Abstract
The selection of suitable storage conditions and monitoring of changes in the wheat grains using suitable parameters are of great importance for the sustainability of technological quality and utilization of the entire stored quantity of wheat grains without waste generation until the next harvest. Wheat grains of three varieties, stored for 12 months at three different conditions of environmental temperature and relative humidity (% RH): (1) 40 ± 1.06 °C; 45 ± 3% RH; (2) 4 ± 1.05 °C; 45 ± 4% RH, (3) 15 ± 8.51 °C; and 67 ± 4% RH, were compared for germination percentage, protein and advanced glycation end products content, oxidoreductive and proteolytic enzyme activity, wet gluten content and falling number. A decrease in the activity of guaiacol peroxidase and polyphenol oxidase, as well as an increase in the content of advanced glycosylation end products and the falling number, was observed in wheat grains during storage conditions at 40 ± 1.06 °C; 45 ± 4% RH. On the other hand, storage of wheat grains at lower temperatures resulted in much lower changes in examined parameters, among which advanced glycation end-product content, peroxidase activity and falling number values were the greatest. Based on the obtained results, it can be assumed that changes in guaiacol peroxidase activity and falling number might be used as indicators of improper wheat grain storage.
1. Introduction
One of the important steps in the global cereal market chain is storage of wheat grains in warehouse facilities, storage rooms or silos. Grains are stored for several weeks to several years for seeding purposes, as raw material for the food industry or for animal feeding. During the grain storage, a number of spontaneous chemical reactions such as free radical generation/oxidation and non-enzymatic glycosylation could take place within cereal grains, leading to molecular damage, fragmentation and/or cross-linking of constitutive proteins, lipids, sugars and nucleic acids, consequently resulting in changes of endogenous enzymes activities, membrane deterioration, chromosome mutations and finally loss of grain viability and death [1,2]. However, the degree of molecular changes within the grains largely depends on the length of storage, temperature, relative humidity and moisture content of the grains. The greater degree of molecular changes within the grains might be expected with prolonged storage, higher storage temperature and relative humidity, as well as higher moisture content of the grains [3,4].
In parallel with the oxidative modification of endogenous biomolecules, all modern theories of ageing point out that non-enzymatic glycosylation and subsequent Maillard reactions are the major players in grain ageing [2,5,6,7,8,9,10,11,12,13,14,15,16,17]. Oxidation and non-enzymatic glycosylation of grain endogenous biomolecules during storage of wheat seeds leads to the increase or decrease in total soluble sugars, decrease in soluble amylose, phytic acid and carotenoids and an increase in ash content. Moreover, these changes also affect the technological and functional quality of the wheat flours including loss of viscoelastic properties of gluten, a slight increase or significant decrease in wet or dry gluten content, decrease in Zeleny sedimentation, hectoliter weight and alveographic W values. Nevertheless, one of the most undesirable negative physiological changes caused by ageing is the reduction or complete loss of grain germination [18,19,20,21,22].
Since wheat is one of the most common cereals used in food production, and there are only a few scientific papers on wheat grain ageing reporting a limited number of parameter changes caused by natural and/or accelerated ageing during storage [20,21,23,24,25,26], there is an obvious need for the more comprehensive examination on the wheat grain ageing during storage which includes the combination of physiological/biological, biochemical and technological parameters. The aforementioned is even more pronounced if one considers inevitable climate changes, and subsequently possible unconditioned wheat grain storage in silos and/or warehouse facilities. If such a scenario occurs, it can lead to the complete uselessness of wheat grains for the production of bakery products intended for human consumption. Therefore, there is a need for the development of additional cost-effective and rapid tests of wheat quality indicators. In this respect, we have investigated the influence of three different storage conditions on the intensity of various biochemical and technological parameter changes in the grains of three different wheat varieties during one-year storage at three different storage conditions.
2. Materials and Methods
2.1. Wheat Grain Storage
Wheat varieties (Triticum aestivum L.) of different protein content, including the Divana (0.1425 g/g grain dry weight), Žitarka (0.1336 g/g grain dry weight) and Srpanjka varieties (0.1052 g/g grain dry weight), were generously supplied by the Agricultural Institute Osijek (Žitarka and Srpanjka) and by the College of Agriculture, Križevci (Divana). Grains of examined wheat varieties, containing 13.7% (Divana) or 13.8% (Žitarka and Srpanjka) of moisture, were packed in multi-wall paper seed storage bags. The bags were sealed using sewing thread, and stored at three different conditions of environmental temperature and relative humidity (% RH): (1) 40 ± 1.06 °C; 45 ± 3% RH; (2) 4 ± 1.05 °C; 45 ± 4% RH; and (3) warehouse conditions with mean average temperature of 15 ± 8.51 °C and mean average relative humidity of 67 ± 4% RH, being dependent on the climate environmental conditions (temperature variation from 2 to 24 °C; relative humidity variation from 62 to 73%). Storage at the above-defined conditions was carried out in a thermostatic incubator Heraeus (Heraeus, Hanau, Germany) (1), in a refrigerator (2), and on a shelf positioned 20 cm from the floor of the unconditioned warehouse (3). Grain samples were taken monthly over a period of 12 months.
2.2. Determination of Germination Percentage
The influence of storage conditions during one year of storage on the germination percentage of wheat grains was performed by the standard laboratory germination test described in the paper of Strelec et al. [27].
2.3. Moisture and Protein Content of Grains
The moisture and protein content of whole wheat grains stored at different storage conditions was measured monthly for one year using Near Infrared Transmission (NIT) using a Foss Tecator 1241 Grain Analyzer (Foss Tecator AB, Höganäs, Sweden) [27].
2.4. Extractable Protein Content, Peptidase and Oxidoreductase Activities
For determination of the extractable protein content and activity of oxidoreductive and proteolytic enzymes, it was necessary to extract proteins and enzymes from differently stored grains. Extractions were performed by Strelec et al. [28]. Extractable protein content was determined using the Bradford method [29] with bovine serum albumin as standard. Aminopeptidase activities were determined using the colorimetric method using amino acid-2-naphthylamides (2NA) of arginine and phenylalanine as substrates, and Fast Blue B Salt as hydrolysis product coupler [30] as previously reported by Strelec et al. [31]. Carboxypeptidase activity was determined using the colorimetric method using N-carbobenzyloxy-L-phenylalanyl-leucine (CBZ-Phe-Leu) as substrate and picrylsulfonic acid as hydrolysis product coupler, according to Waters et al. [32]. Aspartic proteinase activity was measured using 2% (w/v) Hb as substrate according to Voigt et al. [33] with some modification previously reported by Strelec et al. [28]. Catalase activity was determined using a colorimetric assay using a catalase assay kit (Sigma, USA). Glutathione reductase activity was determined by continuous UV assay using a glutathione reductase assay kit (Sigma, Livonia, MI, USA). Peroxidase activity was determined by continuous spectrophotometric assay at 470 nm with H2O2 as substrate and guaiacol as reducing co-substrate [34]. Polyphenol oxidase activity was determined using a colorimetric method according to [35] using L-3,4-dihydroxyphenylalanine as substrate.
2.5. Advanced Glycation End Products Content
Advanced glycation end products (AGE) in stored wheat grains were determined using the fluorescence method previously reported by Strelec et al. [36].
2.6. Wet Gluten Content
The amount of wet gluten content in flours obtained by milling wheat grains stored at defined storage time and conditions was determined by ICC No. 155 [37] performed on the Glutomatic 2200 System and Glutomatic Centrifuge 2015 (Perten, Sweden) as previously described by Strelec et al. [28].
2.7. Falling Number
Falling Number (FN), as an indirect measure of α-amylase activity, was determined in whole-grain flours obtained by milling of wheat grains stored at defined storage time and conditions according to 40 ICC method no. 107/1 [38].
2.8. Statistical Analysis
Differences in temporal changes of examined parameters were analysed for significance using analysis of variance (ANOVA). When ANOVA indicated significant differences in means, post hoc analysis by Duncan’s multiple-range test was performed. The general effects of various storage conditions on the significance of parameter changes for the same variety were calculated using Student’s t-test. Pearson correlation was used for examination of correlation significance between AGE and enzyme activity changes, between AGE and falling number, as well as grain germination and enzyme activity changes. All statistical analyses were performed by statistical software Statistica (TIBCO Statistica® 14.1.0, Stat Soft Inc., Palo Alto, CA, USA) at a level of significance of p < 0.05.
3. Results and Discussion
The present study investigated the effect of three different storage conditions on the biochemical, quality and technological parameters changes of three different wheat varieties during one year of storage. The general effect of storage conditions on the examined parameter changes is shown in Table 1, while the starting values of examined parameters are in Table S1. The major changes during one-year storage of wheat grains of different wheat varieties (germination percentage, dry matter content, catalase activity, guaiacol peroxidase activity polyphenol oxidase activity, advanced glycation end products and falling number) occurred at storage conditions at elevated temperatures (40 ± 1.06 °C; 45 ± 3% RH), while some minor changes of examined parameters could be noticed at lower temperatures (dry matter content; advanced glycation end products), regardless of the examined wheat variety.

Table 1.
General effect of storage conditions on germination, dry matter content, protein concentration, peptidase and oxidoreductase activities, advanced glycation end products content, wet gluten and falling number of ageing wheat varieties during 360 days. Results are presented as mean values ± SD.
One of the key indicators of wheat grain quality is grain germination. The significant loss of germination at 40 ± 1.06 °C could be observed on the 240th day for grains of the Divana and Žitarka variety and on the 270th day for the Srpanjka variety (Figure 1). Divana variety showed the lowest germination (15%) at 40 ± 1.06 °C on the 360th day of storage, while Žitarka and Srpanjka showed 65 and 56% of germination. Decreased germination of wheat grains stored at elevated temperatures might be attributed to a number of molecular and physiological changes within wheat grains during storage including oxidative modification of lipids, proteins and nucleic acids, non-enzymatic glycosylation of proteins and nucleic acids, disruption of the antioxidant defence system, reduction in enzyme activity, and accumulation of toxic substances in grain endosperm [6,23,25,26,27,39,40,41,42,43,44,45,46,47,48,49].
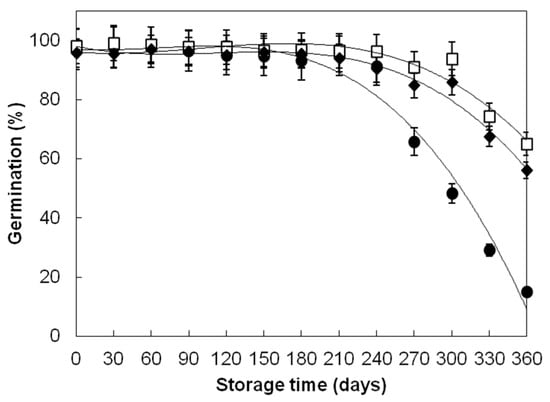
Figure 1.
Germination changes in wheat grains during one-year storage at 40 ± 1.06 °C, 45 ± 3% RH. Varieties: Divana (●), Žitarka (◻), Srpanjka (♦). Results are expressed as mean values ± SD. Vertical bars (SD) smaller than markers are not shown.
Increased temperature of wheat grain storage also led to the increased accumulation of advanced glycation end products of (AGE) as shown in Figure 2. The highest increase in AGE was recorded for grains stored at 40 ± 1.06 °C (Figure 2a), slightly lower for grains stored at 15 ± 8.51 °C in an open-type warehouse (Figure 2b), while for the grains stored at 4 ± 1.05 °C, there were no significant changes of AGE content (Table 1). The differences in the rates of formation and the amount of accumulated advanced glycosylation products in wheat grains stored at the different temperatures clearly show the temperature dependence of AGE formation where the increase from 2- to 4.5-fold was detected by temperature increase from 15 °C to 40 °C, which is congruent with our previous report [36].
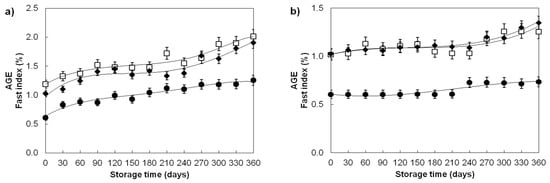
Figure 2.
Advanced glycation end product (AGE) changes in ageing wheat grains during one-year storage at (a) 40 ± 1.06 °C; 45 ± 3% RH and at (b) 15 ± 8.51 °C; 67 ± 4% RH. Varieties: Divana (●), Žitarka (◻), Srpanjka (♦). Results are expressed as mean values ± SD. Vertical bars (SD) smaller than markers are not shown.
Extraction of proteins from differently stored wheat grains by 100 mM KH2PO4 buffer pH 7.0 containing 1 mM EDTA-2Na or by 50 mM Na-Ac buffer pH 5.0 containing 2 mM dithiothreitol revealed that more proteins were extracted by acetate buffer (Table 1), but there were no differences in the extractable protein content changes during the storage. This was quite surprising since the numerous literature data show a decrease in the protein content during the storage/ageing of grains [20,21,43,50,51,52,53,54]. The most probable reason for the observed invariability of protein content during the storage of examined wheat grains (Table 1) is applied storage conditions which obviously prevented enzymatic processes of protein breakdown in the grains.
Numerous scientific studies report various changes in the enzyme activity during the ageing/storage of cereal grains, legumes and industrial plants. When storage conditions of accelerated grain ageing (40–50 °C; 75–100% RH) are applied, increased activity of aspartate protease [43,55] and carboxypeptidase [20,21], as well as decreased activity of glutathione reductase, catalase [40,48] and guaiacol peroxidase [40,54,56] are reported. On the other hand, the application of storage conditions resembling those of natural grain ageing (4–30 °C; 50–75% RH) results in a decrease in aminopeptidase [57], aspartate protease [51], catalase [58] and peroxidase activities [59].
Analysis of oxidoreductase and proteolytic enzyme activity during 360 days of storage of examined wheat varieties showed that all examined enzymes (except guaiacol peroxidase) were quite stable in wheat grains during one-year-ageing at lower temperatures, but significant changes in enzyme activities were observed at 40 ± 1.06 °C (Table 1).
Both aminopeptidases, Arg- and Phe-AP, lost their activity during storage at a temperature of 40 ± 1.06 °C (Figure 3a,b). Significant loss of activities (10%; p < 0.05) occurred on the 150th day in Žitarka, the 180th day in Divana, and on the 270th day in Srpanjka grains, after which AP-activities continuously decreased, and on the 360th day of storage aminopeptidases retained 70–84% of the initial activity dependent on the examined variety. The greatest loss of aminopeptidase activities could be observed for the variety Divana (Arg-AP 25%; Phe-AP 31% loss of activity), and the lowest for the variety Srpanjka (Arg-AP 16%; Phe-AP 23%). Similar changes in AP activity that prefer arginine and phenylalanine cleavage were found for aged barley grains during one-year storage at room temperature [57], while lower loss of AP activity that prefers leucine cleavage was found during natural ageing of flax seeds [51].
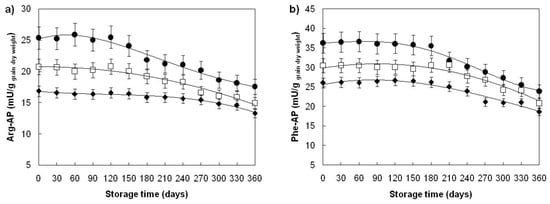
Figure 3.
Aminopeptidase activity changes in ageing wheat grains during one-year storage at 40 ± 1.06 °C, 45 ± 3% RH. Aminopeptidases: (a) arginyl-aminopeptidase, (b) phenylalanyl-aminopeptidase. Varieties: Divana (●), Žitarka (◻), Srpanjka (♦). Results are expressed as mean values ± SD. Vertical bars represent SD.
The activity of aspartic proteinase during one-year storage of examined wheat varieties at different storage conditions (Table 1) was not found significantly changed (p < 0.05). This was different from the reports of Galleschi et al. [21] and Calucci et al. [20] where a four-fold increase in aspartic proteinase activity was recorded during wheat grain storage, as well as with the report of Sammour [51] where a decrease in the activity of aspartate protease was observed during 5-year storage of flax seeds. One of the most probable reasons for the observed lack of changes in the aspartic proteinase activity in differently stored wheat varieties (Table 1) is its localization in protein storage vacuoles of germ, thyroid, aleurone layer and protein bodies of grain endosperm [60,61] which obviously protects it against oxidative processes and non-enzymatic glycosylation.
Similar to the aspartic proteinase, carboxypeptidase II did not show significant activity changes during the one-year storage of examined wheat varieties (Table 1). On the contrary, the reports of Galleschi et al. [21] and Calucci et al. [20] show a four-fold increase in carboxypeptidase activity during accelerated ageing of wheat grains.
Catalase (CAT) and glutathione reductase (GR) are antioxidative enzymes mainly present within living parts of dry grains, and localized within cell cytoplasm, glyoxysomes, peroxisomes and mitochondria [48,62,63], while polyphenol oxidase (PPO) is an oxidoreductase able to insert oxygen in the ortho-position of an existing hydroxyl group in aromatic ring, followed by the oxidation of the diphenol to the corresponding quinine [64]. In wheat grains, PPO is present in the endosperm, bran and germ, being 2–3-fold more abundant in the bran and germ [62]. Investigation of the effect of applied storage conditions on the activity of CAT, GR and PPO of examined wheat grains revealed that all three enzymes showed a decrease in their activity only at 40 ± 1.06 °C (Table 1). Significant loss of catalase activity in wheat grains stored at 40 ± 1.06 °C (10%, p < 0.05) occurred on the 60th day of storage of the Žitarka variety, on the 90th day of Srpanjka, and the 120th day of the Divana variety (Figure 4a). Observed loss of catalase activity continued till the 360th day of storage at which Divana variety retained 52%, Žitarka 67%, and Srpanjka 63% of the initial CAT activity. In the case of glutathione reductase (Figure 4b), loss of enzyme activity occurred on the 210th day of storage in all three varieties and continued until the end of storage, where glutathione reductase retained 82–85% of the initial activity. A significant decrease in PPO activity (10%, p < 0.05) was observed in all three varieties at 30th day of storage (Figure 4c), continued until the 120th day, after which the activity of PPO stabilized, and on the 270th day of storage continued to decline again until 360th day, where PPO retained 56% (Divana), 52% (Žitarka) or 54% (Srpanjka) of its initial activity. The loss of PPO activity during storage of wheat grains, might be attributed to the (a) thermal denaturation of PPO (part of the curve from the 30th to the 270th day), and (b) inactivation of PPO by Maillard reactions (part of the curve from the 270th to the 360th day). When data on the CAT, GR and PPO activity changes during storage (Table 1, Figure 4) are compared with those of the literature, it can be concluded that the observed decrease in catalase and glutathione reductase activity during 360 days of aging of examined wheat varieties stored at 40 ± 1.06 °C are consistent with the results on CAT and GR activity decrease during 360 days of green soybean aging at 33 °C [40], while data on PPO activity changes cannot be confirmed due to the lack of available literature data.
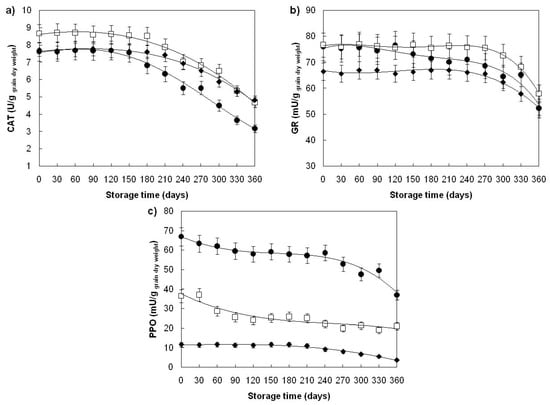
Figure 4.
Catalase (a), glutathione reductase (b) and polyphenol oxidase (c) activity changes in ageing wheat grains during one-year storage at 40 ± 1.06 °C, 45 ± 3% RH. Varieties: Divana (●), Žitarka (◻), Srpanjka (♦). Results are expressed as mean values ± SD. Vertical bars (SD) smaller than markers are not shown.
Contrary to the CAT, GR and PPO, the significant loss of guaiacol peroxidase (POX) activities in the grains of all three examined wheat varieties was observed under all storage conditions (Figure 5a–c). POX activity in grains aged for 360 days at 40 ± 0.6 °C; 45 ± 2% RH decreased exponentially, where a significant decrease in activity (20–25%, p < 0.05) occurred already on the 30th day, while on the 360th day, POX retained only 2–5% of its initial activity. A decrease in the activity of guaiacol peroxidase could be also observed for grains stored at 4 ± 1.05 °C; 45 ± 4% RH and 15 ± 8.51 °C; 67 ± 4% RH. The grains stored at 4 ± 1.05 °C on the 360th day showed a decrease in POX activity from 18 to 26%, and those stored at 15 ± 8.51 °C from 23 to 37% dependent on the examined variety. The observed decrease in POX activity at 40 ± 1.06 °C might be attributed to its thermal instability, while those at lower storage temperatures to the combination of natural denaturation, oxidative modification and/or Maillard reactions. Decreased POX activity has been reported during the accelerated ageing of bamboo seeds [53,54] and corn [2,43], as well as the natural ageing of rice grains [56,59] and green soybeans [40].
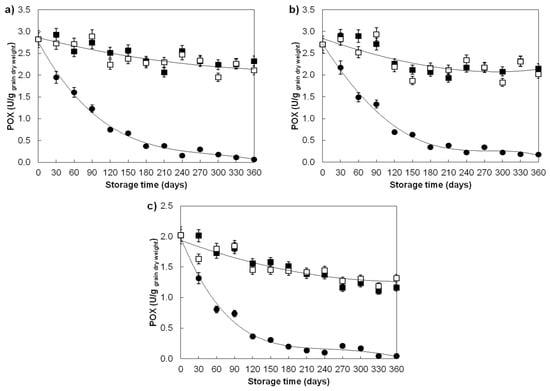
Figure 5.
Guaiacol peroxidase activity changes in ageing wheat grains during one-year storage at different storage conditions. Varieties: (a) Divana, (b) Žitarka, (c) Srpanjka. Storage conditions: 40 ± 1.06 °C; 45 ± 3% RH (●), 4 ± 1.05 °C; 45 ± 4% RH (■), 15 ± 8.51 °C; 67 ± 4% RH (◻). Results are expressed as mean values ± SD. Vertical bars (SD) smaller than markers are not shown.
The technological quality of wheat grains attributable to the quality of wheat flour and bakery products is dependent on the quantity and quality of proteins, degree of starch damage and α-amylase content. These quality parameters are commonly determined by the use of the standard methods of wheat grain analysis including crude protein content, wet gluten content, falling number and Zeleny sedimentation values. However, all these parameters were found to be dependent on wheat grain storage conditions. The quality of wheat flour was found to improve after several weeks of grain storage under appropriate conditions [65,66], but prolonged grain storage at improper storage conditions showed a negative effect on wheat flour quality [67], and consequently quality of bread [68]. The aforementioned can be explained by short- and long-term oxidation reactions occurring during wheat grain storage/ageing. Short-term oxidation (up to one month at appropriate storage conditions) during wheat storage might lead to the formation of disulfide bridges that effectively cross-link gluten proteins resulting in a more stable gluten network enabling better dough strength and elasticity. On the other hand, prolonged wheat grain storage and/or storage at inappropriate conditions might lead to an excessive oxidation of the sulfhydryl groups of gluten proteins and over-crosslinking which make the gluten network rigid and consequently negatively affect dough extensibility, making dough more difficult to handle during bread-making processes.
Table 1 shows the changes in wet gluten content and falling number during one-year ageing of examined wheat varieties. The wet gluten content of examined wheat varieties slightly changed during one-year storage, where significant changes were only detected for the Srpanjka variety stored at 40 ± 1.06 °C; 45 ± 3% RH. On the other hand, one-year storage of examined wheat varieties caused an increase in FN values under all storage conditions, where the intensity of changes was found dependent on the applied storage conditions (Table 1, Figure 6). The greatest changes in FN were observed at 40 ± 1.06 °C; 45 ± 3% RH (Divana; 95%, Žitarka; 70%, and Srpanjka; 86%), and the lowest at 4 ± 1.05 °C; 45 ± 4% RH (increase between 1 and 7%). The observed FN increase during one-year storage of examined wheat varieties was congruent with data on FN changes during wheat flour storage [66], FN increase and α-amylase activity decrease during ageing/storage of different grains [26,41,52,55,56].
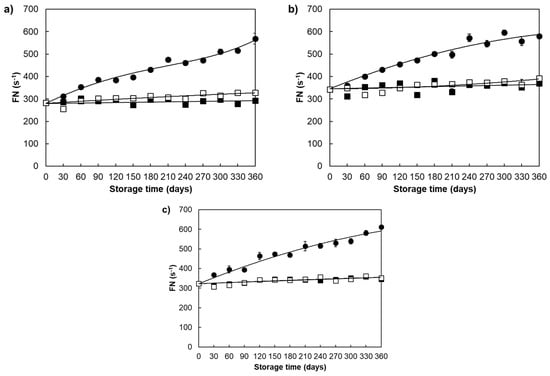
Figure 6.
Falling number changes in ageing wheat grains during one-year storage at different storage conditions. Varieties: (a) Divana, (b) Žitarka, (c) Srpanjka. Storage conditions: 40 ± 1.06 °C; 45 ± 3% RH (●), 4 ± 1.05 °C; 45 ± 4% RH (■), 15 ± 8.51 °C; 67 ± 4% RH (◻). Results are expressed as mean values ± SD. Vertical bars (SD) smaller than markers are not shown.
It is well known that FN correlates with changes in rheological properties, particularly with the dough development time and the mechanical tolerance index, as well as flour water absorption [69]. Moreover, it is designated as an indirect indicator of α-amylase activity and a measure of how far the degradation of starch in the kernel by enzymatic activity has progressed [70], where a high FN value indicates minimal α-amylase activity, while a low FN more substantial enzyme activity [71]. Wheat flour for bread making should have a falling number between 250 and 300 s [72].
Although the determination of FN values is solely based on the measurement of time necessary for a decrease in the viscosity of wheat flour suspension at elevated temperature, mainly attributed to the endogenous α-amylase activity, it should be pointed out that suspension viscosity can be also caused by some other changes during wheat ageing such as degradation of the cell wall structure [73], change in the protein content [74], increase in the soluble protein content, especially high molecular weight glutenin fraction [75] as well as, change in the amount of free fatty acids [76]. Thus, changes in FN values of differently stored wheat grains might reflect much more than α-amylase activity, including multiple parameters, and among them viscosity. Loney and Meredith [77] reported an increase in the peak viscosity of the amylograph during natural (15–20 °C) and accelerated (50 °C) ageing of commercial wheat flours, while several studies on rice grain ageing/storage showed an increase in the viscosity of the aqueous flour suspension during grain ageing/storage [55,56,74,78].
Based on the aforementioned results, it is more than clear, that the majority of examined changes during storage of examined wheat varieties occur at elevated storage conditions (40 ± 1.06 °C; 45 ± 3% RH), rather than lower ones, which was expected. Nevertheless, improper wheat grain storage conditions (such as those of 40 ± 1.06 °C; 45 ± 3% RH) which could be caused by obvious worldwide observed climate changes is a necessity which should be considered as an expected risk in the future, especially considering the use of unconditioned warehouses/silos. Therefore, the examination of “unusual” storage conditions, such as those of 40 ± 1.06 °C, 45 ± 3% RH seems quite reasonable, especially if one considers the necessity of the development of cost-effective and rapid tests of wheat quality indicators.
Table 2 shows correlation coefficients between the most prominently changed parameters during wheat grain storage at 40 ± 1.06 °C; 45 ± 3% RH, including grain germination loss, advanced glycation end product content and activity of examined enzymes (Table 2).

Table 2.
Correlation coefficients between advanced glycation end products, germination percentage, enzyme activities and falling number (FN) of grains of wheat varieties stored at 40 ± 1.06 °C; 45 ± 3% RH.
The higher correlation coefficients were found between the content of AGE and enzyme activity decrease, in comparison to the germination percentage (Table 2). Among them, the highest correlation was found between falling number values and advanced glycation end products, indicating that non-enzymatic glycosylation of wheat grain proteins/enzymes plays a crucial role in endogenous enzyme denaturation. However, some other factors affecting FN determination, such as degradation of the cell wall structure [73], change in the protein content [74], including high molecular weight glutenin fraction [75] and amount of free fatty acids [76] cannot be neglected. The formation of disulfide bonds, which give gluten more strength and elasticity, is promoted by higher temperatures [79] and tends to increase toughness and firmness while reducing the extensibility of the dough [80].
Somewhat lower correlation coefficients were found between germination percentage and examined enzyme activities (Table 2). The highest ones were those for CAT and GR indicating their significant role in grain germination.
4. Conclusions
The present study investigated the effect of three different storage conditions on the biochemical and quality parameter changes of three varieties during one-year storage at three different storage conditions. While the majority of enzymatic changes within the wheat grains occurred at elevated temperatures (40 ± 1.06 °C), the decreased activity of guaiacol peroxidase and increased value of the falling number in wheat grains was found under all examined ageing conditions, indicating POX activity and FN value as potential indicators of improper wheat grain storage. The accumulation of advanced glycosylation end products in wheat grains during storage seems to be the main reason for the observed decrease in enzyme activity and grain germination, as well as in the increase in falling number. Overall, storage of wheat grains at lower temperatures (up to 20 °C) contributes to a better sustainability of the technological quality of wheat grains, which allows its full utilization for the production of flour and quality bakery products until the new harvest.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16031155/s1, Table S1: Germination, dry matter content, protein content, peptidase and oxidoreductase activities, advanced glycation end products content, wet gluten and falling number of wheat varieties at the beginning of storage. Results are presented as mean values ± SD.
Author Contributions
Conceptualization, investigation, methodology, validation, visualization and writing—original draft preparation, I.S. and V.M.; writing—original draft, D.Š.S., J.P. and J.Z.; writing—review and editing, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors gratefully acknowledge the support of the CEEPUS program within the network HR-0306-15-2223, For Safe and Healthy Food in Middle Europe.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Springer: Boston, MA, USA, 1985; ISBN 978-1-4613-5703-2. [Google Scholar]
- McDonald, M.B. Seed Deterioration: Physiology, Repair and Assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Farrant, C.W.V.; Jill, M. Acquisition and Loss of Desiccation Tolerance. In Seed Development and Germination; Routledge: London, UK, 1995; ISBN 978-0-203-74007-1. [Google Scholar]
- Staniek, K.; Nohl, H. Are Mitochondria a Permanent Source of Reactive Oxygen Species? Biochim. Biophys. Acta-Bioenerg. 2000, 1460, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kristal, B.; Yu, B. An Emerging Hypothesis—Synergistic Induction of Aging by Free-Radicals and Maillard Reactions. J. Gerontol. 1992, 47, B107–B114. [Google Scholar] [CrossRef] [PubMed]
- Walters, C. Understanding the Mechanisms and Kinetics of Seed Aging. Seed Sci. Res. 1998, 8, 223–244. [Google Scholar] [CrossRef]
- Ashok, B.T.; Ali, R. The Aging Paradox: Free Radical Theory of Aging. Exp. Gerontol. 1999, 34, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Role of Oxidant Species in Aging. Curr. Med. Chem. 2004, 11, 1105–1112. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The Free Radical Theory of Aging Matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef]
- Levine, R.L.; Stadtman, E.R. Oxidative Modification of Proteins during Aging. Exp. Gerontol. 2001, 36, 1495–1502. [Google Scholar] [CrossRef]
- Berlett, B.S.; Stadtman, E.R. Protein Oxidation in Aging, Disease, and Oxidative Stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef]
- Oliver, C.; Ahn, B.; Moerman, E.; Goldstein, S.; Stadtman, E. Age-Related-Changes in Oxidized Proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Viteri, G.; Carrard, G.; Birlouez-Aragón, I.; Silva, E.; Friguet, B. Age-Dependent Protein Modifications and Declining Proteasome Activity in the Human Lens. Arch. Biochem. Biophys. 2004, 427, 197–203. [Google Scholar] [CrossRef]
- Thorpe, S.R.; Baynes, J.W. Maillard Reaction Products in Tissue Proteins: New Products and New Perspectives. Amino Acids 2003, 25, 275–281. [Google Scholar] [CrossRef]
- Monnier, V. Nonenzymatic Glycosylation, the Maillard Reaction and the Aging Process. J. Gerontol. 1990, 45, B105–B111. [Google Scholar] [CrossRef]
- Dills, W. Protein Fructosylation—Fructose and the Maillard Reaction. Am. J. Clin. Nutr. 1993, 58, 779–787. [Google Scholar] [CrossRef]
- Strelec, I. Aktivnost Aminopeptidaza i Sastav Proteina Sorti Ječma; Prirodoslovno-Matematički Fakultet: Zagreb, Croatia, 2004. [Google Scholar]
- Murat Karaoğlu, M.; Aydeniz, M.; Kotancilar, H.G.; Gerçelaslan, K.E. A Comparison of the Functional Characteristics of Wheat Stored as Grain with Wheat Stored in Spike Form. Int. J. Food Sci. Technol. 2010, 45, 38–47. [Google Scholar] [CrossRef]
- Mezei, Z.; Sipos, P.; Győri, Z. Variations in Quality Parameters of Forage and Medium Quality Winter Wheat Varieties in Storage. Agric. Conspec. Sci. 2007, 72, 221–225. [Google Scholar]
- Calucci, L.; Capocchi, A.; Galleschi, L.; Ghiringhelli, S.; Pinzino, C.; Saviozzi, F.; Zandomeneghi, M. Antioxidants, Free Radicals, Storage Proteins, Puroindolines, and Proteolytic Activities in Bread Wheat (Triticum aestivum) Seeds during Accelerated Aging. J. Agric. Food Chem. 2004, 52, 4274–4281. [Google Scholar] [CrossRef] [PubMed]
- Galleschi, L.; Capocchi, A.; Ghiringhelli, S.; Saviozzi, F.; Calucci, L.; Pinzino, C.; Zandomeneghi, M. Antioxidants, Free Radicals, Storage Proteins, and Proteolytic Activities in Wheat (Triticum durum) Seeds during Accelerated Aging. J. Agric. Food Chem. 2002, 50, 5450–5457. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Biswas, S.K.; Jimenez, L.A.; Torres, M.; Forman, H.J. Glutathione, Stress Responses, and Redox Signaling in Lung Inflammation. Antioxid. Redox Signal. 2005, 7, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Senmandi, S. Triphenyl Tetrazolium Chloride Staining Pattern of Differentially Aged Wheat Seed Embryos. Seed Sci. Technol. 1992, 20, 367–373. [Google Scholar]
- Ganguli, S.; Senmandi, S. Some Physiological Differences between Naturally and Artificially Aged Wheat Seeds. Seed Sci. Technol. 1990, 18, 507–514. [Google Scholar]
- Dell’Aquila, A. Wheat Seed Ageing and Embryo Protein Degradation. Seed Sci. Res. 1994, 4, 293–298. [Google Scholar] [CrossRef]
- Bernal-Lugo, I.; Rodriguez, M.; Gavilanes-Ruiz, M.; Hamabata, A. Reduced Aleurone α-Amylase Production in Aged Wheat Seeds Is Accompanied by Lower Levels of High-Pl α-Amylase Transcripts and Reduced Response to Gibberellic Acid. J. Exp. Bot. 1999, 50, 311–317. [Google Scholar] [CrossRef]
- Strelec, I.; Popović, R.; Ivanišić, I.; Jurković, V.; Jurković, Z.; Ugarčić-Hardi, Ž.; Sabo, M. Influence of Temperature and Relative Humidity on Grain Moisture, Germination and Vigour of Three Wheat Cultivars during One Year Storage. Poljoprivreda 2010, 16, 20–24. [Google Scholar]
- Strelec, I.; Šarkanj, B.; Mrša, V.; Ugarčić-Hardi, Ž. Chemical Composition, Quality Parameters, Exopeptidase and Oxidoreductase Activity Changes During Temporal Development of Wheat Grain Infestation by Sitophilus granarius. J. Food Biochem. 2014, 38, 175–183. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nagatsu, I.; Nagatsu, T.; Yamamoto, T.; Glenner, G.G.; Mehl, J.W. Purification of Aminopeptidase a in Human Serum and Degradation of Angiotensin II by the Purified Enzyme. Biochim. Biophys. Acta BBA-Enzymol. 1970, 198, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Strelec, I.; Vukelić, B.; Vitale, L. Aminopeptidases of Germinated and Non-Germinated Barley. Food Technol. Biotechnol. 2009, 47, 296–303. [Google Scholar]
- Waters, S.P.; Peoples, M.B.; Simpson, R.J.; Dalling, M.J. Nitrogen Redistribution during Grain Growth in Wheat (Triticum aestivum L.). Planta 1980, 148, 422–428. [Google Scholar] [CrossRef]
- Voigt, G.; Biehl, B.; Heinrichs, H.; Voigt, J. Aspartic Proteinase Levels in Seeds of Different Angiosperms. Phytochemistry 1997, 44, 389–392. [Google Scholar] [CrossRef]
- Scebba, F.; Sebastiani, L.; Vitagliano, C. Activities of Antioxidant Enzymes during Senescence of Prunus Armeniaca Leaves. Biol. Plant. 2001, 44, 41–46. [Google Scholar] [CrossRef]
- Okot-Kotber, M.; Liavoga, A.; Yong, K.-J.; Bagorogoza, K. Activation of Polyphenol Oxidase in Extracts of Bran from Several Wheat (Triticum aestivum) Cultivars Using Organic Solvents, Detergents, and Chaotropes. J. Agric. Food Chem. 2002, 50, 2410–2417. [Google Scholar] [CrossRef]
- Ugarčić-Hardi, Ž.; Strelec, I. Accumulation of Amadori and Maillard Products in wheat seeds aged under different storage conditions. Croat. Chem. Acta 2008, 81, 131–137. [Google Scholar]
- 155 Determination of Wet Gluten Quantity and Quality (Gluten Index Ac. to Perten) of Whole Wheat Meal and Wheat Flour (Triticum aestivum). Available online: https://icc.or.at/publications/icc-standards/standards-overview/155-standard-method (accessed on 19 December 2023).
- 107/1 Determination of the Falling Number According to Hagberg—As a Measure of the Degree of Alpha-Amylase Activity in Grain and Flour. Available online: https://icc.or.at/publications/icc-standards/standards-overview/107-1-standard-method (accessed on 19 December 2023).
- Murthy, U.M.N.; Kumar, P.P.; Sun, W.Q. Mechanisms of Seed Ageing under Different Storage Conditions for Vigna radiata (L.) Wilczek: Lipid Peroxidation, Sugar Hydrolysis, Maillard Reactions and Their Relationship to Glass State Transition. J. Exp. Bot. 2003, 54, 1057–1067. [Google Scholar] [CrossRef]
- Murthy, U.M.N.; Liang, Y.H.; Kumar, P.P.; Sun, W.Q. Non-Enzymatic Protein Modification by the Maillard Reaction Reduces the Activities of Scavenging Enzymes in Vigna radiata. Physiol. Plant. 2002, 115, 213–220. [Google Scholar] [CrossRef]
- Ganguli, S.; Senmandi, S. Effects of Aging on Amylase Activity and Scutellar Cell Structure during Imbibition in Wheat Seed. Ann. Bot. 1993, 71, 411–416. [Google Scholar] [CrossRef]
- Dragicevic, V.D.; Sredojevic, S.; Spasic, M.B.; Vrvic, M.M. Ageing-Induced Changes of Reduced and Oxidised Glutathione in Fragments of Maize Seedlings. J. Serb. Chem. Soc. 2003, 68, 911–917. [Google Scholar] [CrossRef]
- Basavarajappa, B.; Shetty, H.; Prakash, H. Membrane Deterioration and Other Biochemical-Changes, Associated with Accelerated Aging of Maize Seeds. Seed Sci. Technol. 1991, 19, 279–286. [Google Scholar]
- Heath, R.; Packer, L. Photoperoxidation in Isolated Chloroplasts. i. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Sun, W.; Leopold, A. The Maillard Reaction and Oxidative Stress during Aging of Soybean Seeds. Physiol. Plant. 1995, 94, 94–104. [Google Scholar] [CrossRef]
- Torres, M.; De Paula, M.; Pérez-Otaola, M.; Darder, M.; Frutos, G.; Martínez-Honduvilla, C.J. Ageing-Induced Changes in Glutathione System of Sunflower Seeds. Physiol. Plant. 1997, 101, 807–814. [Google Scholar] [CrossRef]
- Siegenthaler, P.; Douetorhant, V. Relationship between the Atp Content Measured at 3 Imbibition Times and Germination of Onion Seeds during Storage at 3, 15 and 30 °C. J. Exp. Bot. 1994, 45, 1365–1371. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F.; Come, D. Changes in Malondialdehyde Content and in Superoxide Dismutase, Catalase and Glutathione Reductase Activities in Sunflower Seeds as Related to Deterioration during Accelerated Aging. Physiol. Plant. 1996, 97, 104–110. [Google Scholar] [CrossRef]
- Zhang, M.; Yoshiyama, M.; Nagashima, T.; Nakagawa, Y.; Yoshioka, T.; Esashi, Y. Aging of Soybean Seeds in Relation to Metabolism at Different Relative Humidities. Plant Cell Physiol. 1995, 36, 1189–1195. [Google Scholar]
- Begnami, C.N.; Cortelazzo, A.L. Cellular Alterations during Accelerated Aging of French Bean Seeds. Seed Sci. Technol. 1996, 24, 295–303. [Google Scholar]
- Sammour, R.H. Effect of Ageing on the Major Reserve Molecules and Their Related Enzyme in Natural Aged Seeds of Flax. J. Islam. Acad. Sci. 1989, 2, 247–251. [Google Scholar]
- Petruzzelli, L.; Taranto, G. Wheat Aging—The Contribution of Embryonic and Non-Embryonic Lesions to Loss of Seed Viability. Physiol. Plant. 1989, 76, 289–294. [Google Scholar]
- Ravikumar, R.; Ananthakrishnan, G.; Ganapathi, A.; Appasamy, T. Biochemical Changes Induced by Accelerated Ageing in Bambusa Bambos Seeds. Biol. Plant. 1998, 40, 459–464. [Google Scholar] [CrossRef]
- Ravikumar, R.; Ananthakrishnan, G.; Girija, S.; Ganapathi, A. Seed Viability and Biochemical Changes Associated with Accelerated Ageing in Dendrocalamus Strictus Seeds. Biol. Plant 2002, 45, 153–156. [Google Scholar] [CrossRef]
- Dhaliwal, Y.; Sekhon, K.; Nagi, H. Enzymatic-Activities and Rheological Properties of Stored Rice. Cereal Chem. 1991, 68, 18–21. [Google Scholar]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Ageing of Stored Rice: Changes in Chemical and Physical Attributes. J. Cereal Sci. 2002, 35, 65–78. [Google Scholar] [CrossRef]
- Strelec, I.; Vitale, L. Changes of aminopeptidase activities in barley grain during storage. In Proceedings of the 3rd International Congress ‘Flour-Bread 05’ and 5th Croatian Congress of Cereal Technologists, Opatija, Croatia, 26–29 October 2005; CABI: Wallingford, UK, 2006; p. 63. [Google Scholar]
- Matsukura, U.; Kaneko, S.; Momma, M. Method for measuring the freshness of individual rice grains by means of a color reaction of catalase activity. J. Jpn. Soc. Food Sci. Technol.-Nippon Shokuhin Kagaku Kogaku Kaishi 2000, 47, 523–528. [Google Scholar] [CrossRef]
- Chen, T.F.; Chen, C.L. Analysing the Freshness of Intact Rice Grains by Colour Determination of Peroxidase Activity. J. Sci. Food Agric. 2003, 83, 1214–1218. [Google Scholar] [CrossRef]
- Barrett, A.J.; Rawlings, N.D.; Woessner, J.F. Handbook of Proteolytic Enzymes; Academic Press: Cambridge, MA, USA, 2012; ISBN 978-0-12-382220-8. [Google Scholar]
- Kruger, J.E.; Lineback, D.R. Enzymes and Their Role in Cereal Technology; Stauffer, C.E., Ed.; Amer Assn of Cereal Chemists: St. Paul, MN, USA, 1987; ISBN 978-0-913250-46-4. [Google Scholar]
- Every, D.; Simmons, L.D.; Ross, A.P. Distribution of Redox Enzymes in Millstreams and Relationships to Chemical and Baking Properties of Flour. Cereal Chem. 2006, 83, 315. [Google Scholar] [CrossRef]
- Garcia, R.; Kaid, N.; Vignaud, C.; Nicolas, J. Purification and Some Properties of Catalase from Wheat Germ (Triticum aestivum L.). J. Agric. Food Chem. 2000, 48, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M. Polyphenol Oxidases in Plants and Fungi: Going Places? A Review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Baik, B.-K.; Donelson, T. Postharvest and Postmilling Changes in Wheat Grain and Flour Quality Characteristics. Cereal Chem. 2018, 95, 141–148. [Google Scholar] [CrossRef]
- Hrušková, M.; Machová, D. Changes of Wheat Flour Properties during Short Term Storage. Czech J. Food Sci. 2002, 20, 125–130. [Google Scholar] [CrossRef]
- Wang, L.F.; Flores, R.A. The Effects of Storage on Flour Quality and Baking Performance. Food Rev. Int. 1999, 15, 215–234. [Google Scholar] [CrossRef]
- Hrušková, M.; Škodová, V.; Blažek, J. Wheat Sedimentation Values and Falling Number. Czech J. Food Sci. 2004, 22, 51–57. [Google Scholar] [CrossRef]
- Kibar, H. Influence of Storage Conditions on the Quality Properties of Wheat Varieties. J. Stored Prod. Res. 2015, 62, 8–15. [Google Scholar] [CrossRef]
- Ibanoglu, S. Wheat Washing with Ozonated Water: Effects on Selected Flour Properties. Int. J. Food Sci. Technol. 2002, 37, 579–584. [Google Scholar] [CrossRef]
- Curic, D.; Dugum, J.; Bauman, I. The Influence of Fungal α-Amylase Supplementation on Amylolytic Activity and Baking Quality of Flour. Int. J. Food Sci. Technol. 2002, 37, 673–680. [Google Scholar] [CrossRef]
- Mailhot, W.C.; Patton, J.C. Criteria of Flour Quality. In Wheat Chemistry and Technology; American Association of Cereal Chemists Inc.: St. Paul, MN, USA, 1988; pp. 69–90. [Google Scholar]
- Shibuya, N.; Iwasaki, T. Effect of Cell-Wall Degrading Enzymes on the Cooking Properties of Milled Rice and the Texture of Cooked Rice. J. Jpn. Soc. Food Sci. Technol.-Nippon Shokuhin Kagaku Kogaku Kaishi 1984, 31, 656–660. [Google Scholar] [CrossRef]
- Teo, C.H.; Abd, A.; Cheah, P.B.; Norziah, M.H.; Seow, C.C. On the Roles of Protein and Starch in the Aging of Non-Waxy Rice Flour. Food Chem. 2000, 69, 229–236. [Google Scholar] [CrossRef]
- Wilkes, M.; Copeland, L. Storage of Wheat Grains at Elevated Temperatures Increases Solubilization of Glutenin Subunits. Cereal Chem. 2008, 85, 335–338. [Google Scholar] [CrossRef]
- Salman, H.; Copeland, L. Effect of Storage on Fat Acidity and Pasting Characteristics of Wheat Flour. Cereal Chem. 2007, 84, 600–606. [Google Scholar] [CrossRef]
- Loney, D.; Meredith, P. Note on Amylograph Viscosities of Wheat Flours and Their Starches during Storage. Cereal Chem 1974, 51, 702–705. [Google Scholar]
- Zhou, Z.K.; Robards, K.; Helliwell, S.; Blanchard, C.; Baxterb, G. Rice Ageing. I. Effect of Changes in Protein on Starch Behaviour. Starch-Starke 2003, 55, 162–169. [Google Scholar] [CrossRef]
- Weegels, P.; Verhoek, J.; Degroot, A.; Hamer, R. Effects on Gluten of Heating at Different Moisture Contents. 1. Changes in Functional-Properties. J. Cereal Sci. 1994, 19, 31–38. [Google Scholar] [CrossRef]
- Gonzalez-Torralba, J.; Arazuri, S.; Jaren, C.; Arregui, L.M. Influence of Temperature and r.h. during Storage on Wheat Bread Making Quality. J. Stored Prod. Res. 2013, 55, 134–144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).