Continuous UV/H2O2 Process: A Sustainable Wastewater Treatment Approach for Enhancing the Biodegradability of Aqueous PVA
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Setup and Procedure
2.3. Analytical Methods
2.3.1. UV Lamp Irradiance Measurement
2.3.2. Respirometry
2.3.3. COD Measurement
2.4. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Biodegradability Evaluation of Untreated Aqueous PVA Solutions
3.2. Data-Driven Prediction Model and Analysis of the Variance (ANOVA)
3.3. Overall Process Response Behaviour
3.4. Effect of Individual Variables on the Effluent /COD
3.4.1. Effect of PVA Feed Concentration
3.4.2. Effect of Inlet Concentration
3.4.3. Effect of PVA Feed Flow Rate
3.4.4. Importance of UV Fluence over Reaction Time
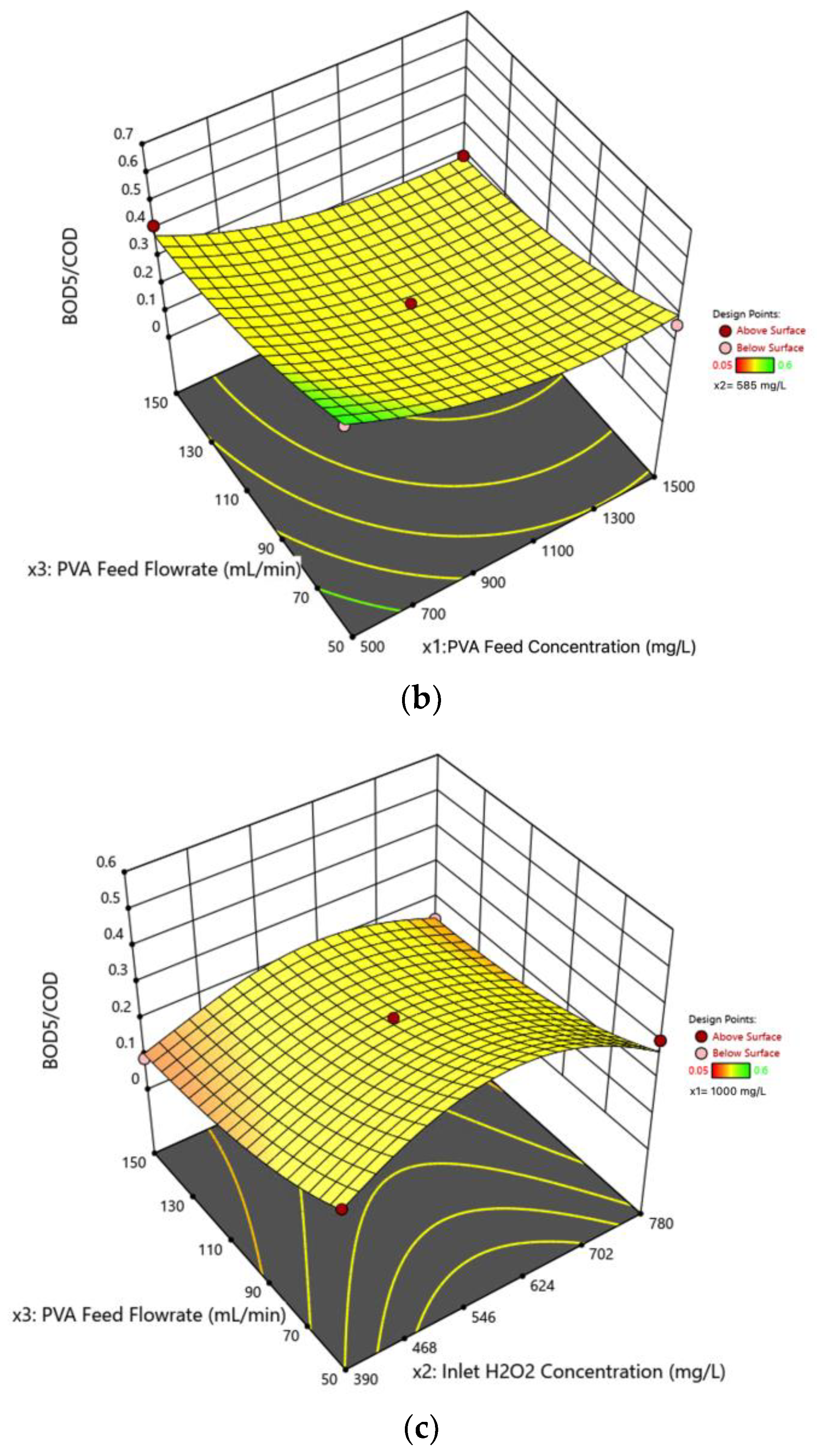
3.5. Interaction Effects of Process Variables on the Effluent /COD
3.6. Optimal Operating Conditions, Experimental Validation, and Comparision with Previous Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdullah, Z.W.; Dong, Y.; Davies, I.J.; Barbhuiya, S. PVA, PVA Blends, and Their Nanocomposites for Biodegradable Packaging Application. Polym-Plast. Technol. Eng. 2017, 56, 1307–1344. [Google Scholar] [CrossRef]
- Hoffmann, J.; Řezníčková, I.; Kozáková, J.; Růžička, J.; Alexy, P.; Bakoš, D.; Precnerová, L. Assessing Biodegradability of Plastics Based on Poly(Vinyl Alcohol) and Protein Wastes. Polym. Degrad. Stab. 2003, 79, 511–519. [Google Scholar] [CrossRef]
- Patil, R.; Bagde, U.S. Development of Novel Bacterial Strains for Enhanced of Plastic Polymers by Protoplast. Asian J. Microbiol. Biotechnol. Environ. Sci. 2016, 18, 239–249. [Google Scholar]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological Degradation of Plastics: A Comprehensive Review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Lim, B.K.H.; Thian, E.S. Biodegradation of Polymers in Managing Plastic Waste—A Review. Sci. Total Environ. 2022, 813, 151880. [Google Scholar] [CrossRef]
- Ghatge, S.; Yang, Y.; Ahn, J.H.; Hur, H.G. Biodegradation of Polyethylene: A Brief Review. Appl. Biol. Chem. 2020, 63, 27. [Google Scholar] [CrossRef]
- Matjašič, T.; Simčič, T.; Medvešček, N.; Bajt, O.; Dreo, T.; Mori, N. Critical Evaluation of Biodegradation Studies on Synthetic Plastics through a Systematic Literature Review. Sci. Total Environ. 2021, 752, 141959. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Y.; Deng, Q.; Liu, X. Water-Soluble Synthetic Polymers: Their Environmental Emission Relevant Usage, Transport and Transformation, Persistence, and Toxicity. Environ. Sci. Technol. 2023, 57, 6387–6402. [Google Scholar] [CrossRef] [PubMed]
- Tchobanoglous, G.; Stensel, H.D.; Tsuchihashi, R.; Burton, F.; Abu-Orf, M.; Bowden, G.; Pfrang, W.; Metcalf & Eddy, Inc. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Al-Sulaiman, A.M.; Khudair, B.H. Correlation Between BOD5 And Cod for Al-Diwaniyah Wastewater Treatment Plants to Obtain the Biodegradability Indices. J. Biotechnol. 2018, 15, 423–427. [Google Scholar]
- Malik, S.N.; Khan, S.M.; Ghosh, P.C.; Vaidya, A.N.; Kanade, G.; Mudliar, S.N. Treatment of Pharmaceutical Industrial Wastewater by Nano-Catalyzed Ozonation in a Semi-Batch Reactor for Improved Biodegradability. Sci. Total Environ. 2019, 678, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Padoley, K.V.; Saharan, V.K.; Mudliar, S.N.; Pandey, R.A.; Pandit, A.B. Cavitationally Induced Biodegradability Enhancement of a Distillery Wastewater. J. Hazard. Mater. 2012, 219–220, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ning, D.; Yang, Y.; Van Nostrand, J.D.; Zhou, J.; Wen, X. Biodegradability of Wastewater Determines Microbial Assembly Mechanisms in Full-Scale Wastewater Treatment Plants. Water Res. 2020, 169, 115276. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chen, L.; Zhang, Y.; Wang, J. Synergistic Effect of Ozonation and Ionizing Radiation for PVA Decomposition. J. Environ. Sci. 2015, 34, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Cao, M.; Wen, H.; Tan, Z.; Jia, S.; Cui, J. Biodegradation of Polyvinyl Alcohol Using Cross-Linked Enzyme Aggregates of Degrading Enzymes from Bacillus Niacini. Int. J. Biol. Macromol. 2019, 124, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Bian, L.; Wang, P. Accelerated Degradation of Polyvinyl Alcohol via a Novel and Cost Effective Heterogeneous System Based on Na2S2O8 Activated by Fe Complex Functionalized Waste PAN Fiber and Visible LED Irradiation. Chem. Eng. J. 2019, 358, 1489–1498. [Google Scholar] [CrossRef]
- Zeeshan, M.H.; Ruman, U.E.; He, G.; Sabir, A.; Shafiq, M.; Zubair, M. Environmental Issues Concerned with Poly (Vinyl Alcohol) (PVA) in Textile Wastewater. In Polymer Technology in Dye-Containing Wastewater. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Khadir, A., Muthu, S.S., Eds.; Springer: Singapore, 2022; pp. 225–236. [Google Scholar] [CrossRef]
- Obaideen, K.; Shehata, N.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Olabi, A.G. The Role of Wastewater Treatment in Achieving Sustainable Development Goals (SDGs) and Sustainability Guideline. Energy Nexus 2022, 7, 100112. [Google Scholar] [CrossRef]
- Demerlis, C.C.; Schoneker, D.R. Review of the Oral Toxicity of Polyvinyl Alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Julinová, M.; Vaňharová, L.; Jurča, M. Water-Soluble Polymeric Xenobiotics–Polyvinyl Alcohol and Polyvinylpyrrolidon–And Potential Solutions to Environmental Issues: A Brief Review. J. Environ. Manag. 2018, 228, 213–222. [Google Scholar] [CrossRef]
- Nigro, L.; Magni, S.; Ortenzi, M.A.; Gazzotti, S.; Della Torre, C.; Binelli, A. Are “Liquid Plastics” a New Environmental Threat? The Case of Polyvinyl Alcohol. Aquat. Toxicol. 2022, 248, 106200. [Google Scholar] [CrossRef]
- Samal, K.; Maiti, K.; Mohanty, K.; Das, C. Ultrafiltration of Aqueous PVA Using Spinning Basket Membrane Module. Water Air Soil Pollut. 2018, 229, 96. [Google Scholar] [CrossRef]
- Manna, M.; Sen, S. Advanced Oxidation Process: A Sustainable Technology for Treating Refractory Organic Compounds Present in Industrial Wastewater. Environ. Sci. Pollut. Res. 2023, 30, 25477–25505. [Google Scholar] [CrossRef]
- Bossmann, S.H.; Oliveros, E.; Göb, S.; Kantor, M.; Göppert, A.; Lei, L.; Yue, P.L.; Braun, A.M. Degradation of Polyvinyl Alcohol (PVA) by Homogeneous and Heterogeneous Photocatalysis Applied to the Photochemically Enhanced Fenton Reaction. Water Sci. Technol. 2001, 44, 257–262. [Google Scholar] [CrossRef]
- Bossmann, S.H.; Oliveros, E.; Göb, S.; Kantor, M.; Göppert, A.; Braun, A.M.; Lei, L.; Lock Yue, P. Oxidative Degradation of Polyvinyl Alcohol by the Photochemically Enhanced Fenton Reaction. Evidences for the Formation of Super-Macromolecules. Prog. React. Kinet. Mech. 2001, 26, 113–137. [Google Scholar] [CrossRef]
- Lin, C.C.; Hsu, S.T. Performance of NZVI/H2O2 Process in Degrading Polyvinyl Alcohol in Aqueous Solutions. Sep. Purif. Technol. 2018, 203, 111–116. [Google Scholar] [CrossRef]
- Bae, W.; Won, H.; Hwang, B.; de Toledo, R.A.; Chung, J.; Kwon, K.; Shim, H. Characterization of Refractory Matters in Dyeing Wastewater during a Full-Scale Fenton Process Following Pure-Oxygen Activated Sludge Treatment. J. Hazard. Mater. 2015, 287, 421–428. [Google Scholar] [CrossRef]
- Giroto, J.A.; Guardani, R.; Teixeira, A.C.S.C.; Nascimento, C.A.O. Study on the Photo-Fenton Degradation of Polyvinyl Alcohol in Aqueous Solution. Chem. Eng. Process. Process Intensif. 2006, 45, 523–532. [Google Scholar] [CrossRef]
- Cataldo, F.; Angelini, G. Some Aspects of the Ozone Degradation of Poly(Vinyl Alcohol). Polym. Degrad. Stab. 2006, 91, 2793–2800. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, J.; Hua, X.; Liang, D.; Dong, D.; Guo, Z.; Zheng, N.; Zhang, L. Catalytic Ozonation for the Degradation of Polyvinyl Alcohol in Aqueous Solution Using Catalyst Based on Copper and Manganese. J. Clean. Prod. 2020, 272, 122856. [Google Scholar] [CrossRef]
- Sun, W.; Chen, L.; Wang, J. Degradation of PVA (Polyvinyl Alcohol) in Wastewater by Advanced Oxidation Processes. J. Adv. Oxid. Technol. 2017, 20, 20170018. [Google Scholar] [CrossRef]
- Hamad, D.; Mehrvar, M.; Dhib, R. Photochemical Kinetic Modeling of Degradation of Aqueous Polyvinyl Alcohol in a UV/H2O2 Photoreactor. J. Polym. Environ. 2018, 26, 3283–3293. [Google Scholar] [CrossRef]
- Lin, C.C.; Lee, L.T. Degradation of Polyvinyl Alcohol in Aqueous Solutions Using UV/Oxidant Process. J. Ind. Eng. Chem. 2015, 21, 569–574. [Google Scholar] [CrossRef]
- Hamad, D.; Dhib, R.; Mehrvar, M. Photochemical Degradation of Aqueous Polyvinyl Alcohol in a Continuous UV/H2O2 Process: Experimental and Statistical Analysis. J. Polym. Environ. 2016, 24, 72–83. [Google Scholar] [CrossRef]
- Lin, C.C.; Lee, L.T.; Hsu, L.J. Degradation of Polyvinyl Alcohol in Aqueous Solutions Using UV-365 Nm/S2O82− Process. Environ. Sci. Technol. 2014, 11, 831–838. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Z.; Yang, Y.; Ke, Q. Heterogeneous Photocatalytic Oxidation of Polyvinyl Alcohol in Water. J. Photochem. Photobiol. A Chem. 2001, 142, 85–89. [Google Scholar] [CrossRef]
- Hsu, L.J.; Lee, L.T.; Lin, C.C. Adsorption and Photocatalytic Degradation of Polyvinyl Alcohol in Aqueous Solutions Using P-25 TiO2. Chem. Eng. J. 2011, 173, 698–705. [Google Scholar] [CrossRef]
- Won, Y.S.; Back, S.O.; Tavakoli, J. Wet Oxidation of Aqueous Polyvinyl Alcohol Solution. Ind. Eng. Chem. Res. 2001, 40, 60–66. [Google Scholar] [CrossRef]
- Chen, G.; Lei, L.; Yue, P.L.; Cen, P. Treatment of Desizing Wastewater Containing Poly(Vinyl Alcohol) by Wet Air Oxidation. Ind. Eng. Chem. Res. 2000, 39, 1193–1197. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.-H.; Park, C.; Shin, E.-B. Electrochemical Oxidation of Polyvinyl Alcohol Using a RuO2/Ti Anode. Desalination 2003, 155, 49–57. [Google Scholar] [CrossRef]
- Huang, K.Y.; Wang, C.T.; Chou, W.L.; Shu, C.M. Removal of Polyvinyl Alcohol in Aqueous Solutions Using an Innovative Paired Photoelectrochemical Oxidative System in a Divided Electrochemical Cell. Int. J. Photoenergy 2015, 2015, 623492. [Google Scholar] [CrossRef]
- Deogaonkar, S.C.; Wakode, P.; Rawat, K.P. Electron Beam Irradiation Post Treatment for Degradation of Non Biodegradable Contaminants in Textile Wastewater. Radiat. Phys. Chem. 2019, 165, 108377. [Google Scholar] [CrossRef]
- Zhang, S.J.; Yu, H.Q.; Ge, X.W.; Zhu, R.F. Optimization of Radiolytic Degradation of Poly(Vinyl Alcohol). Ind. Eng. Chem. Res. 2005, 44, 1995–2001. [Google Scholar] [CrossRef]
- Sun, W.; Tian, J.; Chen, L.; He, S.; Wang, J. Improvement of Biodegradability of PVA-Containing Wastewater by Ionizing Radiation Pre-treatment. Environ. Sci. Pollut. Res. 2012, 19, 3178–3184. [Google Scholar] [CrossRef]
- Asheghmoalla, M.; Mehrvar, M. Integrated and Hybrid Processes for the Treatment of Actual Wastewaters Containing Micropollutants: A Review on Recent Advances. Processes 2024, 12, 339. [Google Scholar] [CrossRef]
- Dhanke, P.; Wagh, S. Treatment of Vegetable Oil Refinery Wastewater with Biodegradability Index Improvement. Mater. Today Proc. 2020, 27, 181–187. [Google Scholar] [CrossRef]
- Ganzenko, O.; Trellu, C.; Oturan, N.; Huguenot, D.; Péchaud, Y.; van Hullebusch, E.D.; Oturan, M.A. Electro-Fenton Treatment of a Complex Pharmaceutical Mixture: Mineralization Efficiency and Biodegradability Enhancement. Chemosphere 2020, 253, 126659. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.T.; Schaar, H.; Saracevic, E.; Krampe, J.; Kreuzinger, N. Effect of Ozonation on the Biodegradability of Urban Wastewater Treatment Plant Effluent. Sci. Total Environ. 2022, 812, 152466. [Google Scholar] [CrossRef]
- Gharibian, S.; Hazrati, H. Towards Practical Integration of MBR with Electrochemical AOP: Improved Biodegradability of Real Pharmaceutical Wastewater and Fouling Mitigation. Water Res. 2022, 218, 118478. [Google Scholar] [CrossRef]
- Ledezma Estrada, A.; Li, Y.Y.; Wang, A. Biodegradability Enhancement of Wastewater Containing Cefalexin by Means of the Electro-Fenton Oxidation Process. J. Hazard. Mater. 2012, 227–228, 41–48. [Google Scholar] [CrossRef]
- Ulucan-Altuntas, K.; Ilhan, F. Enhancing Biodegradability of Textile Wastewater by Ozonation Processes: Optimization with Response Surface Methodology. Ozone Sci. Eng. 2018, 40, 465–472. [Google Scholar] [CrossRef]
- Martins, R.C.; Rossi, A.F.; Quinta-Ferreira, R.M. Fenton’s Oxidation Process for Phenolic Wastewater Remediation and Biodegradability Enhancement. J. Hazard. Mater. 2010, 180, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Yamatsu, A.; Matsumi, R.; Atomi, H.; Imanaka, T. Isolation and Characterization of a Novel Poly(Vinyl Alcohol)-Degrading Bacterium, Sphingopyxis sp. PVA3. Appl. Microbiol. Biotechnol. 2006, 72, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Kim, S.; Choi, K.; Kim, J.O. Degradation of Polyvinyl Alcohol in Textile Waste Water by Microbacterium barkeri KCCM 10507 and Paenibacillus amylolyticus KCCM 10508. Environ. Technol. 2016, 37, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Měrková, M.; Julinová, M.; Houser, J.; Růžička, J. An Effect of Salt Concentration and Inoculum Size on Poly(Vinyl Alcohol) Utilization by Two Sphingomonas Strains. J. Polym. Environ. 2018, 26, 2227–2233. [Google Scholar] [CrossRef]
- Wei, Y.; Fu, J.; Wu, J.; Jia, X.; Zhou, Y.; Li, C.; Dong, M.; Wang, S.; Zhang, J.; Chen, F. Bioinformatics Analysis and Characterization of Highly Efficient Polyvinyl Alcohol (PVA)- Degrading Enzymes from the Novel PVA Degrader Stenotrophomonas rhizophila QL-P4. Appl. Environ. Microbiol. 2017, 84, e01898-17. [Google Scholar] [CrossRef] [PubMed]
- Aldaher, M.; Gengec, E. A Comparative Study among the Technology Classic Fenton and Electro-Fenton for the Degradation of Polyvinyl Alcohol in Textile Industrial Wastewater. J. Water Chem. Technol. 2023, 45, 446–454. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; American Water Works Association: Washington, DC, USA, 2022. [Google Scholar]
- Young, J.C.; Cowan, R.M. Respirometry for Environmental Science and Engineering; SJ Enterprises: Springdale, AR, USA, 2004. [Google Scholar]
- Bioscience Inc. BI-2000 Electrolytic Respirometer User’s Reference Manual and Troubleshooting Guide; Bioscience Inc.: Allentown, PA, USA, 2018. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C.; Runner, G.C. Applied Statistics and Probability for Engineering, 6th ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Hoang, N.T.; Mwazighe, F.M. The Various Effects of Hydrogen Phosphate and Bicarbonate in the Degradation of Some Pollutants in the UV/Chlorine and the UV/H2O2 Processes. J. Water Proc. Eng. 2024, 57, 104646. [Google Scholar] [CrossRef]
- La Manna, P.; De Carluccio, M.; Iannece, P.; Vigliotta, G.; Proto, A.; Rizzo, L. Chelating Agents Supported Solar Photo-Fenton and Sunlight/H2O2 Processes for Pharmaceuticals Removal and Resistant Pathogens Inactivation in Quaternary Treatment for Urban Wastewater Reuse. J. Hazard. Mater. 2023, 452, 131235. [Google Scholar] [CrossRef]
- Mulai, T.; Kumar, J.E.; Kharmawphlang, W.; Sahoo, M.K. Exploring a Substitute for Hydrogen Peroxide in Fenton Process—A Case Study on the COD Removal of Acid Orange 8. Acta Chim. Slov. 2024, 71, 99–109. [Google Scholar] [CrossRef]
- Banayan Esfahani, E.; Mohseni, M. Fluence-Based Photo-Reductive Decomposition of PFAS Using Vacuum UV (VUV) Irradiation: Effects of Key Parameters and Decomposition Mechanism. J. Environ. Chem. Eng. 2022, 10, 107050. [Google Scholar] [CrossRef]
- Oppenländer, T. Photochemical Purification of Water and Air: Advanced Oxidation Processes (AOPs), 1st ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2002. [Google Scholar]
- Elyasi, S.; Taghipour, F. Simulation of UV Photoreactor for Degradation of Chemical Contaminants: Model Development and Evaluation. Environ. Sci. Technol. 2010, 44, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.R.; Cotton, C.A. The Ultraviolet Disinfection Handbook; American Water Works Association: Denver, CO, USA, 2008; ISBN 1583215840. [Google Scholar]
- ISO 15522; Water Quality—Determination of the Inhibitory E Ect of Water Constituents on the Growth of Activated Sludge Microorganisms. International Organisation for Standardization: Geneva, Switzerland, 1999.
- ISO 11348; Water Quality—Determination of the Inhibitory E Ect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test); Part 1: Method Using Freshly Prepared Bacteria; Part 2: Method Using Liquid-Dried Bacteria; Part 3: Method Using Freeze-Dried Bacteria. International Organisation for Standardization: Geneva, Switzerland, 2007.
- OECD. Test No. 209: Activated Sludge, Respiration Inhibition Test (Carbon and Ammonium Oxidation), OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2010; ISBN 9789264070080. [Google Scholar]
- Strotmann, U.; Flores, D.P.; Konrad, O.; Gendig, C. Bacterial Toxicity Testing: Modification and Evaluation of the Luminescent Bacteria Test and the Respiration Inhibition Test. Processes 2020, 8, 1349. [Google Scholar] [CrossRef]
- Strotmann, U.; Thouand, G.; Pagga, U.; Gartiser, S.; Heipieper, H.J. Toward the Future of OECD/ISO Biodegradability Testing-New Approaches and Developments. Appl. Microbiol. Biotechnol. 2023, 107, 2073–2095. [Google Scholar] [CrossRef] [PubMed]
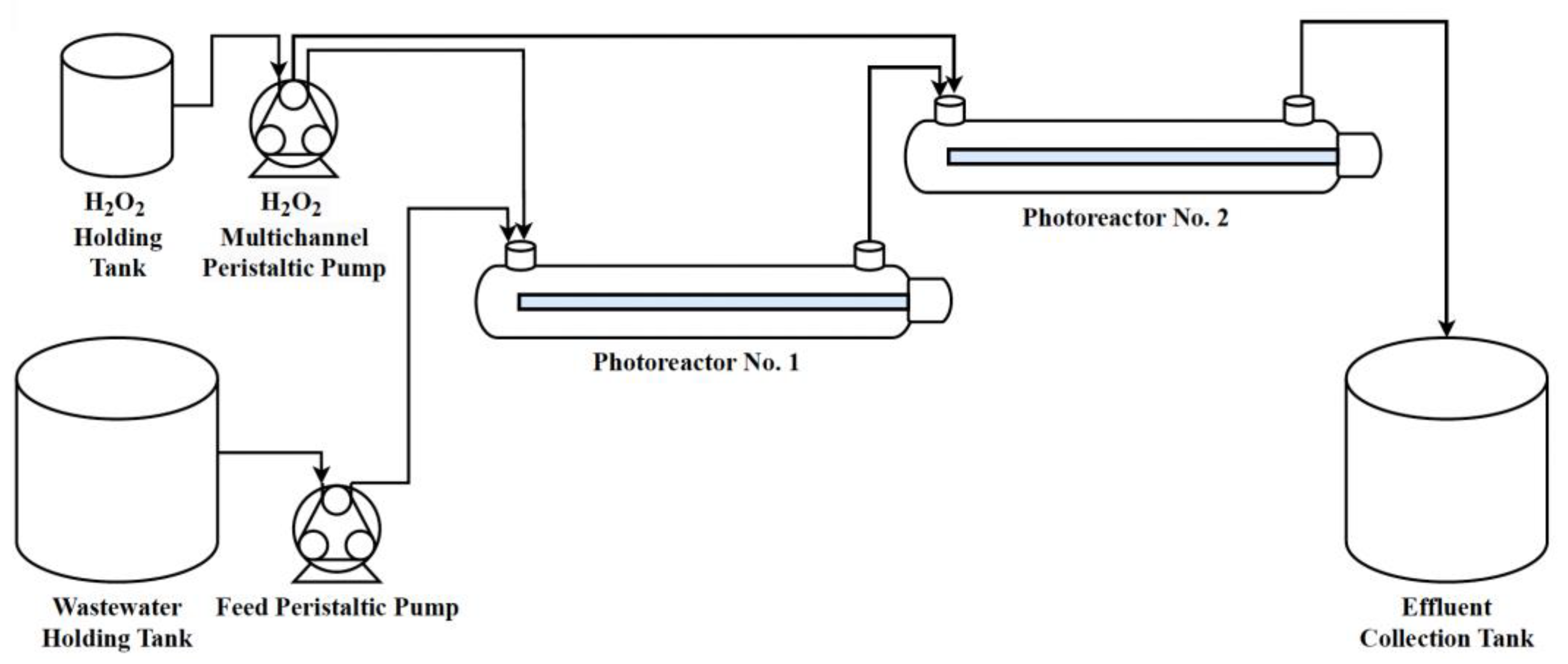
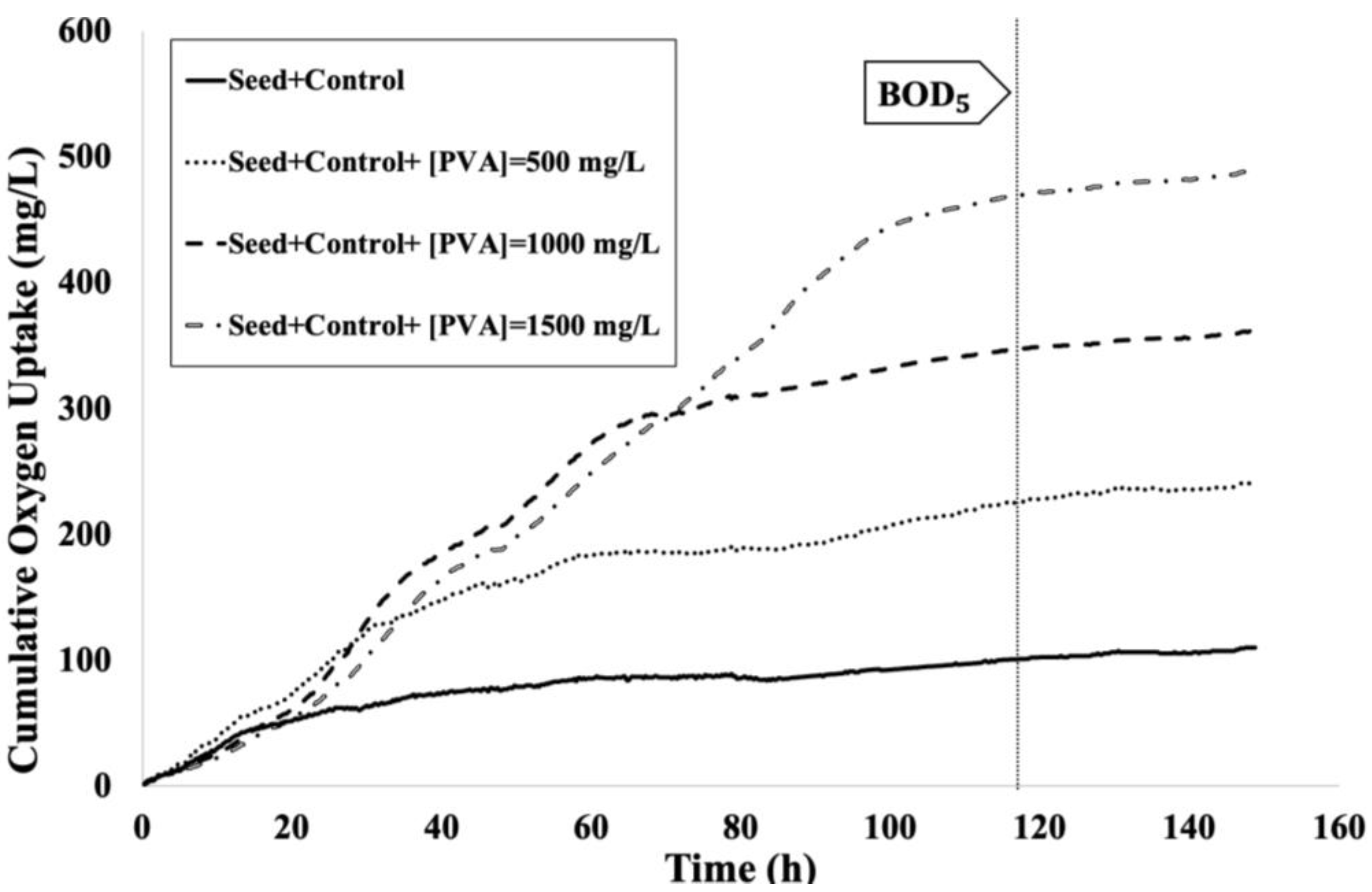
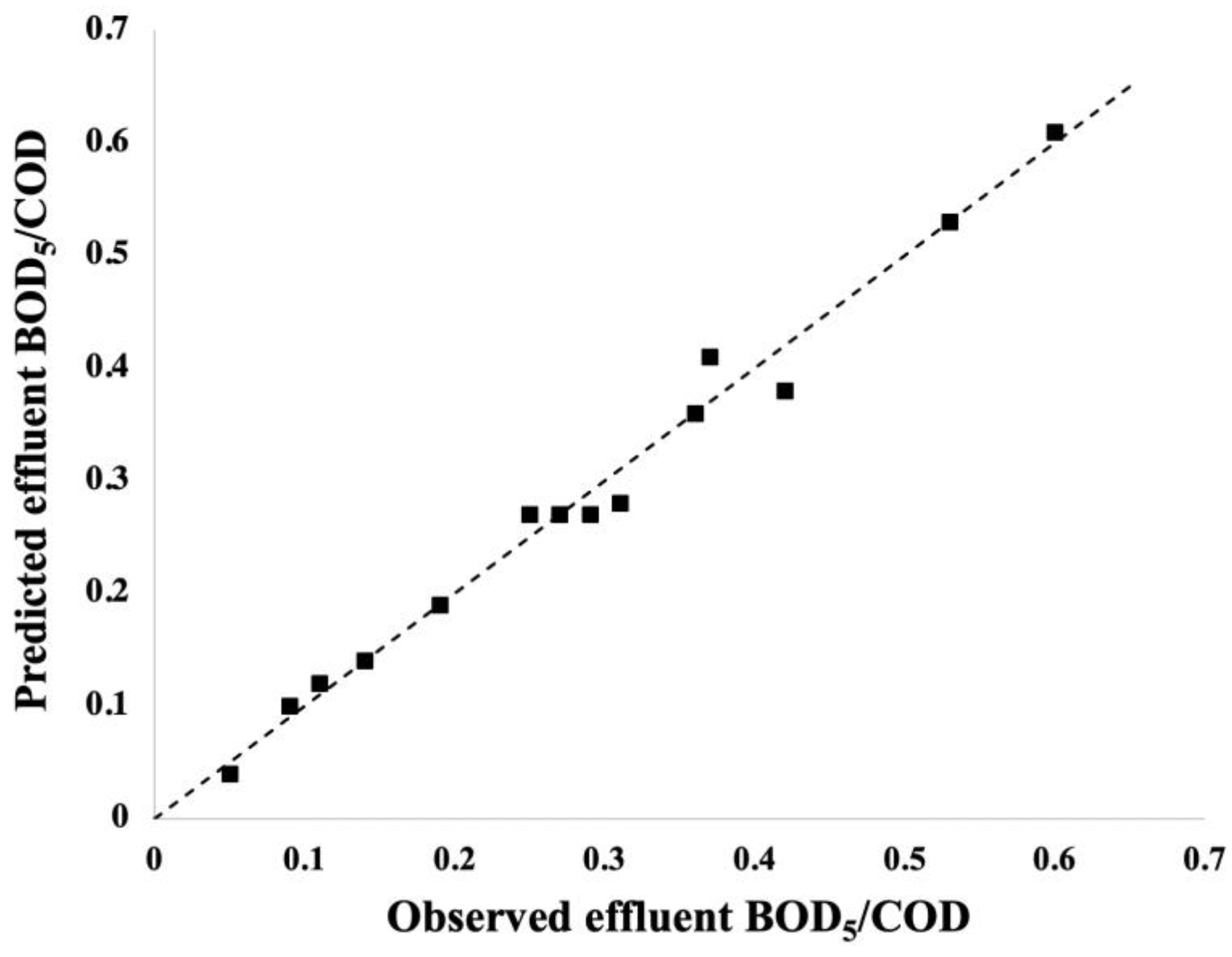

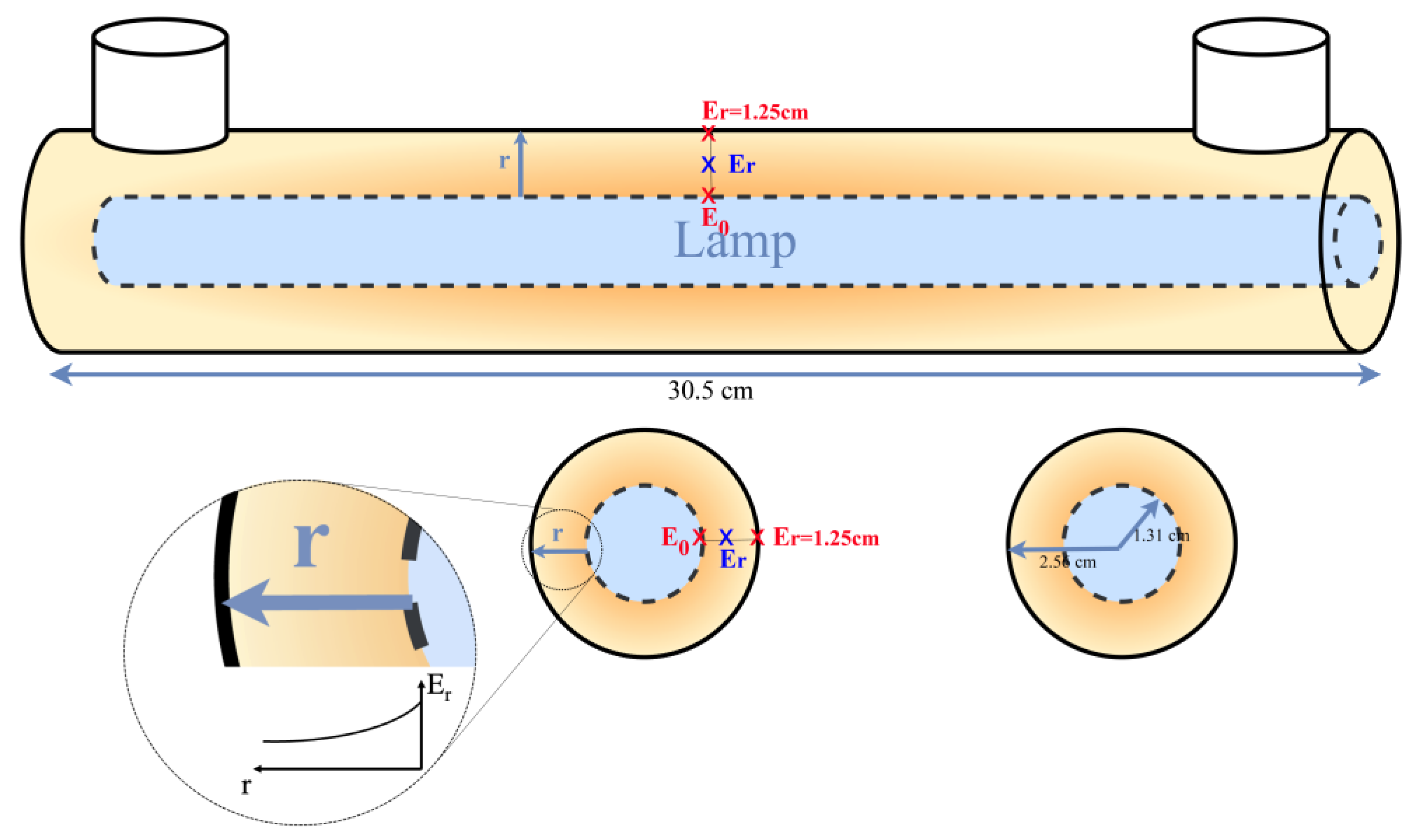
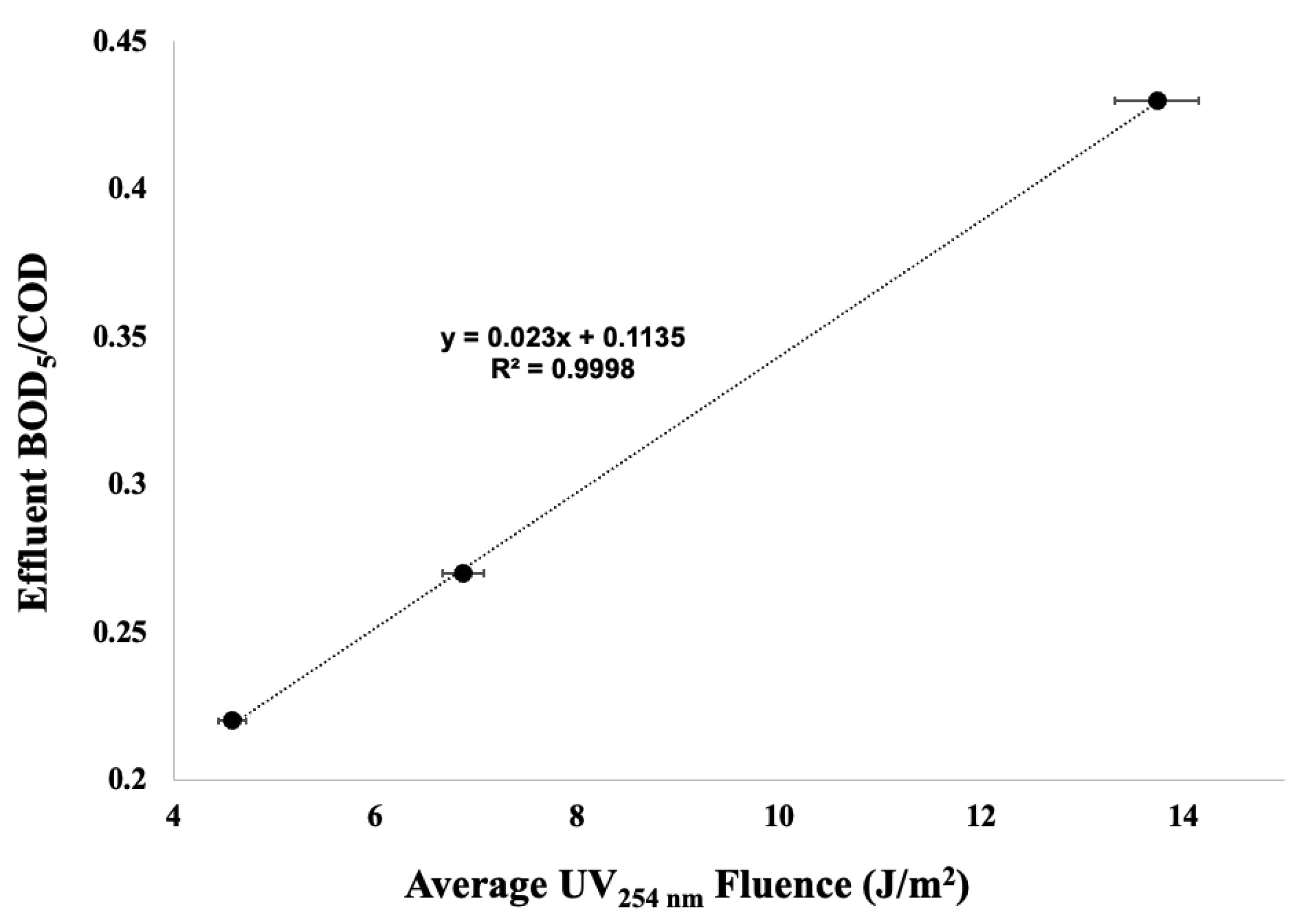

| Trial | PVA Concentration (mg/L) | COD (mg/L) | (mg/L) | /COD |
|---|---|---|---|---|
| 1 | 500 | 850 | 125.81 | 0.15 |
| 2 | 1000 | 1570 | 247.15 | 0.16 |
| 3 | 1500 | 2443 | 370.1 | 0.15 |
| Source | SS a | df b | MS c | F-Value | p-Value | Note |
|---|---|---|---|---|---|---|
| Model | 11.45 | 9 | 1.272 | 42.74 | 0.0003 | Significant |
| 1.633 | 1 | 1.633 | 54.85 | 0.0007 | Significant | |
| 9.053 × | 1 | 9.053 × | 3.042 | 0.1416 | ||
| 1.980 | 1 | 1.980 | 66.53 | 0.0005 | Significant | |
| 5.244 | 1 | 5.244 | 176.2 | <0.0001 | Significant | |
| 8.983 × | 1 | 8.983 × | 0.3018 | 0.6064 | ||
| 2.308 × | 1 | 2.308 × | 0.7754 | 0.4189 | ||
| 0.3659 | 1 | 0.3659 | 12.29 | 0.0172 | Significant | |
| 1.764 | 1 | 1.764 | 59.27 | 0.0006 | Significant | |
| 0.1575 | 1 | 0.1575 | 5.290 | 0.0698 | ||
| Residual | 0.1488 | 5 | 2.976 × | |||
| Lack of fit | 0.1282 | 3 | 4.272 × | 4.137 | 0.2008 | Not significant |
| Pure error | 2.065 × | 2 | 1.033 × | |||
| Cor total d | 11.60 | 14 | ||||
| R2 = 0.9872 | ||||||
| Adjusted R2 = 0.9641 | ||||||
| Predicted R2 = 0.8192 | ||||||
| Adequate precision = 24.87 | ||||||
| Reference | Optimization Objective | PVA Feed Concentration (mg/L) | Concentration (mg/L) | PVA Feed Flow Rate (mL/min) | Response Result |
|---|---|---|---|---|---|
| This study | /COD = 0.5 | 665 | 390 | 59 | 5% of the prediction |
| [35] | Maximizing TOC removal % | 665 | 460 | 50 | 5% of the prediction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parsa, Z.; Dhib, R.; Mehrvar, M. Continuous UV/H2O2 Process: A Sustainable Wastewater Treatment Approach for Enhancing the Biodegradability of Aqueous PVA. Sustainability 2024, 16, 7060. https://doi.org/10.3390/su16167060
Parsa Z, Dhib R, Mehrvar M. Continuous UV/H2O2 Process: A Sustainable Wastewater Treatment Approach for Enhancing the Biodegradability of Aqueous PVA. Sustainability. 2024; 16(16):7060. https://doi.org/10.3390/su16167060
Chicago/Turabian StyleParsa, Zahra, Ramdhane Dhib, and Mehrab Mehrvar. 2024. "Continuous UV/H2O2 Process: A Sustainable Wastewater Treatment Approach for Enhancing the Biodegradability of Aqueous PVA" Sustainability 16, no. 16: 7060. https://doi.org/10.3390/su16167060
APA StyleParsa, Z., Dhib, R., & Mehrvar, M. (2024). Continuous UV/H2O2 Process: A Sustainable Wastewater Treatment Approach for Enhancing the Biodegradability of Aqueous PVA. Sustainability, 16(16), 7060. https://doi.org/10.3390/su16167060









