Soil CO2 and CH4 Dynamics and Their Relationships with Soil Nutrients, Enzyme Activity, and Root Biomass during Winter Wheat Growth under Shallow Groundwater
Abstract
1. Introduction
2. Materials and Methods
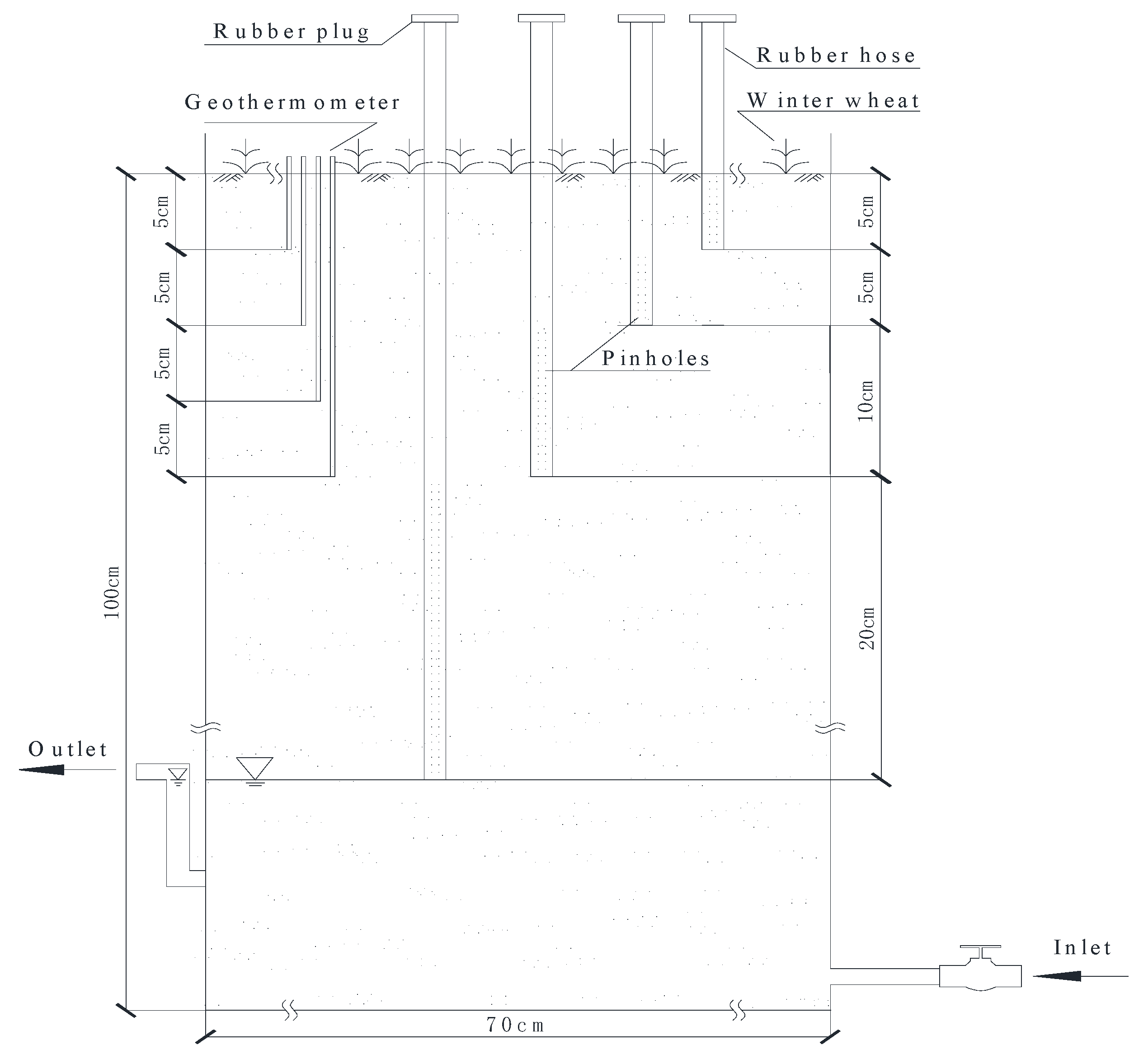
2.1. Experimental Site and Treatments
2.2. Soil Nutrient Determination
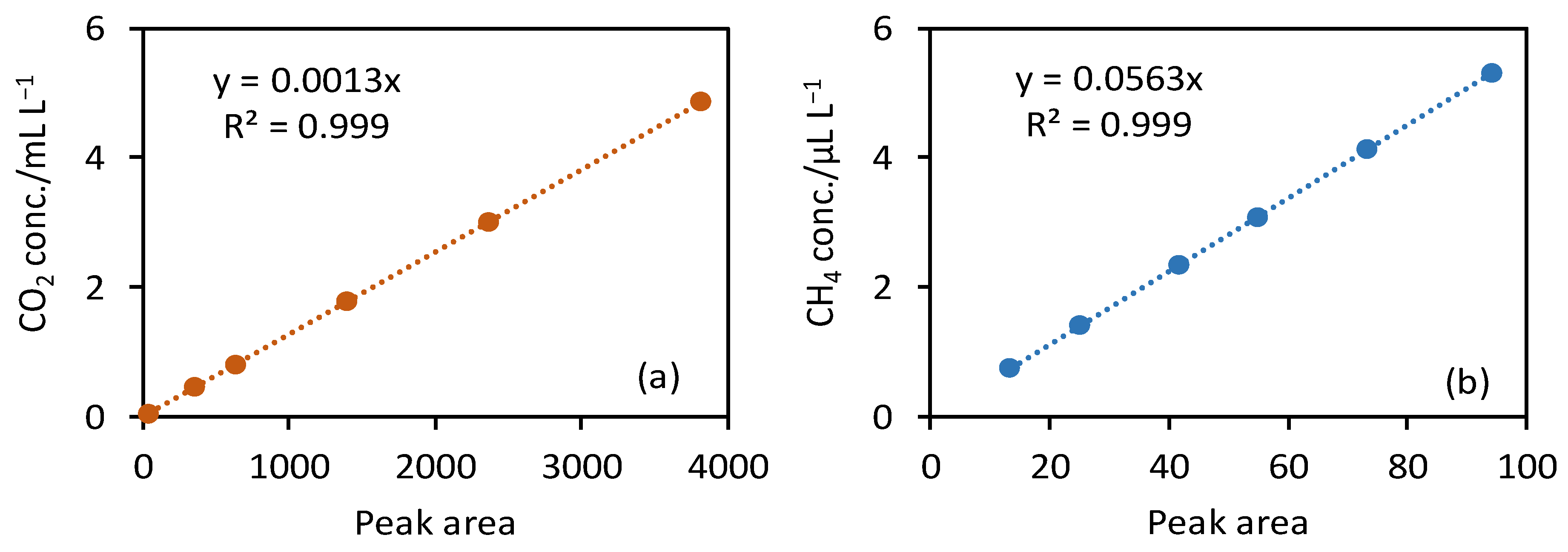
2.3. Gas Collection
2.4. Plant Biometric Analysis
2.5. Statistical Analysis
3. Results
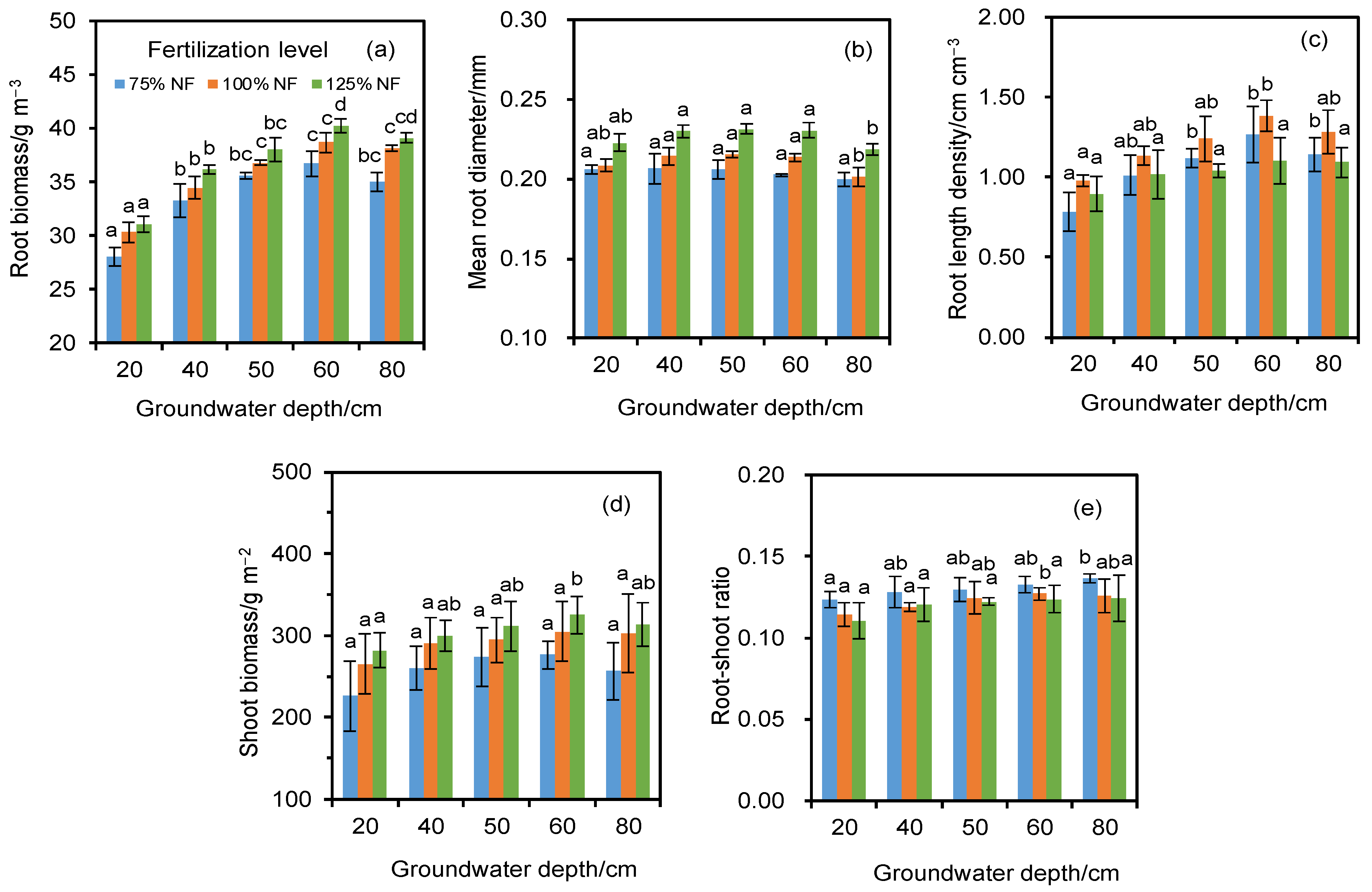
3.1. Root Parameters
3.2. Shoot Biomass and Root:Shoot Ratio
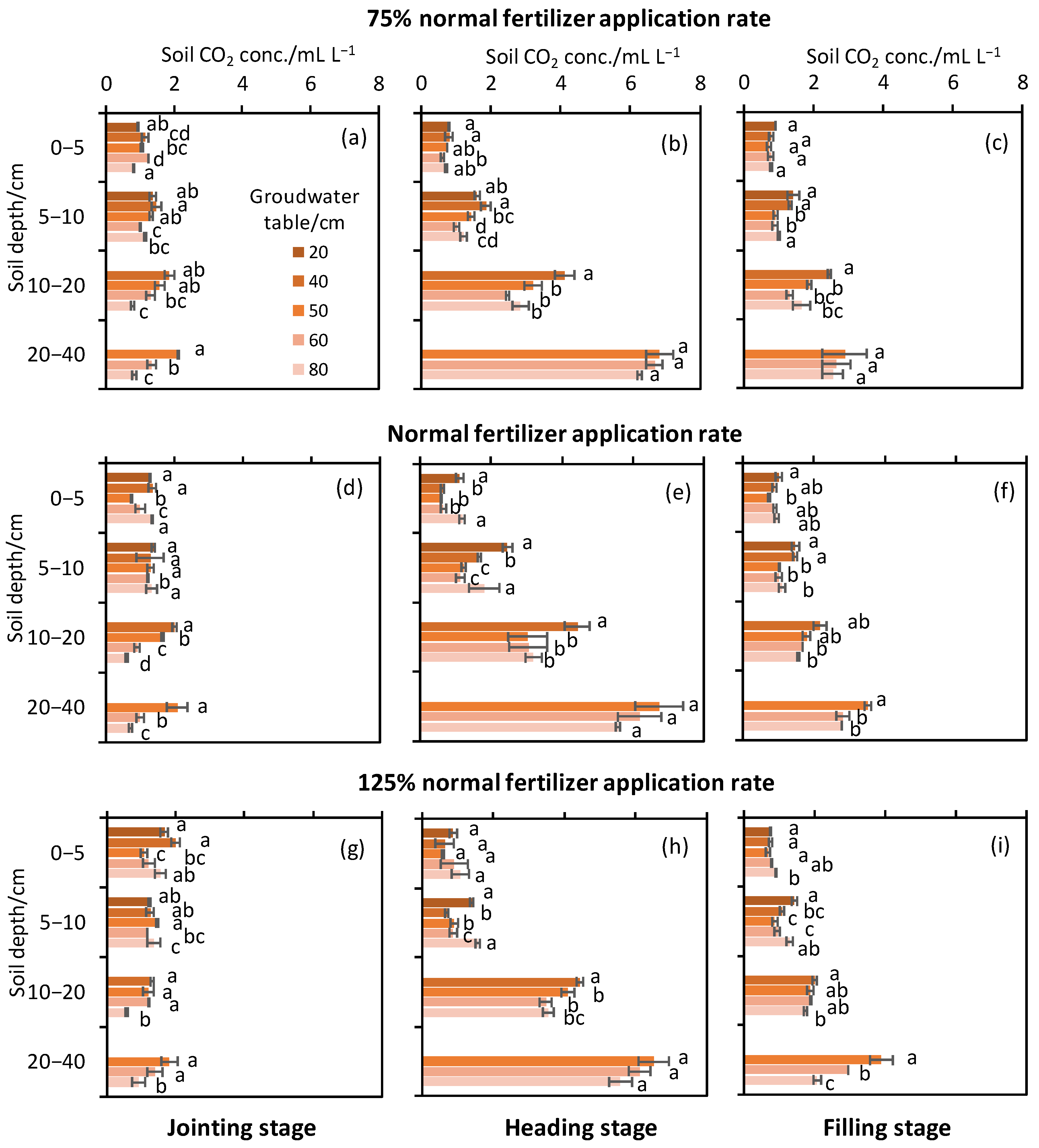
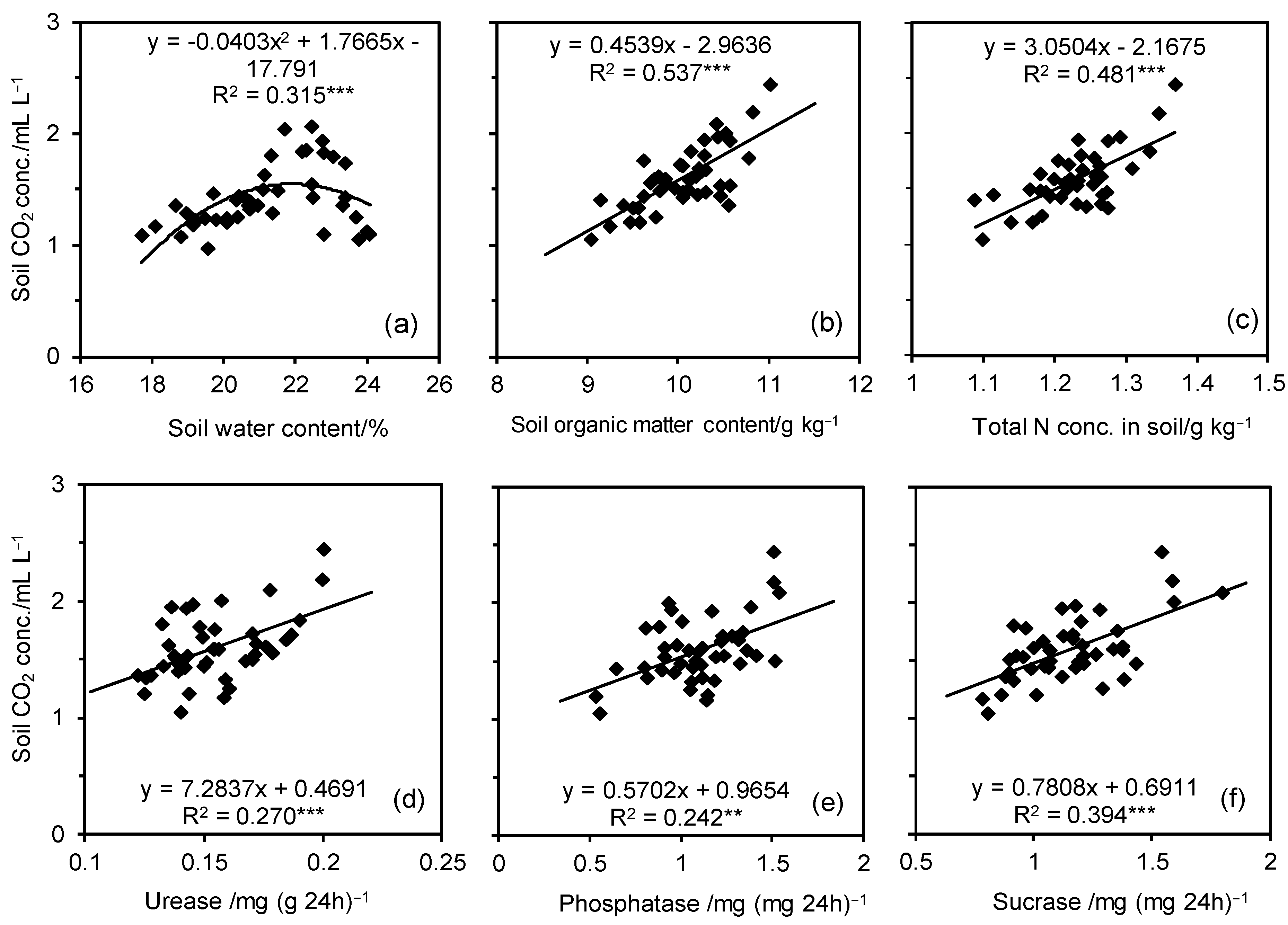
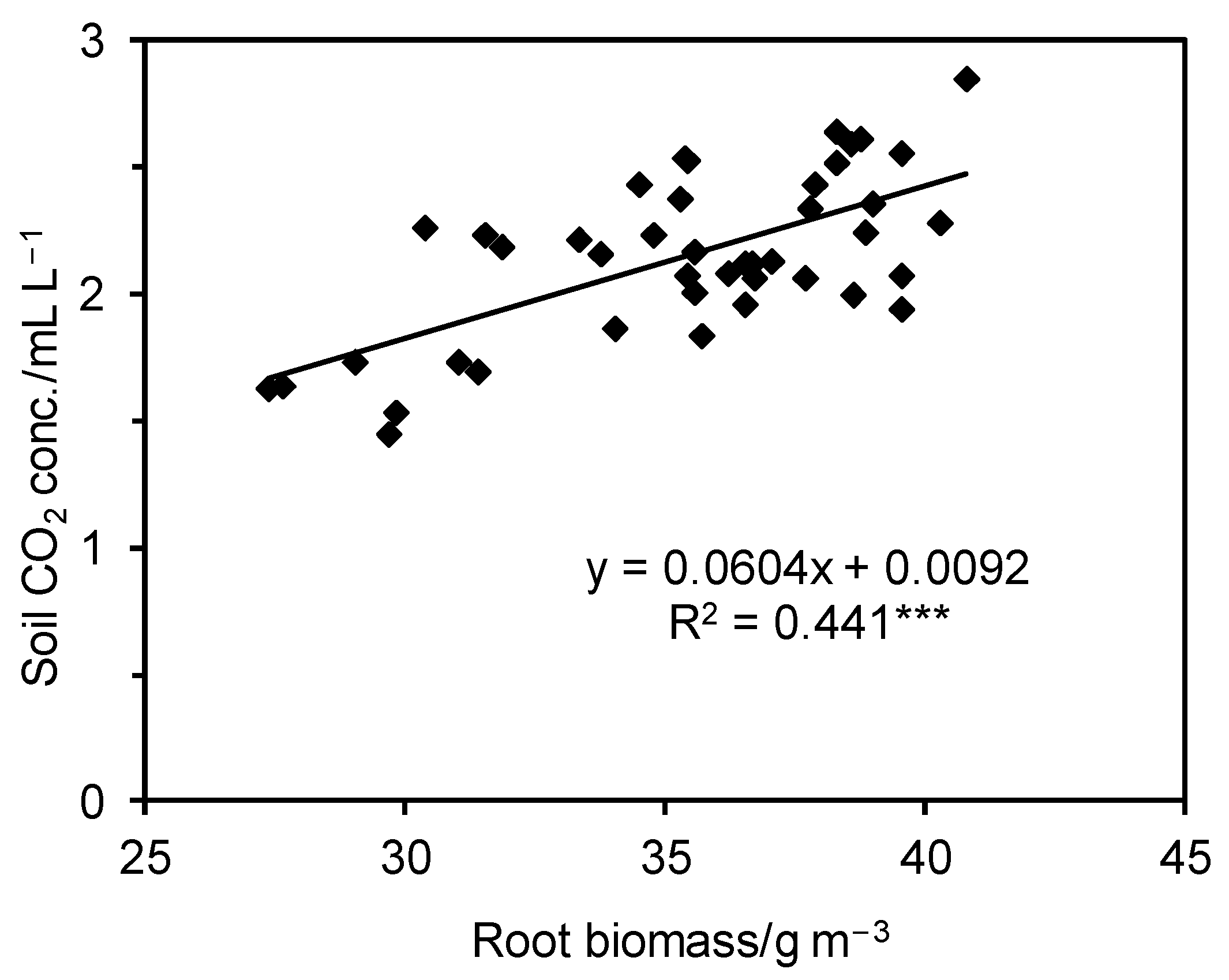
3.3. Soil CO2 Concentration
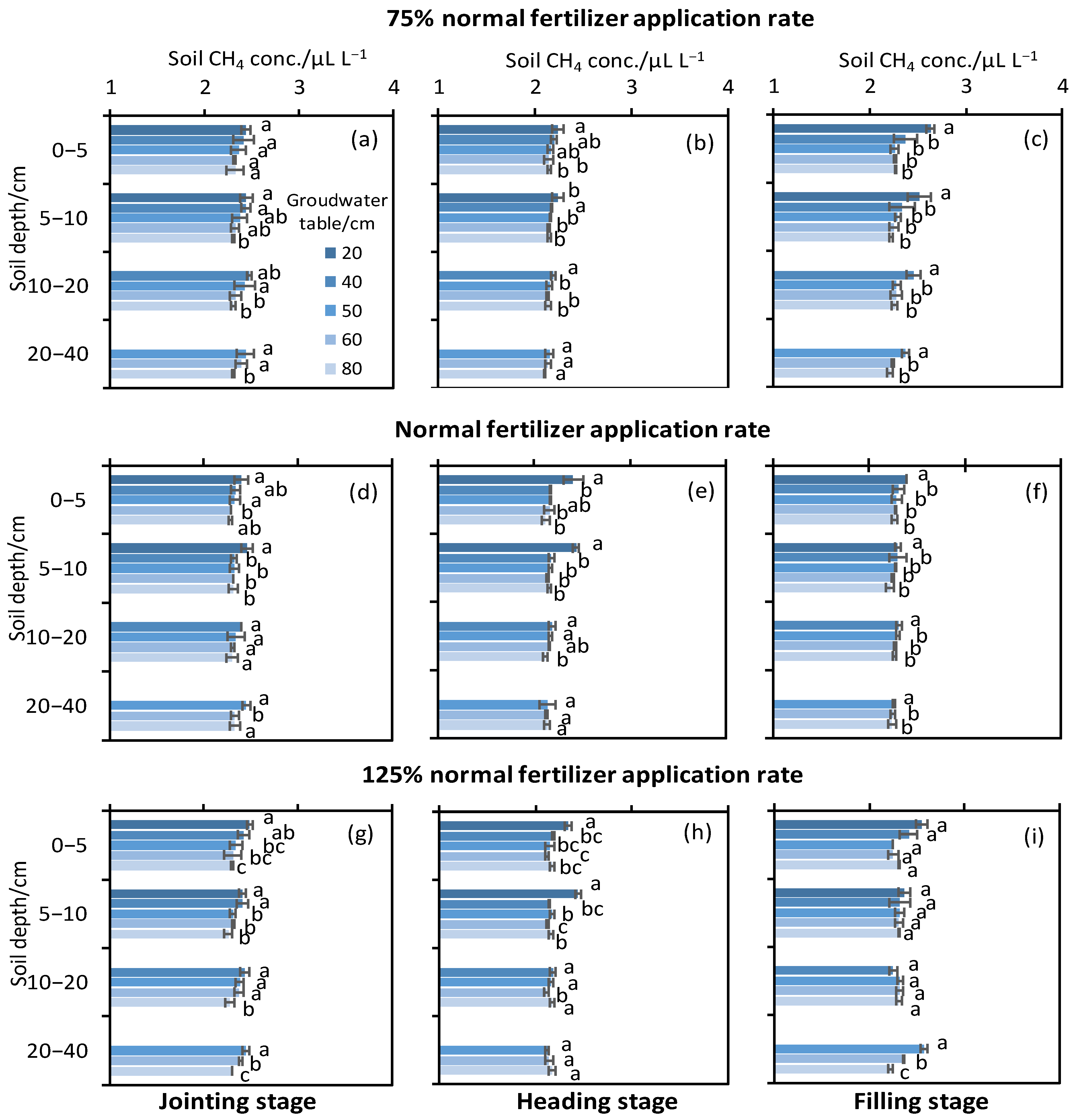
3.4. Soil CH4 Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Phillips, R.P.; Manzoni, S.; Scott, R.L.; Oishi, A.C.; Finzi, A.; Daly, E.; Vargas, R.; Novick, K.A. Changes in photosynthesis and soil moisture drive the seasonal soil respiration-temperature hysteresis relationship. Agric. For. Meteorol. 2018, 259, 184–195. [Google Scholar] [CrossRef]
- Buysse, P.; Flechard, C.R.; Hamon, Y.; Viaud, V. Impacts of water regime and land-use on soil CO2 efflux in a small temperate agricultural catchment. Biogeochemistry 2016, 130, 267–288. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, R.; Wang, Y.; Du, L.; Zhao, M.; Gao, X.; Hu, Y.; Guo, S. Temperature sensitivity of soil respiration to nitrogen and phosphorous fertilization: Does soil initial fertility matter? Geoderma 2018, 325, 172–182. [Google Scholar] [CrossRef]
- Qu, L.; Kitaoka, S.; Koike, T. Factors controlling soil microbial respiration during the growing season in a mature larch plantation in Northern Japan. J. Soils Sediments 2018, 18, 661–668. [Google Scholar] [CrossRef]
- Lou, Y.; Gu, X.; Zhou, W. Effect of elevated UV-B radiation on microbial biomass C and soil respiration in different barley cultivars under field conditions. Water Air Soil Pollut. 2017, 228, 95–103. [Google Scholar] [CrossRef]
- Tong, X.; Li, J.; Nolan, R.H.; Yu, Q. Biophysical controls of soil respiration in a wheat-maize rotation system in the North China Plain. Agric. For. Meteorol. 2017, 246, 231–240. [Google Scholar] [CrossRef]
- Chen, X.; Peng, S.; Chen, C.; Chen, H.Y.H. Water availability regulates tree mixture effects on total and heterotrophic soil respiration: A three-year field experiment. Geoderma 2021, 402, 115259. [Google Scholar] [CrossRef]
- Li, X.; Guo, D.; Zhang, C.; Niu, D.; Fu, H.; Wan, C. Contribution of root respiration to total soil respiration in a semi-arid grassland on the Loess Plateau, China. Sci. Total Environ. 2018, 627, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.; Darenova, E.; Krupková, L.; Pavelka, M. Seasonal and inter-annual variability of soil CO2 efflux in a Norway spruce forest over an eight-year study. Agric. For. Meteorol. 2018, 256–257, 93–103. [Google Scholar] [CrossRef]
- Savage, K.; Davidson, E.A.; Tang, J. Diel patterns of autotrophic and heterotrophic respiration among phenological stages. Glob. Chang. Biol. 2013, 19, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Tomotsune, M.; Yoshitake, S.; Watanabe, S.; Koizumi, H. Separation of root and heterotrophic respiration within soil respiration by trenching, root biomass regression, and root excising methods in a cool-temperate deciduous forest in Japan. Ecol. Res. 2013, 28, 259–269. [Google Scholar] [CrossRef]
- Wertha, M.; Kuzyakov, Y. Root-derived carbon in soil respiration and microbial biomass determined by 14C and 13C. Soil Biol. Biochem. 2008, 40, 625–637. [Google Scholar] [CrossRef]
- Hu, Z.; Islam, A.T.; Chen, S.; Hu, B.; Shen, S.; Wu, Y.; Wang, Y. Effects of warming and reduced precipitation on soil respiration and N2O fluxes from winter wheat-soybean cropping systems. Geoderma 2019, 337, 956–964. [Google Scholar] [CrossRef]
- Zhong, Y.; Yan, W.; Zong, Y.; Shangguan, Z. Biotic and abiotic controls on the diel and seasonal variation in soil respiration and its components in a wheat field under long-term nitrogen fertilization. Field Crops Res. 2016, 199, 1–9. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, J.; Zhou, X.; Li, F. Effects of shallow groundwater table and fertilization level on soil physico-chemical properties, enzyme activities, and winter wheat yield. Agric. Water Manag. 2018, 208, 307–317. [Google Scholar] [CrossRef]
- Cai, Z.C.; Xu, H.; Ma, J. Methane and Nitrous Oxide Emissions from Rice-Based Ecosystem; University of Science and Technology of China Press: Hefei, China, 2009. [Google Scholar]
- Wang, Z.; Zeng, D.; Patrick, W.H. Methane emissions from natural wetlands. Methane Emiss. Nat. Wetl. 1996, 42, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.A.; Turner, D.W.; Gibbs, D.J.; Armstrong, W.; Sivasithamparam, K. Oxygen distribution and movement, respiration and nutrient loading in banana roots (Musa spp. L.) subjected to aerated and oxygen-depleted environments. Plant Soil 2003, 253, 91–102. [Google Scholar] [CrossRef]
- Pulford, I.D.; Tabatabai, M.A. Effect of waterlogging on enzyme activities in soils. Soil Biol. Biochem. 1988, 20, 215–219. [Google Scholar] [CrossRef]
- Cao, R.; Xi, X.; Yang, Y.; Wei, X.; Wu, X.; Sun, S. The effect of water table decline on soil CO2 emission of Zoige peatland on eastern Tibetan Plateau: A four-year in situ experimental drainage. Appl. Soil Ecol. 2017, 120, 55–61. [Google Scholar] [CrossRef]
- Creze, C.M.; Madramootoo, C.A. Water table management and fertilizer application impacts on CO2, N2O and CH4 fluxes in a corn agro-ecosystem. Sci. Rep. 2019, 9, 2692–2704. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, W.; Liu, D.; Yue, S.; Cui, Z.; Chen, X.; Zou, C. Effects of nitrogen management on root morphology and zinc translocation from root to shoot of winter wheat in the field. Field Crops Res. 2014, 161, 38–45. [Google Scholar] [CrossRef]
- Jurasinski, G.; Jordan, A.; Glatzel, S. Mapping soil CO2 efflux in an old-growth forest using regression kriging with estimated fine root biomass as ancillary data. For. Ecol. Manag. 2012, 263, 101–113. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J. The effect of chemical fertilizer on soil organic carbon renewal and CO2 emission—A pot experiment with maize. Plant Soil 2011, 353, 85–94. [Google Scholar] [CrossRef]
- Guan, S. Methods of Soil Enzymology; China Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Dick, R.P. Soil Enzyme Activities as Integrative Indicators of Soil Health; Biological indicators of soil health; CAB International: Wallingford, CT, USA, 1997. [Google Scholar]
- Iovieno, P.; Morra, L.; Leone, A.; Pagano, L.; Alfani, A. Effect of organic and mineral fertilizers on soil respiration and enzyme activities of two Mediterranean horticultural soils. Biol. Fertil. Soils 2009, 45, 555–561. [Google Scholar] [CrossRef]
- Singh, S.P.; Setter, T.L. Effect of waterlogging on element concentrations, growth and yield of wheat varieties under farmer’s sodic field conditions. Proc. Natl. Acad. Sci. USA India Sect. B Biol. Sci. 2017, 87, 513–520. [Google Scholar] [CrossRef]
- Bao, S. Soil Agro-Chemistrical Analysis(Ⅲ); China Agriculture Press: Beijing, China, 2008. [Google Scholar]
- Hodgkinson, L.; Dodd, I.C.; Binley, A.; Ashton, R.W.; White, R.P.; Watts, C.W.; Whalley, W.R. Root growth in field-grown winter wheat: Some effects of soil conditions, season and genotype. Eur. J. Agron. 2017, 91, 74–83. [Google Scholar] [CrossRef] [PubMed]
- de San Celedonio, R.P.; Abeledo, L.G.; Mantese, A.I.; Miralles, D.J. Differential root and shoot biomass recovery in wheat and barley with transient waterlogging during preflowering. Plant Soil 2017, 417, 481–498. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Ma, Z.; Lyu, X.; Liu, T.; Siddique, K.H. Optimum water and nitrogen supply regulates root distribution and produces high grain yields in spring wheat (Triticum aestivum L.) under permanent raised bed tillage in arid northwest China. Soil Tillage Res. 2018, 181, 117–126. [Google Scholar] [CrossRef]
- Becker, S.R.; Byrne, P.F.; Reid, S.D.; Bauerle, W.L.; McKay, J.K.; Haley, S.D. Root traits contributing to drought tolerance of synthetic hexaploid wheat in a greenhouse study. Euphytica 2015, 207, 213–224. [Google Scholar] [CrossRef]
- Ali, S.; Xu, Y.; Ahmad, I.; Jia, Q.; Ma, X.; Ullah, H.; Alam, M.; Adnan, M.; Daur, I.; Ren, X.; et al. Tillage and deficit irrigation strategies to improve winter wheat production through regulating root development under simulated rainfall conditions. Agric. Water Manag. 2018, 209, 44–54. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, L.; Ma, J.; Li, X.; Chen, R. Meta-analysis of the effect of subsurface irrigation on crop yield and water productivity. Sustainability 2023, 15, 15716. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, J.; Jiang, J.; Gu, K.; Zhu, C.; Pan, C.; Yin, W. Effect of soil volumetric water content on the CO2 diffusion coefficient. Sustainability 2023, 15, 1263. [Google Scholar] [CrossRef]
- Xiao, L.; Bao, L.; Ren, L.; Xie, Y.; Wang, H.; Wang, X.; Wang, J.; Qiao, C.; Xiao, X. Appropriate irrigation and fertilization regime restrain indigenous soil key ammonia-oxidizing archaeal and bacterial consortia to mitigate greenhouse gas emissions. Sustainability 2022, 14, 6113. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Q. Effect of waterlogged and aerobic incubation on enzyme activities in paddy soil. Pedosphere 2006, 16, 532–539. [Google Scholar] [CrossRef]
- Liu, L.; Hu, C.; Yang, P.; Ju, Z.; Olesen, J.E.; Tang, J. Effects of experimental warming and nitrogen addition on soil respiration and CH4 fluxes from crop rotations of winter wheat–soybean/fallow. Agric. For. Meteorol. 2015, 207, 38–47. [Google Scholar] [CrossRef]
- Illeris, L.; Michelsen, A.; Jonasson, S. Soil plus root respiration and microbial biomass following water, nitrogen, and phosphorus application at a high arctic semi desert. Biogeochemistry 2003, 65, 15–29. [Google Scholar] [CrossRef]
- Li, L.J.; You, M.Y.; Shi, H.A.; Ding, X.L.; Qiao, Y.F.; Han, X.Z. Soil CO2 emissions from a cultivated Mollisol: Effects of organic amendments, soil temperature, and moisture. Eur. J. Soil Biol. 2013, 55, 83–90. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Li, J.; Schneider, R.; Lamberts, W. Growth dynamics of Chinese wingnut (Pterocarya stenoptera) seedlings and its effects on soil chemical properties under simulated water change in the Three Gorges Reservoir Region of Yangtze River. Environ. Sci. Pollut. Res. 2013, 20, 7112–7123. [Google Scholar] [CrossRef]
- Kering, M.K.; Butler, T.J.; Biermacher, J.T.; Mosali, J.; Guretzky, J.A. Effect of potassium and nitrogen fertilizer on switchgrass productivity and nutrient removal rates under two harvest systems on a low potassium soil. Bioenerg. Res. 2012, 6, 329–335. [Google Scholar] [CrossRef]
- Olmo, M.; Villar, R.; Salazar, P.; Alburquerque, J.A. Changes in soil nutrient availability explain biochar’s impact on wheat root development. Plant Soil 2015, 399, 333–343. [Google Scholar] [CrossRef]
- Hirte, J.; Leifeld, J.; Abiven, S.; Mayer, J. Maize and wheat root biomass, vertical distribution, and size class as affected by fertilization intensity in two long-term field trials. Field Crops Res. 2018, 216, 197–208. [Google Scholar] [CrossRef]
- Jiang, D.; Fan, X.; Dai, T.; Cao, W. Nitrogen fertiliser rate and post-anthesis waterlogging effects on carbohydrate and nitrogen dynamics in wheat. Plant Soil 2008, 304, 301–314. [Google Scholar] [CrossRef]
- Chen, Z.K.; Niu, Y.P.; Ma, H.; Hafeez, A.; Luo, H.H.; Zhang, W.F. Photosynthesis and biomass allocation of cotton as affected by deep-layer water and fertilizer application depth. Photosynthetica 2016, 55, 638–647. [Google Scholar] [CrossRef]
- Zhu, C.; Ma, Y.; Wu, H.; Sun, T.; La Pierre, K.J.; Sun, Z.; Yu, Q. Divergent efects of nitrogen addition on soil respiration in a semiarid grassland. Sci. Rep. 2016, 6, 33541. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, L.; Zhang, C.; Zhao, X.; Bu, W.; Gadow, K.V. Combined effects of nitrogen addition and organic matter manipulation on soil respiration in a Chinese pine forest. Environ. Sci. Pollut. Res. 2016, 23, 22701–22710. [Google Scholar] [CrossRef]
- Bellingrath-Kimura, S.D.; Kishimoto-Mo, A.W.; Oura, N.; Sekikawa, S.; Yonemura, S.; Sudo, S.; Hayakawa, A.; Minamikawa, K.; Takata, Y.; Hara, H. Differences in the spatial variability among CO2, CH4, and N2O gas fluxes from an urban forest soil in Japan. Ambio 2015, 44, 55–66. [Google Scholar] [CrossRef]
- Pires, C.V.; Schaefer, C.; Hashigushi, A.K.; Thomazini, A.; Filho, E.; Mendonca, E.S. Soil organic carbon and nitrogen pools drive soil C-CO2 emissions from selected soils in Maritime Antarctica. Sci. Total Environ. 2017, 596–597, 124–135. [Google Scholar] [CrossRef]
- You, G.; Zhang, Z.; Zhang, R. Temperature adaptability of soil respiration in short-term incubation experiments. J. Soils Sediments 2018, 19, 557–565. [Google Scholar] [CrossRef]
- Xiao, S.; You, H.; You, W.; Liu, J.; Cai, C.; Wu, J.; Ji, Z.; Zhan, S.; Hu, Z.; Zhang, Z.; et al. Rhizosphere and bulk soil enzyme activities in a Nothotsuga longibracteata forest in the Tianbaoyan National Nature Reserve, Fujian Province, China. J. For. Res. 2016, 28, 521–528. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. The role of beneficial microorganisms in soil quality and plant health. Sustainability 2022, 14, 5358. [Google Scholar] [CrossRef]

| Items | Soil CO2 Concentration | Soil CH4 Concentration | Root Biomass | Root Diameter | Root Length Density | Shoot Biomass | Root:Shoot Ratio |
|---|---|---|---|---|---|---|---|
| GD | *** | *** | *** | *** | *** | NS | * |
| FL | NS | NS | *** | *** | *** | ** | NS |
| GD × FL | NS | NS | NS | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Guo, C.; Zhou, X.; Zhu, J.; Li, F. Soil CO2 and CH4 Dynamics and Their Relationships with Soil Nutrients, Enzyme Activity, and Root Biomass during Winter Wheat Growth under Shallow Groundwater. Sustainability 2024, 16, 1396. https://doi.org/10.3390/su16041396
Zhang W, Guo C, Zhou X, Zhu J, Li F. Soil CO2 and CH4 Dynamics and Their Relationships with Soil Nutrients, Enzyme Activity, and Root Biomass during Winter Wheat Growth under Shallow Groundwater. Sustainability. 2024; 16(4):1396. https://doi.org/10.3390/su16041396
Chicago/Turabian StyleZhang, Wenchao, Chen Guo, Xinguo Zhou, Jianqiang Zhu, and Fahu Li. 2024. "Soil CO2 and CH4 Dynamics and Their Relationships with Soil Nutrients, Enzyme Activity, and Root Biomass during Winter Wheat Growth under Shallow Groundwater" Sustainability 16, no. 4: 1396. https://doi.org/10.3390/su16041396
APA StyleZhang, W., Guo, C., Zhou, X., Zhu, J., & Li, F. (2024). Soil CO2 and CH4 Dynamics and Their Relationships with Soil Nutrients, Enzyme Activity, and Root Biomass during Winter Wheat Growth under Shallow Groundwater. Sustainability, 16(4), 1396. https://doi.org/10.3390/su16041396







