Enhancing Biogas Production: An Assessment of Pasteurization Effects on Poultry, Swine, Bovine Manure and Food Waste Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
- Poultry manure from a broiler farm (poultry manure—PM).
- Swine manure from a pig farm (swine manure—SM).
- Cattle manure from a bovine farm (cattle manure—CM).
- Expired or unsuitable for human consumption foodstuffs (FW), including both liquid and solid foods. This category encompasses animal-origin liquids and plant-based foods (e.g., milk, juices, oil) as well as solid foods such as restaurant leftovers, fruits, vegetables, legumes, canned goods, deli meats, and dairy products.
- Inoculum containing all the necessary anaerobic microorganisms (hydrolytic, acidogenic, acetogenic, and methanogenic) to initiate the chemical and enzymatic reactions of the raw materials within the digester.
2.2. Physicochemical Analysis
(0.64×Crude Fiber%DM × 790) + (0.9 × NFE%DM × 790) × DM%]/10,000
2.3. Microbiological Analysis
2.4. Biogas Measurement
2.5. Statistical Analysis
3. Results
Physicochemical Analysis
4. Discussion
5. Conclusions
- Biogas yield enhancement: Pasteurization generally improved biogas yields from poultry manure, swine slurry, and cattle manure. This suggests that pasteurization breaks down complex organic compounds, making them more accessible to anaerobic bacteria and enhancing the digestion process. This can lead to more efficient biogas production from these substrates, optimizing energy recovery from organic waste.
- Food waste considerations: Although food waste showed a slight decrease in biogas yield postpasteurization, this highlights the complexity of its composition. The diverse mix of animal and plant materials in food waste may require tailored pasteurization processes to avoid inhibiting microbial communities crucial for biodegradation. Further research is needed to refine pasteurization techniques for food waste to maximize its biogas potential.
- Preservation of substrate quality: Pasteurization did not alter the physicochemical parameters or metal content of the substrates. This ensures that the structural integrity and elemental composition of the substrates are maintained, preserving their quality for effective biogas production.
- Pathogen reduction: Pasteurization significantly reduced pathogenic loads in the substrates, enhancing the safety of the digestate for agricultural use. This addresses public health concerns by minimizing the risk of pathogen spread through the application of treated organic waste, promoting safer and more sustainable agricultural practices.
- Sustainability and waste management: The study supports the use of pasteurization in anaerobic digestion processes to improve biogas yield and safety. By optimizing the digestion process, it contributes to more efficient waste management and supports renewable energy production, aligning with sustainability goals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papagrigoriou, A.; Zavali, M. Circular Economy and Entrepreneurship in the Agri-Food Sector, 1st ed.; Kallipos, Open Academic Editions: Athens, Greece, 2023. [Google Scholar]
- Regulation, (EU). No 1774/2002 of The European Parliament and of The Council of 3 October 2002 Laying down Health Rules Concerning Animal by-Products Not Intended for Human Consumption. Off. J. Eur. Union 2002, 1–95. [Google Scholar]
- Regulation, (EU). No 142/2011 of 25 February 2011 Implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down Health Rules as Regards Animal by-Products and Derived Products Not Intended for Human consumption and Implementing Council Directive 97/78/EC as Regards Certain Samples and Items from Veterinary Checks at the Border under That Directive. Off. J. Eur. Union 2011, 1–254. [Google Scholar]
- Rafique, R.; Poulsen, T.G.; Nizami, A.S.; Murphy, J.D.; Kiely, G. Effect of Thermal, Chemical and Thermo-Chemical Pre-Treatments to Enhance Methane Production. Energy 2010, 35, 4556–4561. [Google Scholar] [CrossRef]
- Liu, X.; Lendormi, T.; Lanoisellé, J.L. A Review of Hygienization Technology of Biowastes for Anaerobic Digestion: Effect on Pathogen Inactivation and Methane Production. Chem. Eng. Trans. 2018, 70, 529–534. [Google Scholar]
- Gavala, H.N.; Yenal, U.; Skiadas, I.V.; Westermann, P.; Ahring, B.K. Mesophilic and Thermophilic Anaerobic Digestion of Primary and Secondary Sludge. Effect of Pre-Treatment at Elevated Temperature. Water Res. 2003, 37, 4561–4572. [Google Scholar] [CrossRef] [PubMed]
- Edström, M.; Nordberg, A.; Thyselius, L. Anaerobic Treatment of Animal Byproducts from Slaughterhouses at Laboratory and Pilot Scale. Appl. Biochem. Biotech. 2003, 109, 127–138. [Google Scholar] [CrossRef]
- Liu, X.; Souli, I.; Chamaa, M.-A.; Lendormi, T.; Sabourin, C.; Lemée, Y.; Boy, V.; Chaira, N.; Ferchichi, A.; Morançais, P.; et al. Effect of Thermal Pretreatment at 70 °C for One Hour (EU Hygienization Conditions) of Various Organic Wastes on Methane Production under Mesophilic Anaerobic Digestion. AIMS Environ. Sci. 2018, 5, 117–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Kusch-Brandt, S.; Heaven, S.; Banks, C.J. Effect of Pasteurisation on Methane Yield from Food Waste and Other Substrates in Anaerobic Digestion. Processes 2020, 8, 1351. [Google Scholar] [CrossRef]
- Rodríguez-Abalde, A.; Fernández, B.; Silvestre, G.; Flotats, X. Effects of Thermal Pre-Treatments on Solid Slaughterhouse Waste Methane Potential. Waste Manag. 2011, 31, 1488–1493. [Google Scholar] [CrossRef]
- Grim, J.; Malmros, P.; Schnürer, A.; Nordberg, Å. Comparison of Pasteurization and Integrated Thermophilic Sanitation at a Full-Scale Biogas Plant—Heat Demand and Biogas Production. Energy 2015, 79, 419–427. [Google Scholar] [CrossRef]
- Hejnfelt, A.; Angelidaki, I. Anaerobic Digestion of Slaughterhouse By-Products. Biomass Bioenergy 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Verein Deutscher Ingenieure VDI 4630 Fermentation of Organic Materials Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; VDI-Gesellschaft Energie und Umwelt (GEU): Düsseldorf, Germany, 2016.
- Karvonen, N. Operating Instructions. J. Wildl. Rehabil. 2002, 25, 27. [Google Scholar]
- Nathan, A.J.; Scobell, A. APHA AWWA 23rd EDITION. Foreign Aff. 2017, 91, 1689–1699. [Google Scholar]
- ISO 17294-2:2016; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. International Standard: Geneva, Switzerland, 2016.
- CEN/TR 16193:2013; Sludge, Treated Biowaste and Soil—Detection and Enumeration of Escherichia coli. International Standard: Geneva, Switzerland, 2013.
- ISO 7899-2; Water Quality—Detection and Enumeration of Intestinal Enterococci—Part 2: Membrane Filtration Method. International Standard: Geneva, Switzerland, 2000.
- ISO 6579-1:2017; Microbiology of the Food Chain, Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. International Standard: Geneva, Switzerland, 2017.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. International Standard: Geneva, Switzerland, 2017.
- Weik, M.H. Maintenance Manual. Comput. Sci. Commun. Dict. 2000, 46, 969. [Google Scholar]
- Markou, G. Poultry Waste (Manure): A Review of the Main Technologies for Its Management and Utilization. e-J. Sci. Technol. (E-JST) 2016, 1–21. [Google Scholar]
- Sager, M. Trace and Nutrient Elements in Manure, Dung and Compost Samples in Austria. Soil. Biol. Biochem. 2007, 39, 1383–1390. [Google Scholar] [CrossRef]
- Hamilton, D.W. Anaerobic Digestion of Animal Manures: Methane Production Potential of Waste Materials. Bae 2012, 1762, 4–7. [Google Scholar]
- Li, Y.; Zhang, R.; Liu, G.; Chen, C.; He, Y.; Liu, X. Comparison of Methane Production Potential, Biodegradability, and Kinetics of Different Organic Substrates. Bioresour. Technol. 2013, 149, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Liu, X.; Chen, C.; Xiao, X.; Feng, L.; He, Y.; Liu, G. Evaluating Methane Production from Anaerobic Mono- and Co-Digestion of Kitchen Waste, Corn Stover, and Chicken Manure. Energy Fuels 2013, 27, 2085–2091. [Google Scholar] [CrossRef]
- Sakar, S.; Yetilmezsoy, K.; Kocak, E. Anaerobic Digestion Technology in Poultry and Livestock Waste Treatment—A Literature Review. Waste Manag. Res. 2009, 27, 3–18. [Google Scholar] [CrossRef]
- Wijaya, A.S.; Jariyaboon, R.; Reungsang, A.; Kongjan, P. Biochemical Methane Potential (BMP) of Cattle Manure, Chicken Manure, Rice Straw, and Hornwort in Mesophilic Mono-Digestion. Int. J. Integr. Eng. 2020, 12, 1–8. [Google Scholar]
- Orlando, M.-Q.; Borja, V.-M. Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review. Energies 2020, 13, 3573. [Google Scholar] [CrossRef]
- Assefa, A.; Egigu, M.C.; Kebede, A. Thermal And Chemical Pre-Treatments Of Cow Dung And Poultry Litter Enhance Biogas Production In Batch Fermentation. Int. J. Sci. Tech. Res. 2014, 3, 165–170. [Google Scholar]
- Ogunwande, G.A.; Adeagbo, J.A.; Fakuyi, A.O. Effects of Co-Digesting Swine Manure with Chicken Manure on Biogas Production. Ife J. Sci. 2013, 15, 1–8. [Google Scholar]
- Moral, R.; Perez-Murcia, M.D.; Perez-Espinosa, A.; Moreno-Caselles, J.; Paredes, C. Estimation of Nutrient Values of Pig Slurries in Southeast Spain Using Easily Determined Properties. Waste Manag. 2005, 25, 719–725. [Google Scholar] [CrossRef]
- YANG, Z.; HAN, L.; LI, Q.; PIAO, X. Estimating Nutrient Contents of Pig Slurries Rapidly by Measurement of Physical and Chemical Properties. J. Agric. Sci. 2006, 144, 261–267. [Google Scholar] [CrossRef]
- Sommer, S.G.; Hjorth, M.; Leahy, J.J.; Zhu, K.; Christel, W.; Sørensen, C.G.; Sutaryo, S. Pig Slurry Characteristics, Nutrient Balance and Biogas Production as Affected by Separation and Acidification. J. Agric. Sci. 2015, 153, 177–191. [Google Scholar] [CrossRef]
- Søndergaard, M.M.; Fotidis, I.A.; Kovalovszki, A.; Angelidaki, I. Anaerobic Co-Digestion of Agricultural Byproducts with Manure for Enhanced Biogas Production. Energy Fuels 2015, 29, 8088–8094. [Google Scholar] [CrossRef]
- Rodriguez-Perez, A.I.; Sucunza, D.; Pedrosa, M.A.; Garrido-Gil, P.; Kulisevsky, J.; Lanciego, J.L.; Labandeira-Garcia, J.L. Angiotensin Type 1 Receptor Antagonists Protect against Alpha-Synuclein-Induced Neuroinflammation and Dopaminergic Neuron Death. Neurotherapeutics 2018, 15, 1063–1081. [Google Scholar] [CrossRef]
- Ólafsdóttir, S.S.; Jensen, C.D.; Lymperatou, A.; Henriksen, U.B.; Gavala, H.N. Effects of Different Treatments of Manure on Mitigating Methane Emissions during Storage and Preserving the Methane Potential for Anaerobic Digestion. J. Environ. Manag. 2023, 325, 116456. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic Co-Digestion of Animal Manures and Lignocellulosic Residues as a Potent Approach for Sustainable Biogas Production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Vergote, T.L.I.; De Dobbelaere, A.E.J.; Willems, B.; Leenknegt, J.; Buysse, J.; Volcke, E.I.P.; Meers, E. Stability of Thermophilic Pig Manure Mono-Digestion: Effect of Thermal Pre-Treatment and Separation. Front. Energy Res. 2020, 8, 40. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, H.; Xu, W.; He, Q.; Zhou, Q. Enhancement of Biochemical Methane Potential from Excess Sludge with Low Organic Content by Mild Thermal Pretreatment. Biochem. Eng. J. 2013, 70, 127–134. [Google Scholar] [CrossRef]
- Triolo, J.M.; Ward, A.J.; Pedersen, L.; Sommer, S.G. Characteristics of Animal Slurry as a Key Biomass for Biogas Production in Denmark. Biomass Now Sustain. Growth Use 2013, 307–326. [Google Scholar]
- Chastain, J.P.; Camberato, J.J. Dairy Manure Production and Nutrient Content. Confin. Anim. Manure Manag. Certif. Program Man. Dairy Version 2004, 1–16. [Google Scholar]
- Shepherd, M.; Bhogal, A.; Philipps, L.; Jackson, L. The Nutrient Content of Cattle Manures from Organic Holdings in England. Biol. Agric. Hortic. 2002, 20, 229–242. [Google Scholar] [CrossRef]
- Abreu-Junior, C.H.; de Lima Brossi, M.J.; Monteiro, R.T.; Cardoso, P.H.S.; da Silva Mandu, T.; Nogueira, T.A.R.; Ganga, A.; Filzmoser, P.; de Oliveira, F.C.; Firme, L.P.; et al. Effects of Sewage Sludge Application on Unfertile Tropical Soils Evaluated by Multiple Approaches: A Field Experiment in a Commercial Eucalyptus Plantation. Sci. Total Environ. 2019, 655, 1457–1467. [Google Scholar] [CrossRef]
- Hassan Onsa -Khalid Elbadawi Elshafie, M. Increase Methane Concentration in Biogas Productions by Pasteurization Waste. Available online: https://www.academia.edu/111434513/Increase_methane_concentration_in_biogas_productions_by_pasteurization_waste?uc-sb-sw=23703042 (accessed on 7 July 2024).
- Luste, S.; Luostarinen, S.; Sillanpää, M. Effect of Pre-Treatments on Hydrolysis and Methane Production Potentials of by-Products from Meat-Processing Industry. J. Hazard Mater. 2009, 164, 247–255. [Google Scholar] [CrossRef]
- Luste, S.; Luostarinen, S. Enhanced Methane Production from Ultrasound Pre-Treated and Hygienized Dairy Cattle Slurry. Waste Manag. 2011, 31, 2174–2179. [Google Scholar] [CrossRef] [PubMed]
- Izhar, T.N.T.; Zakarya, I.A.; Zaaba, S.K.; Yusof, A.H.M.; Shahril, N.M. A Review of Food Waste Characterization and Treatment in Anaerobic Digestion. IOP Conf. Ser. Earth Environ. Sci. 2021, 646, 012004. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Zain, S.M.; Pramanik, B.K. The Anaerobic Digestion Process of Biogas Production from Food Waste: Prospects and Constraints. Bioresour. Tech. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- Ferdeș, M.; Zăbavă, B.Ș.; Paraschiv, G.; Ionescu, M.; Dincă, M.N.; Moiceanu, G. Food Waste Management for Biogas Production in the Context of Sustainable Development. Energies 2022, 15, 6268. [Google Scholar] [CrossRef]
- Xue, S.; Zhao, N.; Song, J.; Wang, X. Interactive Effects of Chemical Composition of Food Waste during Anaerobic Co-Digestion under Thermophilic Temperature. Sustainability 2019, 11, 2933. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Kulupa, T.; Kubiak, A.; Wolna-Maruwka, A.; Pilarski, K.; Niewiadomska, A. Anaerobic Digestion of Food Waste—A Short Review. Energies 2023, 16, 5742. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, H.; Li, J. Effects of Organic Composition on Mesophilic Anaerobic Digestion of Food Waste. Bioresour. Technol. 2017, 244, 213–224. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Frunzo, L.; Esposito, G.; Lens, P.N.L.; Pirozzi, F. Enhanced Anaerobic Digestion of Food Waste by Thermal and Ozonation Pretreatment Methods. J. Environ. Manag. 2014, 146, 142–149. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.; Unalan, S.; et al. A Critical Review of Pretreatment Technologies to Enhance Anaerobic Digestion and Energy Recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Gómez, X.; Otero, M.; Morán, A. Anaerobic Digestion and Co-Digestion of Slaughterhouse Waste (SHW): Influence of Heat and Pressure Pre-Treatment in Biogas Yield. Waste Manag. 2010, 30, 1780–1789. [Google Scholar] [CrossRef]
| Raw Material | Total Solids (TS) | Volatile Solids (VS) | Fat FM * | Fat DM ** | Protein FM * | Protein DM ** | Crude Fibers FM * | Crude Fibers DM ** | Nitrogen Free Extracts (NFE) | Biogas Yield | Biogas Yield |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % | % | mL/g FM * | mL/g VS | |
| 1st batch | |||||||||||
| Inoculum | 3.3 | 1.9 | |||||||||
| PM | 63.1 | 54.6 | 3.8 | 6.1 | 19.7 | 31.8 | 11.4 | 18.3 | 30.0 | 211 | 394 |
| SM | 2.1 | 1.7 | 0.2 | 5.4 | 1.4 | 40.4 | 0.5 | 15.1 | 19.3 | 7.4 | 440 |
| CM | 6.8 | 5.5 | 0.4 | 4.7 | 1.9 | 23.2 | 1.9 | 22.8 | 31.4 | 24.0 | 436 |
| FW | 32.9 | 30.5 | 6.5 | 19.5 | 6.0 | 18.1 | 2.1 | 6.2 | 48.6 | 248 | 814 |
| PM (p) | 63.3 | 53.6 | 4.0 | 6.5 | 19.3 | 31.0 | 12.0 | 19.2 | 29.5 | 207 | 407 |
| SM (p) | 2.8 | 2.3 | 0.3 | 8.9 | 1.1 | 32.7 | 0.4 | 10.9 | 26.7 | 8.9 | 396 |
| CM (p) | 6.6 | 5.4 | 0.4 | 5.8 | 1.6 | 23.7 | 1.4 | 20.9 | 31.8 | 42.5 | 785 |
| FW (p) | 33.1 | 30.2 | 7.5 | 22.0 | 6.2 | 18.2 | 2.9 | 8.5 | 44.1 | 196 | 648 |
| 2nd batch | |||||||||||
| Inoculum | 3.5 | 2.2 | |||||||||
| PM | 62.0 | 53.4 | 4.0 | 6.5 | 18.7 | 30.1 | 11.9 | 19.1 | 30.5 | 214 | 400 |
| SM | 3.5 | 2.8 | 0.4 | 10.2 | 1.5 | 42.1 | 0.8 | 23.0 | 5.0 | 13.6 | 480 |
| CM | 8.4 | 6.9 | 0.4 | 4.2 | 2.7 | 32.2 | 4.6 | 54.7 | 0.0 | 21.6 | 315 |
| FW | 33.0 | 30.5 | 8.2 | 24.8 | 5.7 | 17.4 | 2.3 | 7.0 | 43.3 | 248 | 814 |
| PM (p) | 62.3 | 53.7 | 3.5 | 5.7 | 19.2 | 30.8 | 12.4 | 19.9 | 29.9 | 206 | 383 |
| SM (p) | 3.3 | 2.6 | 0.3 | 9.4 | 1.3 | 40.2 | 0.9 | 27.8 | 1.8 | 11.7 | 447 |
| CM (p) | 6.8 | 5.6 | 0.3 | 3.9 | 1.1 | 16.6 | <0.30 | 0.7 | 60.9 | 41.8 | 753 |
| FW (p) | 34.0 | 31.6 | 7.6 | 22.4 | 5.1 | 14.9 | 2.4 | 7.2 | 48.3 | 245 | 775 |
| 3rd batch | |||||||||||
| Inoculum | 4.1 | 2.7 | |||||||||
| PM | 57.1 | 48.8 | 3.6 | 6.3 | 19.4 | 33.9 | 12.1 | 21.2 | 24.1 | 185 | 379 |
| SM | 18.4 | 15.2 | 0.4 | 9.4 | 1.3 | 35.5 | 0.3 | 8.4 | 39.2 | 8.6 | 250 |
| CM | 9.3 | 7.5 | 0.6 | 6.6 | 2.7 | 28.7 | 2.6 | 28.1 | 17.5 | 22.3 | 298 |
| FW | 32.3 | 29.9 | 7.8 | 24.1 | 5.8 | 17.9 | 2.5 | 7.9 | 42.8 | 224 | 750 |
| PM (p) | 59.4 | 51.2 | 3.7 | 6.3 | 19.6 | 33.0 | 14.7 | 24.7 | 22.2 | 246 | 481 |
| SM (p) | 3.1 | 2.8 | 0.4 | 13.0 | 1.5 | 49.7 | 0.8 | 27.2 | 1.2 | 8.9 | 320 |
| CM (p) | 13.1 | 11.1 | 0.4 | 3.1 | 1.9 | 14.2 | 2.0 | 15.3 | 52.2 | 35.7 | 320 |
| FW (p) | 34.0 | 31.9 | 6.2 | 18.1 | 4.0 | 11.8 | 1.7 | 5.0 | 58.8 | 199 | 623 |
| 4th batch | |||||||||||
| Inoculum | 4.1 | 2.7 | |||||||||
| PM | 56.9 | 54.6 | 4.0 | 7.1 | 19.8 | 34.8 | 12.1 | 21.3 | 21.7 | 177 | 363 |
| SM | 3.5 | 3.4 | 0.4 | 11.2 | 1.5 | 42.4 | 0.8 | 22.8 | 0.9 | 7.3 | 217 |
| CM | 10.0 | 7.4 | 0.6 | 5.9 | 2.9 | 28.6 | 4.4 | 44.2 | 3.9 | 19.0 | 253 |
| FW | 32.7 | 30.7 | 8.4 | 25.7 | 6.0 | 18.2 | 2.5 | 7.8 | 40.6 | 189 | 633 |
| PM (p) | 58.4 | 54.4 | 4.0 | 6.9 | 19.6 | 33.6 | 14.5 | 24.8 | 20.0 | 201 | 393 |
| SM (p) | 3.7 | 2.8 | 0.4 | 10.6 | 1.5 | 41.3 | 0.8 | 21.1 | 5.5 | 9.5 | 341 |
| CM (p) | 13.7 | 10.6 | 0.4 | 3.2 | 1.9 | 13.9 | 2.1 | 15.5 | 53.5 | 24.0 | 216 |
| FW (p) | 33.2 | 31.9 | 7.9 | 23.7 | 6.2 | 18.6 | 2.9 | 8.8 | 41.2 | 169 | 530 |
| Batch Number | PM | PMp | SM | SMp | CM | CMp | FW | FWp |
|---|---|---|---|---|---|---|---|---|
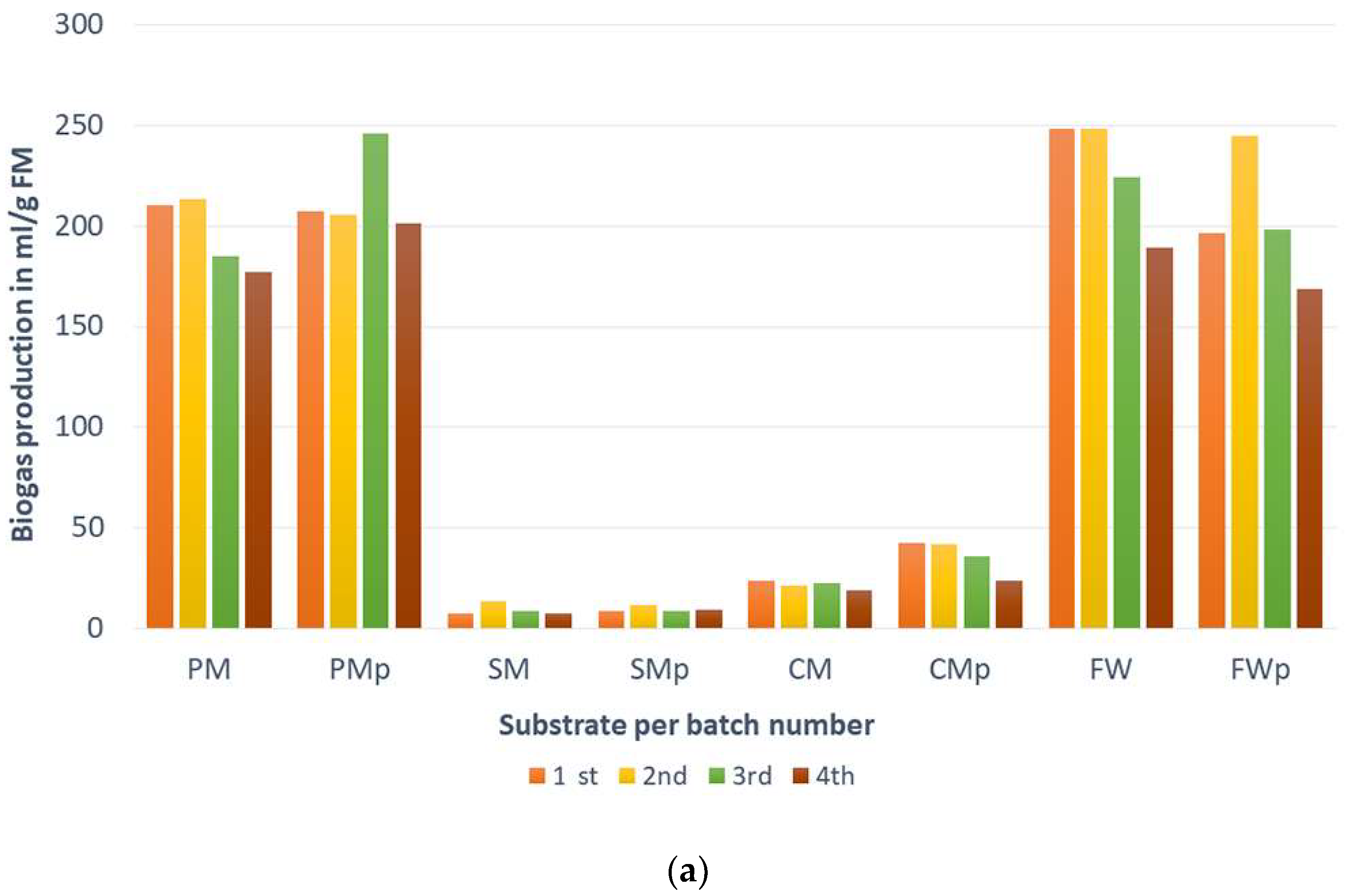
| 1st | 211 | 207 | 7.4 | 8.9 | 24.0 | 42.5 | 248 | 196 |
| 2nd | 214 | 206 | 13.6 | 11.7 | 21.6 | 41.8 | 248 | 245 |
| 3rd | 185 | 246 | 8.6 | 8.9 | 22.3 | 35.7 | 224 | 199 |
| 4th | 177 | 201 | 7.3 | 9.5 | 19.0 | 24.0 | 189 | 169 |
| mean | 197 | 215 | 9.2 | 9.7 | 21.7 | 36.0 | 228 | 202 |
| stdev | 15.9 | 16.2 | 2.6 | 1.2 | 1.8 | 7.4 | 24.3 | 24.3 |
| p-value | 0.12 | 0.07 | 0.02 | 0.11 | ||||
| coefficient of variation | 0.08 | 0.08 | 0.28 | 0.12 | 0.08 | 0.21 | 0.11 | 0.12 |
| Batch Number | PM | PMp | SM | SMp | CM | CMp | FW | FWp |
|---|---|---|---|---|---|---|---|---|
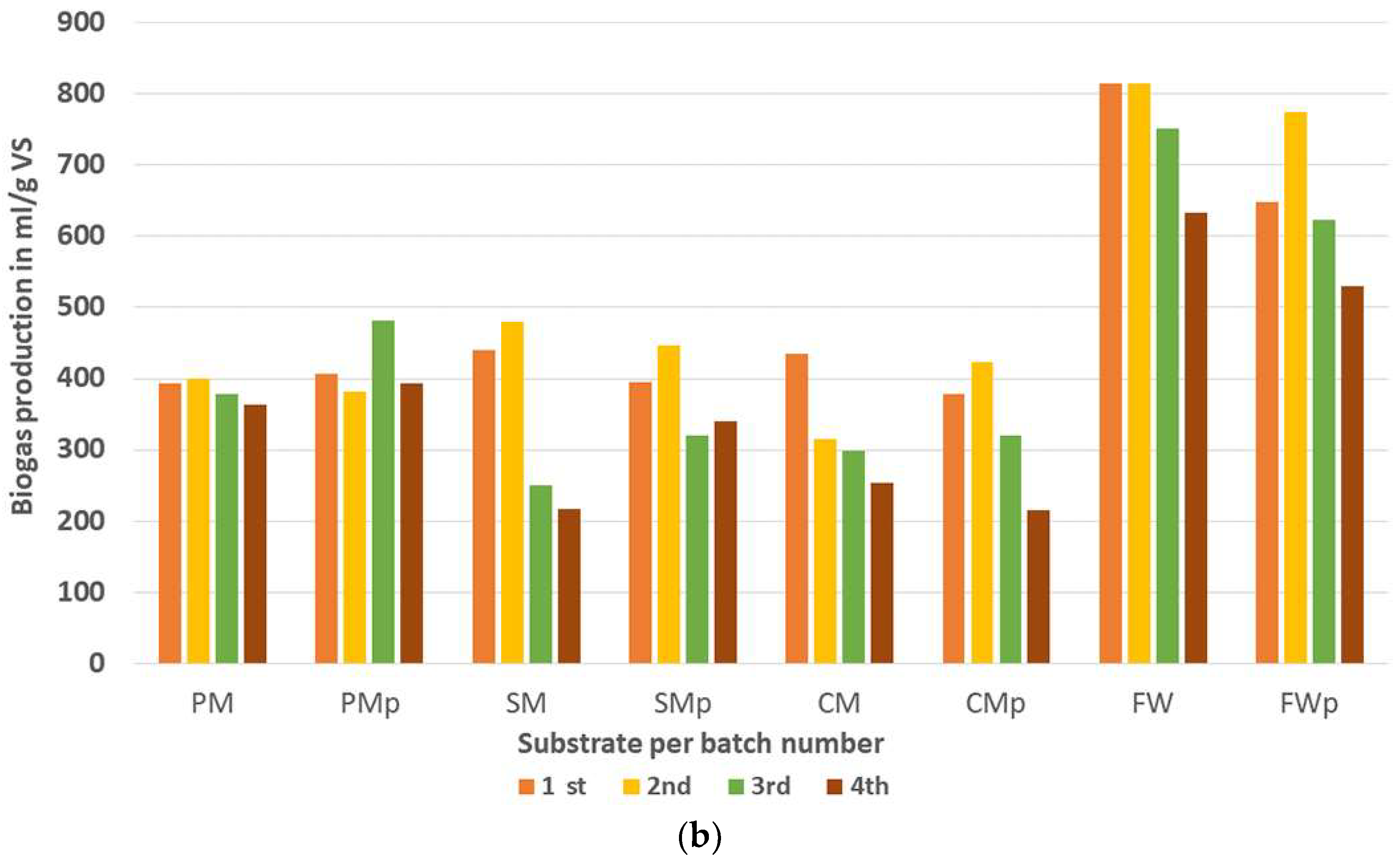
| 1st | 394 | 407 | 440 | 396 | 436 | 378 | 814 | 648 |
| 2nd | 400 | 383 | 480 | 447 | 315 | 423 | 814 | 775 |
| 3rd | 379 | 481 | 250 | 320 | 298 | 320 | 750 | 623 |
| 4th | 363 | 393 | 217 | 342 | 253 | 216 | 633 | 530 |
| mean | 384 | 416 | 347 | 376 | 325 | 334 | 753 | 644 |
| stdev | 14.4 | 38.6 | 115 | 49.4 | 67.5 | 77.8 | 73.9 | 87.4 |
| p-value | 0.12 | 0.35 | 0.44 | 0.04 | ||||
| coefficient of variation | 0.04 | 0.09 | 0.33 | 0.13 | 0.21 | 0.23 | 0.10 | 0.14 |
| Raw Material | Nutrients | Trace Elements | Heavy Metals | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | S | Na | Cu | Zn | Fe | Se | Co | B | Μο | Mn | Al | Ni | Cd | Cr | Pb | As | Hg | |
| g/kg | g/kg | g/kg | g/kg | g/kg | g/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | |
| 1st batch | |||||||||||||||||||||
| PM | 4.4 | 18.7 | 8.7 | 3.9 | 3.3 | 1.2 | 41.9 | 251 | 467 | 0.8 | 0.5 | 45.7 | 3.6 | 385 | 364 | 4.9 | 0.12 | 3.39 | 0.30 | 0.21 | 0.05 |
| SM | 0.5 | 0.6 | 0.7 | 0.3 | 0.2 | 0.2 | 22.7 | 102 | 55.3 | 0.1 | 0.0 | 4.2 | 0.3 | 17.5 | 30.6 | 0.5 | 0.01 | 0.42 | 0.06 | 0.02 | 0.08 |
| CM | 0.5 | 2.1 | 1.7 | 0.8 | 0.2 | 0.7 | 7.3 | 22.7 | 173 | 0.1 | 0.3 | 5.6 | 0.3 | 20.6 | 77.4 | 3.9 | 0.01 | 1.96 | 0.06 | 0.03 | 0.05 |
| FW | 1.0 | 1.8 | 1.6 | 0.3 | 0.6 | 4.0 | 2.8 | 11.9 | 272 | 0.1 | 0.1 | 3.5 | 0.2 | 9.6 | 126 | 0.7 | 0.01 | 1.51 | 0.45 | 0.05 | 0.01 |
| PM (p) | 4.0 | 17.2 | 7.6 | 3.5 | 3.0 | 1.1 | 37.0 | 221 | 400 | 0.7 | 0.4 | 38.8 | 3.1 | 356 | 286 | 4.2 | 0.10 | 2.02 | 0.28 | 0.18 | 0.09 |
| SM (p) | 0.6 | 0.6 | 0.9 | 0.4 | 0.2 | 0.2 | 25.3 | 113 | 59.6 | 0.1 | 0.0 | 4.2 | 0.3 | 18.7 | 26.9 | 0.5 | 0.01 | 0.50 | 0.06 | 0.02 | 0.04 |
| CM (p) | 0.4 | 1.5 | 1.4 | 0.6 | 0.2 | 0.5 | 4.8 | 19.0 | 141 | 0.1 | 0.2 | 4.4 | 0.3 | 17.2 | 64.9 | 2.9 | 0.01 | 2.61 | 0.06 | 0.02 | 0.02 |
| FW (p) | 1.0 | 1.9 | 1.7 | 0.3 | 0.6 | 4.0 | 2.4 | 16.3 | 294 | 0.1 | 0.1 | 4.0 | 0.2 | 10.6 | 123 | 0.6 | 0.01 | 1.25 | 0.47 | 0.05 | 0.13 |
| 2nd batch | |||||||||||||||||||||
| PM | 3.7 | 15.5 | 6.7 | 3.3 | 2.7 | 1.0 | 35.7 | 187 | 400 | 0.6 | 0.4 | 32.7 | 3.2 | 320 | 289 | 4.0 | 0.10 | 1.57 | 0.19 | 0.21 | 0.01 |
| SM | 3.1 | 0.9 | 5.0 | 1.7 | 0.9 | 0.4 | 101 | 467 | 438 | 0.2 | 0.1 | 6.2 | 1.3 | 104 | 102 | 1.7 | 0.03 | 1.28 | 0.23 | 0.08 | 0.10 |
| CM | 0.7 | 1.9 | 2.4 | 1.1 | 0.4 | 0.7 | 10.2 | 31.3 | 277 | 0.1 | 0.4 | 6.3 | 0.5 | 32.1 | 104 | 5.9 | 0.02 | 2.67 | 0.22 | 0.03 | 0.01 |
| FW | 0.9 | 1.7 | 1.4 | 0.3 | 0.5 | 3.7 | 3.9 | 9.4 | 305 | 0.1 | 0.1 | 2.9 | 0.2 | 9.4 | 131 | 0.7 | 0.02 | 1.38 | 0.50 | 0.05 | 0.01 |
| PM (p) | 3.7 | 16.2 | 7.9 | 3.5 | 2.8 | 1.1 | 37.1 | 193 | 412 | 0.6 | 0.4 | 33.7 | 3.2 | 355 | 315 | 4.0 | 0.10 | 1.64 | 0.20 | 0.17 | 0.01 |
| SM (p) | 0.2 | 0.5 | 0.3 | 0.1 | 0.1 | 0.2 | 9.7 | 37.6 | 18.8 | 0.0 | 0.0 | 2.4 | 0.1 | 5.3 | 7.9 | 0.2 | 0.00 | 0.32 | 0.02 | 0.01 | 0.01 |
| CM (p) | 0.4 | 1.5 | 1.3 | 0.6 | 0.3 | 0.5 | 4.8 | 13.1 | 125 | 0.1 | 0.2 | 4.7 | 0.3 | 16.4 | 51.1 | 3.0 | 0.01 | 1.33 | 0.04 | 0.02 | 0.01 |
| FW (p) | 0.8 | 1.4 | 1.2 | 0.2 | 0.5 | 4.1 | 2.4 | 7.4 | 412 | 0.0 | 0.1 | 3.0 | 0.2 | 9.0 | 116 | 0.7 | 0.01 | 1.30 | 0.43 | 0.05 | 0.01 |
| 3rd batch | |||||||||||||||||||||
| PM | 3.9 | 16.4 | 7.7 | 3.6 | 2.9 | 1.1 | 42.9 | 204 | 457 | 0.6 | 0.5 | 36.6 | 3.6 | 362 | 327 | 4.5 | 0.14 | 1.72 | 0.20 | 0.20 | 0.01 |
| SM | 0.3 | 0.4 | 0.4 | 0.2 | 0.1 | 0.2 | 11.7 | 47.7 | 28.8 | 0.0 | 0.0 | 2.3 | 0.2 | 8.7 | 9.9 | 0.3 | 0.00 | 0.17 | 0.02 | 0.01 | 0.03 |
| CM | 0.6 | 2.4 | 2.1 | 1.1 | 0.4 | 0.9 | 9.6 | 22.6 | 225 | 0.1 | 0.4 | 5.2 | 0.4 | 26.7 | 92.1 | 5.1 | 0.02 | 2.40 | 0.06 | 0.03 | 0.01 |
| FW | 0.9 | 1.7 | 1.5 | 0.3 | 0.5 | 3.8 | 4.7 | 10.3 | 307 | 0.1 | 0.1 | 2.5 | 0.2 | 9.9 | 118 | 0.7 | 0.01 | 1.66 | 0.47 | 0.05 | 0.01 |
| PM (p) | 3.9 | 16.2 | 7.2 | 3.4 | 2.8 | 1.1 | 36.8 | 195 | 417 | 0.6 | 0.4 | 33.1 | 3.2 | 346 | 297 | 4.1 | 0.10 | 1.88 | 0.21 | 0.17 | 0.01 |
| SM (p) | 0.4 | 0.8 | 0.6 | 0.3 | 0.2 | 0.3 | 20.2 | 83.9 | 47.4 | 0.1 | 0.0 | 3.2 | 0.3 | 14.5 | 16.9 | 0.5 | 0.01 | 0.22 | 0.04 | 0.02 | 0.01 |
| CM (p) | 0.3 | 1.9 | 0.9 | 0.6 | 0.1 | 0.7 | 3.6 | 9.1 | 86.1 | 0.0 | 0.2 | 3.6 | 0.2 | 11.4 | 34.1 | 2.1 | 0.01 | 0.93 | 0.02 | 0.02 | 0.01 |
| FW (p) | 0.7 | 1.2 | 1.0 | 0.2 | 0.5 | 3.5 | 2.0 | 6.2 | 396 | 0.0 | 0.1 | 2.6 | 0.2 | 7.6 | 315 | 0.8 | 0.01 | 1.18 | 0.35 | 0.04 | 0.01 |
| 4th batch | |||||||||||||||||||||
| PM | 4.0 | 16.9 | 7.7 | 3.6 | 3.0 | 1.1 | 40.2 | 214 | 441 | 0.6 | 0.5 | 38.4 | 3.5 | 356 | 327 | 4.5 | 0.12 | 2.22 | 0.23 | 0.21 | 0.02 |
| SM | 1.3 | 0.6 | 2.0 | 0.7 | 0.4 | 0.3 | 45.1 | 205 | 174 | 0.1 | 0.1 | 4.2 | 0.6 | 43.4 | 47.5 | 0.8 | 0.01 | 0.62 | 0.10 | 0.03 | 0.07 |
| CM | 0.6 | 2.1 | 2.1 | 1.0 | 0.3 | 0.8 | 9.0 | 25.6 | 225 | 0.1 | 0.4 | 5.7 | 0.4 | 26.5 | 91.2 | 5.0 | 0.02 | 2.34 | 0.11 | 0.03 | 0.02 |
| FW | 1.0 | 1.8 | 1.5 | 0.3 | 0.6 | 3.8 | 3.8 | 10.5 | 294 | 0.1 | 0.1 | 3.0 | 0.2 | 9.6 | 125 | 0.7 | 0.01 | 1.51 | 0.47 | 0.05 | 0.01 |
| PM (p) | 3.9 | 16.5 | 7.6 | 3.5 | 2.9 | 1.1 | 36.9 | 203 | 409 | 0.6 | 0.4 | 35.2 | 3.2 | 352 | 299 | 4.1 | 0.10 | 1.85 | 0.23 | 0.17 | 0.04 |
| SM (p) | 0.4 | 0.6 | 0.6 | 0.2 | 0.2 | 0.2 | 18.4 | 78.2 | 41.9 | 0.1 | 0.0 | 3.2 | 0.3 | 12.8 | 17.2 | 0.4 | 0.01 | 0.35 | 0.04 | 0.02 | 0.02 |
| CM (p) | 0.4 | 1.6 | 1.2 | 0.6 | 0.2 | 0.6 | 4.4 | 13.7 | 118 | 0.1 | 0.2 | 4.2 | 0.3 | 15.0 | 50.1 | 2.7 | 0.01 | 1.62 | 0.04 | 0.02 | 0.01 |
| FW (p) | 0.9 | 1.5 | 1.3 | 0.2 | 0.5 | 3.9 | 2.3 | 10.0 | 367 | 0.1 | 0.1 | 3.2 | 0.2 | 9.0 | 184 | 0.7 | 0.01 | 1.24 | 0.42 | 0.05 | 0.05 |
| 1st Batch | 1st Batch after AD | |||||
|---|---|---|---|---|---|---|
| Raw Material | Salmonella in 25 g | E. coli cfu/g | Enterococcus faecalis cfu/g | Salmonella in 25 g | E. coli cfu/g | Enterococcus faecalis cfu/g |
| PM | ND ** | <9.1 | 2.3 × 103 | ND ** | <9.1 | 6.2 × 103 |
| SM | ND ** | 2.5 × 102 | 8.4 × 103 | ND ** | <9.1 | <9.1 |
| CM | ND ** | <9.1 | 1.1 × 103 | ND ** | <9.1 | <9.1 |
| FW | ND ** | <9.1 | 1.3 × 104 | ND ** | <9.1 | 1.0 × 103 |
| PM (p) | ND ** | <9.1 | 2.1 × 102 | ND ** | <9.1 | <9.1 |
| SM (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | <9.1 |
| CM (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | 64 est |
| FW (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | 1.1 × 102 |
| 2nd batch | 2nd batch after AD | |||||
| Raw material | Salmonella in 25 g | E. coli cfu/g | Enterococcus faecalis cfu/g | Salmonella in 25 g | E. coli cfu/g | Enterococcus faecalis cfu/g |
| PM | ND ** | <9.1 | 2.2 × 103 | ND ** | <9.1 | 6.2 × 103 |
| SM | ND ** | 2.5 × 102 | 8.6 × 103 | ND ** | <9.1 | <9.1 |
| CM | D * | <9.1 | 1.0 × 103 | ND ** | <9.1 | <9.1 |
| FW | ND ** | <9.1 | 1.2 × 104 | ND ** | <9.1 | 1.0 × 103 |
| PM (p) | ND ** | <9.1 | 2.0 × 102 | ND ** | <9.1 | <9.1 |
| SM (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | <9.1 |
| CM (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | 64 est |
| FW (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | 1.1 × 102 |
| 3rd batch | 3rd batch after AD | |||||
| Raw material | Salmonella in 25 g | E. coli cfu/g | Enterococcus faecalis cfu/g | Salmonella in 25 g | E. coli cfu/g | Enterococcus faecalis cfu/g |
| PM | ND ** | 45 est | 2.2 × 103 | ND ** | <9.1 | <9.1 |
| SM | ND ** | 1.1 × 103 | 7.3 × 103 | ND ** | <9.1 | <9.1 |
| CM | ND ** | <40 | 5.6 × 103 | ND ** | <9.1 | <9.1 |
| FW | ND ** | <9.1 | 7.2 × 103 | ND ** | <9.1 | <9.1 |
| PM (p) | ND ** | <9.1 | 1.2 × 103 | ND ** | <9.1 | <9.1 |
| SM (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | <9.1 |
| CM (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | <9.1 |
| FW (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | <40 |
| 4th batch | 4th batch after AD | |||||
| Raw material | Salmonella in 25 g | E. coli cfu/g | Enterococcus faecalis cfu/g | Salmonella in 25 g | E. coli cfu/g | Enterococcus faecalis cfu/g |
| PM | ND ** | 1.1 × 103 | 7.3 × 103 | ND ** | <9.1 | <9.1 |
| SM | ND ** | <40 | 5.6 × 103 | ND ** | <9.1 | <9.1 |
| CM | ND ** | <9.1 | 7.2 × 103 | ND ** | <9.1 | <9.1 |
| FW | ND ** | <9.1 | 1.2 × 103 | ND ** | <9.1 | <9.1 |
| PM (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | <9.1 |
| SM (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | <9.1 |
| CM (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | <9.1 |
| FW (p) | ND ** | <9.1 | <9.1 | ND ** | <9.1 | <40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michailidou, I.; Grigoriadou, I.; Sfetsas, T.; Vlachokostas, C.; Arsenos, G.; Lymperopoulos, A. Enhancing Biogas Production: An Assessment of Pasteurization Effects on Poultry, Swine, Bovine Manure and Food Waste Substrates. Sustainability 2024, 16, 7130. https://doi.org/10.3390/su16167130
Michailidou I, Grigoriadou I, Sfetsas T, Vlachokostas C, Arsenos G, Lymperopoulos A. Enhancing Biogas Production: An Assessment of Pasteurization Effects on Poultry, Swine, Bovine Manure and Food Waste Substrates. Sustainability. 2024; 16(16):7130. https://doi.org/10.3390/su16167130
Chicago/Turabian StyleMichailidou, Ioanna, Ifigeneia Grigoriadou, Themistoklis Sfetsas, Christos Vlachokostas, Georgios Arsenos, and Aristotelis Lymperopoulos. 2024. "Enhancing Biogas Production: An Assessment of Pasteurization Effects on Poultry, Swine, Bovine Manure and Food Waste Substrates" Sustainability 16, no. 16: 7130. https://doi.org/10.3390/su16167130
APA StyleMichailidou, I., Grigoriadou, I., Sfetsas, T., Vlachokostas, C., Arsenos, G., & Lymperopoulos, A. (2024). Enhancing Biogas Production: An Assessment of Pasteurization Effects on Poultry, Swine, Bovine Manure and Food Waste Substrates. Sustainability, 16(16), 7130. https://doi.org/10.3390/su16167130









