Abstract
Aralia cordata is called “Udo” and is one of the famous herbaceous perennial plants found in Korea, China, and Japan. In Japan, aerial parts of A. cordata have been consumed. Furthermore, its rhizome and root are utilized as crude drugs known as “dokukatsu” and “wakyoukatsu”, respectively. A. cordata is cultivated as a vegetable in many places in Japan, and one of the production areas is Tokyo. A. cordata made in Tokyo is known as a high-quality “Udo” product (aerial part) using a unique cultivation method, known as “Udo muro”. “Udo muro” blocks light and maintains optimal temperature and humidity throughout the year, facilitating the soft cultivation of udo. However, the roots of A. cordata cultivated in Tokyo are all discarded. Thus, the utilization of the A. cordata root cultivated in Tokyo might lead to sustainability and income improvement for farmers. In this study, we investigated the effect of “Udo muro” with or without cultivation and drying temperature on chlorogenic acid (CA) contents in A. cordata root and compared it with A. cordata produced in other areas (“wakyoukatsu”) by a quantitative analysis of the CA content using high-performance liquid chromatography. The results indicate that the CA content of the roots of A. cordata grown in Tokyo was higher than those grown in other areas. Furthermore, the usefulness of A. cordata root was evaluated using inhibitory activity tests such as nitric oxide production and melanin production using Raw264.7 and B16F10 cell lines, respectively.
1. Introduction
Aralia cordata belongs to the Araliaceae family and is an herbaceous perennial plant distributed in Japan, Korea, and China. In Japan, A. cordata is called “Udo” and has been consumed for a long time. The juice of the stems or leaves has traditionally been used as a tonic and curative for headaches, colds, and rheumatism [1]. A. cordata has been used as traditional folk medicine for the treatment of rheumatism, lumbago, and lameness in Korea. The root is sometimes used in China as a substitute for ginseng (Panax species) [2]. Thus, A. cordata is one of the important plants in Asia used as food and folk medicine. A. cordata is cultivated as a vegetable (“Udo”) in many places in Japan, and one of the production areas is Tokyo. “Udo” made in Tokyo is a high-quality product grown using a unique method, known as “Udo muro” cultivation. “Udo muro” is traditional cultivation facility that originated in Tokyo. There are several forms of “Udo muro”, which include underground pits and semi-underground structures. The predominant form involves cultivating A. cordata in holes dug underground, representing a traditional method in Tokyo. When “Udo” is cultivated in Tokyo, it is first grown in an open field to develop the root (Figure 1A). After that, the root stocks are dug up (Figure 1B) and cultivated using “Udo muro” (Figure 1C), and the elongated stems are harvested in the darkness. The “Udo muro” blocks light and maintains optimal temperature and humidity throughout the year, facilitating the soft cultivation of udo [3,4]. The products are beautiful and white, unlike those cultivated in other regions (Figure 1D), and have a mild taste and are suitable for eating raw and in salads.
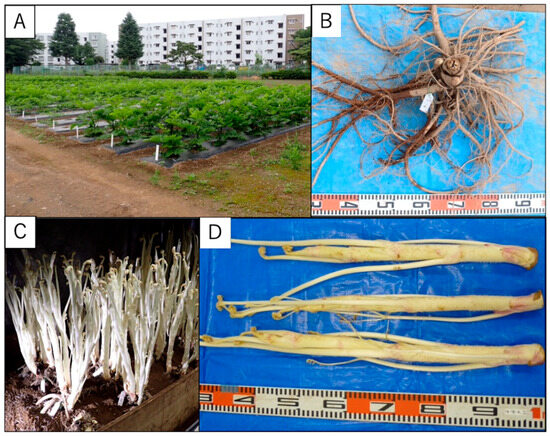
Figure 1.
Cultivation of A. cordata in Tokyo. (A) The open field cultivation of root. (B) The dug-up root stocks. (C) The method of cultivation called “Udo muro”. (D) The aerial part of A. cordata cultivated in Tokyo.
Although the root of A. cordata is inedible, the rhizome and root of A. cordata cultivated in other areas are utilized for crude drugs called “dokukatsu” and “wakyoukatsu”, respectively, in Japan. Although “dokukatsu” is used as a component of Kampo medicine listed in the Japanese pharmacopoeia such as “jūmihaidoku-to” and “dokukatsu-to” [5], “wakyoukatsu” is not. Furthermore, “wakyoukatsu” is generally not used domestically. In other words, the roots of A. cordata cultivated in Tokyo are all discarded. The sufficiency rate for crude drugs is 10% in Japan, and most of it relies on imports from China. Furthermore, due to the aging of farmers and the decline in crude drug prices, there is a possibility that the situation will become even more critical in the future. Crude drugs generally use parts of plants, and unused parts are often discarded. The search for utilization of the unused parts is essential to realizing a sustainable society. In this study, the utilization of A. cordata roots cultivated in Tokyo might lead to sustainability of producing crude drugs and income for farmers.
Some recent studies have reported various components of A. cordata, such as diterpenoids and diacetylenes, as active compounds of inhibitors against nitric oxide production and triacylglycerol biosynthesis [6,7]. Our Comprehensive Medicinal Plant Database (http://mpdb.nibiohn.go.jp, accessed on 22 December 2023) of crude drugs reveals that one of the major components of “dokukatsu” is chlorogenic acid (CA). CA is contained in many foods, such as coffee and black tea [8,9]. CA has been reported to possess many beneficial effects such as anti-inflammatory, analgesic, and antipyretic activities [10]. Some animal examinations have been proposed to investigate the beneficial effects of CA against oxidative stress [11], neuroprotection [12], hypotension [13,14], and myocardial infarction [15] and on metabolic syndromes [16,17,18,19,20]. Recently, CA has also been reported to have anti-cancer activity. Ranjbary et al. reported that CA induced apoptosis and cell-cycle arrest in colorectal cancer cells [21]. Zeng et al. demonstrated that CA demonstrated the potent induction of apoptosis against breast cancer via the NF-kB signaling pathway [22]. Furthermore, there have been reports that CA inhibits epithelial–mesenchymal transition and the invasion of breast cancer through down-regulating LRP6 expression [23]. As mentioned above, recently, a variety of useful effects have been continuously reported for CA, and it is an important naturally occurring ingredient.
We expected that A. cordata produced in Tokyo using “Udo muro” cultivation has unique characteristics with respect to its chemical components. In this study, we investigated the effect with or without “Udo muro” cultivation and drying temperature on CA content in A. cordata root and compared that with A. cordata produced in other areas (“wakyoukatsu”). Furthermore, the usefulness of the root was evaluated using inhibitory activity tests such as nitric oxide (NO) production and melanin production using the Raw264.7 and B16F10 cell lines, respectively.
2. Materials and Methods
2.1. Material and Instruments
All solvents were acquired from FUJIFILM Wako Pure Chemical (Tokyo, Japan). Commercial material of CA was purchased from FUJIFILM Wako Pure Chemical Corporation.
High-performance liquid chromatography (HPLC) was performed using the JASCO LC-NetII/ADC system (JASCO Co., Tokyo, Japan) with a column oven (CO-4060), PDA detector (MD-4015), and pump (PU-4180). The number of cells was measured using the LUNAII automated cell counter (Logos Biosystems, Anyang, Gyeonggi-do, Republic of Korea).
2.2. Cultivation of A. cordata
The cultivation of the root of A. cordata was performed in an open field (gray lowland soil) and a semi-underground softening chamber (temperature 20 °C, humidity 90% or higher, and dark conditions) at the Tokyo Metropolitan Agriculture and Forestry Research Center. In May 2020, seed stocks were planted in the field, and the roots were cultivated using conventional methods. The planting space was 120 cm in row width and 60 cm in plant space. The amount of fertilizer used was N:P:K = 10 kg:20 kg:10 kg per 10a for the base fertilizer and N:K = 6 kg:6 kg per 10a for the top dressing. In late October, the aboveground part was cut and the root stock was dug up. The root stocks were washed to remove any soil that had adhered to them and sterilized by immersing them in a 500-fold diluted solution of benomyl hydrating agent for 30 min. The root stocks were placed in a plastic greenhouse, dried overnight, and then stored in a refrigerator (4 °C). These roots were divided into groups A and B; group B was preserved at a low temperature until the harvest time of group A. Group A root stocks were taken out of the refrigerator in mid-January 2021 and sterilized by instant immersion in an 800-fold diluted solution of metalaxyl M/TPN hydrating agent. The surface of the root stock was air-dried and instantly immersed in a 50 ppm solution of gibberellin. After the root stocks were air-dried, they were cultured in “Udo muro” and thoroughly irrigated. The softened stems were harvested after one month. In mid-February, the roots of group A were harvested from the softened stems, and the root stocks of group B were used in this study.
2.3. Dryness and Extraction of the A. cordata Roots Made in Tokyo and “Wakyoukatsu”
Two varieties of A. cordata roots (‘Yamagata’ and ‘Tama’) cultivated in Tokyo were used in this study (hereinafter referred to as Yam and Tam). These were obtained from Horticultural Science Division, Tokyo Metropolitan Agriculture and Forestry Research Center, Tokyo, Japan. The roots of Yam-A, Yam-B, Tam-A, and Tam-B were dried at 20 °C, 40 °C, and 60 °C for 72 h using a food dryer (TAIKI SANGYO Co. Ltd., Okayama, Japan) and 90 °C for 48 h using Forced Convection Oven (ADVANTEC, Tokyo, Japan). In addition, freeze-dried roots were also prepared. The two kinds of “wakyoukatsu”, which were domestically distributed, were purchased from Tochimoto Tenkaido Co., Ltd. (Osaka, Japan) and Uchida Wakanyaku Ltd. (Tokyo, Japan). Dried roots and two kinds of “wakyoukatsu”, which were cultivated in Gunma prefecture and Korea, were then milled and extracted with MeOH (1 L × 2), using a mantle heater at 60 °C for 2 h and evaporated.
2.4. Quantitative Analysis of CA
Approximately 50 mg of CA was accurately weighed and dissolved in methanol to a final volume of 5 mL. Then, 2.5 mL of the solution was removed, and, to this sample, methanol was added to make exactly 5 mL. This operation was repeated to prepare 4-fold and 8-fold diluted standard solutions. Approximately 50 mg of methanol extracts of the roots of Yam-A, Yam-B, Tam-A, Tam-B, or “wakyoukatsu”, were also accurately weighed and dissolved in methanol to a final volume of 5 mL (n = 3).
The following HPLC conditions were used for the quantitative analysis of CA: Detection: UV (254 nm); column: Waters XBridge C18 (5 mm, 4.6 × 150 mm); and column temperature: 40 °C. The mobile phase was (A) H2O with 0.1% trifluoroacetic acid (TFA) and (B) acetonitrile. The flow rate was 1.0 mL/min. The mobile phase used is described below: initially, 0 min A–B (95:5, v/v), then 0–45 min gradient system to A–B (0:100, v/v), and for 45–60 min, the linear isocratic elution was from A–B (0:100, v/v). Chromatograms were plotted using a photodiode array detector (DAD). Detector responses were measured as peak areas at UV absorbance 254 nm. The injection volume was 5 μL, and triplicate injections were used for each sample. Linearity was obtained for compounds between peak areas and concentrations of 12.5–50 µg/mL with correlation coefficient (r2) = 0.9999.
2.5. Cell-Based Assay
2.5.1. Cell Culture and Preparation of Test Samples
The B16F10 and Raw264.7 cells were obtained from the Japanese Collection of Research Bioresources Cell Bank, National Institute of Biomedical Innovation, Health and Nutrition (Osaka, Japan). B16F10 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (FUJIFILM Wako Pure Chemical) containing 10% fetal bovine serum and 1% penicillin/streptomycin (FUJIFILM Wako Pure Chemical). Raw264.7 cells were cultured in nutrient mixture of F-12 Hum (Sigma Aldrich, Tokyo, Japan) containing 10% fetal bovine serum and 1% penicillin/streptomycin. The methanol extracts of Yam-A, Yam-B, Tam-A, Tam-B, and 2 kinds of “wakyoukatsu” were dissolved in dimethyl sulfoxide (DMSO) at 250 mg/mL.
2.5.2. Inhibitory Activity of Melanin and Nitric Oxide Production
Inhibition of melanin production was assessed using B16F10 cells. The cells were seeded in 24-well plates at 2.5 × 104 cells/well. After 16 h of incubation (37 °C and 5% CO2), the cells were treated with 100 µM 3-isobutyl-1-methylxanthine (IBMX) (FUJIFILM Wako Pure Chemical) and 5 µM α-melanocyte-stimulating hormone (α-MSH) (FUJIFILM Wako Pure Chemical) for melanin induction, and each test sample was added at final concentrations of 62.5, 125, and 250 µg/mL. Albutin was used as positive control (100 µM) and cultured for 48 h. Cells were lysed with 150 μL of 1 N NaOH, followed by boiling at 60 °C for 1 h. The intracellular melanin contents were determined by measuring absorbance at 450 nm using a microplate reader (xMarkTX Microplate Absorbance Spectrophotometer, BIO-RAD, Hercules, CA, USA). Melanin production inhibition rate was presented as a percentage (%) of the control when control was 100%.
The cytotoxicity on B16F10 was measured using the Cell Counting kit-8 (Dojindo Laboratories, Kumamoto, Japan) assay as follows: The cells were seeded in 96-well plates at 2 × 104 cell/well. Each test sample was added at final concentrations of 62.5, 125, and 250 µg/mL. After 24 h incubation, 10 µL of Cell Counting kit-8 solution was added in each well. The cells were incubated for 2 h, and then the absorbance was measured at 450 nm using the microplate reader. Cell viability was calculated as a percentage (%) of the control (DMSO) when control was 100%.
Inhibition of NO production was assessed using Raw264.7 cells. The cells were seeded in 96-well plates at 2.0 × 105 cells/well (200 µL). After 2 h incubation (37 °C and 5% CO2), the cells were treated with 0.06 µg/mL interferon-γ (INF-γ: FUJIFILM Wako Pure Chemical) and 20 µg/mL lipopolysaccharide (LPS: Sigma Aldrich) for NO production; each test sample was added at final concentrations of 62.5, 125, and 250 µg/mL and incubated for 16 h. After incubation, 100 µL of supernatant of each well was divided into the other 96-well pate. The Griess reagent [24] (1% sulfanilamide/0.1% N-(1-naphtyl) ethylene diamine dihydrochloride in 2.5% H3PO4) was mixed with an equal part of cell culture medium of control and extracts from treated RAW 264.7 cells. The color development corresponding to NO level was assessed at 550 nm with a microplate reader. The percentage NO inhibition was determined according to the formula below. Positive control NG-Monomethyl-L-arginine Acetate (L-NMMA) (100 µM) was used. NO inhibitory ratio was calculated as follows: NO inhibitory ratio (%) = (1 − (AS − AN)/(AD − AN)) × 100; AS (Sample + LPS + INF-γ), AD (DMSO + LPS + INF-γ), and AN (non-treatment).
Cell viability of Raw 264.7 cells was measured using the remaining cells after the supernatant was removed. About 10 µL of Cell Counting kit-8 solution was added in each well. The cells were incubated for 2 h, and then the absorbance was measured at 450 nm using a microplate reader. Cell viability was calculated as a percentage (%) of the control (DMSO) when control was 100%.
3. Results and Discussion
3.1. Effect of Cultivation with or without “Udo muro” and of Temperature and Dryness on CA Content of the A. cordata Root Made in Tokyo
Crude drug of “wakyoukatsu” was generally dried. The Japanese Pharmacopoeia normally stipulates that crude drugs should be dried at 60 °C or less. Because it is not known what temperature is used to dry wakyokatsu, several drying temperatures were investigated. Furthermore, certain plant components have been reported to decrease as the drying temperature increases. For example, perillaldehyde and rosmarinic acid contained in perilla herb decreased when dried at 70 °C. The losses were significant even after drying at 30 and 50 °C for two days. Therefore, it is necessary to focus on the drying temperature. To compare the CA content and degree of loss for the roots of A. cordata and “wakyoukatsu”, the roots of A. cordata were dried at 20, 40, 60, and 90 °C. Non-heated dried roots were also prepared through freeze-drying. Then, 10 g of these dried roots were milled and extracted with 100 mL of methanol, and the methanol was evaporated. The quantity of methanol extract is shown in Table 1. The methanol extracts of Yam-A and Yam-B were greater than those of Tam-A and Tam-B. The extract of the freeze-dried root was less than that of the other dried extracts except Tam-B dried at 90 °C.

Table 1.
The quantity of methanol extract of each sample.
In order to compare the effect of cultivation with and without “Udo muro”, an HPLC analysis of CA was conducted on A. cordata root extracts that were freeze-dried and dried at 20, 40, 60, and 90 °C. Each extract was analyzed using HPLC-DAD. The CA content was assessed using peak area measurement with ultra violet (UV) absorbance at 254 nm. The CA content in extracts was evaluated through comparison with the retention time (RT) and UV spectrum of a standard compound. The CA peak area of each sample is shown in Figure 2. In Figure 2A, the comparison between with and without “Udo muro” cultivation of Yam is shown. Yam-A contained about two times more CA than Yam-B. The comparison between Tam-A and Tam-B is shown in Figure 2B. Significant differences in the CA content were not observed regardless of with and without “Udo muro” cultivation in root extracts dried at any temperature. Overall, the CA content tended to decrease with increasing drying temperature. It is suggested that “Udo muro” cultivation increased the CA content of some varieties.
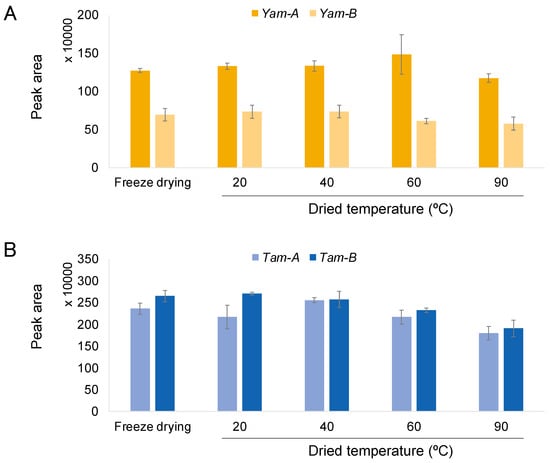
Figure 2.
The comparison of peak areas of each sample, which were analyzed using HPLC. (A) Yam-A and Yam-B, (B) Tam-A and Tam-B.
3.2. The Quantitative Analysis and Comparison of CA Content between Extracts Cultivated in Tokyo and Other Areas
In this study, the crude drug “wakyoukatsu” was used as a comparison sample cultivated in other areas. Two kinds of “wakyoukatsu”, which were cultivated in the Gunma prefecture and Korea, were purchased on the Japanese market. These crude drugs were also extracted with methanol and dissolved in methanol. Authentic standards of CA were also dissolved in methanol and analyzed using HPLC-DAD, and then a standard curve was constructed. The CA content was assessed using peak area measurements and compared using a standard curve, which was plotted using three pure CA solutions. The content of CA is shown in Table 2. The data are expressed as the amount of CA (mg) per 10 g of dry root weight. The CA contents of “wakyoukatsu” cultivated in the Gunma prefecture and Korea were 391.3 ± 11.5 mg and 151.0 ± 0.36 mg, respectively. The CA content in the A. cordata root cultivated in Tokyo was higher than that from Korea regardless of the drying temperature. In the Yam-B, the CA content was almost the same as that of “wakyoukatsu” cultivated in the Gunma prefecture, while the CA content of the other conditions, Tam-A, Tam-B, and Yam-A, were higher than those cultivated in the Gunma prefecture and Korea. These results suggest that one of the characteristics of the root of A. cordata cultivated in Tokyo is high in CA content.

Table 2.
The amount of CA (mg) per 10 g of dry root weight.
3.3. Evaluation of the Extract of A. cordata Cultivated in Tokyo Using Animal Cell Lines
CA has been reported to have inhibitory activity against NO and melanin production. To investigate the usefulness of the root of A. cordata cultivated in Tokyo, its anti-inflammatory and anti-melanogenesis activities were evaluated. The extracts of A. cordata cultivated in Tokyo dried at 40 °C were used because they had the highest CA content. The anti-inflammatory activity was evaluated using Raw264.7 cells. The cells were induced to release NO through LPS and INF-γ with or without the extract samples. The production of NO was measured using the Griess method [24]. The data are presented as percent of inhibition rate. The cell viability is presented as a percent of the control. As a result of the assay (Figure 3), Tam-A and Tam-B exhibited weak inhibitory activity with IC50 at 124.6 µg/mL and over 125 µg/mL, respectively. Yam-A, Yam-B, and two kinds of “wakyoukatsu” showed weak cytotoxicity. In this assay, CA was used at concentrations of 11.3, 22.5, 45, and 90 µg/mL because the test samples of Tam-A and Tam-B at 125 µg/mL included CA production at a concentration of about 70 µg/mL, as per the values calculated using a standard curve. However, CA showed no activity at any concentration. CA inhibits NO production at 0.5 mM (177 μg/mL) [25]. The extract used in this study needs to be approximately 300 µg/mL to contain this CA content. Because the 300 µg/mL extract precipitates when dissolved in the medium, the highest concentration tested was at 250 µg/mL; certain precipitation occurred even at 250 µg/mL. This may have contributed to the high SD. It was difficult to accurately evaluate the activity of the extract. The results suggest that other components have nitric oxide production inhibitory activity in addition to CA.
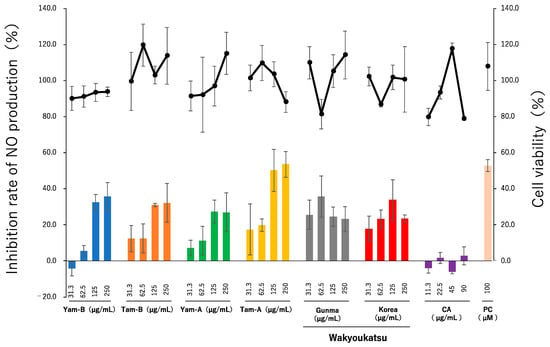
Figure 3.
The inhibitory activity of the extract of A. cordata root cultivated in Tokyo and other production areas (Wakyoukatsu). The NO inhibitory activity is expressed as inhibition rate (bar graph). The cell viability is expressed as a line graph. L-NMMA was used as positive control (PC) at 100 µM.
The anti-melanogenesis activity was evaluated using B16F10 cells. Melanogenesis was induced by 100 µM IBMX and 5 µM α-MSH with or without extract samples. The cell viability was presented as percent of control. As a result of the assay (Figure 4), all extracts exhibited cytotoxicity. However, CA showed anti-melanogenesis activity in a dose-dependent manner without cytotoxicity. It was considered that the activity of CA including that of the root of A. cordata was not cytotoxic. Several examples of agricultural crops exhibiting inhibitory effects on melanin production have been reported. Steamed sweet potato extract suppressed melanogenesis in mouse melanoma B16 cells [26]. Chlorogenic acid and three isochlorogenic acids were identified as the main phenolic constituents of the sweet potato extract. These four phenolic compounds equally suppress melanogenesis. The melanogenesis suppression ratio of the phenolic compounds corresponded to 98% of the total suppressive activity of the steamed sweet potato extract. Furthermore, the spinach extract suppressed melanin formation in B16 cells. This activity was the highest in the high-molecular-weight fraction. A common feature of the activities exhibited by these two crops is that they both have strong antioxidant activity. A. cordata has been reported to exhibit strong antioxidant activity [27]. Therefore, although the anti-melanogenic activity is masked by cytotoxicity, it is thought that by removing the cytotoxic components, it is possible to bring out the original potential. These results suggest that the usefulness of the root extract of A. cordata cultivated in Tokyo is not limited to CA and may depend on other components.
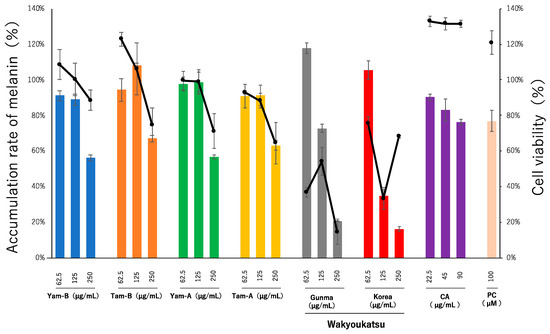
Figure 4.
The anti-melanogenesis activity of the extract of A. cordata root cultivated in Tokyo and other production areas (Wakyoukatsu). The bar and line graphs express accumulation rates of melanin and cell viability, respectively. Albutin was used as positive control (PC) at 100 µM.
4. Conclusions
In this study, the CA content of A. cordata cultivated in Tokyo was evaluated through a quantitative analysis. Furthermore, the effect of “Udo muro” cultivation and drying temperature on the CA content was investigated. As a result of the analysis, the CA content of A. cordata cultivated in Tokyo tended to decrease with increasing drying temperature. The effect of “Udo muro” cultivation on CA content was different among the two breeds. In the Yam, the CA content increased about two times upon “Udo muro” cultivation, while no change was observed in Tam. Because we expected that the root of A. cordata cultivated in Tokyo would contain abundant CA, the biological activity associated with CA was assessed. The inhibitory activity against NO production was observed using the methanol extracts of Tam-A and Tam-B. However, it was suggested that these activities were not caused by CA but by other components. In the anti-melanogenesis assay, the activity was considered hidden from other cytotoxic components. Therefore, it was impossible to evaluate the activity of the extracts. Because all the extracts tested in this study showed cytotoxicity, it is thought that this component is permanently present in the roots of A. cordata. The root has been used as a crude drug in Japan, Korea, and China, and its safety is not an issue. As CA has been shown to be active, the root of A. cordata produced in Tokyo can be expected to be used in the cosmetics field.
Thus, we concluded that the root of A. cordata cultivated in Tokyo is useful because of its high CA content and other beneficial components.
Author Contributions
Conceptualization, H.M. and H.F.; methodology, H.M.; formal analysis, H.M. and H.K.; resources, Y.O. and C.M.; writing—original draft preparation, H.M.; writing—review and editing, H.F. and K.Y.; supervision, N.K. and K.Y.; project administration, N.K. and K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- The Ministry of Health, Labour and Welfare, Japan. MHLW Grants System (2012). Available online: https://mhlw-grants.niph.go.jp/system/files/2011/114011/201129014A/201129014A0006.pdf (accessed on 10 February 2024).
- Qu, F.; Zhou, J.; Zhou, Z.; LI, H.; Burrows, E. Genetic analysis of Aralia cordata Thunb by RAPD. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 17–22. [Google Scholar] [CrossRef]
- Yoshio, O. About “Udo muro” in underground. Kensetsukikaiseko 2017, 69, 86–87. (In Japanese) [Google Scholar]
- Tokyo Udo Monogatari Editorial Committee. Tokyo Udo Story: Magazine Commemorating the 45th Anniversary of the Founding of the Tokyo Udo Producers Association; Tachikawa: Tokyo Udo Producers Association: Tokyo, Japan, 1997; pp. 76–95. [Google Scholar]
- The Ministry of Health, Labour and Welfare, Japan. The Japanese Pharmacopoeia Eighteenth Edition. 2021. Available online: https://www.mhlw.go.jp/content/11120000/000912390.pdf (accessed on 26 February 2024).
- Kim, J.G.; Lee, J.W.; Le, T.P.L.; Han, J.S.; Kwon, H.; Lee, D.; Hong, J.T.; Kim, Y.; Le, M.K.; Hwang, B.Y. Diterpenoids and diacetylenes from roots of Aralia cordata with inhibitory effects on nitric oxide production. J. Nat. Prod. 2021, 84, 230–238. [Google Scholar] [CrossRef]
- Puzerytė, V.; Viškelis, P.; Balčiūnaitienė, A.; Štreimikytė, P.; Viškelis, J.; Urbonavičienė, D. Aralia cordata Thunb. as a source of bioactive compounds: Phytochemical composition and antioxidant activity. Plants 2022, 11, 1704. [Google Scholar] [CrossRef]
- Perrone, D.; Donangelo, R.; Donangelo, C.M.; Farah, A. Modeling weight loss and chlorogenic acids content in coffee during roasting. J. Agric. Food Chem. 2010, 58, 12238–12243. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Zock, P.L.; Katan, M.B. Consumption of high doses of chlorogenic acid, present in coffee, or of black tea increases plasma total homocysteine concentrations in humans. Am. J. Clin. Nutr. 2001, 73, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; De Souza, G.E.P. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Suzuki, O.; Igarashi, K. Protective effects of chlorogenic acid on paraquat-induced oxidative stress in rats. Biosci. Biotechnol. Biochem. 1996, 60, 765–801. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kagawa, D.; Ochiai, R.; Tokimitsu, I.; Saito, I. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. Hypertens. Res. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Suzuki, A.; Fujii, A.; Yamamoto, N.; Yamamoto, M.; Ohminami, H.; Kameyama, A.; Shibuya, Y.; Nishizawa, Y.; Tokimitsu, I.; Saito, I. Improvement of hypertension and vascular dysfunction by hydroxyhydroquinone-free coffee in a genetic model of hypertension. FEBS Lett. 2006, 580, 2317–2322. [Google Scholar] [CrossRef]
- Kanno, Y.; Watanabe, R.; Zempo, H.; Ogawa, M.; Suzuki, J.; Isobe, M. Chlorogenic Acid attenuates ventricular remodeling after myocardial infarction in mice. Int. Heart J. 2012, 54, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez de Sotillo, D.V.; Hadley, M. Chlorogenic acid modifies plasma and liver concentrations of: Cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J. Nutr. Biochem. 2002, 13, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chang, C.; Liu, Y.; Chen, Z. Effect of chlorogenic acid on disordered glucose and lipid metabolism in db/db mice and its mechanism. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2011, 33, 281–286. [Google Scholar] [PubMed]
- Li, S.Y.; Chang, C.Q.; Ma, F.Y.; Yu, C.L. Modulating effects of chlorogenic acid on lipids and glucose metabolism and expression of hepatic peroxisome proliferator-activated receptor-α in golden hamsters fed on high fat diet. Biomed. Environ. Sci. 2009, 22, 122–129. [Google Scholar] [CrossRef]
- Wan, C.W.; Wong, C.N.Y.; Pin, W.K.; Wong, M.H.Y.; Kwok, C.Y.; Chan, R.Y.K.; Yu, P.H.F.; Chan, S.W. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef]
- Karthikesan, K.; Pari, L.; Menon, V. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem. Biol. Interact. 2010, 188, 643–650. [Google Scholar] [CrossRef]
- Ranjbary, A.G.; Bagherzadeh, A.; Sabbaghi, S.S.; Faghihi, A.; Karimi, D.N.; Naji, S.; Kardani, M. Chlorogenic acid induces apoptosis and cell-cycle arrest in colorectal cancer cells. Mol. Biol. Rep. 2023, 50, 9845–9857. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Hao, J.; Zhang, Q.; Jin, R.; Luo, Z.; Yang, X.; Liu, Y.; Lu, Q.; Ouyang, Y.; Guo, H. Chlorogenic Acid Inhibits Epithelial-Mesenchymal Transition and Invasion of Breast Cancer by Down-Regulating LRP6. J. Pharmacol. Exp. Ther. 2023, 384, 254–264. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, S.Y.; Park, L.Y.; Myung, D.S.; Rew, J.R.; Joo, Y.E. Chlorogenic acid suppresses lipopolysaccharide-induced nitric oxide and interleukin-1β expression by inhibiting JAK2/STAT3 activation in Raw 264.7 cels. Mol. Med. Rep. 2017, 16, 9224–9232. [Google Scholar] [CrossRef] [PubMed]
- Shimozono, H.; Kobori, M.; Shinmoto, H.; Tsushida, T. Suppression of the melanogenesis of mouse melanoma B16 cells by sweet potato extract. Nippon Shokuhin Kagaku Kogaku Kaishi 1996, 43, 313–317. [Google Scholar] [CrossRef]
- Jang, J.Y.; Seong, Y.H. Anti-nociceptive and anti-inflammatory effect of an ethanol extract of the leaf and stem of Aralia cordata. Nat. Prod. Sci. 2014, 20, 301–305. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).