Abstract
With the world shifting towards renewable and sustainable resources, polyhydroxyalkanoates (PHAs) have attracted significant interest as an alternative to synthetic plastics. While possessing promising properties suitable for various applications, the production of PHAs has not yet reached a global commercial scale. The main reason is the high cost of production, which represents a major limitation. Sugarcane bagasse (SCB) is an abundant lignocellulosic waste around the world. Its use to produce PHA enhances the feasibility of producing PHAs at commercial scale. However, SCB requires pretreatment and hydrolysis steps to release the sugars prior to the microbial fermentation. The cost associated with these steps poses additional challenges for large-scale production. Another challenge is the release of inhibitors during the pretreatment process which can result in a low PHA yield. The development of a low cost, co-culture strategy for the bioconversion of SCB into PHAs, can represent a pivotal step towards the large-scale production of bioplastics. This review highlights the advancements made in recent years on the microbial production of PHA using SCB as potential feedstock, with a proposed biological strategy and circular economy model.
1. Introduction
Due to their various mechanical properties, plastics are ubiquitously used in daily life and industry. The global production of plastics reached 390.7 million tonnes (Mt) in 2021, including fossil-based (350 Mt), recycled (32.5 Mt) and bio-based plastics (5.9 Mt), with an estimation to reach 760 Mt by 2050 [1]. In general, at the end of life, plastic waste is managed by landfilling and incineration [2,3]. It has been reported that around 80% of plastic waste is sent to landfilling, while 12% is incinerated [4]. However, both disposal methods lead to the release of toxic by-products, threatening ecosystems. Fossil-based plastics consist of 90.2% of the total yield of plastics [1]. This type of plastic is known for its low biodegradability and thus its complete degradation may take years to centuries [5]. Due to plastic’s low biodegradability, together with poor waste management, plastic pollution has become one of the most significant environmental threats impacting both terrestrial and aquatic environments. It is estimated that around 30 million tonnes of plastic waste have leaked into oceans and seas, and a further 109 million tonnes have flowed into rivers, causing potential toxicological and physical risks to the aquatic ecosystem [6,7]. Therefore, as an alternative to fossil fuel-derived plastics, bioplastics would help to tackle the plastic pollution problem.
Recently, bioplastics have attracted attention as the world shifts towards sustainable and renewable resources. Compared to fossil-based plastics, bioplastics are characterised by their biodegradability and sustainability which make them a potential environmentally friendly alternative to petroleum-derived plastics [8]. It has been reported that bioplastics can be completely biodegraded to biomass, carbon dioxide (CO2) and water within 2 months under standard conditions [9], which would in the long term significantly reduce future plastic pollution and its severe impact on the environment.
Polyhydroxyalkanoates (PHAs) are a family of biodegradable polymers synthesised by a broad range of microorganisms, i.e., bacteria and archaea, as an energy storage compound in the form of lipid granules [10]. These biopolymers have similar mechanical characteristics to petroleum-derived plastics in terms of flexibility, elasticity, versality, etc., which make them one of the most investigated classes of bioplastics [11].
Despite the increasing market interest in PHAs and the significant number of studies, the production of PHAs is still limited to pilot scale. According to European bioplastics database [12], bioplastics production represented less than 1% of the total plastic production in 2022. One of the main restrictions for the large-scale production of PHAs is the high cost of production, which is approximately six times higher than the cost of petroleum-derived plastics [13]. The carbon source used as a feedstock is the main reason for the high production cost, accounting for around 50% of the total production cost [14,15]. Therefore, it is necessary to find cheap and efficient alternative substrates to increase the economic feasibility and sustainability of PHA production. Recently, researchers have started to explore the use of different low-cost substrates such as whey [16,17], glycerol [18,19], molasses [20,21], oils [22,23] and wastewater [24] for the biosynthesis of PHAs.
The use of renewable, cheap, and abundantly available feedstocks, such as agricultural waste, is also an option. Agricultural wastes are residues produced in the process of agricultural production and include crop residues, leaf litter, bagasse, sawdust, and peels [25]. This type of waste consists one of the largest categories of waste produced worldwide, with an estimated 998 Mt generated annually [26]. The lignocellulosic residues can be considered as promising feedstocks for PHA production due to their abundant availability, low cost, and lack of competition with human and food supply [27]. Cellulose and hemicellulose are the main compounds of lignocellulosic residues and their hydrolysis releases fermentable sugars [28]. These sugars can be turned into PHAs via biological processes by PHA-producing microorganisms. Several studies have investigated the use of agricultural waste such as food residues, bagasse, straws, corn cob and spent coffee grounds for PHA production [29,30,31,32,33].
One of the most abundant agricultural lignocellulosic wastes in tropical and sub-tropical regions is sugarcane bagasse (SCB). PHAs can be efficiently produced through integration into a sugarcane mill [34]. Besides being a cheap feedstock for PHA production, SCB can also be incinerated to generate the energy required for the production process. Hence, PHA production from SCB is an economically viable option due to the accessibility of a low-cost carbon source and energy. While several reviews discuss the use of different wastes to produce bioplastics, none have fully examined the use of SCB for the biosynthesis of PHA. This review highlights the recent advancements in research on the microbial production of PHA using SCB as potential feedstock. This includes a discussion of the pretreatment strategies, enzymatic hydrolysis, and accumulation of PHA by different microorganisms using this agricultural waste. In addition, the challenges associated with the use of SCB in PHA biosynthesis, which impacts its feasibility at a large scale, are discussed. To overcome these challenges, a biological strategy, co-culture, is suggested and discussed. Finally, an example of a circular economy model is proposed.
2. Structure and Composition of Polyhydroxyalkanoate
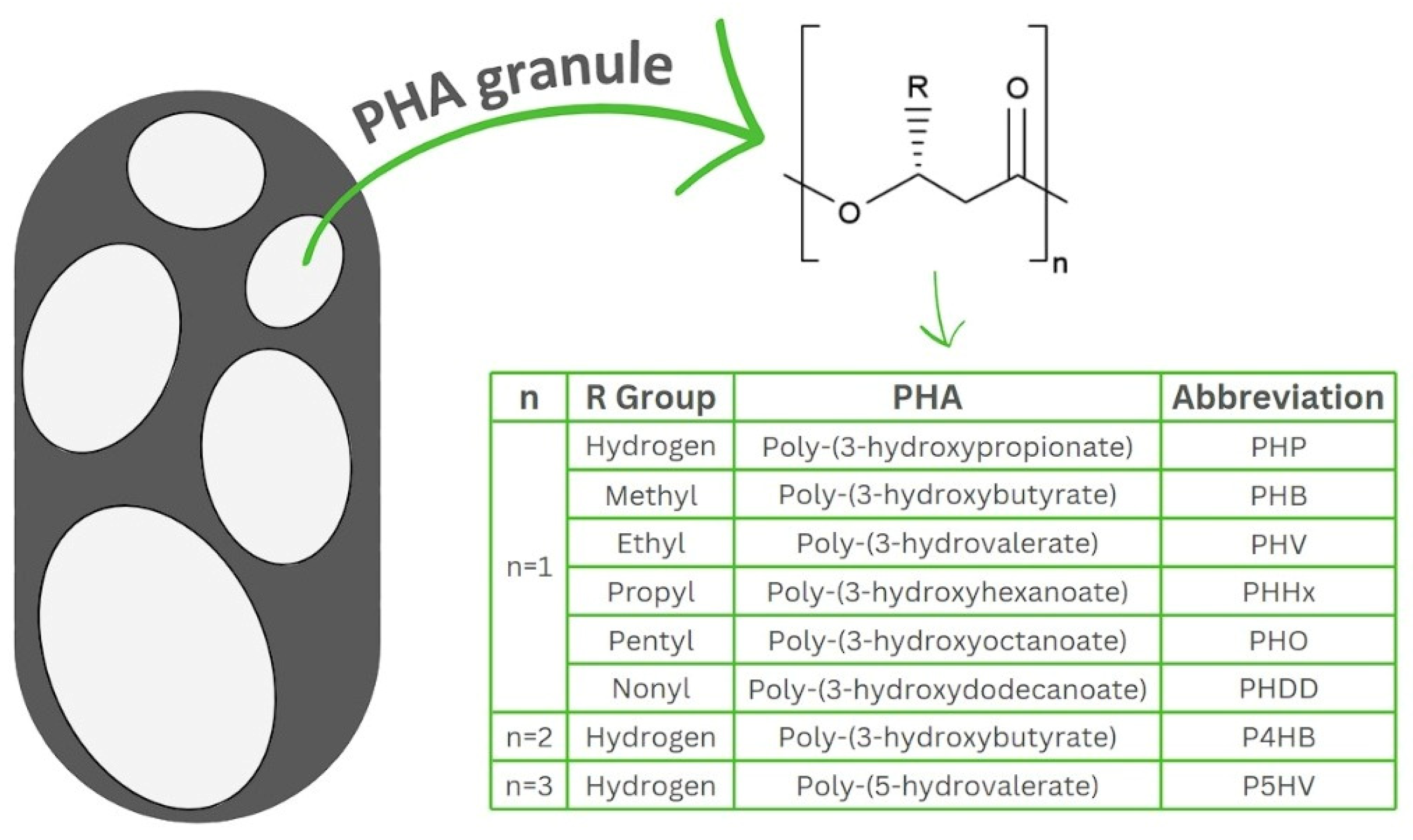
PHAs are biodegradable and biocompatible thermoplastics, soluble in chlorinated solvents, insoluble in water and resistant to hydrolytic attack and UV [35]. These biopolymers are polymerised polyoxoesters of polyesters of hydroxyalkanoates synthesised intracellularly by a wide range of microorganisms in the form of granules, with diameters ranging between 0.2 and 0.5 µm (Figure 1) [10,36,37]. The physical and chemical properties of PHAs, such as melting point, crystallinity, hydrophobicity, etc., differ significantly depending on the composition of the monomers [38]. PHAs also have a wide range of mechanical characteristics which vary from hard to elastic thermoplastics according to the type of feedstock, microbial host and fermentation strategy [38,39,40].
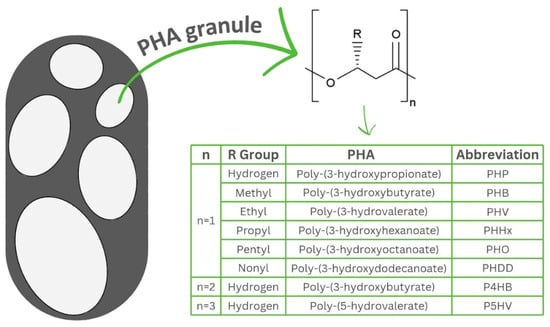
Figure 1.
General structure of polyhydroxyalkanoates accumulated in bacteria in the form of granules. Polyhydroxyalkanoates are classified as short chain length (scl-PHAs), medium chain length (mcl-PHAs) and long chain length (lcl-PHAs). The table insert shows different PHA derivatives.
The classification of PHAs depends on the number of carbon atoms present in their hydroxyacid chain, consisting of three classes including short chain length (scl-PHAs) with 3–5 carbon atoms, medium chain length (mcl-PHAs) with 6–14 carbon atoms and long chain length (lcl-PHAs) with more than 14 carbon atoms in each monomer unit [10,41,42]. Due to differences in structure, the physical and mechanical properties of scl-PHAs differ from mcl-PHAs. For example, scl-PHAs have a significantly higher melting point than mcl-PHAs, which have a greater elasticity than scl-PHAs [43]. More than 150 monomers of PHAs have been reported to date with different structures including saturated, unsaturated, straight, branched and aromatic with poly(3-hydroxybutyrate) (PHB) being the most synthesised monomer [43]. Depending on the type of monomers and composition, PHAs can be classified as homopolyesters with only one monomer type or heteropolyesters with two or more monomer types [44].
3. Microbial Production of Polyhydroxyalkanoates and Different Pathways
PHAs are synthesised by different microorganisms, including bacteria and archaea such as Bacillus, Pseudomonas, Acinetobacter, Legionella, Agrobacterium, Halobacteriaceae, to sustain energy balance in the cell [45]. There are two groups of microbes involved in PHA production [46]. The first group includes growth-associated microorganisms, such as recombinant Escherichia coli, which accumulate PHAs during their exponential phase. The second group consists of non-growth-associated microbes, such as Pseudomonas oleovorans, which synthesise PHAs under stress due to an excess in carbon, limitation of oxygen, nitrogen or phosphorus, and extreme conditions. Classical strain improvement and metabolic engineering have also been broadly applied to generate PHA-producing engineered microorganisms in order to improve PHA production [13].
Among the different PHA-producing bacteria, Burkholderia cepacia and Cupriavidus necator, previously known as Ralstonia eutropha and Wautersia eutropha [47], are known for their ability to accumulate up to 75% and 90% (% of CDM) of their cellular mass as PHA and for using different substrates as carbon sources [48,49]. Several Bacillus species such as Priestia megaterium (previously known as B. megaterium [50]) have also been used to produce PHA due to their high growth rate, absence of lipopolysaccharide cell membrane which facilitates the extraction process, and the ability to produce enzymes that hydrolyse complex substrates for simpler carbon sources [51].
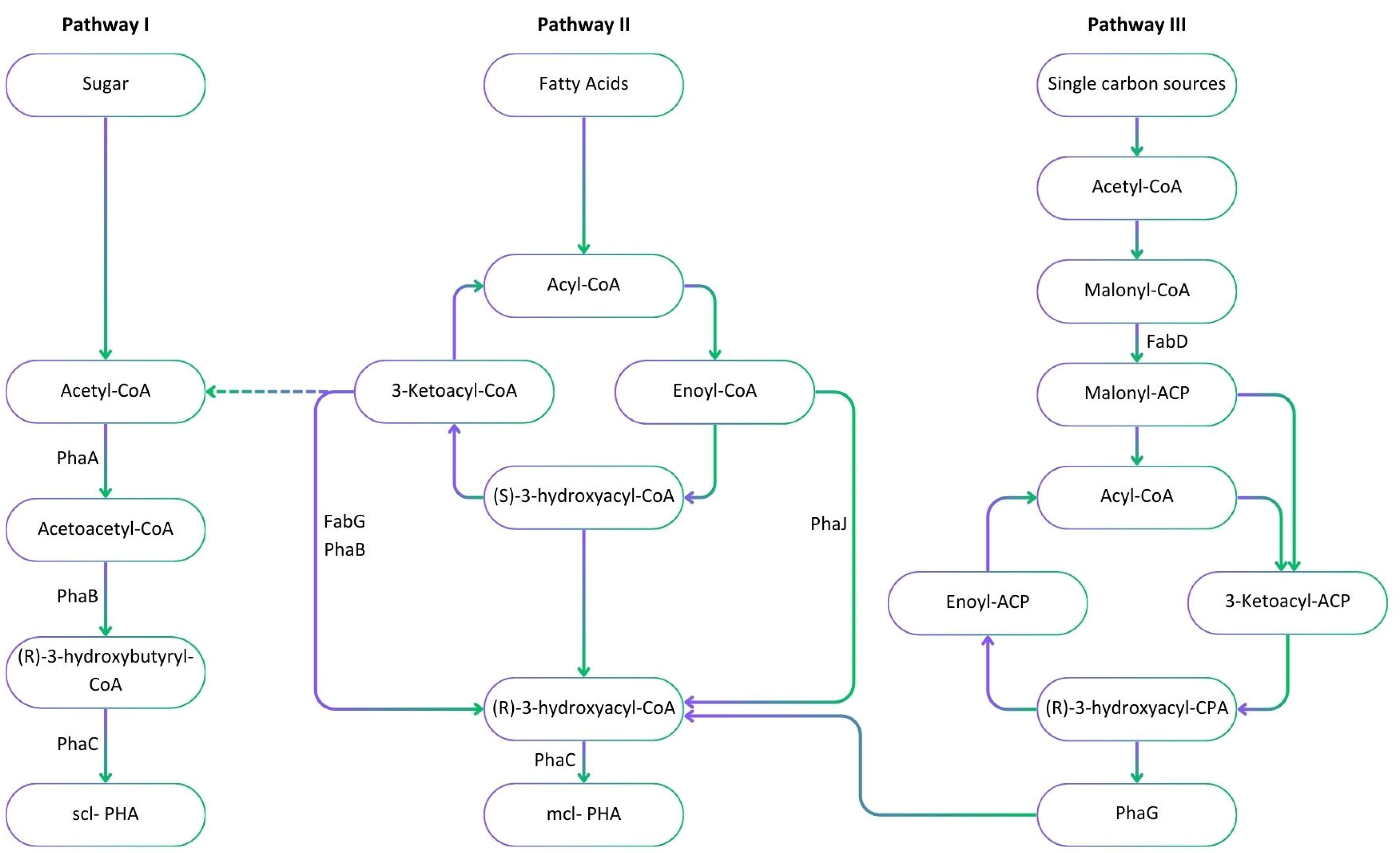
The microbial synthesis of PHA involves three different pathways, which depend on the general metabolism of the microbial host (Figure 2). Scl-PHAs are synthesised by bacteria such as Cupriavidus necator through the glycolysis of sugars via pathway I. This pathway involves three key enzymes including 3-ketothiolase, acetoacetyl-CoA reductase and PHA synthase encoded by three genes, phaA, phaB and phaC, grouped together on the phaCAB operon, respectively. Following the glycolysis of glucose, 3-ketothiolase converts two molecules of acetyl-CoA into one molecule of acetoacetyl-CoA. Acetoacetyl-CoA is then converted into 3-hydroxybutyryl-CoA by acetoacetyl-CoA reductase. Finally, an ester-bond is formed between 3-hydroxybutyryl-CoA molecules to produce a polymer such as PHB [52]. Mcl-PHAs are synthesised via pathway II and III, such as in Pseudomonas sp. through different types of precursors and enzymes. Pathway II involves the production of PHAs from the breakdown of lipids and fatty acids by the β-oxidation cycle [53]. In this pathway, different hydroxyacid monomers are synthesised by the activity of acyl-CoA oxidase, (R)-specific enoyl-CoA hydratase, and 3-ketoacyl-CoA reductase. Polyhydroxyalkanoate synthase then joins the monomer molecules to produce PHA polymers [46,54]. Pseudomonas aeruginosa is an example of bacteria that use pathway II to produce mcl-PHAs [55]. Pathway III emphasises the production of PHAs from simple carbon sources such as methanol and carbon dioxide (CO2) [56]. The phaG gene encodes for an acyl-ACP-CoA transacylase enzyme which is responsible for the transformation of intermediates generated in the fatty acid biosynthesis pathway to COA form [57].
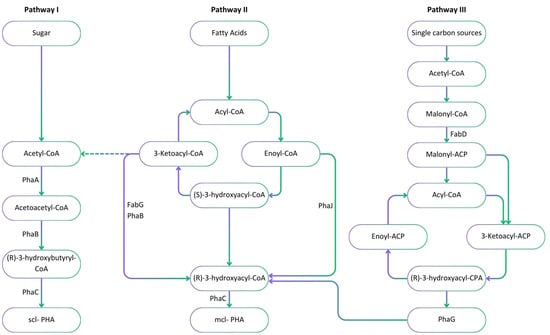
Figure 2.
Metabolic pathways including precursors and enzymes involved in the microbial synthesis of PHAs from sugars through glycolysis (pathway I), fatty acids through β-oxidation (pathway II) and simple carbon sources through the de novo synthesis of fatty acids (pathway III). The major enzymes involved in the production process are PhaA: 3-ketothiolase; PhaB: acetoacetyl-CoA reductase; PhaC: PHA synthase; PhaG: (R)-3-hydroxyacyl-ACP-CoA transferase; PhaJ: (R)-specific enoyl-CoA hydratase; FabD: Malonyl CoA-acyl carrier protein (ACP) transacylase; and FabG: 3-ketoacyl-ACP reductase.
4. Sugarcane Bagasse: An Abundant Substrate for Polyhydroxyalkanoate Production
Sugarcane, Saccharum officinarum, is a major crop in tropical and subtropical regions, with its juice being the main feedstock to produce sugars in sugar mills. With around 1870 Mt produced annually, sugarcane contributes more than 70% of the global sugar demand and is one of the largest feedstocks for biofuels production globally [58].
Numerous waste products are generated during the processing steps of sugarcane including sugarcane straws as harvest residues, bagasse after sugar extraction, molasses, etc. Compared to the world’s other major crops such as wheat, rice and corn, sugarcane produces the highest crop residues yield per unit area and the highest lignocellulosic content, some three to four times higher than other major crops [59]. Sugarcane bagasse (SCB) is obtained after a series of milling steps to extract sugars from sugarcane. Theoretically, for every tonne of sugarcane, 0.3 tonnes of bagasse is generated. This residual waste is considered one of the largest agricultural wastes globally, with an annual production of 513 Mt [60]. A significant fraction of SCB is usually disposed of in an uncontrolled way as waste piles in open lands, resulting in serious environmental problems including the release of unpleasant odours arising from the decomposition of waste, greenhouse gas emissions, land contamination and occasionally self-igniting fires [61]. When not landfilled or disposed of, SCB is inefficiently incinerated for the generation of electricity in sugar mills, which results in a loss of around 65% of its energy content, in addition to the emission of a significant amount of carbon dioxide [62]. In contrast, bagasse can be converted into valuable products due to its high polysaccharide content, which consists of around 60–80% of its wet mass [63]. For example, monosaccharides, resulting from the hydrolysis of polysaccharides in bagasse, can be fermented by microbes into biofuels, biopolymers such as PHAs [64]. Moreover, due to its fibrous nature, SCB can potentially be used in the production of sound adsorbers and thermal insulation [65]. The use of SCB to produce PHAs offers several advantages as it is considered a sustainable source for PHA production that does not compete with food production, which addresses the concern associated with the use of food crops to produce biopolymers [41]. Moreover, the use of such agricultural residues represents a form of waste valorisation, by converting SCB into a valuable bioplastic, thus reducing its negative impact on the environment. In addition, using SCB as a feedstock reduces the dependency on fossil fuels used to produce conventional plastics which consume around 20% of global oil and gas [66]. Overall, the production of PHA using SCB as a carbon source aligns with the concepts of circular economy, sustainability and a transition towards greener products.
5. The Structure and Composition of Sugarcane Bagasse
The structure and composition of SCB have been extensively studied [64,67]. Bagasse is a fibrous waste consisting of about 40–45% fibres, 45–50% water and 2–5% dissolved sugars [68]. The fibres are mostly composed of cellulose (40–50%), hemicellulose (25–35%) and lignin (20–30%) [69]. Cellulose and hemicellulose are embedded in the lignin matrix which helps to improve the rigidity of the bagasse [63]. Cellulose consists of -d-glucose bonded by β-1,4-glycosidic bonds. Due to its high molecular weight and crystallinity, cellulose is not digested by humans and does not dissolve in water [70]. Hemicellulose is an amorphous polysaccharide and is composed mainly of xylose, with other sugars including galactose, mannose, arabinose and rhamnose [69]. Both cellulose and hemicellulose are valuable compounds in bagasse as they represent sustainable sources of fermentable sugars after hydrolysis. However, lignin is a phenolic macromolecule that is resistant to enzymatic degradation [71]. Therefore, the lignin percentage and distribution in SCB are considered major parameters in determining the resistance of bagasse to hydrolysis and thus sugars release [72].
6. The Production Process of Polyhydroxyalkanoates from Sugarcane Bagasse
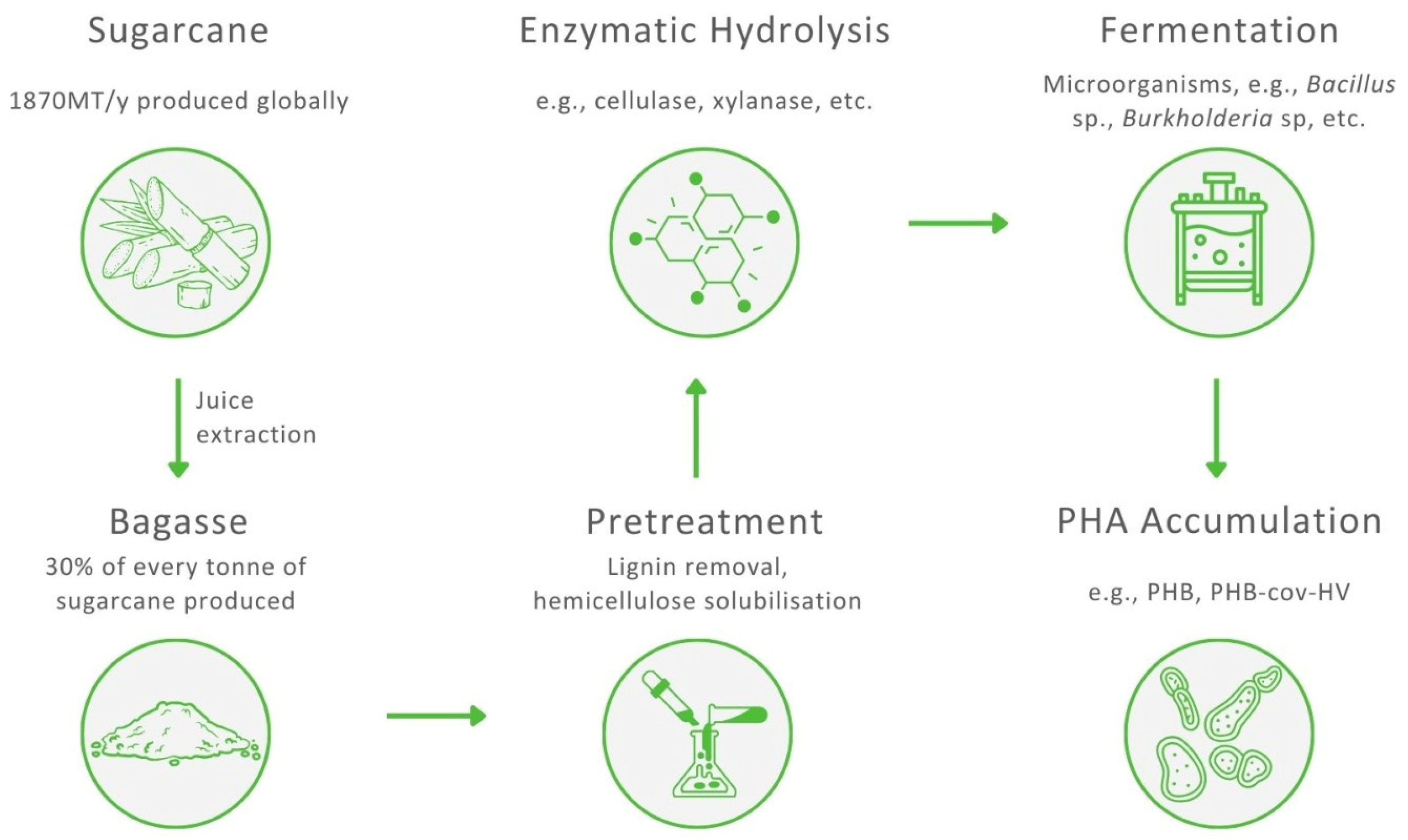
Transforming complex polysaccharides into fermentable sugars is one of the major challenges in the use of SCB as a feedstock to produce PHAs and requires two essential steps: pretreatment and hydrolysis. After hydrolysis, monomeric sugars are fermented by PHA-accumulating bacteria. The pretreatment of SCB is required for the removal of lignin, decrystallisation of glucose and partial depolymerisation of hemicellulose to increase the porosity of bagasse and facilitate the access of enzymes during the hydrolysis step [73]. Consequently, both polysaccharides, cellulose and hemicellulose, can be hydrolysed to monosaccharides, making SCB a potential raw material for the biosynthesis of PHA. Generally, a biorefinery aims to sustainably convert biomass, in an optimal matter, to produce high-value products. To accomplish this, an SCB-based biorefinery should combine several biotransformation processes to utilise each lignocellulosic component of bagasse, resulting in an adequate yield and valorisation of cellulose, hemicellulose, and lignin. It has been reported that with appropriate pretreatment and an efficient enzymatic hydrolysis, 90% of the total reducing sugar yield from a lignocellulosic biomass can be utilised [74]. A general scheme presenting the production of PHA from SCB is shown in Figure 3.
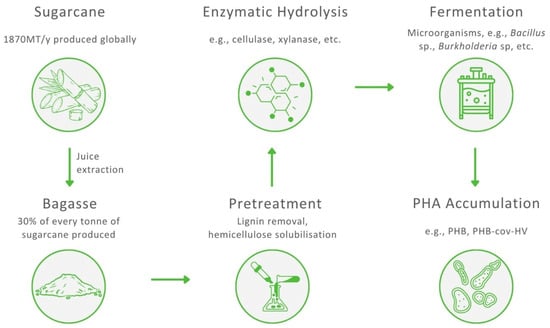
Figure 3.
The process of the production of polyhydroxyalkanoates from sugarcane bagasse.
6.1. Pretreatment of Sugarcane Bagasse
The pretreatment of SCB allows the removal of lignin and the breakdown of the lignocellulosic structure [75]. Pretreatment strategies have been applied to lignocellulosic materials, including SCB, to reduce their recalcitrance and improve the release of fermentable sugars following enzymatic degradation. The pretreatment of lignocellulose may be conducted using chemical, physical or biological processes. Pretreatment is considered effective if it accomplishes the following: (1) enhances the release of fermentable sugars; (2) preserves the structure of carbohydrates; (3) limits the formation of by-products, i.e., inhibitors; and (4) is economically feasible [76]. Many pretreatment methods have been performed on SCB with the most common methods being dilute acid hydrolysis, alkaline pretreatment, steam explosion, organosolv pretreatment, liquid hot water and biological pretreatment. Table 1 shows some examples of different pretreatments for SCB and their outcomes.

Table 1.
Advantages and disadvantages of pretreatments with examples of sugarcane bagasse pretreatments from the literature.
Pre-hydrolysis with dilute acid has been demonstrated to be an effective method for lignocellulosic biomass such as SCB [85]. Sulfuric acid is the most widely used acid to treat bagasse; however, other acids such as hydrochloric, phosphoric, and nitric acid can also be employed [86]. Many studies on dilute acid pretreatment using sulfuric acid have shown the effectiveness of this acid on sugarcane bagasse. For example, Zhao et al. [87] reported that pretreating SCB with 2% sulfuric acid for 2 h at 121 °C allowed the solubilisation of 85% of the hemicellulose and the elimination of 16% of the lignin content. Due to its low cost and convenient application, dilute acid pretreatment is the most used pretreatment for bagasse to produce PHA [88,89,90,91,92,93]. However, the disadvantage of this method is the formation of different types of inhibitors, such as phenolic compounds from lignin degradation, and furfural, hydroxymethylfurfural (HMF) and acetic acid from hemicellulose and cellulose degradation [94]. These inhibitors have negative effects on the microbial fermentation [95].
Alkaline pretreatment is another chemical pretreatment strategy used for SCB. This pretreatment is considered a cost-effective process with less inhibitors produced [96]. Several bases can also be used, such as sodium hydroxide (NaOH) [97,98], potassium hydroxide (KOH) [99], calcium hydroxide (Ca(OH)2) [100], ammonia (NH3) [101] and a combination of NaOH and hydrogen peroxide (H2O2) [102]. Yu, Tan, Sun, Nishimura, Takei, Tang and Kida [98] reported that treating SCB with NaOH (1%) for 10 min at 120 °C removed 67.5% of lignin. Similarly, Zhang et al. [103] reported that using NaOH with H2O2 in the pretreatment of SCB resulted in a significant breakdown of lignin, hence improving the enzymatic digestibility of the bagasse. The main disadvantage of this pretreatment is that the use of sugars released from hemicellulose is more difficult than in the case of dilute acid pretreatment. This is because most of the hemicellulose content remains in the residual bagasse even after alkaline pretreatment, hence the need to add hemicellulolytic enzymes such as xylanase in the following hydrolysis step [104].
It has also been demonstrated that organosolv pretreatment is effective to pretreat bagasse in many studies. Schmatz and Brienzo [105] were able to remove 45.3% of lignin and 72.5% of hemicellulose from SCB after pretreatment with 50% ethanol at 121 °C. Similarly, Zhang et al. [106] reported the effective removal of 75.5% lignin after pretreating bagasse with 60% ethanol and 5% NaOH at 180 °C. This pretreatment employs an organic solvent with high concentrations ranging from 30 to 70% at temperatures of 100–200 °C in the presence or absence of a catalyst [107]. One drawback of organosolv pretreatment is the high cost compared to other leading pretreatments [108].
As a physical pretreatment, steam explosion is an eco-friendly technology which allows the fractionation and recovery of the three main components of SCB in high yield [109]. For instance, Silveira et al. [110] pretreated SCB by steam explosion using a 65 L reactor. The pretreatment resulted in 85% hemicellulose solubilisation, proving the efficiency of this technique. Pitarelo et al. [111] also used steam explosion in the presence of H3PO4 with a concentration of 19 mg g−1 to pretreat SCB. Pretreatment at 180 °C for 5 min was reported to be optimal, with a total sugar yield of 75% after hydrolysis. In addition to steam explosion, liquid hot water is a green technology that can be considered a potential pretreatment method to pretreat SCB. Zhang, You, Lei, Li and Jiang [83] reported that the acetyl-assisted hot water pretreatment of SCB with water for 70 min at 160 °C resulted in 9.8 g L−1 of xylose. Moreover, it has been reported that autohydrolysis can alter the surface morphology of SCB and improve the saccharification efficiency [112].
Biological pretreatment is an environmentally friendly method based on employing suitable cellulolytic and hemicellulolytic enzymes or microorganisms to degrade lignocellulosic biomass [113]. This pretreatment requires less energy and generates fewer inhibitors compared to chemical and physical pretreatments [114]. However, the long biodegradation period limits the further development and use of this pretreatment method by industries [84]. Microorganisms, including fungi and bacteria, isolated from different environments such as soil and lignocellulosic waste can be used for biologically pretreating SCB. To date, few studies evaluating the biological pretreatment of SCB have been reported [84]. However, some studies have shown that the use of fungi enhances the digestibility of polysaccharides, while very few microorganisms are able to fully decompose lignin in SCB [115,116,117]. Microbial consortium pretreatment has been used on lignocellulosic biomass to increase biogas production [118,119]. However, its use to pretreat SCB to increase PHA production has not been fully investigated. This pretreatment method does not require the sterilisation of biomass in the case of using pure culture [85]. Generally, the use of biological pretreatments to treat lignocellulosic biomass is not as effective as chemical pretreatments due to its long degradation time and high selectivity of microbes. Further studies are needed to overcome these issues and enable the use of this green pretreatment efficiently.
6.2. Hydrolysis of Polysaccharides in Treated Sugarcane Bagasse
Following pretreatment, SCB undergoes hydrolysis to transform cellulose and hemicellulose into monomeric sugars to produce PHA. This step facilitates the availability and solubility of the carbon source to be used by bacteria. Generally, acid treatment and enzymatic hydrolysis are the major methods employed. However, enzymatic hydrolysis is favoured over acid hydrolysis as it is environmentally friendly, releases less inhibitors, does not require corrosion resistant equipment, and most importantly, leads to an almost complete hydrolysis of cellulose content in SCB, resulting in a high PHA yield [120,121]. Cellulase is responsible for the hydrolysis of cellulose by cleaving the β-(1–4)-d-glucose. There are three types of cellulases involved in hydrolysis; endoglucanases, exocellobiohydrolases and β-glucosidase [122]. These cellulases break down the cellulose into monosaccharides, mainly glucose molecules. In contrast, many enzymes are required for the hydrolysis of hemicellulose, such as xylanases, arabinofuranosidase and glucuronidase [121]. Due to its branched structure, hemicellulose is readily hydrolysed to mainly xylose, galactose, and arabinose [123]. Several bacterial and fungal microorganisms can hydrolyse polysaccharides from lignocellulosic biomass into fermentable sugars. Bacteria belonging to the genera Streptomyces, Bacillus and Clostridium have been reported as cellulolytic bacteria [124]. In addition, fungi such as Trichoderma, Penicillium and Aspergillus exhibit a wide range of cellulase enzymes which play a key role in the hydrolysis of lignocellulosic biomass [125]. Although research on hydrolysis by microorganisms has been conducted and some progress has been reported, the use of bacteria and fungi is rarely carried out due to the long incubation time required to achieve hydrolysis [126]. Usually, commercial enzymes are applied to achieve an efficient hydrolysis rate.
7. The Status of the Production of Polyhydroxyalkanoates from Bagasse
Studies on PHA production using SCB hydrolysates have focused on optimising both culture conditions and experimental parameters to achieve high yields. Batch culture approaches has been the most applied system reported. While a wide range of biopolymers are produced by bacteria, PHB is the main polymer produced from SCB (Table 2). Several factors affect the yield of PHA from SCB including the microorganism used, the mode of culture (pure, co-, or mixed culture), and experimental parameters such as incubation time, pH, inoculum density, temperature, carbon to nitrogen ratio (C/N) and oxygen concentration [127]. The optimisation of experimental parameters depends on the microorganism and the mode of culture used to produce PHA. Currently only a few microorganisms have been investigated for their potential to produce PHA from SCB (Table 2). Bacillus spp. was used to produce PHB from pre-treated SCB and achieved a polymer content of 56% [30]. In another study, Burkholderia sp. was able to utilise SCB hydrolysate and produced PHB with 49% polymer content [128]. However, a PHB content of 61.5% was achieved when Lysinibacillus sp. utilised SCB hydrolysate with 2% corn steep liquor [129]. Madhumathi et al. [130] reported that, compared to other agrowastes such as molasses, rice bran, wheat bran and whey waste, SCB showed the maximum PHA yield with a concentration of 6.4 g L−1 and an accumulation of about 70% by Bacillus safensis. This can be explained by the high cellulose content in SCB that was converted into glucose, readily utilised by Bacillus safensis.
The efficiency of enzymatic hydrolysis depends on several parameters including the structure of pretreated SCB, enzyme loading and the hydrolysis period [122]. According to several economic analyses, the steps of releasing sugars from lignocellulosic biomass, including pretreatment and hydrolysis (the production and purification of enzymes), contributes to around 45% of the total cost, with the cost of cellulase enzyme being between $0.2 to $0.4 per litre of the final product [120]. The high cost associated with pretreatment and the use of enzymes currently hinders the use of SCB at a large scale for PHA production. Therefore, reducing these significant costs is a key concern for making SCB utilisation a commercially viable process. Catabolite repression is also considered one of the factors responsible for the low yield of PHA, where the presence of one carbon source controls the use of others in the culture medium; that is, bacteria selectively assimilate only one carbon source among many other sources present in the medium, resulting in low productivity [131]. As previously stated, glucose and xylose are the main sugars released following the hydrolysis of SCB. As bacteria generally prefer the C6 sugar, glucose, this causes an accumulation of the C5 sugar, xylose, and other sugars, which results in an inefficient bioconversion of SCB into PHA. An important exception has been reported recently by Kourilova et al. [132] who demonstrated that the thermophilic strain of Schlegelella thermodepolymerans (now Caldimonas thermodepolymerans [133]) prefers xylose over other sugars including glucose, arabinose fructose, galactose, mannose, and lactose and accumulates a considerable amount of PHA using xylose-rich resources. Moreover, the release of inhibitors during the pretreatment of SCB can significantly affect the growth of PHA-producing bacteria [134]. Therefore, further research is required in terms of the optimisation and improvement of PHA synthesis from SCB. Additionally, there is a need to investigate more microbial strains for their ability to use more than one carbon source from the SCB hydrolysate mixture.

Table 2.
Studies in the literature using sugarcane bagasse (SCB) hydrolysates as feedstock to produce polyhydroxyalkanoates (PHA).
Table 2.
Studies in the literature using sugarcane bagasse (SCB) hydrolysates as feedstock to produce polyhydroxyalkanoates (PHA).
| Microorganism | Mode of Culture | Type of PHA | Dry Cell Weight (g L−1) | PHA Accumulation (% CDW) | PHA Titre (g L−1) | Reference |
|---|---|---|---|---|---|---|
| Lysinibacillus sp. RGS | Batch | PHB | 8.7 | 61.5 | 5.3 | [129] |
| Klebsiella pneumoniae G1 | Batch | PHB | 22.5 | 40 | 9 | [135] |
| Bacillus safensis EBT1 | Batch | PHB | 9.2 | 69.5 | 6.4 | [130] |
| Burkholderia sp. F24 | Batch | PHB PHB-co-HV | 9.8 | 49 | 4.72 | [128] |
| Halogeometricum borinquense strain E3 | Batch | PHB-co-HV | 4.2 | 45.7 | 1.9 | [136] |
| Burkholderia sacchari IPT101 | Batch | PHB | 4.4 | 62 | 2.7 | [137] |
| Burkholderia cepacia IPT048 | Batch | PHB | 4.4 | 53 | 2.3 | [137] |
| Bacillus sp. | Batch | PHB | 9 | 55.6 | 5 | [30] |
| Ralstonia eutropha | Batch | PHB | 6 | 65 | 3.9 | [138] |
| Burkholderia glumae MA13 | Batch | PHB | 0.61 | 14.9 | 9 | [139] |
| Bacillus thuringiensis IAM 12077 | Batch | PHB | 10.6 | 39.6 | 4.2 | [140] |
| Bacillus megaterium PNCM 1890 | Batch | PHB | 4.9 | 40.8 | 2 | [141] |
| Bacillus sp. | Batch | PHB | 9 | 55.6 | 5 | [30] |
PHB: poly(3-hydroxybutyrate); PHB-co-HV: poly(3-hydroxybutyrate-co-3-hydroxyvalerate).
8. Co-Culture: A Strategy to Address Polyhydroxyalkanoate Production Challenges
The microbial production of bioplastics using SCB supports the concept of a circular economy. Nevertheless, this review has highlighted some challenges which hinder the feasibility of producing bioplastics from this feedstock at an industrial scale. To address these challenges, co-culture can be applied to enhance PHA production. Co-cultures are biological systems where two or more different microorganisms naturally or artificially grow together within a medium [142]. This biological system has the potential to mitigate some challenges associated with the production of PHA from SCB, as it leads to the tolerance of bacteria against inhibitors released during pretreatment, the promotion of enzymatic hydrolysis and the bioconversion of several sugars into PHA within the culture medium.
Recently, there has been increasing interest in the use of synthetic co-cultures for PHA production [49,143]. However, the use of co-cultures is currently reliant on the use of expensive soluble sugars extracted from different plant biomass [144,145,146], while the use of lignocellulosic feedstocks including SCB remains a significant challenge. Currently there are only few studies that have used co-cultures to produce PHA from lignocellulosic biomass. As an example, Saratale, Cho, Kadam, Ghodake, Kumar, Bharagava, Varjani, Nair, Kim, Shin and Saratale [134] developed a microbial co-culture system of Lysinibacillus sp. RGS and Ralstonia eutropha ATCC 17699 to enhance PHA production using acid pretreated SCB. The co-culture strategy showed higher assimilation of SCB hydrolysates and stimulated bacterial growth compared to individual strains. This study demonstrated that the use of co-culture could result in an effective utilisation of SCB, due to a synergetic effect of the bacterial strains used in the experiment. Another study investigated the use of two bacterial strains: the cellulolytic bacteria Streptomyces sp. SirexAA-E and the PHA-producing bacteria Priestia megaterium NBRC 15308, where neither strain could produce PHA from Miscanthus grass alone. However, co-culturing both strains allowed the production of PHA without any addition of hydrolysis enzymes [147]. Co-culture can also be employed to overcome the catabolite repression of some sugars. In a study conducted by Lee et al. [148], to avoid the inhibiting effect of glucose, Bacillus sp. SM01, a xylose-utilising bacterium was co-cultured with Cupriavidus necator NCIMB 11599, which is known for its inability to assimilate xylose. The study showed an increase in PHA production of 40% compared to monoculture.
Compared to other microbial systems, microbial co-culture is more robust than a monoculture system, while it is less complex than mixed culture systems. Therefore, the co-culture approach is a potential alternative for the efficient bioconversion of lignocellulosic feedstocks into valuable biopolymers [49]. Studies on synthetic co-cultures have primarily focused on the use of a broader range of simple substrates, the neutralisation of toxic by-products and the synthesis of mcl-PHAs [49,144,149,150,151]. However, research on the use of synthetic co-culture systems to produce PHA from complex substrates such as SCB is still at an early stage and needs more investigation in terms of exploring microbial interactions and bioprocess optimisation. In addition to investigating microbial interactions, exploration of the robustness of the co-culture systems and the effect of prolonged co-evolution during their application in the PHA production process is needed. Future studies must address these topics to have better understanding of the applicability of co-culture systems to produce PHA from lignocellulosic biomass such as SCB.
9. Circular Economy Model for Polyhydroxyalkanoate Production
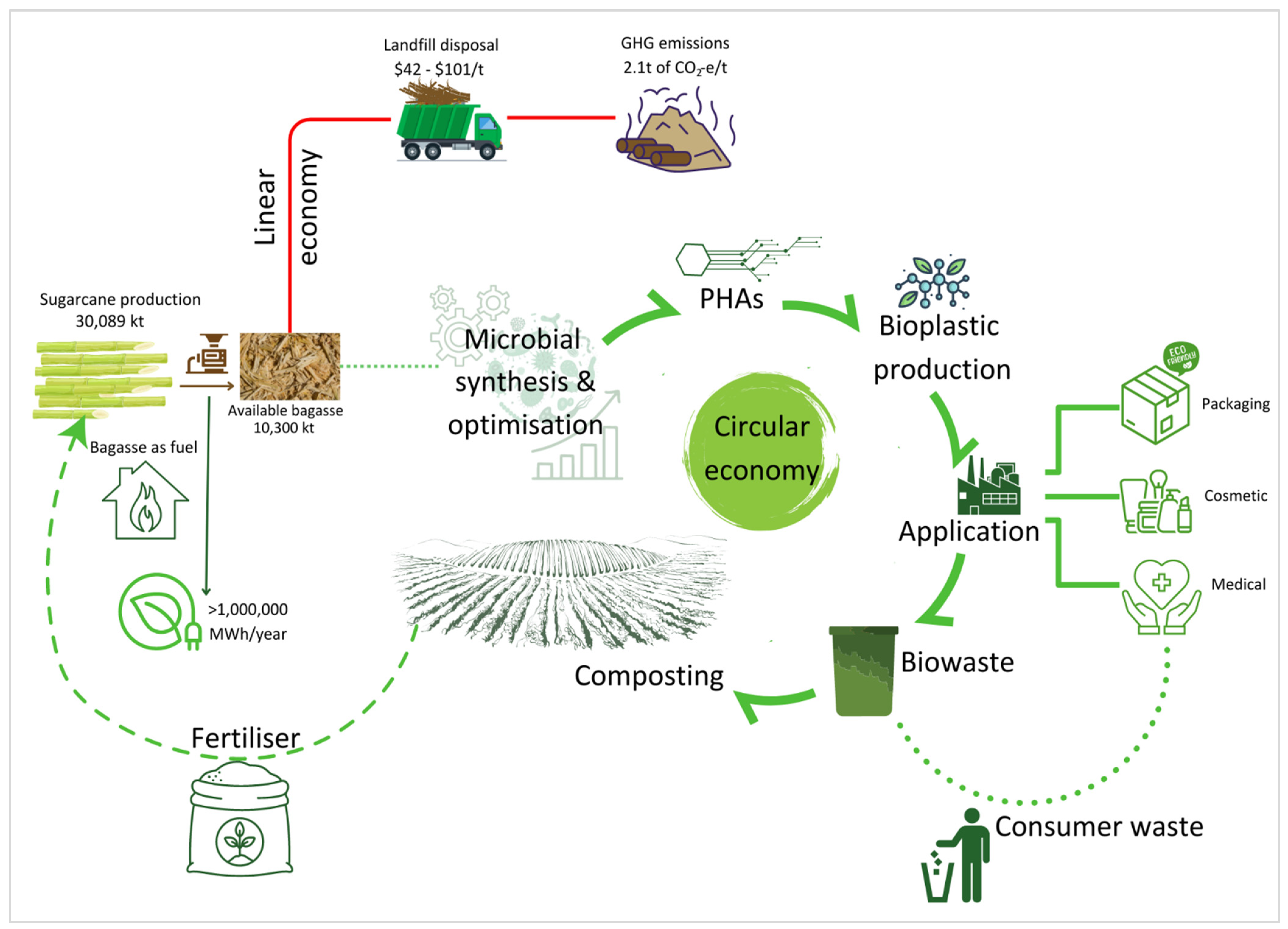
Sugarcane is produced in tropical and subtropical areas in Australia with 95% grown in Queensland and about 5% in northern New South Wales [152]. Based on data provided by the Australian Sugar Milling Council, 30,090 kT of sugarcane was produced and crushed in 2021 (Figure 4) [153]. The milling and processing of sugarcane generated around 10,000 kT of bagasse [154]. Currently, sugarcane bagasse is used as a carbon-neutral fuel source to generate electricity [155]. The use of bagasse as a fuel source is desirable as it can generate more than one million MWh per year, of which 56% is used to power operations and 44% is exported to the grid, capable of powering up to 135,000 households (Figure 4) [156]. However, not all bagasse generated can be used as a fuel source; this is due to the high capital cost involved in establishing co-generation facilities. The current combined capacity of the 28 power stations utilising bagasse as a fuel source is 539 megawatts, with an estimated capital cost of around $1.5 billion [157].
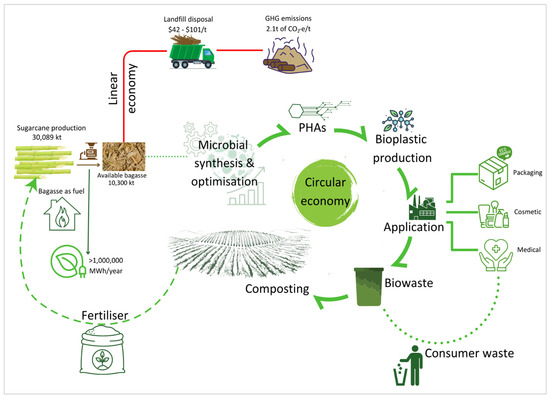
Figure 4.
Comparison of linear economy and circular economy models arising from bagasse generation in Australia. Available bagasse refers to the proportion of bagasse that is not required for fuelling.
It was reported by the National Waste Report that 10,300 kT of bagasse, in the form of available bagasse, was produced in Australia in 2021 [154,157]. While the fate of bagasse was not recorded, bagasse is generally transported off-site and disposed of in landfills, resulting in greenhouse gas (GHG) emissions [155,158]. This can incur high operational expenses as the estimated cost of landfills in Australia is between $42 to $101 per tonne of waste [159]. In addition, the disposal of bagasse into landfills will generate around 2.1 tonnes of CO2-equivalent per tonne of waste [160]. In this current linear economy model, valuable resources arising from bagasse are lost and their disposal into landfills is not environmentally friendly (Figure 4).
The diversion of this valuable resource away from landfills and into PHA production can create a closed-loop, circular economy model for bagasse in Australia and tropical and subtropical regions of the world. Bagasse can undergo biological processes to produce PHAs, which can be turned into environmentally friendly bioplastics. The desirable features of PHAs, such as mechanical properties, biocompatibility, biodegradability and non-toxicity, make them suitable for diverse applications across various sectors including but not limited to industrial, environmental, and biomedical sectors. The application of PHAs in industries involves their use for packaging. For example, a copolymer (poly(3-hydroxybutyrate-co-3-hydroxyvalerate)), marketed as BIOPOL® was produced by Imperial Chemical Industry Biological (ICI), London, UK, and used for the packaging of shampoo bottles and razors as well as disposable cups [161]. Another PHA, poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)(PHBH), is industrially produced under the name of Nodax™ by Danimer Scientific, Bainbridge, GA, USA, and is applied in packaging carpet and compostable bags [38]. Due to its high filler loading ability, PHA can also be used with various natural fibre materials to develop biopolymer composites. For example, studies reported the use of PHB or PHBV with the reinforcement of SCB to produce PHA-based composites [162,163]. The biomedical application of PHA includes its use as a drug carrier, for implants, in tissue engineering, etc. [164]. In terms of environmental application, PHAs can be used as antimicrobial agents to control the outbreak of certain diseases. It has been reported that the use of PHB in aquacultures controls Vibrio infection in shrimp due to its antiadhesive property [165].
The biowaste arising from bioplastic applications, as well as from the consumption of bioplastic-derived products, can be composted. Compostable biopolymers are being developed and demonstrate a high degradation rate [166]. In the last ten years, researchers have explored the role of composting in the biodegradation of bioplastics [167,168,169]. This fertiliser can then be reapplied to sugarcane plantations, closing the waste loop of bagasse.
10. Conclusions
Plastic pollution has increased significantly in recent decades, posing health and environmental risks. Biodegradable plastics are a sustainable alternative to petroleum-derived plastics. One of these bioplastics is PHA, a biopolymer produced by microbes which shows promising chemical and physical properties, biocompatibility, and biodegradability. Despite the increasing market interest in PHAs, their production is not practical due to high production costs. A lack of a suitable carbon source is one of the major constraints for large-scale production. Therefore, finding a cheap carbon source to be used as a feedstock is a potential solution. While the concept of using agricultural waste to produce PHA is not new, innovative and new strategies are needed to reduce the cost associated with the production of PHA and improve the feasibility of producing these biopolymers at large scale. Currently, the selection of an efficient pretreatment of SCB with a high sugar recovery level and less inhibitors is considered a key factor to maximise the PHA yield. Moreover, the selection of the most suitable strains and culture strategies as well as the optimisation of the experimental conditions are crucial to achieve scale-up of PHA production. The development of an eco-friendly strategy employing the co-culture of PHA-producing microorganisms, which simplifies the steps in the conversion of SCB into bioplastics, and reduces the cost of production, would be a significant breakthrough for the PHA industry.
Author Contributions
Conceptualization, S.H.; methodology, S.H.; investigation, S.H.; writing—original draft preparation, S.H. and T.N.; writing—review and editing, S.H., T.N. and A.S.B.; supervision, A.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The first author acknowledges the Vice-Chancellor’s PhD Scholarship (VCPS) received from RMIT University, Australia. Additionally, the first author acknowledges the ARC Industrial Transformation Training Centre for the Transformation of Australia’s Biosolids Resource Award in provision of the scholarship training.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Plastics Europe. Plastics—The Facts 2022; Plastics Europe: Brussels, Belgium, 2022. [Google Scholar]
- Wojnowska-Baryła, I.; Bernat, K.; Zaborowska, M. Plastic Waste Degradation in Landfill Conditions: The Problem with Microplastics, and Their Direct and Indirect Environmental Effects. Int. J. Environ. Res. Public Health 2022, 19, 13223. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Ioakeimidis, C.; Fotopoulou, K.N.; Karapanagioti, H.K.; Geraga, M.; Zeri, C.; Papathanassiou, E.; Galgani, F.; Papatheodorou, G. The degradation potential of PET bottles in the marine environment: An ATR-FTIR based approach. Sci. Rep. 2016, 6, 23501. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Global Plastics Outlook Database; OECD: Paris, France, 2022. [Google Scholar]
- Diggle, A.; Walker, T.R. Environmental and Economic Impacts of Mismanaged Plastics and Measures for Mitigation. Environments 2022, 9, 15. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Producing PHAs in the bioeconomy—Towards a sustainable bioplastic. Sustain. Prod. Consum. 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Hassan, M.A.; Yee, L.-N.; Yee, P.L.; Ariffin, H.; Raha, A.R.; Shirai, Y.; Sudesh, K. Sustainable production of polyhydroxyalkanoates from renewable oil-palm biomass. Biomass Bioenergy 2013, 50, 1–9. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Surendran, A.; Lakshmanan, M.; Chee, J.Y.; Sulaiman, A.M.; Thuoc, D.V.; Sudesh, K. Can Polyhydroxyalkanoates Be Produced Efficiently from Waste Plant and Animal Oils? Front. Bioeng. Biotechnol. 2020, 8, 169. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics Market Data. Available online: https://www.european-bioplastics.org/market/#:~:text=Bioplastics%20market%20data,tonnes%20of%20plastic%20produced%20annually* (accessed on 13 April 2023).
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef]
- Sun, Z.; Ramsay, J.A.; Guay, M.; Ramsay, B.A. Fermentation process development for the production of medium-chain-length poly-3-hyroxyalkanoates. Appl. Microbiol. Biotechnol. 2007, 75, 475–485. [Google Scholar] [CrossRef]
- Kourmentza, C.; Ntaikou, I.; Lyberatos, G.; Kornaros, M. Polyhydroxyalkanoates from Pseudomonas sp. using synthetic and olive mill wastewater under limiting conditions. Int. J. Biol. Macromol. 2015, 74, 202–210. [Google Scholar] [CrossRef]
- Carvalheira, M.; Hilliou, L.; Oliveira, C.S.S.; Guarda, E.C.; Reis, M.A.M. Polyhydroxyalkanoates from industrial cheese whey: Production and characterization of polymers with differing hydroxyvalerate content. Curr. Res. Biotechnol. 2022, 4, 211–220. [Google Scholar] [CrossRef]
- Carvalho, G.; Pedras, I.; Karst, S.M.; Oliveira, C.S.S.; Duque, A.F.; Nielsen, P.H.; Reis, M.A.M. Functional redundancy ensures performance robustness in 3-stage PHA-producing mixed cultures under variable feed operation. N. Biotechnol. 2018, 40, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Mishra, S. Efficient Production of Polyhydroxyalkanoate Through Halophilic Bacteria Utilizing Algal Biodiesel Waste Residue. Front. Bioeng. Biotechnol. 2021, 9, 624859. [Google Scholar] [CrossRef]
- de Paula, F.C.; Kakazu, S.; de Paula, C.B.C.; Gomez, J.G.C.; Contiero, J. Polyhydroxyalkanoate production from crude glycerol by newly isolated Pandoraea sp. J. King Saud Univ. Sci. 2017, 29, 166–173. [Google Scholar] [CrossRef]
- Kiselev, E.G.; Demidenko, A.V.; Zhila, N.O.; Shishatskaya, E.I.; Volova, T.G. Sugar Beet Molasses as a Potential C-Substrate for PHA Production by Cupriavidus necator. Bioengineering 2022, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.Y.; Sohn, Y.J.; Park, S.Y.; Son, J.; Yoo, J.I.; Baritugo, K.-A.; David, Y.; Kang, K.H.; Kim, H.; Choi, J.-i.; et al. Biosynthesis of polyhydroxyalkanoates from sugarcane molasses by recombinant Ralstonia eutropha strains. Korean J. Chem. Eng. 2021, 38, 1452–1459. [Google Scholar] [CrossRef]
- Ruiz, C.; Kenny, S.T.; Narancic, T.; Babu, R.; Connor, K.O. Conversion of waste cooking oil into medium chain polyhydroxyalkanoates in a high cell density fermentation. J. Biotechnol. 2019, 306, 9–15. [Google Scholar] [CrossRef]
- Cruz, M.V.; Araújo, D.; Alves, V.D.; Freitas, F.; Reis, M.A.M. Characterization of medium chain length polyhydroxyalkanoate produced from olive oil deodorizer distillate. Int. J. Biol. Macromol. 2016, 82, 243–248. [Google Scholar] [CrossRef]
- Bagatella, S.; Ciapponi, R.; Ficara, E.; Frison, N.; Turri, S. Production and Characterization of Polyhydroxyalkanoates from Wastewater via Mixed Microbial Cultures and Microalgae. Sustainability 2022, 14, 3704. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Huang, J.; Qu, Z. A review on polyhydroxyalkanoate production from agricultural waste Biomass: Development, Advances, circular Approach, and challenges. Bioresour. Technol. 2021, 342, 126008. [Google Scholar] [CrossRef]
- Costa, P.; Basaglia, M.; Casella, S.; Favaro, L. Polyhydroxyalkanoate Production from Fruit and Vegetable Waste Processing. Polymers 2022, 14, 5529. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Colombo, B.; Favini, F.; Scaglia, B.; Sciarria, T.P.; D’Imporzano, G.; Pognani, M.; Alekseeva, A.; Eisele, G.; Cosentino, C.; Adani, F. Enhanced polyhydroxyalkanoate (PHA) production from the organic fraction of municipal solid waste by using mixed microbial culture. Biotechnol. Biofuels 2017, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes 2016, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Kucera, D.; Pernicová, I.; Kovalcik, A.; Koller, M.; Mullerova, L.; Sedlacek, P.; Mravec, F.; Nebesarova, J.; Kalina, M.; Marova, I.; et al. Characterization of the promising poly(3-hydroxybutyrate) producing halophilic bacterium Halomonas halophila. Bioresour. Technol. 2018, 256, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Oh, M.-K. Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int. J. Biol. Macromol. 2015, 80, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, W.; Wang, H.; Geng, A. Polyhydroxybutyrate production from oil palm empty fruit bunch using Bacillus megaterium R11. Bioresour. Technol. 2013, 147, 307–314. [Google Scholar] [CrossRef]
- Nonato, R.; Mantelatto, P.; Rossell, C. Integrated production of biodegradable plastic, sugar and ethanol. Appl. Microbiol. Biotechnol. 2001, 57, 1–5. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Alvarez, V.; Lazzeri, A. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Pötter, M.; Madkour, M.H.; Mayer, F.; Steinbüchel, A. Regulation of phasin expression and polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha H16. Microbiology 2002, 148, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Dartiailh, C.; Blunt, W.; Sharma, P.K.; Liu, S.; Cicek, N.; Levin, D.B. The Thermal and Mechanical Properties of Medium Chain-Length Polyhydroxyalkanoates Produced by Pseudomonas putida LS46 on Various Substrates. Front. Bioeng. Biotechnol. 2021, 8, 617489. [Google Scholar] [CrossRef] [PubMed]
- Szacherska, K.; Moraczewski, K.; Czaplicki, S.; Oleskowicz-Popiel, P.; Mozejko-Ciesielska, J. Effect of short- and medium-chain fatty acid mixture on polyhydroxyalkanoate production by Pseudomonas strains grown under different culture conditions. Front. Bioeng. Biotechnol. 2022, 10, 951583. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.B.S.; Malafaia, C.B. Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int. J. Biol. Macromol. 2018, 107, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Simon-Colin, C.; Gouin, C.; Lemechko, P.; Kervarec, N.; Guezennec, J. Development of a three-steps derivatization assay for the localization of double bond in monounsaturated monomers of poly-beta-hydroxyalkanoates by GC–MS. J. Chromatogr. B 2012, 900, 64–70. [Google Scholar] [CrossRef]
- Ciesielski, S.; Możejko, J.; Pisutpaisal, N. Plant oils as promising substrates for polyhydroxyalkanoates production. J. Clean. Prod. 2015, 106, 408–421. [Google Scholar] [CrossRef]
- Koller, M. Chemical and Biochemical Engineering Approaches in Manufacturing Polyhydroxyalkanoate (PHA) Biopolyesters of Tailored Structure with Focus on the Diversity of Building Blocks. Chem. Biochem. Eng. Q. 2019, 32, 413–438. [Google Scholar] [CrossRef]
- Koller, M. Chapter 1—Production, properties, and processing of microbial polyhydroxyalkanoate (PHA) biopolyesters. In Microbial and Natural Macromolecules; Das, S., Dash, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 3–55. [Google Scholar]
- Guleria, S.; Singh, H.; Sharma, V.; Bhardwaj, N.; Arya, S.K.; Puri, S.; Khatri, M. Polyhydroxyalkanoates production from domestic waste feedstock: A sustainable approach towards bio-economy. J. Clean. Prod. 2022, 340, 130661. [Google Scholar] [CrossRef]
- Vandamme, P.; Coenye, T. Taxonomy of the genus Cupriavidus: A tale of lost and found. Int. J. Syst. Evol. Microbiol. 2004, 54, 2285–2289. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Pavan, F.A.; Bordignon, S.E.; Aragão, G.M.F.d.; Poletto, P. Polyhydroxybutyrate (PHB) production by Cupriavidus necator from sugarcane vinasse and molasses as mixed substrate. Process Biochem. 2019, 85, 12–18. [Google Scholar] [CrossRef]
- Khatami, K.; Perez-Zabaleta, M.; Cetecioglu, Z. Pure cultures for synthetic culture development: Next level municipal waste treatment for polyhydroxyalkanoates production. J. Environ. Manag. 2022, 305, 114337. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef]
- Vu, D.H.; Wainaina, S.; Taherzadeh, M.J.; Åkesson, D.; Ferreira, J.A. Production of polyhydroxyalkanoates (PHAs) by Bacillus megaterium using food waste acidogenic fermentation-derived volatile fatty acids. Bioengineered 2021, 12, 2480–2498. [Google Scholar] [CrossRef] [PubMed]
- Kniewel, R.; Lopez, O.R.; Prieto, M.A. Biogenesis of Medium-Chain-Length Polyhydroxyalkanoates. In Biogenesis of Fatty Acids, Lipids and Membranes; Geiger, O., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 457–481. [Google Scholar]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and Modifications. Polymer 2021, 212, 123161. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.-C.; Ma, Y.-M.; Chen, G.-Q. Engineering biosynthesis of polyhydroxyalkanoates (PHA) for diversity and cost reduction. Metab. Eng. 2020, 58, 82–93. [Google Scholar] [CrossRef]
- Impallomeni, G.; Ballistreri, A.; Carnemolla, G.M.; Rizzo, M.G.; Nicolò, M.S.; Guglielmino, S.P.P. Biosynthesis and structural characterization of polyhydroxyalkanoates produced by Pseudomonas aeruginosa ATCC 27853 from long odd-chain fatty acids. Int. J. Biol. Macromol. 2018, 108, 608–614. [Google Scholar] [CrossRef]
- Tortajada, M.; da Silva, L.F.; Prieto, M.A. Second-generation functionalized medium-chain-length polyhydroxyalkanoates: The gateway to high-value bioplastic applications. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2013, 16, 1–15. [Google Scholar]
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.; Pandey, A.; Gnansounou, E.; Andrew Lin, K.-Y.; Tsang, D.C.W.; Thakur, I.S. Bacterial polyhydroxyalkanoates: Opportunities, challenges, and prospects. J. Clean. Prod. 2020, 263, 121500. [Google Scholar] [CrossRef]
- Garcia Tavares, R.; Lakshmanan, P.; Peiter, E.; O’Connell, A.; Caldana, C.; Vicentini, R.; Soares, J.S.; Menossi, M. ScGAI is a key regulator of culm development in sugarcane. J. Exp. Bot. 2018, 69, 3823–3837. [Google Scholar] [CrossRef]
- Huang, J.; Khan, M.T.; Perecin, D.; Coelho, S.T.; Zhang, M. Sugarcane for bioethanol production: Potential of bagasse in Chinese perspective. Renew. Sustain. Energy Rev. 2020, 133, 110296. [Google Scholar] [CrossRef]
- Toscano Miranda, N.; Lopes Motta, I.; Maciel Filho, R.; Wolf Maciel, M.R. Sugarcane bagasse pyrolysis: A review of operating conditions and products properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Biriș, S.-Ș. Sustainable Valorization of Waste and By-Products from Sugarcane Processing. Sustainability 2022, 14, 11089. [Google Scholar] [CrossRef]
- Talekar, S.; Ekanayake, K.; Holland, B.; Barrow, C. Food waste biorefinery towards circular economy in Australia. Bioresour. Technol. 2023, 388, 129761. [Google Scholar] [CrossRef]
- Rezende, C.A.; de Lima, M.A.; Maziero, P.; de Azevedo, E.R.; Garcia, W.; Polikarpov, I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 54. [Google Scholar] [CrossRef]
- Pan, S.; Zabed, H.M.; Wei, Y.; Qi, X. Technoeconomic and environmental perspectives of biofuel production from sugarcane bagasse: Current status, challenges and future outlook. Ind. Crops Prod. 2022, 188, 115684. [Google Scholar] [CrossRef]
- Mehrzad, S.; Taban, E.; Soltani, P.; Samaei, S.E.; Khavanin, A. Sugarcane bagasse waste fibers as novel thermal insulation and sound-absorbing materials for application in sustainable buildings. Build. Environ. 2022, 211, 108753. [Google Scholar] [CrossRef]
- Nicholson, S.R.; Rorrer, N.A.; Carpenter, A.C.; Beckham, G.T. Manufacturing energy and greenhouse gas emissions associated with plastics consumption. Joule 2021, 5, 673–686. [Google Scholar] [CrossRef]
- Khoo, R.Z.; Chow, W.S.; Ismail, H. Sugarcane bagasse fiber and its cellulose nanocrystals for polymer reinforcement and heavy metal adsorbent: A review. Cellulose 2018, 25, 4303–4330. [Google Scholar] [CrossRef]
- Sahu, O. Assessment of sugarcane industry: Suitability for production, consumption, and utilization. Ann. Agrar. Sci. 2018, 16, 389–395. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Anannya, F.R. Sugarcane bagasse—A source of cellulosic fiber for diverse applications. Heliyon 2021, 7, e07771. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.X.; Sun, X.F.; Zhao, H.; Sun, R.C. Isolation and characterization of cellulose from sugarcane bagasse. Polym. Degrad. Stab. 2004, 84, 331–339. [Google Scholar] [CrossRef]
- Tomizawa, S.; Chuah, J.-A.; Matsumoto, K.; Doi, Y.; Numata, K. Understanding the Limitations in the Biosynthesis of Polyhydroxyalkanoate (PHA) from Lignin Derivatives. ACS Sustain. Chem. Eng. 2014, 2, 1106–1113. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuels Bioprod. Biorefining 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Xing, Y.; Bu, L.; Wang, K.; Jiang, J. Pretreatment of furfural residues with alkaline peroxide to improve cellulose hydrolysis and characterization of isolated lignin. Cellul. Chem. Technol. 2012, 46, 249. [Google Scholar]
- Xu, Z.; Huang, F. Pretreatment Methods for Bioethanol Production. Appl. Biochem. Biotechnol. 2014, 174, 43–62. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osiboye, O.O.; Toluwalope Odediran, E.; Louis, H. Sustainable synthesis and applications of polyhydroxyalkanoates (PHAs) from biomass. Process Biochem. 2020, 96, 174–193. [Google Scholar] [CrossRef]
- Cruz, G.; Santiago, P.A.; Braz, C.E.M.; Seleghim, P.; Crnkovic, P.M. Investigation into the physical–chemical properties of chemically pretreated sugarcane bagasse. J. Therm. Anal. Calorim. 2018, 132, 1039–1053. [Google Scholar] [CrossRef]
- Jackson de Moraes Rocha, G.; Martin, C.; Soares, I.B.; Souto Maior, A.M.; Baudel, H.M.; Moraes de Abreu, C.A. Dilute mixed-acid pretreatment of sugarcane bagasse for ethanol production. Biomass Bioenergy 2011, 35, 663–670. [Google Scholar] [CrossRef]
- Vieira, S.; Barros, M.V.; Sydney, A.C.N.; Piekarski, C.M.; de Francisco, A.C.; Vandenberghe, L.P.d.S.; Sydney, E.B. Sustainability of sugarcane lignocellulosic biomass pretreatment for the production of bioethanol. Bioresour. Technol. 2020, 299, 122635. [Google Scholar] [CrossRef] [PubMed]
- Nosratpour, M.J.; Karimi, K.; Sadeghi, M. Improvement of ethanol and biogas production from sugarcane bagasse using sodium alkaline pretreatments. J. Environ. Manag. 2018, 226, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.A.L.; Zamora, H.D.Z.; Varão, L.H.R.; Prado, N.S.; Baffi, M.A.; Pasquini, D. Effect of Steam Explosion Pretreatment Catalysed by Organic Acid and Alkali on Chemical and Structural Properties and Enzymatic Hydrolysis of Sugarcane Bagasse. Waste Biomass Valorization 2018, 9, 2191–2201. [Google Scholar] [CrossRef]
- Terán Hilares, R.; Swerts, M.P.; Ahmed, M.A.; Ramos, L.; da Silva, S.S.; Santos, J.C. Organosolv Pretreatment of Sugar Cane Bagasse for Bioethanol Production. Ind. Eng. Chem. Res. 2017, 56, 3833–3838. [Google Scholar] [CrossRef]
- Zhang, W.; You, Y.; Lei, F.; Li, P.; Jiang, J. Acetyl-assisted autohydrolysis of sugarcane bagasse for the production of xylo-oligosaccharides without additional chemicals. Bioresour. Technol. 2018, 265, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.d.S.; Ferraz, A. Biological pretreatment of sugarcane bagasse with basidiomycetes producing varied patterns of biodegradation. Bioresour. Technol. 2017, 225, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Dai, L.; Huang, T.; Jiang, K.; Zhou, X.; Xu, Y. A novel recyclable furoic acid-assisted pretreatment for sugarcane bagasse biorefinery in co-production of xylooligosaccharides and glucose. Biotechnol. Biofuels 2021, 14, 35. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, J.; Chen, H.; Liu, D. The fate of lignin during atmospheric acetic acid pretreatment of sugarcane bagasse and the impacts on cellulose enzymatic hydrolyzability for bioethanol production. Renew. Energy 2018, 128, 200–209. [Google Scholar] [CrossRef]
- Salgaonkar, B.B.; Bragança, J.M. Utilization of sugarcane bagasse by Halogeometricum borinquense strain E3 for biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering 2017, 4, 50. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Sustainable PHA production in integrated lignocellulose biorefineries. New Biotechnol. 2019, 49, 161–168. [Google Scholar] [CrossRef]
- Soares, I.B.; Mendes, K.C.S.; Benachour, M.; Abreu, C.A.M. Evaluation of the effects of operational parameters in the pretreatment of sugarcane bagasse with diluted sulfuric acid using analysis of variance. Chem. Eng. Commun. 2017, 204, 1369–1390. [Google Scholar] [CrossRef]
- Hernández-Salas, J.; Villa-Ramírez, M.; Veloz-Rendón, J.; Rivera-Hernández, K.; González-César, R.; Plascencia-Espinosa, M.; Trejo-Estrada, S. Comparative hydrolysis and fermentation of sugarcane and agave bagasse. Bioresour. Technol. 2009, 100, 1238–1245. [Google Scholar] [CrossRef]
- Rueda, S.G.; Rafael, R.A.; Carlos, G.S.; Aline, C.C.; Maciel Filho, R. Pretreatment of sugar cane bagasse with phosphoric and sulfuric diluted acid for fermentable sugars production by enzymatic hydrolysis. Chem. Eng. Trans. 2010, 20, 321–326. [Google Scholar]
- Rodríguez-Chong, A.; Ramírez, J.A.; Garrote, G.; Vázquez, M. Hydrolysis of sugar cane bagasse using nitric acid: A kinetic assessment. J. Food Eng. 2004, 61, 143–152. [Google Scholar] [CrossRef]
- Kovalcik, A.; Kucera, D.; Matouskova, P.; Pernicova, I.; Obruca, S.; Kalina, M.; Enev, V.; Marova, I. Influence of removal of microbial inhibitors on PHA production from spent coffee grounds employing Halomonas halophila. J. Environ. Chem. Eng. 2018, 6, 3495–3501. [Google Scholar] [CrossRef]
- Kumar, R.; Mago, G.; Balan, V.; Wyman, C.E. Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour. Technol. 2009, 100, 3948–3962. [Google Scholar] [CrossRef] [PubMed]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N.J. Potentials and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Wunna, K.; Nakasaki, K.; Auresenia, J.L.; Abella, L.C.; Gaspillo, P.-a.D. Effect of alkali pretreatment on removal of lignin from sugarcane bagasse. Chem. Eng. Trans. 2017, 56, 1831–1836. [Google Scholar]
- Yu, N.; Tan, L.; Sun, Z.-Y.; Nishimura, H.; Takei, S.; Tang, Y.-Q.; Kida, K. Bioethanol from sugarcane bagasse: Focused on optimum of lignin content and reduction of enzyme addition. Waste Manag. 2018, 76, 404–413. [Google Scholar] [CrossRef]
- Paixão, S.; Ladeira, S.; Silva, T.P.; Arez, B.; Roseiro, J.C.; Martins, M.; Alves, L. Sugarcane bagasse delignification with potassium hydroxide for enhanced enzymatic hydrolysis. RSC Adv. 2016, 6, 1042–1052. [Google Scholar] [CrossRef]
- Grimaldi, M.P.; Marques, M.P.; Laluce, C.; Cilli, E.M.; Sponchiado, S.R.P. Evaluation of lime and hydrothermal pretreatments for efficient enzymatic hydrolysis of raw sugarcane bagasse. Biotechnol. Biofuels 2015, 8, 205. [Google Scholar] [CrossRef]
- Hedayatkhah, A.; Motamedi, H.; Najafzadeh Varzi, H.; Ghezelbash, G.; Amopour Bahnamiry, M.; Karimi, K. Improvement of hydrolysis and fermentation of sugarcane bagasse by soaking in aqueous ammonia and methanolic ammonia. Biosci. Biotechnol. Biochem. 2013, 77, 1379–1383. [Google Scholar] [CrossRef]
- Ayeni, A.; Adeeyo, O.; Ayoola, A. Effective gravimetric characterization for lignocellulosic biomass: Comparison of NaOH-H2O2 and Ca(OH)2-H2O2 oxidation pretreated sugarcane bagasse. Int. J. Sci. Eng. Technol. 2015, 4, 5–9. [Google Scholar]
- Zhang, H.; Huang, S.; Wei, W.; Zhang, J.; Xie, J. Investigation of alkaline hydrogen peroxide pretreatment and Tween 80 to enhance enzymatic hydrolysis of sugarcane bagasse. Biotechnol. Biofuels 2019, 12, 107. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.Y.; Jang, H.M.; Lee, M.W.; Park, J.M. Sequential dilute acid and alkali pretreatment of corn stover: Sugar recovery efficiency and structural characterization. Bioresour. Technol. 2015, 182, 296–301. [Google Scholar] [CrossRef]
- Schmatz, A.A.; Brienzo, M. Butylated Hydroxytoluene Improves Lignin Removal by Organosolv Pretreatment of Sugarcane Bagasse. BioEnergy Res. 2022, 15, 166–174. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, J.; Zhang, H. Sodium hydroxide catalytic ethanol pretreatment and surfactant on the enzymatic saccharification of sugarcane bagasse. Bioresour. Technol. 2021, 319, 124171. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, S.C.; Nakasu, P.Y.S.; Scopel, E.; Araújo, M.F.; Cardoso, L.H.; Costa, A.C.d. Organosolv pretreatment for biorefineries: Current status, perspectives, and challenges. Bioresour. Technol. 2023, 369, 128331. [Google Scholar] [CrossRef] [PubMed]
- Raud, M.; Olt, J.; Kikas, T. N2 explosive decompression pretreatment of biomass for lignocellulosic ethanol production. Biomass Bioenergy 2016, 90, 1–6. [Google Scholar] [CrossRef]
- Silveira, M.H.L.; Chandel, A.K.; Vanelli, B.A.; Sacilotto, K.S.; Cardoso, E.B. Production of hemicellulosic sugars from sugarcane bagasse via steam explosion employing industrially feasible conditions: Pilot scale study. Bioresour. Technol. Rep. 2018, 3, 138–146. [Google Scholar] [CrossRef]
- Pitarelo, A.P.; Fonseca, C.S.d.; Chiarello, L.M.; Gírio, F.M.; Ramos, L.P. Ethanol Production from Sugarcane Bagasse Using Phosphoric Acid-Catalyzed Steam Explosion. J. Braz. Chem. Soc. 2016, 27, 1889–1898. [Google Scholar] [CrossRef]
- Yan, X.; Li, D.; Ma, X.; Li, J. Bioconversion of renewable lignocellulosic biomass into multicomponent substrate via pressurized hot water pretreatment for bioplastic polyhydroxyalkanoate accumulation. Bioresour. Technol. 2021, 339, 125667. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Fungal pretreatment of lignocellulosic biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef]
- Malhotra, M.; Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: Removing obstacles in energy generation. Environ. Sci. Pollut. Res. 2021, 28, 58929–58944. [Google Scholar] [CrossRef]
- Masran, R.; Zanirun, Z.; Bahrin, E.K.; Ibrahim, M.F.; Lai Yee, P.; Abd-Aziz, S. Harnessing the potential of ligninolytic enzymes for lignocellulosic biomass pretreatment. Appl. Microbiol. Biotechnol. 2016, 100, 5231–5246. [Google Scholar] [CrossRef]
- Hartmann, C.; Fontana, R.C.; de Siqueira, F.G.; Camassola, M. Fungal pretreatment of sugarcane bagasse: A green pathway to improve saccharification and ethanol production. BioEnergy Res. 2022, 15, 1130–1143. [Google Scholar] [CrossRef]
- Rencoret, J.; Pereira, A.; del Río, J.C.; Martínez, Á.T.; Gutiérrez, A. Delignification and Saccharification Enhancement of Sugarcane Byproducts by a Laccase-Based Pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 7145–7154. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Tian, M.; Mao, Z.; Tang, L.; Zhang, J.; Zhang, H. Enhancement of methane production from cassava residues by biological pretreatment using a constructed microbial consortium. Bioresour. Technol. 2011, 102, 8899–8906. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Lai, Q.-H.; Lu, Y.; Liu, Z.-D.; Wang, T.-M.; Zhang, C.; Xing, X.-H. Enhanced biohydrogen production from corn stover by the combination of Clostridium cellulolyticum and hydrogen fermentation bacteria. J. Biosci. Bioeng. 2016, 122, 482–487. [Google Scholar] [CrossRef]
- Hui, W.; Jiajia, L.; Yucai, L.; Peng, G.; Xiaofen, W.; Kazuhiro, M.; Zongjun, C. Bioconversion of un-pretreated lignocellulosic materials by a microbial consortium XDC-2. Bioresour. Technol. 2013, 136, 481–487. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Pérez-Rivero, C.; Uwaezuoke, O.J.; Ngwuluka, N.C. From waste to wealth: Upcycling of plastic and lignocellulosic wastes to PHAs. J. Chem. Technol. Biotechnol. 2022, 97, 3217–3240. [Google Scholar] [CrossRef]
- González-Rojo, S.; Díez-Antolínez, R. Production of polyhydroxyalkanoates as a feasible alternative for an integrated multiproduct lignocellulosic biorefinery. Bioresour. Technol. 2023, 386, 129493. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, S.; Ma, M.-G.; Ji, X.-X.; Choi, S.-E.; Si, C. The Kinetics Studies on Hydrolysis of Hemicellulose. Front. Chem. 2021, 9, 781291. [Google Scholar] [CrossRef]
- Liu, L.; Huang, W.-C.; Liu, Y.; Li, M. Diversity of cellulolytic microorganisms and microbial cellulases. Int. Biodeterior. Biodegrad. 2021, 163, 105277. [Google Scholar] [CrossRef]
- Sahu, A.; Manna, M.C.; Bhattacharjya, S.; Thakur, J.K.; Mandal, A.; Rahman, M.M.; Singh, U.B.; Bhargav, V.K.; Srivastava, S.; Patra, A.K.; et al. Thermophilic ligno-cellulolytic fungi: The future of efficient and rapid bio-waste management. J. Environ. Manag. 2019, 244, 144–153. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Mahato, R.P.; Kumar, S.; Singh, P. Production of polyhydroxyalkanoates from renewable resources: A review on prospects, challenges and applications. Arch. Microbiol. 2023, 205, 172. [Google Scholar] [CrossRef]
- Lopes, M.S.G.; Gomez, J.G.C.; Taciro, M.K.; Mendonça, T.T.; Silva, L.F. Polyhydroxyalkanoate biosynthesis and simultaneous remotion of organic inhibitors from sugarcane bagasse hydrolysate by Burkholderia sp. J. Ind. Microbiol. Biotechnol. 2014, 41, 1353–1363. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Ghodake, G.S.; Bharagava, R.N.; Kim, D.S.; Nair, S.; Shin, H.S. Efficient bioconversion of sugarcane bagasse into polyhydroxybutyrate (PHB) by Lysinibacillus sp. and its characterization. Bioresour. Technol. 2021, 324, 124673. [Google Scholar] [CrossRef]
- Madhumathi, R.; Muthukumar, K.; Velan, M. Optimization of Polyhydroxybutyrate Production by Bacillus safensis EBT1. CLEAN Soil Air Water 2016, 44, 1066–1074. [Google Scholar] [CrossRef]
- Lee, S.M.; Cho, D.-H.; Jung, H.J.; Kim, B.; Kim, S.H.; Bhatia, S.K.; Gurav, R.; Jeon, J.-M.; Yoon, J.-J.; Kim, W.; et al. Finding of novel polyhydroxybutyrate producer Loktanella sp. SM43 capable of balanced utilization of glucose and xylose from lignocellulosic biomass. Int. J. Biol. Macromol. 2022, 208, 809–818. [Google Scholar] [CrossRef]
- Kourilova, X.; Pernicova, I.; Sedlar, K.; Musilova, J.; Sedlacek, P.; Kalina, M.; Koller, M.; Obruca, S. Production of polyhydroxyalkanoates (PHA) by a thermophilic strain of Schlegelella thermodepolymerans from xylose rich substrates. Bioresour. Technol. 2020, 315, 123885. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Pei, T.; Du, H.; Feng, G.-D.; Zhu, H. Genome-based taxonomic classification of the closest-to-Comamonadaceae group supports a new family Sphaerotilaceae fam. nov. and taxonomic revisions. Syst. Appl. Microbiol. 2022, 45, 126352. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.-K.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Varjani, S.; Nair, S.; Kim, D.-S.; Shin, H.-S.; et al. Developing Microbial Co-Culture System for Enhanced Polyhydroxyalkanoates (PHA) Production Using Acid Pretreated Lignocellulosic Biomass. Polymers 2022, 14, 726. [Google Scholar] [CrossRef]
- Siripurapu, A.; Kvn, V.; Shivshetty, N.; Poosarla, V.G. Production and characterization of biodegradable polymer-polyhydroxybutyrate from agricultural waste-sugarcane bagasse by the novel marine bacterium Klebsiella pneumoniae G1. Bioresour. Technol. Rep. 2022, 20, 101268. [Google Scholar] [CrossRef]
- Salgaonkar, B.B.; Bragança, J.M. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Halogeometricum borinquense strain E3. Int. J. Biol. Macromol. 2015, 78, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Taciro, M.; Michelin Ramos, M.; Carter, J.; Pradella, J.; Gomez, J. Poly-3-hydroxybutyrate (P3HB) production by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J. Ind. Microbiol. Biotechnol. 2004, 31, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Stahl, H. Microbial utilization and biopolyester synthesis of bagasse hydrolysates. Bioresour. Technol. 2008, 99, 8042–8048. [Google Scholar] [CrossRef] [PubMed]
- de Paula, C.B.C.; de Paula-Elias, F.C.; Rodrigues, M.N.; Coelho, L.F.; de Oliveira, N.M.L.; de Almeida, A.F.; Contiero, J. Polyhydroxyalkanoate Synthesis by Burkholderia glumae into a Sustainable Sugarcane Biorefinery Concept. Front. Bioeng. Biotechnol. 2020, 8, 631284. [Google Scholar] [CrossRef] [PubMed]
- Gowda, V.; Shivakumar, S. Agrowaste-based Polyhydroxyalkanoate (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM 12077. Braz. Arch. Biol. Technol. 2014, 57, 55–61. [Google Scholar] [CrossRef]
- Barrameda, H.J.; Requiso, P.J.; Alfafara, C.G.; Nayve Jr, F.R.P.; Ventura, R.L.G.; Ventura, J.-R.S. Hydrolysate production from sugarcane bagasse using steam explosion and sequential steam explosion-dilute acid pretreatment for polyhydroxyalkanoate fermentation. Bioact. Carbohydr. Diet. Fibre 2023, 30, 100376. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Buitrón, G.; Chairez, I.; Ruiz, H.A. Microbial co-culturing strategies for the production high value compounds, a reliable framework towards sustainable biorefinery implementation—An overview. Bioresour. Technol. 2021, 321, 124458. [Google Scholar] [CrossRef]
- Ai, M.; Zhu, Y.; Jia, X. Recent advances in constructing artificial microbial consortia for the production of medium-chain-length polyhydroxyalkanoates. World J. Microbiol. Biotechnol. 2021, 37, 2. [Google Scholar] [CrossRef]
- Corrado, I.; Petrillo, C.; Isticato, R.; Casillo, A.; Corsaro, M.M.; Sannia, G.; Pezzella, C. The power of two: An artificial microbial consortium for the conversion of inulin into Polyhydroxyalkanoates. Int. J. Biol. Macromol. 2021, 189, 494–502. [Google Scholar] [CrossRef]
- Sawant, S.S.; Salunke, B.K.; Taylor, L.E.; Kim, B.S. Enhanced Agarose and Xylan Degradation for Production of Polyhydroxyalkanoates by Co-Culture of Marine Bacterium, Saccharophagus degradans and Its Contaminant, Bacillus cereus. Appl. Sci. 2017, 7, 225. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Jia, X. Construction of a “nutrition supply–detoxification” coculture consortium for medium-chain-length polyhydroxyalkanoate production with a glucose–xylose mixture. J. Ind. Microbiol. Biotechnol. 2020, 47, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Fox, B.G.; Takasuka, T.E. Consolidated bioprocessing of plant biomass to polyhydroxyalkanoate by co-culture of Streptomyces sp. SirexAA-E and Priestia megaterium. Bioresour. Technol. 2023, 376, 128934. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lee, H.-J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Jeon, J.-M.; Yoon, J.-J.; Bhatia, S.K.; Gurav, R. Screening of the strictly xylose-utilizing Bacillus sp. SM01 for polyhydroxybutyrate and its co-culture with Cupriavidus necator NCIMB 11599 for enhanced production of PHB. Int. J. Biol. Macromol. 2021, 181, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kratzl, F.; Kremling, A.; Pflüger-Grau, K. Streamlining of a synthetic co-culture towards an individually controllable one-pot process for polyhydroxyalkanoate production from light and CO2. Eng. Life Sci. 2023, 23, e2100156. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ai, M.; Jia, X. Optimization of a Two-Species Microbial Consortium for Improved Mcl-PHA Production from Glucose–Xylose Mixtures. Front. Bioeng. Biotechnol. 2022, 9, 794331. [Google Scholar] [CrossRef]
- Wei, Z.; Zhu, Y.; Ai, M.; Liu, C.; Jia, X. Construction and analysis of a Pseudomonas-Saccharomyces microbial consortium for mcl-PHA production from xylose and octanoate. Can. J. Chem. Eng. 2023, 101, 3680–3692. [Google Scholar] [CrossRef]
- Department of Agriculture, Fisheries and Forestry. Sugarcane in Australia. Available online: https://www.agriculture.gov.au/agriculture-land/farm-food-drought/crops/sugar#:~:text=Overview,in%20northern%20New%20South%20Wales (accessed on 9 January 2024).
- Australian Sugar Milling Council. Sugar Industry Summary Statistics; Australian Sugar Milling Council: Brisbane City, Australia, 2022. [Google Scholar]
- National Waste Report 2022. National Waste Report 2022. Available online: https://www.dcceew.gov.au/environment/protection/waste/national-waste-reports/2022 (accessed on 14 April 2023).
- Australian Government Clean Energy Regulator. Bagasse: Recycling Crop Residue; Australian Government Clean Energy Regulator: Canberra, Australia, 2023. [Google Scholar]
- Australian Sugar Milling Council. Sugar Mills a Source of Renewable Power. Available online: https://asmc.com.au/wp-content/uploads/2019/12/ASMC_Factsheet_Sugar-mills-a-source-of-renewable-power_web.pdf (accessed on 30 June 2023).
- Australian Sugar Milling Council. Renewable Energy. Available online: https://asmc.com.au/policy-advocacy/energy-policy/ (accessed on 30 June 2023).
- Recyclopaedia. Bagasse. Available online: https://www.cityservices.act.gov.au/recyclopaedia/items/b/bagasse (accessed on 30 June 2023).
- Department of Climate Change, Energy, the Environment and Water. Heritage and the Arts. The Full Cost of Landfill Disposal in Australia; Department of Climate Change, Energy, the Environment and Water: Canberra, Australia, 2009. [Google Scholar]
- EPA. Emissions Impacts of Landfilling Food Waste; EPA: New South Wales, Australia, 2022. [Google Scholar]
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef]
- Camargo, F.A.; Innocentini-Mei, L.H.; Lemes, A.P.; Moraes, S.G.; Durán, N. Processing and characterization of composites of poly(3-hydroxybutyrate-co-hydroxyvalerate) and lignin from sugar cane bagasse. J. Compos. Mater. 2012, 46, 417–425. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Anburajan, P.; Parvez, A.; Kathirvel, P.; Qi, X. Active polyhydroxybutyrate (PHB)/sugarcane bagasse fiber-based anti-microbial green composite: Material characterization and degradation studies. Appl. Nanosci. 2023, 13, 1187–1199. [Google Scholar] [CrossRef]
- Masood, F.; Yasin, T.; Hameed, A. Polyhydroxyalkanoates—What are the uses? Current challenges and perspectives. Crit. Rev. Biotechnol. 2015, 35, 514–521. [Google Scholar] [CrossRef]
- Kiran, G.S.; Lipton, A.N.; Priyadharshini, S.; Anitha, K.; Suárez, L.E.C.; Arasu, M.V.; Choi, K.C.; Selvin, J.; Al-Dhabi, N.A. Antiadhesive activity of poly-hydroxy butyrate biopolymer from a marine Brevibacterium casei MSI04 against shrimp pathogenic vibrios. Microb. Cell Factories 2014, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, W.A.; Hussain, A.; Lin, C.; Nguyen, M.K. Biodegradation of Different Types of Bioplastics through Composting—A Recent Trend in Green Recycling. Catalysts 2023, 13, 294. [Google Scholar] [CrossRef]
- Javierre, C.; Sarasa, J.; Claveria, I.; Fernandez, A. Study of the biodisintegration on a painted bioplastic material waste. Mater. Plast. 2015, 52, 116–121. [Google Scholar]
- Cafiero, L.M.; Canditelli, M.; Musmeci, F.; Sagnotti, G.; Tuffi, R. Assessment of disintegration of compostable bioplastic bags by management of electromechanical and static home composters. Sustainability 2020, 13, 263. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Aliaga, C.; Fito, C.; Hortal, M. Compostability assessment of nano-reinforced poly (lactic acid) films. Waste Manag. 2016, 48, 143–155. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).