Abstract
Background: Biolubricants represent a category of lubricating substances derived from sustainable sources such as vegetable oils, animal fats, and other bio-based materials. They are considered more environmentally friendly than mineral-based lubricants because they are biodegradable and nontoxic. Biolubricants derived from vegetable oils or animal fats were used as first-generation biolubricants. They have limited performance at extreme temperatures, both high and low, as well as low oxidative stability. Substitution of the double bonds by branching improves the performance and stability of the resulting second-generation biolubricants. Methods: In the past, the production of these compounds has relied on the chemical pathway. This method involves elevated temperatures and inorganic catalysts, leading to the necessity of additional purification steps, which decreases environmental sustainability and energy efficiency. A more environmentally friendly alternative, the enzymatic route, has been introduced, in accordance with the principles of “Green Chemistry”. Results: In this paper, the esterification of 2-methylhexanoic acid with 2-octyl-1-dodecanol and its optimization were developed for the first time. The synthesis was conducted within a jacketed batch reactor connected to a thermostatic bath in a solvent-free reaction medium and using Lipozyme® 435 as biocatalyst. Conclusions: The high viscosity index value of this new hyperbranched ester (>200, ASTM D2270) suggests that it may be an excellent biolubricant to be used under extreme temperature conditions. Regarding sustainability, the main green metrics calculated point to an environmentally friendly process.
1. Introduction
Lubricants, extensively used in industry and in our day-to-day life, are compounds which are mainly obtained from mineral oils [1]. The growing concern for environmental protection has prompted the search for new and more environmentally friendly products with good lubricity characteristics [2]. In this context, lubricants derived from bio-based sources, known as biolubricants, have emerged as promising alternatives poised to supplant traditional mineral oils derived from petroleum [3]. Among the advantages of biolubricants, their relatively high viscosity indexes, high lubricity, lower coefficient of friction, biodegradability, and nontoxicity stand out [2,3,4,5].
In the past, vegetable oils, both natural and synthetic, consisting mainly of esters of linear unsaturated fatty acids were used as first-generation biolubricants [6]. However, they have good cold performance but low oxidative resistance due to unsaturation; therefore, to overcome the latter drawback, unsaturations are replaced by branching, which increases the oxidative stability of the oils and leads to second-generation biolubricants [6,7]. In addition, the introduction of appropriate branching into ester molecules contributes to extending their liquidity to very low temperatures, which improves their cold flow properties. The lowest cloud points are measured when esters have branching in both the acid and alcohol moieties [8].
Recently, different branched esters have been synthesized from Guerbet alcohols, which are primary alcohols with β-branching, unsaturated fatty acids and dicarboxylic acids. A comparison of these new compounds with the analogous commercial petroleum base oils demonstrated that branching improved lubricant performance properties; therefore, Guerbet esters are useful as biolubricant oils because of their liquidity at subzero temperatures, resistance to oxidation, minimal variation in viscosity with respect to temperature, higher flash point and biodegradability [1].
Normally, biolubricants are synthesized by chemical routes from various bio-based sources, including vegetable oils, microbially derived oils, waste cooking oil, etc., which are modified through different pathways of which the most common are esterification and transesterification, estolide formation, epoxidation, and selective hydrogenation [9]. However, using high temperatures and inorganic catalysts has led to downstream purification steps necessary to remove undesired sub-products and solvent residues. This results in a reduction in both environmental sustainability and energy efficiency in the process. Adhering to the principles of “Green Chemistry” [10] and the quest for more eco-friendly methods, an alternative enzymatic route has been introduced. This approach incorporates lower temperatures and eliminates the need for solvents, thereby contributing to a more sustainable process.
To date, biocatalytic synthesis has been extensively developed. The esterification and transesterification of a wide variety of fatty acid-composed materials with different alcohols and poliols (methanol, neopentyl glycol, isoamyl alcohol, trimethylolpropane, pentaerythritol and fusel oil) have been catalyzed by lipases, both free and immobilized, from different sources, in solvent-free systems to yield first-generation biolubricants [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30].
On the other hand, biocatalytic ester production from free fatty acid and branched alcohol has also been widely described in bibliography [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Lipase-catalyzed esterification of linear fatty acids (C5–C18) and diacid (C6–C10) has been successfully performed with different branched alcohols and diols to produce mono branched esters suitable to be used as biolubricants. However, when branched acids are used as substrates, the biocatalytic esterification process is not so easy. In the literature, cetyl 2-ethylhexanoate [52] and 2-ethylhexyl 2-ethylhexanoate [53] synthesis has been described with no success because high lipase concentrations (above 250%) and long times (more than 60 h) were required to obtain relatively low conversions. If ethyl branching in the acid is changed by methyl, the biocatalytic synthesis can be successfully developed to yield 2-ethylhexyl 2-methylhexanoate with a productivity of 203.84 kg product × kg biocatalyst−1 under optimal reaction conditions (70 °C, 10% molar excess of alcohol and six uses of the immobilized enzyme) [54].
Economic considerations are crucial in the development and potential industrial application of biotechnological processes. The cost of enzymes and energy consumption are typically the primary factors affecting profitability. Therefore, economic analysis is essential in determining the optimal operating temperature and assessing the feasibility of biocatalyst reuse, ensuring the economic viability of the process [47,48,50,51,54].
The sustainability of a process is no longer an abstract concept but something measurable through green metrics. Since Sheldon, in 1992, introduced the use of the E factor, different indexes have been developed, depending on how concern for environmental protection and the development of environmentally sustainable processes has increased [55,56,57,58]. Using these metrics, process sustainability can be assessed based on three key aspects: efficiency in the use of resources (mainly materials and energy); environmental, health and safety impacts; and life cycle assessment considerations. For example, the E factor allows the assessment of compliance with the first principle of “Green Chemistry”, related to the prevention of waste generation during production. The process mass intensity (PMI) allows one to check whether the process complies with the principle of atom economy, ensuring the maximal incorporation of all materials utilized in the process into the final product. On the other hand, the determination of the EHSI (Environmental–Health–Safety Index) allows the toxicity and environmental risk of the process associated with the use of a specific solvent in the process to be evaluated.
According to the above-mentioned aspects, this research proposes the development of a sustainable process to produce a new double-branched ester, 2-octyl-1-dodecanoyl 2-methylhexanoate, by lipase-catalyzed synthesis with the commercial immobilized lipase Lipozyme® 435. The reaction scheme is depicted in Figure 1. A solvent-free medium was used to minimize downstream separation steps and waste generation. Biocatalytic reactions are highly affected by both temperature and biocatalyst concentration, so these variables were optimized to reach maximum conversion in the shortest possible time. The characterization of the double-branched ester, obtained through kinematic and dynamic viscosity measurements and determination of the viscosity index, proves that it can be an excellent biolubricant. The assessment of process sustainability includes the examination of parameters such as the E factor, cE factor, atom economy (AE), carbon mass efficiency (CME), and process mass intensity (PMI).
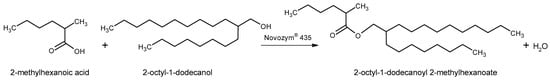
Figure 1.
Process enzymatic esterification scheme involving 2-methylhexanoic acid and 2-octyl-1-dodecanol.
2. Materials and Methods
2.1. Materials
Lipozyme® 435, an immobilized CALB lipase on a hydrophobic acrylic resin carrier with a specified activity of 10,000 PLU/g, was generously provided by Novozymes Spain S.A. 2-methylhexanoic acid (≥99%) and 2-octyl-1-dodecanol (97%) were procured from Sigma-Aldrich, St. Louis, MO, USA. All other reagents utilized in the experiment were of analytical reagent grade.
2.2. Biocatalytic Synthesis
2-Octyl-1-dodecanoyl-2-methylhexanoate was synthesized in a 50 mL jacketed open air batch reactor. All reactions were carried out in a solvent-free medium. A mixture of 2-methyl hexanoic acid and 2-octyl-1-dodecanol in stoichiometric molar ratio (1:1, acid/alcohol; total amount: 20 g) was introduced in the reactor and mixed with a vertical paddle stirrer (Ika Nanostar 7.5 digital, Barcelona, Spain) at 350 rpm. Amounts of 0.25, 0.5 and 1 g (concentrations of 1.25, 2.5 and 5% w/w regarding the substrates) of immobilized derivative and temperatures between 60 and 90 °C were tested. The progress of the reaction was monitored by measuring the acid value of several samples taken from the reactor during the esterification process.
The acid value (AV) is defined as the milligrams of potassium hydroxide required to neutralize the free acids present in 1 g of a sample [59]. The conversion can then be calculated as:
where is the acid value determined at the start of the reaction and the acid value of a sample taken at a given time. The results presented in this paper are the average of three different measurements and the errors were always less than 5%.
2.3. Biocatalyst Reusability
To evaluate biocatalysts’ reuse, different assays of ester synthesis were carried out under the best reaction conditions. After first use, the immobilized derivative was recovered and, without any additional washing, kept cold until the next experiment with new fresh reagents.
2.4. Characterization of the 2-Octyl-1-dodecanoyl 2-Methylhexanoate
The density and viscosity of the obtained ester were measured with a DMA 5000 M density meter/LOVIS 2000 M rolling-ball viscometer from Anton Paar, Madrid, Spain. Measurements were performed at two distinct temperatures, specifically 40 °C and 100 °C. The viscosity index (VI) was calculated according to the ASTM D2270 standard [60], which includes different methodologies if the viscosity index of the substance is lower or higher than 100.
2.5. Energy Consumption
The measurement of current intensity in real time was performed with an ammeter clamp to quantify energy costs.
Initially, it was acknowledged that the standard voltage at the equipment terminals was 220 V. Subsequently, the amount of energy required to maintain the temperature of the reactors through thermostatic baths was determined. To reach the working temperatures and homogenize the reaction mixture, the equipment must be operated continuously for 10 min initially. After this time, the thermostat enters maintenance mode, which reduces energy consumption. Additionally, the power requirements for the mixing devices remain constant and much lower over time.
3. Results and Discussion
3.1. Biocatalyst Amount Influence in the Reaction Evolution
Biocatalysts are employed to enhance the reaction rate without being consumed. Normally, increasing the quantity of the biocatalyst used results in a rise in the reaction rate. However, going overboard might be undesirable as the increase in reaction rate is no longer significant because of the emergence of diffusional restrictions, while the expensive cost associated with a biocatalyst has an adverse effect on the process’s economy [48].
Therefore, it is important to determine the optimal amount of biocatalyst that will provide an adequate reaction rate. With this objective, the esterification reaction was carried out with different amounts of biocatalyst (0.25, 0.5 and 1 g corresponding to concentrations of 1.25, 2.5 and 5% w/w regarding the substrates), at a temperature of 80 °C, following the procedure described in Section 2.2.
The results obtained are depicted in Figure 2, illustrating the conversion’s progression over time during the reaction. The graph illustrates that elevating the concentration of lipase from 1.5 to 2.5% (w/w) produced an increase in conversion from 13.50 to 27.12% during the initial stage of operation (1 h). Such a rise indicated that the reaction speed had doubled. Conversely, raising the concentration of biocatalyst from 2.5 to 5% (w/w) prompted a rate increase of only 1.5 times. The difference was more pronounced when total reaction times were examined. The experiments carried out at higher lipase concentrations achieved conversions of over 95% after just five hours of reaction, while at a concentration of 1.5%, conversion times of over eight hours were required.
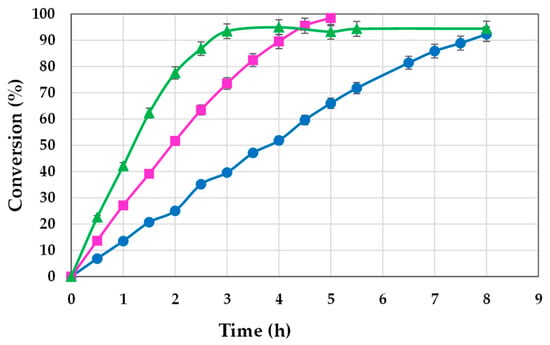
Figure 2.
Influence of lipase concentration on the synthesis of 2-octyl-1-dodecanoyl 2-methylhexanoate at 80 °C (● 1.5%, ■ 2.5%, ▲ 5.0%).
Derived from the obtained results, the optimal concentration of lipase is 2.5% (w/w), as any higher amounts do not enhance the process in terms of conversion or time but rather increase the cost associated with the biocatalyst.
This outcome aligns with findings from other investigations that utilized lipase quantities equivalent to or below 2.5% (w/w) [31,38,42,48,50,54] or moderately exceeding this amount [33,44,46,47,51].
3.2. Temperature’s Influence on the Reaction Evolution
It is widely acknowledged that temperature has a dual significance in this category of reactive systems. On the one hand, it enhances the reaction rate while lowering the viscosity of the mixture, raises solubility, and improves substrate diffusion, which together decrease mass transfer restrictions and facilitate interactions between enzyme particles and substrates [36]. On the other hand, high temperatures can result in the denaturation of the lipase, leading to a loss of activity and stability [61]. Therefore, enzymes typically have an optimal temperature range for maximum efficiency, such as in the case of free CALB lipase, which operates optimally at 50–60 °C [62]. However, immobilization has been shown to increase lipase stability and extend the optimal temperature range to 90 °C [47].
To establish the optimal temperature for Lipozyme® 435 in the synthesis under study, experiments were conducted at diverse temperatures, ranging from 60 to 90 °C. The procedure outlined in Section 2.2 was followed, and the optimal quantity of biocatalyst determined above, 2.5% (w/w), was used. The obtained results are illustrated in Figure 3.
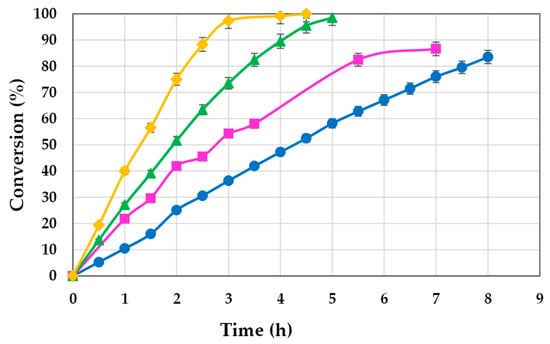
Figure 3.
Influence of temperature on the synthesis of 2-octyl-1-dodecanoyl 2-methylhexanoate with 2.5% of lipase (● 60 °C, ■ 70 °C, ▲ 80 °C, ♦ 90 °C).
It is evident from Figure 3 that an increase in the operating temperature enhances the reaction progression in relation to both the initial rate and the final conversion achieved. At 80 °C, the operation for five hours yielded a conversion of 98.51%, whereas at 90 °C, similar conversion (99.20%) took place in only 4 h. The experiments conducted at 60 and 70 °C exhibited a significantly reduced rate, with markedly lower final conversions attained at longer times (86.66% in 7 h at 70 °C and 79.59% in 7.5 h at 60 °C).
In principle, the optimum temperature could be deemed as 90 °C, owing to the marked reduction in operating times in comparison to the other temperatures tested, which would supposedly improve the profitability of the process. However, the cost savings related to shorter operating times may fail to be offset by the increased energy cost incurred at 90 °C. A detailed cost analysis is therefore necessary to determine the optimum process temperature accurately.
3.3. Optimum Temperature Determination Based on Preliminary Economic Study
As stated previously, to identify the optimum operating temperature, it is essential to conduct an initial economic analysis that considers the expenses linked to energy consumption and those related to substrate and biocatalyst consumption. The examination was conducted based on the production of one kilogram of the final product.
The cost related to substrate consumption was computed using the prices from the supplier, who offers them in bulk with a minimum purchase of 1 kg. Novozymes, the company donating the biocatalyst, was contacted to ascertain its price via internal communication. The energy cost was calculated by measuring the actual energy consumed by the equipment employed (thermostatic bath and overhead stirrer) in accordance with Section 2.5. Furthermore, the energy price of 0.2336 EUR/kWh (the mean value for the latter half of 2022 in Spain) was taken into consideration.
Table 1 presents the expenses associated with each item, and the last row summarizes the total direct production costs for each process.

Table 1.
Substrates, biocatalyst, energy, and total direct costs in the synthesis of 2-octyl-1-dodecanoyl 2-methylhexanoate at 80 and 90 °C.
Since the different trials analyzed used equivalent amounts of substrates and lipase, the expenses related to their use were identical, and the distinctions should be evident in the energy costs. In the analysis of the latter, it was noted that the energy cost was the same for both temperatures in the initial heating phase, but there were disparities during the maintenance stage. However, these differences are not significant enough to make a clear distinction between the total costs (see Table 1). Therefore, based on the results of the economic study, either of the two tested temperatures could be considered optimal.
Finally, a temperature of 80 °C was selected as the optimum, since it is anticipated to result in a higher operational stability of the lipase, as has been reported in the literature [38,46]. This facilitates the potential reutilization of the lipase in subsequent reaction cycles and opens up the possibility of increasing the economic profitability of the process, since, as shown in Figure 4, the expense of lipase consumption is the cost with the most substantial impact on the overall expenditure.
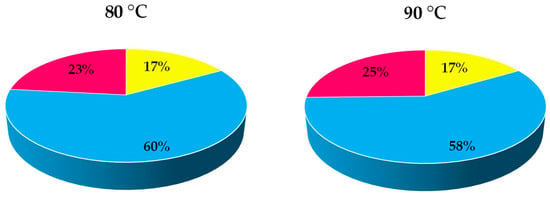
Figure 4.
Contribution of substrates (■), biocatalyst (■), and energy (■) cost in the total direct production cost of 2-octyl-1-dodecanoyl 2-methylhexanoate at 80 and 90 °C.
3.4. The Influence of Biocatalyst Reutilization in the Process Economic Profitability
As stated previously, the cost associated with biocatalyst consumption has the greatest impact on the total direct cost. The most effective way to reduce this cost is to separate and reuse the lipase in successive reaction cycles. This section outlines the study conducted to evaluate the effectiveness of separation and to assess the operational stability of the lipase after consecutive recoveries.
After first use, the immobilized derivative was recovered and, without any additional washing, kept cold until the next experiment with new fresh substrates. Successive assays were conducted as described in Section 2.2 with the optimum temperature (80 °C) and biocatalyst concentration (2.5% w/w). The obtained results are displayed in Figure 5, which illustrates the percentage ratio of conversion achieved after four hours of reaction for each subsequent reuse compared to the initial use.
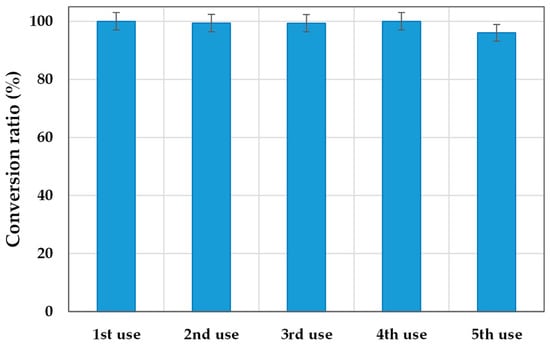
Figure 5.
Reusability of the biocatalyst Lypozyme® 435 under the best operational conditions: 2.5% w/w, 80 °C, and 5 h of reaction in each use.
As can be seen, the figure shows that the biocatalyst retained 96.03% of its initial activity after five consecutive reuses. This significantly increases the productivity of the lipase from 37.9 to 187.29 kg product/kg biocatalyst when reused.
After verifying the feasibility of reusing the lipase for at least five consecutive uses without any significant loss of activity, an economic analysis was conducted, as described in the previous section, to determine the impact of reuse on the process’s profitability. The results obtained are summarized in Table 2.

Table 2.
Substrates, biocatalyst, energy, and total direct costs in the synthesis of 2-octyl-1-dodecanoyl 2-methylhexanoate with lipase reutilization (after 5 uses), at optimal operational conditions (80 °C, 2.5% biocatalyst concentration (w/w)).
When comparing these results to those in Table 1 for the single-use lipase assay at 80 °C, a significant reduction in total cost is observed. The cost decreased by 46.34% from 70.70 to 37.94 EUR/kg product when reusing lipase five times.
Figure 6 illustrates the distribution of substrate, lipase, and energy costs in the final cost and how they vary with lipase reuse. When the biocatalyst is used only once, its cost has the greatest impact on the total cost, with the influence of energy cost being minimal. However, when the lipase is reused, the cost of the biocatalyst decreases and the cost of energy becomes more significant, as it remains a fixed cost associated with the operating time. This study examines the consecutive use of lipase up to five times, but based on the results, it is anticipated that additional consecutive reuses can be performed without a significant loss of activity, resulting in a further reduction in total costs.
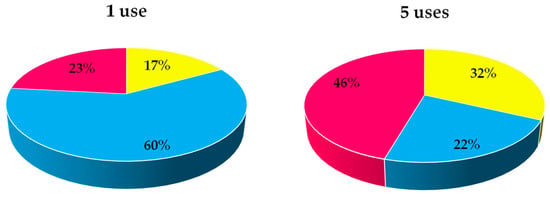
Figure 6.
Influence of lipase reutilization on the distribution of substrates (■), biocatalyst (■), and energy (■) cost in the total direct cost production of 2-octyl-1-dodecanoyl 2-methylhexanoate at 80.
3.5. Analysis of Process Sustainability by Measurements of Green Metrics
When developing a process, it is essential to ensure both its economic viability and environmental sustainability. Biocatalytic processes are often considered environmentally sustainable as they comply with 10 out of the 12 principles of “Green Chemistry” [63]. The catalyst, which is an enzyme, is produced from available renewable resources and is biodegradable, nonhazardous, and nontoxic. Enzymatic reactions are usually conducted in water at ambient temperature and atmospheric pressure, without the need for functional-group activation, protection, and deprotection steps. This leads to more step-economical routes that generate less waste than conventional organic syntheses. Therefore, biocatalytic methods are more environmentally friendly, cost-effective, and sustainable [63].
However, the above considerations provide only qualitative information on the sustainability of a process. To accurately report on the environmental viability of a process, it is necessary to establish quantitative measures. This quantitative information is obtained from the determination of sustainability indicators. A large number of “green metrics” are described in the literature [56,64,65,66,67], with atom economy (AE), E factor (EF), complete E factor (cEF), carbon mass efficiency (CME), and process mass intensity (PMI) being considered very suitable for measuring the sustainability of biocatalytic processes [48,50,51,54,63].
Table 3 shows the different sustainability indices calculated and their corresponding values.

Table 3.
Green metrics determined to analyze biocatalytic synthesis sustainability of 2-octyl-1-dodecanoyl 2-methylhexanoate after five uses of the enzymes.
The AE value of 95.63% indicates that the substrates were mostly incorporated into the desired product with minimal production of side products. This demonstrates a high mass yield and low waste production process. Values close to zero for both E factors (EF 0.0375 and cEF 0.083) indicate that the process generates very little waste and is therefore environmentally friendly.
In addition, the value calculated for the CME (94.75%) highlights the substantial percentage of carbon from the reagents retained in the final product, minimizing the likelihood of it ending up in a waste stream or as emissions. The PMI value (1.082) shows a high mass yield, which is not 100% due to water generation. Both metrics imply a high level of sustainability for the process.
Overall, the values obtained for the different indicators are as expected for a biocatalytic process carried out without solvents and final purification steps and are better than those reported in the literature [56,57,58,61,62,63,64,65,66,67,68,69,70]. It can be concluded that the developed process is highly environmentally sustainable as it occurs with high substrate utilization and low waste and emissions generation.
3.6. The Characterization of 2-Octyl-1-dodecanoyl 2-Methylhexanoate as a Biolubricant
The viscosity index is a key factor in determining a substance’s lubricant performance. It measures the rate of viscosity change due to temperature, with higher values indicating lower viscosity changes. Viscosity itself refers to a lubricant’s resistance to flow and shear, which can vary with temperature. The value of the viscosity index was determined using ASTM D2270 [60], which involves measuring the density, dynamic viscosity, and kinematic viscosity at 40 and 100 °C. The ASTM standard provides different methodologies depending on whether the viscosity index of the substance is below or above 100. Furthermore, it should be noted that the aforementioned procedure is only applicable when the compound has a kinematic viscosity greater than 2 mm2/s at 100 °C.
Table 4 shows the results obtained for the product synthesized (0.985 purity) in a reaction developed at optimal conditions with a 0.985.

Table 4.
Values of density, dynamic viscosity, kinematic viscosity at 40 and 100 °C and viscosity index for the product obtained at optimal operation conditions (0.985 purity).
Upon comparing the viscosity index of the ester under study with those of other biolubricants described in the consulted bibliography [11,12,13,16,17,21,26,29,30], it is evident that the value calculated in this work (204.43) falls within the range of those found in the studies of other researchers (above 150). Therefore, it can be concluded that this ester is good enough for use as a biolubricant because it maintains its viscosity over a broad temperature range, ensuring consistent performance and protection against wear and tear, even in extreme conditions. Moreover, high-viscosity-index biolubricants reduce friction and improve the overall efficiency of hydraulic systems. This can lead to energy savings, especially in heavy machinery where hydraulic systems are a significant source of power consumption.
In addition to its good lubricating properties, and according to the literature [6,7,8], this ester also exhibits high oxidative stability owing to its saturated chains and has good cold flow properties due to the presence of branches in both the acid and alcohol moieties.
4. Conclusions
This work reports the successful synthesis of a new second-generation biolubricant through a lipase-catalyzed reaction between branched acid and alcohol. The enzymatic esterification process was optimized, achieving up to 95% conversion under optimal operating conditions (a stoichiometric molar ratio of 1:1 acid to alcohol, 80 °C, and 2.5% biocatalyst concentration (w/w)). Operational lipase stability was demonstrated after five consecutive reuses as the biocatalyst retained 96.03% of its initial activity. This led to a significant increase in lipase productivity, from 37.9 to 187.29 kg product/kg biocatalyst when reused. Studies on economic and environmental sustainability have shown that the process is both profitable and sustainable; therefore, it could be scaled up for further production. The hyperbranched ester obtained has a high viscosity index (above 200), ensuring good lubricating properties, and a structure that gives it high oxidative stability. Therefore, it can be used as a second-generation biolubricant.
Author Contributions
Conceptualization, M.C.M., F.M. and J.B.; methodology, M.C.M., M.G., M.D.M., S.O.-R., F.M. and J.B.; validation, M.G., M.D.M. and S.O.-R.; formal analysis, M.C.M., M.G., M.D.M. and S.O.-R., F.M. and J.B.; investigation, M.G. and M.D.M.; resources, F.M. and J.B.; data curation, F.M. and M.D.M.; writing—original draft preparation, M.C.M. and M.G.; writing—review and editing, M.D.M. and S.O.-R.; visualization, M.C.M., M.G. and M.D.M.; supervision, F.M. and J.B.; project administration, J.B.; funding acquisition, F.M. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded with support from RTI 2018-094908-B-I00 MCIN/AEI/10.13039/501100011033/ and ERDF “A way of making Europe”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to acknowledge Ramiro Martínez Gutiérrez (Novozymes España S.A.) who kindly provided the biocatalysts. We also wish to thank José Ginés Hernandez Cifre (University of Murcia) for his collaboration in carrying out the density and viscosity measurements and providing the necessary equipment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Waykole, C.S.; Malib, S.N.; Mahalea, D.D.; Pratapa, A.P. Guerbet alcohol esters: Practical synthesis and applications. J. Indian Chem. Soc. 2022, 99, 100304. [Google Scholar] [CrossRef]
- Ribeiro Filho, P.R.C.F.; do Nascimiento, M.R.; Cavalcante, C.L.; de Luna, F.M.T. Synthesis and tribological properties of bio-based lubricants from soybean oil. Biomass Conv. Bioref. 2023, May, 1–13. [Google Scholar] [CrossRef]
- Attia, N.K.; El-Mekkawi, S.A.; Elardy, O.A.; Abdelkader, E.A. Chemical and rheological assessment of produced biolubricants from different vegetable oils. Fuel 2020, 271, 117578. [Google Scholar] [CrossRef]
- Waled, A.A.; Salih, N.; Salimon, J. Synthesis, characterization, tribological and rheological properties of di (2-butyloctyl) dicarboxylate esters for environmentally friendly biolubricant applications. Biointerface Res. Appl. Chem. 2023, 13, 278. [Google Scholar] [CrossRef]
- Carvalho, W.C.A.; Luiz, J.H.H.; Fernandez-Lafuente, R.; Hirata, D.B.; Mendes, A.A. Eco-friendly production of trimethylolpropane triesters from refined and used soybean cooking oils using an immobilized low-cost lipase (Eversa>® Transform 2.0) as heterogeneous catalyst. Biomass Bioenerg. 2021, 155, 106302. [Google Scholar] [CrossRef]
- García-Colomer, A. Diseño, Selección y Producción de Nuevos Biolubricantes. Ph.D. Thesis, Universidad Ramón Llul, Barcelona, Spain, 3 November 2011. [Google Scholar]
- Zhou, F.; Yang, K.; Li, D.; Shi, X. Acid number prediction model of lubricating oil based on mid-infrared spectroscopy. Lubricants 2022, 10, 205. [Google Scholar] [CrossRef]
- O’Lenick, A.J., Jr. Guerbet chemistry. J. Surfactants. Deterg. 2001, 4, 311–315. [Google Scholar] [CrossRef]
- Appiah, G.; Tulashie, S.K.; Akpari, E.E.A.; Rene, E.R.; Dodoo, D. Biolubricant production via esterification and transesterification processes: Current updates and perspectives. Int. J. Energy Res. 2022, 46, 3860–3890. [Google Scholar] [CrossRef]
- Anastas, P.; Zimmerman, J.B. Sustainability requires objectives at the molecular, product, process, and system levels. Environ. Sci. Technol. 2003, 37, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Afifah, A.N.; Syahrullail, S.; Wan Azlee, N.I.; Sidik, N.A.C.; Yahya, W.J.; Rahim, E.A. Biolubricant production from palm stearin through enzymatic transesterification method. Biochem. Eng. J. 2019, 148, 178–184. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Cavalcanti, E.D.C.; da Silva, P.R.; Soares, V.F.; Fernandez-Lafuente, R.; Assunção, C.L.B.; da Silva, J.A.C.; Freire, D.M.G. Enzymatic synthesis of neopentyl glycol-bases biolubricants using biodiesel from soybean and castor bean as raw materials. Renew. Energ. 2020, 148, 689–696. [Google Scholar] [CrossRef]
- de Araujo-Silva, R.; Vieira, A.C.; de Campos Giordano, R.; Fernandez-Lafuente, R.; Tardioli, P.W. Enzymatic synthesis of fatty acid isoamyl monoesters from soybean oil deodorizer distillate: A renewable and ecofriendly base stock for lubricant industries. Molecules 2022, 27, 2692. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.S.; Freire, C.C.C.; Brandão, L.M.S.; Pereira, E.B.; Mendes, A.A.; Pereira, M.M.; Lima, A.S.; Soares, C.M.F. Biolubricant production under zero-waste Moringa oleifera Lam biorefinery approach for boosting circular economy. Ind. Crops Prod. 2021, 167, 113542. [Google Scholar] [CrossRef]
- Cavalcanti, E.D.C.; Aguieiras, E.C.G.; da Silva, P.R.; Duarte, J.G.; Cipolattia, E.P.; Fernandez-Lafuente, R.; da Silva, J.A.C.; Freire, D.M.G. Improved production of biolubricants from soybean oil and different polyols via esterification reaction catalyzed by immobilized lipase from Candida rugosa. Fuel 2018, 215, 705–713. [Google Scholar] [CrossRef]
- Cerón, A.A.; Vilas-Bôas, R.N.; Biaggio, F.C.; de Castro, H.F. Synthesis of biolubricant by transesterification of palm kernel oil with simulated fusel oil: Batch and continuous processes. Biomass Bioenerg. 2018, 119, 166–172. [Google Scholar] [CrossRef]
- Da Silva, A.P.T.; Breddab, E.H.; de Castro, H.F.; da Rósa, P.C.M. Enzymatic catalysis: An environmentally friendly method to enhance the transesterification of microalgal oil with fusel oil for production of fatty acid esters with potential application as biolubricants. Fuel 2020, 273, 117786. [Google Scholar] [CrossRef]
- Fernandes, K.V.; Papadaki, A.; da Silva, J.A.C.; Fernandez-Lafuente, R.; Koutinas, A.A.; Freire, D.M.G. Enzymatic esterification of palm fatty-acid distillate for the production of polyol esters with biolubricant properties. Ind. Crops Prod. 2018, 116, 90–96. [Google Scholar] [CrossRef]
- Fernandes, K.V.; Cavalcanti, E.D.C.; Cipolatti, E.P.; Aguieiras, E.C.G.; Pinto, M.C.C.; Tavares, F.A.; da Silva, P.R.; Lafuente-Fernandez, R.; Arana-Peña, S.; Pinto, J.C.; et al. Enzymatic synthesis of biolubricants from by-product of soybean oil processing catalyzed by different biocatalysts of Candida rugosa lipase. Catal. Today 2021, 362, 122–129. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Miranda, L.P.; Fernandez-Lafuente, R.; Tardioli, P.W. Immobilization of Eversa® transform via CLEA technology converts it in a suitable biocatalyst for biolubricant production using waste cooking oil. Molecules 2021, 26, 193. [Google Scholar] [CrossRef]
- Guedes Júnior, J.G.E.; Mattos, F.R.; Sabi, G.J.; Carvalho, W.C.A.; Luiz, J.H.H.; Cren, C.A.; Fernandez-Lafuente, R.; Mendes, A.A. Design of a sustainable process for enzymatic production of ethylene glycol diesters via hydroesterification of used soybean cooking oil. J. Environ. Chem. Eng. 2022, 10, 107062. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, B.H.; Kim, Y.; Lee, M.W.; Im, D.J.; Kim, I.H. Lipase-mediated synthesis of neopentyl glycol diester using a combination of reduced and standard pressure. J. Am. Oil Chem. Soc. 2021, 98, 1001–1007. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Song, Z.; Yu, K.; He, C.; Zhang, X. Enzyme-catalyzed synthesis and properties of polyol ester biolubricant produced from Rhodotorula glutinis lipid. Biochem. Eng. J. 2021, 173, 108101. [Google Scholar] [CrossRef]
- Papadaki, A.; Fernandes, K.V.; Chatzifragkou, A.; Aguieiras, E.C.G.; da Silva, J.A.C.; Fernandez-Lafuente, R.; Papanikolaou, S.; Koutinas, A.; Freire, D.M.G. Bioprocess development for biolubricant production using microbial oil derived via fermentation from confectionery industry wastes. Bioresour. Technol. 2018, 267, 311–318. [Google Scholar] [CrossRef]
- Sabi, G.J.; Gama, R.S.; Fernandez-Lafuente, R.; Cancino-Bernardi, J.; Mendes, A.A. Decyl esters production from soybean-based oils catalyzed by lipase immobilized on differently functionalized rice husk silica and their characterization as potential biolubricants. Enzym. Microb. Technol. 2022, 157, 110019. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M.; Cirillo, C. Optimized procedure for the preparation of an enzymatic nanocatalyst to produce a bio-lubricant from waste cooking oil. Chem. Eng. J. 2019, 377, 120273. [Google Scholar] [CrossRef]
- Unugul, T.; Kutluk, T.; Kutluk, B.G.; Kapucu, N. Environmentally friendly processes from coffee wastes to trimethylolpropane esters to be considered biolubricants. J. Air Waste Manag. Assoc. 2020, 70, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Bôas, R.N.V.; de Lima, R.; Mendes, A.A.; Freitas, L.; Bento, H.B.S.; de Carvalho, A.K.F.; de Castro, H.F. Batch and continuous production of biolubricant from fusel oil and oleic acid: Lipase screening, reactor system development, and reaction optimization. Chem. Eng. Process. 2021, 168, 108568. [Google Scholar] [CrossRef]
- Wafti, N.S.A.; Yunus, R.; Lau, H.L.N.; Yaw, T.C.S.; Aziz, S.A. Immobilized lipase-catalyzed transesterification for synthesis of biolubricant from palm oil methyl ester and trimethylolpropane. Bioprocess Biosyst Eng. 2021, 44, 2429–2444. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, H.; Song, Y.; Ma, S.; Xiong, W.; Chen, C.; Chen, B.; Zhang, X. Green preparation of branched biolubricant by chemically modifying waste cooking oil with lipase and ionic liquid. J. Clean. Prod. 2020, 274, 122918. [Google Scholar] [CrossRef]
- Gryglewicz, S. Enzyme catalysed synthesis of some adipic esters. J. Mol. Catal. B Enzym. 2001, 15, 9–13. [Google Scholar] [CrossRef]
- He, X.L.; Chen, B.Q.; Tan, T.W. Enzymatic synthesis of 2-ethylhexyl esters of fatty acids by immobilized lipase from Candida sp. 99–125. J. Mol. Catal. B Enzym. 2002, 18, 333–339. [Google Scholar] [CrossRef]
- Gryglewicz, S. Lipase catalysed synthesis of sebacic and phthalic esters. Enzym. Microb. Technol. 2003, 33, 952–957. [Google Scholar] [CrossRef]
- Tan, T.; Chen, B.Q.; Ye, H. Enzymatic synthesis of 2-ethylhexyl palmitate by lipase immobilized on fabric membranes in the batch reactor. Biochem. Eng. J. 2006, 29, 41–45. [Google Scholar] [CrossRef]
- Verma, M.; Chauhan, G.; Kanwar, S. Purification and characterization of a low molecular mass alkaliphilic lipase of Bacillus cereus MTCC 8372. Acta Microbiol. Immunol. Hung. 2008, 55, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Richetti, A.; Leite, S.G.F.; Antunes, O.A.C.; Lerin, L.A.; Dallago, R.M.; Emmerich, D.; di Luccio, M.; Oliveira, J.V.; Treichel, H.; de Oliveira, D. Assessment of process variables on 2-ethylhexyl palmitate production using Novozym 435 as catalyst in a solvent-free system. Bioprocess Biosyst. Eng. 2010, 33, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Richetti, A.; Leite, S.G.F.; Antunes, O.A.C.; de Souza, A.L.F.; Lerin, L.A.; Dallago, R.M.; Paroul, N.; di Luccio, M.; Oliveira, J.V.; Treichel, H.; et al. Optimization of 2-ethylhexyl palmitate production using lipozyme RM IM as catalyst in a solvent-free system. Appl. Biochem. Biotechnol. 2010, 260, 2498–2508. [Google Scholar] [CrossRef] [PubMed]
- Åkerman, C.O.; Hagström, A.E.V.; Mollaahmad, M.A.; Karlsson, S.; Hatti-Kaul, R. Biolubricant synthesis using immobilised lipase: Process optimisation of trimethylolpropane oleate production. Process Biochem. 2011, 46, 2225–2231. [Google Scholar] [CrossRef]
- Brenneis, R.; Baeck, B. Esterification of fatty acids using Candida antarctica lipase A in water-abundant systems. Biotechnol Lett. 2012, 34, 1459–1463, Epub 18 April 2012PMID: 22526422. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, B.; Liu, L.; Tan, T. Synthesis of trimethylolpropane esters with immobilized lipase from Candida sp. 99–125. J. Mol. Catal. B Enzym. 2012, 74, 151–155. [Google Scholar] [CrossRef]
- Tao, Y.; Cui, C.; Shen, H.; Liu, L.; Chen, B.; Tan, T. Enhancing trimethylolpropane esters synthesis through lipase immobilized on surface hydrophobic modified support and appropriate substrate feeding methods. Enzym. Microb. Technol. 2014, 58–59, 60–67. [Google Scholar] [CrossRef]
- Trivedi, J.; Aila, M.; Sharma, C.D.; Gupta, P.; Kaul, S. Clean synthesis of biolubricant range esters using novel liquid lipase enzyme in solvent free medium. SpringerPlus 2015, 4, 165. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Zhang, Y.; Zheng, L.; Huang, H.; Wang, Z. Synthesis of 2-ethylhexyl palmitate catalyzed by enzyme under microwave. Appl. Biochem. Biotechnol. 2018, 185, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, T.; Choi, N.; Kim, B.H.; Oh, S.W.; Kim, I.H. Synthesis of diethylhexyl adipate by Candida antarctica lipase-catalyzed esterification. Process Biochem. 2019, 78, 58–62. [Google Scholar] [CrossRef]
- Lee, A.; Kim, H.; Choi, N.; Yoon, S.W.; Kim, Y.; Kim, H.R.; Kim, I.H. Preparation of diisononyl adipate in a solvent-free system via an immobilized lipase-catalyzed esterification. Enzym. Microb. Technol. 2019, 131, 109340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liang, F.; Hou, H.; Chen, Y.; Liu, X.; Zhu, X. Enzymatic synthesis of a polyol ester from levulinic acid and trimethylolpropane and its tribological behavior as potential biolubricant basestock. Polymers 2020, 12, 2256. [Google Scholar] [CrossRef]
- Murcia, M.D.; Serrano-Arnaldos, M.; Ortega-Requena, S.; Máximo, F.; Bastida, J.; Montiel, M.C. Optimization of a sustainable biocatalytic process for the synthesis of ethylhexyl fatty acids esters. Catal. Today 2020, 346, 98–105. [Google Scholar] [CrossRef]
- Serrano-Arnaldos, M.; Ortega-Requena, S.; Sánchez, J.A.; Hernández, A.; Montiel, M.C.; Máximo, F.; Bastida, J. Sustainable synthesis of branched-chain diesters. J. Biotechnol. 2021, 325, 91–99. [Google Scholar] [CrossRef]
- He, C.; Guo, Z.; Deng, Z.; Li, S.; Zhang, X. Enzyme-catalyzed preparation of polyol ester lubricants and performance research-based on pelargonic acid, oleic acid and trimethylolpropane. Biochem. Eng. J. 2022, 187, 108641. [Google Scholar] [CrossRef]
- Máximo, F.; Asensi, M.; Serrano-Arnaldos, M.; Ortega-Requena, S.; Montiel, C.; Bastida, J. Biocatalytic intensified process for the synthesis of neopentyl glycol dicaprylate/dicaprate. Sustain. Chem. Phar. 2022, 30, 100882. [Google Scholar] [CrossRef]
- Montiel, C.; Gimeno-Martos, S.; Ortega-Requena, S.; Serrano-Arnaldos, M.; Máximo, F.; Bastida, J. Green production of a high-value branched-chain diester: Optimization based on operating conditions and economic and sustainability criteria. Appl. Sci. 2023, 13, 6177. [Google Scholar] [CrossRef]
- Chen, H.C.; Kuo, C.H.; Chen, H.H.; Liu, Y.C.; Shieh, C.J. Optimization of enzymatic synthesis of cetyl 2-ethylhexanoate by Novozym® 435. J. Am. Oil Chem. Soc. 2011, 88, 1917–1923. [Google Scholar] [CrossRef]
- Ghaziaskar, H.S.; Daneshfar, A.; Calvo, L. Continuous esterification or dehydration in supercritical carbon dioxide. Green Chem. 2006, 8, 576–581. [Google Scholar] [CrossRef]
- Montiel, M.C.; Asensi, M.; Gimeno-Martos, S.; Máximo, F.; Bastida, J. Sustainable biocatalytic procedure for obtaining new branched acid esters. Materials 2021, 14, 6847. [Google Scholar] [CrossRef] [PubMed]
- Roschangar, F.; Sheldon, R.A.; Senanayake, C.H. Overcoming barriers to green chemistry in the pharmaceutical industry—The Green Aspiration Level TM concept. Green Chem. 2015, 17, 752. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Lund, C. Green metrics in pharmaceutical development. Curr. Opin. Green Sustain. Chem. 2022, 33, 100564. [Google Scholar] [CrossRef]
- de María, P.B. Biocatalysis, sustainability, and industrial applications: Show me the metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514. [Google Scholar] [CrossRef]
- ASTM D974-02e1; Standard Test Method for Acid and Base Number by Color-Indicator Titration. ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM D2270; Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 °C and 100 °C. ASTM International: West Conshohocken, PA, USA, 2016.
- Wang, X.; Chen, Y.; Ma, Y.; Jin, Q.; Wang, X. Lipozyme 435-catalyzed synthesis of eicosapentaenoyl ethanolamidein a solvent-free system. J. Mol. Catal. B Enzym. 2015, 122, 233–239. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Wang, T.; Geng, H.; Wang, L.; Jiang, L.; Elfalleh, W. Immobilized Candida antarctica lipase B (CALB) on functionalized MCM-41: Stability and catalysis of transesterification of soybean oil and phytosterol. Food Biosci. 2021, 40, 100906. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Hessel, V.; Escribà-Gelonch, M.; Bricout, J.; Tran, N.N.; Anastasopoulou, A.; Ferlin, F.; Valentini, F.; Lanari, D.; Vaccaro, L. Quantitative sustainability assessment of flow chemistry−from simple metrics to holistic assessment. ACS Sustain. Chem. Eng. 2021, 9, 9508–9540. [Google Scholar] [CrossRef]
- Lima-Ramos, J.; Tufvesson, P.; Woodley, J.M. Application of environmental and economic metrics to guide the development of biocatalytic processes. Green Process Synth. 2014, 3, 195–213. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of green chemistry and sustainability: Past, present, and future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor at 30: A passion for pollution prevention. Green Chem. 2023, 25, 1704–1728. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. The green metric evaluation and synthesis of diesel-blend compounds from biomass derived levulinic acid in supercritical carbon dioxide. Biomass Bioenerg. 2016, 84, 12–21. [Google Scholar] [CrossRef]
- Ni, Y.; Holtmann, D.; Hollmann, F. How green is biocatalysis? To calculate is to know. ChemCatChem 2014, 6, 930–943. [Google Scholar] [CrossRef]
- Martínez, A.; Mijangos, G.E.; Romero-Ibarra, I.C.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y.; Gutiérrez, S. A novel green one-pot synthesis of biodiesel from Ricinus communis seeds by basic heterogeneous catalysis. J. Clean. Prod. 2018, 196, 340–349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).