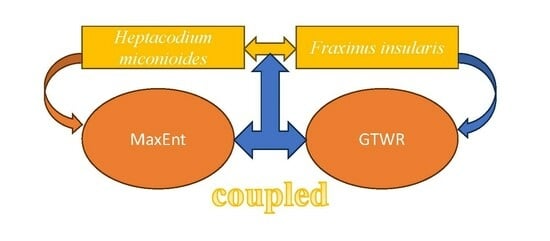
Regional Analysis of the Potential Distribution of Heptacodium miconioides and Its Competitor Species in China
Abstract
1. Introduction
2. Materials and Methods
2.1. ENMTools
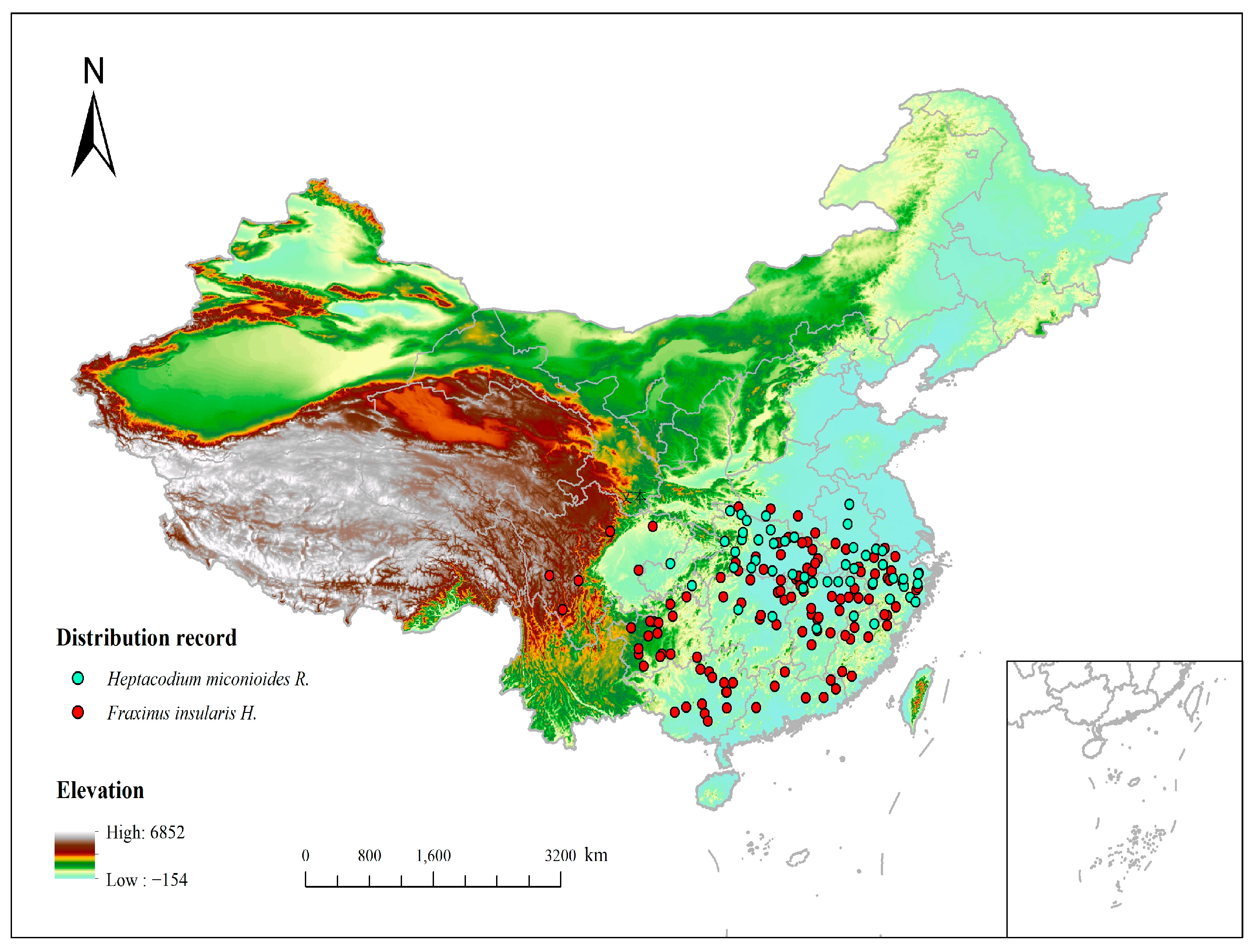
2.2. Species Distribution Data Sources and Processing
2.3. Environmental Data and Processing
2.4. MaxEnt Modeling
2.5. Ecological Niche Overlap and Geographic Distribution Overlap Analysis
2.6. GTWR Model
3. Results
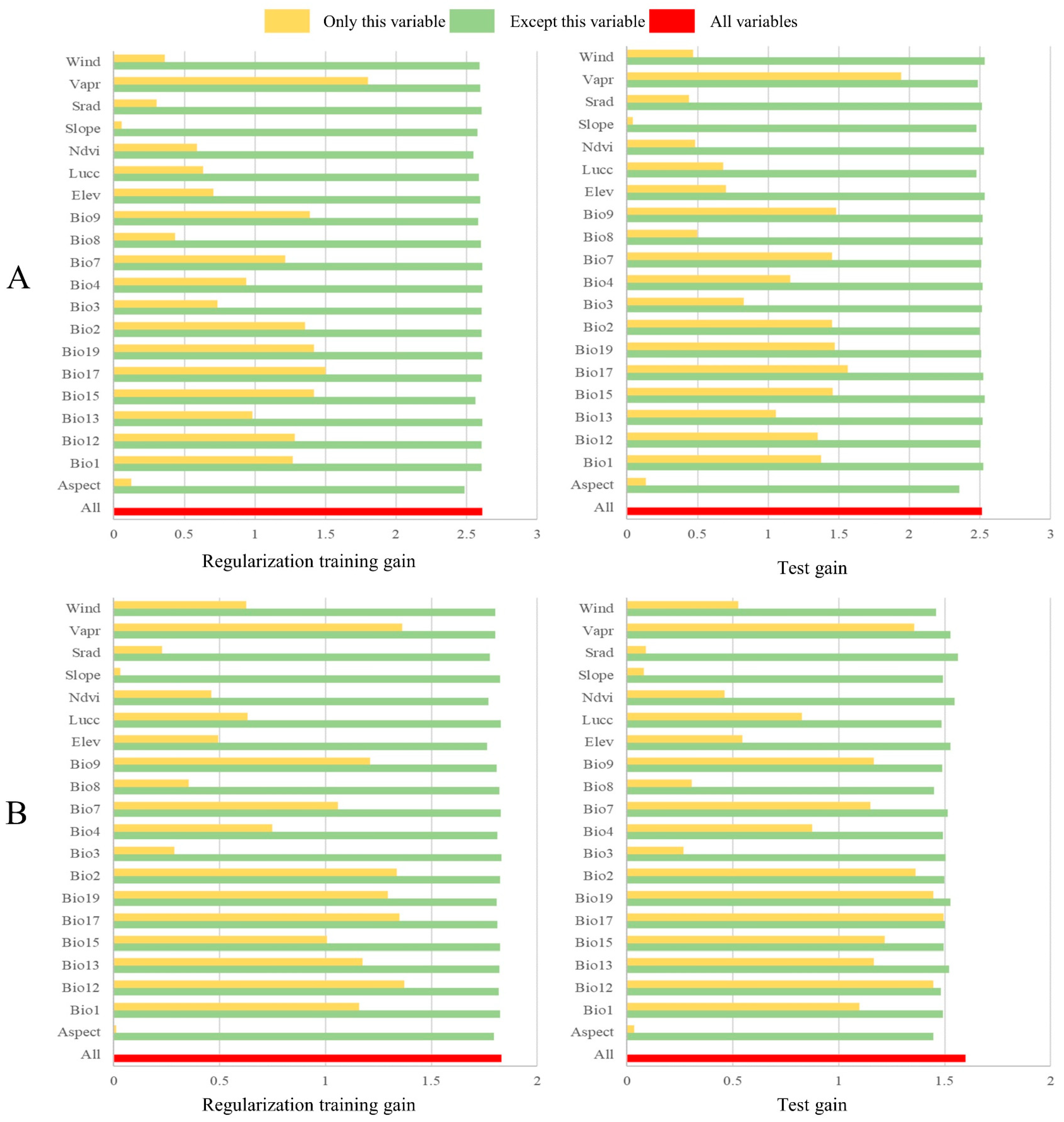
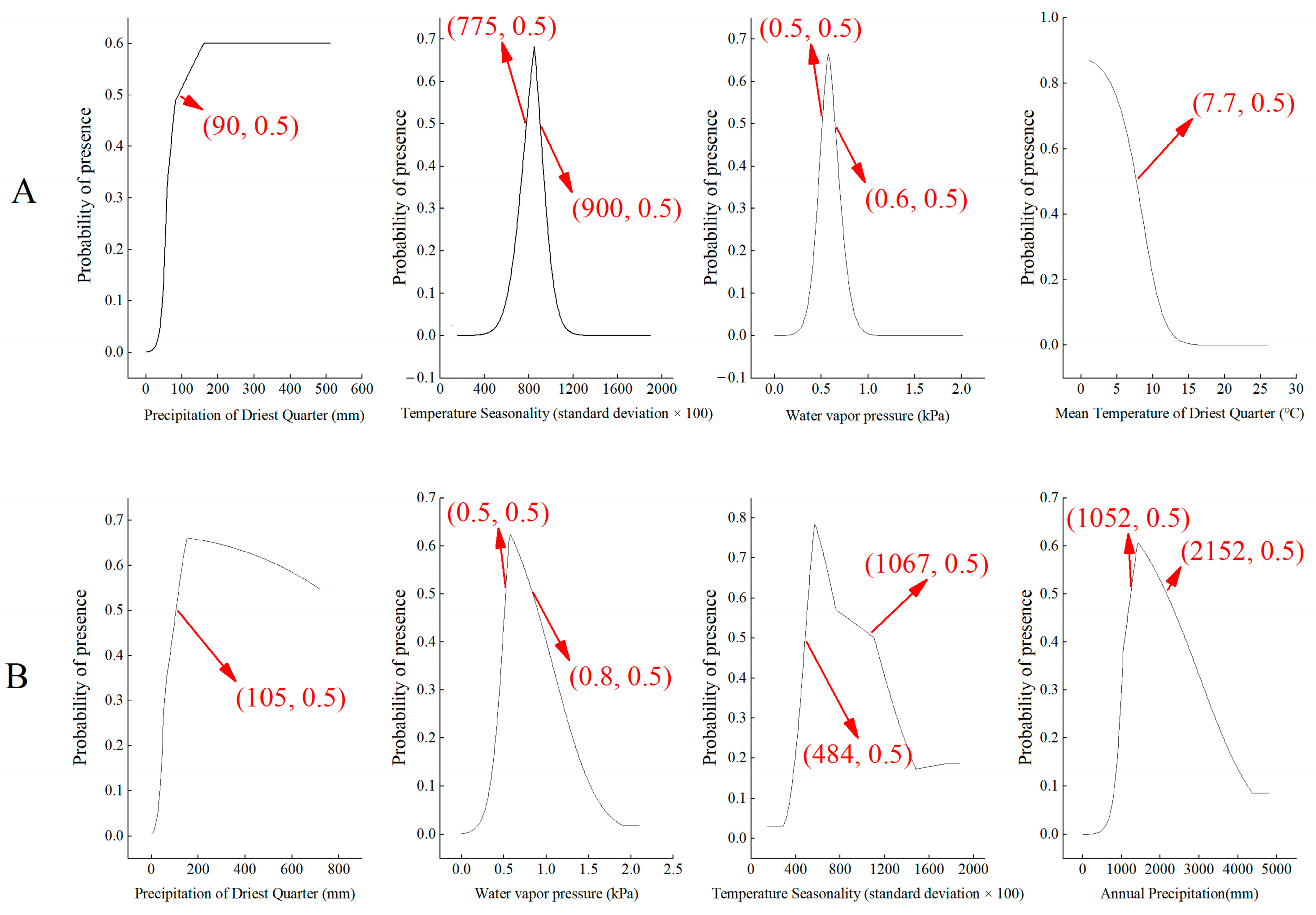
3.1. Environmental Characteristics of Suitable Areas
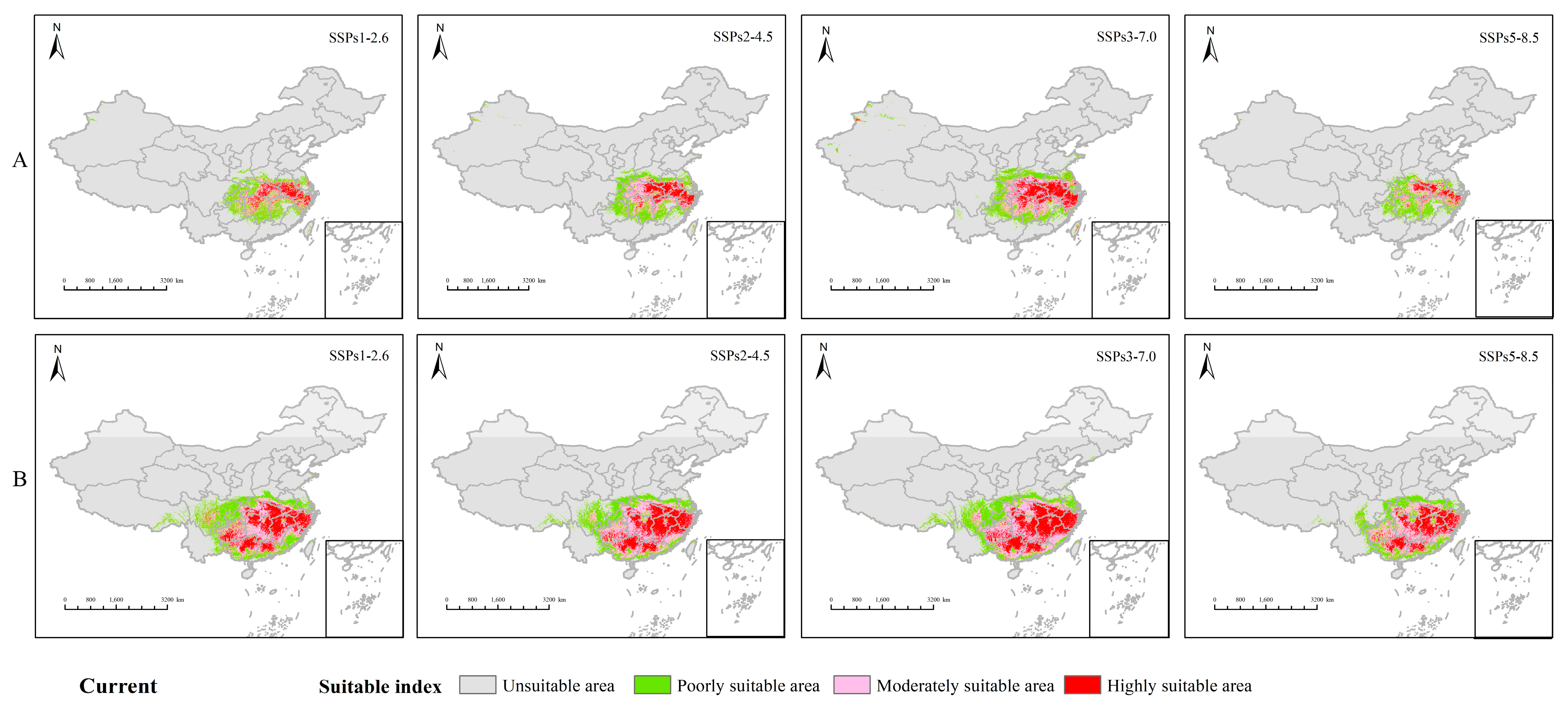
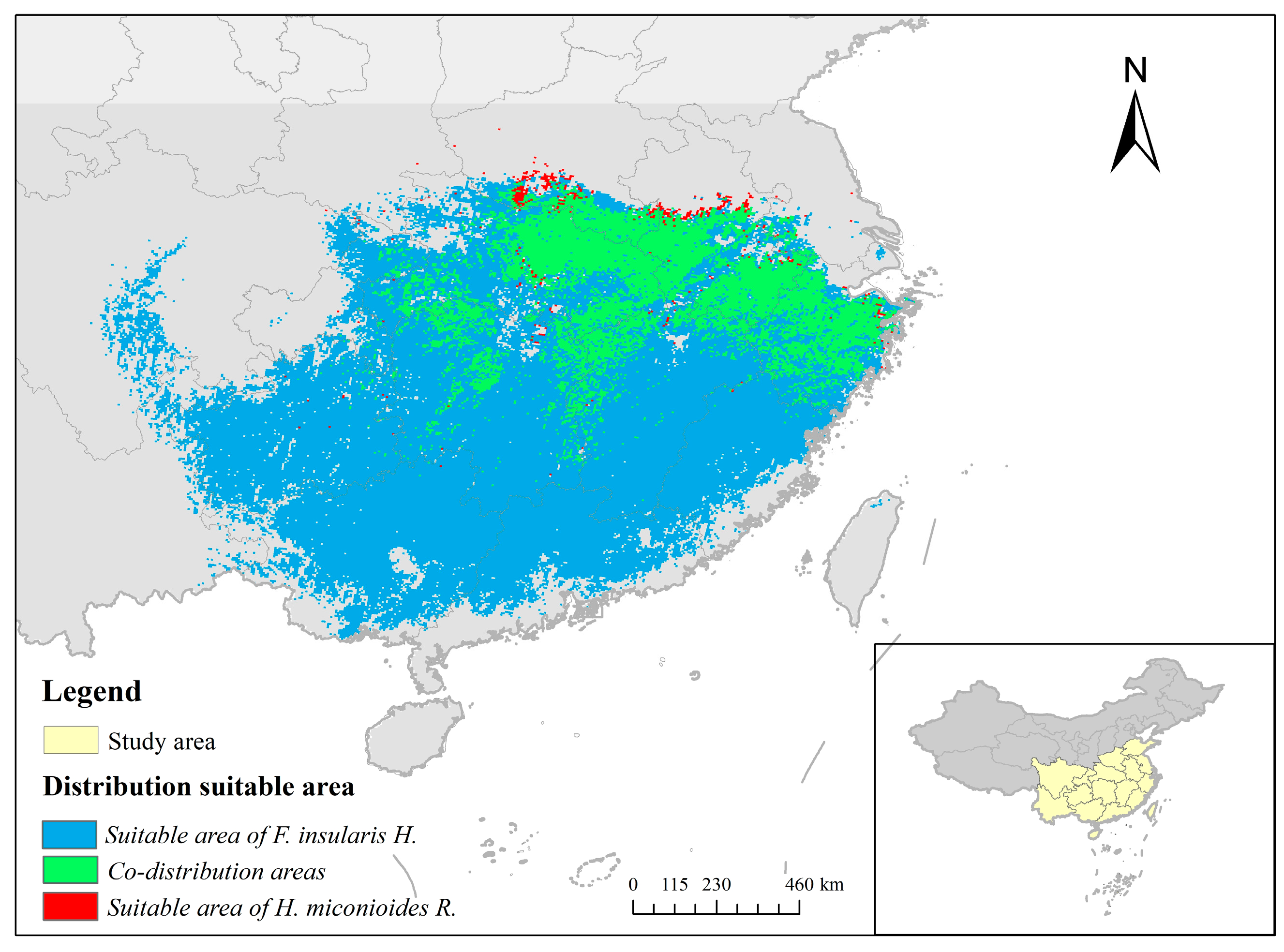
3.2. Analysis of the Distribution Pattern of H. miconioides and F. insularis under Current Environmental Patterns
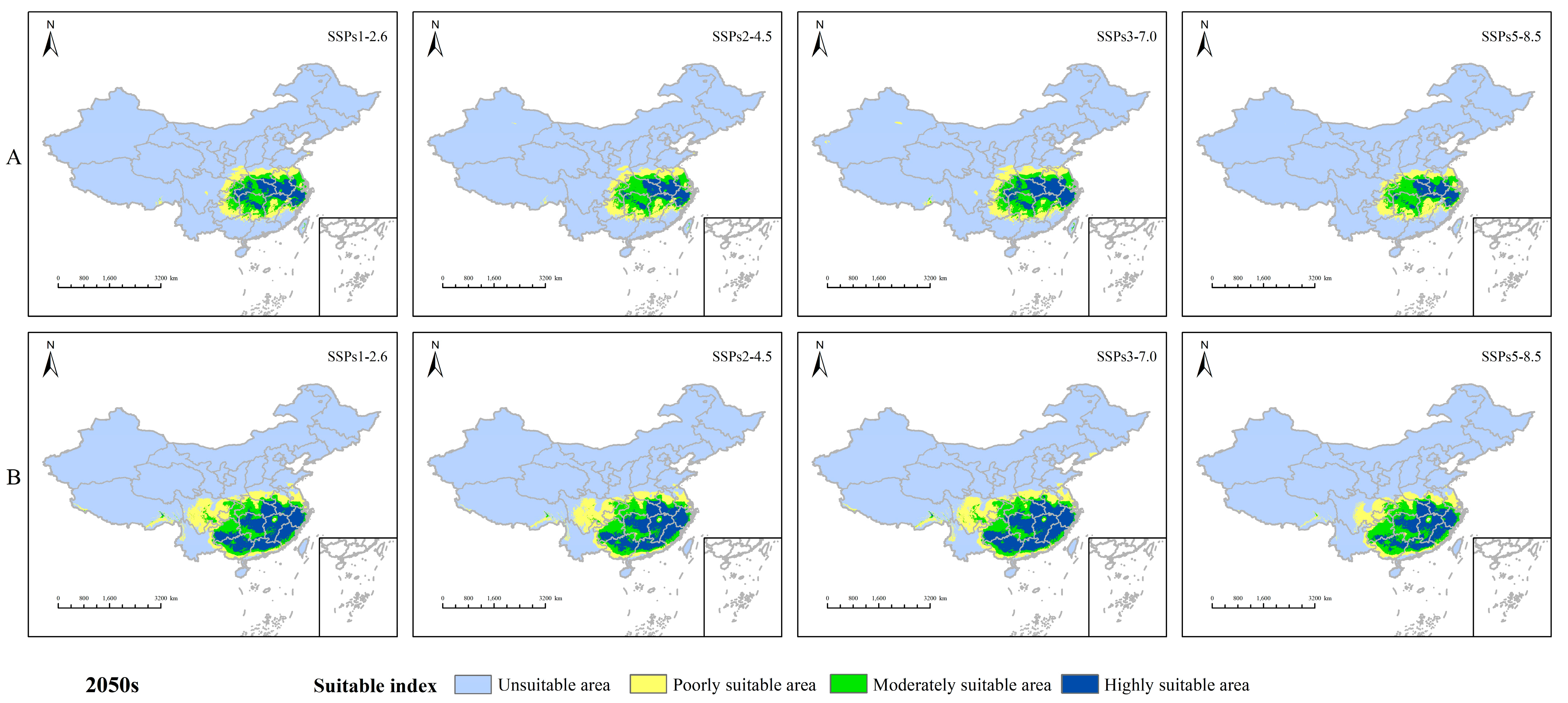
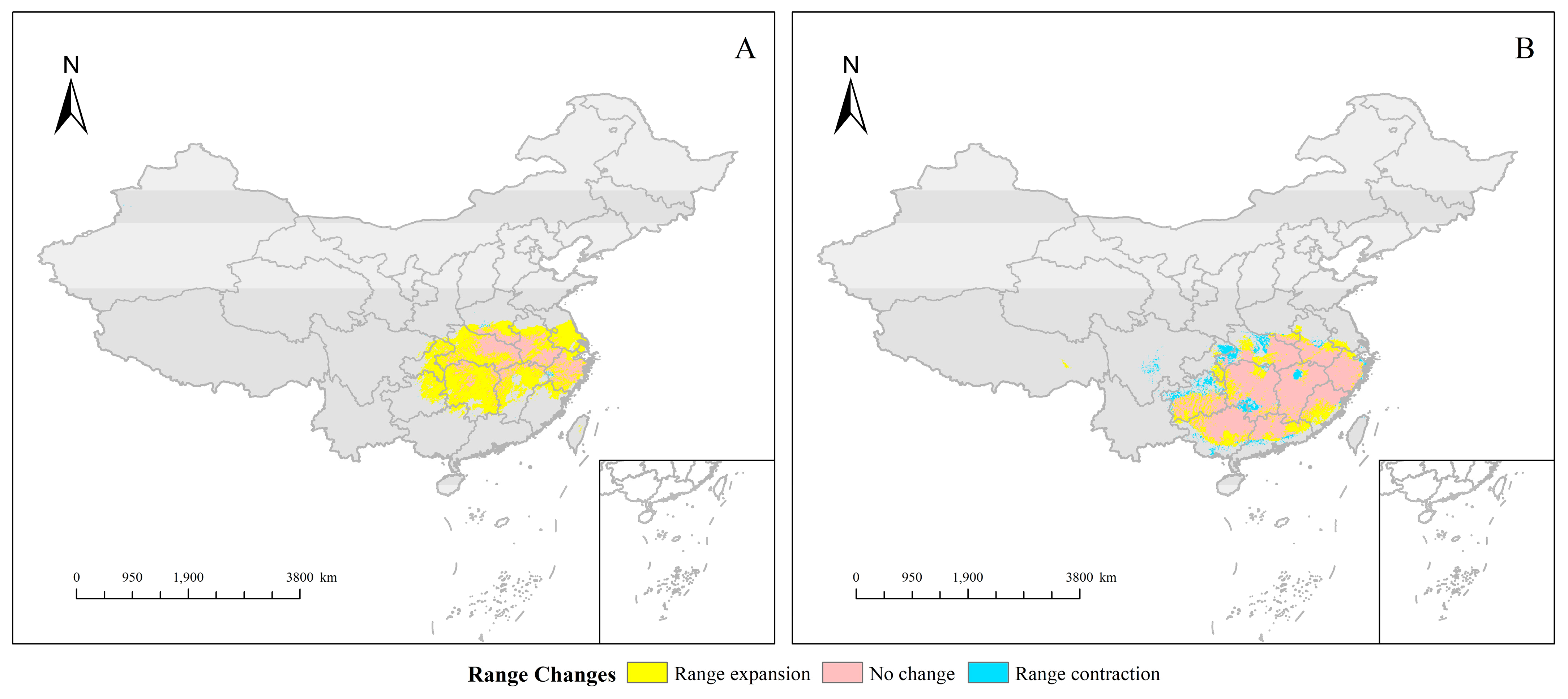
3.3. Habitat Changes under Future Environmental Models
3.4. Ecological Niche and Geographic Distribution Analysis
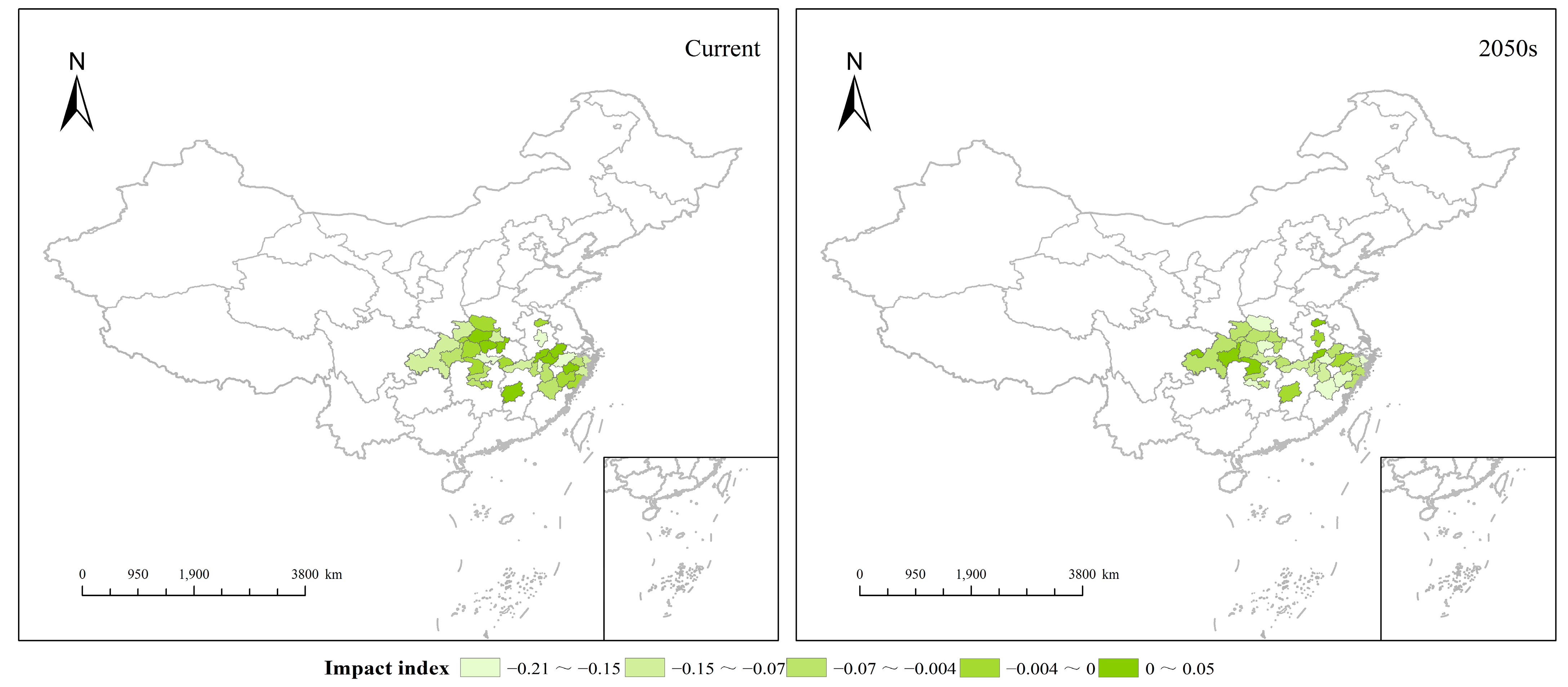
3.5. Temporal and Spatial Effects of F. insularis Wood on H. miconioides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martorell, C.; Freckleton, R.P. Testing the roles of competition, facilitation and stochasticity on community structure in a species-rich assemblage. J. Ecol. 2013, 102, 74–85. [Google Scholar] [CrossRef]
- Zhai, L.; Xie, R.; Li, S.; Zhang, Z. Effects of Nitrogen and Plant Density on Competition between Two Maize Hybrids Released in Different Eras. Agron. J. 2017, 109, 2670. [Google Scholar] [CrossRef]
- You, Y.; Wang, J.; Huang, X.; Tang, Z.; Liu, S.; Sun, O.J. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecol. Evol. 2014, 4, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Huang, Y.; Ma, M.; Wen, Z.; Chen, J.; Chen, C.; Wu, S. Plant trait-based analysis reveals greater focus needed for mid-channel bar downstream from the Three Gorges Dam of the Yangtze River. Ecol. Indic. 2020, 111, 105950. [Google Scholar] [CrossRef]
- Buonincontri, M.P.; Luciano, B.; Smeraldo, S.; Chiusano, M.L.; Pasta, S.; Pasquale, G. Shedding light on the effects of climate and anthropogenic pressures on the disappearance of Fagus sylvatica in the Italian lowlands: Evidence from archaeo-anthracology and spatial analyses. Sci. Total Environ. 2023, 877, 162893. [Google Scholar] [CrossRef]
- Kress, W.J.; Krupnick, G.A. Lords of the biosphere: Plant winners and losers in the Anthropocene. Plants People Planet 2022, 4, 350–366. [Google Scholar] [CrossRef]
- Trtikova, M. Effects of competition and mowing on growth and reproduction of the invasive plant Erigeron annuus at two contrasting altitudes. Bot. Helv. 2009, 119, 1–6. [Google Scholar] [CrossRef]
- Remya, K.; Ramachandran, A.; Jayakumar, S. Predicting the current and future suitable habitat distribution of Myristica dactyloides gaertn. using maxent model in the eastern ghats, India. Ecol. Eng. 2015, 82, 184–188. [Google Scholar] [CrossRef]
- Jones, M.C.; Cheung, L. Multi-model ensemble projections of climate change effects on global marine biodiversity. Ices J. Mar. Sci. 2015, 72, 741–752. [Google Scholar] [CrossRef]
- Subedi, T.R.; Peréz-García, J.M.; Gurung, S.; Baral, H.S.; Virani, M.Z.; Sah, S.A.M.; Anadón, J.D. Global range dynamics of the bearded vulture (Gypaetus barbatus) from the last glacial maximum to climate change scenarios. Ibis 2023, 165, 403–419. [Google Scholar] [CrossRef]
- Boussouf, S.; Fernández, T.; Hart, A.B. Landslide susceptibility mapping using maximum entropy (MaxEnt) and geographically weighted logistic regression (GWLR) models in the Río Aguas catchment (Almería, SE Spain). Nat. Hazards 2023, 117, 207–235. [Google Scholar] [CrossRef]
- Zheng, S.M.; Feng, W.Q.; Wang, S.Q. Elastic properties of high entropy alloys by MaxEnt approach-ScienceDirect. Comput. Mater. Sci. 2018, 142, 332–337. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, X.; Xiang, W.; Chen, L.; Ouyang, S. Predicting potential suitable habitats of Chinese fir under current and future climatic scenarios based on Maxent model. Ecol. Inform. 2021, 64, 101393. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Zhu, B.; Cheng, X.; Liu, Y.; Gao, M.; Kong, R. MaxEnt Modeling Based on CMIP6 Models to Project Potential Suitable Zones for Cunninghamia lanceolata in China. Forests 2021, 12, 752. [Google Scholar] [CrossRef]
- Gao, Z.; Hou, Y.; Zaitchik, B.F.; Chen, Y.; Chen, W. A Two-Step Integrated MLP-GTWR Method to Estimate 1 km Land Surface Temperature with Complete Spatial Coverage in Humid, Cloudy Regions. Remote Sens. 2021, 13, 971. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Liu, Y.; Xu, S.; Zhao, Y.; Chen, Y.; Hong, S. Urban expansion patterns and their driving forces based on the center of gravity-GTWR model: A case study of the Beijing-Tianjin-Hebei urban agglomeration. J. Geogr. Sci. 2020, 30, 297–318. [Google Scholar] [CrossRef]
- Wu, C.; Ren, F.; Hu, W.; Du, Q. Multiscale geographically and temporally weighted regression: Exploring the spatiotemporal determinants of housing prices. Int. J. Geogr. Inf. Sci. 2018, 33, 489–511. [Google Scholar] [CrossRef]
- Zhang, K.; Leeuw, G.D.; Yang, Z.; Chen, X.; Jiao, J. The Impacts of the COVID-19 Lockdown on Air Quality in the Guanzhong Basin, China. Remote Sens. 2020, 12, 3042. [Google Scholar] [CrossRef]
- Lu, H.P.; Cai, Y.W.; Chen, X.Y.; Zhang, X.; Gu, Y.J.; Zhang, G.F. High RAPD but no cpDNA sequence variation in the endemic and endangered plant, Heptacodium miconioides Rehd. (Caprifoliaceae). Genetica 2006, 128, 409–417. [Google Scholar] [CrossRef]
- Jin, Z.X.; Zhang, W.B. The quantitative relation of intraspecific and interspecific competition in endangered plant Heptacodium miconioides. Bulltin Bot. Res. 2004, 24, 53–58. [Google Scholar]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Liu, P.; Yang, Y.S.; Xu, G.; Hao, C. Physiological response of rare and endangered seven-son-flower (Heptacodium miconioides) to light stress under habitat fragmentation. Environ. Exp. Bot. 2006, 57, 32–40. [Google Scholar] [CrossRef]
- LaRue, M.A.; Nielsen, C.K. Modelling potential dispersal corridors for cougars in midwestern North America using least-cost path methods. Ecol. Model. 2007, 212, 372–381. [Google Scholar] [CrossRef]
- Su, K.; Yu, Q.; Yue, D.; Zhang, Q.; Sun, X. Simulation of a forest-grass ecological network in a typical desert oasis based on multiple scenes. Ecol. Model. 2019, 413, 108834. [Google Scholar] [CrossRef]
- Javier, O.; Ari, A.H.; Encinas, M.A.; Francisco, P.; Raghava, G.P.S. Assessing the Primary Data Hosted by the Spanish Node of the Global Biodiversity Information Facility (GBIF). PLoS ONE 2013, 8, e55144. [Google Scholar]
- Harte, J.; Newman, E.A. Maximum information entropy: A foundation for ecological theory. Trends Ecol. Evol. 2014, 29, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Kafash, A.; Ashrafi, S.; Yousefi, M. Modeling habitat suitability of bats to identify high priority areas for field monitoring and conservation. Environ. Sci. Pollut. Res. 2022, 29, 25881–25891. [Google Scholar] [CrossRef]
- Mishra, S.N.; Gupta, H.S.; Kulkarni, N. Impact of climate change on the distribution of Sal species. Ecol. Inform. 2021, 61, 101244. [Google Scholar] [CrossRef]
- Yang, F.; He, D.H. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar]
- Soares, J.R.S.; Ramos, R.S.; Silva, R.S.D.; Neves, D.V.C.; Picano, M.C. Climate change impact assessment on worldwide rain fed soybean based on species distribution models. Trop. Ecol. 2021, 62, 612–625. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, X.; Liu, M.; Liu, X.; Tao, J. Species distribution models of the Spartina alterniflora loisel in its origin and invasive country reveal an ecological niche shift. Front. Plant Sci. 2021, 12, 2159. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Du, Q.; Yan, S.; Cao, Y.; Liu, X.; Guan, D.X.; Ma, L.Q. Geographical distribution of as-hyperaccumulator Pteris vittata in China: Environmental factors and climate changes. Sci. Total Environ. 2022, 803, 149864. [Google Scholar] [CrossRef] [PubMed]
- Ngarega, B.K.; Masocha, V.F.; Schneider, H. Forecasting the effects of bioclimatic characteristics and climate change on the potential distribution of Colophospermum mopane in southern Africa using Maximum Entropy (Maxent). Ecol. Inform. 2021, 65, 101419. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Lucas, J.; Weltersbach, M.S.; Kmle, D.; Strehlow, H.V. Niche overlap among anglers, fishers and cormorants and their removals of fish biomass: A case from brackish lagoon ecosystems in the southern baltic sea. Fish. Res. 2021, 238, 105894. [Google Scholar] [CrossRef]
- Bellamy, C.; Boughey, K.; Hawkins, C.; Reveley, S.; Spake, R.; Williams, C.; Altringham, J.A. sequential multi-level framework to improve habitat suitability modelling. Landsc. Ecol. 2020, 35, 1001–1020. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, Y.; Yang, F.; Shi, Q. Spatial-temporal heterogeneity of green innovation in China. J. Clean. Prod. 2021, 282, 124464. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, W.; Zhou, Q.; Shi, T. Spatiotemporal and driving forces of ecological carrying capacity for high-quality development of 286 cities in China. J. Clean. Prod. 2021, 293, 126186. [Google Scholar] [CrossRef]
- Cui, L.; Li, R.; Zhang, Y.; Meng, Y.; Zhao, Y.; Fu, H. A geographically and temporally weighted regression model for assessing intra-urban variability of volatile organic compounds (vocs) in Yangpu district, Shanghai. Atmos. Environ. 2019, 213, 746–756. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Sarma, K.; Krishna, M.; Tripathi, O.P. Predicting suitable habitats for the vulnerable eastern hoolock gibbon, hoolock leuconedys, in india using the maxent model. Folia Primatol. 2015, 86, 387–397. [Google Scholar] [CrossRef]
- Lautenschleger, A.; Vizentin-Bugoni, J.; Cavalheiro, L.B.; Iserhard, C.A. Morphological matching and phenological overlap promote niche partitioning and shape a mutualistic plant-hawkmoth network. Ecol. Entomol. 2021, 46, 292–300. [Google Scholar] [CrossRef]
- Verdú, J.R.; Oliva, D.; Victoria, C.G.G.; Cortez, V. Differential ecophysiological syndromes explain the partition of the thermal niche resource in coexisting eucraniini dung beetles. Ecol. Entomol. 2022, 47, 689–702. [Google Scholar] [CrossRef]
- Thuiller, W.; Richardson, D.M.; Rouget, M.; Proche, K.; Wilson, J.R.U. Interactions between environment, species traits, and human uses describe patterns of plant invasions. Ecology 2006, 87, 1755–1769. [Google Scholar] [CrossRef]
- Robert, P.; Anderson, A.B.; Israel, G.J.A. Species-specific tuning increases robustness to sampling bias in models of species distributions: An implementation with maxent. Ecol. Model. 2011, 222, 2796–2811. [Google Scholar]
- Rawat, N.; Purohit, S.; Painuly, V.; Negi, G.S.; Bisht, M.P.S. Habitat distribution modeling of endangered medicinal plant Picrorhiza kurroa (Royle ex Benth) under climate change scenarios in Uttarakhand Himalaya, India. Ecol. Inform. 2022, 68, 101550. [Google Scholar] [CrossRef]
- Yi, Y.J.; Cheng, X.; Yang, Z.F.; Zhang, S.H. Maxent modeling for predicting the potential distribution of endangered medicinal plant (H. riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Huntley, B. How plants respond to climate change: Migration rates, individualism and the consequences for plant communities. Ann. Bot. 1991, 67, 15–22. [Google Scholar] [CrossRef]
- Yang, J.T.; Jiang, X.; Chen, H.; Jiang, P.; Liu, M.; Huang, Y. Predicting the potential distribution of the endangered plant magnolia wilsonii using maxent under climate change in China. Pol. J. Environ. Stud. 2022, 31, 4435–4445. [Google Scholar] [CrossRef]
- Jiang, D. Climate change over China with a 2 °C global warming. Chin. J. Atmos. Ences 2012, 10, 121–122. [Google Scholar]
- Zhang, B.; Cadotte, M.W.; Chen, S.; Tan, X.; Han, X. Plants alter their vertical root distribution rather than biomass allocation in response to changing precipitation. Ecology 2019, 100, e02828. [Google Scholar] [CrossRef]
- Brousse, C. A historical and ethnobotanic analysis of the connection between human activities and grassland vegetation. Fourrages 2011, 208, 245–251. [Google Scholar]
- Huang, Y.; Zeng, Y.; Jiang, P.; Chen, H.; Yang, J. Prediction of potential geographic distribution of endangered relict tree species Dipteronia sinensis in China based on maxent and gis. Pol. J. Environ. Stud. 2022, 31, 3597–3609. [Google Scholar] [CrossRef]
- Steinmüller, K. A model of niche overlap and interaction in ecological systems. Biom. J. 2011, 22, 211–228. [Google Scholar] [CrossRef]
- Geange, S.W.; Pledger, S.; Burns, K.C.; Shima, J.S. A unified analysis of niche overlap incorporating data of different types. Methods Ecol. Evol. 2011, 2, 175–184. [Google Scholar] [CrossRef]
- Nunes, L.A.; Pearson, R.G. A null biogeographical test for assessing ecological niche evolution. J. Biogeogr. 2017, 44, 1331–1343. [Google Scholar] [CrossRef]
- Fackovcova, M.G.A. Ecological niche conservatism shapes the distributions of lichens: Geographical segregation does not reflect ecological differentiation. Preslia 2017, 89, 63–85. [Google Scholar] [CrossRef]
- Suárez-Mota, M.E.; Villaseor, J.L. Ecological niche overlap among species of the genus Zaluzania (Asteraceae) from the dry regions of Mexico. Plant Ecol. Evol. 2020, 153, 337–347. [Google Scholar] [CrossRef]
- Kermavnar, J.; Kutnar, L.; Marinsek, A.; Babij, V. Are ecological niche optimum and width of forest plant species related to their functional traits? Flora 2023, 301, 152247. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Hawkins, S.J.; Southward, A.J.; Burrows, M.T. Modeling the response of populations of competing species to climate change. Ecology 2008, 89, 3138–3149. [Google Scholar] [CrossRef]
- Wang, W.; Shen, J.; Luo, Z. Partial survival and extinction in two competing species with impulses. Nonlinear Anal. Real World Appl. 2009, 10, 1243–1254. [Google Scholar] [CrossRef]
- López-Gómez, J. Coexistence and meta-coexistence for competing species. Houst. J. Math. 2003, 29, 483–536. [Google Scholar]
- Çoban, H.O.; Ömer, K.Ö.; Arslan, E.S. MaxEnt Modeling for Predicting the Current and Future Potential Geographical Distribution of Quercus libani Olivier. Sustainability 2020, 12, 2671. [Google Scholar] [CrossRef]
- Ma, X.; Li, A.; Dong, X.P.; Cai, Y.; Ma, M. The Interspecific Competition of Xanthium italicum Moretti Significantly Reduces the Growth of Helianthus annuus and the Yield and Quality of Its Seeds. Pol. J. Environ. Stud. 2023, 32, 199–206. [Google Scholar] [CrossRef]
- Dang, T.K.P. The Discourse of Forest Cover in Vietnam and Its Policy Implications. Sustainability 2022, 14, 10976. [Google Scholar] [CrossRef]
- Pasquale, G.D.; Saracino, A.; Bosso, L.; Russo, D.; Allevato, E. Coastal pine-oak glacial refugia in the Mediterranean basin: A biogeographic approach based on charcoal analysis and spatial modelling. Forests 2020, 11, 673. [Google Scholar] [CrossRef]


| Emission | Description |
|---|---|
| SSP1–2.6 | SSP1 (Low forcing scenario) Upgrade to RCP2.6 scenario based on (Radiative forcing reaches 2.6 W/m2 in 2100) |
| SSP2–4.5 | SSP2 (Medium forcing scenario) Upgrade to RCP4.5 scenario based on (Radia-tive forcing reaches 4.5 W/m2 in 2100) |
| SSP3–7.0 | SSP3 (Medium forcing scenario) New RCP7.0 emission path based on (Radia-tive forcing will reach 7.0 W/m2 in 2100) |
| SSP5–8.5 | SSP5 (High Forcing Scenario) Upgrade to RCP8.5 scenario based on (SSP5 is the only SSP scenario that can achieve radiative forcing to 8.5 W/m2 in 2100) |
| Code | Description |
|---|---|
| Bio1 | Annual Mean Temperature (°C) |
| Bio2 | Mean Diurnal Range (Mean of monthly (max temp–min temp)) (°C) |
| Bio3 | Isothermality (BIO2/BIO7) (×100) |
| Bio4 | Temperature Seasonality (standard deviation × 100) |
| Bio7 | Temperature Annual Range (°C) |
| Bio8 | Mean Temperature in Wettest Quarter (°C) |
| Bio9 | Mean Temperature in Driest Quarter (℃) |
| Bio12 | Annual Precipitation (mm) |
| Bio13 | Precipitation in Wettest Month (mm) |
| Bio15 | Precipitation Seasonality (Coefficient of Variation) |
| Bio17 | Precipitation in Driest Quarter (mm) |
| Bio19 | Precipitation in Coldest Quarter (mm) |
| Wind | Wind speed (m·s−1) |
| Vapr | Water vapor pressure (kPa) |
| Srad | Solar radiation (kJ·m−2·day−1) |
| Elev | Elevation (m) |
| Slo | Slope (°) |
| Asp | Aspect |
| Lucc | Land-Use and Land-Cover Change |
| Ndvi | Normalized Difference Vegetation Index |
| Species | Environmental Factors | Contribution Rate |
|---|---|---|
| H. miconioides | Precipitation of Driest Quarter | 52.8% |
| Temperature Seasonality | 13.1% | |
| Water Vapor Pressure | 10.4% | |
| Mean Temperature of Driest Quarter | 3.5% | |
| F. insularis | Precipitation of Driest Quarter | 49.8% |
| Water vapor pressure | 23.9% | |
| Temperature Seasonality | 4.4% | |
| Annual Precipitation | 3.6% |
| Species | SSP1–2.6 | SSP2–4.5 | SSP3–7.0 | SSP5–8.5 | |
|---|---|---|---|---|---|
| Unsuitable area | A | 462.05 | 428.26 | 369.51 | 519.26 |
| B | 283.01 | 290.31 | 267.74 | 328.81 | |
| Poorly suitable area | A | 240.96 | 239.14 | 236.06 | 242.41 |
| B | 262.17 | 228.09 | 234.05 | 224.74 | |
| Moderately suitable area | A | 161.66 | 180.09 | 198.52 | 136.42 |
| B | 230.31 | 239.81 | 236.06 | 234.91 | |
| Highly suitable area | A | 95.33 | 112.51 | 155.91 | 61.91 |
| B | 184.51 | 201.79 | 222.15 | 171.54 |
| Species | SSP1–2.6 | SSP2–4.5 | SSP3–7.0 | SSP5–8.5 | |
|---|---|---|---|---|---|
| Unsuitable area | A | 377.37 | 389.85 | 362.11 | 458.21 |
| B | 262.65 | 270.33 | 255.74 | 299.71 | |
| Poorly suitable area | A | 218.97 | 223.58 | 216.09 | 335.39 |
| B | 244.89 | 244.89 | 239.81 | 215.42 | |
| Moderately suitable area | A | 196.32 | 211.98 | 201.12 | 135.07 |
| B | 186.55 | 195.47 | 183.27 | 220.03 | |
| Highly suitable area | A | 167.34 | 134.59 | 180.68 | 131.33 |
| B | 266.01 | 249.31 | 281.18 | 224.84 |
| Time | Niche Overlap | Range Overlap | |
|---|---|---|---|
| D | I | ||
| Current | 0.7261 | 0.9188 | 0.5351 |
| 2050s | 0.8048 | 0.9551 | 0.6209 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Liu, M.; Ji, L.; Wang, F. Regional Analysis of the Potential Distribution of Heptacodium miconioides and Its Competitor Species in China. Sustainability 2024, 16, 752. https://doi.org/10.3390/su16020752
Li L, Liu M, Ji L, Wang F. Regional Analysis of the Potential Distribution of Heptacodium miconioides and Its Competitor Species in China. Sustainability. 2024; 16(2):752. https://doi.org/10.3390/su16020752
Chicago/Turabian StyleLi, Le, Minxia Liu, Lanxiang Ji, and Fei Wang. 2024. "Regional Analysis of the Potential Distribution of Heptacodium miconioides and Its Competitor Species in China" Sustainability 16, no. 2: 752. https://doi.org/10.3390/su16020752
APA StyleLi, L., Liu, M., Ji, L., & Wang, F. (2024). Regional Analysis of the Potential Distribution of Heptacodium miconioides and Its Competitor Species in China. Sustainability, 16(2), 752. https://doi.org/10.3390/su16020752