Optimization of a Low-Cost Corona Dielectric-Barrier Discharge Plasma Wastewater Treatment System through Central Composite Design/Response Surface Methodology with Mechanistic and Efficiency Analysis
Abstract
1. Introduction
2. Materials and Methods
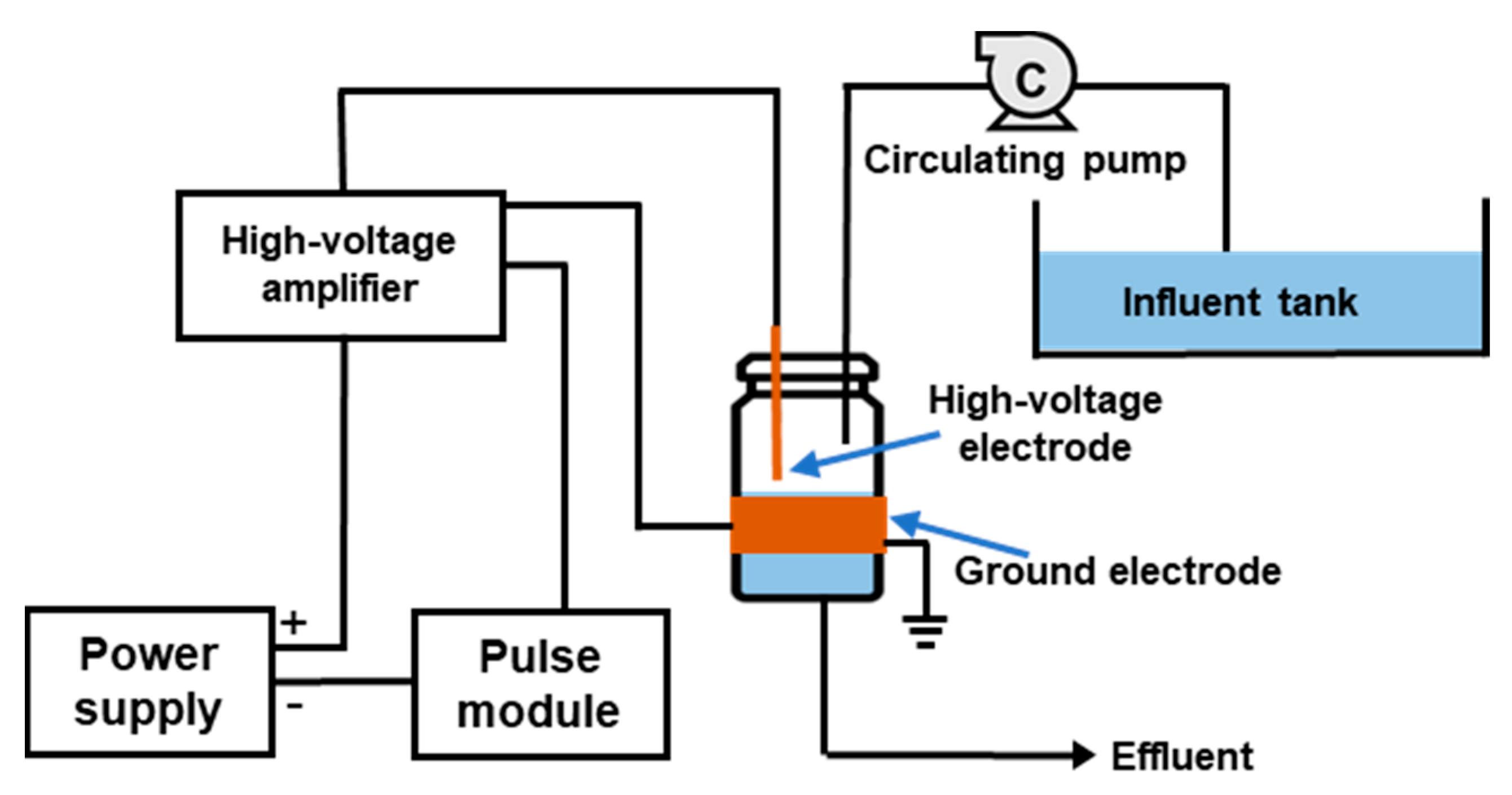
2.1. Experimental Setup
2.2. Methods and Analysis
2.3. Experimental Design
3. Results and Discussion
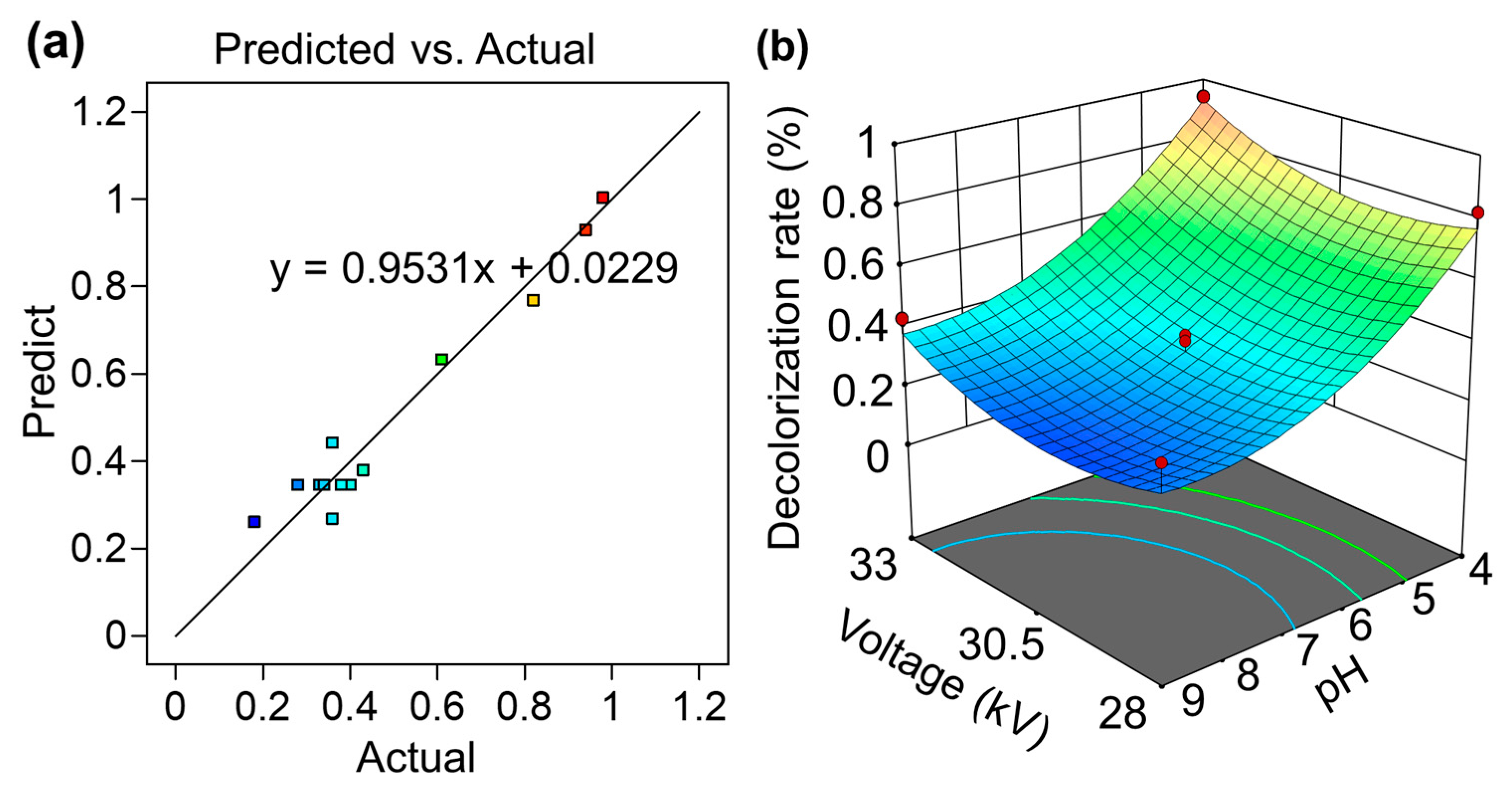
3.1. Model Evaluation and Statistical Analysis
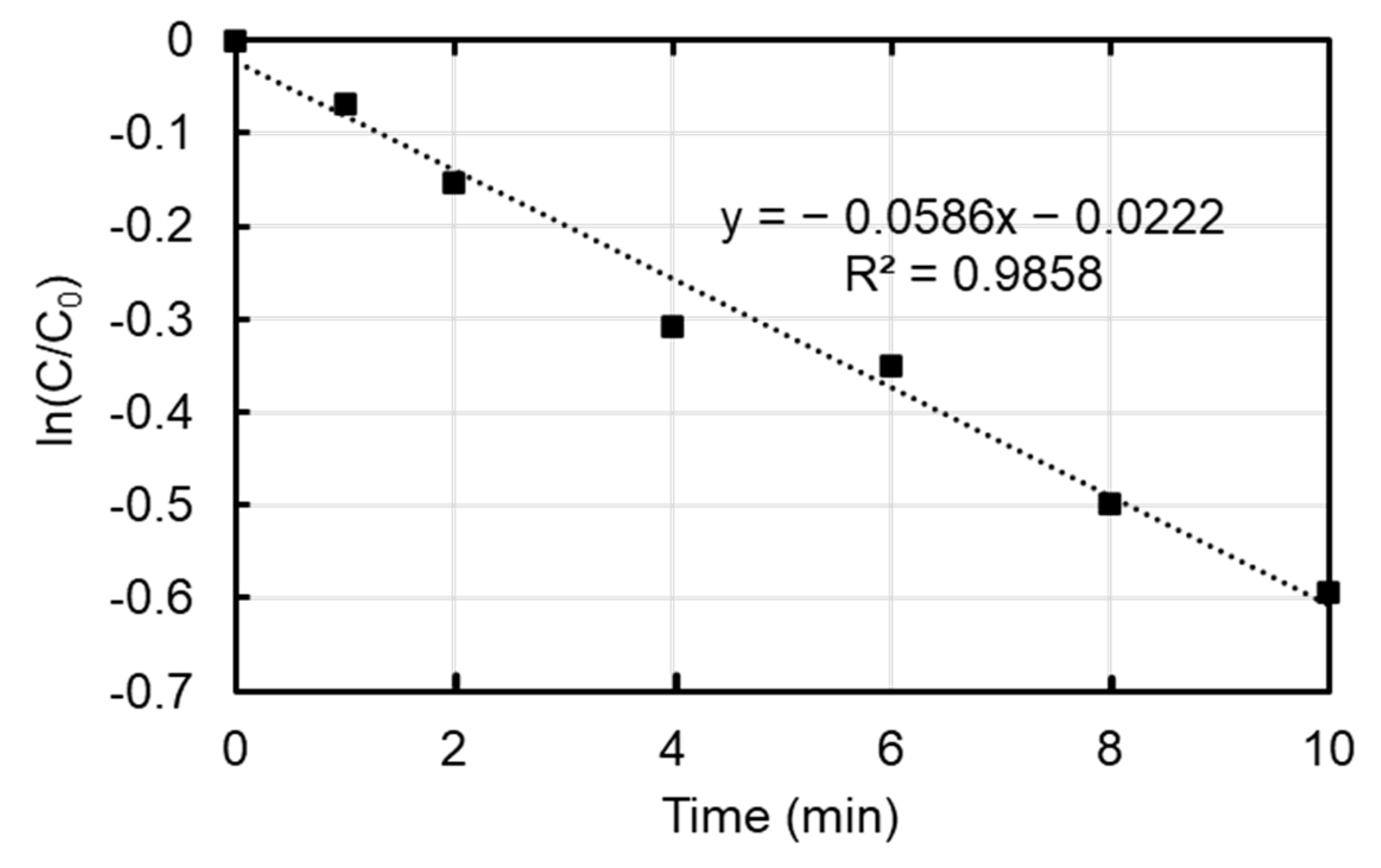
3.2. Reaction Order and Kinetics
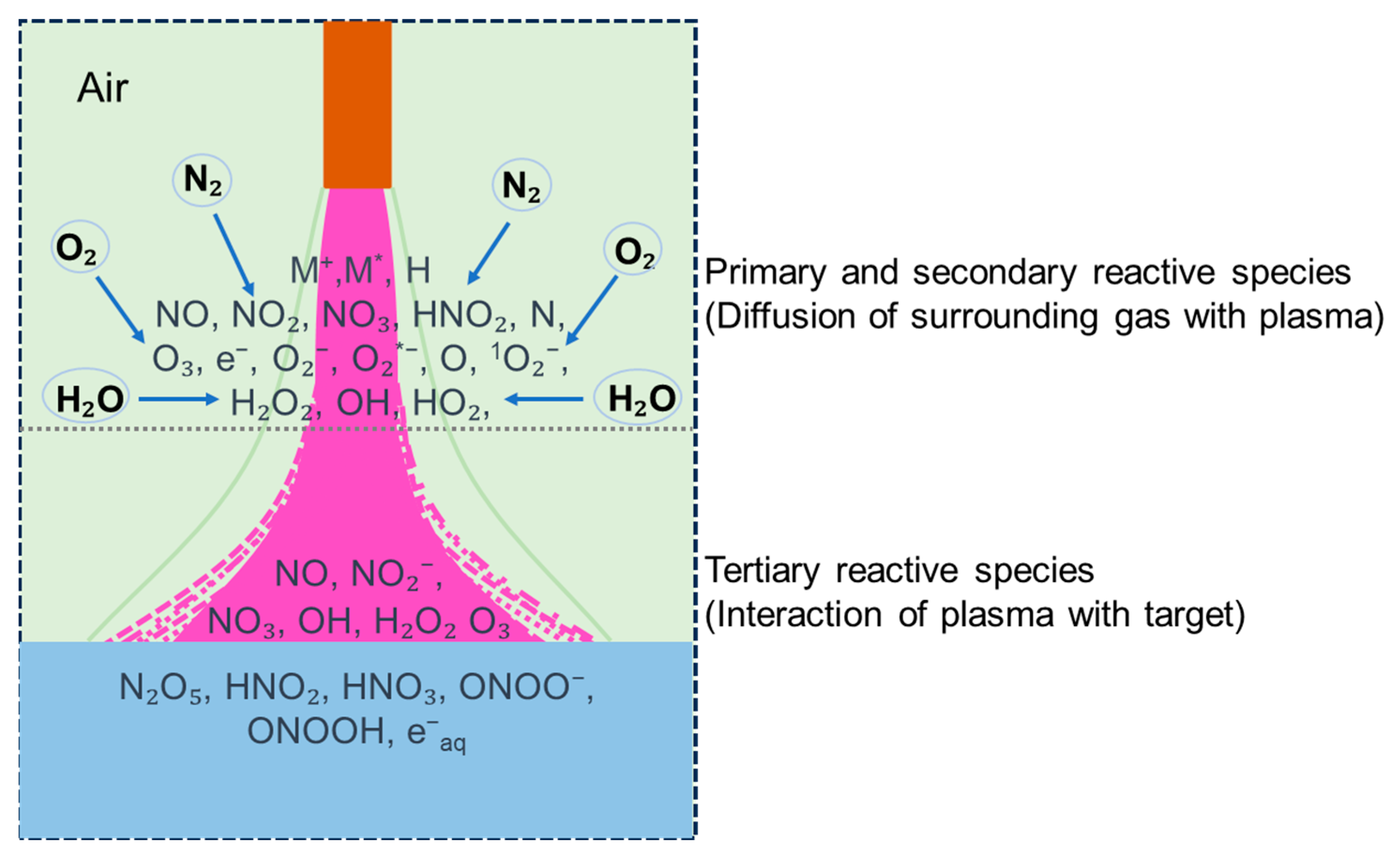
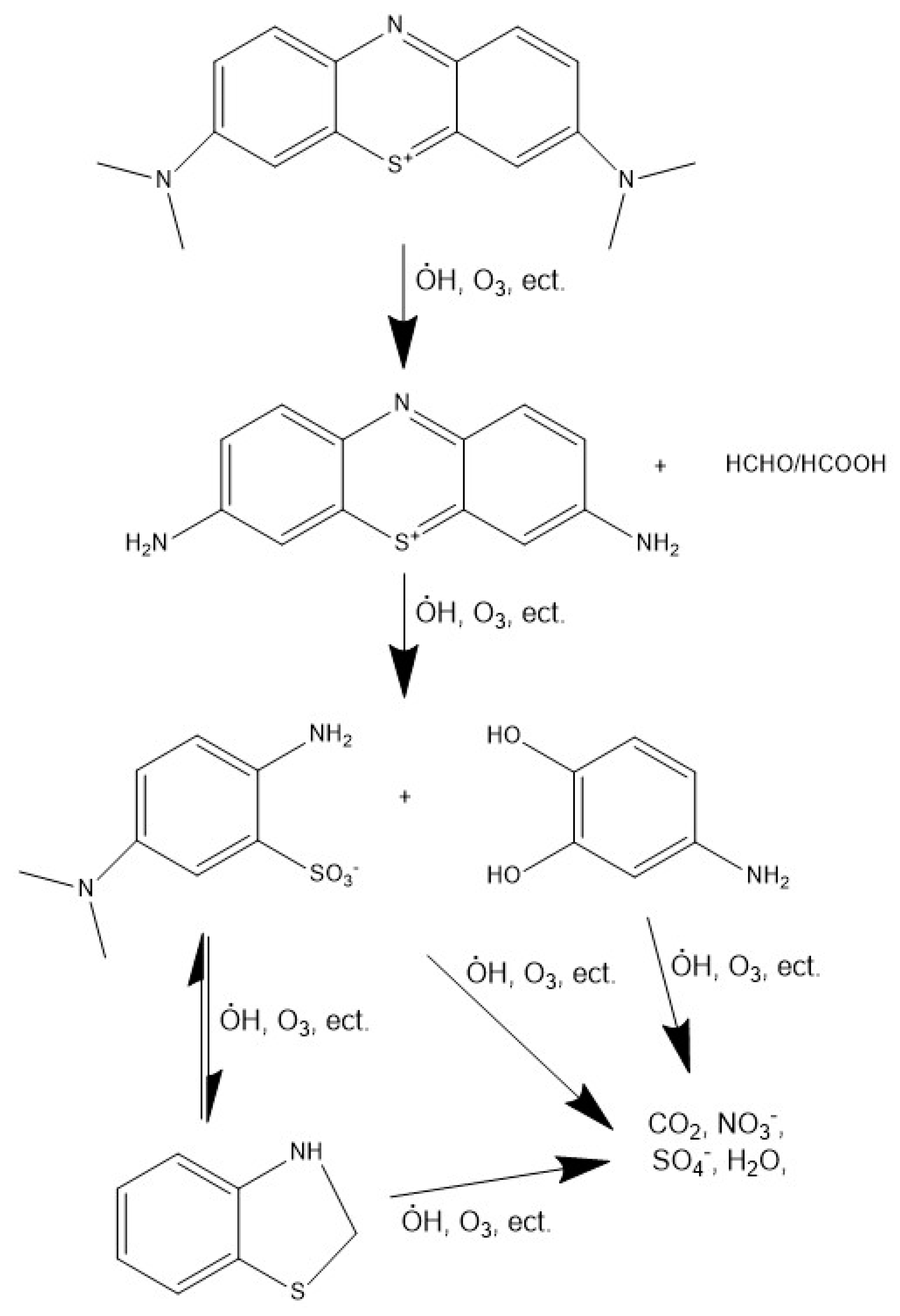
3.3. Mechanism Analysis
3.4. Practical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vaidya, A.A. Environmental Pollution during Chemical Processing of Synthetic Fibers. Colourage 1982, 14, 3–10. [Google Scholar]
- Chung, K.-T.; Stevens, S.E., Jr. Degradation Azo Dyes by Environmental Microorganisms and Helminths. Environ. Toxicol. Chem. 1993, 12, 2121–2132. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Krishna Moorthy, A.; Govindarajan Rathi, B.; Shukla, S.P.; Kumar, K.; Shree Bharti, V. Acute Toxicity of Textile Dye Methylene Blue on Growth and Metabolism of Selected Freshwater Microalgae. Environ. Toxicol. Pharmacol. 2021, 82, 103552. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Env. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, J.; Dan, Z.; Tian, Y.; Duan, N.; Xin, B. Simultaneous Oxidative Degradation of Toxic Acid Wastewater from Production of Nitrocellulose and Release of Mn2+ from Low-Grade MnO2 Ore as Oxidant. J. Chem. Technol. Biotechnol. 2017, 92, 1638–1644. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, Y.; Zhan, Y.; Zhu, J. Degradation of Organic Pollutants in Flocculated Liquid Digestate Using Photocatalytic Titanate Nanofibers: Mechanism and Response Surface Optimization. Front. Agr. Sci. Eng. 2023, 10, 492–502. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced Oxidation Processes (AOPs) Based Wastewater Treatment—Unexpected Nitration Side Reactions—A Serious Environmental Issue: A Review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Okolie, J.A.; Vichare, P.; Rolland, L.; Yaseen, M. Ozone Decontamination of Medical and Nonmedical Devices: An Assessment of Design and Implementation Considerations. Ind. Eng. Chem. Res. 2023, 62, 4191–4209. [Google Scholar] [CrossRef]
- Peroxone: Ozone Peroxide Advanced Oxidation Processes AOP. Spartan Water Treatment. Available online: https://spartanwatertreatment.com/advanced-oxidation-peroxide-ozone/ (accessed on 15 October 2023).
- He, Y.; Sang, W.; Lu, W.; Zhang, W.; Zhan, C.; Jia, D. Recent Advances of Emerging Organic Pollutants Degradation in Environment by Non-Thermal Plasma Technology: A Review. Water 2022, 14, 1351. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar, V.; Joshi, N.C.; Tomar, M.S.; Pathak, B. Cold Plasma Technology: Advanced and Sustainable Approach for Wastewater Treatment. Env. Sci. Pollut. Res. Int. 2021, 28, 65062–65082. [Google Scholar] [CrossRef]
- Saeed, M.O.; Azizli, K.; Isa, M.H.; Bashir, M.J.K. Application of CCD in RSM to Obtain Optimize Treatment of POME Using Fenton Oxidation Process. J. Water Process Eng. 2015, 8, e7–e16. [Google Scholar] [CrossRef]
- Christensen, R. Analysis of Variance, Design, and Regression: Applied Statistical Methods; CRC Press: Boca Raton, FL, USA, 1996; ISBN 978-0-412-06291-9. [Google Scholar]
- Minamoto, C.; Fujiwara, N.; Shigekawa, Y.; Tada, K.; Yano, J.; Yokoyama, T.; Minamoto, Y.; Nakayama, S. Effect of Acidic Conditions on Decomposition of Methylene Blue in Aqueous Solution by Air Microbubbles. Chemosphere 2021, 263, 128141. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Sun, Y.; Gong, S.; Jiang, G.; Zheng, X.; Yu, Z. Degradation of Sulfamethoxazole in Aqueous Solution by Dielectric Barrier Discharge Plasma Combined with Bi2WO6-RMoS2 Nanocomposite: Mechanism and Degradation Pathway. Chemosphere 2019, 222, 872–883. [Google Scholar] [CrossRef]
- Guan, R.; Yuan, X.; Wu, Z.; Jiang, L.; Li, Y.; Zeng, G. Principle and Application of Hydrogen Peroxide Based Advanced Oxidation Processes in Activated Sludge Treatment: A Review. Chem. Eng. J. 2018, 339, 519–530. [Google Scholar] [CrossRef]
- Ahmadi, E.; Shokri, B.; Mesdaghinia, A.; Nabizadeh, R.; Reza Khani, M.; Yousefzadeh, S.; Salehi, M.; Yaghmaeian, K. Synergistic Effects of α-Fe2O3-TiO2 and Na2S2O8 on the Performance of a Non-Thermal Plasma Reactor as a Novel Catalytic Oxidation Process for Dimethyl Phthalate Degradation. Sep. Purif. Technol. 2020, 250, 117185. [Google Scholar] [CrossRef]
- Wang, T.; Qu, G.; Sun, Q.; Liang, D.; Hu, S. Evaluation of the Potential of P-Nitrophenol Degradation in Dredged Sediment by Pulsed Discharge Plasma. Water Res. 2015, 84, 18–24. [Google Scholar] [CrossRef]
- Locke, B.R.; Sato, M.; Sunka, P.; Hoffmann, M.R.; Chang, J.-S. Electrohydraulic Discharge and Nonthermal Plasma for Water Treatment. Ind. Eng. Chem. Res. 2006, 45, 882–905. [Google Scholar] [CrossRef]
- Foster, J.E. Plasma-Based Water Purification: Challenges and Prospects for the Future. Phys. Plasmas 2017, 24, 055501. [Google Scholar] [CrossRef]
- Kim, H.H.; Teramoto, Y.; Negishi, N.; Ogata, A.; Kim, J.H.; Pongrác, B.; Machala, Z.; Gañán-Calvo, A.M. Polarity Effect on the Electrohydrodynamic (EHD) Spray of Water. J. Aerosol Sci. 2014, 76, 98–114. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, R.H.; Hong, Y.J.; Lamichhane, P.; Adhikari, B.C.; Choi, J.; Choi, E.H. Interactions between Atmospheric Pressure Plasma Jet and Deionized Water Surface. Results Phys. 2020, 19, 103569. [Google Scholar] [CrossRef]
- Lamichhane, P.; Paneru, R.; Nguyen, N.L.; Sup Lim, J.; Bhartiya, P.; Chandra Adhikari, B.; Mumtaz, S.; Ha Choi, E. Plasma-Assisted Nitrogen Fixation in Water with Various Metals. React. Chem. Eng. 2020, 5, 2053–2057. [Google Scholar] [CrossRef]
- Attri, P.; Kim, Y.H.; Park, D.H.; Park, J.H.; Hong, Y.J.; Uhm, H.S.; Kim, K.-N.; Fridman, A.; Choi, E.H. Generation Mechanism of Hydroxyl Radical Species and Its Lifetime Prediction during the Plasma-Initiated Ultraviolet (UV) Photolysis. Sci. Rep. 2015, 5, 9332. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.; Lamichhane, P.; Lim, J.S.; Min, B.; Paneru, R.; Weltmann, K.-D.; Choi, E.H. An Atmospheric Pressure Plasma Jet Operated by Injecting Natural Air. Appl. Phys. Lett. 2018, 113, 194101. [Google Scholar] [CrossRef]
- Barjasteh, A.; Dehghani, Z.; Lamichhane, P.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Recent Progress in Applications of Non-Thermal Plasma for Water Purification, Bio-Sterilization, and Decontamination. Appl. Sci. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Palma, D.; Richard, C.; Minella, M. State of the Art and Perspectives about Non-Thermal Plasma Applications for the Removal of PFAS in Water. Chem. Eng. J. Adv. 2022, 10, 100253. [Google Scholar] [CrossRef]
- Merényi, G.; Lind, J. Free Radical Formation in the Peroxynitrous Acid (ONOOH)/Peroxynitrite (ONOO-) System. Chem. Res. Toxicol. 1998, 11, 243–246. [Google Scholar] [CrossRef]
- Manoj Kumar Reddy, P.; Rama Raju, B.; Karuppiah, J.; Linga Reddy, E.; Subrahmanyam, C. Degradation and Mineralization of Methylene Blue by Dielectric Barrier Discharge Non-Thermal Plasma Reactor. Chem. Eng. J. 2013, 217, 41–47. [Google Scholar] [CrossRef]
- Mondal, S.; Reyes, M.E.D.A.; Pal, U. Plasmon Induced Enhanced Photocatalytic Activity of Gold Loaded Hydroxyapatite Nanoparticles for Methylene Blue Degradation under Visible Light. RSC Adv. 2017, 7, 8633–8645. [Google Scholar] [CrossRef]
- Huang, F.; Chen, L.; Wang, H.; Yan, Z. Analysis of the Degradation Mechanism of Methylene Blue by Atmospheric Pressure Dielectric Barrier Discharge Plasma. Chem. Eng. J. 2010, 162, 250–256. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-Merit for the Technical Development and Application of Advanced Oxidation Technologies for Both Electric- and Solar-Driven Systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Advanced Oxidation Processes—With so Many Options, How Can We Gauge Efficiency? Available online: https://wcponline.com/2021/08/15/advanced-oxidation-processes-with-so-many-options-how-can-we-gauge-efficiency/ (accessed on 8 December 2023).
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced Oxidation Processes (AOPs) Involving Ultrasound for Waste Water Treatment: A Review with Emphasis on Cost Estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Tezcanli-Güyer, G.; Ince, N.H. Individual and Combined Effects of Ultrasound, Ozone and UV Irradiation: A Case Study with Textile Dyes. Ultrasonics 2004, 42, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Fung, P.C.; Sin, K.M.; Tsui, S.M. Decolorisation and Degradation Kinetics of Reactive Dye Wastewater by a UV/Ultrasonic/Peroxide System. Color. Technol. 2000, 116, 170–173. [Google Scholar] [CrossRef]
- An, T.; Gu, H.; Xiong, Y.; Chen, W.; Zhu, X.; Sheng, G.; Fu, J. Decolourization and COD Removal from Reactive Dye-Containing Wastewater Using Sonophotocatalytic Technology. J. Chem. Technol. Biotechnol. 2003, 78, 1142–1148. [Google Scholar] [CrossRef]
- Singh, R.K.; Philip, L.; Ramanujam, S. Continuous Flow Pulse Corona Discharge Reactor for the Tertiary Treatment of Drinking Water: Insights on Disinfection and Emerging Contaminants Removal. Chem. Eng. J. 2019, 355, 269–278. [Google Scholar] [CrossRef]
- Fahmy, A.; El-Zomrawy, A.; Saeed, A.M.; Sayed, A.Z.; El-Arab, M.A.E.; Shehata, H.; Friedrich, J. Degradation of Organic Dye Using Plasma Discharge: Optimization, PH and Energy. Plasma Res. Express 2020, 2, 015009. [Google Scholar] [CrossRef]
| Code | Coded and Actual Levels | |||||
|---|---|---|---|---|---|---|
| Factors | Xi | −1.414 (−α) | −1 (Low) | 0 (Center) | 1 (High) | 1.414 (+α) |
| Voltage (kV) | X1 | 27 | 28 | 30.5 | 33 | 34 |
| pH | X2 | 3.0 | 4.00 | 6.5 | 9 | 10.0 |
| Run | Voltage (kV) | pH | Decolorization Rate% |
|---|---|---|---|
| 1 | 30.5 | 6.5 | 28.1 |
| 2 | 28.0 | 9.0 | 36.4 |
| 3 | 33.0 | 9.0 | 43.0 |
| 4 | 34.0 | 6.5 | 61.1 |
| 5 | 30.5 | 6.5 | 40.2 |
| 6 | 27.0 | 6.5 | 36.2 |
| 7 | 30.5 | 6.5 | 32.9 |
| 8 | 30.5 | 6.5 | 33.5 |
| 9 | 33.0 | 4.0 | 94.5 |
| 10 | 28.0 | 4.0 | 81.6 |
| 11 | 30.5 | 10.0 | 18.1 |
| 12 | 30.5 | 6.5 | 37.5 |
| 13 | 30.5 | 3.0 | 97.9 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.7750 | 5 | 0.1550 | 29.59 | 0.0001 | significant |
| X1-Voltage | 0.0368 | 1 | 0.0368 | 7.03 | 0.0329 | |
| X2-pH | 0.5520 | 1 | 0.5520 | 105.37 | <0.0001 | |
| X1 X2 | 0.0006 | 1 | 0.0006 | 0.12 | 0.7399 | |
| X12 | 0.0646 | 1 | 0.0646 | 12.33 | 0.0098 | |
| X22 | 0.1421 | 1 | 0.1421 | 27.13 | 0.0012 | |
| Residual | 0.0367 | 7 | 0.0052 | |||
| Lack of Fit | 0.0280 | 3 | 0.0093 | 4.27 | 0.0972 | not significant |
| Pure Error | 0.0087 | 4 | 0.0022 | |||
| Cor Total | 0.8117 | 12 |
| AOPs | C0 | C | Chemicals Cost (USD) | Energy Density Used (watt/mL) | EE/O (kWh/m3/order) | Total Capital Cost (USD) | Reference |
|---|---|---|---|---|---|---|---|
| US | 20 | 2 | NA | 0.50 | 10,964.69 | 1.83 × 1010 | [36,37] |
| O3 | 20 | 2 | NA | 0.03 | 103.91 | 4.53 × 105 | [36,37] |
| U/S + UV | 20 | 2 | NA | 0.53 | 3698.09 | 5.86 × 109 | [36,37] |
| U/S + O3 | 20 | 2 | NA | 0.53 | 1215.02 | 1.92 × 109 | [36,37] |
| UV + O3 | 20 | 2 | NA | 0.06 | 111.56 | 1.12 × 107 | [36,37] |
| U/S + UV + O3 | 20 | 2 | NA | 0.56 | 989.9 | 1.50 × 109 | [36,37] |
| U/S + H2O2 | 100 | 45.65 | 2.06 × 105 | 0.03 | 43.07 | 5.33 × 108 | [36,38] |
| UV + H2O2 | 100 | 84.35 | 2.06 × 105 | 0.01 | 559.2 | 9.09 × 107 | [36,38] |
| U/S + UV + H2O2 | 100 | 9.13 | 2.06 × 105 | 0.04 | 39.76 | 7.99 × 107 | [36,38] |
| Photocatalysis | 402.6 | 40.26 | 2.52 × 104 | 0.71 | 3654.68 | 2.67 × 108 | [36,39] |
| U/S + photocatalysis | 402.6 | 40.26 | 6.98 × 103 | 0.76 | 1059.08 | 1.11 × 108 | [36,39] |
| Plasma | 20 | 0.7 | NA | 0.33 | 31.82 | 62 | This study |
| Plasma | 10 | 0.1 | NA | 0.15 | 5.79 | 62 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Tian, Y.; Zhan, Y.; Zhu, J. Optimization of a Low-Cost Corona Dielectric-Barrier Discharge Plasma Wastewater Treatment System through Central Composite Design/Response Surface Methodology with Mechanistic and Efficiency Analysis. Sustainability 2024, 16, 605. https://doi.org/10.3390/su16020605
Xiao Y, Tian Y, Zhan Y, Zhu J. Optimization of a Low-Cost Corona Dielectric-Barrier Discharge Plasma Wastewater Treatment System through Central Composite Design/Response Surface Methodology with Mechanistic and Efficiency Analysis. Sustainability. 2024; 16(2):605. https://doi.org/10.3390/su16020605
Chicago/Turabian StyleXiao, Yiting, Yang Tian, Yuanhang Zhan, and Jun Zhu. 2024. "Optimization of a Low-Cost Corona Dielectric-Barrier Discharge Plasma Wastewater Treatment System through Central Composite Design/Response Surface Methodology with Mechanistic and Efficiency Analysis" Sustainability 16, no. 2: 605. https://doi.org/10.3390/su16020605
APA StyleXiao, Y., Tian, Y., Zhan, Y., & Zhu, J. (2024). Optimization of a Low-Cost Corona Dielectric-Barrier Discharge Plasma Wastewater Treatment System through Central Composite Design/Response Surface Methodology with Mechanistic and Efficiency Analysis. Sustainability, 16(2), 605. https://doi.org/10.3390/su16020605






