Advancing the Sustainability of Geopolymer Technology through the Development of Rice Husk Ash Based Solid Activators
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid Activators Synthesis
- The shortest treatment time was required for both treatment types to produce a dry product. More precisely, the temperature and time ranges were chosen to allow for the complete solubilization of the NH reagent and the dehydration of the reactants’ pulp.
- The household microwave oven’s technical features limited the microwave power to certain values (120, 460 and 700 W).
- The value range of SiO2/Na2O molar ratio was chosen taking into account the practice used so far in geopolymerization in relation to alkali silicate. In particular, SiO2/Na2O molar ratios with an upper limit value equal to 2 were applied, since alkali silicates prepared with higher values exhibit very low solubility in water and pH [31].
2.2. Geopolymer Synthesis
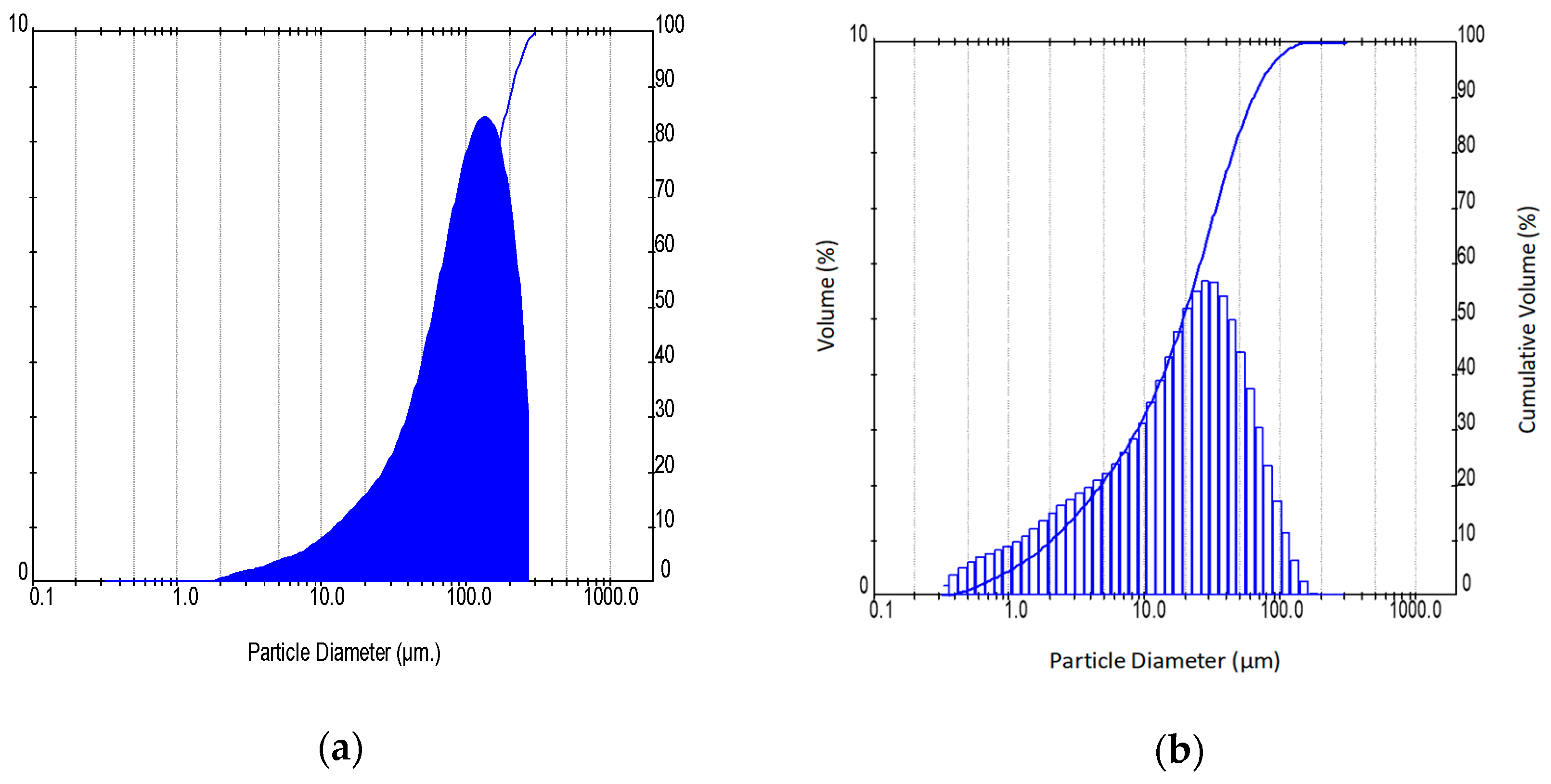
2.3. Characterization Techniques
3. Results
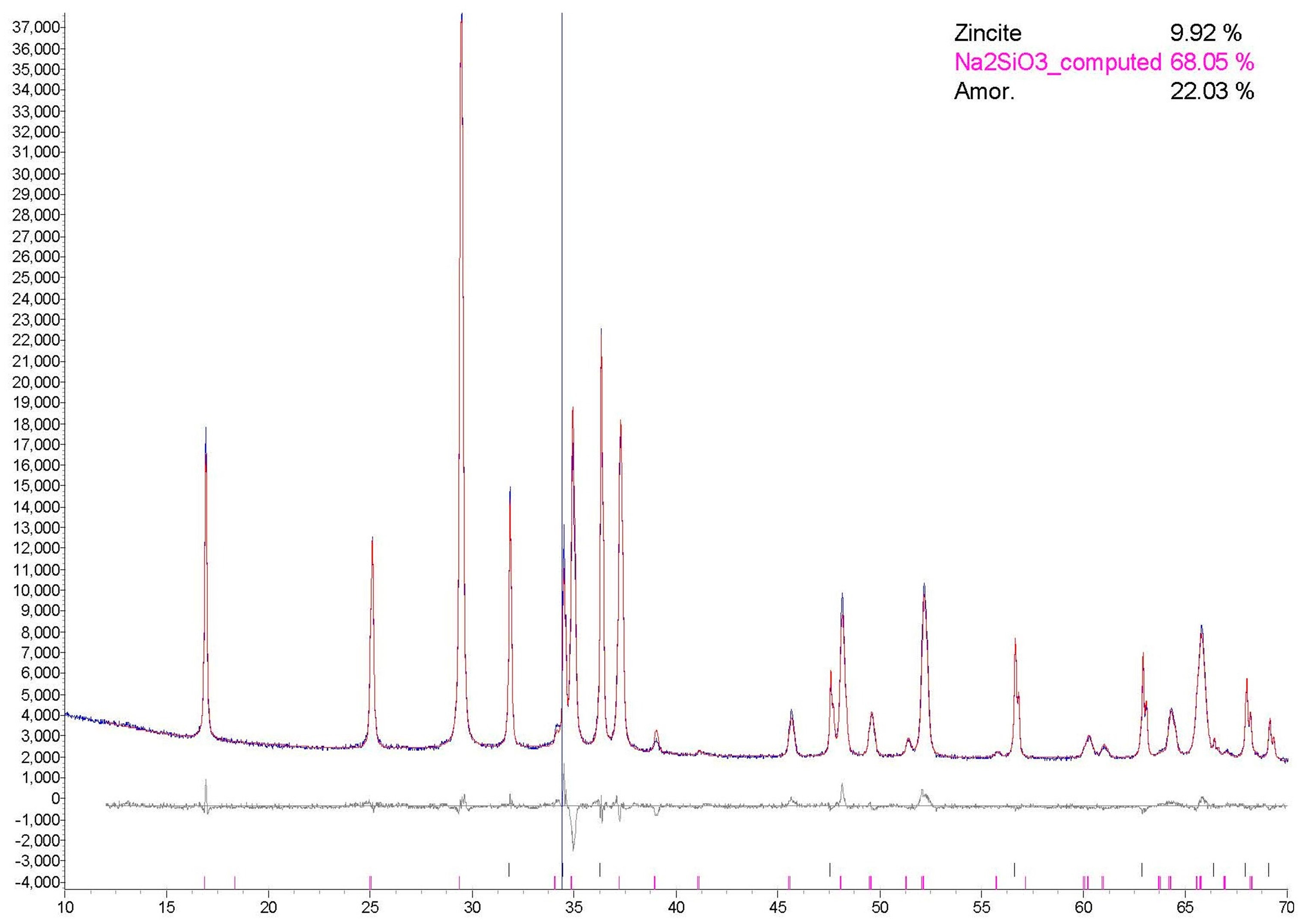
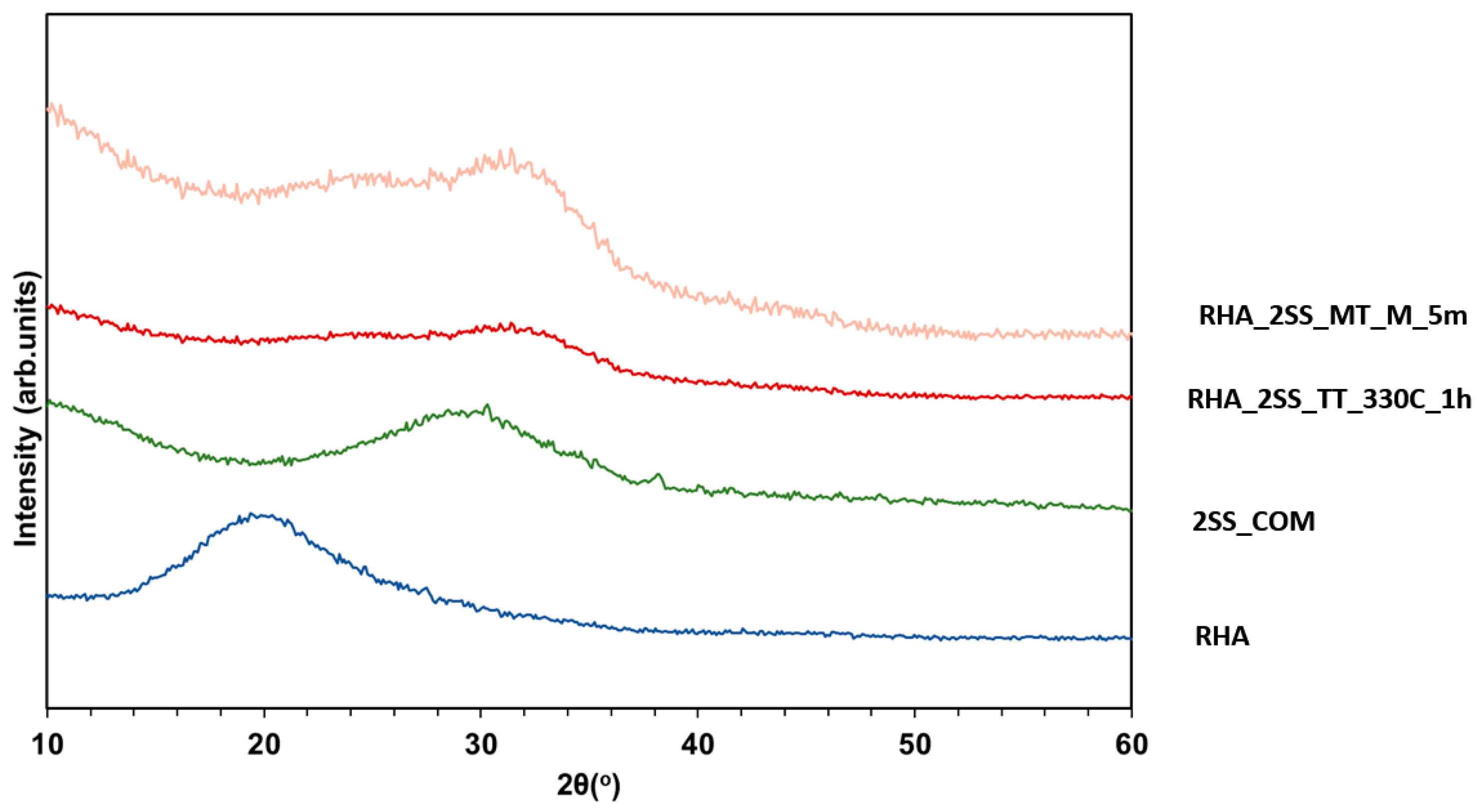
3.1. XRD Analysis
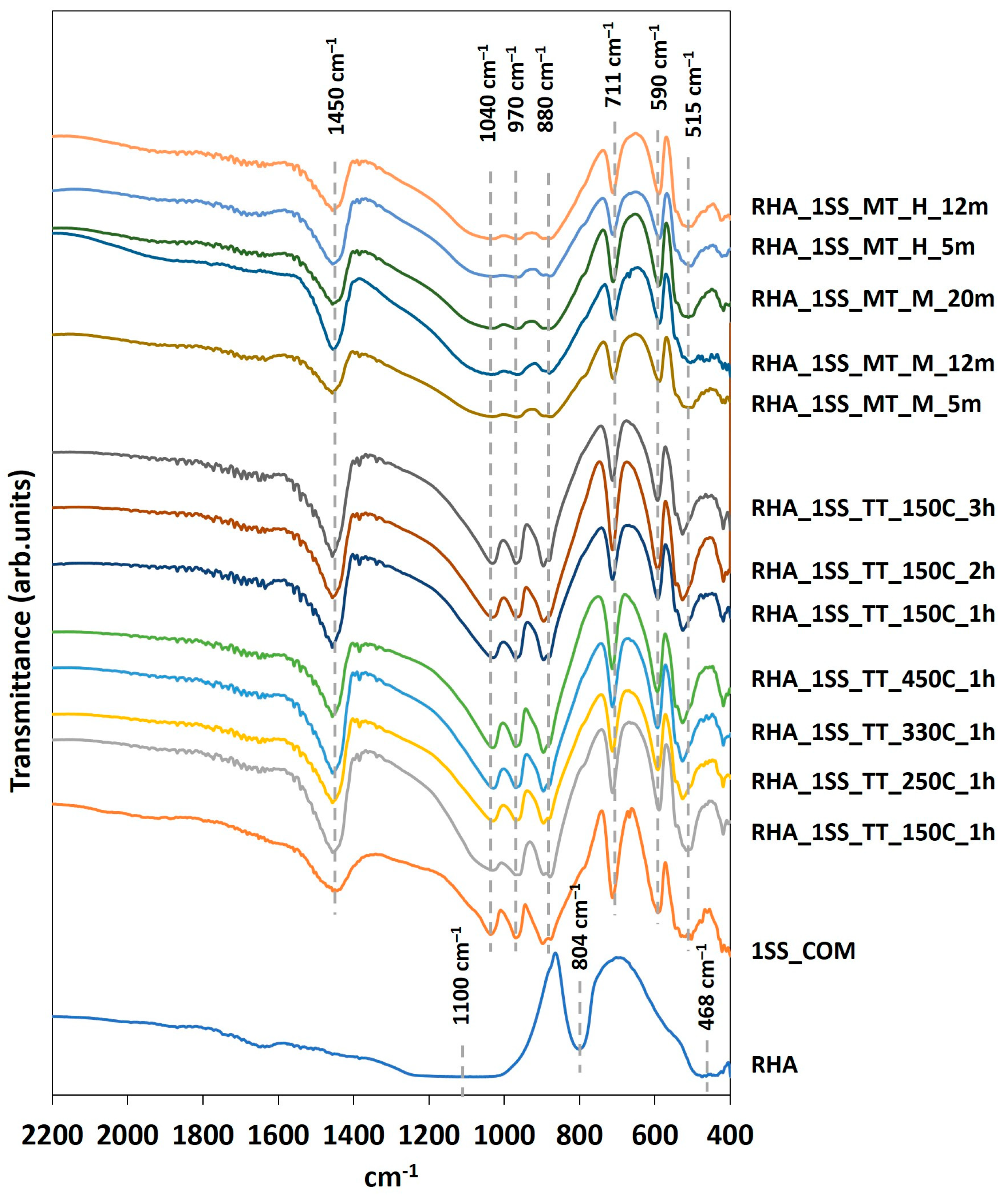
3.2. FTIR Analysis
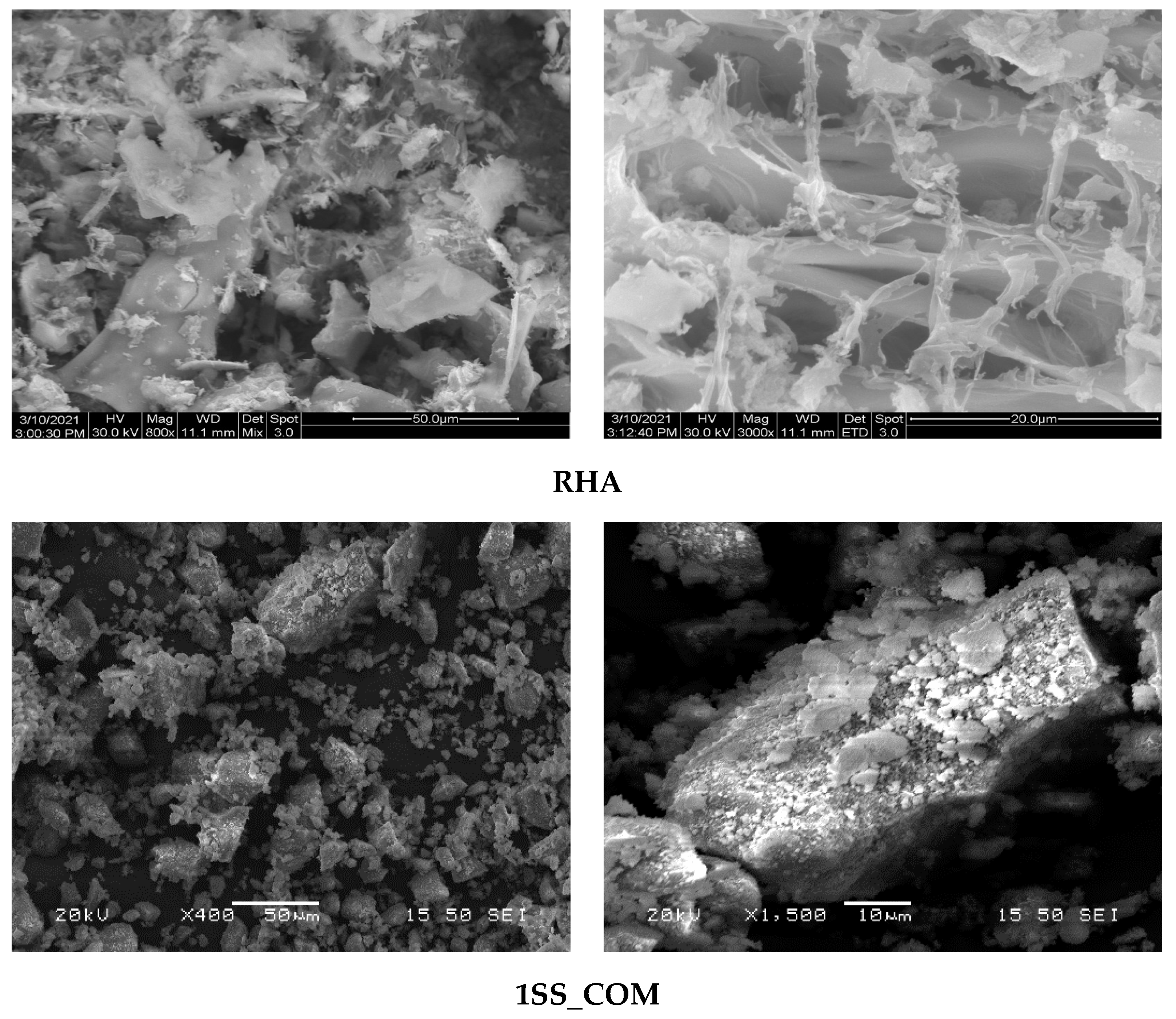
3.3. Microstructural Analysis
3.4. Compressive Strength
4. Conclusions
- A simple synthesis procedure for the preparation of RHA-based solid activators was developed. This procedure involves the mixing of RHA and NH with water to form a pulp and then the treatment of the pulp by either conventional or microwave heating at mild conditions. Indeed, solid activators were successfully prepared by thermal or microwave processing after treatment at 150 °C for 1 h or 460 W for 5 min, respectively.
- The alternative method of microwave treatment is 12 times faster than the average time of conventional heat treatment in a laboratory oven and, therefore, more economically and environmentally advantageous.
- The SiO2/Na2O molar ratio proved to be a crucial factor for the successful preparation of RHA based solid activators. The application of SiO2/Na2O molar ratio equal to 1, successfully converts RHA to activators mainly consisting of crystalline Na2SiO3 (~60–76%) that obtain similar water solubility (>97%), microstructure and branched silica network to the commercial sodium silicate product. Higher SiO2/Na2O molar ratios did not succeed to achieve high conversion yields of RHA.
- The other synthesis parameters (temperature, microwave power and time of processing) do not significantly differentiate the activators’ composition. However, they affect the Na2SiO3 crystalline content. In general, conventional thermal processing produces activators of higher crystalline Na2SiO3 contents (59.9–76.1%) in relation to microwave processing (53.9–70.7%).
- The type of processing had a great impact on the microstructure of the produced solid activators. Microwave processing leads to more porous activators that can be more reactive compared to their counterpart obtained through conventional thermal treatment.
- The efficiency of the developed solid activators was tested through the preparation of geopolymer pastes from FA. The results showed that the RHA activators can effectively replace both the conventional activation solution and the commercial solid activators (sodium silicates and disilicates) of the geopolymer technology, since the prepared geopolymer pastes achieve 90 to 100% of their compressive strength values (62 MPa).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eberhardt, L.C.M.; Birgisdottir, H.; Birkved, M. Potential of Circular Economy in Sustainable Buildings. IOP Conf. Ser. Mater. Sci. Eng. 2019, 471, 092051. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A Review and Prospects for the Minerals Industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an Alternative to Portland Cement: An Overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Rakhimova, N.R. Recent Advances in Blended Alkali-Activated Cements: A Review. Eur. J. Environ. Civ. Eng. 2022, 26, 4596–4618. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications; Geopolymer Institute: Saint-Quenti, France, 2008; ISBN 978-2-9514820-1-2. [Google Scholar]
- Part, W.K.; Ramli, M.; Cheah, C.B. An Overview on the Influence of Various Factors on the Properties of Geopolymer Concrete Derived from Industrial By-Products. Constr. Build. Mater. 2015, 77, 370–395. [Google Scholar] [CrossRef]
- Almalkawi, A.T.; Balchandra, A.; Soroushian, P. Potential of Using Industrial Wastes for Production of Geopolymer Binder as Green Construction Materials. Constr. Build. Mater. 2019, 220, 516–524. [Google Scholar] [CrossRef]
- Ming, L.Y.; En, O.W.; Yong, H.C.; Abdullah, M.M.A.B.; Ween, O.S. Characteristic of One-Part Geopolymer as Building Materials. In Sustainable Waste Utilization in Bricks, Concrete, and Cementitious Materials: Characteristics, Properties, Performance, and Applications; Abdul Kadir, A., Amira Sarani, N., Shahidan, S., Eds.; Lecture Notes in Civil Engineering; Springer: Singapore, 2021; pp. 97–118. ISBN 978-981-334-918-6. [Google Scholar]
- Global Geopolymers for Construction Market—Industry Reports. Available online: https://www.researchreportsworld.com/global-geopolymers-for-construction-market-27262614 (accessed on 12 June 2024).
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-Part Alkali-Activated Materials: A Review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Qin, Y.; Qu, C.; Ma, C.; Zhou, L. One-Part Alkali-Activated Materials: State of the Art and Perspectives. Polymers 2022, 14, 5046. [Google Scholar] [CrossRef]
- Elzeadani, M.; Bompa, D.V.; Elghazouli, A.Y. One Part Alkali Activated Materials: A State-of-the-Art Review. J. Build. Eng. 2022, 57, 104871. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Liu, J.-C.; Wu, B. Mechanical Properties and Reaction Mechanism of One-Part Geopolymer Mortars. Constr. Build. Mater. 2021, 273, 121973. [Google Scholar] [CrossRef]
- Palomo, A.; Maltseva, O.; Garcia-Lodeiro, I.; Fernández-Jiménez, A. Portland Versus Alkaline Cement: Continuity or Clean Break: “A Key Decision for Global Sustainability”. Front. Chem. 2021, 9, 705475. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; He, Y.; Zhu, Y.; Liu, L.; Cui, X. Novel Procedure of CO2 Capture of the CaO Sorbent Activator on the Reaction of One-Part Alkali-Activated Slag. RSC Adv. 2021, 11, 12476–12483. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Provis, J.L.; van Deventer, J.S.J. Thermal Activation of Albite for the Synthesis of One-Part Mix Geopolymers. J. Am. Ceram. Soc. 2012, 95, 565–572. [Google Scholar] [CrossRef]
- Peng, M.X.; Wang, Z.H.; Xiao, Q.G.; Song, F.; Xie, W.; Yu, L.C.; Huang, H.W.; Yi, S.J. Effects of Alkali on One-Part Alkali-Activated Cement Synthesized by Calcining Bentonite with Dolomite and Na2CO3. Appl. Clay Sci. 2017, 139, 64–71. [Google Scholar] [CrossRef]
- Hassan, H.S.; Abdel-Gawwad, H.A.; Vásquez-García, S.R.; Israde-Alcántara, I.; Flores-Ramirez, N.; Rico, J.L.; Mohammed, M.S. Cleaner Production of One-Part White Geopolymer Cement Using Pre-Treated Wood Biomass Ash and Diatomite. J. Clean. Prod. 2019, 209, 1420–1428. [Google Scholar] [CrossRef]
- Peng, M.X.; Wang, Z.H.; Shen, S.H.; Xiao, Q.G. Synthesis, Characterization and Mechanisms of One-Part Geopolymeric Cement by Calcining Low-Quality Kaolin with Alkali. Mater. Struct. 2015, 48, 699–708. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Roles of Hybrid Activators in Improving the Early-Age Properties of One-Part Geopolymer Pastes. Constr. Build. Mater. 2021, 306, 124880. [Google Scholar] [CrossRef]
- Wan-En, O.; Yun-Ming, L.; Li-Ngee, H.; Abdullah, M.M.A.B.; Shee-Ween, O. The Effect of Sodium Carbonate on the Fresh and Hardened Properties of Fly Ash-Based One-Part Geopolymer. IOP Conf. Ser. Mater. Sci. Eng. 2020, 864, 012197. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S.J. Effect of Alumina Release Rate on the Mechanism of Geopolymer Gel Formation. Chem. Mater. 2010, 22, 5199–5208. [Google Scholar] [CrossRef]
- Gluth, G.J.G.; Lehmann, C.; Rübner, K.; Kühne, H.-C. Geopolymerization of a Silica Residue from Waste Treatment of Chlorosilane Production. Mater. Struct. 2013, 46, 1291–1298. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Carcelen-Taboada, V.; Fernández-Jiménez, A.; Palomo, A. Manufacture of Hybrid Cements with Fly Ash and Bottom Ash from a Municipal Solid Waste Incinerator. Constr. Build. Mater. 2016, 105, 218–226. [Google Scholar] [CrossRef]
- Suwan, T.; Fan, M. Effect of Manufacturing Process on the Mechanisms and Mechanical Properties of Fly Ash-Based Geopolymer in Ambient Curing Temperature. Mater. Manuf. Process. 2017, 32, 461–467. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Haruna, S.; Wahab, M.M.A.; Liew, M.S.; Haruna, A. Mechanical and Microstructural Properties of High Calcium Fly Ash One-Part Geopolymer Cement Made with Granular Activator. Heliyon 2019, 5, e02255. [Google Scholar] [CrossRef] [PubMed]
- Haruna, S.; Mohammed, B.S.; Wahab, M.M.A.; Kankia, M.U.; Amran, M.; Gora, A.M. Long-Term Strength Development of Fly Ash-Based One-Part Alkali-Activated Binders. Materials 2021, 14, 4160. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Qiu, J.; Yang, E.-H. Micromechanics-Based Investigation of a Sustainable Ambient Temperature Cured One-Part Strain Hardening Geopolymer Composite. Constr. Build. Mater. 2017, 131, 552–563. [Google Scholar] [CrossRef]
- Çetintaş, R.; Soyer-Uzun, S. Relations between Structural Characteristics and Compressive Strength in Volcanic Ash Based One–Part Geopolymer Systems. J. Build. Eng. 2018, 20, 130–136. [Google Scholar] [CrossRef]
- Panitsa, O.A.; Kioupis, D.; Kakali, G. One-Part Geopolymer Synthesis of Greek Fly Ash. Key Eng. Mater. 2021, 894, 135–142. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F.U.A. Synthesis of Heat and Ambient Cured One-Part Geopolymer Mixes with Different Grades of Sodium Silicate. Ceram. Int. 2015, 41, 5696–5704. [Google Scholar] [CrossRef]
- Zareechian, M.; Siad, H.; Lachemi, M.; Sahmaran, M. Advancements in Cleaner Production of One-Part Geopolymers: A Comprehensive Review of Mechanical Properties, Durability, and Microstructure. Constr. Build. Mater. 2023, 409, 133876. [Google Scholar] [CrossRef]
- Vinai, R.; Soutsos, M. Production of Sodium Silicate Powder from Waste Glass Cullet for Alkali Activation of Alternative Binders. Cem. Concr. Res. 2019, 116, 45–56. [Google Scholar] [CrossRef]
- Panitsa, O.A.; Kioupis, D.; Kakali, G. Thermal and Microwave Synthesis of Silica Fume-Based Solid Activator for the One-Part Geopolymerization of Fly Ash. Environ. Sci. Pollut. Res. 2022, 29, 59513–59523. [Google Scholar] [CrossRef] [PubMed]
- Sturm, P.; Gluth, G.J.G.; Brouwers, H.J.H.; Kühne, H.-C. Synthesizing One-Part Geopolymers from Rice Husk Ash. Constr. Build. Mater. 2016, 124, 961–966. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; van Deventer, J.S.J. Solid Reactant-Based Geopolymers from Rice Hull Ash and Sodium Aluminate. Waste Biomass Valorization 2017, 8, 2131–2140. [Google Scholar] [CrossRef]
- Luukkonen, T.; Sreenivasan, H.; Abdollahnejad, Z.; Yliniemi, J.; Kantola, A.; Telkki, V.-V.; Kinnunen, P.; Illikainen, M. Influence of Sodium Silicate Powder Silica Modulus for Mechanical and Chemical Properties of Dry-Mix Alkali-Activated Slag Mortar. Constr. Build. Mater. 2020, 233, 117354. [Google Scholar] [CrossRef]
- ASTM C109/C109M; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2023.
- Asprogerakas, A.; Koutelia, A.; Kakali, G.; Tsivilis, S. Durability of Fly Ash Geopolymer Mortars in Corrosive Environments, Compared to That of Cement Mortars. Adv. Sci. Technol. 2014, 92, 84–89. [Google Scholar] [CrossRef]
- Panagiotopoulou, C.; Tsivilis, S.; Kakali, G. Application of the Taguchi Approach for the Composition Optimization of Alkali Activated Fly Ash Binders. Constr. Build. Mater. 2015, 91, 17–22. [Google Scholar] [CrossRef]
- ASTM C305; Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency. ASTM International: West Conshohocken, PA, USA, 2023.
- Wiphawadee, P.; Charoen, N.; Claudia, K.; Norbert, V. Influence of Filler from a Renewable Resource and Silane Coupling Agent on the Properties of Epoxidized Natural Rubber Vulcanizates. Available online: https://www.hindawi.com/journals/jchem/2015/796459/ (accessed on 2 November 2023).
- Min, Y.J.; Hong, S.-M.; Kim, S.H.; Lee, K.B.; Jeon, S.G. High-Temperature CO2 Sorption on Na2CO3-Impregnated Layered Double Hydroxides. Korean J. Chem. Eng. 2014, 31, 1668–1673. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Lee, M.S. Infrared Spectra and Thermal Properties of Sodium Silicate Solutions. Korean J. Met. Mater. 2018, 56, 72–78. [Google Scholar]
- Kordatos, K.; Gavela, S.; Ntziouni, A.; Pistiolas, K.N.; Kyritsi, A.; Kasselouri-Rigopoulou, V. Synthesis of Highly Siliceous ZSM-5 Zeolite Using Silica from Rice Husk Ash. Microporous Mesoporous Mater. 2008, 115, 189–196. [Google Scholar] [CrossRef]
- Kordatos, K.; Ntziouni, A.; Iliadis, L.; Kasselouri-Rigopoulou, V. Utilization of Amorphous Rice Husk Ash for the Synthesis of ZSM-5 Zeolite under Low Temperature. J. Mater. Cycles Waste Manag. 2013, 15, 571–580. [Google Scholar] [CrossRef]




| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | TiO2 | P2O5 | MnO | L.O.I. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 40.60 | 20.58 | 3.33 | 11.61 | 1.19 | 0.72 | 0.23 | 0.90 | 0.79 | 0.26 | 0.06 | 16.70 |
| RHA | 90.41 | 0.00 | 0.06 | 1.04 | 3.39 | 0.52 | 0.00 | 0.33 | 0.00 | 1.07 | 0.15 | 4.20 |
| ID of RSAs | RHA (% w/w) | NH (% w/w) | H2O (% w/w) | SiO2/Na2O Molar Ratio | Treatment Method | Temperature (°C) | Power (watt) | Treatment Duration |
|---|---|---|---|---|---|---|---|---|
| RHA_1SS_TT_330C_0.5h | 30.4 | 36.6 | 32.9 | 1 | Thermal | 330 | 0.5 h | |
| RHA_1SS_TT_330C_1h | 30.4 | 36.6 | 32.9 | 1 | Thermal | 330 | - | 1 h |
| RHA_1SS_TT_330C_2h | 30.4 | 36.6 | 32.9 | 1 | Thermal | 330 | - | 2 h |
| RHA_1SS_TT_330C_3h | 30.4 | 36.6 | 32.9 | 1 | Thermal | 330 | - | 3 h |
| RHA_1SS_TT_150C_1h | 30.4 | 36.6 | 32.9 | 1 | Thermal | 150 | - | 1 h |
| RHA_1SS_TT_250C_1h | 30.4 | 36.6 | 32.9 | 1 | Thermal | 250 | - | 1 h |
| RHA_1SS_TT_450C_1h | 30.4 | 36.6 | 32.9 | 1 | Thermal | 450 | - | 1 h |
| RHA_2SS_TT_330C_1h | 46.6 | 28.1 | 25.3 | 2 | Thermal | 330 | - | 1 h |
| RHA_1SS_MT_M_5m | 30.4 | 36.6 | 32.9 | 1 | Microwave | - | 460 | 5 min |
| RHA_1SS_MT_M_12m | 30.4 | 36.6 | 32.9 | 1 | Microwave | - | 460 | 12 min |
| RHA_1SS_MT_M_20m | 30.4 | 36.6 | 32.9 | 1 | Microwave | - | 460 | 20 min |
| RHA_1SS_MT_H_5m | 30.4 | 36.6 | 32.9 | 1 | Microwave | - | 700 | 5 min |
| RHA_1SS_MT_H_12m | 30.4 | 36.6 | 32.9 | 1 | Microwave | - | 700 | 12 min |
| RHA_2SS_MT_M_5m | 46.6 | 28.1 | 25.3 | 2 | Microwave | - | 460 | 5 min |
| MIX ID | FA | Sol | 1SS_COM | 2SS_COM | RSAs | NH | H2O | |
|---|---|---|---|---|---|---|---|---|
| 1SS | 2SS | |||||||
| G2P | 60.4 | 14.8 | - | - | - | - | 9.8 | 14.9 |
| G1P_1SS_COM | 61.3 | - | 15.3 | - | - | - | - | 23.4 |
| G1P_2SS_COM | 63.1 | - | - | 11.6 | - | - | 5.2 | 20.0 |
| G1P_RHA_1SS_TT_150C | 63.4 | - | - | - | 15.8 | - | - | 20.8 |
| G1P_RHA_2SS_TT_150C | 57.8 | - | - | - | - | 10.6 | 4.8 | 26.8 |
| G1P_RHA_1SS_MT_M | 63.9 | - | - | - | 16.0 | - | - | 20.1 |
| G1P_RHA_2SS_MT_M | 58.0 | - | - | - | - | 10.7 | 4.8 | 26.5 |
| Sample ID | Na2SiO3 (%) | Amorphous (%) | Solubility (%) * |
|---|---|---|---|
| Commercial | |||
| 1SS_COM | 50.9 | 49.1 | 99.1 |
| 2SS_COM | - | 100.0 | 99.2 |
| Thermally Treated | |||
| RHA_1SS_TT_330C_0.5h | 59.9 | 40.1 | 98.3 |
| RHA_1SS_TT_330C_1h | 76.1 | 23.9 | 97.1 |
| RHA_1SS_TT_330C_2h | 67.3 | 32.7 | 97.9 |
| RHA_1SS_TT_330C_3h | 66.9 | 33.1 | 98.5 |
| RHA_1SS_TT_150C_1h | 66.8 | 33.2 | 98.3 |
| RHA_1SS_TT_250C_1h | 68.9 | 31.1 | 98.2 |
| RHA_1SS_TT_450C_1h | 75.5 | 24.5 | 96.5 |
| RHA_2SS_TT_330C_1h | - | 100.0 | 10.1 |
| Microwave-treated | |||
| RHA_1SS_MT_M_5m | 56.8 | 43.2 | 95.9 |
| RHA_1SS_MT_H_5m | 58.0 | 42.0 | 99.2 |
| RHA_1SS_MT_M_12m | 57.1 | 42.9 | 99.6 |
| RHA_1SS_MT_H_12m | 70.7 | 29.3 | 98.7 |
| RHA_1SS_MT_M_20m | 53.9 | 46.1 | 98.7 |
| RHA_2SS_MT_M_5m | - | 100.0 | 15.2 |
| RHA | - | 100.0 | 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panitsa, O.A.; Kioupis, D.; Kakali, G. Advancing the Sustainability of Geopolymer Technology through the Development of Rice Husk Ash Based Solid Activators. Sustainability 2024, 16, 7243. https://doi.org/10.3390/su16177243
Panitsa OA, Kioupis D, Kakali G. Advancing the Sustainability of Geopolymer Technology through the Development of Rice Husk Ash Based Solid Activators. Sustainability. 2024; 16(17):7243. https://doi.org/10.3390/su16177243
Chicago/Turabian StylePanitsa, Olga Andriana, Dimitrios Kioupis, and Glikeria Kakali. 2024. "Advancing the Sustainability of Geopolymer Technology through the Development of Rice Husk Ash Based Solid Activators" Sustainability 16, no. 17: 7243. https://doi.org/10.3390/su16177243
APA StylePanitsa, O. A., Kioupis, D., & Kakali, G. (2024). Advancing the Sustainability of Geopolymer Technology through the Development of Rice Husk Ash Based Solid Activators. Sustainability, 16(17), 7243. https://doi.org/10.3390/su16177243







