Abstract
The seed industry plays a crucial role in global food production but it faces a persistent challenge in ensuring the health and quality of seeds, particularly those of tomato and pepper seeds, which represent key seed commodities on the global market. Seeds can serve as potential pathways for the introduction and dissemination of seed-borne bacteria, which may have devastating effects on crop yield, farmers’ remunerability, and food security. Therefore, fungicides and other antimicrobial compounds are extensively used to disinfect the seeds, thus increasing the input of chemicals in the agri-environment. In this review, we address aspects that connect disease epidemiology with seed infection and health, including seed contamination, endophytic colonization, and seed-borne infections. We focused on the main bacterial diseases affecting tomato and pepper seeds by discussing their official seed testing methods as requirements supporting a smooth seed trade. Moreover, we present a survey on the past and recent innovations for seed treatments, focusing on sustainable disinfection methods. Therefore, this review will be a short but indispensable guide for seed technologists and pathologists involved in the production of high-quality seeds, providing indications and suggestions to contrast seed-borne pathogen dissemination and avoid international controversies and complaints by phytosanitary authorities, extension services, and farmers.
1. Introduction
“Seeds are a basic input for all crop production: all farmers need good seed, irrespective of their farming systems and the markets that they focus on” [1]. The seed sector is probably the most important agricultural input for growers to produce crops for food, feed, and non-food uses. Indeed, with the global population expected to reach 9.8 billion by 2050, access to quality seeds is critical for food security and nutrition, as is often stressed by the Food and Agriculture Organization of the United Nations (FAO). The seed industry plays a pivotal role in ensuring crop productivity by strengthening the seed value chain and implementing good seed availability worldwide in terms of improved, well-adapted, productive, nutritious, and resilient genotypes: this appears fundamental in food-insecure parts of the world but also in high-income countries, to ensure correct remuneration to dedicated farmers. According to the International Standards For Phytosanitary Measures (ISPM) 5 [2], seeds (in the botanical sense) are a commodity intended for planting [2] and therefore a living material that farmers may use to produce crop plants, more frequently vegetables or cereals, but also ornamentals and weed species to be used in gardens, parks, and other public green. Seeds are a global commodity: in 2021, the market for commercial seeds grew by 7.1% over 2020, reaching an estimated value of USD 47,242 million [3], with a crucial role in promoting food security: indeed, almost 90% of the world’s food crops are grown from seeds [4]. In 2020, vegetable seeds had a market share of almost 17% in global seed sales of the seed industry; therefore, compared with other seed segments (e.g., maize, soybean, and cotton), the vegetable seed market is highly fragmented [5].
The seed “industry” greatly developed in the last few decades from the use of traditional genotypes restricted in small areas or regions that did not take advantage of any emergent technology for seed production to the breeding of modern cultivars that suits the need of high-yield crop plants, resilient to the challenges posed by a changing climate, and adaptable to the most different environments. Therefore, this jump required the transition to a modern seed production chain through the development and use of technologies that emerged from the fourth agricultural revolution and by the implementation of a multifaceted international regulatory background [6]. Indeed, the structure of the seed industry changed from a high number of small regional enterprises to a few big global players; these are currently able to introduce and implement advanced seed production chains, involving multiple actors and extending to several countries over the five continents (Figure 1). Then, market globalization and the need to pursue food security and safety required a trans-disciplinary approach by such a multistakeholder sector. The current efforts by the seed industry, besides breeding high-yield crop plants, are also directed at the development of sanitation methods to avoid the dissemination of seed-associated pests from area to area and from country to country. An assessment of the impact of such industrial development (e.g., the application of innovative sanitation methods) on countries and society is still missing; indeed, a sustainable modern agriculture technology assessment (SMATA) is desirable [7], for instance, to study the impact of massive production of tomato and pepper seeds on behalf of the major global players in low-income countries, on their society and rural communities. This might greatly help governmental bodies and organizations to make appropriate decisions and advocate the involvement of a larger group of stakeholders.
The international movement of seeds is thoroughly regulated; the main justification for such rules is to avoid the movement of plant pests that are associated with seeds from a region where they are established to areas afar that are still pest-free. Therefore, the FAO standard on the international movement of seeds was approved and published to provide guidance to national plant protection organizations (NPPOs) in identifying, assessing, and managing the pest risk associated with international seed trade [4].
Tomato seeds, and pepper seeds to a lesser extent, can also be considered global seed commodities; for instance, the global tomato seeds market is valued at USD 1.33 billion in 2023 and is expected to be worth USD 1.78 billion by 2027 [8]. Very frequently, trade and plant health are tightly connected when it comes to phytosanitary issues: for instance, in the case of tomato seeds, breeding parental lines is performed in one European country (e.g., The Netherlands), whereas the production of basic seeds is conducted in a second European country (e.g., Spain). Then, basic seeds are treated and manufactured in different European areas or countries to provide seeds to China, where the production of hybrid seeds is commonly performed. From China, large seed lots are again shipped to Europe for treatment as commercial hybrid seeds; finally, commercial packaging, final sale, and use may happen in the Americas (Figure 1).
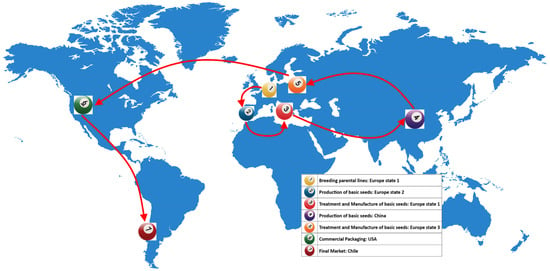
Figure 1.
Trade representation on the movement of vegetable seeds around the globe (adapted from [9]).
Seeds, as any other plant material in trade, may be associated with one or more plant pests; therefore, seeds are known pathways for the introduction and spread of pests into new territories when and wherever suitable hosts and environmental conditions are available. Tomato and pepper seeds are recognized pathways for a large number of pests and pathogens, both regulated and non-regulated. In the European Union, a notification and rapid alert system dealing with the interception of pests (EUROPHYT, https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en, accessed on 21 December 2023) has been in place for several years and is essential for the implementation of preventative measures based on robust and up-to-date data from trade in plants, including seeds. For instance, in 2022, 48 interceptions of tomato seeds infected by the tomato brown rugose fruit virus (ToBRFV) were notified to the European Commission, whereas in 2019, there were only 2 such notifications. The known number of seed-associated and seed-transmitted bacteria affecting tomato and pepper seeds is five: the Gram-positive Clavibacter michiganensis subsp. michiganensis (Cmm) and four Gram-negative Xanthomonads, Xanthomonas euvesicatoria pv. euvesicatoria (Xee), X. e. pv. perforans (Xep), X. hortorum pv. gardneri (Xhg), and X. vesicatoria (Xv). An additional one, Clavibacter capsici (basonym C. michiganensis subsp. capsici; Cc), has been recently reported to affect pepper seeds and not tomato seeds [10]. Finally, Pseudomonas syringae pv. tomato (Pst) is a seed-borne tomato pathogen but it has never been reported to affect pepper. Apart from the latter, all other bacteria are regulated in the European Union; the status of C. capsici is still to be defined, since it is a very recent finding and, presumably, will be regulated as well.
2. Seed Endophytes: Recruitment and Role
Endophytic communities are defined as populations of microorganisms that establish themselves within the internal tissues of plants without causing any apparent harm to the host plant [11]. Nevertheless, this is a debated definition as, theoretically, the microbiota within an apparently healthy plant could consist of a mix of mutualistic, commensal, and latent pathogenic strains [12]. These communities consist of a wide range of taxa, including fungi and bacteria, and have been studied in various plant species and different plant parts, from roots to aerial organs, with the aim of understanding the interactions with their host. Seed-associated microbial communities represent the initial inoculum source for the plant microbiota and assist seed conservation, germination, and seedling development through the production of suitable metabolites that are made available during the early stages of seed revitalization [13]. Most endophytes appear to originate from the plant rhizosphere [14]. Initially, the germinating seeds absorb water that is available in the sowing bed and starts to excrete some exudates that may attract bacteria from the surroundings, particularly from the spermosphere and the rhizoplane; such bacteria may enhance plant growth and vigor [15]. Microorganisms may also be transferred from plants’ vegetative parts to the seeds [16] or through the male gametophyte and, as the seed develops inside the fruit, it colonizes the embryo and then the surrounding endosperm [17]. Additionally, plants recruit endophytes from their phyllosphere as well and, moreover, microbial epiphytes are also recruited horizontally, therefore moving from plant to plant during particular weather conditions like, e.g., rains or showers [18]. Finally, some endophytes may be vertically transmitted through the seed [19].
Seed colonization represents a significant phase in endophyte biology and specific microbial communities (i.e., core seed microbiome) can persist there for years in a dormant state when the appropriate conditions are met; subsequently, such communities will again develop into the new plant originating from the germinating seed [14]. Endophytes found in different plant parts, including roots, stems, leaves, flowers, fruits, and seeds, may show a different taxonomic structure and a different functional behavior [19]. Some of them have been shown to exert beneficial effects on certain plant species, whereas they may exhibit pathogenic behavior towards other plant species. Therefore, the pathogenicity of some endophytes can be influenced by a number of biotic and environmental factors [20,21]. In general, endophytes play an important role in plant health and may positively influence plant growth and productivity; particularly, numerous studies revealed that endophytic microorganisms play a crucial role in controlling plant pathogens by assisting in nutrient availability and uptake, enhancing stress tolerance, and providing disease resistance [22]. Therefore, most of the published research focuses on the taxonomic identification and characterization of putative beneficial microbes colonizing the seeds, in order to (i) describe the seed-associated microbiota; (ii) highlight the significance of seed microbiota in seed quality; (iii) describe the plant colonization and the vertical transmission of seed endophytes; and (iv) verify the application potential of selected beneficial microbes [23].
The microbial communities associated with tomato seeds have been more extensively studied than the corresponding communities associated with pepper seeds: most published research focuses on beneficial endophytes as a significant portion of the total seed microbiome [24,25].
3. Phytopathogenic Bacteria in Tomato and Pepper Seeds
Tomato and pepper seeds are affected by several phytopathogens, mainly viruses and fungi; a few phytopathogenic bacteria are also known to cause important diseases and may be regarded as a global threat to the seed industry since most of them are seed-transmitted. Two Gram-positive bacteria are a true challenge, Cmm and Cc. The first may affect both tomato and pepper seeds, whereas the latter is specifically associated with pepper seeds [26]. Additionally, five Gram-negative bacteria are also known to be associated with tomato and/or pepper seeds; whereas, Pst is specific to tomato seeds. The four Xanthomonads show very different behavior towards their respective hosts: Xv and Xep are pathogenic to tomato seeds only, whereas Xhg and Xee may affect both tomato and pepper seeds [27]. Other phytopathogenic bacteria may infect tomato and pepper seeds, the most important of which are Ralstonia solanacearum and R. syzygii subsp. indonesiensis [28]; despite their importance as causal agents of destructive diseases, they are not known to be seed-transmitted. To understand the routes of seed infection by the phytopathogenic bacteria, the diseases caused by Clavibacters, Pst, and the Xanthomonads are briefly described.
3.1. Clavibacter michiganensis subsp. michiganensis (Smith) Davis et al. and Clavibacter capsici (Oh et al.) Li et al.
Clavibacter michiganensis subsp. michiganensis (Smith) (Cmm) is a Gram-positive bacterium that is the causal agent of bacterial canker and can produce significant yield losses and economic damage to the affected crops: indeed, during severe epidemics, up to 93% of plant deaths and approximately 50% of the decrease in average fruit weight are reported [29,30]. Cmm is considered a high-risk pathogen and is included in the A2 list of quarantine organisms by the European and Mediterranean Plant Protection Organization (EPPO) [31]. Similarly, Cc causes bacterial canker of pepper plants, as initially reported in Korea [32]. Both pathogenic Clavibacters invade and colonize the xylem vessels of their respective hosts, causing characteristic browning along the internal vasculature and the progressive degradation of vascular tissues, including unilateral leaf wilting, marginal leaf necrosis, stem cankers, and plant death [30,31,33]. Both bacteria are commonly recognized as being seed-transmitted, both internally in the seed and on the seed surface. Therefore, seed infection and colonization are essential aspects of bacterial canker epidemiology in tomato and pepper plants. Despite the fact that the rate of disease transmission by seeds is quite low [34], recent studies revealed that, under favorable conditions, one infected seed in 10,000 can give rise to devastating epidemics [35]. When infected seeds are sown, the bacterium can move systemically through the emerging seedlings, leading to cankers as the plants grow [36]. Other sources of primary inoculum are represented by infected plant debris after the harvest, where phytopathogenic bacteria are still viable. Theoretically, secondary inocula may be produced along the growing season, when conducive conditions favor pathogen evasion and dissemination through rains or sprinkler irrigation: in such cases, phytopathogenic bacteria may penetrate their respective hosts through stomata and fruit lenticels. Fruit infection is visible when typical bird’s eye spots are developing on the berries. Finally, Cmm can be easily horizontally transmitted when tomato plants are trimmed and/or pinched off: indeed, trimming blades or even operators’ hands can be easily contaminated by a single infected plant and, therefore, spread the inoculum to further plants during such agronomic practice.
Tomato seed contamination by Cmm may occur systemically, through the vascular tissue, or by its penetration through fruit lenticels and stomata. Then, being the bacterium highly tolerant to desiccation, it can survive on/in seeds for years [37].
3.2. Pseudomonas syringae pv. tomato (Okabe) Young, Dye, and Wilkie
Pseudomonas syringae pv. tomato (Pst) is a Gram-negative bacterium and the causal agent of the bacterial speck of tomato. Pst is considered one of the most significant and widespread pathogens affecting tomato plants [38]. Bacterial speck is a parenchymatic disease and causes significant economic losses for tomato growers worldwide, as it can reduce fruit yield and quality [39]. Pst penetrates its host plant mainly through stomata and lenticels and forms necrotic spots on the sepals and necrosis along the pedicel and colonizes symptomless flowers of different tomato varieties [40]. Typical symptoms of bacterial speck consist of small and dark lesions on leaves, usually surrounded by a yellow halo, provoked by a coronatine toxin, and necrotic spots along stems and on fruits; from necrotic lesions the bacteria can evade the plant as exudates and spread around. The damages caused by Pst can be remarkable in nurseries, greenhouses, and fields during warm and humid conditions [41]. Pst can survive in various environments, such as in plant debris, soil and, as an epiphyte, on leaf surfaces of the host plants or even weeds [42]; indeed, bacterial speck is a polycyclic disease and several secondary inocula provide an excellent means of pathogen spread in the field. While surviving in different environments for extended periods, the bacterium can act as a source of inoculum for new infections and continue its life cycle among susceptible tomato plants [43]. This bacterium is a seed-borne pathogen that primarily survives on the seed surface, attached to the desiccated pulp or internally within the seed; therefore, the bacterium is spread over long distances by infected seeds [44,45].
3.3. Xanthomonads: Xanthomonas vesicatoria (Doidge) Vauterin, Hoste, Kersters, and Swings, X. euvesicatoria pv. euvesicatoria (Jones et al.) Constantin et al.; X. euvesicatoria pv. perforans (Jones et al.) Constantin et al., and X. hortorum pv. gardneri (Jones et al.) Morinière et al.
Xanthomonads are Gram-negative yellow-pigmented bacteria affecting a large number of host plants around the world. Bacterial spot is an economically significant disease affecting tomatoes and different types of peppers. The disease is caused by four distinct bacteria belonging to the Xanthomonas genus: Xanthomonas vesicatoria, X. euvesicatoria pv. euvesicatoria, X. euvesicatoria pv. perforans, and X. hortorum pv. gardneri. Xanthomonads cause necrotic lesions on leaves with polygonal shape, surrounded by chlorotic tissue; symptoms on fruits are scab-like raised whitish lesions, which leads to their decreased market value [46]. Xanthomonads enter their host plants primarily through stomata and lenticels but wounds (e.g., trimming/pinching lesions or hail wounds) can also occasionally provide penetration sites into the host plants. Infection by Xanthomonads can cause yield losses of up to 50%. Barak et al. [47] reported that very few infected plants can lead to severe disease outbreaks. Bacterial spot is primarily a parenchymatic disease; therefore, the Xanthomonads are not supposed to efficiently colonize the xylem vessels and use them to move acropetally/basipetally within the host plants. Xanthomonads are considered seed-borne or seed-associated pathogens and the infected seeds are major means for long-distance dispersal of the diseases. Seeds are associated/contaminated by Xanthomonads, where bacteria can be deposited on the seed surface mixed with the infected pulp; internal seed infections are also reported [48]. Xanthomonads can survive on/in stored seed for up to 10 years [45,46]. Indeed, infested tomato and pepper seeds can serve as a significant source of primary inoculum in transplants and fruit production systems [49,50]. Giovanardi et al. [51] showed that a seed contamination level higher than 100 CFU g−1 is needed for a disease bacterial spot outbreak caused by Xee. Nevertheless, due to the polycyclic nature of the disease, it is important to emphasize that the threshold level may be variable, considering that pepper-growing areas have quite different climatic conditions and/or different agronomic practices (e.g., higher seeding rates). However, the long-distance spread of Xanthomonas spp. can be also associated with the trade of infected transplants harboring latent infections [52].
4. How Phytopathogenic Clavibacters, Pseudomonas syringae pv. Tomato, and Xanthomonads Colonize Tomato and Pepper Seeds
Microbes can be transmitted to and colonize their host plants horizontally, via the environment from a suitable source (e.g., another host plant), or vertically, from within the parent plant to the offspring via the seed [53]. Phytopathogenic bacteria may be transmitted to and colonize their specific host plants in the same way and, as plant endophytes, they may colonize seeds in their different parts, including the embryo, and their precise localization in seeds is consistent with their epidemiology, biology, penetration route, and colonization pattern [54]. The knowledge of the precise location of phytopathogenic bacteria in seeds is pivotal during the selection of an appropriate method for seed disinfection; for instance, phytopathogenic bacteria colonizing the external part of the seed may be easily removed by washing in a disinfecting solution. This sanitation approach is useless when the pathogens are located in the endosperm or the embryo. In general, bacteria can horizontally colonize seeds from the external environment via flowers or fruits: this pathway requires that the pathogen (i.e., Clavibacters, Pst, and Xanthomonads) is able to produce secondary inocula, which are dispersed in the field, e.g., by wind-driven rain droplets or sprinkler irrigation (Figure 2). Then, germs are able to penetrate their respective hosts through fruit lenticels, colonize the pulp extensively, and reach the seeds inside the endocarp; in such cases, phytopathogenic bacteria are mostly located above or under the seed coat and, rarely, in the endosperm [55,56]. Developing seeds can be vertically colonized from the parent plant: Cmm can multiply and acropetally colonizes the xylem vessels, thus reaching the fruit clusters and penetrating the developing fruits through the pedicels and moving until the placental tissue. Alternatively, Cmm penetration through fruit stomata causes the development of circular lesions in the exo- and mesocarp, where the bacteria multiply; such bacteria may then reach the seeds through the endocarp or the locular cavity when the tomatoes ripen, thus infecting the seed testa [57] (Figure 2).
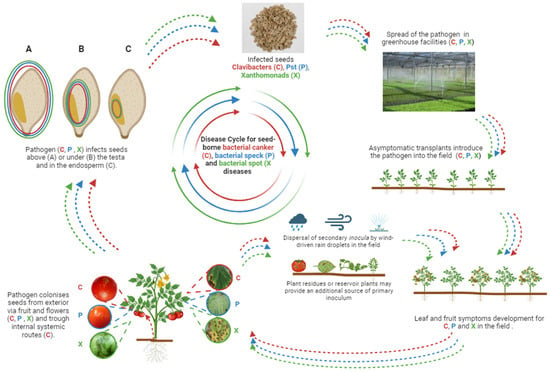
Figure 2.
Graphical representation of disease cycles for seed-borne bacterial canker (C), bacterial speck (P), and bacterial spot (X) diseases on tomato plants.
Theoretically, phytopathogenic bacteria may infect the seed through contaminated pollen: this is considered both horizontal and vertical transmission. Pollen, as a pathway for fruit and seed infections, has been described in a few pathosystems [58]; in particular, Dutta et al. [59] described the penetration of Acidovorax citrulli into watermelon seeds by the pollen germ tubes through the stigmas, thus resulting in the colonization of the ovules. Regretfully, dedicated studies have never been attempted for the tomato/pepper–phytopathogenic bacteria pathosystems.
Then, seeds represent the main source of primary bacterial inoculum in tomato and pepper seedling production facilities: the bacteria are introduced in the glasshouses by infected seeds or, alternatively, disseminated by water, as in the case of Pst and Xanthomonads (in case of poor hygienic conditions). Commercial cultivation of tomatoes and peppers starts with transplanting plantlets in open fields or in protected environments (e.g., tunnels and glasshouses); in such cases, transplants represent the main source of primary inoculum, whereas in contaminated water, plant residues from a previous crop or reservoir plants may provide an additional source of additional inoculum (Figure 2). Therefore, to ensure the highest phytosanitary standards along the tomato/pepper production chains, the key issue is the phytosanitary certification of seeds (mandatory in the case of regulated pathogens) and the possible analysis of transplants [46].
5. Detection of Phytopathogenic Bacteria in Tomato and Pepper Seeds
5.1. Direct Isolation on Agar Media
Direct isolation is a common detection technique in phytobacteriology and it is applied to plant samples to multiply the target bacteria on a solid medium, thus making them visible in the form of a colony (Figure 3). This technique is highly valuable in diagnosing plant bacterial diseases and allows the detection and characterization of specific bacterial isolates. Several media have been developed to support the growth of specific types of bacterial species, while inhibiting the growth of other microbes; they are based on knowledge of the nutritional requirements and physiological tolerances of the target bacterium. For direct isolation, semi-selective media are preferred, to exclude the growth of unwanted microorganisms, such as fungi or saprophytes [60]. For instance, Wilbrink’s medium [61], NSCAA [62], and MXV [63] are three of the numerous agar media commonly used for the isolation of Xanthomonads. Performance criteria for bacterial isolation from seeds have been assessed and, depending on the media and seed sample, the detectable concentrations of the target bacteria may vary from a few dozen up to approximately 104 CFU mL−1 [46]. Differently, for the direct isolation of Cmm, two different agar media, CMM1T and SCMF, performed much better than the previous ones, with an analytical sensitivity of around 25 CFU mL−1 of final concentrate [26].
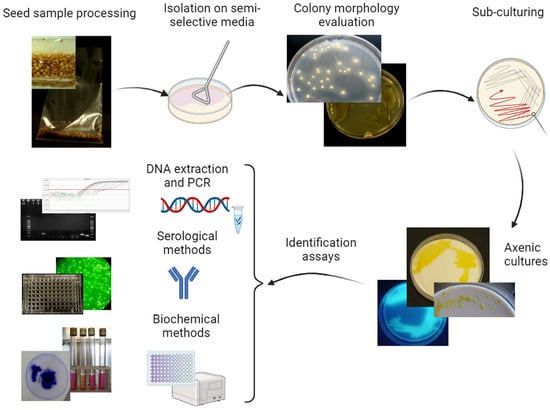
Figure 3.
Scheme for the detection and identification of Clavibacter michiganensis subsp. michiganensis, Clavibacter capsici, Xanthomonas euvesicatoria pv. euvesicatoria, X. e. pv. perforans, X. hortorum pv. gardneri, X. vesicatoria, and Pseudomonas syringae pv. tomato in tomato and pepper seed samples through direct isolation. After sample processing, plating seed extract on specific semi-selective media, sub-culturing pathogen-like colonies on nutritive media, identification of the axenic colonies through molecular, and serological and/or biochemical methods.
Thus, the direct isolation allows the recovery and growth of living bacteria that could be sub-cultured to obtain an axenic culture suitable for microbial identification. Moreover, the bacterial culture can be stored at −80 °C for further studies or strain characterization.
5.2. Serological Detection
The serological methods in plant bacteriology diagnostics were set up, approved by the phytosanitary authorities, and applied worldwide from the late 1970s to the 1990s of the last century, when reliable molecular methods were not available, to speed up the analyses and increase their analytical specificity in pathogen detection from both symptomatic and asymptomatic plant material, including seeds. The most popular serological methods applied are indirect immunofluorescence antibody staining (IFAS), with a sensitivity threshold between 103–104 CFU mL−1, enzyme-linked immunosorbent assay (ELISA), with a sensitivity threshold approximately at 104 CFU mL−1, dot-blot immunobinding assay (DIBA), fluorescent in situ hybridization (FISH), and more recently implemented—from half of the 1990s to half of the 2000s—the immunomagnetic separation (IMS) method. Interestingly, after the development and implementation of PCR, these immunological assays were studied in combination with molecular methods (i.e., PCR-FISH, PCR-ELISA, and PCR-IMS) to improve the detection threshold up to a few colonies per mL [64]. Nowadays, serological methods are used to confirm or confute the results of direct isolation or the response of molecular methods. Cross reactions are the main concern of serological methods, especially ELISA; indeed, unknown seed endophytes or saprobes may share the same epitopes or antigenic determinants, with Clavibacters or Xanthomonads [65,66]. This problem should be tackled by using a second detection method that is based on a different scientific principle, as previously highlighted. Cmm was one of the numerous bacterial pathogens, which was used to produce monoclonal antibodies, aiming its detection in seed samples [64]. Moreover, an IMS method was set up and coupled with an enrichment step, which allowed Cmm detection at low concentrations (approx. 10 CFU mL−1), similarly to pathogen concentrations that can be found in naturally-infected tomato seeds (approx. 10–102 CFU mL−1) [46,67]. Serological methods for the detection of the Xanthomonas species in tomato and pepper seeds are present in the earlier literature; monoclonal antibodies were produced for the detection of Xanthomonas campestris pv. Vesicatoria, the older name that comprised the nowadays three species of the Xanthomonas genus causing bacterial spot in tomato and pepper plants. In that paper, the specificity assay failed by reacting with Pseudomonas gardneri, now called Xanthomonas hortorum pv. Gardneri. Officially, the EPPO Bulletin suggests IFAS or ELISA assays on bacterial axenic cultures as well as identification methods and not only on seed sample extracts as optional screening tests [46]. The National Seed Health System (NSHS) protocol, the only official protocol for the detection and identification of Pst, allows culture identification through biochemical methods and pathogenicity assay (NSHS So. 3.1, 2001; https://seedhealth.org/files/2022/03/So-3.1-Pseudomonas-syringae-pv-tomato-%E2%80%93-tomato-word.doc.pdf, accessed on 10 January 2024). In the recent past, monoclonal antibodies were obtained and used towards the lipopolysaccharide O-chains of Pseudomonas pathovars; the Mab Ps4e reacted against the Pst strain IPGR 140 but also reacted on nine other pathovars, clearly showing its lack of specificity [68].
5.3. Molecular Detection
From the early 1990s, at the beginning of the PCR era up to date, molecular methods have significantly increased the specificity and sensitivity of pathogen detection. Molecular detection methods sped up the analytical procedures that should ensure the phytosanitary quality of seed and, contemporarily, they gave the possibility to significantly increase the number of samples to be analyzed; this appears to be particularly important for seed companies involved in the international trade of seeds. Available PCR-based detection methods are numerous nowadays, from the conventional end-point PCR to the qPCR, to the loop-mediated isothermal amplification (LAMP), and to the last tool available nowadays: the digital droplet PCR (ddPCR). These faster methods are usually combined with pathogen extraction methods based on the use of commercial kits that have improved and standardized the yield and quality of the nucleic acids.
The phytosanitary quality of seeds may be checked at the origin, e.g., by the producing company and/or at the destination, e.g., by an accredited diagnostic laboratory in the importing country. Whereas the producing company usually analyses their own seeds prior to additional manipulations (e.g., addition of dyes, fungicide treatments, and addition of inert pelleting material), commercial seeds lots to be sampled and analyzed by labs in the importing countries are frequently “treated”; it has been noted that any material added to commercial seeds prior to packaging may have a negative impact on the yield and quality of nucleic acids and may strongly inhibit any PCR reaction. Therefore, a nucleic acid cleaning step is highly recommended after the extraction. In general, molecular methods are used twice along the flow charts: as a preliminary assay on the seed extract and as a test to confirm pathogen identity after isolating the putative target bacteria.
For the molecular detection of any phytogenic bacteria possibly present in tomato and pepper seeds, only one sample is required for extraction: the same seed extract is then analyzed, following the respective procedures, to check the possible presence of Clavibacters, Xanthomonads, and Pst; then, there is no need to prepare multiple extracts, thus speeding up the phytosanitary analyses [26,46]. For the detection of Cmm in tomato seeds, molecular methods are officially validated and recently updated. Several PCR adapted protocols are available through probes designed on several conserved regions (e.g., 16S-23S rDNA). Starting from the DNA of an axenic Cmm culture, an end-point duplex-PCR protocol [69] can be applied, as it is also suggested by EPPO [26]; a duplex-qPCR method on Cmm pure culture is also suggested [70]. Although the end-point PCR method may detect low numbers of Cmm cells (approx. 102 CFU g−1 seed) with high specificity, the PCR-based detection from seed extracts is still to be validated [51]. The more updated ISHI-Veg method suggests a multiplex-qPCR protocol that was adapted (with endogenous control primers) from different molecular protocols [70,71]; this protocol may be reliably applied to crude seed extracts and proved its high specificity and sensitivity. Nevertheless, direct isolation and a PCR test on axenic cultures is mandatory to identify and confirm the isolates as Cmm. At present, this validated molecular method is the most recent and reliable tool for Cmm detection directly on seed extracts and it is also approved by NSHS (So 2.1). A tool for the identification of Cmm colonies is the barcoding on a specific 350 bp region in 16S rDNA gene but, more rapidly, a Rep-PCR can be enough to identify the isolates in an axenic culture [26]. An LAMP PCR for the detection of Cmm directly on seed extracts was set up by using primers designed on the micA gene, coding a toxin towards the other subspecies of C. michiganensis [72]. This assay allows the detection of Cmm up to 104 CFU g−1 seed. The study compared the detection ability of the LAMP method with Immunostrip: both were able to detect Cmm in the seed samples. In a previous work, the same authors set up the LAMP method on pure Cmm cultures and, then, they compared the results with the available methods: in some cases, the conventional and the qPCR failed the detection; on the contrary, LAMP PCR detected Cmm in all the samples prepared as well as the avirulent strains [73]. Among the new molecular techniques requiring validation, Morcia et al. [74] set up a ddPCR, conceptually similar to qPCR but more sensitive, which is able to detect Cmm (and the Ralstonia solanacearum species complex, Rssc) directly from symptoms or from stems; nevertheless, the authors did not evaluate the robustness of such method directly on seeds. The primers for the ddPCR were designed on the 16S-23S region and they did not amplify other bacterial pathogens of tomato seeds, such as the Xanthomonads or Pst. For the detection of viable but not culturable (VBNC) Cmm strains, a study was carried out by improving a qPCR [75].
For the detection of Xanthomonads, two duplex end-point PCR methods are suggested, both together, to cover all pathogen species and pathovars causing bacterial spot [46]. The method is specific, using probes designed on sequences from an unknown fragment obtained by the amplified fragment length polymorphism (AFLP), but it cannot be directly applied on seed extracts because of its high detection threshold (approx. 105–106 CFU mL−1). However, it is suggested and adapted by EPPO [46] to perform it on Xanthomonas spp. Axenic cultures [76]; in addition, the primers to detect Xhg can fail on Iranian strains, questioning the reliability of these primers in all geographic areas [52]. The ISHI-Veg method, even if more updated, suggests two multiplex qPCR methods (without reference) on axenic cultures as well. In both official protocols, the direct isolation and the PCR on the axenic culture can validate the analysis; moreover, though it is just recommended, a pathogenicity assay may be performed to confirm the isolate identity. Although not yet validated, a multiplex qPCR was also developed, aiming at the detection of the whole set of Xanthomonas spp. that are described as the causal agents of bacterial spot [77]. The primers were designed on the hrpB7 gene of the Type III Secretion System (T3SS) and they were highly specific but, again, the method was not tested on seed extracts. Moreover, an isothermal reaction, through a recombinase polymerase amplification assay (RPA), was also developed and implemented by using primers designed on the hrpB (or hrcN) gene and evaluated for its use in the field (in situ) on symptomatic plants; such a technique showed a sensitivity threshold of about 106 CFU mL−1, which is acceptable during the analysis of symptomatic tissues, though the primers for Xep did not detect their bacterial target [78].
Pst detection and identification lack validated molecular methods and official guidelines for a diagnostic analysis: the reason is that this organism is not regulated in a large part of the world (EPPO Global Database, https://gd.eppo.int/, accessed on 12 January 2024). Nevertheless, a few methods based on end-point PCR and qPCR are reliable and available; in particular, Zaccardelli et al. [79] designed primers on the hrpZ gene and developed a specific assay to be used on pure cultures, symptomatic material, symptomless transplants, and experimentally contaminated seeds. The calculated detection threshold evaluated on crude extracts of leaves and seeds resulted in approx. 105 and 106 CFU g−1, respectively. A qPCR for Pst detection was studied using a molecular beacon (MB) PCR by Fanelli and coworkers [80]; the method, using primers designed from a Random Amplification of Polymorphic DNA (RAPD) unknown fragment, reached a sensitivity threshold of approx. 102 CFU mL−1, both in a pure culture suspension and in seed extracts. Seeds may contain bacteria in their VBNC state [81]: this mainly because of the long quiescent phase, or because sanitation treatments that temporarily block the culturability of bacteria. Therefore, the isolation might result in the underestimation of bacterial contamination or, possibly, to a false negative detection. This impacts on the evaluation of the efficacy of disinfection methods, where it is not generally possible to discriminate dead from viable phytopathogenic bacteria. Then, molecular methods could be improved by adding propidium monoazide (PMA) as was performed for Cmm, where the authors set up a new qPCR method to detect VBNC Cmm in seed samples [75]. Recently, a qPCR amended with PMA was also set up for the detection of Xanthomonads in tomato seed batches [82].
6. Seed Treatments
Seeds are a recognised and very efficient pathway for the introduction and dissemination of several pests and pathogens [83] and this is particularly true for tomato seeds, as the most important vegetable crop [84]. Therefore, to ensure their phytosanitary quality and allow their safe trade worldwide, seeds should be treated (or disinfected or sanitised) to reduce the presence of pathogens to a minimum [37]. An excellent example of hygiene in tomato seed production and pathogen control of Cmm is published by the international business chain system, Good Seed and Plant Practices (GSPP), to prevent tomato seeds being infected by Cmm [85]. Therefore, seeds are commonly treated/disinfected before commercialization or movement to ensure the production of good seedlings/transplants, to minimise yield losses, to maintain and improve crop quality, and to avoid the spread of harmful organisms [86]. However, the production of healthy and high value tomato and pepper seeds should consider all the phytosanitary challenges. Indeed, seed production, starting from healthy stock seeds tested negative for seed borne pathogens and produced in confined cropping areas, can promote quality and ensure healthy seeds [85]. These production strategies can be also used to reduce the input of copper-based compounds in the field during the cropping season, thus allowing a more sustainable pest management. In fact, bacterial diseases are difficult to manage once they have affected the crops, partly because there are few effective pesticides [87]. Copper (Cu) is a fundamental tool in conventional and integrated pest management (IPM) farming systems, despite its several limitations in the European Union [88]. These limitations on Cu-based pesticides are applied for copper, since a heavy metal accumulates in the environment (soil and water bodies) and, therefore, may have a deleterious impact on biodiversity. Moreover, the presence of copper-resistant or copper-tolerant phytopathogens, such as Cmm [89], Xanthomonas species [90], and Pst [91], is currently raising great concern and is making the control of these bacterial diseases quite cumbersome once they are established in the field. However, another key role in the management of bacterial diseases is played by tolerant/resistant tomato and pepper varieties. Host resistance can limit disease severity, the spread of bacterial secondary inocula in the field throughout the crop season, and, therefore, the use of bactericides [92]. Commercial varieties of tomato seeds with moderate tolerance against Cmm, moderate resistance (IR) against Xanthomonads (i.e., Xee, Xv, and Xep), complete resistance (HR) against Pst (i.e., race 0 and race 1), and of pepper seeds (Capsicum annuum L.) with resistance to bacterial spot are currently available on the market [30,93]. However, the continuous efforts to identify introgressions of resistance are challenging, since the development of Cmm, Pst, and Xanthomonad disease-resistant cultivars stimulates the emergence of new races and species of these pathogens. Moreover, the frequent negative correlation between fruit quality and disease resistance has made the introgression of resistance even more challenging [94].
As a matter of fact, the best way to ensure a fair and safe international trade of seed commodities is to avoid the burden of increasing the use of copper-based pesticides and to allow sustainable and remunerative crop management and yield, making “clean” seeds available to farmers. This goal may be reached by applying a set of disinfection methods along several different sanitation procedures. Physical, chemical, or biological methods for seed treatment have been proposed, from time to time, for eliminating or reducing the bacterial seed-borne inoculum (Table 1) as a primary management strategy to prevent disease outbreaks or epidemics [95].

Table 1.
List of physical, biological, and chemical treatments for tomato and pepper seeds and their disinfection efficacy from phytopathogenic bacteria.
6.1. Tomato and Pepper Seed Extraction Procedures
Botanically, tomatoes and peppers are berries but, though belonging to the same Solanaceae family, they show quite different fruit morphology and structure and such features impact on methods and procedures to extract and obtain seeds. Basically, tomato seeds are attached to the placental tissue surrounded by a thick layer of jelly polysaccharides and immersed in the locular cavity that, in ripe fruits, is filled by such polysaccharide matrix. In peppers, seeds also protrude into the locular cavity but without such a surrounding polysaccharide matrix and, additionally, the locular cavity is and remains empty in ripe fruits. Therefore, after opening ripe peppers (manually or mechanically), seeds are easily detached from the placenta, whereas in tomatoes, the cutting or breaking of fruits results in the production of a juicy mass containing the seeds. Harvested peppers can be kept at room temperature to increase seed maturity; later, the fleshy walls are cut and seeds are manually scraped off. In the case of large batches, unscraped cores or small whole fruits are covered with water and broken up with a mixing blade; then, mature seeds precipitate and the broken pulp, together with the immature seeds, remain floating in the water and can be discarded. Mature seeds are then rinsed and dried at room temperature under circulating air flow [111].
Large scale production of basic, certified, or commercial tomato seeds is, in general, fully mechanized; the ripe (frequently overripe) fruits are mechanically harvested and passed into a crusher that squashes the fruits into a mixture of pulp, juice, and gelatinous seeds. The mixture is then passed into a revolving cylindrical screen that separates the juice and the seeds from the remaining fruit debris through an appropriate mesh. Juice and seeds are collected into separate containers and the gelatinous seeds are then separated from the remaining pulp and cleaned from the polysaccharide gel by (i) fermentation or by (ii) adding 10% solution of sodium carbonate or, alternatively, by (iii) addition of hydrochloric acid. After such extraction treatments, seeds are immediately washed and, finally, dried at room temperature under circulating air flow [112].
As previously described, phytopathogenic bacteria affecting tomato and pepper can be present on the seed coat as well as within the innermost layer of the seed coat, where they are difficult to eradicate by treatments (i.e., chemicals) without damaging the embryo. These sources of infection have been the focus of numerous attempts to devise effective seed treatments for the elimination of bacterial pathogens.
Seed extraction from fruits is the initial challenging step during the seed production chain, where possible cross contaminations of seed borne pathogens may happen [113]. Indeed, bacterial spot (Xanthomonas spp.), bacterial speck (Pst), and bacterial canker (Cmm) produce lesions on the surface of fruits and bacteria growing inside these lesions can contaminate seeds during the seed extraction process [114]. Then, through seed extraction, bacteria can easily contaminate the seed externally; indeed, tomato seeds have numerous epidermal cells that form hairs and crevices on the seed coat that may provide areas and niches in which the pathogen may reside and, later, evade disinfection treatments [115]. Then, the jellified pectinaceous material surrounding seeds, covering the bacteria and protecting them once the seed has dried, are commonly removed through different manual or mechanical handling, such as fermentation, acid extraction, or pectinase addition to the washing solutions; those procedures are then followed by mechanical washing, drying, and winnowing of the inert material from the seed [116]. Another important goal of seed extraction is to facilitate the separation of seeds from the surrounding pulp to increase their nutritive value, germination performance, seed quality parameters [117], and, finally, act as a seed sanitation/disinfection step.
Natural fermentation of tomato pulp at room temperature for 24–72 h and manual seed extraction are commonly used for obtaining seeds [118] and such procedures have been found to decrease the proportion of infected seeds by Cmm in seed lots for many years [119]; regretfully, such an approach is not efficiently applicable for large scale seed production [117]. Nonetheless, Jones et al. [87] reported and stressed that tomato seed extraction by fermentation or acid extraction efficiently reduced the level of seed contamination by killing Xanthomonads. This was confirmed by Giovanardi et al. [51], who proved that the natural fermentation of tomato pulp obtained from symptomatic fruits generated healthy seeds that tested negative by agar plating and standard molecular assays [46]. Similar results, but referred to as Pst, were reported by Chambers and Merrimans [120], where the phytopathogenic bacteria were isolated from tomato seeds mechanically extracted from symptomatic fruits but not from seeds extracted from the pulp mass after either a natural or an acidic fermentation process. Pradhanang and Collier [121] compared different fermentation techniques (i.e., natural fermentation and acidic extraction, where pulp soaked in an equal volume of 5% hydrochloric acid (HCl) solution for 7 to 10 min, followed by immediate seed wash) and showed that this fermentation step was effective in decreasing the bacterial inoculum but still not reliable by itself to eliminate Cmm, unless it is followed by an HCl treatment of dry seeds. More recently, Degwale et al. [117] compared natural fermentation to an HCl-based treatment of tomato seeds; their results highlighted the potential of acid extraction in 2% HCl for 60 min to control seed-borne pathogens, as confirmed by the absence of any detectable microbial epiphytes.
For peppers, due to the anatomical and physiological characteristics of fruits and seeds, the most common seed extraction methods are manual or mechanical, with the possible use of a disinfectant during the extraction [122]. However, the literature is very poor, or even absent, on the possible impact of the different extraction methods on bacterial seedborne pathogens affecting pepper seeds.
6.2. Seed Sanitation Methods and Procedures
The use of “clean” seeds (e.g., sanitized and/or certified) is the most important preventive measure to control seed transmitted diseases, particularly those caused by phytopathogenic bacteria: this approach is considered the simplest yet the cheapest and safest method of managing different seed-associated pests [86]. The need to provide clean seeds to the growers led to decades of research on developing and implementing seed treatments [101,123]. Nonetheless, the efficacy of currently-available treatments for seed-borne bacteria (e.g., thermotherapy, use of chemicals, and application of bio-inoculants) are often partially effective, depending on the location of the pathogen on or in the seed; therefore, the control of seed-transmitted bacterial diseases is still a challenge in both organic and conventional production [124]. Moreover, the application of any seed treatment should take into proper account the methods and procedures used for seed extraction and the specific conditions required to keep the embryo vitality and the quality of seeds; these are fundamental aspects in crop production, since uniform seed germination and high seedling vigor contribute to successful crop establishment and performance [125]. In addition, seed treatments applied during a sanitation procedure (e.g., thermal treatments and application of chemicals) may concurrently suppress the vitality of seed-associated beneficial endophytes, which have a pivotal role in early seedling growth and establishment [22]. These seed endophytes serve as an important link between the microbiome vertical transmission to the next seedling generation as well; therefore, it is important to assess the consequences of seed treatment practices on the whole seed microbiota [126]. Little is known about the unintended adverse effects of seed treatments on non-target beneficial endophytes that may attenuate the net benefit of these practices. Therefore, the mechanisms behind seed treatment-endophyte-seedling growth interactions, which may have a role in the development of sustainable disease control strategies, should be deeply investigated [127].
Finally, treatments should not be applied to pelleted seed, to seeds that have been previously treated, or to old or poor-quality seeds because germination can be significantly reduced by the treatment and/or the pathogen may not be completely eliminated.
6.2.1. Chemical Seed Treatments
Chemical-based seed treatment methods, such as the application of generic disinfectants or bactericides, have been used to reduce the incidence (or the level of contamination) of pathogens in seeds for decades [97]. Chemical treatments are often simpler and more convenient to use, though care must be taken to use the minimum concentration of chemical(s) required for the minimum exposure time as these can also decrease seed vitality [128]. Chemical seed treatments are effective and commonly used for seedborne fungal diseases as well [129]. For the elimination of phytopathogenic bacteria associated to seeds, the most used chemicals for treatment are sodium hypochlorite in an aqueous solution (diluted NaOCl) and different acids [123,130]; these were found to be efficient in disinfecting tomato seed from Cmm [131]. The use of chlorine for the disinfection of tomato and pepper seeds is common but often, the used methods do not consider additional complex factors, which can affect results and compromise seed quality (e.g., the presence of a mass of organic matter, like the tomato pulp). One of the best sources for information on using chlorine as a disinfectant is the “White’s Handbook of Chlorination and Alternative Disinfectants”, a 1062-page compilation of chlorine information [132].
The application of diluted NaOCl in tomato seed treatments can vary from 0.5% to 20% (v/v) in a water mixture and the duration of soaking is from 2 to 40 min [45,133]. In all cases, seeds should be thoroughly rinsed in water after soaking to minimize any negative impact on seed vitality [134]. A chlorine concentration equivalent to less than 1% bleach can kill bacterial spores within 5 to 10 min [135]; therefore, since Clavibacters and Xanthomonads are non-spore forming bacteria, the same concentration is efficiently applied as disinfectant. Finally, McFarquhar [109] showed the potential of diluted NaOCl in disinfecting artificially-contaminated pepper seeds by Xee.
Inorganic and organic acids are the most popular chemicals used in organic seed production for their effectiveness in sanitation treatments for both tomato and pepper seeds [136]. HCl is also commonly used as a sanitizing agent in seed production [131]. In fact, tomato seed can be treated with a diluted solution of HCl at different concentrations (i.e., 1 to 5%) and for different soaking times (i.e., 1 to 60 min), to eliminate seed-borne bacterial pathogens such as Xanthomonas spp., Pst, and Cmm [97].
Other than HCl, acetic and peracetic (peroxyacetic) acids [128,137] and hydrogen peroxide are often used in combination in commercial formulation (e.g., Tsunami 100; Oxidate 2.0) and they were found to reduce pepper (Xanthomonads) and tomato (Cmm, Pst and Xanthomonads) pathogens by 80.3% and 99%, respectively [110,135].
Another common chemical disinfectant for tomato seeds is the trisodium phosphate (TSP). Its application allowed epiphytic bacterial pathogens to be reduced or even sanitized through seed soaking in a 10% aqueous solution (v/v) for 15 min, followed by rinsing in a cold-water bath for 5 min to remove the residual disinfectant prior to seed drying [138].
6.2.2. Physical Seed Treatments
Physical treatments of tomato and pepper seeds include thermal treatments with hot water, aerated steam, hot dry air, ozonization, and ultraviolet (UV) irradiation. These treatments have a number of advantages over other treatments: in most countries they (i) do not require registration or approval, (ii) have a wide spectrum of activity, (iii) do not leave any toxic or polluting residues, and (iv) are considered sustainable and eco-friendly [139].
Phytopathogenic bacteria are, in general, non-sporing and most of them do not grow at a temperature higher than 41 °C; indeed, the most thermophilic phytopathogenic bacteria have an optimum growth temperature between 31–36 °C [140]. Temperatures approaching 48–50 °C are then very deleterious for non-spore forming phytopathogenic bacteria, which are not able to survive; therefore, the most common temperature range for seed disinfection is between 48–55 °C [44,97]. For tomato seeds, no effect on germination was observed when seeds were heat-treated up to 55 °C in humid conditions [96].
Heat Treatments
Heat can be applied to seeds or other plant material as humid treatment (hot water or hot steam), in dry conditions (ventilated oven), or both. Hot water soaking is an old-age practice, which is usually effective for the sanitation of seeds of different plant species [137,141]. Although theoretically easy to perform, a heat treatment can be difficult to conduct because of the need to fine-tune the temperature required to kill the pathogen(s) without impacting the germinating potential, seedling vigor, and shelf life of the seed [128,131,142,143]. Moreover, treatment protocols implemented for small seed lots may be difficult or even impractical for large-scale production and there is no guarantee that all the seeds in a large treatment batch will attain the treatment temperature for the recommended duration [144].
Heat treatment may be applied for agricultural commodities by (i) immersion in hot water, (ii) exposure to vapor heat, and (iii) exposure to hot dry air. Hot water treatments of seeds and plant material are classical thermophysical methods in plant protection and are more eco-friendly and more effective than chemical treatments. In particular, heat can kill bacteria as seed endophytes, whereas chemical treatments are mostly effective against phytopathogens residing on the seed coat or, in a few cases, just below it. A compilation of hot water seed treatment conditions for different vegetables, including tomato and pepper, is publicly available [145]. Several examples of hot water treatment for seed disinfection by bacterial pathogens and their efficacy are listed in Table 1. The main disadvantage of hot water treatments is the need for post-treatment drying.
The application of dry heat to seed batches is equally as effective as wet heat; interestingly, the increase in temperature in dry conditions is higher than in humid conditions: Marinescu [146] demonstrated that 1 h at 53 °C in wet conditions had a potential to disinfect tomato seeds from Cmm equal to 1 h at 80 °C in dry air flow. Dry conditions are less dangerous for seeds and heat exposure can be much longer than in humid conditions: indeed, Murata et al. [99] showed that dry heat treatment at 70 °C for 4 to 6 days allowed the disinfection of tomato seeds from Cmm. Therefore, dry heat treatments, which have been developed and implemented several years ago, are also common practices applied to solanaceous seeds, as reported by Rui-yun et al. [147] and by Akman [148] to control external and internal seed-borne pathogens, such as fungi and bacteria.
Ozone
Ozone, (O3) is a reactive oxygen species (ROS) with a very high antimicrobial potential and ozonization is known to be an excellent method for disinfection, thus ensuring food hygiene, sterilization of utensils, facilities, and warehouses, and sanitation of drinkable water [149]. The strong potential of ozone in eliminating or reducing seed-borne bacteria and fungi in seeds has been demonstrated in recent years. The sterilization performance of O3 and its effects on seed germination closely depend on the concentration and duration of the specific treatment, the type and structure of the seed, and the target pathogen [150].
The mechanism behind the antimicrobial properties of ozone is attributed to either its strong oxidizing capacity or due to the generation of ROS during its decomposition [151]. Glycoproteins and lipids located in the bacterial and fungal cell membranes are destroyed by ozone or its byproducts [100]. The application of ozone to tomato seeds was attempted by Çetinkaya et al. [152]: they demonstrated the efficacy of disinfecting experimentally-inoculated seeds with Cmm and Pst by a gaseous ozone treatment at 90 and 120 min, without any negative effect on seed germination rate. Therefore, ozonization might be a very promising treatment, being cheap and effective, to eliminate phytopathogenic bacteria without affecting seed quality.
UV-C Light Irradiation
Finally, other methods based on physical principles have been implemented for seed treatment: irradiation by UV-C light has been used as a way to sterilize seed surface [153] and there is evidence that some seed-transmitted fungi may be killed by an appropriate UV-C treatment [154]. Regretfully, UV-C irradiation does not have a significant direct effect on seed endophytes, particularly bacteria, despite some authors reporting that a UV-C treatment was able to induce a resistant state in cabbage against Xanthomonas campestris pv. campestris [155] but without showing a direct antibacterial effect against the pathogen. Therefore, such a technique does not suit the purpose of sanitizing/disinfecting seeds as a possible pathway for pathogen dissemination.
6.2.3. Microorganisms and Natural Products
Chemical seed dressing against seed-borne pathogens or against soil microorganisms that may affect seed germination and seedlings’ development is mainly performed against fungi, e.g., Fusarium spp. for cereals [156] or Ascochyta pisi for peas [157]. For seed-borne bacteria, there are far less options for their control [63]. Effective pesticides for seed dressings and fungicides among them are available in the global markets but the increasing trend and consciousness for a more sustainable agriculture are inducing the policy makers of several countries to revoke such active substances for their intended use. For instance, Thiram, a very popular fungicide that was used for several decades for seed dressing, is now prohibited in the European Union [158].
Nowadays, biological seed treatments represent one of the fastest growing sectors in the implementation of seed sanitation strategies worldwide. Bio-inoculants can protect plants against different seed-borne pathogens through numerous biological mechanisms, such as antibiosis, competitive exclusion, or inducing systemic resistance in host plants [159,160,161]. They represent an effective alternative technology to chemical-based plant protection, with the potential for a large-scale application by maintaining seed quality and promoting healthy plant growth in sustainable agricultural systems [162]. Thus, the demand for biological solutions for seed treatment is increasing, also in view of consumers’ acceptance for chemical-free food.
Beneficial microorganisms or natural products can be applied through coatings onto the seed surface for the protection of seeds and seedlings from seed-borne and soil-borne pathogens [163]. For seed treatments, various methods are used, such as seed coating, seed pelleting, seed washing, or seed dressing, which usually consists of applications where liquid bio-inoculants (generally supplemented with adhesives) are sprayed onto the seeds either by hand, rotating drums, or automated seed coaters, followed by drying with forced air using a seed drying equipment.
Recently, the application of bio-inoculants to seeds has increased because of increasing public concern for safety issues and awareness for a healthy environment, sustainable development, and health hazards caused by the excessive use of agrochemicals [164]. However, research on the commercial use of beneficial microorganisms as seed inoculants is limited and there are only a few examples of their commercial development, implementation, and use. Indeed, only few biopesticides formulations for seed coating are currently registered and specifically commercialized in Europe for seed-borne diseases, e.g., Cerall (Pseudomonas chlororaphis) marketed by Serbios srl, Italy, and Mycostop (Streptomyces griseoviridis K61) marketed by CBC Bioplanet, Italy (http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN, accessed on 15 January 2024). The same is also true for botanicals (i.e., plant extracts) as seed treatment products, despite many reports of bactericidal and fungicidal effects of such plant-derived compounds. The reason is possibly due to commercial constraints, like the high development and registration costs in relation to market size [165].
Biopesticides registered for tomato or pepper seeds against seed-borne bacteria, such as Cmm, Pst, or Xanthomonads, are still few or even absent. In the European Union, the regulatory framework for the registration of microbial biocontrol agent derives from that one currently used for chemical pesticides [166]. Therefore, there are still safety issues to be tackled, e.g., the dynamics and fate of such microbes in the environment, their possible classification as generally recognized as safe (GRAS) (https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras, accessed on 16 January 2024), their impact on biodiversity, etc. The cost of producing a thick dossier including the data required by the regulatory institutions may discourage the registration and marketing of biopesticides.
Nevertheless, different studies have been published reporting the potential of plant extracts or microbial biocontrol agents applied as seed bio-inoculants (Table 1). Among these studies, only a limited number of reports include all scales (e.g., laboratory, greenhouse, and commercial fields), even if different seed sanitation methods and procedures showed to be effective for up to 100% in eradicating bacterial pathogens under controlled conditions. For instance, Kasselaki et al. [124] showed that the application of Bacillus sp. as a seed bio-inoculant was able to reduce the presence of Cmm by up to 100%. Concerning natural products, Mbega et al. [101] found that 4 plant extracts (i.e., Aloe vera, Coffea arabica, Glycyrrhiza uralensis, and Yucca schidigera) out of 84 were able to experimentally disinfect tomato seeds inoculated with Xep in both in vitro and in planta assays.
In other studies, the inconsistency of the seed testing results with the in planta performance can be considered as one of the main challenges for the wide application of seeds coated with bio-inoculant (Table 1). Thus, results that clearly demonstrate and validate the efficacy of the delivery system and the microbial application covering all stages of the process are essential. Among other constraints concerning the microbial application techniques, it may be assumed that seeds can be coated only with a limited amount of inoculant, which might be a limiting factor for such treatment, since a threshold of a microbial biological control agent (mBCA) may be needed for a successful biological control. Again, factors influencing microbial viability and survival on seeds are the release of toxic exudates from the seed coat or the incompatibility between the inoculant strains and other seed-applied chemicals [162]. Microbial viability and survival may be improved by the coating materials (e.g., carriers and binders), which could also foster the performance of the target crop [167]. Since different beneficial microorganisms may react differently to coating, the development of coating materials that are compatible with a wide range of bio-inoculants is then crucial to the industry. Finally, the formulation of bio-inoculants should also take into proper consideration the local agriculture conditions and practices (such as soil conditions, use and nature of pesticides applied, fertilizers, and irrigation management) to optimize their performance and survival [162].
7. Conclusions
In this review, we described the epidemiology, seed health assays, and treatments of the most harmful seed-borne phytopathogenic bacteria affecting tomato and pepper seeds. These bacteria are responsible for the emergence or re-emergence of disease epidemics through their movement across international borders and their introduction into new areas, thus limiting crop production and threatening the seed industry worldwide.
According to the Farm to Fork Strategy, part of the European Union Green Deal [168], limitations on chemical pesticides foster the importance of an integrated disease management aimed to avoid the spread of these pathogens and to prevent the establishment of trade barriers between countries. Therefore, the implementation by the seed industry of international science-based standards for phytosanitary measures are essential to maintain seed quality and prevent the risk posed by the short to long dissemination of pathogens. Among these standards, seed production strategies (e.g., GSPP), diagnostic protocols for seed analysis and certification, seed extraction, and treatments are playing a key role for the seed industry. Moreover, non-chemical seed treatments, which include physical methods and microbial applications, together with the use of other antimicrobial agents of natural origin, like plant extracts, meet the requirements of the new agricultural policies and the market’s requests. In fact, the demand for biological seed treatment solutions is increasing in view of consumer acceptance for chemical-free food. Nevertheless, further research is needed to seek for new solutions for innovative, sustainable, and field-applicable IPM strategies against tomato and pepper seedborne phytopathogenic bacteria.
Author Contributions
B.X., D.G., E.B. and E.S. contributed equally to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest. All authors have read and agreed to the published version of the manuscript.
References
- Louwaars, N.P.; Manicad, G. Seed Systems Resilience—An Overview. Seeds 2022, 1, 340–356. [Google Scholar] [CrossRef]
- FAO Glossary of Phytosanitary Terms, ISPM 5. Available online: https://www.fao.org/3/mc891e/mc891e.pdf (accessed on 19 December 2023).
- Seed and GM Crop Market Analysis. Available online: https://www.spglobal.com/ratings/en/research-insights/topics/outlook-2023 (accessed on 17 July 2023).
- Dongyu, Q.U. A Statement by FAO Director-General of FAO. Available online: https://www.fao.org/director-general/speeches/detail/en/c/1321173/ (accessed on 4 November 2023).
- Malhotra, B. Global Vegetable Seeds Market Is Increasingly Fragmented and Diversified; S&P Global—Commodity Insights: London, UK, 2021; Available online: https://www.spglobal.com/commodityinsights/en/ci/research-analysis/global-vegetable-seeds-market.html (accessed on 3 November 2023).
- OECD Seed Schemes. Rules and Regulations; Organization for Economic Co-Operation and Development (OECD). 2023. Available online: https://www.oecd.org/agriculture/seeds/rules-regulations/ (accessed on 14 February 2024).
- Liang, D.; Tang, W.; Fu, Y. Sustainable Modern Agricultural Technology Assessment by a Multistakeholder Transdisciplinary Approach. IEEE Trans. Eng. Manag. 2023, 70, 1061–1075. [Google Scholar] [CrossRef]
- The Business Research Company. Tomato Seed Global Market Report; The Business Research Company: Dublin, Ireland, 2023; Available online: https://www.thebusinessresearchcompany.com/report/tomato-seeds-global-market-report (accessed on 6 November 2023).
- FAO. International Standard for Phytosanitary Measures n°38. International Movement of Seeds. 2018. Available online: http://www.fao.org/3/i7219en/i7219en.pdf (accessed on 11 December 2023).
- Oh, E.J.; Bae, C.; Lee, H.B.; Hwang, I.S.; Lee, H.I.; Yea, M.C.; Yim, K.O.; Lee, S.; Heu, S.; Cha, J.S.; et al. Clavibacter michiganensis subsp. capsici subsp. Nov., Causing Bacterial Canker Disease in Pepper. Int. J. Syst. Evol. Microbiol. 2016, 66, 4065–4070. [Google Scholar] [CrossRef]
- Wilson, D. Endophyte: The Evolution of a Term, and Clarification of Its Use and Definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Chee-Sanford, J.C.; Williams, M.M.; Davis, A.S.; Sims, J.K. Do Microorganisms Influence Seed-Bank Dynamics? Weed Sci. 2006, 54, 575–587. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; van Themaat, E.V.L.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing Structure and Assembly Cues for Arabidopsis Root-Inhabiting Bacterial Microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.B. Microbial Dynamics and Interactions in the Spermosphere. Ann. Rev. Phytopathol. 2004, 42, 271–309. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.K.; Sinclair, J.B. Principles of Seed Pathology; eBook; CRC Press: Boca Raton, FL, USA, 1996; pp. 1–560. ISBN 9780429152856. [Google Scholar] [CrossRef]
- Malfanova, N.; Lugtenberg, B.J.J.; Berg, G. Molecular Microbial Ecology of the Rhizosphere; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Santoyo, G. How Plants Recruit Their Microbiome? New Insights into Beneficial Interactions. J. Adv. Res. 2021, 40, 45–58. [Google Scholar] [CrossRef]
- Herre, E.A.; Knowlton, N.; Mueller, U.G.; Rehner, S.A. The Evolution of Mutualisms: Exploring the Paths between Conflict and Cooperation. Trends Ecol. Evol. 1999, 14, 49–53. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic Potential of Endophytic Bacteria. Curr. Opin. Biotechnol. 2014, 27, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.-J.; Sessitsch, A. Ecology and Genomic Insights into Plant-Pathogenic and Plant-Nonpathogenic Endophytes. Ann. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Silvestri, C.; Ahmad, T.; Hafiz, I.A.; Abbasi, N.A.; Manzoor, A.; Cristofori, V.; Rugini, E. Osmotin: A Cationic Protein Leads to Improve Biotic and Abiotic Stress Tolerance in Plants. Plants 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Samreen, T.; Naveed, M.; Nazir, M.Z.; Asghar, H.N.; Khan, M.I.; Zahir, Z.A.; Kanwal, S.; Jeevan, B.; Sharma, D.; Meena, V.S.; et al. Seed Associated Bacterial and Fungal Endophytes: Diversity, Life Cycle, Transmission, and Application Potential. Appl. Soil Ecol. 2021, 168, 104–161. [Google Scholar] [CrossRef]
- Bergna, A.; Cernava, T.; Rändler, M.; Grosch, R.; Zachow, C.; Berg, G. Tomato Seeds Preferably Transmit Plant Beneficial Endophytes. Phytobiome J. 2018, 2, 183–193. [Google Scholar] [CrossRef]
- Yildirim, K.C.; Orel, D.C.; Okyay, H.; Gursan, M.M.; Demir, I. Quality of Immature and Mature Pepper (Capsicum annuum L.) Seeds in Relation to Bio-Priming with Endophytic Pseudomonas and Bacillus spp. Horticulturae 2021, 7, 75. [Google Scholar] [CrossRef]
- Corrigendum—PM 7/42 (3) Clavibacter michiganensis subsp. michiganensis. EPPO Bull. 2022, 53, 148. [CrossRef]
- PM 7/110 (2) Xanthomonas spp. (Xanthomonas euvesicatoria pv. euvesicatoria, Xanthomonas hortorum pv. gardneri, Xanthomonas euvesicatoria pv. perforans, Xanthomonas vesicatoria) Causing Bacterial Spot of Tomato and Sweet Pepper. EPPO Bull. 2023, 53, 558–579. [CrossRef]
- PM 7/21 (2) Ralstonia solanacearum, R. pseudosolanacearum and R. syzygii (Ralstonia solanacearum species Complex). EPPO Bull. 2018, 48, 32–63. [CrossRef]
- Sen, Y.; van der Wolf, J.; Visser, R.G.F.; van Heusden, S. Bacterial Canker of Tomato: Current Knowledge of Detection, Management, Resistance, and Interactions. Plant Dis. 2015, 99, 4–13. [Google Scholar] [CrossRef]
- Peritore-Galve, F.C.; Tancos, M.A.; Smart, C.D. Bacterial Canker of Tomato: Revisiting a Global and Economically Damaging Seedborne Pathogen. Plant Dis. 2021, 105, 1581–1595. [Google Scholar] [CrossRef]
- Osdaghi, E.; Rahimi, T.; Taghavi, S.M.; Ansari, M.; Zarei, S.; Portier, P.; Briand, M.; Jacques, M.-A. Comparative Genomics and Phylogenetic Analyses Suggest Several Novel Species within the Genus Clavibacter, Including Nonpathogenic Tomato-Associated Strains. Appl. Environ. Microbiol. 2020, 86, e02873-19. [Google Scholar] [CrossRef]
- Yim, K.-O.; Lee, H.-I.; Kim, J.-H.; Lee, S.-D.; Cho, J.-H.; Cha, J.-S. Characterization of Phenotypic Variants of Clavibacter michiganensis subsp. michiganensis Isolated from Capsicum annuum. Eur. J. Plant Pathol. 2012, 133, 559–575. [Google Scholar] [CrossRef][Green Version]
- Nandi, M.; MacDonald, J.; Liu, P.; Weselowski, B.; Yuan, Z. Clavibacter michiganensis subsp. michiganensis: Bacterial Canker of Tomato, Molecular Interactions and Disease Management. Mol. Plant Pathol. 2018, 19, 2036–2050. [Google Scholar] [CrossRef]
- Chang, R.J.; Ries, S.M.; Pataky, J.K. Dissemination of Clavibacter michiganensis subsp. michiganensis by Practices Used to Produce Tomato Transplants. Phytopathology 1991, 81, 1276–1281. [Google Scholar] [CrossRef]
- Jones, J.B.; Zitter, T.A.; Momol, T.M.; Miller, S.A. Compendium of Tomato Diseases and Pests, 2nd ed.; APS Press: St. Paul, MN, USA, 2014; pp. 1–168. ISBN 978-0-89054-434-1. [Google Scholar]
- Quesada-Ocampo, L.M.; Landers, N.A.; Lebeis, A.C.; Fulbright, D.W.; Hausbeck, M.K.; Sen, Y.; Aysan, Y.; Mirik, M.; Ozdemir, D.; Meijer-Dekens, F.; et al. Genetic Structure of Clavibacter michiganensis subsp. michiganensis Populations in Michigan Commercial Tomato Fields. Plant Dis. 2012, 96, 788–796. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Plant Health. Scientific Opinion on the Pest Categorisation of Clavibacter michiganensis subsp. michiganensis (Smith) Davis et al. EFSA J. 2014, 12, 3721. [Google Scholar] [CrossRef]
- El-Fatah, B.A.; Imran, M.; Abo-Elyousr, K.; Mahmoud, A. Isolation of Pseudomonas syringae Pv. Tomato Strains Causing Bacterial Speck Disease of Tomato and Marker-Based Monitoring for Their Virulence. Mol. Biol. Rep. 2023, 50, 4917–4930. [Google Scholar] [CrossRef]
- Wilson, M.; Campbell, H.L.; Ji, P.; Jones, J.B.; Cuppels, D.A. Biological Control of Bacterial Speck of Tomato under Field Conditions at Several Locations in North America. Phytopathology 2002, 92, 1284–1292. [Google Scholar] [CrossRef]
- Vasileva, K.; Ganeva, D.; Bogatzevska, N. Species Composition of the Bacterial Population Colonizing Tomato Flowers. Bulg. J. Agr. Sci. 2022, 28, 677–690. [Google Scholar]
- Cement, A.; Saygili, H.; Horuz, S.; Aysan, Y. Potential of Bacteriophages to Control Bacterial Speck of Tomato (Pseudomonas syringae pv. tomato). Fresenius Env. Bull. 2018, 27, 9366–9373. [Google Scholar]
- Preston, G.M. Pseudomonas syringae pv. tomato: The Right Pathogen, of the Right Plant, at the Right Time. Mol. Plant Pathol. 2000, 1, 263–275. [Google Scholar] [CrossRef]
- Santamaría-Hernando, S.; López-Maroto, A.; Galvez-Roldán, C.; Munar-Palmer, M.; Monteagudo-Cascales, E.; Rodríguez-Herva, J.-J.; Krell, T.; López-Solanilla, E. Pseudomonas syringae pv. tomato Infection of Tomato Plants Is Mediated by GABA and l-Pro Chemoperception. Mol. Plant Pathol. 2022, 23, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Devash, Y.; Bashan, Y.; Okon, Y.; Henis, Y. Survival of Pseudomonas tomato in Soil and Seeds. J. Phytopathol. 1979, 60, 597–601. [Google Scholar] [CrossRef]
- Bashan, Y.; Diab, S.; Okon, Y. Survival of Xanthomonas campestris pv. vesicatoria in pepper seeds and roots in symptomless and dry leaves in non-host plants and in the soil. Plant Soil 1982, 68, 161–170. [Google Scholar] [CrossRef]
- Scortichini, M.; Stefani, E.; Elphinstone, J.G.; Vlami, M.B. PM 7/110 (1) Xanthomonas spp. (Xanthomonas euvesicatoria, Xanthomonas gardneri, Xanthomonas perforans, Xanthomonas vesicatoria) Causing Bacterial Spot of Tomato and Sweet Pepper. EPPO Bull. 2013, 43, 7–20. [Google Scholar] [CrossRef]
- Barak, J.D.; Vancheva, T.; Lefeuvre, P.; Jones, J.B.; Timilsina, S.; Minsavage, G.V.; Vallad, G.E.; Koebnik, R. Whole-Genome Sequences of Xanthomonas euvesicatoria Strains Clarify Taxonomy and Reveal a Stepwise Erosion of Type 3 Effectors. Front. Plant Sci. 2016, 7, 1805. [Google Scholar] [CrossRef]
- Zitter, T.A. Pepper Disease Control It Starts with the Seed; Department of Plant Pathology, Cornell University: Ithaca, NY, USA, 2004; p. 14853. Available online: https://www.northeastipm.org/ipm-in-action/ipmresources/resource-detail/?id=4345 (accessed on 28 October 2023).
- Jones, J.B.; Bouzar, H.; Stall, R.E.; Almira, E.C.; Roberts, P.D.; Bowen, B.W.; Sudberry, J.; Strickler, P.M.; Chun, J. Systematic Analysis of Xanthomonads (Xanthomonas Spp.) Associated with Pepper and Tomato Lesions. Int. J. Syst. Evol. Microbiol. 2000, 50, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Gitaitis, R.; Smith, S.; Langston, D.B. Interactions of Seedborne Bacterial Pathogens with Host and Non-Host Plants in Relation to Seed Infestation and Seedling Transmission. PLoS ONE 2014, 9, e99215. [Google Scholar] [CrossRef] [PubMed]
- Giovanardi, D.; Biondi, E.; Ignjatov, M.; Jevtic, R.; Stefani, E. Impact of Bacterial Spot Outbreaks on the Phytosanitary Quality of Tomato and Pepper Seeds. Plant Pathol. 2018, 67, 1168–1176. [Google Scholar] [CrossRef]
- Osdaghi, E. Xanthomonas euvesicatoria pv. euvesicatoria (Bacterial Spot of Tomato and Pepper). CABI Compend. 2022. [Google Scholar] [CrossRef]
- Frank, A.C.; Guzmán, J.P.S.; Shay, J.E. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Barret, M.; Guimbaud, J.F.; Darrasse, A.; Jacques, M.A. Plant Microbiota Affects Seed Transmission of Phytopathogenic Microorganisms. Mol. Plant Pathol. 2016, 17, 791–795. [Google Scholar] [CrossRef]
- Bashan, Y. Long-Term Survival of Pseudomonas syringae pv. tomato and Xanthomonas campestris pv. vesicatoria in Tomato and Pepper Seeds. Phytopathology 1982, 72, 1143–1144. [Google Scholar] [CrossRef]
- Bashan, Y. Detection of Cutinases and Pectic Enzymes During Infection of Tomato by Pseudomonas syringae pv. tomato. Phytopathology 1985, 75, 940. [Google Scholar] [CrossRef]
- Tancos, M.A.; Chalupowicz, L.; Barash, I.; Manulis-Sasson, S.; Smart, C.D. Tomato Fruit and Seed Colonization by Clavibacter Michiganensis Subsp. Michiganensis through External and Internal Routes. Appl. Environ. Microbiol. 2013, 79, 6948–6957. [Google Scholar] [CrossRef]
- Dutta, B.; Avci, U.; Hahn, S.K.; Hahn, M.G.; Walcott, R. Location of Acidovorax citrulli in Infested Watermelon Seeds Is Influenced by the Pathway of Bacterial Invasion. Phytopathology 2012, 102, 461–468. [Google Scholar] [CrossRef]
- Dutta, B.; Ha, Y.; Lessl, J.T.; Avci, U.; Sparks, A.C.; Johnson, K.L.; Walcott, R.R. Pathways of Bacterial Invasion and Watermelon Seed Infection by Acidovorax citrulli. Plant Pathol. 2015, 64, 537–544. [Google Scholar] [CrossRef]
- Gitaitis, R.D.; Walcott, R. The Epidemiology and Management of Seedborne Bacterial Diseases. Ann. Rev. Phytopathol. 2007, 45, 371–397. [Google Scholar] [CrossRef]
- Koike, H. The Aluminum-Cap Method for Testing Sugarcane Varieties against Leaf Scald Disease. Phytopathology 1965, 55, 317–319. [Google Scholar]
- Schaad, N.W.; Franken, A.A.J.M. ISTA Handbook on Seed Health Testing, Working Sheet No. 50, 2nd ed.; ISTA: Zurich, Switzerland, 1996. [Google Scholar]
- Sijam, K.; Chang, C.J.; Gitaitis, R.D. A Medium for Differentiating Tomato and Pepper Strains of Xanthomonas campestris pv. vesicatoria. Can. J. Plant Pathol. 1992, 14, 182–184. [Google Scholar] [CrossRef]
- Alvarez, A.M. Integrated Approaches for Detection of Plant Pathogenic Bacteria And Diagnosis Of Bacterial Diseases. Ann. Rev. Phytopathol. 2004, 42, 339–366. [Google Scholar] [CrossRef]
- Schaad, N.W. Detection of Seedborne Bacterial Plant Pathogens. Plant Dis. 1982, 66, 885–890. [Google Scholar] [CrossRef]
- Chu, P.; Waterhouse, P.; Martin, R.; Gerlach, W. New Approaches to the Detection of Microbial Plant Pathogens. BGER 1989, 7, 45–112. [Google Scholar] [CrossRef]
- De León, L.; Rodríguez, A.; López, M.M.; Siverio, F. Evaluation of the Efficacy of Immunomagnetic Separation for the Detection of Clavibacter michiganensis subsp. michiganensis in Tomato Seeds. J. Appl. Microbiol. 2008, 104, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Ovod, V.V.; Rudolph, K.; Krohn, K. Serological Classification of Pseudomonas syringae Pathovars Based on Mononoclonal Antibodies Towards the Lipopolysaccharide O-Chains. J. Bacteriol. 1997, 9, 526–531. [Google Scholar] [CrossRef]
- Pastrik, K.H.; Rainey, F.A. Identification and Differentiation of Clavibacter michiganensis Subspecies by Polymerase Chain Reaction-Based Techniques. J. Phytopathol. 1999, 147, 687–693. [Google Scholar] [CrossRef]
- Oosterhof, J.; Berendsen, S. The Development of a Specific Real-Time TaqMan for the Detection of Clavibacter michiganensis subsp. michiganensis. In Proceedings of the APS-IPPC Meeting, Honolulu, HI, USA, 6–10 August 2011; Available online: https://www.apsnet.org/meetings/Documents/2011_Meeting_Abstracts/a11ma777.htm (accessed on 12 December 2023).
- Wu, Y.-D.; Chen, L.-H.; Wu, X.-J.; Shang, S.; Lou, J.; Du, L.-Z.; Zhao, Z. Gram Stain-Specific-Probe-Based Real-Time PCR for Diagnosis and Discrimination of Bacterial Neonatal Sepsis. J. Clin. Microbiol. 2008, 46, 2613–2619. [Google Scholar] [CrossRef]
- Yasuhara-Bell, J.; Baysal-Gurel, F.; Miller, S.A.; Alvarez, A.M. Utility of a Loop-Mediated Amplification Assay for Detection of Clavibacter michiganensis subsp. michiganensis in Seeds and Plant Tissues. Can. J. Plant Pathol. 2015, 37, 260–266. [Google Scholar] [CrossRef]
- Yasuhara-Bell, J.; Kubota, R.; Jenkins, D.M.; Alvarez, A.M. Loop-Mediated Amplification of the Clavibacter michiganensis subsp. michiganensis MicA Gene Is Highly Specific. Phytopathology 2013, 103, 1220–1226. [Google Scholar] [CrossRef]
- Morcia, C.; Piazza, I.; Ghizzoni, R.; Terzi, V.; Carrara, I.; Bolli, G.; Chiusa, G. Molecular Diagnostics in Tomato: Chip Digital PCR Assays Targeted to Identify and Quantify Clavibacter michiganensis subsp. michiganensis and Ralstonia solanacearum in Planta. Horticulturae 2023, 9, 553. [Google Scholar] [CrossRef]
- Han, S.; Jiang, N.; Lv, Q.; Kan, Y.; Hao, J.; Li, J.; Luo, L. Detection of Clavibacter michiganensis subsp. michiganensis in Viable but Nonculturable State from Tomato Seed Using Improved qPCR. PLoS ONE 2018, 13, e0196525. [Google Scholar] [CrossRef] [PubMed]
- Koenraadt, H.; Van Betteray, B.; Germain, R.; Hiddink, G.; Jones, J.B.; Oosterhof, J. Development Of Specific Primers For The Molecular Detection Of Bacterial Spot Of Pepper And Tomato. Acta Hortic. 2009, 808, 99–102. [Google Scholar] [CrossRef]
- Strayer, A.L.; Jeyaprakash, A.; Minsavage, G.V.; Timilsina, S.; Vallad, G.E.; Jones, J.B.; Paret, M.L. A Multiplex Real-Time PCR Assay Differentiates Four Xanthomonas Species Associated with Bacterial Spot of Tomato. Plant Dis. 2016, 100, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Strayer-Scherer, A.; Jones, J.B.; Paret, M.L. Recombinase Polymerase Amplification Assay for Field Detection of Tomato Bacterial Spot Pathogens. Phytopathology 2019, 109, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Zaccardelli, M.; Spasiano, A.; Bazzi, C.; Merighi, M. Identification and in Planta Detection of Pseudomonas syringae pv. tomato Using PCR Amplification of hrpZPst. Eur. J. Plant Pathol. 2005, 111, 85–90. [Google Scholar] [CrossRef]
- Fanelli, V.; Cariddi, C.; Finetti-Sialer, M. Selective Detection of Pseudomonas syringae pv. tomato Using Dot Blot Hybridization and Real-time PCR. Plant Pathol. 2007, 56, 683–691. [Google Scholar] [CrossRef]
- Jiang, N.; Lv, Q.; Xu, X.; Walcott, R.; Li, J.; Luo, L. Induction of the Viable but Nonculturable State in Clavibacter michiganensis subsp. michiganensis and in Planta Resuscitation of the Cells on Tomato Seedlings. Plant Pathol. 2016, 65, 826–836. [Google Scholar] [CrossRef]
- Wang, H.; Wagnon, R.; Moreno, D.; Timilsina, S.; Jones, J.; Vallad, G.; Turechek, W.W. A Long-Amplicon Viability-qPCR Test for Quantifying Living Pathogens That Cause Bacterial Spot in Tomato Seed. Plant Dis. 2022, 106, 1474–1485. [Google Scholar] [CrossRef]
- Denancé, N.; Grimault, V. Seed Pathway for Pest Dissemination: The ISTA Reference Pest List, a Bibliographic Resource in Non-vegetable Crops. EPPO Bull. 2022, 52, 434–445. [Google Scholar] [CrossRef]
- Strider, D.L. Bacterial Canker of Tomato Caused by Corynebacterium michiganense: A Literature Review and Bibliography. Technical Bull. N. Carol. Agric. Exp. Stn. 1969, 193, 1–110. [Google Scholar]
- GSPP Standard for Tomato Seed and Young Plant Production Sites (Valid from 1st June 2022). 2022. Available online: https://www.Gspp.Eu/Images/Documents/GSPP_Standard_V3.3.pdf (accessed on 9 December 2023).
- Management of Seed-Borne Diseases: An Integrated Approach. In Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis & Management; Kumar, R., Gupta, A., Eds.; Springer: Singapore, 2020; pp. 717–745. ISBN 978-981-329-045-7. [Google Scholar]
- Jones, J.B.; Pohronezny, K.L.; Stall, R.E.; Jones, J.P. Survival of Xanthomonas campestris pv. vesicatoria in Florida on Tomato Crop Residue, Weeds, Seeds, and Volunteer Tomato Plants. Phytopathology 1986, 76, 430–434. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2018/1981 of 13 December 2018; OJ L 317, 14.12.2018; European Commission: Brussels, Belgium, 2018; pp. 16–20. [Google Scholar]
- Cooksey, D.A. Genetics of Bactericide Resistance in Plant Pathogenic Bacteria. Ann. Rev. Phytopathol. 1990, 28, 201–219. [Google Scholar] [CrossRef]
- Jones, J.B.; Lacy, G.H.; Bouzar, H.; Stall, R.E.; Schaad, N.W. Reclassification of the Xanthomonads Associated with Bacterial Spot Disease of Tomato and Pepper. Syst. Appl. Microbiol. 2004, 27, 755–762. [Google Scholar] [CrossRef]
- Griffin, K.; Gambley, C.F.; Brown, P.H.; Li, Y. Copper-Tolerance in Pseudomonas syringae pv. tomato and Xanthomonas spp. and the Control of Diseases Associated with These Pathogens in Tomato and Pepper. A Systematic Literature Review. Crop Prot. 2017, 96, 144–150. [Google Scholar] [CrossRef]
- Stall, R.E.; Jones, J.B.; Minsavage, G.V. Durability of resistance in tomato and pepper to Xanthomonads causing bacterial spot. Ann. Rev. Phytopathol. 2009, 47, 265–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Gao, Z.; Yang, W. Breeding for resistance to tomato bacterial diseases in China: Challenges and prospects. Hort. Plant J. 2018, 4, 193–207. [Google Scholar] [CrossRef]
- Adhikari, P.; Adhikari, T.B.; Louws, F.J.; Panthee, D.R. Advances and challenges in bacterial spot resistance breeding in tomato (Solanum lycopersicum L.). Int. J. Mol. Sci. 2020, 21, 1734. [Google Scholar] [CrossRef] [PubMed]
- Van Der Plank, J.E. Plant Diseases: Epidemics and Control. Soil Sci. 1964, 98, 279. [Google Scholar] [CrossRef]
- Bryan, M.K. Studies on Bacterial Canker of Tomato. J. Agric. Res. 1930, 41, 825–851. [Google Scholar]
- Shoemaker, P.B.; Echandi, E. Seed and Plant Bed Treatments for Bacterial Canker of Tomato. Plant Dis. Rep. 1976, 60, 163–166. [Google Scholar]
- Pyke, N.B.; Milne, K.S.; Neilson, H.F. Tomato Seed Treatments for the Control of Bacterial Speck. N. Z. J. Exp. Agr. 1984, 12, 161–164. [Google Scholar] [CrossRef]
- Murata, A.; Numata, I. Heat Endurance of Corynebacterium michiganense and Tomato Seeds to Dry Seeds. Proc. Kanto Pl. Protocol Soc. 1970, 17, 55–56. [Google Scholar]
- Cho, M.; Kim, J.; Kim, J.Y.; Yoon, J.; Kim, J.-H. Mechanisms of Escherichia Coli Inactivation by Several Disinfectants. Water Res. 2010, 44, 3410–3418. [Google Scholar] [CrossRef]
- Mbega, E.R.; Mortensen, C.N.; Mabagala, R.B.; Wulff, E.G. The Effect of Plant Extracts as Seed Treatments to Control Bacterial Leaf Spot of Tomato in Tanzania. J. Gen. Plant Pathol. 2012, 78, 277–286. [Google Scholar] [CrossRef]
- Kotan, R.; Dadasoğlu, F.; Karagoz, K.; Cakir, A.; Ozer, H.; Kordali, S.; Cakmakci, R.; Dikbas, N. Antibacterial Activity of the Essential Oil and Extracts of Satureja hortensis against Plant Pathogenic Bacteria and Their Potential Use as Seed Disinfectants. Sci. Hortic. 2013, 153, 34–41. [Google Scholar] [CrossRef]
- Karabuyuk, F.; Aysan, Y. Aqueous Plant Extracts as Seed Treatments on Tomato Bacterial Speck Disease. Acta Hortic. 2018, 1207, 193–196. [Google Scholar] [CrossRef]
- Umesha, S.; Kavitha, R. Prevalence of Bacterial Spot in Tomato Fields of Karnataka and Effect of Biological Seed Treatment on Disease Incidence. Crop Prot. 2006, 25, 375–381. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E. Protection of Tomato Seedlings against Infection by Pseudomonas syringae Pv. Tomato by Using the Plant Growth-Promoting Bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2002, 68, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Kritzman, G.A. Chemi-Thermal Treatment for Control of Seedborne Bacterial Pathogens of Tomato. Phytoparasitica 1993, 21, 101–109. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E. Reduction of Bacterial Speck (Pseudomonas syringae pv. tomato) of Tomato by Combined Treatments of Plant Growth-Promoting Bacterium, Azospirillum brasilense, Streptomycin Sulfate, and Chemo-Thermal Seed Treatment. Eur. J. Plant Pathol. 2002, 108, 821–829. [Google Scholar] [CrossRef]
- Sanogo, S.; Clary, M. Bacterial Leaf Spot of Chile Pepper: A Short Guide for Growers. New Mexico State University, College of Agriculture and Home Economics: Las Cruces, NM, USA. 2008. Available online: https://pubs.nmsu.edu/research/horticulture/NMCA30/index.html (accessed on 21 December 2023).
- McFarquhar, J. Organic Seed Treatments for the Reduction of Xanthomonas euvesicatoria on Tomato Seed. Master’s Thesis, University of Georgia, Athens, GA, USA, 2015. Available online: http://getd.libs.uga.edu/pdfs/mcfarquhar_judith_201505_ms.pdf (accessed on 12 December 2023).
- Mtui, H.D.; Bennett, M.A.; Maerere, A.P.; Miller, S.A.; Kleinhenz, M.D.; Sibuga, K.P. Effect of Seed Treatments and Mulch on Seedborne Bacterial Pathogens and Yield of Tomato (Solanum lycopersicum Mill.) in Tanzania. J. Anim. Plant Sci. 2010, 8, 1006–1015. [Google Scholar]
- Lordon, M. Sweet Pepper Breeding and Seed Saving Guide, Department of Agriculture: USDA, Minnesota. 2022. Available online: https://seedalliance.org/crops/peppers/ (accessed on 25 November 2023).
- Welbaum, G. Tomato. Publisher: VCE Publication, Department of Horticulture, Virginia Tech: Blacksburg, VA. 2021. Available online: https://pubs.ext.vt.edu/426/426-418/mobile-version.html (accessed on 25 November 2023).
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; ISBN 9780120445653. [Google Scholar]
- Ryan, R.P.; Vorhölter, F.-J.; Potnis, N.; Jones, J.B.; Van Sluys, M.-A.; Bogdanove, A.J.; Dow, J.M. Pathogenomics of Xanthomonas: Understanding Bacterium-Plant Interactions. Nat. Rev. Microbiol. 2011, 9, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Sauer, D.B.; Burroughs, R. Disinfection of Seed Surfaces with Sodium Hypochlorite. Phytopathology 1986, 76, 745–749. [Google Scholar] [CrossRef]
- Raval, A.; Sasidharan, N.; Rao, K. Effect of seed extraction procedures on seed quality parameters in tomato. Adv. Life Sci. 2016, 5, 9020–9024. [Google Scholar]
- Degwale, A.; Tesfa, T.; Meseret, B.; Fantaw, S. Seed Extraction Methods Affect the Physiological Quality of Tomato Seed and Developing Seedlings. Int. J. Veg. Sci. 2022, 29, 16–24. [Google Scholar] [CrossRef]
- Agarwal, V.K.; Sinclair, J.B. Principles of Seed Pathology, 2nd ed.; Lewis Publisher: Boca Raton, FL, USA, 1997; ISBN 978-0-87371-670-3. [Google Scholar]
- Ercolani, G.L. Effettività e Misura Della Trasmissione Di Xanthomonas vesicatoria e Di Corynebacterium michiganense Attraverso Il Seme Del Pomodoro. Ind. Conserve 1968, 43, 14–22. [Google Scholar]
- Chambers, S.; Merriman, P. Perennation and Control of Pseudomonas Tomato in Victoria. Crop Pasture Sci. 1975, 26, 657–663. [Google Scholar] [CrossRef]
- Pradhanang, P.M.; Collier, G. How Effective Is Hydrochloric Acid Treatment to Control Clavibacter michiganensis subsp. michiganensis Contamination in Tomato Seed. Acta Hortic. 2009, 808, 81–85. [Google Scholar] [CrossRef]
- McCormack, J. Pepper Seed Production. 2005. Available online: https://www.carolinafarmstewards.org/wp-content/uploads/2012/05/PepperSeedProductionVer1_2.pdf (accessed on 10 January 2024).
- Carisse, O.; Ouimet, A.; Toussaint, V.; Philion, V. Philion Evaluation of the Effect of Seed Treatments, Bactericides, and Cultivars on Bacterial Leaf Spot of Lettuce Caused by Xanthomonas campestris pv. vitians. Plant Dis. 2000, 84, 295–299. [Google Scholar] [CrossRef]
- Kasselaki, A.-M.; Goumas, D.E.; Tamm, L.; Fuchs, J.; Fuchs, J.G.; Cooper, J.; Leifert, C. Effect of Alternative Strategies for the Disinfection of Tomato Seed Infected with Bacterial Canker (Clavibacter michiganensis subsp. michiganensis). NJAS Wagening. J. Life Sci. 2011, 58, 145–147. [Google Scholar] [CrossRef]
- Cardarelli, M.; Woo, S.L.; Rouphael, Y.; Colla, G. Seed Treatments with Microorganisms Can Have a Biostimulant Effect by Influencing Germination and Seedling Growth of Crops. Plants 2022, 11, 259. [Google Scholar] [CrossRef]
- Ayesha, M.S.; Suryanarayanan, T.S.; Nataraja, K.N.; Prasad, S.R.; Shaanker, R.U. Seed Treatment with Systemic Fungicides: Time for Review. Front. Plant Sci. 2021, 12, 654512. [Google Scholar] [CrossRef]
- Vasanthakumari, M.M.; Shridhar, J.; Madhura, R.J.; Nandhitha, M.; Kasthuri, C.; Janardhana, B.; Nataraja, K.N.; Ravikanth, G.; Shaanker, R.U. Role of endophytes in early seedling growth of plants: A test using systemic fungicide seed treatment. Plant Physiol. Rep. 2019, 24, 86–95. [Google Scholar] [CrossRef]
- van der Wolf, J.M.; Birnbaum, Y.E.; van der Zouwen, P.S.; Groot, S.P.C.; Groot, S.P.C. Disinfection of Vegetable Seed by Treatment with Essential Oils, Organic Acids and Plant Extract. Seed Sci. Technol. 2008, 36, 76–88. [Google Scholar] [CrossRef]
- Maude, R.B. Seedborne Diseases and Their Control: Principles and Practice; CAB International: Wallingford, UK, 1996; pp. 1–280. [Google Scholar]
- Chun, S.-C.; Schneider, R.W.; Cohn, M.A. Sodium Hypochlorite: Effect of Solution pH on Rice Seed Disinfestation and Its Direct Effect on Seedling Growth. Plant Dis. 1997, 81, 821–824. [Google Scholar] [CrossRef]
- Fatmi, M.; Schaad, N.W.; Bolkan, H.A. Seed Treatments for Eradicating Clavibacter Michiganensis Subsp. Michiganensis from Naturally Infected Tomato Seeds. Plant Dis. 1991, 75, 383–385. [Google Scholar] [CrossRef]
- Black & Veatch Corporation. White’s Handbook of Chlorination and Alternative Disinfectants, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–1062. [Google Scholar]
- Ritchie, D.F.; Averre, C.W. Bacterial Spot of Pepper and Tomato. Phytopathology 1996, 86, 952–958. [Google Scholar] [CrossRef]
- Khah, E.M.; Passam, H.C. Sodium Hypochlorite Concentration, Temperature, and Seed Age Influence Germination of Sweet Pepper. Hortscience 1992, 27, 821–823. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Inf. Contr. Hosp. Epidemiol. 2008, 18, 240–264. [Google Scholar]
- Le Buanec, B. Organic Seed; APSA Technological Report No. 39; APSA: Seoul, Republic of Korea, 2004. [Google Scholar]
- Borgen, A.; Nielsen, B. Effect of seed treatment with acetic acid in control of seed borne diseases. In Proceedings of the BCPC Symposium No. 76: “Seed Treatment: Challenges & Opportunities”, Farnham, 76, British Crop Protection Council, Wishaw, UK, 26–27 February 2001; Biddle, A.J., Ed.; Available online: http://orgprints.org/1116/1/acidBCPC.htm (accessed on 21 December 2023).
- Kuhar, T.P.; Rideout, S.L.; Reiter, M.S. Southeastern U.S. Vegetable Crop Handbook; Highland AG: Mulberry, FL, USA, 2021. [Google Scholar] [CrossRef]
- Scott, G.; Almasrahi, A.; Mansoorkhani, F.M.; Rupar, M.; Dickinson, M.; Shama, G. Hormetic UV-C Seed Treatments for the Control of Tomato Diseases. Plant Pathol. 2019, 68, 700–707. [Google Scholar] [CrossRef]
- Schaad, N.W. Emerging Plant Pathogenic Bacteria and Global Warming. In Pseudomonas syringae Pathovars and Related Pathogens–Identification, Epidemiology and Genomics; Fatmi, M., Collmer, A., Iacobellis, N.S., Mansfield, J.W., Murillo, J., Schaad, N.W., Ullrich, M., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 369–379. [Google Scholar] [CrossRef]
- McGee, D.C. Seed Pathology: Its Place in Modern Seed Production. Plant Dis. 1981, 65, 638–642. [Google Scholar] [CrossRef]
- Goode, M.J.; Sasser, M. Prevention--the Key to Controlling Bacterial Spot and Bacterial Speck of Tomato. Plant Dis. 1980, 64, 831–834. [Google Scholar] [CrossRef]
- Nega, E.; Ulrich, R.; Werner, S.; Jahn, M. Hot Water Treatment of Vegetable Seed—An Alternative Seed Treatment Method to Control Seed Borne Pathogens in Organic Farming. J. Plant Dis. Prot. 2003, 110, 220–234. [Google Scholar]
- du Toit, L.J.; Hernandez-Perez, P. Efficacy of Hot Water and Chlorine for Eradication of Cladosporium variabile, Stemphylium botryosum, and Verticillium dahliae from Spinach Seed. Plant Dis. 2005, 89, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.T. Hot Water Seed Treatment Protocols. 2013. Available online: https://cpb-us-e1.wpmucdn.com/blogs.cornell.edu/dist/1/7446/files/2021/04/Hot-Water-Seed-Treatment-Tables.pdf (accessed on 13 December 2023).
- Marinescu, G. Recherches Sur La Desinfection Des Semences de Tomates Contaminees Par Corynebacterium Michiganense (E. F. Smith). Jensen. Bull. Acad. Sci. Agric. For. 1975, 5, 101–109. [Google Scholar]
- Rui-yun, L.; Lin, Y.; Hua, C.; Wei, L.; Shu-peng, Y.; Fu-xin, G. Effect of Dry-Heat Treatment of Different Temperature on Germination of Vegetable Seed. China Veg. 2011, 1, 67–71. [Google Scholar]
- Akman, Z. Comparison of High Temperature Tolerance in Maize, Rice and Sorghum Seeds by Plant Growth Regulators. J. Anim. Vet. Adv. 2009, 8, 358–361. [Google Scholar]
- Pérez-Calvo, M. Chapter 9—Sanitation with Ozone. In Gases Agro-Food Process, 1st ed.; Cachon, R., Girardon, P., Voilley, A., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Mohan, N.; Patel, K.; Padmanabhan, K.; Ananthi, S. Ozone for Plant Pathological Applications. Ozone Sci. Eng. 2005, 27, 499–502. [Google Scholar] [CrossRef]
- Bocci, V.; Borrelli, E.; Travagli, V.; Zanardi, I. The Ozone Paradox: Ozone Is a Strong Oxidant as Well as a Medical Drug. Med. Res. Rev. 2009, 29, 646–682. [Google Scholar] [CrossRef]
- Çetinkaya, N.; Pazarlar, S.; Paylan, İ.C. Ozone Treatment Inactivates Common Bacteria and Fungi Associated with Selected Crop Seeds and Ornamental Bulbs. Saudi J. Biol. Sci. 2022, 29, 103480. [Google Scholar] [CrossRef]
- Czarnek, K.; Tatarczak-Michalewska, M.; Dreher, P.; Rajput, V.D.; Wójcik, G.; Gierut-Kot, A.; Szopa, A.; Blicharska, E. UV-C Seed Surface Sterilization and Fe, Zn, Mg, Cr Biofortification of Wheat Sprouts as an Effective Strategy of Bioelement Supplementation. Int. J. Mol. Sci. 2023, 24, 10367. [Google Scholar] [CrossRef]
- Falconí, C.E.; Yánez-Mendizábal, V. Efficacy of UV-C Radiation to Reduce Seedborne Anthracnose (Colletotrichum acutatum) from Andean Lupin (Lupinus mutabilis). Plant Pathol. 2018, 67, 831–838. [Google Scholar] [CrossRef]
- Brown, J.E.; Lu, T.Y.; Stevens, C.; Khan, V.A.; Lu, J.Y.; Wilson, C.L.; Collins, D.J.; Wilson, M.A.; Igwegbe, E.C.K.; Chalutz, E.; et al. The Effect of Low Dose Ultraviolet Light-C Seed Treatment on Induced Resistance in Cabbage to Black Rot (Xanthomonas campestris pv. campestris). Crop Prot. 2001, 20, 873–883. [Google Scholar] [CrossRef]
- Shude, S.P.N.; Yobo, K.S.; Mbili, N.C. Progress in the Management of Fusarium Head Blight of Wheat: An Overview. S. Afr. J. Sci. 2020, 116, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Maude, R.B.; Kyle, A.M. Seed Treatments with Benomyl and Other Fungicides for the Control of Ascochyta pisi on Peas. Ann. Appl. Biol. 1970, 66, 37–41. [Google Scholar] [CrossRef]
- European Commission. Implementing Regulation (EU) 2018/1500 of 9 October 2018 Concerning the Non-Renewal of Approval of the Active Substance Thiram, and Prohibiting the Use and Sale of Seeds Treated with Plant Protection Products Containing Thiram, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending Commission Implementing Regulation (EU) No. 540/2011; OJ L 254, 10.10.2018; European Commission: Brussels, Belgium, 2018; pp. 1–3. [Google Scholar]
- Sain, S.K.; Pandey, A.K. Biological Spectrum of Trichoderma harzianum Rifai Isolates to Control Fungal Diseases of Tomato (Solanum lycopersicon L.). Arch. Phytopathol. Plant Prot. 2016, 49, 507–521. [Google Scholar] [CrossRef]
- Chandel, S.; Allan, E.J.; Woodward, S. Biological Control of Fusarium oxysporum f. sp. lycopersici on Tomato by Brevibacillus brevis. J. Phytopathol. 2010, 158, 470–478. [Google Scholar] [CrossRef]
- Jambhulkar, P.P.; Sharma, P. Promotion of Rice Seedling Growth Characteristics by Development and Use of Bioformulation of Pseudomonas fluorescens. Indian J. Agric. Sci. 2013, 83, 136–142. [Google Scholar]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial Seed Coating: An Attractive Tool for Sustainable Agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Bejarano, A.; Puopolo, G. Bioformulation of Microbial Biocontrol Agents for a Sustainable Agriculture. In Progress in Biological Control; Springer: Cham, Switzerland, 2020; pp. 275–293. [Google Scholar] [CrossRef]
- Jambhulkar, P.P.; Sharma, P.; Yadav, R. Delivery Systems for Introduction of Microbial Inoculants in the Field. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; pp. 199–218. [Google Scholar] [CrossRef]
- Mandiriza, G.; Kritzinger, Q.; Aveling, T.A.S. The Evaluation of Plant Extracts, Biocontrol Agents and Hot Water as Seed Treatments To Control Black Rot Of Rape In South Africa. Crop Prot. 2018, 114, 129–136. [Google Scholar] [CrossRef]
- Grimaldi, A.; Galy, R. An Overview of European Regulatory of Biopesticides. 4th International Symposium on Biological Control of Bacterial Plant Diseases. J. Plant Pathol. 2019, 101, 849–883. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Frontiers in Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Farm to Fork Strategy; For a Fair, healthy and Environmentally-Friendly Food System; 23 Pages; European Commission: Brussels, Belgium, 2020; Available online: https://food.ec.europa.eu/system/files/2020-05/f2f_action-plan_2020_strategy-info_en.pdf (accessed on 22 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).