Microwave-Assisted Production of 5-Hydroxymethylfurfural from Fructose Using Sulfamic Acid as a Green Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
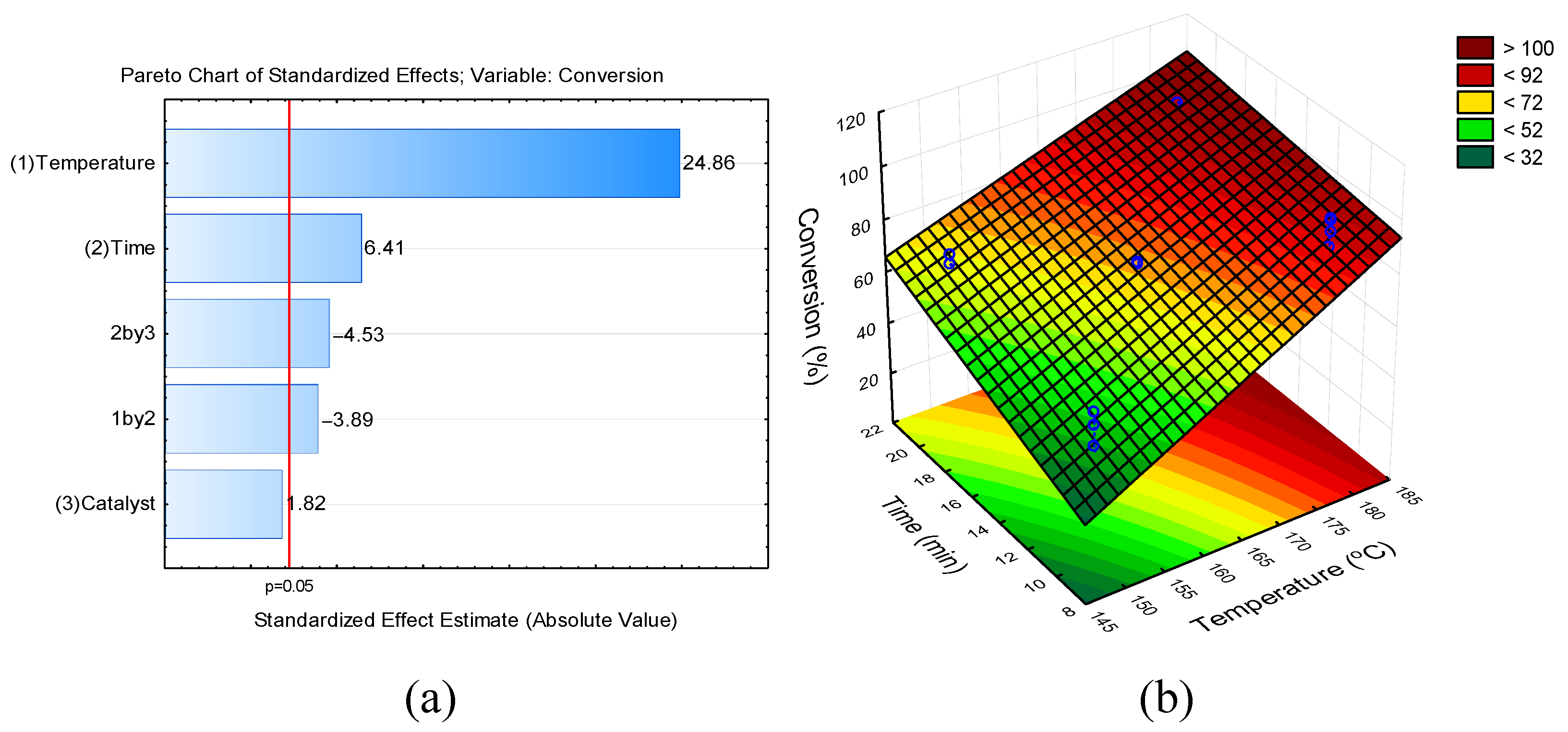
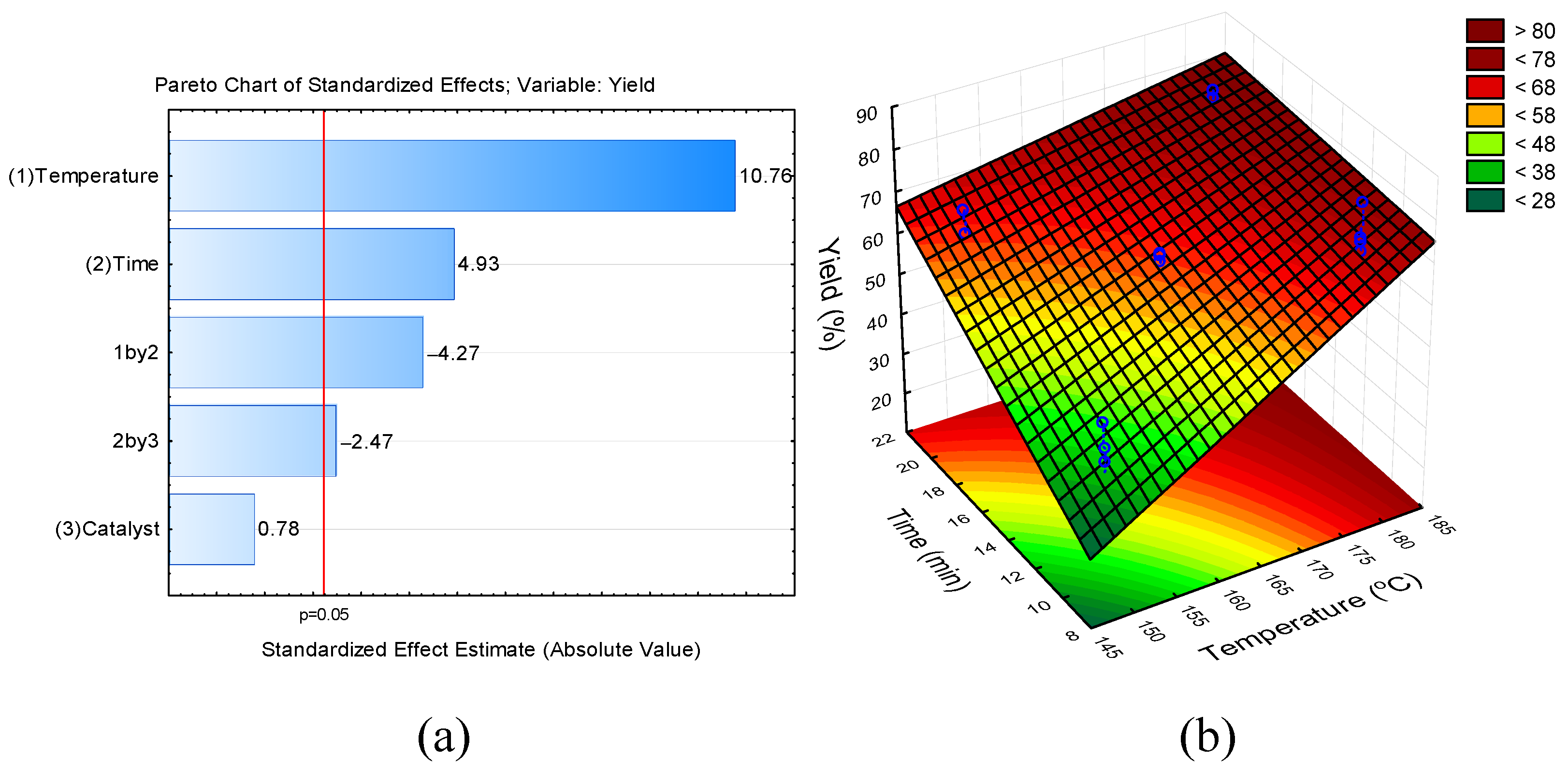
2.2. Experimental Design
2.3. Conversion, Yield, and Selectivity Measurement
2.4. Production of 5-HMF under the Optimized Conditions
2.5. Statistical Analysis
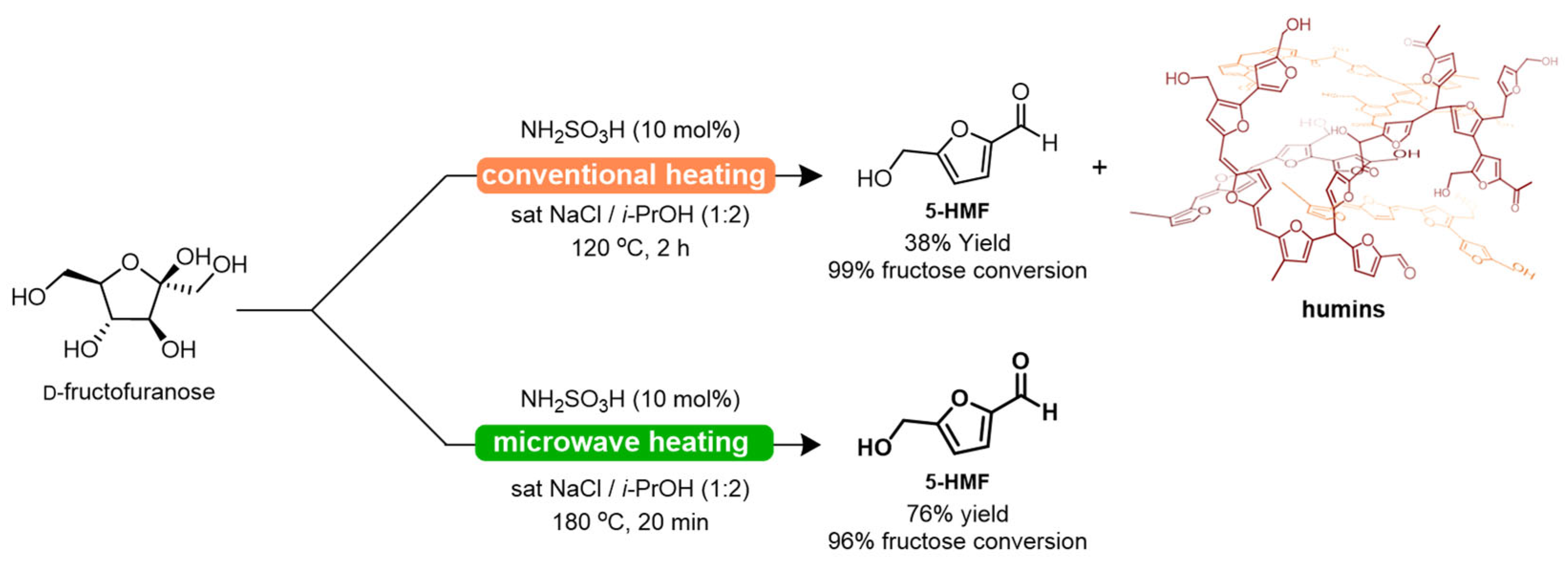
3. Results and Discussion
Conversion of Fructose into 5-HMF
| Run | Temperature (°C) | Time (min) | Catalyst (mol%) | Conversion (%) (mean ± SD) | Yield (%) (mean ± SD) |
|---|---|---|---|---|---|
| 1 | 150 (−1) | 10 (−1) | 10 (−1) | 42.04 ± 3.54 e | 37.22 ± 3.28 c |
| 2 | 150 (−1) | 10 (−1) | 20 (+1) | 54.58 ± 3.45 b | 45.96 ± 4.29 cd |
| 3 | 150 (−1) | 20 (+1) | 10 (−1) | 68.54 ± 2.45 cd | 64.61 ± 5.29 ab |
| 4 | 150 (−1) | 20 (+1) | 20 (+1) | 60.04 ± 6.38 bc | 58.20 ± 6.46 bd |
| 5 | 180 (+1) | 10 (−1) | 10 (−1) | 89.32 ± 4.16 a | 71.33 ± 2.16 ab |
| 6 | 180 (+1) | 10 (−1) | 20 (+1) | 96.47 ± 0.83 a | 76.59 ± 6.52 a |
| 7 | 180 (+1) | 20 (+1) | 10 (−1) | 96.75 ± 1.06 a | 75.81 ± 4.99 a |
| 8 | 180 (+1) | 20 (+1) | 20 (+1) | 96.84 ± 2.31 a | 74.94 ± 3.49 a |
| 9 | 165 (0) | 15 (0) | 15 (0) | 73.74 ± 0.57 d | 62.61 ± 1.04 ab |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, Q.-S.; Li, X.-L.; Xu, H.-J.; Fu, Y. Conversion of 5-Hydroxymethylfurfural to Chemicals: A Review of Catalytic Routes and Product Applications. Fuel Process. Technol. 2020, 209, 106528. [Google Scholar] [CrossRef]
- Chacón-Huete, F.; Messina, C.; Cigana, B.; Forgione, P. Diverse Applications of Biomass-Derived 5-Hydroxymethylfurfural and Derivatives as Renewable Starting Materials. ChemSusChem 2022, 15, e202200328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Dumont, M.J. Advances in Polymer Precursors and Bio-Based Polymers Synthesized from 5-Hydroxymethylfurfural. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1478–1492. [Google Scholar] [CrossRef]
- Fulignati, S.; Licursi, D.; Di Fidio, N.; Antonetti, C.; Raspolli Galletti, A.M. Novel Challenges on the Catalytic Synthesis of 5-Hydroxymethylfurfural (HMF) from Real Feedstocks. Catalysts 2022, 12, 1664. [Google Scholar] [CrossRef]
- Torres-Olea, B.; Fúnez-Núñez, I.; García-Sancho, C.; Cecilia, J.A.; Moreno-Tost, R.; Maireles-Torres, P. Influence of Lewis and Brønsted Acid Catalysts in the Transformation of Hexoses into 5-Ethoxymethylfurfural. Renew. Energy 2023, 207, 588–600. [Google Scholar] [CrossRef]
- Messori, A.; Fasolini, A.; Mazzoni, R. Advances in Catalytic Routes for the Homogeneous Green Conversion of the Bio-Based Platform 5-Hydroxymethylfurfural. ChemSusChem 2022, 15, e202200228. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ding, Y.; Dai, J.; Long, Y.; Hu, Z.-T. Synthesis of 5-Hydroxymethylfurfural from Dehydration of Biomass-Derived Glucose and Fructose Using Supported Metal Catalysts. Green Synth. Catal. 2021, 2, 187–197. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Zou, F.; Zhong, Y.; Du, N.; Huang, X. Room-Temperature Ionic Liquid System Converting Fructose into 5-Hydroxymethylfurfural in High Efficiency. ACS Sustain. Chem. Eng. 2015, 3, 3338–3345. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Xie, X.; Huang, C.; Yang, S. Dehydration of Fructose, Sucrose and Inulin to 5-Hydroxymethylfurfural over Yeast-Derived Carbonaceous Microspheres at Low Temperatures. RSC Adv. 2019, 9, 9041–9048. [Google Scholar] [CrossRef]
- Körner, S.; Albert, J.; Held, C. Catalytic Low-Temperature Dehydration of Fructose to 5-Hydroxymethylfurfural Using Acidic Deep Eutectic Solvents and Polyoxometalate Catalysts. Front. Chem. 2019, 7, 661. [Google Scholar] [CrossRef]
- Velasco Calderón, J.C.; Arora, J.S.; Mushrif, S.H. Mechanistic Investigation into the Formation of Humins in Acid-Catalyzed Biomass Reactions. ACS Omega 2022, 7, 44786–44795. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, Y.; Liao, Y.; Wang, H.; Liu, Q.; Ma, L.; Wang, C. Advances in Understanding the Humins: Formation, Prevention and Application. Appl. Energy Combust. Sci. 2022, 10, 100062. [Google Scholar] [CrossRef]
- Delbecq, F.; Len, C. Recent Advances in the Microwave-Assisted Production of Hydroxymethylfurfural by Hydrolysis of Cellulose Derivatives—A Review. Molecules 2018, 23, 1973. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.S.; Woodley, J.M.; Riisager, A. Efficient Microwave-Assisted Synthesis of 5-Hydroxymethylfurfural from Concentrated Aqueous Fructose. Carbohydr. Res. 2009, 344, 2568–2572. [Google Scholar] [CrossRef]
- Kappe, C.O.; Pieber, B.; Dallinger, D. Microwave Effects in Organic Synthesis: Myth or Reality? Angew. Chem. Int. Ed. 2013, 52, 1088–1094. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Catalytic Dehydration of Fructose into 5-Hydroxymethylfurfural by Ion-Exchange Resin in Mixed-Aqueous System by Microwave Heating. Green Chem. 2008, 10, 799. [Google Scholar] [CrossRef]
- De, S.; Dutta, S.; Saha, B. Microwave Assisted Conversion of Carbohydrates and Biopolymers to 5-Hydroxymethylfurfural with Aluminium Chloride Catalyst in Water. Green Chem. 2011, 13, 2859–2868. [Google Scholar] [CrossRef]
- Gomes, G.R.; Pastre, J.C. Microwave-Assisted HMF Production from Water-Soluble Sugars Using Betaine-Based Natural Deep Eutectic Solvents (NADES). Sustain. Energy Fuels 2020, 4, 1891–1898. [Google Scholar] [CrossRef]
- Baker-Fales, M.; Chen, T.Y.; Vlachos, D.G. Scale-up of Microwave-Assisted, Continuous Flow, Liquid Phase Reactors: Application to 5-Hydroxymethylfurfural Production. Chem. Eng. J. 2023, 454, 139985. [Google Scholar] [CrossRef]
- Chen, W.; Malhotra, A.; Yu, K.; Zheng, W.; Plaza-Gonzalez, P.J.; Catala-Civera, J.M.; Santamaria, J.; Vlachos, D.G. Intensified Microwave-Assisted Heterogeneous Catalytic Reactors for Sustainable Chemical Manufacturing. Chem. Eng. J. 2021, 420, 130476. [Google Scholar] [CrossRef]
- Duan, Y.; Yuan, P.; Huang, S.; Wang, L.; Deng, J.; Yu, S.; Xie, Q.; Nie, Y. Experimental Study on Reactor Scale-up for Microwave-Assisted Pyrolysis of Methyl Ricinoleate. Chem. Eng. Process. Process Intensif. 2023, 184, 109293. [Google Scholar] [CrossRef]
- Buttress, A.J.; Hargreaves, G.; Ilchev, A.; Monti, T.; Sklavounou, A.; Katrib, J.; Martin-Tanchereau, P.; Unthank, M.G.; Irvine, D.J.; Dodds, C.D. Design and Optimisation of a Microwave Reactor for Kilo-Scale Polymer Synthesis. Chem. Eng. Sci. X 2019, 2, 100022. [Google Scholar] [CrossRef]
- Spillane, W.; Malaubier, J.B. Sulfamic Acid and Its N- and O-Substituted Derivatives. Chem. Rev. 2014, 114, 2507–2586. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.; Baghernejad, B.; Oskooie, H. Application of Sulfamic Acid in Organic Synthesis-A Short Review. Curr. Org. Chem. 2009, 13, 1002–1014. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Sulfamic Acid Is an Environment-Friendly Alternative Electrolyte for Industrial Acid Cleaning and Corrosion Inhibition: A Mini Review. Corros. Rev. 2022, 40, 119–126. [Google Scholar] [CrossRef]
- Moraes, M.C.; Lenardão, E.J.; Barcellos, T. Synthesis of C4-Substituted Coumarins via Pechmann Condensation Catalyzed by Sulfamic Acid. Insights into the Reaction Mechanism by HRMS Analysis. Arkivoc 2021, 2021, 151–163. [Google Scholar] [CrossRef]
- Arora, G.; Gupta, R.; Yadav, P.; Dixit, R.; Srivastava, A.; Sharma, R.K. Ultrasonically-Mediated One-Pot Synthesis of Substituted Imidazoles via Sulfamic Acid Functionalized Hollow Magnetically Retrievable Solid-Acid Catalyst. Curr. Res. Green Sustain. Chem. 2021, 4, 100050. [Google Scholar] [CrossRef]
- Bhirud, J.D.; Patil, R.D.; Narkhede, H.P. Sulfamic Acid Catalyzed Synthesis of New 3,5-[(Sub)Phenyl]-1H-Pyrazole Bearing N1-Isonicotinoyl: And Their Pharmacological Activity Evaluation. Bioorganic Med. Chem. Lett. 2020, 30, 127558. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, X.; Shen, Y.; Yi, Y.; Wang, B.; Xu, F.; Sun, R. Conversion of Bamboo Fiber into 5-Hydroxymethylfurfural Catalyzed by Sulfamic Acid with Microwave Assistance in Biphasic System. Ind. Crops Prod. 2015, 70, 266–271. [Google Scholar] [CrossRef]
- Lu, Y.; He, Q.; Peng, Q.; Chen, W.; Cheng, Q.; Song, G.; Fan, G. Directional Synthesis of Furfural Compounds from Holocellulose Catalyzed by Sulfamic Acid. Cellulose 2021, 28, 8343–8354. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Fu, X.; Tang, D.; Li, H.; Hu, P.; Zhu, L.; Hu, C. Insights into the NaCl-Induced Formation of Soluble Humins during Fructose Dehydration to 5-Hydroxymethylfurfural. Ind. Eng. Chem. Res. 2022, 61, 5786–5796. [Google Scholar] [CrossRef]
- Gomes, F.N.D.C.; Pereira, L.R.; Ribeiro, N.F.P.; Souza, M.M.V.M. Production of 5-Hydroxymethylfurfural (HMF) via Fructose Dehydration: Effect of Solvent and Salting-Out. Braz. J. Chem. Eng. 2015, 32, 119–126. [Google Scholar] [CrossRef]
- Galaverna, R.; Breitkreitz, M.C.; Pastre, J.C. Conversion of D-fructose to 5-(hydroxymethyl)furfural: Evaluating batch and continuous flow conditions by design of experiments and in-line FTIR monitoring. ACS Sustain. Chem. Eng. 2018, 6, 4220–4230. [Google Scholar] [CrossRef]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and Limitations of Microwave Reactors: From Chemical Synthesis to the Catalytic Valorization of Biobased Chemicals. ACS Sustain. Chem. Eng. 2019, 7, 3–21. [Google Scholar] [CrossRef]
- Barham, J.P.; Koyama, E.; Norikane, Y.; Ohneda, N.; Yoshimura, T. Microwave Flow: A Perspective on Reactor and Microwave Configurations and the Emergence of Tunable Single-Mode Heating Toward Large-Scale Applications. Chem. Rec. 2019, 19, 188–203. [Google Scholar] [CrossRef]
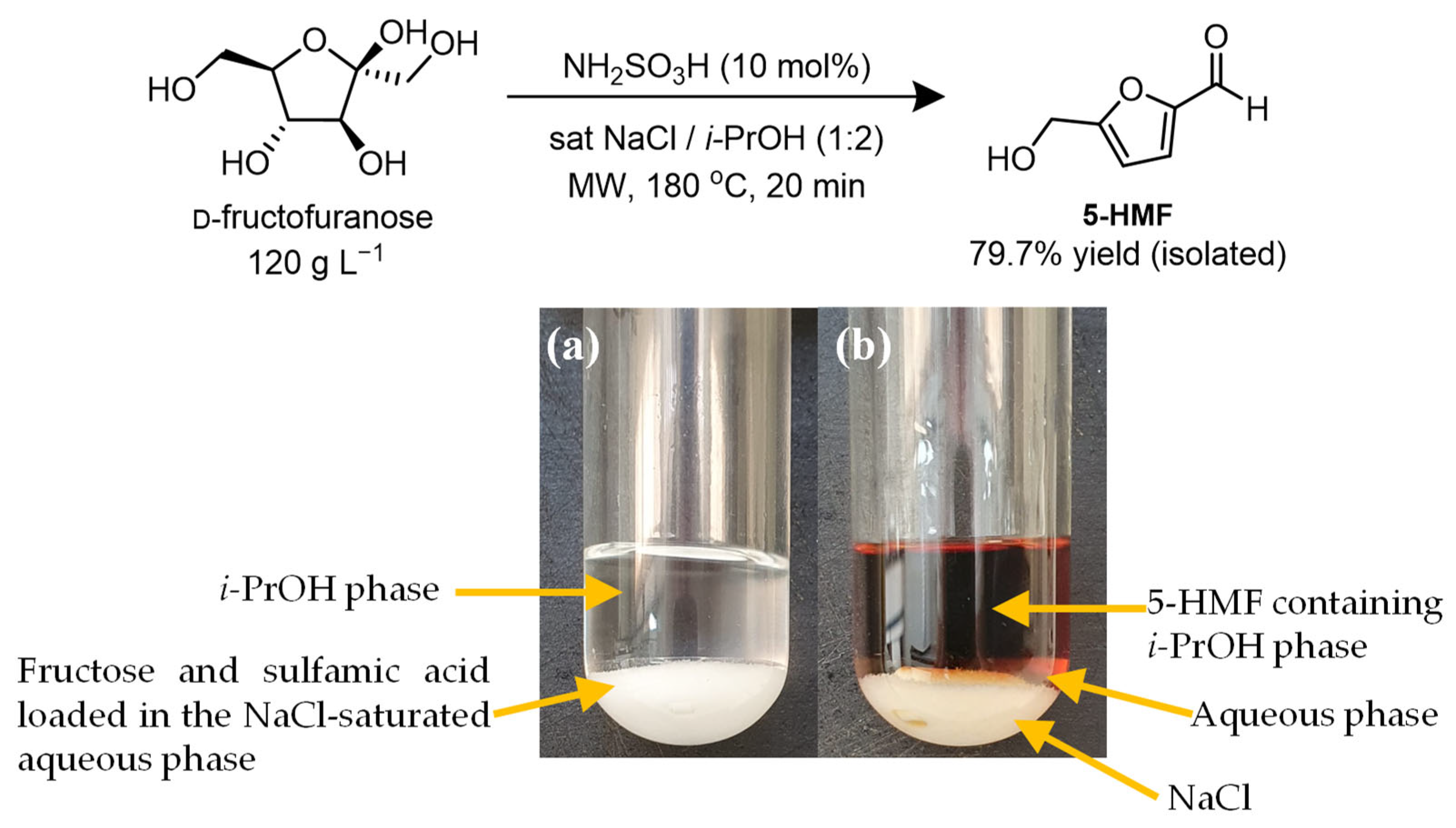
| Entry | Fructose Concentration (g L−1) | Conversion (%) (Mean ± SD) | Yield (%) (Mean ± SD) | Selectivity (%) (Mean ± SD) |
|---|---|---|---|---|
| 1 | 30 | 98.13 ± 0.39 a | 78.98 ± 0.17 a | 77.50 ± 0.34 a |
| 2 | 60 | 96.84 ± 7.08 a | 76.52 ± 6.86 a | 74.41 ± 7.01 a |
| 3 | 120 | 91.15 ± 6.98 a | 80.34 ± 8.41 a | 73.20 ± 8.23 a |
| 4 | 150 | 94.03 ± 0.25 a | 60.79 ± 1.18 b | 57.16 ± 1.78 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoncello Molon, V.; Ferreira, B.L.; Colombo Tedesco, C.; Delagustin, M.G.; Barcellos, T. Microwave-Assisted Production of 5-Hydroxymethylfurfural from Fructose Using Sulfamic Acid as a Green Catalyst. Sustainability 2024, 16, 858. https://doi.org/10.3390/su16020858
Bertoncello Molon V, Ferreira BL, Colombo Tedesco C, Delagustin MG, Barcellos T. Microwave-Assisted Production of 5-Hydroxymethylfurfural from Fructose Using Sulfamic Acid as a Green Catalyst. Sustainability. 2024; 16(2):858. https://doi.org/10.3390/su16020858
Chicago/Turabian StyleBertoncello Molon, Vinícius, Bruno Luís Ferreira, Carolina Colombo Tedesco, Maria Gabriele Delagustin, and Thiago Barcellos. 2024. "Microwave-Assisted Production of 5-Hydroxymethylfurfural from Fructose Using Sulfamic Acid as a Green Catalyst" Sustainability 16, no. 2: 858. https://doi.org/10.3390/su16020858
APA StyleBertoncello Molon, V., Ferreira, B. L., Colombo Tedesco, C., Delagustin, M. G., & Barcellos, T. (2024). Microwave-Assisted Production of 5-Hydroxymethylfurfural from Fructose Using Sulfamic Acid as a Green Catalyst. Sustainability, 16(2), 858. https://doi.org/10.3390/su16020858








