Unravelling the Role of Biochemical Compounds within the Hydrothermal Liquefaction Process of Real Sludge Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Nature and Characterisation
2.2. Hydrothermal Liquefaction Tests
2.3. Characterisation of HTL Products
3. Results and Discussion
3.1. Chemical and Energetic Properties of Sludges
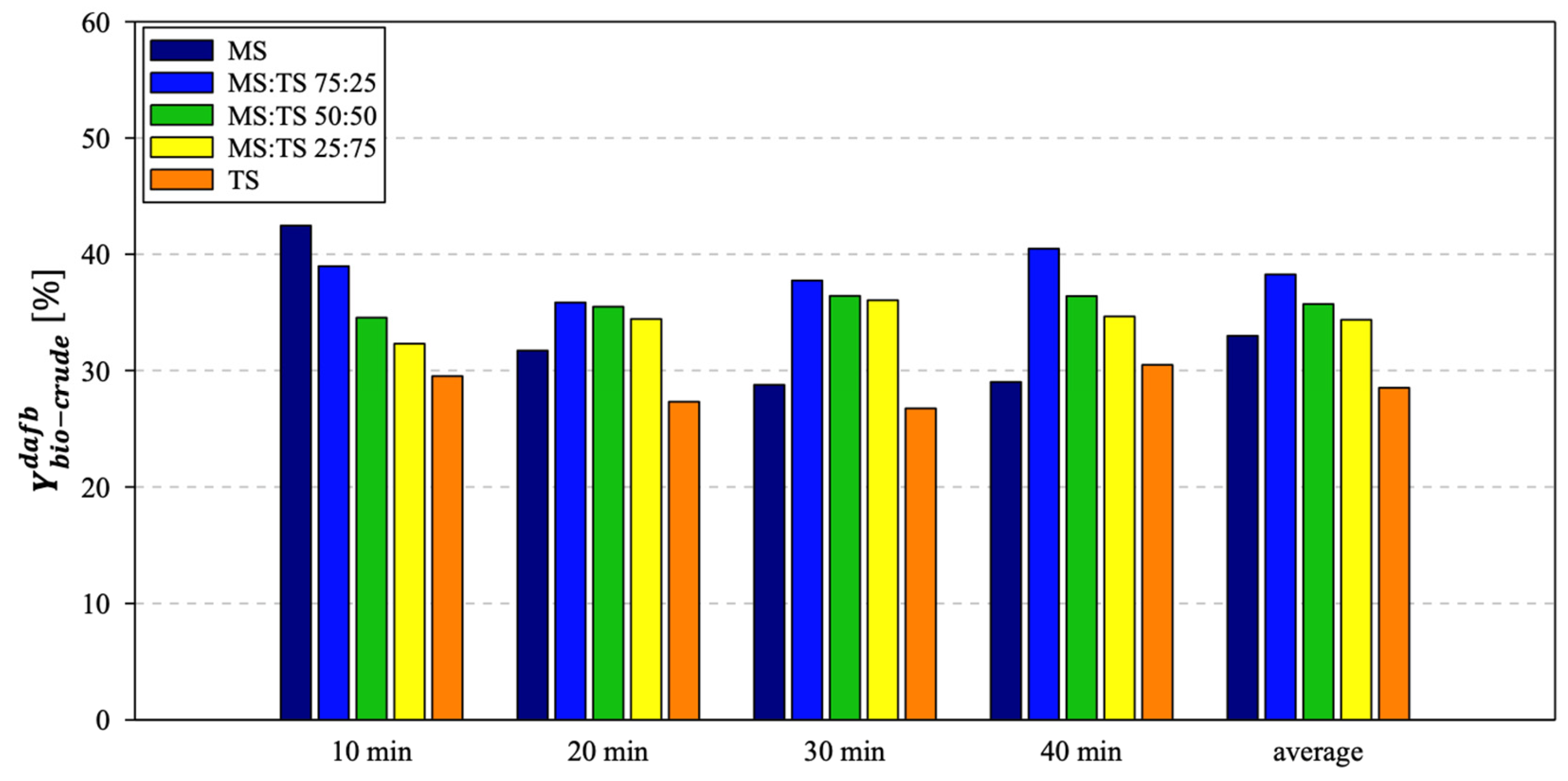
3.2. HTL Results: Yields of Phases and Energetic Properties
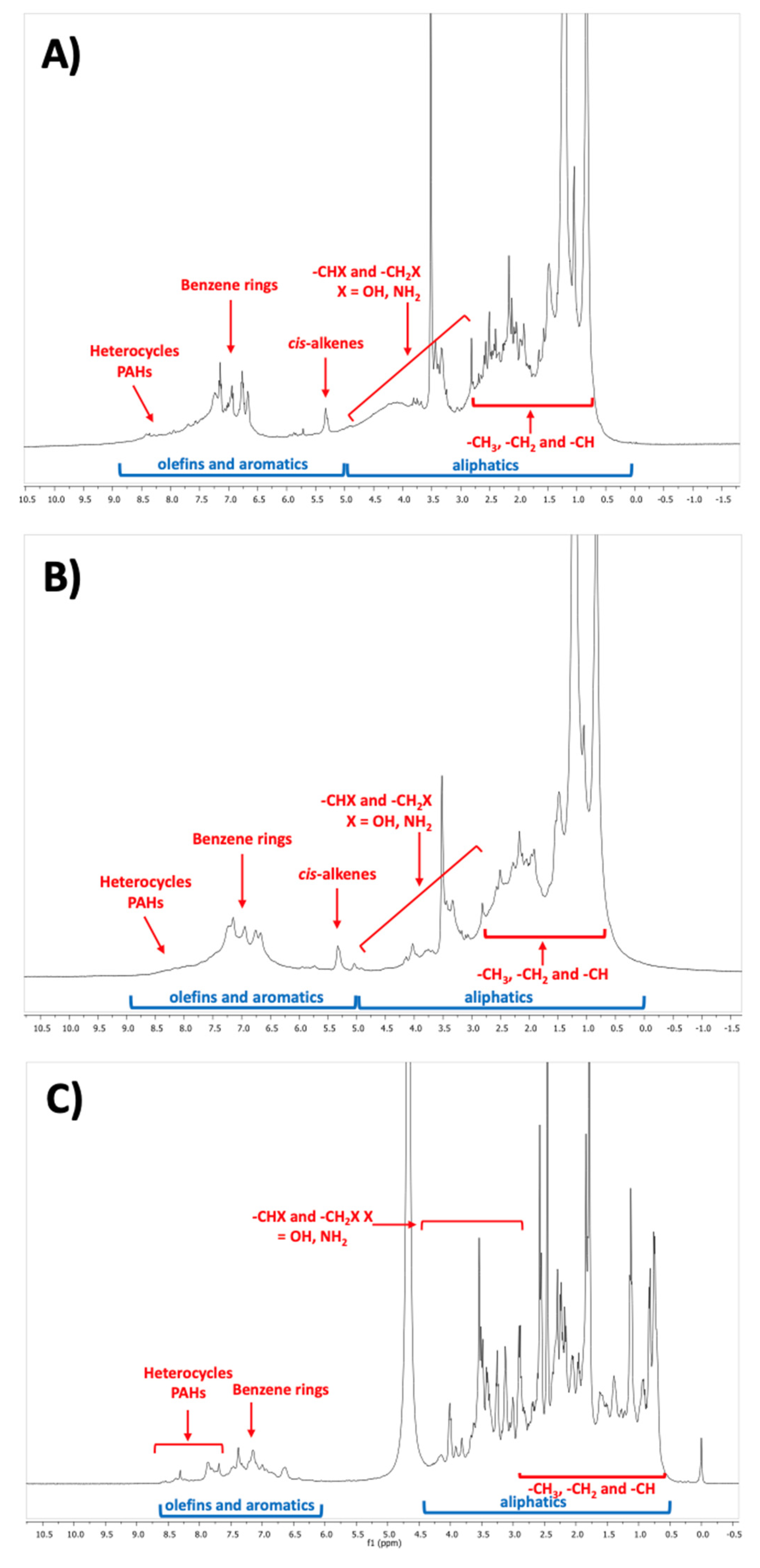
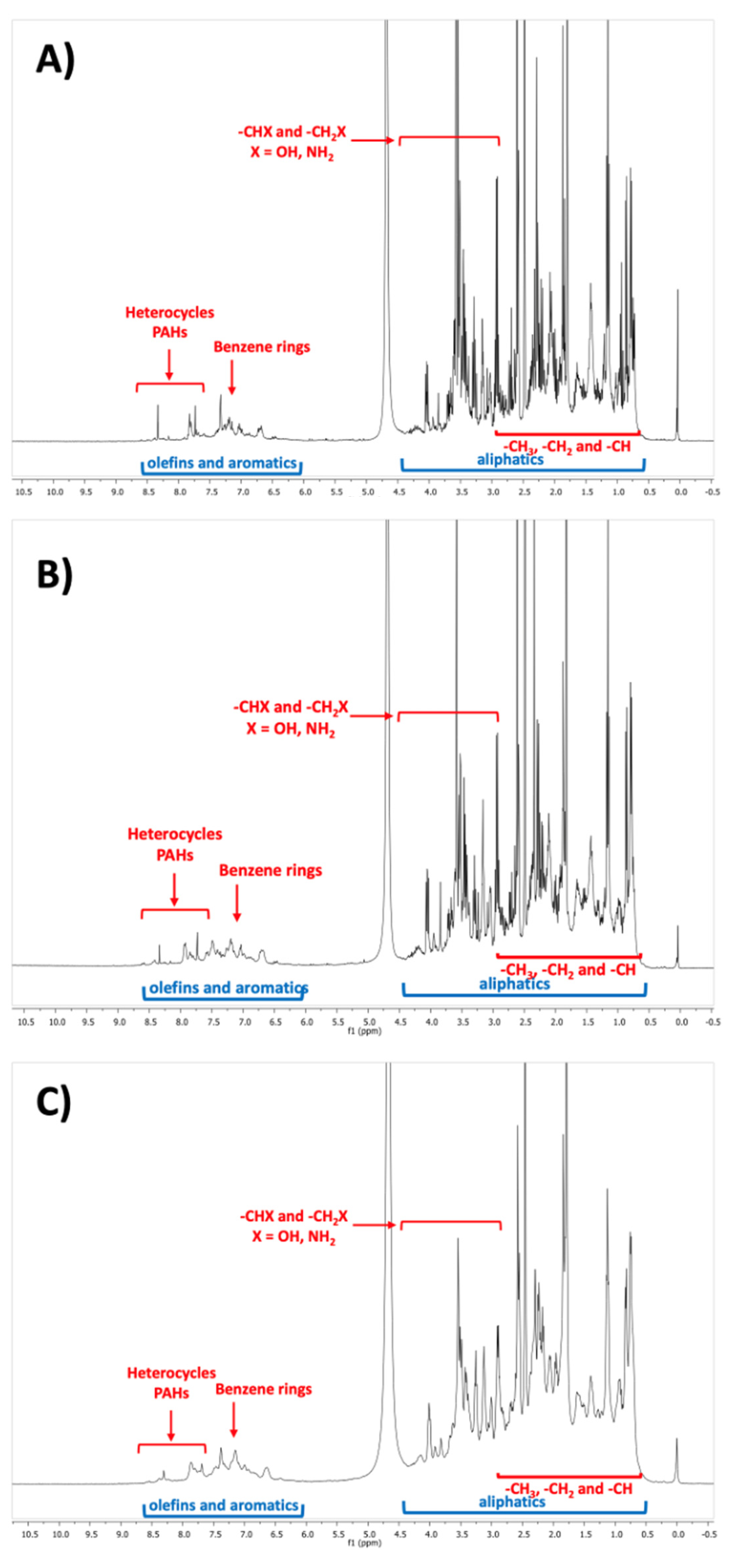
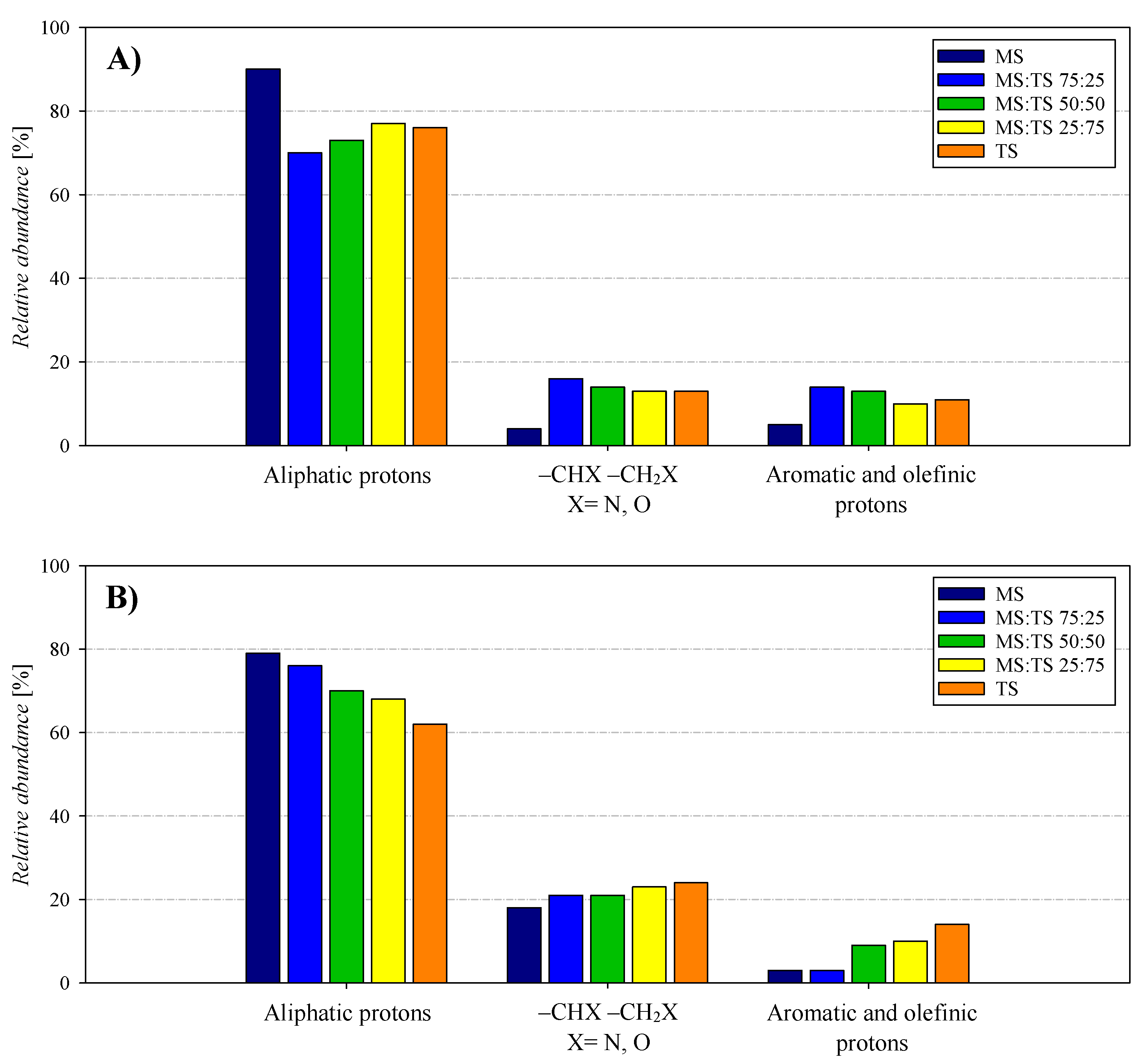
3.3. Chemical Properties of Bio-Crude and Aqueous Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balsamo, M.; Montagnaro, F.; Anthony, E.J. Socio-economic parameters affect CO2 emissions and energy consumption—An analysis over the United Nations Countries. Curr. Opin. Green Sustain. Chem. 2023, 40, 100740. [Google Scholar] [CrossRef]
- Ahanchi, M.; Jafary, T.; Yeneneh, A.M.; Rupani, P.F.; Shafizadeh, A.; Shahbeik, H.; Pan, J.; Tabatabaei, M.; Aghbashlo, M. Review on waste biomass valorization and power management systems for microbial fuel cell application. J. Clean. Prod. 2022, 380, 134994. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Berni, M.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Anaerobic digestion process: Technological aspects and recent developments. Int. J. Environ. Sci. Technol. 2018, 15, 2033–2046. [Google Scholar] [CrossRef]
- Kunatsa, T.; Xia, X. A review on anaerobic digestion with focus on the role of biomass co-digestion, modelling and optimisation on biogas production and enhancement. Bioresour. Technol. 2022, 344, 126311. [Google Scholar] [CrossRef]
- Lazaroiu, G.; Pop, E.; Negreanu, G.; Pisa, I.; Mihaescu, L.; Bondrea, A.; Berbece, V. Biomass combustion with hydrogen injection for energy applications. Energy 2017, 127, 351–357. [Google Scholar] [CrossRef]
- Hao, D.; Xuguang, J.; Lv, G.; Yong, C.; Jianhua, Y. Co-combustion of tannery sludge in a commercial circulating fluidized bed boiler. Waste Manag. 2015, 46, 227–233. [Google Scholar]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass pyrolysis: A review on recent advancements and green hydrogen production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef] [PubMed]
- Udayanga, W.D.C.; Veksha, A.; Giannis, A.; Lim, T.T. Pyrolysis derived char from municipal and industrial sludge: Impact of organic decomposition and inorganic accumulation on the fuel characteristics of char. Waste Manag. 2019, 83, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Del Grosso, M.; Sridharan, B.; Tsekos, C.; Klein, S.; de Jong, W. A modelling based study on the integration of 10 MWth indirect torrefied biomass gasification, methanol and power production. Biomass Bioenergy 2020, 136, 105529. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels production by biomass gasification: A review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Di Lauro, F.; Migliaccio, R.; Ruoppolo, G.; Balsamo, M.; Montagnaro, F.; Imperiale, E.; Caracciolo, D.; Urciuolo, M. Tannery sludge gasification in a fluidized bed for its energetic valorization. Ind. Eng. Chem. Res. 2022, 61, 16972–16979. [Google Scholar] [CrossRef]
- Zaccariello, L.; Montagnaro, F. Fluidised bed gasification of biomasses and wastes to produce hydrogen-rich syn-gas—A review. J. Chem. Technol. Biotechnol. 2023, 98, 1878–1887. [Google Scholar] [CrossRef]
- Brachi, P.; Chirone, R.; Miccio, M.; Ruoppolo, G. Fluidized bed torrefaction of commercial wood pellets: Process performance and solid product quality. Energy Fuels 2018, 32, 9459. [Google Scholar] [CrossRef]
- Adams, P.; Bridgwater, T.; Lea-Langton, A.; Ross, A.; Watson, I. Chapter 8—Biomass Conversion Technologies. In Greenhouse Gas Balances of Bioenergy Systems; Thornley, P., Adams, P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 107–139. [Google Scholar]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Li, P.; Shi, X.; Wang, X.; Song, J.; Fang, S.; Bai, J.; Zhang, G.; Chang, C.; Pang, S. Bio-oil from biomass fast pyrolysis: Yields, related properties and energy consumption analysis of the pyrolysis system. J. Clean. Prod. 2021, 328, 129613. [Google Scholar] [CrossRef]
- Klüpfel, C.; Herklotz, B.; Biller, P. Influence of processing conditions and biochemical composition on the hydrothermal liquefaction of digested urban and agricultural wastes. Fuel 2023, 352, 129016. [Google Scholar] [CrossRef]
- Parkhey, P. Biomethanol: Possibilities towards a bio-based economy. Biomass Convers. Biorefinery 2022, 12, 1877–1887. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Li, A. Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review. Renew. Sustain. Energy Rev. 2019, 108, 423–440. [Google Scholar] [CrossRef]
- Liu, H.; Basar, I.A.; Eskicioglu, C. Hydrothermal liquefaction for sludge-to-energy conversion: An evaluation of biocrude production and management of waste streams. Energy 2023, 281, 128268. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Prestigiacomo, C.; Scialdone, O.; Galia, A. Hydrothermal liquefaction of wet biomass in batch reactors: Critical assessment of the role of operating parameters as a function of the nature of the feedstock. J. Supercrit. Fluids 2022, 189, 105689. [Google Scholar] [CrossRef]
- Thomsen, L.B.S.; Carvalho, P.N.; dos Passos, J.S.; Anastasakis, K.; Bester, K.; Biller, P. Hydrothermal liquefaction of sewage sludge; energy considerations and fate of micropollutants during pilot scale processing. Water Res. 2020, 183, 116101. [Google Scholar] [CrossRef]
- Tai, L.; de Caprariis, B.; Scarsella, M.; De Filippis, P.; Marra, F. Improved quality bio-crude from hydrothermal liquefaction of oak wood assisted by zero-valent metals. Energy Fuels 2021, 35, 10023–10034. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef]
- Teri, G.; Luo, L.; Savage, P.E. Hydrothermal treatment of protein, polysaccharide, and lipids alone and in mixtures. Energy Fuels 2014, 28, 7501–7509. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef] [PubMed]
- Brindhadevi, K.; Anto, S.; Rene, E.R.; Sekar, M.; Mathimani, T.; Chi, N.T.L.; Pugazhendhi, A. Effect of reaction temperature on the conversion of algal biomass to bio-oil and biochar through pyrolysis and hydrothermal liquefaction. Fuel 2021, 285, 119106. [Google Scholar] [CrossRef]
- He, S.; Zhao, M.; Wang, J.; Cheng, Z.; Yan, B.; Chen, G. Hydrothermal liquefaction of low-lipid algae Nannochloropsis sp. and Sargassum sp.: Effect of feedstock composition and temperature. Sci. Total Environ. 2020, 712, 135677. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.; Liu, T.; Yang, L.; Li, H.; Peng, H.; Jiang, S.; Wang, X.; Leng, L. Machine learning aided bio-oil production with high energy recovery and low nitrogen content from hydrothermal liquefaction of biomass with experiment verification. Chem. Eng. J. 2021, 425, 130649. [Google Scholar] [CrossRef]
- Peterson, A.A.; Lachance, R.P.; Tester, J.W. Kinetic evidence of the Maillard reaction in hydrothermal biomass processing: Glucose–glycine interactions in high-temperature, high-pressure water. Ind. Eng. Chem. Res. 2010, 49, 2107–2117. [Google Scholar] [CrossRef]
- Fan, Y.; Hornung, U.; Dahmen, N. Hydrothermal liquefaction of sewage sludge for biofuel application: A review on fundamentals, current challenges and strategies. Biomass Bioenergy 2022, 165, 106570. [Google Scholar] [CrossRef]
- Shah, A.A.; Toor, S.S.; Conti, F.; Nielsen, A.H.; Rosendahl, L.A. Hydrothermal liquefaction of high ash containing sewage sludge at sub and supercritical conditions. Biomass Bioenergy 2020, 135, 105504. [Google Scholar] [CrossRef]
- dos Passos, J.S.; Matayeva, A.; Biller, P. Synergies during hydrothermal liquefaction of cow manure and wheat straw. J. Environ. Chem. Eng. 2022, 10, 108181. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.S.; Yang, L. A review on hydrothermal co-liquefaction of biomass. Appl. Energy 2019, 250, 926–945. [Google Scholar] [CrossRef]
- Thomsen, L.B.S.; Anastasakis, K.; Biller, P. Hydrothermal liquefaction potential of wastewater treatment sludges: Effect of wastewater treatment plant and sludge nature on products distribution. Fuel 2024, 355, 129525. [Google Scholar] [CrossRef]
- Subramanya, S.M.; Rios, N.; Kollar, A.; Stofanak, R.; Maloney, K.; Waltz, K.; Powers, L.; Rane, C.; Savage, P.E. Statistical models for predicting oil composition from hydrothermal liquefaction of biomass. Energy Fuels 2023, 37, 6619–6628. [Google Scholar] [CrossRef]
- Chacón-Parra, A.D.; Hall, P.A.; Lewis, D.M.; Glasius, M.; van Eyk, P.J. Elucidating the Maillard reaction mechanism in the hydrothermal liquefaction of binary model compound mixtures and spirulina. ACS Sustain. Chem. Eng. 2022, 10, 10989–11003. [Google Scholar] [CrossRef]
- Tito, E.; Pipitone, G.; Monteverde Videla, A.H.A.; Bensaid, S.; Pirone, R. Exploring HTL pathways in carbohydrate–protein mixture: A study on glucose–glycine interaction. Biomass Convers. Biorefinery 2023, 13, 16385–16404. [Google Scholar] [CrossRef]
- Fan, Y.; Hornung, U.; Dahmen, N.; Kruse, A. Hydrothermal liquefaction of protein-containing biomass: Study of model compounds for Maillard reactions. Biomass Convers. Biorefinery 2018, 8, 909–923. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Hartree, E.F. Determination of protein: A modification of the lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Di Lauro, F.; Balsamo, M.; Solimene, R.; Salatino, P.; Montagnaro, F. Hydrothermal liquefaction process to obtain sludge-derived bio-fuels: Setup of the experimental apparatus and preliminary tests. Chem. Eng. Trans. 2022, 92, 475–480. [Google Scholar]
- Di Lauro, F.; Balsamo, M.; Solimene, R.; Alfieri, M.L.; Manini, P.; Migliaccio, R.; Salatino, P.; Montagnaro, F. Characterization of biocrude produced by hydrothermal liquefaction of municipal sewage sludge in a 500 mL batch reactor. Ind. Eng. Chem. Res. 2024, 63, 955–967. [Google Scholar] [CrossRef]
- Fan, Y.; Meyer, L.; Gong, M.; Krause, B.; Hornung, U.; Dahmen, N. Understanding the fate of nitrogen during catalytic hydrothermal liquefaction of sewage sludge. Fuel 2023, 339, 126948. [Google Scholar] [CrossRef]
- Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Liquid biofuels from the organic fraction of municipal solid waste: A review. Renew. Sustain. Energy Rev. 2019, 110, 298–314. [Google Scholar] [CrossRef]
- Lu, J.; Fan, G.; Yang, M.; Wu, Y. Origin of carbon in the bio-oil from hydrothermal liquefaction of protein and glucose via isotopic labeling. Fuel 2022, 308, 121941. [Google Scholar] [CrossRef]
- Qiu, Y.; Aierzhati, A.; Cheng, J.; Guo, H.; Yang, W.; Zhang, Y. Biocrude oil production through the Maillard reaction between leucine and glucose during hydrothermal liquefaction. Energy Fuels 2019, 33, 8758–8765. [Google Scholar] [CrossRef]
- Basar, I.A.; Liu, H.; Carrere, H.; Trably, E.; Eskicioglu, C. A review on key design and operational parameters to optimize and develop hydrothermal liquefaction of biomass for biorefinery applications. Green Chem. 2021, 23, 1404–1446. [Google Scholar] [CrossRef]



| Municipal Sludge (MS) | Tannery Sludge (TS) | |
|---|---|---|
| Proximate analysis [% wt.] | ||
| Moisture | 12.68 ± 0.01 | 18.46 ± 0.31 |
| Volatile matter | 56.36 ± 0.03 | 50.13 ± 0.06 |
| Fixed carbon | 9.54 ± 0.11 | n.d. |
| Ash | 21.43 ± 0.18 | 31.41 ± 0.43 |
| Ultimate analysis [% wt.], dry basis | ||
| C | 34.55 ± 0.32 | 33.61 ± 0.14 |
| H | 4.87 ± 0.24 | 5.10 ± 0.19 |
| N | 5.90 ± 0.07 | 2.44 ± 0.05 |
| S | 0.77 ± 0.04 | 4.07 ± 0.03 |
| Cl | 0.61 ± 0.02 | 0.35 ± 0.03 |
| O | 28.76 | 15.91 |
| Heating value [MJ/kg], dry basis | ||
| HHV | 16.67 ± 0.40 | 14.90 ± 0.31 |
| LHV | 15.57 | 13.75 |
| Macro-components [% wt.], dry basis | ||
| Lipids | 2.4 ± 0.10 | 5.3 ± 0.20 |
| Proteins | 25.0 ± 0.10 | 29.4 ± 0.90 |
| Carbohydrates | 59.0 ± 0.03 | 21.0 ± 0.02 |
| Lipids (L) | Proteins (P) | Carbohydrates (C) | L:P:C | |
|---|---|---|---|---|
| MS | 2.4 | 25.0 | 59.0 | 1:10.4:24.6 |
| MS:TS 75:25 | 3.1 | 26.1 | 49.5 | 1:8.4:16.0 |
| MS:TS 50:50 | 3.8 | 27.2 | 40.0 | 1:7.2:10.5 |
| MS:TS 25:75 | 4.6 | 28.3 | 30.5 | 1:6.2:6.6 |
| TS | 5.3 | 29.4 | 21.0 | 1:5.6:4.0 |
| HHVbio-crude [MJ/kg] | [%], Equation (8) | ER [%], Equation (5) | |
|---|---|---|---|
| MS:TS 75:25, t = 10 min | 29.42 ± 0.81 | 28.1 | 50.94 |
| MS:TS 75:25, t = 30 min | 28.57 ± 1.63 | 27.1 | 47.70 |
| # | Time (min) | Compound | [M + H]+ (m/z) | TS | MS:TS 25:75 | MS:TS 50:50 | MS:TS 75:25 | MS |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.5 | p-aminophenol | 110 | w/o | w | w | w/o | w |
| 2 | 5 | cresol | 109 | w | w | w | w | - |
| 3 | 5.2 | xylene | 107 | - | - | w | w | - |
| 4 | 5.9 | hydroxymethylfuran-carboxylic acid | 143 | - | w | w | w | - |
| 5 | 7.1 | hydroxymethylfurfural | 126 | w | w | w | w | w/o |
| 6 | 7.8 | dimethylindole | 146 | - | w | w | w | w |
| 7 | 7.9 | quinoline | 130 | w/o | w/o | w/o | w/o | w/o |
| 8 | 8.5 | aminovaleraldehyde | 102 | - | o | o | o | - |
| 9 | 8.8 | methyl syringol | 169 | w/o | w/o | w/o | w/o | w |
| 10 | 9.1 | phenylethylamine | 122 | o | o | o | o | - |
| 11 | 9.5 | gallic acid | 171 | w | w | w | w/o | w |
| 12 | 10 | ferulic acid | 195 | w/o | w/o | w/o | w/o | w |
| 13 | 10.2 | methyl guaiacol | 139 | w/o | w/o | w/o | w/o | w |
| 14 | 11 | p-coumaric acid | 165 | o | o | o | w/o | w/o |
| 15 | 11.1 | tryptamine | 161 | o | o | o | o | - |
| 16 | 11.5 | ethyl pyrrolidinone | 114 | w/o | w/o | w/o | w/o | w |
| 17 | 12 | phenylalanine | 166 | o | o | o | o | - |
| 18 | 12.5 | phenylpropionic acid | 197 | w/o | w | w | w | w |
| 19 | 15.3 | naphthylamine | 144 | o | o | o | o | - |
| 20 | 16.7 | cinnamic acid | 149 | w | w | w | w | - |
| 21 | 17.3 | diphenylamine | 170 | o | o | o | o | - |
| 22 | 17.5 | carboxyethyllysine | 219 | w | w | w | w | - |
| 23 | 18 | homovanillic acid | 183 | w/o | w/o | w/o | w/o | w/o |
| 24 | 18.5 | sinapil alcohol | 211 | w/o | w/o | w/o | w/o | w/o |
| 25 | 19 | coniferilic alcohol | 181 | o | w/o | w/o | w/o | w/o |
| 26 | 20 | p-coumaryl alcohol | 151 | - | o | o | o | w/o |
| 27 | 20.5 | argpyrimidine | 255 | - | - | w | w | - |
| 28 | 20.8 | trimethylbenzonitrile | 146 | - | w | w | w | - |
| 29 | 22 | carboxyethylarginine | 247 | w/o | w/o | w/o | w/o | - |
| 30 | 22.2 | fluorene | 167 | - | w/o | w/o | w/o | - |
| 31 | 23.2 | carboxymethylarginine | 233 | - | - | w | w | - |
| 32 | 26.4 | N-fructosyllysine | 309 | w | w | w | w | w |
| 33 | 30.5 | p-nitrocatechol | 156 | w/o | w/o | w/o | w/o | w/o |
| 34 | 31 | 2-phenylpyridine | 156 | w/o | w/o | w/o | w/o | w/o |
| 35 | 38.4 | cellobiose | 343 | w | w | w | w | w |
| 36 | 41.7 | hexadecylamine | 242 | o | o | o | o | - |
| 37 | 43.4 | phenanthrene | 179 | w | w | w | w | w/o |
| 38 | 45.5 | octadecylamine | 270 | o | o | o | o | - |
| 39 | 47 | methyl palmitate | 271 | - | o | o | o | o |
| 40 | 49.4 | methyl dodecanamide | 214 | o | o | o | o | o |
| 41 | 50 | methyl stearate | 313 | o | o | o | o | o |
| 42 | 50.7 | pyrene | 203 | - | o | o | o | w/o |
| 43 | 52 | N,N-dimethylstearamide | 312 | o | o | o | o | o |
| 44 | 52.2 | dibenzoanthracene | 279 | w/o | w/o | w/o | w/o | w/o |
| 45 | 52.5 | stigmasterol | 413 | w/o | w/o | w/o | w/o | - |
| 46 | 52.7 | tetradecanamide | 228 | o | o | o | o | o |
| 47 | 53.5 | pentadecane | 213 | o | o | o | o | - |
| 48 | 55 | tribenzocoronene | 425 | w/o | w/o | w/o | w/o | o |
| 49 | 55.8 | methyl oleate | 297 | o | o | o | o | o |
| 50 | 56 | hexadecanamide | 256 | o | o | o | o | o |
| 51 | 58 | heptadecane | 214 | o | o | o | o | - |
| 52 | 59 | ethyl oleate | 311 | o | o | o | o | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balsamo, M.; Di Lauro, F.; Alfieri, M.L.; Manini, P.; Salatino, P.; Montagnaro, F.; Solimene, R. Unravelling the Role of Biochemical Compounds within the Hydrothermal Liquefaction Process of Real Sludge Mixtures. Sustainability 2024, 16, 1770. https://doi.org/10.3390/su16051770
Balsamo M, Di Lauro F, Alfieri ML, Manini P, Salatino P, Montagnaro F, Solimene R. Unravelling the Role of Biochemical Compounds within the Hydrothermal Liquefaction Process of Real Sludge Mixtures. Sustainability. 2024; 16(5):1770. https://doi.org/10.3390/su16051770
Chicago/Turabian StyleBalsamo, Marco, Francesca Di Lauro, Maria Laura Alfieri, Paola Manini, Piero Salatino, Fabio Montagnaro, and Roberto Solimene. 2024. "Unravelling the Role of Biochemical Compounds within the Hydrothermal Liquefaction Process of Real Sludge Mixtures" Sustainability 16, no. 5: 1770. https://doi.org/10.3390/su16051770
APA StyleBalsamo, M., Di Lauro, F., Alfieri, M. L., Manini, P., Salatino, P., Montagnaro, F., & Solimene, R. (2024). Unravelling the Role of Biochemical Compounds within the Hydrothermal Liquefaction Process of Real Sludge Mixtures. Sustainability, 16(5), 1770. https://doi.org/10.3390/su16051770












