Abstract
The presented review concerns the cross-disciplinary approaches to the subject of blue food and blue colourants, the socio-cultural aspects of blue food and beverage consumption, human health effects, environmental impact, and economic aspects. Blue colour in relation to food is not only about improving visual appeal, to which the addition of food colouring is usually limited when the food is coloured in some way that does not encourage eating. It is also the rich and complex sociological side related to food, that is, not only the food itself but also the background, dishware, and light, depending on whether we want to encourage—to increase consumption—or discourage—to, for example, reduce the amount of food eaten for dietary purposes. The negative side of consuming and disposing of synthetic dyes and the health-promoting aspects of natural dyes are also mentioned, with the economic and environmental aspects of sourcing natural dyes being discussed. The food industry uses blue dyes not only for consumption, but also for food quality control, taking advantage of the pH-dependent colour change properties of the compound.
1. Introduction
Colour signals the edibility and nutritional value of food [1]. According to the FDA (U.S. Food and Drug Administration), a colour additive is “any dye, pigment or substance which when added or applied to a food, drug or cosmetics, or the human body, is capable (alone or through reactions with other substances) of imparting a colour”. These may be preparations obtained from natural raw materials (vegetable, animal, mineral, or other source) by selective extraction, isolation, or otherwise derived, with or without an intermediate or final change in the identity of pigments [2]. Based on their use, two groups can be distinguished among food colourants:
- -
- food colourants with a limited permissible daily intake, the maximum levels of which are contained in the relevant legislation (mainly synthetic colourants),
- -
- colourants used in line with the quantum satis rule (comprising mainly colourants of natural origin), which have no defined maximum level of use and should be used at the lowest dose necessary to achieve the intended technological effect, applying the principles of good manufacturing practice.
Dyes are used in foods to improve visual appeal. The addition of colourants to foods is thought to have occurred in Egyptian cities, where confectionery manufacturers cca 1500 BC added natural extracts and wine to enhance the appearance of the product [3,4]. However, it is difficult to speak of such use in the case of the colour blue, where a colour different from the natural one is deliberately used.
The blue colour of food has always signified otherness, but over time the meaning, the sense of otherness, has changed. In the past, blue meant rotten—mouldy; later, with the development of the dye industry, it took on the meaning of ‘artificial’ [5]. Currently, it has its adherents, especially among young people; the distinctiveness of blue food is perceived positively as unique. Trends over the last few years have shown the increasing popularity of blue food. It seems that acceptance, however, only applies to a certain group of foods. Certain blue foods are already considered ‘acceptable’, like sweets, candies, cakes, and beverages, and receive significant attention from consumers who are delighted by the colour difference [5,6]. However, consumers still associate blue foods with artificial colouring [6,7].
It appears that determinants of food choice may be linked to affective memory and genetic factors [8,9]. For young people, the blue colour of food can be associated with messages passed down in childhood fairy tales and cartoons, in which blue berries, such as gummiberries (Gummi Bears) and Smurf berries (Smurfs), had magical properties. This fact is not entirely dissimilar to reality, as the intensely blue-coloured berries are characterised by a high content of anthocyanins, which have strong antioxidant effects and interesting properties. When deciding between using natural and synthetic food colourings, it is important to consider the complex benefit-loss balance. From the manufacturer’s and retailer’s point of view, colourants should, above all, be stable enough to avoid significant degradation during distribution and sale, both the ingredient and the food or beverage, which may include storage from days to years under refrigerated or ambient conditions.
General causes of degradation include heat, light, oxygen, acid, and oxidants like ascorbic acid or trace metals. Producers may have additional demands, such as water- or oil-solubility for defined applications [10].
The issues involved in an interdisciplinary approach to the links between economic, environmental, and social relationships are often complex, interrelated, and defined by the characteristics of each compound. Figure 1 presents the most important sustainable correlations between aspects of the use of natural blue food colourants, according to the main pillars of sustainability with the inclusion and attempted positioning of health aspects and properties (Figure 1). Before considering the socio-cultural, economic, and ecological contexts, a brief review of the current state of knowledge on the origin and properties of blue food dyes is necessary to explore aspects that will show us the turning points, which are also linked to health-promoting aspects. To increase the clarity of the work, Figure 2 illustrates a summary of the content of the review (Figure 2).
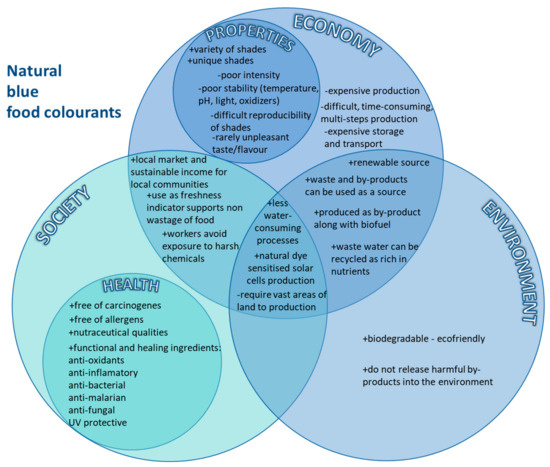
Figure 1.
Correlations between sustainability aspects of natural blue food colourants, (+) advantages, (-) disadvantages.

Figure 2.
Summary of review content.
2. Origin of Blue Pigments—Food Colourants
Dyes can be of natural and synthetic origin, and blue colour is not always strictly determined by the presence of blue dye in a material. The colour perceived as blue in animals, plants, algae, fungi, and bacteria or as a result of human activities in the manipulation of natural products has two key sources: (i) structural colour caused by the reflection of blue light from nanoscale structures (iridescent phenomenon) and (ii) molecular colour due to red-light absorbers [11].
Nature offers a wide variety of colours [10], but blue is the last colour to appear in the natural world and the last to be developed and produced in the Anthropocene [11]. It is known that blue colours are infrequent in nature because the electronic configurations required for photon absorption at 560–700 nm are complex and rarely occur [12]. Colour systems utilised by liverworts (furanoflavylium), mosses and ferns (3-deoxyanthocyanins), and angiosperms (anthocyanins) are chemically identical, although only the anthocyanins are capable of producing the colour blue [11].
The food industry is seeking more and more colourings of natural origin. It is often the case that a pigment changes colour depending on the pH, as is the case with anthocyanins, whose shade is determined by structural changes that occur under the influence of pH. Under acidic conditions, anthocyanins appear pink or red, while in an alkaline environment, they become blue [13].
There also exist dyes that turn blue when the relevant reaction has occurred. Fruits of Genipa americana L. and Gardenia jasminoides Ellis contain the iridoid glycoside geniposide, which releases the aglycone genipin after enzymatic hydrolysis by β-glucosidase [14]. While genipin is colourless, when it reacts with primary amines and proteins in an aerobic environment, it forms a water-soluble blue pigment that is stable when exposed to light [15] and low pH (3.0–4.0) conditions [16].
Pigments can also turn blue after appropriate treatment, such as thermal degradation. An example of such a pigment is bikaverin, a red polyketide derived from the secondary metabolism of fungi, mainly of the genus Fusarium [17]. Similarly, chamazulene, a component of the volatile oil of chamomile (Matricaria chamomilla L.) and yarrow (Achillea millefolium L.), turns blue after the thermal degradation of its colourless precursor during distillation [6].
2.1. Synthetic Blue Pigments
The food industry makes extensive use of artificial dyes to restore the colour lost during food processing and storage, enhance the current colour, minimise colour differences in batches, and present the food more attractively to consumers [9,18]. To ensure food has an attractive appearance, food manufacturers use synthetic or natural dyes. Synthetic colours were first produced in the mid-19th century, and due to their low production cost, high heat resistance, and chemical stability, they rapidly became popular as food colourings [3,19,20]. Artificial dyes are divided into two main groups, azo and triphenylmethane [21]. Currently, three artificial colourants are used in the food industry (Figure 3, Table 1): blue dyes (E131 and E133), belonging to the triphenylmethane group, and indigo dye (E132) [9,22].
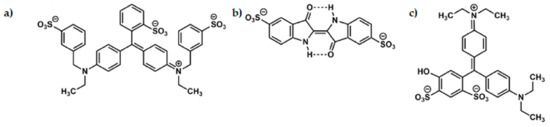
Figure 3.
(a) Brilliant blue FCF, (b) indigo carmine, and (c) patent blue V.

Table 1.
Synthetic dye properties [3,23,24].
During the past century, these additives have most often been associated with toxicological risks to human health. Many substances have already been banned in some countries due to experimental confirmations of carcinogenic effects. Still, the food industry uses them as they are cheaper, more stable, and brighter than natural dyes [9,18].
Patent blue (INS 131, E131, Cl food blue 5) is a sky-blue synthetic food colourant with a triphenylmethane group. It is water soluble and slightly soluble in ethanol, in an aqueous solution, and pH-dependent: deep blue in alkaline to weak acidic media and yellow-orange in strong acidic media [23].
Indigo carmine (INS 132, E132) is a synthetic dye, soluble in water but sparingly soluble in ethanol. Originally extracted from a shrub species (Indigofera tinctoria), they are currently commonly obtained by chemical synthesis. It is very sensitive to light and oxidising agents such as bleach [23].
Brilliant blue (INS 133 or E133, Cl food blue 2, acid blue 9) is soluble in water and glycerol but only slightly soluble in ethanol. It is very sensitive to light and oxidising agents such as bleach [23].
All above-mentioned synthetic dyes are used in the food industry, especially in flavoured drinks, decorations and coatings for pastry products, pastry and fine bakery products, confectionery including breath-refreshing and chewing gum, flavoured fermented milk products, edible cheese rinds, desserts, edible ices, several types of preserves of red fruits, seasonings, snacks, and alcoholic beverages [23,25].
2.2. Natural Blue Pigments
Naturally coloured foods in yellow, red, and green can be found on the market. Naturally blue foods are mainly blue mould cheeses namely the so-called ‘blue-veined’ cheeses, which include French Roquefort (sheep’s milk cheese, mould produced by Penicillium roqueforti) [26], English Stilton, and Italian Gorgonzola (cow’s milk cheese, Penicillium glaucum), boasting of a history of more than a thousand years [27]. In addition, naturally blue foods include blue corn (Zea mays L.) and purple sweet potato (Ipomoea batatas L.) [28] varieties such as Purple Majesty [29], Vitelotte [30], Ayamurasaki [31], and Congo [32]. However, in regard to the blue colourants, their use is limited, and natural sources have low stability [6]. At present, only anthocyanins (INS 163) and phycocyanins (INS 134-spirulina) have regulatory approval for their use as blue colourants [5].
Despite the wide range of natural pigments that are used in the food industry, anthocyanins (ANCs), carotenoids, phycobiliproteins, betalains, and chlorophylls remain the most frequently applied [33]. Edible natural pigments not only add colour but also carry the potential for health benefits as bioactive substances [34].
Naturally occurring blue pigments belong to anthocyanins and eight major structural classes: azulenes, quinones, tetrapyrrole, phenazine, indole and pyridine alkaloids, metalloproteins, and organometallics [35].
Natural blue pigments can be categorised according to their origin into those produced by representatives of all five kingdoms of organisms, i.e., animals, plants, prokaryotes [36], fungi, and protists [10,17,37,38,39] and pigments of mineral origin.
2.2.1. Animals
In animals, blue colours usually arise from the optical effects of blue light reflected by nanoscale structures rather than from the occurrence of true blue pigment molecules [10,17]. The most exemplary are the blue wings of the butterfly Morpho sp. [40] and the blue feathers of the bird Sialia sp. However, they live mainly in Central and South America. In our part of the globe, i.e., in Poland, it is sometimes possible to observe a butterfly whose wing colour is also based on the same phenomenon. It is a rare purple emperor butterfly (Figure 4b). Similarly, poisonous tree frogs have few natural enemies, and the colour is a deterrent (Figure 4a). The deterrent mechanism is also used by the male moor frog (Rana arvalis) during mating season [41].

Figure 4.
Blue animals: (a) blue poison arrow frogs Dendrobates tinctorius ‘azureus’ (Zamość Zoo, SE, Poland, photo: A. Szmagara); (b) in sunlight, many shades of blue and purple can be seen on the wings of the purple emperor butterfly (Apatura iris) when viewed from an angle (Lublin, SE Poland, photo: A. Szmagara).
Some animals, like crustaceans and arthropods, have blue blood due to the respiratory protein haemocyanin instead of the iron-based haemoglobin [42]. The haemocyanin chromophore contains a binuclear copper active site that reversibly binds oxygen molecules [10].
The α-crustacyanin is an example of a blue-coloured pigment found in the carapace of lobsters (Nephrops) Leach, 1814 [43], and β-crustacyanin (blue) is found in lobsters (Homarus gammarus) [44].
From echinoderms, specifically from the vivid blue skin of calcified starfish called “blue star” (Linckia laevigata L.), a blue carotenoprotein called “linckiacyanin” (λmax = 612 nm) can be isolated [45].
In ancient times, indigo dye was isolated from the hypobranchial gland of the sea snail Hexaplex trunculus (known as Murex trunculus, Phyllonotus trunculus) [46].
One instance of tetrapyrroles are biliverdins, some of the few blue pigments that occur in the animal kingdom and are responsible for the bluing of bruises [10].
Of these blue pigments of animal origin, a few azulene chromophore-based compounds have been identified in marine organisms like the gorgonians Paramuricea chamaeleon [47], Euplexaura erecta [48], and Anthogorgia sp. [49] and the marine sponge Dysidea sp. [10].
2.2.2. Plants
Blue in nature is seemingly popular, but not among flowers [50]. Indeed, flowers perceived by humans as being blue are infrequent (Figure 5), representing, according to various authors, about 7% [51] to <10% of the nearly 300,000 known flowering plant species [52]. Blue flowers are phylogenetically limited, only occurring in 372 out of 14,038 genera of angiosperms worldwide, and in 53 out of 406 families [53]. Moreover, blue is much more common among biotically pollinated flowers than among wind-pollinated flowers, possibly suggesting that investigating the rarity of blue flowers requires a deeper understanding of how animals perceive these colours [50].

Figure 5.
Examples of flowers perceived as blue: (a) Pulmonaria officinalis, (b) Omphalodes verna, (c) Veronica chamaedrys, (d) Hyacinthus orientalis, (e) Myosotis sylvatica, (f) Geranium pratense, (g) Hydrangea macrophylla, (h) Scilla siberica, (i) Brunnera macrophylla, (j) Iris pallida, (k) Vinca minor, (l) Veronica persica, (m) Clematis alpina, (n) Viola tricolor, (o) Muscari armeniacum, (p) Cichorium intybus, and (q) Hepatica nobilis (photo: A. Szmagara).
The adaptive value of blue flowers should be enhanced by nutrient richness or other factors, abiotic and biotic, that can reduce additional costs of blue pigment synthesis [50]. They display key roles in plant propagation and defence mechanisms [54]. The subsequent production and localisation of anthocyanins in root, stem, and especially leaf tissues can allow the plant to develop resistance to environmental stresses like radiation, cold temperatures, and drought [55]. Such an example is research under which Centaurea cyanus cell cultures were evidently protected by anthocyanins from UVB-induced DNA damage [56].
The blue colour in plants is mainly related to anthocyanin content (Table 2). Customer demand for food products containing natural food ingredients and colours has prompted a global demand mainly for anthocyanins [57]. In general, health and nutritional supplements demand multipurpose properties, and anthocyanin is one of them. Anthocyanins are water-soluble pH-dependent plant pigments. Colour variation is affected by changing structural forms. When pH increases from acidic conditions, the flavylium cations (which normally look red, pH ≤ 3) become deprotonated, lose their colour (pH 3–6), and finally form quinonoidal bases (purple-blue; pH ≥ 6) [58]. Blue colour expression by anthocyanins may also result from co-pigmentation and/or the chelation of metal ions by the pigment, a mechanism widely observed in floral systems [58].
Among the anthocyanin pigments, the blue colour is characterised by delphinidin present in fruit: blackcurrants, blueberries, elderberries, and grapes [39,59], and typically these fruits are the most commonly used sources of anthocyanins in the food industry [60]. Anthocyanins are present also in other parts of plants, in flowers, leaves, and stems.
Anthocyanins extracted from butterfly pea (blue pea, Clitoria ternatea L.) flowers (Figure 6) are a prospective food colourant [61] and were investigated in rice and yoghurt drinks [62], in muffins [63], functional beverages [64], and powder drinks [65]. Clitoria flower extract at pH 5–8 and shikonin from gromwell roots (Lithospermum erythrorhizon) in alkaline solutions (pH 10–12) express blue hues [66]. The anthocyanins of red cabbage and purple sweet potato showed colours similar to indigo carmine (E132) at a pH of 8 [67].

Figure 6.
Infusion of dried butterfly pea flowers (Clitoria ternatea L.) (A. Szmagara).
Anthocyanin complexes with flavones and metal cations have been indicated as responsible for the blue colours of many floral systems [68]. In addition, certain nutrients, in particular aluminium Al3+ and Fe3+ cations, combined with a low soil pH ≥ 2.5 [69], may lead to a colour change from violet to blue in certain plants [50,55,58].
Commelinin, from Asiatic dayflowers, is a blue pigment that is stable in concentrated solutions at pH ≥ 2.4; when diluted, the molecule readily dissociates and loses its blue colour [68]. Pires and colleagues employed pigment extracted from cornflower (Centaurea cyanus L.) flowers, which contain high amounts of anthocyanins (TAC = 26 μg/g) to improve yoghurt [70].
Trichotomine and its glycosides are bis(indiol) alkaloids isolated from Kusagi berries (Clerodendron trichotomum Thun.), a shrub native to Asia. In addition to high absorption at 660 nm (pH5) and similarity to brilliant blue (E133), they appear to be stable and safe [10,71]. For reasons linked to the difficulty of large-scale cultivation and the low amounts of pigment in the fruit, the berries have not been commercially exploited, and as a compound that does not have a long history of safe consumption, it requires a comprehensive safety assessment before approval and commercialisation [8].
Gardenia sp. and Genipa americana L. are sources of iridoids, pigments that occur both in free and glycosylated forms (genipin and geniposide). Genipin, after being exposed to oxygen, reacts with primary amino acids and protein hydrolysates to form water-soluble blue pigments [72].
Vaccinium bracteatum leaves are also rich in iridoids, and their aqueous extracts were used by natives of the south of the Yangtze River as the materials of ‘Wu mi’ to dye rice into a blue colour, which turned dark blue after cooking [73].
Another blue compound with an extended quinone structure, ventilein A (λmax = 645 nm), was isolated as a minor constituent from Ventilago calyculata root bark [74] and from Ventilago goughii [10,75].
Azulenes belong to the class of plant-derived colourants, bicyclic azure-blue aromatic pigments, resulting from the fusion of a cyclopentadiene and a cycloheptatriene ring. They include, inter alia, chamazulene, a constituent of the volatile oil of some perennial herbs, like chamomile (Matricaria chamomilla L.) and yarrow (Achillea millefolium L.), which is a blue-coloured derivative resulting from the thermal decomposition of a colourless precursor during distillation. The use of azulenes as food colourants is constrained due to the slight water solubility and odour similar to naphthalene [6].
The blue colour can be related to blue copper proteins, which may be present, among others, where they usually have biosynthetic functions involving electron transfer [10,76]. Examples include stellacyanin [77] and plastocyanin [78], which are type I copper-binding proteins (cupredoxins) common in vascular plants. Other proteins of this type include cuscacyanin and phytocyaninin from cucumber [79] and umecyanin from horseradish root [80].

Table 2.
Selected natural blue pigments produced by plants.
Table 2.
Selected natural blue pigments produced by plants.
| Compound | Plant | λmax | ε | References |
|---|---|---|---|---|
| azulene | Artemisia sp. (oil) | 576 | 362 | [10] |
| diosindigo A | Diospyros sp. (tree; heartwood) | 697 | 28,180 | [10,81] |
| 2,12′-bis(hamazulenyl) | Ajania fruticulosa (oil) | 657 | 132 | [10] |
| indigotin | Indigofera tinctoria (Indigo plant) | 610 | 22,140 | [10] |
| genipocyanin G1 | Gardenia jasminoides, Genipa americana | 595 | 43,700 | [10] |
| guaiazulene | Matricaria chamomilla (oil) | 648 | 407 | [10] |
| lactarazulene | Artemisia sp. (oil) | 604 | 871 | [10] |
| oenin | Vitis vinifera skin, aged wine | 538 | 16,000 | [10] |
| portisin A | Vitis vinifera; aged wine | 587 | 82,900 | [10] |
| shikonin | Lithospermum erythrorhizon (roots) | n/a | n/a | [66] |
| ternatin | butterfly pea (Clitoria ternatea L.) | 548 | n/a | [82] |
| trichotomine | Clerodendron trichotomum (fruit callus) | 658 | 70,000 | [10] |
| ventilein A | Ventilago calyculata (root bark) Ventilago goughii | 645 | n/a | [10] |
where: λmax, wavelength of maximum absorption, ε, molar extinction coefficient (or molar absorptivity) in M−1 cm−1, n/a—data not available.
2.2.3. Protists
Blepharisma japonicum is one of the few protists that possesses a pigment. The pigments it produces, i.e., blepharins such as oxyblepharin A and analogues, are red, but their photoreaction products are blue (λmax = 592) [10,83].
2.2.4. Fungi
Fungi and microorganisms produce a diverse range of blue compounds (Table 3), usually as a response to environmental stress or predators [10]. Moulds are a source of pigments that can be exploited by the food industry. This is consistent with the changing market preferences of consumers, who need a source to replace synthetic pigments [84]. Filamentous fungi are among the natural sources currently being investigated for pigment production. Fusarium graminearum produces, inter alia, a blue pigment (6–O–demethyl– 5–deoxybostrycoidin anthrone) [85], and Lactarius sp. produces azulenes (blue) [84,85].
The usage of fungal pigments in food matrices also depends on their stability to pH fluctuations, heat, and UV light, which are attributes typically occurring in food processing [86].
Fungal species producing blue pigments are also found in extreme environments—a fungus of the genus Periconia isolated from a hypersaline environment (Puerto Rico) subjected to high solar radiation has been reported to produce a still unidentified and unusual pigment [87], and Antarctomyces pellizariae, a snow-dwelling ascomycete found on Robert Island, in the South Shetland Islands, Antarctica, produced a blue pigment [88].

Table 3.
Natural blue pigments produced by fungi.
Table 3.
Natural blue pigments produced by fungi.
| Compound | Organism | λmax | ε | References |
|---|---|---|---|---|
| albatrellin | Alabtrellus flettii (Basidiomycete) | 535 | 3162 | [10] |
| candidine | Candida lipolytica | 573 | 12,880 | [10] |
| corticin A | Corticium caeruleum | 565 | 6900 | [10] |
| guaiazulene | Lactarius sp. | 648 | 407 | [10] |
| lactarazulene | Lactarius sp. | 604 | 871 | [10] |
| sanguinone A | Mycena sanguinolenta | 578 | 437 | [10] |
| scleroderris blue | Gremmeniella abietina | 612 | 50,000 | [10] |
| variegatic acid anion | Basidiomycete fungi | 605 | n/a | [10] |
| Blue pigments (unnamed) | some fungi such as Penicillin sp. and Hypocrea sp. | n/a | n/a | [10,89] |
where: λmax, wavelength of maximum absorption, ε, molar extinction coefficient (or molar absorptivity) in M−1 cm−1, n/a—data not available.
Notwithstanding the listed benefits, there is a danger of mycotoxins associated with the use of fungal pigments in the food industry, as some fungi synthesise pigments together with mycotoxins. The presence of mycotoxins in pigments limits their use as additives [85,87]. For example, the EU and the US ban the consumption of Monascus pigments that are generated with the nephrotoxic and hepatotoxic citrinin mycotoxin, questioning their safe use [90], although Monascus red-coloured foods are consumed by over 1 billion Asians [37,87].
The development of fungal pigment production on a feasible scale and with industrial perspectives in mind is an approach worthy of consideration. Selecting efficient fungal strains with minimal/zero mycotoxin co-production and lower energy requirements is a crucial driver for production relevance [91]. Furthermore, major biological issues such as ensuring fungal strain stability, reducing mycotoxin co-production, and inducing higher pigment production titres can be addressed through the genetic engineering of fungal strains [92].
Anthocyanins, which are derived from red-pigmented fruit, are often used in the food industry as colouring agents, and among these, there are fungal infections with mycotoxin-producing microorganisms, including patulin [93].
2.2.5. Prokaryotes
The existence of pigments has been recorded throughout the microbial world, including bacteria, fungi, yeasts, algae, and protozoa [94]. These microorganisms can be isolated/cultivated from a variety of environmental sources, such as water, soil, plants, insects, and animals [94].
Pigmented species are numerous in the prokaryotic world (Table 4). The presence of blue pigments is echoed in the names of many genera and species of heterotrophic bacteria through the use of Greek, Latin, or neo-Latin terms, including Vibrio azureus, Gemmobacter caeruleus, Rheinheimera coerulea, Actinoallomurus caesius, Saccharomonospora cyanea, and Ciceribacter lividus. Phototrophic prokaryotes that use light absorbed by pigments can also exhibit a blue colour, as reflected in names such as Cyanothece [36]. Properties that include the colony colour and the presence of distinct pigments can be used for the taxonomic characterisation of prokaryotes [95], and the pigments may even be implicated as potential biosignatures of extraterrestrial life in astrobiology [96].
Carotenoids are rarely blue [36], like in the case of certain marine organisms. Examples include the caroteno-protein asteriarubin found in the starfish Asterias rubens (with astaxanthin derivatives) [97], crustacyanin known from Homarus gammarus (also with astaxanthin) [98], and marennine produced by the diatom Haslea ostrearia. Diatoms with blue tips, recorded as H. ostrearia, have been reported from almost all seas and oceans [99], in both the northern and southern hemispheres [100], for example, inhabiting oyster ponds on the Atlantic Coast (France) [101]. Moreover, other diatoms, like H. provincialis in the Mediterranean Sea [102] and Haslea karadagensis on the Crimean coast of the Black Sea (Ukraine) [100] produce blue pigments. A marennine-like blue pigment is also produced by the diatom Haslea nusantara found in the tropical region of the Southern Hemisphere in the Java Sea (Indonesia) [103], and cosmopolitan species of the blue diatom Haslea silbo, found on both sides of the Atlantic Ocean [104]. Some microalgae, which can grow in both saltwater or freshwater, and phytoplankton provide a rich source of blue phytobiliproteins [14,45]. Structurally, phycocyanins are bioactive peptides, classified based on their protein and pigment contents [105]. The best-known cyanobacterium is Spirulina spp., which has a high phycocyanin content of up to 20% dw.

Table 4.
Natural blue pigments produced by prokaryotes.
Table 4.
Natural blue pigments produced by prokaryotes.
| Compound | Organism | λmax | ε | References |
|---|---|---|---|---|
| actinorhodin | Streptomyces coelicolor | 640 | 25,300 | [10] |
| akashin A | Streptomyces sp. GW 48/1497 (marine) | 619 | 16,232 | [10] |
| ammosamide A | Streptomyces CNR-698 (marine) | 584 | 5200 | [10] |
| anthracyclinone-blue B | Streptomyces galilaeus mutant | 608 | 28,890 | [10,106] |
| bactobilin | Clostridium tetanomorphum | 633 | n/a | [10] |
| benthocyanin A | Streptomyces prunicolor | 638 | 16,200 | [10] |
| blue pigment (unnamed) | Arthrobacter sp., Nocardia sp. | 648 | 17,380 | [10] |
| daunorubicin | Streptomyces peucetius | 530 | 6000 | [10] |
| glaukothalin | Rheinheimera sp. HP1 (marine γ-Proteobacteria) | 636 | 32,360 | [10,107] |
| granaticin B | Streptomyces violaceoruber | 630 | 8910 | [10] |
| indigoidine | Corynebacterium insidiosum | 602 | 23,400 | [10,108] |
| indochrome A | Arthrobacter polychromogenes | 570 | 38,100 | [10] |
| kyanomycin | Nonomuria sp | 600 | 11,480 | [109] |
| lavanducyanin | Streptomyces aeriouvifer | 705 | 1700 | [10] |
| lemonnierin | Pseudomonas lemonnieri | 625 | 56,230 | [10] |
| marennine | Haslea ostrearia (marine diatom) | 672 | 7200 | [10,101] |
| N,N-dodecylindigoidine | Shewanella violacea DSS12 (marine) | 636 | n/a | [10] |
| phenazinomycin | Streptomyces sp. WK-2057 mycelia | 745 | 6600 | [10] |
| phycocyanobilin | Spirulina sp., common among cyanobacteria | 604 | 17,100 | [10] |
| prodeoxyviolacein | Chromobacterium violaceum | 609 | 25,000 | [10] |
| prodigiosin tetrapyrrole | Serratia marcescens, Hahella sp. (marine) | 588 | n/a | [10] |
| pyocyanin | Pseudomonas aeruginosa | 745 | 5800 | [10] |
| spirulina | Spirulina platensis (Arthrospira platensis) | 604 | 17,100 | [3] |
where: λmax, wavelength of maximum absorption, ε, molar extinction coefficient (or molar absorptivity) in M−1 cm−1, n/a—data not available.
The main limitation of phycocyanin is its low thermal resistance, resulting in a fading colour [110]. Thermostable phycocyanin from the red microalga Cyanidioschyzon merolae is a new natural blue food colourant [111]. Cyanobacteria Aphanizomenon sp. produce a blue pigment called phycocyanin, which is used in the food and beverage industry [19,110].
The bacteria produce a whole palette of colour shades, among them blue pigments with market potential: Corynebacterium insidiosum—indigoidine, Erwinia chrysanthemi, and Vogesella indigofera [19,92,108,112].
Among actinomycetes, Streptomyces coelicolor is well known to produce the blue pigment actinorhodin [20,108], a pigment used in the food industry as an ice cream colourant. Other soil-born Streptomyces sp., i.e., S. vietnamensis [113], S. shaanxiensis [114], S. caeruleatus [115], and Streptomyces sp. A1013Y [116], are also responsible for producing blue pigments.
The first reported study concerning microbial indigo production was conducted in 1928, using Pseudomonas strains isolated from soil [117]. Since then, many microorganisms have been shown to be able to produce indigo [17], especially the genera Pseudomonas, like P. fluorescens [118] and Acinetobacter [119]. For example, the blue pigment pyocyanin, extracted from Pseudomonas aeruginosa [108,112], when assessed for its utilisation as a food colouring with agar, gave a pleasant colour at 25 mg mL−1 [120].
Another pigment is glaukothalin produced by Rheinheimera sp. isolated from the Wadden Sea (Germany) and Øresund (Denmark) [107].
Indigoidine is a pigment from the carotenoid group [121], which is synthesised by very few microorganisms [88], namely Erwinia chrysanthemi [122], Phaeobacter sp. [123], marine Pseudomonas nigrifaciens [124], Rhodosporidium toruloides [125], Streptomyces chromofuscus [126], and Vogesella indigofera [127].
Natural anthraquinoid compounds are rarely blue under acidic conditions, but there are a few exceptions, which include the microbial metabolites of anthracyclinone-blue B from the Streptomyces galilaeus mutant [106] and kyanomycin obtained from Nonomuria sp. [109].
Among alga-derived sources, Porphyridium aerugineum red microalga-derived blue colour (λmax = 620 nm) has been used for acidic beverages. These pigments display a stable blue colour (not typical of blue colours from Cyanobacteria) at pH 4.0–5.0 for 1 month at room temperature or up to 40 min at 60 °C [33]. It can be successfully assayed in acidic, non-heat-treated carbonated beverages (Pepsi® Blue) or low-grade alcohol beverages (Bacardi Breezer®) [128].
2.2.6. Minerals
Minerals have long been used as pigments in food, cosmetics, and art. Currently, no blue minerals are used in the food industry. Only one mineral pigment with deep blue tones, ultramarine blue, is used in animal feed [58].
3. Health
The usage of natural pigments in place of synthetic ones is increasingly popular, as the former are widely regarded as safer, healthier, and more environmentally friendly [21,129]. Because of growing health awareness, replacing artificial colourants with natural alternatives is a prime challenge for the food industry [6].
3.1. Positive Health Benefits
Natural pigments not only offer colour but also provide prospective health benefits as important bioactive compounds [130].
The consumption of extracted pigments has been associated with human health promoting effects [45,131], but the bioaccessibility and bioavailability of pigments are determined by their chemical characteristics and the principal digestive processes.
An in vitro study with bacterial strains showed that after incubation with black rice anthocyanins, Bifidobacteria and Lactobacillus increased and pH values decreased [132]. Similarly, Sun et al. [133] found that after incubation of peonidine derivatives with various bacterial strains, pH values decreased and growth rates of B. bifidum, B. adolescentis, B. infantis, and L. acidophilus increased. They concluded that the metabolism of anthocyanins by microbiota bacteria results in a decrease in pH and provides a suitable medium for the proliferation of probiotic bacteria [134]. In order to enhance the effect of the anthocyanins and prevent their premature degradation, as well as modulate the gut microbiota, they can be supplied to the body after appropriate treatment, i.e., encapsulation with different materials [63,135].
Anthocyanins may not only enhance beneficial bacteria in gut microbiota but may also decrease several types of harmful bacteria. Flores et al. [136] found that Clostridium histolyticum was strongly reduced after colonic fermentation of some anthocyanins, i.e., cyanidin and delphinidin derivatives.
Factors affecting anthocyanin bioavailability include food processing (↑steam-blanching, ↑juice processing—milling, mashing, pressing, pasteurisation, ↓fermentation, ↓microwave cooking, ↓conservation, jam, squeeze), food matrix, hydrolysis by various enzymes in the small intestine and metabolism, and intestinal bacterial enzymatic activity in the gut microbiota [134].
Pigments can be used as antioxidants, anticancer agents, and antimicrobial agents [92]. Anthocyanins are known for their health-promoting properties, and these beneficial properties also include diabetes prevention [57]. The beneficial effects of an aqueous extract of Clitoria ternatea were studied in vivo in a mouse model of obesity and metabolic syndrome. The extract alleviated oxidative stress and inflammatory mediators. Moreover, the plasma leptin, free fatty acids, low-density lipoprotein cholesterol, and hepatic malondialdehyde levels were decreased. The extract significantly reduced total cholesterol and alleviated insulin resistance. The results showed that the aqueous extract of C. ternatea petals contains bioactive anthocyanins, which enforce significant hypolipidemic and anti-inflammatory effects by promoting reverse cholesterol transport in mice [137].
Iridoid genipin shows antimicrobial, anti-inflammatory, and anticancer properties, while geniposide (glycosylated form of genipin) has been described as a regulator of insulin and exerts protective effects in asthma [138].
The iridoid glucosides present in Vaccinium bracteatum Thunb. leaves [73] are responsible for the dark blue colour and most of the health-beneficial properties in the traditional food, ‘Wu mi’. According to a millennia-old custom, VBT leaves are harvested in the spring around Tombsweeping Day to make the cereal food ‘Wu mi’. The conventional process involves using the fresh leaves to homogenise and extract juice to soak rice, which is dyed dark blue after an soaking overnight. This process and product, ‘Wu mi’, was recorded in Shizhen Li’s Compendium of Materia Medica as a functional food with qi-beneficial effects to improve eyesight, revitalise the body, maintain agerasia, and prolong life. Currently, there is a trend to use ‘Wu mi’ as a health food for diabetic patients because no drastic symptoms of glycaemic response were observed in diabetic patients after consuming ‘Wu mi’ compared to rice. Moreover, the antimicrobial activity of Vaccinium bracteatum Thunb. Leaves, traditionally used for food preservation in China, was verified [139]. The aqueous extracts can successfully inhibit the growth of Escherichia coli, Staphylococcus aureus, and Bacillus subtilis.
In addition to their colourant properties, bacterial and fungal pigments exhibit numerous biological activities such as antioxidant, antimicrobial, and anticancer effects [92]. Bioactive compounds of bacterial isolates such as pyocyanin (blue-green) serve as novel compounds with antioxidative, antimicrobial, antiviral, antitumor, antiprotozoa, antioxidant, anticancer, and immunomodulatory activities and also reduce allergy risks [108,130]. Pyocyanin has been used as a bio-control agent and possesses antibacterial and antifungal actions [92,140]. This dye can inhibit the development of Escherichia coli [140], P. aeruginosa, Staphylococcus aureus, S. saprophyticus, and Enterococcus faecalis [108] and has proven antimicrobial activity towards Citrobacter sp., which is common in urinary tract infections and wounds [19].
Algae or cyanobacteria are famous for their bioactive ingredients and dietary components [105]. Phycocyanin peptides show health-promoting properties, such as immunomodulatory [141], anti-inflammatory, anti-cancer [142,143], antidiabetic [144], neuroprotective [145], hepatoprotective [146], and antioxidant [147] effects [105].
Marennine pigments are natural blue pigments that exhibit different biological actions—e.g., antioxidant and free radical scavenging properties [101] and antimicrobial and antiproliferative properties—with great potential for food applications [99]. Marennine, the structure of which stays unsolved [148], displays an antiproliferative effect on the growth of lung malignant cells in cell models [149], and exhibits antiviral [150] and anticoagulant properties [151].
3.2. Negative Health Effects
All three synthetic blue pigments (E131, E132, and E133) are water-soluble and anionic, and belong to the group of non-azoic dyes [152]. E131 and E133 are triphenylmethane dyes while E132 is pyrrole-based.
Although these dyes are used in monitored doses evaluated to guarantee consumers’ safety, their purity specifications allow concentrations of impurities such as aromatic non-sulphonated amines, with genotoxic and carcinogenic effects, and can be as high as 100 mg/kg of dye [5].
The synthetic dye brilliant blue (BB; E133) is a triphenylmethane dye, not an azo dye, but can induce reproductive and neurological disorders, and can also cause allergic reactions [153]. Allergic reactions were also reported after the use of the food dye patent blue (E131) [154].
Unfortunately, most artificially coloured foods, such as sweets, confectionery, ice cream, snacks, breakfast cereals, and cakes, are consumed daily by children [152]. Amounts in the range of 0.3–33 mg in sweets, 9.4–41.3 mg in breakfast cereals, 1.9–6 mg in ice cream, and 2.8–14.4 mg in snacks can be consumed daily by children [155]. It is even possible for a child to consume, from a variety of sources, cumulative amounts of colourings well above the recommended ADI levels, as each food may contain colourings at the maximum allowable concentration [5]. There are no reports of side effects in children caused by the aforementioned blue colourants. A large survey called the ‘Southampton study’ found behavioural disturbances, irritability, anxiety, and sleep disturbances after ingesting large amounts of artificial colourants and included six of the most common colourants, the so-called ‘Southampton six’, but none of the blue colours [156]. Allergic reactions may occur, including reactions caused by immune (immediate and late hypersensitivity) and non-immune (intolerance) mechanisms, which arise more rapidly in children. In addition, the long-term intake of these colourants can have prolonged consequences owing to the accumulation of these substances in the body [21,152]. The Acceptable Daily Intake (ADI) factors for individual synthetic dyes are (EU/US) [mg/kg bw] E131—(5/-); E132—(5/2.5); and E133—(6/12) [152].
Brilliant blue is reported to be marginally absorbable by the human gastrointestinal tract and is found unchanged in human faeces [57,157]. However, both brilliant blue and patent blue are absorbed via the oral mucosa, reaching the bloodstream. The studies by Lucova et al. [158] on the blue hues E131 (patent blue V) and E133 (brilliant blue) showed that both were able to pervade the bloodstream through the back of the tongue and, in slightly damaged skin, can infiltrate epithelial cells and reach the circulatory system without passing through the digestive system, making them more harmful as their opportunities for decomposition become minimal. Therefore, the use of these colourants in lollipops, chewing gum, sucking candies, and topical products should be strongly avoided [57].
There are reports of detrimental effects after chronic exposure to a high dose of brilliant blue by decreasing haemoglobin, haematocrit, and red blood cell count [9,157], as well as hepatocellular damage, kidney failure, and decreased sperm production as an effect of patent blue [158,159,160,161]. In vitro experiments on the effects of brilliant blue on serum and lymphocyte cell lines have shown that this dye has mutagenic and carcinogenic capacities [160] and causes retinal thinning [162].
Research has been reported on the issue of patent blue colourant binding to human serum albumin [157].
Brilliant blue represents the only approved food colouring that crosses the blood-brain barrier [29]. In an in vitro test, blue no. 1 (brilliant blue) inhibited neurite growth and worked in synergism with L-glutamic acid, which suggests the neurotoxicity potential. This is especially worrying for foetuses and children younger than 6 months of age in whom the blood-brain barrier is not fully developed [18]. blue no. 2 (indigo carmine) was found to be incapable of crossing the blood-brain barrier [18].
On the other hand, very few toxicological studies have been reported for natural food colourants, and only when they are adulterated by other synthetic food ingredients [57].
4. Quality Control—Food Safety Markers
The food industry uses blue dyes not only for consumption but also for food quality control, taking advantage of the pH-dependent colour change properties of the compound.
Freshness indicators are a type of smart packaging that offers non-invasive and non-destructive real-time monitoring of food quality and safety [163]. Based on research issued by FAO, approximately one-third of the total food produced for human consumption worldwide is wasted annually, costing an estimated USD 1 trillion. In industrialised countries (i.e., North America and European countries), food losses occur mainly at the distribution and consumption stages [164]. Freshness indicators have a promising role not only in protecting consumers from food poisoning but also in minimising food waste and increasing sustainability by offering more dynamic “use-by” dates [163,165].
As losses are particularly acute in the meat, fish, and poultry sectors—causing wastage of more than half of the total production—the need for the specific monitoring of these high-value products is highly desirable. The spoilage of these products is predominantly driven by microorganisms, which produce various metabolites (i.e., CO2, H2S, TVB-Ns) [166]. When these compounds begin to accumulate in the headspace of the package, a change in pH follows over time, which can be detected with a suitable pH-sensitive indicator. Spoilage metabolites react with the pH-sensitive dyes trapped in a matrix, resulting in a visible colour change readily differentiated by consumers [167]. The synthetic dyes phenol red (PR) and bromothymol blue (BTB) are used as real-time CO2-sensitive freshness indicators in the three-layer system using cellulose-based binder, which was developed to determine the freshness/spoilage of chicken breasts [168], as well as the freshness of shrimp [169].
Of the approximately one-third of food produced worldwide that is lost or wasted, it is estimated that fruits and vegetables account for between 40% and 50% of the total [170]. Blue dyes, like methylene blue, are also used as nano-fibre smart indicators for the direct monitoring of fruit freshness [171].
To date, the development of freshness indicators based on synthetic or natural pH-sensitive dyes has been reported [172,173,174]. While natural dyes are non-toxic, stability issues hinder the application of natural dyes in smart labels for the food packaging industry, not to mention that natural dyes are often applied with natural matrices (e.g., starch, chitosan) that are not commercially used [175,176].
The recent literature describes commonly used natural dyes for more sustainable smart packaging [66,177,178,179]. An excellent example of such pigments are water-soluble anthocyanins that exhibit the ability to change colour with pH change and, in addition, have strong antimicrobial and antioxidant properties, making them a suitable agent for active packaging [180]. Colour changes are due to a modification of the chemical structure of the phenolic substances in anthocyanins as a result of pH changes [181] and thus can be effectively used in the development of pH-sensitive smart packaging to assess food freshness and quality [182,183].
The freshness of food depends not only on the storage time but also on the temperature. Fresh food spoils easily due to the increase in contaminants at a certain temperature. Extreme temperatures and their fluctuations have a major impact on the shelf life of refrigerated food products. Monitoring and controlling the storage temperature of food products, such as fish, for example, is crucial as temperature largely determines the rate of microbial activity. The result, i.e., the growth of harmful microorganisms, can be the same in both cases, i.e., too high a temperature or too long a storage time. Temperature fluctuations can be encountered at any point in the supply chain. Therefore, monitoring and recording the temperature history of these products from production to loading, unloading, temperature cycling in cold stores, storage exposures, and domestic transport to consumption is very important. Solutions capable of assessing the temperature history of foods are known as time-temperature indicators (TTIs), whose mechanism of action in most microbial TTIs is through irreversible colour change. The dye produced by Pantoea agglomerans may have a potentially interesting application as a food temperature indicator. The normally yellow cells of P. agglomerans secrete a water-soluble dark blue dye into the environment of the agar medium at temperatures above 10 °C. Furthermore, this dye turns pink under acidic conditions [184].
Determining the freshness of food also relies on O2 and CO2 measurements [185], as CO2 inhibits the growth of bacteria and fungi, while it can also lower the pH in the food environment. The antimicrobial effect of CO2 is due to its ability to create an anaerobic environment, which prevents enzymatic decarboxylation. The accumulation of CO2 can also interfere with the membrane permeability of some microorganisms. Several microbial metabolites can affect the food pH, so controlling the amount of CO2 and monitoring pH changes can be an effective method of identifying food spoilage and is crucial for extending the shelf life of food products. Modified atmosphere packaging (MAP) for non-reactive foods typically consists of a low O2 concentration (0–2%) and a high CO2 concentration (20–80%).
Furthermore, moisture content is an essential indicator to be observed when testing food quality, as increased moisture content provides a favourable growth environment for microorganisms and fungi, thus becoming a safety issue for product consumption. In addition to microbial growth and the associated changes in CO2 content and pH level, moisture also results in a shorter shelf life due to the decomposition of the dry product, causing it to soften and become damp [185].
Natural colourants have been successfully used for the intelligent packaging of meat products [57] such as beef, pork, and poultry (red radish extract [186], purple sweet potatoes [182] and grape skin for chilled pork [187], blueberry [188] and red cabbage [189] for sausage [190], roselle (Hibiscus sabdariffa) for pork [183]), seafood and fish products such as shrimp and fish fillets (red cabbage for fish [191], red cabbage for prawns [192], butterfly pea flower (Clitoria ternatea) [193] and black rice bran for seafood [194]), and milk and dairy products (red cabbage for cheese [195], purple sweet potato for milk [13], grape for milk [196], red cabbage [197] and anthocyanins for milk [198]).
5. Ecological Aspects
The agri-food industry has become a world-leading sector, producing large quantities of highly coloured effluents [199].
Most of the colourants are water-soluble, making their isolation and treatment much more difficult and sometimes impossible by traditional coagulation, filtration, or adsorption methods, and they have been undegradable, persistent, and prevalent in the environment for many years [57]. Therefore, the treatment and reuse of industrial water is an important and urgent necessity. Drhimer and co-workers presented a method for removing brilliant blue by photocatalytic degradation with the presence of TiO2 as a catalyst and its further recycling. The results showed 98% degradation of brilliant blue at the laboratory scale and 93.3% and 75% at the pilot-scale using recirculation reactors with flow rates of 800 and 200 L·h−1, respectively [199].
In view of the harmfulness of brilliant blue to natural habitats, methods are being developed to remove it from the environment. Yousefi et al. investigated the oxidative degradation of brilliant blue FCF in the presence of zero-valent iron as a catalyst, and the degradation took place over 30 min [153] When using green synthesised silver nanoparticles (AgNPs) as a nanocatalyst for BB reductive degradation, the reaction was twice as fast [200].
On the other hand, natural colourants are biodegradable and non-toxic [57]. Natural dyes are eco-friendly and do not release harmful by-products into the environment. Furthermore, natural pigments are a renewable resource, and, in addition, waste and by-products can also be used as a source. Sometimes, however, the production of colourants like anthocyanins takes place using a method for utilising fruit agro-waste after juice production [177], such as blackberry residues [54,201], blueberry pomace [188], or a mixture of their extracts [180], which are substantial sources of natural colourants due to their high anthocyanin content. This has understandable ecological implications. Also, processed garlic and its blue-green discolourations, which are derivatives of pyrrole compounds, may be the source of pigments [58,202]. The reasoning behind these pigments is strictly related to a decrease in the content of the compound thiosulfinate, which also results in a reduction in the aroma of allium. Eight compounds were also identified as blue or green and are usually tri- or tetrapyrroles [202]. Microbial pigments can also be produced quickly and easily in a culture medium, which can even be waste [130].
Using waste to produce pigments and thus contributing to a reduction in waste is very important. However, scientists are trying to go one step further and use natural blue dyes to directly reduce waste. The possibility of using the microalgae Spirulina sp., which produce a natural blue dye, to degrade plastics such as PET (polyethylene terephthalate) and PP (polypropylene) in freshwater environments is being explored [203].
From an ecological point of view, even for natural pigments, the method of extraction is also very important. The spontaneous release of marennin by microorganisms of microalgae makes this natural pigment of great interest for industrial applications, since usually long extraction procedures using environmentally harmful organic solvents and various separation techniques are required to isolate the pigments from plants or flowers and result in the generation of organic waste from unused plant parts [99].
Natural pigments are produced from microalgae grown in bodies of water, such as open ponds, raceways, and natural lakes, especially in tropical and subtropical regions, and unfortunately require vast areas of land for production. A favourable solution can be combination with biofuel production [204]. Of course, sustainable practises and conditions, such as locations outside priority areas for biodiversity and carbon storage (e.g., tropical rainforests) prevent deforestation and promote sustainable biofuel production practises [205]. These technological improvements include the development of biorefinery systems, in which high-value co-products such as pigments and proteins [206] are extracted along with biofuels [207,208], and the recycling of water and nutrients from cultivation (e.g., through anaerobic digestion) [209].
Particular attention should be paid to the fact that the overexploitation of algae from their natural sites can lead to their total exploitation, as well as to possible environmental degradation and ecological disasters, as sand from the seabed is maintained by the algae and, when they are not present, the sand is washed away.
In addition to colourants, microbial pigments are used as bioindicators, e.g., for the detection of heavy metals. For example, Vogesella indigofera produces a blue pigment (indigoidine) under normal environmental growth conditions; however, after exposure to heavy metals such as harmful hexavalent chromium, no pigment production is observed [92,210].
Pigments are used to produce solar cells sensitised with natural dye. Natural pigments, such as anthocyanins, also have found very interesting applications as natural dye-sensitised solar cells (NDSSCs) [211,212]. They are based on a thick nanoporous layer of a semiconductor, titanium dioxide (TiO2), deposited by screen printing on a photoanode [213]. The TiO2 layer provides a large surface area available for the adsorption of the dye, which must have properties such as the ability to adsorb onto the semiconductor surface and absorb photons. The sensitiser absorbs incident photons and is excited from the ground state to the excited state. Among the existing types of photosensitisers for this type of solar cell, organic dyes show an advantage due to low cost, easy manipulation, and environmentally friendly properties over ruthenium [214,215] and platinum complexes [216], which show high toxicity and are rare in nature, revealing difficult, expensive, and environmentally unfriendly production.
6. Economical Aspects
Due to their environmentally friendly nature and less cumbersome production techniques, naturally produced dyes have become an effective substitute for toxic synthetic dyes [57].
For both natural and synthetic dyes, the total cost must be considered, from the beginning of the production chain—acquisition or breeding, extraction, purification, transportation, and storage—to its use, the effects on the human body, and disposal/degradation in the environment. Also important are stability in light, acidic-alkaline conditions, and oxidative agents and colour intensity.
However, since the colour blue is rare in nature, only a few blue pigments are currently available. Currently, only phycocyanin, anthocyanin, metal chelates, and pigments derived from genipin are used industrially to produce blue hues [6]. For example, phycocyanin produced by the cyanobacterium Arthrospira (Spirulina) platensis reached a global market size of $348 million in 2018, and the market is estimated to reach $779 million by 2026 [129].
Natural dyes rarely match the intensity of artificial dyes. Most natural and synthetic colour additives have εmax values of 105 or 106 [217]. Colourants with lower εmax values must be used in higher concentrations to achieve the desired appearance, thus increasing costs and the risk of off-flavour [10]. The blue colourants with the highest ε values are blue no. 1 (brilliant blue) = 134,000, blue no. 2 (indigo carmine) = 7920, phycocyanin = 17,100, and trichotomine = 70,000 [10].
Colourants must also be stable enough to avoid significant degradation during the distribution and sales of both the ingredient and the food or beverage, which may include storage from days to years under refrigerated or ambient conditions. The most typical causes of instability include heat, light, oxygen, acid, and exposure to oxidants such as ascorbic acid or trace metals [10]. In the case of the natural colourant spirulina, the colour of the pigment is not affected by pH or light, but it is sensitive to heat [57]. This means that phycocyanin is not stable enough for most food and beverage applications [10]. But there are applications in which this natural dye with a bright blue colour is essential: fermented milk products, ice creams, soft drinks, milkshakes, desserts, and sweet cake decorations [57].
The extraction of natural dye from finished materials is time-consuming and labour-intensive. This can be demonstrated in detail by the example of spirulina extraction. The extraction process involves breaking down the cell wall, extracting the water-soluble protein-pigment complex, and then concentrating, and if necessary, purifying it [6]. Pigment losses of approximately 50% have been reported at elevated temperatures (>50 °C), regardless of the drying method (e.g., spray, oven, sun drying). Maceration at temperatures below 30 °C and air circulation through the suspension increase the extraction yield and prevent unpleasant odours [218]. Optimal pigment recovery has been achieved by repeated freeze-thaw cycles using fresh wet materials [219]. The residual cells are removed by filtration or centrifugation. Its use as a food colouring requires further concentration of the crude extract by vacuum distillation at moderate temperatures. Nevertheless, additional sterile filtration is recommended for food safety reasons. The addition of sugar improves the stability of the pigment during the heating process [218]. To increase the purity from ∼0.8 in the crude extract to more than 4, ammonium sulphate precipitation, ultrafiltration, charcoal adsorption with or without the addition of chitosan, and different chromatographic purification steps have been used [220]. However, these methods are time- and cost-intensive, as they involve a large number of processing steps. An option for purification on a larger scale can be aqueous two-phase extraction with the use of a polyethylene glycol/potassium phosphate mixture [221,222].
Spirulina is mainly produced by Arthrospira platensis (commonly named Spirulina platensis), the photoautotropic cyanobacteria cultured in open ponds and natural lakes, especially in tropical and subtropical regions. Despite the generally high phycocyanin content (60–70 mg/g), productivity is restricted because the biomass is strongly dependent on optimal light conditions [6,220]. In addition, there is a risk of foreign organism contamination, which presents problems in achieving the hygiene standards demanded for food applications.
Eriksen [220] recommended another organism, a unicellular rhodophyte, Galdieria sulphuraria, evaluated earlier as a potential human food source, as an appropriate alternative to spirulina production. Despite the pigment content being relatively low (10–25 mg/g), heterotrophic cultivation in the dark enables large-scale axenic production, resulting in a high biomass and thus a much higher phycocyanin yield. However, pigment extraction is hampered by the difficulty in breaking down cellulose-rich cell walls, with the consequence that G. sulphuraria has not yet been used for commercial spirulina production.
The economically favourable solution seems to be the production of spirulina as a high-value byproduct in the production of biofuel from microalgae [206,207,208]. Also beneficial is the recycling of water and nutrients from crops (e.g., through anaerobic digestion) [209], the development of more efficient harvesting techniques [223], and co-location with free sources of nutrients or CO2 from industry [224].
In order to increase the yield and production of natural colourants, a suitable extraction procedure must be chosen. Different extraction techniques to extract natural pigments were described in detail in [54], and include traditional processes that are simple, economical, and straightforward to use, like Soxhlet extraction, maceration, and hydrodistillation. In addition, non-traditional extraction methods, often known as green extraction techniques, have recently emerged as a viable alternative to conventional extraction as they are less solvent- and time-consuming. Other new technologies, such as ultra-high pressure, vacuum cavitation, high-voltage electrical discharge, ohmic heating, pulsed electric fields, mechanical-chemical methods, and high-pressure homogenisation, have proven to be very effective methods for the extraction of plant pigments. The most common extraction methods for the recovery of anthocyanins from natural sources include solid-liquid extraction, supercritical fluid extraction, ultrasound-assisted extraction, and microwave-assisted extraction [57,225].
The criterion choice of suitable pigment extraction method is not only the yield and price but also the critical external factor affecting pigment stability and potential application [226]. Newer methods, such as ultrasound-assisted extraction, which uses cavitation effects and shear forces, significantly reduces extraction time but requires strict control to prevent the violation of anthocyanin structural integrity [227]. Microwave-assisted extraction, which is known for its efficiency, uses natural ionic conduction and dipole relaxation to accelerate solvent temperature, reduce viscosity, and facilitate anthocyanin diffusion also reduces extraction time. Strict control of parameters is necessary to prevent structural damage. Supercritical carbon dioxide extraction, which is a state-of-the-art technology with properties between gas and liquid, offers advantages such as high efficiency, environmentally friendly processing, safety, and minimal contamination. This method is particularly suited to heat-sensitive substances such as anthocyanins, but the cost of equipment and technological investment limit large-scale industrial applications [228].
The latest combined methods, such as ultrasound-assisted enzymatic extraction and ultrasound-assisted deep eutectic solvent extraction, seamlessly integrate enzymatic and deep eutectic solvent processes. Enzymatic methods using cellulase and pectinase increase anthocyanin yields, while ultrasonic processing further improves extraction efficiency [229].
Moreover, some pigment stabilisation methods are used. Increasing the stability of anthocyanins can be achieved either by modifying the structure of the molecule (intrinsic factors) through copigmentation, acylation, and biosynthesis or by controlling environmental factors (extrinsic factors) [230]. Encapsulation is an advanced technology that embeds bioactive substances in solid particles or liquid vesicles. In this way, it provides precise control over the release of these bioactive substances while providing benefits such as masking unwanted odours, improving stability, and maintaining bioactivity. Anthocyanins, which are light- and heat-sensitive, benefit greatly from encapsulation techniques to maintain their stability and extend their shelf life [231]. Various methods of encapsulation have been employed, including spray drying, freeze-drying, ionic gelation, liposome entrapment, nanoencapsulation, yeast encapsulation, phase separation, emulsification, complexation, molecular inclusion, and supercritical antisolvent precipitation [54].
Microcapsules, liposomes, and nanoparticles are commonly used for anthocyanin encapsulation [232]. Often composite materials, such as mixtures of carbohydrates with proteins and polysaccharides, are used to obtain the expected encapsulation properties. Typical materials for anthocyanins include carbohydrates, proteins, and water-soluble plant gums, as well as hydrocolloids, each of which offers unique advantages such as solubility and stability [135,233].
It also seems to be economically and commercially advantageous to produce pigments from microorganisms, e.g., carotenoids and Monascus pigments, because microbial growth conditions can be easily controlled [57]. Natural microbial pigments play a significant role as food colourings due to their low-cost production, easier extraction, high yield, and lack of raw material shortages and seasonal variations [92,234].
Marennine, produced by the diatom Haslea ostrearia, appears to be a good candidate for a new colourant for food and beverage products, especially with very good stability of the blue colour (λmax = 620 nm) in acidic conditions (pH 1–6) [129] and at pH 8 (λmax = 677 nm) [99], and with stability in the presence of common food antioxidants and preservatives such as ascorbic acid and sodium sulfite [99]. Indeed, most blue natural colourants lose their blue shades in an acidic medium: anthocyanins turn purple-pink at low pH, natural blue anthraquinone, except for anthracyclinone-blue B and kyanomycin, and quinoid dyes become red or orange, and phycocyanin from spirulina is unstable [10]. The shade of purified marennine blue is unaffected by low pH, and remains unaltered even after acidic hydrolysis in harsh conditions (100 °C, 16 h) [129].
Another natural pigment, jagua blue (from the fruit of Genipa americana L.) may be a potential new source of blue pigment. In the pH range of 3.6–5.0, the shade of jagua blue solutions was similar to that of blue no. 2 (indigo carmine) but jagua blue showed significantly higher stability during storage (t1/2 = 86–105 days) than blue no. 2 (t1/2 ≤ 9 days) and was less susceptible to acidic pH 3.6 (t1/2 = 86 days) than spirulina (t1/2 = 70 days) [235].
The cost of sourcing natural colours becomes the basis for adulteration—so-called economically motivated adulteration, fraud, or unfair competition [236]. Adulteration of food with illegal colours has been reported as a public safety concern [237], and there have been cases of the adulteration of many different types of food products in recent years [238]. Natural and artificial colouring agents can be visually identical, so any detection method would need to be able to provide information about the chemical composition of the colourants that are present. Hence, the need for food control to confirm the use of the correct/declared colours by analytical methods. Existing methods for the detection of artificial colourants include chemical determination by chromatographic methods such as HPLC [239], mass spectrometry [240], and capillary electrophoresis [241]. These methods typically require labour-intensive sample preparation techniques and specialised equipment and training. An alternative method is surface-enhanced Raman spectroscopy (SERS) [236].
The development of cost-effective and viable technologies for the preparation of natural food colourings and their use in food is currently a great challenge and a major need. Their incorporation into food products is very difficult because they are chemically unstable and show poor bioavailability. During food fortification, efficient technologies are needed to prevent the degradation of pigments and reserve their bioaccessibility in the human gastrointestinal system. There are good perspectives for the inclusion of plant pigments in the food industry. Encapsulation (micro- and nano-) is an excellent process to enhance its bioaccessibility, digestibility, and controlled and targeted release [45,54,57].
Stability can also be achieved by immobilising pigments in solids, such as zeolite matrices [45,242]. Stability studies of ‘Maya Blue’ (a mixture of indigo and palygorskite clay) have been conducted in the context of the development of hybrid pigments adsorbed in nanoporous solids such as mesoporous silica, zeolites, layered double hydroxides, and smectite, which allowed a degree of control over the photochemical and photophysical properties of the pigments, depending on the guest-host interaction, with the key parameters being the matching of pore size to the dimensions of the guest molecule [242,243]. This approach is not always ideal for food applications but is suitable for environmentally friendly applications.
One of the leading candidates for a replacement for brilliant blue is trichotomine (a natural colourant) from Kusagi berries from the Asian shrub Clerodendron trichotomum [10].
7. Socio-Cultural Aspects
7.1. Food Colour
Colour represents a crucial role in the acceptability of foods to enhance their actual appearance and quality [54]. The colour of food not only provides information about its edibility but also its palatability [1]. The evaluation of the hedonic reward from food is highly susceptible to suggestion [244].
The red colour of food is very common in nature and is typical of ripe fruits and fresh meat. In contrast, there are not many naturally occurring blue-coloured foods, and sometimes “blue” even means inedible (such as mould) (Figure 7) [244]. Therefore, it is not surprising that the colour red is considered appetising, while blue acts as an appetite suppressant [1,245,246]. In the past, the colour blue in food meant: watch out, don’t touch, don’t move, don’t eat. The blue colour of food meant that the food was spoilt, harmful, and dangerous, and this was associated with the presence of mould. The repellent effect of the blue colour is still used today and has remained, for example, in the colouring of granulated molluscicide agents (i.e., metaldehyde, Snacol 5 GB, BROS) to make the colouring repellent to other animals.
Figure 7.
Blue mould on food (photo: A. Szmagara).
Food colour promotes psychological effects. The uncommon blue colour brings the sensation of artificial food and is not satiable. Studies by Suzuki et al. [247] proved this effect. The authors observed tasters’ behaviour when eating identical soups with different colours: yellow, white, and blue. They aimed to verify whether the colour could affect the feeling of satiety. After ingestion, blue soups showed significantly lower satiety rates than white and yellow soups. The authors concluded that food colour might be associated with the expected flavour and produce different effects depending on their experiences. Since blue foods are rare, the visual signs of the blue colour may have caused cognitive unfamiliarity due to the difficulty of associating colours with some type of sensory experience [5].
Paakki and co-workers [32] investigated the consumer preference by comparing “traditional” yellow potatoes and blue potatoes to verify the feeling that blue food evokes. Only 28% of consumers selected blue potatoes due to their desire to try new and unusual things. Tasters who preferred blue potatoes tended to be more neophilic, responding positively to the consumption and application of atypical colours in food, variety, and trend-seeking, and younger than those choosing yellow potatoes [5,32].
Sometimes food products are given distinctive colours to help attract consumer attention [248]. This is likely to have been the case with the striking blue ‘Bolt from the blue’ drink introduced by Innocent Smoothies [29].
Moreover, the same colour of a drink can have a completely different meaning when shown in a plastic bathroom cup as opposed to a cocktail glass. In the former case, a blue-coloured drink can be interpreted as being associated with mouthwash and therefore associated with the taste of mint, whereas when the same colour is seen in a cocktail glass, it can be interpreted as signifying the orange flavour of blue curaçao [1].
Blue dyes are often added to candy, so the candy colour combinations most appealing to children were examined, as well as children and adolescents’ acceptance of single candy colours and two-colour combinations [5,249], and in all combinations blue played an essential role in attracting them.
Galetović and co-workers [250] added phytobiliproteins from cyanobacteria to milk beverages, achieving satisfactory sensory acceptability, which increased after information that the blue-coloured dairy beverage had antioxidant properties. Natural blue-coloured products may attract consumers because of their unusual, differentiated colour, evoking a feeling of “novelty” and providing enticement to eat [251]. The association of “artificial” or “less filling” is also expected to be reduced for blue foods [6,32].
7.2. Background—Packing, Dishware, and Light
Cognitive thoughts about colours are stored in memory along with associated mental concepts or experiences, which may result from everyday knowledge of similar products and colours or learnt stereotypes and symbolic meanings of colours [252]. A cool colour, such as blue, evokes sedative emotions [253] and is the colour of peace and hope [254]. In contrast, blue should also not be forgotten in relation to defining sadness, loneliness, and depression.
Very interesting aspects concerning the impact of the colour blue are described, which do not relate to the food itself but to its surroundings. These concerned the colour of the packaging, the colour of the dishware, and also the lighting.
Few natural foods have blue colouring, but this shade is very commonly found on packaging [255]. Some research explores the relationship between packaging colour and product attributes [256]. For instance, warm-coloured packaging might be closely associated with the tastiness or sweetness of food, while cool colours might relate to the freshness or health attributes of the product [257]. Huang and Lu [252] researched consumer preferences and observed that products in blue packaging were associated with higher healthy perceptions and were more likely to be purchased than products in red packaging. Similarly, a study by Hallez and colleagues [258] examined how packaging design in warm or cool colours and the presence or absence of nutrition and environmental claims influenced consumer evaluations. They showed that green and blue packaging designs, representing cool colours, led to higher health and sustainability perceptions [258,259].
Pereira [260], when investigating the symbolic function of blue on food packaging, found that this colour is the second most recurrent. It is a typical colour for food products that incorporate the idea of diet in their design, reflecting a context in which the blue colour represents the concepts of health and beauty promoted by today’s society as part of a wider process of promoting values and symbols. Its most common role was to indicate specific nutritional qualities associated with the concept of healthy eating, symbolising the ideas of moderation, restraint, and rationality. The blue colour’s role was to indicate characteristics related to healthy eating, reduction in ingredients (“light”, “zero”, “decaffeinated” and “skimmed”, but mainly associated with a reduced fat content), or even to highlight premium products from the others. The study shows that meaning-making is based on the relationship of opposition between the colour blue and the warm colours characteristic of the food world. Blue was also the second most dominant colour in dairy packaging since this colour conveys calmness, reliability, and hygiene [261], as well as freshness and coldness [262,263]. Therefore, the blue colour should be used when the intention is to convey the idea to the consumer that it is a high value-added product because of rigorous quality control correlated with a healthy-looking product, or because it is a food that stands out from others of the same class.
The impact of the background is also the impact of the dishware. Even the blue colour of the vessel can discourage consumption [7]. More precisely, according to Crumpacker [264], ‘the term blue plate special became popular during the Great Depression because restaurant owners found that diners were satisfied with smaller portions of food if it was served on blue plates’.
Some studies have determined the effect of the colour of light (effect of light) on the desire to eat the products underneath. Cho and colleagues [265] reported that blue lighting significantly reduced food intake for breakfast in studied Swedish men but not in women when compared to yellow and white lighting. The overall intensity of the taste and the overall impression of the food did not differ significantly between the three lighting colours. This study provides empirical evidence that lighting colour can modulate meal size. In particular, blue lighting can reduce the amount of food consumed in men without reducing food acceptability [244,265]. Several other studies have also reported that blue lighting impairs people’s perception (or the eye appeal) of various fruits and vegetables [266,267].
7.3. Thermal Perception and Feeling Thirsty
The colour of food is known to modulate not only consumers’ motivation to eat but also to influence the thermal perception of food. Suzuki and co-workers [247], conducting a study on a female group, found that the blue colour of soup significantly decreased willingness to eat, ratings of palatability and comfort, but also the heat judgement of the meal, and significantly increased anxiety feelings compared to the white and yellow soups. This study provides new evidence that the colours of hot food can modulate postprandial satiety, thermal sensations, and peripheral temperature. Such an effect of colour may be useful for dietary strategies for people who need to control their appetite.
It is also known that the colour of the dish influences the perception of the temperature of the food being served [247]. With regard to foods consumed hot or warm, serving them in a blue dish will discourage eating, which can be used to control appetite. In the case of cold drinks, the blue colour of the dish further influences the consumer’s feelings, intensifying the sensation of cold. A cold beverage item was evaluated as more “thirst-quenching” when served in a blue glass than in a green, yellow, or red glass” [268], and a hot beverage was perceived as the warmest when served in a red cup, followed by yellow, green, and blue cups [263]. Thus, the colour (warm or cold) of a beverage and its container has been shown to affect the perception of the temperature of the beverage [247].
In relation to taste, the colour blue is known to be associated with the perception of a salty taste. In general, sweet is widely linked with reddish/pink colours, sour with yellow/green, salty with white/blue, and bitter with black [1,269].
There is also an additional troublesome aspect related to the natural origin of dyes, specifically, the dye can have an unpleasant taste or odour that will be an obstacle to its use in food. Such a situation is reported in the case of spirulina, which is obtained from algae. That said, blue spirulina (going under the brand name Blue Majik), one of the blue dyes that is currently the most popular, can taint the food to which it is added with an unpleasant fishy taste [270]. Attempts have even been made to adapt production methods to remove the unpleasant fishy smell/taste through the use of basil leaf extract [271].
8. Politics and Governance—Regulations
Before the 19th century, food was mostly prepared at home. Only occasionally and by wealthy people were dyes extracted from animals, vegetables, or minerals used as food decoration [272,273]. After the Industrial Revolution, food was increasingly processed at a large scale, and new technologies, including preservation, altered the natural appearance of foods [3]. Therefore, inexpensive and stable synthetic and mineral dyes with high colouring power and light shades were excessively used for a wide range of food products. Some of these dyes, such as indigo, had toxic properties. To restrain their overuse in the United States (US), a list of approved food colourants was published in 1906 (US Food and Drug Act) [272]. Among the blue dyes on this list was indigotine. In 1929, a further blue dye, brilliant blue, was added to the list.
In 1960, the Colour Additives Amendment included the Delaney clause banning additives that cause cancer in humans or animals [274]. In the UK, several colours were forbidden in 1923, and a legally binding list of permitted colours was established in 1957 [272]. The Joint Expert Committee on Food Additives (JECFA), co-managed by the FAO and WHO, was established as early as 1956 and has since comprehensively reviewed 1500 substances, including food colourants, setting standards for safety assessment worldwide. The WHO’s International Programme on Chemical Safety (IPCS) also assesses the health effects of chemicals in food.
Currently, colourings are probably the most strictly regulated food additives worldwide [273]. In most countries, the use of food additives is regulated by strict rules. However, despite global cooperation and harmonisation efforts, regulations vary from country to country. Legislation determines which substances can be used, their source, purity, in which foods, and the concentrations that can be added. However, legislation is based on the traditional local use of the additive. Worldwide, the two main authorities regulating food additives are the European Food Safety Authority (EFSA) in the European Union (EU) and the US Food and Drug Administration (FDA) in the United States [275]. Since 1956, the Joint Expert Committee on Food Additives (JECFA), which acts as an international scientific committee of experts responsible for assessing the risks associated with the consumption of additives, has made recommendations to the Food and Agriculture Organisation (FAO), the World Health Organisation (WHO), and 51 member countries of both organisations [276]. The food category system comprises 16 main categories, for a total of 266 categories, including subcategories [277]. The JECFA assigns all additives an acceptable value in the relevant category.
The introduction of new natural colours is often significant for local people in their place of origin. For example, the list of colours awaiting adoption at stage 5/8 currently includes the natural blue dye of plant origin, jagua (genipin-glycine) blue (INS 183) [278], and the record is annotated with the comment that Colombia has expressed their appreciation for the conclusions regarding the use of jagua (genipin-glycine) blue (INS 183), highlighting the significant benefits of its inclusion in the GSFA for indigenous communities in their country and the Latin American region, while recognising jagua (genipin-glycine) blue (INS 183) as a valuable resource and stressing that its inclusion in the GSFA will open up new commercial opportunities and drive biodiversity conservation and the adoption of sustainable agricultural practises.
As trade becomes increasingly global, sourcing from different continents, regional and national regulations can become trade barriers that increase transaction costs, or more specifically compliance costs, leading to negative economic impacts. In addition to the economic aspects, technical barriers can impede the global use of all available food through free trade to reduce hunger and poverty [279].
In both the EU and the US, only approved dyes can be used in food. However, the regulations for food dyes in the EU and the US are different and embedded in two very different legal frameworks. As a result, the definition of food colourant, approval requirements, approved colourants and their specifications, restrictions on their use, and responsibilities for rulemaking and compliance monitoring are different. The main regulations governing the use of colours in food in the EU and the US are listed in Table 5.

Table 5.
Adequate Daily Intake (ADI) values of synthetic dyes.
Food exporters and importers are perplexed since the same food colour may be allowed in one country but banned in another [283]. Patent blue V in the USA, Australia, and New Zealand is banned, but in the EU it is used as E131 in food. Indigo carmine is used in the EU (E132) and US (FD&C No. 2) but is already banned in Japan, Australia, and Norway. Brilliant blue is currently still permitted in the EU (E133) and US (FD&C No. 1) with different ADI values (Table 5). However, at the national level, some European countries (Austria, Belgium, France, Norway, Sweden, Switzerland, and Germany) have already banned its use [284,285]. For ease of identification, each pigment is given a colour index name (C.I.) and a colour index number (C.I. No.) (Table 6). The colour index number conveys some information about the chemical composition of the pigment, as some pigment classes have specific number ranges [286]. In 1989, the CCFA created the International Numbering System (INS) to provide an international numeric system for identifying additives in the list of food ingredients. It is also an alternative to using a specific name, often long and complicated [9,275].

Table 6.
Artificial and natural dyes authorised in various countries (modified, according to [9,217]).
Phycocyanin, a pigment found in spirulina species discussed earlier in this review, is the most stable natural dye for the blue shade. However, it is not permitted as a raw material for dye production in the US and EU. Spirulina sp. is classified by the FDA as a cyanobacterium and is considered a food rather than a source of dye; therefore, it does not comply with the regulations of 21CFR 73.260 [3]. Due to conflictual restrictions imposed on some natural dyes in different countries, the approval and commercialisation of safe and new dyes is usually delayed. Therefore, the joint regulatory authority can issue international standard codes for natural colourants to ensure consumer safety and health.
The principal markets of food-grade biocolourants are in the US, EU, Japan, and the emerging markets are in China, India, and South Korea. Developing countries such as India and China can play an important role in supplying natural colours either in processed forms or as raw materials to the EU markets due to their favourable climatic and production conditions coupled with the growth in their middle-income families. More research supporting the safety of food products could influence regulation in the US, Asia, and the EU [57].
9. Perspectives
The search for sustainable and harmless colourings for food and beverages continues. Currently, one of the biggest challenges in the colourant industry is obtaining natural blue colourants. Because, in line with consumers’ preferences, interest in blue food products is not waning, food and beverage manufacturers continue to look for natural blue colour as an alternative to synthetic dyes. Both for food intended for direct consumption and for monitoring the freshness of products. Some blue dyes have been discovered whose structure has not yet been determined [10] and properties are not known in detail, so their use in food is not yet possible.
Unfortunately, the use of NPs is limited by their intrinsic molecular instability, as well as their higher cost compared to synthetic pigments and the need for higher concentrations to achieve equivalent colour intensity [45,287]. However, as mentioned earlier, they are associated with serious stability and pH limitation issues. For example, acidic foods that use temperatures above 60 °C during processing cannot use anthocyanins as a blue pigment [5]. The stability of the natural pigments determines the use, even for different products in a related food group, as in a study of the fortification of yoghurt and fermented milk with the natural blue pigments, during which better results were obtained in the case of yoghurt for butterfly pea extract, while in the case of fermented milk for spirulina [288].
A prospect may be the production of blue pigments using genetic modification. For example, genetically modified blue silkworms capable of producing high levels of natural blue pigment (indigoidin) in the posterior silk gland have been successfully obtained [289], as have blue flowers obtained through genetic modification rather than artificial dyeing. Many ornamental plants grown for high-volume cut flowers, such as rose and chrysanthemum, lily, carnation, and gerbera, lack key genes for the production of the blue pigment delphinidin or do not have an intracellular environment suitable for blue colour [290]. Blue flowers, such as rose and chrysanthemum, were developed using genetic engineering at the end of the 20th century [11,291]. After studying the flavonoid profiles of hundreds of rose cultivars, suitable hosts were selected for high delphinidin accumulation to produce a blue flower colour. Overexpression of the viola F3′5′H gene resulted in the accumulation of delphinidin to 95% of all anthocyanidins and resulted in a new blue colour [11,292]. Also, dahlia, phalaenopsis, lily, and chrysanthemum are examples of these genetically modified blue flowers [290].
Consumer awareness of the multifaceted benefits of food colourants will encourage the commercialisation of safe and novel food colourants. Many consumers are unaware that in 2008, Nestle replaced the blue colour in candy with a colour derived from spirulina [57,293].
The socio-cultural aspects presented are of great importance in working towards an increasingly healthy society (obesity-related metabolic diseases), dwindling food resources, and therefore the need to eat smaller portions of food. It is worth building on previous findings about the effect of blue surroundings and dishes on reducing appetite.
Similarly, the blue colour of the vessels influencing the satisfaction of the sensation of thirst can have both positive and negative implications when we seemingly feel less thirsty, but in fact the correct level of hydration is not provided to the organism.
The health-promoting properties of plants such as Clitoria have great potential, and interest in colour can be used to encourage regular consumption of infusions with health-promoting properties.
Furthermore, in addition to looking for new sources, better methods of obtaining natural dyes from already known sources still need to be developed. For example, spray-drying, freeze-drying, encapsulation, and coatings improve the colour of anthocyanins [57].
There is undoubtedly an advantage of natural dyes over synthetic ones, but among the natural ones, those of microbial origin seem to be the most economically viable [94]. For several reasons, successively: dyes of animal origin—the necessity of breeding, the difficulty of extraction, of plant origin—cultivation dependent on year-round availability, external factors, weather, climate of plant growth, i.e., temperature and humidity, natural disasters and human factors related to plant harvesting, protection against pathogens and pests, multi-stage extraction, stability, and water-solubility of the pigment [130]. The exploitation of plants on a large scale may lead to the loss of valuable species. Microorganisms such as fungi, bacteria, algae, and actinomycetes are a reliable and readily available alternative source of natural pigments [287]. Microorganisms are advantageous over plants for biopigment production in terms of availability, labour and cost efficiency (easy and rapid multiplication in a low-cost medium), yield, stability, easy downstream processing, and weather- and season-independent growth [92,294]. Microbial culture can be achieved by solid and submerged fermentation on natural raw materials or industrial organic wastes. Microbial pigments not only act as colouring agents in various food and cosmetic industries but also have anticancer, antioxidant, anti-inflammatory, and antimicrobial properties [112]. The main disadvantage of dyes of fungal origin is the possibility of contamination with mycotoxins. Moreover, bacteria have numerous advantages over fungi for pigment production, including a shorter life cycle and relatively easy genetic modification [19,92,295]. However, research on bacterial blue pigments is limited, probably because few bacteria are capable of producing blue pigment [295].
The authorised artificial colours, indigo carmine (E132) and brilliant blue (E133), are used in so many groups of food, in foodstuffs for everyday use, even where one would not expect them to be at all, and even in foods considered to be popular such as pickled cucumbers (together with natural colours such as chlorophyll copper complex, annatto extract, turmeric, beta-carotene, oleoresin of paprika, and other authorised artificial colours such as fast green FCF, tartrazine, sunset yellow FCF) at 300 mg/kg singly or in combination [278]. In addition, synthetic blue dyes, E132 and E133, are still permitted in various food groups: dairy and dairy-based desserts, bakery products of various types, and vegetable purées. Although a natural substitute, the algae-derived colour E134, is already permitted in these same groups, at specific maximum concentration levels (GMP—Good Manufacture Practise).
Of the natural colours, the following are permitted as food colourants: anthocyanins (INS 163) [296]—Table 7, with the last of which, 163 (xi)—butterfly pea flower extract, which was introduced in 2021 [297] and, from 2023, spirulina extract (INS 134), which can be used in the indicated food category under the conditions of good manufacturing practise (GMP).

Table 7.
Permitted colourants of the anthocyanin group [296].
Moreover, the plant-derived colour INS 183—jagua (genipin-glycine) blue is on the legislative path [278] at step 5 of 8. The legislative pathway for the approval of new blue colours, i.e., gardenia blue (INS 165) is currently underway ([278] in: Appendix XI, Part A: List of substances used as food additives proposed for evaluation by JECFA). Proposed by Japan, the gardenia blue dye is intended to add or restore colour to food. It thus imparts colour to food, thereby improving the organoleptic properties of that food, which would otherwise not be coloured or whose colour has been affected by processing and needs to be restored. The proposed maximum use levels are based on the amount of colour required technologically to achieve the desired effect in different foods. The use of blue gardenia pigments in foods is still not legal in the United States or European Union but is in Japan, China, and Korea [10,58,298].
It is sometimes found that natural colourings (INS 163) are used or proposed in much higher quantities than synthetic ones in the same food group (Table 8).

Table 8.
Maximum food colourant (allowed and under legislation process) levels in different food groups, according to the JECFA [277,278].
10. Conclusions
Colour is an important part of consumers’ perception of food and an important factor in determining its taste. Therefore, the food industry uses natural or synthetic dyes to make processed foods more appealing to consumers. Colourant is added to compensate for colour loss due to processing or storage, or to compensate for differences in natural colour. Dye is also added to products without natural colour, such as confectionery and sugar-based soft drinks, to make them attractive to consumers and to match their expectations. However, consumer preference for naturally derived colours—which are closely linked to the image of healthy, safe, and good-quality products—has risen sharply as a large number of synthetic colours have recognised side effects on human health.
The subject of blue food colourants fits into the three traditional pillars of sustainability: environmental, social, and economic, and is expanded by another: the human aspect, culture, and safety.
Currently, it is necessary to link this theme in particular with the health-related aspects of sustainability. It seems most necessary to introduce changing consumption and production patterns. Blue pigments have not only colourful properties but need to be chosen for their rich health-promoting, antioxidant, anticancer, and antidiabetic properties, which are now important for a large group of people, and which are not described in detail in this review.
From an economic point of view, the calculus may not be obvious at first sight since synthetic dyes are apparently cheap, but considering the overall balance, the choice is clear. There are several blue natural dyes that could become good substitutes for synthetic dyes (spirulina, marennine, trichotomine, genipin, and clitoria), those in the approval process, and many that are still waiting to be tested for use in the food industry. It is necessary to select specific pigments in order to obtain at the same time specific health benefits tailored to individual needs, also using modern techniques of non-destructive extraction of the most valuable components and improving the stability and bioavailability of the pigment.
Aspects related to the sustainability of natural pigments and stability in the presence of agents that could potentially cause decomposition also include attempts to advance technological solutions (encapsulation) for their use by increasing bioavailability. More research is required to assess the chemical composition of food colourants derived from natural sources. Furthermore, the stability of the food colourants has to be considered when they are used in food products with varied pH and temperature ranges.
At the same time, lower-cost production solutions are being sought, such as production from waste or the production of pigment as a valuable by-product.
Sociological and cultural aspects related to the possibility of reducing consumption, not only through the disincentive blue colour of the food itself but also the blue surroundings of the food, are also irrelevant and undeniably important, especially for diabetics and those promoting a healthy lifestyle.
The aspect of quality control of food using blue dyes is also very important, as is the environmental aspect of using one group of blue dyes (anthocyanins) to build solar cells.
It should also be remembered that blue is one of the primary colours and is sometimes used, including in the food industry, to create derived colours, especially green (in combination with yellow dyes, natural or synthetic, sometimes also harmful).
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We gratefully acknowledge the professional and useful comments of the editor and the reviewers.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Spence, C. On the Psychological Impact of Food Colour. Flavour 2015, 4, 21. [Google Scholar] [CrossRef]
- FDA. 21 CFR Part 70-Color Additives; Food Drug Administration: Jersey City, NJ, USA, 2023. [Google Scholar]
- Downham, A.; Collins, P. Colouring Our Foods in the Last and next Millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Meggos, H. Food Colors-An International Perspective. Manuf. Confect. 1995, 75, 59. [Google Scholar]
- Neves, M.I.L.; Silva, E.K.; Meireles, M.A.A. Natural Blue Food Colorants: Consumer Acceptance, Current Alternatives, Trends, Challenges, and Future Strategies. Trends Food Sci. Technol. 2021, 112, 163–173. [Google Scholar] [CrossRef]
- Buchweitz, M. 17—Natural Solutions for Blue Colors in Food. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 355–384. ISBN 978-0-08-100371-8. [Google Scholar]
- Spence, C. What Is so Unappealing about Blue Food and Drink? Int. J. Gastron. Food Sci. 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Lee, S.-M.; Lee, K.-T.; Lee, S.-H.; Song, J.-K. Origin of Human Colour Preference for Food. J. Food Eng. 2013, 119, 508–515. [Google Scholar] [CrossRef]
- Mota, I.G.C.; Neves, R.A.M.D.; Nascimento, S.S.D.C.; Maciel, B.L.L.; Morais, A.H.D.A.; Passos, T.S. Artificial Dyes: Health Risks and the Need for Revision of International Regulations. Food Rev. Int. 2023, 39, 1578–1593. [Google Scholar] [CrossRef]
- Newsome, A.G.; Culver, C.A.; van Breemen, R.B. Nature’s Palette: The Search for Natural Blue Colorants. J. Agric. Food Chem. 2014, 62, 6498–6511. [Google Scholar] [CrossRef]
- Pina, F.; Basílio, N.; Parola, A.J.; Melo, M.J.; Oliveira, J.; de Freitas, V. The Triumph of the Blue in Nature and in Anthropocene. Dye. Pigment. 2023, 210, 110925. [Google Scholar] [CrossRef]
- Newsome, A.G.; Murphy, B.T.; van Breemen, R.B. Isolation and Characterization of Natural Blue Pigments from Underexplored Sources. In Physical Methods in Food Analysis; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1138, pp. 105–125. ISBN 978-0-8412-2884-9. [Google Scholar]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and Characterization of Intelligent Starch/PVA Films for Simultaneous Colorimetric Indication and Antimicrobial Activity for Food Packaging Applications. Carbohydr. Polym. 2017, 157, 842–849. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Gani, A.; Noor, N. Valorization of Natural Colors as Health-Promoting Bioactive Compounds: Phytochemical Profile, Extraction Techniques, and Pharmacological Perspectives. Food Chem. 2021, 362, 130141. [Google Scholar] [CrossRef] [PubMed]
- Paik, Y.-S.; Lee, C.-M.; Cho, M.-H.; Hahn, T.-R. Physical Stability of the Blue Pigments Formed from Geniposide of Gardenia Fruits: Effects of PH, Temperature, and Light. J. Agric. Food Chem. 2001, 49, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Neri-Numa, I.A.; Angolini, C.F.F.; Bicas, J.L.; Ruiz, A.L.T.G.; Pastore, G.M. Iridoid Blue-Based Pigments of Genipa americana L.(Rubiaceae) Extract: Influence of PH and Temperature on Color Stability and Antioxidant Capacity during In Vitro Simulated Digestion. Food Chem. 2018, 263, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.d.; Bicas, J.L. Natural Blue Pigments and Bikaverin. Microbiol. Res. 2021, 244, 126653. [Google Scholar] [CrossRef] [PubMed]
- Kobylewski, S.; Jacobson, M.F. Toxicology of Food Dyes. Int. J. Occup. Environ. Health 2012, 18, 220–246. [Google Scholar] [CrossRef]
- Devi, M.; Ramakrishnan, E.; Deka, S.; Parasar, D.P. Bacteria as a Source of Biopigments and Their Potential Applications. J. Microbiol. Methods 2024, 219, 106907. [Google Scholar] [CrossRef]
- Manikprabhu, D.; Lingappa, K. γ Actinorhodin a Natural and Attorney Source for Synthetic Dye to Detect Acid Production of Fungi. Saudi J. Biol. Sci. 2013, 20, 163–168. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In Vivo Antioxidant Activity of Phenolic Compounds: Facts and Gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- König, J. 2—Food Colour Additives of Synthetic Origin. In Colour Additives for Foods and Beverages; Scotter, M.J., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 35–60. ISBN 978-1-78242-011-8. [Google Scholar]
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food Colour Additives: A Synoptical Overview on Their Chemical Properties, Applications in Food Products, and Health Side Effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef]
- Bevziuk, K.; Chebotarev, A.; Fizer, M.; Klochkova, A.; Pliuta, K.; Snigur, D. Protonation of Patented Blue V in Aqueous Solutions: Theoretical and Experimental Studies. J. Chem. Sci. 2018, 130, 12. [Google Scholar] [CrossRef]
- EU. Commission Regulation (EU) No 1129/2011 of 11 November 2011 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by Establishing a Union List of Food Additives. Off. J. Eur. Union L 2011, L295, 1–177. [Google Scholar]
- Cleere, M.M.; Novodvorska, M.; Geib, E.; Whittaker, J.; Dalton, H.; Salih, N.; Hewitt, S.; Kokolski, M.; Brock, M.; Dyer, P.S. New Colours for Old in the Blue-Cheese Fungus Penicillium roqueforti. NPJ Sci. Food 2024, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- López-Díaz, T.M.; Alegría, Á.; Rodríguez-Calleja, J.M.; Combarros-Fuertes, P.; Fresno, J.M.; Santos, J.A.; Flórez, A.B.; Mayo, B. Blue Cheeses: Microbiology and Its Role in the Sensory Characteristics. Dairy 2023, 4, 410–422. [Google Scholar] [CrossRef]
- Hernández-Santos, B.; Lerdo-Reyes, A.A.; Téllez-Morales, J.A.; Rodríguez-Miranda, J. Chemical Composition, Techno-Functional Properties, and Bioactive Components of Blends of Blue Corn/Purple Sweet Potato for Its Possible Application in the Food Industry. J. Food Meas. Charact. 2023, 17, 1909–1920. [Google Scholar] [CrossRef]
- Spence, C. What’s the Story with Blue Steak? On the Unexpected Popularity of Blue Foods. Front. Psychol. 2021, 12, 638703. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.N.; Rodrigues, R.M.; Genisheva, Z.; Oliveira, H.; de Freitas, V.; Teixeira, J.A.; Vicente, A.A. Effects of Ohmic Heating on Extraction of Food-Grade Phytochemicals from Colored Potato. LWT 2016, 74, 493–503. [Google Scholar] [CrossRef]
- Tensiska, T.; Marta, H.; Cahyana, Y.; Amirah, N.S. Application of Encapsulated Anthocyanin Pigments from Purple Sweet Potato (Ipomoea Batatas L.) in Jelly Drink. KnE Life Sci. 2017, 2, 482–493. [Google Scholar] [CrossRef]
- Paakki, M.; Sandell, M.; Hopia, A. Consumer’s Reactions to Natural, Atypically Colored Foods: An Investigation Using Blue Potatoes. J. Sens. Stud. 2016, 31, 78–89. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Yañez, J.; Mojica, L.; Luna-Vital, D.A. Technological Applications of Natural Colorants in Food Systems: A Review. Foods 2021, 10, 634. [Google Scholar] [CrossRef]
- Pailliè-Jiménez, M.E.; Stincone, P.; Brandelli, A. Natural Pigments of Microbial Origin. Front. Sustain. Food Syst. 2020, 4, 590439. [Google Scholar] [CrossRef]
- Xu, F.; Gage, D.; Zhan, J. Efficient Production of Indigoidine in Escherichia Coli. J. Ind. Microbiol. Biotechnol. 2015, 42, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Jehlička, J.; Edwards, H.G.M.; Oren, A. Analysis of Brown, Violet and Blue Pigments of Microorganisms by Raman Spectroscopy. TrAC Trends Anal. Chem. 2022, 146, 116501. [Google Scholar] [CrossRef]
- Dufossé, L. Chapter 19—Current and Potential Natural Pigments from Microorganisms (Bacteria, Yeasts, Fungi, and Microalgae). In Handbook on Natural Pigments in Food and Beverages, 2nd ed.; Schweiggert, R., Ed.; Woodhead Publishing: Sawston, UK, 2024; pp. 419–436. ISBN 978-0-323-99608-2. [Google Scholar]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Şahin, E. Chapter 4—Natural Dyes and Pigments in Food and Beverages. In Renewable Dyes and Pigments; Ul Islam, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 49–76. ISBN 978-0-443-15213-9. [Google Scholar]
- Singh, T.; Pandey, V.K.; Dash, K.K.; Zanwar, S.; Singh, R. Natural Bio-Colorant and Pigments: Sources and Applications in Food Processing. J. Agric. Food Res. 2023, 12, 100628. [Google Scholar] [CrossRef]
- de Campos Vidal, B. Butterfly Scale Form Birefringence Related to Photonics. Micron 2011, 42, 801–807. [Google Scholar] [CrossRef]
- Sztatecsny, M.; Preininger, D.; Freudmann, A.; Loretto, M.-C.; Maier, F.; Hödl, W. Don’t Get the Blues: Conspicuous Nuptial Colouration of Male Moor Frogs (Rana arvalis) Supports Visual Mate Recognition during Scramble Competition in Large Breeding Aggregations. Behav. Ecol. Sociobiol. 2012, 66, 1587–1593. [Google Scholar] [CrossRef]
- Magnus, K.A.; Ton-That, H.; Carpenter, J.E. Recent Structural Work on the Oxygen Transport Protein Hemocyanin. Chem. Rev. 1994, 94, 727–735. [Google Scholar] [CrossRef]
- Quarmby, R.; Nordens, D.A.; Zagalsky, P.F.; Ceccaldi, H.J.; Daumas, R. Studies on the Quaternary Structure of the Lobster Exoskeleton Carotenoprotein, Crustacyanin. Comp. Biochem. Physiol. Part B Comp. Biochem. 1977, 56, 55–61. [Google Scholar] [CrossRef]
- Nishida, Y.; Berg, P.C.; Shakersain, B.; Hecht, K.; Takikawa, A.; Tao, R.; Kakuta, Y.; Uragami, C.; Hashimoto, H.; Misawa, N. Astaxanthin: Past, Present, and Future. Mar. Drugs 2023, 21, 514. [Google Scholar] [CrossRef]
- Jurić, S.; Jurić, M.; Król-Kilińska, Ż.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, Stability, Encapsulation and Application of Natural Pigments in Foods. Food Rev. Int. 2022, 38, 1735–1790. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; De Villemereuil, V.; Magiatis, P.; Polychronopoulos, P.; Vougogiannopoulou, K.; Skaltsounis, A. Identification of the Coloring Constituents of Four Natural Indigoid Dyes. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1491–1502. [Google Scholar] [CrossRef]
- Imre, S.; Thomson, R.; Yalhi, B. Linderazulene, a New Naturally Occurring Pigment from the Gorgonian Paramuricea Chamaeleon. Experientia 1981, 37, 442–443. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. The Nature and Role of Pigments of Marine Invertebrates. Nat. Prod. Rep. 2006, 23, 223–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yu, S.; van Ofwegen, L.; Proksch, P.; Lin, W. Anthogorgienes A–O, New Guaiazulene-Derived Terpenoids from a Chinese Gorgonian Anthogorgia Species, and Their Antifouling and Antibiotic Activities. J. Agric. Food Chem. 2012, 60, 112–123. [Google Scholar] [CrossRef]
- Dyer, A.G.; Jentsch, A.; Burd, M.; Garcia, J.E.; Giejsztowt, J.; Camargo, M.G.G.; Tjørve, E.; Tjørve, K.M.C.; White, P.; Shrestha, M. Fragmentary Blue: Resolving the Rarity Paradox in Flower Colors. Front. Plant Sci. 2021, 11, 618203. [Google Scholar] [CrossRef] [PubMed]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P. TRY Plant Trait Database—Enhanced Coverage and Open Acces. Glob. Change Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, D. Nature’s Palette: The Science of Plant Color; University of Chicago Press: Chicago, IL, USA, 2010; ISBN 978-0-226-47105-1. [Google Scholar]
- Gottsberger, G.; Gottlieb, O.R. Blue Flower Pigmentation and Evolutionary Advancement. Biochem. Syst. Ecol. 1981, 9, 13–18. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural Colorants from Plant Pigments and Their Encapsulation: An Emerging Window for the Food Industry. LWT 2022, 153, 112527. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Takahashi, A.; Takeda, K.; Ohnishi, T. Light-Induced Anthocyanin Reduces the Extent of Damage to DNA in UV-Irradiated Centaurea cyanus Cells in Culture. Plant Cell Physiol. 1991, 32, 541–547. [Google Scholar] [CrossRef]
- Renita, A.A.; Gajaria, T.K.; Sathish, S.; Kumar, J.A.; Lakshmi, D.S.; Kujawa, J.; Kujawski, W. Progress and Prospective of the Industrial Development and Applications of Eco-Friendly Colorants: An Insight into Environmental Impact and Sustainability Issues. Foods 2023, 12, 1521. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.-M.; Onofrei, C.; Turturică, M.; Bahrim, G.; Râpeanu, G.; Stănciuc, N. The Kinetics of Thermal Degradation of Polyphenolic Compounds from Elderberry (Sambucus nigra L.) Extract. Food Sci. Technol. Int. 2018, 24, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, A.; Ayyıldız, S. Food Additives: Colorants. In Science within Food: Up-to-Date Advances on Research and Educational Ideas; Food Science Series N°1; Formatex Research Center: Badajoz, Spain, 2017; pp. 87–94. ISBN 978-84-947512-1-9. [Google Scholar]
- Netravati Gomez, S.; Pathrose, B.; Mini, R.N.; Meagle, J.P.; Kuruvila, B. Comparative Evaluation of Anthocyanin Pigment Yield and Its Attributes from Butterfly Pea (Clitorea ternatea L.) Flowers as Prospective Food Colorant Using Different Extraction Methods. Future Foods 2022, 6, 100199. [Google Scholar] [CrossRef]
- Nikijuluw, C.; Andarwulan, N. Color Characteristic of Butterfly Pea (Clitoria ternatea L.) Anthocyanin Extracts and Brilliant Blue. Sci. Repos. 2013. [Google Scholar]
- Ab Rashid, S.; Tong, W.Y.; Leong, C.R.; Abdul Ghazali, N.M.; Taher, M.A.; Ahmad, N.; Tan, W.-N.; Teo, S.H. Anthocyanin Microcapsule from Clitoria ternatea: Potential Bio-Preservative and Blue Colorant for Baked Food Products. Arab. J. Sci. Eng. 2021, 46, 65–72. [Google Scholar] [CrossRef]
- Lakshan, S.A.T.; Jayanath, N.Y.; Abeysekera, W.P.K.M.; Abeysekera, W.K.S.M. A Commercial Potential Blue Pea (Clitoria ternatea L.) Flower Extract Incorporated Beverage Having Functional Properties. Evid.-Based Complement. Altern. Med. 2019, 2019, e2916914. [Google Scholar] [CrossRef]
- Marpaung, A.M.; Lee, M.; Kartawiria, I.S. The Development of Butterfly Pea (Clitoria ternatea) Flower Powder Drink by Co-Crystallization. Indones. Food Sci. Technol. J. 2020, 3, 34–37. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.-J.; Rhim, J.-W. Effect of Blended Colorants of Anthocyanin and Shikonin on Carboxymethyl Cellulose/Agar-Based Smart Packaging Film. Int. J. Biol. Macromol. 2021, 183, 305–315. [Google Scholar] [CrossRef]
- Ahmadiani, N. Anthocyanin Based Blue Colorants. Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2012. [Google Scholar]
- Yoshida, K.; Mori, M.; Kondo, T. Blue Flower Color Development by Anthocyanins: From Chemical Structure to Cell Physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Giusti, M.M. Bathochromic and Hyperchromic Effects of Aluminum Salt Complexation by Anthocyanins from Edible Sources for Blue Color Development. J. Agric. Food Chem. 2014, 62, 6955–6965. [Google Scholar] [CrossRef]
- Pires, T.C.; Dias, M.I.; Barros, L.; Barreira, J.C.; Santos-Buelga, C.; Ferreira, I.C. Incorporation of Natural Colorants Obtained from Edible Flowers in Yogurts. LWT 2018, 97, 668–675. [Google Scholar] [CrossRef]
- Koda, T.; Ichi, T.; Odake, K.; Furuta, H.; Sekiya, J. Blue Pigment Formation by Clerodendron trichotomum Callus. Biosci. Biotechnol. Biochem. 1992, 56, 2020–2022. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Vardanega, R.; Meireles, M.A.A. Extraction of Natural Blue Colorant from Genipa americana L. Using Green Technologies: Techno-Economic Evaluation. Food Bioprod. Process. 2019, 114, 132–143. [Google Scholar] [CrossRef]
- Fan, M.; Li, T.; Li, Y.; Qian, H.; Zhang, H.; Rao, Z.; Wang, L. Vaccinium bracteatum Thunb. as a Promising Resource of Bioactive Compounds with Health Benefits: An Updated Review. Food Chem. 2021, 356, 129738. [Google Scholar] [CrossRef] [PubMed]
- Hanumaiah, T.; Marshall, D.S.; Rao, B.; Rao, J.; Rao, K.; Thomson, R.H. Naphthoquinone-Lactones and Extended Quinones from Ventilago Calyculata. Phytochemistry 1985, 24, 2669–2672. [Google Scholar] [CrossRef]
- Jammula, S.; Pepalla, S.; Rao, K.; Rao, P. Chemical Components from Ventilago goughii, Gamble. Acta Cienc. Indica Chem. 1993, 19, 36. [Google Scholar]
- Nersissian, A.; Immoos, C.; Hill, M.; Hart, P.; Williams, G.; Herrmann, R.; Valentine, J. Uclacyanins, Stellacyanins, and Plantacyanins Are Distinct Subfamilies of Phytocyanins: Plant-Specific Mononuclear Blue Copper Proteins. Protein Sci. A Publ. Protein Soc. 1998, 7, 1915–1929. [Google Scholar] [CrossRef]
- Hart, P.J.; Eisenberg, D.; Nersissian, A.M.; Valentine, J.S.; Herrmann, R.G.; Nalbandyan, R.M. A Missing Link in Cupredoxins: Crystal Structure of Cucumber Stellacyanin at 1.6 Å Resolution. Protein Sci. 1996, 5, 2175–2183. [Google Scholar] [CrossRef]
- Guss, J.M.; Freeman, H.C. Structure of Oxidized Poplar Plastocyanin at 1·6 Å Resolution. J. Mol. Biol. 1983, 169, 521–563. [Google Scholar] [CrossRef]
- Guss, J.M.; Merritt, E.; Phizackerley, R.P.; Freeman, H. The Structure of a Phytocyanin, the Basic Blue Protein from Cucumber, Refined at 1.8 Å Resolution. J. Mol. Biol. 1996, 262, 686–705. [Google Scholar] [CrossRef]
- Paul, K.; Stigbrand, T. Umecyanin, a Novel Intensely Blue Copper Protein from Horseradish Root. Biochim. Biophys. Acta 1970, 221, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Sankaram, A.V.; Reddy, V.V.N.; Sidhu, G.S. A Pentacyclic Quinone Diosindigo B from the Heartwood of Diospyros Melanoxylon. Phytochemistry 1981, 20, 1093–1096. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant Capacities and Total Phenolic Contents of 62 Fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Spitzner, D.; Höfle, G.; Klein, I.; Pohlan, S.; Ammermann, D.; Jaenicke, L. On the Structure of Oxyblepharismin and Its Formation from Blepharismin. Tetrahedron Lett. 1998, 39, 4003–4006. [Google Scholar] [CrossRef]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and Colorants from Filamentous Fungi. In Fungal Metabolites; Springer: Berlin/Heidelberg, Germany, 2017; pp. 499–568. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal Pigments and Their Prospects in Different Industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef]
- Molelekoa, T.B.J.; da Silva, L.S.; Regnier, T.; Augustyn, W. Application and Stability of Fungal Pigments Using Jelly Sweets as a Food Model System. Int. J. Food Sci. Technol. 2023, 58, 6761–6774. [Google Scholar] [CrossRef]
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.; Sutthiwong, N. Filamentous Fungi Are Large-Scale Producers of Pigments and Colorants for the Food Industry. Curr. Opin. Biotechnol. 2014, 26, 56–61. [Google Scholar] [CrossRef]
- Sajjad, W.; Din, G.; Rafiq, M.; Iqbal, A.; Khan, S.; Zada, S.; Ali, B.; Kang, S. Pigment Production by Cold-Adapted Bacteria and Fungi: Colorful Tale of Cryosphere with Wide Range Applications. Extremophiles 2020, 24, 447–473. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Stadler, M.; Voglmayr, H. Blue Pigment in Hypocrea caerulescens Sp. Nov. and Two Additional New Species in Sect. Trichoderma. Mycologia 2012, 104, 925–941. [Google Scholar] [CrossRef]
- Carvalho, J.C.d.; Oishi, B.O.; Pandey, A.; Soccol, C.R. Biopigments from Monascus: Strains Selection, Citrinin Production and Color Stability. Braz. Arch. Biol. Technol. 2005, 48, 885–894. [Google Scholar] [CrossRef]
- Meruvu, H.; Dos Santos, J.C. Colors of Life: A Review on Fungal Pigments. Crit. Rev. Biotechnol. 2021, 41, 1153–1177. [Google Scholar] [CrossRef] [PubMed]
- Narsing Rao, M.P.; Xiao, M.; Li, W.-J. Fungal and Bacterial Pigments: Secondary Metabolites with Wide Applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef] [PubMed]
- Sadok, I.; Szmagara, A.; Krzyszczak, A. Validated QuEChERS-Based UHPLC-ESI-MS/MS Method for the Postharvest Control of Patulin (Mycotoxin) Contamination in Red-Pigmented Fruits. Food Chem. 2023, 400, 134066. [Google Scholar] [CrossRef]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial Pigments as Natural Color Sources: Current Trends and Future Perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678. [Google Scholar] [CrossRef]
- Oren, A. Characterization of Pigments of Prokaryotes and Their Use in Taxonomy and Classification. In Methods in Microbiology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 38, pp. 261–282. ISBN 0580-9517. [Google Scholar]
- Schwieterman, E.W.; Cockell, C.S.; Meadows, V.S. Nonphotosynthetic Pigments as Potential Biosignatures. Astrobiology 2015, 15, 341–361. [Google Scholar] [CrossRef]
- Bernhard, K.; Englert, G.; Meister, W.; Vecchi, M.; Renstrøm, B.; Liaaen-Jensen, S. Carotenoids of the Carotenoprotein Asteriarubin. Optical Purity of Asterinic Acid. Helv. Chim. Acta 1982, 65, 2224–2229. [Google Scholar] [CrossRef]
- Britton, G.; Weesie, R.J.; Askin, D.; Warburton, J.D.; Gallardo-Guerrero, L.; Jansen, F.J.; de Groot, H.J.M.; Lugtenburg, J.; Cornard, J.-P.; Merlin, J.-C. Carotenoid Blues: Structural Studies on Carotenoproteins. Pure Appl. Chem. 1997, 69, 2075–2084. [Google Scholar] [CrossRef]
- Gabed, N.; Verret, F.; Peticca, A.; Kryvoruchko, I.; Gastineau, R.; Bosson, O.; Séveno, J.; Davidovich, O.; Davidovich, N.; Witkowski, A.; et al. What Was Old Is New Again: The Pennate Diatom Haslea ostrearia (Gaillon) Simonsen in the Multi-Omic Age. Mar. Drugs 2022, 20, 234. [Google Scholar] [CrossRef]
- Gastineau, R.; Davidovich, N.A.; Bardeau, J.-F.; Caruso, A.; Leignel, V.; Hardivillier, Y.; Jacquette, B.; Davidovich, O.I.; Rincé, Y.; Gaudin, P.; et al. Haslea Karadagensis (Bacillariophyta): A Second Blue Diatom, Recorded from the Black Sea and Producing a Novel Blue Pigment. Eur. J. Phycol. 2012, 47, 469–479. [Google Scholar] [CrossRef]
- Pouvreau, J.-B.; Morancais, M.; Taran, F.; Rosa, P.; Dufossé, L.; Guérard, F.; Pin, S.; Fleurence, J.; Pondaven, P. Antioxidant and Free Radical Scavenging Properties of Marennine, a Blue-Green Polyphenolic Pigment from the Diatom Haslea Ostrearia (Gaillon/Bory) Simonsen Responsible for the Natural Greening of Cultured Oysters. J. Agric. Food Chem. 2008, 56, 6278–6286. [Google Scholar] [CrossRef] [PubMed]
- Gastineau, R.; Hansen, G.; Davidovich, N.A.; Davidovich, O.; Bardeau, J.-F.; Kaczmarska, I.; Ehrman, J.M.; Leignel, V.; Hardivillier, Y.; Jacquette, B. A New Blue-Pigmented Hasleoid Diatom, Haslea provincialis, from the Mediterranean Sea. Eur. J. Phycol. 2016, 51, 156–170. [Google Scholar] [CrossRef]
- Prasetiya, F.S.; Sunarto, S.; Bachtiar, E.; Agung, M.U.K.; Nathanael, B.; Pambudi, A.C.; Lestari, A.D.; Astuty, S.; Mouget, J.-L. Effect of the Blue Pigment Produced by the Tropical Diatom Haslea nusantara on Marine Organisms from Different Trophic Levels and Its Bioactivity. Aquac. Rep. 2020, 17, 100389. [Google Scholar] [CrossRef]
- Gastineau, R.; Hansen, G.; Poulin, M.; Lemieux, C.; Turmel, M.; Bardeau, J.-F.; Leignel, V.; Hardivillier, Y.; Morançais, M.; Fleurence, J.; et al. Haslea silbo, A Novel Cosmopolitan Species of Blue Diatoms. Biology 2021, 10, 328. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Samborska, K.; Lee, C.C.; Tomas, M.; Capanoglu, E.; Tarhan, Ö.; Taze, B.; Jafari, S.M. Phycocyanin, a Super Functional Ingredient from Algae; Properties, Purification Characterization, and Applications. Int. J. Biol. Macromol. 2021, 193 Pt B, 2320–2331. [Google Scholar] [CrossRef]
- Eckardt, K.; Tresselt, D.; Ihn, W.; Schumann, G.; Eritt, I.; Sedmera, P.; Novak, J. Anthracyclinone-blue A and B, New Natural Anthracyclinones Containing Nitrogen in the Molecules: Isolation, Chemical Structures and Biosynthesis. J. Basic Microbiol. 1991, 31, 371–376. [Google Scholar] [CrossRef]
- Grossart, H.-P.; Thorwest, M.; Plitzko, I.; Brinkhoff, T.; Simon, M.; Zeeck, A. Production of a Blue Pigment (Glaukothalin) by Marine Rheinheimera spp. Int. J. Microbiol. 2009, 2009, 701735. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Renuka Devi, P. Bacterial Pigments: Sustainable Compounds With Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 100. [Google Scholar] [CrossRef]
- Pfefferle, C.-M.; Breinholt, J.; Olsen, C.E.; Kroppenstedt, R.M.; Wellington, E.M.; Gürtler, H.; Fiedler, H.-P. Kyanomycin, a Complex of Unusual Anthracycline-Phospholipid Hybrids from Nonomuria Species. J. Nat. Prod. 2000, 63, 295–298. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Giaroni, C.; Baj, A.; Bolognese, F. Bacterial Pigments: A Colorful Palette Reservoir for Biotechnological Applications. Biotechnol. Appl. Biochem. 2022, 69, 981–1001. [Google Scholar] [CrossRef]
- Rahman, D.Y.; Sarian, F.D.; van Wijk, A.; Martinez-Garcia, M.; van der Maarel, M.J.E.C. Thermostable Phycocyanin from the Red Microalga Cyanidioschyzon merolae, a New Natural Blue Food Colorant. J. Appl. Phycol. 2017, 29, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Venil, C.K.; Lakshmanaperumalsamy, P. An Insightful Overview on Microbial Pigment, Prodigiosin. Electron. J. Biol. 2009, 5, 49–61. [Google Scholar]
- Zhu, H.; Guo, J.; Yao, Q.; Yang, S.; Deng, M.; Phuong, L.T.B.; Hanh, V.T.; Ryan, M.J. Streptomyces vietnamensis Sp. Nov., a Streptomycete with Violet-Blue Diffusible Pigment Isolated from Soil in Vietnam. Int. J. Syst. Evol. Microbiol. 2007, 57, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.B.; Wang, X.Y.; Fang, H.; Ma, Y.N.; Tang, J.; Tang, M.; Wei, G.H. Streptomyces shaanxiensis Sp. Nov., a Blue Pigment-Producing Streptomycete from Sewage Irrigation Soil. Int. J. Syst. Evol. Microbiol. 2012, 62, 1725–1730. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, J.; Yao, Q.; Yang, S.; Deng, M.; Li, T. Streptomyces caeruleatus Sp. Nov., with Dark Blue Diffusible Pigment. Int. J. Syst. Evol. 2011, 61, 507–511. [Google Scholar] [CrossRef]
- Zhu, Y.; Shang, X.; Yang, L.; Zheng, S.; Liu, K.; Li, X. Purification, Identification and Properties of a New Blue Pigment Produced from Streptomyces Sp. A1013Y. Food Chem. 2020, 308, 125600. [Google Scholar] [CrossRef]
- Gray, P. The Formation of Indigotin from Indol by Soil Bacteria. In Proceedings of the Royal Society of London, Series B, Containing Papers of a Biological Character; Royal Society: London, UK, 1928; Volume 102, pp. 263–280. [Google Scholar] [CrossRef]
- Andreani, N.A.; Carraro, L.; Martino, M.E.; Fondi, M.; Fasolato, L.; Miotto, G.; Magro, M.; Vianello, F.; Cardazzo, B. A Genomic and Transcriptomic Approach to Investigate the Blue Pigment Phenotype in Pseudomonas Fluorescens. Int. J. Food Microbiol. 2015, 213, 88–98. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Fan, J.; Zhang, Z.; Ma, Q.; Peng, X. Indigoids Biosynthesis from Indole by Two Phenol-Degrading Strains, Pseudomonas Sp. PI1 and Acinetobacter Sp. PI2. Appl. Biochem. Biotechnol. 2015, 176, 1263–1276. [Google Scholar] [CrossRef]
- Nawaz, A.; Chaudhary, R.; Shah, Z.; Dufossé, L.; Fouillaud, M.; Mukhtar, H.; ul Haq, I. An Overview on Industrial and Medical Applications of Bio-Pigments Synthesized by Marine Bacteria. Microorganisms 2020, 9, 11. [Google Scholar] [CrossRef]
- Abel, G.; Amobonye, A.; Bhagwat, P.; Pillai, S. Diversity, Stability and Applications of Mycopigments. Process Biochem. 2023, 133, 270–284. [Google Scholar] [CrossRef]
- Reverchon, S.; Rouanet, C.; Expert, D.; Nasser, W. Characterization of Indigoidine Biosynthetic Genes in Erwinia chrysanthemi and Role of This Blue Pigment in Pathogenicity. J. Bacteriol. 2002, 184, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Cude, W.N.; Mooney, J.; Tavanaei, A.A.; Hadden, M.K.; Frank, A.M.; Gulvik, C.A.; May, A.L.; Buchan, A. Production of the Antimicrobial Secondary Metabolite Indigoidine Contributes to Competitive Surface Colonization by the Marine Roseobacter Phaeobacter Sp. Strain Y4I. Appl. Environ. Microbiol. 2012, 78, 4771–4780. [Google Scholar] [CrossRef] [PubMed]
- Soliev, A.B.; Hosokawa, K.; Enomoto, K. Bioactive Pigments from Marine Bacteria: Applications and Physiological Roles. Evid.-Based Complement. Altern. Med. 2011, 2011, 670349. [Google Scholar] [CrossRef] [PubMed]
- Wehrs, M.; Gladden, J.M.; Liu, Y.; Platz, L.; Prahl, J.-P.; Moon, J.; Papa, G.; Sundstrom, E.; Geiselman, G.M.; Tanjore, D.; et al. Sustainable Bioproduction of the Blue Pigment Indigoidine: Expanding the Range of Heterologous Products in R. toruloides to Include Non-Ribosomal Peptides. Green Chem. 2019, 21, 3394–3406. [Google Scholar] [CrossRef]
- Yu, D.; Xu, F.; Valiente, J.; Wang, S.; Zhan, J. An Indigoidine Biosynthetic Gene Cluster from Streptomyces Chromofuscus ATCC 49982 Contains an Unusual IndB Homologue. J. Ind. Microbiol. Biotechnol. 2013, 40, 159–168. [Google Scholar] [CrossRef]
- Day, P.A.; Villalba, M.S.; Herrero, O.M.; Arancibia, L.A.; Alvarez, H.M. Formation of Indigoidine Derived-Pigments Contributes to the Adaptation of Vogesella Sp. Strain EB to Cold Aquatic Iron-Oxidizing Environments. Antonie Leeuwenhoek 2017, 110, 415–428. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Chidambara Murthy, K.N.; Ravishankar, G.A. Microorganisms and Microalgae as Sources of Pigments for Food Use: A Scientific Oddity or an Industrial Reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Francezon, N.; Herbaut, M.; Bardeau, J.-F.; Cougnon, C.; Bélanger, W.; Tremblay, R.; Jacquette, B.; Dittmer, J.; Pouvreau, J.-B.; Mouget, J.-L.; et al. Electrochromic Properties and Electrochemical Behavior of Marennine, a Bioactive Blue-Green Pigment Produced by the Marine Diatom Haslea Ostrearia. Mar. Drugs 2021, 19, 231. [Google Scholar] [CrossRef]
- Di Salvo, E.; Lo Vecchio, G.; De Pasquale, R.; De Maria, L.; Tardugno, R.; Vadalà, R.; Cicero, N. Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients 2023, 15, 1923. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural Colorants: Pigment Stability and Extraction Yield Enhancement via Utilization of Appropriate Pretreatment and Extraction Methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and Prebiotics Activity of Anthocyanins from Black Rice (Oryza sativa L.) In Vitro. PLoS ONE 2018, 13, e0195754. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and Prebiotic Activity of Five Peonidin-Based Anthocyanins Extracted from Purple Sweet Potato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. [Google Scholar] [CrossRef] [PubMed]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, Y.; Tao, Y.; Li, D.; Xie, G.; Show, P.L.; Lee, S.Y. In Vitro Gastrointestinal Digestion and Fecal Fermentation Reveal the Effect of Different Encapsulation Materials on the Release, Degradation and Modulation of Gut Microbiota of Blueberry Anthocyanin Extract. Food Res. Int. 2020, 132, 109098. [Google Scholar] [CrossRef]
- Flores, G.; Ruiz del Castillo, M.L.; Costabile, A.; Klee, A.; Bigetti Guergoletto, K.; Gibson, G.R. In Vitro Fermentation of Anthocyanins Encapsulated with Cyclodextrins: Release, Metabolism and Influence on Gut Microbiota Growth. J. Funct. Foods 2015, 16, 50–57. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Xie, Y.; Li, N.; Liu, Y.; Wen, J.; Zhang, M.; Feng, W.; Huang, J.; Guo, Y.; et al. Clitoria Ternatea Blue Petal Extract Protects against Obesity, Oxidative Stress, and Inflammation Induced by a High-Fat, High-Fructose Diet in C57BL/6 Mice. Food Res. Int. 2022, 162, 112008. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Tarone, A.G.; Tosi, M.M.; Maróstica Júnior, M.R.; Meireles, M.A.A. Extraction of Bioactive Compounds from Genipap (Genipa americana L.) by Pressurized Ethanol: Iridoids, Phenolic Content and Antioxidant Activity. Food Res. Int. 2017, 102, 595–604. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, L.; Liu, Y.; Shi, L.; Wan, S.; Wang, L. Evaluation of Antimicrobial Activity of Water-Soluble Flavonoids Extract from Vaccinium bracteatum Thunb. Leaves. Food Sci. Biotechnol. 2019, 28, 1853–1859. [Google Scholar] [CrossRef]
- Jayaseelan, S.; Ramaswamy, D.; Dharmaraj, S. Pyocyanin: Production, Applications, Challenges and New Insights. World J. Microbiol. Biotechnol. 2014, 30, 1159–1168. [Google Scholar] [CrossRef]
- Chen, H.-W.; Yang, T.-S.; Chen, M.-J.; Chang, Y.-C.; Wang, E.I.-C.; Ho, C.-L.; Lai, Y.-J.; Yu, C.-C.; Chou, J.-C.; Chao, L.K.-P.; et al. Purification and Immunomodulating Activity of C-Phycocyanin from Spirulina platensis Cultured Using Power Plant Flue Gas. Process Biochem. 2014, 49, 1337–1344. [Google Scholar] [CrossRef]
- Hamdan, N.T.; Jwad, B.A.A.A.A.; Jasim, S.A. Synergistic Anticancer Effects of Phycocyanin and Citrullus Colocynthis Extract against WiDr, HCT-15 and HCT-116 Colon Cancer Cell Lines. Gene Rep. 2021, 22, 100972. [Google Scholar] [CrossRef]
- Wen, Y.; Wen, P.; Hu, T.-G.; Linhardt, R.J.; Zong, M.-H.; Wu, H.; Chen, Z.-Y. Encapsulation of Phycocyanin by Prebiotics and Polysaccharides-Based Electrospun Fibers and Improved Colon Cancer Prevention Effects. Int. J. Biol. Macromol. 2020, 149, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and Characterization of Phycocyanin from Spirulina platensis and Evaluation of Its Anticancer, Antidiabetic and Antiinflammatory Effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Park, J.S.; Luo, L.; Kwon, Y.S.; Lee, H.C.; Jung Shim, H.; Kim, I.-D.; Lee, J.-K.; Shin, H.S. Assessment of C-Phycocyanin Effect on Astrocytes-Mediated Neuroprotection against Oxidative Brain Injury Using 2D and 3D Astrocyte Tissue Model. Sci. Rep. 2015, 5, 14418. [Google Scholar] [CrossRef]
- Gammoudi, S.; Athmouni, K.; Nasri, A.; Diwani, N.; Grati, I.; Belhaj, D.; Bouaziz-Ketata, H.; Fki, L.; El Feki, A.; Ayadi, H. Optimization, Isolation, Characterization and Hepatoprotective Effect of a Novel Pigment-Protein Complex (Phycocyanin) Producing Microalga: Phormidium VersicolorNCC-466 Using Response Surface Methodology. Int. J. Biol. Macromol. 2019, 137, 647–656. [Google Scholar] [CrossRef]
- Fratelli, C.; Burck, M.; Amarante, M.C.A.; Braga, A.R.C. Antioxidant Potential of Nature’s “Something Blue”: Something New in the Marriage of Biological Activity and Extraction Methods Applied to C-Phycocyanin. Trends Food Sci. Technol. 2021, 107, 309–323. [Google Scholar] [CrossRef]
- Singh, J.; Meehnian, H.; Gupta, P.; Verma, M. Food Colours: The Potential Sources of Food Adulterants and Their Food Safety Concerns. In Biotechnological Approaches in Food Adulterants; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 79–101. ISBN 978-0-367-36986-6. [Google Scholar]
- Carbonnelle, D.; Pondaven, P.; Morançais, M.; Massé, G.; Bosch, S.; Jacquot, C.; Briand, G.; Robert, J.; Roussakis, C. Antitumor and Antiproliferative Effects of an Aqueous Extract from the Marine Diatom Haslea ostrearia (Simonsen) against Solid Tumors: Lung Carcinoma (NSCLC-N6), Kidney Carcinoma (E39) and Melanoma (M96) Cell Lines. Anticancer Res. 1999, 19, 621–624. [Google Scholar]
- Gastineau, R.; Pouvreau, J.-B.; Hellio, C.; Morançais, M.; Fleurence, J.; Gaudin, P.; Bourgougnon, N.; Mouget, J.-L. Biological Activities of Purified Marennine, the Blue Pigment Responsible for the Greening of Oysters. J. Agric. Food Chem. 2012, 60, 3599–3605. [Google Scholar] [CrossRef]
- Bergé, J.-P.; Bourgougnon, N.; Alban, S.; Pojer, F.; Billaudel, S.; Chermann, J.-C.; Robert, J.; Franz, G. Antiviral and Anticoagulant Activities of a Water-Soluble Fraction of the Marine Diatom Haslea Ostrearia. Planta Medica 1999, 65, 604–609. [Google Scholar] [CrossRef]
- Feketea, G.; Tsabouri, S. Common Food Colorants and Allergic Reactions in Children: Myth or Reality? Food Chem. 2017, 230, 578–588. [Google Scholar] [CrossRef]
- Yousefi, M.; Ghanbari, F.; Zazouli, M.; Madihi Bidgoli, S. Brilliant Blue FCF Degradation by Persulfate/Zero Valent Iron: The Effects of Influencing Parameters and Anions. Desalination Water Treat. 2017, 70, 364–371. [Google Scholar] [CrossRef]
- Chadwick, B.L.; Hunter, M.L.; Evans, M.T.; Hunter, B. Allergic Reaction to the Food Dye Patent Blue. Br. Dent. J. 1990, 168, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.J.; Burgess, J.R.; Stochelski, M.A.; Kuczek, T. Amounts of Artificial Food Dyes and Added Sugars in Foods and Sweets Commonly Consumed by Children. Clin. Pediatr. 2015, 54, 309–321. [Google Scholar] [CrossRef] [PubMed]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food Additives and Hyperactive Behaviour in 3-Year-Old and 8/9-Year-Old Children in the Community: A Randomised, Double-Blinded, Placebo-Controlled Trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health Safety Issues of Synthetic Food Colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef]
- Lucová, M.; Hojerová, J.; Pažoureková, S.; Klimová, Z. Absorption of Triphenylmethane Dyes Brilliant Blue and Patent Blue through Intact Skin, Shaven Skin and Lingual Mucosa from Daily Life Products. Food Chem. Toxicol. 2013, 52, 19–27. [Google Scholar] [CrossRef]
- EFSA. Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the Re-evaluation of Brilliant Blue FCF (E 133) as a Food Additive. EFSA J. 2010, 8, 1853. [Google Scholar] [CrossRef]
- Kus, E.; Eroğlu, H. Genotoxic and Cytotoxic Effects of Sunset Yellow and Brilliant Blue, Colorant Food Additives, on Human Blood Lymphocytes. Pak. J. Pharm. Sci. 2015, 28, 227–230. [Google Scholar]
- Mahmoud, N. Toxic Effects of the Synthetic Food Dye Brilliant Blue on Liver, Kidney and Testes Functions in Rats. J. Egypt. Soc. Toxicol. 2006, 34, 77–84. [Google Scholar]
- Jindal, A.; Pathengay, A.; Mithal, K.; Chhablani, J.; Pappuru, R.K.; Flynn, H. Macular Toxicity Following Brilliant Blue G-Assisted Macular Hole Surgery-a Report of Three Cases. Nepal. J. Ophthalmol. A Biannu. Peer-Rev. Acad. J. Nepal Ophthalmic Soc. Nepjoph 2014, 6, 98–101. [Google Scholar] [CrossRef]
- Kuswandi, B.; Wicaksono, Y.; Jayus; Abdullah, A.; Heng, L.Y.; Ahmad, M. Smart Packaging: Sensors for Monitoring of Food Quality and Safety. Sens. Instrum. Food Qual. Saf. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Blakeney, M. Food Loss and Food Waste: Causes and Solutions; Edward Elgar Publishing: Cheltenham, UK, 2019; ISBN 978-1-78897-539-1. [Google Scholar]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a Food Spoilage Indicator for Monitoring Freshness of Skinless Chicken Breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, S.; Lee, K.; Baek, S.; Seo, J. Development of a PH Indicator Composed of High Moisture-Absorbing Materials for Real-Time Monitoring of Chicken Breast Freshness. Food Sci. Biotechnol. 2017, 26, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Karaca, I.M.; Haskaraca, G.; Ayhan, Z.; Gültekin, E. Development of Real Time-PH Sensitive Intelligent Indicators for Monitoring Chicken Breast Freshness/Spoilage Using Real Packaging Practices. Food Res. Int. 2023, 173, 113261. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, Q.; Zeng, X.; Chen, X.; Li, M.; Wu, X.; Liu, Y.; Zheng, Y.; Xiang, J.; Wang, C.; et al. Novel PH-Responsive Indicator Films Based on Bromothymol Blue-Anchored Chitin for Shrimp Freshness Monitoring. Int. J. Biol. Macromol. 2023, 253, 127052. [Google Scholar] [CrossRef]
- Parsafar, B.; Ahmadi, M.; Jahed Khaniki, G.R.; Shariatifar, N.; Rahimi Foroushani, A. The Impact of Fruit and Vegetable Waste on Economic Loss Estimation. Glob. J. Environ. Sci. Manag. 2023, 9, 871–884. [Google Scholar] [CrossRef]
- Zhang, W.; An, H.; Sun, X.; Du, H.; Li, Y.; Yang, M.; Zhu, Z.; Wen, Y. Dual-Functional Smart Indicator for Direct Monitoring Fruit Freshness. Food Packag. Shelf Life 2023, 40, 101192. [Google Scholar] [CrossRef]
- Luo, X.; Zaitoon, A.; Lim, L.-T. A Review on Colorimetric Indicators for Monitoring Product Freshness in Intelligent Food Packaging: Indicator Dyes, Preparation Methods, and Applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2489–2519. [Google Scholar] [CrossRef]
- Obaidi, A.A.; Karaca, I.M.; Ayhan, Z.; Haskaraca, G.; Gultekin, E. Fabrication and Validation of CO2-Sensitive Indicator to Monitor the Freshness of Poultry Meat. Food Packag. Shelf Life 2022, 34, 100930. [Google Scholar] [CrossRef]
- Shaik, M.I.; Azhari, M.F.; Sarbon, N.M. Gelatin-Based Film as a Color Indicator in Food-Spoilage Observation: A Review. Foods 2022, 11, 3797. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Tajik, H.; Moradi, M. Fabrication and Characterization of Alizarin Colorimetric Indicator Based on Cellulose-Chitosan to Monitor the Freshness of Minced Beef. Sens. Actuators B Chem. 2019, 285, 519–528. [Google Scholar] [CrossRef]
- Smolander, M.; Hurme, E.; Latva-Kala, K.; Luoma, T.; Alakomi, H.-L.; Ahvenainen, R. Myoglobin-Based Indicators for the Evaluation of Freshness of Unmarinated Broiler Cuts. Innov. Food Sci. Emerg. Technol. 2002, 3, 279–288. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and Intelligent Biodegradable Packaging Films Using Food and Food Waste-Derived Bioactive Compounds: A Review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Chi, W.; Cao, L.; Sun, G.; Meng, F.; Zhang, C.; Li, J.; Wang, L. Developing a Highly PH-Sensitive ĸ-Carrageenan-Based Intelligent Film Incorporating Grape Skin Powder via a Cleaner Process. J. Clean. Prod. 2020, 244, 118862. [Google Scholar] [CrossRef]
- Talukder, S.; Mendiratta, S.K.; Kumar, R.R.; Agrawal, R.K.; Soni, A.; Luke, A.; Chand, S. Jamun Fruit (Syzgium cumini) Skin Extract Based Indicator for Monitoring Chicken Patties Quality during Storage. J. Food Sci. Technol. 2020, 57, 537–548. [Google Scholar] [CrossRef]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Ščetar, M.; Uzelac, V.D.; Galić, K. Development and Evaluation of a Novel Antioxidant and PH Indicator Film Based on Chitosan and Food Waste Sources of Antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Shahid, M.; Shahid-ul-Islam; Mohammad, F. Recent Advancements in Natural Dye Applications: A Review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent PH Indicator Film Composed of Agar/Potato Starch and Anthocyanin Extracts from Purple Sweet Potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an Intelligent PH Film Based on Biodegradable Polymers and Roselle Anthocyanins for Monitoring Pork Freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Fujikawa, H.; Akimoto, R. New Blue Pigment Produced by Pantoea agglomerans and Its Production Characteristics at Various Temperatures. Appl. Environ. Microbiol. 2011, 77, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Su, H.-M.; Imani, S.M.; Alkhaldi, K.M.; Filipe, C.D.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Chayavanich, K.; Thiraphibundet, P.; Imyim, A. Biocompatible Film Sensors Containing Red Radish Extract for Meat Spoilage Observation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117601. [Google Scholar] [CrossRef] [PubMed]
- Golasz, L.B.; Silva, J.d.; Silva, S.B.d. Film with Anthocyanins as an Indicator of Chilled Pork Deterioration. Food Sci. Technol. 2013, 33, 155–162. [Google Scholar] [CrossRef]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of Blueberry Residue Incorporated Cassava Starch Film as PH Indicator in Different Simulants and Foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Vo, T.-V.; Dang, T.-H.; Chen, B.-H. Synthesis of Intelligent PH Indicative Films from Chitosan/Poly(Vinyl Alcohol)/Anthocyanin Extracted from Red Cabbage. Polymers 2019, 11, 1088. [Google Scholar] [CrossRef]
- Wardana, A.A.; Widyaningsih, T.D. Development of Edible Films from Tapioca Starch and Agar, Enriched with Red Cabbage (Brassica oleracea) as a Sausage Deterioration Bio-Indicator; IOP Publishing: Bristol, UK, 2017; Volume 109, p. 012031. [Google Scholar]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/Corn Starch Blend Films with Extract from Brassica oleraceae (Red Cabbage) as a Visual Indicator of Fish Deterioration. LWT-Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Anugrah, D.S.B.; Darmalim, L.V.; Sinanu, J.D.; Pramitasari, R.; Subali, D.; Prasetyanto, E.A.; Cao, X.T. Development of Alginate-Based Film Incorporated with Anthocyanins of Red Cabbage and Zinc Oxide Nanoparticles as Freshness Indicator for Prawns. Int. J. Biol. Macromol. 2023, 251, 126203. [Google Scholar] [CrossRef]
- Narayanan, G.P.; Radhakrishnan, P.; Baiju, P. Fabrication Of Butterfly Pea Flower Anthocyanin-Incorporated Colorimetric Indicator Film Based On Gelatin/Pectin For Monitoring Fish Freshness. Food Hydrocoll. Health 2023, 4, 100159. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an Intelligent Film Based on Chitosan/Oxidized Chitin Nanocrystals Incorporating Black Rice Bran Anthocyanins for Seafood Spoilage Monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Zandraa, O.; Pummerová, M.; Sáha, P. Essential Oil Based PVP-CMC-BC-GG Functional Hydrogel Sachet for ‘Cheese’: Its Shelf Life Confirmed with Anthocyanin (Isolated from Red Cabbage) Bio Stickers. Foods 2020, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, L. Preparation of a Visual PH-Sensing Film Based on Tara Gum Incorporating Cellulose and Extracts from Grape Skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active Chitosan/PVA Films with Anthocyanins from Brassica Oleraceae (Red Cabbage) as Time-Temperature Indicators for Application in Intelligent Food Packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Wang, S.; Li, R.; Han, M.; Zhuang, D.; Zhu, J. Intelligent Active Films of Sodium Alginate and Konjac Glucomannan Mixed by Lycium Ruthenicum Anthocyanins and Tea Polyphenols for Milk Preservation and Freshness Monitoring. Int. J. Biol. Macromol. 2023, 253, 126674. [Google Scholar] [CrossRef]
- Drhimer, F.; Rahmani, M.; Regraguy, B.; El Hajjaji, S.; Mabrouki, J.; Amrane, A.; Fourcade, F.; Assadi, A.A. Treatment of a Food Industry Dye, Brilliant Blue, at Low Concentration Using a New Photocatalytic Configuration. Sustainability 2023, 15, 5788. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B. Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes. Nanomaterials 2020, 10, 202. [Google Scholar] [CrossRef]
- da Fonseca Machado, A.P.; Rezende, C.A.; Rodrigues, R.A.; Barbero, G.F.; e Rosa, P.d.T.V.; Martínez, J. Encapsulation of Anthocyanin-Rich Extract from Blackberry Residues by Spray-Drying, Freeze-Drying and Supercritical Antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Block, E. Garlic and Other Alliums: The Lore and the Science; RSC Publishing: Cambridge, UK, 2010; ISBN 978-0-85404-190-9. [Google Scholar]
- Khoironi, A.; Anggoro, S.; Sudarno, S. Evaluation of the Interaction among Microalgae Spirulina Sp, Plastics Polyethylene Terephthalate and Polypropylene in Freshwater Environment. J. Ecol. Eng. 2019, 20, 161–173. [Google Scholar] [CrossRef]
- Correa, D.F.; Beyer, H.L.; Possingham, H.P.; García-Ulloa, J.; Ghazoul, J.; Schenk, P.M. Freeing Land from Biofuel Production through Microalgal Cultivation in the Neotropical Region. Environ. Res. Lett. 2020, 15, 094094. [Google Scholar] [CrossRef]
- De Man, R.; German, L. Certifying the Sustainability of Biofuels: Promise and Reality. Energy Policy 2017, 109, 871–883. [Google Scholar] [CrossRef]
- Mathimani, T.; Pugazhendhi, A. Utilization of Algae for Biofuel, Bio-Products and Bio-Remediation. Biocatal. Agric. Biotechnol. 2019, 17, 326–330. [Google Scholar] [CrossRef]
- Chia, S.R.; Chew, K.W.; Show, P.L.; Yap, Y.J.; Ong, H.C.; Ling, T.C.; Chang, J. Analysis of Economic and Environmental Aspects of Microalgae Biorefinery for Biofuels Production: A Review. Biotechnol. J. 2018, 13, 1700618. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garcia, L.; Adjallé, K.; Barnabé, S.; Raghavan, G. Microalgae Biomass Production for a Biorefinery System: Recent Advances and the Way towards Sustainability. Renew. Sustain. Energy Rev. 2017, 76, 493–506. [Google Scholar] [CrossRef]
- González-González, L.M.; Correa, D.F.; Ryan, S.; Jensen, P.D.; Pratt, S.; Schenk, P.M. Integrated Biodiesel and Biogas Production from Microalgae: Towards a Sustainable Closed Loop through Nutrient Recycling. Renew. Sustain. Energy Rev. 2018, 82, 1137–1148. [Google Scholar] [CrossRef]
- Gu, J.-D.; Cheung, K. Phenotypic Expression of Vogesella indigofera upon Exposure to Hexavalent Chromium, Cr6+. World J. Microbiol. Biotechnol. 2001, 17, 475–480. [Google Scholar] [CrossRef]
- Calogero, G.; Bartolotta, A.; Di Marco, G.; Di Carlo, A.; Bonaccorso, F. Vegetable-Based Dye-Sensitized Solar Cells. Chem. Soc. Rev. 2015, 44, 3244–3294. [Google Scholar] [CrossRef]
- Lucioli, S.; Di Bari, C.; Forni, C.; Di Carlo, A.; Barrajón-Catalán, E.; Micol, V.; Nota, P.; Teoli, F.; Matteocci, F.; Frattarelli, A.; et al. Anthocyanic Pigments from Elicited in Vitro Grown Shoot Cultures of Vaccinium corymbosum L., Cv. Brigitta Blue, as Photosensitizer in Natural Dye-Sensitized Solar Cells (NDSSC). J. Photochem. Photobiol. B Biol. 2018, 188, 69–76. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-Sensitized Solar Cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Amiri, O.; Salavati-Niasari, M.; Mir, N.; Beshkar, F.; Saadat, M.; Ansari, F. Plasmonic Enhancement of Dye-Sensitized Solar Cells by Using Au-Decorated Ag Dendrites as a Morphology-Engineered. Renew. Energy 2018, 125, 590–598. [Google Scholar] [CrossRef]
- Amiri, O.; Salavati-Niasari, M.; Farangi, M.; Mazaheri, M.; Bagheri, S. Stable Plasmonic-Improved Dye Sensitized Solar Cells by Silver Nanoparticles Between Titanium Dioxide Layers. Electrochim. Acta 2015, 152, 101–107. [Google Scholar] [CrossRef]
- Wu, W.; Xu, X.; Yang, H.; Hua, J.; Zhang, X.; Zhang, L.; Long, Y.; Tian, H. D–π–M–π–A Structured Platinum Acetylide Sensitizer for Dye-Sensitized Solar Cells. J. Mater. Chem. 2011, 21, 10666–10671. [Google Scholar] [CrossRef]
- Sharma, V.; McKone, H.T.; Markow, P.G. A Global Perspective on the History, Use, and Identification of Synthetic Food Dyes. J. Chem. Educ. 2011, 88, 24–28. [Google Scholar] [CrossRef]
- Christiansen, C.; Heyde, A.; Schiffelbein, O. Protein-Rich Spirulina Extracts. PCT Patent 2012104091, 9 August 2012. [Google Scholar]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina Sp: Influence of Processing of Biomass on Phycocyanin Yield, Analysis of Efficacy of Extraction Methods and Stability Studies on Phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Eriksen, N.T. Production of Phycocyanin—A Pigment with Applications in Biology, Biotechnology, Foods and Medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.; Chethana, S.; Madhusudhan, M.C.; Raghavarao, K.S.M.S. Fractionation and Purification of the Phycobiliproteins from Spirulina Platensis. Bioresour. Technol. 2008, 99, 7393–7396. [Google Scholar] [CrossRef] [PubMed]
- Rito-Palomares, M.; Nuñez, L.; Amador, D. Practical Application of Aqueous Two-Phase Systems for the Development of a Prototype Process for c-Phycocyanin Recovery from Spirulina maxima. J. Chem. Technol. Biotechnol. 2001, 76, 1273–1280. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S. Microalgae Harvesting Techniques: A Review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Judd, S.J.; Al Momani, F.; Znad, H.; Al Ketife, A. The Cost Benefit of Algal Technology for Combined CO2 Mitigation and Nutrient Abatement. Renew. Sustain. Energy Rev. 2017, 71, 379–387. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; del Pilar Sanchez-Camargo, A.; Benvenutti, L.; Ferro, D.M.; Dias, J.L.; Ferreira, S.R.S. High-Pressure Fluid Technologies: Recent Approaches to the Production of Natural Pigments for Food and Pharmaceutical Applications. Trends Food Sci. Technol. 2021, 118, 850–869. [Google Scholar] [CrossRef]
- Wijesekara, T.; Xu, B. A Critical Review on the Stability of Natural Food Pigments and Stabilization Techniques. Food Res. Int. 2024, 179, 114011. [Google Scholar] [CrossRef]
- Azman, E.M.; Charalampopoulos, D.; Chatzifragkou, A. Acetic Acid Buffer as Extraction Medium for Free and Bound Phenolics from Dried Blackcurrant (Ribes nigrum L.) Skins. J. Food Sci. 2020, 85, 3745–3755. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and Purification of Anthocyanins: A Review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, G.; Khan, M.A.; Yan, Z.; Beta, T. Ultrasonic-Assisted Enzymatic Extraction and Identification of Anthocyanin Components from Mulberry Wine Residues. Food Chem. 2020, 323, 126714. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z. A Comprehensive Review on Innovative and Advanced Stabilization Approaches of Anthocyanin by Modifying Structure and Controlling Environmental Factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef]
- Pieczykolan, E.; Kurek, M.A. Use of Guar Gum, Gum Arabic, Pectin, Beta-Glucan and Inulin for Microencapsulation of Anthocyanins from Chokeberry. Int. J. Biol. Macromol. 2019, 129, 665–671. [Google Scholar] [CrossRef]
- Ji, Y. Synthesis of Porous Starch Microgels for the Encapsulation, Delivery and Stabilization of Anthocyanins. J. Food Eng. 2021, 302, 110552. [Google Scholar] [CrossRef]
- Weiss, V.; Okun, Z.; Shpigelman, A. Utilization of Hydrocolloids for the Stabilization of Pigments from Natural Sources. Curr. Opin. Colloid Interface Sci. 2023, 68, 101756. [Google Scholar] [CrossRef]
- Malik, K.; Tokas, J.; Goyal, S. Microbial Pigments: A Review. Int. J. Microb. Res. Technol. 2012, 1, 361–365. [Google Scholar]
- Brauch, J.; Zapata, S.; Buchweitz, M.; Aschoff, J.; Carle, R. Jagua Blue Derived from Genipa Americana L. Fruit: A Natural Alternative to Commonly Used Blue Food Colorants? Food Res. Int. 2016, 89, 391–398. [Google Scholar] [CrossRef]
- Gukowsky, J.C.; Xie, T.; Gao, S.; Qu, Y.; He, L. Rapid Identification of Artificial and Natural Food Colorants with Surface Enhanced Raman Spectroscopy. Food Control 2018, 92, 267–275. [Google Scholar] [CrossRef]
- Johnson, R. Food Fraud and Economically Motivated Adulteration of Food and Food Ingredients; Library of Congress: Washington, DC, USA, 2014. [Google Scholar]
- Tarantelli, T.; Sheridan, R. Toxic Industrial Colorants Found in Imported Foods; State Department of Agriculture & Markets Food Laboratory: New York, NY, USA, 2011. [Google Scholar]
- Yoshioka, N.; Ichihashi, K. Determination of 40 Synthetic Food Colors in Drinks and Candies by High-Performance Liquid Chromatography Using a Short Column with Photodiode Array Detection. Talanta 2008, 74, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, S.; Fang, K.; Wang, Y.; Yang, Y.; Han, C.; Shen, Y. Rapid Determination of 93 Banned Industrial Dyes in Beverage, Fish, Cookie Using Solid-Supported Liquid-Liquid Extraction and Ultrahigh-Performance Liquid Chromatography Quadrupole Orbitrap High-Resolution Mass Spectrometry. Food Chem. 2022, 388, 132976. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.; Gratzfeld-Hüsgen, A. Analysis of Synthetic Dyes in Food Samples by Capillary Zone Electrophoresis. In Hewlett R Packard Application Note; HP Company: Palo Alto, CA, USA, 1995. [Google Scholar]
- Teepakakorn, A.P.; Bureekaew, S.; Ogawa, M. Adsorption-Induced Dye Stability of Cationic Dyes on Clay Nanosheets. Langmuir 2018, 34, 14069–14075. [Google Scholar] [CrossRef] [PubMed]
- Dejoie, C.; Martinetto, P.; Dooryhee, E.; Van Elslande, E.; Blanc, S.; Bordat, P.; Brown, R.; Porcher, F.; Anne, M. Association of Indigo with Zeolites for Improved Color Stabilization. Appl. Spectrosc. 2010, 64, 1131–1138. [Google Scholar] [CrossRef]
- Schlintl, C.; Schienle, A. Effects of Coloring Food Images on the Propensity to Eat: A Placebo Approach with Color Suggestions. Front. Psychol. 2020, 11, 589826. [Google Scholar] [CrossRef]
- Spence, C.; Levitan, C.A.; Shankar, M.U.; Zampini, M. Does Food Color Influence Taste and Flavor Perception in Humans? Chemosens. Percept. 2010, 3, 68–84. [Google Scholar] [CrossRef]
- Wadhera, D.; Capaldi-Phillips, E.D. A Review of Visual Cues Associated with Food on Food Acceptance and Consumption. Eat. Behav. 2014, 15, 132–143. [Google Scholar] [CrossRef]
- Suzuki, M.; Kimura, R.; Kido, Y.; Inoue, T.; Moritani, T.; Nagai, N. Color of Hot Soup Modulates Postprandial Satiety, Thermal Sensation, and Body Temperature in Young Women. Appetite 2017, 114, 209–216. [Google Scholar] [CrossRef]
- Garber, L.L.J.; Hyatt, E.M.; Starr, R.G., Jr. Placing Food Color Experimentation into a Valid Consumer Context. J. Food Prod. Mark. 2001, 7, 3–24. [Google Scholar] [CrossRef]
- Moskowitz, H. Children and “Tween” Acceptance of Single Candy Colors and Two-Color Combinations. J. Sens. Stud. 2002, 17, 115–120. [Google Scholar] [CrossRef]
- Galetović, A.; Seura, F.; Gallardo, V.; Graves, R.; Cortés, J.; Valdivia, C.; Núñez, J.; Tapia, C.; Neira, I.; Sanzana, S.; et al. Use of Phycobiliproteins from Atacama Cyanobacteria as Food Colorants in a Dairy Beverage Prototype. Foods 2020, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lu, J. Eat with Your Eyes: Package Color Influences the Perceptions of Food Taste and Healthiness Moderated by External Eating. Mark. Manag. 2015, 25, 71–87. [Google Scholar]
- Su, J.; Wang, S. Influence of Food Packaging Color and Foods Type on Consumer Purchase Intention: The Mediating Role of Perceived Fluency. Front. Nutr. 2024, 10, 1344237. [Google Scholar] [CrossRef]
- Kaya, N.; Epps, H.H. Relationship between Color and Emotion: A Study of College Students. Coll. Stud. J. 2004, 38, 396–406. [Google Scholar]
- Muniz, V.; Ribeiro, I.; Beckmam, K.; Godoy, R. The Impact of Color on Food Choice. Braz. J. Food Technol. 2023, 26, e2022088. [Google Scholar] [CrossRef]
- Theben, A.; Gerards, M.; Folkvord, F. The Effect of Packaging Color and Health Claims on Product Attitude and Buying Intention. Int. J. Environ. Res. Public Health 2020, 17, 1991. [Google Scholar] [CrossRef]
- Baptista, I.Y.F. The Flavor of the Color and the Texture of the Shape: Effects of Visual Aspects on Expectation and Perception of Chocolates. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 2022. [Google Scholar]
- Hallez, L.; Vansteenbeeck, H.; Boen, F.; Smits, T. Persuasive Packaging? The Impact of Packaging Color and Claims on Young Consumers’ Perceptions of Product Healthiness, Sustainability and Tastiness. Appetite 2023, 182, 106433. [Google Scholar] [CrossRef]
- Steiner, K.; Florack, A. The Influence of Packaging Color on Consumer Perceptions of Healthfulness: A Systematic Review and Theoretical Framework. Foods 2023, 12, 3911. [Google Scholar] [CrossRef]
- Pereira, C.P.d.A. A Cor Do Infinito e a Beleza Inatingível: Sobre a Função Simbólica Do Azul Em Embalagens de Alimento. Blucher Des. Proc. 2014, 1, 58–66. [Google Scholar] [CrossRef][Green Version]
- Guilhon, D.; Castro, E.; Silva, V. Perfil Cromático de Embalagens de Produtos Lácteos–Um Estudo Preliminar; Sociedade Brasileira de Design da Informação: Curitiba, Brazil, 2021; pp. 131–148. [Google Scholar]
- Berthold, A.; Guion, S.; Siegrist, M. The Influence of Material and Color of Food Packaging on Consumers’ Perception and Consumption Willingness. Food Humanit. 2024, 2, 100265. [Google Scholar] [CrossRef]
- Guéguen, N.; Jacob, C. Coffee Cup Color and Evaluation of a Beverage’s “Warmth Quality”. Color Res. Appl. 2014, 39, 79–81. [Google Scholar] [CrossRef]
- Crumpacker, B. The Sex Life of Food: When Body and Soul Meet to Eat; Thomas Dunne Books: New York, NY, USA, 2006; ISBN 0-312-34207-1. [Google Scholar]
- Cho, S.; Han, A.; Taylor, M.H.; Huck, A.C.; Mishler, A.M.; Mattal, K.L.; Barker, C.A.; Seo, H.-S. Blue Lighting Decreases the Amount of Food Consumed in Men, but Not in Women. Appetite 2015, 85, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Suk, H.-J.; Park, G.L.; Kim, Y. Bon Appétit! An Investigation about the Best and Worst Color Combinations of Lighting and Food. J. Lit. Art. Stud. 2012, 2, 559–566. [Google Scholar]
- Yang, F.; Cho, S.; Seo, H.-S. Effects of Light Color on Consumers’ Acceptability and Willingness to Eat Apples and Bell Peppers. J. Sens. Stud. 2016, 31, 3–11. [Google Scholar] [CrossRef]
- Guéguen, N. The Effect of Glass Colour on the Evaluation of a Beverage’s Thirst-Quenching Quality. Curr. Psychol. Lett. Behav. Brain Cogn. 2003, 11, 1–6. [Google Scholar] [CrossRef]
- Motoki, K.; Spence, C.; Velasco, C. When Visual Cues Influence Taste/Flavour Perception: A Systematic Review. Food Qual. Prefer. 2023, 111, 104996. [Google Scholar] [CrossRef]
- Spence, C. On the Manipulation, and Meaning(s), of Color in Food: A Historical Perspective. J. Food Sci. 2023, 88, A5–A20. [Google Scholar] [CrossRef]
- Agustini, T.; Amalia, U.; Dewi, E.; Kurniasih, R. Application of Basil Leaf Extracts to Decrease Spirulina Platensis off-Odour in Increasing Food Consumption. Int. Food Res. J. 2019, 26, 1789–1794. [Google Scholar]
- Burrows, A. Palette of Our Palates: A Brief History of Food Coloring and Its Regulation. Compr. Rev. Food Sci. Food Saf. 2009, 8, 394–408. [Google Scholar] [CrossRef]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of Food Colour Regulations in the EU and the US: A Review of Current Provisions. Food Addit. Contam. Part A 2017, 34, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.C. Food Law and Regulation for Non-Lawyers: A US Perspectives, Food Science Text Series; 1st ed.; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-12472-8. [Google Scholar]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Joint FAO/WHO Expert Committee on Food Additives. In Evaluation of Certain Food Additives: Eighty-Second Report of the Joint FAO/WHO Expert Committee on Food Additives, 82nd ed.; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-121000-3. [Google Scholar]
- CXS 192-1995; Codex Alimentarius International Food Standards, General Standard for Food Additives. Food and Agriculture Organization-World Health Organization: Geneva, Switzerland, 2023; pp. 1–535.
- JECFA. Codex Alimentarius Commisssion, JOINT FAO/WHO Food Standards Programme Codex Alimentarius Commission. In Proceedings of the Report of the Fifty Fourth Session of the Codex Committee on Food Additives, Chengdu, China, 22–26 April 2024; pp. 1–196. [Google Scholar]
- Magnuson, B.; Munro, I.; Abbot, P.; Baldwin, N.; López-Garcia, R.; Ly, K.; McGirr, L.; Roberts, A.; Socolovsky, S. Review of the Regulation and Safety Assessment of Food Substances in Various Countries and Jurisdictions. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 1147–1220. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific Opinion on the Re-evaluation of Indigo Carmine (E 132) as a Food Additive. EFSA J. 2014, 12, 3768. [Google Scholar] [CrossRef]
- EFSA. Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific Opinion on the Re-evaluation of Patent Blue V (E 131) as a Food Additive. EFSA J. 2013, 11, 2818. [Google Scholar]
- Food and Agriculture Organization. Compendium of Food Additive Specifications. In Joint FAO/WHO Expert Committee on Food Additives (JECFA); Food and Agriculture Organization: Rome, Italy, 2016. [Google Scholar]
- Oplatowska-Stachowiak, M.; Elliott, C.T. Food Colors: Existing and Emerging Food Safety Concerns. Crit. Rev. Food Sci. Nutr. 2015, 57, 524–548. [Google Scholar] [CrossRef]
- Okafor, S.N.; Obonga, W.; Ezeokonkwo, M.A.; Nurudeen, J.; Orovwigho, U.; Ahiabuike, J. Assessment of the Health Implications of Synthetic and Natural Food Colourants—A Critical Review. Pharm. Biosci. J. 2016, 4, 01–11. [Google Scholar] [CrossRef]
- Pereira, H.; Deuchande, T.; Fundo, J.F.; Leal, T.; Pintado, M.E.; Amaro, A.L. Painting the Picture of Food Colouring Agents: Near-Ubiquitous Molecules of Everyday Life—A Review. Trends Food Sci. Technol. 2024, 143, 104249. [Google Scholar] [CrossRef]
- Lomax, S.Q.; Learner, T. A Review of the Classes, Structures, and Methods of Analysis of Synthetic Organic Pigments. J. Am. Inst. Conserv. 2006, 45, 107–125. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D. Natural Food Pigments and Colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Gamage, G.C.V.; Goh, J.K.; Choo, W.S. Application of Anthocyanins from Blue Pea Flower in Yoghurt and Fermented Milk: An Alternate Natural Blue Colour to Spirulina. Int. J. Gastron. Food Sci. 2024, 37, 100957. [Google Scholar] [CrossRef]
- Jia, L.; Lu, W.; Hu, D.; Feng, M.; Wang, A.; Wang, R.; Sun, H.; Wang, P.; Xia, Q.; Ma, S. Genetically Engineered Blue Silkworm Capable of Synthesizing Natural Blue Pigment. Int. J. Biol. Macromol. 2023, 235, 123863. [Google Scholar] [CrossRef] [PubMed]
- Noda, N. Recent Advances in the Research and Development of Blue Flowers. Breed. Sci. 2018, 68, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Azadi, P.; Bagheri, H.; Nalousi, A.M.; Nazari, F.; Chandler, S.F. Current Status and Biotechnological Advances in Genetic Engineering of Ornamental Plants. Biotechnol. Adv. 2016, 34, 1073–1090. [Google Scholar] [CrossRef]
- Katsumoto, Y.; Fukuchi-Mizutani, M.; Fukui, Y.; Brugliera, F.; Holton, T.; Karan, M.; Nakamura, N.; Yonekura-Sakakibara, K.; Togami, J.; Pigeaire, A.; et al. Engineering of the Rose Flavonoid Biosynthetic Pathway Successfully Generated Blue-Hued Flowers Accumulating Delphinidin. Plant Cell Physiol. 2007, 48, 1589–1600. [Google Scholar] [CrossRef]
- Scotter, M. Emerging and Persistent Issues with Artificial Food Colours: Natural Colour Additives as Alternatives to Synthetic Colours in Food and Drink. Qual. Assur. Saf. Crops Foods 2011, 3, 28–39. [Google Scholar] [CrossRef]
- Joshi, V.K.; Attri, D.; Bala, A.; Bhushan, S. Microbial Pigments. Indian J. Biotechnol. 2003, 2, 362–369. [Google Scholar]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial Pigments and Their Applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- CXG 36-1989; Codex Alimentarius-Class Names and the International Numbering System for Food Additives. Food and Agriculture Organization-World Health Organization: Geneva, Switzerland, 2023.
- Department of Health and Human Services Food and Drug Administration. Listing of Color Additives Exempt From Certification: Butterfly Pea Flower Extract-Federal Register; Food and Drug Administration: Silver Spring, MD, USA, 2021; Volume 86 FR 49230, pp. 49230–49234. [Google Scholar]
- Jespersen, L.; Strømdahl, L.D.; Olsen, K.; Skibsted, L.H. Heat and Light Stability of Three Natural Blue Colorants for Use in Confectionery and Beverages. Eur. Food Res. Technol. 2005, 220, 261–266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).