems in Vegetable Production Using Chitin and Chitosan as Plant Biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, F.; Li, C.; An, H.; Wan, T.; Zhang, P. Application of Chitosan and Its Derivative Polymers in Clinical Medicine and Agriculture. Polymers 2022, 14, 958. [Google Scholar] [CrossRef]
- El-Araby, A.; Janati, W.; Ullah, R.; Ercisli, S.; Errachidi, F. Chitosan, Chitosan Derivatives, and Chitosan-Based Nanocomposites: Eco-Friendly Materials for Advanced Applications (a Review). Front. Chem. 2024, 11, 1327426. [Google Scholar] [CrossRef] [PubMed]
- Pichyangkura, R.; Chadchawan, S. Biostimulant Activity of Chitosan in Horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Jiao, Q.; Shen, F.; Fan, L.; Song, Z.; Zhang, J.; Song, J.; Fahad, S.; Liu, F.; Zhao, Y.; Tian, Z.; et al. Chitosan Regulates the Root Architecture System, Photosynthetic Characteristics and Antioxidant System Contributing to Salt Tolerance in Maize Seedling. Agriculture 2024, 14, 304. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; AL-Huqail, A.A.; AL-Harbi, M.S.; Ali, E.F.; Wang, J.; Ding, Z.; Rekaby, S.A.; Ghoneim, A.M.; Eissa, M.A. Mechanisms of Chitosan Nanoparticles in the Regulation of Cold Stress Resistance in Banana Plants. Nanomaterials 2021, 11, 2670. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.F.; El-Shehawi, A.M.; Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Moussa, M.M.; Hassan, F.a.S. A Vital Role of Chitosan Nanoparticles in Improvisation the Drought Stress Tolerance in Catharanthus roseus (L.) through Biochemical and Gene Expression Modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Nascimento, C.D.V.; Simmons, R.W.; Feitosa, J.P.d.A.; Dias, C.T.d.S.; Costa, M.C.G. Potential of Superabsorbent Hydrogels to Improve Agriculture under Abiotic Stresses. J. Arid Environ. 2021, 189, 104496. [Google Scholar] [CrossRef]
- Abdallah, A. The Effect of Hydrogel Particle Size on Water Retention Properties and Availability under Water Stress. Int. Soil Water Conserv. Res. 2019, 7, 275–285. [Google Scholar] [CrossRef]
- Zhou, B.; Renkuan, L.; Li, Y.; Tao, G.; Peiling, Y.; Ji, F.; Weimin, X.; Zhichao, Z. Water-absorption Characteristics of Organic–Inorganic Composite Superabsorbent Polymers and Its Effect on Summer Maize Root Growth. J. Appl. Polym. Sci. 2012, 126, 423–435. [Google Scholar] [CrossRef]
- Misiewicz, J.; Lejcuś, K.; Dąbrowska, J.; Marczak, D. The Characteristics of Absorbency Under Load (AUL) for Superabsorbent and Soil Mixtures. Sci. Rep. 2019, 9, 18098. [Google Scholar] [CrossRef] [PubMed]
- Guancha-Chalapud, M.A.; Serna-Cock, L.; Tirado, D.F. Hydrogels Are Reinforced with Colombian Fique Nanofibers to Improve Techno-Functional Properties for Agricultural Purposes. Agriculture 2022, 12, 117. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M. A Review on Nanocomposite Hydrogels and Their Biomedical Applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Ingram, D.L.; Yeager, T.H. Effects of Irrigation Frequency and A Water-Absorbing Polymer Amendment on Ligustrum Growth and Moisture Retention by a Container Medium. J. Environ. Hortic. 1987, 5, 19–21. [Google Scholar] [CrossRef]
- Situ, Y.; Yang, Y.; Huang, C.; Liang, S.; Mao, X.; Chen, X. Effects of Several Superabsorbent Polymers on Soil Exchangeable Cations and Crop Growth. Environ. Technol. Innov. 2023, 30, 103126. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, C.; Wu, Y.; Han, W.; Song, J.; Bai, W. Effects of Different Concentrations of Super-Absorbent Polymers on Soil Structure and Hydro-Physical Properties Following Continuous Wetting and Drying Cycles. J. Integr. Agric. 2022, 21, 3368–3381. [Google Scholar] [CrossRef]
- Azevedo, G.T.d.O.S.; de Souza, A.M.; de Azevedo, G.B.; Teodoro, P.E.; Sousa, J.R.L. de Influence of Fertilizer and Hydrogel on Physical-Chemical Attributes of Substrate for Seedling Production. Biosci. J. 2019, 35, 1399–1407. [Google Scholar] [CrossRef]
- Nejatzadeh, F. Investigation of Super Absorbent Polymers and Zinc Sulfate on The Yield and Yield Components of Calendula officinalis L. Acta Sci. Pol. Hortorum Cultus 2019, 18, 105–113. [Google Scholar] [CrossRef]
- Achtenhagen, J.; Kreuzig, R. Laboratory Tests on the Impact of Superabsorbent Polymers on Transformation and Sorption of Xenobiotics in Soil Taking C-14-Imazalil as an Example. Sci. Total Environ. 2011, 409, 5454–5458. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Saensupo, S.; Na-iam, Y.; Klomsa-Ard, P.; Sriroth, K. Effects of Superabsorbent Polymer on Soil Water Content and Sugarcane Germination and Early Growth in Sandy Soil Conditions. Sugar Tech 2018, 21, 444–450. [Google Scholar] [CrossRef]
- Tomášková, I.; Resnerová, K.; Trombik, J.; Bláha, J.; Pastierovič, F.; Macků, J. Potential of Hydrogel Treatment in Forest Regeneration: Impact on Growth and Vitality of Central European Tree Species. Front. For. Glob. Chang. 2023, 6, 1251041. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, B. 1—Introduction to Polymeric Gels. In Polymeric Gels; Pal, K., Banerjee, I., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2018; pp. 3–27. ISBN 978-0-08-102179-8. [Google Scholar]
- Entry, J.A.; Sojka, R.E.; Watwood, M.; Ross, C. Polyacrylamide Preparations for Protection of Water Quality Threatened by Agricultural Runoff Contaminants. Environ. Pollut. 2002, 120, 191–200. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Sun, H.; Ye, F.; Liu, W.; Luo, S. Polyacrylamide Molecular Formulation Effects on Erosion Control of Disturbed Soil on Steep Rocky Slopes. Can. J. Soil Sci. 2011, 91, 917–924. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Sun, X.; Miao, L.; Shi, W.; Wu, L.; Yuan, J. Erosion Resistance of Treated Dust Soils Based on the Combined Enzymatically Induced Carbonate Precipitation and Polyacrylic Acid. Biogeotechnics 2023, 1, 100050. [Google Scholar] [CrossRef]
- Larson, R.J.; Bookland, E.A.; Williams, R.T.; Yocom, K.M.; Saucy, D.A.; Freeman, M.B.; Swift, G. Biodegradation of Acrylic Acid Polymers and Oligomers by Mixed Microbial Communities in Activated Sludge. J. Environ. Polym. Degrad. 1997, 5, 41–48. [Google Scholar] [CrossRef]
- Caulfield, M.J.; Qiao, G.G.; Solomon, D.H. Some Aspects of the Properties and Degradation of Polyacrylamides. Chem. Rev. 2002, 102, 3067–3084. [Google Scholar] [CrossRef]
- Guezennec, A.G.; Michel, C.; Bru, K.; Touze, S.; Desroche, N.; Mnif, I.; Motelica-Heino, M. Transfer and Degradation of Polyacrylamide-Based Flocculants in Hydrosystems: A Review. Environ. Sci. Pollut. Res. Int. 2015, 22, 6390–6406. [Google Scholar] [CrossRef]
- Suzuki, J.; Hukushima, K.; Suzuki, S. Effect of Ozone Treatment upon Biodegradability of Water-Soluble Polymers. Environ. Sci. Technol. 1978, 12, 1180–1183. [Google Scholar] [CrossRef]
- Stahl, J.D.; Cameron, M.D.; Haselbach, J.; Aust, S.D. Biodegradation of Superabsorbent Polymers in Soil. Environ. Sci. Pollut. Res. 2000, 7, 83–88. [Google Scholar] [CrossRef]
- Sutherland, G.R.; Haselbach, J.; Aust, S.D. Biodegradation of Crosslinked Acrylic Polymers by a White-Rot Fungus. Environ. Sci. Pollut. Res. Int. 1997, 4, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Smagin, A.V.; Sadovnikova, N.B.; Belyaeva, E.A.; Korchagina, C.V. Biodegradability of Gel-Forming Superabsorbents for Soil Conditioning: Kinetic Assessment Based on CO2 Emissions. Polymers 2023, 15, 3582. [Google Scholar] [CrossRef]
- Sojka, R.E.; Bjorneberg, D.L.; Entry, J.A.; Lentz, R.D.; Orts, W.J. Polyacrylamide in Agriculture and Environmental Land Management. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2007; Volume 92, pp. 75–162. [Google Scholar]
- Azzam, R.A.I. Agricultural Polymers Polyacrylamide Preparation, Application and Prospects in Soil Conditioning. Commun. Soil Sci. Plant Anal. 1980, 11, 767–834. [Google Scholar] [CrossRef]
- Lakshmikandan, M.; Sivaraman, K.; Elaiya Raja, S.; Vasanthakumar, P.; Rajesh, R.P.; Sowparthani, K.; Jebasingh, S.E.J. Biodegradation of Acrylamide by Acrylamidase from Stenotrophomonas acidaminiphila MSU12 and Analysis of Degradation Products by MALDI-TOF and HPLC. Int. Biodeterior. Biodegrad. 2014, 94, 214–221. [Google Scholar] [CrossRef]
- Petka-Poniatowska, K.; Sroka, P.; Tarko, T.; Duda, A. The Acrylamide Degradation by Probiotic Strain Lactobacillus Acidophilus LA-5. Foods 2022, 11, 365. [Google Scholar] [CrossRef]
- Shanker, R.; Ramakrishna, C.; Seth, P.K. Microbial Degradation of Acrylamide Monomer. Arch. Microbiol. 1990, 154, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Shukor, M.Y.; Gusmanizar, N.; Azmi, N.A.; Hamid, M.; Ramli, J.; Shamaan, N.A.; Syed, M.A. Isolation and Characterization of an Acrylamide-Degrading Bacillus Cereus. J. Environ. Biol. 2009, 30, 57–64. [Google Scholar] [PubMed]
- Mai, C.; Schormann, W.; Majcherczyk, A.; Hüttermann, A. Degradation of Acrylic Copolymers by White-Rot Fungi. Appl. Microbiol. Biotechnol. 2004, 65, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Naseem, S.; Gilani, S.R.; Durrani, A.I. Optimization of Swelling and Mechanical Behavior of Acer Platanoides Cellulose Combo Hydrogel. Kuwait J. Sci. 2024, 51, 100177. [Google Scholar] [CrossRef]
- Smith, E.A.; Prues, S.L.; Oehme, F.W. Environmental Degradation of Polyacrylamides. 1. Effects of Artificial Environmental Conditions: Temperature, Light, and pH. Ecotoxicol. Environ. Saf. 1996, 35, 121–135. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Biodegradable Superabsorbent Hydrogel for Water Holding in Soil and Controlled-Release Fertilizer. J. Appl. Polym. Sci. 2020, 137, 48495. [Google Scholar] [CrossRef]

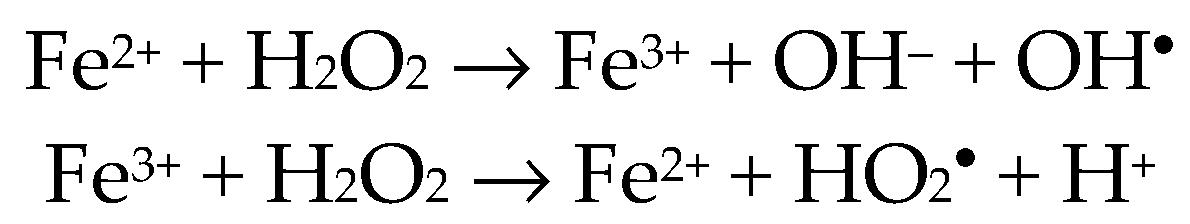

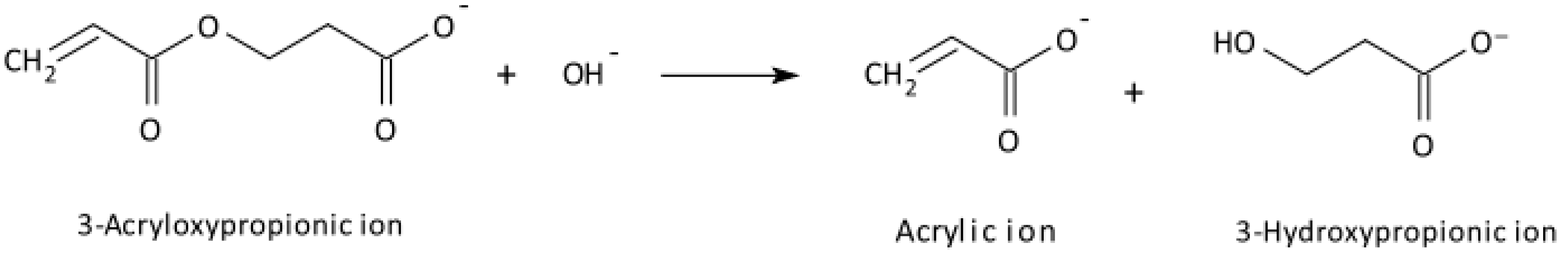
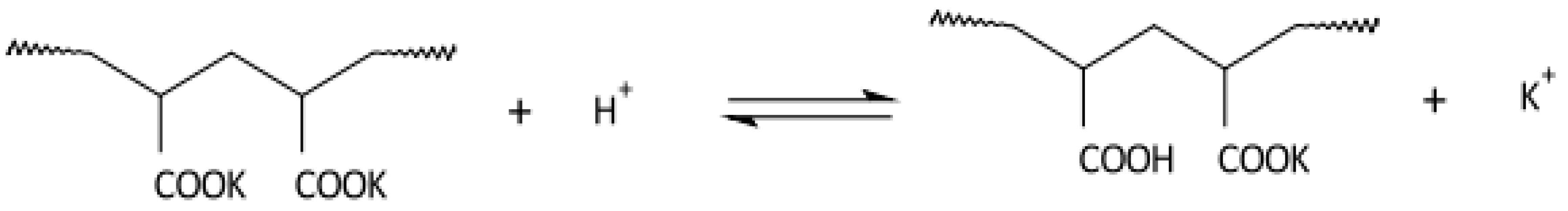
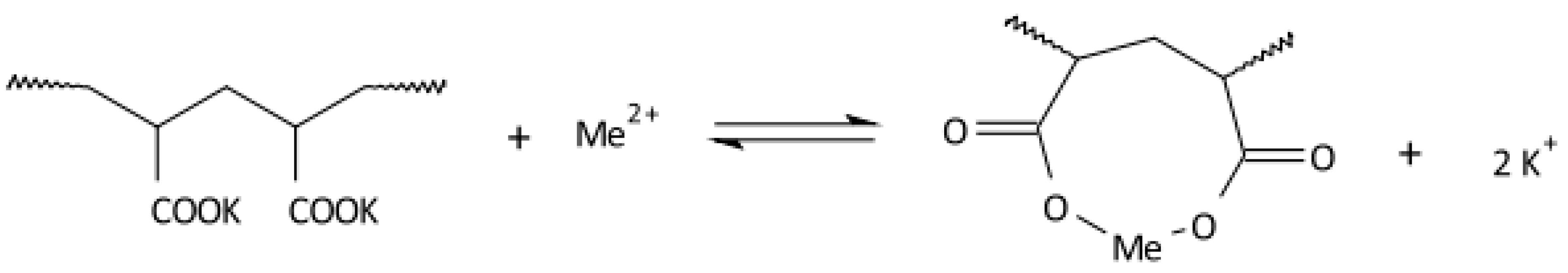
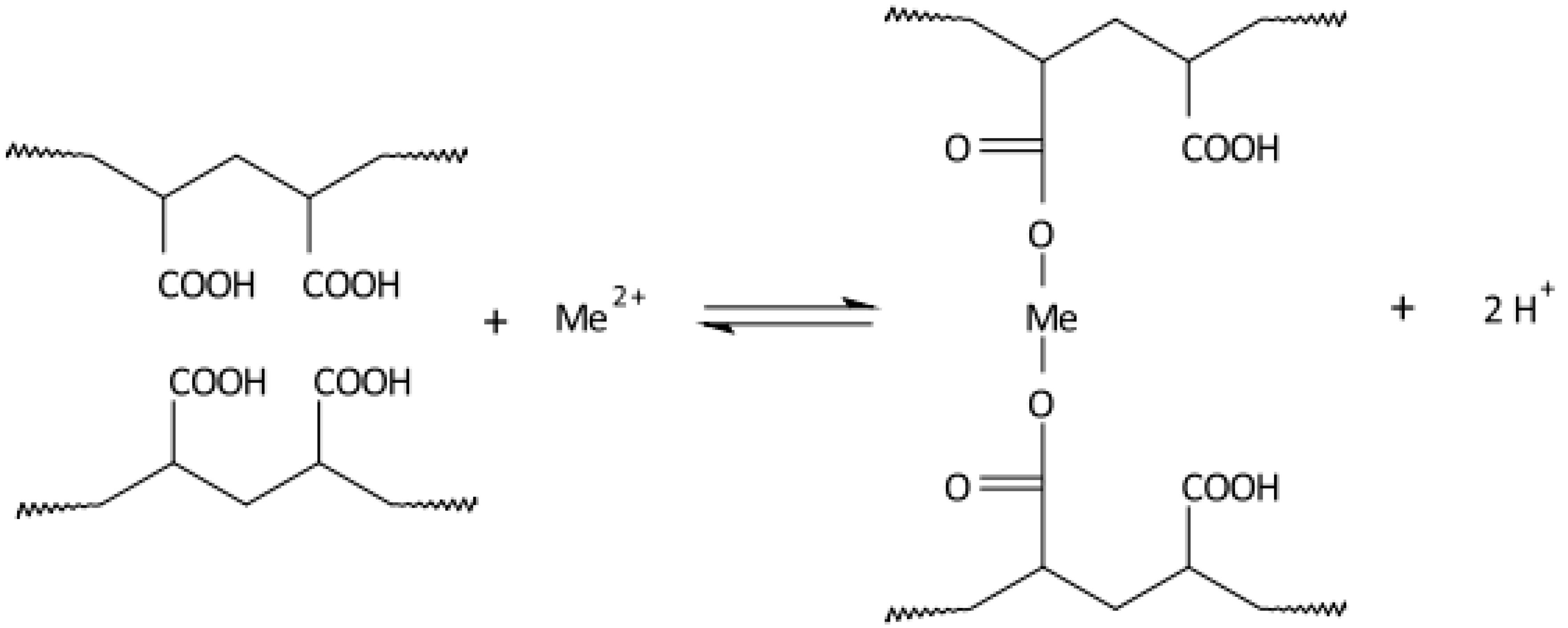
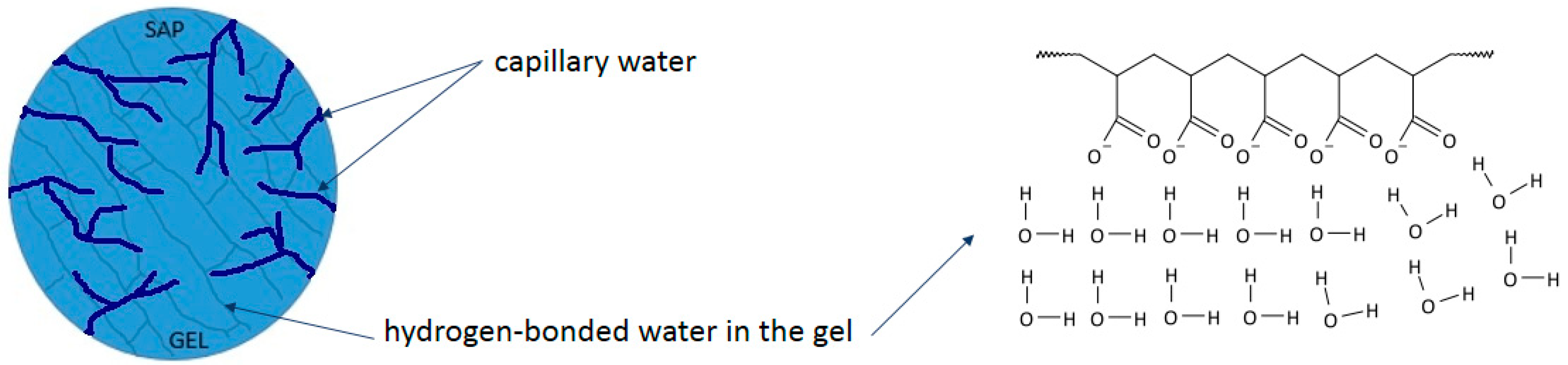
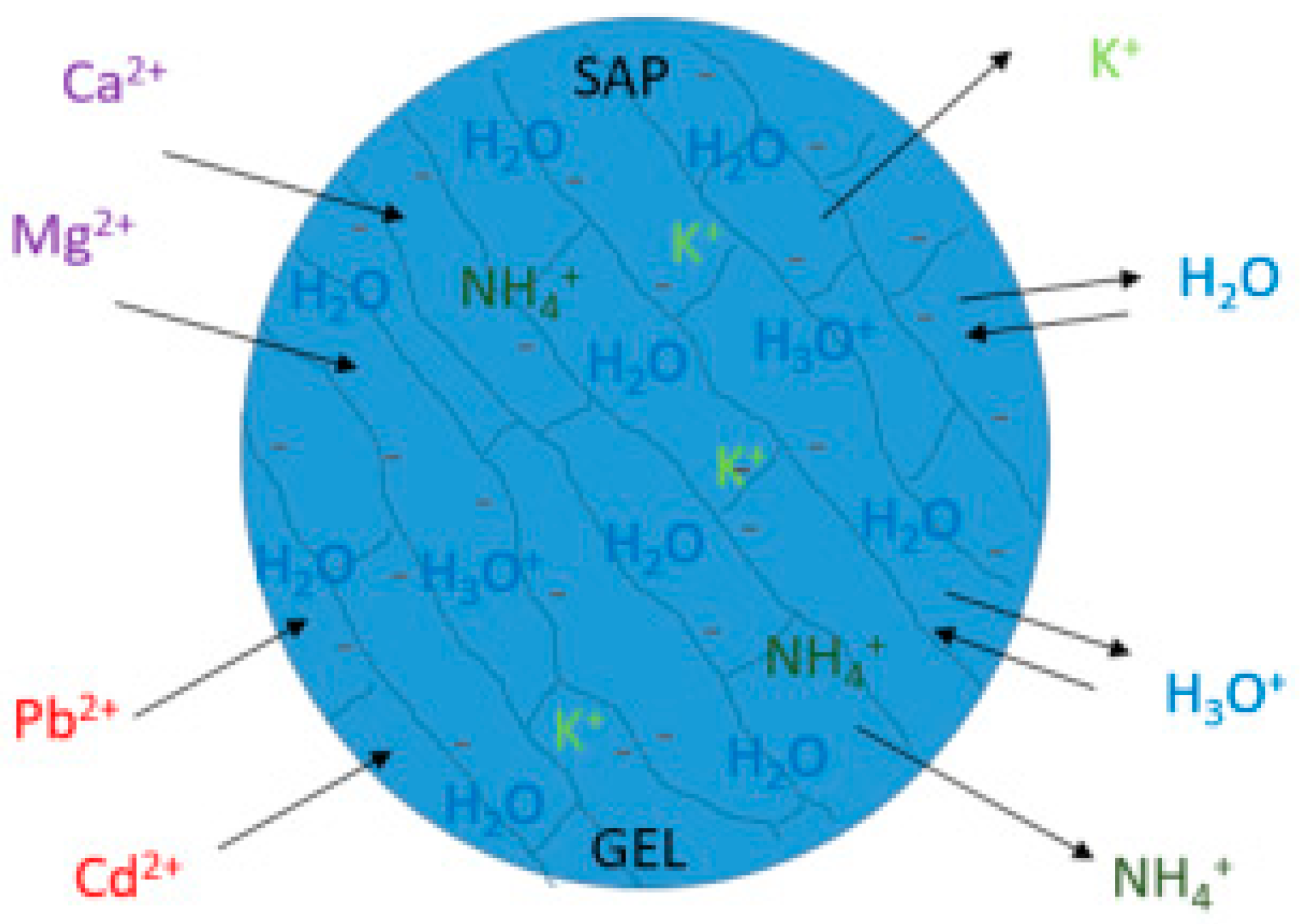
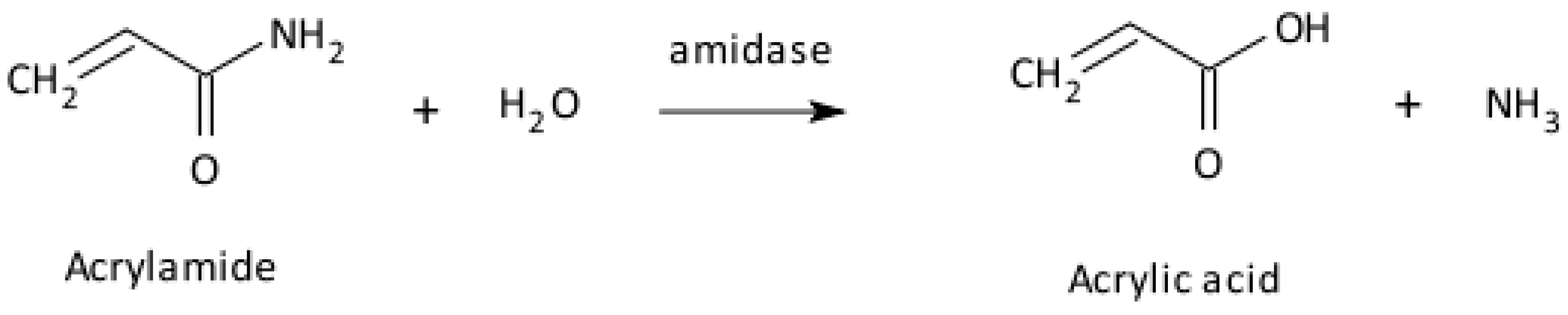
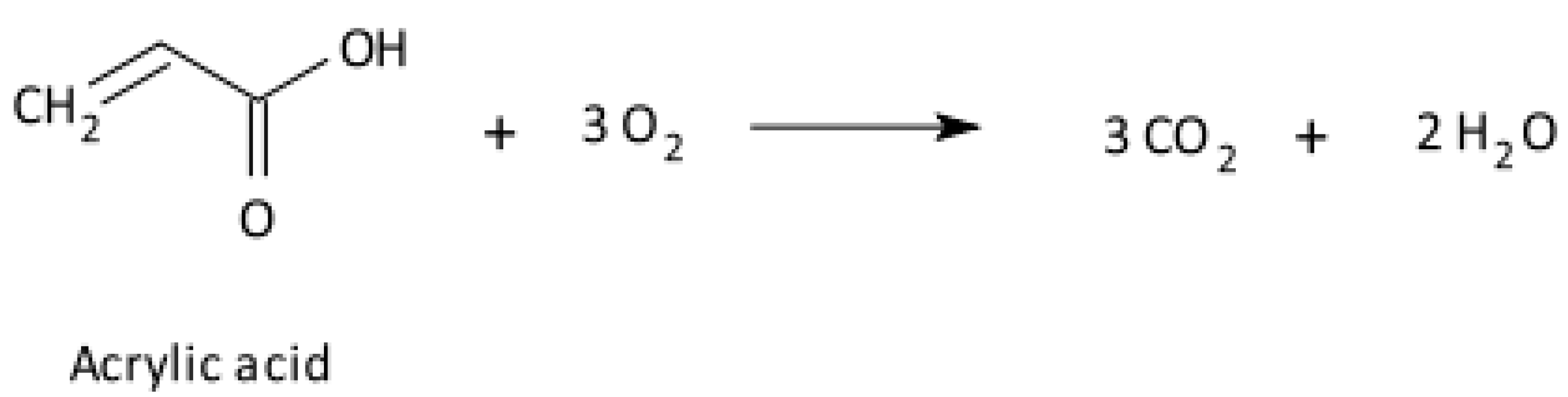
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sroka, K.; Sroka, P. Superabsorbent Hydrogels in the Agriculture and Reclamation of Degraded Areas. Sustainability 2024, 16, 2945. https://doi.org/10.3390/su16072945
Sroka K, Sroka P. Superabsorbent Hydrogels in the Agriculture and Reclamation of Degraded Areas. Sustainability. 2024; 16(7):2945. https://doi.org/10.3390/su16072945
Chicago/Turabian StyleSroka, Katarzyna, and Paweł Sroka. 2024. "Superabsorbent Hydrogels in the Agriculture and Reclamation of Degraded Areas" Sustainability 16, no. 7: 2945. https://doi.org/10.3390/su16072945
APA StyleSroka, K., & Sroka, P. (2024). Superabsorbent Hydrogels in the Agriculture and Reclamation of Degraded Areas. Sustainability, 16(7), 2945. https://doi.org/10.3390/su16072945







